Chapter 2 Rationale and Objective
|
|
|
- Shana Bennett
- 5 years ago
- Views:
Transcription
1 Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44
2 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the human arena has been focused on the optimization of drug delivery. Traditionally, these delivery systems have been drug specific, revolving around a particular drug and its clinical application. However, the past two decades have witnessed the emergence of numerous drug delivery technologies that have developed independently of any specific compound or diseased condition 118 (Rathbone MJ et al, 2003). An ideal dosage regimen for the treatment of any disease is the one, which attains therapeutic concentration of a drug molecule in plasma and or at the site of action in predetermined time interval and is subsequently required to be maintained during treatment. Conventional dosage forms have many limitations like patient noncompliance, chances of missing the dose because of frequent administration, peak-valley concentration profile, fluctuating blood levels, gastro intestinal (GI) irritation, first pass metabolism, degradation in GI acidity etc. Extended release drug delivery systems can be used to overcome the above mentioned limitations. Extended release drug delivery systems are designed to deliver drugs specific target tissues at the right amount, at and for the right amount of time and with minimum of side effects. These systems help to achieve prolonged therapeutic effect by continuously releasing the drug over an extended period of time after administration of a single dose. The blood level oscillations by multiple dosing of conventional dosage forms are reduced as a more even and effective drug level is maintained. The safety margin of high potency drugs can be increased and incidence of both local and systemic side effects can be reduced in sensitive patients. Objective of Extended Release Drug Delivery systems The fundamental need and principles underlying the development and use of modified drug delivery systems for humans include. Improved clinical effectiveness. The relative efficiency of reduced dosing regimens (i.e. improved patient compliance). Improved patient handling (e.g. nursing or outpatient visits for repeat drug administration) and medical care (e.g. handling of adverse events or side effects). SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 45
3 Need for generic development Many diseases are still major culprits for morbidity and mortality. There are not enough effective and sufficient drugs to control these diseases. However, to launch new molecules as drugs is too costly a proposition. Hence to recover cost of development, new molecule is allowed to be patented for some time. After patent expiry, other companies may launch same molecule as generic version. To make drug therapy more cost effective, it is necessary to reduce cost of drug development while taking care of safety and efficacy of drug product. Thus, generic drugs have to pass limited clinical trial in the form of comparative bioavailability or bioequivalence study. This accelerates the process of development and takes care of safety issues. This view is also supported by regulatory authorities. This makes drug therapy more cost effective, while taking care of safety and efficacy of drug product. Gliclazide is a second generation hypoglycemic sulfonylurea mainly used in the treatment of type II Diabetes mellitus. It is drug of choice as it has good tolerance, low incidence of hypoglycemia, low rate of secondary failure, inhibits platelet aggregation and increases fibrinolysis. Gliclazide - Need for extended release formulation Gliclazide is usually marketed in the base form. Gliclazide has absorption in gastrointestinal tract. Its oral bioavailability is in the range of 40-60% and shows linear response with the dose increment. Drugs that have absorption limited to the gastrointestinal tract coupled with poor absorption in the large intestine and colon, such drugs are usually regarded as inappropriate candidates for formulation into oral controlled delivery systems. This limitation on absorption (for example, in the gastrointestinal tract) is referred to as the absorption window. The gastrointestinal tract functions to propel ingested material from the stomach (where digestion takes place) into the small intestine (where absorption principally occurs) and on to the large intestine (where water is absorbed/secreted as part of body fluid regulation processes). Residence time for non-digestible materials in the stomach depends on whether one is dealing with a fed or fasting subject. Typical gastric emptying times for particular material (greater than a few millimeters in diameter) vary from a few tens of minutes in the fasted state to a few hours in the fed state. Transit times through the small intestine are consistently of the order of 3-4 hours. Oral controlled release delivery systems function by releasing their payload of the drug over an extended period of time following short period in the regions of SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 46
4 the gastrointestinal tract where good absorption of certain drugs is poor or non-existent, still releasing its contained drug albeit with a significant percentage of its payload still to be delivered. Drug when released from the dosage form in the circumstances described will not be absorbed. Thus, administration of a drug subject to a window of absorption in a conventional controlled release delivery system can lead to sub therapeutic blood levels and ineffective treatment of the disease state for which the drug was invented. Maintained or even improved bioavailability from an extended release dosage form that releases gliclazide at a rate likely to provide the desired plasma levels of drug for an extended time period might, however, be possible from a dosage form that has extended residence time in the upper gastrointestinal tract, resisting mechanisms that promote normal transit time for solid materials. The distinct advantages of gliclazide if it is an extended release product is it reduces the number of daily doses and increases patient compliance. Gliclazide being an extended release preparation can also avoid loading dose. This commonly occurs with conventional oral formulations when large doses are given which may cause sudden release and absorption of a large amount of drug. Gliclazide is released in smaller doses, and hence ensures increased bioavailability and decreased side effects. In contrast, conventional gliclazide has less bioavailability since absorption decreases as it passes through the lower part of small intestine. Conventional gliclazide has an oral bioavailability of 40-60% and gastrointestinal absorption is apparently complete within 6 hours of ingestion. Plasma t 1/2 is 10 hours. Hence it has to be given 2-3 times a day, whereas gliclazide extended release tablet being a controlled release formulation is released in small quantities in intestine where drug has better absorption and has a prolonged duration of action. The bioavailability increases to almost 100%. As gliclazide is released slowly, side effects like flatulence, abdominal discomfort, are less unlike plain gliclazide tablets. An inverse relationship was observed between the dose ingested and elative absorption with therapeutic doses ranging from mg suggesting the involvement of an active, storable absorption process. Thus extended release formulation of gliclazide can not only optimize the daily requirement of gliclazide but can also reduce the need of a higher dose. SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 47
5 Objective The primary objective of the project is to develop a stable, non-infringing extended release formulation which can be marketed as a generic product in the regulated market. To develop and evaluate a stable gliclazide extended release tablet formulation using three different technologies: i. Matrix technology ii. Melt granulation technology iii. Multiparticulate unit dose technology To carry out bio-equivalence studies for the developed formulation in human subjects (fasting condition). Pharmacoeconomics of the developed formulation for the comparison of the cost of one drug formulated with different technologies. SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 48
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS. Pharmaceutical Manufacturing-4
 CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
D9G : Oro-Mucosal Dosage Forms Development Background Paper
 D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
7. SUMMARY, CONCLUSION AND RECOMMENDATIONS
 211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
Revised European Guideline on PK and Clinical Evaluation of Modified Release Dosage Forms
 1st MENA Regulatory Conference on Bioequivalence, Biowaivers, Bioanalysis and Dissolution Jordan September 23 24, 2013 Revised European Guideline on PK and Clinical Evaluation of Modified Release Dosage
1st MENA Regulatory Conference on Bioequivalence, Biowaivers, Bioanalysis and Dissolution Jordan September 23 24, 2013 Revised European Guideline on PK and Clinical Evaluation of Modified Release Dosage
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
CHAPTER - IV OBJECTIVES OF THE STUDY
 CHAPTER - IV OBJECTIVES OF THE STUDY 4.1 BACKGROUND Human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) commonly referred to as HIV & AIDS have emerged as being
CHAPTER - IV OBJECTIVES OF THE STUDY 4.1 BACKGROUND Human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) commonly referred to as HIV & AIDS have emerged as being
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS
 CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
Routes of drug administration
 Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Suppository Chapter Content
 10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
Chapter 1 Introduction
 Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Interchangeable Drug Products - Additional Criteria
 Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
GSC CODEX MESSAGE CCFA48/2016/25
 FORM FOR THE SUBMISSION OF SUBSTANCES TO BE EVALUATED BY JECFA In completing this form, only brief information is required. The form may be retyped if more space is needed under any one heading provided
FORM FOR THE SUBMISSION OF SUBSTANCES TO BE EVALUATED BY JECFA In completing this form, only brief information is required. The form may be retyped if more space is needed under any one heading provided
FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES
 FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES 1 SCOPE In pursuance of section 47 of the Food and Drugs Law 1992, P.N.D.C.L 305B, as amended by Act 523, 1996, these
FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES 1 SCOPE In pursuance of section 47 of the Food and Drugs Law 1992, P.N.D.C.L 305B, as amended by Act 523, 1996, these
Tablets that melt in the mouth:
 Tablets that melt in the mouth: formulations for the future Dr Afzal R Mohammed Email: a.u.r.mohammed@aston.ac.uk A. R. Mohammed Slides are for personal use only. They are NOT for reproduction or publication
Tablets that melt in the mouth: formulations for the future Dr Afzal R Mohammed Email: a.u.r.mohammed@aston.ac.uk A. R. Mohammed Slides are for personal use only. They are NOT for reproduction or publication
Pharmaceutics I صيدالنيات 1. Unit 2 Route of Drug Administration
 Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
EVALUATION OF EFFERVESCENT FLOATING TABLETS. 6.7 Mathematical model fitting of obtained drug release data
 EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
Introducing Pharmacokinetics and Pharmacodynamics. Janice Davies Pharmacist Room 23 Maudland Building
 Introducing Pharmacokinetics and Pharmacodynamics Janice Davies Pharmacist Room 23 Maudland Building JADavies5@uclan.ac.uk 1 elearn 2 DVD Any problems / questions? 3 Learning outcomes Define and discuss
Introducing Pharmacokinetics and Pharmacodynamics Janice Davies Pharmacist Room 23 Maudland Building JADavies5@uclan.ac.uk 1 elearn 2 DVD Any problems / questions? 3 Learning outcomes Define and discuss
Determination of bioavailability
 Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
Lipid Based Matrices as Colonic Drug Delivery System for Diflunisal (In-vitro, In-vivo study)
 Lipid Based Matrices as Colonic Drug Delivery System for Diflunisal (In-vitro, In-vivo study) Presented by Dr. AHMED ATEF DONIA, PH. D., Lecturer of Pharmaceutical Technology, Faculty of Pharmacy, Tanta
Lipid Based Matrices as Colonic Drug Delivery System for Diflunisal (In-vitro, In-vivo study) Presented by Dr. AHMED ATEF DONIA, PH. D., Lecturer of Pharmaceutical Technology, Faculty of Pharmacy, Tanta
Tablet is a major category of solid dosage forms which are widely used worldwide. Extensive information is required to prepare tablets with good
 TABLET PRODUCTİON Tablet is a major category of solid dosage forms which are widely used worldwide. Extensive information is required to prepare tablets with good quality at high standards. Based on preformulation
TABLET PRODUCTİON Tablet is a major category of solid dosage forms which are widely used worldwide. Extensive information is required to prepare tablets with good quality at high standards. Based on preformulation
BIOPHARMACEUTICS and CLINICAL PHARMACY
 11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
An introduction to Liposomal Encapsulation Technology
 An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
Basic Concepts of TDM
 TDM Lecture 1 5 th stage What is TDM? Basic Concepts of TDM Therapeutic drug monitoring (TDM) is a branch of clinical pharmacology that specializes in the measurement of medication concentrations in blood.
TDM Lecture 1 5 th stage What is TDM? Basic Concepts of TDM Therapeutic drug monitoring (TDM) is a branch of clinical pharmacology that specializes in the measurement of medication concentrations in blood.
innovative products. faster to market. reliably supplied.
 innovative products. faster to market. reliably supplied. consumer health E DEVELOPMENT DELIVERY SUPPLY We create unique, tailored consumer health solutions to help your brand grow. With more differentiated
innovative products. faster to market. reliably supplied. consumer health E DEVELOPMENT DELIVERY SUPPLY We create unique, tailored consumer health solutions to help your brand grow. With more differentiated
What is Nanotechnology?
 Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Available Online through Research Article
 ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
The BCS: Where Do We Go from Here?
 The BCS: Where Do We Go from Here? Jennifer Dressman,* James Butler, John Hempenstall, and Christos Reppas Since the Biopharmaceutics Classification System (BCS) was introduced several years ago, it has
The BCS: Where Do We Go from Here? Jennifer Dressman,* James Butler, John Hempenstall, and Christos Reppas Since the Biopharmaceutics Classification System (BCS) was introduced several years ago, it has
Current Challenges and Opportunities in Demonstrating Bioequivalence
 Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
BCS: Dissolution Testing as a Surrogate for BE Studies
 BCS: Dissolution Testing as a Surrogate for BE Studies Dirk M Barends National Institute of Public Health and the Environment The Netherlands APV / IKEV Seminar on Bioavailability and Bioequivalence, Istanbul,
BCS: Dissolution Testing as a Surrogate for BE Studies Dirk M Barends National Institute of Public Health and the Environment The Netherlands APV / IKEV Seminar on Bioavailability and Bioequivalence, Istanbul,
ADVANCES IN ORAL INSULIN DELIVERY
 ADVANCES IN ORAL INSULIN DELIVERY Zakieh I. Al-Kurdi, Babur Z. Chowdhry, Nidal A. Qinna, Mahmoud M.H. Al Omari and Adnan A. Badwan 2 ND MENA REGULATORY CONFERENCE ON BIOEQUIVALENCE, BIOWAIVERS, BIOANALYSIS,
ADVANCES IN ORAL INSULIN DELIVERY Zakieh I. Al-Kurdi, Babur Z. Chowdhry, Nidal A. Qinna, Mahmoud M.H. Al Omari and Adnan A. Badwan 2 ND MENA REGULATORY CONFERENCE ON BIOEQUIVALENCE, BIOWAIVERS, BIOANALYSIS,
Chapter 5. The Actions of Drugs. Origins of Drugs. Names of Drugs. Most drugs come from plants or are chemically derived from plants
 Chapter 5 The Actions of Drugs Origins of Drugs Most drugs come from plants or are chemically derived from plants Names of Drugs Chemical name: Complete chemical description of the molecule Example: N'-[2-[[5-(dimethylaminomethyl)-2-furyl]
Chapter 5 The Actions of Drugs Origins of Drugs Most drugs come from plants or are chemically derived from plants Names of Drugs Chemical name: Complete chemical description of the molecule Example: N'-[2-[[5-(dimethylaminomethyl)-2-furyl]
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Understand the physiological determinants of extent and rate of absorption
 Absorption and Half-Life Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand Objectives Understand the physiological determinants of extent and rate of absorption
Absorption and Half-Life Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand Objectives Understand the physiological determinants of extent and rate of absorption
Corso di Laurea Magistrale in Chimica e Tecnologia Farmaceutiche
 Universita degli Studi di Milano Corso di Laurea Magistrale in Chimica e Tecnologia Farmaceutiche Tecnologia e Legislazione Farmaceutiche II- 9 CFU Prof. Andrea Gazzaniga Rilascio Modificato via Orale
Universita degli Studi di Milano Corso di Laurea Magistrale in Chimica e Tecnologia Farmaceutiche Tecnologia e Legislazione Farmaceutiche II- 9 CFU Prof. Andrea Gazzaniga Rilascio Modificato via Orale
Controlled Delivery of Biologics NBC 22 June 2009
 Controlled Delivery of Biologics NBC 22 June 2009 ivery System Overview, Chris Rhodes, Amylin ulatory Perspectives, Mei-Ling Chen,FDA tained Circulation Strategies, Tim Riley, Nektar nsdermal Microporation,
Controlled Delivery of Biologics NBC 22 June 2009 ivery System Overview, Chris Rhodes, Amylin ulatory Perspectives, Mei-Ling Chen,FDA tained Circulation Strategies, Tim Riley, Nektar nsdermal Microporation,
Biowaiver Study on Prednisolone Tablets 5 mg in Three Different Brands. Marketed in Sudan. Safaa Mohamed *, Tilal Elsaman
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9 [4]:8-114 [http://scholarsresearchlibrary.com/archive.html] ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9 [4]:8-114 [http://scholarsresearchlibrary.com/archive.html] ISSN 0975-5071 USA CODEN: DPLEB4
Modified release drug delivery system for antiepileptic drug (Formulation development and evaluation).
 TITLE OF THE THESIS / RESEARCH: Modified release drug delivery system for antiepileptic drug (Formulation development and evaluation). INTRODUCTION: Epilepsy is a common chronic neurological disorder characterized
TITLE OF THE THESIS / RESEARCH: Modified release drug delivery system for antiepileptic drug (Formulation development and evaluation). INTRODUCTION: Epilepsy is a common chronic neurological disorder characterized
Assessing bioavailability/bioaccessibility of the dietary supplement components
 Assessing bioavailability/bioaccessibility of the dietary supplement components By Dr. David D. Kitts Food, Nutrition and Health, University of British Columbia May 15, 2018 OVERVIEW FACT: Nutrients/Toxins
Assessing bioavailability/bioaccessibility of the dietary supplement components By Dr. David D. Kitts Food, Nutrition and Health, University of British Columbia May 15, 2018 OVERVIEW FACT: Nutrients/Toxins
Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution)
 Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution) This document should not be treated as a comprehensive guideline; it serves as a
Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution) This document should not be treated as a comprehensive guideline; it serves as a
Factors responsible for the impaired bioavailability and instability rifampicin FDC
 Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
ASPIRIN. Session Two of TIP Assignment
 ASPIRIN Session Two of TIP Assignment History Behind Aspirin Development 2 Pain relief is something that has been sought after since the ancient Greeks and Egyptians used bark and dried leaves of the poplar
ASPIRIN Session Two of TIP Assignment History Behind Aspirin Development 2 Pain relief is something that has been sought after since the ancient Greeks and Egyptians used bark and dried leaves of the poplar
A Layperson s Guide to Pediatric Formulation Development
 A Layperson s Guide to Pediatric Formulation Development Karen C. Thompson PhD Senior Principal Scientist Preclinical Development Merck Research Laboratory West Point, Pennsylvania Martin J. Gartland PhD
A Layperson s Guide to Pediatric Formulation Development Karen C. Thompson PhD Senior Principal Scientist Preclinical Development Merck Research Laboratory West Point, Pennsylvania Martin J. Gartland PhD
EUDRATEC GRS. Your floating solution for an improved absorption in the stomach.
 EUDRATEC GRS Your floating solution for an improved absorption in the stomach. L+L 16-01-375 Flyer EUDRATEC GRS, 6 Seiten, DIN-A5.indd 02. Dec 2016; 11:44 Uhr EUDRATEC GRS unique gastro-retentive system
EUDRATEC GRS Your floating solution for an improved absorption in the stomach. L+L 16-01-375 Flyer EUDRATEC GRS, 6 Seiten, DIN-A5.indd 02. Dec 2016; 11:44 Uhr EUDRATEC GRS unique gastro-retentive system
Clinical Trials A Practical Guide to Design, Analysis, and Reporting
 Clinical Trials A Practical Guide to Design, Analysis, and Reporting Duolao Wang, PhD Ameet Bakhai, MBBS, MRCP Statistician Cardiologist Clinical Trials A Practical Guide to Design, Analysis, and Reporting
Clinical Trials A Practical Guide to Design, Analysis, and Reporting Duolao Wang, PhD Ameet Bakhai, MBBS, MRCP Statistician Cardiologist Clinical Trials A Practical Guide to Design, Analysis, and Reporting
Drugs, Society and Behavior
 SOCI 270 Drugs, Society and Behavior Professor Kurt Reymers, Ph.D. Chapter 5 The Actions of Drugs 2011 McGraw-Hill Higher Education. All rights reserved. 1. Drug Facts a. Sources: Most drugs come from
SOCI 270 Drugs, Society and Behavior Professor Kurt Reymers, Ph.D. Chapter 5 The Actions of Drugs 2011 McGraw-Hill Higher Education. All rights reserved. 1. Drug Facts a. Sources: Most drugs come from
Gastro Retentive Drug Delivery System
 Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
The solid choice for Omega-3
 The solid choice for Omega-3 The highest-load powder in its class. Superior bioavailability, stability and compressibility. Enabling smaller, simpler and more satisfying supplements. Unmatched properties
The solid choice for Omega-3 The highest-load powder in its class. Superior bioavailability, stability and compressibility. Enabling smaller, simpler and more satisfying supplements. Unmatched properties
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
BASIC PHARMACOKINETICS
 BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
5 Application of the ESR online-method for the monitoring of nanocapsule digestion
 5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
Microneedles for Drug Delivery via Gastrointestinal Tract. Maleeha Akram 08-arid-1772 Ph.D. Zoology
 Microneedles for Drug Delivery via Gastrointestinal Tract Maleeha Akram 08-arid-1772 Ph.D. Zoology 1 Contents Gastrointestinal tract Histology of GI tract Absorption through GI tract Drug delivery History
Microneedles for Drug Delivery via Gastrointestinal Tract Maleeha Akram 08-arid-1772 Ph.D. Zoology 1 Contents Gastrointestinal tract Histology of GI tract Absorption through GI tract Drug delivery History
Medical Policy. MP Ingestible ph and Pressure Capsule
 Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Unigel TM Case Study: Extending Soft Gelatin Capsule Benefits to Novel Fixed Dose Combinations
 Unigel TM Case Study: Extending Soft Gelatin Capsule Benefits to Novel Fixed Dose Combinations Diego Monterroza, H. M. Sc, Corporate Manager, R&D Procaps S.A. Stand # 30A46 OUTLINE Fixed Dose Combinations
Unigel TM Case Study: Extending Soft Gelatin Capsule Benefits to Novel Fixed Dose Combinations Diego Monterroza, H. M. Sc, Corporate Manager, R&D Procaps S.A. Stand # 30A46 OUTLINE Fixed Dose Combinations
Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation
 January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
Gastrointestinal Tract Imaging. Objectives. Reference. VMB 960 April 6, Stomach Small Intestine Colon. Radiography & Ultrasound
 Gastrointestinal Tract Imaging VMB 960 April 6, 2009 Stomach Small Intestine Colon Objectives Radiography & Ultrasound Contrast Examination of the Small Intestine Reference Chapters 45 47 Pages 750 805
Gastrointestinal Tract Imaging VMB 960 April 6, 2009 Stomach Small Intestine Colon Objectives Radiography & Ultrasound Contrast Examination of the Small Intestine Reference Chapters 45 47 Pages 750 805
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PREFACE. Saskatchewan is participating in the Common Drug Review (CDR).
 PREFACE OBJECTIVES The Drug Plan has been established to: provide coverage to Saskatchewan residents for quality pharmaceutical products of proven therapeutic effectiveness; reduce the direct cost of prescription
PREFACE OBJECTIVES The Drug Plan has been established to: provide coverage to Saskatchewan residents for quality pharmaceutical products of proven therapeutic effectiveness; reduce the direct cost of prescription
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization
 Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
Completion of the development of a formulation: Requirements for compliance check vs. requirements for Marketing Authorisation
 Completion of the development of a formulation: Requirements for compliance check vs. requirements for Marketing Authorisation Workshop on Paediatric Formulations For Assessors in National Regulatory Agencies
Completion of the development of a formulation: Requirements for compliance check vs. requirements for Marketing Authorisation Workshop on Paediatric Formulations For Assessors in National Regulatory Agencies
VICTOSA and Renal impairment DR.R.S.SAJAD
 VICTOSA and Renal impairment DR.R.S.SAJAD February 2019 Main effect of GLP-1 is : Stimulating glucose dependent insulin release from the pancreatic islets. Slow gastric emptying Inhibit inappropriate
VICTOSA and Renal impairment DR.R.S.SAJAD February 2019 Main effect of GLP-1 is : Stimulating glucose dependent insulin release from the pancreatic islets. Slow gastric emptying Inhibit inappropriate
Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn
 University of Groningen Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn IMPORTANT NOTE: You are advised to consult
University of Groningen Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn IMPORTANT NOTE: You are advised to consult
Bioequivalence Requirements: USA and EU
 Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
Drug CHAPTER 2. Pharmacologic Principles. NDEG 26A Eliza Rivera-Mitu, RN, MSN. Pharmacology. Drug Names. Pharmacologic Principles. Drug Names (cont'd)
 CHAPTER 2 Pharmacologic Principles NDEG 26A Eliza Rivera-Mitu, RN, MSN Drug Any chemical that affects the physiologic processes of a living organism Pharmacology The study or science of drugs Drug Names
CHAPTER 2 Pharmacologic Principles NDEG 26A Eliza Rivera-Mitu, RN, MSN Drug Any chemical that affects the physiologic processes of a living organism Pharmacology The study or science of drugs Drug Names
Uncovering Encapsulation Differences
 Uncovering Encapsulation Differences All encapsulates are not created equal Dr. Jeff Elliott Balchem Western Technical Services Lipid encapsulation has proven to be a valuable tool to protect nutrients
Uncovering Encapsulation Differences All encapsulates are not created equal Dr. Jeff Elliott Balchem Western Technical Services Lipid encapsulation has proven to be a valuable tool to protect nutrients
Pharmacokinetic Phase
 RSPT 2217 Principles of Drug Action Part 2: The Pharmacokinetic Phase Gardenhire Chapter 2; p. 14-25 From the Text Common Pathways for Drug Box 2-3; page 18 Plasma Half-lives of Common Drugs Table 2-4;
RSPT 2217 Principles of Drug Action Part 2: The Pharmacokinetic Phase Gardenhire Chapter 2; p. 14-25 From the Text Common Pathways for Drug Box 2-3; page 18 Plasma Half-lives of Common Drugs Table 2-4;
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE
 UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
Diabetes Oral Agents Pharmacology. University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
Diabetes Oral Agents Pharmacology University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 Learning Objectives Understand the role of the utilization of free
Posology. Posology and Dosage Regimen: Factors Affecting Drug Dosage: 08/09/ ) Age: 1. Young s Rule, based on age:
 Posology and Dosage Regimen: Posology Posology: (Derived from the Greek posos- how much, and logos- science) is the branch of medicine/pharmacy dealing with doses. Posology is a branch of medical science
Posology and Dosage Regimen: Posology Posology: (Derived from the Greek posos- how much, and logos- science) is the branch of medicine/pharmacy dealing with doses. Posology is a branch of medical science
Got Absorption? Maximizing Nutrition Through Liposomal Technology. Jim Cartmill President & CEO Let s Talk Health, Inc.
 Got Absorption? Maximizing Nutrition Through Liposomal Technology Jim Cartmill President & CEO Let s Talk Health, Inc. letstalkhealth.com blog.letstalkhealth.com letstalkhealth.com/facebook The Hope &
Got Absorption? Maximizing Nutrition Through Liposomal Technology Jim Cartmill President & CEO Let s Talk Health, Inc. letstalkhealth.com blog.letstalkhealth.com letstalkhealth.com/facebook The Hope &
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition
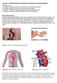 Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Lecture 1: Physicochemical Properties of Drugs and Drug Disposition Key objectives: 1. Be able to explain the benefits of oral versus IV drug administration 2. Be able to explain the factors involved in
Introduction to. Pharmacokinetics. University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 Introduction to 1 Pharmacokinetics University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 2 Learning objectives Understand compartment models and how they effects
Introduction to 1 Pharmacokinetics University of Hawai i Hilo Pre-Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 2 Learning objectives Understand compartment models and how they effects
LAO Aripiprazole OW Long Acting Oral aripiprazole Once Weekly
 LAO Aripiprazole OW Long Acting Oral aripiprazole Once Weekly An alternative option to Long Acting Injectables (LAI) in the rapidly expanding market for prevention of psychotic relapse Zysis Management
LAO Aripiprazole OW Long Acting Oral aripiprazole Once Weekly An alternative option to Long Acting Injectables (LAI) in the rapidly expanding market for prevention of psychotic relapse Zysis Management
WORKING PARTY ON HERBAL MEDICINAL PRODUCTS FINAL PROPOSAL FOR A CORE DATA FOR ISPAGHULA HUSK
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use 27 March 2003 EMEA/HMPWP/15/00 WORKING PARTY ON HERBAL MEDICINAL PRODUCTS FINAL PROPOSAL FOR A CORE DATA
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use 27 March 2003 EMEA/HMPWP/15/00 WORKING PARTY ON HERBAL MEDICINAL PRODUCTS FINAL PROPOSAL FOR A CORE DATA
Advanced functional coating solutions for nutraceuticals
 Advanced functional coating solutions for nutraceuticals About Evonik In 2017, our more than 36,000 Evonik employees produced sales of 14,4 billion and an operating result (EBITDA) of 2.36 billion. As
Advanced functional coating solutions for nutraceuticals About Evonik In 2017, our more than 36,000 Evonik employees produced sales of 14,4 billion and an operating result (EBITDA) of 2.36 billion. As
Scottish Medicines Consortium
 Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium ketoprofen/, 100mg/20mg; 200mg/20mg modified release capsules (Axorid ) No. (606/10) Meda Pharmaceuticals 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan
 1 5 th Joint Conference of Taiwan and Japan on Medical Products Regulation Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan Mr. Yoshihiko Sano Deputy Director,
1 5 th Joint Conference of Taiwan and Japan on Medical Products Regulation Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan Mr. Yoshihiko Sano Deputy Director,
MCT AND THE ROLES NUTRITION
 MCT AND THE ROLES NUTRITION Nguyen Hoang Nhut Hoa Department of Nutrition Children's Hospital 2 OBJECTIVES Structure Absorption and metabolic Effects of MCT in the treatment of certain diseases Demand
MCT AND THE ROLES NUTRITION Nguyen Hoang Nhut Hoa Department of Nutrition Children's Hospital 2 OBJECTIVES Structure Absorption and metabolic Effects of MCT in the treatment of certain diseases Demand
5.1 Summary. Summary & Conclusions
 5.1 Summary The thesis can be summarized as follows: 1. Introduction- It is the first chapter of the thesis. It describes about the importance of generic products, its role in pharmaco-economics of India.
5.1 Summary The thesis can be summarized as follows: 1. Introduction- It is the first chapter of the thesis. It describes about the importance of generic products, its role in pharmaco-economics of India.
Fonterra Probiotics: From guts to glory
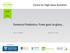 Fonterra Probiotics: From guts to glory James Dekker April 16, 2015 Host Institution Probiotic bacteria Live micro-organisms which, when administered in adequate amounts, confer a health benefit on the
Fonterra Probiotics: From guts to glory James Dekker April 16, 2015 Host Institution Probiotic bacteria Live micro-organisms which, when administered in adequate amounts, confer a health benefit on the
Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
 SYNOPSIS Issue Date: 27 April 2009 Document No.: EDMS-PSDB-9908562:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient Johnson & Johnson Pharmaceutical Research & Development,
SYNOPSIS Issue Date: 27 April 2009 Document No.: EDMS-PSDB-9908562:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient Johnson & Johnson Pharmaceutical Research & Development,
Valproate Case 3: Formulations Jose de Leon, MD
 Valproate Case 3: Formulations 2-12-16 Jose de Leon, MD 3.Valproate Case 3 Described in J Clin Psychiatry 2004;65:724-5 http://www.ncbi.nlm.nih.gov/pubmed/15163266 Pharmacological explanation provided
Valproate Case 3: Formulations 2-12-16 Jose de Leon, MD 3.Valproate Case 3 Described in J Clin Psychiatry 2004;65:724-5 http://www.ncbi.nlm.nih.gov/pubmed/15163266 Pharmacological explanation provided
PERFORMANCE & MUSCLE GAIN
 PERFORMANCE & MUSCLE GAIN... Nutrition is 100% Responsible for Exercise-Induced Results Safely Enhance Training Outcomes For Experienced Athletes & Exercisers CreatineXXL Speed recovery, improve performance
PERFORMANCE & MUSCLE GAIN... Nutrition is 100% Responsible for Exercise-Induced Results Safely Enhance Training Outcomes For Experienced Athletes & Exercisers CreatineXXL Speed recovery, improve performance
DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects
 1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
ANNEX II SCIENTIFIC CONCLUSIONS AND GROUNDS FOR POSITIVE OPINION
 ANNEX II SCIENTIFIC CONCLUSIONS AND GROUNDS FOR POSITIVE OPINION 3 Scientific conclusions Overall summary of the scientific evaluation of Okrido and associated names (see Annex I) Okrido is an oral solution
ANNEX II SCIENTIFIC CONCLUSIONS AND GROUNDS FOR POSITIVE OPINION 3 Scientific conclusions Overall summary of the scientific evaluation of Okrido and associated names (see Annex I) Okrido is an oral solution
Development of the Rodent Gastrointestinal Tract:
 White Paper Development of the Rodent Gastrointestinal Tract: The regulation of the development of the GI tract is unique and complex. With respect to the GI tract there are several considerations that
White Paper Development of the Rodent Gastrointestinal Tract: The regulation of the development of the GI tract is unique and complex. With respect to the GI tract there are several considerations that
ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE
 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
Chapter 20 The Digestive System Exam Study Questions
 Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Gastrointestinal-Specific and General Physiology Issues in Pediatrics: Implications for Pediatric Formulation Development
 Gastrointestinal-Specific and General Physiology Issues in Pediatrics: Implications for Pediatric Formulation Development Andrew E. Mulberg, MD, FAAP Division of Gastroenterology and Inborn Errors Products
Gastrointestinal-Specific and General Physiology Issues in Pediatrics: Implications for Pediatric Formulation Development Andrew E. Mulberg, MD, FAAP Division of Gastroenterology and Inborn Errors Products
International Journal of Medicine and Pharmaceutical Research
 International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
Conferencia Inaugural
 Conferencia Inaugural UNDERSTANDING GASTRIC DIGESTION TO DEVELOP NEW FOODS FOR HEALTH R. Paul Singh Distinguished Professor of Food Engineering Department of Biological and Agricultural Engineering University
Conferencia Inaugural UNDERSTANDING GASTRIC DIGESTION TO DEVELOP NEW FOODS FOR HEALTH R. Paul Singh Distinguished Professor of Food Engineering Department of Biological and Agricultural Engineering University
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON PSYLLIUM SEED (PLANTAGO AFRA ET PLANTAGO INDICA, SEMEN)
 European Medicines Agency Evaluation of Medicines for Human Use London, 24 October 2005 Doc. Ref. EMEA/HMPC/340865/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 24 October 2005 Doc. Ref. EMEA/HMPC/340865/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON
A matter of timing. ensure optimal delivery for acid-sensitive products. capsugel.com
 A matter of timing ensure optimal delivery for acid-sensitive products capsugel.com The preferred choice for you and your customers Capsugel DRcaps capsules offer nutritional supplement makers a dosage
A matter of timing ensure optimal delivery for acid-sensitive products capsugel.com The preferred choice for you and your customers Capsugel DRcaps capsules offer nutritional supplement makers a dosage
SEE THE BIG PICTURE OF YOUR GI HEALTH. PillCam SB System. A simple way to evaluate the small bowel
 SEE THE BIG PICTURE OF YOUR GI HEALTH PillCam SB System A simple way to evaluate the small bowel PATIENT-FRIENDLY SMALL BOWEL VISUALIZATION If you or a loved one suffers from gastrointestinal (GI) bleeding,
SEE THE BIG PICTURE OF YOUR GI HEALTH PillCam SB System A simple way to evaluate the small bowel PATIENT-FRIENDLY SMALL BOWEL VISUALIZATION If you or a loved one suffers from gastrointestinal (GI) bleeding,
