Lung Cancer Clinical Data Standards
|
|
|
- Jeffry Robbins
- 5 years ago
- Views:
Transcription
1 For reference only Do Not Use For more information, contact; Lung Cancer Clinical Data Standards June 2008 National Clinical Dataset Development Programme (NCDDP) Support Team Information Services Area 74A Gyle Square 1 South Gyle Crescent Edinburgh EH12 9EB Tel: to: NCDDPsupportteam@isd.csa.scot.nhs.uk Website:
2 Section 1 - Overview & Background... 4 Overview... 4 Lung Cancer Data Standards... 5 Background to NCDDP... 5 Clinical Terminology... 5 Date Recording... 6 Generic Data Item Table Section 2 - Socio-Environmental Detail(s) Social and Personal Circumstances Asbestos Exposure Section 3 - Clinical / Care Process Assessment(s), Examination(s) and Investigation(s) Clinical Symptoms at Presentation (Lung Cancer) Clinical Signs Pulmonary Function Test Pulmonary Function Test Result Complications of Condition (Lung Cancer) Co-Morbidity Superior Vena Cava Obstruction (SVCO) Diagnosed Invasive Diagnostic Procedure Type (Lung Cancer) Specimen Type (Lung Cancer) Body Surface Area Finding(s) and Result(s) Extent of Disease (Small Cell Carcinoma of Lung) TNM Tumour Classification (Clinical) (Lung Cancer) TNM Nodal Classification (Clinical) (Lung Cancer) TNM Metastases Classification (Clinical) (Lung Cancer) TNM Stage Group (Non-small Cell Carcinoma of Lung) TNM Tumour Classification (Clinical) (Pleural Mesothelioma) TNM Nodal Classification (Clinical) (Pleural Mesothelioma) TNM Metastases Classification (Clinical) (Pleural Mesothelioma) Care Planning, Management and Outcome(s) Patient Involved in Care Plan Carer Involved in Care Plan Care Plan - Reason Patient Not Involved Care Plan - Reason Carer Not Involved Treatment(s) and Intervention(s) Thoracic Procedure (Lung Cancer) Sclerosant Agent Lymph Node Stations Thoracic Surgery Lymph Node Station Related Activity Tumour Visible at Bronchoscopy Endobronchial Treatment Type Conformal Shaping Technique Multileaf Collimator Leaf Resolution On-Treatment Verification Method Chemotherapy Total Number of Cycles Planned Chemotherapy Agent (Lung Cancer) Lung Cancer Clinical Data Standards 2
3 3.34 Chemotherapy Cycle Frequency Biological Agent (Lung Cancer) Photosensitising Agent Clinical Trial Name Care Journey and Encounter Treatment Summary Forwarded To Appendix 1 - Working Group Appendix 2 - Consultation Distribution List Lung Cancer Clinical Data Standards 3
4 Section 1 - Overview & Background Overview The Scottish Cancer Group supports the development of Lung Cancer Data Standards for NHS Scotland in order to ensure a national approach to the collection of clinical and non-clinical data items relating to cancer. The aim is to ensure intercompatibility of national clinical information systems, and to support the implementation of an electronic integrated health record. Cancer already has a range of individual national data sets for audit, screening and registration and NCDDP standards build on these systems. National data standards will support data sharing and allow secondary use of data for these purposes. Some NCDDP cancer core standards and site-specific standards for breast, endometrial and head & neck cancers have already been published. However a need for further cancer lung standards was identified. A multi-disciplinary Clinical Working Group, established in May 2007 and supported by the National Clinical Dataset Development Programme (NCDDP) Support Team in Information Services Division (ISD), carried out the development of these lung cancer data standards. The Lung Cancer Data Standards will: Define common data items recommended for collection in a wide variety of clinical settings Support the exchange of patient information between healthcare providers Support the consistent recording of patient information throughout NHS Scotland It is important to emphasise that these are data standards rather than a dataset. This means that the individual data items included in this document are not required to be recorded together in a single clinical information system. However where it is considered appropriate to record a particular data item as part of the record of care, the information should be recorded in accordance with the nationally agreed standard. We are now asking for feedback from the wider clinical community in order to ensure that these data standards are fit for purpose and ready for inclusion in the national Health and Social Care Data Dictionary. We invite all interested organisations and individuals to take part in this consultation by completing the attached Consultation Response Form and then returning it to NCDDPsupportteam@isd.csa.scot.nhs.uk. Comments on all or a part of the document are welcome. Some background information on the NCDDP and Lung Cancer Clinical Data Standard development can be found below. If you have any further queries, please go to our website or contact NCDDPsupportteam@isd.csa.scot.nhs.uk. Lung Cancer Clinical Data Standards 4
5 Lung Cancer Data Standards The membership of the Lung Cancer Clinical Data Standards Working Group is shown in appendix 1. This group agreed the inclusion of individual data items using the following criteria: 1. Is the data item one that would reasonably be expected to be collected for all patient s lung cancer? 2. Is the data item necessary for the healthcare of cancer patients? 3. Is the data item one that is likely to be shared among health care professionals? Once consultation is complete the Lung Cancer Clinical Data Standards will be submitted to the NCDDP Programme Board for formal approval as a national standard, and then passed to the ehealth National Clinical Information Steering Group and the NHS Scotland Information Standards Group for endorsement. Once approved the Lung Cancer Clinical Data Standards will be freely and widely available through publication in the Health and Social Care Data Dictionary ( As far as possible they are UK compatible. It is expected that the Lung Cancer Clinical Data Standards will be implemented within existing and emerging national clinical information systems and commercially procured national products, as well as being available to commercial developers to ensure the ability of their systems to support national information requirements. Background to NCDDP The National Clinical Dataset Development Programme (NCDDP) supports clinicians to develop sets of interoperable national datasets to facilitate the implementation of the integrated care records across NHS Scotland. These standards will: Support direct patient care, by reflecting current best practice guidance Facilitate effective communication between health care professionals Improve data quality and support secondary data requirements where possible including data to support clinical governance Be freely and widely available through publication in the web-based Health & Social Care Data Dictionary Incorporate agreed national clinical definitions and implement national terminology Be UK compatible where possible The Chief Medical Officer established the programme in 2003 to support clinicians developing national clinical data standards, initially to support the national priority areas. These standards are an essential element of the Electronic Health Record, a central aim of the National e-health Strategy. More information can be found on our website Clinical Terminology The strategic standard for clinical terminology in NHS Scotland is SNOMED-Clinical Terms. This means that over the next few years, clinical information systems will progress to record clinical data using this international standard. Lung Cancer Clinical Data Standards 5
6 Date Recording It is good record-keeping practice always to identify the date of recording of any clinical information. It is expected that all clinical information systems should include date stamping as standard functionality; therefore the Lung Cancer Standards do not deal with this issue. In many clinical situations, the date of an event, investigation, etc is required for clinical purposes and should be visible to the health care professional. This date may not be the same as the date on which the data are entered onto the system. In these instances the system must allow the health care professional to enter whichever date is appropriate. These issues must be addressed during system specification and development. The date format for storage and management within a system should conform to the 1Government Data Standards Catalogue format: CCYY-MM-DD. However, this does not preclude entry or display of data on the user interface using the traditional DD-MM-CCYY format. An example of a date & time in correct format is: T19:20:30+01:00 (CCYY-MM-DDThh: mmtzd) It is recommended that a time should always be recorded with the appropriate date and not on its own, however it may not be necessary to display the date along with the time. This is of particular importance where any calculations or analyses are likely to be performed. Automated times recorded by IT systems should include all elements of the time, i.e. hour, minutes and seconds, and are expected to be actual. Where times are entered manually, it is likely that only the hours and minutes will be required, although in some circumstances only hours may be required. Time, or any element of the time (hours, minutes or seconds) may be actual or estimated. In some circumstances only an actual time may be acceptable, whilst in others an estimated time may be allowed. In the latter situation, it may be necessary to identify whether the time recorded is actual or estimated. Times identified as actual may be used in calculations and analyses. Times marked as estimated should be treated with caution and the implications of undertaking any calculations or analyses should be considered in the particular context within which the time is recorded or to be made subsequent use of. Where an estimated time is allowed, the appropriate degree of verification detail required should be decided, again dependent on the context in which it is recorded and how the time is to be used. Government Data Standards Catalogue 1. All times must be expressed in the 24 hour clock format, e.g. one minute past midnight is 00:01: Values of any element less than 10 should be entered with a zero in the first position. 3. All times for UK transactions/events will be assumed to be GMT. 4. Systems should record whether the time is Coordinated Universal Time or British Summer Time in the Time zone designator. This will allow time elapsed to be calculated correctly, for example for A&E waiting times Lung Cancer Clinical Data Standards 6
7 Generic Data Item Table 1 Generic Data Items Person Family Name Person Given Name Person Birth Date Person Sex at Birth Location Code Health Record Identifier CHI Number Associated Professional - Identifier - Group - Role ASA Status Discussed by Care Team Reason not Discussed by Care Team Route of Administration Imaging Modality Contrast Agent Administered Status Procedure (Clinical Imaging) Cancer Generic Data Items Date of Referral (Cancer) Referral Source (Cancer) Urgency of Referral (Cancer) Date Referral Received (Cancer) Definition That part of a person s name which is used to describe family, clan, tribal group or marital association. The forename or given name of a person. The date of which a person was born or is officially deemed to have been born, as recorded on the Birth Certificate. This is a factual statement, as far as is known, about the phenotypic (biological) sex of the person at birth. This is the reference number of any building or set of buildings where events pertinent to NHS Scotland take place. Locations include hospitals, health centres, GP surgeries, clinics, NHS board offices, nursing homes, schools and patients/clients home. A patient Health Record Identifier is a code (set of characters) used to uniquely identify a patient within a health register or a health records system e.g. PAS. The Community Health Index (CHI) is a population register, which is used in Scotland for health care purposes. The CHI number uniquely identifies a person on the index. Associated Professionals are those individuals who are involved with the client/ patient in a professional capacity e.g. consultant, social worker, occupational therapist etc. The ASA PS classification globally assesses the degree of "sickness" or "physical state" prior to selecting the anaesthetic or prior to performing surgery. This denotes whether the proposed management and treatment of the patient was discussed by the care/ multidisciplinary team. The reason why the patient was not discussed by a care/ multidisciplinary team. A description of the way in which a drug or preparation is given or used. A high level description of the types of imaging modality available under radiology services. An indicator of whether or not a contrast agent was administered for an imaging procedure. A record of the specific procedure performed during the current episode of care. This includes diagnostic and therapeutic procedures. Definition The date on which a referral is made by a primary care or hospital clinician for symptoms that led to a diagnosis of cancer. The route by which the patient was referred for investigation of signs or symptoms that led to a diagnosis of cancer. The urgency of referral as assigned by the referring primary care clinician. It is also applied for direct emergency referral and formal risk based triage. The date on which a healthcare service receives a referral for investigation of signs or symptoms that lead to a diagnosis of cancer. 1 All data items in the table above are existing nationally approved data standards, which can be found in the Health and Social care Data Dictionary. Lung Cancer Clinical Data Standards 7
8 Family History of Cancer (Cancer) Referred to Clinical Genetics Service Date First Seen by Associated Professional (Cancer) Date Histo/Cytopathological specimen taken Histo/Cytopathology report number Histo/Cytopathology investigation report date Site of Origin of Primary tumour Tumour Type (Morphology of Tumour) (Cancer) Most Valid Basis of Diagnosis Date of Diagnosis (Cancer) Synchronous Tumour Indicator (Cancer) Anatomical Imaging Site (Cancer) Date of Imaging (Cancer) Imaging Results (Cancer) Date Discussed by Care Team (Cancer) Seen by Specialist Palliative Care (Cancer) Date First Seen by Specialist Palliative Care (Cancer) Reason Not Seen by Specialist Palliative Care (Cancer) Type of First Treatment (Cancer) Date of First Treatment Reason(s) for Delay in Starting First Treatment Treatment Status {Cancer} Reason Treatment Not Delivered {Cancer} Date of Surgery {Cancer} Associated Professional Grade (Most Senior Operating Surgeon) (Cancer) Presentation Type Deep Vein Thrombosis (DVT) Prophylaxis Antibiotic Prophylaxis (Preoperative) Surgical Procedure Intent Surgical Procedure {Cancer} Past occurrence and/or present existence of cancer in first, second or third degree relatives of the patient. A record of whether or not the patient has been referred to a clinical genetics service to manage the potential risk of disease. Date of first seeing responsible Associated Professional is the date on which an Associated Professional first sees a patient for investigation or management of cancer following referral from primary or secondary healthcare. This is the date the histo/ cytopathological specimen was taken. The reference number of the histo/cytopathology specimen. The date that the result of the specimen was reported by the pathology laboratory. The anatomical site of origin of the primary tumour according to the International Classification of Diseases for Oncology (ICD-O(3)). The morphology of the tumour according to the International Classification of Diseases for Oncology (ICD-O(3)). The best evidence in support of the diagnosis of cancer. The date on which there was first confirmation of the diagnosis of cancer whether by histology, cytology, immunology, cytogenetics or clinical (including radiological) methods. A record of the presence of multiple tumours at the same time. A classification of the part of the body that is subject to the imaging or radio diagnostic event. The date that imaging was undertaken for the purposes of diagnosing cancer. A record of the findings of an imaging investigation. This denotes the date the care team meeting was held to discuss the management of the patient's care. A record of whether the patient was seen by a member of the specialist palliative care team as a result of diagnosis of their cancer. The date the patient was first seen by a member of the specialist palliative care team as described below. The reason why the patient was not seen by a specialist in palliative care. The first specific treatment modality administered to a patient. The date the type of first cancer treatment was given to the patient. The reason for delay in the investigation, diagnosis and treatment of patients with cancer attributable to the patient and/or the system. This denotes whether or not the patient had a specific treatment delivered for the treatment of their cancer. The reason why the treatment was not performed The date on which the operation for cancer was performed. The clinical grade of the most senior clinician participating in the operation. How the patient presented for surgery. The measures (mechanical or pharmacological) taken to prevent deep vein thrombosis following cancer surgery. The delivery of a single dose of antibiotics immediately prior to surgery or other invasive procedure to prevent infection arising from surgery. The purpose of the surgical procedure to be carried out. The surgical procedure(s) performed for investigation and Lung Cancer Clinical Data Standards 8
9 Date Treatment Started (Cancer) Date Treatment Completed (Cancer) External Beam Radiotherapy Course Type (Cancer) Anatomical Treatment Site (Cancer) External Beam Radiotherapy Dose: Total Planned (Cancer) External Beam Radiotherapy Dose: Total Administered (Cancer) External Beam Radiotherapy Fractions: Total Planned (Cancer) External Beam Radiotherapy Fractions: Total Administered (Cancer) Chemotherapy Type (Cancer) Chemotherapy Course Number (Cancer) Biological Therapy Type (Cancer) Participant in Clinical Trial Clinical Trial Formal Entry Date Date of Clinical Status Assessment Recurrence Indicator (Cancer) Primary Tumour Status {Cancer} Nodal Status {Cancer} Metastatic Status {Cancer} Date Recurrence First Suspected {Cancer} Date of Cancer Proven Recurrence {Cancer} Person Death Date Underlying Cause of Death Treatment Related Morbidity (Cancer) treatment of cancer. This also includes nodal and reconstructive surgery performed on the patient for treatment of cancer. The date cancer treatment course commenced. The date cancer treatment course ended. The type of course of external beam radiotherapy administered for the treatment of the cancer. The part(s) of the body to which therapy is directed. The total Target Applied Dose (recorded in Gy) planned to be administered between the start and completion dates of a course of external beam radiotherapy. The Target Applied Dose (TAD) actually given (recorded in Gy) between the start and completion dates recorded for the course of external beam radiotherapy. The number of radiation treatments planned for any individual course of therapy (described by the start and completion dates of External Beam Radiotherapy). The number of radiation treatments actually given for any individual course of therapy (described by the start and completion dates of External Beam Radiotherapy). The type of course of cytotoxic drugs administered for the treatment of the cancer. Cytotoxic drugs are drugs which destroy cells. The chronological course number of chemotherapy. The type of course of immunotherapy administered for the treatment of the cancer. A record of whether or not the patient received treatment within the context of a clinical trial. The date that the patient formally entered into the named clinical trial. The date the patient was seen for clinical status assessment. An indicator of the certainty of the recurrence of cancer. The status of the primary tumour at follow-up contact. The status of any nodal metastases at follow-up contact. The status of any distant metastases at follow-up contact. The date the recurrence was first suspected. The date of diagnosis of recurrence of the patient s cancer. The date on which a person died or is officially deemed to have died, as recorded on the Death Certificate The underlying cause of death as recorded by GRO(S) in Part I of the death certificate. An indication of the extent of morbidity that is directly related to all cancer treatment modalities. Lung Cancer Clinical Data Standards 9
10 Section 2 - Socio-Environmental Detail(s) Social and Personal Circumstances 2.1 Asbestos Exposure Definition: An indication of whether or not the patient has ever been exposed to asbestos. Code Value 00 No 01 Yes Duration Duration (Descriptive) Lung Cancer Clinical Data Standards 10
11 Section 3 - Clinical / Care Process Assessment(s), Examination(s) and Investigation(s) 3.1 Clinical Symptoms at Presentation (Lung Cancer) Main source of standard: Scottish Referral Guidelines for Suspected Cancer, SIGN Guideline 80: Management of Patients With Lung Cancer. Definition: The type of lung cancer related symptom(s) reported by the patient or their representative at presentation. Field length: 3 Code Value Sub- Sub-Value Explanatory Notes Code 01 Cough 02 Breathlessness 03 Haemoptysis 04 Wheeze 05 Hoarseness 06 Stridor 07 Anorexia 08 Weight Loss (Significant) Significant weight loss is usually >10% loss of weight in the past six months which is not attributable to lifestyle changes (e.g. dieting) or other medical conditions. 09 Pain A Pain suggestive of a primary tumour B Pain suggestive of metastases Z Other (specify) 10 Fatigue 96 Not applicable Asymptomatic 98 Other (specify) Duration (Symptom) Anatomical Site Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 98 Other (specify) and Z sub-values Other (specify) with free text fields for recording other values of this item. Lung Cancer Clinical Data Standards 11
12 3.2 Clinical Signs Definition: The type of clinical signs observed when the patient is examined clinically. Code Value Explanatory Notes 01 Hoarseness 02 Stridor 03 Finger Clubbing/ Hypertrophic Pulmonary Osteoarthropathy HPOA 04 Signs of Superior Vena Cava Obstruction SVCO 05 Signs of Metastases E.g. brain, bone, liver or skin 06 Lymphadenopathy 07 Localising chest signs 08 Recent weight loss 09 Horner s Syndrome 96 Not applicable 98 Other (specify) Clinical Examination Performed Date of Clinical Status Assessment {Cancer} Anatomical Site Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. 3.3 Pulmonary Function Test Definition: The type of pulmonary function test. Lung Cancer Clinical Data Standards 12
13 Code Value Explanatory Notes 01 Forced Expiratory Volume (FEV1) The maximal volume of gas, in litres, which can be expelled from the lungs in the first second of a forced expiration from a position of full inspiration. 02 Forced Vital Capacity (FVC) The maximal volume of gas, in litres, that can be expelled from the lungs during a forced and complete expiration from a position of full inspiration. 03 Lung Membrane Permeability Transfer Coefficient for Carbon Monoxide (KCO) 04 Lung Transfer Factor for Carbon Monoxide (TLCO) 98 Other (Specify) Dates and Times of Laboratory Processes and Procedures Pulmonary Function Test Result The Lung Transfer Factor for Carbon Monoxide (TLCO) divided by the alveolar volume at the time of measurement. The rate of transfer of carbon monoxide between alveoli and the erythrocytes in the alveolar capillaries, the units being quantity of carbon monoxide per unit time per unit pressure difference (gradient) between two sites. Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. Lung Cancer Clinical Data Standards 13
14 3.4 Pulmonary Function Test Result Definition: The result of a pulmonary function test. Format: Numeric (nn.nn) Field length: 5 N/A Sub-data items: Units of measurement Code Value Explanatory Notes 01 Litres e.g. units of measurement for Forced Expiratory Volume (FEV1), Forced Vital Capacity 02 mmol x min-1 x kpa -1 e.g. units of measurement for Lung Transfer Factor for Carbon Monoxide mmol x min-1 x kpa -1 x L -1 e.g. units of measurement for Lung 03 Membrane Permeability Transfer Coefficient for Carbon Monoxide Assessment Code Value 01 Absolute value 02 Predicted value Dates and Times of Laboratory Processes and Procedures Pulmonary Function Test Further information: Percentage of predicted value may be calculated as Absolute value/predicted value * 100%. Lung Cancer Clinical Data Standards 14
15 3.5 Complications of Condition (Lung Cancer) Definition: Any lung cancer specific complications, which may require additional healthcare intervention. Field length: 3 Code Value Sub-Code Sub-Value Explanatory notes 01 Hyponatraemia 02 Superior Vena Cava SVCO Obstruction 03 Recurrent Laryngeal Nerve Palsy 04 HyperCalcaemia 05 Pleural Effusion 06 Spinal Cord Compression 07 Cerebral Metastases A Solitary cerebral metastasis When diagnosed by MRI scan. B Multiple cerebral metastases 08 Pneumonia 09 Major Haemoptysis Requiring hospital admission. 10 Pathological fracture of bone 11 Other Metastatic complications (specify) 96 Not applicable 98 Other non-metastatic complications (specify) Anatomical Site Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 11 Other Metastatic complications (specify) and code 98 Other non-metastatic complications (specify) with a free text field for recording other values of these items. Lung Cancer Clinical Data Standards 15
16 3.6 Co-Morbidity Definition: Any disease or condition that exists or develops alongside the main disease or condition and affects the management of the patient.. Code Value Explanatory Notes 01 Chronic obstructive pulmonary disease e.g. COPD, Emphysema 02 Interstitial lung disease 03 Coronary Artery Disease/Ischaemic Heart Disease CAD/IHD 04 Cerebrovascular disease 05 Dementia 06 Renal Impairment 07 Diabetes 08 Peripheral vascular disease PVD 09 Other malignancy (specify) 98 Other (specify) Treatment Related Morbidity Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 09 Other malignancy (specify) and code 98 Other (specify) with a free text field for recording other values of these items. 3.7 Superior Vena Cava Obstruction (SVCO) Diagnosed Common name: SVCO diagnosed Definition: An indication of whether or not the patient has been diagnosed with Superior Vena Cava Obstruction. Code Value Explanatory Notes 00 No 01 Yes Clinical Signs (Lung Cancer) Lung Cancer Clinical Data Standards 16
17 3.8 Invasive Diagnostic Procedure Type (Lung Cancer) Main source of standard: The Royal College of Pathologists, Dataset for Lung Cancer Histopathology Reports (2 nd edition), September Definition: The type of invasive diagnostic procedure performed for a potential diagnosis of cancer. Field length: 3 Code Value Subcode 01 Endoscopic A procedures B C D E 02 Imaging guided procedures 03 Biopsy of metastatic site 04 Pleural procedures 05 Mediastinoscopy 06 Mediastinotomy 07 Thoracotomy 08 Thoracoscopy Z A B C D Z A B Z A Sub-value Rigid bronchoscopy Fibreoptic bronchoscopy Transbronchial needle aspiration (TBNA) Endobronchial Ultrasound (EBUS) Endoscopic Ultrasound (EUS) Other (specify) Transthoracic CT guided biopsy Transthoracic Ultrasound guided biopsy Transthoracic fluoroscopic guided biopsy Ultrasound guided needle aspiration of neck nodes Other (specify) Aspiration Closed (needle) biopsy Other (specify) Medical Thoracoscopy Explanatory Notes B Surgical Thoracoscopy VATS 96 Not applicable 98 Other (specify) Date Histo/Cytopathological Specimen Taken Anatomical Site Recording guidance: IT systems should allow for the recording of multiple values for this item. This item may occur more than once throughout a patient s record. Users may wish to augment code 98 Other (specify) and Z sub-values Other (specify) with free text fields for recording other values of these items. Lung Cancer Clinical Data Standards 17
18 3.9 Specimen Type (Lung Cancer) Main source of standard: The Royal College of Pathologists, Dataset for Lung Cancer Histopathology Reports (2 nd edition), September Definition: The type of specimen reported. Field length: 3 Code Value Subcode 01 Cytological A specimen B C D E Z 02 Biopsy A specimen B C D Z 03 Surgical A specimen B C D Z 96 Not applicable 98 Other (specify) Sub-value Bronchial washings Bronchial brushings Sputum Fluid Aspirate Cellular Aspirate Other (specify) Bronchial Pleural Liver Mediastinal lymph node Other (specify) Wedge Segment Lobe Whole lung Other (specify) Explanatory Notes Date Histo/Cytopathological Specimen Taken Anatomical Site Invasive Diagnostic Procedure Type (Lung Cancer) Thoracic Surgical Procedure (Lung Cancer) Recording guidance: IT systems should allow for the recording of multiple values for this item. This item may occur more than once throughout a patient s record. Users may wish to augment code 98 Other (specify) and Z sub-values Other (specify) with free text fields for recording other values of these items. Lung Cancer Clinical Data Standards 18
19 3.10 Body Surface Area Main source of standard: British National Formulary Definition: The patient s body surface area measured in m², using the Dubois formula. Format: Numeric (n.nn) Field length: 4 N/A Height Weight Further Information: The Dubois formula is: Body surface area (m²) = x (patient height in cm) x (patient weight in kg) The Dubois formula is from: Du Bois D and Du Bois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17: Lung Cancer Clinical Data Standards 19
20 Finding(s) and Result(s) 3.11 Extent of Disease (Small Cell Carcinoma of Lung) Main source of standard: The Royal College of Pathologists, Dataset for Lung Cancer Histopathology Reports (2 nd edition), September Definition: The extent of the tumour of the lung. Code Value Explanatory Notes 01 Limited Disease Disease confined to one hemithorax including involvement of ipsi-and-/or contralateral hilar, mediastinal or supraclavicular lymph nodes. Patients with ipsilateral pleural effusion, regardless of pleural cytology, should be included in this group. 02 Extensive Disease Any disease beyond the definition of limited stage. 96 Not applicable Recording guidance: This is only applicable where the tumour is diagnosed as small cell carcinoma of the lung. Lung Cancer Clinical Data Standards 20
21 3.12 TNM Tumour Classification (Clinical) (Lung Cancer) Common name: Clinical TNM Tumour Classification (Lung Cancer) Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The size and extent of the tumour as determined by pre-treatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 00 TNM Classification T0 No evidence of primary tumour 01 TNM Classification Tis Carcinoma in situ (CIS) 02 TNM Classification T1 Tumour up to 3cm, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e. not the main bronchus). 03 TNM Classification T2 Tumour with any of the of the following: Tumour >3 cm Involves main bronchus, 2cm or more distal to the carina Invades visceral pleura Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung. 04 TNM Classification T3 Tumour of any size that directly invades any of the following: chest wall (including superior sulcus tumours), diaphragm, mediastinal pleura, parietal pericardium; or tumour in the main bronchus < 2cm distal to the carina (The uncommon superficial spreading tumour of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1.) but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung. 05 TNM Classification T4 Tumour of any size that invades any of the following: Mediastinum, heart, great vessels, trachea, oesophagus, vertebral body, carina; separate tumour nodule(s) in the same lobe; tumour with malignant pleural effusion (Most pleural effusions with lung cancer are due to tumour. In a few patients, however, multiple cytopathological examination of pleural fluid are negative for tumour, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgement dictate that the effusion is not related to the tumour, the effusion should be excluded as a staging element and the patient should be classified as T1, T2, or T3). 06 TNM Classification TX Primary tumour cannot be assessed Lung Cancer Clinical Data Standards 21
22 TNM Nodal Classification (Clinical) (Lung Cancer) TNM Metastases Classification (Clinical) (Lung Cancer) Further information: Clinical TNM is derived from all the clinical, radiological and biochemical results prior to treatment. The TNM system is base on the assessment of three components (T tumour, N node and M metastases) and the addition of numbers after the letter components to indicate the extent of the malignant disease. In the case of multiple simultaneous tumours in one or two lungs, the tumour with the highest T category should be used for classification TNM Nodal Classification (Clinical) (Lung Cancer) Common name: Clinical TNM Nodal Classification (Lung Cancer). Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The extent of regional lymph node metastases as determined by pretreatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 00 TNM Classification N0 No regional lymph nodes metastasis. 01 TNM Classification N1 Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension. (Node stations 10-14). 02 TNM Classification N2 Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s). (Node stations 1-9). 03 TNM Classification N3 Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s). 04 TNM Classification NX Regional lymph nodes cannot be assessed (e.g. previously removed). Related Data items: TNM Tumour Classification (Clinical) (Lung Cancer) TNM Metastases Classification (Clinical) (Lung Cancer) Lung Cancer Clinical Data Standards 22
23 3.14 TNM Metastases Classification (Clinical) (Lung Cancer) Common name: Clinical TNM Metastases Classification (Lung Cancer). Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The extent of metastatic spread of the tumour as determined by pretreatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 00 TNM Classification M0 No distant metastasis. 01 TNM Classification M1 Distant metastasis, includes separate tumour nodule(s) in a different lobe (ipsilateral or contralateral). 02 TNM Classification MX Distant metastasis cannot be assessed. TNM Nodal Classification (Clinical) (Lung Cancer) TNM Tumour Classification (Clinical) (Lung Cancer) 3.15 TNM Stage Group (Non-small Cell Carcinoma of Lung) Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The lung cancer stage group, coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 01 Stage 0 Carcinoma in Situ Tis N0 M0 02 Stage Ia T1 N0 M0 03 Stage Ib T2 N0 M0 04 Stage IIa T1 N1 M0 04 Stage IIb T2 N1 M0 T3 N0 M0 05 Stage IIIa T1,T2 N2 M0 T3 N1,N2 M0 06 Stage IIIb Any T N3 M0 T4 Any N M0 07 Stage IV Any T Any N M1 Lung Cancer Clinical Data Standards 23
24 Recording guidance: This is only applicable where the tumour is diagnosed as non-small cell carcinoma of the lung TNM Tumour Classification (Clinical) (Pleural Mesothelioma) Common name: Clinical TNM Tumour Classification (Pleural Mesothelioma) Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The size and extent of the tumour as determined by pre-treatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Field length: 3 Code Value Subcode Sub-value Explanatory Notes 00 TNM Classification T0 No evidence of primary tumour 01 TNM Classification T1 A No involvement of Tumour involves ipsilateral parietal visceral pleura (mediastinal, diaphragmatic) pleura. B Focal involvement of visceral pleura Tumour involves ipsilateral parietal (mediastinal, diaphragmatic) pleura. 02 TNM Classification T2 Tumour involves any ipsilateral pleural surfaces, with at least one of the following: Confluent visceral pleural tumour (including fissure). Invasion of diaphragmatic muscle. Invasion of lung parenchyma. 03 TNM Classification T3 Tumour involves any ipsilateral pleural surfaces, with at least one of the following: Invasion of endothoracic fascia. Invasion into mediastinal fat. Solitary focus of tumour invading soft tissues of the chest wall. Non-transmural involvement of the pericardium. 04 TNM Classification T4 Tumour involves any ipsilateral pleural surfaces, with at least one of the following: Lung Cancer Clinical Data Standards 24
25 Diffuse or multifocal invasion of soft tissues of chest wall. Any involvement of rib. Invasion through diaphragm to peritoneum. Invasion of any mediastinal organ(s). Direct extension to contralateral pleura. Invasion in to the spine. Extension to internal surface of pericardium. Pericardial effusion with positive cytology. Invasion of myocardium. Invasion of brachial plexus. 05 TNM Classification TX Primary tumour cannot be assessed TNM Nodal Classification (Clinical) (Pleural Mesothelioma) TNM Metastases Classification (Clinical) (Pleural Mesothelioma) Further information: Clinical TNM is derived from all the clinical, radiological and biochemical results prior to treatment. The TNM system is base on the assessment of three components (T tumour, N node and M metastases) and the addition of numbers after the letter components to indicate the extent of the malignant disease TNM Nodal Classification (Clinical) (Pleural Mesothelioma) Common name: Clinical TNM Nodal Classification (Pleural Mesothelioma). Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The extent of regional lymph node metastases as determined by pretreatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 00 TNM Classification N0 No regional lymph nodes metastasis. 01 TNM Classification N1 Metastasis in ipsilateral bronchopulmonary and/or hilar lymph node(s). 02 TNM Classification N2 Metastasis in subcarinal lymph node(s) and/or internal mammary or mediastinal lymph node(s). Lung Cancer Clinical Data Standards 25
26 03 TNM Classification N3 Metastasis in contralateral mediastinal, internal mammary, or hilar node(s) and /or ipsilateral or contralateral supraclavicular or scalene lymph node(s). 04 TNM Classification NX Regional lymph nodes cannot be assessed (e.g. previously removed). Related Data items: TNM Tumour Classification (Clinical) (Pleural Mesothelioma) TNM Metastases Classification (Clinical) (Pleural Mesothelioma) 3.18 TNM Metastases Classification (Clinical) (Pleural Mesothelioma) Common name: Clinical TNM Metastases Classification (Pleural Mesothelioma). Main source of standard: TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, UICC, 2002). Definition: The extent of metastatic spread of the tumour as determined by pretreatment investigations (not pathological), coded according to the official TNM Classification (TNM Classification of Malignant Tumours, Sixth Edition, 2002). Code Value Explanatory Notes 00 TNM Classification M0 No distant metastasis. 01 TNM Classification M1 Distant metastasis. 02 TNM Classification MX Distant metastasis cannot be assessed. TNM Nodal Classification (Clinical) (Pleural Mesothelioma) TNM Tumour Classification (Clinical) (Pleural Mesothelioma) Lung Cancer Clinical Data Standards 26
27 Care Planning, Management and Outcome(s) 3.19 Patient Involved in Care Plan Common name: Care Management Plan Patient Involvement Definition: An indication of whether or not the patient was involved in discussing their care plan. Code Value Explanatory Notes 00 No 01 Yes Care Plan - Reason Patient Not Involved Further information: A care plan is a document which details the care and treatment that a patient/user receives, and identifies who delivers the care and treatment. Recording guidance: If the patient was involved in discussions but declined involvement in care decisions code 01 Yes Carer Involved in Care Plan Common name: Care Management Plan Carer Involvement Definition: An indication of whether or not the patient s carer was involved in discussing the care plan of the patient s condition. Code Value Explanatory Notes 00 No 01 Yes Care Plan - Reason Carer Not Involved Lung Cancer Clinical Data Standards 27
28 Further information: A care plan is a document which details the care and treatment that a patient/user receives, and identifies who delivers the care and treatment Care Plan - Reason Patient Not Involved Definition: The reason why the patient was not involved in discussing the care plan of their condition. Code Value Explanatory notes 00 No reason given 01 Not indicated e.g. Patient unconscious. 02 Clinical decision e.g. learning disability, dementia. 95 Patient declined 98 Other (specify) Patient Involved in Care Plan Further information: A care plan is a document which details the care and treatment that a patient/user receives, and identifies who delivers the care and treatment. Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item Care Plan - Reason Carer Not Involved Definition: The reason why the carer was not involved in discussing the care plan of the patient s condition. Code Value Explanatory notes 00 No reason given 01 Patient consent not obtained 02 Carer declined 98 Other (specify) e.g. Carer unable to comprehend care plan process. Lung Cancer Clinical Data Standards 28
29 Carer Involved in Care Plan Further information: A care plan is a document which details the care and treatment that a patient/user receives, and identifies who delivers the care and treatment. This data item is not intended to indicate the responsibility of involving a carer. Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. Lung Cancer Clinical Data Standards 29
30 Treatment(s) and Intervention(s) 3.23 Thoracic Procedure (Lung Cancer) Common name: Thoracic Procedure. Definition: The thoracic procedure(s) performed for the treatment of cancer. Field length: 3 02 Segmentectomy 03 Lobectomy / Bi-lobectomy 04 Pneumonectomy B A B C Sub-Value Single Wedge Resection Multiple Wedge Resection Code Value Sub- Code 01 Wedge Resection A Extrapericardial Pneumonect omy Intrapericardial Pneumonect omy Extrapleural Pneumonect omy Explanatory Notes e.g. Pleural Mesothelioma treatment. 05 Sleeve Resection 06 Lung resection with resection of chest wall (not identifying which lobe resection) 07 Carinal resection 08 Open and close Thoracotomy 09 Pleurectomy e.g. Decortification, Pleural Mesothelioma treatment. 10 Video-Assisted Thorascopic Surgery 11 Pleurodesis A Medical Pleurodesis 12 Drain insertion 96 Not applicable 98 Other (specify) Anatomical Site Cancer Surgery Information {Cancer} B Surgical Pleurodesis Lung Cancer Clinical Data Standards 30
31 Date of Surgery {Cancer} Surgical Procedure {Cancer} Surgical Procedure Intent {Cancer} Invasive Diagnostic Procedure Type (Lung Cancer) Specimen Type (Lung Cancer) Further information: This standard provides a short list of procedures. Further detail may be recorded using Snomed-CT. Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item Sclerosant Agent Common name: Pleurodesis sclerosant. Main source of standard: SIGN Guideline 80, Management of patients with lung cancer. Definition: The type of sclerosant agent used for pleurodesis treatment of the patient. Code Value Explanatory Notes 01 Talc 02 Kaolin 02 Tetracycline 03 Bleomycin 04 Povidone iodine 96 Not applicable 98 Other (specify) Thoracic Procedure (Lung Cancer) Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. Lung Cancer Clinical Data Standards 31
32 3.25 Lymph Node Stations Thoracic Surgery Definition: The lymph node station(s) assessable during thoracic surgery. Code Value Explanatory Notes 01 Node station 2 Upper paratracheal nodes 02 Node station 3 Prevascular and retrotracheal nodes 03 Node station 4 Lower paratracheal nodes 04 Node station 5 Subaortic or aorticopulmonary window nodes 05 Node station 6 Paraaortic (ascending aortic or phrenic) nodes 06 Node station 7 Subcarinal nodes 07 Node station 8 Paraesophageal nodes 08 Node station 9 Pulmonary ligament nodes 09 Node station 10 Hilar nodes 10 Node station 11 Interlobar nodes 11 Node stations 12 to 14 Intrapulmonary nodes 96 Not applicable This data item is inapplicable/not relevant to the person or not required. 98 Other (specify) Laterality {Cancer} Lymph Node Station Related Decision Further Information: Station 1 would normally be assessed at mediastinoscopy which occurs in the staging process before resection. Pathologist will normally report on intrapulmonary nodes, but not split them in to stations 12,13 and 14. Recording guidance: IT systems should allow for the recording of multiple values for this item. Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. Lung Cancer Clinical Data Standards 32
33 3.26 Lymph Node Station Related Activity Definition: The activity performed regarding the lymph node station(s) assessable during surgery. Code Value 01 Sampled 02 Inspected 03 Not inspected 96 Not applicable 98 Other (specify) Lymph Node Stations Thoracic Surgery Recording guidance: IT systems should allow for the recording of multiple values for this item Tumour Visible at Bronchoscopy Common name: Bronchoscopy Tumour Visualised Definition: An indication of whether or not any endobronchial tumour was visible at bronchoscopy. Code Value Explanatory Notes 00 No 01 Yes 02 Equivocal Lung Cancer Clinical Data Standards 33
34 3.28 Endobronchial Treatment Type Main source of standard: SIGN Guideline 80, Management of patients with lung cancer. Definition: The type of endobronchial treatment administered to the patient. Code Value Explanatory Notes 01 Photodynamic Therapy PDT 02 Brachytherapy 03 Electrocautery Diathermy 04 Cryotherapy 05 Stents 06 Laser therapy 07 Bronchoscopic Debulking 96 Not applicable 98 Other (specify) Anatomical Treatment Site {Cancer} Cancer Care Plan Intent {Cancer} Surgical Procedure Intent Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item. Lung Cancer Clinical Data Standards 34
35 3.29 Conformal Shaping Technique Definition: The conformal shaping technique used in the delivery of external beam radiotherapy treatment to the patient. Code Value Explanatory Notes 01 Multileaf Collimator MLC 02 Custom-made alloy blocks 03 Standard, regular lead blocks 96 Not applicable 98 Other (specify) Anatomical Treatment Site {Cancer} External Beam Radiotherapy Treatment Delivery Multileaf Collimator Leaf Resolution Recording guidance: Users may wish to augment code 98 Other (specify) with a free text field for recording other values of this item Multileaf Collimator Leaf Resolution Definition: The Multileaf Collimator Leaf Resolution of the external beam radiotherapy treatment machine, measured in millimetres. Format: Numeric N/A Conformal Shaping Technique Recording Guidance: Multileaf Collimator resolution may not be constant across the whole field. In this case record the resolution of the finest (smallest) leafs. Lung Cancer Clinical Data Standards 35
Slide 1. Slide 2. Slide 3. Investigation and management of lung cancer Robert Rintoul. Epidemiology. Risk factors/aetiology
 Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer
 THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer [Based on WOSCAN NSCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED
THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer [Based on WOSCAN NSCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED
GUIDELINES FOR CANCER IMAGING Lung Cancer
 GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
Bronchogenic Carcinoma
 A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
An Update: Lung Cancer
 An Update: Lung Cancer Andy Barlow Consultant in Respiratory Medicine Lead Clinician for Lung Cancer (West Herts Hospitals NHS Trust) Lead for EBUS-Harefield Hospital (RB&HFT) Summary Lung cancer epidemiology
An Update: Lung Cancer Andy Barlow Consultant in Respiratory Medicine Lead Clinician for Lung Cancer (West Herts Hospitals NHS Trust) Lead for EBUS-Harefield Hospital (RB&HFT) Summary Lung cancer epidemiology
Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010
 LSU HEALTH SCIENCES CENTER NSCLC Guidelines Feist-Weiller Cancer Center Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010 Initial Evaluation/Intervention: 1. Pathology Review 2. History and Physical
LSU HEALTH SCIENCES CENTER NSCLC Guidelines Feist-Weiller Cancer Center Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010 Initial Evaluation/Intervention: 1. Pathology Review 2. History and Physical
Collaborative Stage. Site-Specific Instructions - LUNG
 Slide 1 Collaborative Stage Site-Specific Instructions - LUNG In this presentation, we are going to review the AJCC Cancer Staging criteria for the lung primary site. Slide 2 Reading Assignments As each
Slide 1 Collaborative Stage Site-Specific Instructions - LUNG In this presentation, we are going to review the AJCC Cancer Staging criteria for the lung primary site. Slide 2 Reading Assignments As each
AJCC-NCRA Education Needs Assessment Results
 AJCC-NCRA Education Needs Assessment Results Donna M. Gress, RHIT, CTR Survey Tool 1 Survey Development, Delivery, Analysis THANKS to NCRA for the following work Developed survey with input from partners
AJCC-NCRA Education Needs Assessment Results Donna M. Gress, RHIT, CTR Survey Tool 1 Survey Development, Delivery, Analysis THANKS to NCRA for the following work Developed survey with input from partners
ACRIN NLST 6654 Primary Lung Cancer. F1/F2 Interval: to (mm-dd-yyyy) 1. Date of diagnosis: (mm-dd-yyyy)
 No. F1/F2 Interval: - - 20 to - - 20 (mm-dd-yyyy) 1. Date of diagnosis: - - 20 (mm-dd-yyyy) 2. Samples recorded: ZP Number S-Number 1) 2) 3) 4) (Refer to Form PX, Column 1. In the rare instance of a diagnosis
No. F1/F2 Interval: - - 20 to - - 20 (mm-dd-yyyy) 1. Date of diagnosis: - - 20 (mm-dd-yyyy) 2. Samples recorded: ZP Number S-Number 1) 2) 3) 4) (Refer to Form PX, Column 1. In the rare instance of a diagnosis
The 8th Edition Lung Cancer Stage Classification
 The 8th Edition Lung Cancer Stage Classification Elwyn Cabebe, M.D. Medical Oncology, Hematology, and Hospice and Palliative Care Valley Medical Oncology Consultants Director of Quality, Medical Oncology
The 8th Edition Lung Cancer Stage Classification Elwyn Cabebe, M.D. Medical Oncology, Hematology, and Hospice and Palliative Care Valley Medical Oncology Consultants Director of Quality, Medical Oncology
North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma
 THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
Surgical management of lung cancer
 Surgical management of lung cancer Nick Roubos FRACS Cardiothoracic Surgeon Box Hill Hospital, Epworth Eastern Thoracic Oncology Non Small Cell Lung Cancer (NSCLC) Small Cell Lung Cancer Mesothelioma Pulmonary
Surgical management of lung cancer Nick Roubos FRACS Cardiothoracic Surgeon Box Hill Hospital, Epworth Eastern Thoracic Oncology Non Small Cell Lung Cancer (NSCLC) Small Cell Lung Cancer Mesothelioma Pulmonary
Mediastinal Staging. Samer Kanaan, M.D.
 Mediastinal Staging Samer Kanaan, M.D. Overview Importance of accurate nodal staging Accuracy of radiographic staging Mediastinoscopy EUS EBUS Staging TNM Definitions T Stage Size of the Primary Tumor
Mediastinal Staging Samer Kanaan, M.D. Overview Importance of accurate nodal staging Accuracy of radiographic staging Mediastinoscopy EUS EBUS Staging TNM Definitions T Stage Size of the Primary Tumor
Thoracic Surgery; An Overview
 Thoracic Surgery What we see Thoracic Surgery; An Overview James P. Locher, Jr, MD Methodist Cardiovascular and Thoracic Surgery Lung cancer Mets Fungus and TB Lung abcess and empyema Pleural based disease
Thoracic Surgery What we see Thoracic Surgery; An Overview James P. Locher, Jr, MD Methodist Cardiovascular and Thoracic Surgery Lung cancer Mets Fungus and TB Lung abcess and empyema Pleural based disease
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
Collecting Cancer Data: Lung
 Collecting Cancer Data: Lung NAACCR 2011 2012 Webinar Series 2/2/2012 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this
Collecting Cancer Data: Lung NAACCR 2011 2012 Webinar Series 2/2/2012 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this
Lung Cancer Clinical Guidelines: Surgery
 Lung Cancer Clinical Guidelines: Surgery 1 Scope of guidelines All Trusts within Manchester Cancer are expected to follow this guideline. This guideline is relevant to: Adults (18 years and older) with
Lung Cancer Clinical Guidelines: Surgery 1 Scope of guidelines All Trusts within Manchester Cancer are expected to follow this guideline. This guideline is relevant to: Adults (18 years and older) with
Lung 8/7/14. Collecting Cancer Data: Lung NAACCR Webinar Series. August 7, 2014
 Collecting Cancer Data: Lung 2013 2014 NAACCR Webinar Series August 7, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Collecting Cancer Data: Lung 2013 2014 NAACCR Webinar Series August 7, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Role of Surgery in Management of Non Small Cell Lung Cancer. Dr. Ahmed Bamousa Consultant thoracic surgery Prince Sultan Military Medical City
 Role of Surgery in Management of Non Small Cell Lung Cancer Dr. Ahmed Bamousa Consultant thoracic surgery Prince Sultan Military Medical City Introduction Surgical approach Principle and type of surgery
Role of Surgery in Management of Non Small Cell Lung Cancer Dr. Ahmed Bamousa Consultant thoracic surgery Prince Sultan Military Medical City Introduction Surgical approach Principle and type of surgery
Seventh Edition of the Cancer Staging Manual and Stage Grouping of Lung Cancer. Quick Reference Chart and Diagrams
 CHEST Special Features Seventh Edition of the Cancer Staging Manual and Stage Grouping of Lung Cancer Quick Reference Chart and Diagrams Omar Lababede, MD ; Moulay Meziane, MD ; and Thomas Rice, MD, FCCP
CHEST Special Features Seventh Edition of the Cancer Staging Manual and Stage Grouping of Lung Cancer Quick Reference Chart and Diagrams Omar Lababede, MD ; Moulay Meziane, MD ; and Thomas Rice, MD, FCCP
Adam J. Hansen, MD UHC Thoracic Surgery
 Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
Breast Cancer Data Standards
 For reference only Do Not Use For more information, contact; cdsis@nhs.net Breast Cancer Data Standards These are standards current on 21st May 2007 National Clinical Dataset Development Programme (NCDDP)
For reference only Do Not Use For more information, contact; cdsis@nhs.net Breast Cancer Data Standards These are standards current on 21st May 2007 National Clinical Dataset Development Programme (NCDDP)
GROUP 1: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases. Including: Excluding:
 GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
Lung /1/16. Please submit all questions concerning webinar content through the Q&A panel. Reminder:
 1 NAACCR 2015-2016 Webinar Series Collecting Cancer Data: Lung NAACCR 2015 2016 Webinar Series Presented by: Angela Martin amartin@naaccr.org Jim Hofferkamp jhofferkamp@naaccr.org Q&A Please submit all
1 NAACCR 2015-2016 Webinar Series Collecting Cancer Data: Lung NAACCR 2015 2016 Webinar Series Presented by: Angela Martin amartin@naaccr.org Jim Hofferkamp jhofferkamp@naaccr.org Q&A Please submit all
Case Scenario 1. The patient agreed to a CT guided biopsy of the left upper lobe mass. This was performed and confirmed non-small cell carcinoma.
 Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Larry Tan, MD Thoracic Surgery, HSC. Community Cancer Care Educational Conference October 27, 2017
 Larry Tan, MD Thoracic Surgery, HSC Community Cancer Care Educational Conference October 27, 2017 To describe patient referral & triage for the patient with suspected lung cancer To describe the initial
Larry Tan, MD Thoracic Surgery, HSC Community Cancer Care Educational Conference October 27, 2017 To describe patient referral & triage for the patient with suspected lung cancer To describe the initial
Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600
 Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Understanding surgery
 What does surgery for lung cancer involve? Surgery for lung cancer involves an operation, which aims to remove all the cancer from the lung. Who will carry out my operation? In the UK, we have cardio-thoracic
What does surgery for lung cancer involve? Surgery for lung cancer involves an operation, which aims to remove all the cancer from the lung. Who will carry out my operation? In the UK, we have cardio-thoracic
NAACCR Webinar Series 1
 Collecting Cancer Data: Lung 2013 2014 NAACCR Webinar Series August 7, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Collecting Cancer Data: Lung 2013 2014 NAACCR Webinar Series August 7, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
MEDIASTINAL STAGING surgical pro
 MEDIASTINAL STAGING surgical pro Paul E. Van Schil, MD, PhD Department of Thoracic and Vascular Surgery University of Antwerp, Belgium Mediastinal staging Invasive techniques lymph node mapping cervical
MEDIASTINAL STAGING surgical pro Paul E. Van Schil, MD, PhD Department of Thoracic and Vascular Surgery University of Antwerp, Belgium Mediastinal staging Invasive techniques lymph node mapping cervical
Lung Cancer Imaging. Terence Z. Wong, MD,PhD. Department of Radiology Duke University Medical Center Durham, NC 9/9/09
 Lung Cancer Imaging Terence Z. Wong, MD,PhD Department of Radiology Duke University Medical Center Durham, NC 9/9/09 Acknowledgements Edward F. Patz, Jr., MD Jenny Hoang, MD Ellen L. Jones, MD, PhD Lung
Lung Cancer Imaging Terence Z. Wong, MD,PhD Department of Radiology Duke University Medical Center Durham, NC 9/9/09 Acknowledgements Edward F. Patz, Jr., MD Jenny Hoang, MD Ellen L. Jones, MD, PhD Lung
LUNG STAGING FORM LATERALITY: LEFT RIGHT BILATERAL
 LUNG STAGING FORM LATERALITY: LEFT RIGHT BILATERAL ( ) Tx Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging
LUNG STAGING FORM LATERALITY: LEFT RIGHT BILATERAL ( ) Tx Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging
Lung Cancer Case Study
 Lung Cancer Case Study Presented by s GP Education Programme 2 Part One Initial presentation 60 year old lady, presents with a 6 week history of right sided chest pain. The pain is like a dull ache, but
Lung Cancer Case Study Presented by s GP Education Programme 2 Part One Initial presentation 60 year old lady, presents with a 6 week history of right sided chest pain. The pain is like a dull ache, but
Charles Mulligan, MD, FACS, FCCP 26 March 2015
 Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
NICE Quality Standards and COF
 NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
6 th Reprint Handbook Pages AJCC 7 th Edition
 6 th Reprint Handbook Pages AJCC 7 th Edition AJCC 7 th Edition Errata for 6 th Reprint Table 1 Handbook No Significant Staging Clarifications for 6 th Reprint AJCC 7 th Edition Errata for 6 th Reprint
6 th Reprint Handbook Pages AJCC 7 th Edition AJCC 7 th Edition Errata for 6 th Reprint Table 1 Handbook No Significant Staging Clarifications for 6 th Reprint AJCC 7 th Edition Errata for 6 th Reprint
WHITE PAPER - SRS for Non Small Cell Lung Cancer
 WHITE PAPER - SRS for Non Small Cell Lung Cancer I. Introduction This white paper will focus on non-small cell lung carcinoma with sections one though six comprising a general review of lung cancer from
WHITE PAPER - SRS for Non Small Cell Lung Cancer I. Introduction This white paper will focus on non-small cell lung carcinoma with sections one though six comprising a general review of lung cancer from
The Itracacies of Staging Patients with Suspected Lung Cancer
 The Itracacies of Staging Patients with Suspected Lung Cancer Gerard A. Silvestri, MD,MS, FCCP Professor of Medicine Medical University of South Carolina Charleston, SC silvestri@musc.edu Staging Lung
The Itracacies of Staging Patients with Suspected Lung Cancer Gerard A. Silvestri, MD,MS, FCCP Professor of Medicine Medical University of South Carolina Charleston, SC silvestri@musc.edu Staging Lung
Case Conference: Post-Operative Radiotherapy for Non-Small Cell Lung Cancer. Doug Rahn 6/1/12
 Case Conference: Post-Operative Radiotherapy for Non-Small Cell Lung Cancer Doug Rahn 6/1/12 Outline I. Presentation of Case II. Epidemiology III. Staging IV. Review of Literature V. Recommendations VI.
Case Conference: Post-Operative Radiotherapy for Non-Small Cell Lung Cancer Doug Rahn 6/1/12 Outline I. Presentation of Case II. Epidemiology III. Staging IV. Review of Literature V. Recommendations VI.
Endobronchial valve insertion to reduce lung volume in emphysema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
Lung cancer LUNG CANCER. Box 1 Clinical signs
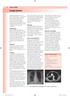 22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
FDG PET/CT in Lung Cancer Read with the experts. Homer A. Macapinlac, M.D.
 FDG PET/CT in Lung Cancer Read with the experts Homer A. Macapinlac, M.D. Patient with suspected lung cancer presents with left sided chest pain T3 What is the T stage of this patient? A) T2a B) T2b C)
FDG PET/CT in Lung Cancer Read with the experts Homer A. Macapinlac, M.D. Patient with suspected lung cancer presents with left sided chest pain T3 What is the T stage of this patient? A) T2a B) T2b C)
Lung Cancer - Suspected
 Lung Cancer - Suspected Shared Decision Making Lung Cancer: http://www.enhertsccg.nhs.uk/ Patient presents with abnormal CXR Lung cancer - clinical presentation History and Examination Incidental finding
Lung Cancer - Suspected Shared Decision Making Lung Cancer: http://www.enhertsccg.nhs.uk/ Patient presents with abnormal CXR Lung cancer - clinical presentation History and Examination Incidental finding
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
and Strength of Recommendations
 ASTRO with ASCO Qualifying Statements in Bold Italics s patients with T1-2, N0 non-small cell lung cancer who are medically operable? 1A: Patients with stage I NSCLC should be evaluated by a thoracic surgeon,
ASTRO with ASCO Qualifying Statements in Bold Italics s patients with T1-2, N0 non-small cell lung cancer who are medically operable? 1A: Patients with stage I NSCLC should be evaluated by a thoracic surgeon,
Lung Cancer. Definition of lung cancer Malignancy arising from lung tissue
 Lung Cancer Definition of lung cancer Malignancy arising from lung tissue Epidemiology of lung cancer Commonest malignancy in western world Commonest cause of death in men and women in the UK Approximately
Lung Cancer Definition of lung cancer Malignancy arising from lung tissue Epidemiology of lung cancer Commonest malignancy in western world Commonest cause of death in men and women in the UK Approximately
LungStage. Bringing machine learning to Nuclear Medicine and Lung Cancer using Big Data, Machine Learning and Multicenter Studies
 LungStage Bringing machine learning to Nuclear Medicine and Lung Cancer using Big Data, Machine Learning and Multicenter Studies Medical Team: Bram Stieltjes, MD PhD; Alex Sauter, MD; Gregor Sommer, MD;
LungStage Bringing machine learning to Nuclear Medicine and Lung Cancer using Big Data, Machine Learning and Multicenter Studies Medical Team: Bram Stieltjes, MD PhD; Alex Sauter, MD; Gregor Sommer, MD;
Lung cancer. The diagnosis and treatment of lung cancer. Issued: April NICE clinical guideline 121. guidance.nice.org.
 Lung cancer The diagnosis and treatment of lung cancer Issued: April 2011 NICE clinical guideline 121 guidance.nice.org.uk/cg121 NICE has accredited the process used by the Centre for Clinical Practice
Lung cancer The diagnosis and treatment of lung cancer Issued: April 2011 NICE clinical guideline 121 guidance.nice.org.uk/cg121 NICE has accredited the process used by the Centre for Clinical Practice
The Imaging Journey of Patients with Malignant Pleural Mesothelioma: Experience of a Tertiary Mesothelioma MDT
 The Imaging Journey of Patients with Malignant Pleural Mesothelioma: Experience of a Tertiary Mesothelioma MDT V. Lam, J. Brozik, A. J. Sharkey, A. Bajaj, D. T. Barnes Glenfield Hospital, Leicester, United
The Imaging Journey of Patients with Malignant Pleural Mesothelioma: Experience of a Tertiary Mesothelioma MDT V. Lam, J. Brozik, A. J. Sharkey, A. Bajaj, D. T. Barnes Glenfield Hospital, Leicester, United
PLACE LABEL HERE. Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form. RTOG Study No.
 Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form RTOG Study No. 0813 Case # Name RTOG Patient ID INSTRUCTIONS: Submit this form at the appropriate followup
Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form RTOG Study No. 0813 Case # Name RTOG Patient ID INSTRUCTIONS: Submit this form at the appropriate followup
Lung Cancer Resection
 Lung Cancer Resection Introduction The occurrence of lung cancer has increased dramatically over the last 50 years. Your health care provider may have recommended an operation to remove your lung cancer.
Lung Cancer Resection Introduction The occurrence of lung cancer has increased dramatically over the last 50 years. Your health care provider may have recommended an operation to remove your lung cancer.
Lung. 10/24/13 Chest X-ray: 2.9 cm mass like density in the inferior lingular segment worrisome for neoplasm. Malignancy cannot be excluded.
 Lung Case Scenario 1 A 54 year white male presents with a recent abnormal CT of the chest. The patient has a history of melanoma, kidney, and prostate cancers. 10/24/13 Chest X-ray: 2.9 cm mass like density
Lung Case Scenario 1 A 54 year white male presents with a recent abnormal CT of the chest. The patient has a history of melanoma, kidney, and prostate cancers. 10/24/13 Chest X-ray: 2.9 cm mass like density
Non small cell Lung Cancer
 Non small cell Lung Cancer The 13th refresher course for residents in radiation oncology Jiraporn Setakornnukul, M.D. Radiation oncology division, Radiology department Siriraj Hospital, Mahidol University
Non small cell Lung Cancer The 13th refresher course for residents in radiation oncology Jiraporn Setakornnukul, M.D. Radiation oncology division, Radiology department Siriraj Hospital, Mahidol University
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS
 Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Endoscopic UltraSound (EUS) Endoscopic Mucosal Resection (EMR) Moishe Liberman Director C.E.T.O.C.
 Endoscopic UltraSound (EUS) Endoscopic Mucosal Resection (EMR) Moishe Liberman Director C.E.T.O.C. Division of Thoracic Surgery Centre Hospitalier de l Université de Montréal Research Grants: Disclosures
Endoscopic UltraSound (EUS) Endoscopic Mucosal Resection (EMR) Moishe Liberman Director C.E.T.O.C. Division of Thoracic Surgery Centre Hospitalier de l Université de Montréal Research Grants: Disclosures
Lung Carcinoid Tumor Early Detection, Diagnosis, and Staging
 Lung Carcinoid Tumor Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that can
Lung Carcinoid Tumor Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that can
Early-stage locally advanced non-small cell lung cancer (NSCLC) Clinical Case Discussion
 Early-stage locally advanced non-small cell lung cancer (NSCLC) Clinical Case Discussion Pieter Postmus The Clatterbridge Cancer Centre Liverpool Heart and Chest Hospital Liverpool, United Kingdom 1 2
Early-stage locally advanced non-small cell lung cancer (NSCLC) Clinical Case Discussion Pieter Postmus The Clatterbridge Cancer Centre Liverpool Heart and Chest Hospital Liverpool, United Kingdom 1 2
The International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project, Data Elements
 Page 1 Contents 1.1. Registration... 2 1.2. Patient Characteristics... 3 1.3. Laboratory Values at Diagnosis... 5 1.4. Lung Cancers with Multiple Lesions... 6 1.5. Primary Tumour Description... 10 1.6.
Page 1 Contents 1.1. Registration... 2 1.2. Patient Characteristics... 3 1.3. Laboratory Values at Diagnosis... 5 1.4. Lung Cancers with Multiple Lesions... 6 1.5. Primary Tumour Description... 10 1.6.
Lung Cancer. Current Therapy JEREMIAH MARTIN MBBCh FRCSI MSCRD
 Lung Cancer Current Therapy JEREMIAH MARTIN MBBCh FRCSI MSCRD Objectives Describe risk factors, early detection & work-up of lung cancer. Define the role of modern treatment options, minimally invasive
Lung Cancer Current Therapy JEREMIAH MARTIN MBBCh FRCSI MSCRD Objectives Describe risk factors, early detection & work-up of lung cancer. Define the role of modern treatment options, minimally invasive
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
Guideline for the Diagnosis of Breast Cancer
 Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
Guideline for the Diagnosis of Breast Cancer Version History Version Date Brief Summary of Change Issued 2.0 May 2007 Approved by the Governance Committee 2.0 25.11.08 Discussed at the NSSG 2.1 5.12.08
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
MEDIASTINAL LYMPH NODE METASTASIS IN PATIENTS WITH CLINICAL STAGE I PERIPHERAL NON-SMALL-CELL LUNG CANCER
 MEDIASTINAL LYMPH NODE METASTASIS IN PATIENTS WITH CLINICAL STAGE I PERIPHERAL NON-SMALL-CELL LUNG CANCER Tsuneyo Takizawa, MD a Masanori Terashima, MD a Teruaki Koike, MD a Hideki Akamatsu, MD a Yuzo
MEDIASTINAL LYMPH NODE METASTASIS IN PATIENTS WITH CLINICAL STAGE I PERIPHERAL NON-SMALL-CELL LUNG CANCER Tsuneyo Takizawa, MD a Masanori Terashima, MD a Teruaki Koike, MD a Hideki Akamatsu, MD a Yuzo
National Optimal Lung Cancer Pathway
 National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
Chapter 8. Other Important Tests and Procedures. Mosby items and derived items 2011, 2006 by Mosby, Inc., an affiliate of Elsevier Inc.
 Chapter 8 Other Important Tests and Procedures 1 Introduction Additional important diagnostic studies include: Sputum examination Skin tests Endoscopic examination Lung biopsy Thoracentesis Hematology,
Chapter 8 Other Important Tests and Procedures 1 Introduction Additional important diagnostic studies include: Sputum examination Skin tests Endoscopic examination Lung biopsy Thoracentesis Hematology,
UICC TNM 8 th Edition Errata
 UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
UICC TNM 8 th Edition Errata ions are in italics Page 28 Oropharynx p16 positive Pathological Stage II,T2 N2 M0 T3 N0,N1 M0 Stage II,T2 N2 M0 T3,T4 N0,N1 M0 Page 61 Oesophagus Adenocarcinoma Pathological
Video-Mediastinoscopy Thoracoscopy (VATS)
 Surgical techniques Video-Mediastinoscopy Thoracoscopy (VATS) Gunda Leschber Department of Thoracic Surgery ELK Berlin Chest Hospital, Berlin, Germany Teaching Hospital of Charité Universitätsmedizin Berlin
Surgical techniques Video-Mediastinoscopy Thoracoscopy (VATS) Gunda Leschber Department of Thoracic Surgery ELK Berlin Chest Hospital, Berlin, Germany Teaching Hospital of Charité Universitätsmedizin Berlin
LYMPHATIC DRAINAGE IN THE HEAD & NECK
 LYMPHATIC DRAINAGE IN THE HEAD & NECK Like other parts of the body, the head and neck contains lymph nodes (commonly called glands). Which form part of the overall Lymphatic Drainage system of the body.
LYMPHATIC DRAINAGE IN THE HEAD & NECK Like other parts of the body, the head and neck contains lymph nodes (commonly called glands). Which form part of the overall Lymphatic Drainage system of the body.
Histopathology of NSCLC, IHC markers and ptnm classification
 ESMO Preceptorship on Non-Small Cell Lung Cancer November 15 th & 16 th 2017 Singapore Histopathology of NSCLC, IHC markers and ptnm classification Prof Keith M Kerr Department of Pathology, Aberdeen University
ESMO Preceptorship on Non-Small Cell Lung Cancer November 15 th & 16 th 2017 Singapore Histopathology of NSCLC, IHC markers and ptnm classification Prof Keith M Kerr Department of Pathology, Aberdeen University
SIGN 137 Management of lung cancer. A national clinical guideline February Evidence
 SIGN 137 Management of lung cancer A national clinical guideline February 2014 Evidence KEY TO EVIDENCE STATEMENTS AND GRADES OF RECOMMENDATIONS LEVELS OF EVIDENCE 1 ++ High quality meta-analyses, systematic
SIGN 137 Management of lung cancer A national clinical guideline February 2014 Evidence KEY TO EVIDENCE STATEMENTS AND GRADES OF RECOMMENDATIONS LEVELS OF EVIDENCE 1 ++ High quality meta-analyses, systematic
Evaluation of Cancer Outcomes Barwon South West Registry
 Evaluation of Cancer Outcomes Barwon South West Registry Data Request Form Applicant details Applicant name: Position: Email: Project start date: Date: Telephone: Project completion date: Project details
Evaluation of Cancer Outcomes Barwon South West Registry Data Request Form Applicant details Applicant name: Position: Email: Project start date: Date: Telephone: Project completion date: Project details
National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia
 National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia Patient Details Surname: NHS number: Forename: Postcode: Sex: Male Female
National Oesophago-Gastric Cancer Audit New Patient Registration sheet Patients with Oesophageal High Grade Glandular Dysplasia Patient Details Surname: NHS number: Forename: Postcode: Sex: Male Female
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma
 Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
CT Depiction of Regional Nodal Stations for Lung Cancer Staging
 ownloaded from www.ajronline.org by 37.44.204.189 on 11/24/17 from IP address 37.44.204.189. opyright RRS. For personal use only; all rights reserved T epiction of Regional Nodal Stations for Lung ancer
ownloaded from www.ajronline.org by 37.44.204.189 on 11/24/17 from IP address 37.44.204.189. opyright RRS. For personal use only; all rights reserved T epiction of Regional Nodal Stations for Lung ancer
Lung cancer forms in tissues of the lung, usually in the cells lining air passages.
 Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Thoracoscopy for Lung Cancer
 Thoracoscopy for Lung Cancer Introduction The occurrence of lung cancer has increased dramatically over the last 50 years. Your doctor may have recommended an operation to remove your lung cancer. The
Thoracoscopy for Lung Cancer Introduction The occurrence of lung cancer has increased dramatically over the last 50 years. Your doctor may have recommended an operation to remove your lung cancer. The
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Lung Cancer. This reference summary will help you better understand lung cancer and the treatment options that are available.
 Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Guide to Understanding Lung Cancer
 Guide to Understanding Lung Cancer Lung cancer is the second most common cancer overall for men and women in the U.S., with an estimated 222,500 new cases in 2017. However, lung cancer is the most common
Guide to Understanding Lung Cancer Lung cancer is the second most common cancer overall for men and women in the U.S., with an estimated 222,500 new cases in 2017. However, lung cancer is the most common
The diagnosis and treatment of lung cancer
 The diagnosis and treatment of lung cancer NICE guideline Second draft for consultation, September 2004 If you wish to comment on the recommendations, please make your comments on the full version of the
The diagnosis and treatment of lung cancer NICE guideline Second draft for consultation, September 2004 If you wish to comment on the recommendations, please make your comments on the full version of the
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012
 Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Audit Report. Lung Cancer Quality Performance Indicators. Patients diagnosed April 2014 March Published: May 2016
 NORTH OF SCOTLAND PLANNING GROUP Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: May 2016 Mr Hardy Remmen
NORTH OF SCOTLAND PLANNING GROUP Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: May 2016 Mr Hardy Remmen
Small Cell Lung Cancer
 Small Cell Lung Cancer Small cell lung cancer (SCLC) affects 15% of all lung cancer patients. SCLC is the most aggressive type of lung cancer. It may be treated with chemotherapy and radiation. SCLC has
Small Cell Lung Cancer Small cell lung cancer (SCLC) affects 15% of all lung cancer patients. SCLC is the most aggressive type of lung cancer. It may be treated with chemotherapy and radiation. SCLC has
Table 1 Major Classifications of Lung Cancer. Non Small Cell Lung Cancer Adenocarcinoma
 VIII LUNG CANCER Jeffrey Crawford, m.d. Definition and Classifications Bronchogenic carcinoma of the lung lung cancer comprises a group of malignant neoplasms that arise from bronchial epithelium. The
VIII LUNG CANCER Jeffrey Crawford, m.d. Definition and Classifications Bronchogenic carcinoma of the lung lung cancer comprises a group of malignant neoplasms that arise from bronchial epithelium. The
Interventional Pulmonology
 Interventional Pulmonology The Division of Thoracic Surgery Department of Cardiothoracic Surgery New York Presbyterian/Weill Cornell Medical College p: 212-746-6275 f: 212-746-8223 https://weillcornell.org/eshostak
Interventional Pulmonology The Division of Thoracic Surgery Department of Cardiothoracic Surgery New York Presbyterian/Weill Cornell Medical College p: 212-746-6275 f: 212-746-8223 https://weillcornell.org/eshostak
Small Cell Lung Cancer Early Detection, Diagnosis, and Staging
 Small Cell Lung Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that
Small Cell Lung Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that
Respiratory Interactive Session. Elaine Borg
 Respiratory Interactive Session Elaine Borg Case 1 Respiratory Cytology 55 year old gentleman Anterior mediastinal mass EBUS FNA Case 1 Respiratory Cytology 55 year old gentleman with anterior mediastinal
Respiratory Interactive Session Elaine Borg Case 1 Respiratory Cytology 55 year old gentleman Anterior mediastinal mass EBUS FNA Case 1 Respiratory Cytology 55 year old gentleman with anterior mediastinal
Imaging of Lung Cancer: A Review of the 8 th TNM Staging System
 Imaging of Lung Cancer: A Review of the 8 th TNM Staging System Travis S Henry, MD Associate Professor of Clinical Radiology Cardiac and Pulmonary Imaging Section University of California, San Francisco
Imaging of Lung Cancer: A Review of the 8 th TNM Staging System Travis S Henry, MD Associate Professor of Clinical Radiology Cardiac and Pulmonary Imaging Section University of California, San Francisco
Chest and cardiovascular
 Module 1 Chest and cardiovascular A. Doss and M. J. Bull 1. Regarding the imaging modalities of the chest: High resolution computed tomography (HRCT) uses a slice thickness of 4 6 mm to identify mass lesions
Module 1 Chest and cardiovascular A. Doss and M. J. Bull 1. Regarding the imaging modalities of the chest: High resolution computed tomography (HRCT) uses a slice thickness of 4 6 mm to identify mass lesions
Chest X-ray Interpretation
 Chest X-ray Interpretation Introduction Routinely obtained Pulmonary specialist consultation Inherent physical exam limitations Chest x-ray limitations Physical exam and chest x-ray provide compliment
Chest X-ray Interpretation Introduction Routinely obtained Pulmonary specialist consultation Inherent physical exam limitations Chest x-ray limitations Physical exam and chest x-ray provide compliment
FEE RULES RADIATION ONCOLOGY FEE SCHEDULE CONTENTS
 Tel: +27-21-9494060 Fax: +27-21-9494112 E-mail: leon.gouws@cancercare.co.za FEE RULES RADIATION ONCOLOGY FEE SCHEDULE CONTENTS 1. EXTERNAL BEAM RADIATION... 2 2. PLANNING OF TREATMENT... 2 3. DELIVERY
Tel: +27-21-9494060 Fax: +27-21-9494112 E-mail: leon.gouws@cancercare.co.za FEE RULES RADIATION ONCOLOGY FEE SCHEDULE CONTENTS 1. EXTERNAL BEAM RADIATION... 2 2. PLANNING OF TREATMENT... 2 3. DELIVERY
THORACIK RICK. Lungs. Outline and objectives Richard A. Malthaner MD MSc FRCSC FACS
 THORACIK RICK Outline and objectives Lungs Management of a solitary lung nodule Mediastinum Management of a mediastinal mass Pleura Management of a pleural fluid & pneumothorax Esophagus & Stomach Management
THORACIK RICK Outline and objectives Lungs Management of a solitary lung nodule Mediastinum Management of a mediastinal mass Pleura Management of a pleural fluid & pneumothorax Esophagus & Stomach Management
Audit Report. Lung Cancer Quality Performance Indicators. Patients diagnosed January December Published: November 2017
 Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed January December 2016 Published: November 2017 Hardy Remmen NOSCAN Lung Cancer MCN Clinical
Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed January December 2016 Published: November 2017 Hardy Remmen NOSCAN Lung Cancer MCN Clinical
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
THORACIC MALIGNANCIES
 THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
OBJECTIVES. Solitary Solid Spiculated Nodule. What would you do next? Case Based Discussion: State of the Art Management of Lung Nodules.
 Organ Imaging : September 25 2015 OBJECTIVES Case Based Discussion: State of the Art Management of Lung Nodules Dr. Elsie T. Nguyen Dr. Kazuhiro Yasufuku 1. To review guidelines for follow up and management
Organ Imaging : September 25 2015 OBJECTIVES Case Based Discussion: State of the Art Management of Lung Nodules Dr. Elsie T. Nguyen Dr. Kazuhiro Yasufuku 1. To review guidelines for follow up and management
Video-assisted thoracoscopic surgery in lung cancer staging
 Review Article on Thoracic Surgery Page 1 of 7 Video-assisted thoracoscopic surgery in lung cancer staging Frederico Krieger Martins, Guilherme Augusto Oliveira, Juliano Cé Coelho, Márcio Chmelnitsky Kruter,
Review Article on Thoracic Surgery Page 1 of 7 Video-assisted thoracoscopic surgery in lung cancer staging Frederico Krieger Martins, Guilherme Augusto Oliveira, Juliano Cé Coelho, Márcio Chmelnitsky Kruter,
Innovations in Lung Cancer Diagnosis and Surgical Treatment
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
