LIVER-SPECIFIC DISTRIBUTION OF ROSUVASTATIN IN RATS: COMPARISON WITH PRAVASTATIN AND SIMVASTATIN
|
|
|
- Randell Franklin
- 6 years ago
- Views:
Transcription
1 /02/ $7.00 DRUG METABOLISM AND DISPOSITION Vol. 30, No. 11 Copyright 2002 by The American Society for Pharmacology and Experimental Therapeutics 729/ DMD 30: , 2002 Printed in U.S.A. LIVER-SPECIFIC DISTRIBUTION OF ROSUVASTATIN IN RATS: COMPARISON WITH PRAVASTATIN AND SIMVASTATIN KEN-ICHI NEZASA, KAZUTAKA HIGAKI, TADAHIKO MATSUMURA, KAZUHIRO INAZAWA, HIROSHI HASEGAWA, MASAYUKI NAKANO, AND MASAHIRO KOIKE Developmental Research Laboratories, Shionogi and Co., Ltd., Osaka, Japan (K.N., T.M., K.I., H.H., M.N., M.K.); and Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Okayama University, Okayama, Japan (K.H.) (Received March 3, 2002; accepted July 31, 2002) This article is available online at ABSTRACT: 1 Abbreviations used are: HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; CL uptake, clearance uptake; C p,t, plasma concentration of total radioactivity at time t; AUC, area under the curve; Sg total, total area of silver grains; Sg mean, mean area of each silver grain; OATP, organic anion transporters. Address correspondence to: Ken-ichi Nezasa, Developmental Research Laboratories, Shionogi and Co., Ltd., 3-1-1, Futaba-cho, Toyonaka, Osaka , Japan. kenichi.nezasa@shionogi.co.jp Rosuvastatin is a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. The liver is the target organ for the lipid-regulating effect of rosuvastatin; therefore liver-selective uptake of this drug is a desirable property. The aim of this study was to investigate, and compare with pravastatin and simvastatin, the tissuespecific distribution of rosuvastatin. Bolus intravenous doses (5 mg/kg) of radiolabeled rosuvastatin, pravastatin, and simvastatin were administered to rats, and initial uptake clearance (CL uptake )in various tissues was calculated. Hepatic CL uptake of rosuvastatin (0.885 ml/min/g tissue) was significantly (p < 0.001) larger than that of pravastatin (0.703 ml/min/g tissue), and rosuvastatin was taken up by the hepatic cells more selectively and efficiently than pravastatin. Hepatic CL uptake of simvastatin (1.24 ml/min/g tissue) was significantly larger than that of rosuvastatin (p < 0.01) and pravastatin (p < 0.001). However, adrenal CL uptake of simvastatin (1.55 ml/min/g tissue) was larger than hepatic CL uptake, and simvastatin was distributed to other tissues more easily than rosuvastatin. Microautoradiography of the liver, spleen, and adrenal was undertaken 5 min after administration of the study drugs; distribution was quantified by counting the number of silver grains. After administration of rosuvastatin and pravastatin, silver grains were distributed selectively in the intracellular space of the liver, but more rosuvastatin ( particles/mm 2 ) than pravastatin ( particles/mm 2 ) tended to distribute to the liver. Simvastatin was less liver-specific (it also distributed to the spleen and adrenal). The results of this study indicated that rosuvastatin was taken up by hepatic cells more selectively and more efficiently than pravastatin and simvastatin. Rosuvastatin (Crestor), calcium bis[( )-(3R,5S,6E)-7-[4-(pfluorophenyl)-6-isopropyl-2-(N-methylmethanesulfonamido)-5-pyrimidinyl]-3,5-dihydroxy-6-heptenoate], is a new and highly effective inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA 1 ) reductase (the rate-limiting enzyme in cholesterol biosynthesis). The drug, hereafter referred to as rosuvastatin, which was originally synthesized at Shionogi and Co., Ltd. (Watanabe et al., 1997), has completed phase III clinical development at AstraZeneca for the treatment of patients with dyslipidemia. In clinical trials, rosuvastatin (1 to 80 mg) produced significant dose-dependent reductions in low-density lipoprotein cholesterol (up to 65%), total cholesterol, and apolipoprotein B (Olsson et al., 2001). Reductions in triglycerides and increases in high-density lipoprotein cholesterol were consistently observed with rosuvastatin, and the drug was well tolerated (Olsson et al., 2001). The liver is the target organ for the lipid-regulating effect of rosuvastatin; therefore liver-selective uptake of this drug is a desirable property. The aim of the present study was to investigate, and compare with pravastatin and simvastatin, the tissue-specific distribution of rosuvastatin in rats. Experimental Procedures Materials. [ 14 C]Rosuvastatin (1.15 MBq/mg), [ 14 C]pravastatin (1.17 MBq/ mg), and [ 14 C]simvastatin (1.85 MBq/mg) were synthesized at Shionogi and Co., Ltd. (Osaka, Japan). The radiochemical purities were 99.4, 99.0, and 98.6%, respectively. The chemical structure of these HMG-CoA reductase inhibitors is shown in Fig. 1. All other chemicals were of analytical grade and commercially available. Animals. Male rats of Jcl:Sprague-Dawley strain were purchased from CLEA Japan, Inc. (Osaka, Japan). The rats were 10-weeks old, and their weight ranged between 317 and 405 g. Prior to study, the rats acclimatized for 1 week in the animal room at Shionogi and Co., Ltd. (room temperature 23 1 C; relative humidity 55 10%); free access to water and laboratory food (CA-1; CLEA Japan, Inc.) was permitted. Preparation of the HMG-CoA Reductase Inhibitors. Dosing solutions (5 mg/ml) were prepared immediately before administration of the HMG-CoA reductase inhibitors to the rats. [ 14 C]Rosuvastatin and [ 14 C]pravastatin were dissolved in physiological saline. [ 14 C]Simvastatin was dissolved in a mixture of N,N-dimethylacetamide, HCO60 (hydrogenated castor oil 60), and physiological saline (2:1:4, by volume). In Vivo Experiment. The rats were anesthetized by intraperitoneal administration of pentobarbital sodium (50 mg/kg) and incised along the abdominal midline. A bolus intravenous dose (5 mg/kg) of [ 14 C]rosuvastatin, [ 14 C]pravastatin, or [ 14 C]simvastatin dosing solution was then administered through the tail vein. At 15, 30, 45, 60, 90, 120, 150, 180, 240, and 300 s after administration of the HMG-CoA reductase inhibitor, blood (8 10 ml) was drawn from the 1158
2 LIVER-SPECIFIC DISTRIBUTION OF ROSUVASTATIN 1159 FIG. 1.Chemical structures of [ 14 C]rosuvastatin, [ 14 C]pravastatin, and [ 14 C]simvastatin. The asterisk denotes the labeled position. Molecular weights of rosuvastatin, pravastatin, and simvastatin are 500, 447, and 419, respectively. abdominal vena cava for exactly 60 s. The rats were killed by exsanguination and the following tissues and organs collected: eyeball, cerebrum, cerebellum, thyroid, lung, heart, adrenal, testis, prostate, spleen, kidney, liver, and ileum. Handling of Samples for Calculation of Uptake Clearance. Blood samples were centrifuged at 13,000g for 30 s, and the plasma harvested. A 100- l aliquot of the plasma sample was weighed in a counting vial. Pico-Fluor 40 (PerkinElmer Life Sciences, Boston, MA) 10 ml was then added, and the radioactivity measured by a liquid scintillation counter (Tri-Carb 2200-CA; PerkinElmer Life Sciences). A sample of each tissue/organ (blotted on filter paper to remove as much blood as possible) was weighed in a counting vial. Soluene-350 (2 ml; PerkinElmer Life Sciences) was added to solubilize the sample, which was then bleached with 200 l of hydrogen peroxide solution. Pico-fluor 40 (10 ml) was added, and the radioactivity measured by a liquid scintillation counter (Tri-Carb 2200-CA). Calculation of Uptake Clearance. The uptake clearance was calculated according to the following differential equation (Kim et al., 1988; Yanai et al., 1990). dx tissue,t /dt CL uptake C p,t where X tissue,t denotes the tissue concentration of total radioactivity at time t, CL uptake denotes uptake clearance, and C p,t denotes the plasma concentration of the total radioactivity at time t. By integrating eq. 1 with time and dividing both sides of the equation by C p,t, eq. 2 is obtained. X tissue,t /C p,t CL uptake AUC 0-t /C p,t The slope of the linear line obtained by plotting AUC 0-t /C p,t against X tissuet / C p,t (an integration plot) corresponds to CL uptake (Kim et al., 1988, Yanai et al., 1990). Thus, the AUC 0-t value was calculated by the trapezoidal rule, and the AUC 0-t /C p,t values were plotted against the X tissue,t /C p,t values. The CL uptake value was then calculated by linear regression analysis. The data points actually employed in the calculation of the CL uptake values occurred s after administration of the HMG-CoA reductase inhibitor. If the number of data points on the line was small, saturation of tissue uptake was considered to have occurred early after administration. Handling of Samples for Microautoradiography. Samples of the liver and spleen and the whole adrenal were excised and rapidly frozen by liquid nitrogen. Sections (10 m) were then prepared, lyophilized, and mounted on glass slides coated with photosensitive emulsion (NTB-3; Kodak, Rochester, NY). After exposure in a shaded box for 1 month at 4 C, the glass slides were developed for 2 min at 17 C with Dektol (Kodak) and stained with methyleneblue-basic fuchsin for microscopic observation. Quantitative Microautoradiography. Input of image data and software for imaging analysis. For quantitative measurement, three different visible fields of the tissue picture ( m 2 in size; object lens, 40) were taken using a digital camera (HC-2000; Fuji Photo Film Co., Ltd., Tokyo, Japan) connected to a light microscope. For imaging analysis, Optimas version 6 (Optimas Corporation, Bothell, WA) software was used. Measurement of the outer and inner areas of cell. The unstained area was regarded as the outer area of the cell (cellular interstitial part and blood vessel). By setting the threshold level of the stained color, separation of the outer area of the cell from the inner area of the cell was achieved and each area (S cell ) was measured. Measurement of the total area of silver grains. To discriminate the silver grains, the threshold level of the color was set again for each area (outer and inner) of the cell, and the total area of silver grains (Sg total ) in each was measured. Measurement of the mean area of each silver grain. In the case that more than two particles of silver grains are present as a mass, this mass is counted as one particle. Therefore, the mean area of each silver grain (Sg mean ) was calculated according to the following procedure. Using the outer area of the liver cells showing good separation of each silver grain and having negligible mass of silver grains, the total number of the silver grains (N total ) present in the outer parts of the cells as well as Sg total were measured, and Sg mean was calculated according to eq. 3. Sg mean Sg total /N total The mean area of each silver particle (subsequently found to be m 2 ) was obtained as the mean value of the three visible fields for each drug (a total of nine visible fields). Measurement of the number of silver grains in each unit area of the outer and inner parts of the cell. The number of silver grains in each unit area (N) was calculated (particles/mm 2 ) for the outer and inner parts of the cell according to eq. 4. N Sg total /Sg mean 1/S cell Statistical Analysis. An analysis of variance was performed to determine the statistical significance between groups using Bartlett s test. Significant differences between the means were examined using Tukey s method. Comparison of regression lines was performed by calculating the standard error of the difference in slope according to Student s t test. Statistical significance between observed data and data calculated by linear regression were determined by Pearson s method, which is used to estimate the significance for the linear correlation. Results Uptake Clearance. Figure 2 shows the integration plots for uptake by the liver, kidney, spleen, and adrenal following administration of [ 14 C]rosuvastatin (Fig. 2A), [ 14 C]pravastatin (Fig. 2B), and [ 14 C]simvastatin (Fig. 2C). The CL uptake values (i.e., the slopes of the integration plots) are summarized in Fig. 3. The linear correlation was statistically significant in all tissues examined except for kidney for pravastatin, in which the correlation coefficient was relatively low (r 0.767). Following administration of [ 14 C]rosuvastatin, the liver showed the
3 1160 NEZASA ET AL. FIG. 2.Integration plots of [ 14 C]rosuvastatin (plot A), [ 14 C]pravastatin (plot B), and [ 14 C]simvastatin (plot C). The data points are from single animals. Closed circle, liver; open circle, kidney; triangle, spleen; square, adrenal. The statistical significance of the linear correlation was determined as follows: plot A, p 0.01 (liver, kidney, and adrenal), p 0.05 (spleen); plot B, p 0.01 (liver, spleen, and adrenal); plot C; p 0.01 (all tissues). highest CL uptake value (0.885 ml/min/g tissue). The kidney showed the second highest CL uptake value (0.186 ml/min/g tissue; approximately 20% of that in the liver), followed by the ileum (0.021 ml/min/g tissue; approximately 2% of that in the liver). CL uptake values for the lung and heart were and ml/min/g tissue, FIG. 3.Comparison of uptake clearance (CL uptake ) in tissues and organs after intravenous administration of [ 14 C]rosuvastatin, [ 14 C]pravastatin, and [ 14 C]simvastatin. Statistical significance was examined in liver, kidney, spleen, and adrenal. #, significantly different from pravastatin (#, p 0.05; ###, p 0.001);, significantly different from simvastatin (, p 0.05;, p 0.01;, p 0.001). respectively (approximately 1% of that in the liver). CL uptake values for other tissues and organs were extremely low ( 0.5% of that in the liver). These results demonstrate that uptake of rosuvastatin by the liver was very selective. Following administration of [ 14 C]pravastatin, the liver showed the highest CL uptake value (0.703 ml/min/g tissue). The kidney showed the second highest CL uptake value (0.390 ml/min/g tissue; approximately 55% of that in the liver), followed by the lung (0.022 ml/min/g tissue; approximately 3% of that in the liver) and the heart (0.013 ml/min/g tissue; approximately 2% of that in the liver). CL uptake values for other tissues and organs were very low ( 2% of that in the liver). Following administration of [ 14 C]simvastatin, the adrenal showed the highest CL uptake value (1.55 ml/min/g tissue; approximately 25% higher than that in the liver). The liver showed the second highest CL uptake value (1.24 ml/min/g tissue) followed by the heart, kidney, thyroid, and lung (0.995, 0.911, 0.785, and ml/min/g tissue, respectively; approximately 80 50% of that in the liver). The CL uptake values for other tissues and organs were low ( 25% of that in the liver). Microautoradiography. The microautoradiograms obtained from the liver, spleen, and adrenal are shown in Fig. 4. In the liver (Fig. 4a), a large number of silver grains were observed for all drugs. In the spleen (Fig. 4b), silver grains were only observed for simvastatin. In the adrenal (Fig. 4c), silver grains were also only observed for simvastatin (to at least the same extent as in the liver). The number of silver grains distributed to each tissue is shown in Table 1. In the intracellular space of the liver there were particles/mm 2 for rosuvastatin, particles/mm 2 for pravastatin, and particles/mm 2 for simvastatin. Thus
4 LIVER-SPECIFIC DISTRIBUTION OF ROSUVASTATIN 1161 FIG. 4.Microautoradiograms of [ 14 C]rosuvastatin (A), [ 14 C]pravastatin (B), and [ 14 C]simvastatin (C) in rat liver (a), spleen (b), and adrenal (c). Arrows indicate some of the silver grains. the number of silver grains distributed to the liver following administration of rosuvastatin and simvastatin tended to be greater than that following administration of pravastatin, although it was not statistically significant. In the extracellular space of the liver, there was no difference in the number of silver grains between the drugs. In the spleen there were almost no silver grains in the inner or outer parts of the cell following administration of rosuvastatin and pravastatin. However, there were a number of silver grains ( particles/mm 2 ) in the inner part of the cell following administration of simvastatin. In the adrenal there were almost no silver grains in the inner or outer parts of the cell following administration of rosuvastatin and
5 1162 NEZASA ET AL. TABLE 1 Distribution of silver grains in rat tissues following intravenous administration of [ 14 C]rosuvastatin, [ 14 C]pravastatin, and [ 14 C]simvastatin Each value represents the mean standard deviation of three different optical fields. Tissue HMG-CoA Reductase Inhibitor Number of Silver Grains ( 10 5 particles/ mm 2 ) Intracellular Extracellular Liver Rosuvastatin Pravastatin Simvastatin Spleen Rosuvastatin *** * Pravastatin *** * Simvastatin Adrenal Rosuvastatin *** *** Pravastatin *** *** Simvastatin * p 0.05 vs. simvastatin; *** p vs. simvastatin. pravastatin. However, the number of silver grains in both the inner and outer parts of the cell was marked following administration of simvastatin. The number of silver grains in the inner part of the cell was particles/mm 2, which was the highest value of all the tissues investigated. Discussion The liver is the target organ for the lipid-regulating effect of HMG-CoA reductase inhibitors; therefore liver-selective uptake of these drugs is a desirable property. In the present study, we investigated, and compared with pravastatin and simvastatin, the tissuespecific distribution of rosuvastatin in rats. The hepatic CL uptake of rosuvastatin (0.885 ml/min/g tissue) was significantly (p 0.001) larger than that of pravastatin (0.703 ml/min/g tissue). [The hepatic CL uptake value for pravastatin is comparable to values reported previously, 0.44 ml/min/g tissue (Yamazaki et al., 1993) and 0.59 ml/min/g tissue (Yamazaki et al., 1996).] Furthermore, the CL uptake values of pravastatin in the kidney, testis, thyroid, eyeball, prostate, and spleen were 2- to 4-times larger than those of rosuvastatin, indicating that rosuvastatin was taken up by the hepatic cells more selectively and more efficiently than pravastatin. The microautoradiographic data showed that both rosuvastatin and pravastatin were distributed selectively in the intracellular space of the liver, but more rosuvastatin than pravastatin tended to distribute to the liver. The hepatic CL uptake of simvastatin (1.24 ml/min/g tissue) was significantly larger than that of rosuvastatin (p 0.01) and pravastatin (p 0.001). However, the CL uptake values of simvastatin in the other tissues were much larger than those of rosuvastatin, indicating that simvastatin distributed to other tissues more easily than rosuvastatin. The microautoradiographic data also showed that simvastatin distributed to several tissues and was less liver-specific. The results of this study support the previous finding that pravastatin has a higher liver-selectivity than simvastatin (Koga et al., 1995; van Vliet et al., 1995). However, the number of silver grains in the spleen following administration of pravastatin was much less than that of simvastatin, and this differs from previous findings (Koga et al., 1992). Transport of pravastatin to hepatocytes is proposed to occur mainly via a Na -independent anion transporter in the sinusoidal membrane, due to the negative charge and hydrophilicity of pravastatin (Komai et al., 1992; Yamazaki et al., 1993). In contrast, passive diffusion may contribute largely to the transport of simvastatin because of its high lipophilicity (Komai et al., 1992). Therefore, pravastatin might be distributed less than simvastatin to tissues/organs without that putative transport mechanism (e.g., the spleen). The results of this study highlighted quite large differences in distribution to the extrahepatic tissues between these two compounds. It has been speculated that rosuvastatin is selectively taken up by the liver via the organic anion transport system (possibly a Na - independent anion transporter), due to the negative charge of rosuvastatin. A recent study involving oocytes expressing OATP-C or OATP-A suggested that rosuvastatin is a substrate for OATP-C but not for OATP-A (Brown et al., 2001). The n-octanol-water partition coefficient of rosuvastatin is 1.46 at ph 7.0, which is closer to that of pravastatin (0.59) than that of simvastatin (48400) (Serajuddin et al., 1991). The high lipophilicity of simvastatin has been linked with side effects such as myopathy (Pierno et al., 1999). Furthermore, compared with pravastatin, a hydrophilic compound, the risk of myopathy with simvastatin was shown to be higher when using a urethane infusion method (Matsuyama et al., 2002). Generally, the specific distribution of drugs to the target organ contributes to the increase of clinical effect and the decrease of toxicity in the other organs. In the clinical dose-ranging trial (1 80 mg) of rosuvastatin for 6 weeks, myopathy, which can be associated with elevated creatine kinase levels, is relatively infrequent (Olsson et al., 2001). However, there are serious events associated with statin therapy that appear to be more common at higher doses of the other statins (Maron et al., 2000). The high hydrophilicity of rosuvastatin may explain the minor nonspecific distribution to extrahepatic tissues and may be consistent with low toxicity. In the clinical trial for 12-week dosing, 5 and 10 mg of rosuvastatin reduced low-density lipoprotein cholesterol by 42 and 49%, respectively, compared with a 28% reduction with 20 mg of pravastatin and 37% with 20 mg of simvastatin, and the effect of rosuvastatin was significantly larger than either pravastatin or simvastatin (Paoletti et al., 2001). It has been reported that rosuvastatin exhibited inhibition of cholesterol synthesis with an IC 50 of 0.16 nm in rat hepatocytes and was significantly more potent than both pravastatin and simvastatin (McTaggart et al., 2001). In addition to this higher potency, our findings, the more selective distribution to the liver, also contribute to a greater clinical effect of rosuvastatin over both pravastatin and simvastatin. In conclusion, the results of this study indicated that rosuvastatin was taken up by the hepatic cells more selectively and more efficiently than pravastatin and simvastatin. Acknowledgments. We thank T. Nagasaki, Y. Katsuyama, and M. Segawa for synthesizing and purifying [ 14 C]rosuvastatin, [ 14 C]pravastatin, and [ 14 C]simvastatin. References Brown CDA, Windass A, Bleasby K, and Lauffart B (2001) Rosuvastatin is a high affinity substrate of hepatic organic anion transporter OATP-C (abstract). Atheroscler Suppl 2:90. Kim DC, Sugiyama Y, Satoh H, Fuwa T, Iga T, and Hanano M (1988) Kinetic analysis of the receptor dependent uptake of human epidermal growth factor by the tissues of the rat in vivo. J Pharm Sci 77: Koga T, Fukuda K, Shimada Y, Fukami M, Koike H, and Tsujita Y (1992) Tissue selectivity of pravastatin sodium, lovastain and simvastatin. Eur J Biochem 209: Koga T, Kikuchi T, Miyazaki A, and Koike H (1995) Tissue-selective inhibition of sterol synthesis in mice by pravastatin sodium after a single or repeated oral administrations. Lipids 30: Komai T, Shigehara E, Tokui T, Koga T, Ishigami M, Kuroiwa C, and Horiuchi S (1992) Carrier-mediated uptake of pravastatin by rat hepatocytes in primary culture. Biochem Pharmacol 43: Maron DJ, Fazio S, and Linton MF (2000) Current perspectives on statins. Circulation 101: Matsuyama K, Nakagawa K, Nakai A, Konishi Y, Nishikata M, Tanaka H, and Uchida T (2002) Evaluation of myopathy risk for HMG-CoA reductase inhibitors by urethane infusion method. Biol Pharm Bull 25: McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, and Warwick M (2001) Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy- 3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 87 (Suppl):28B 32B. Olsson AG, Pears J, McKellar J, Mizan J, and Raza A (2001) Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol 88: Paoletti R, Fahmy M, Mahla G, Mizan J, and Southworth H (2001) ZD4522 is superior to
6 LIVER-SPECIFIC DISTRIBUTION OF ROSUVASTATIN 1163 pravastatin and simvastatin in reducing low density lipoprotein cholesterol, enabling more hypercholesterolemic patients to achieve target low density lipoprotein cholesterol guidelines (abstract). J Am Coll Cardiol 37 (Suppl A):291A. Pierno S, De Luca A, Liantonio A, Camerino C, and Conte Camerino D (1999) Effects of HMG-CoA reductase inhibitors on excitation-contraction coupling of rat skeletal muscle. Eur J Pharmacol 364: Serajuddin ATM, Ranadive SA, and Mahoney EM (1991) Relative lipophilicities, solubilities and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin and simvastatin. J Pharm Sci 80: van Vliet A, Van Thiel G, Huisman RH, Moshage H, Yap SH, and Cohen LH (1995) Different effects of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors on sterol synthesis in various human cell types. Biochem Biophys Acta 1254: Watanabe M, Koike H, Ishida T, Okada T, Seo S, and Hirai K (1997) Synthesis and biological activity of methanesulfonamide pyrimidine- and N-methanesulfonyl pyrrole-substituted 3,5- dihydroxy-6-heptenoates, a novel series of HMG-CoA reductase inhibitors. Bioorg Med Chem 5: Yamazaki M, Suzuki H, Hanano M, Tokui T, Komai T, and Sugiyama Y (1993) Na - independent multispecific anion transporter mediates active transport of pravastatin into rat liver. Am J Physiol 264:G36 G44. Yamazaki M, Tokui T, Ishigami M, and Sugiyama Y (1996) Tissue-selective uptake of pravastatin in rats: contribution of a specific carrier-mediated uptake system. Biopharm Drug Dispos 17: Yanai S, Sugiyama Y, Iga T, Fuwa T, and Hanano M (1990) Kinetic analysis of the downregulation of epidermal growth factor receptors in rats in vivo. Am J Physiol 258:C593 C598.
Pharmacologic Characteristics of Statins
 Clin. Cardiol. Vol. 26 (Suppl. III), III-32 III-38 (2003) Pharmacologic Characteristics of Statins JAMES M. MCKENNEY, PHARM.D. National Clinical Research, Inc., and School of Pharmacy, Virginia Commonwealth
Clin. Cardiol. Vol. 26 (Suppl. III), III-32 III-38 (2003) Pharmacologic Characteristics of Statins JAMES M. MCKENNEY, PHARM.D. National Clinical Research, Inc., and School of Pharmacy, Virginia Commonwealth
Statin inhibition of HMG-CoA reductase: a 3-dimensional view
 Atherosclerosis Supplements 4 (2003) 3/8 www.elsevier.com/locate/atherosclerosis Statin inhibition of HMG-CoA reductase: a 3-dimensional view Eva Istvan * Department of Molecular Microbiology, Howard Hughes
Atherosclerosis Supplements 4 (2003) 3/8 www.elsevier.com/locate/atherosclerosis Statin inhibition of HMG-CoA reductase: a 3-dimensional view Eva Istvan * Department of Molecular Microbiology, Howard Hughes
Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia
 ISPUB.COM The Internet Journal of Cardiovascular Research Volume 3 Number 1 Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia V Save, N Patil, G Rajadhyaksha Citation V Save,
ISPUB.COM The Internet Journal of Cardiovascular Research Volume 3 Number 1 Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia V Save, N Patil, G Rajadhyaksha Citation V Save,
Lack of effect of ketoconazole on the pharmacokinetics of rosuvastatin in healthy subjects
 Blackwell Science, LtdOxford, UKBCPBritish Journal of Clinical Pharmacology0306-5251Blackwell Publishing 200254Original ArticleCo-administration of rosuvastatin and ketoconazolek. J. Cooper Lack of effect
Blackwell Science, LtdOxford, UKBCPBritish Journal of Clinical Pharmacology0306-5251Blackwell Publishing 200254Original ArticleCo-administration of rosuvastatin and ketoconazolek. J. Cooper Lack of effect
Characterization and method development for estimation and validation of Rosuvastatin Calcium by UV visible spectrophotometry
 International Journal of Theoretical & Applied Sciences, 1(1): 48-53(2009) Characterization and method development for estimation and validation of Rosuvastatin Calcium by UV visible spectrophotometry
International Journal of Theoretical & Applied Sciences, 1(1): 48-53(2009) Characterization and method development for estimation and validation of Rosuvastatin Calcium by UV visible spectrophotometry
Introduction. Paul D. Martin, 1 Patrick D. Mitchell 2 & Dennis W. Schneck Blackwell Science Ltd Br J Clin Pharmacol, 54,
 Blackwell Science, LtdOxford, UKBJCPBritish Journal of Clinical Pharmacology0306-5251Blackwell Science, 200254Original ArticleEffects of rosuvastatin after a.m. or p.m. dosingp. D. Martin et al. Pharmacodynamic
Blackwell Science, LtdOxford, UKBJCPBritish Journal of Clinical Pharmacology0306-5251Blackwell Science, 200254Original ArticleEffects of rosuvastatin after a.m. or p.m. dosingp. D. Martin et al. Pharmacodynamic
Original paper. Abstract. Abdullah S. Asia 1*, Al-Mahdi A. Modar 2, Hadi M. Ali 3
 Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
ANTIHYPERLIPIDEMIA. Darmawan,dr.,M.Kes,Sp.PD
 ANTIHYPERLIPIDEMIA Darmawan,dr.,M.Kes,Sp.PD Plasma lipids consist mostly of lipoproteins Spherical complexes of lipids and specific proteins (apolipoproteins). The clinically important lipoproteins, listed
ANTIHYPERLIPIDEMIA Darmawan,dr.,M.Kes,Sp.PD Plasma lipids consist mostly of lipoproteins Spherical complexes of lipids and specific proteins (apolipoproteins). The clinically important lipoproteins, listed
Antihyperlipidemic Drugs
 Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Kristina Strutt, MBBS; Richard Caplan, PhD*; Howard Hutchison, MD*; Aaron Dane, MSc; James Blasetto, MD* Circ J 2004; 68:
 Circ J 2004; 68: 107 113 More Western Hypercholesterolemic Patients Achieve Japan Atherosclerosis Society LDL-C Goals With Rosuvastatin Therapy Than With Atorvastatin, Pravastatin, or Simvastatin Therapy
Circ J 2004; 68: 107 113 More Western Hypercholesterolemic Patients Achieve Japan Atherosclerosis Society LDL-C Goals With Rosuvastatin Therapy Than With Atorvastatin, Pravastatin, or Simvastatin Therapy
Cellular uptake of fluvastatin, an inhibitor of HMG-CoA reductase, by rat cultured hepatocytes and human aortic endothelial cells
 Cellular uptake of fluvastatin, an inhibitor of HMG-CoA reductase, by rat cultured hepatocytes and human aortic endothelial cells Masakatsu Ohtawa, 1 Naoki Masuda, 1 Izumi Akasaka, 1 Akinori Nakashima,
Cellular uptake of fluvastatin, an inhibitor of HMG-CoA reductase, by rat cultured hepatocytes and human aortic endothelial cells Masakatsu Ohtawa, 1 Naoki Masuda, 1 Izumi Akasaka, 1 Akinori Nakashima,
Human Liver-Specific Organic Anion Transporter, LST-1, Mediates Uptake of Pravastatin by Human Hepatocytes
 0022-3565/01/2973-861 867$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 297, No. 3 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3460/896005
0022-3565/01/2973-861 867$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 297, No. 3 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3460/896005
Atsuhiro Miyazaki 1, Tadashi Koieyama 2, Yukio Shimada 3, Takashi Kikuchi 4, Kayoko Ito 5, Naomi Kasanuki 2, and Teiichiro Koga 6.
 22 Journal of Atherosclerosis and Thrombosis Original Article Vol. 11, No. 1 Pravastatin Sodium, an Inhibitor of HMG-CoA Reductase, Decreases HDL Cholesterol by Transfer of Cholesteryl Ester from HDL to
22 Journal of Atherosclerosis and Thrombosis Original Article Vol. 11, No. 1 Pravastatin Sodium, an Inhibitor of HMG-CoA Reductase, Decreases HDL Cholesterol by Transfer of Cholesteryl Ester from HDL to
Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats
 J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
J. Biosci., Vol. 12, Number 2, June 1987, pp. 137 142. Printed in India. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats P. VALSALA and P. A. KURUP Department
 Effect of a Selenium Analogue of [L Title Transport of Candida pelliculosa (C Dedicated to Professor Masaya Okano Retirement) Author(s) Shimizu, Eiichi; Yamana, Ryutaro; T Kenji Citation Bulletin of the
Effect of a Selenium Analogue of [L Title Transport of Candida pelliculosa (C Dedicated to Professor Masaya Okano Retirement) Author(s) Shimizu, Eiichi; Yamana, Ryutaro; T Kenji Citation Bulletin of the
SLCO1B1 Pharmacogenetic Competency
 SLCO1B1 Pharmacogenetic Competency Updated on 6/2015 Pre-test Question # 1 Which of the following is not currently a recognized SLCO1B1 phenotype? a) Low function b) Normal function c) Intermediate function
SLCO1B1 Pharmacogenetic Competency Updated on 6/2015 Pre-test Question # 1 Which of the following is not currently a recognized SLCO1B1 phenotype? a) Low function b) Normal function c) Intermediate function
Targeting of Coenzyme Q10 Solubilized with Soy Lecithin to Heart of Guinea Pigs
 J. Nutr. Sci. Vitaminol., 31, 115-120, 1985 Communication Targeting of Coenzyme Q10 Solubilized with Soy Lecithin to Heart of Guinea Pigs Masahiro TAKADA, Teruaki YUZURIHA, Kouichi KATAYAMA, Chiyuki YAMATO,1
J. Nutr. Sci. Vitaminol., 31, 115-120, 1985 Communication Targeting of Coenzyme Q10 Solubilized with Soy Lecithin to Heart of Guinea Pigs Masahiro TAKADA, Teruaki YUZURIHA, Kouichi KATAYAMA, Chiyuki YAMATO,1
DRUG INTERACTION BETWEEN SIMVASTATIN AND ITRACONAZOLE IN MALE AND FEMALE RATS
 0090-9556/01/2907-1068 1072$3.00 DRUG METABOLISM AND DISPOSITION Vol. 29, No. 7 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 331/915884 DMD 29:1068 1072, 2001 Printed
0090-9556/01/2907-1068 1072$3.00 DRUG METABOLISM AND DISPOSITION Vol. 29, No. 7 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 331/915884 DMD 29:1068 1072, 2001 Printed
Univ.-Doz. Prof. Dr. W. Renner
 Pharmacogenetics of statin-inducedinduced myopathies Wilfried Renner Medical University Graz Clinical Institute of Medical and Chemical Laboratory Diagnostics It started with a fungus 1973: Isolation of
Pharmacogenetics of statin-inducedinduced myopathies Wilfried Renner Medical University Graz Clinical Institute of Medical and Chemical Laboratory Diagnostics It started with a fungus 1973: Isolation of
Human LDL Receptor / LDLR ELISA Pair Set
 Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States
 Bull. Natl. Inst. Health Sci., 123 Notes # Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States Journal of American Medical Association (JAMA) Statin HMG
Bull. Natl. Inst. Health Sci., 123 Notes # Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States Journal of American Medical Association (JAMA) Statin HMG
Setting The study setting was secondary care. The economic analysis was conducted in the UK.
 Rosuvastatin is cost-effective in treating patients to low-density lipoprotein-cholesterol goals compared with atorvastatin, pravastatin and simvastatin: analysis of the STELLAR trial Hirsch M, O'Donnell
Rosuvastatin is cost-effective in treating patients to low-density lipoprotein-cholesterol goals compared with atorvastatin, pravastatin and simvastatin: analysis of the STELLAR trial Hirsch M, O'Donnell
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Take-Home Exam Distributed: October 16, 2013, at 1:30 p.m. Due: October 21, 2013, at 10:00 a.m.
 20.201 Take-Home Exam Distributed: October 16, 2013, at 1:30 p.m. Due: October 21, 2013, at 10:00 a.m. Directions: This take-home exam is to be completed without help from any other individual except:
20.201 Take-Home Exam Distributed: October 16, 2013, at 1:30 p.m. Due: October 21, 2013, at 10:00 a.m. Directions: This take-home exam is to be completed without help from any other individual except:
COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010
 COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010 Office of Clinical Research Training and Medical Education National
COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010 Office of Clinical Research Training and Medical Education National
B. Patient has not reached the percentage reduction goal with statin therapy
 Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Life Needs Energy. The Rules (Laws of Thermodynamics) 1) energy can not be created or destroyed, but it can be changed from one form to another
 Intro to Metabolism Learning Outcomes Explain laws governing energy and energy transfers. Describe enzymes and how they work. Explain what is meant by selectively permeable. Explain the differences between
Intro to Metabolism Learning Outcomes Explain laws governing energy and energy transfers. Describe enzymes and how they work. Explain what is meant by selectively permeable. Explain the differences between
CHOLESTAGEL 625 mg Genzyme
 CHOLESTAGEL 625 mg Genzyme 1. NAME OF THE MEDICINAL PRODUCT Cholestagel 625 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 625 mg colesevelam hydrochloride (hereafter
CHOLESTAGEL 625 mg Genzyme 1. NAME OF THE MEDICINAL PRODUCT Cholestagel 625 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 625 mg colesevelam hydrochloride (hereafter
Development and Validation of a UV-Spectrophotometric Method for Quantification of Atorvastatin in Tablets
 Journal of PharmaSciTech 0; ():4-40 Research Article Development and Validation of a UV-Spectrophotometric Method for Quantification of Atorvastatin in Tablets Ghanty S*, Sadhukhan N, Mondal A Gupta College
Journal of PharmaSciTech 0; ():4-40 Research Article Development and Validation of a UV-Spectrophotometric Method for Quantification of Atorvastatin in Tablets Ghanty S*, Sadhukhan N, Mondal A Gupta College
DYSLIPIDEMIA PHARMACOLOGY. University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 DYSLIPIDEMIA PHARMACOLOGY University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 LEARNING OBJECTIVES Know normal cholesterol levels Understand what the role
DYSLIPIDEMIA PHARMACOLOGY University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 LEARNING OBJECTIVES Know normal cholesterol levels Understand what the role
Rosuvastatin is transferred into human breast milk: A case report.
 *Manuscript track changes off Click here to view linked References Rosuvastatin is transferred into human breast milk: A case report. Aletta E Schutte PhD, 1 Elizabeth A Symington MDiet, 2 Jan L du Preez
*Manuscript track changes off Click here to view linked References Rosuvastatin is transferred into human breast milk: A case report. Aletta E Schutte PhD, 1 Elizabeth A Symington MDiet, 2 Jan L du Preez
LDL Uptake Flow Cytometry Assay Kit
 LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Anti Hyperlipidemic Drugs. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Anti Hyperlipidemic Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Lipoproteins Macromolecular complexes in the blood that transport lipids Apolipoproteins
Anti Hyperlipidemic Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Lipoproteins Macromolecular complexes in the blood that transport lipids Apolipoproteins
July 2007 Biol. Pharm. Bull. 30(7) (2007)
 July 2007 Biol. Pharm. Bull. 30(7) 1237 1241 (2007) 1237 Prediction of a 1 -Adrenoceptor Occupancy in the Human Prostate from Plasma Concentrations of Silodosin, Tamsulosin and Terazosin to Treat Urinary
July 2007 Biol. Pharm. Bull. 30(7) 1237 1241 (2007) 1237 Prediction of a 1 -Adrenoceptor Occupancy in the Human Prostate from Plasma Concentrations of Silodosin, Tamsulosin and Terazosin to Treat Urinary
Reducing low-density lipoprotein cholesterol treating to target and meeting new European goals
 European Heart Journal Supplements (2004) 6 (Supplement A), A12 A18 Reducing low-density lipoprotein cholesterol treating to target and meeting new European goals University of Sydney, Sydney, NSW, Australia
European Heart Journal Supplements (2004) 6 (Supplement A), A12 A18 Reducing low-density lipoprotein cholesterol treating to target and meeting new European goals University of Sydney, Sydney, NSW, Australia
Rosuvastatin 5 mg, 10 mg and 20 mg Tablet
 Rosuvastatin 5 mg, 10 mg and 20 mg Tablet Description is a preparation of Rosuvastatin. Rosuvastatin is a member of the drug class of statins, used in combination with exercise, diet, and weight-loss to
Rosuvastatin 5 mg, 10 mg and 20 mg Tablet Description is a preparation of Rosuvastatin. Rosuvastatin is a member of the drug class of statins, used in combination with exercise, diet, and weight-loss to
Antihyperlipidemic Drugs
 Antihyperlipidemic Drugs Lipid disorders: Disorders of lipid metabolism are manifest by elevation of the plasma concentrations of the various lipid and lipoprotein fractions (total and LDL cholesterol,
Antihyperlipidemic Drugs Lipid disorders: Disorders of lipid metabolism are manifest by elevation of the plasma concentrations of the various lipid and lipoprotein fractions (total and LDL cholesterol,
JLR Papers In Press. Published on October 16, 2003 as Manuscript D JLR200
 JLR Papers In Press. Published on October 16, 2003 as Manuscript D300024-JLR200 A method of direct measurement for the enzymatic determination of cholesterol esters Toshimi Mizoguchi 1, Toshiyuki Edano,
JLR Papers In Press. Published on October 16, 2003 as Manuscript D300024-JLR200 A method of direct measurement for the enzymatic determination of cholesterol esters Toshimi Mizoguchi 1, Toshiyuki Edano,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Selecting the Primary Emollient to Enhance the Vitamin E- Acetate Skin Penetration
 Selecting the Primary Emollient to Enhance the Vitamin E- Acetate Skin Penetration JOSHITA DJAJADISASTRA 1, Sasanti Tarini 2, Sugiyono 1 1 Department of Pharmacy Faculty of Mathematics and Sciences, University
Selecting the Primary Emollient to Enhance the Vitamin E- Acetate Skin Penetration JOSHITA DJAJADISASTRA 1, Sasanti Tarini 2, Sugiyono 1 1 Department of Pharmacy Faculty of Mathematics and Sciences, University
BASIC PHARMACOKINETICS
 BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
Management of Post-transplant hyperlipidemia
 Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Determination of bioavailability
 Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Introduction Hyperlipidemia hyperlipoproteinemia Primary hyperlipidemia (Familial) Secondary hyperlipidemia (Acquired)
 Introduction Hyperlipidemia, or hyperlipoproteinemia, is the condition of abnormally elevated levels of any or all lipids and/or lipoproteins in the blood. Hyperlipidemias are divided in primary and secondary
Introduction Hyperlipidemia, or hyperlipoproteinemia, is the condition of abnormally elevated levels of any or all lipids and/or lipoproteins in the blood. Hyperlipidemias are divided in primary and secondary
Cholesterol Management Roy Gandolfi, MD
 Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using THlwater
 Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using THlwater Debra J. Jeske and John M. Dietschy' Department of Internal Medicine, University of Texas
Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using THlwater Debra J. Jeske and John M. Dietschy' Department of Internal Medicine, University of Texas
Application of a Physiologically Based Pharmacokinetic Model to Predict OATP1B1-Related Variability in Pharmacodynamics of Rosuvastatin
 Original Article Citation: CPT Pharmacometrics Syst. Pharmacol. (), e; doi:./psp.. ASCPT All rights reserved -/ Application of a Physiologically Based Pharmacokinetic Model to Predict OATPB-Related Variability
Original Article Citation: CPT Pharmacometrics Syst. Pharmacol. (), e; doi:./psp.. ASCPT All rights reserved -/ Application of a Physiologically Based Pharmacokinetic Model to Predict OATPB-Related Variability
SIMVASTATIN AND EZETIMIBE IN THE DRUG PRODUCTS
 SIMVASTATIN AND EZETIMIBE IN THE DRUG PRODUCTS T.V.KAMALI Ph.D. Research Scholar of Bharathiar University Coimbatore- 641 046 Dr. T. ANANDABASKARAN Assistant Professor, Department of Chemistry, Annamalai
SIMVASTATIN AND EZETIMIBE IN THE DRUG PRODUCTS T.V.KAMALI Ph.D. Research Scholar of Bharathiar University Coimbatore- 641 046 Dr. T. ANANDABASKARAN Assistant Professor, Department of Chemistry, Annamalai
Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more
 Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
Lecture-4 Many drugs have both lipophilic and hydrophilic chemical substituents. Those drugs that are more lipid soluble tend to traverse cell membranes more easily than less lipid-soluble or more water-soluble
Human LDL ELISA Kit. Innovative Research, Inc.
 Human LDL ELISA Kit Catalog No: IRKTAH2582 Lot No: SAMPLE INTRODUCTION Human low-density lipoprotein (LDL) transports cholesterol from the liver to tissues where it is incorporated into cell membranes.
Human LDL ELISA Kit Catalog No: IRKTAH2582 Lot No: SAMPLE INTRODUCTION Human low-density lipoprotein (LDL) transports cholesterol from the liver to tissues where it is incorporated into cell membranes.
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The LDL Receptor, LDL Uptake, and the Free Cholesterol Pool
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM The, LDL Uptake, and the Free Cholesterol Pool I. Michael Brown and Joseph Goldstein A. Studied families with familial hypercholesterolemia. B. Defined the relationship
Cellular control of cholesterol. Peter Takizawa Department of Cell Biology
 Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
Cellular control of cholesterol Peter Takizawa Department of Cell Biology Brief overview of cholesterol s biological role Regulation of cholesterol synthesis Dietary and cellular uptake of cholesterol
DEVELOPMENT OF UV SPECTROPHOTOMETRIC METHOD FOR THE ESTIMATION OF EZETIMIBE FROM TABLET FORMULATION
 Int. J. Chem. Sci.: 13(2), 2015, 1051-1056 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT OF UV SPECTROPHOTOMETRIC METHOD FOR THE ESTIMATION OF EZETIMIBE FROM TABLET FORMULATION KHEMCHAND GUPTA
Int. J. Chem. Sci.: 13(2), 2015, 1051-1056 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT OF UV SPECTROPHOTOMETRIC METHOD FOR THE ESTIMATION OF EZETIMIBE FROM TABLET FORMULATION KHEMCHAND GUPTA
Antihyperlipidemic drugs
 Antihyperlipidemic drugs The clinically important lipoproteins are LDL low density lipoprotein, VLDL very low density lipoprotein, HDL high density lipoprotein. Hyperlipidemia may caused 1. by individual
Antihyperlipidemic drugs The clinically important lipoproteins are LDL low density lipoprotein, VLDL very low density lipoprotein, HDL high density lipoprotein. Hyperlipidemia may caused 1. by individual
Quantitative estimation of Rosuvastatin in bulk and tablet dosage form by using area under curve method
 Original research article ISSN 2321-0125 www.jpbs-online.com Quantitative estimation of Rosuvastatin in bulk and tablet dosage form by using area under curve method P. S. Jain*, N. K. Kale, S. J. Surana
Original research article ISSN 2321-0125 www.jpbs-online.com Quantitative estimation of Rosuvastatin in bulk and tablet dosage form by using area under curve method P. S. Jain*, N. K. Kale, S. J. Surana
Investigating Transporter-Mediated Drug-Drug Interactions Using a Physiologically Based Pharmacokinetic Model of Rosuvastatin
 Citation: CPT Pharmacometrics Syst. Pharmacol. (2017) 6, 228 238; VC 2017 ASCPT All rights reserved doi:10.1002/psp4.12168 ORIGINAL ARTICLE Investigating Transporter-Mediated Drug-Drug Interactions Using
Citation: CPT Pharmacometrics Syst. Pharmacol. (2017) 6, 228 238; VC 2017 ASCPT All rights reserved doi:10.1002/psp4.12168 ORIGINAL ARTICLE Investigating Transporter-Mediated Drug-Drug Interactions Using
Pharmacological Activities of Glycyrrhetinic Acid Derivatives: Analgesic and Anti-Type IV Allergic Effects
 3888 Vol. 35 (1987) Chem. Pharm. Bull. 35( 9 )3888-3893(1987) Pharmacological Activities of Glycyrrhetinic Acid Derivatives: Analgesic and Anti-Type IV Allergic Effects HIDEO INOUE,*, a TAKEO MORI,a SHOJI
3888 Vol. 35 (1987) Chem. Pharm. Bull. 35( 9 )3888-3893(1987) Pharmacological Activities of Glycyrrhetinic Acid Derivatives: Analgesic and Anti-Type IV Allergic Effects HIDEO INOUE,*, a TAKEO MORI,a SHOJI
>>> Oral Formulation Optimization. Introduction. A Tiered Approach for Identifying Enabling Formulations
 Application Note #28-DMPK-3 >>> Oral Formulation Optimization Introduction Among the criteria required of compounds advancing from drug discovery programs, adequate systemic exposure (plasma concentrations
Application Note #28-DMPK-3 >>> Oral Formulation Optimization Introduction Among the criteria required of compounds advancing from drug discovery programs, adequate systemic exposure (plasma concentrations
International Journal of Research and Development in Pharmacy and Life Sciences. Research Article
 International Journal of Research and Development in Pharmacy and Life Sciences Available online at http//www.ijrdpl.com February - March, 214, Vol. 3, No.2, pp 943-948 ISSN: 2278-238 Research Article
International Journal of Research and Development in Pharmacy and Life Sciences Available online at http//www.ijrdpl.com February - March, 214, Vol. 3, No.2, pp 943-948 ISSN: 2278-238 Research Article
J. Nutr. Sci. Vitaminol., 38, , Note. in Tissues
 J. Nutr. Sci. Vitaminol., 38, 517-521, 1992 Note A Simple Enzymatic Quantitative in Tissues Analysis of Triglycerides Hiroshi DANNO, Yuu JINCHO, Slamet BUDIYANTO, Yuji FURUKAWA, and Shuichi KIMURA Laboratory
J. Nutr. Sci. Vitaminol., 38, 517-521, 1992 Note A Simple Enzymatic Quantitative in Tissues Analysis of Triglycerides Hiroshi DANNO, Yuu JINCHO, Slamet BUDIYANTO, Yuji FURUKAWA, and Shuichi KIMURA Laboratory
It is the policy of health plans affiliated with PA Health & Wellness that Vytorin is medically necessary when the following criteria are met:
 Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd.
 rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Supporting Information. Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively
 S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
Short Communication. Abstract. Introduction
 Short Communication JPP, 6: 76 7 The Authors JPP Royal Pharmaceutical Society Received November 8, Accepted February, DOI./j.-758..76.x ISSN -57 Relationship between lipophilicity and absorption from the
Short Communication JPP, 6: 76 7 The Authors JPP Royal Pharmaceutical Society Received November 8, Accepted February, DOI./j.-758..76.x ISSN -57 Relationship between lipophilicity and absorption from the
Kinetic characterization of rat hepatic uptake of 16 actively transported drugs. Yoshiyuki Yabe, Aleksandra Galetin and J Brian Houston
 DMD Fast Forward. Published on July 5, 2011 as doi:10.1124/dmd.111.040477 Kinetic characterization of rat hepatic uptake of 16 actively transported drugs Yoshiyuki Yabe, Aleksandra Galetin and J Brian
DMD Fast Forward. Published on July 5, 2011 as doi:10.1124/dmd.111.040477 Kinetic characterization of rat hepatic uptake of 16 actively transported drugs Yoshiyuki Yabe, Aleksandra Galetin and J Brian
Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients
 Antiviral Therapy 12:1127 1132 Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients Manon van der Lee 1,2 *, Raaj Sankatsing 3, Emile Schippers
Antiviral Therapy 12:1127 1132 Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients Manon van der Lee 1,2 *, Raaj Sankatsing 3, Emile Schippers
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin
 Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE
 California Avocado Society 1968 Yearbook 52: 102-108 SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE Yoshio Kikuta Present address: Department of Botany, Faculty of Agriculture,
California Avocado Society 1968 Yearbook 52: 102-108 SEASONAL CHANGES OF AVOCADO LIPIDS DURING FRUIT DEVELOPMENT AND STORAGE Yoshio Kikuta Present address: Department of Botany, Faculty of Agriculture,
Slide 1. Slide 2. Slide 3. Drug Action and Handling. Lesson 2.1. Lesson 2.1. Drug Action and Handling. Drug Action and Handling.
 Slide 1 Drug Action and Handling Chapter 2 1 Slide 2 Lesson 2.1 Drug Action and Handling 1. Differentiate dose, potency, and efficacy in the context of the actions of drugs. 2. Explain the pharmacologic
Slide 1 Drug Action and Handling Chapter 2 1 Slide 2 Lesson 2.1 Drug Action and Handling 1. Differentiate dose, potency, and efficacy in the context of the actions of drugs. 2. Explain the pharmacologic
T Eley, Y-H Han, S-P Huang, B He, W Li, W Bedford, M Stonier, D Gardiner, K Sims, P Balimane, D Rodrigues, RJ Bertz
 IN VIVO AND IN VITRO ASSESSMENT OF ASUNAPREVIR (ASV; BMS-650032) AS AN INHIBITOR AND SUBSTRATE OF ORGANIC ANION TRANSPORT POLYPEPTIDE (OATP) TRANSPORTERS IN HEALTHY VOLUNTEERS T Eley, Y-H Han, S-P Huang,
IN VIVO AND IN VITRO ASSESSMENT OF ASUNAPREVIR (ASV; BMS-650032) AS AN INHIBITOR AND SUBSTRATE OF ORGANIC ANION TRANSPORT POLYPEPTIDE (OATP) TRANSPORTERS IN HEALTHY VOLUNTEERS T Eley, Y-H Han, S-P Huang,
Pharmacokinetics One- compartment Open Model Lec:2
 22 Pharmacokinetics One- compartment Open Model Lec:2 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 1 Outline Introduction
22 Pharmacokinetics One- compartment Open Model Lec:2 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 1 Outline Introduction
Chemical Level Of Organization
 Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
Chemical Level Of Organization List the Four Chemical Elements that Make Up Most of the Body s Mass Oxygen Carbon Hydrogen Nitrogen Distinguish Between Organic and Inorganic Compounds Organic Compounds:
A mong the 20 leading prescription
 Safety and Statins: Pharmacologic and Clinical Perspectives Michael B. Bottorff, PharmD A mong the 20 leading prescription drugs in the United States, 3 agents atorvastatin (Lipitor), simvastatin (Zocor),
Safety and Statins: Pharmacologic and Clinical Perspectives Michael B. Bottorff, PharmD A mong the 20 leading prescription drugs in the United States, 3 agents atorvastatin (Lipitor), simvastatin (Zocor),
Atorvastatin route of administration
 Atorvastatin route of administration 8-3-2017 Atorvastatin Calcium. Atorvastatin Calcium Combinations; Routes.. Petrella G et al. Effects of simvastatin and atorvastatin administration on. 1-3-2017 Atorvastatin
Atorvastatin route of administration 8-3-2017 Atorvastatin Calcium. Atorvastatin Calcium Combinations; Routes.. Petrella G et al. Effects of simvastatin and atorvastatin administration on. 1-3-2017 Atorvastatin
Simvastatin 40 mg equivalent
 Simvastatin 40 mg equivalent medications equivalent to Simvastatin is available on the Drugs.com website. Simvastatin (Zocor ): 10 mg : Equivalent Dosages - 3: Atorvastatin (Lipitor. 40 mg : Equivalent
Simvastatin 40 mg equivalent medications equivalent to Simvastatin is available on the Drugs.com website. Simvastatin (Zocor ): 10 mg : Equivalent Dosages - 3: Atorvastatin (Lipitor. 40 mg : Equivalent
Scientific conclusions
 Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
PHA First Exam Fall 2013
 PHA 5127 First Exam Fall 2013 On my honor, I have neither given nor received unauthorized aid in doing this assignment. Name Question Set/Points I. 30 pts II. III. IV 20 pts 20 pts 15 pts V. 25 pts VI.
PHA 5127 First Exam Fall 2013 On my honor, I have neither given nor received unauthorized aid in doing this assignment. Name Question Set/Points I. 30 pts II. III. IV 20 pts 20 pts 15 pts V. 25 pts VI.
Ezetimibe: a selective inhibitor of cholesterol absorption
 European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
Ezetimibe: a selective inhibitor of cholesterol absorption
 European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
European Heart Journal Supplements (2001) 3 (Supplement E), E6 E10 Ezetimibe: a selective inhibitor of cholesterol absorption Dipartimento di Scienze Farmacologiche, Universita degli Studi di Milano, Milano,
DEVELOPMENT OF IN VITRO-IN VIVO CORRELATION FOR ENCAPSULATED METOPROLOL TARTRATE
 Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 70 No. 4 pp. 743ñ747, 2013 ISSN 0001-6837 Polish Pharmaceutical Society DEVELOPMENT OF IN VITRO-IN VIVO CORRELATION FOR ENCAPSULATED METOPROLOL TARTRATE
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 70 No. 4 pp. 743ñ747, 2013 ISSN 0001-6837 Polish Pharmaceutical Society DEVELOPMENT OF IN VITRO-IN VIVO CORRELATION FOR ENCAPSULATED METOPROLOL TARTRATE
Title. Author(s)Hayakawa, Mineji; Fujita, Itaru; Iseki, Ken; Gando, CitationASAIO Journal, 55(3): Issue Date Doc URL. Rights.
 Title The Administration of Ciprofloxacin During Continuou Author(s)Hayakawa, Mineji; Fujita, Itaru; Iseki, Ken; Gando, CitationASAIO Journal, 55(3): 243-245 Issue Date 2009-05 Doc URL http://hdl.handle.net/2115/43035
Title The Administration of Ciprofloxacin During Continuou Author(s)Hayakawa, Mineji; Fujita, Itaru; Iseki, Ken; Gando, CitationASAIO Journal, 55(3): 243-245 Issue Date 2009-05 Doc URL http://hdl.handle.net/2115/43035
Chapter 13 worksheet
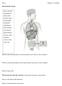 Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
EP A1 (19) (11) EP A1. (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC
 (19) (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 13(4) EPC (11) EP 2 07 001 A1 (43) Date of publication: 01.07.2009 Bulletin 2009/27 (21) Application number: 07834499.1 (22) Date
(19) (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 13(4) EPC (11) EP 2 07 001 A1 (43) Date of publication: 01.07.2009 Bulletin 2009/27 (21) Application number: 07834499.1 (22) Date
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition
 Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Differences in the Fate of Methylmercury between Mice with and without Hair
 450 Journal of Health Science, 52(4) 450 454 (2006) Differences in the Fate of Methylmercury between Mice with and without Hair Tatsumi Adachi*, a, b and Takashi Kuwana a, c a Department of Basic Medical
450 Journal of Health Science, 52(4) 450 454 (2006) Differences in the Fate of Methylmercury between Mice with and without Hair Tatsumi Adachi*, a, b and Takashi Kuwana a, c a Department of Basic Medical
Pharmacokinetics of ibuprofen in man. I. Free and total
 Pharmacokinetics of ibuprofen in man. I. Free and total area/dose relationships Ibuprofen kinetics were studied in 15 subjects after four oral doses. Plasma levels of both total and free ibuprofen were
Pharmacokinetics of ibuprofen in man. I. Free and total area/dose relationships Ibuprofen kinetics were studied in 15 subjects after four oral doses. Plasma levels of both total and free ibuprofen were
Metabolism and Kinetics of Adrenocortical Steroids. -A Consideration on Corticosteroid Therapy-
 Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
General Principles of Pharmacology and Toxicology
 General Principles of Pharmacology and Toxicology Parisa Gazerani, Pharm D, PhD Assistant Professor Center for Sensory-Motor Interaction (SMI) Department of Health Science and Technology Aalborg University
General Principles of Pharmacology and Toxicology Parisa Gazerani, Pharm D, PhD Assistant Professor Center for Sensory-Motor Interaction (SMI) Department of Health Science and Technology Aalborg University
Cholesterol (blood, plasma, serum)
 1 Cholesterol (blood, plasma, serum) 1 Name and description of analyte 1.1 Name of analyte Cholesterol (plasma; also blood, serum) 1.2 Alternative names 2,15-dimethyl-14-(1,5-dimethylhexyl)tetracyclo[8.7.0.0
1 Cholesterol (blood, plasma, serum) 1 Name and description of analyte 1.1 Name of analyte Cholesterol (plasma; also blood, serum) 1.2 Alternative names 2,15-dimethyl-14-(1,5-dimethylhexyl)tetracyclo[8.7.0.0
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins
 Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
Unit IV Problem 3 Biochemistry: Cholesterol Metabolism and Lipoproteins - Cholesterol: It is a sterol which is found in all eukaryotic cells and contains an oxygen (as a hydroxyl group OH) on Carbon number
TCP Transl Clin Pharmacol
 TCP 2017;25(1):10-14 http://dx.doi.org/10.12793/tcp.2017.25.1.10 Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers Seol Ju
TCP 2017;25(1):10-14 http://dx.doi.org/10.12793/tcp.2017.25.1.10 Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers Seol Ju
A cost-effectiveness model of alternative statins to achieve target LDL-cholesterol levels Maclaine G D, Patel H
 A cost-effectiveness model of alternative statins to achieve target LDL-cholesterol levels Maclaine G D, Patel H Record Status This is a critical abstract of an economic evaluation that meets the criteria
A cost-effectiveness model of alternative statins to achieve target LDL-cholesterol levels Maclaine G D, Patel H Record Status This is a critical abstract of an economic evaluation that meets the criteria
Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General
 Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General For the detection and quantitation of human OxLDL in plasma, serum or other biological fluid samples Cat. No. KT-959 For Research Use Only.
Human Oxidized LDL ELISA Kit (MDA-LDL Quantitation), General For the detection and quantitation of human OxLDL in plasma, serum or other biological fluid samples Cat. No. KT-959 For Research Use Only.
Drug regulation of serum lipids
 Drug regulation of serum lipids Foundations of Biomedical Science MEDS90001 Dr Michelle Hansen Pharmacology & Therapeutics mjhansen@unimelb.edu.au References Katzung, Basic & Clinical Pharmacology Ch 35
Drug regulation of serum lipids Foundations of Biomedical Science MEDS90001 Dr Michelle Hansen Pharmacology & Therapeutics mjhansen@unimelb.edu.au References Katzung, Basic & Clinical Pharmacology Ch 35
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date:
 Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
1.* Dosage. A. Adults
 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors [Developed, November 1994; Revised, October 1996; September 1997; September 1998; October 1999; November 1999; August 2000; September
3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors [Developed, November 1994; Revised, October 1996; September 1997; September 1998; October 1999; November 1999; August 2000; September
STATINS ARE DANGEROUS DRUGS. Statins actually increase the risk of heart attacks and strokes. (!!!)
 STATINS ARE DANGEROUS DRUGS Statins actually increase the risk of heart attacks and strokes. (!!!) Statins cause Type 2 Diabetes. Statins cause memory loss and cognitive decline. Statins destroy muscle
STATINS ARE DANGEROUS DRUGS Statins actually increase the risk of heart attacks and strokes. (!!!) Statins cause Type 2 Diabetes. Statins cause memory loss and cognitive decline. Statins destroy muscle
Simultaneous Estimation of Ezetimibe and Lovastatin by Derivative Spectroscopy
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 894-899, July-Sept 2009 Simultaneous Estimation of Ezetimibe and Lovastatin by Derivative Spectroscopy S.J.RAJPUT
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 894-899, July-Sept 2009 Simultaneous Estimation of Ezetimibe and Lovastatin by Derivative Spectroscopy S.J.RAJPUT
