ATC/DDD classification
|
|
|
- Joel Conley
- 5 years ago
- Views:
Transcription
1 The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measurin unit are tools for exchanin and comparin data on dru use at international, national or local levels. The ATC/DDD system has become the old standard for international dru utilization research. It is maintained by the WHO Collaboratin Centre for Dru Statistics Methodoloy in Oslo, Norway. Visit for more information. (temporary) The followin s and DDDs were areed at the meetin of the WHO International Workin Group for Dru Statistics Methodoloy in March Comments or objections to the decisions from the meetin should be forwarded to the WHO Collaboratin Centre for Dru Statistics Methodoloy before 1 September If no objections are received before this date, the new s and DDDs will be considered final and included in the January 2019 version of the ATC/DDD Index. New ATC 5 th level codes abemaciclib aniotensin II apalutamide avatrombopa candesartan, amlodipine and hydrochlorothiazide cannabidiol cefpodoxime and beta-lactamase inhibitor copanlisib doravirine edaravone fexinidazole fostamatinib emiliptin and rosuvastatin hydroen peroxide ibalizumab lamivudine, tenofovir disoproxil and doravirine leuprorelin and bicalutamide metformin, saxaliptin and dapaliflozin L01XE50 C01CX09 L02BB05 B02BX08 C09DX06 N03AX24 J01DD64 L01XX61 J05AG06 N07XX14 P01CA03 B02BX09 A10BH52 D11AX25 J05AX23 J05AR24 L02AE51 A10BD25 276
2 Temporary New ATC 5 th level codes (continued) omidenepa prasterone talazoparib telotristat trifarotene S01EX06 G03XX01 L01XX60 A16AX15 D10AD06 New ATC level codes (other than 5 th levels) Other sex hormones and modulators of the enital system G03XX Chane of Previous New dimethyl fumarate N07XX09 L04AX07 Chanes of ATC level names Previous New Aniotensin II antaonists, plain Aniotensin II receptor blockers (ARBs), plain C09C Aniotensin II antaonists, plain Aniotensin II receptor blockers (ARBs), plain C09CA Aniotensin II antaonists, Aniotensin II receptor blockers (ARBs), combinations combinations C09D Aniotensin II antaonists and Aniotensin II receptor blockers (ARBs) diuretics and diuretics C09DA Aniotensin II antaonists and Aniotensin II receptor blockers (ARBs) calcium channel blockers and calcium channel blockers C09DB Aniotensin II antaonists, other Aniotensin II receptor blockers (ARBs), combinations other combinations C09DX fluoromethylcholine (18F) fluorocholine (18F) V09IX07 combinations potassium (different salts in combination) A12BA30 New DDDs DDD unit Adm.R* artemether 0.28 R P01BE02 artesunate 0.28 R P01BE03 benralizumab 0.54 m P R03DX10 chenodeoxycholic acid 1 O A05AA01 cladribine 0.34 m O L04AA40 dupilumab 21.4 m P D11AH05 277
3 Temporary New DDDs (continued) DDD unit Adm.R* emicizumab 15 m P B02BX06 ertuliflozin 10 m O A10BK04 uselkumab 1.79 m P L04AC16 letermovir 0.48 O,P J05AX18 ocrelizumab 3.29 m P L04AA36 patiromer calcium 8.4 O V03AE09 rolapitant 0.18 O A04AD14 telotristat 0.75 O A16AX15 * Administration Route: O = oral; P = parenteral. Chanes of DDDs Previous DDD New DDD DDD Unit Adm.R.* DDD Unit Adm.R.* aprepitant 1 95 m P 150 m P A04AD12 aprepitant 95 m O 165 m O A04AD12 temocillin 2 P 4 P J01CA17 vasopressin (aripressin) 4 U P 40 U P H01BA01 * Administration Route: P = parenteral. 1 expressed as fosaprepitant WHO Collaboratin Centre for Dru Statistics Methodoloy Oslo, May
4 (final) The followin s and DDDs were areed at the meetin of the WHO International Workin Group for Dru Statistics Methodoloy in October These are considered final and included in the January 2019 version of the ATC/DDD Index. New ATC 5 th level codes ambrisentan and tadalafil atazanavir and ritonavir atezolizumab avelumab caplacizumab cefteram cladribine 1 clenbuterol and ambroxol crisaborole crofelemer cytarabine and daunorubicin dacomitinib dapivirine delafloxacin doxepin emapalumab enasidenib encorafenib enisamium iodide epacadostat eravacycline ertuliflozin fexapotide floxuridine alcanezumab lecaprevir and pibrentasvir ibandronic acid and colecalciferol icotinib imidazolyl ethanamide pentandioic acid indocyanine reen ipraliflozin isoniazid, sulfamethoxazole, trimethoprim and pyridoxine kaocel lanadelumab C02KX52 J05AR23 L01XC32 L01XC31 B01AX07 J01DD18 L04AA40 R03CC63 D11AH06 A07XA06 L01XY01 L01XE47 G01AX17 J01MA23 D04AX01 L04AA39 L01XX59 L01XE46 J05AX17 L01XX58 J01AA13 A10BK04 G04CX04 L01BC09 N02CX08 J05AP57 M05BB09 L01XE48 J05AX21 V04CX01 A10BK05 J04AM08 J05AX22 B06AC05 279
5 Final New ATC 5 th level codes (continued) letermovir lorlatinib lusutrombopa medroxyproesterone and estradiol meropenem and vaborbactam metformin and ertuliflozin naldemedine neratinib norestimate and ethinylestradiol ozanimod patisiran peramivir riboflavin rifampicin, ethambutol and isoniazid roxadustat sitaliptin and ertuliflozin tebipenem pivoxil tildrakizumab tilorone tosufloxacin uramustine vainal rin with proestoen valbenazine valsartan and nebivolol J05AX18 L01XE44 B02BX07 G03AA17 J01DH52 A10BD23 A06AH05 L01XE45 G03AB09 L04AA38 N07XX12 J05AH03 S01XA26 J04AM07 B03XA05 A10BD24 J01DH06 L04AC17 J05AX19 J01MA22 L01AD08 G02BB02 N07XX13 C09DX05 1 Oral formulations indicated for multiple sclerosis. Parenteral formulations are classified in L01BB04. Chane of ATC level name Previous New dinutuximab dinutuximab beta L01XC16 New DDDs DDD Unit Adm.R* atazanavir and ritonavir O J05AR23 baricitinib 4 m O L04AA37 cariprazine 3 m O N05AX15 cefroxadine 2.1 O J01DB11 cefteram 0.4 O J01DD18 ceftriaxone and beta-lactamase inhibitor 2 2 P J01DD63 colistin 9 MU O A07AA10 280
6 Final New DDDs (continued) DDD Unit Adm.R* delafloxacin delafloxacin O P J01MA23 J01MA23 delamanid 0.2 O J04AK06 desfesoterodine 3.5 m O G04BD13 droxidopa 1 O C01CA27 eluxadoline 0.2 O A07DA06 enisamium iodide 1.5 O J05AX17 estradiol 1.53 m TD spray G03CA03 faropenem 0.75 O J01DI03 follitropin delta 12 mc P G03GA10 arenoxacin 0.4 O J01MA19 emifloxacin 0.32 O J01MA15 imidazolyl ethanamide pentandioic acid 90 m O J05AX21 insulin larine and lixisenatide 40 U 3 P A10AE54 kaocel 60 m O J05AX22 kanamycin 3 O A07AA08 lomefloxacin 0.4 O J01MA07 mepyramine theophyllinacetate 0.6 O R03DA12 midecamycin 1.2 O J01FA03 misoprostol 0.2 m O G02AD06 naldemedine 0.2 m O A06AH05 nusinersen 0.1 m P M09AX07 pyrimethamine, combinations 75 m 4 P P01BD51 reslizumab 7.1 m P R03DX08 rimantadine 0.2 O J05AC02 sarilumab 14.3 m P L04AC14 secnidazole 2 O P01AB07 tebipenem pivoxil 0.56 O J01DH06 thyroid land preparations 0.1 O H03AA05 tilorone O J05AX19 tosufloxacin 0.45 O J01MA22 * Administration Route: O = oral; P = parenteral. 1 refers to atazanavir. 2 refers to ceftriaxone. 3 refers to insulin larine. 4 refers to pyrimethamine. 281
7 Final Chanes of DDDs Previous DDD New DDD DDD Unit Adm.R.* DDD Unit Adm.R.* amoxicillin amoxicillin 1 1 O P O P J01CA04 J01CA04 amoxicillin and betalactamase inhibitor 1 1 O 1.5 O J01CR02 ampicillin 2 P 6 P J01CA01 cefepime 2 P 4 P J01DE01 ciprofloxacin 0.5 P 0.8 P J01MA02 colistin 3 MU P 9 MU P J01XB01 meropenem 2 P 3 P J01DH02 * Administration Route: O = oral; P = parenteral. 1 New ATC level name valid from WHO Collaboratin Centre for Dru Statistics Methodoloy Oslo, May
ATC/DDD classification
 WHO Dru Information Vol. 31, No. 4, 2017 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measurin unit are tools for exchanin
WHO Dru Information Vol. 31, No. 4, 2017 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measurin unit are tools for exchanin
ATC/DDD classification
 The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or
The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or
ATC/DDD classification
 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international,
ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international,
ATC/DDD Classification
 (Temporary) The following anatomical therapeutic codes (ATC), defined daily doses (DDD) and alterations were considered by the WHO International Working Group for Drug Statistics Methodology at its meeting
(Temporary) The following anatomical therapeutic codes (ATC), defined daily doses (DDD) and alterations were considered by the WHO International Working Group for Drug Statistics Methodology at its meeting
Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use
 3 August 206 EMA/52958/206 Information Management Division Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use August 206 This document lists information
3 August 206 EMA/52958/206 Information Management Division Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use August 206 This document lists information
Anatomical Therapeutic Chemical Classification (ATC) & And Defined Daily Dose (DDD) Principles for classifying and quantifying drug use
 Anatomical Therapeutic Chemical Classification (ATC) & And Defined Daily Dose (DDD) Principles for classifying and quantifying drug use Margaret Oluka Course outline/objectives 1. Introduction: drug classification
Anatomical Therapeutic Chemical Classification (ATC) & And Defined Daily Dose (DDD) Principles for classifying and quantifying drug use Margaret Oluka Course outline/objectives 1. Introduction: drug classification
Outline. Definitions. ATC Anatomical Therapeutic Chemical classification
 The Anatomical Therapeutic Chemical Classification and the Defined Daily Dose: Classifying and Quantifying Drug Use Hanne Strøm Department of Pharmacoepidemiology ICPE, Chicago August 2011 : Preconference
The Anatomical Therapeutic Chemical Classification and the Defined Daily Dose: Classifying and Quantifying Drug Use Hanne Strøm Department of Pharmacoepidemiology ICPE, Chicago August 2011 : Preconference
«Belmedpreparaty» Minsk, Republic of Belarus
 Dear Sirs, «Belmedpreparaty» Minsk, Republic of Belarus «Belmedpreparaty» offers cooperation to wholesale distributors working on the pharmaceutical markets. Our enterprise is the leading pharmaceutical
Dear Sirs, «Belmedpreparaty» Minsk, Republic of Belarus «Belmedpreparaty» offers cooperation to wholesale distributors working on the pharmaceutical markets. Our enterprise is the leading pharmaceutical
Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use
 7 January 2016 EMA/6499/2016 Information Management Division Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use January 2016 This document lists
7 January 2016 EMA/6499/2016 Information Management Division Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use January 2016 This document lists
CURRENT WORK PLAN New monographs for inclusion in The International Pharmacopoeia and revision of related monographs
 page 1 CURRENT WORK PLAN New monographs for inclusion in The International Pharmacopoeia and revision of related monographs 2011 The following categories are included in this work programme: 1. Medicines
page 1 CURRENT WORK PLAN New monographs for inclusion in The International Pharmacopoeia and revision of related monographs 2011 The following categories are included in this work programme: 1. Medicines
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/321/5886/263/dc1 Supporting Online Material for Drug Target Identification Using Side-Effect Similarity Monica Campillos, Michael Kuhn, Anne-Claude Gavin, Lars Juhl
www.sciencemag.org/cgi/content/full/321/5886/263/dc1 Supporting Online Material for Drug Target Identification Using Side-Effect Similarity Monica Campillos, Michael Kuhn, Anne-Claude Gavin, Lars Juhl
New Drugs of ,3,5,7,10,14,16,20,22,24,26,28,30,32,34,36,38,40,41,43,45-86 Brand Name/ Generic Name/ Approved Indication
 1 New Drugs of 2017 1,3,5,7,10,14,16,20,22,24,26,28,30,32,34,36,38,40,41,43,45-86 Brand Name/ Generic Name/ Approved Indication Company Aliqopa Copanlisib Dihydrochloride Intravenous Bayer HealthCare Pharmaceuticals
1 New Drugs of 2017 1,3,5,7,10,14,16,20,22,24,26,28,30,32,34,36,38,40,41,43,45-86 Brand Name/ Generic Name/ Approved Indication Company Aliqopa Copanlisib Dihydrochloride Intravenous Bayer HealthCare Pharmaceuticals
Clinical Overview of Innovative New Drug Approvals in 2017
 Reddy: Clinical Overview of Innovative New Drug Approvals in 2017 4225 International Journal of Pharmaceutical Sciences and Nanotechnology Review Article Clinical Overview of Innovative New Drug Approvals
Reddy: Clinical Overview of Innovative New Drug Approvals in 2017 4225 International Journal of Pharmaceutical Sciences and Nanotechnology Review Article Clinical Overview of Innovative New Drug Approvals
1. PROCEDURE FOR THIS INVITATION TO EOI 2. APIS INCLUDED IN THE 14TH INVITATION. Guidance Document 11 April 2017
 To support national and global efforts to increase access to and the affordability of care and treatment of HIV/AIDS, hepatitis B and C, tuberculosis, malaria, neglected tropical diseases, influenza, diarrhoea
To support national and global efforts to increase access to and the affordability of care and treatment of HIV/AIDS, hepatitis B and C, tuberculosis, malaria, neglected tropical diseases, influenza, diarrhoea
HCPWP feedback from CHMP
 HCPWP feedback from CHMP Presented by: Fátima Ventura (CHMP) 26 September 2018 An agency of the European Union Summary CHMP opinions (Apr 2018 Sep 2018) New medicines Other issues related to authorised
HCPWP feedback from CHMP Presented by: Fátima Ventura (CHMP) 26 September 2018 An agency of the European Union Summary CHMP opinions (Apr 2018 Sep 2018) New medicines Other issues related to authorised
Guidelines for ATC classification and DDD assignment 2019
 Guidelines for ATC classification and DDD assignment 2019 ISSN 1726-4898 ISBN 978-82-8082-974-0 Suggested citation: WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification
Guidelines for ATC classification and DDD assignment 2019 ISSN 1726-4898 ISBN 978-82-8082-974-0 Suggested citation: WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification
Registered Products in BG
 Product name INN Pharmaceutical form Pharmaceutical Package size ATC code Prescription strength status Acnederm Tretinoin cream 0,5 mg/g 30g D10AD01 Adrenalin Epinephrine 1 mg/ml C01CA 24 Allergosan Chloropyramine
Product name INN Pharmaceutical form Pharmaceutical Package size ATC code Prescription strength status Acnederm Tretinoin cream 0,5 mg/g 30g D10AD01 Adrenalin Epinephrine 1 mg/ml C01CA 24 Allergosan Chloropyramine
Health Partners Medicare Special Formulary Updates
 March 2018 Effective Date Brand Name Generic Name Type of Change 90 / 30 DAYS tenofovir disoproxil fumarate tenofovir disoproxil fumarate clobetasol propionate tenofovir disoproxil fumarate Previous Value
March 2018 Effective Date Brand Name Generic Name Type of Change 90 / 30 DAYS tenofovir disoproxil fumarate tenofovir disoproxil fumarate clobetasol propionate tenofovir disoproxil fumarate Previous Value
(Samt mulig andre indikasjoner i fremtiden.) Oppført på listen Depatuximab mafodotin Treatment of glioblastoma (GBM) nov.18. Virus type 1 (HIV-1)
 Nye legemidler som ikke har markedsføringstillatelse For legemidler oppført på listen gjelder retningslinjens vilkår uavhengig av indikasjon for bruken. Oppdatert: 13.11.2018 Orphan medicinal product (*
Nye legemidler som ikke har markedsføringstillatelse For legemidler oppført på listen gjelder retningslinjens vilkår uavhengig av indikasjon for bruken. Oppdatert: 13.11.2018 Orphan medicinal product (*
LUNCH AND LEARN. May 11, CE Activity Information & Accreditation
 LUNCH AND LEARN May 11, 2018 Featured Speaker: Mary Lynn Moody, B.S. Pharm. Assistant Dean for Business Development Clinical Associate Professor Drug Information and Prior Authorization Group Department
LUNCH AND LEARN May 11, 2018 Featured Speaker: Mary Lynn Moody, B.S. Pharm. Assistant Dean for Business Development Clinical Associate Professor Drug Information and Prior Authorization Group Department
Drug utilization patterns among elderly hospitalized patients on poly-pharmacy in Punjab, Pakistan
 Sarwar et al. Journal of Pharmaceutical Policy and Practice (2017) 10:23 DOI 10.1186/s40545-017-0112-z RESEARCH Drug utilization patterns among elderly hospitalized patients on poly-pharmacy in Punjab,
Sarwar et al. Journal of Pharmaceutical Policy and Practice (2017) 10:23 DOI 10.1186/s40545-017-0112-z RESEARCH Drug utilization patterns among elderly hospitalized patients on poly-pharmacy in Punjab,
LUNCH AND LEARN. April 13, CE Activity Information & Accreditation
 LUNCH AND LEARN New Drugs of 2017: Part 1 April 13, 2018 Featured Speaker: Mary Lynn Moody, B.S. Pharm. Assistant Dean for Business Development Clinical Associate Professor Drug Information and Prior Authorization
LUNCH AND LEARN New Drugs of 2017: Part 1 April 13, 2018 Featured Speaker: Mary Lynn Moody, B.S. Pharm. Assistant Dean for Business Development Clinical Associate Professor Drug Information and Prior Authorization
3a o6m:ectbeha rropbqka c rrpe)j;met
 3a o6m:ectbeha rropbqka c rrpe)j;met ",l.j;octabka Ha JieKapcTBeHH rrpo)j;ykth 1a HY~HTe Ha I(CMII-CT.3aropa rrpe3 2015 r." IIpHJIOLKeHHe.NH Me~yuapot~,uo.N! ATC uenatehtho HaUMeHOBaHHSI JleKapcTBeua opMa
3a o6m:ectbeha rropbqka c rrpe)j;met ",l.j;octabka Ha JieKapcTBeHH rrpo)j;ykth 1a HY~HTe Ha I(CMII-CT.3aropa rrpe3 2015 r." IIpHJIOLKeHHe.NH Me~yuapot~,uo.N! ATC uenatehtho HaUMeHOBaHHSI JleKapcTBeua opMa
ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS GIT PRODUCTS
 SR. NO 1 ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS Paracetamol 500 mg, Phenylephrine HCL 5 mg With Chlorpheniramine Maleate 2 mg & Caffeine 30 mg Tablets 2 Salbutamol Tablets BP 2 mg 3 Salbutamol Tablets
SR. NO 1 ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS Paracetamol 500 mg, Phenylephrine HCL 5 mg With Chlorpheniramine Maleate 2 mg & Caffeine 30 mg Tablets 2 Salbutamol Tablets BP 2 mg 3 Salbutamol Tablets
Centurion Laboratories Pvt. Ltd.
 Centurion Laboratories Pvt. Ltd. P-2, Bio-tech Park, Manjusar, Savli, Baroda - 391780 (Guj) INDIA. www.centurionlaboratories.com,www.centurionremedies.com,sales@centurionremedies.com Centurion Laboratories
Centurion Laboratories Pvt. Ltd. P-2, Bio-tech Park, Manjusar, Savli, Baroda - 391780 (Guj) INDIA. www.centurionlaboratories.com,www.centurionremedies.com,sales@centurionremedies.com Centurion Laboratories
Appendix 4: Renal impairment
 Appendix 4: Renal impairment Reduced renal function may cause problems with drug therapy for the following reasons: 1. The failure to excrete a drug or its metabolites may produce toxicity. 2. The to some
Appendix 4: Renal impairment Reduced renal function may cause problems with drug therapy for the following reasons: 1. The failure to excrete a drug or its metabolites may produce toxicity. 2. The to some
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Svanström H, Pasternak B, Hviid A. Use of azithromycin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Svanström H, Pasternak B, Hviid A. Use of azithromycin and
PA Category Name Code(s) Additional Notes ABA. Applied Behavioral Analysis stage 3*
 ABA BEHAVIORAL HEALTH CHEMICAL DEPENDENCY Applied Behavioral Analysis stage 3* Neuropsychological Testing Chemical Dependency/Substance Abuse* (MA Only) 0373T H2020 96116 96112 96113 96121 96130 96131
ABA BEHAVIORAL HEALTH CHEMICAL DEPENDENCY Applied Behavioral Analysis stage 3* Neuropsychological Testing Chemical Dependency/Substance Abuse* (MA Only) 0373T H2020 96116 96112 96113 96121 96130 96131
TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS. Reviewed Items. August 2016
 TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS Reviewed Items August 2016 SUMMARY OF CHANGES TO THE TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS (JUNE 2016) M03AX01
TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS Reviewed Items August 2016 SUMMARY OF CHANGES TO THE TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS (JUNE 2016) M03AX01
Miriam O Shea *, Mary Teeling and Kathleen Bennett
 O Shea et al. BMC Health Services Research 2013, 13:23 RESEARCH ARTICLE Open Access The prevalence and ingredient cost of chronic comorbidity in the Irish elderly population with medication treated type
O Shea et al. BMC Health Services Research 2013, 13:23 RESEARCH ARTICLE Open Access The prevalence and ingredient cost of chronic comorbidity in the Irish elderly population with medication treated type
TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS. April 2016
 TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS April 2016 TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS ATC CODE MEDICINE INDICATION RECOMMENDATION A ALIMENTARY TRACT
TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS April 2016 TERTIARY AND QUATERNARY LEVEL ESSENTIAL MEDICINES RECOMMENDATIONS ATC CODE MEDICINE INDICATION RECOMMENDATION A ALIMENTARY TRACT
Laurajo Ryan, PharmD, MSc, BCPS Clinical Associate Professor
 NEW DRUG UPDATE 2018 Laurajo Ryan, PharmD, MSc, BCPS Clinical Associate Professor RyanL@uthscsa.edu Bryson Duhon, PharmD, BCPS Clinical Assistant Professor Duhon@austin.utexas.edu Accreditation New Drug
NEW DRUG UPDATE 2018 Laurajo Ryan, PharmD, MSc, BCPS Clinical Associate Professor RyanL@uthscsa.edu Bryson Duhon, PharmD, BCPS Clinical Assistant Professor Duhon@austin.utexas.edu Accreditation New Drug
Supplementary Materials for
 Supplementary Materials for Williamson T, Green ME, Birtwhistle R, et al. Validating the 8 CPCSSN case definitions for chronic surveillance in a primary care database of electronic health records. Ann
Supplementary Materials for Williamson T, Green ME, Birtwhistle R, et al. Validating the 8 CPCSSN case definitions for chronic surveillance in a primary care database of electronic health records. Ann
INDEX THERAPEUTIC CLASS OF DRUGS
 BHARAT PARENTERALS LIMITED PRODUCT LIST OF BPL AS PER THERAPEUTIC CLASSIFICATION AS ON NOVEMBER 2016 SR NO. INDEX THERAPEUTIC CLASS OF DRUGS PAGE NO 1 ANTIMALARIAL 2 2 GASTROINESTINALTRACT/ALIMENTARY 3
BHARAT PARENTERALS LIMITED PRODUCT LIST OF BPL AS PER THERAPEUTIC CLASSIFICATION AS ON NOVEMBER 2016 SR NO. INDEX THERAPEUTIC CLASS OF DRUGS PAGE NO 1 ANTIMALARIAL 2 2 GASTROINESTINALTRACT/ALIMENTARY 3
Appendix 5: Hepatic impairment
 Appendix 5: Hepatic impairment Liver disease may alter the response to drugs. However, the hepatic reserve appears to be large and liver disease has to be severe before important changes in drug metabolism
Appendix 5: Hepatic impairment Liver disease may alter the response to drugs. However, the hepatic reserve appears to be large and liver disease has to be severe before important changes in drug metabolism
Cover story. Austedo Ingrezza Brineura Alunbrig Rydapt Tymlos Imfinzi Radicava Kevzara Baxdela Bevyxxa
 Cover story Downloaded via 148.251.232.83 on March 9, 2019 at 14:34:33 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. Trulance Parsabiv Emflaza
Cover story Downloaded via 148.251.232.83 on March 9, 2019 at 14:34:33 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. Trulance Parsabiv Emflaza
Medicine Price Data Collection Form. Use a separate form for each medicine outlet
 (Read this to the study participant) Price Data Collection Form Use a separate form for each medicine outlet "Boston University in collaboration with and IPA is conducting a study on the availability and
(Read this to the study participant) Price Data Collection Form Use a separate form for each medicine outlet "Boston University in collaboration with and IPA is conducting a study on the availability and
Rx PRODUCTS. Rx PRODUCTS PORTFOLIO
 Rx PRODUCTS Rx PRODUCTS PORTFOLIO STRAGEN Rx PRODUCTS When the generic drug market is often synonym of blockbuster race and cost cutting, STRAGEN has always been engaged in the development of Selected
Rx PRODUCTS Rx PRODUCTS PORTFOLIO STRAGEN Rx PRODUCTS When the generic drug market is often synonym of blockbuster race and cost cutting, STRAGEN has always been engaged in the development of Selected
1. Procedure for this Invitation to EOI. 2. Medicinal products included on the 14 th Invitation. Guidance Document 14 May 2016
 To support national and global efforts to increase access to and the affordability of HIV/AIDS-related care and treatment together with UNICEF, UNAIDS and UNITAID, invite applicants for selected pharmaceutical
To support national and global efforts to increase access to and the affordability of HIV/AIDS-related care and treatment together with UNICEF, UNAIDS and UNITAID, invite applicants for selected pharmaceutical
Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011)
 Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011) What s new in hypertension? NICE has issued an updated Clinical
Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011) What s new in hypertension? NICE has issued an updated Clinical
ASEBP and ARTA TARP Drugs and Reference Price by Categories
 ASEBP Pantoprazole Sodium 40 mg (generic) $0.2016 ASEBP Dexlansoprazole 30 mg Dexlansoprazole 60 mg Esomeprazole 10 mg Esomeprazole 20 mg Esomeprazole 40 mg Lansoprazole 15 mg Lansoprazole 30 mg Omeprazole
ASEBP Pantoprazole Sodium 40 mg (generic) $0.2016 ASEBP Dexlansoprazole 30 mg Dexlansoprazole 60 mg Esomeprazole 10 mg Esomeprazole 20 mg Esomeprazole 40 mg Lansoprazole 15 mg Lansoprazole 30 mg Omeprazole
5 Infections. To be used in conjunction with NICE guidance, The British National Formulary for adults and/or children and
 5 Infections To be used in conjunction with NICE guidance, The British National Formulary for adults and/or children and Southend University Hospital, Antibiotic Guidelines Index 5.1 Antibacterial drugs
5 Infections To be used in conjunction with NICE guidance, The British National Formulary for adults and/or children and Southend University Hospital, Antibiotic Guidelines Index 5.1 Antibacterial drugs
PMPRB ANNUAL REPORT 2011
 PMPRB ANNUAL REPORT 2011 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO THE PMPRB 2011* JANUARY 1 DECEMBER 31, 2011 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES
PMPRB ANNUAL REPORT 2011 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO THE PMPRB 2011* JANUARY 1 DECEMBER 31, 2011 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES
ischemic stroke, transient ischemic attack, or peripheral artery embolism
 Appendix Table S1: ICD- 8/1 codes and ATC- codes Population Acute myocardial infarction Defined from primary inpatient diagnoses codes PCI Defined from procedure codes Non- valvular atrial fibrillation
Appendix Table S1: ICD- 8/1 codes and ATC- codes Population Acute myocardial infarction Defined from primary inpatient diagnoses codes PCI Defined from procedure codes Non- valvular atrial fibrillation
MEDICINES CATALOG NOVEMBER 2018 GLOBAL DRUG FACILITY (GDF)
 NOVEMBER 2018 MEDICINES CATALOG GLOBAL DRUG FACILITY (GDF) Ensuring an uninterrupted supply of quality-assured, affordable tuberculosis (TB) medicines and diagnostics to the world. stoptb.org/gdf Stop
NOVEMBER 2018 MEDICINES CATALOG GLOBAL DRUG FACILITY (GDF) Ensuring an uninterrupted supply of quality-assured, affordable tuberculosis (TB) medicines and diagnostics to the world. stoptb.org/gdf Stop
DOSSIER LIST. tlo_seledyn.pdf :38:01 CMY K CM MY CY
 tlo_seledyn.pdf 1 22.08.2017 14:38:01 C M Y CM MY CY CMY K DOSSIER LIST tlo_seledyn.pdf 1 22.08.2017 14:38:01 PRODUCT NAME PHARMACEUTICAL FORM STRENGTH REFERENCE THERAPEUTIC CLASS ALIMENTARY TRACT & METABOLISM
tlo_seledyn.pdf 1 22.08.2017 14:38:01 C M Y CM MY CY CMY K DOSSIER LIST tlo_seledyn.pdf 1 22.08.2017 14:38:01 PRODUCT NAME PHARMACEUTICAL FORM STRENGTH REFERENCE THERAPEUTIC CLASS ALIMENTARY TRACT & METABOLISM
Supplementary Online Content
 Supplementary Online Content Lin Y-S, Chen Y-L, Chen T-H, et al. Comparison of Clinical Outcomes Among Patients With Atrial Fibrillation or Atrial Flutter Stratified by CHA 2 DS 2 -VASc Score. JAMA Netw
Supplementary Online Content Lin Y-S, Chen Y-L, Chen T-H, et al. Comparison of Clinical Outcomes Among Patients With Atrial Fibrillation or Atrial Flutter Stratified by CHA 2 DS 2 -VASc Score. JAMA Netw
COMMERCIAL APIs. S. No Molecule Name Therapeutic Category USDMF EDMF CEP IH
 1 Abacavir Sulphate Antiretroviral - * - 2 Abiraterone Acetate Oncology - - - 3 Albendazole Anti-infective - - - 4 Albuterol Sulphate Respiratory - - 5 Alendronate Sodium Trihydrate Metabolic Disorder
1 Abacavir Sulphate Antiretroviral - * - 2 Abiraterone Acetate Oncology - - - 3 Albendazole Anti-infective - - - 4 Albuterol Sulphate Respiratory - - 5 Alendronate Sodium Trihydrate Metabolic Disorder
PMPRB ANNUAL REPORT 2008
 PMPRB ANNUAL REPORT 2008 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO PMPRB 2008* JANUARY 1 DECEMBER 31, 2008 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES LIMITED
PMPRB ANNUAL REPORT 2008 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO PMPRB 2008* JANUARY 1 DECEMBER 31, 2008 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES LIMITED
Supplementary Online Content
 Supplementary Online Content Jørgensen ME, Hlatky MA, Køber L, et al. β-blocker associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. Published online
Supplementary Online Content Jørgensen ME, Hlatky MA, Køber L, et al. β-blocker associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. Published online
Invitation to Manufacturers 16 August 2017
 Invitation to Manufacturers 16 August 2017 Manufacturers of Antiretroviral (HIV/AIDS), Antihepatitis B and C, Antituberculosis and Antimalarial medicines Are Invited to Submit An Expression of Interest
Invitation to Manufacturers 16 August 2017 Manufacturers of Antiretroviral (HIV/AIDS), Antihepatitis B and C, Antituberculosis and Antimalarial medicines Are Invited to Submit An Expression of Interest
МИНИСТЕРСТВО НА ЗДРАВЕОПАЗВАНЕТО
 МИНИСТЕРСТВО НА ЗДРАВЕОПАЗВАНЕТО Наредба 25 от 1999 г. за оказване на спешна медицинска помощ Промяна, обн. ДВ 18/04.03.2014 Чл. 10. (1) Центровете за спешна медицинска помощ закупуват, поддържат в наличност
МИНИСТЕРСТВО НА ЗДРАВЕОПАЗВАНЕТО Наредба 25 от 1999 г. за оказване на спешна медицинска помощ Промяна, обн. ДВ 18/04.03.2014 Чл. 10. (1) Центровете за спешна медицинска помощ закупуват, поддържат в наличност
The NDAC (Antimicrobial, Antiparasitic, Antifungal & Antiviral) deliberated the proposals on and recommended the following:-
 Recommendation:- The NDAC (Antimicrobial, Antiparasitic, Antifungal & Antiviral) deliberated the proposals on 23.08.2013 and recommended the following:- Agenda no. Drug Recommendations 1 2 Raltegravir,
Recommendation:- The NDAC (Antimicrobial, Antiparasitic, Antifungal & Antiviral) deliberated the proposals on 23.08.2013 and recommended the following:- Agenda no. Drug Recommendations 1 2 Raltegravir,
Orphan Drug List. Drug Name Dosage Form Route of Admin ATC Code Description List Name. Amyl Nitrite INHALER INHALATION V۰۳AB
 Orphan Drug List - Drug Name Dosage Form Route of Admin ATC Code Description List Name Alprostadil ۲۰ mcg INJECTION, POWDER PARENTERAL CEA Alprostadil ۲۰ mcg INJECTION, SOLUTION PARENTERAL CEA Alteplase
Orphan Drug List - Drug Name Dosage Form Route of Admin ATC Code Description List Name Alprostadil ۲۰ mcg INJECTION, POWDER PARENTERAL CEA Alprostadil ۲۰ mcg INJECTION, SOLUTION PARENTERAL CEA Alteplase
Innovative Products Available. OTC Products Available
 Innovative Products Benfotiamine film-coated tablets 150 mg Cholecalciferol film-coated tablets 22400 I.U. Diltiazem rectal ointment 2% Agents for Treatment of Hemorrhoids and Fissures Blood and Blood
Innovative Products Benfotiamine film-coated tablets 150 mg Cholecalciferol film-coated tablets 22400 I.U. Diltiazem rectal ointment 2% Agents for Treatment of Hemorrhoids and Fissures Blood and Blood
Riesbeck's Pharmacy Reward Club Generic Medication List February 2018 $4 30 Day Supply
 Allergy, Cold & Flu Antibiotic Treatments Arthritis & Pain Benzonatate 100mg cap 14 42 Diphenhydramine HCl Cap 50 MG 30 90 Diphenhydramine HCl Inj 50MG/ML 1 3 Diphenhydramine HCl Liquid 12.5 MG/5ML 720ml
Allergy, Cold & Flu Antibiotic Treatments Arthritis & Pain Benzonatate 100mg cap 14 42 Diphenhydramine HCl Cap 50 MG 30 90 Diphenhydramine HCl Inj 50MG/ML 1 3 Diphenhydramine HCl Liquid 12.5 MG/5ML 720ml
WHO Model List of Essential Medicines
 WHO Model List of Essential Medicines 20th List (March 2017) (Amended August 2017) Status of this document This is a reprint of the text on the WHO Medicines website http://www.who.int/medicines/publications/essentialmedicines/en/
WHO Model List of Essential Medicines 20th List (March 2017) (Amended August 2017) Status of this document This is a reprint of the text on the WHO Medicines website http://www.who.int/medicines/publications/essentialmedicines/en/
UPHP Advantage (HMO) (H ) and UPHP Choice (HMO) (H ) Updates
 March, 2018 03/01/2018 clindacin p clindamycin phosphate 03/01/2018 glatiramer acetate 03/01/2018 glatiramer acetate 03/01/2018 oseltamivir phosphate 03/01/2018 oseltamivir phosphate 03/01/2018 ADACEL
March, 2018 03/01/2018 clindacin p clindamycin phosphate 03/01/2018 glatiramer acetate 03/01/2018 glatiramer acetate 03/01/2018 oseltamivir phosphate 03/01/2018 oseltamivir phosphate 03/01/2018 ADACEL
ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS ALISKIREN 150MG TABLET RASILAZ RENIN INHIBITOR
 S/N ME OF MEDICATIONS BRANDED/GENERIC DRUG CLASS * UNIT PRICE RANGE (SGD$) 1 ACEBUTOLOL HCL 100MG CAPSULE SECTRAL BETA BLOCKERS 0.50 2 ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS 0.33 3 ALISKIREN
S/N ME OF MEDICATIONS BRANDED/GENERIC DRUG CLASS * UNIT PRICE RANGE (SGD$) 1 ACEBUTOLOL HCL 100MG CAPSULE SECTRAL BETA BLOCKERS 0.50 2 ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS 0.33 3 ALISKIREN
February - March - April 2017 Selected Content Updates. Approved to treat chorea associated with Huntington disease
 Selected Content for Customer Newsletter and Content Link February March April 2017 FDA Approvals new monographs and patient medication instructions Abaloparatide (Tymlos ) Approved to reduce the risk
Selected Content for Customer Newsletter and Content Link February March April 2017 FDA Approvals new monographs and patient medication instructions Abaloparatide (Tymlos ) Approved to reduce the risk
A Prospective Drug Utilization Study and Pharmacoeconomic Analysis of Critically Ill Patients in Acute Medical Care Unit of a Tertiary Care Hospital
 ISSN 2395-3411 Available online at www.ijpacr.com 128 Research Article A Prospective Drug Utilization Study and Pharmacoeconomic Analysis of Critically Ill Patients in Acute Medical Care Unit of a Tertiary
ISSN 2395-3411 Available online at www.ijpacr.com 128 Research Article A Prospective Drug Utilization Study and Pharmacoeconomic Analysis of Critically Ill Patients in Acute Medical Care Unit of a Tertiary
PMPRB ANNUAL REPORT 2009
 PMPRB ANNUAL REPORT 2009 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO PMPRB 2009* JANUARY 1 DECEMBER 31, 2009 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES LIMITED
PMPRB ANNUAL REPORT 2009 PATENTED DRUG PRODUCTS FOR HUMAN USE REPORTED TO PMPRB 2009* JANUARY 1 DECEMBER 31, 2009 DIN/GP Brand Name Generic Name ATC Dosage Form Comments Status ABBOTT LABORATORIES LIMITED
Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents*
 Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents* Cardiac Drug(s) Enzyme/ Action Chemotherapy Drug Effect of Drug- Drug Interaction Suggested Oncologist Management Suggested
Table 1: Drug-Drug Interactions of Common Cardiac Drugs and Chemotherapeutic Agents* Cardiac Drug(s) Enzyme/ Action Chemotherapy Drug Effect of Drug- Drug Interaction Suggested Oncologist Management Suggested
HYPERTENSION IN CKD. LEENA ONGAJYOOTH, M.D., Dr.med RENAL UNIT SIRIRAJ HOSPITAL
 HYPERTENSION IN CKD LEENA ONGAJYOOTH, M.D., Dr.med RENAL UNIT SIRIRAJ HOSPITAL Stages in Progression of Chronic Kidney Disease and Therapeutic Strategies Complications Normal Increased risk Damage GFR
HYPERTENSION IN CKD LEENA ONGAJYOOTH, M.D., Dr.med RENAL UNIT SIRIRAJ HOSPITAL Stages in Progression of Chronic Kidney Disease and Therapeutic Strategies Complications Normal Increased risk Damage GFR
Pharmaceuticals in the environment: The relevance of point sources. Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research
 Pharmaceuticals in the environment: The relevance of point sources Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research 25th April 2008 1 Background: Pharmaceuticals as contaminants
Pharmaceuticals in the environment: The relevance of point sources Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research 25th April 2008 1 Background: Pharmaceuticals as contaminants
WHO Model List (revised April 2002)
 Essential Medicines Explanatory Notes The complementary list presents essential medicines for priority diseases which are efficacious, safe and cost-effective but not necessarily affordable, or for which
Essential Medicines Explanatory Notes The complementary list presents essential medicines for priority diseases which are efficacious, safe and cost-effective but not necessarily affordable, or for which
ADRs caused by potential. only. Patients (n, %) 132 (49.6%) 111 (41.7%) 10 (3.8%) 2 (0.8%) 9 (3.4%) 2 (0.8%)
 Journal: Drug Safety Study title: Self-medication with OTC and prescribed drugs causing ADR-related hospital admissions results of a prospective, long-term multi-centre study Authors: 1 S. Schmiedl*, 2
Journal: Drug Safety Study title: Self-medication with OTC and prescribed drugs causing ADR-related hospital admissions results of a prospective, long-term multi-centre study Authors: 1 S. Schmiedl*, 2
Inventory of paediatric therapeutic needs
 12 December 2012 EMA/PDCO/287222/2012 Human Medicines Development and Evaluation Infectious diseases Agreed by PDCO November 2012 Adopted by PDCO for release for consultation 7 December 2012 Start of public
12 December 2012 EMA/PDCO/287222/2012 Human Medicines Development and Evaluation Infectious diseases Agreed by PDCO November 2012 Adopted by PDCO for release for consultation 7 December 2012 Start of public
Appendix 1: Interactions
 Appendix 1: Interactions Two or more drugs given at the same time may interact with each other. The interaction may be potentiation or antagonism of one drug by another, or occasionally some other. Drug
Appendix 1: Interactions Two or more drugs given at the same time may interact with each other. The interaction may be potentiation or antagonism of one drug by another, or occasionally some other. Drug
Registered Products in BG
 Registered Products in BG (*) en la columna Package size elimine los empaquetamientos que no desee y en la columna Units / Year indique las cantidades deseadas. Nº Product name INN Pharmaceutical form
Registered Products in BG (*) en la columna Package size elimine los empaquetamientos que no desee y en la columna Units / Year indique las cantidades deseadas. Nº Product name INN Pharmaceutical form
Active Pharmaceutical Ingredient (API) List List Updated 03/08/2018
 5-Fluorouracil HPLC/ UHPLC Yes USP 4017 30 5mL 2 7-keto DHEA HPLC/ UHPLC Yes Medisca 4258 180 5mL 1 Acetaminophen HPLC/ UHPLC Yes USP 4406 5mL 2 Acetylcysteine HPLC/ UHPLC Yes USP 4255 60 5mL 1 Adenosine
5-Fluorouracil HPLC/ UHPLC Yes USP 4017 30 5mL 2 7-keto DHEA HPLC/ UHPLC Yes Medisca 4258 180 5mL 1 Acetaminophen HPLC/ UHPLC Yes USP 4406 5mL 2 Acetylcysteine HPLC/ UHPLC Yes USP 4255 60 5mL 1 Adenosine
EFFECTIVE 01/02/2018 TYPHIM VI 25 MCG/0.5 ML VIAL. - Added to Tier 1. typhoid vaccine vi capsular polysaccharide
 EFFECTIVE 01/02/2018 TYPHIM VI 25 MCG/0.5 ML VIAL typhoid vaccine vi capsular polysaccharide PAGE 1 LAST UPDATED 09/2018 EFFECTIVE 01/05/2018 atazanavir sulfate 150 mg capsule atazanavir sulfate 200 mg
EFFECTIVE 01/02/2018 TYPHIM VI 25 MCG/0.5 ML VIAL typhoid vaccine vi capsular polysaccharide PAGE 1 LAST UPDATED 09/2018 EFFECTIVE 01/05/2018 atazanavir sulfate 150 mg capsule atazanavir sulfate 200 mg
Dr Diana R Holdright. MD, FRCP, FESC, FACC, MBBS, DA, BSc. Consultant Cardiologist HYPERTENSION.
 Dr Diana R Holdright MD, FRCP, FESC, FACC, MBBS, DA, BSc. Consultant Cardiologist HYPERTENSION www.drholdright.co.uk Blood pressure is the pressure exerted on the walls of the arteries when the heart pumps;
Dr Diana R Holdright MD, FRCP, FESC, FACC, MBBS, DA, BSc. Consultant Cardiologist HYPERTENSION www.drholdright.co.uk Blood pressure is the pressure exerted on the walls of the arteries when the heart pumps;
Added, Removed or Changed. Added, Removed or Changed
 One mission: you s March 8, 2018 Blue Cross of Idaho reviews its formularies (covered drug lists) periodically to allow members access to new drugs and to provide safe, cost effective options for your
One mission: you s March 8, 2018 Blue Cross of Idaho reviews its formularies (covered drug lists) periodically to allow members access to new drugs and to provide safe, cost effective options for your
Table A1: Fifty Most Often Repeated Oral Drug Product Shortages (35 ingredients), Canada,
 1 Appendix Commentary 515 Appendix: Assessing Canada s Drug Shortage Problem Table A1: Fifty Most Often Repeated Oral Drug Product Shortages (35 ingredients), Canada, 2013 16 Drug Name Dosage Therapeutic
1 Appendix Commentary 515 Appendix: Assessing Canada s Drug Shortage Problem Table A1: Fifty Most Often Repeated Oral Drug Product Shortages (35 ingredients), Canada, 2013 16 Drug Name Dosage Therapeutic
Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005)
 Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005) Streptococcus pneumoniae (SP) Blood Culture Isolates Penicillin intermediate Penicillin Cefotaxime 336
Antibiotic Resistance Pattern of Blood and CSF Culture Isolates At NHLS Academic Laboratories (2005) Streptococcus pneumoniae (SP) Blood Culture Isolates Penicillin intermediate Penicillin Cefotaxime 336
INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]
![INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY] INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]](/thumbs/85/92112268.jpg) Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
Chapter 2 ~ Cardiovascular system
 Chapter 2 ~ Cardiovascular System: General Section 1 of 6 Chapter 2 ~ Cardiovascular system 2.1 Positive inotropic drugs 2.1.1 Cardiac glycosides DIGOXIN 2.2 Diuretics Elixir 50micrograms in 1ml Injection
Chapter 2 ~ Cardiovascular System: General Section 1 of 6 Chapter 2 ~ Cardiovascular system 2.1 Positive inotropic drugs 2.1.1 Cardiac glycosides DIGOXIN 2.2 Diuretics Elixir 50micrograms in 1ml Injection
Chapter 5 ~ Infections
 Chapter 5 ~ Infections: Special Section 1 of 6 Chapter 5 ~ Infections Please refer to The Hillingdon Hospitals NHS Trust Antibiotic Guidelines, Policy number 233, and Surgical Prophylaxis Policy, Policy
Chapter 5 ~ Infections: Special Section 1 of 6 Chapter 5 ~ Infections Please refer to The Hillingdon Hospitals NHS Trust Antibiotic Guidelines, Policy number 233, and Surgical Prophylaxis Policy, Policy
Adnova Healthcare Pvt. Ltd. - Product List
 1 Aceclofenac 100mg 2 Aceclofenac 100mg + Thiocolchicoside 4mg 3 Aceclofenac 100mg+ Paracetamol 325mg + Chlorzoxazone 250mg 4 Aceclofenac 100mg + Paracetamol 325mg + Serratiopeptidase 10mg 5 Aceclofenac
1 Aceclofenac 100mg 2 Aceclofenac 100mg + Thiocolchicoside 4mg 3 Aceclofenac 100mg+ Paracetamol 325mg + Chlorzoxazone 250mg 4 Aceclofenac 100mg + Paracetamol 325mg + Serratiopeptidase 10mg 5 Aceclofenac
(To be Published in Part II, Section 3, Sub-section (ii) of the Gazette of India, Extraordinary)
 (To be Published in Part II, Section 3, Sub-section (ii) of the Gazette of India, Extraordinary) Government of India Ministry of Chemicals and Fertilizers Department of Pharmaceuticals National Pharmaceutical
(To be Published in Part II, Section 3, Sub-section (ii) of the Gazette of India, Extraordinary) Government of India Ministry of Chemicals and Fertilizers Department of Pharmaceuticals National Pharmaceutical
Drug Utilization Evaluation of Antihypertensives in A Superspeciality Hospital
 Research Article Drug Utilization Evaluation of Antihypertensives in A Superspeciality Hospital Mahalaxmi Mohan Department of Pharmacology, MGVs Pharmacy College, Department of Pharmacology, Nashik-422003,
Research Article Drug Utilization Evaluation of Antihypertensives in A Superspeciality Hospital Mahalaxmi Mohan Department of Pharmacology, MGVs Pharmacy College, Department of Pharmacology, Nashik-422003,
2018 OPEN FORMULARY Updates
 January, 2018 01/01/2018 OPDIVO nivolumab 01/01/2018 KEVZARA sarilumab 01/01/2018 KEVZARA sarilumab 01/01/2018 AIMOVIG AUTOINJECTOR,AIMOVIG AUTOINJECTOR (2 PACK) erenumab-aooe 01/02/2018 glucose in water
January, 2018 01/01/2018 OPDIVO nivolumab 01/01/2018 KEVZARA sarilumab 01/01/2018 KEVZARA sarilumab 01/01/2018 AIMOVIG AUTOINJECTOR,AIMOVIG AUTOINJECTOR (2 PACK) erenumab-aooe 01/02/2018 glucose in water
Hypertension, Hyperlipidemia and Obesity. Mi-CCSI
 Hypertension, Hyperlipidemia and Obesity Mi-CCSI Objectives Review the prevalence of hypertension, hyperlipidemia and obesity Correlation of the 3 conditions Discuss why it is important to treat these
Hypertension, Hyperlipidemia and Obesity Mi-CCSI Objectives Review the prevalence of hypertension, hyperlipidemia and obesity Correlation of the 3 conditions Discuss why it is important to treat these
Drug safety analysis in a real-life cohort of Parkinson s disease patients with polypharmacy
 Drug safety analysis in a real-life cohort of Parkinson s disease patients with polypharmacy Saskia Müller-Rebstein 1, 2, Claudia Trenkwalder 1, Jens Ebentheuer 1, Wolfgang H Oertel 3, Carsten Culmsee
Drug safety analysis in a real-life cohort of Parkinson s disease patients with polypharmacy Saskia Müller-Rebstein 1, 2, Claudia Trenkwalder 1, Jens Ebentheuer 1, Wolfgang H Oertel 3, Carsten Culmsee
Supplementary Data. Supplementary Table S2. Antiretroviral Therapies Taken with Ledipasvir/Sofosbuvir
 Supplementary Data Statistical Analysis Due to the limited number of patients with acute kidney injury and concern for model overfitting, covariates included in multivariable logistic regression analyses
Supplementary Data Statistical Analysis Due to the limited number of patients with acute kidney injury and concern for model overfitting, covariates included in multivariable logistic regression analyses
Soliqua 100/33. (insulin glargine, lixisenatide) New Product Slideshow
 Soliqua 100/33 (insulin glargine, lixisenatide) New Product Slideshow Introduction Brand name: Soliqua 100/33 Generic name: Insulin glargine (rdna origin), lixisenatide Pharmacological class: Human insulin
Soliqua 100/33 (insulin glargine, lixisenatide) New Product Slideshow Introduction Brand name: Soliqua 100/33 Generic name: Insulin glargine (rdna origin), lixisenatide Pharmacological class: Human insulin
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 March 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
WEB ANNEX J. TABLE OF DRUG INTERACTIONS WITH ANTIRETROVIRAL DRUGS
 WEB ANNEX J. TABLE OF DRUG INTERACTIONS WITH ANTIRETROVIRAL DRUGS In: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on
WEB ANNEX J. TABLE OF DRUG INTERACTIONS WITH ANTIRETROVIRAL DRUGS In: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on
What in the World is Functional Medicine?
 What in the World is Functional Medicine? An Introduction to a Systems Based Approach of Chronic Disease Meneah R Haworth, FNP-C Disclosure v I am a student of the Institute for Functional Medicine. They
What in the World is Functional Medicine? An Introduction to a Systems Based Approach of Chronic Disease Meneah R Haworth, FNP-C Disclosure v I am a student of the Institute for Functional Medicine. They
MIC Test Strip for antimicrobial susceptibility testing
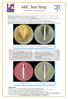 for antimicrobial susceptibility testing Liofilchem Liofilchem Quantitative assay for determining the Minimum Inhibitory Concentration (M.I.C.) is a quantitative assay for determining the Minimum Inhibitory
for antimicrobial susceptibility testing Liofilchem Liofilchem Quantitative assay for determining the Minimum Inhibitory Concentration (M.I.C.) is a quantitative assay for determining the Minimum Inhibitory
Chapter / Section / Drug
 2 Cardiovascular System 2.1 Positive inotropic drugs Digoxin Digoxin specific antibody ( DigiFab ) 2.2 Diuretics 2.2.1 Thiazides and related diuretics Indapamide (1 st Line) Bendroflumethiazide Metolazone
2 Cardiovascular System 2.1 Positive inotropic drugs Digoxin Digoxin specific antibody ( DigiFab ) 2.2 Diuretics 2.2.1 Thiazides and related diuretics Indapamide (1 st Line) Bendroflumethiazide Metolazone
Antihypertensive Agents Part-2. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Antihypertensive Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Agents that block production or action of angiotensin Angiotensin-converting
Antihypertensive Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Agents that block production or action of angiotensin Angiotensin-converting
Dr Narender Goel MD (Internal Medicine and Nephrology) Financial Disclosure: None, Conflict of Interest: None
 Dr Narender Goel MD (Internal Medicine and Nephrology) drnarendergoel@gmail.com Financial Disclosure: None, Conflict of Interest: None 12 th December 2013, New York Visit us at: http://kidneyscience.info/
Dr Narender Goel MD (Internal Medicine and Nephrology) drnarendergoel@gmail.com Financial Disclosure: None, Conflict of Interest: None 12 th December 2013, New York Visit us at: http://kidneyscience.info/
UPDATE Georgia Medicaid
 March 26, 2015 UPDATE Georgia Medicaid Preferred Drug List Dear Provider: At the March 05, 2015 WellCare Pharmacy & Therapeutics Committee meeting, it was decided that the following changes will be made
March 26, 2015 UPDATE Georgia Medicaid Preferred Drug List Dear Provider: At the March 05, 2015 WellCare Pharmacy & Therapeutics Committee meeting, it was decided that the following changes will be made
WHO Technical Report Series The Selection and Use of Essential Medicines
 WHO Technical Report Series The Selection and Use of Essential Medicines Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2017 (including the 20th WHO Model List of Essential
WHO Technical Report Series The Selection and Use of Essential Medicines Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2017 (including the 20th WHO Model List of Essential
Medicines for high blood pressure
 Patient Information: Medicines Medicines for high blood pressure Health & care information you can trust The Information Standard Certified Member Working together for better patient information What is
Patient Information: Medicines Medicines for high blood pressure Health & care information you can trust The Information Standard Certified Member Working together for better patient information What is
New Product to Market: Epidiolex. New Product to Market: Ajovy Magellan Health, Inc. All rights reserved.
 The following tables list the Agenda items as well as the that are scheduled to be presented and reviewed at the March 21, 2019 meeting of the Pharmacy and Therapeutics Advisory Committee. Epidiolex Ajovy
The following tables list the Agenda items as well as the that are scheduled to be presented and reviewed at the March 21, 2019 meeting of the Pharmacy and Therapeutics Advisory Committee. Epidiolex Ajovy
Excessive daytime sleepiness and antipathogen drug consumption in the elderly: a test of the immune theory of sleep
 Supplementary Information Excessive daytime sleepiness and antipathogen drug consumption in the elderly: a test of the immune theory of sleep Berticat Claire 1, Thomas Fréderic 2, Dauvilliers Yves 3, 4,
Supplementary Information Excessive daytime sleepiness and antipathogen drug consumption in the elderly: a test of the immune theory of sleep Berticat Claire 1, Thomas Fréderic 2, Dauvilliers Yves 3, 4,
