RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
|
|
|
- Felix Harrington
- 5 years ago
- Views:
Transcription
1 RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi Article no: N1723 R-Biopharm AG, An der neuen Bergstraße 17, D Darmstadt, Germany Phone: +49 (0) / Fax: +49 (0)
2 1. Intended use For in vitro diagnostic use. RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi Test is an immunochromatographic rapid assay for the qualitative determination of Cryptosporidium parvum and / or Giardia lamblia and / or Entamoeba histolytica (sensu lato) in stool samples. 2. Summary and explanation of the test Giardia lamblia is an intestinal flagellate. The morphologically characteristic Trophozoites only survive for a short time outside the host organism. Transmission takes place via the highly infectious cysts. Because it is spread world-wide, Giardia lamblia has become an important cause of chronic diarrhoeas, particularly in the case of problems in emporiatrics. The infection occurs after the ingestion of cysts in contaminated food and water. In communal facilities with inadequate hygiene, the infection usually occurs via the faecal-oral route from person to person. This mode of transmission is particularly common among children and in kindergartens, as well as among male homosexuals and prison inmates. The infection can also be passed on from children to parents. Unlike infants, older children who are infected can be free of the symptoms. Nevertheless, they excrete the cysts and can infect other humans. The symptoms of Giardiasis (Lambliasis) are acute or chronic diarrhoea. The incubation time is between 3 and 42 days. The method most frequently used to diagnose Giardiasis in the past has been the detection of cysts in the stool by microscopy, which can only be carried out by experienced personnel. The investigations also have to be carried out over a long period of time because the excretion of cysts fluctuates greatly. Cryptosporidium parvum is a parasite which is very common in animals and occurs as an important pathogenic organism in domestic animals and particularly in calves. However, infections in humans are now observed in many countries more frequently than was previously assumed. In tropical developing countries, the parasite is often endemic and causes diarrhoea epidemics among children. With immunocompetent patients, the disease manifests itself as self-healing gastroenteritis. The diarrhoea lasts between 3 and 10 days and may be accompanied by fever and gastrointestinal symptoms such as nausea and pain, which resembles those of giardiasis (lambliasis). The symptoms and effects are substantially more serious with immunoincompetent patients, where diarrhoea persists and is very severe. The infection can be transmitted from animal to humans via contaminated water and from human to human. Members of communal facilities, children in kindergartens and the high-risk groups, homosexual men and patients infected with HIV, are particularly at risk. In the past, the methods most frequently used for the diagnosis of cryptosporidiosis were the microscopic detection of Oocysts in the stool or the microscopic examination of small intestine biopsy samples which can only be carried out by experienced personnel. RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
3 Across the world, up to 500 million people are infected with Entamoeba histolytica (sensu lato) every year. Molecular-genetic investigations have shown that the protozoa, which has been given the name Entamoeba histolytica and is identified using conventional diagnostic methods, consists of two morphologically indistinguishable species: the pathogenic species, Entamoeba histolytica sensu stricto and the non-pathogenic species (according to current knowledge), Entamoeba dispar. Roughly 90% of people with Entamoeba infections have E. dispar. The approximately million cases of amoebic colitis or hepatic abscess which result in 80,000 deaths every year are caused by E. histolytica. The life cycle of the Entamoeba is relatively straightforward. The infection is caused by the oral ingestion of cysts with four nuclei. In the small intestine, these develop into the single nucleus form of the parasite, the trophozoite (forma minuta), which multiplies and differentiates predominantly in the large intestine. Encapsulation is probably triggered by the environment in the lower region of the large intestine. Besides the cysts, trophozoites are only found in stools with accelerated intestinal passage. The clinical symptoms of amoebiasis are triggered by the invasion of the parasite from the lumen of the bowels into the mucous membrane of the colon. Trophozoites with phagocytised erythrocytes are frequently found at the same time. These trophozoites are known as forma magna because of their size. The symptoms of invasion into the mucous membrane of the intestine are diarrhoea, dysentery or even amebomas. The complications which may occur after disseminate dispersion are hepatic abscesses, pulmonary abscesses or, in very rare cases, even cerebral abscesses which, if untreated, usually end in death. The clinical symptoms of the acute intestinal form of amoebiasis are cramp-like abdominal pains with nausea and severe diarrhoea with bloody and slimy stools. The acute stage can develop into a chronic stage with occasional diarrhoea alternating with constipation, abdominal pain, nausea and vomiting. Completely symptom-free cyst elimination has also been described. Approximately 10% of cases of acute amoebic dysentery result in extra-intestinal complications such as hepatic abscesses or the invasion of other organs. With extra-intestinal amoebiasis, a serological determination of antibodies is indicated. Intestinal amoebiasis can be diagnosed by using complicated microscopic procedures for determining the cysts and trophozoites in the stools. However, since the parasite density can be very small, it can be assumed that the sensitivity of this method for a single stool investigation is only 75%, even with experienced personnel. The method also involves the risk of confusing the Entamoeba with cells of the intestine epithelium, granulocytes, macrophages and fungi. Sensitive immunological test procedures with specific antibodies against antigens of Entamoeba have a great advantage. The diagnostic method is not dependent on subjective evaluation and is more sensitive since components which can no longer be identified by their morphology are RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
4 also used in the determination. Only the invasive form of Entamoeba causes the formation of antibodies. Since antibody titers can usually be detected with the onset of the clinical symptoms, a specific antibody determination can be used to identify E. histolytica. This also offers the possibility of differentiating between the size of the titers of intestinal and extraintestinal amoebiasis, which is crucial for deciding on the choice of therapy. An important alternative method to microscopy for the detection of Entamoeba histolytica, Giardia lamblia and Cryptosporidium parvum is the quick immunochromatographic rapid assay described in the following which, because it uses monoclonal antibodies, is equivalent to the microscopy investigation procedures in terms of sensitivity and specificity. The test is quick and simple to perform and does not require specially trained microbiologists to carry it out. 3. Test principle This rapid assay is a single-step immunochromatographic lateral-flow test, where specific antibodies which are directed against each parasite attach themselves to green (Entamoeba specific), red (Giardia specific) or blue (Cryptosporidia specific) latex particles. Other specific antibodies against the three pathogens are firmly bound to the membrane. The stool sample is first suspended in the extraction buffer and then precipitated. An aliquot portion of the clear supernatant of the sample is placed on the test field. The sample with the coloured latex particles, to which the antigen is attached if present if the test is positive, then passes through the membrane and is bonded to the specific collection bands. A green and/or red and/or blue band appears, depending on the antigens present in the sample. 4. Reagents provided There are enough reagents in the pack for 20 determinations. Cassette 20 det. 20 individually packed test cassettes Diluent 26 ml Extraction buffer, ready for use; contains 0.1 % sodium azide Pipet 25 pieces Bag containing 25 disposable pipettes RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
5 5. Storage instructions The pack can be stored at 2-30 C and can be used until the printed expiry date. After the expiry date, the quality guarantee is no longer valid. Likewise, the usability of the cassettes cannot be guaranteed once the external packaging of the individual cassette has been damaged. 6. Materials required but not provided - Test tubes for stool suspension - Vortex mixer (optional) - Micropipette (200 µl µl) - Waste container containing 0.5% sodium hypochlorite solution 7. Precautions for users For in vitro diagnostic use only. This test must only be carried out by trained laboratory personnel. The guidelines for working in medical laboratories must be followed and the instructions for carrying out the test strictly adhered to. The extraction buffer contains sodium azide as a preservative. These substances must not be allowed to come into contact with the skin or mucous membrane. Samples or reagents must not be pipetted by mouth and contact with injured skin or mucous membranes must be prevented. When handling the samples, wear disposable gloves and when the test is finished, wash your hands. Do not smoke, eat or drink in areas where samples are being used. All reagents and materials which come into contact with potentially infectious samples must be treated with suitable disinfectants (e.g. sodium hypochlorite) in exactly the same way as the samples themselves or autoclaved for at least one hour at 121 C. 8. Sample collection and storage Stool samples must be collected in clean containers without any additives and stored at 2-8 C before beginning the test. If stored for more than 3 days, the sample must be frozen at -20 C. In this case, the sample must be completely thawed out and brought to room temperature before testing begins. Avoid freezing and thawing the sample repeatedly. If rectal swabs have to be used, make sure that sufficient stool material (approx. 50 mg) is collected to carry out the test. RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
6 9. Test procedure 9.1. General information The samples, extraction buffer and test cassettes must be brought to room temperature (20-25 C) before using. The test cassettes must only be removed from the external packaging shortly before they are used. Once used, the cassettes must not be used again. The test must not be carried out in direct sunlight. Do not pour reagents back into vials since this may lead to contamination of the reagent Preparing the samples Place 1 ml Extraction Buffer Diluent in a labelled test tube. With the liquid stool sample, pipette Pipet 100 µl (up to just above the second thickening) of the sample and suspend it in the buffer which was placed in the tube beforehand. With solid stool samples, suspend 50 mg of the sample in the buffer. The sample must then be well homogenised. This can be achieved either by repeated suction and ejection of the suspension using the disposable pipette Pipet or, alternatively, by mixing on a vortex mixer. Afterwards, allow the homogeneous suspension to settle for at least 3 minutes until a clear supernatant is formed Testing the sample When removed from the external packing, first lay the test cassette Cassette on a level mat. After this, pipette 200 µl (micropipette) or 4 drops (disposable pipette) Pipet of the clear supernatant of the stool suspension into the round opening of the test cassette. Make sure that the liquid flows through the membrane unimpeded. Any particles pipetted at the same time can cause an obstruction and must be removed beforehand. The test result can be read off after 10 minutes. 10. Quality control signs of reagent expiry The test must only be evaluated if the test cassette is intact before the sample suspension is pipetted into it and no colour changes or bands are visible on the membrane. In addition to this, at least the crimson control band must be visible after the test incubation. If this does not appear, the following must be checked before repeating the test: - Expiry date of the test cassettes and the extraction buffer being used - Correct test procedure - Contamination of the extraction buffer If the control band is still not visible after repeating the test with a new test cassette, please contact the manufacturer or your local R-Biopharm distributor. RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
7 11. Evaluation and interpretation A maximum of four bands should appear in the following order, as seen from the sampleapplication site: one blue ( 1 = Test band 1), one red ( 2 = Test band 2), one green ( 3 = Test band 3) and one crimson (C = control band) band. If the crimson control band is missing, the test is invalid and cannot be evaluated! The following interpretations are possible: - Cryptosporidia positive: blue and crimson bands are visible. - Giardia positive: red and crimson bands are visible. - Entamoeba positive: green and crimson bands are visible. The three specific test bands may also appear in any combination with the crimson control band, depending on which of the three pathogens are present in the sample. - Negative: only the crimson control band appears. - Not valid: no visible band or a combination other than the one described above or other changes in band colour. Likewise, any changes in band colour which appear after 10 minutes or later are also without any diagnostic value and must not be used for evaluation. 12. Limitations of the method RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi detects the antigens of Cryptosporidium parvum and / or Giardia lamblia and / or Entamoeba histolytica (sensu lato) in stool samples. The test cannot be used to derive a relationship between the intensity of the specific visible bands and the occurrence or severity of clinical symptoms. The results obtained must always be interpreted in combination with the clinical picture. A positive result does not rule out the presence of another infectious pathogen. A negative result does not necessarily mean that there is no infection with Cryptosporidiae, Lambliae or Entamoebae. This can be due to intermittent excretion of the pathogen or to the quantity of antigens in the sample being too small. If the patient is anaemic or is suspected as being infected by the pathogens being looked for, another stool sample should be tested after four weeks. An excess of stool sample can cause brownish bands to appear instead of the specifically coloured bands. These brownish bands do not have any diagnostic value. In such cases, it will be necessary to repeat the test with a smaller stool quantity or dilute the suspension already prepared further (clear supernatant after precipitation) in order to clarify whether the pathogens being looked for are in the sample and have been masked by too much stool matrix. RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
8 13. Performance characteristics Clinical comparison study In a multi-centre study involving five different institutions, a total of 252 stool samples (which had been determined beforehand using different methods and kept frozen for later use) were thawed and analysed using the RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi rapid assay. The individual results are listed in Table 1. The average sensitivity and specificity have been calculated from the individual results from the five validation centres. Table 1 Results from a multi-centre study using the RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi rapid assay Samples Specific Parasite Test Band total pos. negative Cryptosporidium Giardia Entamoeba Reference no other method parasites Sens. Spec. Sens. Spec. Sens. Spec. Microscopy Microscopy Microscopy Microscopy / PCR Elisa Total % 93.3% 91.9% 99.5% 84.8% 87.4% Cross reactivity None of the following specified intestine parasites led to a cross reaction in RIDA QUICK Cryptosporidium/Giardia/ Entamoeba Combi: Entamoeba coli Blastocystis hominis Chilomastix mesnili Endolimax nana Entamoeba nana Entamoeba hartmannii Hymenolepsis nana Isospora belli Isospora felis Jodamoeba buetschlii RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
9 References 1. Black, R. E. et al.: Giardiasis in day-care centers: Evidence of person-to-person transmission. Pediatrics 60 (No. 4), (1977). 2. Craun, G. F.: Waterborne Giardiasis in the United States: A review. Am. J. Pub. Health 69 (No. 8), (1979). 3. Nask, T. E. et al.: Experimental human infections with Giardia lamblia. J. Infect. Dis. 156 (No. 6), (1987). 4. Smith, H. V. et al.: Giardia and Giardiasis: What's in a name? Microbiol. Eur. 3 (No. 1), (1995). 5. Thompson, R. C. A., Reynoldson, J. A.: Giardia and Giardiasis. Adv. Parasitol. 32, (1993) 6. Xiao, L.: Giardia infection in farm animals. Parasitology today 10 (No. 11), (1994). 7. Schunk, M. et al.: Detection of Giardia lamblia and Entamoeba histolytica in stool samples by two enzyme immunoassays. Eur. J. Clin. Microbiol. Infect. Dis. 20, (2001) 8. Clavel, A.: Evaluation of the optimal number of fecal specimens in the diagnosis of cryptosporidiosis in AIDS and immunocompetent patients. Eur. Journal Clin. Microbiol. Infect. Dis. 14, (1995). 9. Current, W. L., Garcia, L. S.: Cryptosporidiosis. Clinics in Laboratory Medicine 11 (No. 4), (1991). 10. Current, W. L., Garcia, L. S.: Cryptosporidiosis. Clin. Microbiol. Rev. 4 (No. 3), (1991). 11. Flanigan, T. P.: Human immunodeficiency virus infection and cryptosporidiosis: Protective immune responses. Am. J. Trop. Med. Hyg. 50 (5) Suppl., (1994). 12. Guarino, A. et al.: Human intestinal cryptosporidiosis: secretory diarrhea and enterotoxic activity in Caco-2 cells. J. Infect. Dis. 171, (1995). 13. Hayes, E. B. et al.: Large community outbreak of cryptosporidiosis due to contamination of a filtered public water supply. New. Engl. J. Med. 320 (No. 21), (1989). 14. Le Chevallier, M. W. et al.: Giardia and Cryptosporidium spp. in filtered drinking water supplies. Appl. Environ. Microbiol. 57 (No. 9), (1991). 15. Mc. Anulty, J. M. et al.: A community wide outbreak of cryptosporidiosis associated with swimming at a wave pool. Jama 272 (No. 20), (1994). 16. Bracha, R. et al.: Differentiation of clinical isolates of Entamoeba histolytica by using specific DNA probes. Clin. Microbiol. 28 (No. 4), (1990). 17. Citronberg, R. J., Semel, J. D.: Severe vaginal infection with Entamoeba histolytica in a woman who recently returned from Mexico: Case report and review. Clin. Infect. Dis. 20, (1995). RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
10 18. Espinosa-Cantellano, M., Martinez-Palomo, A.: Entamoeba histolytica: Mechanism of surface receptor capping. Exp. Parasitol. 79, (1994). 19. Katzwinkel-Wladarsch, S. et al.: Direct amplification and differentiation of pathogenic and nonpathogenic Entamoeba histolytica DNA from stool specimen. Am. J. Trop. Med.-Hyg. 51 (1), (1994). 20. Kean, B. H. et al.: Epidemic of amoebiasis and giardiasis in a biased population. Brit. J. Ven. Dis. 55, (1979). 21. Mannweiler, E.: Immundiagnostik der Amöbiasis. Der Mikrobiologe 5. Jg., Heft 6, (1995). 22. Ohnishi, K. et al.: Brain abscess due to infection with Entamoeba histolytica. Am. J. Trop. Med. Hyg. 51 (2), (1994). 23. Petter, R. et al.: Characterization of two distinct gene transcripts for ribosomal protein L 21 from pathogenic and nonpathogenic strains of Entamoeba histolytica. Gene 150, (1994). 24. Reed, Sh. L.: New Concepts regarding the pathogenesis of amebiasis. Clin. Infect. Dis. 21 (Suppl. 2), (1995). 25. Strachan, W. D. et al.: Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet 12, (1988). 26. van Lunzen, J., Tannich, E., Burchard, G.-D.: Amöbenruhr und Amöbenleberabszeß. Deutsches Ärzteblatt 93, Heft 2, (1996). RIDA QUICK Cryptosporidium/Giardia/Entamoeba Combi
RIDA QUICK Entamoeba. Article no: N 1703
 RIDA QUICK Entamoeba Article no: N 1703 R-Biopharm AG, Landwehrstr. 54, D-64293 Darmstadt, Germany Tel: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic use.
RIDA QUICK Entamoeba Article no: N 1703 R-Biopharm AG, Landwehrstr. 54, D-64293 Darmstadt, Germany Tel: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic use.
RIDA QUICK Entamoeba. Article no: N1702
 RIDA QUICK Entamoeba Article no: N1702 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Phone: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic
RIDA QUICK Entamoeba Article no: N1702 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Phone: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic
RIDA QUICK Rotavirus. Article no: N0902
 RIDA QUICK Rotavirus Article no: N0902 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Phone: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic
RIDA QUICK Rotavirus Article no: N0902 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Phone: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 1. Intended use For in vitro diagnostic
Rapid-VIDITEST. Rota-Adeno Blister. One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual
 Rapid-VIDITEST Rota-Adeno Blister One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual INTENDED USE: The Rapid-VIDITEST Rota-Adeno Blister test is a rapid chromatographic immunoassay
Rapid-VIDITEST Rota-Adeno Blister One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual INTENDED USE: The Rapid-VIDITEST Rota-Adeno Blister test is a rapid chromatographic immunoassay
Rotavirus Test Kit. Instructions For Use. Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA
 Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Catalog # OKDA00123 SUMMARY AND EXPLANATION
 Aviva Systems Biology 5754 Pacific Center Blvd., Suite 201, San Diego, CA 92121, USA Tel: 858-552-6979 Fax: 858-552-6975 www.avivasysbio.com Email: info@avivasysbio.com Giardia lamblia ELISA Kit Catalog
Aviva Systems Biology 5754 Pacific Center Blvd., Suite 201, San Diego, CA 92121, USA Tel: 858-552-6979 Fax: 858-552-6975 www.avivasysbio.com Email: info@avivasysbio.com Giardia lamblia ELISA Kit Catalog
Rapid-VIDITEST FOB Blister
 Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST Enterovirus
 Rapid-VIDITEST Enterovirus A rapid one step Enterovirus Card test for the qualitative detection of Enterovirus antigens in human feces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
Rapid-VIDITEST Enterovirus A rapid one step Enterovirus Card test for the qualitative detection of Enterovirus antigens in human feces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Cryptosporidium Veterinary
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Bacillary Dysentery (Shigellosis)
 Bacillary Dysentery (Shigellosis) An acute bacterial disease involving the large and distal small intestine, caused by the bacteria of the genus shigella. Infectious agent Shigella is comprised of four
Bacillary Dysentery (Shigellosis) An acute bacterial disease involving the large and distal small intestine, caused by the bacteria of the genus shigella. Infectious agent Shigella is comprised of four
Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Giardiasis. Table of Contents
 Table of Contents Case Definition... Error! Bookmark not defined. Reporting Requirements... 2 Etiology... Error! Bookmark not defined. Clinical Presentation... Error! Bookmark not defined. Diagnosis...
Table of Contents Case Definition... Error! Bookmark not defined. Reporting Requirements... 2 Etiology... Error! Bookmark not defined. Clinical Presentation... Error! Bookmark not defined. Diagnosis...
Rapid-VIDITEST Shigella dysenteriae
 Rapid-VIDITEST Shigella dysenteriae One step Shigella dysenteriae test for the qualitative detection of Shigella dysenteriae in faeces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
Rapid-VIDITEST Shigella dysenteriae One step Shigella dysenteriae test for the qualitative detection of Shigella dysenteriae in faeces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
HAV IgG/IgM Rapid Test
 HAV IgG/IgM Rapid Test Cat. No.:DTS649 Pkg.Size: Intended use CD HAV IgG/IgM Rapid Test is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM
HAV IgG/IgM Rapid Test Cat. No.:DTS649 Pkg.Size: Intended use CD HAV IgG/IgM Rapid Test is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM
E. Histolytica IgG ELISA Kit
 E. Histolytica IgG ELISA Kit Catalog Number KA3193 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
E. Histolytica IgG ELISA Kit Catalog Number KA3193 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Rapid-VIDITEST. Astrovirus Card
 Rapid-VIDITEST Astrovirus Card One step Astrovirus Card Test. Instruction manual INTENDED USE: The Rapid-VIDITEST Astrovirus Card is a one step coloured chromatographic immunoassay for the qualitative
Rapid-VIDITEST Astrovirus Card One step Astrovirus Card Test. Instruction manual INTENDED USE: The Rapid-VIDITEST Astrovirus Card is a one step coloured chromatographic immunoassay for the qualitative
Crypto / Giardia Ag Combo (Fecal) ELISA
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Rapid-VIDITEST Swine Flu
 Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Sure-Vue Signature Cryptosporidium/Giardia Test
 SAMPLE PROCEDURE This procedure is provided to Fisher Healthcare customers to assist with the development of laboratory procedures. This document was derived from, and was current with, the instructions
SAMPLE PROCEDURE This procedure is provided to Fisher Healthcare customers to assist with the development of laboratory procedures. This document was derived from, and was current with, the instructions
Rapid-VIDITEST. Rota-Adeno. One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual
 Rapid-VIDITEST Rota-Adeno One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420
Rapid-VIDITEST Rota-Adeno One Step Rotavirus and Adenovirus Antigen Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420
E. Histolytica IgG (Amebiasis)
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Amebiasis Ab E. histolytica IgG
 Code: HP003 96 Test distribuito da: Amebiasis Ab E. histolytica IgG Amebiasis E. histolytica lgg test is an enzyme immunoassay for the detection of specific lgg antibodies against Anti - Entamoeba histolytica
Code: HP003 96 Test distribuito da: Amebiasis Ab E. histolytica IgG Amebiasis E. histolytica lgg test is an enzyme immunoassay for the detection of specific lgg antibodies against Anti - Entamoeba histolytica
PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia)
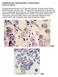 PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia) A biopsy was performed on a 27-year-old man with no known travel history, presenting with a perianal ulcer. The specimen was preserved in formalin
PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia) A biopsy was performed on a 27-year-old man with no known travel history, presenting with a perianal ulcer. The specimen was preserved in formalin
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Amoebiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
August 2011 Amoebiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
Rapid-VIDITEST. Helicobacter pylori. One step Helicobacter pylori Blister test. Instruction manual
 Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
ccess safe drinking wa r is everyone s right Protozoans that cause diarrheal disease
 ccess safe drinking wa r is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
ccess safe drinking wa r is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions For Use For In Vitro Diagnostic Use
 Medical Chemical Corp., 19430 Van Ness Avenue, Torrance, California 90501 (310)787-6800, Fax (310)787-4464 Catalog # MCC-C/G - DFA, 75 Test PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions
Medical Chemical Corp., 19430 Van Ness Avenue, Torrance, California 90501 (310)787-6800, Fax (310)787-4464 Catalog # MCC-C/G - DFA, 75 Test PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions
RIDA GENE Parasitic Stool Panel II real-time PCR
 RIDA GENE Parasitic Stool Panel II real-time PCR Art. Nr.: PG1725 100 Reactions For in vitro diagnostic use. -20 C R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Tel.: +49 (0) 61
RIDA GENE Parasitic Stool Panel II real-time PCR Art. Nr.: PG1725 100 Reactions For in vitro diagnostic use. -20 C R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Tel.: +49 (0) 61
Cryptosporidium spp. ELISA Kit
 Cryptosporidium spp. ELISA Kit Catalog Number KA2094 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Cryptosporidium spp. ELISA Kit Catalog Number KA2094 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
TYPES OF ORGANISM RELATIONSHIPS
 TYPES OF ORGANISM RELATIONSHIPS Normal Flora. Normal flora consists of microorganisms that are normally and consistently found in or on the body in the absence of disease. Symbiosis. This is the close
TYPES OF ORGANISM RELATIONSHIPS Normal Flora. Normal flora consists of microorganisms that are normally and consistently found in or on the body in the absence of disease. Symbiosis. This is the close
Giardia lamblia (flagellates)
 Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
Rapid-VIDITEST Calprotectin
 Rapid-VIDITEST Calprotectin One Step Calprotectin Card Test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz INTENDED
Rapid-VIDITEST Calprotectin One Step Calprotectin Card Test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz INTENDED
Rubella Latex Agglutination Test
 Rubella Latex Agglutination Test Cat. No.:DLAT1088 Pkg.Size:30T Intended use The Rubella Latex Agglutination Test is a rapid latex particle agglutination test for the qualitative and semi-quantitative
Rubella Latex Agglutination Test Cat. No.:DLAT1088 Pkg.Size:30T Intended use The Rubella Latex Agglutination Test is a rapid latex particle agglutination test for the qualitative and semi-quantitative
Foodborne Disease in the Region of Peel
 Foodborne Disease in the Region of Peel HIGHLIGHTS The incidence of selected foodborne diseases was generally higher in Peel than in Ontario between 1993 and 22. A higher incidence was observed in Peel
Foodborne Disease in the Region of Peel HIGHLIGHTS The incidence of selected foodborne diseases was generally higher in Peel than in Ontario between 1993 and 22. A higher incidence was observed in Peel
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Giardiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
August 2011 Giardiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
Rapid-VIDITEST. Influenza A
 Rapid-VIDITEST Influenza A (One step Influenza A Card test for the detection of Influenza type A antigen from human nasopharyngeal specimens (swab, nasopharyngeal wash and aspirate). Instruction manual
Rapid-VIDITEST Influenza A (One step Influenza A Card test for the detection of Influenza type A antigen from human nasopharyngeal specimens (swab, nasopharyngeal wash and aspirate). Instruction manual
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: ABON HIV 1/2/O Tri-Line Human Immunodeficiency Virus Rapid Test Device Number: PQDx 0141-051-00 Abstract ABON HIV 1/2/O Tri-Line Human
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: ABON HIV 1/2/O Tri-Line Human Immunodeficiency Virus Rapid Test Device Number: PQDx 0141-051-00 Abstract ABON HIV 1/2/O Tri-Line Human
CDIA TM Rubella IgG/IgM Rapid Test Kit
 CDIA TM Rubella IgG/IgM Rapid Test Kit Cat.No: DTSJZ024 Lot. No. (See product label) Intended Use The CDIA TM Rubella IgG/IgM Rapid Test Kit is a rapid chromatographic immunoassay for the qualitative detection
CDIA TM Rubella IgG/IgM Rapid Test Kit Cat.No: DTSJZ024 Lot. No. (See product label) Intended Use The CDIA TM Rubella IgG/IgM Rapid Test Kit is a rapid chromatographic immunoassay for the qualitative detection
Entamoeba histolytica/e. dispar. A. Haghighi,
 Entamoeba histolytica/e. dispar A. Haghighi, Wednesday, February 14, 2018 Classification of Protozoa? The protozoa are generally unicellular and may be divided for convenience, into four distinct groups
Entamoeba histolytica/e. dispar A. Haghighi, Wednesday, February 14, 2018 Classification of Protozoa? The protozoa are generally unicellular and may be divided for convenience, into four distinct groups
Giardia lamblia/ Cryptosporidium spp. ELISA Kit
 Giardia lamblia/ Cryptosporidium spp. ELISA Kit Catalog Number KA2095 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background...
Giardia lamblia/ Cryptosporidium spp. ELISA Kit Catalog Number KA2095 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background...
Giardia lamblia ELISA Kit
 Giardia lamblia ELISA Kit Catalog Number KA2093 96 assay Version: 01 Intend for research use only www.abnova.com Introduction and Background A. Intended Use Giardia lamblia ELISA Kit is an Enzyme-Linked
Giardia lamblia ELISA Kit Catalog Number KA2093 96 assay Version: 01 Intend for research use only www.abnova.com Introduction and Background A. Intended Use Giardia lamblia ELISA Kit is an Enzyme-Linked
Fecal H. pylori Antigen Rapid Test (Strip)
 Fecal H. pylori Antigen Rapid Test (Strip) Cat. No.:DTS526 Pkg.Size: Intended use This H. pylori Antigen Rapid Test is intended for the direct qualitative detection of the presence of H. pylori antigen
Fecal H. pylori Antigen Rapid Test (Strip) Cat. No.:DTS526 Pkg.Size: Intended use This H. pylori Antigen Rapid Test is intended for the direct qualitative detection of the presence of H. pylori antigen
American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX
 Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A. Real-Time RT-PCR Detection.
 For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
For in vitro Veterinary Diagnostics only. Kylt Rotavirus A Real-Time RT-PCR Detection www.kylt.eu DIRECTION FOR USE Kylt Rotavirus A Real-Time RT-PCR Detection A. General Kylt Rotavirus A products are
Hepatitis C Virus (HCV) Antibody Test
 Hepatitis C Virus (HCV) Antibody Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HCV Specimen: Serum/Plasma * Please read the instructions carefully before use INTENDED USE Hepatitis
Hepatitis C Virus (HCV) Antibody Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HCV Specimen: Serum/Plasma * Please read the instructions carefully before use INTENDED USE Hepatitis
HBeAg and HBeAg Ab ELISA Kit
 HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 17 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 17 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
Recent Diagnostic Methods for Intestinal Parasitic Infections
 Recent Diagnostic Methods for Intestinal Parasitic Infections By Dr. Doaa Abdel Badie Salem Lecturer of Medical Parasitology, Mansoura Faculty of Medicine Agenda Intestinal parasites. Traditional Diagnostic
Recent Diagnostic Methods for Intestinal Parasitic Infections By Dr. Doaa Abdel Badie Salem Lecturer of Medical Parasitology, Mansoura Faculty of Medicine Agenda Intestinal parasites. Traditional Diagnostic
Anton van Leeuwenhoek. Protozoa: This is what he saw in his own stool sample. Morphology 10/14/2009. Protozoans that cause diarrheal disease
 Access to safe drinking water is everyone s right Anton van Leeuwenhoek Protozoa: Protozoans that cause diarrheal disease This is what he saw in his own stool sample 1. Giardia lamblia 2. Entameba histolytica
Access to safe drinking water is everyone s right Anton van Leeuwenhoek Protozoa: Protozoans that cause diarrheal disease This is what he saw in his own stool sample 1. Giardia lamblia 2. Entameba histolytica
MALARIA P. FALCIPARUM / P. VIVAX
 MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
ELISA TEST FOR DETECTION OF BLASTOCYSTIS SPP. IN HUMAN FAECES: COMPARISON OF THREE METHODS
 ELISA TEST FOR DETECTION OF BLASTOCYSTIS SPP. IN HUMAN FAECES: COMPARISON OF THREE METHODS István Kucsera, Mónika Molnár, Eszter Gályász, József Danka, Erika Orosz National Center for Epidemiology, Department
ELISA TEST FOR DETECTION OF BLASTOCYSTIS SPP. IN HUMAN FAECES: COMPARISON OF THREE METHODS István Kucsera, Mónika Molnár, Eszter Gályász, József Danka, Erika Orosz National Center for Epidemiology, Department
Access to safe drinking water is everyone s right. Protozoans that cause diarrheal disease
 Access to safe drinking water is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
Access to safe drinking water is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
Amoebiasis. (Amoebic dysentery)
 Amoebiasis (Amoebic dysentery) Causative agent: Entamoeba histolytica Amoebiasis Harbouring of protozoa E. histolytica inside the body with or without disease only 10% of infected develop disease two types
Amoebiasis (Amoebic dysentery) Causative agent: Entamoeba histolytica Amoebiasis Harbouring of protozoa E. histolytica inside the body with or without disease only 10% of infected develop disease two types
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
H.Pylori IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Outline EP1201. NEHA 2012 AEC June 2012
 Food and Water Borne Enteric Protozoa: Environmental Health Perspectives Stephanie M. Fletcher, PhD (c) NEHA AEC & Exhibition San Diego June 30, 2012 Outline Introduction Overview of the Epidemiology of
Food and Water Borne Enteric Protozoa: Environmental Health Perspectives Stephanie M. Fletcher, PhD (c) NEHA AEC & Exhibition San Diego June 30, 2012 Outline Introduction Overview of the Epidemiology of
H.Pylori IgG Cat # 1503Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
A New Multiplex Real-time PCR Assay For Detection Of Intestinal Parasites
 A New Multiplex Real-time PCR Assay For Detection Of Intestinal Parasites Dr. Andreas Simons Worldwide provider of diagnostic assay solutions Offers a variety of test kit methodologies R-Biopharm Headquarters
A New Multiplex Real-time PCR Assay For Detection Of Intestinal Parasites Dr. Andreas Simons Worldwide provider of diagnostic assay solutions Offers a variety of test kit methodologies R-Biopharm Headquarters
Hepatitis A virus IgM ELISA Kit
 Hepatitis A virus IgM ELISA Kit Catalog Number KA0285 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Hepatitis A virus IgM ELISA Kit Catalog Number KA0285 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Triiodothyronine (T3) ELISA
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol
 IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol (Cat. No.:EK-310-85) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED
IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol (Cat. No.:EK-310-85) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED
Mouse/Rat THYROXINE (T4) ELISA Catalog No (96 Tests)
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Mouse/Rat Thryroxine Kit is intended for the detection of total T4 in mouse/rat serum or plasma. SUMMARY AND EXPLANATION
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Mouse/Rat Thryroxine Kit is intended for the detection of total T4 in mouse/rat serum or plasma. SUMMARY AND EXPLANATION
Giardiasis Surveillance Protocol
 Provider Responsibilities 1. Report all cases to your local health department by completing the provider section of the WVEDSS form within the timeframe indicated: Sporadic case of - should be reported
Provider Responsibilities 1. Report all cases to your local health department by completing the provider section of the WVEDSS form within the timeframe indicated: Sporadic case of - should be reported
Rapid-VIDITEST. FOB+Tf. One step Fecal Occult Blood Card Test. Instruction manual
 Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
IVD information *Droppers for the sensitized and control cells. Not for use other than dispensing the sensitized and control cells.
 In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
H.pylori IgA Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
IV2-113E Use by. Invitron Glargine ELISA Kit REF LOT IVD. Definitions. English. For in-vitro diagnostic use. Instructions for use.
 Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
Chymotrypsin ELISA Kit
 Chymotrypsin ELISA Kit Cat. No.:DEIA10041 Pkg.Size:96T Intended use The Chymotrypsin ELISA Kit is a sandwich Enzyme Immuno Assay intended for the quantitative determination of Chymotrypsin in stool. General
Chymotrypsin ELISA Kit Cat. No.:DEIA10041 Pkg.Size:96T Intended use The Chymotrypsin ELISA Kit is a sandwich Enzyme Immuno Assay intended for the quantitative determination of Chymotrypsin in stool. General
Chlamydia trachomatis (CHLa)Test Kit
 Chlamydia trachomatis (CHLa)Test Kit Instructions For Use Format: Cassette Specimen: Urethral/Genital Swab Catalog Number: VEL-001-CHLa * Please read the instructions carefully before use INTENDED USE
Chlamydia trachomatis (CHLa)Test Kit Instructions For Use Format: Cassette Specimen: Urethral/Genital Swab Catalog Number: VEL-001-CHLa * Please read the instructions carefully before use INTENDED USE
cytoplasm contains two 2 nuclei and two parabasal bodies (Figure 7).
 Dr. Jabar Etaby Lecture one GIARDIASIS (lambliasis) Etiology: Giardia lamblia (flagellate) Epidemiology: It has worldwide distribution and is not uncommon in South Carolina. It is the most frequent protozoan
Dr. Jabar Etaby Lecture one GIARDIASIS (lambliasis) Etiology: Giardia lamblia (flagellate) Epidemiology: It has worldwide distribution and is not uncommon in South Carolina. It is the most frequent protozoan
Rapid-VIDITEST. Strep A Card. One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture.
 Rapid-VIDITEST Strep A Card One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec,
Rapid-VIDITEST Strep A Card One step Strep A Card for the detection of Group A Streptococcal antigen from throat swabs or culture. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec,
25(OH) Vitamin D ELISA (BD-220BA), 192 Tests
 INTENDED USE The 25-hydroxy (25-OH) Vitamin D ELISA is intended for the quantitative determination of total 25-OH Vitamin D in human serum and Plasma. SUMMARY AND EXPLANATION Vitamin D is a steroid hormone
INTENDED USE The 25-hydroxy (25-OH) Vitamin D ELISA is intended for the quantitative determination of total 25-OH Vitamin D in human serum and Plasma. SUMMARY AND EXPLANATION Vitamin D is a steroid hormone
Rapid-VIDITEST. Influenza A+B
 Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
1. Intended Use New Influenza A virus real time RT-PCR Panel is used for the detection of universal influenza A virus, universal swine Influenza A vir
 New Influenza A Virus Real Time RT-PCR Kit User Manual LT028310RRFY - 1 - 1. Intended Use New Influenza A virus real time RT-PCR Panel is used for the detection of universal influenza A virus, universal
New Influenza A Virus Real Time RT-PCR Kit User Manual LT028310RRFY - 1 - 1. Intended Use New Influenza A virus real time RT-PCR Panel is used for the detection of universal influenza A virus, universal
See external label. 2 C-30 C for test card and Sample tube Cat.# OneStep Rotavirus Antigen RapiCard InstaTest.
 CORTEZ DIAGNOSTICS, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 USA Tel: (818) 591-3030 Fax: (818) 591-8383 E-mail: onestep@rapidtest.com Web site: www.rapidtest.com See external label
CORTEZ DIAGNOSTICS, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 USA Tel: (818) 591-3030 Fax: (818) 591-8383 E-mail: onestep@rapidtest.com Web site: www.rapidtest.com See external label
H. pylori IgM. Cat # H. pylori IgM ELISA. ELISA: Enzyme Linked Immunosorbent Assay. ELISA - Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
HAV IgM Rapid Test(Cassette)
 HAV IgM Rapid Test(Cassette) Cat. No.:DTS586 Pkg.Size:25 T Intended use The HAV IgM Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of IgM antibody to Hepatitis A
HAV IgM Rapid Test(Cassette) Cat. No.:DTS586 Pkg.Size:25 T Intended use The HAV IgM Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of IgM antibody to Hepatitis A
Dr. Jabar Etaby Lecture GIARDIASIS(lambliasis) Etiology: Giardia lamblia (flagellate)
 Dr. Jabar Etaby Lecture Two GIARDIASIS(lambliasis) Etiology: Giardia lamblia (flagellate) Epidemiology: It has worldwide distribution and is not uncommon in South Carolina. It is the most frequent protozoan
Dr. Jabar Etaby Lecture Two GIARDIASIS(lambliasis) Etiology: Giardia lamblia (flagellate) Epidemiology: It has worldwide distribution and is not uncommon in South Carolina. It is the most frequent protozoan
ENG MYCO WELL D- ONE REV. 1.UN 29/09/2016 REF. MS01283 REF. MS01321 (COMPLETE KIT)
 ENG MYCO WELL D- ONE MYCO WELL D-ONE System for the presumptive identification and antimicrobial susceptibility test of urogenital mycoplasmas, Gardnerella vaginalis, Trichomonas vaginalis, Candida albicans
ENG MYCO WELL D- ONE MYCO WELL D-ONE System for the presumptive identification and antimicrobial susceptibility test of urogenital mycoplasmas, Gardnerella vaginalis, Trichomonas vaginalis, Candida albicans
Insulin (Porcine/Canine) ELISA
 Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
PREVALENCE OF DIFFERENT PROTOZOAN PARASITES IN PATIENTS VISITING AT ICDDR B HOSPITAL, DHAKA
 J. Asiat. Soc. Bangladesh, Sci. 39(1): 117-123, June 213 PREVALENCE OF DIFFERENT PROTOZOAN PARASITES IN PATIENTS VISITING AT ICDDR B HOSPITAL, DHAKA SHAHELA ALAM, HAMIDA KHANUM 1, RIMI FARHANA ZAMAN AND
J. Asiat. Soc. Bangladesh, Sci. 39(1): 117-123, June 213 PREVALENCE OF DIFFERENT PROTOZOAN PARASITES IN PATIENTS VISITING AT ICDDR B HOSPITAL, DHAKA SHAHELA ALAM, HAMIDA KHANUM 1, RIMI FARHANA ZAMAN AND
Treponema Pallidum (TP) Antibody Test
 Treponema Pallidum (TP) Antibody Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-TP Specimen: Serum/Plasma * Please read the instructions carefully before use INTENDED USE Velotest
Treponema Pallidum (TP) Antibody Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-TP Specimen: Serum/Plasma * Please read the instructions carefully before use INTENDED USE Velotest
CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol
 CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol (Cat. No.:EK-310-91) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE The
CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol (Cat. No.:EK-310-91) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE The
Prevalence of Intestinal Parasites in HIV Seropositive Patients with and without Diarrhoea and its Correlation with CD4 Counts
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 10 (2016) pp. 527-532 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.510.058
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 10 (2016) pp. 527-532 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.510.058
DRG International, Inc., USA Fax: (908)
 Please use only the valid version of the package insert provided with the kit. 1 INTENDED USE The human Annexin V ELISA is an enzyme-linked immunosorbent assay for determination of human Annexin V. The
Please use only the valid version of the package insert provided with the kit. 1 INTENDED USE The human Annexin V ELISA is an enzyme-linked immunosorbent assay for determination of human Annexin V. The
CONTROL OF VIRAL GASTROENTERITIS OUTBREAKS IN CALIFORNIA LONG-TERM CARE FACILITIES
 CONTROL OF VIRAL GASTROENTERITIS OUTBREAKS IN CALIFORNIA LONG-TERM CARE FACILITIES California Department of Health Services Division of Communicable Disease Control In Conjunction with Licensing and Certification
CONTROL OF VIRAL GASTROENTERITIS OUTBREAKS IN CALIFORNIA LONG-TERM CARE FACILITIES California Department of Health Services Division of Communicable Disease Control In Conjunction with Licensing and Certification
Parasitology. Lab. Amoeba
 Parasitology. Lab. Kingdom : Protista Subkingdom : Protozoa Phylum : Sacromastigophora Subphylum : Sarcodina Superclass : Rhizopoda Class : Lobosea Order : Amoebida Amoeba Protozoa Amoebae geneus Entamoeba
Parasitology. Lab. Kingdom : Protista Subkingdom : Protozoa Phylum : Sacromastigophora Subphylum : Sarcodina Superclass : Rhizopoda Class : Lobosea Order : Amoebida Amoeba Protozoa Amoebae geneus Entamoeba
Helicobacter pylori Antigen Test
 Helicobacter pylori Antigen Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HP(s) Specimen: Fecal Specimen * Please read the instructions carefully before use INTENDED USE Helicobacter
Helicobacter pylori Antigen Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HP(s) Specimen: Fecal Specimen * Please read the instructions carefully before use INTENDED USE Helicobacter
H. pylori IgM CLIA kit
 H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
Influenza A IgG ELISA
 Influenza A IgG ELISA For the qualitative determination of IgG-class antibodies against Influenza A virus in human serum or plasma (citrate, heparin). For Research Use Only. Not For Use In Diagnostic Procedures.
Influenza A IgG ELISA For the qualitative determination of IgG-class antibodies against Influenza A virus in human serum or plasma (citrate, heparin). For Research Use Only. Not For Use In Diagnostic Procedures.
FinTest TM IgG4 Screen 88 ELISA Kit
 FinTest TM IgG4 Screen 88 ELISA Kit Cat. No.:DEIA6227 Pkg.Size:96T Intended use The Kit is for the quantitative determination of IgG4 antibodies against 88 Food Allergens in human serum, plasma and capillary
FinTest TM IgG4 Screen 88 ELISA Kit Cat. No.:DEIA6227 Pkg.Size:96T Intended use The Kit is for the quantitative determination of IgG4 antibodies against 88 Food Allergens in human serum, plasma and capillary
Human Cytomegalovirus IgM ELISA Kit
 Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Amebiasis rev Jan 2018
 rev Jan 2018 BASIC EPIDEMIOLOGY Infectious Agent Entamoeba histolytica, a protozoan parasite. The trophozoite is the active form of the parasite which causes symptoms. Cysts are the infectious form which
rev Jan 2018 BASIC EPIDEMIOLOGY Infectious Agent Entamoeba histolytica, a protozoan parasite. The trophozoite is the active form of the parasite which causes symptoms. Cysts are the infectious form which
Human HBcAb IgM ELISA kit
 Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Cortisol (Sheep) ELISA Kit
 Cortisol (Sheep) ELISA Kit Catalog Number KA0919 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
Cortisol (Sheep) ELISA Kit Catalog Number KA0919 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
WYANDOT COUNTY 2016 COMMUNICABLE DISEASE REPORT
 WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
