NEO PEDICLE SCREW SYSTEM V. 2.0
|
|
|
- Patrick Bradford
- 5 years ago
- Views:
Transcription
1 NEO PEDICLE SCREW SYSTEM V. 2.0 Neo Medical S.A. Route de Lausanne 157A 1096 Villette Switzerland 1250 Important Information on the NEO PEDICLE SCREW SYSTEM CAUTION: USA law restricts this device to sale by or on the order of a licensed healthcare practitioner. Carefully read all instructions and be familiar with the surgical technique(s) prior to using this product. PURPOSE The NEO Pedicle Screw System is intended to help provide immobilization, correction and stabilization of spinal segments as an adjunct to fusion of the thoracic, lumbar, and/or sacral spine. DESCRIPTION The NEO Pedicle Screw System comprises a variety of sizes of rods, screws, as well as instruments. They are all delivered sterile and ready to use. They can be locked together into a variety of configurations which are going to be tailor-made for each individual case. The NEO Pedicle Screw System consists of screws which differ in length and diameter and connecting rods which differ in length. The system includes the relevant instruments which are disposable and delivered sterile. All the system components are made of materials compliant with current ISO and/or ASTM standards. Screw and rod are made of titanium-alloy (Ti-6AI-4vELI) and comply with ISO or ASTM-F136. The screws are delivered pre-mounted on a screw extender including a tissue dilator and are sterile. The rods are delivered sterile. The size and form of the devices is adjusted to the morphology of the body and the operation technique. The system includes a removal kit which should be used for revision surgery. The procedure for use is described in the surgical technique. The T-handle option should be used in case an important torque is required to remove the set screw with the solid screw driver. The T- handle is delivered sterile and can immediately be used. The system can be used via an open or minimally invasive posterior approach. Implants and instruments of the NEO Pedicle Screw System are single use device and should never be reused under any circumstances. LIMITED WARRANTY AND DISCLAIMER Neo Medical s products are sold with a limited warranty to the original purchaser against defects in workmanship and materials. Any other express or implied warranties, including warranties of merchantability or fitness, are hereby disclaimed. SINGLE USE / DISPOSABLE MEDICAL DEVICES The implants of the NEO Pedicle Screw System are for single use. The instruments of the NEO Pedicle Screw System are for single use as well and fully disposable. It is forbidden to reuse or to try to re-sterilize any part of the NEO Pedicle Screw System as certain technical characteristics of the system are not compatible with it. The reuse of any part of the system may lead to a risk for the patient. An attempt to reprocess, clean, sterilize and or disinfect the system might lead to infection or toxic reaction. Furthermore it may, negatively impact the performance and characteristics of parts of the system. After the use all instruments need to be decontaminated and disposed according to local laws and regulations regarding infectious waste. INDICATIONS The NEO Pedicle Screw System is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion. The system is intended for posterior, non-cervical fixation for the following indications: degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e., fracture or dislocation), spinal stenosis, tumor, pseudarthrosis, and/or failed previous fusion. The Instruments are to be used for the implantation of the above mentioned medical devices. 1 (6)
2 CONTRAINDICATIONS Contraindications include, but are not limited to: Active infectious process or significant risk of infection (immunocompromise). Signs of local inflammation. Fever or leukocytosis. Morbid obesity. Pregnancy. Mental illness. Grossly distorted anatomy caused by congenital abnormalities. Any other medical or surgical condition which would preclude the potential benefit of spinal implant surgery, such as the presence of congenital abnormalities, elevation of sedimentation rate unexplained by other diseases, elevation of white blood count (WBC), or a marked left shift in the WBC differential count. Suspected or documented metal allergy or intolerance. Any case not needing a bone graft and fusion. Any case where the implant components selected for use would be too large or too small to achieve a successful result. Any patient having inadequate tissue coverage over the operative site or inadequate bone stock or quality. Any patient in which implant utilization would interfere with anatomical structures or expected physiological performance. Pediatric patients or where the patient still has general skeletal growth. Any patient unwilling to follow postoperative instructions. Any case not described in the indications. NOTA BENE: Although not absolute contraindications, conditions to be considered as potential factors for not using this device include: Severe bone resorption. Osteomalacia Severe osteoporosis. POTENTIAL ADVERSE EVENTS All of the possible adverse events associated with spinal fusion surgery without instrumentation are possible. With instrumentation, a listing of potential adverse events includes, but is not limited to: Early or late loosening of any or all of the components. Disassembly, bending, and/or breakage of any or all of the components. Foreign body (allergic) reaction to implants, debris, corrosion products (from crevice, fretting, and/or general corrosion), including metallosis, staining, tumor formation, and/or autoimmune disease. Pressure on the skin from component parts in patients with inadequate tissue coverage over the implant possibly causing skin penetration, irritation, fibrosis, necrosis, and/or pain. Bursitis. Tissue or nerve damage caused by improper positioning and placement of implants or instruments. Post-operative change in spinal curvature, loss of correction, height, and/or reduction. Infection. Dural tears, pseudomeningocele, fistula, persistent CSF leakage, meningitis. Loss of neurological function (e.g., sensory and/or motor), including paralysis (complete or incomplete), dysesthesias, hyperesthesia, anesthesia, paresthesia, appearance of radiculopathy, and/or the development or continuation of pain, numbness, neuroma, spasms, sensory loss, tingling sensation, and/or visual deficits. Cauda equina syndrome, neuropathy, neurological deficits (transient or permanent), paraplegia, paraparesis, reflex deficits, irritation, arachnoiditis, and/or muscle loss. Urinary retention or loss of bladder control or other types of urological system compromise. Scar formation possibly causing neurological compromise or compression around nerves and/or pain. Fracture, microfracture, resorption, damage, or penetration of any spinal bone (including the sacrum, pedicles, and/or vertebral body) and/or bone graft or bone graft harvest site at, above, and/or below the level of surgery. Retropulsed graft. Herniated nucleus pulposus, disc disruption or degeneration at, above, or below the level of surgery. Non-union (or pseudarthrosis), delayed union, and mal-union. Cessation of any potential growth of the operated portion of the spine. Loss of or increase in spinal mobility or function. Inability to perform the activities of daily living. 2 (6)
3 Bone loss or decrease in bone density, possibly caused by stresses shielding. Graft donor site complications including pain, fracture, or wound healing problems. Ileus, gastritis, bowel obstruction, loss of bowel control, or other types of gastrointestinal system compromise. Hemorrhage, hematoma, occlusion, seroma, edema, hypertension, embolism, stroke, excessive bleeding, phlebitis, wound necrosis, wound dehiscence, damage to blood vessels, or other types of cardiovascular system compromise. Reproductive system compromise, including sterility, loss of consortium, and sexual dysfunction. Development of respiratory problems, e.g. pulmonary embolism, atelectasis, bronchitis, pneumonia, etc. Change in mental status. Death. Note: Additional surgery may be necessary to correct some of these potential adverse events. WARNING The safety and effectiveness of pedicle screw spinal systems have been established only for spinal conditions with significant mechanical instability or deformity requiring fusion with instrumentation. These conditions are significant mechanical instability of the thoracic, lumbar, and sacral spine secondary to degenerative spondylolisthesis with objective evidence of neurologic impairment, fracture, dislocation, spinal tumor, and failed previous fusion (pseudarthrosis). The safety and effectiveness of this device for any other conditions are unknown. The implants are not prostheses. In the absence of fusion, the instrumentation and/or one or more of its components can be expected to pull out, bend, or fracture as a result of exposure to every day mechanical stresses. The Neo T-Handle Kit is designed to be used with both the cannulated screw driver in the instrument kit and the solid screw driver in the removal kit of the NEO Pedicle Screw System. Do not use the cannulated screw driver in the pedicle screw instrument kit for revision surgery. Do not use any of the NEO Pedicle Screw System implant components with components from any other system or manufacturer unless specifically allowed to do so in this or another NEO MEDICAL document. PRECAUTIONS The implantation of pedicle screw spinal systems should be performed only by experienced spinal surgeons with specific training in the use of this pedicle screw spinal system because this is a technically demanding procedure presenting a risk of serious injury to the patient. A successful result is not always achieved in every surgical case. This fact is especially true in spinal surgery where many extenuating circumstances may compromise the results. This device system is not intended to be the sole means of spinal support. Use of this product without a bone graft or in cases that develop into a non-union will not be successful. No spinal implant can withstand body loads without the support of bone. In this event, bending, loosening, disassembly, and/or breakage of the device(s) will eventually occur. Preoperative and operating procedures, including knowledge of surgical techniques, good reduction, and proper selection and placement of the implants are important considerations in the successful utilization of the system by the surgeon. Further, the proper selection and compliance of the patient will greatly affect the results. Patients who smoke have been shown to have an increased incidence of nonunions. These patients should be advised of this fact and warned of this consequence. Obese, malnourished, and/or alcohol abuse patients are also poor candidates for spine fusion. Patients with poor muscle and bone quality and/or nerve paralysis are also poor candidates for spine fusion. MANAGING COMPLICATIONS Best practice to avoid complications and situations potentially leading to adverse events are described in the surgical technique. IMPLANT SELECTION The selection of the proper size, shape and design of the implant for each patient is crucial to the success of the procedure. Metallic surgical implants are subject to repeated stresses in use, and their strength is limited by the need to adapt the design to the size and shape of human bones. Unless great care is taken in patient selection, proper placement of the implant, and postoperative management to minimize stresses on the implant, such stresses may cause metal fatigue and consequent breakage, bending, or loosening of the device before the healing process is complete, which may result in further injury or the need to remove the device prematurely. DEVICE FIXATION For both cases where an open surgery approach or a percutaneous posterior approach is used, refer to the NEO Pedicle Screw System surgical technique. NEO Pedicle Screw System instrumentation contains rods and implants of various diameters, which are intended to be used with device specific instruments. For the set-screw driver, always hold the assembly with the Counter Torque Handle. Tighten and use the torque limiting capability of the set-screw driver to leave the assembly at optimum fixation security. When done according the NEO Pedicle Screw System surgical technique, further re-tightening is not necessary and not recommended. AFTER THE SET-SCREW DRIVER TORQUE LIMITING CAPABILITY HAS BEEN USED, RE-ADJUSTMENT IS NOT POSSIBLE UNLESS THE SET-SCREW IS REMOVED AND REPLACED WITH A NEW SET-SCREW AND SET-SCREW DRIVER. 3 (6)
4 PREOPERATIVE Only patients that meet the criteria described in the indications section should be selected. Patient conditions and/or pre dispositions such as those addressed in the aforementioned contraindications should be avoided. Care should be used in the handling and storage of the implant components. The implants should not be scratched or otherwise damaged. Implants and instruments should be protected during storage, especially from corrosive environments. An adequate inventory of implants should be available at the time of surgery, normally a quantity in excess of what is expected to be used. Since mechanical parts are involved, the surgeon should be familiar with the various components before using the equipment and should personally verify that the necessary items are available before the surgery. The NEO Pedicle Screw System components (described in the DESCRIPTION section) are not to be combined with the components from another manufacturer. Additional components should be available in case of an unexpected need. INTRAOPERATIVE Extreme caution should be used around the spinal cord and nerve roots. Damage to the nerves will cause loss of neurological functions. Breakage, slippage, or misuse of instruments or implant components may cause injury to the patient or operative personnel. Use great care to ensure that the implant surfaces are not scratched or notched, since such actions may reduce the functional strength of the construct. Utilize an imaging system to facilitate surgery. To insert a screw properly, a guide wire should first be used, followed by a sharp tap or self-tapping screw. Caution: Be careful that the guide-wire, if used, is not inserted too deep, becomes bent, and/or breaks. Ensure that the guide-wire does not advance during tapping or screw insertion. Remove the guide-wire and make sure it is intact. Failure to do so may cause the guide wire or part of it to advance through the bone and into a location that may cause damage to underlying structures. Caution: Do not overtap or use a screw that is either too long or too large. Overtapping, using an incorrectly sized screw, or accidentally advancing the guidewire during tap or screw insertion, may cause nerve damage, hemorrhage, or the other possible adverse events listed elsewhere in this package insert. If screws are being inserted into spinal pedicles, use as large a screw diameter as will fit into each pedicle. Caution: The safety and effectiveness of this device has not been established when used in conjunction with bone cement or for use in patients with poor bone quality (e.g., osteoporosis, osteopenia). This device is intended only to be used with saline or radiopaque dye. Bone graft must be placed in the area to be fused and graft material must extend from the upper to the lower vertebrae being fused. Before closing, all the set screws must be tightened according to the NEO Pedicle Screw System surgical technique. POSTOPERATIVE The physician s postoperative directions and warnings to the patient, and the corresponding patient compliance, are extremely important. Detailed instructions on the use and limitations of the device should be given to the patient. If partial weight-bearing is recommended or required prior to firm bony union, the patient must be warned that bending, loosening and/or breakage of the device(s) are complications which may occur as a result of excessive or early weight-bearing or muscular activity. The risk of bending, loosening, or breakage of a temporary internal fixation device during postoperative rehabilitation may be increased if the patient is active, or if the patient is debilitated or demented. The patient should be warned to avoid falls or sudden jolts in spinal position. To allow the maximum chances for a successful surgical result, the patient or devices should not be exposed to mechanical vibrations or shock that may loosen the device construct. The patient should be warned of this possibility and instructed to limit and restrict physical activities, especially lifting and twisting motions and any type of sport participation. The patient should be advised not to smoke tobacco, utilize nicotine products, or to consume alcohol or non-steroidals or anti-inflammatory medications such as aspirin during the bone graft healing process. The patient should be advised of their inability to bend or rotate at the point of spinal fusion and taught to compensate for this permanent physical restriction in body motion. Failure to immobilize a delayed or non-union of bone will result in excessive and repeated stresses on the implant. By the mechanism of fatigue, these stresses can cause the eventual bending, loosening, or breakage of the device(s). It is important that immobilization of the spinal surgical site be maintained until firm bony union is established and confirmed by roentgenographic examination. If a state of nonunion persists or if the components loosen, bend, and/or break, the device(s) should be revised and/or removed immediately before serious injury occurs. The patient must be adequately warned of these hazards and closely supervised to ensure cooperation until bony union is confirmed. As a precaution, before patients with implants receive any subsequent surgery (such as dental procedures), prophylactic antibiotics may be considered, especially for high-risk patients. The NEO Pedicle Screw System implants are temporary internal fixation devices. Internal fixation devices are designed to stabilize the operative site during the normal healing process. After the spine is fused, these devices serve no functional purpose and may be removed. While the final decision on implant removal is, of course, up to the surgeon and patient, in most patients removal is indicated because the implants are not intended to transfer or support forces developed during normal activities. If the device is not removed following completion of its intended use, one or more of the following complications may occur: (1) Corrosion, with localized tissue reaction or pain; (2) Migration of implant position, possibly resulting in injury; (3) Risk of additional injury from postoperative trauma; (4) Bending, loosening and breakage, which could make removal impractical or difficult; (5) Pain, discomfort, or abnormal sensations due to the presence of the device; (6) Possible increased risk of infection; (7) Bone loss due to stress shielding; and (8) Potential unknown and/or 4 (6)
5 unexpected long term effects such as carcinogenesis. Implant removal should be followed by adequate postoperative management to avoid fracture, re-fracture, or other complications. Any retrieved devices should be treated in such a manner that reuse in another surgical procedure is not possible. As with all orthopedic implants, the NEO Pedicle Screw System components must not be reused under any circumstances. Non-clinical testing and MRI simulations were performed to evaluate the entire family of the Neo Pedicle Screw System. Non-clinical testing demonstrated that the entire family of the Neo Pedicle Screw System is MR Conditional. A patient with a device from this family can be scanned safely in an MR system under the following conditions: - Static magnetic field of 1.5-Tesla and 3-Tesla, only - Maximum spatial gradient magnetic field of 4,000-gauss/cm (40-T/m)(extrapolated) or less - Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2-W/kg for 15 minutes of scanning (i.e., per pulse sequence) in the Normal Operating Mode of operation for the MR system - Under the scan conditions defined, the Neo Pedicle Screw System is expected to produce a maximum temperature rise of 3.2 C after 15-minutes of continuous scanning (i.e., per pulse sequence). Artifact Information In non-clinical testing, the image artifact caused by the Neo Pedicle Screw System extends approximately 10-mm from this device when imaged using a gradient echo pulse sequence and a 3-Tesla MR system. REVISION The removal kit should be used for revision surgery. The procedure for use is described in the surgical technique Do not use the cannulated screw driver in the pedicle screw instrument kit for revision surgery. PACKAGING All components of the NEO Pedicle Screw System are supplied sterile and ready to use, the contents are sterile unless the package is damaged, opened, or the expiration date on the device label has passed. Caution: Packages for each of the components should be intact upon receipt. All boxes should be carefully checked to ensure that there is no damage prior to use. Damaged packages or products should not be used, and should be returned to the local distributor or to NEO MEDICAL S.A. Caution: Before use the product expiration date must always be checked and not used if expired. PRODUCT COMPLAINTS Any health care professional (e.g., customer or user of this system of products) who has any complaints or who has experienced any dissatisfaction in the product quality, identity, durability, reliability, safety, effectiveness and/or performance, should notify the official distributor of NEO MEDICALS.A.). Further, if any of the implanted spinal system component(s) ever malfunctions (i.e., does not meet any of its performance specifications or otherwise does not perform as intended), or is suspected of doing so, the distributor should be notified immediately. If any NEO MEDICAL S.A. product ever malfunctions and may have caused or contributed to the death or serious injury of a patient, the distributor should be notified immediately by telephone, FAX, or written correspondence. When filing a complaint, please provide the component(s) name and number, lot number(s), your name and address, the nature of the complaint, and notification of whether a written report from the distributor is requested. FURTHER INFORMATION Recommended directions for use of this system (surgical operative techniques) are available at no charge upon request. If further information is needed or required, please contact NEO MEDICAL S.A. EXPLANATION OF SYMBOLS 5 (6)
6 6 (6)
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
A U X I L I A R Y C O N N E C T O R S Surgical Technique
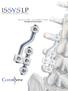 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Doc. No.: IU/3 for. Rev. No.: 07 Adler Spine Implants
 0434 Page 1 of 6 Instructions use For use by an Accredited Orthopaedic Surgeon only Purpose Adler spine implants that include the Zeta Spine System, the OneLock Spine System, the Titan Interbody Spacer
0434 Page 1 of 6 Instructions use For use by an Accredited Orthopaedic Surgeon only Purpose Adler spine implants that include the Zeta Spine System, the OneLock Spine System, the Titan Interbody Spacer
PITBULL. Interspinous Process Decompressiong Device
 PITBULL Interspinous Process Decompressiong Device PITBULL R Interspinous Process Fixation Device indication for use The PITBULL system is intended to be used to help provide immobilization and stabilization
PITBULL Interspinous Process Decompressiong Device PITBULL R Interspinous Process Fixation Device indication for use The PITBULL system is intended to be used to help provide immobilization and stabilization
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
3 Operative Technique U.S. EDITION
 3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
3 A N T E R I O R C E R V I C A L P L AT E S Y S T E M 3 Operative Technique U.S. EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 12 INSTRUCTIONS FOR USE 16 PART NUMBERS Orthofix Spinal
Alamo T Transforaminal Lumbar Interbody System Surgical Technique
 Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Transforaminal Lumbar Interbody System Surgical Technique Table of Contents Indications and Device Description.............. 1 Alamo T Implant Features and Instruments...........2 Surgical Technique......................
Alamo C. Cervical Interbody System Surgical Technique. An Alliance Partners Company
 Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
Cervical Interbody System Surgical Technique Table of Contents Indications for Use................................1 Device Description............................... 1 Alamo C Instruments..............................
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Tempus Anterior Cervical Plate System
 Anterior Cervical Plate System Table of contents Introduction.... 3 Preoperative planning... 6 Patient positioning and exposure... 6 Implant selection and preparation.... 7 Screw hole preparation.... 10
Anterior Cervical Plate System Table of contents Introduction.... 3 Preoperative planning... 6 Patient positioning and exposure... 6 Implant selection and preparation.... 7 Screw hole preparation.... 10
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Zimmer Facet Screw System Surgical Technique
 Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Zimmer Facet Screw System Surgical Technique 2 Zimmer Facet Screw System Surgical Technique Zimmer Facet Screw System Surgical Technique Description, Indications & Contraindications...3 Surgical Technique...4
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
QIN4432TRICREV01. IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage. Version 1 1 STERILE PRODUCT
 IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
IMPORTANT PRODUCT INFORMATION FOR TRITANIUM C Cervical Cage STERILE PRODUCT DESCRIPTION The Stryker Spine Tritanium C Anterior Cervical Cage is a hollow, ring shaped titanium alloy (Ti-6Al- 4V) interbody
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
VTI INTERLINK PEDICLE SCREW SYSTEM
 VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
VTI INTERLINK PEDICLE SCREW SYSTEM SURGICAL TECHNIQUE FORWARD THINKING FOR THE BACK. DEVICE DESCRIPTION The VTI InterLink Pedicle Screw System is comprised of polyaxial pedicle screws in various diameters
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Y o u r Id e a s En g i n e e r e d t o Li f e
 ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
ISSYS LP Spinal Fixation System Surgical Guide Y o u r Id e a s En g i n e e r e d t o Li f e In t r o d u c t i o n ISSYS LP Sp i n a l Fixation System The foundation of the ISSYS LP Spinal Fixation System
Hinged Laminoplasty System surgical technique
 BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
POSTERIOR CERVICAL FUSION
 AN INTRODUCTION TO PCF POSTERIOR CERVICAL FUSION This booklet provides general information on the Posterior Cervical Fusion (PCF) surgical procedure for you to discuss with your physician. It is not meant
AN INTRODUCTION TO PCF POSTERIOR CERVICAL FUSION This booklet provides general information on the Posterior Cervical Fusion (PCF) surgical procedure for you to discuss with your physician. It is not meant
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
HydraLok. Operative Technique. Polyaxial Pedicle Screw System
 HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
HydraLok Operative Technique Polyaxial Pedicle Screw System Table of Contents Introduction...1 OPERATIVE TECHNIQUE OVERVIEW...2 DETAILED OPERATIVE TECHNIQUE...4 LOCATE AND PREPARE THE PEDICLE...4 PROBE
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL
 ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Minimally Invasive Spinal System AVATAR SURGICAL TECHNIQUE GUIDE. Designs For Life. Designs For Life.
 Designs For Life. Minimally Invasive Spinal System SURGICAL TECHNIQUE GUIDE Designs For Life. Minimally Invasive Spinal System CONTENTS Introduction 1 1 Implants 3 Instruments 4 Surgical Technique 7 Product
Designs For Life. Minimally Invasive Spinal System SURGICAL TECHNIQUE GUIDE Designs For Life. Minimally Invasive Spinal System CONTENTS Introduction 1 1 Implants 3 Instruments 4 Surgical Technique 7 Product
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
ORION Anterior Cervical Plate System
 ORION Anterior Cervical Plate System Surgical Technique as described by: Gary L. Lowery, M.D., Ph.D. ORION Anterior Cervical Plate System Surgical Technique As described by Gary L. Lowery, MD, PhD Preliminary
ORION Anterior Cervical Plate System Surgical Technique as described by: Gary L. Lowery, M.D., Ph.D. ORION Anterior Cervical Plate System Surgical Technique As described by Gary L. Lowery, MD, PhD Preliminary
X-spine Systems, Inc. X90 Pedicle Screw System
 X-spine Systems, Inc. X90 Pedicle Screw System GENERAL INFORMATION The X90 Pedicle Screw System consists of rods, pedicle screws, cross bar connectors and hand instruments. Various forms and sizes of these
X-spine Systems, Inc. X90 Pedicle Screw System GENERAL INFORMATION The X90 Pedicle Screw System consists of rods, pedicle screws, cross bar connectors and hand instruments. Various forms and sizes of these
PATIENT: DOB: TODAY S DATE:
 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand this material. I also understand that if certain sections are not
1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand this material. I also understand that if certain sections are not
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
Surgical Technique. Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion
 Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
Surgical Technique Apache Posterior Lumbar Interbody Fusion Apache Transforaminal Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 5 Disc Exposure 5 Disc and
ISSYS LP Spinal Fixation Systems Package Insert
 ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
ISSYS LP Spinal Fixation Systems Package Insert SPINAL ELEMENTS ISSYS LP Spinal Fixation System ISSYS LP Polyaxial Pedicle Screw System - ISSYS LP Monoaxial Screw System CAUTION: Federal law (U.S.A.) restricts
 Visit our website on www.biotech-medical.com The DLP - Dorso-Lumbar Polyaxial Screw System has been designed to address the pathologies of the thoracolumbar spine. The DLP System contains a wide range
Visit our website on www.biotech-medical.com The DLP - Dorso-Lumbar Polyaxial Screw System has been designed to address the pathologies of the thoracolumbar spine. The DLP System contains a wide range
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System consists of rods (straight and curved), lock screws, and
TiLock 2 Spinal System. Surgical Technique
 TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
TiLock 2 Spinal System Surgical Technique Table of Contents Page Preoperative Planning 4 Pedicle Preparation 5 Probe 5 Tap Pedicle 6 Screw Options 7 Screw Insertion 8 Aligning the Windows 9 Rod Insertion
Thunderbolt. surgical technique. MIS Pedicle Screw System. Where Nimble and Secure Intersect
 Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
Thunderbolt TM MIS Pedicle Screw System Where Nimble and Secure Intersect surgical technique i www.choicespine.com System Features Dovetail set screw: Minimizes head splay and cross-threading Secure connection
ACDF. Anterior Cervical Discectomy and Fusion. An introduction to
 An introduction to ACDF Anterior Cervical Discectomy and Fusion This booklet provides general information on ACDF. It is not meant to replace any personal conversations that you might wish to have with
An introduction to ACDF Anterior Cervical Discectomy and Fusion This booklet provides general information on ACDF. It is not meant to replace any personal conversations that you might wish to have with
VIPER PRIME System Cadaver Time Study
 VIPER PRIME System Cadaver Time Study White Paper August 24, 2017 1. INTRODUCTION Minimally invasive surgical techniques to perform spinal stabilization have gained popularity in recent years due to the
VIPER PRIME System Cadaver Time Study White Paper August 24, 2017 1. INTRODUCTION Minimally invasive surgical techniques to perform spinal stabilization have gained popularity in recent years due to the
TiLock XT Minimally Invasive Surgery (MIS) Pedicle Screw System
 Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
Minimally Invasive Surgery (MIS) Pedicle Screw System Surgical Technique Guide 2 Minimally Invasive Surgery (MIS) Pedicle Screw System The Genesys Spine Minimally Invasive Surgery (MIS) Pedicle Screw System
Ascent. Surgical Technique. Breakthrough Thinking. Posterior Occipital Cervico-Thoracic (POCT) System. Germany U.S.A.
 Fusion Motion Preservation Biologics Surgical Technique Breakthrough Thinking Fusion I Motion Preservation I Biologics Posterior Occipital Cervico-Thoracic (POCT) System U.S.A. Corporate Headquarters Blackstone
Fusion Motion Preservation Biologics Surgical Technique Breakthrough Thinking Fusion I Motion Preservation I Biologics Posterior Occipital Cervico-Thoracic (POCT) System U.S.A. Corporate Headquarters Blackstone
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
SECURIS SPINAL Fixation System Package Insert
 SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
SECURIS SPINAL Fixation System Package Insert AMENDIA SECURIS SPINAL Fixation System CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. NON STERILE
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
ProDisc-L Total Disc Replacement. IDE Clinical Study.
 ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
Patient Information. ADULT SCOLIOSIS Information About Adult Scoliosis, Symptoms, and Treatment Options
 Patient Information ADULT SCOLIOSIS Information About Adult Scoliosis, Symptoms, and Treatment Options Table of Contents Anatomy of the Spine...2 What is Adult Scoliosis...4 What are the Causes of Adult
Patient Information ADULT SCOLIOSIS Information About Adult Scoliosis, Symptoms, and Treatment Options Table of Contents Anatomy of the Spine...2 What is Adult Scoliosis...4 What are the Causes of Adult
Thoracolumbar Spinal System NAUTILUS SURGICAL TECHNIQUE GUIDE
 NAUTILUS SURGICAL TECHNIQUE GUIDE NAUTILUS CONTENTS Introduction 1 1 Implants 3 Instruments 6 Surgical Technique 10 Product Information 17 NAUTILUS NAUTILUS Thoracolumbar Spinal System The NAUTILUS Thoracolumbar
NAUTILUS SURGICAL TECHNIQUE GUIDE NAUTILUS CONTENTS Introduction 1 1 Implants 3 Instruments 6 Surgical Technique 10 Product Information 17 NAUTILUS NAUTILUS Thoracolumbar Spinal System The NAUTILUS Thoracolumbar
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION AN INTRODUCTION TO
 AN INTRODUCTION TO MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION This booklet is designed to inform you about the Maximum Access Surgery (MAS ) Transforaminal Lumbar Interbody
AN INTRODUCTION TO MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION This booklet is designed to inform you about the Maximum Access Surgery (MAS ) Transforaminal Lumbar Interbody
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Features & Benefits. 1) Wide Bone Graft Space. Anterior Margin 3mm Posterior Margin 1.5mm. Marker Margin
 ALIF Cage System LnK ALIF Cage System The LnK ALIF Cage can make solid inter-body fusion with optimized anatomical position. Broad bone graft area also makes perfect fusion. It can be used with additional
ALIF Cage System LnK ALIF Cage System The LnK ALIF Cage can make solid inter-body fusion with optimized anatomical position. Broad bone graft area also makes perfect fusion. It can be used with additional
DO YOU SUFFER FROM SACROILIAC (SI) JOINT PAIN? TM SI Joint Stabilization and Fusion. Patient Information
 DO YOU SUFFER FROM SACROILIAC (SI) JOINT PAIN? TM SI Joint Stabilization and Fusion Patient Information TABLE OF CONTENTS About SACROILIAC (SI) JOINT Instability Causes of SI joint Instability and Pain
DO YOU SUFFER FROM SACROILIAC (SI) JOINT PAIN? TM SI Joint Stabilization and Fusion Patient Information TABLE OF CONTENTS About SACROILIAC (SI) JOINT Instability Causes of SI joint Instability and Pain
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
EasyStep. Operative technique
 Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
