MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps
|
|
|
- Eugenia Stevens
- 6 years ago
- Views:
Transcription
1 POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 02/13/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder contract. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This guideline is solely for explaining correct procedure reporting and does not imply coverage and reimbursement. DESCRIPTION Continuous Glucose Monitoring Systems (CGMS) These devices measure glucose levels in interstitial fluid at programmable intervals. These readings help detect any patterns or trends with a patient s glucose levels and are intended to assist in calculating the insulin dosage needed to manage glycemic control. CGMS use sensors that are inserted under the skin in the abdomen, arm or buttocks and work by extracting glucose from the interstitial fluid, measuring and recording the glucose level and converting these measurements into equivalent blood glucose readings. Insulin Pumps The purpose of the insulin pump is to provide an accurate, continuous, controlled delivery of insulin, which can be regulated by the user to achieve intensive glucose control objectives and to prevent the metabolic complications of hypoglycemia, hyperglycemia and diabetic ketoacidosis. Long-term Continuous Glucose Monitoring Systems (CGMS) These devices are owned by individuals and provide real-time glucose values that allow users to track patterns and possibly identify episodes of low and high blood glucose levels. The data can be downloaded to PC software and stored for historical analysis. The system will purportedly alert the user if a glucose level falls below or rises above a preset value. Examples of FDA approved long-term CGMS include, but may not be limited to: Guardian REAL-Time Dexcom G4 PLATINUM On May 22, 2015, the FDA approved this externally-worn glucose sensor that continuously measures and displays glucose values. In addition to reporting glucose values every 5 minutes, the system reports trending information in real-time for up to seven days. ipro2 Professional with Enlite Sensor On June 17, 2016, the FDA approved this system that measures glucose levels in fluid under the skin for up to six days. Glucose values are stored by the ipro2 recorder for use by a health care provider to monitor a patient's glucose management. The values cannot be viewed in real time by the user. Dexcom G5 Mobile On December 20, 2016, the FDA approved expanded indications for the Dexcom G5 Mobile to include replacement of fingerstick blood glucose testing for diabetes treatment decisions in people 2 years of age and older. FreeStyle Libre Flash On September 27, 2017, the FDA approved the FreeStyle Libre Flash Glucose Monitoring System, the first continuous glucose monitoring system that can be used by adult patients to make diabetes treatment decisions without calibration using a blood sample from a fingerstick. Remote Glucose Monitoring An integrated wireless communication system that is built into the receiver, enabling remote monitoring capabilities and sharing of data through a compatible internet-accessible device application. An example of a remote glucose monitor device includes, but may not be limited to: Dexcom SHARE Implantable Interstitial Glucose Sensors These sensors are intended for long term use (e.g., 90 days) and are implanted subcutaneously in the upper arm to measure glucose in the interstitial fluid. The measurement is then relayed to the smart transmitter. The measurement and display of glucose values is done automatically without the need for user intervention. Currently there are no devices that have received FDA approval.
2 - 2 - NO FDA APPROVED PRODUCTS External Insulin Pumps A standard portable external insulin infusion pump is a small battery-operated pump about the size of a personal pager that is filled with insulin, and is connected to thin tubing ending in a needle. The needle is inserted into the skin around the abdomen and supplies a regulated dose of insulin to the user for a day or more at a time. The pump may be carried in a pocket, or in a case worn attached to a belt fastened around a consumer s waist. Examples of FDA approved external insulin pumps include, but may not be limited to: Accu-Chek Aviva Combo System or Spirit Combo Insulin Pump Systems (Roche Diagnostics, Indianapolis, IN) Combined insulin pump with finger stick blood glucose meter for the treatment of insulin requiring diabetes and for the quantitative measurement of glucose in fresh capillary whole blood from the finger OmniPod Insulin Management System (Insulet Corporation, Boston, MA) A wireless insulin pump that consists of a disposable insulin pod and Personal Diabetes Manager that includes a built-in FreeStyle glucose meter. The pod is filled with insulin by the patient and replaced every 72 hours. Animas OneTouch Ping (Animas Corp., Frazer, PA) Insulin pump with a OneTouch Ping Meter Remote for diabetics requiring continuous subcutaneous insulin delivery and measurement of glucose Solo MicroPump t:flex Insulin Delivery System (Tandem Diabetes Care, Inc., San Diego CA) A t:slim predicate device intended for the subcutaneous delivery of insulin for individuals 12 years of age and greater. The t:flex includes a 4.8 ml cartridge vs. 3.0 ml cartridge in the t:slim. V-Go Disposable Insulin Delivery Device A fully disposable, non-electronic device for the delivery of basal-bolus insulin therapy for adults with diabetes. It provides a continuous preset basal rate of insulin and allows for on-demand bolus dosing around mealtimes. It is applied to the skin daily for one 24-hour period. Implantable Insulin Pumps These pumps are surgically implanted rather than worn externally to deliver insulin via intraperitoneal or intravenous routes. Currently there are not any devices that have received FDA approval for use outside of a clinical trial. NO FDA APPROVED PRODUCTS Combined External Insulin Pumps with Continuous Glucose Monitoring Systems (CGMS) A device that integrates an insulin pump with real-time continuous glucose monitoring and is not intended to replace finger sticks. These systems incorporate features including predictive alerts that give early warnings so action can be taken to prevent dangerous high or low blood glucose events. Examples of FDA approved combined external insulin pumps with CGMS include, but may not be limited to: Animas Vibe Insulin Pump Intended for the continuous subcutaneous infusion of insulin for the management of insulin-requiring diabetes. Minimed Paradigm Real-Time Insulin Pump (Medtronic Minimed, Northridge, CA) For the management of diabetes mellitus in persons requiring continuous delivery of insulin (MMT-523/723 for adults and MMT-523K/723K for ages 7 17 years) o mysentry This remote monitoring system is an optional monitoring device for the MiniMed Paradigm REAL-Time Revel System. The mysentry consists of a remote outpost and monitor. Blood glucose levels collected by the CGM are sent to the remote (wireless) monitor, which also has alarms to alert the user of high or low blood glucose levels. t:slim Micro-Delivery Insulin Pump (Tandem Diabetes Care, Inc., San Diego CA) This pump provides a subcutaneous delivery of insulin for the management of diabetes mellitus in persons requiring insulin, for individuals 12 years of age and greater Combined External Insulin Pumps and CGMS with Suspend on Low Feature Suspend on low is the first step towards an artificial pancreas device system (APDS). This technology combines CGMS with an insulin pump which allows the user to set a low blood sugar threshold value. When the CGM sensor detects the preset low
3 - 3 - glucose threshold, insulin delivery is suspended. Examples of combined external insulin pumps with CGMS with suspend on low feature include, but may not be limited to: MiniMed 530G System This system is intended for automatic, continuous delivery of basal insulin (at user selectable rates) and manual administration of insulin boluses (in user selectable amounts) in persons, 16 years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. This device has SmartGuard technology which automatically stops insulin delivery (for up to two hours) when sensor glucose values reach a preset level and when the individual does not respond to the suspend on low alarm. o MiniMed Connect Compatible with MiniMed 530G with Enlite and MiniMed Paradigm Real-Time insulin pump. This optional wireless device can be used to access continuous glucose monitor sensor data. Information can be viewed using an internet application through a smart device or via a browser accessible website and can be shared as needed. MiniMed 630G This system is intended for automatic, continuous delivery of basal insulin (at user selectable rates) and manual administration of insulin boluses (in user selectable amounts) for persons sixteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed 630G system includes SmartGuard which can be programmed to temporarily suspend delivery of insulin for up to two hours when the sensor glucose value falls below a predefined threshold value. The MiniMed 630G system with SmartGuard consists of the following devices: MiniMed 630G insulin pump, Enlite sensor, One-Press serter, Guardian Link transmitter system, CareLink USB and Contour NEXT Link 2.4 wireless glucose meter. Hybrid Closed Loop System Along with the suspend on low feature mentioned above, this newly FDA approved system has a suspend before low feature that purportedly stops insulin delivery when the sensor is predicted to reach a low limit and resumes after sensor glucose levels recover. An example of a hybrid closed loop system includes, but may not be limited to: MiniMed 670G This system is intended for automatic, continuous delivery of basal insulin (at user selectable rates) and manual administration of insulin boluses (in user selectable amounts) for Type 1 diabetes mellitus (T1DM) in persons 14 years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed 670G System includes advanced SmartGuard technology, which can be programmed to automatically adjust delivery of basal insulin based on CGM sensor glucose values and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The MiniMed 670G system consists of the following devices: MiniMed 670G insulin pump, the Guardian Link 3 transmitter, the Guardian Sensor 3, One-Press serter and the Contour NEXT Link 2.4 wireless glucose meter. Artificial Pancreas or Bi-hormonal Bionic Endocrine Pancreas Fully automated, closed-loop glucose management systems with a continuous glucose monitor and an insulin pump programmed with a computer algorithm that calculates insulin and glucagon doses from the CGM readings and tells the pump to deliver or temporarily suspend or reduce insulin based upon specified thresholds of measured glucose levels. POLICY Continuous glucose monitoring systems (CGMS) with or without an external insulin pump (A9276, A9277, A9278, K0553, K0554, S1030, S1031) require prior authorization. External insulin pumps (e.g., Accu-Chek Spirit, Animas OneTouch Ping, t:flex Insulin Delivery System) (E0784) do not require prior authorization. Disposable external insulin pumps (A9274) with wireless communication capability to a hand-held control unit (e.g., OmniPod) do not require prior authorization. Disposable insulin infusion devices/pumps (e.g., V-GO, Omnipod ) are covered under Medicare Part D (prescription) for Elite. Nonprogrammable disposable insulin delivery systems (e.g., V-Go Disposable Insulin Delivery Device) are non-covered for HMO, PPO, Individual Marketplace, & Advantage. Remote glucose monitoring (e.g., the Dexcom SHARE) does not warrant separate reimbursement. Non-Covered:
4 - 4 - Implantable interstitial glucose sensors (0446T-0448T) Implantable insulin pumps Combined external insulin pumps and CGMS with suspend on low feature (eg, MiniMed 530G, MiniMed 630G) Hybrid closed loop system (eg, MiniMed 670G) Artificial pancreas device systems (S1034-S1037) or bi-hormonal bionic endocrine pancreas MiniMed Connect mysentry remote monitoring system HMO, PPO, Individual Marketplace, Elite, Advantage Paramount has determined that the use of FDA approved continuous glucose monitoring systems (CGMS) for longterm use (over 3 days) with or without an external insulin pump is covered under the following guidelines: 1. Approved with prior authorization for Type 1 diabetes when evidence of hypoglycemia exists on 3 consecutive days OR 2. Recurrent episodes of severe hypoglycemia (blood glucose less than 80mg/dl) and hypoglycemia unawareness despite appropriate modifications in insulin regimen and compliance with frequent selfmonitoring OR 3. Women who are pregnant with uncontrolled diabetes mellitus HbA1c >9% with fluctuating blood sugar mg/dl. The requested device must be prescribed according to its FDA approved clearance and guideline information. FDA approved external insulin pumps are medically necessary for members with type 1 diabetes mellitus documented by a C-peptide level less than 0.5 and when all of the following medical necessity criteria are met: 1. The member has completed a diabetes education program within the last twenty four months of being prescribed an insulin infusion pump. 2. The member has been on a program of multiple daily injections of insulin (i.e., at least 3 injections per day), with frequent self-adjustments of insulin dose, for at least 6 months before initiation of the insulin infusion pump. 3. The member had documented frequency that is kept in the member's medical record of glucose self-testing an average of at least 4 times per day during the 2 months before initiation of the insulin infusion pump. 4. The member is at high risk for preventable complications of diabetes. Early signs of diabetic complications include micro-albuminuria and/or documented in the member's medical record persistent difficulty in achieving optimal control of blood sugar levels despite good compliance with an intensive multiple injection regimens. 5. The member needs to meet at least one of the following criteria in order to be eligible for an external insulin infusion pump: Glycated hemoglobin level (HbA1c) > 7.0% History of recurring hypoglycemia Wide fluctuations in blood glucose before mealtime Dawn phenomenon with fasting blood sugars frequently exceeding 200 mg/dl History of severe glycemic excursions Paramount provides no additional reimbursement for a wireless transmission feature that is integrated into a continuous glucose monitor (e.g., Dexcom SHARE) because it is considered a convenience feature. Disposable insulin infusion devices/pumps (e.g., V-GO, Omnipod ) are coverable under Medicare Part D (prescription) as they are not covered under Medicare Part B (medical/dme). Some insulin pumps are covered under Part B as Durable Medical Equipment (DME). Disposable insulin pumps are not durable, and, therefore, are not covered under Part B. Non-Coverage Determination External insulin pumps are non-covered if any of the following contraindications exist: 1. Member has non-insulin dependent (NIDDM or IR-NIDDM, Type II) diabetes, even if insulin is taken 2. Member has end-stage complications such as renal failure 3. Member is unable, because of behavioral, psychological problems or functional ability, to technically operate the pump and perform frequent blood glucose monitoring 4. Member is being prescribed pump therapy to be used for convenience purposes
5 - 5 - Paramount does not cover any of the following because each is considered experimental, investigational or unproven: implantable interstitial glucose sensors (0446T-0448T) Nonprogrammable disposable insulin delivery systems (e.g., V-Go disposable insulin delivery device) Implantable insulin pumps Combined external insulin pumps and CGMS with suspend on low feature (eg, MiniMed 530G, MiniMed 630G) Hybrid closed loop system (eg, MiniMed 670G) Artificial pancreas device systems (S1034-S1037) or bi-hormonal bionic endocrine pancreas MiniMed Connect mysentry remote monitoring system Paramount does not cover the following because each is considered a convenience item and not medically necessary: Replacement of a currently functioning insulin pump for the sole purpose of receiving the most recent insulin pump technology (i.e., upgrading for improved technology) Additional software or hardware required for downloading data to a device such as personal computer, smart phone, or tablet to aid in self-management of diabetes mellitus Dispensing The following components are considered "inclusive" with any external (portable) continuous insulin infusion pump rental or purchase payment made by the department on behalf of a member and cannot be submitted to the department for separate reimbursement: 1. Any supporting wires, power supply, cables, attachment kits, or disposable items associated with the operation of the pump 2. Pump education, training, monitoring, or counseling in support of the member's ordered treatment 3. Maintenance, repair, or cleaning charges in association with the three-month trial rental period 4. Delivery, set-up, or pick-up charges The provider of the standard portable external insulin infusion pump must assure that the member utilizing the device is properly instructed on how to use the device in support of his or her ordered treatment and is aware of and understands any emergency procedures regarding the use of the pump. The provider must maintain written documentation regarding the member's instruction on the use of the pump in the provider's records. When purchasing an external insulin infusion pump, the member must be provided with a product warranty that covers any required maintenance or repairs for duration of at least one year and commences on the date the infusion pump was authorized for purchase. CODING/BILLING INFORMATION The appearance of a code in this section does not necessarily indicate coverage. Codes that are covered may have selection criteria that must be met. Payment for supplies may be included in payment for other services rendered. HCPCS CODES A4230 Infusion set for external infusion pump non-needle cannula type A4231 Infusion set for external infusion pump needle type A4232 Syringe with needle for external pump A9274 External ambulatory insulin delivery system, disposable, each, includes all supplies and accessories A9276 Sensor - invasive (e.g. subcutaneous) disposable for use with interstitial continuous glucose monitoring system, one unit = 1 day supply A9277 Transmitter - external for use with interstitial continuous glucose monitoring system A9278 Receiver (monitor) - external for use with interstitial continuous glucose monitoring system E0784 External ambulatory infusion pump, insulin K0552 Supplies for external drug infusion pump, syringe type cartridge, each K0553 Supply allowance for therapeutic continuous glucose monitor (CGM), includes all supplies and accessories, 1 month supply = 1 unit of service (Code effective 07/01/2017) K0554 Receiver (monitor); dedicated, for use with therapeutic continuous glucose monitoring system (Code effective 07/01/2017) K0601 Replacement battery for external infusion pump owned by patient, silver oxide, 1.5 volt, each K0602 Replacement battery for external infusion pump owned by patient, silver oxide, 3 volt, each K0603 Replacement battery for external infusion pump owned by patient, alkaline, 1.5 volt, each K0604 Replacement battery for external infusion pump owned by patient, lithium, 3.6 volt, each K0605 Replacement battery for external infusion pump owned by patient, lithium, 4.5 volt, each
6 - 6 - S1030 Continuous noninvasive glucose monitoring device, purchase S1031 Continuous noninvasive glucose monitoring device rental including sensor, sensor replacement, and download to monitor S1034 Artificial Pancreas Device System (eg, Low Glucose Suspend [LGS] Feature) Including Continuous Glucose Monitor, Blood Glucose Device, Insulin Pump And Computer Algorithm That Communicates With All Of The Devices S1035 Sensor; Invasive (eg, Subcutaneous), Disposable, For Use With Artificial Pancreas Device System S1036 Transmitter; External, For Use With Artificial Pancreas Device System S1037 Receiver (Monitor); External, For Use With Artificial Pancreas Device System CPT CODES 0446T Creation of subcutaneous pocket with insertion of implantable interstitial glucose sensor, including system activation and patient training (Effective 01/01/17) 0447T Removal of implantable interstitial glucose sensor from subcutaneous pocket via incision (Effective 01/01/17) 0448T Removal of implantable interstitial glucose sensor with creation of subcutaneous pocket at different anatomic site and insertion of new implantable sensor, including system activation (Effective 01/01/17) TAWG REVIEW DATES: Long term Continuous Blood Glucose Monitoring Services - 01/14/2009, 02/18/2009, 02/10/2010, 02/09/2011, 04/11/2012, 05/15/2013, 04/18/2014, 02/26/2015, 02/26/2016 External Insulin Pumps - 07/11/2012 Artificial Pancreas Device Systems (APDS) 12/19/2014, 02/26/2016 REVISION HISTORY EXPLANATION 06/01/09: Updated 06/15/09: Clarification of verbiage 09/01/11: Updated 09/24/11: Replacement clarification 06/01/12: Updated 07/11/12: Added Exception for OmniPod coverage per TAWG approval. 02/11/14: Policy reviewed and updated to reflect most current clinical evidence per Medical Policy Steering Committee. 04/18/14: It was determined that Long Term Continuous Blood Glucose Monitoring Services will continue to be covered with prior authorization for all product lines. Policy reviewed and updated to reflect most current clinical evidence per TAWG Committee. 09/09/14: Disposable external insulin pumps (A9274) with wireless communication capability to a hand-held control unit (e.g., OmniPod) are covered without prior authorization for all product lines. Policy reviewed and updated to reflect most current clinical evidence per Medical Policy Steering Committee. 12/19/14: Added HCPCS codes S1034, S1035, S1036 and S1037. It was determined by TAWG that Artificial Pancreas Device Systems (APDS) will be non-covered for all product lines. Policy reviewed and updated to reflect most current clinical evidence per TAWG Committee. 02/26/15: Added verbiage, The DME supplier must meet eligibility and/or credentialing requirements as defined by the Plan to be eligible for reimbursement. Changes made to current criteria. Policy reviewed and updated to reflect most current clinical evidence per TAWG Committee. 10/09/15: Changed AND to OR so criteria is no. 1 OR no. 2 OR no. 3 per administrative direction. 02/26/16: Policy reviewed and updated to reflect most current clinical evidence per TAWG Committee. 04/11/17: Combined this policy with PG0156 External Insulin Pumps. Added effective 01/01/17 new codes 0446T- 0448T as non-covered. Policy reviewed and updated to reflect most current clinical evidence per TAWG Committee. 07/11/17: Added effective 07/01/17 new codes K0553 & K0554 as covered with prior authorization for HMO, PPO, Individual Marketplace, & Elite per CMS guidelines and non-covered for Advantage per ODM guidelines. Policy reviewed and updated to reflect most current clinical evidence per Medical Policy Steering Committee. 10/10/17: Added Dexcom G4 PLATINUM, ipro2 Professional with Enlite Sensor, & FreeStyle Libre Flash Glucose Monitoring System to examples of FDA approved long-term CGMS. Policy reviewed and updated to reflect most current clinical evidence per Medical Policy Steering Committee. 02/13/18: Disposable insulin infusion devices/pumps (e.g., V-GO, Omnipod) are covered through the pharmacy benefit (Medicare Part D) for Elite. Policy reviewed and updated to reflect most current clinical evidence per Medical Policy Steering Committee. 03/22/18: Effective 01/01/18 codes K0553 & K0554 are now covered for Advantage per ODM guidelines. REFERENCES/RESOURCES Centers for Medicare and Medicaid Services, CMS Manual System and other CMS publications and services Ohio Department of Medicaid
7 - 7 - American Medical Association, Current Procedural Terminology (CPT ) and associated publications and services Centers for Medicare and Medicaid Services, Healthcare Common Procedure Coding System, HCPCS Release and Code Sets Industry Standard Review Hayes, Inc
MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps
 POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 05/24/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 05/24/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps
 POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 07/10/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 07/10/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
Approved by: Integrated Health Quality Management Subcommittee Effective Date: Department of Origin: Integrated Healthcare Services.
 Reference #: MC/L008 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS)
Reference #: MC/L008 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS)
06/13/17. A. Completed a comprehensive diabetes education program within the past two years; and
 Reference #: MC/L011 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA
Reference #: MC/L011 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA
Corporate Medical Policy
 Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Insulin Pumps and Continuous Glucose Sensors- Embracing Technology. Susan Cavalier, BS, RN, CDE Manager, Diabetes Educator Sanford Diabetes Education
 Insulin Pumps and Continuous Glucose Sensors- Embracing Technology Susan Cavalier, BS, RN, CDE Manager, Diabetes Educator Sanford Diabetes Education Diabetes Management Tools in 1974 Insulin pump history
Insulin Pumps and Continuous Glucose Sensors- Embracing Technology Susan Cavalier, BS, RN, CDE Manager, Diabetes Educator Sanford Diabetes Education Diabetes Management Tools in 1974 Insulin pump history
Abbott FreeStyle Libre Pro System
 , the Professional CGM Abbott FreeStyle Libre Pro Reader Kit includes: Reader, USB cable, interactive tutorial on USB, and power adapter, user s manual, Quick Start Guide, Quick Reference Guide Kit includes:
, the Professional CGM Abbott FreeStyle Libre Pro Reader Kit includes: Reader, USB cable, interactive tutorial on USB, and power adapter, user s manual, Quick Start Guide, Quick Reference Guide Kit includes:
The Growing Future of Diabetes: Insulin Pump Therapy in Type 1 and 2 Diabetes
 The Growing Future of Diabetes: Insulin Pump Therapy in Type 1 and 2 Diabetes Sarah Dombrowski, PharmD, BCACP Pennsylvania Pharmacists Association 10/20/18 1 Objectives At the completion of this activity,
The Growing Future of Diabetes: Insulin Pump Therapy in Type 1 and 2 Diabetes Sarah Dombrowski, PharmD, BCACP Pennsylvania Pharmacists Association 10/20/18 1 Objectives At the completion of this activity,
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Abbott FreeStyle Libre Pro System
 Professional CGM Abbott FreeStyle Libre Pro Reader Kit includes: Reader, USB cable, interactive tutorial on USB, and power adapter, user s manual, Quick Start Guide, Quick Reference Guide Kit includes:
Professional CGM Abbott FreeStyle Libre Pro Reader Kit includes: Reader, USB cable, interactive tutorial on USB, and power adapter, user s manual, Quick Start Guide, Quick Reference Guide Kit includes:
Insulin Pumps - External
 Insulin Pumps - External Policy Number: Original Effective Date: MM.01.004 04/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/20174/1/2018 Section: DME Place(s) of
Insulin Pumps - External Policy Number: Original Effective Date: MM.01.004 04/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/20174/1/2018 Section: DME Place(s) of
Continuous Glucose Monitoring (CGM)
 Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP
 NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP No commercial support or sponsorship was received for this project I have nothing to disclose OBJECTIVES Identify at
NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP No commercial support or sponsorship was received for this project I have nothing to disclose OBJECTIVES Identify at
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
Diabetes Technology Update. Sarah Konigsberg, MD Diabetes & Endocrine Assoc. April 7, 2018
 Diabetes Technology Update Sarah Konigsberg, MD Diabetes & Endocrine Assoc. April 7, 2018 Disclosures None No future technologies are FDA approved Continuous Glucose Monitors Continuous Glucose Monitors
Diabetes Technology Update Sarah Konigsberg, MD Diabetes & Endocrine Assoc. April 7, 2018 Disclosures None No future technologies are FDA approved Continuous Glucose Monitors Continuous Glucose Monitors
Policy and Procedure DEPARTMENT: Medical Management
 PAGE: 1 of 5 SCOPE: Louisiana Healthcare Connections (Plan) and Member Services Departments. PURPOSE: To provide guidelines for the authorization of ambulatory insulin pumps. WORK PROCESS: 1. Purchase
PAGE: 1 of 5 SCOPE: Louisiana Healthcare Connections (Plan) and Member Services Departments. PURPOSE: To provide guidelines for the authorization of ambulatory insulin pumps. WORK PROCESS: 1. Purchase
MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection
 POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
Continuous Glucose Monitoring Devices Pharmacy Policy
 Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
Diabetes and Technology. Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE
 Diabetes and Technology Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE Disclosure Speaker s Bureau: Janssan Pharmaceuticals Current Technology V-Go by Valeritas Continuous Sensors (personal
Diabetes and Technology Saturday, September 9, 2017 Aimee G sell, APRN, ANP-C, CDE Disclosure Speaker s Bureau: Janssan Pharmaceuticals Current Technology V-Go by Valeritas Continuous Sensors (personal
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING SYSTEMS/ EXTERNAL INSULIN PUMP THERAPY FOR DIABETES EFFECTIVE DATE: 08/17/17
 MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 11 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 11 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
External Insulin Pumps Corporate Medical Policy
 File Name: External Insulin Pumps File Code: UM.DME.02 Origination: 04/2006 Last Review: 11/2018 Next Review: 11/2019 Effective Date: 04/01/2019 External Insulin Pumps Corporate Medical Policy Description/Summary
File Name: External Insulin Pumps File Code: UM.DME.02 Origination: 04/2006 Last Review: 11/2018 Next Review: 11/2019 Effective Date: 04/01/2019 External Insulin Pumps Corporate Medical Policy Description/Summary
Updates in Diabetes Technology
 Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
10:20 AM March 5, B 100% 235 u. 124 mg/dl 3 HRS INSULIN ON BOARD:
 DEXCOM G5 MOBILE CGM COMPATIBLE B 100% 235 u INSULIN ON BOARD: OPTIONS 10:20 AM March 5, 2017 400 350 300 250 200 150 100 50 1.1 u 1:09 hrs BOLUS 124 mg/dl 3 HRS The pump that gets updated, not outdated.
DEXCOM G5 MOBILE CGM COMPATIBLE B 100% 235 u INSULIN ON BOARD: OPTIONS 10:20 AM March 5, 2017 400 350 300 250 200 150 100 50 1.1 u 1:09 hrs BOLUS 124 mg/dl 3 HRS The pump that gets updated, not outdated.
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
The Realities of Technology in Type 1 Diabetes
 The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond)
 Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
Artificial Pancreas Device System (APDS)
 Medical Policy Manual Durable Medical Equipment, Policy No. 77 Artificial Pancreas Device System (APDS) Next Review: October 2019 Last Review: October 2018 Effective: November 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Durable Medical Equipment, Policy No. 77 Artificial Pancreas Device System (APDS) Next Review: October 2019 Last Review: October 2018 Effective: November 1, 2018 IMPORTANT REMINDER
CONTINUOUS OR INTERMITTENT GLUCOSE MONITORING IN INTERSTITIAL FLUID
 FLUID Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
FLUID Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
Predicts and helps prevent lows with. zero fingersticks NOW FEATURING BASAL-IQ TECHNOLOGY
 Predicts and helps prevent lows with * zero fingersticks NOW FEATURING BASAL-IQ TECHNOLOGY Basal-IQ Technology is not a substitute for active self-management of your diabetes. Please see back cover for
Predicts and helps prevent lows with * zero fingersticks NOW FEATURING BASAL-IQ TECHNOLOGY Basal-IQ Technology is not a substitute for active self-management of your diabetes. Please see back cover for
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
State of California Health and Human Services Agency Department of Health Care Services
 State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson
 DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson March 3 rd, 2018 Who is this guy? Accelerating Progress 1 Oh that guy! Accelerating Progress 2 STATE OF T1D CARE
DIABETES TECHNOLOGY: WHERE ARE WE NOW AND WHERE ARE WE GOING? Presented by: Tom Brobson March 3 rd, 2018 Who is this guy? Accelerating Progress 1 Oh that guy! Accelerating Progress 2 STATE OF T1D CARE
OBJECTIVES THE PAST INNOVATIVE TECHNOLOGY & DIABETES DIABETES MIS MANAGEMENT IMPROVEMENTS IN DIABETES MANAGEMENT
 OBJECTIVES INNOVATIVE TECHNOLOGY & DIABETES TRISHA PORRETTI, RN, BSN, CDE PWD FOR 25 YEARS Identify important safety features and differences of 3 Continuous Glucose Monitoring (CGM) systems Identify important
OBJECTIVES INNOVATIVE TECHNOLOGY & DIABETES TRISHA PORRETTI, RN, BSN, CDE PWD FOR 25 YEARS Identify important safety features and differences of 3 Continuous Glucose Monitoring (CGM) systems Identify important
CareLink. software REPORT REFERENCE GUIDE. Management Software for Diabetes
 CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
Objectives. The Problem. We ve come a LONG way, Innovations in Diabetes Care and Management. Barbara Walz, RN, BSN, CDE April 26, 2018
 Objectives Innovations in Diabetes Care and Management Barbara Walz, RN, BSN, CDE April 26, 2018 1. Briefly discuss the history of diabetes management over the past 100 years 2. Describe the impact of
Objectives Innovations in Diabetes Care and Management Barbara Walz, RN, BSN, CDE April 26, 2018 1. Briefly discuss the history of diabetes management over the past 100 years 2. Describe the impact of
Coverage Guidelines. Continuous Glucose Monitors (CGMs)
 Coverage Guidelines Continuous Glucose Monitors (CGMs) Disclaimer: Please note that Baptist Health Plan Coverage Guidelines may be updated throughout the year. A printed version may not be most up to date
Coverage Guidelines Continuous Glucose Monitors (CGMs) Disclaimer: Please note that Baptist Health Plan Coverage Guidelines may be updated throughout the year. A printed version may not be most up to date
Report Reference Guide
 Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier?
 Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier? Alan B Schorr DO FAAIM FACE www.sugardoc.com abs@sugardoc.com Disclosures
Advances in Technology in the Treatment of Diabetes Mellitus 2017 How far have we come-how far are we going? Is there a final frontier? Alan B Schorr DO FAAIM FACE www.sugardoc.com abs@sugardoc.com Disclosures
Continuous Glucose Monitoring
 Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
Continuous Glucose Monitoring Information about fully-subsidised continuous glucose monitoring for children and young people with type 1 diabetes Continuous glucose monitoring (CGM) can help in managing
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING SYSTEMS/ EXTERNAL INSULIN PUMP THERAPY FOR DIABETES EFFECTIVE DATE: 08/17/17, 08/16/18
 MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 12 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
MEDICAL POLICY SUBJECT: CONTINUOUS GLUCOSE MONITORING PAGE: 1 OF: 12 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
Subject Index. Breastfeeding, self-monitoring of blood glucose 56
 Subject Index Animas Vibe 86, 130 Artificial pancreas clinical studies inpatient studies 175 180 outpatient studies outcome assessment 182, 183 technology 180, 181 telemedicine 182 components glucose sensor
Subject Index Animas Vibe 86, 130 Artificial pancreas clinical studies inpatient studies 175 180 outpatient studies outcome assessment 182, 183 technology 180, 181 telemedicine 182 components glucose sensor
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes. pump. pump
 Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
With new technology available to
 Insulin Pump Therapy: Who, Why, and How Alyssa Kanagaki Greenleaf, BA, MHS, PA-C With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among
Insulin Pump Therapy: Who, Why, and How Alyssa Kanagaki Greenleaf, BA, MHS, PA-C With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among
UNDERSTANDING THE BASIC FEATURES AND MANAGEMENT IN THE SCHOOL SETTING CHRISTINE HERTLER RN BSN CDE & MARY MCCARTHY RN CDE
 UNDERSTANDING THE BASIC FEATURES AND MANAGEMENT IN THE SCHOOL SETTING CHRISTINE HERTLER RN BSN CDE & MARY MCCARTHY RN CDE The insulin pump Replaces injections Delivers insulin through a soft cannula
UNDERSTANDING THE BASIC FEATURES AND MANAGEMENT IN THE SCHOOL SETTING CHRISTINE HERTLER RN BSN CDE & MARY MCCARTHY RN CDE The insulin pump Replaces injections Delivers insulin through a soft cannula
Today s Goals 10/6/2017. New Frontiers in Diabetes Technology. Disclosures
 New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs.
 INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. Waterproof to a depth
INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. Waterproof to a depth
Insulin Pumps Available in Canada
 Insulin Pumps Available in Canada A Comparison from WaltzingTheDragon.ca *Accu-Chek Animas Ping/Vibe Medtronic Minimed 630G Omnipod ABOUT FORM: Is the insulin pump Tubing- Free? Is the pump? Or Water-Resistant?
Insulin Pumps Available in Canada A Comparison from WaltzingTheDragon.ca *Accu-Chek Animas Ping/Vibe Medtronic Minimed 630G Omnipod ABOUT FORM: Is the insulin pump Tubing- Free? Is the pump? Or Water-Resistant?
Company Overview February 26, 2019
 Company Overview February 26, 2019 SAFE HARBOR Cautionary Note Regarding Forward-Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
Company Overview February 26, 2019 SAFE HARBOR Cautionary Note Regarding Forward-Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs.
 INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. MORE TIME HERE TO USING
INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. MORE TIME HERE TO USING
Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets
 Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
Continuous Glucose Monitoring (CGM)
 Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 08/22/2018 Effective Date: 08/22/2018 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 08/22/2018 Effective Date: 08/22/2018 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Cowen Investor Conference March confidently live life with ease
 Cowen Investor Conference March 2018 confidently live life with ease Forward-Looking Statements This presentation contains certain forward-looking statements that involve risks and uncertainties that could
Cowen Investor Conference March 2018 confidently live life with ease Forward-Looking Statements This presentation contains certain forward-looking statements that involve risks and uncertainties that could
Advances in Diabetes Care Technologies
 Advances in Diabetes Care Technologies 1979 2015 Introduction Roughly 20% to 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the U.S. FDA
Advances in Diabetes Care Technologies 1979 2015 Introduction Roughly 20% to 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the U.S. FDA
Canaccord Growth Conference August confidently live life with ease
 Canaccord Growth Conference August 2018 confidently live life with ease Forward-Looking Statements This presentation contains forward-looking statements that are not purely historical regarding Senseonics
Canaccord Growth Conference August 2018 confidently live life with ease Forward-Looking Statements This presentation contains forward-looking statements that are not purely historical regarding Senseonics
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes Individuals: With type 1 diabetes
 Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Animas Vibe System World-class insulin pumping meets world-class CGM*
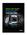 Animas Vibe System World-class insulin pumping meets world-class CGM* Fully integrated with Dexcom G4 PLATINUM CGM* *Continuous glucose monitoring. My Animas pump has really put me in the driver s seat
Animas Vibe System World-class insulin pumping meets world-class CGM* Fully integrated with Dexcom G4 PLATINUM CGM* *Continuous glucose monitoring. My Animas pump has really put me in the driver s seat
Comparative table of insulin pumps
 Comparative table of insulin pumps Pumps reviewed: insight (Roche Veo 640G Mylife OmniPod Mylife S () The first stop for professional medicines advice www.sps.nhs.uk Authored May 2018 Insight OmniPod S
Comparative table of insulin pumps Pumps reviewed: insight (Roche Veo 640G Mylife OmniPod Mylife S () The first stop for professional medicines advice www.sps.nhs.uk Authored May 2018 Insight OmniPod S
MEDICAL POLICY Acupuncture
 POLICY: PG0382 ORIGINAL EFFECTIVE: 12/13/16 LAST REVIEW: 06/12/18 MEDICAL POLICY Acupuncture GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual
POLICY: PG0382 ORIGINAL EFFECTIVE: 12/13/16 LAST REVIEW: 06/12/18 MEDICAL POLICY Acupuncture GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual
Technology for Diabetes: 101 Basic Rules of the Road. Karen Hamon RN, BSN, CDE Stephen Stone MD, FAAP Neil H. White, MD, CDE
 Technology for Diabetes: 101 Basic Rules of the Road Karen Hamon RN, BSN, CDE Stephen Stone MD, FAAP Neil H. White, MD, CDE Quick Pump Facts! o Constant insulin supply o Pager-sized mini-computer worn
Technology for Diabetes: 101 Basic Rules of the Road Karen Hamon RN, BSN, CDE Stephen Stone MD, FAAP Neil H. White, MD, CDE Quick Pump Facts! o Constant insulin supply o Pager-sized mini-computer worn
RELEASED. first steps. Icon Icon name What it means
 Icon Icon name What it means Connection The connection icon appears green when the Sensor feature is on and your transmitter is successfully communicating with your pump. The connection icon appears gray
Icon Icon name What it means Connection The connection icon appears green when the Sensor feature is on and your transmitter is successfully communicating with your pump. The connection icon appears gray
Diabetic Equipment and Supplies
 Diabetic Equipment and Supplies Chapter.1 Enrollment..................................................................... -2.2 Benefits, Limitations, and Authorization Requirements...........................
Diabetic Equipment and Supplies Chapter.1 Enrollment..................................................................... -2.2 Benefits, Limitations, and Authorization Requirements...........................
RELEASED. Clearing your active insulin
 To clear all your settings: 1. Make sure the pump is not connected to your body. 2. Go to the Manage Settings screen. Menu > Utilities > Manage Settings 3. Simultaneously press and hold and until the Manage
To clear all your settings: 1. Make sure the pump is not connected to your body. 2. Go to the Manage Settings screen. Menu > Utilities > Manage Settings 3. Simultaneously press and hold and until the Manage
Clinical Policy Title: Artificial pancreas device system
 Clinical Policy Title: Artificial pancreas device system Clinical Policy Number: 08.02.07 Effective Date: April 1, 2016 Initial Review Date: November 18, 2015 Most Recent Review Date: February 6, 2018
Clinical Policy Title: Artificial pancreas device system Clinical Policy Number: 08.02.07 Effective Date: April 1, 2016 Initial Review Date: November 18, 2015 Most Recent Review Date: February 6, 2018
COMPANY OVERVIEW. July 30, 2018
 COMPANY OVERVIEW July 30, 2018 SAFE HARBOR & NON-GAAP FINANCIAL MEASURES Forward Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
COMPANY OVERVIEW July 30, 2018 SAFE HARBOR & NON-GAAP FINANCIAL MEASURES Forward Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid
 Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid Policy #: 038 Latest Review Date: December 2016 Category: DME Policy Grade: B Background/Definitions: As a general
Name of Policy: Continuous or Intermittent Monitoring of Glucose in the Interstitial Fluid Policy #: 038 Latest Review Date: December 2016 Category: DME Policy Grade: B Background/Definitions: As a general
Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011
 Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011 Information posted May 13, 2011 Effective for dates of service on or after July 1, 2011, the benefit criteria
Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011 Information posted May 13, 2011 Effective for dates of service on or after July 1, 2011, the benefit criteria
WHEN YOUR PANCREAS IS NOT A HAPPY CAMPER A PRESENTATION ON DIABETES MANAGEMENT IN THE CAMP SETTING AMANDA COSCHI, BSCN, RN, CDE
 WHEN YOUR PANCREAS IS NOT A HAPPY CAMPER A PRESENTATION ON DIABETES MANAGEMENT IN THE CAMP SETTING AMANDA COSCHI, BSCN, RN, CDE MAY 5, 2018 OBJECTIVES Strong understanding of diabetes and its management
WHEN YOUR PANCREAS IS NOT A HAPPY CAMPER A PRESENTATION ON DIABETES MANAGEMENT IN THE CAMP SETTING AMANDA COSCHI, BSCN, RN, CDE MAY 5, 2018 OBJECTIVES Strong understanding of diabetes and its management
Designed with your patients lives in mind
 The Accu-Chek Insight diabetes therapy system Designed with your patients lives in mind With pre-filled insulin cartridge Designed for easy patient training The Accu-Chek Insight diabetes therapy system
The Accu-Chek Insight diabetes therapy system Designed with your patients lives in mind With pre-filled insulin cartridge Designed for easy patient training The Accu-Chek Insight diabetes therapy system
DIABETES & ENDOCRINE DIABETES TECHNOLOGY: HOW TO STAY CURRENT WITH ONGOING TECHNOLOGY ADVANCEMENT
 DIABETES TECHNOLOGY: HOW TO STAY CURRENT WITH ONGOING TECHNOLOGY ADVANCEMENT Min-Jye Chen, MD Endocrinology Diabetes Management of the School-Aged Child Provided by Texas Children s Hospital Provider #18-267764-A
DIABETES TECHNOLOGY: HOW TO STAY CURRENT WITH ONGOING TECHNOLOGY ADVANCEMENT Min-Jye Chen, MD Endocrinology Diabetes Management of the School-Aged Child Provided by Texas Children s Hospital Provider #18-267764-A
Diabetes Management, Equipment and Supplies
 Coverage Summary Diabetes Management, Equipment and Supplies Policy Number: D-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 11/01/2006 Approved by: UnitedHeatlhcare Medicare
Coverage Summary Diabetes Management, Equipment and Supplies Policy Number: D-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 11/01/2006 Approved by: UnitedHeatlhcare Medicare
THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE
 THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE Indicated for type 1 patients 14 and over. Prescription required. WARNING: Medtronic performed an evaluation of the MiniMed 670G system and determined that it
THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE Indicated for type 1 patients 14 and over. Prescription required. WARNING: Medtronic performed an evaluation of the MiniMed 670G system and determined that it
DISCOVER THE POWER OF CONNECTION MINIMED 640G
 DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Has your child just been diagnosed with insulin dependent diabetes? Or perhaps they ve been on multiple daily injection
DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Has your child just been diagnosed with insulin dependent diabetes? Or perhaps they ve been on multiple daily injection
COMPANY OVERVIEW. April 26, 2018
 COMPANY OVERVIEW April 26, 2018 SAFE HARBOR & NON-GAAP FINANCIAL MEASURES Forward Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
COMPANY OVERVIEW April 26, 2018 SAFE HARBOR & NON-GAAP FINANCIAL MEASURES Forward Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
MEDICAL POLICY: Enteral and Parenteral Nutrition
 POLICY: PG0114 ORIGINAL EFFECTIVE: 02/15/07 LAST REVIEW: 08/14/18 MEDICAL POLICY: Enteral and Parenteral Nutrition GUIDELINES This policy does not certify benefits or authorization of benefits, which is
POLICY: PG0114 ORIGINAL EFFECTIVE: 02/15/07 LAST REVIEW: 08/14/18 MEDICAL POLICY: Enteral and Parenteral Nutrition GUIDELINES This policy does not certify benefits or authorization of benefits, which is
High rate of non-adherence to insulin pump: over prescription, overuse or misuse? A population-based case-cohort study.
 High rate of non-adherence to insulin pump: over prescription, overuse or misuse? A population-based case-cohort study. Dr. Eugene Merzon MD; Ilia Merhasin, MBA; Dr. Avivit Golan-Cohen MD ; Dr. Shmuel
High rate of non-adherence to insulin pump: over prescription, overuse or misuse? A population-based case-cohort study. Dr. Eugene Merzon MD; Ilia Merhasin, MBA; Dr. Avivit Golan-Cohen MD ; Dr. Shmuel
People with type 1 diabetes and Do It Yourself (DIY) technology solutions ABOUT THIS POSITION STATEMENT
 Position Statement People with type 1 diabetes and Do It Yourself (DIY) technology solutions ABOUT THIS POSITION STATEMENT Diabetes Australia believes that people with diabetes should have choice and access
Position Statement People with type 1 diabetes and Do It Yourself (DIY) technology solutions ABOUT THIS POSITION STATEMENT Diabetes Australia believes that people with diabetes should have choice and access
For more comprehensive information, please refer to the t:connect Application User Guide available online at: Getting Started Guide.
 Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
DISCOVER THE POWER OF CONNECTION MINIMED 640G
 DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Have you just been diagnosed with insulin dependent diabetes? Perhaps you ve been on multiple daily injection therapy
DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Have you just been diagnosed with insulin dependent diabetes? Perhaps you ve been on multiple daily injection therapy
Figure 2.1: Glucose meter
 CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
NDSS Helpline
 Continuous GLUCOSE MONITORING A guide to using CGM for children and young people with type 1 diabetes NDSS Helpline 1300 136 588 ndss.com.au The National Diabetes Services Scheme is an initiative of the
Continuous GLUCOSE MONITORING A guide to using CGM for children and young people with type 1 diabetes NDSS Helpline 1300 136 588 ndss.com.au The National Diabetes Services Scheme is an initiative of the
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools
 Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
Non-Invasive Glucose Monitoring. Kevin Walls April 12, :30 PM-7:30 PM.
 Non-Invasive Glucose Monitoring Kevin Walls April 12, 2018 6:30 PM-7:30 PM. Technology is evolving Technology is evolving at such a rapid rate it s hard to keep up. Imagine living a healthier life without
Non-Invasive Glucose Monitoring Kevin Walls April 12, 2018 6:30 PM-7:30 PM. Technology is evolving Technology is evolving at such a rapid rate it s hard to keep up. Imagine living a healthier life without
Performance-powered. The OneTouch. Ping insulin pump and meter-remote.
 Performance-powered. The OneTouch Ping insulin pump and meter-remote. I We don t just deliver insulin. We deliver outstanding clinical performance. P36337_OTP_DetAid_OmniPodUpdate_r12.indd 1 OneTouch Ping.
Performance-powered. The OneTouch Ping insulin pump and meter-remote. I We don t just deliver insulin. We deliver outstanding clinical performance. P36337_OTP_DetAid_OmniPodUpdate_r12.indd 1 OneTouch Ping.
Report Reference Guide. THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1
 Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Advances Towards the Bionic Pancreas.
 Advances Towards the Bionic Pancreas. Ron Brazg MD, FACE Rainier Clinical Research Center Renton, WA DISCLOSURES Rainier Clinical Research Center is an independent research facility not directly affiliated
Advances Towards the Bionic Pancreas. Ron Brazg MD, FACE Rainier Clinical Research Center Renton, WA DISCLOSURES Rainier Clinical Research Center is an independent research facility not directly affiliated
ADVANCES IN DIABETES TECHNOLOGY: A FOCUS ON CONTINUOUS GLUCOSE MONITORING 9:15 10:15 AM
 ADVANCES IN DIABETES TECHNOLOGY: A FOCUS ON CONTINUOUS GLUCOSE MONITORING 9:15 10:15 AM ACPE UAN: 0107-9999-18-124-L01-P 0.1 CEU/1 hr Activity Type: Knowledge-Based Learning Objectives for Pharmacists:
ADVANCES IN DIABETES TECHNOLOGY: A FOCUS ON CONTINUOUS GLUCOSE MONITORING 9:15 10:15 AM ACPE UAN: 0107-9999-18-124-L01-P 0.1 CEU/1 hr Activity Type: Knowledge-Based Learning Objectives for Pharmacists:
Basics of Continuous Subcutaneous Insulin Infusion Therapy. Lubna Mirza, MD Norman Endocrinology Associates 2018
 Basics of Continuous Subcutaneous Insulin Infusion Therapy Lubna Mirza, MD Norman Endocrinology Associates 2018 Preamble Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients
Basics of Continuous Subcutaneous Insulin Infusion Therapy Lubna Mirza, MD Norman Endocrinology Associates 2018 Preamble Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients
5/16/2018. Integrating Diabetes Technology Into your Practice : Insulin Pump Therapy. Diosclosures. What exactly is an Insulin Pump?
 Integrating Diabetes Technology Into your Practice : Insulin Pump Therapy Lucia M. Novak, MSN, ANP BC, BC ADM, CDTC Director, Riverside Diabetes Center, Riverside Medical Associates Riverdale, MD Adjunct
Integrating Diabetes Technology Into your Practice : Insulin Pump Therapy Lucia M. Novak, MSN, ANP BC, BC ADM, CDTC Director, Riverside Diabetes Center, Riverside Medical Associates Riverdale, MD Adjunct
MEDICAL POLICY Drug Testing
 POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 11/13/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
POLICY: PG0069 ORIGINAL EFFECTIVE: 01/01/11 LAST REVIEW: 11/13/18 MEDICAL POLICY Drug Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each
CGM and Closing The Loop
 CGM and Closing The Loop Dualities Research: Helmsely Charitable Trust, ADA, JDRF, NIDDK Consulting: Abbott Diabetes Care, Roche, Intarcia, Valeritas, Adocia, Big Foot Like With Pumps, We ve Come A Long
CGM and Closing The Loop Dualities Research: Helmsely Charitable Trust, ADA, JDRF, NIDDK Consulting: Abbott Diabetes Care, Roche, Intarcia, Valeritas, Adocia, Big Foot Like With Pumps, We ve Come A Long
MINIMED 640G WITH SMARTGUARD GIVES YOU CONFIDENCE TO TAKE CONTROL OF YOUR DIABETES
 MINIMED 640G WITH SMARTGUARD GIVES YOU CONFIDENCE OF YOUR DIABETES HAVE YOU CONSIDERED THE REAL IMPACT HYPOS HAVE ON YOUR LIFE? AN ESTIMATED 1 HOUR EVERY DAY IS LOST TO THE MANAGEMENT OF 43% OF PEOPLE
MINIMED 640G WITH SMARTGUARD GIVES YOU CONFIDENCE OF YOUR DIABETES HAVE YOU CONSIDERED THE REAL IMPACT HYPOS HAVE ON YOUR LIFE? AN ESTIMATED 1 HOUR EVERY DAY IS LOST TO THE MANAGEMENT OF 43% OF PEOPLE
WOULD YOU LIKE TO REDUCE THE FEAR OF HYPOGLYCAEMIA FOR YOUR PATIENTS?
 WOULD YOU LIKE TO REDUCE THE FEAR OF HYPOGLYCAEMIA FOR YOUR PATIENTS? THE ONLY SYSTEM CLINICALLY PROVEN TO REDUCE HYPOGLYCAEMIA MINIMED 640G SYSTEM WITH SMARTGUARD TECHNOLOGY 1 HYPOGLYCAEMIA IS CHALLENGING
WOULD YOU LIKE TO REDUCE THE FEAR OF HYPOGLYCAEMIA FOR YOUR PATIENTS? THE ONLY SYSTEM CLINICALLY PROVEN TO REDUCE HYPOGLYCAEMIA MINIMED 640G SYSTEM WITH SMARTGUARD TECHNOLOGY 1 HYPOGLYCAEMIA IS CHALLENGING
Pumps & Sensors made easy. OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University
 Pumps & Sensors made easy OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University DeFronzo RA. Diabetes. 2009;58:773-795. Ominous Octet Relationship of b-cell Dysfunction and Development
Pumps & Sensors made easy OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University DeFronzo RA. Diabetes. 2009;58:773-795. Ominous Octet Relationship of b-cell Dysfunction and Development
THE ONLY SYSTEM^ CLINICALLY PROVEN TO REDUCE HYPOGLYCAEMIA
 THE ONLY SYSTEM^ CLINICALLY PROVEN TO REDUCE HYPOGLYCAEMIA MINIMED 640G system^ with SmartGuard technology HYPOGLYCAEMIA IS CHALLENGING FOR GLYCAEMIC CONTROL 1 AND quality of life 2 Achieving good glucose
THE ONLY SYSTEM^ CLINICALLY PROVEN TO REDUCE HYPOGLYCAEMIA MINIMED 640G system^ with SmartGuard technology HYPOGLYCAEMIA IS CHALLENGING FOR GLYCAEMIC CONTROL 1 AND quality of life 2 Achieving good glucose
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA
 BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Continuous Glucose Monitoring System. Receiver Setup
 Continuous Glucose Monitoring System Receiver Setup Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Receiver What do I do with it? Review your readings. Set and receive
Continuous Glucose Monitoring System Receiver Setup Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Receiver What do I do with it? Review your readings. Set and receive
Medical Policy An independent licensee of the Blue Cross Blue Shield Association.
 Insulin Pump Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association. Title: Insulin Pump Professional Original Effective Date: January 1, 1999 Revision Date(s): June
Insulin Pump Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association. Title: Insulin Pump Professional Original Effective Date: January 1, 1999 Revision Date(s): June
