United States Patent (19) Friese et al.
|
|
|
- Louisa Stewart
- 5 years ago
- Views:
Transcription
1 United States Patent (19) Friese et al. 11 Patent Number: (45) Date of Patent: Feb. 27, SULFITED FATS AS OILING AGENTS FOR LEATHER AND SKINS 75) Inventors: Hans-Herbert Friese, Monheim; Friedrich Pieper, Langenfeld-Richrath; Reinhard Bosse, Duessledorf, all of Fed. Rep. of Germany 73) Assignee: 21 Appl. No.: 5,909 (22) Filed: Oct. 11, 1988 Henkel Kommanditgesellschaft auf Aktien, Dusseldorf, Fed. Rep. of Germany Related U.S. Application Data 62 Division of Ser. No. 53,998, May 26, 1987, Pat. No. (30) Foreign Application Priority Data May 26, 1986 DE Fed. Rep. of Germany Int. Cl."... C14C5/00; CO7C 143/90 52 U.S. Cl.... 8/94.23; 260/400; 8/94.21; 8/ ) Field of Search... 8/94.22, 94.21, 94.23; 260/ References Cited U.S. PATENT DOCUMENTS 4,800,045 1/1989 Friese et al /400 FOREIGN PATENT DOCUMENTS /1986 Fed. Rep. of Germany. Primary Examiner-Paul Lieberman Assistant Examiner-John F. McNally Attorney, Agent, or Firm-Ernest G. Szoke; Wayne C. Jaeschke; Henry E. Millson, Jr. 57 ABSTRACT Sulfited fats prepared by oxidation of fats with oxygen containing gas mixtures and simultaneous or subsequent sulfitation using alkali and/or ammonium hydrogen sulfites, wherein the fats used are mixtures containing (A) fats having iodine numbers below about 100 and (B) fatty acid esters having iodine numbers of from about 60 to about 100 and containing from 12 to 24 carbon atoms in the linear or branched, natural and/or synthetic fatty acid residue and from 1 to 5 carbon atoms in the monofunctional alcohol resi due the ratio by weight of (A) to (B) being from about 9:1 to about 1:4. 11 Claims, No Drawings
2 - w 1. SULFITED FATS AS OILNGAGENTS FOR LEATHER AND SKINS This application is a division of application Ser. No. 053,998, filed May 26, 1987, now Pat. No. 4,800,045. BACKGROUND OF THE INVENTION 1. Field of the Invention This invention relates to sulfited fats difficultly sulfitable fats having iodine numbers below 100, to their use as oiling agents for leather and skins and to a process for sulfiting fats having iodine numbers below Statement of Related Art Sulfited fats used as oiling agents for leather and skins are produced by oxidation of fats with oxygen-contain ing gas mixtures, for example air, and simultaneous or subsequent sulfitation with alkali and/or ammonium hydrogen sulfite. For fats to be sulfited at all, it is abso lutely essential to oxidize them before or during sulfita tion by introduction of oxygen-containing gas mixtures. However, since the oxidizability of fats decreases with decreasing iodine number, relatively highly unsaturated fats and oils, i.e. those having iodine numbers above about 100 and preferably above about 130, are normally used (cf. for example "Rauchwarenherstellung und Pelzkonfektion', VEB Fachbuchverlag Leipzig 1970, page 116). Sulfited fats sea-animal oils having high iodine numbers, for example whale oil or fish oil, or from vegetable oils having high iodine numbers, for example soya oil, form readily water-emulsifiable sub stances of high emulsion stability which are particularly suitable for oiling leather and skins. If, by contrast, difficultly sulfitable fats having an iodine number below 100, for example sperm oil, are subjected to oxidizing sulfitation, the oxidation velocity of the fats decreases drastically and products difficult to emulsify in water and having poor emulsion stability are obtained. In order nevertheless to be able to use these sulfitation products for oiling leather and skins, they have to be mixed with emulsifiers or with water-emulsifiable fats which are capable of co-emulsifying the difficulty emul sifiable sulfitation products. STATEMENT OF THE INVENTION Other than in the operating examples, or where oth erwise indicated, all numbers expressing quantities of ingredients or reaction conditions used herein are to be understood as modified in all instances by the term about. An object of the present invention is to provide sul fited fats difficultly sulfitable fats having iodine numbers below 100 which form readily water emulsifiable products of high emulsion stability and which are suitable for oiling leather and skins. It has now surprisingly been found that difficultly sulfitable fats having iodine numbers below 100, for example coconut oil, palm kernel oil, palm oil, tallow, lard, neat's-foot oil, sperm oil, whale oil, triolein, oleic acid, wax esters, fatty acid monoglycerides, fatty acid diglycerides, and/or fatty acid triglycerides, form readily water-emulsifiable sulfitation products of high emulsion stability providing they are mixed before the oxidizing sulfitation step with fatty acid esters having iodine numbers of from 60 to 100 in a ratio by weight of fat to ester of from 9:1 to 1:4. Suitable fatty acid esters contain from 12 to 24 carbon atoms in the fatty acid residue, which is of natural and/or synthetic origin and which may be linear or branched, and from 1 to 5 car bon atoms in the monofunctional alcohol residue. Fatty acid esters containing unsaturated fatty acid residues can be used either individually or in combination with fatty acid esters containing saturated fatty acid residues. Accordingly, the present invention relates to sulfited fats prepared by oxidation of fats with oxygen-contain ing gas mixtures and simultaneous or subsequent sulfita tion using alkali and/or ammonium hydrogen sulfites, wherein the fats used are mixtures containing (A) fats having iodine numbers below about 100, and (B) fatty acid esters having iodine numbers from 60 to 100 and containing from 12 to 24 carbon atoms in the linear or branched, natural and/or synthetic fatty acid residue and from 1 to 5 carbon atoms in the monofunctional alcohol residue; the ratio of weight of A to B being from 9:1 to 1:4. Difficultly sulfitable fats having iodine numbers below 100, and preferably of from 7 to 95, are prefera bly mixed with fatty acid esters having iodine numbers of from 60 to 100 in a ratio by weight of fat to fatty acid ester of from 4:1 to 2:3. below 100 include, for example, fats and oils containing unsaturated fatty acids and/or fatty acid esters, natural and/or synthetic unsaturated fatty acids containing from 12 to 24 carbon atoms, if desired in combination with natural and/or synthetic saturated fatty acids con taining from 12 to 24 carbon atoms, natural and/or synthetic unsaturated fatty acid esters containing from 12 to 24 carbon atoms both in the fatty acid residue and in the alcohol residue, if desired in combination with the analogous natural and/or synthetic saturated fatty acid esters, synthetic fatty acid monoglycerides prepared from unsaturated fatty acids containing from 12 to 24 carbon atoms, if desired in combination with the analo gous saturated, synthetic fatty acid monoglycerides, synthetic fatty acid diglycerides and triglycerides pre pared from unsaturated or unsaturated/saturated fatty acids containing from 12 to 24 carbon atoms and mix tures of fats from the above-mentioned groups. The preferred difficultly sulfitable fats having iodine num bers below 100 and preferably of from 7 to 95 include coconut oil, palm kernel oil, palm oil, tallow, lard, neat's-foot oil, sperm oil, whale oil, triolein. oleic acid, wax esters, fatty acid monoglycerides, fatty acid diglyc erides, fatty acid triglycerides, and mixtures thereof. below 100 and preferably of from 7 to 95 are preferably mixed with fatty acid esters having iodine numbers of from 70 to 85. It is possible to use both unsaturated fatty acid esters and also mixtures of unsaturated and satu rated fatty acid esters having iodine numbers of from 60 to 100 and preferably of from 70 to 85. Suitable fatty acid esters having iodine numbers of from 60 to 100 and preferably of from 70 to 85 contain from 12 to 24, preferably from 12 to 18, and more pref erably from 16 to 18 carbon atoms in the fatty acid residue and from 1 to 5 carbon atoms in the monofunc tional alcohol residue. The fatty acid residue may be linear or branched. Fatty acid methyl, ethyl, isopropyl, and/or isobutyl esters are preferably used as the fatty acid esters. Fat mixtures containing difficultly sulfitable fats hav ing iodine numbers below 100 and preferably of from 7 to 95, and fatty acid esters having iodine numbers of
3 3 from 60 to 100, and preferably of from 70 to 85, are sulfited with 0.5 to 2.5 moles of alkali and/or ammo nium hydrogen sulfites or alkali and/or ammonium hydrogen sulfites and alkali and/or ammonium sulfites in the form of aqueous solutions or suspensions, prefera bly in the form of saturated aqueous solutions, at tem peratures of from 70 to 110 C., and preferably at tem peratures of from 85 to 95 C. The present invention also relates to the use of the sulfited fats according to the invention, if desired in 10 combination with other oiling agents known per se and/or with anionic and/or nonionic emulsifiers, as oiling agents for leather and skins. Suitable oiling agents which may be used together with the sulfited fats according to the invention are, for example, sulfated fats, sulfonated fats, sulfited fats pre pared from high-iodine fats, sulfochlorinated fats or phosphated fats, neutral oils, and mixtures thereof. Suitable anionic and/or nonionic emulsifiers are, for example, alkylene oxide adducts of fatty alcohols, fatty acids, fatty acid amides, fatty amines, and/or alkyl phe nols and sulfuric acid and/or phosphoric acid esters thereof, fatty alcohol sulfates, alkyl sulfates and/or alkylbenzene sulfonates. Oiling agents containing sulfited fats according to the invention are used in quantities of from 1 to % by weight, based on the pelt or sharing weight, and prefer ably in quantity of from 1 to 2% by weight during chrome tanning, and in quantities of from 4 to 16% by weight during the main tanning process or, depending 30 on the type of leather or skin, in concentrations of from 2 to 10 g/l liquor and preferably in concentrations of from 5 to 7 g/l liquor. In oiling agents consisting of several oiling agents and, optionally, emulsifiers, the sulfited fats according to the invention are present in a quantity of from 10 to 95% by weight. The oiling temperature is in the range of from ' to 75' C. and preferably in the range of from to 50 C. The present invention also relates to a process for sulfiting fats having iodine numbers below about 100, 40 characterized in that (A) fats having iodine numbers below about 100 are mixed with (B) fatty acid esters having iodine numbers of from about 60 to about 100 and containing from 12 to 24 carbon atoms in the linear or branched, natural and/or synthetic fatty acid residue 45 and from 1 to 5 carbon atoms in the monofunctional alcohol residue, the ratio by weight of (A) to (B) being from about 9:1 to about 1:4, and the resulting fatty mix tures are oxidized in known manner with oxygen-con taining gas mixtures and then sulfited using alkali and 50 /or ammonium hydrogen sulfites, if desired while more oxygen-containing gas mixture is passed through. It is preferred to use sulfite fat mixtures containing fats having iodine numbers below about 100 and prefer ably of from 7 to 95 and fatty acid esters having iodine 55 numbers of from 60 to 100 in a ratio by weight of from 4:1 to 2:3. below 100, which are suitable as adducts for the process according to the invention for sulfiting fats having io 60 dine numbers below 100, are those described above. According to the invention, the difficultly sulfitable fats having iodine numbers below 100 and preferably of from 7 to 95 are mixed before the oxidizing sulfitation with fatty acid esters having iodine numbers of from 60 to 100 and preferably of from 70 to 85 in a ratio by weight of fat to fatty acid ester of from 9:1 to 1:4 and preferably of from 4:1 to 2:3. Suitable fatty acid esters 4 having iodine numbers of from 60 to 100 and preferably of form 70 to 85 contain from 12 to 24, preferably from 12 to 18 and more preferably from 16 to 18 carbon atoms in the fatty acid residue and from 1 to 5 carbon atoms in the monofunctional alcohol residue. The fatty acid residue may be linear or branched. It is possible to use both unsaturated fatty acid esters and also mixtures of saturated and unsaturated fatty acid esters. The fatty acid esters can be prepared by various methods, for example by transesterification of fats and oils with monofunctional alcohols containing from 1 to 5 carbon atoms or by esterification of fatty acids of natural and /or synthetic origin with monofunctional alcohols con taining from 1 to 5 carbon atoms. Methanol, ethanol, isopropanol and/or isobutanol are preferably used as alcohol components for the preparation of fatty acid esters. In the process of the invention for sulfiting fats hav ing iodine numbers below 100, the fat mixtures are oxi dized at 70 to 0 C., and preferably at 80 to 1 C. by passing an oxygen-containing fat mixture through the fats, which are then sulfited, optionally while more oxygen-containing gas mixture is passed through, at 70 to 110 C. and preferably at 85 to 95 C. with 0.5 to 2.5 moles of alkali and/or ammonium hydrogen sulfites or alkali and/or ammonium hydrogen sulfites and alkali and/or ammonium sulfites/kg fat mixture used in the form of an aqueous solution or suspension, preferably in the form of a saturated aqueous solution. Sulfitation is preferably carried out with 0.5 to 2 moles alkali and/or ammonium hydrogen sulfites or in admixture with alkali and/or ammonium sulfites/kg fat mixture used. The sulfiting agents are mixed with the oxidized fat mixtures by adding the sulfiting agent in the form of an aqueous solution or suspension and preferably in the form of a saturated aqueous solution to the oxidized fat mixture or by stirring the oxidized fat mixture into an aqueous solution or suspension and preferably into a saturated aqueous solution of the sulfiting agent. The sulfited fats according to the invention are distin guished by high water emulsifiability with excellent emulsion stability. They are therefore particularly suit able for oiling leather and skins. Compared with sulfitation products high-iodine fats, for example fish oil, the sulfitation products according to the invention show distinctly better light stability. Thus, the light fastness of leathers, as measured in accordance with DIN 54004, oiled with fish oil sulfite reaches stage 1 of the 8-stage cotton blue scale while leathers treated with the sulfited fats accord ing to the invention reach stage 4 or stage 5. On the scale in question, stage 1 signifies very poor light stabil ity and stage 8 very high light stability. The invention will be illustrated but not limited by the following examples. EXAMPLES I.no. =iodine number, AS =active substance, mins.- = minutes, h. = hours. Unless otherwise stated, "%" means % by weight' while "g/1' means "g/1 liquor'. Example g tallow (I.no. 52) were mixed with 400 g C16-C18 fatty acid methylester (I.no. 80). The resulting mixture was oxidized at 90 C. by passing air through, and was then sulfited at 95 C. with 1.5 moles sodium hydrogen sulfite per kg fat mixture used in the form of
4 5 an aqueous 40% by weight sodium hydrogen sulfite solution. On completion of sulfitation, the product was cooled to room temperature. Example 2 500g palm kernel oil (I.no. 17) were mixed with 500 g. C16-C18 fatty acid methylester (I.no. 80), oxidized at 110 C. by passing air through and then sulfited at 95 C. with a mixture containing 1.2 moles sodium hydrogen sulfite and 0.6 mole sodium sulfite/kg fat mixture used in the form of a saturated aqueous solution. On comple tion of sulfitation, the product was cooled to room temperature. Example g filtered lard (I.no. 87) were mixed with 300 g C12-C18 fatty acid methylester (I.no. 78), oxidized at 85 C. by passing air through and then sulfited at 90 C. with 1.3 moles sodium hydrogen sulfite/kg fat mixture used in the form of an aqueous, 40% by weight sodium hydrogen sulfite solution. On completion of sulfitation, the product was cooled to room temperature. Example 4 A mixture of 550 g triolein (I.no. 92) and 450 g C16-C18 fatty acid methylester (I.no. 88) was subjected to oxidizing sulfitation in the same way as in Example 1. Example 5 A mixture of 500 g technical oleic acid (I.no. 85) and 500 g C16-C18 fatty acid isopropylester (I.no. 80) was subjected to oxidizing sulfitation in the same way as in Example 2. Example g technical oleyloleate (I.no 91) were mixed with 400 g C16-C18 fatty acid methylester (I.no. 85), oxidized at 1 C. by passing air through and then sulfited at 90 C. with 2.0 moles sodium hydrogen sulfite/kg fat mix ture used in the form of an aqueous, 40% by weight sodium hydrogen sulfite solution. On completion of sulfitation, the product was cooled to room tempera ture. Example 7 A mixture of 700 g neat's-foot oil (I.no. 85) and 300 g C16-C18 fatty acid methylester (I.no. 80) was subjected to oxidizing sulfitation in the same as in Example 2. Example 8 Chrome-taned leathers were oiled with 7% by weight AS, based on sharing weight, of the sulfited fat mixtures of Examples 1 to 7 and with sulfited fish oil fish oil (I.no. 1) by oxidation with air at 90 C. and sulfitation at 95 C. with 1.5 moles sodium hydrogen sulfite/kg fish oil in the form of an aqueous, 40% by weight sodium hydrogen sulfite solution. After finishing in the usual way, the leathers are tested for their fastness to light in accordance with DIN The higher the light stability of the oiling agents, the higher the stage number on the 8-stage cotton blue scale. Table 1 shows the results of the individual mea SuteentS. TABLE 1. Oiling agent Example 1 Fastness to light (as measured in accordance with DIN 54004) stage TABLE 1-continued Fastness to light (as measured in Oiling agent accordance with DIN 54004) Example 2 stage 5 Example 3 Example 4 Example 5 stage 4 Example 6 Example 7 Sulfited fish oil stage i APPLICATION EXAMPLE A Use during chrome tanning. All quantities based on pelt weight. Starting material: pelts of South-German origin. Pickling: Tanning and oiling: 100% water C. 8% sodium chloride 0.6% formic acid (85%) diluted 1:10 0.7% sulfuric acid (98%) diluted 1:0 ph ca % AS containing 95 parts by weight sulfitate, 60% lard, I.no. 80 and 40% C12-C24 fatty acid methylester, I.no. 85 and 5 parts by weight C6-C18 fatty alcohol polyglycolether 8% commerical chrome tanning agent containing % Cr2O3, basification with 0.5% MgO ph sharing, retanning, reoiling in the usual way. 10 mins, 3 h. 7 h. APPLICATION EXAMPLE B Oiling of upper leathers. All quantities based on sharing weight. Starting material: hide wet blue, sharing thickness 1.7 I. Washing: Neutralization Retanning Oiling: 0% water 40 C. 10 mins. fresh liquor 100% water 40 C. 0.5% sodium formate Rinsing with water at 60 C. 100% water 60 C. 0.5% commercial neutralizing tanning agent 4% commercial resin tanning agent 3% commercial synthetic 40 mins. full tanning agent 3% AS sulfitate 50% triolein, I.no. 86 and 50% fatty acid isopropylester, 45 mins. I.no. 80 3% AS commercial fish oil sulfate 0.5% AS C6-C18 alkyl sulfate 0.5% formic acid (85%) diluted 1:10 hoard up leather and
5 7 -continued finish in the usual way. 8 -continued then hoard up leather and finish in the usual way. APPLICATION EXAMPLE C Oiling of apparel leather. All quantities based on sharing weight. Starting material: hide wet blue, sharing thickness Washing: 0% water 40 C. 10 mins. fresh liquor Chrome 100% water 40 C. retanning: 3% commercial chrome tanning agent containing % Cr2O3, 3% commercial synthetic full tanning agent 1.5% sodium aluminium silicate Oiling: 3% AS sulfitate, 45% lard, I.no. 85, 45% fatty acid methylester, I.no. 80, 10% glycerol monooleate, I.no. 75, 0.2% AS tallow alcohol poly glycolether 45 mins. 1% AS C2-C18 alkylsulfate 30 Neutralization: 2% sodium hydrogen carbonate 60 mins. rinsing 60' C. dyeing, retanning, reoiling and finishing in the usual way. APPLICATION EXAMPLED Oiling of sheepskin suede. Starting material: pickled sheepskins Liquor ratio: 1: 40 Chrome tanning: bath temperature C. Tanning and oiling 40 g/l sodium chloride 0.5 g/l AS C12-C8 alkyl sulfate 4 g/l AS sulfitate, prepared 45 from 60% technical oleyloleate, I.no. 84, 40% C12-C24 fatty acid methylester, I.no g/l AS C16-C18 fatty alcohol 50 polyglycolether 4 g/l commercial chrome tanning agent containing % Cr3O3, 4 g/l commercial chrome tanning agent 55 4 g/l commercial chrome tanning 5 h. agent move liquor back and forth 1 g/l sodium hydrogen carbonate overnight 1 g/l sodium hydrogen carbonate 4 h. ph I claim: 1. An oiling agent for the treatment of leather and skins comprising I. from about 10 to about 95% by weight of an oxi dized sulfited fat mixture comprising A. at least one sulfited fat having an iodine number before sulfitation below about 100; and B. at least one sulfited fatty acid ester having an iodine number before sulfitation of from about 60 to about 100, and containing from 12 to 24 car bon atoms in a linear or branched, natural or synthetic fatty acid residue, and wherein the ester residue is from a monofunctional C1-C5 alcohol; wherein the ratio by weight of A to B is from about 9:1 to about 1:4; and II. one or more of a sulfated fat, a sulfonated fat, a sulfochlorinated fat, a phosphated fat, a neutral oil, an anionic emulsifier, and a nonionic emulsifier. 2. A method for the treatment of leather or skins agent of claim 1 at a temperature in the range of from 3. An oiling agent according to claim 1 wherein in component I the ratio of A to B is from about 4:1 to about 2:3. 4. An oiling agent according to claim 1 wherein in component IA the iodine number before sulfitation is from about 7 to about An oiling agent according to claim 1 wherein com ponent I A is at least one sulfited fat selected from the group; coconut oil, palm kernel oil, palm oil, tallow, lard, neat's-foot oil, sperm oil, whale oil, triolein, oleic acid, wax esters, fatty acid monoglycerides, fatty acid diglycerides, and fatty acid triglycerides. 6. An oiling agent according to claim 1 wherein in component IB the iodine number before sulfitation is from about 70 to about An oiling agent according to claim 1 wherein in component IB the fatty acid residue contains from 12 to 18 carbon atoms. 8. An oiling agent according to claim 7 wherein the fatty acid residue contains from 16 to 18 carbon atoms. 9. An oiling agent according to claim 1 wherein in component IB the ester is a methyl, ethyl, isopropyl, or isobutyl ester. 10. A method for the treatment of leather or skins agent of claim 5 at a temperature in the range of from 11. A method for the treatment of leather or skins agent of claim 6 at a temperature in the range of from
51) Int. Cl... C07H 1700; CO7H 15/00;
 USOO59519A United States Patent (19) 11 Patent Number: Desai et al. () Date of Patent: Aug. 31, 1999 54 PROCESS FOR THE PREPARATION OF 5,681,948 10/1997 Miller et al.... 536/1 SUCROSE FATTY ACID ESTERS
USOO59519A United States Patent (19) 11 Patent Number: Desai et al. () Date of Patent: Aug. 31, 1999 54 PROCESS FOR THE PREPARATION OF 5,681,948 10/1997 Miller et al.... 536/1 SUCROSE FATTY ACID ESTERS
(12) United States Patent (10) Patent No.: US 6,440,428 B1
 USOO64.40428B1 (12) United States Patent (10) Patent No.: US 6,440,428 B1 Leander et al. (45) Date of Patent: *Aug. 27, 2002 (54) PODOPHYLLOTOXIN PREPARATION 4,235,889 A 11/1980 Evers... 514/863 CONTAINING
USOO64.40428B1 (12) United States Patent (10) Patent No.: US 6,440,428 B1 Leander et al. (45) Date of Patent: *Aug. 27, 2002 (54) PODOPHYLLOTOXIN PREPARATION 4,235,889 A 11/1980 Evers... 514/863 CONTAINING
73 Assignee: Henkel Kommanditgesellschaft auf t 5. E. E. Eas s
 USOO586971.6A United States Patent (19) 11 Patent Number: Pi Subirana et al. () Date of Patent: Feb. 9, 1999 54 PROCESS FOR THE PRODUCTION OF 4,370,272 1/1983 Wechsler et al.... 2/4 ESTERQUATS 5,437,801
USOO586971.6A United States Patent (19) 11 Patent Number: Pi Subirana et al. () Date of Patent: Feb. 9, 1999 54 PROCESS FOR THE PRODUCTION OF 4,370,272 1/1983 Wechsler et al.... 2/4 ESTERQUATS 5,437,801
(12) United States Patent (10) Patent No.: US 7,781,618 B2
 USOO7781.618B2 (12) United States Patent (10) Patent No.: Makimura et al. (45) Date Patent: Aug. 24, 2010 (54) UNSATURATED ALIPHATIC PRIMARY (56) References Cited AMINE AND PRODUCTION METHOD THEREOF U.S.
USOO7781.618B2 (12) United States Patent (10) Patent No.: Makimura et al. (45) Date Patent: Aug. 24, 2010 (54) UNSATURATED ALIPHATIC PRIMARY (56) References Cited AMINE AND PRODUCTION METHOD THEREOF U.S.
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
(19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
III. United States Patent (19) 11 Patent Number: 5,135,768. Campbell et al. (45) Date of Patent: Aug. 4, 1992
 III USOO535768A United States Patent (19) 11 Patent Number: 5,135,768 Campbell et al. (45) Date of Patent: Aug. 4, 1992 (54) NON-DAIRY CREAMS AND PROCESS OF 4,107,343 8/1978 Petricca... 426/570 MAKING
III USOO535768A United States Patent (19) 11 Patent Number: 5,135,768 Campbell et al. (45) Date of Patent: Aug. 4, 1992 (54) NON-DAIRY CREAMS AND PROCESS OF 4,107,343 8/1978 Petricca... 426/570 MAKING
. United States Patent [11] 3,615,682
![. United States Patent [11] 3,615,682 . United States Patent [11] 3,615,682](/thumbs/89/100156418.jpg) . United States Patent [11] 3,615,682 72 Inventors Glenn D. La Baw Greenwich, Conn.; David P. Kidger, Glen Rock; Fred Wanderveer, Mahwah, N.J. Appl. No. 666,566 Filed Sept. 7, 1967 21 ) 22) 45) 73 Patented
. United States Patent [11] 3,615,682 72 Inventors Glenn D. La Baw Greenwich, Conn.; David P. Kidger, Glen Rock; Fred Wanderveer, Mahwah, N.J. Appl. No. 666,566 Filed Sept. 7, 1967 21 ) 22) 45) 73 Patented
IIIHIIII. United States Patent (19) Inayoshi et al. 11) Patent Number: 5,494,695 45) Date of Patent: Feb. 27, 1996
 United States Patent (19) Inayoshi et al. (54) CUSTARD CREAM 75 Inventors: Kuniaki Inayoshi, Sennan; Sayoko Yabuuchi, Matsubara, both of Japan 73) Assignee: Fuji Oil Company Limited, Osaka, Japan 21 Appl.
United States Patent (19) Inayoshi et al. (54) CUSTARD CREAM 75 Inventors: Kuniaki Inayoshi, Sennan; Sayoko Yabuuchi, Matsubara, both of Japan 73) Assignee: Fuji Oil Company Limited, Osaka, Japan 21 Appl.
United States Patent (19)
 United States Patent (19) Sagi et al. 11 Patent Number: () Date of Patent: Jun. 13, 1989 54 HARD BUTTER COMPOSITION CONTAINING TRIGLYCERIDES, AND PROCESS FOR PRODUCTION THEREOF 75 Inventors: Nobuo Sagi,
United States Patent (19) Sagi et al. 11 Patent Number: () Date of Patent: Jun. 13, 1989 54 HARD BUTTER COMPOSITION CONTAINING TRIGLYCERIDES, AND PROCESS FOR PRODUCTION THEREOF 75 Inventors: Nobuo Sagi,
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
FATS & OILS GLOSSARY
 FATS & OILS GLOSSARY Antioxidant A substance that slows or interferes with the reaction of a fat or oil with oxygen. The addition of antioxidants to fats or foods containing them retard rancidity and increases
FATS & OILS GLOSSARY Antioxidant A substance that slows or interferes with the reaction of a fat or oil with oxygen. The addition of antioxidants to fats or foods containing them retard rancidity and increases
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS
 THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
United States Patent (19) Powers et al.
 United States Patent (19) Powers et al. (54) 75) 73 22 21 52 (51) 58) TREATMENT OF TALL OIL FATTY ACDS Inventors: John R. Powers; Frances M. Miller, both of Charleston, S.C. Assignee: Westvaco Corporation,
United States Patent (19) Powers et al. (54) 75) 73 22 21 52 (51) 58) TREATMENT OF TALL OIL FATTY ACDS Inventors: John R. Powers; Frances M. Miller, both of Charleston, S.C. Assignee: Westvaco Corporation,
United States Patent (19)
 United States Patent (19) Naganuma et al. (54) 75 73) 21) 22) (51) (52) 58 56 DEODORIZING AND SMELL REMOVING COMPOSITION AND METHOD OF USING SAME Inventors: Yoshinori Naganuma, Hoya; Haruhiko Arai, Narashino,
United States Patent (19) Naganuma et al. (54) 75 73) 21) 22) (51) (52) 58 56 DEODORIZING AND SMELL REMOVING COMPOSITION AND METHOD OF USING SAME Inventors: Yoshinori Naganuma, Hoya; Haruhiko Arai, Narashino,
(12) United States Patent (10) Patent No.: US 6,656,358 B2
 USOO668B2 (12) United States Patent (10) Patent No.: US 6,6,8 B2 May et al. () Date of Patent: Dec. 2, 2003 (54) METHOD FOR THE CHROMATOGRAPHIC 5,972,7 A * 10/1999 Yamaguchi... 424/1 SOLATION OF VITAMIN
USOO668B2 (12) United States Patent (10) Patent No.: US 6,6,8 B2 May et al. () Date of Patent: Dec. 2, 2003 (54) METHOD FOR THE CHROMATOGRAPHIC 5,972,7 A * 10/1999 Yamaguchi... 424/1 SOLATION OF VITAMIN
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
III IIII IIII. United States Patent (19) Hamann et al. 5,464,565. Nov. 7, Patent Number: (45) Date of Patent:
 United States Patent (19) Hamann et al. (54) PROCESS FOR THE PREPARATION OF HIGHILY CONCENTRATED FREE-FLOWING AQUEOUS SOLUTIONS OF BETAINES (75) Inventors: Ingo Hamann, Bad Orb; Hans-Jirgen Köhle, Schluchtern;
United States Patent (19) Hamann et al. (54) PROCESS FOR THE PREPARATION OF HIGHILY CONCENTRATED FREE-FLOWING AQUEOUS SOLUTIONS OF BETAINES (75) Inventors: Ingo Hamann, Bad Orb; Hans-Jirgen Köhle, Schluchtern;
Calderglen High School CfE Higher Chemistry. Nature s Chemistry Esters, Fats and Oils. Page 1 of 11
 Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
EUROPEAN QUALIFYING EXAMINATION 2006
 EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
IIII IIHill. United States Patent (19) Shelley et al. 11 Patent Number: 5,505, Date of Patent: Apr. 9, 1996
 United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
Chemical Effect on Concrete Concrete Treated with Penetron. Acetic Acid to 30% Disintegrates Slowly S/E. Acetone Liquid loss by penetration N/E
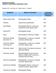 PENETRON SYSTEMS CHEMICAL RESISTANCE/CORROSION CHART Symbols: = No Effect = Slight Effect E = Effected Chemical Effect on Concrete Concrete Treated with Penetron Acetic Acid to 30% Disintegrates Slowly
PENETRON SYSTEMS CHEMICAL RESISTANCE/CORROSION CHART Symbols: = No Effect = Slight Effect E = Effected Chemical Effect on Concrete Concrete Treated with Penetron Acetic Acid to 30% Disintegrates Slowly
60 Provisional application No. 60/030,168, Nov. 13, administration to plants. The fertilizer additives include
 USOO59970A United States Patent (19) 11 Patent Number: 5,997,0 Dean () Date of Patent: Dec. 7, 1999 54 FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METALIONS 1433 8/1980 Germany...
USOO59970A United States Patent (19) 11 Patent Number: 5,997,0 Dean () Date of Patent: Dec. 7, 1999 54 FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METALIONS 1433 8/1980 Germany...
United States Patent Office 3,376,229
 United States Patent ffice Patenied Apr. 2, 1968 SYNTHETIC DETERGENT BAR Robert A. Haass, Ridgewood, and Vincent Lamberti, Teataeck, N.Y., assignors to Lever Brothers Company, New York, N.Y., a corporation
United States Patent ffice Patenied Apr. 2, 1968 SYNTHETIC DETERGENT BAR Robert A. Haass, Ridgewood, and Vincent Lamberti, Teataeck, N.Y., assignors to Lever Brothers Company, New York, N.Y., a corporation
UNITED STATES PATENT of FICE
 Patented Jan. 4, 1949 UNITED STATES PATENT of FICE The present invention relates to an improved process of preparing epoxy compounds in good yields from unsaturated aliphatic compounds. These epoxy compounds
Patented Jan. 4, 1949 UNITED STATES PATENT of FICE The present invention relates to an improved process of preparing epoxy compounds in good yields from unsaturated aliphatic compounds. These epoxy compounds
UNITED STATES PATENT OFFICE
 Patented Aug. 18, 1942 2,293,676 UNITED STATES PATENT OFFICE 2,293,676 METHOD OF SEPARATING FATTY ACDS 8 Claims. This invention relates to a method of separat ing mixed higher fatty acids one from another.
Patented Aug. 18, 1942 2,293,676 UNITED STATES PATENT OFFICE 2,293,676 METHOD OF SEPARATING FATTY ACDS 8 Claims. This invention relates to a method of separat ing mixed higher fatty acids one from another.
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
CHAPTER 5 CONCLUSIONS
 CHAPTER 5 CONCLUSIONS /166/ 5.0 Summary The leather industry uses a quite number of chemicals during various steps involved in the conversion of raw hides and skins to finished leather. Surfactants are
CHAPTER 5 CONCLUSIONS /166/ 5.0 Summary The leather industry uses a quite number of chemicals during various steps involved in the conversion of raw hides and skins to finished leather. Surfactants are
SECTION III ANIMAL OR VEGETABLE FATS AND OILS AND THEIR CLEAVAGE PRODUCTS; PREPARED EDIBLE FATS; ANIMAL OR VEGETABLE WAXES CHAPTER 15
 SECTION III ANIMAL OR VEGETABLE FATS AND OILS AND THEIR CLEAVAGE PRODUCTS; PREPARED EDIBLE FATS; ANIMAL OR VEGETABLE WAXES CHAPTER 15 ANIMAL OR VEGETABLE FATS AND OILS AND THEIR CLEAVAGE PRODUCTS; PREPARED
SECTION III ANIMAL OR VEGETABLE FATS AND OILS AND THEIR CLEAVAGE PRODUCTS; PREPARED EDIBLE FATS; ANIMAL OR VEGETABLE WAXES CHAPTER 15 ANIMAL OR VEGETABLE FATS AND OILS AND THEIR CLEAVAGE PRODUCTS; PREPARED
APPENDIX 1 CALCULATION OF THE MOLAR RATIO (METHANOL TO OIL)
 243 APPENDIX 1 CALCULATION OF THE MOLAR RATIO (METHANOL TO OIL) The molar ratio (oil to methanol) for transesterification process is calculated as follows. Step1: Calculation of molecular weight of pure
243 APPENDIX 1 CALCULATION OF THE MOLAR RATIO (METHANOL TO OIL) The molar ratio (oil to methanol) for transesterification process is calculated as follows. Step1: Calculation of molecular weight of pure
3.1.3 Lipids. Source: AQA Spec
 alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
United States Patent Office 3,636,017
 United States Patent Office Patented Jan. 18, 1972 PRODUCING LACTYLIC ACID ESTERS OF FATTY ACDS Stanley Eng, Mentor, Ohio, assignor to Glyco Chemicals, Inc., New York, N.Y. No Drawing. Continuation-in-part
United States Patent Office Patented Jan. 18, 1972 PRODUCING LACTYLIC ACID ESTERS OF FATTY ACDS Stanley Eng, Mentor, Ohio, assignor to Glyco Chemicals, Inc., New York, N.Y. No Drawing. Continuation-in-part
(12) United States Patent
 (12) United States Patent Yamato USOO6605575B1 (10) Patent No.: (45) Date of Patent: Aug. 12, 2003 (54) CUTTING FLUID COMPOSITION (75) Inventor: Naoya Yamato, Kawasaki (JP) (73) Assignee: Ajinomoto Co.,
(12) United States Patent Yamato USOO6605575B1 (10) Patent No.: (45) Date of Patent: Aug. 12, 2003 (54) CUTTING FLUID COMPOSITION (75) Inventor: Naoya Yamato, Kawasaki (JP) (73) Assignee: Ajinomoto Co.,
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 0236558A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0236558 A1 Tran et al. (43) Pub. Date: (54) EMULSIONS USEFUL IN BEVERAGES Publication Classification (51)
(19) United States US 2011 0236558A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0236558 A1 Tran et al. (43) Pub. Date: (54) EMULSIONS USEFUL IN BEVERAGES Publication Classification (51)
Understanding Ingredients. Fats and Oils
 Understanding Ingredients Fats and Oils Topics Types of Fats and Oils Structures of Fats and Oils Nutritive Value of Fats and Oils Choice and Storage of Fats and Oils Uses of Fats and Oils in Cooking /
Understanding Ingredients Fats and Oils Topics Types of Fats and Oils Structures of Fats and Oils Nutritive Value of Fats and Oils Choice and Storage of Fats and Oils Uses of Fats and Oils in Cooking /
(12) United States Patent
 (12) United States Patent Teoh et al. USOO65664B1 (10) Patent No.: () Date of Patent: May 20, 2003 (54) ELASTOMERIC GLOVES (75) Inventors: Seng Chin Teoh, Penang (MY); Seong Fong Chen, Penang (MY) (73)
(12) United States Patent Teoh et al. USOO65664B1 (10) Patent No.: () Date of Patent: May 20, 2003 (54) ELASTOMERIC GLOVES (75) Inventors: Seng Chin Teoh, Penang (MY); Seong Fong Chen, Penang (MY) (73)
(12) United States Patent
 (12) United States Patent Vinson et al. USOO6281181B1 (10) Patent No.: () Date of Patent: *Aug. 28, 2001 (54) LIGHT-DUTY LIQUID OR GEL DISHWASHING DETERGENT COMPOSITIONS COMPRISING MID-AN BRANED SURFACTANTS
(12) United States Patent Vinson et al. USOO6281181B1 (10) Patent No.: () Date of Patent: *Aug. 28, 2001 (54) LIGHT-DUTY LIQUID OR GEL DISHWASHING DETERGENT COMPOSITIONS COMPRISING MID-AN BRANED SURFACTANTS
C11D. Definition statement. Relationships with other classification places. References. Limiting references CPC - C11D
 C11D DETERGENT COMPOSITIONS (preparations specially adapted for washing the hair A61Q 5/02, A61K 8/00; methods or apparatus for disinfection or sterilisation A61L; special washing compositions for cleaning
C11D DETERGENT COMPOSITIONS (preparations specially adapted for washing the hair A61Q 5/02, A61K 8/00; methods or apparatus for disinfection or sterilisation A61L; special washing compositions for cleaning
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
CH 3. Lipids CHAPTER SUMMARY
 H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
United States Patent (19) Matsumoto et al..
 United States Patent (19) Matsumoto et al.. (54) WATER SOLUBLE PACKAGING FILM 75) Inventors: Takayuki Matsumoto; Tsutomu Suzuki; Yoshiyuki Tsukamoto, all of Toyohashi; Hitoshi Nakamura, Aichi, all of Japan
United States Patent (19) Matsumoto et al.. (54) WATER SOLUBLE PACKAGING FILM 75) Inventors: Takayuki Matsumoto; Tsutomu Suzuki; Yoshiyuki Tsukamoto, all of Toyohashi; Hitoshi Nakamura, Aichi, all of Japan
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0042173 A1 SAUER et al. US 20170042173A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (86) (30) LIQUID EDIBLE FRYING MEDIUM
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0042173 A1 SAUER et al. US 20170042173A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (86) (30) LIQUID EDIBLE FRYING MEDIUM
United States Patent Office 3,355,298
 United States Patent Office 3,3,298 Patented Nov. 28, 1967. 3,3,298 PROCESS OF MAKING CHEMICALLY ACDFED SOUR CREAM TYPE PRODUCTS Ira Loter, Fair Lawn, N.J., assignor to Nopco Chemical Company, Newark,
United States Patent Office 3,3,298 Patented Nov. 28, 1967. 3,3,298 PROCESS OF MAKING CHEMICALLY ACDFED SOUR CREAM TYPE PRODUCTS Ira Loter, Fair Lawn, N.J., assignor to Nopco Chemical Company, Newark,
Detergent Complex Based on Palm Stearin
 Detergent Complex Based on Palm Stearin PIPOC 2007, Malaysia August 29, 2007 Agenda About Trivedi Group Basic Requirements and Process Chemistry Products and Formulations Cost Structure Summary Global
Detergent Complex Based on Palm Stearin PIPOC 2007, Malaysia August 29, 2007 Agenda About Trivedi Group Basic Requirements and Process Chemistry Products and Formulations Cost Structure Summary Global
63 continuationin-part of ser, No. 932,164, Nov. 18, 4ttorney, 4gent, or Firm Rupert B. Hurley, Jr.
 United States Patent 19 11 Patent Number: Kirk et al. 4 Date of Patent: Jan. 9, 1990 14 PROCESS FOR MAKING ASPRAY-DRIED, (6) References Cited DIRECTLY.COMPRESSIBLE VITAMIN POWDER COMPRISING UNHYDROLYZED
United States Patent 19 11 Patent Number: Kirk et al. 4 Date of Patent: Jan. 9, 1990 14 PROCESS FOR MAKING ASPRAY-DRIED, (6) References Cited DIRECTLY.COMPRESSIBLE VITAMIN POWDER COMPRISING UNHYDROLYZED
United States Patent 19 Mazzola
 United States Patent 19 Mazzola I USOO544,375A 11 Patent Number: 5,443,751 45 Date of Patent: Aug. 22, 1995 54 (75) 73 21 22 63 (51) 52 (58) POWDER DETERGENT COMPOSITION FOR COLD WATER WASHING OF FABRICS
United States Patent 19 Mazzola I USOO544,375A 11 Patent Number: 5,443,751 45 Date of Patent: Aug. 22, 1995 54 (75) 73 21 22 63 (51) 52 (58) POWDER DETERGENT COMPOSITION FOR COLD WATER WASHING OF FABRICS
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Alkenes.
 Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
teachers notes Answers to questions on Pupil sheets AO2: 1. A bar chart is the most suitable method of displaying the information.
 teachers notes AO2.1 AO2. Fats This exercise revises information on the structure of fats and encourages pupils to improve their diet with respect to fats. Some pupils may be over concerned with their
teachers notes AO2.1 AO2. Fats This exercise revises information on the structure of fats and encourages pupils to improve their diet with respect to fats. Some pupils may be over concerned with their
Coatings and Specialty Applications
 Coatings and Specialty Applications INTRODUCTION AdvancedChemicalConcepts,Inc.isthepremiersourcefordistributingCoatingsandSpecialty Chemicals,offeringinsightandacomprehensiverangeofmaterialstomeetyourneeds.We
Coatings and Specialty Applications INTRODUCTION AdvancedChemicalConcepts,Inc.isthepremiersourcefordistributingCoatingsandSpecialty Chemicals,offeringinsightandacomprehensiverangeofmaterialstomeetyourneeds.We
(12) United States Patent (10) Patent No.: US 6,365,596 B1
 USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
MIGLYOL Gel B, Gel T, 840 Gel B
 MIGLYOL Gel B, Gel T, 840 Gel B INCI: Gel B: Caprylic/Capric Triglyceride, Stearalkonium Hectorite, Propylene Carbonate Gel T: Caprylic/Capric Triglyceride, Stearalkonium Bentonite, Propylene Carbonate
MIGLYOL Gel B, Gel T, 840 Gel B INCI: Gel B: Caprylic/Capric Triglyceride, Stearalkonium Hectorite, Propylene Carbonate Gel T: Caprylic/Capric Triglyceride, Stearalkonium Bentonite, Propylene Carbonate
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
(12) United States Patent (10) Patent No.: US 6,500,974 B2
 USOO974B2 (12) United States Patent (10) Patent No.: Thengumpillil et al. () Date of Patent: Dec. 31, 2002 (54) PROCESS FOR THE PREPARATION OF A () Prior Publication Data MONOGLYCERIDE US 2002/01209 A1
USOO974B2 (12) United States Patent (10) Patent No.: Thengumpillil et al. () Date of Patent: Dec. 31, 2002 (54) PROCESS FOR THE PREPARATION OF A () Prior Publication Data MONOGLYCERIDE US 2002/01209 A1
QUESTION 1 Fats and oils vary in their degree of solubility in aqueous solutions. Give a reason for this observation.
 QUESTIN 1 Fats and oils vary in their degree of solubility in aqueous solutions. Give a reason for this observation. QUESTIN Why are fatty acids such as palmitic acid, insoluble in water, while ethanoic
QUESTIN 1 Fats and oils vary in their degree of solubility in aqueous solutions. Give a reason for this observation. QUESTIN Why are fatty acids such as palmitic acid, insoluble in water, while ethanoic
III.I.I.I.IIII. United States Patent (19) Candau et al. 5,603,940 Feb. 18, OIL-IN-WATEREMULSION WHICH MAYBE
 United States Patent (19) Candau et al. III.I.I.I.IIII US0053940A 11 Patent Number: () Date of Patent: Feb. 18, 1997 54 OIL-IN-WATEREMULSION WHICH MAYBE USED FOR OBTAINING A CREAM 75 Inventors: Didier
United States Patent (19) Candau et al. III.I.I.I.IIII US0053940A 11 Patent Number: () Date of Patent: Feb. 18, 1997 54 OIL-IN-WATEREMULSION WHICH MAYBE USED FOR OBTAINING A CREAM 75 Inventors: Didier
United States Patent (19)
 United States Patent (19) Yajima 54 75 (73) 21 22 (51 (52) (58) (56) STABLIZED OL AND FAT POWDER Inventor: Assignee: Mizuo Yajima, Tokyo, Japan Asama Chemical Co., Ltd., Tokyo, Japan Appl. No.: 297,831
United States Patent (19) Yajima 54 75 (73) 21 22 (51 (52) (58) (56) STABLIZED OL AND FAT POWDER Inventor: Assignee: Mizuo Yajima, Tokyo, Japan Asama Chemical Co., Ltd., Tokyo, Japan Appl. No.: 297,831
United States Patent Office
 United States Patent ffice PAQUE LIQUID DETERGENT CMPSITIN William C. Krumrei, Springdale, and Joseph Blinka, Cin cinnati, hio, assignors to The Procter & Gable CoR pany, Cincinnati, hio, a corporation
United States Patent ffice PAQUE LIQUID DETERGENT CMPSITIN William C. Krumrei, Springdale, and Joseph Blinka, Cin cinnati, hio, assignors to The Procter & Gable CoR pany, Cincinnati, hio, a corporation
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 2004O156999A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0156999 A1 Biddulph et al. (43) Pub. Date: (54) BLACK TRIVALENT CHROMIUM CHROMATE CONVERSION COATING (75)
(19) United States US 2004O156999A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0156999 A1 Biddulph et al. (43) Pub. Date: (54) BLACK TRIVALENT CHROMIUM CHROMATE CONVERSION COATING (75)
(12) United States Patent
 (12) United States Patent Braun USOO64.871B2 (10) Patent No.: () Date of Patent: Apr. 29, 2003 (54) OXIDATIVE HAIR DYE PRECURSOR COMPOSITIONS CONTAINING 4-5-DIAMINOPYRAZOLE, 5-AMINO-2-METHYLPHENOLAND M-PHENYLENEDLAMINE
(12) United States Patent Braun USOO64.871B2 (10) Patent No.: () Date of Patent: Apr. 29, 2003 (54) OXIDATIVE HAIR DYE PRECURSOR COMPOSITIONS CONTAINING 4-5-DIAMINOPYRAZOLE, 5-AMINO-2-METHYLPHENOLAND M-PHENYLENEDLAMINE
(12) United States Patent (10) Patent No.: US 7,790,190 B2
 US007790190B2 (12) United States Patent () Patent No.: US 7,790,190 B2 Papas et al. () Date of Patent: Sep. 7, 20 (54) AQUEOUSEMULSIONS OF LIPOPHILE 4,289,638 A 9, 1981 Svenson... 5,8 SOLUBLIZED WITH VITAMINE
US007790190B2 (12) United States Patent () Patent No.: US 7,790,190 B2 Papas et al. () Date of Patent: Sep. 7, 20 (54) AQUEOUSEMULSIONS OF LIPOPHILE 4,289,638 A 9, 1981 Svenson... 5,8 SOLUBLIZED WITH VITAMINE
United States Patent 19 Veits
 United States Patent 19 Veits USOO5744634A 11 Patent Number: 45 Date of Patent: 5,744,634 Apr. 28, 1998 54 PROCESS FOR PRODUCING 2-KETO-L- GULONCACD ESTERS (75) Inventor: Joachim Weits, Rheinfelden, Germany
United States Patent 19 Veits USOO5744634A 11 Patent Number: 45 Date of Patent: 5,744,634 Apr. 28, 1998 54 PROCESS FOR PRODUCING 2-KETO-L- GULONCACD ESTERS (75) Inventor: Joachim Weits, Rheinfelden, Germany
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 20070237860A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0237860 A1 Abu-Ali et al. (43) Pub. Date: (54) EDIBLE ADHESIVE COATINGS FOR MULTI-COMPONENT FOOD PRODUCTS
(19) United States US 20070237860A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0237860 A1 Abu-Ali et al. (43) Pub. Date: (54) EDIBLE ADHESIVE COATINGS FOR MULTI-COMPONENT FOOD PRODUCTS
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 20060051474A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0051474 A1 Furlong et al. (43) Pub. Date: (54) (76) (21) (22) (60) PROTEIN-ENRICHED NUT BUTTER COMPOSITIONS
(19) United States US 20060051474A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0051474 A1 Furlong et al. (43) Pub. Date: (54) (76) (21) (22) (60) PROTEIN-ENRICHED NUT BUTTER COMPOSITIONS
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
(12) United States Patent
 (12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
(12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
(12) United States Patent (10) Patent No.: US 8.426,654 B2
 USOO8426654B2 (12) United States Patent () Patent No.: US 8.426,654 B2 B0ensch et al. (45) Date of Patent: Apr. 23, 2013 (54) METHOD FOR THE PRODUCTION OF E. bi A 3.28. push tal eoka et al. FATTY ALCOHOLS
USOO8426654B2 (12) United States Patent () Patent No.: US 8.426,654 B2 B0ensch et al. (45) Date of Patent: Apr. 23, 2013 (54) METHOD FOR THE PRODUCTION OF E. bi A 3.28. push tal eoka et al. FATTY ALCOHOLS
General Compatibility List for WST Flexible Tank Materials
 2-Cycle Oil 2-Cycle Outboard Marine Oil 2-Ethylhexanol (Aliphatic Alcohol) Adhesive Aliphatic Glycol Ether Alkyl Amide Propyl Betaine Alkyl Benzene Alkyl Phenolic Glycol Ether Alkylate Aluminium Polychloride
2-Cycle Oil 2-Cycle Outboard Marine Oil 2-Ethylhexanol (Aliphatic Alcohol) Adhesive Aliphatic Glycol Ether Alkyl Amide Propyl Betaine Alkyl Benzene Alkyl Phenolic Glycol Ether Alkylate Aluminium Polychloride
Interesterification. 4.1 Introduction. Chapter 4. Efforts have been made to improve the low-temperature properties by blending the
 Chapter 4 Interesterification 4.1 Introduction Efforts have been made to improve the low-temperature properties by blending the vegetable oils with diluents such as poly α olefin, diisodecyl adipate, and
Chapter 4 Interesterification 4.1 Introduction Efforts have been made to improve the low-temperature properties by blending the vegetable oils with diluents such as poly α olefin, diisodecyl adipate, and
Quality Considerations and Control Factors for Homebrewing Biodiesel. John Bush
 Quality Considerations and Control Factors for Homebrewing Biodiesel John Bush John@boulderbiodiesel.com www.boulderbiodiesel.com Quality? Modern Diesel Engines are very sensitive to fuel quality issues.
Quality Considerations and Control Factors for Homebrewing Biodiesel John Bush John@boulderbiodiesel.com www.boulderbiodiesel.com Quality? Modern Diesel Engines are very sensitive to fuel quality issues.
22. The Fischer Esterification
 22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
22. The Fischer Esterification A. Background Esters are an incredibly important functional group in organic chemistry. Esters are typically very pleasant smelling molecules and are therefore frequently
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160376526A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0376526 A1 Smith (43) Pub. Date: (54) POTASSIUM SOAPS THAT CAN BE A61O 19/10 (2006.01) THICKENED WITH CHLORIDE
(19) United States US 20160376526A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0376526 A1 Smith (43) Pub. Date: (54) POTASSIUM SOAPS THAT CAN BE A61O 19/10 (2006.01) THICKENED WITH CHLORIDE
M1.(a) 1. Fewer children / less likely that children with asthma eat fish; Accept converse.
 M.(a). Fewer children / less likely that children with asthma eat fish; Accept converse.. Fewer children / less likely that children with asthma eat oily fish; MP and Allow use of numbers.. Little / only
M.(a). Fewer children / less likely that children with asthma eat fish; Accept converse.. Fewer children / less likely that children with asthma eat oily fish; MP and Allow use of numbers.. Little / only
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
USOO A United States Patent Patent Number: 5,704,961 Hudson 45) Date of Patent: Jan. 6, 1998
 III USOO5704961A United States Patent 19 11 Patent Number: Hudson ) Date of Patent: Jan. 6, 1998 (54) CORROSION INHIBITORS FOR LIQUID 3,931,029 1/1976 Dutton et al.... 2/76 FERTLIZERS 4,113,498 9/1978
III USOO5704961A United States Patent 19 11 Patent Number: Hudson ) Date of Patent: Jan. 6, 1998 (54) CORROSION INHIBITORS FOR LIQUID 3,931,029 1/1976 Dutton et al.... 2/76 FERTLIZERS 4,113,498 9/1978
The four levels of protein structure are: primary structure, secondary structure, tertiary structure, and quaternary structure.
 Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166282 A1 Martinez et al. US 20140166282A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (60) FUNCTIONALIZED HYDROGEN SULFIDE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166282 A1 Martinez et al. US 20140166282A1 (43) Pub. Date: (54) (71) (72) (73) (21) (22) (60) FUNCTIONALIZED HYDROGEN SULFIDE
July 21, 1959 S. KARYS ETAL 2,895,976 PROCESS FOR FRACTIONATION OF FATTY ACID MIXTURES SOAPS OF MXED SATURATED AND .JNSATURATED FATTY ACDS
 July 21, 199 S. KARYS ETAL PROCESS FOR FRACTIONATION OF FATTY ACID MIXTURES Filed July 9, 196 2 Sheets-Sheet l SOAPS OF MXED SATURATED AND.JNSATURATED FATTY ACDS AQUEOUS 0AP AS, FREE h m ( -% SOLUTION
July 21, 199 S. KARYS ETAL PROCESS FOR FRACTIONATION OF FATTY ACID MIXTURES Filed July 9, 196 2 Sheets-Sheet l SOAPS OF MXED SATURATED AND.JNSATURATED FATTY ACDS AQUEOUS 0AP AS, FREE h m ( -% SOLUTION
(19) United States (12) Reissued Patent (10) Patent Number:
 (19) United States (12) Reissued Patent () Patent Number: USOORE44719E Schweitzer et al. () Date of Reissued Patent: Jan. 21, 2014 (54) PROCESS FOR PRODUCING A E. '93; A1 23. TRGLYCERIDE EP 1477O7O 11,
(19) United States (12) Reissued Patent () Patent Number: USOORE44719E Schweitzer et al. () Date of Reissued Patent: Jan. 21, 2014 (54) PROCESS FOR PRODUCING A E. '93; A1 23. TRGLYCERIDE EP 1477O7O 11,
SPECIALITIES FOR TECHNICAL APPLICATIONS. Rofacer
 SPECIALITIES FOR TECHNICAL APPLICATIONS Rofacer State-of-the-Art Technology Designed for High-Standard Products Ester, Ether, Sugar Derivatives and Other Specialities By 1931, Deutsche Hydrierwerke (DHW)
SPECIALITIES FOR TECHNICAL APPLICATIONS Rofacer State-of-the-Art Technology Designed for High-Standard Products Ester, Ether, Sugar Derivatives and Other Specialities By 1931, Deutsche Hydrierwerke (DHW)
Carbon s unique bonding pattern arises from the hybridization of the electrons.
 Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Ulllted States Patent [19] [11] Patent Number: 4,997,881
![Ulllted States Patent [19] [11] Patent Number: 4,997,881 Ulllted States Patent [19] [11] Patent Number: 4,997,881](/thumbs/91/105614517.jpg) Ulllted States Patent [19] [11] Patent Number: 4,997,881 Patel et a1. [45] Date of Patent: Mar. 5, 1991 [54] POLY(ALKYL VINYL ETHER)POLYOLEFIN [56] References Cited COPOLYMERS U.S. PATENT DOCUMENTS [75]
Ulllted States Patent [19] [11] Patent Number: 4,997,881 Patel et a1. [45] Date of Patent: Mar. 5, 1991 [54] POLY(ALKYL VINYL ETHER)POLYOLEFIN [56] References Cited COPOLYMERS U.S. PATENT DOCUMENTS [75]
United States Patent Nies et al.
 United States Patent Nies et al. (15) 3,649,172 (45) Mar. 14, 1972 54 72 73 22 21 63 52 51 ZINC BORATE OF LOW HYDRATION AND METHOD FOR PREPARING SAME Inventors: Nelson P. Nies, Laguna Beach; Richard W.
United States Patent Nies et al. (15) 3,649,172 (45) Mar. 14, 1972 54 72 73 22 21 63 52 51 ZINC BORATE OF LOW HYDRATION AND METHOD FOR PREPARING SAME Inventors: Nelson P. Nies, Laguna Beach; Richard W.
March 19, 1957 J. T. PATTON, JR 2,786,080 STABILIZED POLYOXYALKYLENE COMPOSITIONS
 March 19, 1957 J. T. PATTON, JR STABILIZED POLYOXYALKYLENE COMPOSITIONS Filed Nov. 25, 1953 FIGURE I 2 Sheets-Sheet l PERCENT CARBONYL DEVELOPED IN POLYOXYALKYLENE COMPOUNDS AT OO C. 3. Polyoxypropylene
March 19, 1957 J. T. PATTON, JR STABILIZED POLYOXYALKYLENE COMPOSITIONS Filed Nov. 25, 1953 FIGURE I 2 Sheets-Sheet l PERCENT CARBONYL DEVELOPED IN POLYOXYALKYLENE COMPOUNDS AT OO C. 3. Polyoxypropylene
(12) United States Patent
 (12) United States Patent Defense USOO64O7271B1 (10) Patent No.: (45) Date of Patent: US 6,7,271 B1 Jun. 18, 2002 (54) METHOD FOR ELIMINATING METALS FROM FATTY SUBSTANCES AND GUMS ASSOCATED WITH SAID METALS
(12) United States Patent Defense USOO64O7271B1 (10) Patent No.: (45) Date of Patent: US 6,7,271 B1 Jun. 18, 2002 (54) METHOD FOR ELIMINATING METALS FROM FATTY SUBSTANCES AND GUMS ASSOCATED WITH SAID METALS
CORROSION INHIBITORS CATALOGUE
 CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
(12) United States Patent (10) Patent No.: US 8,414,943 B2
 USOO8414943B2 (12) United States Patent (10) Patent No.: Wijngaarden et al. (45) Date of Patent: Apr. 9, 2013 (54) PROCESS FOR PRODUCING A PALM OIL (56) References Cited PRODUCT (75) Inventors: Leendert
USOO8414943B2 (12) United States Patent (10) Patent No.: Wijngaarden et al. (45) Date of Patent: Apr. 9, 2013 (54) PROCESS FOR PRODUCING A PALM OIL (56) References Cited PRODUCT (75) Inventors: Leendert
(12) United States Patent
 (12) United States Patent Yanagawa et al. USOO6462231B1 (10) Patent No.: () Date of Patent: Oct. 8, 2002 (54) METHOD OF PRODUCING CARBOXYLIC ACID AND ALCOHOL (75) Inventors: Masatoshi Yanagawa, Niigata-ken
(12) United States Patent Yanagawa et al. USOO6462231B1 (10) Patent No.: () Date of Patent: Oct. 8, 2002 (54) METHOD OF PRODUCING CARBOXYLIC ACID AND ALCOHOL (75) Inventors: Masatoshi Yanagawa, Niigata-ken
United States Patent (19)
 United States Patent (19) Fung (54) DENTAL CROWN 76) Inventor: John Fung, 627 George Street, Sydney, NSW 2000, Australia (21) Appl. No.: 969,186 (22) PCT Filed: Jul. 5, 1991 (86). PCT No.: PCT/AU91/00300
United States Patent (19) Fung (54) DENTAL CROWN 76) Inventor: John Fung, 627 George Street, Sydney, NSW 2000, Australia (21) Appl. No.: 969,186 (22) PCT Filed: Jul. 5, 1991 (86). PCT No.: PCT/AU91/00300
Lec 4a- BPK 110 Human Nutrition: Current Iss.
 Lec 4a- BPK 110 Human Nutrition: Current Iss. TOPICS FOR Lec 4a: 1. Introduction to Lipids 2. Lipid Structure 3. Saturated vs. Unsaturated Fatty Acid Chains 4. Phospholipids and Sterols (Other Lipids)
Lec 4a- BPK 110 Human Nutrition: Current Iss. TOPICS FOR Lec 4a: 1. Introduction to Lipids 2. Lipid Structure 3. Saturated vs. Unsaturated Fatty Acid Chains 4. Phospholipids and Sterols (Other Lipids)
MODULE TOPIC PERIODS 1 Oils and fats, soaps & detergents 13 2 Pulp and Paper Explosives and insecticides Sugar, starch and leather 13
 COURSE TITLE : ORGANIC TECHNOLOGY COURSE CODE : 5072 COURSE CATEGORY : A PERIODS/ WEEK : 4 PERIODS/ SEMESTER : 52 CREDIT : 4 TIME SCHEDULE MODULE TOPIC PERIODS 1 Oils and fats, soaps & detergents 13 2
COURSE TITLE : ORGANIC TECHNOLOGY COURSE CODE : 5072 COURSE CATEGORY : A PERIODS/ WEEK : 4 PERIODS/ SEMESTER : 52 CREDIT : 4 TIME SCHEDULE MODULE TOPIC PERIODS 1 Oils and fats, soaps & detergents 13 2
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 (19) United States US 2002O151753A1 (12) Patent Application Publication (10) Pub. No.: US 2002/0151753 A1 Fremy (43) Pub. Date: (54) METHOD FOR MAKING DIMETHYL (30) Foreign Application Priority Data DSULPHIDE
(19) United States US 2002O151753A1 (12) Patent Application Publication (10) Pub. No.: US 2002/0151753 A1 Fremy (43) Pub. Date: (54) METHOD FOR MAKING DIMETHYL (30) Foreign Application Priority Data DSULPHIDE
New generation of phosphate-esters for MWF: balancing performance, labeling and economics.
 New generation of phosphate-esters for MWF: balancing performance, labeling and economics. Claude-Emmanuel Hédoire 1), 1) Solvay Novecare, Aubervilliers, France 1 Introduction Phosphate-esters are well
New generation of phosphate-esters for MWF: balancing performance, labeling and economics. Claude-Emmanuel Hédoire 1), 1) Solvay Novecare, Aubervilliers, France 1 Introduction Phosphate-esters are well
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
FROM SKIN TO LEATHER IN DENSE PRESSURIZED CO 2
 FROM SKIN TO LEATHER IN DENSE PRESSURIZED CO 2 C. Perre (1) A-L Hans (2) M. Dedieu (3) O. Gand (4) L. Saldinari (5) L. Dutel (6) (1) CEA VRH DEN/DTE/SLP BP111 26700 PIERRELATTE (France) e-mail : christian.perre@cea.fr
FROM SKIN TO LEATHER IN DENSE PRESSURIZED CO 2 C. Perre (1) A-L Hans (2) M. Dedieu (3) O. Gand (4) L. Saldinari (5) L. Dutel (6) (1) CEA VRH DEN/DTE/SLP BP111 26700 PIERRELATTE (France) e-mail : christian.perre@cea.fr
Studies on the Urea-Dewaxing of Lubricating Oils*
 Studies on the Urea-Dewaxing of Lubricating Oils* Naoki Yata** Summery: A behavior of activators and inhibitors of the urea adduct formation was investigated, and these two factors seemed to be of primary
Studies on the Urea-Dewaxing of Lubricating Oils* Naoki Yata** Summery: A behavior of activators and inhibitors of the urea adduct formation was investigated, and these two factors seemed to be of primary
Attention is drawn to the following places, which may be of interest for search:
 A23D EDIBLE OILS OR FATS, e.g. MARGARINES, SHORTENINGS, COOKING OILS (animal feeding-stuffs A23K 10/00-A23K 20/30, A23K 30/00-A23K 50/90; foods or foodstuffs containing edible oils or fats A21D, A23C,
A23D EDIBLE OILS OR FATS, e.g. MARGARINES, SHORTENINGS, COOKING OILS (animal feeding-stuffs A23K 10/00-A23K 20/30, A23K 30/00-A23K 50/90; foods or foodstuffs containing edible oils or fats A21D, A23C,
United States Patent (19) Groesch et al.
 United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
(12) United States Patent (10) Patent No.: US 7,785,823 B2
 USOO7785823B2 (12) United States Patent (10) Patent No.: US 7,785,823 B2 Sim et al. (45) Date of Patent: Aug. 31, 2010 (54) METHOD FOR SELECTIVE SEPARATION OF (56) References Cited FREE-ASTAXANTHN FROM
USOO7785823B2 (12) United States Patent (10) Patent No.: US 7,785,823 B2 Sim et al. (45) Date of Patent: Aug. 31, 2010 (54) METHOD FOR SELECTIVE SEPARATION OF (56) References Cited FREE-ASTAXANTHN FROM
Ulllted States Patent [19] [11] Patent Number: 5,997,600
![Ulllted States Patent [19] [11] Patent Number: 5,997,600 Ulllted States Patent [19] [11] Patent Number: 5,997,600](/thumbs/86/93631727.jpg) US005997600A Ulllted States Patent [19] [11] Patent Number: 5,997,600 Dean [45] Date of Patent: Dec. 7, 1999 [54] FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METAL IONS 143365 8/1980
US005997600A Ulllted States Patent [19] [11] Patent Number: 5,997,600 Dean [45] Date of Patent: Dec. 7, 1999 [54] FERTILIZER COMPOSITIONS INCLUDING FOREIGN PATENT DOCUMENTS CHELATED METAL IONS 143365 8/1980
United States Patent (19)
 United States Patent (19) Farmer, Jr. et al. 54 (75) 73 21 22 (51) 52) 58 (56) PROCESS FOR PRODUCING SODUMAND ZINC PYRTHIONE Inventors: Douglas A. Farmer, Jr., Madison; Lawrence E. Katz, Orange, both of
United States Patent (19) Farmer, Jr. et al. 54 (75) 73 21 22 (51) 52) 58 (56) PROCESS FOR PRODUCING SODUMAND ZINC PYRTHIONE Inventors: Douglas A. Farmer, Jr., Madison; Lawrence E. Katz, Orange, both of
