Pre-loading of insulin syringes for adult patients to administer at home
|
|
|
- Scott Anthony
- 5 years ago
- Views:
Transcription
1 Title: Directorate: Responsible for review: Ratified by: Diabetes - Pre-loading of insulin syringes for adult patients to administer at home Operations Samantha Rosindale, Diabetes Lead Champion Lynda Price, Interim Head of Medicines Optimisation Care and Clinical Policies Sub Group 1917 Ref No: Version 2.0 Due for Review: June 2017 Applicability: All Registered Nurses and Prescribers Table of Contents 1. Purpose Introduction Roles and Responsibilities Principles of Practice for the Pre-Loading of Insulin in Syringes Contraindications Training Monitoring and Auditing References Equality and Diversity Further Information... 7 Appendix A: Process of syringes being pre-loaded for the patient on the first occasion... 8 Appendix B. Standard Operating Procedure (SOP) for the assessment of a patient to have insulin prepared in advance of administration Document Control Information Mental Capacity Act Quality Impact Assessment (QIA) Purpose There are potential legal and safety complexities associated with the practice of pre-loading insulin syringes in order to enable people to self-administer in their own homes that health care practitioners MUST be aware of. Firstly, the preparation of pre-loaded syringes represents a form of secondary dispensing which, under the terms of the Medicines Act (1968), is classified as an unlicensed activity in all four UK countries. Consequently, health care organisations and practitioners must take full responsibility for the safety of this activity. Version 2.0 April, 2015 Page 1 of 24
2 Secondly, the safety of individuals using pre-loaded insulin syringes, as well as the storage, stability and sterility of insulin once drawn up in the syringe, is of paramount importance (Rosindale, 2014). For this reason Torbay & Southern Devon Health and care NHS Trust and the RCN (2015) recommends nurses should operate within the framework of this pre-loaded insulin syringe clinical policy which details the responsibilities of every individual involved in the practice (employing organisation/patient/gp/community nurse) and standardises how nurses perform the preparation of syringes (essential to assure vicarious liability protection for nurses undertaking this practice). Practitioners are also directed to review the recently updated NMC Standards for medicines management (NMC, 2014) The purpose of this policy is to: 1.1 To promote patient safety. 1.2 To ensure that Registered Nurses (RN s) are aware of the potential risks of pre-loading insulin syringes for later use by a patient. 1.3 To provide a clear and consistent framework across Torbay & Southern Devon Health and Care NHS Trust for the appropriate assessment and management of a person with diabetes who cannot safely prepare their own insulin dose. 1.4 The Nursing & Midwifery Council (NMC) standards for medicines management 14 (2010) state that registrants must not prepare substances for injection in advance of their immediate use. The Department of Health (DoH)/Medicines & Healthcare products Regulatory Agency (MHRA) advise against pre-loading medication for injection at a later time (Rosindale 2014). 1.5 To support RN s to provide insulin therapy as detailed in this policy which is classified as secondary dispensing (and thus not covered in the Medicines Act 1968), however takes note of Royal College of Nursing guidance (2015) Advance preparation of insulin syringes for adult patients to administer at home. 1.6 The National Patient Safety Agency (NPSA) are unaware of any reports where insulin syringes prepared in advance by nurses in the community and given expiry dates of greater than 24 hours have caused serious harm due to infection and contamination issues (Rosindale 2014). However a Rapid Response Report has been issued for the safer prescribing and administration of insulin (2010). 2 Introduction 2.1 This policy is supported by a risk assessment and Standard Operating Procedure (SOP) for the pre-loading of insulin for patients to self-administer later. It stresses the necessary principles of practice including patient assessment and review, ensures other methods of insulin administration have been considered, together with contra-indications, and details appropriate insulin storage requirements and record keeping. 2.2 Diabetes mellitus is a chronic condition. Patients require long-term medication to control blood glucose levels and reduce the risk of associated complications. For some patients the prescribed treatment is regular insulin injections. 2.3 There are a number of patients with diabetes who cannot convert to using an insulin pen for independent self-administration of insulin because of manual dexterity, lack of strength, Version 2.0 April, 2015 Page 2 of 24
3 personal preference or reluctance to change. As a result many patients are unable to draw up their own insulin and need community nurse support, although they are able to inject independently using a syringe once or twice a day. The preparation of insulin injections by community nurses for patients to administer in their own homes at a later time has been the practice for many years. In this way each patient can administer their insulin at the correct time in relation to their meals. This preserves the individual s independence (Rosindale 2014). 2.4 The pre-loading of insulin into syringes is an unlicensed activity that falls outside of the Medicines Act (Rosindale 2014) and adherence to this policy protects the patient, and provides legal protection for the Registered Nurse and this organisation under vicarious liability. The Royal College of Nursing (RCN 2015) advises that this activity must be seen as the FINAL option, and only considered when all other options have been exhausted. 2.5 Within the context of this policy the definition of pre-loading an insulin syringe refers to insulin that has been withdrawn from a 10ml vial, using an insulin syringe that is marked in one or two unit graduations. It is recommended that 8 mm needles be used when using an insulin syringe. 2.6 No other type of syringe should ever be used for insulin administration. 3 Roles and Responsibilities 3.1 This policy covers all RN s employed by Torbay and Southern Devon Health & Care NHS Trust who are required to treat patients with diabetes mellitus within their own home. 3.2 It relates specifically to the patient who is able to safely administer the correct dose of insulin at the correct time, but is unable to draw up insulin or utilise standard commercially available insulin preparations. 3.3 It is the responsibility of every Trust employed RN who is required to treat patients with diabetes mellitus to be familiar with this policy and procedure. 3.4 RN to positively identify the patient (obtaining confirmation of name and DOB) and establish allergy status. 3.5 All RNs involved in the administration of insulin, as in all other areas of their practice, will be responsible for maintaining and updating their knowledge and practice. The e-learning module on the Safe use of Insulin is a mandatory requirement every 2 years with a pass mark of 80%. 3.6 RNs are responsible for the initial and continued assessment of patients who are selfadministering and have continued responsibility for recognising and acting upon changes in a patient s condition with regards to safety of the patient and others. The Nursing & Midwifery Council (NMC) & Mental Capacity Act 2005 state that for a patient to be able to self-administer the patient should be assessed as being at Level 3 which is defined as the patient accepts full responsibility for the storage and administration of the medicinal products. The level should be documented in the patient s records (Standard 9 NMC Standards for medicines management 2010). 3.7 Patients must be allowed to decide whether they will agree to treatment in this way and this should be documented in the patient case notes. 3.8 RN s in administering any medicines, in assisting with administration or over-seeing any selfadministration must assess the patient s suitability and understanding of how to use an appropriate compliance aid safely (Standard 16 NMC 2010). Version 2.0 April, 2015 Page 3 of 24
4 3.9 RN s are accountable to ensure that the patient is competent to carry out the task (Standard 17 NMC 2010) RN s are responsible for implementing this policy. Pre-loading insulin syringes must not be delegated to non-registered staff Pre-loaded syringes may not be prepared by one registered nurse for another health professional or skilled not registered to administer Under no circumstances may RN s mix and pre-load different insulins in the same syringe for administration at a later time (There is no longer a need to mix insulins due to the availability of suitable manufacturer s preparations) Registered nurses are responsible for recognising any limitations in their knowledge and competence and declining any duties they do not feel able to perform in a skilled and safe manner (NMC 2015). 4 Principles of practice for the pre-loading of insulin in syringes 4.1 Pre-loading of insulin should only be recommended when alternative methods of delivery are not possible and after appropriate risk assessment as outlined in Appendices A, B & C. 4.2 RN s should be aware of the alternative injection devices available and discuss the patient s needs and preferred options with the Senior Diabetes Specialist Nurse (SDSN) and General Practitioner. 4.3 Pre-loading of insulin for injection must only begin following a full written risk assessment, involving the SDSN, and the ruling out of alternative methods of administration. A thorough assessment of the patients understanding of the insulin regime, their ability to manage it and the support available between community nurse visits, must be undertaken using: Appendix B: Standard Operating Procedure (SOP) for the assessment of a patient to have Insulin prepared in advance of administration. Followed by completion of Appendix C: Risk Assessment Form Advanced Preparation of Insulin into syringes for a patient to administer at a later date. 4.4 The risk assessment form in Appendix C should be undertaken every 3 months, or sooner if the patient s condition changes. 4.5 The patient should always be consulted about their insulin administration and informed consent obtained regarding the care provided. 4.6 On completion of the risk assessment the RN should decide on the appropriate number of days that the insulin syringes that can be prepared for and left with the patient. It is important that the RN considers all aspects of social and healthcare for their patient in this decision. This number and reason for the decision should be recorded in Appendix C. The maximum number of days that insulin syringes can be left pre-loaded is seven (RCN 2015, Rosindale 2014). Advice may be sought from the SDSN. Each time a nurse pre-loads syringes Appendix D Documentation Form for the advanced preparation of insulin in syringes should be completed. 4.7 Pre-loaded insulin syringes must be labelled individually and stored in a wipe-able, labelled, sealable, hinge-lidded container (see Appendix B). If the patient is having a different type of insulin or dose at another time of the day a different storage container should be used. To prevent any confusion the containers should be either different colours or shapes. Version 2.0 April, 2015 Page 4 of 24
5 4.8 The patient s fridge should be visibly clean and free of debris. The insulin should be stored in the fridge door or top shelf to prevent cross-contamination from other food items. 4.9 It is recommended that insulin is most stable when stored at a temperature of 2 and 8 Celsius. Never allow insulin to freeze Unopened insulin can be kept in these conditions until the expiry date. An opened vial of insulin kept in these conditions should be discarded after 28 days. The date that the vial was opened must be written on the vial and in Appendix D Patients in residential care must have their pre-loaded insulin syringes stored as outlined in but in a locked fridge Arrangements must be made to ensure that the monitoring of diabetes control is undertaken. Capillary blood glucose monitoring may be undertaken by the patient themselves, a family member/friend using their own glucometer. A full written assessment should be undertaken to check patient or family member confident and competent to undertake this procedure. The meter should also be checked weekly with the relevant quality control solution that is provided by the relevant meter company to ensure that it is accurate. Accuracy can also be checked by comparing a capillary blood glucose result with a venous glucose sample on a weekly basis. An Hba1c every 3 months is also required to evaluate the level of diabetes control Liaise with SDSN as required for advice if circumstances change The SDSN will quality assure that the risk assessment & SOP are being completed as outlined in this policy in Appendices B,C and D using Appendix E every six months to ensure that this type of care remains appropriate for the individual An overview of the processes involved in points is outlined in Appendix A. 5 Contraindications 5.1 Insulin Lantus (Glargine) must not be pre-loaded into insulin syringes. 5.2 Very variable capillary blood sugar recordings. 5.3 Lack of satisfactory storage conditions in the patient s home. 5.4 Unpredictable mental state or declining cognitive ability. 5.5 Pre-filled insulin cartridges and commercially available pre-loaded pens must not be used to withdraw insulin in order to comply with this policy. Only 10ml vials are permissible to be used. 5.7 If any of the points are found, then this is to be reported to the GP or Devon Doctors (when out of hours) and the advanced preparation of insulin in syringes should cease and arrangements made for the community nursing team to visit at the required intervals. A clinical incident form should be completed. 6 Training 6.1 All staff involved with the care of these patients will receive a training session delivered through the SDSN. This session will cover a detailed presentation of the policy, the responsibilities of Version 2.0 April, 2015 Page 5 of 24
6 the RN completing the risk assessment and practical aspects of dispensing insulin into syringes. Support and advice may also be sought from the Medicines Optimisation Team. 6.2 There will be annual training offered to the staff concerned by the SDSN to update staff and feedback any observations from the 6 monthly Quality Assurance assessments. 6.3 All clinical staff should be made aware of this policy at induction (new staff) by their Zone Leads and specific medicines management training where appropriate. 6.4 All RNs involved in the administration of insulin, as in all other areas of their practice, will be responsible for maintaining and updating their knowledge and practice. The e-learning module on the Safe use of Insulin is a mandatory requirement every 2 years with a pass mark of 80%. 6.4 All community nurses involved in this practice will insulin administration will undertake the MERIT 1 day module helping people with diabetes to continue insulin therapy. 7. Monitoring and Auditing 7.1 The SDSN will quality assure that the risk assessment & SOP are being completed as outlined in this policy in Appendices B,C and D using Appendix E every six months to ensure that this type of care remains appropriate for the individual. 7.2 All patients receiving insulin by this methodology will be recorded and held on a register by the SDSN. This should be available to the Provider Safety Group at their request. 7.3 This policy will be reviewed in two years through the Torbay and Southern Devon Health and Care NHS Trust Care and Clinical Policies Sub Group. 8 References Nursing and Midwifery Council (NMC) (2010) Standards for medicines management. Nursing and Midwifery Council (2015) The Code. Professional standards of practice and behaviour for nurses and midwives. National Patient Safety Agency (2010) Rapid response Report. Safer Administration of Insulin. NPSA/2010/RRR013 Parliament (1968) Medicines Act 1968, London: Stationery Office. Parliament (2005) Mental Capacity Act 2005, London: Stationery Office. Rosindale S (2014) Pre-loading of insulin syringes for people with diabetes to administer at home: new solution to an old practice, Diabetes and Primary Care, 16 (3), pp Royal College of Nursing (2015) Advance preparation of insulin syringes for adult patients to administer at home.2 nd Edition. Version 2.0 April, 2015 Page 6 of 24
7 9 Equality and Diversity 9.1 This document complies with the South Devon Healthcare Foundation Trust and Torbay and Southern Devon Health and Care NHS Trust Equality and Diversity statements. 10 Further Information 10.1 Links to Administration of Insulin policy This policy has referenced as an example of best practice in the RCN national guidance document Advance preparation of insulin syringes for adult patients to administer at home. 2 nd Edition. Version 2.0 April, 2015 Page 7 of 24
8 Appendix A: Process of syringes being pre-loaded for the patient on the first occasion 1.patient unable to administer insulin with a pen device 5. Pre-load syringes using appendix D documentation form at every visit 2. read appendix B to understand rationale for Standard Operating Procedure (SOP) & Risk assessment 4. After risk assessment completed discuss with Diabetes Lead Champion 3. Undertake risk assessment in appendix C On-going Process of syringes being pre-loaded for the patient 1.patient unable to administer insulin with a pen device 4. Diabetes lead Champion to Quality Assure Risk Assessment & SOP are completed in line with this policy every 6 months using appendix E 2. Pre-load syringes using appendix D documentation form every time 3. Undertake risk assessment in appendix C every 3 months Version 2.0 April, 2015 Page 8 of 24
9 Appendix B Standard Operating Procedure (SOP) for the assessment of a patient to have insulin prepared in advance of administration 1. Mental capacity and physical capability Activity Rationale Responsibility Assess the mental capacity and physical capability of the patient / carers to self-administer insulin. The patient should be at Level 3 which is defined as the patient accepts full responsibility for the storage and administration of the medicinal products. The level should be documented in the patient s records. Reduce risk of medication error NMC Standards for Medicines Management (2010) Care plan must be updated as a minimum every 3 months to meet needs of patient Zone lead/registered nurse Registered nurses should be aware that the Mental Capacity Act 2005 requires all those working with potentially incapacitated people to assess the individual s capacity at a particular moment about a particular decision. Any change in the patient s condition would necessitate a review of their self-administration status; for example, risk of self-harm. Reduce risk of medication error 2. Consent Activity Rationale Responsibility Discuss risks and benefits of self-administration with the patient. Support available between community nurse visits. To gain informed consent and document in patient s record Zone lead/registered nurse Where patients consent to self-administration of their medicines the following points must be considered:- Patients share the responsibility for their actions, relating to self- administration of their medicines. If children have access to the fridge that the patient ensures that the syringes are kept out of their reach and/or a child proof fridge lock is in situ. Patients can withdraw consent at any time. Patients are empowered to make informed choices Safeguarding of Children Patient s retain right to withdraw consent Zone lead/registered nurse Version 2.0 April, 2015 Page 9 of 24
10 3. Ensure most appropriate product has been prescribed Activity Rationale Responsibility Have you discussed a potential alternative insulin preparation/device with a Senior Diabetes Specialist Nurse (SDSN) in order to avoid this situation? Note: this discussion must have taken place before the assessment can continue further to ensure only the most appropriate patients are involved with this way of insulin self-administration. Unable to preload/use pen devices due to manual dexterity, poor vision, peripheral neuropathy, physical weakness to deliver insulin dose in insulin pen device, patient preference or reluctance to change Zone lead/registered nurse 4. Contra-indications Activity Rationale Responsibility Are there any contra-indications for the pre-loading of insulin syringes: Lantus Insulin (Insulin Glargine) must not be preloaded up for later administration as it becomes cloudy. Only use 10ml vials to withdraw insulin into syringes. Do not withdraw from pre-loaded pens or 3ml cartridges. Is the fridge in working order? Is the fridge visibly clean? Is the fridge free from debris? Patient suffers from an unpredictable mental state or declining cognitive ability. Very variable capillary blood glucose readings. If any of the above points are found, then these need to be reported to the GP or Devon Doctors (when out of hours) and the advanced preparation of insulin in syringes should cease and arrangements made for the community nursing team to visit at the required intervals. Complete incident form. Risk of deterioration of insulin To ensure appropriate storage conditions There would be a risk to patient safety Zone lead/registered nurse 5. Education of patient Activity Rationale Responsibility Information / education given and supervision should be tailored to meet individual patient need to enable the patient to administer the right dose, at the right time using the correct technique. To ensure safe administration of insulin Zone lead/ registered nurse Version 2.0 April, 2015 Page 10 of 24
11 The following information should be provided to the patient before commencing self-administration: The name of the medicine. Why they are taking it, dose and frequency. Re-suspending each pre-loaded syringe, between the hands to warm the insulin, at least 20 times prior to injection, if using a cloudy insulin. Inject at a 90 angle into sub-cutaneous tissue using a pinch-up technique into abdomen, outer thigh or buttocks as decided in care plan. Rotation within an injection site or between different injection sites. Common side effects and what to do if they occur e.g. hypoglycaemia. Any special instructions. How to obtain further supplies. How to store the medication. Frequency of visits & contact number for between visits. Recognition of error and procedure to follow. Correct disposal of sharps at the point of use. Plan of care documented and agreed with patient and nurse to ensure adequate support, monitoring of diabetes control and wellbeing. To comply with NMC Standards for medicines management (2010) Injecting cold insulin can be painful and it is not absorbed so effectively To demonstrate partnership working Zone lead/registered nurse One lead/registered nurse 6. Preparation of insulin and pre-loading insulin into syringe Activity Rationale Responsibility Nurses must not mix different insulins in the same syringe for administration at a later time (There is no longer a need to mix insulins due to the availability of suitable manufacturer s preparations) Pre loaded insulin should be left up to a maximum of seven days Document the advanced preparation of insulin on the PCT Community Nursing Record Sheets, ensuring full details are recorded, including batch number, type of insulin and expiry date are recorded To reduce risk of administration errors RCN and NMC policy To comply with Torbay and Southern Devon Health and Care NHS Trust documentation record keeping All registered nurses All registered nurses All registered nurses Version 2.0 April, 2015 Page 11 of 24
12 Equipment required: Plan of care. Sharps box (obtained from Torbay Council). Insulin syringes 30, 50 or 100 unit syringes (depending on which is most appropriate for the dose), needle length should be no more than 8 millimetres (8mm). Relevant insulin vial. Wipe-able, Sealable, hinged, Labelled container/s for storage of pre-filled syringes. Alcohol swab. Syringe labels Read and check plan of care. Check all previous pre-loaded syringes have been administered and safely disposed of. An adverse event form should be completed to investigate why the syringes had not been used. Explain procedure to patient, ensuring consent obtained. Prepare clean working surface and wipe with a Clinell wipe. Collect equipment required; check insulin for expiry date and against instructions of care plan. Wash hands. Prepare equipment and re-suspend (by rocking back and forth) insulin at least 20 times if using a cloudy insulin. Do not shake as you will damage the insulin suspension. If using a clear insulin there is no need to re-suspend. Swab insulin vial with an alcohol wipe and allow to dry. Draw up insulin in presence of patient as follows for each syringe using a clean procedure to prevent contamination: To ensure adequate preparation of procedure To reduce risk of administration errors To risk of cross infection To ensure safe administration of insulin All registered nurses Remove needle cover and pull back plunger to measure an amount of air equivalent to the amount of insulin prescribed. With insulin vial standing upright, insert the needle through the centre of the rubber cap and push down plunger. Invert the insulin vial. Pull back plunger until slightly more than correct dose is drawn up. Expel any air bubbles back into vial. Re-check correct prescribed dose has been drawn up and remove needle from vial. Carefully re-sheath needle (there is no risk of contaminated needle stick injury as needle is sterile in event of a needle stick injury the syringe must be safely discarded). To comply with NMC Standards for medicines management (2010) Version 2.0 April, 2015 Page 12 of 24
13 6.23 Label each syringe with patient name, insulin name, date of preparation, initials of RN Label each container with patient name, insulin name, insulin dose, time of administration, number of syringes, date, name and signature of registered nurse. Store pre-filled syringes with needles slightly elevated, within a labelled container as described earlier in main body of the fridge (away from freezer section or the back of the fridge). To ensure best practice in the preparation of insulin prior to injection All registered nurses 6.26 Dispose of clinical waste and wash hands Complete nursing notes ensuring date, time, insulin type/dose, batch number and number of syringes pre-filled are recorded If any pre-loaded syringes have not been used within the designated period, they must be disposed of. 7. Safe storage of pre-drawn insulin Activity Rationale Responsibility 7.1 The needle should be stored approximately at 45 o to prevent blockage by suspended substances in the insulin. Needle blockage by suspended insulin All registered nurses 7.2 Pre-filled syringes should be labelled and stored in a protective container. If there are different doses then use more than one container. Store in the main body of the fridge (away from the freezer section or the back of the fridge) between 2 8 degrees. The container should be clearly labelled with the following information: Date Name of patient Pre-loaded dose Number of syringes Name of insulin preparation Time of insulin administration e.g. before breakfast, before evening meal Route (subcutaneous) Instructions for administration e.g. just before or 30 minutes before food at times agreed with the patient and documented in the nursing notes Who has prepared the syringes To promote safe storage of pre-loaded insulin To ensure best practice in the administration of medicines To reduce risk of medication errors To reduce medication error To meet patients individual needs Version 2.0 April, 2015 Page 13 of 24
14 7.3 Separate containers should be used for insulin to be delivered at different times of day, particularly if the syringe contains a different dosage or type of insulin (RCN,2015) To promote standardisation of labels used All registered nurses When each new insulin vial is opened, the date and time must be recorded on the vial and in the patient s notes. Any insulin remaining in vial after 28 days should be discarded. Patients should have a vial in use and a backup vial at all times to ensure that vials are used methodically. The patient should be advised of the correct storage (as above) and advised to notify the district nurse team ASAP if their fridge stops working or the fridge freezes. To promote safe storage of insulin All registered nurses Patient/carer Version 2.0 April, 2015 Page 14 of 24
15 Appendix C: RISK ASSESSMENT FORM advanced preparation of insulin into syringes for a patient to administer at a later date. To be completed every three months Name of patient NHS number Date of birth GP Community nursing team Type of diabetes How long has the patient been insulin treated? Range of blood glucose readings: Last Hba1c result and date: Date risk assessment completed: Date of review for risk assessment Full name of nurse completing risk assessment form Signature Version 2.0 April, 2015 Page 15 of 24
16 1. Assessment of patients suitability for self-administration of insulin a) Have you assessed the mental and physical capability of the patient / carer to self-administer insulin? Yes No Potential risk Not known Seek advice b) Is the patient at Level 1? = The registered nurse is responsible for the safe storage of the insulin and the supervision of the administration process ensuring that the patient understands the insulin product being administered. c) Is the patient at level 2? = The registered nurse is responsible for the storage of the insulin. At administration time the patient will ask the nurse to open the cabinet/locker. The patient will then self-administer the insulin under the supervision of the nurse. d) Is the patient at level 3? = The patient accepts full responsibility for the storage and administration of the medicinal products. Note: the patient should be at Level 3 for this assessment to continue further. e) Is the level documented in the patient s records? f) Risk associated with disability sensory and or physical? g) Risk of self-harm? 2. Consent Yes No Potential risk a) Have you discussed the risks and benefits of self-administration with the patient? b) Do children have access the fridge? Not Known Seek advice Version 2.0 April, 2015 Page 16 of 24
17 c) If yes, have you advised the patient to ensure that the syringes are kept out of the reach of children? d) Has a fridge lock been purchased and in situ? e) Provided information about support available between community nurse visits? f) Is the patient happy to receive this service? 3. Ensure the most appropriate product has been prescribed? Yes No Potential risk Not Known Seek advice Have you discussed a potential alternative insulin preparation/device with a Senior Diabetes Specialist Nurse (SDSN) in order to avoid this situation? Note: this discussion must have taken place before the assessment can continue further. 4. CONTRA-INDICATIONS Are there any contra-indications for the pre-loading of insulin syringes:- a) Is the patient on Lantus Insulin (Insulin Glargine)? b) Is the fridge in working order? c) Is the fridge visibly clean? d) Is the fridge free from debris? e) Patient suffers from an unpredictable mental state or declining cognitive ability? f) Blood glucose showing wide variations? g) Is their current insulin in a pre-filled cartridge/pen? If any of the points a - g are found, then this needs to be reported to the SDSN, GP or Devon Doctors (when out of hours) and the advanced preparation of insulin in syringes should cease and arrangements made for the community nursing team to visit at the required intervals. Version 2.0 April, 2015 Page 17 of 24
18 5. Education of patient Yes No Potential risk Has the patient received the following information before commencing self- administration:- a) The name of the insulin? b) Why they are taking it? c) Dose and frequency? d) Shown how to re-suspend each pre-loaded syringe, between their hands to warm the insulin, at least 20 times prior to injection, if using a cloudy insulin? e) Inject at a 90 angle into subcutaneous tissue using a pinchup technique into abdomen, outer thigh or buttocks as documented in the care plan? f) Shown how to rotate within an injection site or between different injection sites? g) Educated how to manage hypoglycaemia and what to do if it occurs? h) How to obtain further supplies? i) How to store the insulin? j) Frequency of visits & contact number for between visits? k) Recognition of error and procedure to follow? l) Correct disposal of sharps at the point of use? m) Is the plan of care documented and agreed with patient? n) Nurse to ensure adequate support, monitoring of diabetes control and wellbeing. Not Known Seek advice 6. Summary of risks identified Summarise risks identified, including intuition and patient s awareness and experience of risk factors. 7. Would pre-loading of insulin promote independence and meet patient need? Yes No Potential risk Not known Seek advice 8. Is there a need for any further assessments? Version 2.0 April, 2015 Page 18 of 24
19 Appendix D: Documentation form for the advanced preparation of insulin in syringes The needle should be stored approximately at 45 o to prevent blockage by suspended substances in the insulin Pre-filled syringes should be stored in a labeled protective container in the main body of the fridge (away from the freezer section or the back of the fridge) between 2 8 Celsius. Date Name of patient NHS number Date of birth GP Community nursing team Name of registered nurse who has prepared the syringes: Name of insulin preparation (morning): Name of insulin preparation (evening): Pre-loaded dose before breakfast Pre-loaded dose before evening meal Number of syringes (maximum 7 days to be pre-loaded) Syringes labelled with: Patient name insulin name date of preparation initials of registered nurse Number of containers (separate containers should be used for insulin to be delivered at different times of day, particularly if the syringe contains a different dosage or type of insulin (RCN 2015) Container labelled with: patient name insulin name insulin dose time of administration number of syringes Date Name and signature of registered nurse. Version 2.0 April, 2015 Page 19 of 24
20 Instructions for administration for example, just before or 30 minutes before food at times agreed with the patient? Date vial of insulin opened discard any unused insulin in vial after 28 days Batch number of insulin vial Expiry date of insulin Does the fridge appear to be in good working order? Documented in the nursing notes? Anymore insulin/supplies need reordering? Patients should have a vial in use and a backup vial at all times to ensure that vials are used methodically. Signature of registered nurse Date and time Date of next visit Version 2.0 April, 2015 Page 20 of 24
21 Appendix E: Senior Diabetes Specialist Nurse 6 month quality assurance for policy Implementation 1. Assessment of patients suitability for self-administration of insulin Yes No Potential risk Not known Seek advice Has an assessment of the mental and physical capability of the patient / carer to self-administer insulin been documented? Is the patient at level 3? = The patient accepts full responsibility for the storage and administration of the medicinal products. Is the level documented in the patient s records? Risk associated with disability sensory and or physical? 2. Consent Yes No Potential risk Not known Seek advice Has the risks and benefits of selfadministration been discussed with the patient? Provided information about support available between community nurse visits? Is the patient happy with this service? 3. CONTR-INDICATIONS Yes No Potential risk Are there any contra-indications for the pre-loading of insulin syringes:- Is the patient on Lantus Insulin (Insulin Glargine)? Is the fridge in working order? Is the fridge visibly clean? Is the fridge free from debris? Protective container/s to store syringes? Blood glucose showing wide variations? Is the insulin being drawn from a pre-filled cartridge/pen? Issues/ comments back to RN s involved Date of next 6 month quality assurance visit Signature of Senior Diabetes Specialist Nurse Date and time Not known Seek advice Version 2.0 April, 2015 Page 21 of 24
22 11. Document Control Information This is a controlled document and should not be altered in any way without the express permission of the author or their representative. Please note this document is only valid from the date approved below, and checks should be made that it is the most up to date version available. If printed, this document is only valid for the day of printing. Ref No: Document title: Pre-loading of insulin syringes for adult patients to administer at home Purpose of document: Safe professional practice Date of issue: June 2015 Next review date: June 2017 Version: 2.0 Last review date: April 2015 Author: Samantha Rosindale, Diabetes Lead Champion Directorate: Operations Equality Impact: The guidance contained in this document is intended to be inclusive for all patients within the clinical group specified, regardless of age, disability, gender, gender identity, sexual orientation, race and ethnicity & religion or belief Committee(s) approving the Care & Clinical policies document: Date approved: Links or overlaps with other policies: All SDHCFT Trust Strategies, policies and procedure documents Have you considered using Equality Impact Assessment? Does this document have implications regarding the Care Act? If yes please state: Please select Yes No Does this document have training implications? If yes please state: Does this document have financial implications? If yes please state: Is this document a direct replacement for another? If yes please state which documents are being replaced: Document Amendment History Date Version no. Amendment summary Ratified by: October new Care & clinical policies November Re-badged Care & clinical policies April updated Care & clinical policies Version 2.0 April, 2015 Page 22 of 24
23 12. The Mental Capacity Act 2005 The Mental Capacity Act provides a statutory framework for people who lack capacity to make decisions for themselves, or who have capacity and want to make preparations for a time when they lack capacity in the future. It sets out who can take decisions, in which situations, and how they should go about this. It covers a wide range of decision making from health and welfare decisions to finance and property decisions Enshrined in the Mental Capacity Act is the principle that people must be assumed to have capacity unless it is established that they do not. This is an important aspect of law that all health and social care practitioners must implement when proposing to undertake any act in connection with care and treatment that requires consent. In circumstances where there is an element of doubt about a person s ability to make a decision due to an impairment of or disturbance in the functioning of the mind or brain the practitioner must implement the Mental Capacity Act. The legal framework provided by the Mental Capacity Act 2005 is supported by a Code of Practice, which provides guidance and information about how the Act works in practice. The Code of Practice has statutory force which means that health and social care practitioners have a legal duty to have regard to it when working with or caring for adults who may lack capacity to make decisions for themselves. The Act is intended to assist and support people who may lack capacity and to discourage anyone who is involved in caring for someone who lacks capacity from being overly restrictive or controlling. It aims to balance an individual s right to make decisions for themselves with their right to be protected from harm if they lack the capacity to make decisions to protect themselves. (3) All Trust workers can access the Code of Practice, Mental Capacity Act 2005 Policy, Mental Capacity Act 2005 Practice Guidance, information booklets and all assessment, checklists and Independent Mental Capacity Advocate referral forms on icare Infection Control All staff will have access to Infection Control Policies and comply with the standards within them in the work place. All staff will attend Infection Control Training annually as part of their mandatory training programme. Version 2.0 April, 2015 Page 23 of 24
24 13. Quality Impact Assessment (QIA) Please select Who may be affected by this document? Patient / Service Users Visitors / Relatives General Public Voluntary / Community Groups Trade Unions GPs NHS Organisations Police Councils Carers Staff Other Statutory Agencies Others (please state): Does this document require a service redesign, or substantial amendments to an existing process? no If you answer yes to this question, please complete a full Quality Impact Assessment. Are there concerns that the document could adversely impact on people and aspects of the Trust under one of the nine strands of diversity? no Age Disability Gender re-assignment Pregnancy and maternity Marriage and Civil Partnership Race, including nationality and ethnicity Religion or Belief Sex Sexual orientation If you answer yes to any of these strands, please complete a full Quality Impact Assessment. If applicable, what action has been taken to mitigate any concerns? Who have you consulted with in the creation of this document? Note - It may not be sufficient to just speak to other health & social care professionals. Patients / Service Users Visitors / Relatives General Public Voluntary / Community Groups Trade Unions GPs NHS Organisations Police Councils Carers Staff Details (please state): Other Statutory Agencies Version 2.0 April, 2015 Page 24 of 24
Pre-Loaded Insulin Syringe Policy
 Pre-Loaded Insulin Syringe Policy Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet. Please visit the intranet for the latest version. Purpose of Agreement
Pre-Loaded Insulin Syringe Policy Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet. Please visit the intranet for the latest version. Purpose of Agreement
Advanced Preparation of Insulin Syringes for Adult Patients to Administer at Home
 Advanced Preparation of Insulin Syringes for Adult Patients to Administer at Home Guidance for nurses Third edition CLINICAL PROFESSIONAL RESOURCE ADVANCED PREPARATION OF INSULIN SYRINGES FOR ADULT PATIENTS
Advanced Preparation of Insulin Syringes for Adult Patients to Administer at Home Guidance for nurses Third edition CLINICAL PROFESSIONAL RESOURCE ADVANCED PREPARATION OF INSULIN SYRINGES FOR ADULT PATIENTS
ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME
 STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
STANDARD OPERATING PROCEDURE ADVANCE PREPARATION OF INSULIN FOR PATIENTS TO ADMINISTER AT HOME First Issued July 08 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote
Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit
 Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit Document Summary The purpose of this policy is to guide and promote
Assistant Practitioner and Health Care Assistants Administration of Insulin, via Pen device, by Delegation Training and competency Toolkit Document Summary The purpose of this policy is to guide and promote
ADMINISTRATION OF INSULIN
 STANDARD OPERATING PROCEDURE ADMINISTRATION OF INSULIN Issue History First issued April 2012 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote the safe administration
STANDARD OPERATING PROCEDURE ADMINISTRATION OF INSULIN Issue History First issued April 2012 Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote the safe administration
PROCEDURE FOR BLOOD GLUCOSE MONITORING
 PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
Developed By Name Signature Date
 Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Administration of Injectable Medication by Healthcare Assistants (Band 3) Standard Operating Procedure
 Administration of Injectable Medication by Healthcare Assistants (Band 3) Standard Operating Procedure DOCUMENT CONTROL: Version: 1 Ratified by: Quality Assurance Sub Committee Date ratified: 4 September
Administration of Injectable Medication by Healthcare Assistants (Band 3) Standard Operating Procedure DOCUMENT CONTROL: Version: 1 Ratified by: Quality Assurance Sub Committee Date ratified: 4 September
Developed By Name Signature Date
 Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
Introduction. Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients)
 Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
This guideline was adapted in collaboration with Dr Georgina Walker and the Palliative Care Team at Rowcroft Hospice. With thanks.
 Title: Directorate: Responsible for review: Ratified by: DIABETES, MANAGEMENT OF IN PALLIATIVE CARE PATIENTS General Medicine Diabetes Specialist Nurse Service Delivery Unit Clinical Director of Pharmacy
Title: Directorate: Responsible for review: Ratified by: DIABETES, MANAGEMENT OF IN PALLIATIVE CARE PATIENTS General Medicine Diabetes Specialist Nurse Service Delivery Unit Clinical Director of Pharmacy
The Administration of Insulin to patients at home and in Community Hospitals
 The Administration of Insulin to patients at home and in Community Hospitals Partners in Care Document Ratification This is a controlled document and should not be altered in any way without the express
The Administration of Insulin to patients at home and in Community Hospitals Partners in Care Document Ratification This is a controlled document and should not be altered in any way without the express
1. Purpose of this document
 Guideline Ref No: 0900 Version 4 Title: Document Author: Ratified by: Specialist Orthopaedic Physiotherapist Care and Clinical Policies Group Date: 13 February 2017 Date: 19 April 2017 Review date: 5 May
Guideline Ref No: 0900 Version 4 Title: Document Author: Ratified by: Specialist Orthopaedic Physiotherapist Care and Clinical Policies Group Date: 13 February 2017 Date: 19 April 2017 Review date: 5 May
Adminstration of Insulin to Patients at Home and in Community Hospitals
 1929 Version 2 The Administration of Insulin to patients at home and in Community Hospitals Date: 26 January 2017 Adminstration of Insulin to Patients at Home and in Community Hospitals Page 1 of 7 Partners
1929 Version 2 The Administration of Insulin to patients at home and in Community Hospitals Date: 26 January 2017 Adminstration of Insulin to Patients at Home and in Community Hospitals Page 1 of 7 Partners
SAFE HANDLING OF VACCINES
 STANDARD OPERATING PROCEDURE SAFE HANDLING OF VACCINES Issue History Issue Version One Purpose of Issue/Description of Change Planned Review Date To ensure vaccines are stored in accordance with manufacturers
STANDARD OPERATING PROCEDURE SAFE HANDLING OF VACCINES Issue History Issue Version One Purpose of Issue/Description of Change Planned Review Date To ensure vaccines are stored in accordance with manufacturers
Template Standard Operating Procedure For: Handling of Midazolam and other controlled drugs in Dental Practices
 Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
Name of Dental Practice : Objectives To ensure implementation of the regulations and guidance on safe and secure handling of midazolam and other controlled drugs (CDs) Scope To cover all aspects of obtaining
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Pre-filled Patient Controlled Analgesia (PCA) syringes
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
Assessment of Mental Capacity and Best Interest Decisions
 Standard Operating Procedure 1 (SOP 1) Assessment of Mental Capacity and Best Interest Decisions Why we have a procedure? This Standard Operating Procedure (SOP) is required to set out how a person s capacity
Standard Operating Procedure 1 (SOP 1) Assessment of Mental Capacity and Best Interest Decisions Why we have a procedure? This Standard Operating Procedure (SOP) is required to set out how a person s capacity
PATIENT INFORMATION. D iabetes. What You Need to Know About. Insulin
 PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
Guidelines for managing the health care needs of children and young persons with diabetes in education
 Guidelines for managing the health care needs of children and young persons with diabetes in education Contents Page: Page Number 1.0 Introduction 1 2.0 The Care Management Plan 2 2.1 Blood Glucose monitoring
Guidelines for managing the health care needs of children and young persons with diabetes in education Contents Page: Page Number 1.0 Introduction 1 2.0 The Care Management Plan 2 2.1 Blood Glucose monitoring
Latex and Occupational Dermatitis Policy Incorporating Glove Selection
 Latex and Occupational Dermatitis Policy Incorporating Glove Selection DOCUMENT CONTROL: Version: 3 Ratified by: Risk Management Sub Group Date ratified: 17 July 2013 Name of originator/author: Health
Latex and Occupational Dermatitis Policy Incorporating Glove Selection DOCUMENT CONTROL: Version: 3 Ratified by: Risk Management Sub Group Date ratified: 17 July 2013 Name of originator/author: Health
Standard operating procedures for preparation and administration of intramuscular injections. No Action Rationale
 Standard operating procedures for preparation and administration of intramuscular injections Preparation Overview No Action Rationale 1 Collect and check all equipment 2 Check that the packaging of all
Standard operating procedures for preparation and administration of intramuscular injections Preparation Overview No Action Rationale 1 Collect and check all equipment 2 Check that the packaging of all
Trust Policy 218 Ionising Radiation Safety Policy
 Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
INSULIN MANAGEMENT POLICY
 INSULIN MANAGEMENT POLICY To be read in conjunction with the Hypoglycaemia Management Policy for adult Patients and the Countywide Standard Operating Procedure for Implementing the Insulin Passport/Safety
INSULIN MANAGEMENT POLICY To be read in conjunction with the Hypoglycaemia Management Policy for adult Patients and the Countywide Standard Operating Procedure for Implementing the Insulin Passport/Safety
Document Details Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No Local Ref (optional) Main points the
 Document Details Title Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No 1506-41174 Local Ref (optional) Main points the Active immunisation against infection caused by Hepatitis
Document Details Title Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No 1506-41174 Local Ref (optional) Main points the Active immunisation against infection caused by Hepatitis
Policy for the safe administration of Insulin
 Policy for the safe administration of Insulin This is a working document and any changes that become necessary to this policy must be Notified in writing to the Medicine Management Group via the Chief
Policy for the safe administration of Insulin This is a working document and any changes that become necessary to this policy must be Notified in writing to the Medicine Management Group via the Chief
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline
 SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
Management of AIDS/HIV Infected Healthcare Workers Policy
 Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
Protocol (Clinical) Country Health SA Local Health Network. Title: Subcutaneous Insulin Administration in Hospital and Aged Care
 Protocol (Clinical) Title: Subcutaneous Insulin Administration in Hospital and Aged Care Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services
Protocol (Clinical) Title: Subcutaneous Insulin Administration in Hospital and Aged Care Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services
MS Society Safeguarding Adults Policy and Procedure (Scotland)
 MS Society Safeguarding Adults Policy and Procedure (Scotland) Safeguarding Adults Policy The phrase adult support and protection is used instead of safeguarding in Scotland. However for consistency across
MS Society Safeguarding Adults Policy and Procedure (Scotland) Safeguarding Adults Policy The phrase adult support and protection is used instead of safeguarding in Scotland. However for consistency across
Document Details. Patient Group Direction
 Document Details Title Patient Group Direction (PGD) Hepatitis B Vaccine (Engerix ) Trust Ref No 1505-41182 Local Ref (optional) Main points the Immunisation against Hepatitis B document covers Who is
Document Details Title Patient Group Direction (PGD) Hepatitis B Vaccine (Engerix ) Trust Ref No 1505-41182 Local Ref (optional) Main points the Immunisation against Hepatitis B document covers Who is
CABINET PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND
 CABINET Report No: 105/2017 PUBLIC REPORT 16 May 2017 PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND Report of the Director of Public Health Strategic Aim: Safeguarding Key Decision:
CABINET Report No: 105/2017 PUBLIC REPORT 16 May 2017 PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND Report of the Director of Public Health Strategic Aim: Safeguarding Key Decision:
To provide nursing staff with guidelines for the safe and appropriate administration of insulin.
 SUBJECT: ADMINISTRATION OF INSULIN This cancels NP 513 dated 3/1/07 1. PURPOSE: COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Medications POLICY NUMBER: 513 Effective Date: June
SUBJECT: ADMINISTRATION OF INSULIN This cancels NP 513 dated 3/1/07 1. PURPOSE: COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Medications POLICY NUMBER: 513 Effective Date: June
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC17 SINGLE USE AND SINGLE PATIENT USE MEDICAL
Policy for Supporting. Children and Young People with. Diabetes in Education
 Policy for Supporting Children and Young People with Diabetes in Education December 2011 POLICY FOR SUPPORTING CHILDREN AND YOUNG PEOPLE WITH DIABETES IN EDUCATION Contents 1. Introduction 2. Before a
Policy for Supporting Children and Young People with Diabetes in Education December 2011 POLICY FOR SUPPORTING CHILDREN AND YOUNG PEOPLE WITH DIABETES IN EDUCATION Contents 1. Introduction 2. Before a
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
PLEASE READ THIS USER MANUAL BEFORE USE
 USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
THE RESPONSIBLE PHARMACIST REGULATIONS
 THE RESPONSIBLE PHARMACIST REGULATIONS A SUMMARY OF THE RESPONSES TO PUBLIC CONSULTATION ON PROPOSALS FOR THE CONTENT OF THE REGULATIONS DH INFORMATION READER BOX Policy HR / Workforce Management Planning
THE RESPONSIBLE PHARMACIST REGULATIONS A SUMMARY OF THE RESPONSES TO PUBLIC CONSULTATION ON PROPOSALS FOR THE CONTENT OF THE REGULATIONS DH INFORMATION READER BOX Policy HR / Workforce Management Planning
Procedure for the prescribing and administration of insulin
 Procedure for the prescribing and administration of insulin Author: Lilian Baxendale and Heather Beadle Designation: Clinical Governance Pharmacists Version: Version 3 Date: December 2016 Date Approved:
Procedure for the prescribing and administration of insulin Author: Lilian Baxendale and Heather Beadle Designation: Clinical Governance Pharmacists Version: Version 3 Date: December 2016 Date Approved:
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0. March 2018
 Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
Blood Glucose and Hyperglycaemia Management in Hospital for Adults with Diabetes Clinical Guideline V2.0 March 2018 Page 1 of 8 Summary flow chart for monitoring of blood glucose if >11mmol/L For Adults
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients
 TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
Consultation Group: Dr Amalia Mayo, Paediatric Consultant. Review Date: March Uncontrolled when printed. Version 2. Executive Sign-Off
 Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES. Insulin
 PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES Insulin This medicine has been proven to be safe and effective, but it can cause serious injury if a mistake happens while taking it. This means
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES Insulin This medicine has been proven to be safe and effective, but it can cause serious injury if a mistake happens while taking it. This means
GG&C PGD ref no: 2016/1408 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT
 GG&C PGD ref no: 2016/1408 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Active immunisation against disease
GG&C PGD ref no: 2016/1408 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Active immunisation against disease
PROCEDURE REF NO SABP/EXECUTIVE BOARD/0017
 PROCEDURE REF NO SABP/EXECUTIVE BOARD/0017 NAME OF PROCEDURE: Medicines Procedure: Patient Group Directions for the Administration of Hepatitis B Vaccine for SABP Drug and Alcohol Teams. PURPOSE OF PROCEDURE:
PROCEDURE REF NO SABP/EXECUTIVE BOARD/0017 NAME OF PROCEDURE: Medicines Procedure: Patient Group Directions for the Administration of Hepatitis B Vaccine for SABP Drug and Alcohol Teams. PURPOSE OF PROCEDURE:
NO SMOKING POLICY. Organisational
 NO SMOKING POLICY Policy Title State previous title where relevant. State if Policy New or Revised Policy Strand Org, HR, Clinical, H&S, Infection Control, Finance For clinical policies only - state index
NO SMOKING POLICY Policy Title State previous title where relevant. State if Policy New or Revised Policy Strand Org, HR, Clinical, H&S, Infection Control, Finance For clinical policies only - state index
1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of acamprosate.
 SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
SHARED CARE GUIDELINE FOR ACAMPROSATE 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the safe and appropriate prescription and administration of
Things you need to know about injections
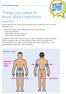 www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
Care Homes - Homely Remedies Protocol
 Care Homes - Homely Remedies Protocol A homely remedy is a medicine for the short-term treatment of a minor ailment, such as indigestion, a cough, mild to moderate pain or constipation, and can be obtained
Care Homes - Homely Remedies Protocol A homely remedy is a medicine for the short-term treatment of a minor ailment, such as indigestion, a cough, mild to moderate pain or constipation, and can be obtained
School Hearing Screening Policy
 School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
School Hearing Screening Policy V2.1 1st August 2017 Page 1 of 13 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership and Responsibilities...
PATIENT GROUP DIRECTION PROCEDURE
 PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE. Chief Pharmacist. Chief Pharmacist
 REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use
 Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
PH52. Audit tool for the implementation of. NICE public health guidance 52 Needle and syringe programmes
 PH52 Audit tool for the implementation of NICE public health guidance 52 Needle and syringe programmes Introduction This audit tool has been developed to help you audit implementation of, and compliance
PH52 Audit tool for the implementation of NICE public health guidance 52 Needle and syringe programmes Introduction This audit tool has been developed to help you audit implementation of, and compliance
making a referral for breast imaging Standard Operating Procedure
 Document Control Title Reporting Radiographer Author Directorate Surgery Date Version Issued 0.1 May 2016 Status Draft Author s job title Reporting Radiographer Department Breast Imaging Comment / Changes
Document Control Title Reporting Radiographer Author Directorate Surgery Date Version Issued 0.1 May 2016 Status Draft Author s job title Reporting Radiographer Department Breast Imaging Comment / Changes
Country Health SA Local Health Network. Title: Subcutaneous Insulin Administration in the Community Setting
 Protocol (Clinical) Title: Subcutaneous Insulin Administration in the Community Setting Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services Approved
Protocol (Clinical) Title: Subcutaneous Insulin Administration in the Community Setting Protocol developed by: CHSALHN Diabetes Service Protocol Sponsor: CHSALHN, Executive Director, Medical Services Approved
Safeguarding Children and Young People Policy
 Safeguarding Children and Young People Policy Policy Summary This policy outlines our commitment to keeping the children and young people who engage with the Red Cross safe. It outlines the expectations
Safeguarding Children and Young People Policy Policy Summary This policy outlines our commitment to keeping the children and young people who engage with the Red Cross safe. It outlines the expectations
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN
 SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
SHARED CARE GUIDELINE FOR BUCCAL MIDAZOLAM FOR THE TREATMENT OF PROLONGED SEIZURES IN CHILDREN 1. Aim/Purpose of this Guideline 1.1. This guideline applies to medical, nursing and pharmacy staff in the
Olanzapine Long-Acting Injection (Zypadhera ) - Guidelines for Prescribing and Administration (Version 3 May 2015)
 1. Key Points Olanzapine Long-Acting Injection (Zypadhera ) - Guidelines for Prescribing and Administration (Version 3 May 2015) 1.1 Olanzapine long acting injection (LAI) is indicated for the maintenance
1. Key Points Olanzapine Long-Acting Injection (Zypadhera ) - Guidelines for Prescribing and Administration (Version 3 May 2015) 1.1 Olanzapine long acting injection (LAI) is indicated for the maintenance
Tony Gray Head of Safety and Security. Tony Gray Head of Safety and Security. Contents Section Description Page No.
 Health and Safety Policy Practice Guidance Note Latex Sensitivity V04 Date Issued Planned Review Issue 1 Oct 17 Oct 2020 HS-PGN-10 Part of NTW(O)20 Health and Safety Policy Author/Designation Tony Gray
Health and Safety Policy Practice Guidance Note Latex Sensitivity V04 Date Issued Planned Review Issue 1 Oct 17 Oct 2020 HS-PGN-10 Part of NTW(O)20 Health and Safety Policy Author/Designation Tony Gray
Audit support for continuous subcutaneous insulin infusion for the treatment of diabetes mellitus (review of technology appraisal guidance 57)
 Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Administering Rescue Medication into Children for Prolonged Seizures
 Standard Operating Procedure 10 (SOP 10) Administering Rescue Medication into Children for Prolonged Seizures Why we have a procedure? Black Country Partnership NHS Trust (hereafter referred to as the
Standard Operating Procedure 10 (SOP 10) Administering Rescue Medication into Children for Prolonged Seizures Why we have a procedure? Black Country Partnership NHS Trust (hereafter referred to as the
Education and Training Committee 15 November 2012
 Education and Training Committee 15 November 2012 Review of the process of approval of hearing aid dispenser pre-registration education and training programmes. Executive summary and recommendations Introduction
Education and Training Committee 15 November 2012 Review of the process of approval of hearing aid dispenser pre-registration education and training programmes. Executive summary and recommendations Introduction
SFHDiabIPT01. Assess the suitability of insulin pump therapy for an individual with Type 1 diabetes. Overview
 Assess the suitability of insulin pump therapy for an individual with Type Overview This standard covers the activities associated with assessing the suitability of insulin pump therapy for individuals
Assess the suitability of insulin pump therapy for an individual with Type Overview This standard covers the activities associated with assessing the suitability of insulin pump therapy for individuals
Diabetes is a lifelong, chronic. Survey on the quality of diabetes care in prison settings across the UK. Keith Booles
 Survey on the quality of diabetes care in prison settings across the UK Article points 1. The Royal College of Nursing Diabetes Forum conducted an audit of prisons within the UK to determine the level
Survey on the quality of diabetes care in prison settings across the UK Article points 1. The Royal College of Nursing Diabetes Forum conducted an audit of prisons within the UK to determine the level
Alcohol and Substance Policy
 Alcohol and Substance Policy Lead Manager Responsible Director Approved by Kenneth Fleming, Head of Health & Safety Anne MacPherson, Director of Human Resource and Organisational Development Health & Safety
Alcohol and Substance Policy Lead Manager Responsible Director Approved by Kenneth Fleming, Head of Health & Safety Anne MacPherson, Director of Human Resource and Organisational Development Health & Safety
Ward/Unit/Team managers to carry out an occupational skin disease risk assessment for their area
 Policy Title: Prevention and Management of Occupational Skin Disease Reference and Version No: RM26 Version 3 Author and Job Title: Jude Cooper Head of Occupational Health and Wellbeing Executive Lead
Policy Title: Prevention and Management of Occupational Skin Disease Reference and Version No: RM26 Version 3 Author and Job Title: Jude Cooper Head of Occupational Health and Wellbeing Executive Lead
Safeguarding Business Plan
 Safeguarding Business Plan 2015-2018 Contents 1. Introduction 2. The Care Act 3. Organisational Development 4. Vision, Values and Strategic Objectives 5. Financial Plan 6. Appendix A Action Plan 7. Appendix
Safeguarding Business Plan 2015-2018 Contents 1. Introduction 2. The Care Act 3. Organisational Development 4. Vision, Values and Strategic Objectives 5. Financial Plan 6. Appendix A Action Plan 7. Appendix
PATIENT GROUP DIRECTION. Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif )
 PATIENT GROUP DIRECTION Administration of: Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif ) By: Practice Nurses In: General Practice It is the responsibility of the professional working under
PATIENT GROUP DIRECTION Administration of: Oral (live attenuated) Typhoid Vaccine (Ty21a) (Vivotif ) By: Practice Nurses In: General Practice It is the responsibility of the professional working under
Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0
 Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0 11 October 2017 Table of Contents 1. Introduction.. 3 2. Purpose of this Policy/Procedure. 3 3. Scope
Procedure for Subcutaneous Injection of Insulin or GLP1 Analogue in Adults Using a Pen Device V2.0 11 October 2017 Table of Contents 1. Introduction.. 3 2. Purpose of this Policy/Procedure. 3 3. Scope
OWNER MANUAL Diabetes in Cats
 www.mypetonline.co.uk/petdiabetes OWNER MANUAL Diabetes in Cats DIABETES IN CATS What is Diabetes? Glucose (blood sugar) is derived from the food your cat eats and provides the energy body cells need to
www.mypetonline.co.uk/petdiabetes OWNER MANUAL Diabetes in Cats DIABETES IN CATS What is Diabetes? Glucose (blood sugar) is derived from the food your cat eats and provides the energy body cells need to
KEEPING SAFE WITH INSULIN THERAPY
 KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
KEEPING SAFE WITH INSULIN THERAPY kk WHY IS THIS LEAFLET FOR YOU? Insulin treatment improves the quality of life in many people and saves the lives of others. It is used to lower blood glucose levels.
USER GUIDE. Melanotan must not be used if you are:
 USER GUIDE This document is for guidance purposes only and in no way replaces any official statements or other legal guidance documentation. This document does not claim to be correct or complete, no one
USER GUIDE This document is for guidance purposes only and in no way replaces any official statements or other legal guidance documentation. This document does not claim to be correct or complete, no one
PROCEDURE Mental Capacity Act. Number: E 0503 Date Published: 20 January 2016
 1.0 Summary of Changes This document has been redrafted and should be read in full by all officers and staff engaged in providing any response to the public concerning all aspects of Mental Health. This
1.0 Summary of Changes This document has been redrafted and should be read in full by all officers and staff engaged in providing any response to the public concerning all aspects of Mental Health. This
Brook Green Centre for Learning. Policy and Guidance for Supporting Pupils with Medical Needs
 Brook Green Centre for Learning Policy and Guidance for Supporting Pupils with Medical Needs This document was written in line with recommendations made in the DfE s information pack Supporting Pupils
Brook Green Centre for Learning Policy and Guidance for Supporting Pupils with Medical Needs This document was written in line with recommendations made in the DfE s information pack Supporting Pupils
Acknowledgement goes to WHO for use of their hand washing technique
 How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
Complementary Therapy Policy - CLP011
 Complementary Therapy Policy - CLP011 Page 1 of 14 Table of Contents Why we need this Policy... 3 What the Policy is trying to do... 3 Which stakeholders have been involved in the creation of this Policy...
Complementary Therapy Policy - CLP011 Page 1 of 14 Table of Contents Why we need this Policy... 3 What the Policy is trying to do... 3 Which stakeholders have been involved in the creation of this Policy...
Service Level Agreement for the Provision of Level 1 Substance Misuse Services from a Community Pharmacy under contract to NHS Grampian
 Service Level Agreement for the Provision of Level 1 Substance Misuse Services from a Community Pharmacy under contract to NHS Grampian 1. Introduction The provision of Substance Misuse (SM) services through
Service Level Agreement for the Provision of Level 1 Substance Misuse Services from a Community Pharmacy under contract to NHS Grampian 1. Introduction The provision of Substance Misuse (SM) services through
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
Lilly. Disposable Insulin Delivery Device User Manual
 1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
GG&C PGD ref no: 2017/1427 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT
 GG&C PGD ref no: 2017/1427 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Active immunisation against rotavirus.
GG&C PGD ref no: 2017/1427 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: Active immunisation against rotavirus.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
A patient guide to the use of insulin for diabetes
 A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
COMPETENT AUTHORITY (UK) MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES
 COMPETENT AUTHORITY (UK) 10 EC MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES (CUSTOM MADE DEVICES) Updated March 2008 CONTENTS PAGE Introduction 3 Definition of dental
COMPETENT AUTHORITY (UK) 10 EC MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES (CUSTOM MADE DEVICES) Updated March 2008 CONTENTS PAGE Introduction 3 Definition of dental
Instructions for Use. BASAGLAR KwikPen. insulin glargine injection (100 units/ml, 3 ml pen)
 Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical Commissioning Group
 BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
Consent to research. A draft for consultation
 Consent to research A draft for consultation 1 Consent to research About the guidance Our guidance Consent: patients and doctors making decisions together (2008) 1 sets out the principles of good practice
Consent to research A draft for consultation 1 Consent to research About the guidance Our guidance Consent: patients and doctors making decisions together (2008) 1 sets out the principles of good practice
Standard Operating Procedure for Use of Opioid Transdermal Patches in homes within NHS Sutton CCG
 Standard Operating Procedure for Use of Opioid Transdermal Patches in homes within NHS Sutton CCG Introduction This procedure is intended to encourage good practice in the management of opioid transdermal
Standard Operating Procedure for Use of Opioid Transdermal Patches in homes within NHS Sutton CCG Introduction This procedure is intended to encourage good practice in the management of opioid transdermal
SINGLE USE MEDICAL DEVICES POLICY
 SINGLE USE MEDICAL DEVICES POLICY First Issued by/date BWW /BKW March 2001 Issue Version Purpose of Issue/Description of Change Planned Review Date 2 Update May 2011 Named Responsible Officer:- Approved
SINGLE USE MEDICAL DEVICES POLICY First Issued by/date BWW /BKW March 2001 Issue Version Purpose of Issue/Description of Change Planned Review Date 2 Update May 2011 Named Responsible Officer:- Approved
Specialised Services Commissioning Policy. CP29: Bariatric Surgery
 Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Falls The Assessment, Prevention and Management of Patient Falls (Adult Services) 1.34
 SECTION: 1 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.34 NATURE AND SCOPE: SUBJECT (Title): POLICY AND PROCEDURE - TRUST WIDE FALLS: THE ASSESSMENT, PREVENTION AND MANAGEMENT OF PATIENT
SECTION: 1 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.34 NATURE AND SCOPE: SUBJECT (Title): POLICY AND PROCEDURE - TRUST WIDE FALLS: THE ASSESSMENT, PREVENTION AND MANAGEMENT OF PATIENT
GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT
 GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: For active immunisation of individuals
GG&C PGD ref no: 2016/1338 YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT Clinical Condition Indication: For active immunisation of individuals
SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector
 SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
Diabetes Competencies for Community Nurses
 Diabetes Competences for Community Nurses Lothian Health Board: August 2012 Lindsey Aniello, and Jill Little, Diabetes Specialist Nurses Judy Hamilton, District Nurse COMPETENCY DESCRIPTOR 1 Participates,
Diabetes Competences for Community Nurses Lothian Health Board: August 2012 Lindsey Aniello, and Jill Little, Diabetes Specialist Nurses Judy Hamilton, District Nurse COMPETENCY DESCRIPTOR 1 Participates,
SFHDiabPT03 Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy
 Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy Overview This standard concerns the activities of helping an individual with diabetes understand
Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy Overview This standard concerns the activities of helping an individual with diabetes understand
Guidelines for the Manual Evacuation of Faeces
 Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
Control of Substances Hazardous to Health (COSHH) Regulations 2002
 Control of Substances Hazardous to Health (COSHH) Regulations 2002 1. Purpose This document provides guidance to staff on how to comply with the COSHH Regulations 2002 (as amended) and in particular informs
Control of Substances Hazardous to Health (COSHH) Regulations 2002 1. Purpose This document provides guidance to staff on how to comply with the COSHH Regulations 2002 (as amended) and in particular informs
Multi-Agency Safeguarding Training. Prospectus April March 2019
 Multi-Agency Safeguarding Training Prospectus April 2018- March 2019 Author: Barbara Morris, Workforce Development Lead - Childrens Barbara.morris@northtyneside.gov.uk Contents Click on the course heading
Multi-Agency Safeguarding Training Prospectus April 2018- March 2019 Author: Barbara Morris, Workforce Development Lead - Childrens Barbara.morris@northtyneside.gov.uk Contents Click on the course heading
