The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 26 November 2008
|
|
|
- Jacob Walker
- 5 years ago
- Views:
Transcription
1 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: ) Applicant: MEDA PHARMA imiquimod ATC Code: D06BB10 List I Date of marketing authorisation (MA): 18 September 1998 (centralised procedure) MA Variation: - 13 July 2004 (extension of indication to basal cell carcinomas) - 24 April 2007 (extension of indication to actinic keratoses) Reason for request: inclusion on the list of medicines reimbursed by National Insurance and approved for use by hospitals in the extension of indication: Clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adult patients when size or number of lesions limit the efficacy and/or acceptability of cryotherapy and if other topical treatment options are contraindicated or less appropriate Medical, Economic and Public Health Assessment Division 1
2 1 CHARACTERISTICS OF THE MEDICINAL PRODUCT 1.1. Active ingredient Imiquimod 1.2. Indications Imiquimod is indicated for the topical treatment of: - External genital and perianal warts (condylomata acuminata) in adults. - Small superficial basal cell carcinomas (sbcc) in adults Extension of indication: Clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adult patients when size or number of lesions limit the efficacy and/or acceptability of cryotherapy and if other topical treatment options are contraindicated or less appropriate 1.3. Dosage and method of administration External genital warts in adults: imiquimod cream should be applied 3 times per week (for example: Monday, Wednesday and Friday or Tuesday, Thursday and Saturday) prior to normal sleeping hours and must remain in contact with the skin for 6 to 10 hours. Imiquimod cream treatment should continue until the clearance of visible genital or perianal warts or for a maximum of 16 weeks per episode of warts. Superficial basal cell carcinomas in adults: apply imiquimod cream for 6 weeks, 5 times per week (example: Monday to Friday) prior to normal sleeping hours, and leave on the skin for approximately eight hours. Actinic keratosis in adults: treatment should be initiated and monitored by a physician. Imiquimod cream should be applied 3 times per week (for example: Monday, Wednesday and Friday) for 4 weeks prior to normal sleeping hours and must remain in contact with the skin for about 8 hours. Sufficient cream should be applied to cover the whole treatment area. After a 4-week treatment-free period, clearance of AKs should be assessed. The maximum recommended dose is one sachet. The maximum recommended treatment duration is 8 weeks. An interruption of dosing should be considered if intense local inflammatory reactions occur or if an infection is observed at the treatment site. In this latter case, other appropriate measures should be taken. Each treatment period should not be extended beyond 4 weeks due to missed doses or rest periods. If the treated lesion(s) show an incomplete response at the follow-up examination at 4-8 weeks after the second treatment period, a different therapy should be used. 2
3 2 SIMILAR MEDICINAL PRODUCTS 2.1. ATC Classification (2008) D: Dermatological medicinal products D06: Antibiotics and chemotherapeutics for dermatological use D06B: Chemotherapeutics for topical use D06BB: Antivirals D06BB10: Imiquimod 2.2. Medicines in the same therapeutic category ALDARA is the only medicinal product in its pharmacotherapeutic class indicated in adults for local treatment of external genital and perianal warts, small superficial basal cell carcinomas and certain actinic keratoses Medicines with a similar therapeutic aim Other medicinal products belonging to another pharmacotherapeutic class and used in the treatment of actinic keratosis: - EFUDIX (5-fluorouracil): indicated in pre-epitheliomatous keratosis - METVIXIA (photodynamic therapy) not reimbursed in actinic keratosis (substantial actual benefit in this indication: opinion of 28 March 2007): indicated in flat or nonhyperkeratotic and non-pigmented actinic keratosis of the face and scalp. Other non-pharmacological treatments: cryotherapy (reference treatment for actinic keratosis), radiotherapy, CO 2 laser, curettage-electrocoagulation. 3 ANALYSIS OF AVAILABLE DATA 3.1. Efficacy Among the studies submitted by Meda Pharma, only the placebo- and active comparatorcontrolled phase III studies using the dosing regimen adopted in the MA will be described below: - 2 placebo-controlled studies with a 1 year extension phase - 1 study versus 5-FU and cryotherapy Placebo-controlled studies Studies 1473-IMIQ and 1487-IMIQ Randomised, double-blind, placebo (excipient)-controlled, phase III studies comparing 5% imiquimod with placebo in adult patients with actinic keratosis (AK) of the head or neck. Main inclusion criteria: - Age: 18 years or more. - Clinically typical, visible but discrete, nonhyperkeratotic, nonhypertrophic AK lesions (4 to 8 for study 1473-IMIQ and 5 to 9 for study 1487-IMIQ) located within a contiguous 25 cm ² treatment area on the scalp or face (but not both). 3
4 - Clinical diagnosis made by a qualified dermatologist and, for study 1487-IMIQ, confirmed by histopathological examination of a 3 to 4-mm biopsy taken before treatment from a representative lesion. Samples were read by two dermatopathologists blind to the treatment received in a centralised laboratory, using a standard definition planned in the protocol. A consensual diagnosis had to be reached in the case of disagreement. - No treatment of any other actinic keratosis lesions outside the treatment area before the end of the study. Exclusion criteria: - Prior treatment of the targeted area by imiquimod; - The following prior systemic treatments: anti-cancer drugs, retinoids (during the 6 months before inclusion); interferons/interferon inducers, immunomodulators, immunosuppressive agents, cytotoxic drugs or drugs with major organ toxicity, investigational medicinal products, corticosteroids (during the 4 weeks before inclusion); vitamin A (during the 2 weeks before inclusion); - Prior topical treatments at any site on the head or face: PUVA therapy, UVB therapy, laser abrasion, dermabrasion, chemical peels (during the 6 months before inclusion); retinoids, 5-FU, masoprocol, photodynamic therapy (during the 4 weeks before inclusion); cryodestruction or chemodestruction, surgical excision, curettage, biopsy, corticosteroids, NSAIDs (during the 4 weeks before inclusion or if healing not complete). Treatments compared and study regimen: - imiquimod 5% cream (250-mg sachets) - placebo (excipient). Application 3 times weekly (Monday, Wednesday and Friday) prior to sleeping hours during a first 4-week cycle followed by a 4-week follow-up period without treatment. Evaluation of lesions 8 weeks after the start of treatment (after 4 weeks of follow-up): - If the treated lesions had completely cleared, the patient was considered to have completed the study. - If the lesions persisted: second cycle of 4 weeks of treatment followed by 4 weeks without treatment. The patients were seen again after 16 weeks: if lesions were still seen during the visit, a final evaluation was performed 4 weeks later without additional treatment (week 20). In the case of local intolerance, a treatment-free period was prescribed at the investigator's discretion, though this did not modify the duration of the treatment cycles. Primary endpoints: - proportion of patients with complete clearance of AKs after the first cycle of treatment; - proportion of patients with complete clearance of AKs after the full treatment (1 or 2 cycles according to the clinical course of each patient). Complete clearance was defined as no visible AK lesions during the clinical assessment (and no histological evidence in study 1487-IMIQ) over the treatment area. Results: Baseline patient characteristics: After randomisation, 246 patients were included in study 1473-IMIQ and 259 in study IMIQ. The median age of patients was 65 years in study 1473-IMIQ and 73 years in study IMIQ. Patients in the two studies were mainly men with Fitzpatrick skin phototype I to III and had previously been treated for actinic keratosis lesions. The median number of lesions was 6, except for the excipient group in study 1487-IMIQ where the median was 7. 4
5 Results for primary endpoints: In the two studies, a statistically significant difference was demonstrated in favour of imiquimod compared to placebo for the proportion of patients with complete clearance of AK lesions after 1 or 2 cycles of treatment and on the proportion of those with complete lesion clearance after a single cycle (see table 1). Table 1: Results for the primary endpoints (ITT population) - studies 1473-IMIQ and 1487-IMIQ Study Imiquimod Placebo p Complete clearance rate after a single cycle: % (n/n) 1473-IMIQ 26.8 (33/123) 4.1 (5/123) < IMIQ 37.2 (48/129) 0.8 (1/130) < Complete clearance rate after 1 or 2 cycles: % (n/n) 1473-IMIQ 53.7 (66/123) 14.6 (18/123) < IMIQ 55.0 (71/129) 2.3 (3/130) < Long-term, placebo-controlled, follow-up studies: 1-year recurrence rate Studies 1518-IMIQ and 1524-IMIQ were extensions of studies 1473-IMIQ and 1487-IMIQ respectively. Their primary objective was to evaluate the recurrence rate after 1 year of follow-up without treatment. The patients included in these follow-up studies were those who had become clinically cleared after 1 or 2 cycles of treatment. Recurrence was defined by the presence of at least 1 AK lesion in the treated area (recurrent lesion or new lesion) during the visit at 1 year or by any therapeutic intervention related to actinic keratosis or squamous cell carcinoma. In study 1518-IMIQ, there were 27/310 recurrent lesions (i.e. 8.7% of lesions) in 23/59 patients (or 39.0%) in the group initially treated by imiquimod and 6/62 recurrent lesions (i.e. 9.7% of lesions) in 8/14 patients (i.e. 57.0%) in the group initially given placebo. In study 1524-IMIQ, there were 14/427 recurrent lesions (i.e. 3.3% of lesions) in the group initially treated by imiquimod in 12/69 patients (17.4%) and 0/18 recurrent lesions in 0/3 patients in the group initially treated with placebo. NB: these results are given as an indication insofar as the design of this study did not permit statistical analysis. 5
6 Study versus active comparators Study 1436-IMIQ 1 Randomised, open-label study comparing the efficacy of 5% imiquimod cream with those of 5-FU cream and cryotherapy, in 75 adults patients with actinic keratosis located on the head or neck. The primary objective of this study was to evaluate oncogenic markers after treatment (4 to 8 weeks after treatment depending on the treatment). Assessment of the clinical, histological and cosmetic outcome and the safety evaluation were secondary objectives. Inclusion criteria: - At least 5 actinic keratosis lesions confirmed by pathological examination. - Located in a single anatomical area of not more than 50 cm 2. - Located on the head, neck or décolleté. - No topical treatment for actinic keratosis during the 2 weeks before the start of the study. Exclusion criteria: treatment of the AK lesion during the 2 months before inclusion or presence of a carcinomatous lesion in the treatment area (a biopsy of any suspect lesion had to be made before deciding whether to include the patient). Groups and study design: Because of the different regimens and methods of administration, treatments were administered open-label: - Imiquimod (26 patients): 3 applications per week for 4 weeks then, where necessary, a second 4-week cycle after a 4-week treatment-free period; maximum of 12 sachets per cycle; a 1-week treatment-free period could be decided if an inflammatory reaction occurred during a cycle; - 5-FU (24 patients): 2 applications per day for 4 weeks, with a treatment-free period of not more than 1 week during therapy in the case of severe inflammation; - Cryotherapy (25 patients): 1 application of liquid nitrogen on the lesions for from 20 to 40 seconds, followed by a second application 15 days later where necessary. Then follow-up of patients for 12 months without treatment for the actinic keratosis on the treatment area. A 4-mm thick biopsy specimen was taken at inclusion from the treatment area and an area of healthy skin. The biopsy was repeated after the end of treatment, on a lesion in the case of persistence of visible lesions, or at the same place as the first in the case of clearance of any lesion. A clinical evaluation with photographs was performed on inclusion and at the end of treatment, then 6 and 12 months later. Primary efficacy endpoint: quantitative changes in mutated p53 and p16ink4a oncogene markers during the last post-treatment examination (6 weeks after the last cryotherapy session, 4 weeks after the last application of 5-FU and 8 weeks after the last application of imiquimod). Secondary endpoints: - Proportion of patients with 100% clinical and histologically confirmed clearance of lesions 4 weeks after the end of the treatment and during the last post-treatment examination (6 weeks after the last cryotherapy session, 4 weeks after the last application of 5-FU and 8 weeks after the last application of imiquimod); - Proportion of patients with no recurrence of treated lesions, evaluated at 6 and 12 months; 1 Krawtchenko N et al. A randomised study of topical 5% imiquimod vs topical 5-fluorouracile vs cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol 2007;157(2):
7 - Proportion of patients with sustained clearance (no recurring or new lesions) of the treated field, evaluated at 6 and 12 months. Results: Patient characteristics: The mean age of included patients was 71 ± 7 years (range: 57 to 88) and they were mainly men (61 men and 14 women). The mean number of AK lesions was 7.9 in the imiquimod and cryotherapy groups and 8.3 in the 5-FU group. The mean cumulative area of AK lesions was 95 cm² in the imiquimod group, 75 cm² in the 5-FU group and 49 cm² in the cryotherapy group. Results for the primary endpoint: No assessment of the oncogene markers could be performed in eight patients as the sample was insufficient. All the other patients had oncogene markers at the initial biopsy. During the assessment at the last post-treatment visit, a statistically significant difference in terms of regression or disappearance of the mutated p53 and p16ink4a markers was observed between the 3 treatment groups (see table 2). Table 2: Changes in oncogene markers- study 1436-IMIQ Imiquimod N=26 5-FU N=24 Cryotherapy N=25 N % n % n % Analysis of mutated p53 marker Worsening No change Disappearance/reduction ND Analysis of p16ink4a marker: Worsening No change Disappearance/reduction ND p Result for secondary endpoints Table 3: Result for the secondary endpoints - study 1436-IMIQ Complete clearance of lesions at last post-treatment clinical examination Complete clearance of lesions at last post-treatment histological examination Recurrence of treated lesions at 12 months Presence of new lesions at 12 months Imiquimod N=26 5-FU N=24 Cryotherapy N=25 n % n % n % p 7
8 Remarks: 1. Although field cancerisation theory suggests that it may be useful to study oncogene markers to evaluate the efficacy of a medicinal product in actinic keratosis, this cannot replace an evaluation based on clinical and histological criteria. 2. This study had 2 primary endpoints without correction of the α risk. 3. The statistical analysis of these results included no pairwise analysis of groups. They therefore do not permit to draw conclusion about the superiority of imiquimod compared to 5-FU and cryotherapy 3.2. Adverse effects Studies 1473-IMIQ and 1487-IMIQ versus placebo In these two studies, the main adverse events reported by patients treated with imiquimod were local skin reactions such as pruritis, burns, erythema, pain, inflammation and swelling. These were observed in approximately 22% of patients (22.0% and 22.5%) and in approximately 5% of patients on placebo (5.7% and 4.6%). Pruritis was the most frequent adverse events (14.6% and 12.4% with imiquimod versus 0% and 1.5% with placebo). In studies 1473-IMIQ and 1487-IMIQ, erythema, excoriations, crusts and, for study IMIQ, mild to moderate erosions/ulcerations were the most commonly observed adverse events in patients receiving imiquimod (see tables 4 and 5 page 10). In the placebo group of these two studies, erythema and symptoms of desquamation/ichthyosis/mild dryness were mainly observed. Local reactions were more severe after 4 weeks of treatment and were generally milder during the second treatment cycle than during the first. Three patients on imiquimod stopped treatment because of local skin reactions, and no patient discontinued placebo. Systemic reactions, which were less frequent than local reactions, were also observed: myalgia (2%), fatigue (1.6%) and nausea (1.6%). Study 1436-IMIQ versus 5-FU and cryotherapy Local reactions were observed in the 3 treatment groups including symptoms such as pain/burns/tingling, oedema, erythema, crusts and desquamation/dryness of the skin. These adverse events were mainly mild to moderate, however, severe reactions were sometimes observed at the start of imiquimod treatment (2/26 patients with erythema and 4 with crusts) and with 5-FU (5/24 patients with pain/burn/tingling, 1 with oedema and 15 with erythema). In the imiquimod group, the local reactions regressed during time and the percentage of patients with at least one local reaction varied from 96% to 30% at 6 months and 17% at 12 months. After 12 months, there remained 1 case of pain, 3 cases of erythema and 1 case of desquamation/dryness of the skin. In the 5-FU and cryotherapy groups, the percentage of patients with at least one local reaction remained high throughout the study: from 100% to 75% at 6 months and 88% at 12 months for 5-FU, with in particular erythema and desquamation/dryness of the skin, and from 100% to 92% at 6 months and 96% at 12 months for cryotherapy, with mainly pain, erythema, crusts and desquamation/dryness of the skin. 8
9 Table 4: Local skin reactions assessed by the investigator - study 1473-IMIQ Type of reaction Severity Imiquimod N = 123 Erythema 7 (5.7%) 31 (25.2%) 65 (52.8%) 20 (16.3%) Desquamation / ichthyosis / dryness Excoriation / crusting Oedema Erosion / ulceration Blisters Oozing / exudation 9 (7.3%) 64 (52.0%) 47 (38.2%) 3 (2.4%) 29 (23.6%) 31 (25.2%) 43 (35.0%) 20 (16.3%) 67 (54.5%) 42 (34.1%) 14 (11.4%) 72 (58.5%) 27 (22.0%) 22 (17.9%) 2 (1.6%) 108 (87.8%) 13 (10.6%) Table 5: Local skin reactions assessed by the investigator - study 1487-IMIQ Erythema Type of reaction Desquamation / ichthyosis / dryness Excoriation / crusting Oedema Erosion / ulceration Blisters Oozing / exudation Severity Imiquimod N = (3.1%) 21 (16.3%) 64 (49.6%) 40 (31.0%) 8 (6.2%) 55 (42.6%) 51 (39.5%) 15 (11.6%) 15 (11.6%) 31 (24.0%) 52 (40.3%) 31 (24.0%) 39 (30.2%) 51 (39.5%) 30 (23.3%) 9 (7.0%) 34 (26.4%) 40 (31.0%) 41 (31.8%) 14 (10.9%) 81 (62.8%) 32 (24.8%) 14 (10.9%) 2 (1.6%) 47 (36.4%) 38 (29.5%) 38 (29.5%) 6 (4.7%) Placebo N = (17.1%) 79 (64.2%) 23 (18.7%) 31 (25.2%) 74 (60.2%) 18 (14.6%) 90 (73.2%) 28 (22.8%) 5 (1%) 116 (94.3%) 7 (5.7%) 116 (94.3%) 6 (4.9%) 123 (100%) 122 (99.2%) Placebo N = (29.2%) 65 (50.0%) 27 (20.8%) 34 (26.2%) 63 (48.5%) 32 (24.6%) 65 (50.0%) 52 (40.0%) 11 (8.5%) 2 (1.5%) 120 (92.3%) 6 (4.6%) 4 (3.1%) 108 (81.3%) 17 (13.1%) 4 (3.1%) 129 (99.2%) 124 (95.4%) 4 (3.1%) 9
10 For the whole study population (n=75), 6 patients reported 14 adverse events other than local reactions including 7 that occurred during the treatment period and 3 that were considered to be treatment-related: - Imiquimod: 1 worsening of hypertension (related to treatment) and 1 cutaneous inflammation (possibly related to treatment) - Cryotherapy: 1 worsening of hypertension (probably related to treatment) During the follow-up period, 2 patients in the cryotherapy groups had to undergo excision of a basal cell carcinoma (outside the treated field) and 1 patient an excision of an actinic keratosis lesion. In the imiquimod group, 1 patient was hospitalised for excision of a skin lesion (outside the treated field) and 1 patient had to undergo excision of a squamous cell carcinoma (outside the treated field) Conclusion The efficacy and safety of imiquimod 5% cream in the treatment of actinic keratosis were evaluated in 2 main placebo (excipient)-controlled studies and in one study versus active comparators, 5-FU cream and cryotherapy, in adult patients with multiple, typical, nonhyperkeratotic and nonhypertrophic keratosis actinic lesions on the head or neck. In the 3 studies, the patients treated by imiquimod received 1 cycle of treatment or, where necessary, 2 cycles separated by a 4-week follow-up period without treatment. A treatment cycle comprised 3 applications of imiquimod per week for 4 weeks. In the study versus active comparators, the patients treated by 5-FU had 2 applications per day for 4 weeks and the patients treated by cryotherapy had 1 application of liquid nitrogen during 20 to 40 seconds on the lesions, followed by a second application where necessary 15 days later. In the randomised, double-blind, placebo-controlled studies, the diagnosis and evaluation after treatment were clinical and, for study 1487-IMIQ, confirmed by biopsy. A statistically significant difference was demonstrated in favour of imiquimod compared to placebo for: - the proportion of patients with total clearance of lesions after a single treatment cycle (26.8% vs 4.1% in study 1473-IMIQ and 37.2% vs 0.8% in study 1487-IMIQ, p<0.0001); - the proportion of patients with total clearance of lesions after 1 or 2 cycles (53.7% vs 14.6% in study 1473-IMIQ and 55.0% vs 2.3% in study 1487-IMIQ). The study versus active comparators did not permit to conclude about the superiority, in terms of efficacy, of imiquimod compared to 5-FU or cryotherapy. The primary endpoints were neither clinical nor histological and there was no pairwise comparison of groups. In terms of safety, the main adverse events reported by patients treated with imiquimod were local skin reactions such as pruritis, burns, erythema, pain, inflammation, swelling, dryness, crusting, erosions and ulcerations. Local reactions were more severe during the 4th week of treatment and were generally milder during the second treatment cycle than during the first. Systemic reactions were also reported with imiquimod, though these were less frequent than the local reactions: myalgia, fatigue and nausea. Three systemic adverse events were considered to be related to treatment in the study versus active comparators: - Imiquimod: 1 worsening of hypertension (treatment-related) and 1 skin inflammation (possibly related to treatment) - Cryotherapy: 1 worsening of hypertension (probably related to treatment) Overall, the efficacy of imiquimod was demonstrated versus placebo in terms of complete clearance of actinic keratosis lesions in patient with multiple, typical, nonhyperkeratotic and nonhypertrophic lesions on the scalp or face. The main adverse effects observed with imiquimod were generally mild to moderate local reactions regressing during a 2nd cycle of treatment. 10
11 4 TRANSPARENCY COMMITTEE CONCLUSIONS 4.1. Actual benefit In the extension of indication: Actinic keratoses are lesions occurring on sun-exposed areas of the skin usually in the elderly. Multiple lesions are frequently observed and these may progress to skin cancer (squamous cell carcinoma) in the absence of effective treatment. This proprietary drug is intended to provide curative treatment. Public health benefit: Actinic keratoses are a low public health burden as although these lesions are relatively frequent, they often have a good prognosis. Improving the management of actinic keratosis is not a public health need. There are alternative pharmacological and non-pharmacological therapies. The clinical trial data discussed here show that this proprietary drug is not expected to have an impact in terms of morbidity for patients with actinic keratoses. Consequently, given our current knowledge and taking into account other therapies available at this time, the proprietary drug ALDARA is not expected to benefit public health in this indication. The efficacy/safety ratio is high. Cryotherapy is the reference treatment for superficial and localised forms of actinic keratoses. This proprietary drug provides second-line treatment in clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adult patients when the size or number of lesions limit the efficacy and/or acceptability of cryotherapy and if other topical treatment options are contraindicated or less appropriate The actual benefit of ALDARA 5%, cream is substantial in this indication Improvement in actual benefit ALDARA 5% cream does not provide an improvement in actual benefit (IAB V) in the treatment of superficial actinic keratoses of the face or scalp in adults but represents an additional means of therapy Therapeutic use (Ref 2, 3, 4, 5, 6 ) Actinic keratoses are dermatological disorders mainly caused by exposure to ultraviolet light. The onset of these skin lesions may therefore be prevented by avoiding exposure to natural or artificial ultraviolet light or to other physical or chemical precipitating factors (ionising radiation). 2 Dubertret et al. Thérapeutique dermatologique. Médecine Sciences Flamarion (2001) 3 Skin Cancer (PDQ ): Treatment Health Professional Version. National Cancer Institute (2006). 4 Saurat J.-H. et al. Dermatologie et infections sexuellement transmissibles (édition MASSON, 4 th edition) 5 de Berker D. et al. on behalf of the British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for the management of actinic keratoses. British Journal of Dermatology 2007;156: Guidelines for the management of actinic keratoses. European Dermatologic Forum Guidelines. 11
12 The goal of treatment is the destruction of the lesions and the prevention of recurrences by regular monitoring after treatment. Actinic keratoses are usually treated by cryotherapy which is considered to be the reference treatment when there is a small number of superficial lesions. Cryotherapy is a simple and rapid technique that does not require any specific equipment. If squamous cell carcinoma cannot be ruled out, a biopsy must be performed before destruction with nitrogen. Surgery is sometimes used for large lesions and this may be followed by a graft if the treatment area is extensive. Topical 5-FU and mechanical dermabrasion are used for multiple keratoses. Vaporisation of the lesions by CO 2 laser and curettage-electrocoagulation provide alternative treatment options. Photodynamic therapy with METVIXIA, cream is an alternative to cryotherapy in the case of multiple superficial and non-pigmented lesions of the face and/or scalp, though this treatment is not reimbursed in this indication. Therapeutic use of ALDARA: ALDARA is used as second-line treatment in clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses of the face or scalp in immunocompetent adult patients when the size or number of lesions limit the efficacy and/or acceptability of cryotherapy and if other topical treatment options are contraindicated or less appropriate 4.4. Target Population In actinic keratosis, the target population of ALDARA is defined by patients with superficial (nonhyperkeratotic) and nonhypertrophic lesions of the face or scalp when there are a large number of lesions and if other topical treatment options are contraindicated or less appropriate. There are few epidemiological data about actinic keratosis. British 7,8 and Irish 9 data demonstrated a prevalence of actinic keratosis of about 19% to 24% in patients aged over 60 years. In the Irish study, the prevalence was estimated to be 13.6% in men and 7.6% in women aged over 21 years. By extrapolating these values to the French population (INED data 2006), the population of patients with actinic keratosis may be estimated to be between 3 and 5 million people. However, this estimation should be regarded with caution because of differences in exposure to sunlight and because the UK and Irish populations have different phototypes to the French population. According to the study of Frost 10 (1994), lesions are located on the face in 20 to 60% of cases, which would reduce the population to 0.6 to 3 million people. No epidemiological data are available to estimate the proportion of superficial actinic keratosis lesions. According to data provided in the Transparency committee opinion for METVIXIA, approximately 90% of the lesions are considered nonhyperkeratotic (grades 1 and 2). Consequently, the population of patients with superficial actinic keratosis of the face and neck, may be estimated, with the above reservation, to be between 0.5 and 2.7 million people. Only a fraction of this population would probably be treated by ALDARA insofar as 7 Harvey I., Frankel S, Marks R et al. Non-melanoma skin cancer and solar keratoses. 1. Method and descriptive results of the South Wales skin cancer study. Br J Cancer 1996; 74: Memon AA, Tomenson JA, Bothwell J, Friedman PS. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol 2000; 142: O Beirn SFO, Judge P, Maccon CF. Skin cancer in County Galway, Ireland. In: Proceedings of Sixth National Cancer Conference, sponsored by the American Cancer Society Inc. And the National Cancer Institute, September Philadelphia: Lippincott, 1970: Frost CA, Green AC. Epidemiology of solar keratoses. Br J dermatol, 1994; 24:
13 cryotherapy remains the reference treatment and ALDARA is a second-line treatment when other topical treatments cannot be used or are less appropriate. As an indication, approximately 90% of patients with superficial actinic keratosis are treated by cryotherapy and approximately 30% of patients receiving 5-FU have irritative adverse effects which prevent the continuation of treatment (expert opinion) Transparency Committee recommendations The Transparency Committee recommends inclusion on the list of medicines reimbursed by National Insurance and on the list of medicinal products approved for use by hospitals and various public services in the extension of indication Packaging: appropriate for the prescription conditions Reimbursement rate: 65% 13
Opinion 6 March 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
Opinion 26 June 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A.
 fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Aldara. Aldara (imiquimod) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
TRANSPARENCY COMMITTEE Opinion 17 September 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 September 2014 VEREGEN 10%, ointment Tube of 15 g (CIP: 34009 222 531 2 1) Applicant: EXPANSCIENCE INN ATC Code
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS
 BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
Developing the next generation of dermatology products to treat serious skin diseases
 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
AWMSG SECRETARIAT ASSESSMENT REPORT. Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel. Reference number: 1392 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 January 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
Corporate Medical Policy
 Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Extreme dermatoheliosis: How to approach the severely sun damaged patient
 Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
Skin disorders. Basal cell carcinoma December 2009 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig ...
 December 29 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig.................................................. ABSTRACT INTRODUCTION: (BCC) is the most common form of skin cancer, predominantly affecting
December 29 Anthony Ormerod, Sanjay Rajpara, and Fiona Craig.................................................. ABSTRACT INTRODUCTION: (BCC) is the most common form of skin cancer, predominantly affecting
Diagnosis and Management of Actinic Keratosis (AKs)
 Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 13 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 31 January 2007 BUCCOBET 0.1 mg, oromucosal tablet B/50 (CIP: 3741470 ) Applicant: DB PHARMA Betamethasone valerate
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 31 January 2007 BUCCOBET 0.1 mg, oromucosal tablet B/50 (CIP: 3741470 ) Applicant: DB PHARMA Betamethasone valerate
The no t-so-simple cutaneous wart
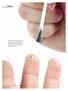 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Richard Turner Consultant Dermatologist
 Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Clinical characteristics
 Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest risk.
 Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
TRANSPARENCY COMMITTEE OPINION. 21 October 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ. Övermark, Meri.
 https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
Skin lesions The Good and the Bad. Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry
 Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
TRANSPARENCY COMMITTEE OPINION. 31 January Date of marketing authorisation: 22 March 2005 (centralised marketing authorisation)
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 31 January 2007 ALOXI 250 µg solution for injection B/1 CIP 375,482-8 Applicant: THERABEL LUCIEN PHARMA palonosetron
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 31 January 2007 ALOXI 250 µg solution for injection B/1 CIP 375,482-8 Applicant: THERABEL LUCIEN PHARMA palonosetron
AWMSG SECRETARIAT ASSESSMENT REPORT. 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel. Reference number: 1074 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel Reference number: 1074 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
AWMSG SECRETARIAT ASSESSMENT REPORT 5-aminolaevulinic acid (Ameluz ) 78 mg/g gel Reference number: 1074 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
TRANSPARENCY COMMITTEE OPINION. 14 February 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 14 February 2007 GLIVEC 100 mg, capsule B/120 capsules (CIP: 358 493-5) GLIVEC 100 mg, capsule B/180 capsules (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 14 February 2007 GLIVEC 100 mg, capsule B/120 capsules (CIP: 358 493-5) GLIVEC 100 mg, capsule B/180 capsules (CIP:
The future of Skin Cancer Treatment
 The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee
 I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
PRODUCT INFORMATION METVIX
 PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
Osteopathic Dermatology
 Osteopathic Dermatology Teodor Huzij, DO, FACN Reagan Anderson, DO, FAOCD, FASMS, Certificate of Added Qualification for Mohs Surgery, MPH, MCS, Program Director for RVU/CDI Dermatology Residency Program
Osteopathic Dermatology Teodor Huzij, DO, FACN Reagan Anderson, DO, FAOCD, FASMS, Certificate of Added Qualification for Mohs Surgery, MPH, MCS, Program Director for RVU/CDI Dermatology Residency Program
Clinical Study Report Synopsis
 This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALDARA 5% cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 12.5 mg of imiquimod in 250 mg cream (5 %).
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALDARA 5% cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 12.5 mg of imiquimod in 250 mg cream (5 %).
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
New Medicines Committee Briefing May 2015
 New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 ERBITUX 2 mg/ml, Solution for infusion 1 bottle of 50 ml (CIP: 565 806 9) Applicant : MERCK LIPHA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 ERBITUX 2 mg/ml, Solution for infusion 1 bottle of 50 ml (CIP: 565 806 9) Applicant : MERCK LIPHA
NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.
![NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.](/thumbs/89/100134179.jpg) ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
1) Photodynamic therapy with topical 5 aminolevulinic acid is considered medically necessary and is covered for the treatment of:
 Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 May 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2012 ANAPEN 0.50 mg/0.3 ml, solution for injection in pre-filled syringe 1 pre-filled syringe (glass), box of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2012 ANAPEN 0.50 mg/0.3 ml, solution for injection in pre-filled syringe 1 pre-filled syringe (glass), box of
Periocular skin cancer
 Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
Cutaneous Malignancies: A Primer COPYRIGHT. Marissa Heller, M.D.
 Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 5 January 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE. Opinion. 1 October 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 1 October 2008 EFFEXOR SR 37.5 mg prolonged-release capsule B/30 (CIP: 346 563-3) EFFEXOR SR 75 mg prolonged-release
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 1 October 2008 EFFEXOR SR 37.5 mg prolonged-release capsule B/30 (CIP: 346 563-3) EFFEXOR SR 75 mg prolonged-release
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare
 camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
camellia sinensis (green tea) leaf extract 10% ointment (Catephen ) SMC No. (1133/16) Kora Healthcare 04 March 2016 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
Dr A Anzarut, MSc, CIP, MD, FRCSC
 Dr A Anzarut, MSc, CIP, MD, FRCSC Plastic and Cosmetic Surgery ASSH Fellowship trained hand surgeon 201 2763 Beverly Street, Duncan, British Columbia Tel: (250) 597 2064 Fax: (250) 597-1297 PATIENT INFORMATION
Dr A Anzarut, MSc, CIP, MD, FRCSC Plastic and Cosmetic Surgery ASSH Fellowship trained hand surgeon 201 2763 Beverly Street, Duncan, British Columbia Tel: (250) 597 2064 Fax: (250) 597-1297 PATIENT INFORMATION
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 November 2006 TAXOTERE 20 mg, concentrate and solvent for infusion in single-dose vials of 7 ml, individually packed
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 November 2006 TAXOTERE 20 mg, concentrate and solvent for infusion in single-dose vials of 7 ml, individually packed
TRANSPARENCY COMMITTEE OPINION. 18 March 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 March 2009 REQUIP LP 2 mg extended-release tablet Box of 21 tablets (CIP: 379 214-8) Box of 28 tablets (CIP: 379
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 March 2009 REQUIP LP 2 mg extended-release tablet Box of 21 tablets (CIP: 379 214-8) Box of 28 tablets (CIP: 379
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
The legally binding text is the original French version TRANSPARENCY COMMITTEE. Opinion. 20 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 20 February 2008 DUROGESIC 12 micrograms/hour (2.1 mg/5.25 cm²), transdermal patch Box of 5 sachets (CIP: 369 851-5)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 20 February 2008 DUROGESIC 12 micrograms/hour (2.1 mg/5.25 cm²), transdermal patch Box of 5 sachets (CIP: 369 851-5)
Interesting Case Series. Aggressive Tumor of the Midface
 Interesting Case Series Aggressive Tumor of the Midface Adrian Frunza, MD, Dragos Slavescu, MD, and Ioan Lascar, MD, PhD Bucharest Emergency Clinical Hospital, Bucharest University School of Medicine,
Interesting Case Series Aggressive Tumor of the Midface Adrian Frunza, MD, Dragos Slavescu, MD, and Ioan Lascar, MD, PhD Bucharest Emergency Clinical Hospital, Bucharest University School of Medicine,
TRANSPARENCY COMMITTEE OPINION. 26 April 2006
 TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
EXTERNAL ANOGENITAL WARTS
 EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
EXTERNAL ANOGENITAL WARTS Whats new HPV vaccination is now available to MSM under the age of 45 years attending Sexual Health Services. This vaccination is recommended even if a prior/current history of
Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine
![Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine](/thumbs/83/88391058.jpg) APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
General information about skin cancer
 Skin Cancer General information about skin cancer Key points Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin. There are different types of cancer that start in
Skin Cancer General information about skin cancer Key points Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin. There are different types of cancer that start in
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD
 A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
Clinical Policy: Benign Skin Lesion Removal Reference Number: CP.MP.HN150
 Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Medical Policy. MP Dermatologic Applications of Photodynamic Therapy
 Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 September 2007 RELENZA 5mg/dose, inhalation powder, in single-dose containers 20 single-dose containers with an
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 September 2007 RELENZA 5mg/dose, inhalation powder, in single-dose containers 20 single-dose containers with an
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALDARA 5% cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 12.5 mg of imiquimod in 250 mg cream (5 %).
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALDARA 5% cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 12.5 mg of imiquimod in 250 mg cream (5 %).
Living Beyond Cancer Skin Cancer Detection and Prevention
 Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Topical immunomodulation. Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University
 Topical immunomodulation Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University Vitiligo Atopic dermatitis Seborrheic dermatitis Oral lichen planus Anogenital warts Bowen s disease actinic
Topical immunomodulation Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University Vitiligo Atopic dermatitis Seborrheic dermatitis Oral lichen planus Anogenital warts Bowen s disease actinic
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 May 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2007 RECTOGESIC 4 mg/g, rectal ointment B/1 (CIP 376 537-0) Applicant : PROSTRAKAN PHARMA SAS Glyceryl trinitrate
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2007 RECTOGESIC 4 mg/g, rectal ointment B/1 (CIP 376 537-0) Applicant : PROSTRAKAN PHARMA SAS Glyceryl trinitrate
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 GRAZAX 75 000 SQ-T, oral lyophilisate B/30 (CIP: 378 011-6) B/100 (CIP code: 378 012-2) B/90 (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 GRAZAX 75 000 SQ-T, oral lyophilisate B/30 (CIP: 378 011-6) B/100 (CIP code: 378 012-2) B/90 (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 16 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 REMOVAB 10 microgram concentrate for infusion solution Carton containing 1 pre-filled syringe (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 REMOVAB 10 microgram concentrate for infusion solution Carton containing 1 pre-filled syringe (CIP:
TRANSPARENCY COMMITTEE OPINION. 15 October 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 TANDEMACT 30 mg/20 mg tablets Box of 30 (CIP: 386 566-3) Box of 90 (CIP: 386 568-6) TANDEMACT 30 mg/4
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 TANDEMACT 30 mg/20 mg tablets Box of 30 (CIP: 386 566-3) Box of 90 (CIP: 386 568-6) TANDEMACT 30 mg/4
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 11 April 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Skin Cancer in Organ Transplant Recipients Challenges and Opportunities
 Disclosure Skin Cancer in Organ Transplant Recipients Challenges and Opportunities Investigator: DUSA Pharmaceuticals, Inc. Investigator: Genentech Consultant: Gerson Lehrman Group Sarah Tuttleton Arron,
Disclosure Skin Cancer in Organ Transplant Recipients Challenges and Opportunities Investigator: DUSA Pharmaceuticals, Inc. Investigator: Genentech Consultant: Gerson Lehrman Group Sarah Tuttleton Arron,
Alcohol should be avoided for 3 days prior to surgery and 2 days after the procedure.
 Mohs Surgery Information Packet Be sure to bring the following to your appointment: Insurance Card Insurance Referral ( If required by your insurance) Name and address of your primary care provider as
Mohs Surgery Information Packet Be sure to bring the following to your appointment: Insurance Card Insurance Referral ( If required by your insurance) Name and address of your primary care provider as
Economic analysis of treatments for external condyloma acuminatum Fagnani F
 Economic analysis of treatments for external condyloma acuminatum Fagnani F Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
Economic analysis of treatments for external condyloma acuminatum Fagnani F Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each abstract
AWMSG SECRETARIAT ASSESSMENT REPORT. Green tea leaf extract (Catephen ) 10% Ointment. Reference number: 2739 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
AWMSG SECRETARIAT ASSESSMENT REPORT Green tea leaf extract (Catephen ) 10% Ointment Reference number: 2739 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology Centre
TRANSPARENCY COMMITTEE OPINION. 27 January 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
TRANSPARENCY COMMITTE OPINION. 19 December 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTE OPINION 19 December 2007 ATRIANCE 5 mg/ml, Solution for Infusion Pack of 6 vials (571 348-9) Applicant: GlaxoSmithKline nelarabine
The legally binding text is the original French version TRANSPARENCY COMMITTE OPINION 19 December 2007 ATRIANCE 5 mg/ml, Solution for Infusion Pack of 6 vials (571 348-9) Applicant: GlaxoSmithKline nelarabine
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 REBETOL 200 mg capsules Pack of 84 (CIP code: 351 971.9) Pack of 112 (CIP code: 373 277.8) Pack of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 REBETOL 200 mg capsules Pack of 84 (CIP code: 351 971.9) Pack of 112 (CIP code: 373 277.8) Pack of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 19 December 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 December 2007 METHADONE AP-HP 1mg, gelatin-coated capsule Box of 7 (CIP: 379 146-2) METHADONE AP-HP 5mg, gelatin-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 December 2007 METHADONE AP-HP 1mg, gelatin-coated capsule Box of 7 (CIP: 379 146-2) METHADONE AP-HP 5mg, gelatin-coated
Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally
 University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
University of Dundee Allergic contact dermatitis to topical prodrugs used in photodynamic therapy Cordey, Helen; Ibbotson, Sally Published in: Photodermatology, Photoimmunology & Photomedicine DOI: 10.1111/phpp.12252
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 NAVELBINE 20 mg, soft capsules B/1 (CIP: 365 948-4) NAVELBINE 30 mg, soft capsules B/1 (CIP: 365 949-0)
Product Information ALDARA TM
 Product Information ALDARA TM NAME OF THE MEDICINE is 1-(2-methylpropyl)-1H-imidazo [4,5-c]quinolin-4-amine. It is an odourless, white to off-white crystalline solid. has a molecular formula of C 14 H
Product Information ALDARA TM NAME OF THE MEDICINE is 1-(2-methylpropyl)-1H-imidazo [4,5-c]quinolin-4-amine. It is an odourless, white to off-white crystalline solid. has a molecular formula of C 14 H
TRANSPARENCY COMMITTEE OPINION. 4 November 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
NHS. Photodynamic therapy for non-melanoma skin tumours (including premalignant and primary non-metastatic skin lesions)
 NHS National Institute for Health and Clinical Excellence Issue date: February 2006 Photodynamic therapy for non-melanoma skin (including premalignant and primary non-metastatic skin lesions) Understanding
NHS National Institute for Health and Clinical Excellence Issue date: February 2006 Photodynamic therapy for non-melanoma skin (including premalignant and primary non-metastatic skin lesions) Understanding
Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses
 Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Clinical Trial Report Synopsis. Efficacy and Safety of LEO in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up
 Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
TOPICAL TREATMENT OF ACTINIC KERATOSIS
 TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 16 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 14 December 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 14 December 2011 INCIVO 375 mg, film-coated tablet B/4 bottles of 42 tablets (CIP code: 217 378-5) B/1 bottle of 42
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 14 December 2011 INCIVO 375 mg, film-coated tablet B/4 bottles of 42 tablets (CIP code: 217 378-5) B/1 bottle of 42
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 7 January 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
