Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0
|
|
|
- Frederica Floyd
- 5 years ago
- Views:
Transcription
1 Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V October 2017
2 Table of Contents 1. Introduction Purpose of this Policy/Procedure Scope 4 4. Definitions/Glossary Ownership and Responsibilities Role of the Managers Role of the Specialist Staff Role of Individual Staff Standards and Practice Dissemination and Implementation Monitoring Compliance and Effectiveness Updating and Review Equality and Diversity 10 Appendix 1. Governance Information Appendix 2. Initial Equality Impact Assessment Form Appendix 3. Appendix 4. Assessment for Self-Administration of Subcutaneous Insulin for Adults with Diabetes.. Competency Assessment Document Subcutaneous Injection of Insulin/GLP Using a Pre-filled Pen Device Page 2 of 18
3 1. Introduction 1.1. Diabetes is a serious life-long health condition that occurs when the amount of glucose in the blood is too high because the body cannot use it properly. If left untreated, high blood glucose levels can cause serious health complications There are two main types of Diabetes: Type 1 and Type 2. Everyone with Type 1 Diabetes, and some people with Type 2 Diabetes, need to take Insulin (a hormone produced by the pancreas) to control blood glucose levels. Some people with Type 2 Diabetes need to take a GLP1 analogue (a hormone produced by the gut) to control blood glucose levels The use of Insulin pens is very common in the UK as an alternative to traditional Insulin vials and Insulin syringes. It is the preferred method of administration by patients who use Insulin. GLP1 is only available in a pen device Insulin pen devices can be reusable with refillable Insulin pen cartridges or prefilled and disposable. GLP1 pen devices are prefilled and disposable. Pen devices are intended for the subcutaneous administration of multiple Insulin/GLP1 doses to the same patient using a new pen needle for each dose. They should always be used in accordance with manufacturer instructions Insulin pens offer several significant advantages over Insulin syringes: ease of handling for those with visual or fine motor skills impairments, improved accuracy as an accurate dose can be pre-set on the dosage dial, and less injection pain This version supersedes any previous versions of this document. 2. Purpose of this Policy/Procedure 2.1. The injection is to be performed by the patient under supervision of nursing staff in all clinical areas. All patients who are self-administering Insulin or GLP1 must have the Trust`s form Registered Nurse Assessment for Patient Self-Administration of Insulin/GLP1 Via a Pen Device completed (CHA see Appendix 3) and filed in the current section of the patient s medical notes If the patient is unable to administer their own Insulin injection using a pen device staff can administer the Insulin using an Insulin safety syringe and Insulin vial and the correct sharps disposal. The patient s usual Insulin is to be prescribed on EPMA in an Insulin vial for this purpose. NB Insulin must not be drawn up from a 3 ml Insulin pen cartridge using a syringe. Only Insulin vials are to be used to withdraw Insulin for subcutaneous injection using an Insulin safety syringe If the patient is unable to administer their own Insulin/GLP1 using a pen device and the Insulin/GLP1 is not available in a vial, staff can administer using a prefilled pen device and use the correct sharps disposal method. Staff who undertake subcutaneous injection using a prefilled pen device must have received training and have demonstrated competency in this procedure (see Appendix 4). Page 3 of 18
4 3. Scope 3.1. This procedure applies to adults with Diabetes and is for Trust-wide use. It applies to all registered nursing staff regardless of grade that are undertaking patient supervision of self-administration or administering a prescribed Insulin/GLP analogue subcutaneous injection within the Royal Cornwall Hospitals Trust The purpose of the document is to ensure that all staff perform the procedure in accordance with best practice guidelines. Registered staff have a duty of care to supervise the patient in this procedure. Individual staff must ensure they are competent to supervise or administer the injection, undertaking the procedure to the standards within this document. 4. Definitions / Glossary EPMA Electronic Prescribing and Medicines Administration GLP1 Glucagon-Like Peptide 1 TTOs To Take Out (prescriptions in hospital discharge summary) 5. Ownership and Responsibilities 5.1. The strategic and operational roles responsible for the development, implementation, management and compliance of the procedure are shown below The lead professions for this procedure are: Consultant Nurse for Infection Prevention and Control Diabetes Specialist Pharmacist RCHT Diabetes Inpatient Specialist Nurse Team CPFT/RCHT 5.3. Role of the Managers Line Managers are responsible for: Identifying registered staff members who will become cascade trainers for their area. Ensuring all staff in their area who undertake patient supervision of selfadministration or who are administering a prescribed Insulin/GLP analogue subcutaneous injection have received training and have had the competency assessment document (Appendix 4) completed and filed in their personal file. Ensuring adequate supplies of patient pen needles are available in their area. Removing Insulin safety pen needles from stock in their area. Ensuring they have adequate supplies of one litre sharps bins (Unit 4 ordering code: FSL315.) available in their area. Ensuring all staff in their area who undertakes patient supervision of selfadministration or who are administering a prescribed Insulin/GLP analogue subcutaneous injection follow this procedure. Monitoring compliance with the procedure Role of the Specialist Staff Ward Pharmacists are responsible for: Ensuring the correct Insulin preparations are prescribed on EPMA and for TTOs during the patient s inpatient stay as per this procedure. Page 4 of 18
5 Ensuring adequate supplies of the correct Insulin preparation have been ordered/are available in the ward area for use. Ensuring, for those patients self-administering Insulin/GLP1, that the Trust s Self-Administration of Insulin/GLP1 form (CHA2976) has been completed and filed in the patient s current section of the medical notes. Liaising with registered ward staff to ensure this form is completed and correctly filed if this is absent and the patient is self-administering Insulin/GLP1. Monitoring compliance with the procedure. Diabetes Specialist Pharmacist and Medical Safety Pharmacist are responsible for: Communication and dissemination of this procedure and any associated information across all three Trust sites. Ensure adequate on-going supplies of Insulin preparations are available in pharmacy to order to avoid Insulin omissions and errors. Monitoring and compliance with the procedure. Auditing practice and producing reports. Nurse Consultant for Infection Prevention and Control is responsible for: Communication of this procedure across all three Trust sites and liaison with the Trust s senior nursing team. Monitoring and compliance with the procedure regarding safe use of sharps. Clinical Nurse Specialist Diabetes In-patient Team is responsible for: The development, updating and ratification (via their governance structure) of this procedure document and any accompanying documentation. Delivering initial cascade training to ward representatives and their competency assessment in the procedure. Communication of this procedure to the Trusts Diabetes Link Nurse group. Liaising with nursing staff to advocate that the Trusts self-administration of Insulin/GLP1 form (CHA2976) has been completed and filed in the patient s current section of the medical notes Role of Individual Staff All staff members are responsible for: Compliance with the procedure. Ensuring that if they undertake patient supervision of self-administration or they administer a prescribed Insulin/GLP analogue subcutaneous injection that they have received the correct training and have had the competency assessment document (Appendix 4) completed. Ensuring their clinical skills are current and up to-date in order to facilitate the procedure. Completing the Trust s Self-Administration of Insulin/GLP1 form (CHA2976) for each patient that is self-administering and file in the patient s current section of the medical notes. Ensuring safe use of sharps as per this procedure. Page 5 of 18
6 6. Standards and Practice Equipment EPMA Prescription Cartridge of prescribed Insulin and appropriate reusable pen device, i.e. NovoPen is using NovoNordisk Insulin, HumaPen if using Lilly Insulin, Autopen24/Clikstar if using Aventis Insulin, Autopen if using C P Pharmaceutical Insulin Or appropriate disposable prefilled Insulin or GLP1 Analogue pen device Pen needle 6mm or less Pen user guide supplied with each box of pens Named patient one litre sharps bin with designated pen needle remover. (Unit 4 ordering code: FSL315.) available Procedures A and B A - If the patient is UNABLE to perform their own Insulin injection using a pen device: 1. Patient s usual Insulin needs to be prescribed on EPMA in vials (their usual Insulin and Insulin pen device needs to be prescribed and then suspended on EPMA to avoid error on discharge). Staff can administer the prescribed Insulin using an Insulin safety syringe, drawing up the Insulin from an Insulin vial. Staff to dispose of the Insulin safety syringe as per the Trust s Sharps Policy. NB - On discharge, if the patient is able to self-administer their own Insulin again, they will need their usual Insulin and Insulin pen device prescribed as TTO s. They are not to be discharged home with Insulin vials and Insulin syringes. 2. If the patient s usual Insulin/GLP1 is not available in a vial, staff can administer the Insulin/GLP1 using a prefilled pen device and patientuse pen needles following the procedure below for Prefilled Insulin/GLP1 Pen Device. 3. After administration, the pen needle needs to be removed using the designated pen needle removing section on the one litre sharps bin (Unit 4 ordering code: FSL315.) available Each patient will need an individual named sharps bin for this method of disposal that is to be stored in the patient s bedside locker with the temporary closure in use. B - If the patient IS ABLE to perform their own Insulin/GLP1 injection using a pen device OR registered staff needs to administer Insulin/GLP1 using a prefilled pen device: 1. Wash hands and assemble required equipment as above and take to patient. 2. Explain procedure to patient and ask patient to wash hands. 3(a) For Prefilled Insulin/GLP1 Pen Devices: i) Twist and pull off cap from main body of pen and put to one side. Page 6 of 18
7 ii) iii) Identify the disposable prefilled pen contains the correct injectable solution and check expiry date. NB Insulin and GLP1 analogue that has been kept out of the fridge has an expiry date within four weeks. Check prefilled pen is intact and that it has no crystals. 3(b) For Reusable Insulin Pen Devices (refer to individual pen guides for specific instructions): i) Twist and pull off cap from main body of pen and put to one side. ii) iii) iv) Unscrew the Insulin cartridge holder which will provide two sections. Identify correct Insulin cartridge has been obtained and check expiry date of cartridge. Ensure Insulin cartridge is matched with the correct Insulin company pen device. v) Check cartridge is intact and that it has no crystals. vi) Using instruction manual which accompanies pen device, ensure piston rod in mechanical section is inside pen. vii) Put Insulin cartridge into the Insulin cartridge holder section. The screw cap end goes first. viii) Screw the mechanical section and the Insulin cartridge holder tightly together. For All Pen Devices (after completing the appropriate instructions as above): 4. Use a new pen needle for each injection (single use only). 5. Take protective tab off the pen needle, push and screw onto the screw cap end of the pen device. Pull off outer and inner needle caps. 6. For cloudy or pre-mixed Insulin e.g. Humulin M3, Insulatard, Novomix 30, gently rock and roll the pen between the palms of your hand 10 times and then turn pen up and down 10 times from one end to the other. Repeat until Insulin is uniform and cloudy. 7. Perform air shot/pen needle prime for all pen devices except Exenatide (Byetta) and Lyxumia (Lixisenatide) where you should refer to the specific pen guide. Check the dose selector is set at zero. Dial 2-4 clicks (for Novopen 4, disposable Lilly pens and Lyxumia GLP1 analogue pen pull the end injection button out before dialling). With pen needle pointed upward, press the plunger all the way in. A drop of solution should appear. If not, repeat procedure until a drop of solution Page 7 of 18
8 appears. This ensures the pen needle is now primed ready for use. This procedure needs to be repeated prior to every injection. 8. Check that dose selector has returned to zero. Dial the required dose by turning the dose selector to the specified amount as prescribed. 9. Select injection site, e.g. lower abdomen, upper outer arm or upper outer thigh. Support injection site for injection. These sites should be rotated daily. 10. Administer as subcutaneous injection 90º angle to skin surface. Depress plunger all the way down. Ensure dose selector has returned to zero. Wait for a count of 10 seconds before removing the pen needle from the skin. 11. Patient only to remove the pen needle from the pen device and dispose of sharp as per local Infection Control Policy. Staff administering Insulin/GLP1 using a prefilled pen device will need to remove the pen needle using the designated pen needle removing section on the one litre sharps bin. Each patient will need an individual, named, one litre sharps bin for sharp disposal that is to be stored in the patient s bedside locker with the temporary closure in use. 12. Complete EPMA prescription and complete nursing documentation. 13. Insulin/GLP analogue that is in use is to be kept out of fridge, at room temperature, for up to four weeks. Please ensure Insulin cartridge/vial/prefilled pen is dated once commenced. 14. Patients pen devices and diabetes equipment should ideally be kept with the patient securely. All individual pen devices should be clearly labelled with the patient s details: name, date of birth, CR number. 7. Dissemination and Implementation 7.1. Dissemination The procedure will be disseminated to the Trust s Ward Managers at the Ward Managers breakfast meeting via a safety briefing document. Ward Managers will disseminate the procedure to all their ward staff using the safety briefing document at their ward safety briefings. Clinical Nurse Specialist Diabetes will disseminate the procedure to the Trust Diabetes Link Nurse group via a link nurse meeting and . The Specialist Diabetes Pharmacist will disseminate the procedure to the Trust s Ward Pharmacists and Pharmacy Department via scheduled continuous professional development lunchtime teaching, at the Wednesday morning Pharmacists meeting and Friday morning dispensary meeting. The Consultant Nurse for Infection Prevention and Control will disseminate the procedure to the Trust s Sharps Safety Group and to the Trust s Senior Nurses. The procedure will be placed on the Trust s electronic document library. Page 8 of 18
9 7.2. Training Cascade training for cascade trainers will be delivered by the Clinical Nurse Specialist Diabetes Inpatient Team at a Ward Managers breakfast meeting. Clinical Nurse Specialist Diabetes Inpatient Team will deliver cascade training to the Trust s Diabetes Link Nurse Group. Trained cascade trainers will train staff at ward level which will be an on-going process. Diabetes Specialist Pharmacist will implement this procedure with the Ward Pharmacists on relevant wards via the regular pharmacy meetings as detailed above. 8. Monitoring compliance and effectiveness Element to be monitored Lead Tool Frequency Reporting arrangements Acting on recommendations and Lead(s) Registered nurse self-assessment form for patient selfadministration of subcutaneous Insulin (CHA2976) has been completed by ward pharmacists. Compliance with the relevant process above for patients seen at a Diabetes Team Review. Ward Pharmacists on review of Individual Patients who are receiving Insulin. Patient Documentation. Registered nurse self-assessment form for patient self-administration of subcutaneous Insulin (CHA2976). Registered nurse self-assessment form for patient selfadministration of subcutaneous Insulin (CHA2976) should be reviewed by ward pharmacists. Adult in-patients with Diabetes who are reviewed/referred to by the specialist Diabetes team. Non-compliance will be reported to the responsible ward/area manager. Non-compliance resulting in an adverse patient event will be reported via Datix. Every patient who is self-administering their own Insulin needs the Registered Nurse self-assessment form for patient self-administration of subcutaneous Insulin (CHA2976) to be completed and filed in the patient s current section of the medical notes. Ward/area managers will undertake subsequent recommendations and action planning for any or all deficiencies and recommendations within reasonable timeframes for their areas. The Specialist Diabetes Pharmacist, Medical Safety Pharmacist, Ward Pharmacists, Nurse Consultant for Infection Prevention and Control and the Clinical Nurse Specialist Diabetes In-Patient Team will undertake any Trust wide recommendations and action planning for any or all deficiencies and recommendations within reasonable timeframes. Page 9 of 18
10 Change in practice and lessons to be shared Lesson learned or changes to practice will be shared with all the relevant stakeholders. 9. Updating and Review 9.1. This procedure will be updated every three years unless practice or legislation changes necessitate the need to review earlier. 10. Equality and Diversity This document complies with the Royal Cornwall Hospitals NHS Trust service Equality and Diversity statement which can be found in the 'Equality, Diversity & Human Rights Policy' or the Equality and Diversity website Equality Impact Assessment The Initial Equality Impact Assessment Screening Form is at Appendix 2. Page 10 of 18
11 Appendix 1. Governance Information Document Title Date Issued/Approved: 11 October 2017 Date Valid From: June 2017 Date Valid To: June 2020 Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults using a Pen Device Directorate / Department responsible (author/owner): Medical Directorate Kim Sleeman, Clinical Nurse Specialist Diabetes Contact details: Brief summary of contents Procedure for subcutaneous injection of Insulin or GLP1 in Adults Analogue using a pen device Suggested Keywords: Target Audience Executive Director responsible for Policy: Diabetes RCHT PCH CFT KCCG Medical Director Date revised: June 2017 This document replaces (exact title of previous version): Approval route (names of committees)/consultation: Divisional Manager confirming approval processes Guideline for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults using a Pen Device Diabetes In-Patient Specialist Nurses, Diabetes Specialist Pharmacist Medical Safety Pharmacist Nurse Consultant Infection Prevention and Control RCHT Sharps Safety Group. Medical Directorate Governance Lead Name and Post Title of additional signatories Name and Signature of Divisional/Directorate Governance Lead confirming approval by specialty and divisional management meetings Signature of Executive Director giving approval Publication Location (refer to Policy on Policies Approvals and Ratification): Not Required {Original Copy Signed} Name: {Original Copy Signed} Internet & Intranet Intranet Only Page 11 of 18
12 Document Library Folder/Sub Folder Links to key external standards Related Documents: Training Need Identified? Clinical/Endocrine and Diabetes/ National Patient Safety Alert Safe Use of Insulin 2011 Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 National Patient Safety Alert - risk of severe harm and death due to withdrawing Insulin from pen devices (Nov 2016) Novo Pen 4 user manual (2008) NovoNordisk Flex Pen user guide (2006) NovoNordisk Lantus SoloStar Information For Patients how to deliver Lantus. Sanofi Aventis (July 2007) Diabetes UK (2009) Lyxumia pre filled pen guide (2013) - Aventis Yes: All registered staff need competency assessment in Subcutaneous injection of Insulin/ GLP1 using a Pre-filled Pen Device. Ward Pharmacist need to be briefed on their roles and responsibilities regarding the implementation, monitoring and compliance with this procedure. Version Control Table Date Version No Summary of Changes Changes Made by (Name and Job Title) June 2013 V1.0 Initial Issue Kim Bull, Clinical Nurse Specialist Diabetes June 2017 V2.0 Document changed from guideline to procedure and other updates. Kim Sleeman, Clinical Nurse Specialist Diabetes All or part of this document can be released under the Freedom of Information Act 2000 This document is to be retained for 10 years from the date of expiry. This document is only valid on the day of printing Controlled Document This document has been created following the Royal Cornwall Hospitals NHS Trust Policy on Document Production. It should not be altered in any way without the express permission of the author or their Line Manager. Page 12 of 18
13 Appendix 2. Initial Equality Impact Assessment Form Name of the strategy / policy /proposal / service function to be assessed: Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue Using a Pen Device in Adults Directorate and service area: Is this a new or existing Policy? Medical Previously a guideline and now a policy Name of individual completing assessment: Telephone: Kim Sleeman 1. Policy Aim* Who is the strategy / policy / proposal / service function aimed at? To provide detailed guidance on the procedure of subcutaneous injection of Insulin or GLP Analogue using a pen device in adults 2. Policy Objectives* To provide a consistent approach to the management of adult patients requiring subcutaneous injection of Insulin or GLP Analogue using a pen Device To maintain patient safety and improve outcomes for adult patients with Diabetes 3. Policy intended Outcomes* 4. *How will you measure the outcome? 5. Who is intended to benefit from the policy? 6a Who did you consult with b). Please identify the groups who have been consulted about this procedure. What was the outcome of the consultation? Consistent management of Diabetes at RCHT sites regarding the procedure for subcutaneous injection of Insulin or GLP Analogue using a pen device in adults Audit Datix reporting Review of medical notes as required All adult patients with Diabetes who require subcutaneous injection of Insulin or GLP Analogue using a pen device Workforce Patients Local External groups organisations x x x x Other Diabetes In-Patient Specialist Nurses Consultant Nurse Infection Control Diabetes Pharmacist Medical Safety Pharmacist RCHT Sharps Safety group Comments taken into consideration and procedure amended as needed Page 13 of 18
14 7. The Impact Please complete the following table. If you are unsure/don t know if there is a negative impact you need to repeat the consultation step. Are there concerns that the policy could have differential impact on: Equality Strands: Yes No Unsure Rationale for Assessment / Existing Evidence Age x Sex (male, female, trans-gender / gender reassignment) Race / Ethnic communities /groups Disability - Learning disability, physical impairment, sensory impairment, mental health conditions and some long term health conditions. Religion / other beliefs x x x x For capacity to consent, refer to Consent Form Appendix 4 Marriage and x Civil partnership Pregnancy and x maternity Sexual x Orientation, Bisexual, Gay, heterosexual, Lesbian You will need to continue to a full Equality Impact Assessment if the following have been highlighted: You have ticked Yes in any column above and No consultation or evidence of there being consultation- this excludes any policies which have been identified as not requiring consultation. or Major this relates to service redesign or development 8. Please indicate if a full equality analysis is recommended. Yes No x 9. If you are not recommending a Full Impact assessment please explain why. Revision of existing guidance updated from a guideline to a procedure. Signature of policy developer / lead manager / director Kim Sleeman Names and signatures of members carrying out the Screening Assessment Human Rights, Equality & Inclusion Lead Date of completion and submission 10/10/17 Page 14 of 18
15 Keep one copy and send a copy to the Human Rights, Equality and Inclusion Lead C/o Royal Cornwall Hospitals NHS Trust, Human Resources Department, Knowledge Spa, Truro, Cornwall, TR1 3HD This EIA will not be uploaded to the Trust website without the signature of the Human Rights, Equality & Inclusion Lead. A summary of the results will be published on the Trust s web site. Signed Date Page 15 of 18
16 Appendix 3 Assessment for Self-Administration of Subcutaneous Insulin/GLP1 for Adults with Diabetes Does the patient have a confirmed diagnosis of diabetes? Yes No Does the patient usually selfadminister their Insulin? Yes No Has a medical decision been made that the patient is NOT suitable for self-administration of insulin No The patient is not appropriate for self-assessment Complete the relevant box on the Assessment form (CHA2976) Yes (To be used in conjunction with RCHT PROCEDURE FOR SUBCUTANEOUS INJECTION OF INSULIN OR GLP ANALOGUE USING A PEN DEVICE) 1. Check that the patient knows the correct name of their Insulin/GLP1, correct dose and correct administration time (e.g. Novomix 30, 18 units, breakfast and evening meal. A response such as blue pen, 9 clicks twice a day is not an acceptable answer) 2. Observe the patient dialling the correct dose (using their usual pen device) 3. Observe the patient administering their own injection (using their usual pen device) 4. If the patient has successfully completed ALL1-3 steps the patient is considered safe to self-administer their own Insulin/GLP1 using a pen device 5. If the patient has not successfully completed any one of the 1 3 steps the patient is NOT considered safe to self-administer their own Insulin/GLP1 6. Complete the Registered Nurse Assessment for Self Administration of Subcutaneous Insulin/GLP1 form (CHA2976) and file in the current section of the patients medical notes. Appendix 4 Page 16 of 18
17 Appendix 4. Competency Assessment Document - Subcutaneous Injection of Insulin/GLP1 in Adults Using a Pre-filled Pen Device Staff Name Ward/Area (Cc to Personal File) Procedure Wash and dry hands Review patient s blood glucose Check the patient s EPMA prescription chart to ascertain the following are correct: Patient name Insulin/GLP dose (written in units for Insulin or mcg/mgs for GLP dependant on which GLP is prescribed) Rationale Compliance with infection control strategies To ensure that blood glucose is within acceptable ranges and that the patient is not Hypoglycaemic To ensure the patient is given the correct drug in the prescribed dose, by the correct route and at the correct time Date of Training & Signature of Trainer Date of Assessment Assessor Signature Date/time of administration Route and method Doctor s signature Gathers equipment: Pre-filled pen device One litre Sharps bin (that contains the designated pen needle remover), non-sterile gloves. To ensure all equipment is in-date and ready to use Checks Insulin is in date Checks that pre-filled pen device has been stored at room temperature and not for more than 28 days Checks that any Insulin contains no crystals Explains and discusses procedure with the patient and obtains verbal consent To ensure the patient can give valid consent Page 17 of 18
18 Procedure Twists and pulls the cap off the pre-filled pen device Checks that the prefilled pen device contents are intact and has no crystals Takes the protective paper tab off the pen needle. Screws the needle onto the screw cap end of the pre-filled pen device (new needle at the start of every injection) For cloudy Insulin Rock and Roll the pre-filled pen device in the palm of their hand 10 times Removes the outer and inner needle caps, and holds pen between thumb and forefinger of dominant hand as if holding a dart Dials up two units (air shot/pen needle prime) and discards, ensuring that the prefilled pen device dose selector has returned to zero Selects injection site e.g. lower abdomen, upper outer thigh or upper outer arm (this should be rotated daily) Inserts the pen needle at an angle of 90 degrees into the chosen injection site. Injects by pressing down on the prefilled pen device plunger until the dose selector returns to zero. Once administered count to ten before the pen needle is withdrawn from patients skin Disposes of all sharps and clinical waste safely, in accordance with local guidelines. Do not replace the pen needle cap Pre-filled pen device cap to be replaced onto the prefilled pen device. The prefilled pen device that is in use can be stored at room temperature for up-to 28 days Records the administration correctly Rationale To prepare pre-filled pen device To ensure safety prior to administration To prepare for injecting To ensure that the Insulin is correctly mixed To enable a quick, smooth, sub cutaneous injection To ensure priming of the prefilled pen device pen needle. Insulin/GLP should be administered subcutaneously. Prevents Lypohypetrophy development Correct administration of the Insulin/GLP To prevent needlestick injury and blood borne virus contamination Correct storage of Insulin/GLP To maintain accurate records Date of training & Signature of Trainer Date of Assessment Assessor Signature Keep one copy and send a copy to the Human Rights, Equality and Inclusion Lead, C/o Royal Cornwall Hospitals NHS Trust, Human Resources Department, Knowledge Spa, Truro, Cornwall, TR1 3HD A summary of the results will be published on the Trust s web site. Signed Date Page 18 of 18
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0. March 2018
 Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1.
 CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1. Patient requires nebulised Pentamidine Ensure equipment listed is available Ensure HEPA filtered room in Haematology Clinic
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF NEBULISED PENTAMIDINE Summary. 1. Patient requires nebulised Pentamidine Ensure equipment listed is available Ensure HEPA filtered room in Haematology Clinic
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol
 Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline
 SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
School Hearing Screening Policy
 School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary. Page 1 of 12 Recurrent LGD at any point- discuss at MDT And consider RFA Patients with LGD should have a repeat endoscopy in 6 months.
CLINICAL GUIDELINE FOR THE MANAGEMENT OF BARRETT S OESOPHAGUS Summary. Page 1 of 12 Recurrent LGD at any point- discuss at MDT And consider RFA Patients with LGD should have a repeat endoscopy in 6 months.
DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0
 DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0 1. Aim/Purpose of this Guideline This guideline is relevant to all medical and nursing staff caring for children with Pyloric
DIAGNOSIS AND MANAGEMENT OF PYLORIC STENOSIS IN CHILDREN CLINICAL GUIDELINE V3.0 1. Aim/Purpose of this Guideline This guideline is relevant to all medical and nursing staff caring for children with Pyloric
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN
 SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary.
 CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary. Yes Is patient prescribed ifosfamide or cyclophosphamide >1g/m 2? Chemotherapy prescription on Aria should
CLINICAL GUIDELINE FOR THE ADMINISTRATION OF MESNA WITH IFOSFAMIDE AND CYCLOPHOSPHAMIDE Summary. Yes Is patient prescribed ifosfamide or cyclophosphamide >1g/m 2? Chemotherapy prescription on Aria should
METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0
 METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline To provide guidance on the prevention of metabolic bone disease in the neonate. All
METABOLIC BONE DISEASE OF PREMATURITY NEONATAL CLINICAL GUIDELINE V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline To provide guidance on the prevention of metabolic bone disease in the neonate. All
1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of acamprosate.
 SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients)
 CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients) 1. Aim/Purpose of this Guideline 1.1. Systemic cancer treatments and immunological
CLINICAL GUIDELINE FOR MANAGEMENT OF NEUTROPENIC SEPSIS IN ADULT CANCER PATIENTS (this guideline excludes haematology patients) 1. Aim/Purpose of this Guideline 1.1. Systemic cancer treatments and immunological
MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES
 MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES 1. Aim/Purpose of this Guideline All clinical staff working in the Division of women,
MANAGEMENT OF THE BLADDER IN THE POSTOPERATIVE PERIOD FOLLOWING UNCOMPLICATED GYNAECOLOGICAL SURGERY CLINICAL GUIDELINES 1. Aim/Purpose of this Guideline All clinical staff working in the Division of women,
Start. What is the serum phosphate concentration? Moderate Hypophosphataemia mmol/l. Replace using oral. phosphate. (See section 3.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0
 PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0 Page 1 of 8 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff caring for a child with Preseptal
PRESEPTAL AND ORBITAL CELLULITIS IN CHILDREN- CLINICAL GUIDELINE V3.0 Page 1 of 8 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical and nursing staff caring for a child with Preseptal
Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation Clinical Guideline V5.0
 Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation November 2018 Summary. Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia
Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia or Intravenous Sedation November 2018 Summary. Fasting for Adults (including Young Adults Age 16+ years) who require Anaesthesia
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOKALAEMIA
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
HYPOSPADIAS NEONATAL CLINICAL GUIDELINE. 1. Aim/Purpose of this Guideline. 2. The Guidance
 HYPOSPADIAS NEONATAL CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all staff managing the initial care of infants born with hypospadias. It includes assessment and
HYPOSPADIAS NEONATAL CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all staff managing the initial care of infants born with hypospadias. It includes assessment and
Policy for the safe administration of Insulin
 Policy for the safe administration of Insulin This is a working document and any changes that become necessary to this policy must be Notified in writing to the Medicine Management Group via the Chief
Policy for the safe administration of Insulin This is a working document and any changes that become necessary to this policy must be Notified in writing to the Medicine Management Group via the Chief
ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME
 STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018
 Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
Hypoglycaemia in Adults with Diabetes Clinical Guideline V5.0. March 2018
 March 2018 Page 1 of 11 Adult with Diabetes and blood glucose < 4 mmol/l Treatment as per Guideline: Nursing / Clinical Staff Patient reviewed as per guideline guidance notes: Nursing / Clinical Staff
March 2018 Page 1 of 11 Adult with Diabetes and blood glucose < 4 mmol/l Treatment as per Guideline: Nursing / Clinical Staff Patient reviewed as per guideline guidance notes: Nursing / Clinical Staff
Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0
 Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0 November 2018 Summary Prescribing, monitoring and administration of Captopril and Enalapril FOR STAFF PATIENTS Medical and Nursing
Captopril and Enalapril (Ace Inhibitor) Therapy Clinical Guideline V1.0 November 2018 Summary Prescribing, monitoring and administration of Captopril and Enalapril FOR STAFF PATIENTS Medical and Nursing
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS
 CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of gallstones pathology in adults. It has been benchmarked against
CLINICAL GUIDELINE FOR MANAGEMENT OF GALLSTONES PATHOLOGY IN ADULTS 1. Aim/Purpose of this Guideline This guideline is for the management of gallstones pathology in adults. It has been benchmarked against
GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE
 GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE V 1.5 2017 Screening - Clinical Guideline Page 1 of
GESTATIONAL DIABETES MELLITUS AND SUBSEQUENT MANAGEMENT OF CONFIRMED GESTATIONAL DIABETES MELLITUS (GDM) AND SELECTIVE SCREENING - CLINICAL GUIDELINE V 1.5 2017 Screening - Clinical Guideline Page 1 of
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.1 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. The
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HIGH BLOOD GLUCOSE LEVELS AND SICK DAYS FOR INSULIN PUMP USERS UNDER THE PAEDIATRIC DIABETES SERVICE. V4.1 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. The
Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall
 Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall Page 1 of 15 Summary Patient clinically assessed and reviewed by a Consultant Cardiologist, Cardiology
Clinical guideline for the introduction of Sacubitril Valsartan in primary and secondary care in Cornwall Page 1 of 15 Summary Patient clinically assessed and reviewed by a Consultant Cardiologist, Cardiology
SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline. 2. The Guidance
 SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of modafinil.
SHARED CARE GUIDELINE FOR MODAFINIL 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of modafinil.
CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAXIS IN INFANTS AND CHILDREN UNDER SIXTEEN YEARS OF AGE V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAIS IN INFANTS AND CHILDREN UNDER SITEEN YEARS OF AGE V3.0 Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide
CLINICAL GUIDELINE FOR THE MANAGEMENT OF ANAPHYLAIS IN INFANTS AND CHILDREN UNDER SITEEN YEARS OF AGE V3.0 Page 1 of 16 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide
SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline
 SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy
SHARED CARE GUIDELINE FOR RIFAXIMIN FOR PREVENTING EPISODES OF OVERT HEPATIC ENCEPHALOPATHY IN ADULT PATIENTS 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy
MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE
 MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the assessment and management of infants with hypotension. All involved will benefit from
MANAGEMENT OF NEONATAL HYPOTENSION CLINICAL GUIDELINE 1. Aim/Purpose of this Guideline 1.1. To provide guidance on the assessment and management of infants with hypotension. All involved will benefit from
HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
ADMINISTRATION OF INSULIN
 STANDARD OPERATING PROCEDURE ADMINISTRATION OF INSULIN Issue History First issued April 2012 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote the safe administration
STANDARD OPERATING PROCEDURE ADMINISTRATION OF INSULIN Issue History First issued April 2012 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote the safe administration
Introduction. Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients)
 Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0
 CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. Tuberculosis is a notifiable disease in the UK and although incidence is low it remains a Public
CLINICAL GUIDELINE FOR NEONATAL BCG VACCINATION V3.0 Page 1 of 11 1. Aim/Purpose of this Guideline 1.1. Tuberculosis is a notifiable disease in the UK and although incidence is low it remains a Public
Suspected Pulmonary embolus Ambulatory Pathway. Document Title. Date Issued/Approved: Date Valid From: 11/11/17. Date Valid To: 11/05/18
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide)
 PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
Lilly. Disposable Insulin Delivery Device User Manual
 1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
PLEASE READ THIS USER MANUAL BEFORE USE
 USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
CLINICAL PROCEDURE FOR THE SAFE REMOVAL OF FEMORAL ARTERIAL SHEATHS USING A DIGITAL APPROACH 1. Aim/Purpose of this Guideline
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
Acknowledgement goes to WHO for use of their hand washing technique
 How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
Pre-loading of insulin syringes for adult patients to administer at home
 Title: Directorate: Responsible for review: Ratified by: Diabetes - Pre-loading of insulin syringes for adult patients to administer at home Operations Samantha Rosindale, Diabetes Lead Champion Lynda
Title: Directorate: Responsible for review: Ratified by: Diabetes - Pre-loading of insulin syringes for adult patients to administer at home Operations Samantha Rosindale, Diabetes Lead Champion Lynda
Cap Clip Rubber Seal Plunger Pen Body Dose Window
 Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Pre-filled Patient Controlled Analgesia (PCA) syringes
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide guidance on caring for children who are receiving epidural
CLINICAL GUIDELINE FOR AN EPIDURAL INFUSION IN CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. The purpose of this guideline is to provide guidance on caring for children who are receiving epidural
PROCEDURE FOR BLOOD GLUCOSE MONITORING
 PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit
 Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit Document Summary The purpose of this policy is to guide and promote
Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit Document Summary The purpose of this policy is to guide and promote
SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector
 SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018
 Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018 1. Aim/Purpose of this Guideline This guideline applies to all medical and nursing staff
Prevention and Treatment of Mucositis in Children and Young People with Cancer Clinical Guideline V3.1 December 2018 1. Aim/Purpose of this Guideline This guideline applies to all medical and nursing staff
Instructions for Use. HUMALOG KwikPen. insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen
 1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY
 This applies to adult patients only CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY Key: General Notes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Yes Patient already taking phenytoin?
This applies to adult patients only CLINICAL GUIDELINE FOR THE USE OF PHENYTOIN IN EPILEPSY Key: General Notes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Yes Patient already taking phenytoin?
Instructions for Use. BASAGLAR KwikPen. insulin glargine injection (100 units/ml, 3 ml pen)
 Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
HUMULIN 70/30 KwikPen
 1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
What do you need to know before you go home?
 What do you need to know before you go home? What is Insulin Types of Insulin Injection Sites How to Inject Insulin Correctly Low Blood Sugar and Treatment Sick Day Management After leaving the Hospital:
What do you need to know before you go home? What is Insulin Types of Insulin Injection Sites How to Inject Insulin Correctly Low Blood Sugar and Treatment Sick Day Management After leaving the Hospital:
Injectable Therapies in Diabetes
 Injectable Therapies in Diabetes Diabetes Specialist Nurse Linda Burns Learning Outcomes Understand the place of injectible therapies in diabetes Understand when patients may require insulin therapy Consider
Injectable Therapies in Diabetes Diabetes Specialist Nurse Linda Burns Learning Outcomes Understand the place of injectible therapies in diabetes Understand when patients may require insulin therapy Consider
Pre-Loaded Insulin Syringe Policy
 Pre-Loaded Insulin Syringe Policy Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet. Please visit the intranet for the latest version. Purpose of Agreement
Pre-Loaded Insulin Syringe Policy Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet. Please visit the intranet for the latest version. Purpose of Agreement
Autopen 24 is an easy to use insulin injection pen, designed for use with 3.0ml sanofi aventis insulin cartridges. Compatible with all pen needles*.
 Instructions for use English Autopen 24 is an easy to use insulin injection pen, designed for use with 3.0ml sanofi aventis insulin cartridges. Compatible with all pen needles*. PLEASE FOLLOW THESE STEP-BY-STEP
Instructions for use English Autopen 24 is an easy to use insulin injection pen, designed for use with 3.0ml sanofi aventis insulin cartridges. Compatible with all pen needles*. PLEASE FOLLOW THESE STEP-BY-STEP
Things you need to know about injections
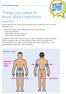 www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
Procedure for the prescribing and administration of insulin
 Procedure for the prescribing and administration of insulin Author: Lilian Baxendale and Heather Beadle Designation: Clinical Governance Pharmacists Version: Version 3 Date: December 2016 Date Approved:
Procedure for the prescribing and administration of insulin Author: Lilian Baxendale and Heather Beadle Designation: Clinical Governance Pharmacists Version: Version 3 Date: December 2016 Date Approved:
DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4
 DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians and the Joint Obstetric/Diabetes
DIABETES IN PREGNANCY, TYPE 1 AND TYPE 2 DIABETES MELLITUS (DM), CLINICAL GUIDELINE FOR MIDWIVES V1.4 1 1. Aim/Purpose of this Guideline To provide guidance to Midwives, Obstetricians and the Joint Obstetric/Diabetes
DO NOT TRANSFER TO A SYRINGE SEVERE OVERDOSE CAN RESULT
 HP8824 Instructions for Use ENTUZITY KwikPen (insulin injection, human biosynthetic) 500 units/ml, 3 ml pen www.lilly.ca IMPORTANT Know your dose of ENTUZITY insulin. The Pen delivers your dose in insulin
HP8824 Instructions for Use ENTUZITY KwikPen (insulin injection, human biosynthetic) 500 units/ml, 3 ml pen www.lilly.ca IMPORTANT Know your dose of ENTUZITY insulin. The Pen delivers your dose in insulin
Giving Yourself an Insulin Injection With the Lantus SoloStar Pen
 PATIENT & CAREGIVER EDUCATION Giving Yourself an Insulin Injection With the Lantus SoloStar Pen This information describes how to prepare and give yourself an insulin injection (shot) with the Lantus SoloStar
PATIENT & CAREGIVER EDUCATION Giving Yourself an Insulin Injection With the Lantus SoloStar Pen This information describes how to prepare and give yourself an insulin injection (shot) with the Lantus SoloStar
How to use your Pergoveris pre-filled pen
 How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
INSULIN INJECTION KNOW-HOW
 0-1- INSULIN INJECTION KNOW-HOW Learning how to Congratulations for making the move to insulin therapy. It won t be long before you start enjoying better blood sugar control, more energy, and a host of
0-1- INSULIN INJECTION KNOW-HOW Learning how to Congratulations for making the move to insulin therapy. It won t be long before you start enjoying better blood sugar control, more energy, and a host of
KEEPING SAFE WITH INSULIN THERAPY
 KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE. Chief Pharmacist. Chief Pharmacist
 REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
PLEASE READ THESE INSTRUCTIONS BEFORE USE
 Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0
 MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all medical and nursing
MUCOSITIS IN CHILDREN AND YOUNG PEOPLE WITH CANCER- CLINICAL GUIDELINE FOR PREVENTION AND TREATMENT V3.0 Page 1 of 10 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all medical and nursing
PATIENT INFORMATION. D iabetes. What You Need to Know About. Insulin
 PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients
 TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
Education for self administration of intravenous therapy HOME IV THERAPY. 30 minute - Baxter Pump Tobramycin
 HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
Protocol (Clinical) Country Health SA Local Health Network. Title: Subcutaneous Insulin Administration in Hospital and Aged Care
 Protocol (Clinical) Title: Subcutaneous Insulin Administration in Hospital and Aged Care Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services
Protocol (Clinical) Title: Subcutaneous Insulin Administration in Hospital and Aged Care Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services
Acutely Painful testes
 2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
INSTRUCTIONS FOR USE. Norditropin NordiFlex Somatropin (rdna origin) injection 30 mg/3 ml Prefilled Pen
 INSTRUCTIONS FOR USE Norditropin NordiFlex Somatropin (rdna origin) injection 30 mg/3 ml Prefilled Pen Using the disposable Norditropin NordiFlex 30 mg/3 ml Prefilled Pen Norditropin NordiFlex 30 mg/3
INSTRUCTIONS FOR USE Norditropin NordiFlex Somatropin (rdna origin) injection 30 mg/3 ml Prefilled Pen Using the disposable Norditropin NordiFlex 30 mg/3 ml Prefilled Pen Norditropin NordiFlex 30 mg/3
GET TO KNOW YOUR PEN. Needle cap. Rubber seal. Insulin scale. Pen cap. Cartridge holder Plunger. Insulin name. Dose window Dose. pointer.
 GET TO KNOW YOUR PEN You ve taken an important step by adding Toujeo (insulin glargine injection) U-3 to your diabetes treatment plan. Toujeo is a long-acting insulin used to treat adults with type and
GET TO KNOW YOUR PEN You ve taken an important step by adding Toujeo (insulin glargine injection) U-3 to your diabetes treatment plan. Toujeo is a long-acting insulin used to treat adults with type and
Osteoblasts (cells which form new bone) Osteoclasts (cells which break down old bone)
 Clinical guideline for the administration of Bisphosphonates and other drugs affecting bone metabolism in Haematology and Oncology patients 1. Aim/Purpose of this Guideline 1.1. To provide education to
Clinical guideline for the administration of Bisphosphonates and other drugs affecting bone metabolism in Haematology and Oncology patients 1. Aim/Purpose of this Guideline 1.1. To provide education to
Vial. A healthcare provider should show you how to inject MYALEPT before you use it for
 MYALEPT (metreleptin) for injection Instructions for Use MYALEPT (MAI-uh-lept) (metreleptin) for injection Vial A healthcare provider should show you how to inject MYALEPT before you use it for 8 Do not
MYALEPT (metreleptin) for injection Instructions for Use MYALEPT (MAI-uh-lept) (metreleptin) for injection Vial A healthcare provider should show you how to inject MYALEPT before you use it for 8 Do not
Spirometry Clinical Guideline V2.0. May 2018
 Spirometry Clinical Guideline V2.0 May 2018 Spirometry Clinical Guideline V2.0 1 Summary REFERRAL FOR SPIROMETRY QUALITY ASSURED SPIROMETRY PERFORMED BY TECHNICIAN TRAINED AND ASSESSED AS COMPETENT SPIROMETRY
Spirometry Clinical Guideline V2.0 May 2018 Spirometry Clinical Guideline V2.0 1 Summary REFERRAL FOR SPIROMETRY QUALITY ASSURED SPIROMETRY PERFORMED BY TECHNICIAN TRAINED AND ASSESSED AS COMPETENT SPIROMETRY
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial
 Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
insulin and injections
 insulin and injections Insulin is a hormone made by the beta cells in the pancreas. Insulin allows sugar to go from the bloodstream into the body s cells and be used for energy. Insulin lowers blood sugar.
insulin and injections Insulin is a hormone made by the beta cells in the pancreas. Insulin allows sugar to go from the bloodstream into the body s cells and be used for energy. Insulin lowers blood sugar.
SHARED CARE GUIDELINE FOR TREATMENT OF DEMENTIA 1. Aim/Purpose of this Guideline
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
Trust Policy 218 Ionising Radiation Safety Policy
 Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
MYALEPT (MAI-uh-lept) (metreleptin) for injection for subcutaneous use
 .3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
.3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0 Clinical Guideline Template Page 1 of 14 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
CLINICAL GUIDELINE FOR THE MANAGEMENT OF CONVULSIVE STATUS EPILEPTICUS IN CHILDHOOD V3.0 Clinical Guideline Template Page 1 of 14 1. Aim/Purpose of this Guideline 1.1. This guideline applies to all nursing
The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline V5.0 December 2018
 The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline December 2018 Summary GP suspects diabetes Under 16 years or still in School Year 11 NO Refer Adults YES Section
The Management of Children and Young People with Newly Presenting Diabetes Clinical Guideline December 2018 Summary GP suspects diabetes Under 16 years or still in School Year 11 NO Refer Adults YES Section
A Fact Sheet for Parents and Carers Insulin and Diabetes
 A Fact Sheet for Parents and Carers Insulin and Diabetes In type 1 diabetes the body stops producing insulin. Insulin therapy is essential in the treatment of type 1 diabetes, together with a healthy eating
A Fact Sheet for Parents and Carers Insulin and Diabetes In type 1 diabetes the body stops producing insulin. Insulin therapy is essential in the treatment of type 1 diabetes, together with a healthy eating
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary. Start. End. Key: Na + below normal range ( mmol/L) Symptomatic?
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary Key: General tes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Na + below normal range (135 145mmol/L) Refer to endocrinology
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPONATRAEMIA Summary Key: General tes ED/MAU/SRU/Acute GP/Amb-Care GP/SWASFT In-patient wards Start Na + below normal range (135 145mmol/L) Refer to endocrinology
Important Safety Information for Adlyxin (lixisenatide) injection
 A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
DONGBAO PEN (ELECTRONIC) INSTRUCTION MANUAL
 DONGBAO PEN (ELECTRONIC) INSTRUCTION MANUAL Foreword The new Dongbao Pen, which is made by Disetronic Medical Systems AG, is an injection aid incorporating the latest advances in technology. It is easy
DONGBAO PEN (ELECTRONIC) INSTRUCTION MANUAL Foreword The new Dongbao Pen, which is made by Disetronic Medical Systems AG, is an injection aid incorporating the latest advances in technology. It is easy
NovoPen Echo User guide
 NovoPen Echo User guide Read this user guide carefully before you use your NovoPen Echo for insulin delivery the first time Find a quick guide at the back of this manual NovoPen Echo Pen Insulin window
NovoPen Echo User guide Read this user guide carefully before you use your NovoPen Echo for insulin delivery the first time Find a quick guide at the back of this manual NovoPen Echo Pen Insulin window
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use
 Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
