Pharmacy Prior Authorization Growth Hormone - Clinical Guidelines
|
|
|
- Aron Warner
- 5 years ago
- Views:
Transcription
1 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents: Nte: Prvider must submit chart ntes that include the fllwing dcumentatin: weight, height, grwth velcity, and lab values (Grwth Hrmne (GH) levels, insulin-like grwth factr-1 (IGF-1) / insulin-like grwth factr-binding 3 (IGFBP-3)), stim test results, bne age Grwth Hrmne will be apprved fr members wh meet all f the fllwing criteria at initiatin f treatment: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist Infant is less than 4 mnths f age and has grwth hrmne deficiency; OR Histry f nenatal hypglycemia assciated with pituitary disease; OR Diagnsis f pan hyppituitarism; OR Histry f irradiatin, surgery r trauma t hypthalamic-pituitary area; OR A defined central nervus system (CNS) pathlgy cnfirmed by magnetic resnance imaging (MRI) r cmputed tmgraphy (CT); OR Nte: magnetic resnance imaging (MRI)/cmputed tmgraphy (CT) shuld be dne t exclude a brain tumr (fr example, cranipharyngima). Members with grwth hrmne deficiency (GHD) have an abnrmality f the pituitary gland (fr example: ectpic bright spt, empty r small sella) Diagnsis f Pediatric grwth hrmne (GH) deficiency cnfirmed by fllwing: Member must meet ne f the fllwing: Height: Height is greater than 2 standard deviatin (SD) belw mid parental height (prjected height); OR Height is greater than 2.25 standard deviatin (SD) belw ppulatin mean fr age and gender OR Grwth Velcity: Grwth Velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender OR Delayed skeletal maturatin (delayed bne age cnfirmed by X-ray): Bne age (BA) cmpared t chrnlgical age (CA) is equal t r greater than 2 standard deviatin (SD) belw mean fr age and gender (fr example: delayed mre than r equal t 2 years cmpared with chrnlgical age) Member must meet ONE f fllwing labratry results: Member has undergne tw prvcative grwth hrmne (GH) stimulatin test (fr example: Arginine, Clnidine, Glucagn, Insulin, Levdpa, grwth hrmne-releasing hrmne (GhRh)) grwth hrmne (GH) respnse values are less than 10 mcg/l; 1 P age
2 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines OR One abnrmal grwth hrmne (GH) test is sufficient fr children with defined central nervus system (CNS) pathlgy, multiple pituitary hrmne deficiency (MPHD), histry f irradiatin, r a genetic defect affecting the grwth hrmne (GH) axis; OR Member is less than 1 year f age IGF-1 (insulin-like grwth factr) r insulin-like grwth factr-binding 3 (IGFBP-3) is belw the age and gender adjusted nrmal range as prvided by the physician s lab Epiphyses are pen (cnfirmatin f pen grwth plates in members ver 12 years f age) Other pituitary hrmne deficiencies (fr example: hypthyridism, chrnic ischemic disease) have been ruled ut Prader-Willi Syndrme (PWS): Member must meet the fllwing fr initial apprval: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist; Diagnsis f Prader-Willi Syndrme (deletin in chrmsmal 15q11.2-q13 regin, maternal uniparental dismy in chrmsme 15, imprinting defects r translcatins invlving chrmsme 15) Grwth velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender Epiphyses are pen (cnfirmatin f pen grwth plates in members ver 12 years f age) Turner Syndrme (TS, gnadal Dysgenesis): Member must meet the fllwing fr initial apprval: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist Diagnsis f Turner Syndrme (karytype shwing a 45, XO gentype) Member is Female (greater than 2 years f age) and Bne age is less than 14 years Grwth velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender Epiphyses are pen (cnfirmatin f pen grwth plates in members ver 12 years f age) Nnan Syndrme (NS): (Nrditrpin nly) Member must meet the fllwing fr initial apprval: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist Diagnsis f Nnan Syndrme Epiphyses are pen (cnfirmatin f pen grwth plates in members ver 12 years f age) 2 P age
3 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Bne age (BA) cmpared t chrnlgical age (CA) is equal t r greater than 2 standard deviatin (SD) belw mean fr age and gender (fr example: delayed mre than r equal t 2 years cmpared with chrnlgical age); OR Grwth velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender Shrt stature with SHOX (shrt stature hmebx-cntaining gene) deficiency (SHOXD): (Humatrpe nly) Member must meet the fllwing fr initial apprval: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist Diagnsis f pediatric grwth failure with shrt-stature hmebx (SHOX) gene deficiency as cnfirmed by genetic testing Epiphyses are pen (cnfirmatin f pen grwth plates in members ver 12 years f age) Bne age (BA) cmpared t chrnlgical age (CA) is equal t r greater than 2 standard deviatin (SD) belw mean fr age and gender (fr example: delayed mre than r equal t 2 years cmpared with chrnlgical age); OR Grwth velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender Grwth failure assciated with Chrnic Renal Insufficiency (CRI) r Chrnic Kidney disease (CKD) (up t the time f renal transplantatin): (Nutrpin nly) Member must meet the fllwing fr initial apprval: Must be prescribed by r in cnsultatin with a Pediatric Endcrinlgist, Pediatric Nephrlgist r Endcrinlgist Diagnsis f pediatric grwth failure due t chrnic renal insufficiency (e.g., serum creatinine is less than 30 mg/dl, up t the time f renal transplant) Bne age (BA) cmpared t chrnlgical age (CA) is equal t r greater than 2 standard deviatin (SD) belw mean fr age and gender (fr example: delayed mre than r equal t 2 years cmpared with chrnlgical age); OR Grwth velcity (GV) is greater than 2 standard deviatin (SD) belw ppulatin mean fr age and gender Nte: Prir t initiatin f grwth hrmne (GH) treatment, existing metablic derangements such as malnutritin, zinc deficiency, and secndary hyperparathyridism shuld be crrected. Grwth failure in Children Small fr Gestatinal Age (SGA): Nte: Prvider must submit chart ntes with that include the fllwing dcumentatin: Gestatinal Age (GA), birth weight, height, and grwth chart Member must meet the fllwing fr initial apprval: Member is greater than 2 years f age Diagnsis f small fr gestatinal age (SGA) (fetal grwth retardatin), child wh failed t catch 3 P age
4 Initial Apprval duratin: 12 mnths Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines up grwth in first 24 mnths f life (by 2 years f age) r with n catch up grwth using a 0-36 mnth grwth chart and shwing: Member is belw the 3 rd percentile fr gestatinal age (mre than 2 standard deviatin (SD) belw ppulatin mean) fr birth weight and length; Member s height remains belw the 3 rd percentile (mre than 2 standard deviatin (SD) belw ppulatin age and gender) Renewal criteria fr grwth hrmne (GH) therapy in Children: Nte: Prvider must submit dcumentatin fr renewal: previus height, current height and expected adult height gal. Member must meet the fllwing fr renewal apprval: Dcumentatin supprting psitive respnse t therapy: Height increase f at least 2.5cm/year (pst-pubertal grwth rate) r 4.5cm/year (pre-pubertal grwth rate) Expected final height is nt achieved Bne age is less than16 years fr males; less than 14 years fr female; Grwth (epiphyseal) plates are still pen Fr children with Prader Willi Syndrme (PWS): Dcumentatin supprting psitive respnse t therapy (fr example: increase in ttal lean bdy mass, decrease in fat mass); OR abve renewal requirements. Renewal Apprval duratin: 12 mnths Discntinuatin criteria fr grwth hrmne (GH) therapy in Children: Expected final adult height has been reached; OR Member had pr respnse t treatment, generally defined as an increase in grwth velcity (GV) f less than 50% frm baseline in the 1 st year f therapy; OR Increase in height velcity is less than 2 cm ttal grwth in 1 year f therapy; OR Epiphyseal fusin has ccurred; r There are persistent and uncrrectable prblems with adherence t treatment II. Transitin Phase Adlescent members Member must meet the fllwing fr initial apprval: Member has attained expected adult height Clsed epiphyses n bne radigraph Member is at high risk f grwth hrmne (GH) deficiency due t childhd-nset grwth hrmne deficiency (COGHD) frm ne f fllwing: Hypthalamic-pituitary structural defect r tumr; OR At least 3 deficiency f anterir pituitary hrmnes (fr example: fllicle-stimulating hrmne (FSH)/luteinizing hrmne (LH), thyrid-stimulating hrmne (TSH), adrencrtictrpic hrmne 4 P age
5 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines (ACTH), Prlactin), pan hyppituitarism; OR Genetic cause f grwth hrmne (GH) Insulin-like grwth factr (IGF-1) is belw the age and gender adjusted nrmal range as prvided by the physician s lab OR Member has stpped grwth hrmne (GH) therapy fr at least ne mnth diagnsis f grwth hrmne deficiency (GHD) has been recnfirmed by ONE f the fllwing: One lw insulin-like grwth factr-1 (IGF-1)/ insulin-like grwth factr-binding 3 (IGFBP-3) and ne grwth hrmne (GH) stim test with grwth hrmne (GH) peak value f greater than 10 mcg/ml; OR Tw grwth hrmne (GH) stim tests with grwth hrmne (GH) peak value f greater than 10 mcg/ml Nte: Transitin Phase: Defined as perid f life starting in late puberty and ending with full adult maturatin (frm mid t late teenage years until 6-7 years after achievement f final height) Adlescent: is a persn between ages f 10 and 19 as defined by the Wrld Health Organizatin (WHO) There is n prven benefit t cntinuing grwth hrmne (GH) treatment in adulthd fr childhd grwth hrmne (GH) treatment f cnditins ther than grwth hrmne deficiency (fr example: Turner s syndrme) Initial Apprval duratin: 12 mnths Renewal criteria fr Transitin Phase Adlescent members: Nte: Prvider must submit dcumentatin fr renewal: chart ntes, insulin-like grwth factr-1 (IGF-1) levels Member must meet the fllwing fr renewal apprval: Dcumentatin supprting psitive respnse t therapy (fr example: increased in ttal lean bdy mass, increased exercise capacity r increased insulin-like grwth factr-1 (IGF-1) levels) Renewal Apprval duratin: 12 mnths III. Adult Grwth Hrmne Deficiency: Nte: Prvider must submit dcumentatin supprting diagnsis, stim test results, insulin-like grwth factr-1 (IGF- 1) levels. Member must meet the fllwing fr initial apprval: Diagnsis f childhd-nset grwth hrmne deficiency (COGHD); OR Diagnsis f adult-nset grwth hrmne deficiency (AOGHD); Dcumentatin supprting hrmne deficiency is due t hypthalamic-pituitary disease frm rganic r knwn causes (fr example: damage frm surgery, cranial irradiatin, head trauma, r subarachnid hemrrhage); 5 P age
6 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Member has undergne ONE prvcative grwth hrmne (GH) stimulatin tests (fr example: insulin tlerance test (ITT), Arginine+ grwth hrmne-releasing hrmne (GHRH), glucagn, Arginine) cnfirming adult grwth hrmne (GH) deficiency ne f fllwing peak values: Insulin tlerance test (ITT) is less than r equal t 5 ng/ml Arginine+ grwth hrmne-releasing hrmne (GHRH): less than r equal t 11 ng/ml if bdy mass index (BMI) less than 25 kg/m2;less than r equal t 8 ng/ml if bdy mass index (BMI) greater than r equal t 25 and less than30 kg/m2; less than r equal t 4 ng/ml if bdy mass index (BMI) greater than r equal t 30 kg/m2 Glucagn: less than r equal t 3 ng/ml Arginine: less than r equal t 0.4 ng/ml Nte: Insulin tlerance test (ITT) is gld standard stimulatin test agent. Insulin tlerance test (ITT) is cntraindicated with crnary artery disease, seizures, abnrmal electrcardigram (EKG) with histry f Ischemic heart disease r cardivascular disease, and nt apprpriate fr thse greater than age 60 years. Others shuld be used when Insulin tlerance test (ITT) is cntraindicated. Glucagn has mre diagnstic accuracy. Arginine alne is rarely used. If arginine is used alne, a secnd stimulatin test may be required depending n insulin-like grwth factr-1 (IGF-1) levels. If the insulin-like grwth factr-1 (IGF-1) is subnrmal with presentatin f a hypthalamic disrder then ne stim test is required. If the insulin-like grwth factr-1 (IGF-1) is nrmal with hypthalamic disrder then tw stim tests are required. OR; Member has at least 3 deficiency f anterir pituitary hrmnes (fr example: fllicle-stimulating hrmne (FSH)/luteinizing hrmne (LH), thyrid-stimulating hrmne (TSH), adrencrtictrpic hrmne (ACTH), Prlactin), pan hyppituitarism; IGF-1 (insulin-like Grwth factr) is belw the age and gender adjusted nrmal range as prvided by the physician s lab Initial Apprval duratin: 12 mnths Renewal criteria fr Adult Grwth Hrmne deficiency: Nte: Prvider must submit dcumentatin fr renewal: chart ntes, insulin-like grwth factr-1 (IGF-1) levels Member must meet the fllwing criteria fr renewal apprval: Dcumentatin supprting psitive respnse t therapy (fr example: increased in ttal lean bdy mass, increased exercise capacity r increased insulin-like grwth factr-1 (IGF-1) levels) Renewal Apprval duratin: 12 mnths 6 P age
7 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines HIV-assciated Cachexia r Wasting: (Serstim nly) Nte: Prvider must submit dcumentatin f bdy mass index (BMI), weight, and ideal bdy weight (IBW) (prir t initiatin and after initiatin f Serstim fr renewals) Member must meet the fllwing fr initial apprval: Prescribed by r in cnsultatin with an infectius Disease r Human Immundeficiency Virus (HIV) Specialist; Currently n antiretrviral therapy; Inadequate respnse, intlerable side effects r cntraindicatin t megestrl acetate r drnabinl; Member has nt had weight lss due t ther causes (fr example: depressin, mycbacterium avium cmplex (MAC), chrnic infectius diarrhea, r malignancy with exceptin f Kapsi s sarcma limited t skin r mucus membranes); Bdy mass index (BMI) less than 20 kg/m2 prir t initiating therapy with Serstim; OR Unintentinal weight lss f mre than 10% ver last 12 mnths r mre than 5% ver the last 6 mnths; OR Member weights less than 90% f the lwer limit f ideal bdy weight (IBW) Initial apprval duratin: 3 mnths Renewal criteria Member must meet the fllwing fr renewal apprval: Dcumentatin supprting psitive respnse t therapy (bdy mass index (BMI) has imprved r stabilized) Currently n antiretrviral therapy Renewal Apprval duratin: 9 mnths (Maximum ttal: 48 weeks) Shrt Bwel Syndrme: (Zrbtive nly) Member must meet the fllwing fr apprval: Diagnsis f Shrt Bwel syndrme; Member is 18 years r lder Member is currently receiving specialized nutritin supprt (e.g., intravenus (IV) parenteral nutritin, fluid, and micrnutrient supplements) ; Member has nt previusly received 4 weeks f treatment with Zrbtive Initial Apprval: 4 weeks. Nte that treatment with Zrbtive will nt be apprved beynd 4 weeks as administratin fr mre than 4 weeks has nt been adequately studied. Cverage criteria fr a Nn-preferred grwth hrmne (GH) prduct: Member must meet ONE f fllwing criteria: Inadequate respnse, an intlerable side effect r has cntraindicatin t preferred agent; OR Inability t use vial frmulatin due t disability (fr example: visual/physical impairment); OR 7 P age
8 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines There is n preferred prduct apprpriate fr the cnditin being treated Pharmacy Prir Authrizatin Grwth Hrmne- Clinical Guidelines Nn Cverage Criteria: Use nt apprved by the Fd and Drugs Administratin (FDA) and the use is unapprved and nt supprted by the literature r evidence as an accepted ff-label use Amytrphic lateral sclersis Anablic therapy t enhance bdy mass r strength fr prfessinal, recreatinal r scial reasns Idipathic Shrt Stature (ISS)* Anti-aging Burn injuries Chrnic catablic states, including inflammatry bwel disease, pharmaclgic gluccrticid administratin, and respiratry failure Cnstitutinal delay f grwth and develpment Insulin-like grwth factr-i (insulin-like grwth factr-1 (IGF-1)) deficiency (als knwn as neursecretry defect) Russell-Silver syndrme (that des nt result in small fr gestatinal age) Stem Cell mbilizatin Traumatic brain injury Wund healing *Aetna des nt cnsider Idipathic shrt stature (ISS) an illness, disease r injury therefre it is nt a cvered plan benefit. 8 P age
9 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Additinal Infrmatin: Examples f Hypthalamic/Pituitary/CNS disrders: Cngenital structural abnrmalities: 1. Optic nerve hypplasia/septic-ptic dysplasia 2. Agenesis f crpus callsum 3. Empty sella syndrme 4. Ectpic psterir pituitary 5. Pituitary aplasia/hypplasia 6. Pituitary stalk defect 7. Vascular malfrmatins Cngenital genetic abnrmalities: Mutatins in grwth hrmne-releasing hrmne (GHRH) receptr, grwth hrmne (GH) gene, grwth hrmne (GH) receptr r pituitary transcriptin factrs Acquired structural abnrmalities (r causes f hypthalamic/pituitary damage): 1. Central Nervus System (CNS) tumrs/neplasms (fr example: cranipharyngima, glima, pituitary adenma) 2. Cysts (Rathke cleft cyst r arachnid cleft cyst) 3. Surgery 4. Radiatin 5. Chemtherapy 6. Central Nervus System (CNS) infectins 7. Centra Nervus System (CNS) infractin (e.g., Sheehan s syndrme) 8. Inflammatry lesins (e.g., autimmune hypphysitis) 9. Infiltrative lesins (e.g., sarcidsis, histicytsis) 10. Head trauma/traumatic brain injury 11. Aneurysmal subarachnid hemrrhage Pituitary Hrmnes (ther than grwth hrmnes) 1. ACTH (adrencrtictrpic hrmne) 2. ADH (Antidiuretic hrmne) 3. FSH (fllicle stimulating hrmne) 4. LH (luteinizing hrmne) 5. Oxytcin 6. Prlactin 7. TSH (thyrid stimulating hrmne) Appendix Grwth Charts: Grwth charts fr infants, children and adlescents are psted at the fllwing internet sites: Centers fr Disease Cntrl and Preventin: (This link includes grwth charts with curves dwn t 2 standard deviatins (apprximately 3rd percentile)). 9 Page
10 Height Velcity Tables: Figure 1: Height Velcity (cm/year) -- Girls Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Age 3rd Percentile 10th Percentile 25th Percentile P a g e
11 Figure 2: Height Velcity (cm/year) -- Bys Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Age 3rd Percentile 10th Percentile 25th Percentile P a g e
12 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines Surce: Interplated frm data frm Tanner and Davies (1995). Cnversin Factr: 1 centimeter (cm) = inches (in). 1 in = 2.54 cm. Nte: Assuming a nrmal distributin, 1 standard deviatin belw the mean is apprximately equal t the 16th percentile, 2 standard deviatins belw the mean is equal t the 2nd percentile, and 3 standard deviatins belw the mean is equal t the 1/10th (0.1) percentile. Weight fr Gestatinal Age Table: Small fr gestatinal age is generally defined as weight fr gestatinal age belw the 10th percentile at birth. A chart indicating the 10th and 90th percentiles f weight fr gestatinal age at birth is presented in Pediatrics 2014;133;844 (Talge et al 2014) Table-1 and Table-2 and is available at the fllwing website: Nrmal Results f a grwth hrmne (GH) Stimulatin Test (using arginine, glucagn r insulin):available at: Nrmal peak value -- at least 10 ng/ml Indeterminate -- 5 t 10 ng/ml Subnrmal -- 5 ng/ml References: 1. Ck D, Yuen K, Biller B et al. American Assciatin f Clinical Endcrinlgist Medical Guidelines fr Clinical Practice fr Grwth Hrmne use in Grwth Hrmne Deficient Adults and Transitin Patients-2009 Update. Endcrine Practice, 2009; 15(Suppl 2): Accessed nline Aug Gharib H, Ck DM, et.al. American assciatin f clinical endcrinlgists medical guidelines fr clinical practice fr grwth hrmne use in adults and children update. Endcrine Practice, 2003; 9(1): Richmnd EJ, Rgl AD. Diagnsis f grwth hrmne deficiency in children. UpTDate www. uptdate.cm. Accessed n 08/ Snyder, P. Grwth hrmne deficiency in adults. UpTDate. Accessed n 08/ Mandy, G. Small fr gestatinal age infant. UpTDate. Accessed n 08/ Gld Standard, Inc. Nrditrpin. Clinical pharmaclgy [database nline] Available at Accessed April Nrditrpin [Prescribing Infrmatin]. Bagsvaerd, Denmar: Nv Nrdisk; Feb Accesses April 8, P a g e
13 Pharmacy Prir Authrizatin Grwth Hrmne - Clinical Guidelines 8. Nutrpin [Prescribing Infrmatin]. San Francisc, CA: Genentech; Dec Accessed April 8, Saizen [Prescribing Infrmatin]. Rckland, MA: EMD Sern Inc.; Dec Accessed April 8, Serstim [Prescribing Infrmatin]. Rckland, MA: EMD Sern Inc.;Dec Accessed April 8, Zrbtive[Prescribing Infrmatin]. Rckland, MA: EMD Sern Inc.; May Accessed April 8, Omnitrpe [Prescribing Infrmatin]. Princetn, NJ: Sandz, Inc.; Dec Accessed April 8, Humatrpe [Prescribing Infrmatin]. Indianaplis, IN: Lilly USA, LLC;Dec Accessed April 8, Gentrpin [Prescribing Infrmatin]. Belgium N.V., Puurs, Belgium: Pfizer Manufacturing; Dec Accessed April 8, Zmactn [Prescribing Infrmatin]. Parsippany, NJ: Ferring Pharmaceuticals; Jan Accessed April 8, Mlitch M, Clemmns DR, Malzwski S et al.; The Endcrine Sciety s Guideline. Evaluatin and Treatment f Adult Grwth Hrmne Deficiency: An Endcrine Sciety Clinical Practice Guideline. Clin Endcrinl Metab. 2006; 91(5): Wilsn T, Rse S, Chenp et al.; Update f Guidelines fr the use f Grwth Hrmne in Children: The Lawsn Wilkins Pediatric Endcrinlgy Sciety Drug and Therapeutics Cmmittee. Jurnal f Pediatrics, Accessed nline Aug Gld Standard, Inc. Zmactn. Clinical pharmaclgy [database nline] Available at Accessed Sep, Rse SR, Ck DM, Fine MJ. Grwth Hhmne therapy guidelines: Clinical and managed care perspectives. Am J Pharm Benefits. 2014;6(5):e134-e146. Accessed nline April Talge NM, Mudd LM, Sikrskii A, Bass O. United States birtb weight reference crrected fr implausible gestatinal age estimates. Pediatrics 2014; 133; DOI: /peds Barstw C, Rerucha C. Evaluatin f shrt and tall stature in children. Am Fam Physician Jul 1;92(1): P a g e
Pharmacy Prior Authorization Growth Hormone- Clinical Guidelines
 Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents: Nte: Prvider must submit chart ntes that include the fllwing
Gentrpin, Humatrpe, Nrditrpin, Nutrpin, Omnitrpe, Saizen, Serstim, smatrpin, Zrbtive, Zmactn I. Grwth Hrmne Deficiency in Children and Adlescents: Nte: Prvider must submit chart ntes that include the fllwing
AETNA BETTER HEALTH Prior Authorization guideline for Growth Hormone
 AETNA BETTER HEALTH Prir Authrizatin guideline fr Grwth Hrmne Grwth Hrmne and related agents Frmulary:, Omnitrpe vials Nn-Frmulary - Gentrpin, Humatrpe,,Saizen, Serstim, Tev-Trpin, Valtrpin, Zrbtive (smatrpin),
AETNA BETTER HEALTH Prir Authrizatin guideline fr Grwth Hrmne Grwth Hrmne and related agents Frmulary:, Omnitrpe vials Nn-Frmulary - Gentrpin, Humatrpe,,Saizen, Serstim, Tev-Trpin, Valtrpin, Zrbtive (smatrpin),
Pharmacy Prior Authorization Growth Hormone- Clinical Guidelines. Serostim Zorbtive somatropin
 Pharmacy Prir Authrizatin Grwth Hrmne- Clinical Guidelines Gentrpin Humatrpe Nrditrpin Nutrpin Omnitrpe Saizen Serstim Zmactn Zrbtive smatrpin General Criteria fr Apprval: Omnitrpe vial frmulatin is the
Pharmacy Prir Authrizatin Grwth Hrmne- Clinical Guidelines Gentrpin Humatrpe Nrditrpin Nutrpin Omnitrpe Saizen Serstim Zmactn Zrbtive smatrpin General Criteria fr Apprval: Omnitrpe vial frmulatin is the
Clinical Policy: Somatropin (Recombinant Human Growth Hormone) Reference Number: ERX.SPA.14 Effective Date:
 Clinical Plicy: Smatrpin (Recmbinant Human Grwth Hrmne) Reference Number: ERX.SPA.14 Effective Date: 07.01.16 Last Review Date: 05.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant
Clinical Plicy: Smatrpin (Recmbinant Human Grwth Hrmne) Reference Number: ERX.SPA.14 Effective Date: 07.01.16 Last Review Date: 05.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant
Drug Therapy Guidelines
 Drug Therapy Guidelines Applicable Medical Benefit Effective: 8/15/18 Pharmacy- Frmulary 1 x Next Review: 6/19 Pharmacy- Frmulary 2 x Date f Origin: 10/99 Grwth Stimulating Drugs: Nrditrpin Flexpr, Gentrpin,
Drug Therapy Guidelines Applicable Medical Benefit Effective: 8/15/18 Pharmacy- Frmulary 1 x Next Review: 6/19 Pharmacy- Frmulary 2 x Date f Origin: 10/99 Grwth Stimulating Drugs: Nrditrpin Flexpr, Gentrpin,
AETNA BETTER HEALTH Prior Authorization guideline for Growth Hormone Agents
 AETNA BETTER HEALTH Prior Authorization guideline for Growth Hormone Agents Growth Hormone and related agents Formulary: Omnitrope vials Non-Formulary: Genotropin, Humatrope, Saizen, Serostim, Tev-Tropin,
AETNA BETTER HEALTH Prior Authorization guideline for Growth Hormone Agents Growth Hormone and related agents Formulary: Omnitrope vials Non-Formulary: Genotropin, Humatrope, Saizen, Serostim, Tev-Tropin,
Pharmacy Prior Authorization Growth Hormone- Clinical Guidelines
 Genotropin, Humatrope, Norditropin, Nutropin, Omnitrope, Saizen, Serostim, somatropin, Zorbtive, Zomacton I. Growth Hormone Deficiency in Children and Adolescents: Note: Provider must submit chart notes
Genotropin, Humatrope, Norditropin, Nutropin, Omnitrope, Saizen, Serostim, somatropin, Zorbtive, Zomacton I. Growth Hormone Deficiency in Children and Adolescents: Note: Provider must submit chart notes
2. Has this plan authorized this medication in the past for this member (i.e., previous authorization is on file under this plan)?
 Pharmacy Prior Authorization AETA BETTER HEALTH KETUCK Growth Hormone (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Pharmacy Prior Authorization AETA BETTER HEALTH KETUCK Growth Hormone (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and
Topic 12: Endocrine System. Function: Group of glands that produces regulatory chemicals ( )
 Tpic 12: Endcrine System Functin: Grup f glands that prduces regulatry chemicals ( ) Hrmnes: Chemical messengers released directly int the bldstream that regulate: *May have wide-spread effects r nly affect
Tpic 12: Endcrine System Functin: Grup f glands that prduces regulatry chemicals ( ) Hrmnes: Chemical messengers released directly int the bldstream that regulate: *May have wide-spread effects r nly affect
Aetna Better Health of Virginia
 Genotropin Nutropin Serostim Zomacton Humatrope Omnitrope Zorbtive somatropin Norditropin Saizen General Criteria for Approval: Omnitrope vial formulation is the preferred Growth Hormone product; consideration
Genotropin Nutropin Serostim Zomacton Humatrope Omnitrope Zorbtive somatropin Norditropin Saizen General Criteria for Approval: Omnitrope vial formulation is the preferred Growth Hormone product; consideration
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Ancillary Symposia Request for Proposals Partner with the Endocrine Society to Educatte the Endocrine Community.
 Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
General Approval Criteria for ALL Growth Hormone agents: (ALL criteria must be met)
 Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
Growth Hormone Agents Prior Authorization Criteria for Louisiana Fee for Service and MCO Medicaid Recipients Page 1 of 7 Preferred Agents Somatropin Pen (Norditropin ) Somatropin Pen (Nutropin AQ ) Non-Preferred
Drug Therapy Guidelines
 Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
GROWTH HORMONE THERAPY
 GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 03/01/201804/01/2019 POLICY A. INDICATIONS The indications below including
GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 03/01/201804/01/2019 POLICY A. INDICATIONS The indications below including
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES. ANTI-OBESITY AGENTS Generic Brand HICL GCN Exception/Other QSYMIA 32515, 32744, 32746, 32745
 Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
GROWTH HORMONE THERAPY
 GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below including FDA-approved
GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below including FDA-approved
GROWTH HORMONE THERAPY
 GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 12/30/201601/01/2018TBD03/01/2018 POLICY A. INDICATIONS The indications
GROWTH HORMONE THERAPY Line(s) of Business: HMO; PPO; QUEST Integration Original Effective Date: 05/21/1999 Current Effective Date: 12/30/201601/01/2018TBD03/01/2018 POLICY A. INDICATIONS The indications
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
2. Is the request for Humatrope? Y N [If no, skip to question 6.]
![2. Is the request for Humatrope? Y N [If no, skip to question 6.] 2. Is the request for Humatrope? Y N [If no, skip to question 6.]](/thumbs/90/101419747.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH FLORIDA Growth Hormone Agents This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date.
Pharmacy Prior Authorization AETA BETTER HEALTH FLORIDA Growth Hormone Agents This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date.
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Rituxan (rituximab) Effective Date: 10/01/2015. Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage
 Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
PRIOR AUTHORIZATION CRITERIA
 DRUG CLASS BRAND NAME (generic) PRI AUTHIZATION CRITERIA TESTOSTERONE PRODUCTS (BRAND AND GENERIC) ANDRODERM (teststerne transdermal patch) ANDROGEL AXIRON (teststerne tpical slutin) DELATESTRYL (teststerne
DRUG CLASS BRAND NAME (generic) PRI AUTHIZATION CRITERIA TESTOSTERONE PRODUCTS (BRAND AND GENERIC) ANDRODERM (teststerne transdermal patch) ANDROGEL AXIRON (teststerne tpical slutin) DELATESTRYL (teststerne
ASSESSMENT OF PITUITARY FUNCTION IN PATIENTS WITH SERUM PROLACTIN LEVELS GREATER THAN 100 NG/ML*t
 FERTILITY AND STERILITY Cpyright 1979 The American Fertility Sciety Vl. 32, N.2, August 1979 Printed in U.s.A. ASSESSMENT OF PITUITARY FUNCTION IN PATIENTS WITH SERUM PROLACTIN LEVELS GREATER THAN 100
FERTILITY AND STERILITY Cpyright 1979 The American Fertility Sciety Vl. 32, N.2, August 1979 Printed in U.s.A. ASSESSMENT OF PITUITARY FUNCTION IN PATIENTS WITH SERUM PROLACTIN LEVELS GREATER THAN 100
o Prostanoids/prostacyclin therapies (oral and inhaled) o Inhaled agents: Ventavis, Tyvaso Page 1 of 5 Revised 02/17/17
 Request fr Prir Authrizatin Pulmnary Arterial Hypertensin (PAH) Agents (Oral and Inhaled) Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 All requests fr Pulmnary Arterial
Request fr Prir Authrizatin Pulmnary Arterial Hypertensin (PAH) Agents (Oral and Inhaled) Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 All requests fr Pulmnary Arterial
APPENDIX A Certification of Advanced Disease:
 APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
PHARMACY POLICY STATEMENT Indiana Medicaid
 DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Indiana Medicaid Zomacton (somatropin) Must
DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Indiana Medicaid Zomacton (somatropin) Must
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
First Name. Specialty: Fax. First Name DOB: Duration:
 Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
Acquired Optic Neuropathies
 Acquired Optic Neurpathies Jseph J. Pizzimenti, OD, FAAO Carl J. Pelin, OD, FAAO GOAL The gal f this presentatin is t prvide the participant with useful clinical infrmatin in the diagnsis and treatment
Acquired Optic Neurpathies Jseph J. Pizzimenti, OD, FAAO Carl J. Pelin, OD, FAAO GOAL The gal f this presentatin is t prvide the participant with useful clinical infrmatin in the diagnsis and treatment
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
HUMAN GROWTH HORMONE GENOTROPIN
 Drug Prior Authorization Guideline HUMAN GROWTH HORMONE GENOTROPIN (somatropin) PA9728 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes Additional Information: Medicare
Drug Prior Authorization Guideline HUMAN GROWTH HORMONE GENOTROPIN (somatropin) PA9728 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes Additional Information: Medicare
Chronic Fatigue Syndrome
 Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Drug Therapy Guidelines
 Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
Weight Assessment and Counseling for Children and Adolescents (NQF 0024)
 Weight Assessment and Cunseling fr Children and Adlescents (NQF 0024) EMeasure Name Weight Assessment and EMeasure Id Pending Cunseling fr Children and Adlescents Versin Number 1 Set Id Pending Available
Weight Assessment and Cunseling fr Children and Adlescents (NQF 0024) EMeasure Name Weight Assessment and EMeasure Id Pending Cunseling fr Children and Adlescents Versin Number 1 Set Id Pending Available
Request for Prior Authorization Growth Hormone (Norditropin
 Request for Prior Authorization Growth Hormone (Norditropin, Nutropin/AQ ) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Growth Hormone require a
Request for Prior Authorization Growth Hormone (Norditropin, Nutropin/AQ ) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Growth Hormone require a
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Drug Therapy Guidelines
 Drug Therapy Guidelines Applicable* Hereditary Angiedema (HAE) Agents: Berinert (C1 esterase inhibitr [human]), Cinryze (C1 esterase inhibitr [human]), Haegarda (C1 esterase inhibitr [human]) Kalbitr (ecallantide),
Drug Therapy Guidelines Applicable* Hereditary Angiedema (HAE) Agents: Berinert (C1 esterase inhibitr [human]), Cinryze (C1 esterase inhibitr [human]), Haegarda (C1 esterase inhibitr [human]) Kalbitr (ecallantide),
PHARMACY POLICY STATEMENT Indiana Medicaid
 DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Indiana Medicaid Norditropin (somatropin)
DRUG NAME BILLING CODE BENEFIT TYPE SITE OF SERVICE ALLOWED COVERAGE REQUIREMENTS LIST OF DIAGNOSES CONSIDERED NOT MEDICALLY NECESSARY PHARMACY POLICY STATEMENT Indiana Medicaid Norditropin (somatropin)
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
Diabetes Mellitus Lab Tests (Screening, Diagnosis & Monitoring)
 Rule Categry: Medical ` Ref: N: 2013-MN-0012 Versin Cntrl: Versin N. 1.1 Effective Date: December 2013 Revisin Date: December 2014 Diabetes Mellitus Lab Tests (Screening, Diagnsis & Mnitring) Adjudicatin
Rule Categry: Medical ` Ref: N: 2013-MN-0012 Versin Cntrl: Versin N. 1.1 Effective Date: December 2013 Revisin Date: December 2014 Diabetes Mellitus Lab Tests (Screening, Diagnsis & Mnitring) Adjudicatin
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache
 MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
HIP REPLACEMENT SURGERY (ARTHROPLASTY)
 Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
The estimator, X, is unbiased and, if one assumes that the variance of X7 is constant from week to week, then the variance of X7 is given by
 ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
High Performance Network Quality Criteria for Designation
 Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Humatrope*, Norditropin*, Genotropin, Nutropin, Nutropin AQ, Omnitrope, Saizen, Zomacton (aka. Tev-Tropin)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.12 Subject: Growth Hormone Pediatric Page: 1 of 6 Last Review Date: September 15, 2016 Growth Hormone
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.12 Subject: Growth Hormone Pediatric Page: 1 of 6 Last Review Date: September 15, 2016 Growth Hormone
Diabetes: HbA1c Poor Control (NQF 0059)
 Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Growth Hormone and related agents
 Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Growth Hormone and related agents Revised April 2014 Growth
Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Non-Formulary Prior Authorization guideline for Growth Hormone and related agents Revised April 2014 Growth
CDC Influenza Technical Key Points February 15, 2018
 CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Imaging tests allow the cancer care team to check for cancer and other problems inside the body.
 IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
Iowa Early Periodic Screening, Diagnosis and Treatment Care for Kids Program Provider Training
 Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Public consultation on the NHMRC s draft revised Australian alcohol guidelines for low-risk drinking
 Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
THROUGH 1979, immunosuppressive
 Five-Year Survival After Liver Transplantatin THROUGH 1979, immunsuppressive therapy fr liver transplantatin at ur center was with aathiprine (r cyclphsphamide) and sterids, t which antilymphcyte glublin
Five-Year Survival After Liver Transplantatin THROUGH 1979, immunsuppressive therapy fr liver transplantatin at ur center was with aathiprine (r cyclphsphamide) and sterids, t which antilymphcyte glublin
Male patients with pain, swelling or erythema please refer to the Acute Painful Scrotum pathway Female patients
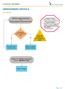 UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
Drug Therapy Guidelines
 Applicable* Medical Benefit x Effective: 2/15/19 Pharmacy- Frmulary 1 Next Review: 12/19 Pharmacy- Frmulary 2 Date f Origin: 4/1/05 Pharmacy- Frmulary 3/Exclusive Review Dates: 4/1/05, 2/1/06, 10/15/06,
Applicable* Medical Benefit x Effective: 2/15/19 Pharmacy- Frmulary 1 Next Review: 12/19 Pharmacy- Frmulary 2 Date f Origin: 4/1/05 Pharmacy- Frmulary 3/Exclusive Review Dates: 4/1/05, 2/1/06, 10/15/06,
Clinical Policy: Corticotropin (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date:
 Clinical Plicy: (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date: 10.01.16 Last Review Date: 02.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal infrmatin.
Clinical Plicy: (H.P. Acthar) Reference Number: ERX.SPA.72 Effective Date: 10.01.16 Last Review Date: 02.18 Revisin Lg See Imprtant Reminder at the end f this plicy fr imprtant regulatry and legal infrmatin.
DRAFT Policy for the Management of Ear Wax
 Clinical Cmmissining Grup (CCG) Treatment Plicy NHS Birmingham and Slihull Clinical Cmmissining Grup NHS Sandwell and West Birmingham Clinical Cmmissining Grup DRAFT Plicy fr the Management f Ear Wax 1
Clinical Cmmissining Grup (CCG) Treatment Plicy NHS Birmingham and Slihull Clinical Cmmissining Grup NHS Sandwell and West Birmingham Clinical Cmmissining Grup DRAFT Plicy fr the Management f Ear Wax 1
Genotropin, Norditropin, Nutropin, Nutropin AQ, Humatrope, Saizen,
 Blue Cross Blue Shield of Vermont and The Vermont Health Plan Prior Approval Guidelines Human Growth Hormone Somatropin (Genotropin, Norditropin, Nutropin, Nutropin AQ, Humatrope, Serostim, Saizen, Zomacton/TevTropin,
Blue Cross Blue Shield of Vermont and The Vermont Health Plan Prior Approval Guidelines Human Growth Hormone Somatropin (Genotropin, Norditropin, Nutropin, Nutropin AQ, Humatrope, Serostim, Saizen, Zomacton/TevTropin,
XX Abraxane 100 MG SUSR (CELGENE CORP)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
PBTC-026: A Feasibility Study of SAHA Combined with Isotretinoin and Chemotherapy in Infants with Embryonal Tumors of the Central Nervous System
 PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
Growth Hormone!gents. WA.PHAR.50 Growth Hormone Agents
 Growth Hormone!gents WA.PHAR.50 Growth Hormone Agents Background: Human growth hormone, also known as somatotropin, is produced in the anterior lobe of the pituitary gland. This hormone plays an important
Growth Hormone!gents WA.PHAR.50 Growth Hormone Agents Background: Human growth hormone, also known as somatotropin, is produced in the anterior lobe of the pituitary gland. This hormone plays an important
WCPT awards programme 2015
 WCPT awards prgramme 2015 The WCPT awards prgramme recgnises utstanding cntributins and leadership by individual physical therapists and grups t the prfessin and/r glbal health at an internatinal level.
WCPT awards prgramme 2015 The WCPT awards prgramme recgnises utstanding cntributins and leadership by individual physical therapists and grups t the prfessin and/r glbal health at an internatinal level.
SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY
 EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY Sme 50% f cancers that affect wmen are lcated in the breast r in the genital rgans. Gynaeclgical
EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY Sme 50% f cancers that affect wmen are lcated in the breast r in the genital rgans. Gynaeclgical
PROVIDER ALERT. Comprehensive Diagnostic Evaluation (CDE) Guidelines to Access the Applied Behavior Analysis (ABA) Benefit.
 Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
New Exception Status Benefits
 FEBRUARY 2019 Nva Sctia Frmulary Updates New Exceptin Status Benefits Prcysbi (cysteamine bitartrate) Nucala (meplizumab) Ocaliva (betichlic acid) Ravicti (glycerl phenylbutyrate) Taltz (ixekizumab) Criteria
FEBRUARY 2019 Nva Sctia Frmulary Updates New Exceptin Status Benefits Prcysbi (cysteamine bitartrate) Nucala (meplizumab) Ocaliva (betichlic acid) Ravicti (glycerl phenylbutyrate) Taltz (ixekizumab) Criteria
o Procedures performed o Diagnoses Identified o Certain devices/equipment/supplies acquired for patient
 Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Growth Hormone Therapy
 Growth Hormone Therapy Policy Number: Original Effective Date: MM.04.011 05/21/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/23/2014 Section: Prescription Drugs Place(s)
Growth Hormone Therapy Policy Number: Original Effective Date: MM.04.011 05/21/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/23/2014 Section: Prescription Drugs Place(s)
2017 CMS Web Interface
 CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
A fake medicine that passes itself off as a real, authorised medicine. (1)
 Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
CLINICAL PRACTICE GUIDELINE
 Pages: 2 f 11 V. GUIDELINE: The fllwing guidelines are cnsistent with the 2018 Standards f Medical Care in Diabetes frm the American Diabetes Assciatin (ADA). Sme specificatins fr HEDIS measures f diabetes
Pages: 2 f 11 V. GUIDELINE: The fllwing guidelines are cnsistent with the 2018 Standards f Medical Care in Diabetes frm the American Diabetes Assciatin (ADA). Sme specificatins fr HEDIS measures f diabetes
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
CIGNA HealthCare Prior Authorization Form - Growth Hormone Medications -
 Pharmacy Services Phone: (800)244-6224 Fax: (800)390-9745 CIGNA HealthCare Prior Authorization Form - Growth Hormone Medications - Notice: Failure to complete this form in its entirety may result in delayed
Pharmacy Services Phone: (800)244-6224 Fax: (800)390-9745 CIGNA HealthCare Prior Authorization Form - Growth Hormone Medications - Notice: Failure to complete this form in its entirety may result in delayed
Prior Authorization Criteria Form This form applies to Paramount Commercial Members Only. Non-Preferred Growth Hormone Products
 Prior Authorization Criteria Form This form applies to Paramount Commercial Members Only Criteria: P0078 Approved: 3/2017 Reviewed: Non-Preferred Growth Hormone Products Complete/review information, sign
Prior Authorization Criteria Form This form applies to Paramount Commercial Members Only Criteria: P0078 Approved: 3/2017 Reviewed: Non-Preferred Growth Hormone Products Complete/review information, sign
