Combined therapy trial with interferon alpha-2a
|
|
|
- Britton Spencer
- 5 years ago
- Views:
Transcription
1 Genitourin Med 1996;72: Department of Genitourinary Medicine, Royal Victoria Hospital, Belfast, BT12 6BA D K B Armstrong R D Maw W W Dinsmore Gynaecology Department, University Hospital, Copenhagen, Denmark J Blaakaer Hospital Naval Marcilio Dias, Rio de Janeiro, Brazil M A G Correa Hudkliniken, Regionsjukhuset, Orebro, Sweden L Falk The Sir Mortimer B Davis Jewish General Hospital, Montreal, Canada A S Ferenczy Hopital du Saint- Sacrement, Quebec, Canada M Fortier Princess Alexandra Hospital, Woolloongabba, Queensland, Australia I Frazer St George Hospital, Kogarah, New South Wales, Australia C Law Gynaecology Department, Odense Hospital, Odense, Denmark B M Moller Hospital Das Clinicas, San Paulo, Brazil N Oyakawa Correspondence to: Dr D K B Armstrong. Accepted for publication 14 November 1995 Combined therapy trial with interferon alpha-2a and ablative therapy in the treatment of anogential warts D K B Armstrong, R D Maw, W W Dinsmore, J Blaakaer, M A G Correa, L Falk, A S Ferenczy, M Fortier, I Frazer, C Law, B M Moller, N Oyakawa Objective: To determine whether the combination of systemically administered interferon alpha-2a and ablative surgery for the treatment of genital and/or perianal warts produces a 30% or greater improvement in lasting response rate compared with a control group receiving a combination of placebo and ablative therapy. Design: Randomised, triple-blind, placebo-controlled trial using 1 or 3 MIU of interferon alpha-2a or placebo administered subcutaneously three times weekly for 10 weeks in combination with ablative surgery. Setting: International, multicentre study in 10 genitourinary medicine clinics. Patients: Two hundred and fifty patients with anogenital warts. Main outcome measures: Lasting response at week 38. Results: Standard efficacy analysis at week 38 showed a lasting response in 51% (35/68) of 3 MIU interferon-treated patients, 48% (30/63) of 1 MIU interferon-treated patients and 43% (29/67) of placebo-treated patients. Conclusions: With the doses and regime described, treatment with interferon alpha-2a in combination with ablative therapy is not significantly superior in the treatment of anogenital warts than placebo and ablative therapy. (Genitourin Med 1996;72: ) Keywords: Human papillomavirus; interferon alpha-2a Introduction Anogenital warts are increasing in incidence and conventional management with chemical and physical ablative therapy is often associated with unsatisfactory response rates and a high incidence of recurrent disease.'-' Ablative therapy aims to remove clinically visible condylomata but latent human papillomavirus (HPV) infection in adjacent apparently normal epithelium may remain and be responsible for the high recurrence rates.4 Inclusion of a wider margin of apparently normal epithelium around lesions undergoing ablative treatment is recommended as one means of attempting to address this problem but in addition and perhaps more theoretically attractive is the use of a systemically administered agent which may act at all such sites of infection. The known antiviral, antiproliferative and immunomodulatory properties of interferons (IFNs) make such agents attractive adjuvant treatment to be used in conjunction with standard "debulking" ablative therapies. IFN treatment, however, is associated with characteristic adverse effects, involves significant expense and may necessitate more visits to the clinic to administer. It is important therefore that clear and significant clinical benefit be demonstrated if such agents are to be prescribed. This study was designed to test the hypothesis that the combination of subcutaneous IEN alpha-2a at a dose of 3 MIU three times weekly for 10 weeks and physical ablative treatment would produce a 30% or more improvement in lasting response rates at week 38 in patients with external genital 103 and/or perianal warts when compared with ablative therapy and placebo. The lasting response rate of a 1 MIU dosage and the tolerability of such treatment were also to be evaluated. Patients and methods This study was conducted in 10 centres in six countries after study approval was obtained from each local medical ethics committee. Patient selection Patients aged 18 years or older with clinically visible (without application of acetic acid) condylomata acuminata on the external genitalia and/or perianal area, likely to be cleared by ablative therapy within six weeks, who had not received treatment within one month of study entry were eligible. Patients were excluded if they had internal genital or anal canal lesions only, had had previous interferon treatment, were HIV antibody positive or had a history of immunomodulatory therapy within the last 12 months and/or therapy duration of more than three months. In addition, patients were excluded if they had evidence of malignant neoplastic disease (except intraepithelial neoplasia), a history of neurological disease, renal failure or autoimmune disease or an active sexually transmitted disease other than genital/perianal warts. Women who were pregnant, lactating or were not practicing an adequate form of contraception were also ineligible. Witnessed oral or written informed consent was obtained from all patients.
2 104 Armstrong, Maw, Dinsmore, Blaakaer, Correa, Falk, et al Study design This was an international, multicentre, randomised, triple-blind, placebo-controlled combination therapy trial. Eligible patients were stratified according to sex and randomised to receive 3 MIU or 1 MIU of IFN alpha-2a (Roferon(R), AF Hoffmann-La Roche, Basel, Switzerland) or matched placebo injections subcutaneously three times per week for 10 consecutive weeks in combination with ablative therapy. Ablative therapy began at day 1, with first injection, and continued, if required, until week 6. Ablative therapy options included diathermy, electric cautery, diathermic loop or laser as viewed most appropriate by each investigator. The ablative therapy included 5 mm of the surrounding apparently normal epithelium. At the end of the 10 week treatment period, all patients with complete clearance of their lesions were followed up for 28 weeks after the end of treatment or until recurrence. Those with only a partial response at week 10 were withdrawn and treated as felt most appropriate by the investigator. Assessment schedule A pre-study screen to include documentation of demographic details, duration of warts, and previous treatments and confirmation of eligibility for study entry was completed within two weeks of commencement of study treatment. Necessary haematological and biochemical measures were checked and HIV antibody testing and pregnancy tests performed. A representative biopsy specimen was taken for histology and HPV DNA typing. Analysis was performed at a central laboratory and was blind to treatment assignment. Detection of HPV DNA in biopsy tissue was performed by in-situ DNA hybridisation (HPV 6, 11, 16, 18, 31, 33). All negative samples were further analysed by polymerase chain reaction techniques. A baseline assessment was performed immediately prior to the first injection and ablative treatment on day 1 to include a description of location, type and number of lesions and estimated operability. An assessment of disease state was carried out at the end of weeks 4, 6 and 10. Any adverse events, intercurrent illnesses or changes in concomitant medication were noted. The number and type of ablative treatments was recorded at the end of week 6. Patients with a partial response only at week 10 assessment were withdrawn. Patients with a complete response at week 10 were seen every four weeks during the 28 weeks of follow-up or until there was recurrence. Patients noting a recurrence between week 10 and week 38 were asked to return to the trial centre immediately where the next monthly assessment was performed. They were subsequently withdrawn and treatment was at the discretion of the supervising physician. Laboratory investigations Blood was taken for haemoglobin concentration, white blood cell count, platelet count, creatinine and AST during the pre-study screen, at weeks 0, 4, 10 and 14. In the event of unexplained or unexpected test value abnormalities, the tests were repeated immediately and followed up until the results returned to the normal range and/or an adequate explanation of the abnormality was found. Criteria for exclusion from efficacy analysis Patients were excluded from standard analysis of efficacy if they violated entry criteria, became pregnant, missed three consecutive injections or did not receive a minimum of 26 injections, or in the case of recurrence before week 10 a minimum number defined by the week of recurrence. The use of systemic or topical treatment likely to interfere with evaluation of efficacy or loss to follow-up also constituted grounds for withdrawal from standard analysis of efficacy. An intent-to-treat analysis included all patients who received study medication and had at least one post-baseline assessment. All such patients were included in the analysis of safety. Statistical analysis Sample size was calculated to detect a 30% difference in response rates between placebo and IFN alpha-2a (3 MIU)- treated patients with 80% power using a twosided test at the 5% level of significance. Analysis was undertaken without knowledge of the treatment code. The hypothesis had assumed a lasting response would be achieved in approximately 70% of patients treated with 3 MIU Interferon alpha-2a and 40% of those treated with placebo. This assumption proved invalid and therefore only descriptive statistics were used. The frequency of responders, the response rate and the 95% confidence interval defined by the Pearson-Clopper limits were calculated for the standard analysis population and the intent-to-treat population. Results In total, 250 patients were recruited in 10 centres. Two hundred and forty-nine received at least one injection. Of these, 81 were randomised to placebo, 83 to IFN alpha-2a (3 MIU) and 85 to IFN alpha-2a (1 MIU). Demographic data and pre-study characteristics There were no significant differences between treatment groups in age, weight, height, sex distribution, race, sexual orientation or smoking habit. The groups were comparable for previous treatments for their anogenital warts and for concomitant diseases and medication. Table 1 summarises pretreatment lesion characteristics which showed no notable differences between treatment groups. The majority of internal warts involved the vagina in all treatment groups with smaller numbers affecting the cervix, anal canal, urethral meatus and urethra. Histopathology and HPV typing data Tables 2 and 3 outline the microscopic morphology and HPV DNA types detected respectively in biopsy specimens taken. The predominant microscopic morphology was
3 Combined therapy trial with interferon alpha-2a and ablative therapy in the treatment of anogenital warts 105 Table 1 Pre-treatment characteristics Placebo IFN (1 MIU) IFN (3 MIU) Parameter N = 81 N = 85 N = 83 Duration of disease median (months) range Operability-No (%) operable 71 (88) 79 (93) 80 (96) difficult 7 (9) 5 (6) 1 (1) at limit 3 (4) 1 (1) 2 (2) Number of external (17) 17 (20) 21 (26) lesions-no (%) (25) 16 (19) 22 (27) (11) 15 (18) 7 (9) (7) 4 (5) 7 (9) >20 6(7) 11 (13) 6(7) not countable 26 (32) 21 (25) 19 (23) Location of external genital 52 (64) 54 (64) 50 (61) lesions-no (%) perianal 7 (9) 7 (8) 7 (9) both 22 (27) 23 (27) 25 (30) Presence of internal yes 27 (33) 19 (22) 23 (28) lesions-no (%) no 54 (67) 66 (78) 60 (72) Table 2 Microscopic morphology of biopsies Placebo IFN (1 MIU) IFN (3 MIU) Total Classification n (%o) n (%) n (oo) n (%) Papillary 50 (62 5) 56 (66.7) 52 (62.7) 158 (64.0) Flat 11 (13-8) 12 (14-3) 10 (12-0) 33 (13-4) Pigmentedpapulosis 18 (22 5) 15 (17-9) 20 (24.1) 53 (21-5) Bowenoidpapulosis 1 (1.2) 0 1 (1.2) 2 (0.8) Pigmented naevus 0 1 (1-2) 0 1 (0.4) Total Table 3 HPV DNA typing of biopsies Placebo IFN (1 MIU) IFN (3 MIU) Total Type n (%) n (%) n (%) n (%) 6 29 (36 7) 36 (42.9) 34 (41-0) 99 (40.2) (27.9) 22 (26.2) 18 (21.7) 62 (25.2) 18 1 (1-3) 0 4 (48) 5 (2.0) 31 1 (1-3) 2 (24) 2 (24) 5 (20) Other 3 (3.8) 2 (2.4) 6 (7.2) 11 (4.5) Negative 23 (29-1) 22 (26.2) 19 (22.9) 64 (26.0) Total Table 4 Overall assessment of response to treatment Placebo IFN (1 MIU) IFN (3 MIU) All centres n (Oo) (95%OCI) n (%) (95%0CI) n (O%) (95%oCI) N Lasting response 29 (43) (31-56) 30 (48) (35-61) 35 (51) (39-64) Treatment failures 38 (57) 33 (52) 33 (49) papillary. The individual whose biopsy specimen showed a pigmented naevus was excluded from efficacy analysis. There was a low frequency of dysplasia with four cases of mild dysplasia in the 3 MIU IFN group, three in the 1 MIU IFN group and one in the placebo-treated group. Only two cases of moderate dysplasia were detected with one case in the 3 MIU IFN group and one case in the placebo-treated group. No cases of severe dysplasia were detected. HPV types 6 and 11 were most commonly detected. The three treatment groups were comparable. Efficacy analysis The primary efficacy analysis was to determine the number of patients who had a lasting response at week 38, i.e. complete clearance of all clinically visible lesions and no recurrence thereafter up to and including week 38. Treatment failures included both patients who showed only partial clearance of lesions by week 10 or recurrence prior to or at week 38. Table 4 summarises the standard analysis of efficacy of the three treatment groups. At week 38, 35 of the 68 evaluable patients in the 3 MIU IFN group (51%), 30 of the 63 patients in the 1 MIU group (48%) and 29 of the 67 placebo patients (43%) had lasting responses. The 33 treatment failures (49%) in the 3 MIU IFN group included 10 with a partial response at week 10 and 23 patients with recurrences. Thirty-three patients (52%) in the 1 MIU were treatment failures with nine showing partial response at week 10 and 24 patients noting recurrence. In the placebo group the 38 patients (57%) classified as treatment failures comprised 14 with a partial response at week 10 and 24 with recurrences at or before week 38. The results of an intent-to-treat analysis including all patients who had received treatment did not differ substantially from the results of the above standard efficacy analysis. As a result of small numbers of patients at each centre it was not possible to draw clear conclusions as to any effect of the centre on overall response rates. Clearly the combination of 3 MIU of IFN alpha-2a and ablative therapy did not produce an improvement of 30% in lasting response rate in excess of that produced by placebo and ablative therapy. Hence the test hypothesis is invalid. Ablative therapy The proportions of patients treated with each type of ablative therapy and the number of ablative treatments given were similar in all three treatment groups. The majority of patients received one or two treatments for external lesions and a single treatment for internal lesions. Safety analysis One hundred and eighteen (47%) of the 249 patients who received IFN or placebo experienced adverse effects. Forty-three of these were in the 1 MIU IFN group, 42 in the 3 MIU group and 33 in the placebo group. The most frequently reported adverse events in the IFN-treated patients were headaches, fatigue, flu-like symptoms, local erythema, dizziness and myalgia. The most common adverse events in placebo-treated patients were headache, fatigue, dizziness, nasal congestion and flu-like symptoms. The majority of adverse events were of mild severity and most were considered by the investigator to be probably or possibly related to the study drug. Laboratory data All HIV antibody tests were negative. There were no clinically relevant changes in median haemoglobin, creatinine or AST levels during the study. Median leucocyte count fell in both 3 MIU and 1 MIU IFN groups at week 4 (by 1.2 x 103/l and 1.4 x 103/l respectively) but in each case counts returned close to pretreat-
4 106 Armstrong, Maw, Dinsmore, Blaakaer, Correa, Falk, et al ment levels by week 14. Median platelet levels declined slightly in both IFN-treated groups at week 4. In the 1 MIU IFN group the platelet count remained at this lower level at week 14 (270.5 x 103/L compared with a pretreatment level of 283 x 103/1) but in the 3 MIU group returned to near pretreatment levels. Discussion HPV infection of the anogenital epithelium is a multicentric process and in addition to the production of clinically visible condylomata acuminata may produce latent and clinically inapparent "disease". It is this phenomenon which may explain the often inadequate response rates and unacceptably high recurrence rates with currently available conventional chemical and physical ablative therapies.'-3 Interferons are known to possess antiviral, anti-proliferative and immunomodulatory effects and as such were thought to represent attractive agents in the treatment of HPV infection. Early trials with IFN monotherapy showed somewhat conflicting results and comparison between studies is difficult because of variations in study design, IFN subtype, route of administration, nature of warts treated and endpoint measures of efficacy chosen. Some degree of clinical efficacy has been demonstrated by studies using parenteral and intra-lesional routes of administration in both primary and recalcitrant warts5-1 but several of these studies involved relatively small numbers of patients, were open and uncontrolled. One large randomised open study, however, compared systemically administered IFN alpha-2a with podophyllin in the treatment of primary anogenital warts and found IFN to be a significantly less effective form of monotherapy. 12 Indeed it is the general consensus that IFN monotherapy is not superior to currently available standard therapeutic techniques. " The potential therapeutic benefit of a combination of a standard chemical or physical ablative treatment and IFN therapy has subsequently been addressed. Douglas et al demonstrated an improvement in complete response rate in patients treated with a combination of intralesional IFN alpha-2b and podophyllin versus podophyllin alone.'3 Several other authors have also shown a beneficial adjuvant effect of systemic IFN therapy when used in combination with ablative therapy for recalcitrant anogenital warts.'1'6 It is notable, however, that several of these reports have methodological limitations in terms of patient numbers, limited controls and duration of follow-up. Recently three large well conducted randomised, placebo-controlled combination therapy trials have failed to demonstrate a significant improvement in either complete response or recurrence rates when systemic interferon alpha-2a was combined with laser surgery,'7 cryotherapy'8 or podophyllin.'9 This present study was evaluating the effect of adjuvant systemic IFN alpha-2a in combination with ablative surgery on the rate of lasting response, that is persistence of complete clearance, up to and including 28 weeks after cessation of IFN therapy. Interferon therapy is associated with well described side effects and involves additional expenditure. It follows that clear clinical benefit should be demonstrated before such treatment could be justified. It was considered that in order for such adjuvant treatment to be regarded as therapeutically useful a lasting response rate of at least 30% over and above the placebo response was necessary. The number of patients recruited was sufficient to supply the required statistical power to test our initial hypothesis. The treatment groups were comparable for prestudy lesion characteristics and the histopathology and HPV DNA findings are similar to other large series with a predominance of papillary microscopic morphology, types 6 and 11 HPV DNA and infrequent evidence of dysplasia."2 1' As such our study population would appear to be representative. With a lasting response rate of 51% in the 3 MIU IFN group compared with 43% in the placebo treated group clearly our test hypothesis was invalid. An intermediate value of 48% was obtained with the 1 MIU IFN dose. Complete response rates at week 10 were also comparable between treatment groups. The most frequently reported adverse events were flu-like symptoms and were generally mild. In summary we conclude that although well tolerated, interferon alpha-2a, given at 1 MIU or 3 MIU three times per week in combination with ablative surgery was not found to be superior in the treatment of anogenital warts when compared with treatment with ablative therapy plus placebo. 1 Gabriel G, Thin RNT. Treatment of anogenital wartscomparison of trichloracetic acid and podophyllin versus podophyllin alone. BrJ7 Venereal Dis 1983;59: Stone KM, Becker TM, Hadgu A, Kraus SJ. Treatment of external genital warts: a randomised clinical trial comparing podophyllin, cryotherapy and electrodessication. Genitourin Med 1990;66: Ling MR. Therapy of genital human papillomavirus infections, part II: Methods of treatment. Int J Dermatol 1992;31: Ferenczy A, Mitao M, Nagai N, et al. Latent papillovirus and recurring genital warts. N Engl J Med 1985;313: Welander CE, Homesley HD, Smiles KA, Peets EA. Intralesional interferon alfa-2b for the treatment of genital warts. Am3t Obstet Gynecol 1990;162: Reichman RC, Oakes D, Bonnez W, et al. Treatment of condyloma acuminatum with three different interferons administered intra-lesionally. Ann Intern Med 1988;108: Gall SA, Hughes CE, Mounts P, et al. Efficacy of human lymphoblastoid interferon in the therapy of resistant condyloma acuminata. Obstet Gynecol 1986;67: Eron U, Judson F, Tucker S, et al. Interferon therapy for condyloma acuminata. N EngJ Med 1986;315: Friedman-Kien AE, Eron U, Canant M, et al. Natural interferon alfa for treatment of condylomata acuminata. JAAIA 1988;259: Kirby PK, Kiviat N, Beckman A, et al. Tolerance and efficacy of recombinant human interferon gamma in the treatment of refractory genital warts. Am J Med 1988; 85: Reichman RC, Oakes D, Bonnez W, et al. Treatment of condyloma acuminata with three different interferonalpha preparations administered parenterally: A doubleblind, placebo-controlled trial. J Infect Dis 1990;162: Anon. A comparison of interferon alfa-2a and podophyllin in the treatment of primary condylomata acuminata. Genitourin Med 1991;67: Douglas JM, Eron LJ, Judson FN, et al. A randomised trial of combination therapy with intralesional interferon alpha-2b and podophyllin versus podophyllin alone for therapy of anogenital warts. JInfect Dis 1990;162:52-9.
5 Combined therapy trial with interferon alpha-2a and ablative therapy in the treatment of anogenital warts Petersen CS, Bjerring P, Larsen J, et al. Systemic interferon alpha-2b increases the cure rate in laser treated patients with multiple persistent genital warts: a placebocontrolled study. Genitourin Med 1991;67: Hopfl RM, Sandbichler M, Zelger BWH, Conrad FG, Fritsch PO. Adjuvant treatment of recalcitrant genitoanal warts with systemic recombinant interferon-alpha-2c. Acta Derm Venereol 1992;72: Reid R, Greenberg MD, Pizzuti DJ, et al. Superficial laser vulvectomy-enhanced by adjuvant systemic interferon. Am Jf Obstet Gynecol 1992;166: Anon. Randomised placebo-controlled double-blind combined therapy with laser surgery and systemic interferon alpha-2a in the treatment of anogenital condylomata acuminata. Infect Dis 1993;167: Handley JM, Homer T, Maw RD, Lawther H, Dinsmore WW. Subcutaneous interferon alpha 2a combined with cryotherapy vs cryotherapy alone in the treatment of primary anogenital warts: a randomised observer blind placebo-controlled study. Genitourin Med 1991;67: Armstrong DKB, Maw RD, Dinsmore WW, et al. Randomised, double blind, parallel group study to compare subcutaneous interferon alfa-2a plus podophyllin with placebo plus podophyllin in the treatment of primary condylomata acuminata. Genitourin Med 1994;70: Genitourin Med: first published as /sti on 1 April Downloaded from on 1 November 2018 by guest. Protected by copyright.
Anogenital warts: epidemiology, treatment and association with cervical atypia
 The Ulster Medical Journal, Volume 56, No. 2, pp. 104-108, October 1987. Anogenital warts: epidemiology, treatment and association with cervical atypia W W Dinsmore, T Horner, H Chambers, R D Maw Accepted
The Ulster Medical Journal, Volume 56, No. 2, pp. 104-108, October 1987. Anogenital warts: epidemiology, treatment and association with cervical atypia W W Dinsmore, T Horner, H Chambers, R D Maw Accepted
Richard Gilson, Jonathan Ross, Raymond Maw, David Rowen, Christopher Sonnex, Charles Lacey
 A multi-centre, randomised, double-blind, placebo-controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Richard Gilson, Jonathan Ross,
A multi-centre, randomised, double-blind, placebo-controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Richard Gilson, Jonathan Ross,
EXTERNAL ANOGENITAL WARTS
 EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
Economic analysis of treatments for external condyloma acuminatum Fagnani F
 Economic analysis of treatments for external condyloma acuminatum Fagnani F Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
Economic analysis of treatments for external condyloma acuminatum Fagnani F Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human Papillomavirus
 Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Cervical human papilloma virus (HPV) infection, exophytic or subclinical, was present in 58.3% and 77-2% of females in the
 Genitourin Med 1991;67:297-302 Subcutaneous interferon alpha 2a combined with vs alone in the treatment of primary anogenital warts: a randomised observer blind placebo controlled study J M Handley, T
Genitourin Med 1991;67:297-302 Subcutaneous interferon alpha 2a combined with vs alone in the treatment of primary anogenital warts: a randomised observer blind placebo controlled study J M Handley, T
Study Number: Title: Rationale: Phase: Study Period Study Design: Centres: Indication Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco
 Anal cytology and anal cancer in HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco April 10, 2010 Disclosures Merck and Co: Research grant support, advisory boards
Anal cytology and anal cancer in HIV-infected men and women. Joel Palefsky, M.D. University of California, San Francisco April 10, 2010 Disclosures Merck and Co: Research grant support, advisory boards
Treatment of cutaneous and mucosal HPV lesions with Interferon (IFN) recombinant human -Α2b in pediatric patients.
 Research Article http://www.alliedacademies.org/journal-dermatology-research-skin-care/ Treatment of cutaneous and mucosal HPV lesions with Interferon (IFN) recombinant human -Α2b in pediatric patients.
Research Article http://www.alliedacademies.org/journal-dermatology-research-skin-care/ Treatment of cutaneous and mucosal HPV lesions with Interferon (IFN) recombinant human -Α2b in pediatric patients.
Human Papillomavirus
 Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION. Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata
 Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Human Papillomavirus (HPV) in Patients with HIV.
 Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Human Papillomavirus (HPV) in Patients with HIV www.hivguidelines.org Purpose of the Guideline Increase the numbers of NYS residents with HIV who are screened for HPV-related dysplasia and managed effectively.
Genital Human Papillomavirus (HPV) Infections
 Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
Genital Human Papillomavirus (HPV) Infections January 2008 Etiology... 1 Epidemiology... 1 Prevention... 2 Diagnosis... 3 Management... 6 Treatment... 7 Consideration for Other STIs... 9 Reporting and
NVESTIGATION Abstract: INTRODUCTION
 236 INVESTIGATION s A prospective, open, comparative study of 5% potassium hydroxide solution versus cryotherapy in the of genital warts in men* Caio Lamunier de Abreu Camargo 1 Walter Belda Junior 1 Luiz
236 INVESTIGATION s A prospective, open, comparative study of 5% potassium hydroxide solution versus cryotherapy in the of genital warts in men* Caio Lamunier de Abreu Camargo 1 Walter Belda Junior 1 Luiz
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare
 camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts
 82 Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts Jeong-Hun Park, M.D., Young-Keun Kim, M.D., Gwang-Seong Choi, M.D. Department of Dermatology,
82 Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts Jeong-Hun Park, M.D., Young-Keun Kim, M.D., Gwang-Seong Choi, M.D. Department of Dermatology,
Dermatology Research
 Research Article Research Article Dermatology Research Therapy with Pegylated Interferon or Combined with in Condyloma Acuminata. Phase iii Clinical Trial Israel Alfonso Trujillo *, Tomás Tabilo Bocic,
Research Article Research Article Dermatology Research Therapy with Pegylated Interferon or Combined with in Condyloma Acuminata. Phase iii Clinical Trial Israel Alfonso Trujillo *, Tomás Tabilo Bocic,
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Make Love Not Warts Genital Warts
 Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Patients who achieved the primary criterion for response i.e.: complete clearance or a reduction
 MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
SCCPS Scientific Committee Position Paper on HPV Vaccination
 SCCPS Scientific Committee Position Paper on HPV Vaccination Adapted from Joint Statement (March 2011) of the: Obstetrical & Gynaecological Society of Singapore (OGSS) Society for Colposcopy and Cervical
SCCPS Scientific Committee Position Paper on HPV Vaccination Adapted from Joint Statement (March 2011) of the: Obstetrical & Gynaecological Society of Singapore (OGSS) Society for Colposcopy and Cervical
Pathology of the Cervix
 Pathology of the Cervix Thomas C. Wright Pathology of the Cervix Topics to Consider Burden of cervical cancer 1 Invasive Cervical Cancer Cervical cancer in world Second cause of cancer death in women Leading
Pathology of the Cervix Thomas C. Wright Pathology of the Cervix Topics to Consider Burden of cervical cancer 1 Invasive Cervical Cancer Cervical cancer in world Second cause of cancer death in women Leading
trial update clinical
 trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
HPV-related papillomatous-condylomatous lesions in female anogenital area
 HPV-related papillomatous-condylomatous lesions in female anogenital area Theo Panoskaltsis MD, FRCOG, CCST (UK) Epidemiology Anal cancer is increasing in both men and women Groups at risk: - HIV (+)
HPV-related papillomatous-condylomatous lesions in female anogenital area Theo Panoskaltsis MD, FRCOG, CCST (UK) Epidemiology Anal cancer is increasing in both men and women Groups at risk: - HIV (+)
Condylomata acuminata in children: report of four cases
 Genitourin Med 1985; 61: 338-342 Condylomata acuminata in children: report of four cases MOHAMMED AMEEN SAIT AND B R GARG From the Department of Dermatology and Sexually Transmitted Disease, Jawaharlal
Genitourin Med 1985; 61: 338-342 Condylomata acuminata in children: report of four cases MOHAMMED AMEEN SAIT AND B R GARG From the Department of Dermatology and Sexually Transmitted Disease, Jawaharlal
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent
National Cervical Screening Program MBS Item Descriptors
 National Cervical Screening Program MBS Item Descriptors Item Item descriptor 73070 A test, including partial genotyping, for oncogenic human papillomavirus that may be associated with cervical pre-cancer
National Cervical Screening Program MBS Item Descriptors Item Item descriptor 73070 A test, including partial genotyping, for oncogenic human papillomavirus that may be associated with cervical pre-cancer
What is a Pap smear?
 Pap smear What is a Pap smear? A Pap smear is a test that checks for changes in the cells of your cervix. The cervix is the lower part of the uterus that opens into the vagina. Developed over forty years
Pap smear What is a Pap smear? A Pap smear is a test that checks for changes in the cells of your cervix. The cervix is the lower part of the uterus that opens into the vagina. Developed over forty years
Woo Dae Kang, Ho Sun Choi, Seok Mo Kim
 Is vaccination with quadrivalent HPV vaccine after Loop Electrosurgical Excision Procedure effective in preventing recurrence in patients with High-grade Cervical Intraepithelial Neoplasia (CIN2-3)? Chonnam
Is vaccination with quadrivalent HPV vaccine after Loop Electrosurgical Excision Procedure effective in preventing recurrence in patients with High-grade Cervical Intraepithelial Neoplasia (CIN2-3)? Chonnam
WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16
 WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 05/01/95 ANY MISSING OR INCOMPLETE TEST RESULTS
WOMEN S INTERAGENCY HIV STUDY LABORATORY - PELVIC EXAM STUDIES TREATMENT FORM FORM L16 ID LABEL HERE ---> - - - VISIT #: FORM COMPLETED BY: VERSION DATE: 05/01/95 ANY MISSING OR INCOMPLETE TEST RESULTS
Human Papilloma Viruses HPV Testing and Treatment of STDs
 Human Papilloma Viruses HPV Testing and Treatment of STDs New York State Collaborative Efforts in Medical Evaluations of Child and Adolescent Sexual Offenses. Child and Adolescent Sexual Offense Post-Assault
Human Papilloma Viruses HPV Testing and Treatment of STDs New York State Collaborative Efforts in Medical Evaluations of Child and Adolescent Sexual Offenses. Child and Adolescent Sexual Offense Post-Assault
immunocytochemical and histologic study of epithelium adjacent to anogenital warts in patients
 Department of Genitourinary Medicine, Royal Victoria Hospital, Belfast BT 6BA J M Handley R D Maw T Homer H Lawther W W Dinsmore Department of Pathology, Royal Victoria Hospital, Belfast M Walsh Accepted
Department of Genitourinary Medicine, Royal Victoria Hospital, Belfast BT 6BA J M Handley R D Maw T Homer H Lawther W W Dinsmore Department of Pathology, Royal Victoria Hospital, Belfast M Walsh Accepted
The society for lower genital tract disorders since 1964.
 The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
Human Papillomaviruses and Cancer: Questions and Answers. Key Points. 1. What are human papillomaviruses, and how are they transmitted?
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Human Papillomaviruses
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$
 !"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
Human Papillomavirus. Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita
 Human Papillomavirus Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita What is Genital HPV Infection Human papillomavirus is
Human Papillomavirus Kathryn Thiessen, ARNP, ACRN The Kansas AIDS Education and Training Center The University of Kansas School of Medicine Wichita What is Genital HPV Infection Human papillomavirus is
The Management of Minor Degrees of Cervical Dysplasia Associated with the Human Papilloma Virus
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 64 (1991), 591-597 The Management of Minor Degrees of Cervical Dysplasia Associated with the Human Papilloma Virus JOHN A. CARMICHAEL, M.D., C.M., F.R.C.S.C. Division
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 64 (1991), 591-597 The Management of Minor Degrees of Cervical Dysplasia Associated with the Human Papilloma Virus JOHN A. CARMICHAEL, M.D., C.M., F.R.C.S.C. Division
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Scottish Medicines Consortium
 Scottish Medicines Consortium romiplostim, 250 microgram vial of powder for solution for subcutaneous injection (Nplate ) No. (553/09) Amgen 08 May 2009 (Issued 4 September 2009) The Scottish Medicines
Scottish Medicines Consortium romiplostim, 250 microgram vial of powder for solution for subcutaneous injection (Nplate ) No. (553/09) Amgen 08 May 2009 (Issued 4 September 2009) The Scottish Medicines
Clinically Microscopically Pathogenesis: autoimmune not lifetime
 Vulvar Diseases: Can be divided to non-neoplastic and neoplastic diseases. The neoplastic diseases are much less common. Of those, squamous cell carcinoma is the most common. most common in postmenopausal
Vulvar Diseases: Can be divided to non-neoplastic and neoplastic diseases. The neoplastic diseases are much less common. Of those, squamous cell carcinoma is the most common. most common in postmenopausal
Cancer Research Group Version Date: November 5, 2015 NCI Update Date: January 15, Schema. L O Step 1 1,2
 Cancer esearch roup ev. 6/14, 2/15, 1/16 Step 2 Schema 5 Arm A: (7 weeks) Step 1 1,2 N Accrual: 515 S Arm S ransoral esection dissections S A N D M Z 4 ntermediate isk 7 Stratify: = 10 pk-yr vs. > 10 pk-yr
Cancer esearch roup ev. 6/14, 2/15, 1/16 Step 2 Schema 5 Arm A: (7 weeks) Step 1 1,2 N Accrual: 515 S Arm S ransoral esection dissections S A N D M Z 4 ntermediate isk 7 Stratify: = 10 pk-yr vs. > 10 pk-yr
Safety profile of Liraglutide: Recent Updates. Mohammadreza Rostamzadeh,M.D.
 Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
GSK Medication: Study No.: Title: Rationale: Phase: Study Period Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Drug Class Monograph
 Drug Class Monograph Class: Chronic Hepatitis B Drug: Baraclude (entecavir), Epivir (lamivudine), Hepsera (adefovir), Intron A (interferon alfa- 2b), Pegasys (peginterferon alfa-2a), Tyzeka (telbivudine),
Drug Class Monograph Class: Chronic Hepatitis B Drug: Baraclude (entecavir), Epivir (lamivudine), Hepsera (adefovir), Intron A (interferon alfa- 2b), Pegasys (peginterferon alfa-2a), Tyzeka (telbivudine),
F.C. Shakhtatinskaya, L.S. Namazova-Baranova, V.K. Tatochenko, D.A. Novikova, T.E. Tkachenko
 F.C. Shakhtatinskaya, L.S. Namazova-Baranova, V.K. Tatochenko, D.A. Novikova, T.E. Tkachenko Scientific Center of Children s Health, Moscow, Russian Federation Human Papilloma Virus. Prevention of HPV-Associated
F.C. Shakhtatinskaya, L.S. Namazova-Baranova, V.K. Tatochenko, D.A. Novikova, T.E. Tkachenko Scientific Center of Children s Health, Moscow, Russian Federation Human Papilloma Virus. Prevention of HPV-Associated
ANAL CONDYLOMA. 1. What are anal condylomata?
 ANAL CONDYLOMA 1. What are anal condylomata? Anal condylomata (or condylomata acuminata) is a very uncomfortable and relatively frequent problem that is normally localized in the perianal area, although
ANAL CONDYLOMA 1. What are anal condylomata? Anal condylomata (or condylomata acuminata) is a very uncomfortable and relatively frequent problem that is normally localized in the perianal area, although
The promise of HPV vaccines for Cervical (and other genital cancer) prevention
 The promise of HPV vaccines for Cervical (and other genital cancer) prevention CONTROVERSIES IN OBSTETRICS GYNECOLOGY & INFERTILITY Barcelona March 27 F. Xavier Bosch Catalan Institute of Oncology CURRENT
The promise of HPV vaccines for Cervical (and other genital cancer) prevention CONTROVERSIES IN OBSTETRICS GYNECOLOGY & INFERTILITY Barcelona March 27 F. Xavier Bosch Catalan Institute of Oncology CURRENT
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
Estimated New Cancers Cases 2003
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz Estimated New Cancers Cases 2003 Images removed due to copyright reasons.
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz Estimated New Cancers Cases 2003 Images removed due to copyright reasons.
TRANSPARENCY COMMITTEE Opinion 17 September 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
Can erythropoietin treatment during antiviral drug treatment for hepatitis C be cost effective?
 Below Are Selected Good Abstracts from Digestive Disease Week 2006 Meeting Published in Gut. 2006 April; 55(Suppl 2): A1 A119. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1859999/?tool=pmcentrez Can erythropoietin
Below Are Selected Good Abstracts from Digestive Disease Week 2006 Meeting Published in Gut. 2006 April; 55(Suppl 2): A1 A119. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1859999/?tool=pmcentrez Can erythropoietin
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two
 Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
The HPV Immunisation Programme in NZ. Chris Millar Senior Advisor Immunisation Ministry of Health
 The HPV Immunisation Programme in NZ Chris Millar Senior Advisor Immunisation Ministry of Health chris_millar@moh.govt.nz 4 September 2015 Background of NZ s HPV Immunisation Programme Aim: To protect
The HPV Immunisation Programme in NZ Chris Millar Senior Advisor Immunisation Ministry of Health chris_millar@moh.govt.nz 4 September 2015 Background of NZ s HPV Immunisation Programme Aim: To protect
Hepatitis B Prior Authorization Policy
 Hepatitis B Prior Authorization Policy Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review of medical literature,
Hepatitis B Prior Authorization Policy Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review of medical literature,
Sexually transmitted infections (in women)
 Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
Sexually transmitted infections (in women) Timothy Kremer, MD Assistant Professor, Department of Obstetrics and Gynecology University of North Texas Health Science Center Last official CDC guidelines:
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary
 Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
The HPV Vaccine: A Sheep in Wolf's Clothing
 The HPV Vaccine: A sheep in wolf s clothing Jessica Francis, MD Medical College of Wisconsin Nothing to disclose Disclosures Objectives To review the effects of HPV To review recommendations regarding
The HPV Vaccine: A sheep in wolf s clothing Jessica Francis, MD Medical College of Wisconsin Nothing to disclose Disclosures Objectives To review the effects of HPV To review recommendations regarding
Leukocyte Ultrafiltrate
 Infectious Diseases in Obstetrics and Gynecology 8:120-123 (2000) (C) 2000 Wiley-Liss, Inc. Immunotherapy of Gynaecological High-Risk Human Papilloma Virus Infection With Human Leukocyte Ultrafiltrate
Infectious Diseases in Obstetrics and Gynecology 8:120-123 (2000) (C) 2000 Wiley-Liss, Inc. Immunotherapy of Gynaecological High-Risk Human Papilloma Virus Infection With Human Leukocyte Ultrafiltrate
HUMAN PAPILLOMAVIRUS. About Human papillomavirus
 HUMAN PAPILLOMAVIRUS Hpv A common virus that affects both males and females that could result in genital warts or cancer. Diagnosis Male & Female About Human papillomavirus Human papillomavirus, or HPV,
HUMAN PAPILLOMAVIRUS Hpv A common virus that affects both males and females that could result in genital warts or cancer. Diagnosis Male & Female About Human papillomavirus Human papillomavirus, or HPV,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e
 Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 62: Caring for Clients With Sexually Transmitted Diseases Slide 1 Epidemiology Introduction Study of the occurrence, distribution, and causes
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 62: Caring for Clients With Sexually Transmitted Diseases Slide 1 Epidemiology Introduction Study of the occurrence, distribution, and causes
HPV-Associated Cancers
 HPV-Associated Cancers HPV Vaccination Summit: Debunking Myths and Preventing Cancer Thursday, February 2, 2017 St. Luke s Anderson Center Chris Johnson, MPH Cancer Data Registry of Idaho Outline Human
HPV-Associated Cancers HPV Vaccination Summit: Debunking Myths and Preventing Cancer Thursday, February 2, 2017 St. Luke s Anderson Center Chris Johnson, MPH Cancer Data Registry of Idaho Outline Human
Understanding Your Pap Test Results
 Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
The no t-so-simple cutaneous wart
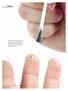 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
AWMSG SECRETARIAT ASSESSMENT REPORT. Green tea leaf extract (Catephen ) 10% Ointment. Reference number: 2739 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
Human papillomavirus: Beware the infection you can t see
 THEME: STIs Human papillomavirus: Beware the infection you can t see Stella Heley BACKGROUND Genital human papillomavirus (HPV) is a common sexually transmitted infection. In the first 10 years of sexual
THEME: STIs Human papillomavirus: Beware the infection you can t see Stella Heley BACKGROUND Genital human papillomavirus (HPV) is a common sexually transmitted infection. In the first 10 years of sexual
DISCLOSURES. None of the planners or presenters of this session have disclosed any conflict or commercial interest
 Latest HPV Science Holly B. Fontenot, PhD, RN, WHNP-BC Assistant Professor, Boston College Director, WHNP Program Adjunct Faculty, The Fenway Institute NP, Fenway Health/Sidney Borum Health Center DISCLOSURES
Latest HPV Science Holly B. Fontenot, PhD, RN, WHNP-BC Assistant Professor, Boston College Director, WHNP Program Adjunct Faculty, The Fenway Institute NP, Fenway Health/Sidney Borum Health Center DISCLOSURES
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Penile and scrotal condyloma acuminatum in a three-year-old boy: A rare case report
 PEDIATRIC UROLOGY CASE REPORTS DOI: 10.14534/PUCR.2016418816 DOI: Open Access Penile and scrotal condyloma acuminatum in a three-year-old boy: A rare case report Hulya Ozturk 1, Gulzade Ozyalvacli 2, Mervan
PEDIATRIC UROLOGY CASE REPORTS DOI: 10.14534/PUCR.2016418816 DOI: Open Access Penile and scrotal condyloma acuminatum in a three-year-old boy: A rare case report Hulya Ozturk 1, Gulzade Ozyalvacli 2, Mervan
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Per the study design and a limitation of this analysis, swabs were not tested for HPV types 6
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 SUPPLEMENTARY MATERIAL Progression, regression, and clearance of HPV6/11 infections Per the study design and a limitation of this analysis, swabs
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 SUPPLEMENTARY MATERIAL Progression, regression, and clearance of HPV6/11 infections Per the study design and a limitation of this analysis, swabs
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
Should Anal Pap Smears Be a Standard of Care in HIV Management?
 Should Anal Pap Smears Be a Standard of Care in HIV Management? Gordon Dickinson, M.D., FACP Professor of Medicine and Chief Infectious Diseases, Miller School of Medicine Short Answer: NO But 15-20 HPV
Should Anal Pap Smears Be a Standard of Care in HIV Management? Gordon Dickinson, M.D., FACP Professor of Medicine and Chief Infectious Diseases, Miller School of Medicine Short Answer: NO But 15-20 HPV
Scottish Medicines Consortium
 Scottish Medicines Consortium eltrombopag, 25mg and 50mg film-coated tablets (Revolade ) No. (625/10) GlaxoSmithKline UK 09 July 2010 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium eltrombopag, 25mg and 50mg film-coated tablets (Revolade ) No. (625/10) GlaxoSmithKline UK 09 July 2010 The Scottish Medicines Consortium (SMC) has completed its assessment
Clinical Pathological Conference. Malignant Melanoma of the Vulva
 Clinical Pathological Conference Malignant Melanoma of the Vulva History F/48 Chinese Married Para 1 Presented in September 2004 Vulval mass for 2 months Associated with watery and blood stained discharge
Clinical Pathological Conference Malignant Melanoma of the Vulva History F/48 Chinese Married Para 1 Presented in September 2004 Vulval mass for 2 months Associated with watery and blood stained discharge
Efficacy of Levetiracetam: A Review of Three Pivotal Clinical Trials
 Epilepsia, 42(Suppl. 4):31 35, 2001 Blackwell Science, Inc. International League Against Epilepsy Efficacy of : A Review of Three Pivotal Clinical Trials Michael Privitera University of Cincinnati Medical
Epilepsia, 42(Suppl. 4):31 35, 2001 Blackwell Science, Inc. International League Against Epilepsy Efficacy of : A Review of Three Pivotal Clinical Trials Michael Privitera University of Cincinnati Medical
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Silgard, suspension for injection. Silgard, suspension for injection in a pre-filled syringe. Human Papillomavirus Vaccine
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Silgard, suspension for injection. Silgard, suspension for injection in a pre-filled syringe. Human Papillomavirus Vaccine
Anogenital Warts. Questions & Answers
 Anogenital Warts Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital switchboard - 0141 810 3151 Ph.
Anogenital Warts Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital switchboard - 0141 810 3151 Ph.
HPV & RELATED DISEASES
 GAY MEN, HPV & ANAL CANCER THEBOTTOMLINE.ORG.AU HPV & RELATED DISEASES WHAT IS HPV? The Human Papilloma Virus (HPV) is not one virus, but a family of about 200 different ones that cause common warts, genital
GAY MEN, HPV & ANAL CANCER THEBOTTOMLINE.ORG.AU HPV & RELATED DISEASES WHAT IS HPV? The Human Papilloma Virus (HPV) is not one virus, but a family of about 200 different ones that cause common warts, genital
The HPV Vaccination Programme Early intervention in cancer prevention Northern Ireland
 The HPV Vaccination Programme Early intervention in cancer prevention Northern Ireland Immunisations Very cost effective intervention Give vaccine before exposure to disease UK has life course approach
The HPV Vaccination Programme Early intervention in cancer prevention Northern Ireland Immunisations Very cost effective intervention Give vaccine before exposure to disease UK has life course approach
VIN/VAIN O C T O B E R 3 RD J M O R G A N
 VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
VIN/VAIN O C T O B E R 3 RD 2 0 1 8 J M O R G A N Vaginal Intraepithelial Neoplasia VAIN I, II, III Incidence 0.1/100,000 women in US Mean age 50s (J Womens Health (Larchmt) 2009:18:1731) (J Obstet Gynaecol
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Cytyc Corporation - Case Presentation Archive - June 2003
 ThinPrep General Cytology History: Asymptomatic 35 Year Old Male Specimen type: Anal Cytology - This specimen was collected using a Dacron swab under proctoscopic visualization. This case was provided
ThinPrep General Cytology History: Asymptomatic 35 Year Old Male Specimen type: Anal Cytology - This specimen was collected using a Dacron swab under proctoscopic visualization. This case was provided
Treatment of Cervical Intraepithelial Neoplasia. Case. How would you manage this woman?
 Treatment of Cervical Intraepithelial Neoplasia Karen Smith-McCune Professor, Department of Obstetrics, Gynecology and Reproductive Sciences I have no conflicts of interest Case How would you manage this
Treatment of Cervical Intraepithelial Neoplasia Karen Smith-McCune Professor, Department of Obstetrics, Gynecology and Reproductive Sciences I have no conflicts of interest Case How would you manage this
Risk for anogenital cancer and other cancer among women hospitalized with gonorrhea
 Acta Obstet Gynecol Scand 2001; 80: 757 761 Copyright C Acta Obstet Gynecol Scand 2001 Printed in Denmark All rights reserved Acta Obstetricia et Gynecologica Scandinavica ISSN 0001-6349 ORIGINAL ARTICLE
Acta Obstet Gynecol Scand 2001; 80: 757 761 Copyright C Acta Obstet Gynecol Scand 2001 Printed in Denmark All rights reserved Acta Obstetricia et Gynecologica Scandinavica ISSN 0001-6349 ORIGINAL ARTICLE
Can HPV, cervical neoplasia or. HIV transmission?
 Interactions between HPV and HIV: STIs and HIV shedding, regulation of HPV by HIV, and HPV VLP influence upon HIV Jennifer S. Smith Department of Epidemiology pd University of North Carolina Can HPV, cervical
Interactions between HPV and HIV: STIs and HIV shedding, regulation of HPV by HIV, and HPV VLP influence upon HIV Jennifer S. Smith Department of Epidemiology pd University of North Carolina Can HPV, cervical
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
HUMAN PAPILLOMAVIRUS VACCINES AND CERVICAL CANCER
 HUMAN PAPILLOMAVIRUS VACCINES AND CERVICAL CANCER Virology The Human Papillomavirus (HPV) is a relatively small virus, belonging to the family Papillomaviridae, containing circular double-stranded DNA
HUMAN PAPILLOMAVIRUS VACCINES AND CERVICAL CANCER Virology The Human Papillomavirus (HPV) is a relatively small virus, belonging to the family Papillomaviridae, containing circular double-stranded DNA
UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA. Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA
 UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA DISCLAIMER Please note: The views expressed within this presentation
UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA DISCLAIMER Please note: The views expressed within this presentation
Intron A. Intron A (interferon alfa-2b) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Intron A Page: 1 of 6 Last Review Date: June 19, 2015 Intron A Description Intron A (interferon
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Intron A Page: 1 of 6 Last Review Date: June 19, 2015 Intron A Description Intron A (interferon
PRODUCT CIRCULAR. GARDASIL [Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine]
![PRODUCT CIRCULAR. GARDASIL [Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine] PRODUCT CIRCULAR. GARDASIL [Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine]](/thumbs/95/122763989.jpg) PRODUCT CIRCULAR [Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine] I. THERAPEUTIC CLASS GARDASIL is a recombinant, quadrivalent vaccine that protects against Human Papillomavirus
PRODUCT CIRCULAR [Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine] I. THERAPEUTIC CLASS GARDASIL is a recombinant, quadrivalent vaccine that protects against Human Papillomavirus
2. SYNOPSIS Name of Sponsor/Company:
 in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
Additional file 2: Details of cohort studies and randomised trials
 Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
I have no financial interests in any product I will discuss today.
 Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Prevalence Of Precancerous Lesions Of The Uterine Cervix
 Prevalence Of Precancerous Lesions Of The Uterine Cervix 1 / 6 2 / 6 3 / 6 Prevalence Of Precancerous Lesions Of INTRODUCTION. Gastric intestinal metaplasia is an intermediate precancerous gastric lesion
Prevalence Of Precancerous Lesions Of The Uterine Cervix 1 / 6 2 / 6 3 / 6 Prevalence Of Precancerous Lesions Of INTRODUCTION. Gastric intestinal metaplasia is an intermediate precancerous gastric lesion
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
What is the Safety and Efficacy of Vaccinating the Male Gender to Prevent HPV Related Neoplastic Disorders in Both the Male and Female Genders
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 What is the Safety and Efficacy of Vaccinating
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 What is the Safety and Efficacy of Vaccinating
GSK Medicine Study Number: Title: Rationale Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and nonapproved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and nonapproved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
