ANALYTICAL SCIENCES FEBRUARY 2014, VOL The Japan Society for Analytical Chemistry
|
|
|
- Brian Barnett
- 5 years ago
- Views:
Transcription
1 ANALYTICAL SCIENCES FEBRUARY 2014, VOL The Japan Society for Analytical Chemistry Special Reviews New Trend in the LC Separation Analysis of Pharmaceuticals High Performance Separation by Ultra High-performance Liquid Chromatography (UHPLC) with Core-shell Particle C18 Columns Hiroyuki NISHI and Kumi NAGAMATSU Laboratory of Analytical Chemistry, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Yasuda Women s University, Yasuhigashi, Asaminami, Hiroshima , Japan This article presents a mini-review of the recent results in the ultra high-performance liquid chromatography (UHPLC) separation of pharmaceuticals by our group. High performance UHPLC separation employing core-shell particle C18 columns was demonstrated. High performance (high theoretical plate number of approximately 20000/10 cm, low theoretical plate height of 5 μm) was obtained without any specific devices in the conventional HPLC apparatus, only through changing detector sampling times and the inner diameter of the connecting tube. High theoretical plate numbers with low column back pressure obtained by the core-shell particle columns enabled fast separation of the analytes. Methanol, which gives high column pressure drops in the reversed-phase mode HPLC compared with acetonitrile, can be used without any trouble. One analysis of the purity testing of diltiazem hydrochloride was performed within 100 s. One analysis in the photostability testing of mecobalamin (vitamin B 12 analogue) was successful within 180 s. Keywords Ultra high-performance liquid chromatography (UHPLC), separation, core-shell particle, pharmaceuticals, diltiazem (Received September 6, 2013; Accepted October 15, 2013; Published February 10, 2014) 1 Introduction UHPLC Separation UHPLC separation by the core-shell particle columns 2 2 HPLC system for UHPLC separation 2 3 Comparison between the conventional columns (5 μm) and the UHPLC columns (<<3 μm) 3 Application to the Separation of Pharmaceuticals Separation of diltiazem and its related compounds 3 2 Separation of water-soluble vitamins 3 3 Separation of NSAIDs and cold medicines 4 Conclusion and Perspectives Ackowledgements References Introduction The most versatile analytical method used in the quality evaluation of pharmaceuticals is chromatography, especially HPLC. From the 1970s, excellent reversed-phase packing materials such as μbondapak C18 or Nucleosil C18 have been available, leading to rapid progress in HPLC analysis. 1 In those Hiroyuki NISHI received his Ph.D. degree from Kyoto University in He worked at Analytical Research Laboratories, Tanabe Seiyaku Co., Ltd. ( ) as a Researcher (1982), Chief Researcher ( ), Section Manager ( ), and Department Manager ( ). In 2008, he moved to Yasuda Women s University as a Professor. His current interest is the development of highly sensitive, selective separation methods employing UHPLC and capillary electrophresis. Kumi NAGAMATSU received her Master degree in Graduate School of Medical Sciences from Kyushu University in She worked at Yasuda Women s University as an assistant from 2011 to She is now attending Nagasaki University for a doctor s course. Her research area is the development of precise and robust screening methods of toxic drugs in forensic and clinical samples by GC-MS. To whom correspondence should be addressed. nishi-h@yasuda-u.ac.jp K. N. present address: Department of Clinical Pharmacy, Course of Pharmaceutical Sciences, Graduate School of Biomedical Sciences, Nagasaki University, 1-14 Bunkyo-machi, Nagasaki , Japan.
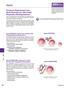
2 206 ANALYTICAL SCIENCES FEBRUARY 2014, VOL. 30 Fig. 1 Schematic illustration of conventional totally porous particles (5 μm) and some commercially available core-shell particles of 2.6 μm. Core size: 1.9 μm (Kinetex), 1.6 μm (Sunslhell). Shell size: 0.35 μm (Kinetex), 0.5 μm (Sunshell). days, 10 and 7 μm particles for the reversed-phase mode, named 10C18 and 7C18 respectively, were the most popular packing materials. Since then, 5 μm (5C18) has become the popular particle size in HPLC analysis. For example, The testing methods and the specifications of most pharmaceuticals in The Japanese Pharmacopoeia 16th edition (JP16), which lists a large number of important or well-used pharmaceuticals, adopts HPLC purity testing (so-called related substance testing) and HPLC assay by the 5C18 columns. 2 Among with the HPLC investigations, development of high performance (high theoretical plate number (N), low theoretical plate height (H)), high selectivity, and high sensitivity (low detection limit) systems has progressed. For high performance, smaller particle size of the packing materials gives a lower H value, according to the van Deemter equation 3 (see below). In 2004, an ultra high-pressure HPLC system with smaller particle packing materials (< 2 μm) was introduced to the market, based on the above concept. 4 Small particle size leads to a dramatic increase of the column pressure drops. This system is called ultra performance liquid chromatography (UPLC) 4 or ultra fast liquid chromatography (UFLC) depending on the system maker. At the present time, ultra high-performance liquid chromatography abbreviated as UHPLC is accepted worldwide, high speed and high performance separation analysis has been demonstrated by UHPLC. The use of packing materials with around 2 μm particle size leads to high column pressure drops. For example, particle size of 2.2 μm (3 mm f 5 cm) under the following conditions: a mobile phase mixture of methanol (MeOH)/water = /40, flow rate of 1 ml/min, and column temperature of 40 C results in a pressure drop of about 300 kg/cm 2. Compared with kg/cm 2 in the conventional analytical columns (5 μm, mm f 15 cm), very high pressure was monitored. High pressure is not good for the packing materials and the apparatus, even though both are usable under high pressure conditions. Recently, new packing materials called core-shell particles have appeared on the market. 5 Core-shell particles enable high performance separation with lower column pressure drops, compared with the smaller totally porous particle packing materials previously employed in UHPLC. Core-shell particles are superficially porous silica particles, which were developed by Kirkland in 1969, and were called pellicular type silica in those days. 6 In that paper, Kirkland reported novel particles composed of a 30-μm solid core encircled by a roughly 0.5-μm porous layer. However, due to low performance (low N) and low sample loadability, this pellicular type material was not used widely and converted to the totally porous 10C18 particle. In 2000, Kirkland et al. 7 reported 5 μm core-shell particles composed of a 4-μm solid core encircled by a roughly 0.5-μm porous layer. Then in 2007, this group developed 2.7 μm core-shell particles composed of a 1.7-μm solid core encircled by a roughly 0.5-μm porous layer. 8 This particle column shows high N values obtained by the sub-2 μm totally porous particle with much lower column pressure drops. This column has drawn attention in recent UHPLC analysis activities. Since 2010, reports on the applications using this type of core-shell particle column have increased. For example, a fast analysis of amines and amino acids was successfully performed within 7 min. 9 In this mini-review, some of our group s recent results regarding the fast analysis of pharmaceuticals by employing core-shell particle C18 columns are shown Our group uses a conventional HPLC apparatus with changes to the connecting tubes and some other parameters of the detector, other than the columns (i.e., core-shell particle). In line with the trend toward the use of smaller particle packing materials, columns for chiral separation and conventional HPLC having a small particle size (3 μm) have become commercially available. High performance enantiomer separations have been successfully achieved using 3 μm chiral columns UHPLC Separation 2 1 UHPLC separation by the core-shell particle columns The structure of a core-shell particle is shown in Fig. 1. This particle reported by Kirkland in 2007, 8 has a 1.7-μm solid core encircled by a roughly 0.5-μm porous layer. Particle size distribution of this particle is quite low (standard deviation (SD), approximately 6%), compared with conventional commercially available packing materials (SD approximately 20%). 18 Separation performance in HPLC can be described by the van Deemter s equation, which shows a relationship between H and linear velocity (u): H = Ad p + B(D m/u) + C(d p2 /D m)u. (1) H, d p, u, and D m are the theoretical plate height, particle diameter
3 ANALYTICAL SCIENCES FEBRUARY 2014, VOL Table 1 Comparison of the performance between the conventional columns and the UHPLC columns Column a d p/μm f/mm Column length/ cm Mobile phase MeOH, % N of Bu paraben t R of Bu paraben/ min Rs (ipr vs. npr) Pressure/ kg cm 2 Fig. 2 Total A Total B Core A Core A Core B (A) (B) (C) a. Total A, Phenomenex Gemini C18; Total B, Shim-pack XR-ODS; Core A, Sunshell C18; Core B, Kinetex C18. Conditions: column temperature, 40 C; flow rate, 1.0 ml/min. of packing materials, linear velocity of the mobile phase, and diffusion coefficient of an analyte in the mobile phase, respectively. A is a term regarding eddy effusion, B is a term concerning diffusion into a column axis direction, and C is a term concerning mass transfer between the stationary phase and the mobile phase, and that in the particle inside. According to this equation, d p in A and C terms largely influences H, i.e., the smaller d p, the smaller H values (higher N values). Core-shell particles of 2.6 μm shown in Fig. 1, which are much smaller in size than the conventional 5 μm totally porous particles, give low H values as shown in van Deemter s equation. Other than this small d p effect, an important factor in the core-shell particle, the narrow distribution range of the particle size largely contributes to low H values. A narrow distribution range of the particle size enables minute packing, leading to narrow gaps between the particles. Therefore, eddy effusion is limited, and consequently a small A term is obtained. On the other hand, the particle having a wide particle size distribution can not be packed minutely and shows a large A term. Furthermore, a non porous solid core, where an analyte can not diffuse inside, contributes to the small diffusion in the core-shell particle. As mentioned above, the B term is diffusion to the column axis direction and the C term is a term of the mass transfer in the particle. Diffusion in both terms is concerned in a thin layer (i.e., superficially porous 0.5 μm layer) of the coreshell particle. This thin layer gives a short transfer time or short transfer distance, leading to smaller B and C terms. Higher performance of the core-shell particle compared with the totally porous particle of the same size can be ascribed to the above mentioned factors. 2 2 HPLC system for UHPLC separation To show the high performance of UHPLC, it is important to properly set HPLC parameters other than the columns. The inside diameter (i.d.) of the connecting tubes between the injection port and the column, and those between the column and the detector should be smaller than that of the conventional ones. Use of low cell volume (micro-cell) in the detector is desirable. The detector s sampling time also should be optimized (fast sampling time). Apparatus for exclusive UHPLC use, these devices and parameters are loaded from the first time. However, our group used a conventional HPLC apparatus, the Prominense (Shimadzu, Kyoto, Japan), which can be used up to 40 MPa. We used the device with some modification. A photodiode array detector (PDA) was used for the detection and the monitoring of the UV spectra of each analyte. Samples of 0.1% concentration (1 mg/ml) were prepared by MeOH and mixed or diluted with a mobile phase or water to the desirable concentration (usually 0.01%). Approximately 5 10 μl was injected by an auto-injector SIL-20AC. First, connecting tubes between the injection port and the column, and the column and the detector were changed to 0.1 mm i.d. Then, performance of the system was investigated by changing the detector response (time constant, default 640 ms, available ms) and sampling cycle (default value 640 ms, available ms). The slit width used was 8 nm. The samples used were a mixture of methyl (Me), ethyl (Et), propyl (npr), isopropyl (ipr), and butyl (Bu) paraben. The separation was performed by the core-shell column (Kinetex C18, mm f 10 cm, 2.6 μm) with MeOH/water = 70/30 as a mobile phase. The detector cell used was the normal cell (cell volume, 10 μl). The obtained N values of Bu paraben (latest eluted peak at around 2 min) were 9500 at 640 ms and at 80 ms. Very low H around 5 μm was obtained. 10 Satisfacrory high performance was obtained even in the normal cell, although it is possible to obtain higher performance and a very fast analysis when a micro-cell is used. In conclusion, we decided to use the conventional HPLC with a normal cell after changing both detector response and sampling time from 640 to 80 ms, considering the versatile usage of the system. Resolution (Rs), N and H were calculated by using the software equipped with the apparatus (LC solution, Shimadzu). 2 3 Comparison between the conventional columns (5 μm) and the UHPLC columns (<<3 μm) Performance of the conventional column with totally porous particle (Phenomenex Gemini-NX C18, mm f 15 cm, 5 μm) and the UHPLC column with totally porous particle (Shim-pack XR-ODS, 3 mm f 5 cm, 2.2 μm) was compared by using a mixture of five parabens as a test sample and the mixture of MeOH/water = /40 as a mobile phase. The HPLC apparatus and the parameters used were the same as those mentioned above. Results are shown in Table Data indicates that almost the same performance (Rs, N, and H) was obtained in one-third the analytical time by the UHPLC column. However, column back pressures were 75 kg/cm 2 at the Phenomenex Gemini-NX C18 column, and 280 kg/cm 2 at the Shim-pack XR-ODS column. Next, the same analysis was performed employing core-shell particle columns (Kinetex C18, Sunshell C18, mm f 10 cm, 2.6 μm). The same performance as with 2.2 μm particle was obtained by the core-shell column judging from the Rs between two parabens (ipr and npr) (Table 1). Typical separation of parabens by HPLC and UHPLC is shown in Fig. 2. Column back pressures of 280 kg/cm 2 for 2.2 μm particle at 40 C was reduced to 230 kg/cm 2 at 50 C. 11
4 208 ANALYTICAL SCIENCES FEBRUARY 2014, VOL. 30 Fig. 3 Separation of (A) diltiazem and its related compounds and (B) purity testing of diltiazem API by UHPLC with 2.6 μm core-shell particle column (Sunshell C18, mm f 10 cm). Conditions: flow rate, 1.0 ml/min; mobile phase, 0.05 mol/l phosphate buffer (ph 3.0)/ MeOH = 35/65; column temperature, 40 C; detection, UV 254 nm. Desacetyl diltiazem, diltiazem, desacetyl chlorodiltiazem, chlorodiltiazem. 3 Application to the Separation of Pharmaceuticals Fig. 2 Separation of paraben mixtures by (A) the conventional HPLC with 5 μm totally porous particle (Phenomenex Gemini 5C18, mm f 15 cm), (B) UHPLC with 2.2 μm totally porous particle (Shim-pack XR-ODS, 3 mm f 5 cm) and (C) UHPLC with 2.6 μm core-shell particle (Kinetex C18, mm f 10 cm). Conditions: flow rate, 1.0 ml/min; mobile phase, (A) and (B) MeOH %, (C) MeOH 65%; column temperature, 40 C; detection, UV 254 nm. Rs shown are the value between npr and ipr parabens. First eluted peak was uracil use as t o marker. 3 1 Separation of diltiazem and its related compounds Separation of diltiazem and its analogues was investigated by employing core-shell particle columns. Diltiazem (Herbesser ) is a Ca-channel blocker used for the treatment of hypertension in more than 100 countries. Columns used were Kinetex C18 and Sunshell C18 ( mm i.d. 10 cm, 2.6 μm) under the following conditions: flow rate 1.0 ml/min, column temperature 40 C, and mobile phase of phosphate buffer (ph 3.0)/MeOH. Some chromatograms are shown in Fig. 3. Results of the purity testing are summarized in Table 2. Separation of the desacetylform of diltiazem and dilitiazem was successful within 100 s, resulting in a fast purity testing. 11 Samples, active pharmaceutical ingredients (API), and some formulations, were stored in colorless glass bottles at room temperature for over 5 years. This indicates that diltiazem and its formulations are very stable.
5 ANALYTICAL SCIENCES FEBRUARY 2014, VOL Table 2 Results of the purity testing of diltiazem API and its formulations by UHPLC with the core-shell particle column API a Formulation (tablets) a Lot No. Impurity, % b Lot No. Impurity, % b A B C A B C a. Stored at room temperature for more than 5 years. b. Only desacetyl form was detected. Fig. 5 Photostability of mecobalamin (MeB 12). Sample solution was injected at every 5 min interval. Square symbol indicates MeB 12 and circle symbol indicates OHB 12, which is a photo-decomposed product of MeB 12. Diltiazem API is included in the JP16 and the specification of the related substances is not more than 0.3%. 2 The method employed in JP16 is the reversed-phase HPLC with 5C18. According to the JP16 method, one analysis needs around 20 min. In the case of the quality evaluation test (both HPLC related substances and HPLC assay) for five lots of newly produced tablets, many solutions need to be injected. For example, standard solutions, sample solutions, solutions for the system suitability test, etc. A total of 30 analyses are required. This means 30 times 20 min = 0 min (10 h). In this case, the quality control laboratory at the tablet maker usually operates the HPLC system all-night. Checking of the raw data (chromatograms), summarizing of the results etc. are performed the following day. However, with the use of the developed UHPLC method, 30 times 2 min = min (1 h). Analysis time required will be reduced to one tenth, enabling completion of the test within one day. First, the HPLC apparatus is operated, then the solutions are prepared and injected, and this is followed by the data collection and documentation to be finished within the same day. There is no need to operate the system all-night. Furthermore, one analysis can be completed in 1 min using a core-shell particle (2.6 μm) column of 5 cm in length for much faster analysis. 12 Fig. 4 Separation of (A) vitamin B 2, vitamin B 12 and its analogues (a standard solution) and (B) OHB 12 and B 12 (just after the dissolution of MeB 12 API) by UHPLC with 2.6 μm core-shell particle C18 column. Conditions: Kinetex C18 ( mm f 10 cm); flow rate, 1.0 ml/min; mobile phase, (A) ACN 15%, (B) ACN 18%; column temperature, 40 C; detection, UV 254 nm. 3 2 Separation of water-soluble vitamins Separation of 10 water soluble vitamins was investigated by employing core-shell particle columns. Columns used were Kinetex C18, Sunshell C18, and Capcellcore C18 ( mm f 10 cm, 2.7 or 2.6 μm) under the following conditions: flow rate 1.0 ml/min, column temperature 40 C, and mobile phase of phosphate buffer (ph 5.2)/acetonitrile (ACN). No difference was observed in the selectivity between the buffer of ph 2.5 and ph 5.2, and between MeOH and ACN. Five of 10 water soluble vitamins (vitamin C, vitamin B 1, vitamin B 6, nicotinic acid, and nicotinamide), which are very hydrophilic, were successfully separated within 5 min, except vitamin C (no retention, almost eluted at t 0), by employing 1% ACN mobile phase (chromatogram not shown). The other five water soluble vitamins, including vitamin B 2 and vitamin B 12 (no elution under the conditions mentioned above, i.e., 1% ACN mobile phase), were separated within 7.5 min by using 15 18% ACN mobile phase. Typical
6 210 ANALYTICAL SCIENCES FEBRUARY 2014, VOL. 30 chromatograms of the separation of vitamin B 2 (B 2), vitamin B 2 phosphate (B 2-P), vitamin B 12 (B 12) and its analogues are shown in Fig. 4. Mecobalamin (MeB 12) is well known as an unstable vitamin for light irradiation. As shown in Fig. 4, even just after dissolution, the decomposed product hydroxocobalamin (OHB 12) was observed. Within 1 h under irradiation by room light (about 3000 lux), almost all B 12 transferred to OHB 12 as shown in Fig. 5. One analysis can be finished within 180 s, the method enables frequent sampling (sampling interval 5 min) of the sample solution. The other five highly hydrophilic water soluble vitamins, including vitamin C, mentioned above were successfully separated within 5 min by employing a C30 column (3 μm, mm f 15 cm) (chromatogram not shown). The mobile phase was a diluted trifluoroacetic acid (1 1000) Separation of NSAIDs and cold medicines The separation of 12 cold medicines and nonsteroidal antiinflammatory drugs (NSAIDs) was investigated by employing core-shell particle C18 columns. 15 Columns used were Kinetex C18, Sunshell C18, and Capcellcore C18 ( mm f 10 cm, 2.7 or 2.6 μm) under the following conditions: flow rate 1.0 ml/min, column temperature 40 C, and mobile phase of phosphate buffer (ph 2.5)/MeOH. Selectivity in MeOH was found to be better than ACN. The mobile phase used for 6 cold medicines was MeOH 30%, and that for six NSAIDs was MeOH 70%. All 12 drugs were separated by employing a gradient elution mode within approximately 8 min. Separation examples are shown in Fig A mixture of 11 NSAIDs (Fig. 6) was also successful within roughly 6 min except co-elution of indometacin and dicrofenac. In the separation of parabens and diltiazems, no difference was found in separation selectivity. However, retention of chlorpheniramine, a basic cold medicine (antihistamine), differed among three core-shell particle columns as sometimes experienced in the use of the conventional reversed-phase columns. 4 Conclusion and Perspectives In the HPLC separation analysis of pharmaceuticals, by employing core-shell particle columns or small 3 μm particle columns in the conventional HPLC apparatus with some modifications, it was found that high performance (high N or low H) can be obtained, leading to fast analysis. High performance with low column back pressure, compared with the smaller particle size and totally porous UHPLC columns, 18 is the largest merit of the core-shell particle column. Low column back pressure enables the use of the conventional HPLC apparatus. From the viewpoint of the routine (cost) or versatility of analysis, core-shell particle columns with the conventional HPLC apparatus seem to be the most suitable because high performance can be obtained without any special devices, such as microcell or special UHPLC system. At this time, however, the variety of core-shell particle columns is limited to the C18 types. Especially, core-shell particle chiral columns are not available at present. However, chiral columns of 3 μm particle size became commercially available a few years ago. 19 As an example, separation of enantiomers of naproxen, one of the NSAIDs, by a reversed-phase chiral column with 3 μm particle is shown in Fig Baseline separation of enantiomers was successful within approximately 7 min, leading to fast optical purity testing. Resolution (Rs) between the enantiomers was Enantiomer separation is an important problem for pharmaceutical companies. Development of novel chiral columns is still ongoing. 20 Markedly high performance and Fig. 6 Separation of cold medicines and NSAIDs by UHPLC with 2.6 μm core-shell particle column (Sunshell C18, mm f 10 cm). (A) Separation of six cold medicines and six NSAIDs by a gradient elution, (B) separation of 11 NSAIDs by an isocratic mode (MeOH 70%). Conditions: flow rate, 1.0 ml/min; mobile phase, (A) 0 min, 0.05 mol/l phosphate buffer (ph 3.0)/MeOH = 70/30; 1.00 min, curve gradient code 10; 1.01 min, 0.02 mol/l phosphate buffer (ph 3.0)/ MeOH = 20/80; 10.0 min, controller stop, (B) 0.05 mol/l phosphate buffer (ph 3.0)/MeOH = 30/70; column temperature, 40 C; detection, UV 210 nm. Order of retention times: (A) maleic acid, acetoaminophen, methylephedrine, caffeine, aspirin, chlorpheniramine, ethenzamide, pranoprofen, ketoprofen, naproxen, flurbiprofen, ibuprofen, mefenamic acid (13 peaks), (B) pranoprofen, loxoprofen, ketoprofen, naproxen, phenylbutazone, flurbiprofen, indomethacin + dicrofenac, ibuprofen, mefenamic acid, torfenamic acid (10 peaks). high speed enantiomer separation could be obtained with chiral core-shell particle columns with around 3 μm particle size. Therefore, development of chiral core-shell columns is highly expected. In the near future, columns used for the routine analysis of pharmaceuticals is expected to shift from the totally porous 5C18 to core-shell particle C18 with particle size of
7 ANALYTICAL SCIENCES FEBRUARY 2014, VOL Fig. 7 Separation of enantiomers of naproxen by high performance chiral column Chiralpak AS-3R (3 μm, mm f 15 cm). Conditions: flow rate, 1.0 ml/min; mobile phase, 0.05 mol/l phosphate buffer (ph 3.0)/ACN = /40; column temperature, 40 C; detection, UV 254 nm. around 3 μm. A paper on the characteristics of the core-shell particle published recently provides additional information Acknowledgements This work was partly supported by a Grant-in-Aid for Scientific Research (c) (No ) from the Japan Society for the Promotion of Science (JSPS). H. N. thanks Shimadzu Co. (Kyoto, Japan) for the gift of Shim-pack XR-ODS column. 6 References 1. H. Hatano, ed., Application of High-Speed Liquid Chromatography, Kagaku no Ryoiki, 1976, Zokan Vol. 109, Nankodo. 2. The Japanese Pharmacopoeia 16th, Jihou-sha, J. J. Van Deemter, F. J. Zuyderweg, and A. Klinkenberg, Chem. Eng. Sci., 1956, 5, ACQUITY UPLC system, Waters, C. Sanchez and T. Farkas, Am. Lab., 2012, 44, J. J. Kirkland, J. Chromatogr. Sci., 1969, 7, J. J. Kirkland, F. A. Truszkowski, C. H. Diks, and G. S. Engel, J. Chromatogr. A, 2000, 890, J. J. Kirkland, T. J. Langlois, and J. J. DeStefano, Am. Lab., 2007, 39, S. Iijima, Y. Sato, M. Bounoshita, T. Miyaji, D. J. Tognarelli, and M. Saito, Anal. Sci., 2013, 29, K. Nagamatsu, M. Nishimura, and H. Nishi, J. Yasuda Women s Univ., 2012, 40, K. Nagamatsu and H. Nishi, J. Yasuda Women s Univ., 2013, 41, M. Doi, K. Nagamatsu, and H. Nishi, in Proceedings of The Japan Society for Analytical Chemistry (JSAC) the 62nd Annual Conference, ed. JSAC, 2013, Osaka, Japan, K. Nagamatsu, M. Doi, Y. Mukaiyama, A. Miyawaki, and H. Nishi, in Proceedings of the Japan Society for Analytical Chemistry (JSAC) the 73rd Analytical Symposium, ed. JSAC, 2013, Hakodate, Japan, S. Tayamoto, A. Miyawaki, and H. Nishi, in Proceedings of the Japan Society for Analytical Chemistry (JSAC) the 62nd Annual Conference, ed. JSAC, 2013, Osaka, Japan, K. Nagamatsu, C. Morita, and H. Nishi, in Proceedings of the 25th Symposium on Biomedical-Analytical Sciences (BMAS2012), ed. BMAS, 2012, Tokyo, Japan, H. Mukaiyama, A. Miyawaki, and H. Nishi, in Proceedings of the Japan Society for Analytical Chemistry (JSAC) the 62nd Annual Conference, ed. JSAC, 2013, Osaka, Japan, K. Nagamatsu, M. Tanaka, and H. Nishi, in Proceedings of the Japan Society for Analytical Chemistry (JSAC) the 72nd Analytical Symposium, ed. JSAC, 2012, Kagoshima, Japan, M. Lee, J. Park, M. Lim, S. J. Seong, J. Lee, J. J. Seo, Y. Park, H. W. Lee, and Y. Yoon, Anal. Sci., 2012, 28, DAICEL CHIRAL COLUMNS Brochure No. 5 (2012). 20. G. M. Peng, S. Q. Wu, W. G. Zhang, J. Fan, S. R. Zheng, G. Xu, and Q. Xu, Anal. Sci., 2013, 29, N. Nagae and T. Tsukamoto, Chromatography, 2013, 34, 41.
APPLICATIONS TN Overview of Kinetex 2.6 µm Core-Shell Technology
 Determination of Impurities and Related Substances for Acetylsalicylic Acid (Ph. Eur. Monograph 9): Increased Sensitivity and Resolution, Faster Analysis, and Reduced Solvent Usage Using Kinetex 2.6 µm
Determination of Impurities and Related Substances for Acetylsalicylic Acid (Ph. Eur. Monograph 9): Increased Sensitivity and Resolution, Faster Analysis, and Reduced Solvent Usage Using Kinetex 2.6 µm
SUMMARY, CONCLUSION & RECOMMENDATIONS
 196 Chapter-5 SUMMARY, CONCLUSION & RECOMMENDATIONS 197 CHAPTER 5 5.1 Summary, Conclusion and Recommendations Summary and Conclusion are drawn based on the work carried out by the author on development
196 Chapter-5 SUMMARY, CONCLUSION & RECOMMENDATIONS 197 CHAPTER 5 5.1 Summary, Conclusion and Recommendations Summary and Conclusion are drawn based on the work carried out by the author on development
RITONAVIRI COMPRESSI RITONAVIR TABLETS. Final text for addition to The International Pharmacopoeia (July 2012)
 July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
Ultra High Performance Liquid Chromatograph. Nexera X2 C196-E079
 Ultra High Performance Liquid Chromatograph Nexera X2 C196-E79 Maximizing the Potential of UHPLC/HPLC Analyses Intelligence Intelligent data solutions, including a new i -PDeA peak calculation method for
Ultra High Performance Liquid Chromatograph Nexera X2 C196-E79 Maximizing the Potential of UHPLC/HPLC Analyses Intelligence Intelligent data solutions, including a new i -PDeA peak calculation method for
HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 1290 Infinity II LC
 HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 129 Infinity II LC Application Note Small Molecule Pharmaceuticals Authors Gerd Vanhoenacker, Mieke Steenbeke, Koen Sandra,
HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 129 Infinity II LC Application Note Small Molecule Pharmaceuticals Authors Gerd Vanhoenacker, Mieke Steenbeke, Koen Sandra,
Core-Shell / Solid core Particles: A Practical Substitute to Sub-2 Micron Particles: An overview
 Core-Shell / Solid core Particles: A Practical Substitute to Sub-2 Micron Particles: An overview ABSTRACT: S. M. Ramdharane 1, K. B. Vyas 2, K. S. Nimavat 3 * 1 Pacific Academy of Higher Education & Research
Core-Shell / Solid core Particles: A Practical Substitute to Sub-2 Micron Particles: An overview ABSTRACT: S. M. Ramdharane 1, K. B. Vyas 2, K. S. Nimavat 3 * 1 Pacific Academy of Higher Education & Research
ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS
 ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS Tanya Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Some of the most challenging methods
ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS Tanya Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Some of the most challenging methods
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):138-144 Simultaneous RP HPLC determination of Latanoprost
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):138-144 Simultaneous RP HPLC determination of Latanoprost
Phenyl-Hexyl. UHPLC Columns. lternate, complementary selectivity to C18 and C8 bonded phases
 Phenyl-Hexyl UHPLC Columns lternate, complementary selectivity to A C8 and C8 bonded phases articularly recommended for P compounds containing aromatic groups xcellent bonded phase stability for E durable,
Phenyl-Hexyl UHPLC Columns lternate, complementary selectivity to A C8 and C8 bonded phases articularly recommended for P compounds containing aromatic groups xcellent bonded phase stability for E durable,
ISSN: ; CODEN ECJHAO E-Journal of Chemistry 2011, 8(3),
 ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010)
 June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Comparison of a UPLC Method across Multiple UHPLC Systems
 Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 1290 Infinity LC System
 Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 129 Infinity LC System Application Note Pharmaceutical and Chemical Analysis
Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 129 Infinity LC System Application Note Pharmaceutical and Chemical Analysis
Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system
 Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system A practical guide on how to use parameters to increase peak capacity Application Note Pharmaceutical and Chemical Author
Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system A practical guide on how to use parameters to increase peak capacity Application Note Pharmaceutical and Chemical Author
A Validated Chiral Liquid Chromatographic Method for The Enantiomeric Separation of Dapoxetine Hydrochloride
 Received on 15/05/2012; Revised on 22/05/2012; Accepted on 09/06/2012 A Validated Chiral Liquid Chromatographic thod for The Enantiomeric Separation of Dapoxetine Hydrochloride T.Rohith 1 and S. Ananda
Received on 15/05/2012; Revised on 22/05/2012; Accepted on 09/06/2012 A Validated Chiral Liquid Chromatographic thod for The Enantiomeric Separation of Dapoxetine Hydrochloride T.Rohith 1 and S. Ananda
Available online at Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (3):157-161 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (3):157-161 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
F. Al-Rimawi* Faculty of Science and Technology, Al-Quds University, P.O. Box 20002, East Jerusalem. Abstract
 JJC Jordan Journal of Chemistry Vol. 4 No.4, 2009, pp. 357-365 Development and Validation of Analytical Method for Fluconazole and Fluconazole Related Compounds (A, B, and C) in Capsule Formulations by
JJC Jordan Journal of Chemistry Vol. 4 No.4, 2009, pp. 357-365 Development and Validation of Analytical Method for Fluconazole and Fluconazole Related Compounds (A, B, and C) in Capsule Formulations by
Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate
 Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate Application Note Pharmaceutical QA/QC Author Siji Joseph Agilent Technologies, Inc. Bangalore,
Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate Application Note Pharmaceutical QA/QC Author Siji Joseph Agilent Technologies, Inc. Bangalore,
International Journal of Pharma and Bio Sciences
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACEUTICAL ANALYSIS DEVELOPMENT AND VALIDATION OF LIQUID CHROMATOGRAPHIC METHOD FOR ESTIMATION OF ESCITALOPRAM OXALATE IN TABLET DOSAGE
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACEUTICAL ANALYSIS DEVELOPMENT AND VALIDATION OF LIQUID CHROMATOGRAPHIC METHOD FOR ESTIMATION OF ESCITALOPRAM OXALATE IN TABLET DOSAGE
Shuguang Li, Jason Anspach, Sky Countryman, and Erica Pike Phenomenex, Inc., 411 Madrid Ave., Torrance, CA USA PO _W
 Simple, Fast and Accurate Quantitation of Human Plasma Vitamins and Their Metabolites by Protein Precipitation Combined with Columns Using HPLC-UV, HPLC-FLD or LC/MS/MS Shuguang Li, Jason Anspach, Sky
Simple, Fast and Accurate Quantitation of Human Plasma Vitamins and Their Metabolites by Protein Precipitation Combined with Columns Using HPLC-UV, HPLC-FLD or LC/MS/MS Shuguang Li, Jason Anspach, Sky
Am I getting the very best value from my UHPLC analyses?
 Am I getting the very best value from my UHPLC analyses? Stephen Luke LC Columns Product Manager 1 Primary reasons for UHPLC use Very fast Very high resolution Column 2.1 x 50 mm Column 2.1 x 150 mm Run
Am I getting the very best value from my UHPLC analyses? Stephen Luke LC Columns Product Manager 1 Primary reasons for UHPLC use Very fast Very high resolution Column 2.1 x 50 mm Column 2.1 x 150 mm Run
A HIGH PERFORMANCE LIQUID CHROMATOGRAPHIC ASSAY FOR LERCANIDIPINE HYDROCHLORIDE
 Int. J. Chem. Sci.: 6(1), 2008, 441-446 A HIGH PERFORMANCE LIQUID CHROMATOGRAPHIC ASSAY FOR LERCANIDIPINE HYDROCHLORIDE S. APPALA RAJU, ARVIND B. KARADI and SHOBHA MANJUNATH HKES s College of Pharmacy,
Int. J. Chem. Sci.: 6(1), 2008, 441-446 A HIGH PERFORMANCE LIQUID CHROMATOGRAPHIC ASSAY FOR LERCANIDIPINE HYDROCHLORIDE S. APPALA RAJU, ARVIND B. KARADI and SHOBHA MANJUNATH HKES s College of Pharmacy,
A New Stability-Indicating and Validated RP-HPLC Method for the Estimation of Liraglutide in Bulk and Pharmaceutical Dosage Forms
 OPEN ACCESS Eurasian Journal of Analytical Chemistry ISSN: 1306-3057 2017 12(2):31-44 DOI 10.12973/ejac.2017.00152a A New Stability-Indicating and Validated RP-HPLC Method for the Estimation of Liraglutide
OPEN ACCESS Eurasian Journal of Analytical Chemistry ISSN: 1306-3057 2017 12(2):31-44 DOI 10.12973/ejac.2017.00152a A New Stability-Indicating and Validated RP-HPLC Method for the Estimation of Liraglutide
Stability indicating RP-HPLC method development and validation of Etizolam and Propranolol hydrochloride in pharmaceutical dosage form
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
CHAPTER INTRODUCTION OF DOSAGE FORM AND LITERATURE REVIEW
 132 CHAPTER 6 DEVELOPMENT AND VALIDATION OF A STABILITY-INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF PARACETAMOL, TRAMADOL HYDROCHLORIDE AND DOMPERIDONE IN A COMBINED DOSAGE FORM 6.1 INTRODUCTION
132 CHAPTER 6 DEVELOPMENT AND VALIDATION OF A STABILITY-INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF PARACETAMOL, TRAMADOL HYDROCHLORIDE AND DOMPERIDONE IN A COMBINED DOSAGE FORM 6.1 INTRODUCTION
International Journal of Pharma and Bio Sciences DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 120 PFP
 Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 1 PFP Application Note Clinical Research Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd Abstract
Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 1 PFP Application Note Clinical Research Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd Abstract
Analysis of Common Sweeteners and Additives in Beverages with the PerkinElmer Flexar FX-15 System Equipped with a PDA Detector
 application Note Liquid Chromatography Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Common Sweeteners and Additives in Beverages with the PerkinElmer Flexar FX-15 System Equipped
application Note Liquid Chromatography Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Common Sweeteners and Additives in Beverages with the PerkinElmer Flexar FX-15 System Equipped
Maximizing Efficiency Using Agilent InfinityLab Poroshell 120 Columns
 Maximizing Efficiency Using Agilent InfinityLab Poroshell 12 Columns 1, Plates in Less Than Minutes Using Coupled Column Technology Application Note Food, Environmental, Chemical, Pharmaceutical Authors
Maximizing Efficiency Using Agilent InfinityLab Poroshell 12 Columns 1, Plates in Less Than Minutes Using Coupled Column Technology Application Note Food, Environmental, Chemical, Pharmaceutical Authors
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2014, 5(5):91-98 ISSN: 0976-8688 CODEN (USA): PSHIBD A novel RP-HPLC method development and validation of Perindopril Erbumine in
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2014, 5(5):91-98 ISSN: 0976-8688 CODEN (USA): PSHIBD A novel RP-HPLC method development and validation of Perindopril Erbumine in
Hyderabad, India. Department of Pharmaceutical Chemistry, Glocal University, Saharanpur, India.
 International Journal On Engineering Technology and Sciences IJETS RP-HPLC Method development and validation for the Simultaneous Estimation of Metformin and Empagliflozine in Tablet Dosage Form Shaik
International Journal On Engineering Technology and Sciences IJETS RP-HPLC Method development and validation for the Simultaneous Estimation of Metformin and Empagliflozine in Tablet Dosage Form Shaik
Tenofovir disoproxil fumarate (Tenofoviri disoproxili fumaras)
 C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
Aeris. Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations. Aeris PEPTIDE. Aeris WIDEPORE
 Aeris Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations Aeris is a specialized line of reversed phase core-shell UHPLC columns, built exclusively for the ultra-high performance
Aeris Precision Engineered Core- Shell Particles for Ultra-High Resolution BioSeparations Aeris is a specialized line of reversed phase core-shell UHPLC columns, built exclusively for the ultra-high performance
Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit
 Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit Application Note General Chromatography, Food Industry Authors John W. Henderson Jr., Judy Berry, Anne Mack, William Long Agilent
Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit Application Note General Chromatography, Food Industry Authors John W. Henderson Jr., Judy Berry, Anne Mack, William Long Agilent
RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form
 RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form S. LAVANYA* 1, SK. MANSURA BEGUM 1, K. NAGAMALLESWARA RAO 2, K. GAYATHRI DEVI 3 Department of pharmaceutical
RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form S. LAVANYA* 1, SK. MANSURA BEGUM 1, K. NAGAMALLESWARA RAO 2, K. GAYATHRI DEVI 3 Department of pharmaceutical
Available online Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):171-176 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Reverse phase high performance liquid chromatography
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):171-176 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Reverse phase high performance liquid chromatography
Comprehensive Two-Dimensional HPLC and Informative Data Processing for Pharmaceuticals and Lipids
 PO-CON1576E Comprehensive Two-Dimensional HPLC and Informative Data Processing for Pharmaceuticals and Lipids HPLC 2015 PSB-MULTI-06 Yoshiyuki WATABE, Tetsuo IIDA, Daisuke NAKAYAMA, Kanya TSUJII, Saki
PO-CON1576E Comprehensive Two-Dimensional HPLC and Informative Data Processing for Pharmaceuticals and Lipids HPLC 2015 PSB-MULTI-06 Yoshiyuki WATABE, Tetsuo IIDA, Daisuke NAKAYAMA, Kanya TSUJII, Saki
Scholars Research Library. Der Pharmacia Lettre, 2015, 7 (5):44-49 (
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (5):44-49 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (5):44-49 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC FOR ESTIMATION OF ZOLPIDEM TARTARATE
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ramalakshmi et al. SJIF Impact Factor 6.647 Volume 7, Issue 2, 1010-1018 Research Article ISSN 2278 4357 METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ramalakshmi et al. SJIF Impact Factor 6.647 Volume 7, Issue 2, 1010-1018 Research Article ISSN 2278 4357 METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC
Impurity Profiling of Carbamazepine by HPLC/UV
 Application Note: 52049 Impurity Profiling of Carbamazepine by HPLC/UV Terry Zhang, Guifeng Jiang, Thermo Fisher Scientific, San Jose, CA, USA Key Words Accela Hypersil GOLD Carbamazepine Drug Analysis
Application Note: 52049 Impurity Profiling of Carbamazepine by HPLC/UV Terry Zhang, Guifeng Jiang, Thermo Fisher Scientific, San Jose, CA, USA Key Words Accela Hypersil GOLD Carbamazepine Drug Analysis
ACE Excel UHPLC Columns
 Excel UHPLC Columns info@ace-hplc.com Advanced Chromatography Technologies Ltd www.ace-hplc.com Australia & New Zealand contact: infor@winlab.com.au or call + 0 Outline Introduction Key Product Points
Excel UHPLC Columns info@ace-hplc.com Advanced Chromatography Technologies Ltd www.ace-hplc.com Australia & New Zealand contact: infor@winlab.com.au or call + 0 Outline Introduction Key Product Points
Probing for Packaging Migrants in a Pharmaceutical Impurities Assay Using UHPLC with UV and Mass Detection INTRODUCTION
 Probing for Packaging Migrants in a Pharmaceutical Impurities Assay Using UHPLC with UV and Mass Detection Michael Jones Waters Corporation, Wilmslow, UK APPLICATION BENEFITS The ACQUITY Arc System is
Probing for Packaging Migrants in a Pharmaceutical Impurities Assay Using UHPLC with UV and Mass Detection Michael Jones Waters Corporation, Wilmslow, UK APPLICATION BENEFITS The ACQUITY Arc System is
A New USP Tool The Class Monograph
 CHPA Regulatory, Scientific & Quality Conference Washington DC, May 13, 2014 Quality Session 4 A New USP Tool The Class Monograph Alan R. Potts, Ph.D. Principal Scientific Liaison-Chemical Medicines The
CHPA Regulatory, Scientific & Quality Conference Washington DC, May 13, 2014 Quality Session 4 A New USP Tool The Class Monograph Alan R. Potts, Ph.D. Principal Scientific Liaison-Chemical Medicines The
Hyper-fast & Super-rugged
 Hyper-fast & Super-rugged UHPLC & HPLC columns FALL 2013 PRODUCT CATALOG Hyper-fast & Super-rugged UHPLC & HPLC columns he introduction of HALO columns, developed using innovative Fused-Core particle
Hyper-fast & Super-rugged UHPLC & HPLC columns FALL 2013 PRODUCT CATALOG Hyper-fast & Super-rugged UHPLC & HPLC columns he introduction of HALO columns, developed using innovative Fused-Core particle
RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms
 Asian Journal of Chemistry Vol. 22, No. 7 (2010), 5067-5071 RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms A. LAKSHMANA RAO*, G. TARAKA RAMESH and J.V.L.N.S. RAO Department of Pharmaceutical
Asian Journal of Chemistry Vol. 22, No. 7 (2010), 5067-5071 RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms A. LAKSHMANA RAO*, G. TARAKA RAMESH and J.V.L.N.S. RAO Department of Pharmaceutical
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
 Int. J. Chem. Sci.: 11(4), 2013, 1607-1614 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
Int. J. Chem. Sci.: 11(4), 2013, 1607-1614 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page:
 IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Method development and validation of tenofovir disoproxil fumerate and emtricitabine in combined tablet
IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Method development and validation of tenofovir disoproxil fumerate and emtricitabine in combined tablet
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR ESTIMATION OF LACOSAMIDE IN BULK AND ITS PHARMACEUTICAL FORMULATION
 http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
Separation of Macrocyclic Lactones (Avermectins) on FLARE C18 MM & FLARE C18+ Columns
 Separation of Macrocyclic Lactones (Avermectins) on FLARE C8 MM & FLARE C8+ Columns Introduction Diamond Analytics Technical Note: T05- Avermectins are a series of 6-membered macrocyclic lactone derivatives
Separation of Macrocyclic Lactones (Avermectins) on FLARE C8 MM & FLARE C8+ Columns Introduction Diamond Analytics Technical Note: T05- Avermectins are a series of 6-membered macrocyclic lactone derivatives
Restek Ultra II HPLC Columns
 Restek Ultra II HPLC Columns THE Choice for All U(HPLC) Systems November 2009 1 www.chromtech.net.au, sales@chromtech.net.au, Tel: 03 9762 2034, Fax: 03 9761 1169 Topics for Today Introducing Restek Ultra
Restek Ultra II HPLC Columns THE Choice for All U(HPLC) Systems November 2009 1 www.chromtech.net.au, sales@chromtech.net.au, Tel: 03 9762 2034, Fax: 03 9761 1169 Topics for Today Introducing Restek Ultra
Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation
 Oriental Journal of Chemistry Vol. 24(2), 537-544 (2008) Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation P.N. DALVI,
Oriental Journal of Chemistry Vol. 24(2), 537-544 (2008) Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation P.N. DALVI,
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC
 ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
Determination of enantiomeric purity of Timolol Maleate by Supercritical Fluid Chromatography
 Determination of enantiomeric purity of Timolol Maleate by Supercritical Fluid Chromatography Dr. Damian Connolly, (PMBRC, WIT) & Mr. Adrian Marley (Allergan Pharmaceuticals, Westport) PMBRC 3 rd Analytical
Determination of enantiomeric purity of Timolol Maleate by Supercritical Fluid Chromatography Dr. Damian Connolly, (PMBRC, WIT) & Mr. Adrian Marley (Allergan Pharmaceuticals, Westport) PMBRC 3 rd Analytical
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD ESTIMATION OF TOLVAPTAN IN BULK PHARMACEUTICAL FORMULATION
 http://www.rasayanjournal.com Vol.4, No.1 (2011), 165-171 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR AND ITS PHARMACEUTICAL FORMULATION V. Kalyana Chakravarthy * and
http://www.rasayanjournal.com Vol.4, No.1 (2011), 165-171 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR AND ITS PHARMACEUTICAL FORMULATION V. Kalyana Chakravarthy * and
Converting a CHP Method for Insulin to Agilent Poroshell 120 Columns
 Converting a CHP Method for Insulin to Agilent Poroshell Columns Application Note Biopharm Author Rongjie Fu Agilent Technologies (Shanghai) Co., Ltd. 4 Ying Lun Road Shanghai 3 China Abstract A regulatory
Converting a CHP Method for Insulin to Agilent Poroshell Columns Application Note Biopharm Author Rongjie Fu Agilent Technologies (Shanghai) Co., Ltd. 4 Ying Lun Road Shanghai 3 China Abstract A regulatory
REVERSE PHASE HPLC METHOD FOR THE ANALYSIS OF ALFUZOSIN HYDROCHLORIDE IN PHARMACEUTICAL DOSAGE FORMS
 Int. J. Chem. Sci.: 6(1), 2008, 399-404 REVERSE PHASE HPLC METHOD FOR THE ANALYSIS OF ALFUZOSIN HYDROCHLORIDE IN PHARMACEUTICAL DOSAGE FORMS S. APPALA RAJU, ARVIND B. KARADI and SHOBHA MANJUNATH HKES s
Int. J. Chem. Sci.: 6(1), 2008, 399-404 REVERSE PHASE HPLC METHOD FOR THE ANALYSIS OF ALFUZOSIN HYDROCHLORIDE IN PHARMACEUTICAL DOSAGE FORMS S. APPALA RAJU, ARVIND B. KARADI and SHOBHA MANJUNATH HKES s
Development and validation of stability indicating RP-LC method for estimation of calcium dobesilate in pharmaceutical formulations
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
APPLICATIONS TN µm Core-Shell Particle. (continued on page 2) Page 1 of 8. For additional technical notes, visit
 Kinetex 5 µm Core-Shell Columns Deliver Significant Improvements in Chromatographic Efficiency and Resolution versus Traditional Fully Porous 5 µm Columns Philip J. Koerner and Jeff Layne Phenomenex, Inc.,
Kinetex 5 µm Core-Shell Columns Deliver Significant Improvements in Chromatographic Efficiency and Resolution versus Traditional Fully Porous 5 µm Columns Philip J. Koerner and Jeff Layne Phenomenex, Inc.,
IJRPC 2013, 3(2) Nagamallika et al. ISSN: INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A VALIDATED STABILITY INDICATING RP-UPLC METHOD FOR SIMULTANEOUS DETERMINATION OF WATER SOLUBLE
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A VALIDATED STABILITY INDICATING RP-UPLC METHOD FOR SIMULTANEOUS DETERMINATION OF WATER SOLUBLE
ISSN (Print)
 Scholars Academic Journal of Pharmacy (SAJP) Sch. Acad. J. Pharm., 2014; 3(3): 240-245 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com
Scholars Academic Journal of Pharmacy (SAJP) Sch. Acad. J. Pharm., 2014; 3(3): 240-245 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com
ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) ARTESUNATI COMPRESSI ARTESUNATE TABLETS
 December 2009 ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
December 2009 ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets
 Journal of PharmaSciTech 0; ():- Research Article Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets * Sayyed Hussain,
Journal of PharmaSciTech 0; ():- Research Article Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets * Sayyed Hussain,
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Analytical and Preparative SFC Columns
 Analytical and Preparative SFC Columns Best Performance for Supercritical Fluid Chromatography Sepax SFC-Cyano... Sepax SFC-Amino... Sepax SFC-Pyridine... Sepax SFC-SCX... 5 Sepax SFC-Diol... 6 Sepax SFC-Silica...
Analytical and Preparative SFC Columns Best Performance for Supercritical Fluid Chromatography Sepax SFC-Cyano... Sepax SFC-Amino... Sepax SFC-Pyridine... Sepax SFC-SCX... 5 Sepax SFC-Diol... 6 Sepax SFC-Silica...
Sepax Technologies, Inc.
 Sepax Technologies, Inc. Sepax Technologies, Inc. develops and manufactures products in the area of chemical and biological separations, biosurfaces and proteomics. Sepax product portfolio includes ) liquid
Sepax Technologies, Inc. Sepax Technologies, Inc. develops and manufactures products in the area of chemical and biological separations, biosurfaces and proteomics. Sepax product portfolio includes ) liquid
Application Note. Separation of clindamycin phosphate and process impurities. Summary. Introduction. Category Pharmaceutical analysis Matrix
 Application Note Separation of clindamycin phosphate and process impurities Category Pharmaceutical analysis Matrix Drugs Method UHPLC Keywords Antibiotics, process impurities, quality control Analytes
Application Note Separation of clindamycin phosphate and process impurities Category Pharmaceutical analysis Matrix Drugs Method UHPLC Keywords Antibiotics, process impurities, quality control Analytes
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016.
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Aspartame This monograph was also published in: Compendium of Food Additive
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Aspartame This monograph was also published in: Compendium of Food Additive
Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by RP-HPLC method
 International Journal of Chemical and Pharmaceutical Sciences 2017, Mar., Vol. 8 (1) ISSN: 0976-9390 IJCPS Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by
International Journal of Chemical and Pharmaceutical Sciences 2017, Mar., Vol. 8 (1) ISSN: 0976-9390 IJCPS Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by
Sanjog Ramdharane 1, Dr. Vinay Gaitonde 2
 JPSBR: Volume 5, Issue 2: 2015 (151-155) ISS. 2271-3681 A ew Gradient RP- HPLC Method for Quantitative Analysis of : (3-luoro-4- Morpholin-4-yl-Phenyl)-Carbamic Acid Methyl Ester and its Related Substances
JPSBR: Volume 5, Issue 2: 2015 (151-155) ISS. 2271-3681 A ew Gradient RP- HPLC Method for Quantitative Analysis of : (3-luoro-4- Morpholin-4-yl-Phenyl)-Carbamic Acid Methyl Ester and its Related Substances
The One Minute Chromatographer
 The One Minute Chromatographer Agilent Technologies, Inc. 2013 Page 1 Sub 1 Minute Separations with RRHD Columns Agilent ZORBAX RRHD SB-C18, 2.1 x 100mm, 1.8 µm H2O (0.05% Formic acid) / 2-98 % Acetonitrile
The One Minute Chromatographer Agilent Technologies, Inc. 2013 Page 1 Sub 1 Minute Separations with RRHD Columns Agilent ZORBAX RRHD SB-C18, 2.1 x 100mm, 1.8 µm H2O (0.05% Formic acid) / 2-98 % Acetonitrile
The high efficiency of sub-2 µm UHPLC columns combined with the low pressure and high speed of monolith columns.
 The high efficiency of sub-2 µm UHPLC columns combined with the low pressure and high speed of monolith columns. Fast High peak capacity Low back pressure PROTEIN PEPTIDE UHPLC COLUMNS Put these new, high-performance
The high efficiency of sub-2 µm UHPLC columns combined with the low pressure and high speed of monolith columns. Fast High peak capacity Low back pressure PROTEIN PEPTIDE UHPLC COLUMNS Put these new, high-performance
YMC-Actus. Columns for Semi-preparative HPLC. Selectivity Durability Loadability
 YMC-Actus Columns for Semi-preparative HPLC Selectivity Durability Loadability Fast semi-preparative chromatography Semi-preparative chromatography is the link between analytical HPLC and preparative LC.
YMC-Actus Columns for Semi-preparative HPLC Selectivity Durability Loadability Fast semi-preparative chromatography Semi-preparative chromatography is the link between analytical HPLC and preparative LC.
Separation of Vitamin D and Vitamin D Metabolites on FLARE C18 MM (Mixed Mode) HPLC Column
 Diamond Analytics Technical Note: T131-1 Separation of Vitamin D and Vitamin D Metabolites on FLARE C1 MM (Mixed Mode) HPLC Column Introduction In this technical note, the versatility of the diamond-based
Diamond Analytics Technical Note: T131-1 Separation of Vitamin D and Vitamin D Metabolites on FLARE C1 MM (Mixed Mode) HPLC Column Introduction In this technical note, the versatility of the diamond-based
Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin in Pharmaceutical Dosage Form using RP-HPLC Method
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION OF RISPERIDONE AND TRIHEXYPHENIDYL HYDROCHLORIDE FROM BULK AND DOSAGE FORMS.
 Research article Hygeia.J.D.Med.vol.3 (1), 2011, 29-33. www.hygeiajournal.com HYGEIA JOURNAL FOR DRUGS AND MEDICINES ISSN 2229 3590 (online) ISSN 0975 6221 (print) AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION
Research article Hygeia.J.D.Med.vol.3 (1), 2011, 29-33. www.hygeiajournal.com HYGEIA JOURNAL FOR DRUGS AND MEDICINES ISSN 2229 3590 (online) ISSN 0975 6221 (print) AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION
A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS
 A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS By Shun-Hsin Liang and Frances Carroll Abstract Vitamin K₁ and K₂ analysis is typically complex and time-consuming because these
A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS By Shun-Hsin Liang and Frances Carroll Abstract Vitamin K₁ and K₂ analysis is typically complex and time-consuming because these
New Solvent Grade Targeted for Trace Analysis by UHPLC-MS
 New Solvent Grade Targeted for Trace Analysis by UHPLC-MS Subhra Bhattacharya, Deva H. Puranam, and Stephen C. Roemer Thermo Fisher Scientific Fisher Chemical, One Reagent Lane, Fair Lawn, NJ Material
New Solvent Grade Targeted for Trace Analysis by UHPLC-MS Subhra Bhattacharya, Deva H. Puranam, and Stephen C. Roemer Thermo Fisher Scientific Fisher Chemical, One Reagent Lane, Fair Lawn, NJ Material
A simple validated RP-HPLC method for quantification of sumatriptan succinate in bulk and pharmaceutical dosage form
 IJPAR Vol.4 Issue 1 Jan-Mar-2015 Journal Home page: ISSN: 2320-2831 Research article Open Access A simple validated RP-HPLC method for quantification of sumatriptan succinate in bulk and pharmaceutical
IJPAR Vol.4 Issue 1 Jan-Mar-2015 Journal Home page: ISSN: 2320-2831 Research article Open Access A simple validated RP-HPLC method for quantification of sumatriptan succinate in bulk and pharmaceutical
Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector
 application Note UHPLC Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector Introduction Foods
application Note UHPLC Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector Introduction Foods
[Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY
![[Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY [Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY](/thumbs/74/70091865.jpg) TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY Camille Chatenay, 1,2 Fabien Bévalot, 1,2 Cyril Mounier, 1 Jean Michel Prévosto 1 1 Hôpital d Instruction des Armées Desgenettes,
TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY Camille Chatenay, 1,2 Fabien Bévalot, 1,2 Cyril Mounier, 1 Jean Michel Prévosto 1 1 Hôpital d Instruction des Armées Desgenettes,
LC/MS Analysis of Various Hydrophilic Compounds Using a Polymer-Based Amino Column - Shodex TM HILICpak TM VG-50 2D
 LC/MS Analysis of Various Hydrophilic Compounds Using a Polymer-Based Amino Column - Shodex TM HILICpak TM VG-50 2D Introduction Components of pharmaceutical products and food products often include high
LC/MS Analysis of Various Hydrophilic Compounds Using a Polymer-Based Amino Column - Shodex TM HILICpak TM VG-50 2D Introduction Components of pharmaceutical products and food products often include high
NOVEL RP-HPLC METHOD. B.Lakshmi et a. concentration range KEY INTRODUCTION. Diltiazem is used to. . It works by of contractionn of the. (dilate).
 B.Lakshmi et a ISSN-2319-2119 al, The Experiment, January. 2013 Vol..6(4), 365-371 A NOVEL RP-HPLC METHOD FOR THE DETERMINATION OF DILTIAZEM IN PHARMACEUTICAL DRUG PRODUCTS ABSTRACT High resolution RP-HPLC
B.Lakshmi et a ISSN-2319-2119 al, The Experiment, January. 2013 Vol..6(4), 365-371 A NOVEL RP-HPLC METHOD FOR THE DETERMINATION OF DILTIAZEM IN PHARMACEUTICAL DRUG PRODUCTS ABSTRACT High resolution RP-HPLC
Simultaneous Estimation of Gemcitabine Hydrochloride and Capecitabine Hydrochloride in Combined Tablet Dosage Form by RP-HPLC Method
 ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1212-1217 Simultaneous Estimation of Gemcitabine Hydrochloride and Capecitabine Hydrochloride in Combined Tablet Dosage
ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1212-1217 Simultaneous Estimation of Gemcitabine Hydrochloride and Capecitabine Hydrochloride in Combined Tablet Dosage
Rapid and sensitive UHPLC screening of additives in carbonated beverages with a robust organic acid column
 APPLICATION NOTE 21673 Rapid and sensitive UHPLC screening of additives in carbonated beverages with a robust organic acid column Authors Aaron Lamb and Brian King, Thermo Fisher Scientific, Runcorn, UK
APPLICATION NOTE 21673 Rapid and sensitive UHPLC screening of additives in carbonated beverages with a robust organic acid column Authors Aaron Lamb and Brian King, Thermo Fisher Scientific, Runcorn, UK
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
EMTRICITABINE AND TENOFOVIR TABLETS
 September 2010 RESTRICTED EMTRICITABINE AND TENOFOVIR TABLETS Draft proposal for The International Pharmacopoeia (September2010) REVISED DRAFT FOR COMMENT This document was provided by a quality control
September 2010 RESTRICTED EMTRICITABINE AND TENOFOVIR TABLETS Draft proposal for The International Pharmacopoeia (September2010) REVISED DRAFT FOR COMMENT This document was provided by a quality control
J Pharm Sci Bioscientific Res (4): ISSN NO
 Development and Validation of Analytical Methods for Simultaneous Estimation of Pregabalin and Amitriptyline Hydrochloride in their Combined Marketed Dosage form ABSTRACT: Nikhilkumar Patel, Gurjit Kaur,
Development and Validation of Analytical Methods for Simultaneous Estimation of Pregabalin and Amitriptyline Hydrochloride in their Combined Marketed Dosage form ABSTRACT: Nikhilkumar Patel, Gurjit Kaur,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2*
 Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Selectivity Comparison of Agilent Poroshell 120 Phases in the Separation of Butter Antioxidants
 Selectivity Comparison of Agilent Poroshell 1 Phases in the Separation of Butter Antioxidants Application Note Food Testing & Agriculture Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd. Abstract
Selectivity Comparison of Agilent Poroshell 1 Phases in the Separation of Butter Antioxidants Application Note Food Testing & Agriculture Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd. Abstract
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com VALIDATED RP-HPLC METHOD FOR DETERMINATION OF BROMHEXINE
Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com VALIDATED RP-HPLC METHOD FOR DETERMINATION OF BROMHEXINE
Simultaneous Analysis of Active Pharmaceutical Ingredients and Their Counter-Ions Using a Mixed-Mode Column
 P-CN54E Simultaneous Analysis of Active Pharmaceutical Ingredients and Their Counter-Ions Using a Mixed-Mode Column Pittcon 5 9-6P Kenichiro Tanaka, William Hedgepeth, Yuki Sato Shimadzu Scientific Instruments,
P-CN54E Simultaneous Analysis of Active Pharmaceutical Ingredients and Their Counter-Ions Using a Mixed-Mode Column Pittcon 5 9-6P Kenichiro Tanaka, William Hedgepeth, Yuki Sato Shimadzu Scientific Instruments,
APPLICATIONS TN Dramatically Improve Existing 5 µm and 3 µm Fully Porous Methods with Kinetex 5 µm Core-shell Technology.
 Dramatically Improve Existing 5 µm and 3 µm Fully Porous Methods with Kinetex 5 µm Core-shell Technology Jeff Layne, Phenomenex, Inc. Phenomenex, Inc., 411 Madrid Ave., Torrance, CA 951 USA With virtually
Dramatically Improve Existing 5 µm and 3 µm Fully Porous Methods with Kinetex 5 µm Core-shell Technology Jeff Layne, Phenomenex, Inc. Phenomenex, Inc., 411 Madrid Ave., Torrance, CA 951 USA With virtually
APPLICATIONS Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns
 Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns Michael McGinley, Deborah Jarrett, and Jeff Layne Phenomenex, Inc., 411 Madrid Avenue, Torrance, CA 91 USA Aeris WIDEPORE
Improving Intact Biogeneric Protein Separations with Aeris WIDEPORE Core-Shell Columns Michael McGinley, Deborah Jarrett, and Jeff Layne Phenomenex, Inc., 411 Madrid Avenue, Torrance, CA 91 USA Aeris WIDEPORE
Pankti M. Shah et al, Asian Journal of Pharmaceutical Technology & Innovation, 04 (17); 2016; 07-16
 Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 30-03-2016 Accepted on: 01-04-2016 Published on: 15-04-2016 Corresponding Author: *Pankti M. Shah,
Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 30-03-2016 Accepted on: 01-04-2016 Published on: 15-04-2016 Corresponding Author: *Pankti M. Shah,
Development and Validation of a RPLC Method for the Determination of 2-Phenoxyethanol in Senselle Lubricant Formulation
 Research Paper Development and Validation of a RPLC Method for the Determination of 2-Phenoxyethanol in Senselle Lubricant Formulation G. A. SHABIR*, T. K. BRADSHAW, G. Q. SHAR 1 AND S. A. ARAIN 2 Oxford
Research Paper Development and Validation of a RPLC Method for the Determination of 2-Phenoxyethanol in Senselle Lubricant Formulation G. A. SHABIR*, T. K. BRADSHAW, G. Q. SHAR 1 AND S. A. ARAIN 2 Oxford
Mass-Based Purification of Natural Product Impurities Using an Agilent 1260 Infinity II Preparative LC/MSD System
 Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
Meteoric Core. Core-Shell Columns for UHPLC & HPLC. Easy Method Optimization! Excellent Chromatography with Fast Separation!
 Meteoric Core Easy Method Optimization! Excellent Chromatography with Fast Separation! Core Porous shell layer Meteoric Core Particle Image Structure Schöttmannshof 19 46539 Dinslaken Phone +49 (0)2064
Meteoric Core Easy Method Optimization! Excellent Chromatography with Fast Separation! Core Porous shell layer Meteoric Core Particle Image Structure Schöttmannshof 19 46539 Dinslaken Phone +49 (0)2064
Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms
 Asian Journal of Chemistry Vol. 21, No. 2 (2009), 829-833 Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms B.V.V.S. JAGADEESH, S. SATYANARAYANA RAJU, V.JAYATHIRTHA RAO and J.V.L.N.
Asian Journal of Chemistry Vol. 21, No. 2 (2009), 829-833 Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms B.V.V.S. JAGADEESH, S. SATYANARAYANA RAJU, V.JAYATHIRTHA RAO and J.V.L.N.
LC Columns - Exceed the limit. A premium inert range of LC columns delivering optimal peak shape. ProteCol -P PEEK lined
 ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
ProteCol LC Columns - Exceed the limit A premium inert range of LC columns delivering optimal peak shape. ProteCol -G Glass lined ProteCol -P PEEK lined B C A D E F A Column end cap B PEEK frit housing
Estimation of Emtricitabine in Tablet Dosage Form by RP-HPLC
 Asian Journal of Chemistry Vol. 21, No. 8 (9), 5979-5983 Estimation of Emtricitabine in Tablet Dosage Form by RP-HPLC V. KIRAN KUMAR* and N. APPALA RAJU Department of Pharmaceutical Analysis, Sree Chaitanya
Asian Journal of Chemistry Vol. 21, No. 8 (9), 5979-5983 Estimation of Emtricitabine in Tablet Dosage Form by RP-HPLC V. KIRAN KUMAR* and N. APPALA RAJU Department of Pharmaceutical Analysis, Sree Chaitanya
