TRACRIUM GlaxoSmithKline
|
|
|
- Barry Sutton
- 5 years ago
- Views:
Transcription
1 TRACRIUM GlaxoSmithKline Atracurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: A sterile solution containing 10 mg atracurium besylate per ml, without an antimicrobial preservative, supplied in ampoules. Multi-dose vial: Injection containing atracurium besylate 10 mg per ml with 0.9% w/v benzyl alcohol as an antimicrobial preservative, supplied in vials. PHARMACEUTICAL FORM Solution for injection or infusion. CLINICAL PARTICULARS Indications TRACRIUM is a highly selective, competitive or nondepolarising neuromuscular blocking agent which is used as an adjunct to general anaesthesia to enable tracheal intubation to be performed and to relax skeletal muscles during surgery or controlled ventilation during a wide range of medical procedures. TRACRIUM injection is also used to facilitate mechanical ventilation in Intensive Care Unit (ICU) patients. The multi-dose vial contains benzyl alcohol 0.9% w/v as an antimicrobial preservative and is intended for multiple use in one or more patients. Dosage and Administration IN COMMON WITH ALL NEUROMUSCULAR BLOCKING AGENTS MONITORING OF NEURO- MUSCULAR FUNCTION IS RECOMMENDED DURING THE USE OF TRACRIUM IN ORDER TO INDIVIDUALISE DOSAGE REQUIREMENTS. Use by injection in adults TRACRIUM is administered by i.v. injection. The dosage range for adults is 0.3 to 0.6 mg/kg (depending on the duration of full block required) and will provide adequate relaxation for 15 to 35 min. Endotracheal intubation can usually be accomplished within 90 seconds from the i.v. injection of 0.5 to 0.6 mg/kg. Full block can be prolonged with supplementary doses of 0.1 to 0.2 mg/kg as required. Successive supplementary dosing does not give rise to accumulation of neuromuscular blocking effect. Spontaneous recovery from the end of full block occurs in about 35 min as measured by the restoration of the tetanic response to 95% of normal neuromuscular function. The neuromuscular block produced by TRACRIUM can be rapidly reversed by standard doses of anticholinesterase agents, such as neostigmine and edrophonium, accompanied or preceded by atropine, with no evidence of recurarisation. Use as an infusion in adults After an initial bolus dose of 0.3 to 0.6 mg/kg, TRACRIUM can be used to maintain neuromuscular block during long surgical procedures by administration as a continuous infusion at rates of 0.3 to 0.6 mg/kg/h. TRACRIUM can be administered by infusion during cardiopulmonary bypass surgery at the recommended infusion rates. Induced hypothermia to a body temperature of 25oC to 26oC reduces the rate of inactivation of TRACRIUM, therefore full neuromuscular block may be maintained by approximately half the original infusion rate at these low temperatures. TRACRIUM (multi-dose vials and injection) is compatible with the following infusion solutions for the times stated below. Infusion Solution Sodium Chloride I.V. Infusion BP (0.9% w/v) Glucose I.V. Infusion BP (5% w/v) Ringer s Injection USP Period of Stability 24 h
2 Infusion Solution Sodium Chloride (0.18% w/v) and Glucose (4% w/v) I.V. Infusion BP Compound Sodium Lactate I.V. Infusion BP (Hartmann s Solution for Injection) Period of Stability When diluted in these solutions to give TRACRIUM concentrations of 0.5 mg/ml and above, the resultant solutions will be stable in daylight for the stated periods at temperatures of up to 30oC. Use in children The dosage in children over the age of 1 month is the same as that in adults on a bodyweight basis. Use in the elderly TRACRIUM may be used at standard dosage in elderly patients. It is recommended, however, that the initial dose be at the lower end of the range and that it be administered slowly. Use in patients with reduced renal and/or hepatic function TRACRIUM may be used at standard dosage at all levels of renal or hepatic function, including endstage failure. Use in patients with cardiovascular disease In patients with clinically significant cardiovascular disease, the initial dose of TRACRIUM should be administered over a period of 60 seconds. Use in Intensive Care Unit (ICU) patients (TRACRIUM injection only) After an optional initial bolus dose of TRACRIUM of 0.3 to 0.6 mg/kg, TRACRIUM can be used to maintain neuromuscular block by administering a continuous infusion at rates of between 11 and 13 micrograms/kg/min (0.65 to 0.78 mg/kg/h). However, there is wide inter-patient variability in dosage requirements. Dosage requirements may change with time. Infusion rates as low as 4.5 micrograms/kg/min (0.27 mg/kg/h) or as high as 29.5 micrograms/kg/ min (1.77 mg/kg/h) are required in some patients. The rate of spontaneous recovery from neuromuscular block after infusion of TRACRIUM in ICU 4 h patients is independent of the duration of administration. Spontaneous recovery to a train-of-four ratio greater than 0.75 (the ratio of the height of the fourth to the first twitch in a train-of-four) can be expected to occur in approximately 60 min. A range of 32 to 108 min has been observed in clinical trials. Contraindications Injection: - Atracurium is contraindicated in patients known to be hypersensitive to atracurium, cisatracurium or benzenesulfonic acid. Multi-dose vial: - Atracurium (Multi-dose vial) is contraindicated in patients known to be hypersensitive to atracurium, cisatracurium, benzenesulfonic acid or benzyl alcohol. Warnings and Precautions IN COMMON WITH ALL THE OTHER NEURO- MUSCULAR BLOCKING AGENTS TRACRIUM PARALYSES THE RESPIRATORY MUSCLES AS WELL AS OTHER SKELETAL MUSCLES BUT HAS NO EFFECT ON CONSCIOUSNESS. TRACRIUM SHOULD BE ADMINISTERED ONLY WITH ADEQUATE GENERAL ANAESTHESIA AND ONLY BY OR UNDER THE CLOSE SUPERVISION OF AN EXPERIENCED ANAESTHETIST WITH ADEQUATE FACILITIES FOR ENDOTRACHEAL INTUBATION AND ARTIFICIAL VENTILATION. The potential for histamine release exists in susceptible patients during TRACRIUM administration. Caution should be exercised in administering TRACRIUM to patients with a history suggestive of an increased sensitivity to the effects of histamine. Caution should also be exercised when administering TRACRIUM to patients who have shown hypersensitivity to other neuromuscular blocking agents since cross-sensitivity between neuromuscular blocking agents has been reported (see Contraindications). TRACRIUM does not have significant vagal or ganglionic blocking properties in the recommended dosage range. Consequently, TRACRIUM has no clinically
3 significant effects on heart rate in the recommended dosage range and it will not counteract the bradycardia produced by many anaesthetic agents or by vagal stimulation during surgery. In common with other non-depolarising neuromuscular blocking agents, increased sensitivity to TRACRIUM may be expected in patients with myasthenia gravis, other forms of neuromuscular disease and severe electrolyte imbalance. TRACRIUM should be administered over a period of 60 seconds to patients who may be unusually sensitive to falls in arterial blood pressure, for example those who are hypovolaemic. TRACRIUM is inactivated by high ph and so must not be mixed in the same syringe with thiopentone or any alkaline agent. When a small vein is selected as the injection site, TRACRIUM should be flushed through the vein with physiological saline after injection. When other anaesthetic drugs are administered through the same in-dwelling needle or cannula as TRACRIUM it is important that each drug is flushed through with an adequate volume of physiological saline. TRACRIUM is hypotonic and must not be administered into the infusion line of a blood transfusion. Studies in malignant hyperthermia in susceptible animals (swine) and clinical studies in patients susceptible to malignant hyperthermia indicate that TRACRIUM does not trigger this syndrome. In common with other non-depolarising neuromuscular blocking agents, resistance may develop in patients suffering from burns. Such patients may require increased doses dependent on the time elapsed since the burn injury and the extent of the burn. Intensive Care unit (ICU) Patients: When administered to laboratory animals in high doses, laudanosine, a metabolite of atracurium, has been associated with transient hypotension and in some species, cerebral excitatory effects. Although seizures have been seen in ICU patients receiving TRACRIUM, a causal relationship to laudanosine has not been established (see Adverse Reactions). Benzyl alcohol is used as an antimicrobial preservative in many parenteral drug formulations, including TRACRIUM multi-dose vial. Because of reports linking the use of parenteral drug formulations containing benzyl alcohol to morbidity and mortality amongst low weight neonates such formulations should be used with caution in neonates and theoretically, in other patient groups suspected of possessing a reduced ability to metabolise benzyl alcohol. Interactions The neuromuscular block produced by TRACRIUM may be increased by the concomitant use of inhalational anaesthetics such as halothane, isoflurane and enflurane. In common with all non-depolarising neuromuscular blocking agents the magnitude and/or duration of a non-depolarising neuromuscular block may be increased as a result of interaction with: - antibiotics:including the aminoglycosides, polymyxins, spectinomycin, tetracyclines, lincomycin and clindamycin - antiarrhythmic drugs: propranolol, calcium channel blockers, lignocaine, procainamide and quinidine - diuretics: frusemide and possibly mannitol, thiazide diuretics and acetazolamide - magnesium sulphate - ketamine - lithium salts - ganglion blocking agents: trimetaphan, hexamethonium. Rarely, certain drugs may aggravate or unmask latent myasthenia gravis or actually induce a myasthenic syndrome; increased sensitivity to TRACRIUM would be consequent on such a development. Such drugs include various antibiotics, beta-blockers (propranolol, oxprenolol), antiarrhythmic drugs (procainamide, quinidine), antirheumatic drugs (chloroquine, D-penicillamine), trimetaphan, chlorpromazine, steroids, phenytoin and lithium. The onset of non-depolarising neuromuscular block is likely to be lengthened and the duration of block shortened in patients receiving chronic anticonvulsant therapy.
4 The administration of combinations of non-depolarising neuromuscular blocking agents in conjunction with TRACRIUM may produce a degree of neuromuscular blockade in excess of that which might be expected were an equipotent total dose of TRACRIUM administered. Any synergistic effect may vary between different drug combinations. A depolarising muscle relaxant such as suxamethonium chloride should not be administered to prolong the neuromuscular blocking effects of non-depolarising agents such as TRACRIUM, as this may result in a prolonged and complex block which can be difficult to reverse with anti-cholinesterase drugs. Pregnancy and Lactation Fertility studies have not been performed. Animal studies have indicated that atracurium has no significant effects on foetal development. In common with all neuromuscular blocking agents, TRACRIUM should be used during pregnancy only if the potential benefit to the mother outweighs any potential risk to the foetus. TRACRIUM is suitable for maintenance of muscle relaxation during Caesarean section as it does not cross the placenta in clinically significant amounts following recommended doses. It is not known whether atracurium is excreted in human milk. Effects on Ability to Drive and Use Machines No data. Adverse Reactions Adverse reactions are listed below by system organ class and frequency. Frequencies are defined as: very common: 1 in 10 common: 1 in 100 and <1 in 10 uncommon: 1 in 1,000 and <1 in 100 rare: 1 in 10,000 and <1 in 1,000 very rare: <1/10,000. Very common, common and uncommon frequencies were determined from clinical trial data. Rare and very rare frequencies were generally derived from spontaneous data. The frequency classification Not known has been applied to those reactions where a frequency could not be estimated from the available data. Clinical Trial Data Vascular Disorders Events which have been attributed to histamine release are indicated by a hash (#). Common: Hypotension (mild, transient)#, Skin flushing# Respiratory, thoracic and mediastinal disorders Events which have been attributed to histamine release are indicated by a hash (#). Uncommon: Bronchospasm# Postmarketing Data Immune system disorders Very rare Anaphylactic reaction, anaphylactoid reaction Very rarely, severe anaphylactoid or anaphylactic reactions have been reported in patients receiving atracurium in conjunction with one or more anaesthetic agents. Nervous system disorder Not known Seizures There have been reports of seizures in ICU patients who have been receiving atracurium concurrently with several other agents. These patients usually had one or more medical conditions predisposing to seizures (e.g. cranial trauma, cerebral oedema, viral encephalitis, hypoxic encephalopathy, uraemia). A causal relationship to laudanosine has not been established. In clinical trials, there appears to be no correlation between plasma laudanosine concentration and the occurrence of seizures. Musculoskeletal and connective tissue disorders Not known Myopathy, muscle weakness There have been some reports of muscle weakness and/or myopathy following prolonged use of muscle relaxants in severely ill patients in the ICU. Most patients were receiving concomitant corticosteroids. These events have been seen infrequently in association with atracurium and a causal relationship has not been established.
5 Overdose Prolonged muscle paralysis and its consequences are the main signs of overdosage. It is essential to maintain a patent airway together with assisted positive pressure ventilation until spontaneous respiration is adequate. Full sedation will be required since consciousness is not impaired. Recovery may be hastened by the administration of anticholinesterase agents accompanied by atropine or glycopyrrolate, once evidence of spontaneous recovery is present. PHARMACOLOGICAL PROPERTIES Pharmacodynamics Atracurium is a highly selective, competitive or nondepolarising neuromuscular blocking agent. TRACRIUM has no direct effect on intra-ocular pressure, and is therefore suitable for use in ophthalmic surgery. Pharmacokinetics Atracurium is inactivated by Hofmann elimination, a non-enzymatic process which occurs at physiological ph and temperature, and by ester hydrolysis catalysed by non-specific esterases. Tests with plasma from patients with low levels of pseudocholinesterase show that the inactivation of atracurium proceeds unaffected. Variations in the blood ph and body temperature of the patient within the physiological range will not significantly alter the duration of action of atracurium. The termination of the neuromuscular blocking action of TRACRIUM is not dependent on its hepatic or renal metabolism or excretion. Its duration of action, therefore, is unlikely to be affected by impaired renal, hepatic or circulatory function. Special Patient Populations Haemofiltration and haemodiafiltration have a minimal effect on plasma levels of atracurium and its metabolites, including laudanosine. The effects of haemodialysis and haemoperfusion on plasma levels of atracurium and its metabolites are unknown. Concentrations of metabolites are higher in ICU patients with abnormal renal and/or hepatic function (see Warnings and Precautions). These metabolites do not contribute to neuromuscular block. Pre-clinical Safety Data Atracurium has been evaluated in three short-term mutagenicity tests. It was not mutagenic in either the in vitro Ames salmonella assay at concentrations up to 1000 micrograms/plate or in an in vivo rat bone marrow assay at doses up to those which resulted in neuromuscular blockade. In a second in vitro test, the mouse lymphoma assay, mutagenicity was not observed at doses up to 60 micrograms/ ml which killed up to 50% of the treated cells but it was moderately mutagenic at concentrations of 80 micrograms/ml in the absence of metabolising agent and weakly mutagenic at very high concentrations (1200 micrograms/ml) when metabolising enzymes were added. At both concentrations over 80% of the cells were killed. In view of the nature of human exposure to TRACRIUM, the mutagenic risk to patients undergoing surgical relaxation with TRACRIUM must be considered negligible. Carcinogenicity studies have not been performed. PHARMACEUTICAL PARTICULARS List of Excipients Multi-dose vial contains benzyl alcohol. Incompatibilities No data. Shelf Life The expiry date is indicated on the packaging. Special Precautions for Storage Short periods at temperatures up to 30oC are permissible but ONLY to allow transportation or temporary storage outside of a cold store. It is estimated that an 8% loss of potency would occur if TRACRIUM injection was stored at 30oC for 1 month. Injection: Store at temperatures between 2oC and 8oC. Protect from light. Do not freeze.
6 Any unused TRACRIUM injection from opened ampoules should be discarded. Nature and Contents of Container As registered locally. Instructions for Use/Handling TRACRIUM is compatible with the following infusion solutions for the times stated below. Infusion Solution Sodium Chloride I.V. Infusion BP (0.9% w/v) Glucose I.V. Infusion BP (5% w/v) Ringer s Injection USP Sodium Chloride (0.18% w/v) and Glucose (4% w/v) I.V. Infusion BP Compound Sodium Lactate I.V. Infusion BP (Hartmann s Solution for Injection) Period of Stability 24 h When diluted in these solutions to give TRACRIUM concentrations of 0.5 mg/ml and above, the resultant solutions will be stable in daylight for the stated periods at temperatures of up to 30oC. TRACRIUM multi-dose vial contains 0.9% w/v benzyl alcohol as an antimicrobial preservative and is intended for multiple use in one or more patients. It is good clinical practice to discard any partly used vials at the end of an operating day. Not all presentations are available in every country. Version number: GDS16/IPI03 Date of issue: 30 March 2006 TRACRIUM is a trademark of: the GlaxoSmithKline group of companies 4 h
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory TRACRIUM. Atracurium Besylate Injection IP
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory TRACRIUM Atracurium Besylate Injection IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Atracurium Besylate
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory TRACRIUM Atracurium Besylate Injection IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Atracurium Besylate
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ACURMIL 25 mg/2.5 ml solution for intravenous injection ACURMIL 50 mg/5 ml solution for intravenous injection 2. QUALITATIVE AND QUANTITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ACURMIL 25 mg/2.5 ml solution for intravenous injection ACURMIL 50 mg/5 ml solution for intravenous injection 2. QUALITATIVE AND QUANTITATIVE
PRODUCT INFORMATION. TRACRIUM INJECTION atracurium besylate DESCRIPTION:
 PRODUCT INFORMATION TRACRIUM INJECTION (atracurium besylate) NAME OF THE DRUG: atracurium besylate DESCRIPTION: TRACRIUM (atracurium besylate) is an intermediate-duration, non-depolarising, skeletal muscle
PRODUCT INFORMATION TRACRIUM INJECTION (atracurium besylate) NAME OF THE DRUG: atracurium besylate DESCRIPTION: TRACRIUM (atracurium besylate) is an intermediate-duration, non-depolarising, skeletal muscle
MIVACRON GlaxoSmithKline
 MIVACRON GlaxoSmithKline Mivacurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: Sterile solution containing 2 mg mivacurium per ml as mivacurium chloride, without an antimicrobial preservative,
MIVACRON GlaxoSmithKline Mivacurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: Sterile solution containing 2 mg mivacurium per ml as mivacurium chloride, without an antimicrobial preservative,
PRODUCT INFORMATION CISATRACURIUM PFIZER NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY. Cisatracurium besylate
 PRODUCT INFORMATION CISATRACURIUM PFIZER Cisatracurium besylate NAME OF THE MEDICINE The chemical name of cisatracurium besylate is (1R,1 R,2R,2 R,)-2,2 -(3,11-dioxo-4,10- dioxatridecamethylene) bis (1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-
PRODUCT INFORMATION CISATRACURIUM PFIZER Cisatracurium besylate NAME OF THE MEDICINE The chemical name of cisatracurium besylate is (1R,1 R,2R,2 R,)-2,2 -(3,11-dioxo-4,10- dioxatridecamethylene) bis (1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-
A Nondepolarizing Neuromuscular Blocking (NMB) Agent
 DOSING GUIDE A Nondepolarizing Neuromuscular Blocking (NMB) Agent Easy to remember dosing for the 0.20 mg/kg adult intubating doses of NIMBEX 1 *: For every 10 kg, give 1 ml of NIMBEX (2 mg/ml concentration)
DOSING GUIDE A Nondepolarizing Neuromuscular Blocking (NMB) Agent Easy to remember dosing for the 0.20 mg/kg adult intubating doses of NIMBEX 1 *: For every 10 kg, give 1 ml of NIMBEX (2 mg/ml concentration)
PACKAGE LEAFLET: INFORMATION FOR THE USER. Atracurium Besilate 10 mg/ml Solution for Injection The active substance is atracurium besilate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Atracurium Besilate 10 mg/ml Solution for Injection The active substance is atracurium besilate Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER Atracurium Besilate 10 mg/ml Solution for Injection The active substance is atracurium besilate Read all of this leaflet carefully before you start using this
Please refer to the Summary of Product Characteristics (SPC) for further details on this product.
 [Aspen Logo] Information for the Physician Nimbex cisatracurium Please refer to the Summary of Product Characteristics (SPC) for further details on this product. Trade name of the medicinal product Nimbex
[Aspen Logo] Information for the Physician Nimbex cisatracurium Please refer to the Summary of Product Characteristics (SPC) for further details on this product. Trade name of the medicinal product Nimbex
Core Safety Profile. Pharmaceutical form(s)/strength: Solution for Injection CZ/H/PSUR/0005/002 Date of FAR:
 Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
NEW ZEALAND DATA SHEET. Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE Pancuronium Bromide B.P. Injection 2 mg/ml PRESENTATION Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide. INDICATIONS
NEW ZEALAND DATA SHEET NAME OF MEDICINE Pancuronium Bromide B.P. Injection 2 mg/ml PRESENTATION Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide. INDICATIONS
ULTIVA GlaxoSmithKline
 ULTIVA GlaxoSmithKline Remifentanil QUALITATIVE AND QUANTITATIVE COMPOSITION Remifentanil for injection is a sterile, endotoxin-free, preservative-free, white to off white, lyophilised powder, to be reconstituted
ULTIVA GlaxoSmithKline Remifentanil QUALITATIVE AND QUANTITATIVE COMPOSITION Remifentanil for injection is a sterile, endotoxin-free, preservative-free, white to off white, lyophilised powder, to be reconstituted
FOR REPRESENTATIVE EDUCATION
 Neuromuscular Blockade in the ICU NIMBEX Indication 1 NIMBEX (cisatracurium besylate) is indicated as an adjunct to general anesthesia to facilitate tracheal intubation in adults and in pediatric patients
Neuromuscular Blockade in the ICU NIMBEX Indication 1 NIMBEX (cisatracurium besylate) is indicated as an adjunct to general anesthesia to facilitate tracheal intubation in adults and in pediatric patients
200 mg/20 ml (10 mg/ml) in single-dose vials (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NIMBEX safely and effectively. See full prescribing information for NIMBEX. NIMBEX (cisatracurium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NIMBEX safely and effectively. See full prescribing information for NIMBEX. NIMBEX (cisatracurium
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VECURE 1. Product Name VECURE 10 mg, powder for injection. 2. Qualitative and Quantitative Composition VECURE 10 mg, 1 vial contains 10 mg vecuronium bromide. When reconstituted
NEW ZEALAND DATA SHEET VECURE 1. Product Name VECURE 10 mg, powder for injection. 2. Qualitative and Quantitative Composition VECURE 10 mg, 1 vial contains 10 mg vecuronium bromide. When reconstituted
PRODUCT MONOGRAPH. Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium. Non-depolarizing Skeletal Neuromuscular Blocking Agent
 PRODUCT MONOGRAPH Pr CISATRACURIUM BESYLATE INJECTION Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium Non-depolarizing Skeletal Neuromuscular Blocking Agent This drug should be administered
PRODUCT MONOGRAPH Pr CISATRACURIUM BESYLATE INJECTION Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium Non-depolarizing Skeletal Neuromuscular Blocking Agent This drug should be administered
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
NEW ZEALAND DATA SHEET. Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution containing 2.5 mg/ml Neostigmine methylsulphate.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
ULTIVA. Remifentanil hydrochloride
 ULTIVA Remifentanil hydrochloride QUALITATIVE AND QUANTITATIVE COMPOSITION Remifentanil for injection is a sterile, preservative-free, white to off white, lyophilised powder, to be reconstituted before
ULTIVA Remifentanil hydrochloride QUALITATIVE AND QUANTITATIVE COMPOSITION Remifentanil for injection is a sterile, preservative-free, white to off white, lyophilised powder, to be reconstituted before
New Zealand Data Sheet DBL TM Rocuronium Bromide Injection
 New Zealand Data Sheet DBL TM Rocuronium Bromide Injection NAME OF THE MEDICINE DBL TM Rocuronium Bromide Injection 50 mg/5 ml Vial PRESENTATION Rocuronium bromide is a quaternary aminosteroid and an analogue
New Zealand Data Sheet DBL TM Rocuronium Bromide Injection NAME OF THE MEDICINE DBL TM Rocuronium Bromide Injection 50 mg/5 ml Vial PRESENTATION Rocuronium bromide is a quaternary aminosteroid and an analogue
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
08-15 NORCURON ORGANON
 08-15 NORCURON ORGANON 1. NAME OF THE MEDICINAL PRODUCT Norcuron 4 mg, powder for solution for injection Norcuron 10 mg, powder for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Norcuron
08-15 NORCURON ORGANON 1. NAME OF THE MEDICINAL PRODUCT Norcuron 4 mg, powder for solution for injection Norcuron 10 mg, powder for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Norcuron
Package leaflet: Information for the user
 Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
P-RMS: NO/H/PSUR/0009/001
 Core Safety Profile Active substance: Propofol Pharmaceutical form(s)/strength: Emulsion for injection, infusion, 10mg/ml Emulsion for infusion, 20mg/ml P-RMS: NO/H/PSUR/0009/001 Date of FAR: 30.06.2011
Core Safety Profile Active substance: Propofol Pharmaceutical form(s)/strength: Emulsion for injection, infusion, 10mg/ml Emulsion for infusion, 20mg/ml P-RMS: NO/H/PSUR/0009/001 Date of FAR: 30.06.2011
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
VENTOLIN RESPIRATOR SOLUTION
 VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Rocuronium bromide 10 mg/ml solution for injection/ infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution of Rocuronium
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Rocuronium bromide 10 mg/ml solution for injection/ infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution of Rocuronium
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
DATA SHEET. Remifentanil hydrochloride for injection1mg and 2mg (remifentanil base) vials
 DATA SHEET 1. PRODUCT NAME ULTIVA Remifentanil hydrochloride for injection1mg and 2mg (remifentanil base) vials 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance Remifentanil hydrochloride 1mg
DATA SHEET 1. PRODUCT NAME ULTIVA Remifentanil hydrochloride for injection1mg and 2mg (remifentanil base) vials 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance Remifentanil hydrochloride 1mg
DATA SHEET. Product Summary. 1. Trade Name of Medicinal Product. Protamine Sulphate Injection BP. 2. Qualitative and Quantitative Composition
 DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
ZOFRAN INJECTION GlaxoSmithKline
 ZOFRAN INJECTION GlaxoSmithKline Ondansetron QUALITATIVE AND QUANTITATIVE COMPOSITION Each 1 ml of aqueous solution contains 2 mg ondansetron as hydrochloride dihydrate. PHARMACEUTICAL FORM A clear, colourless,
ZOFRAN INJECTION GlaxoSmithKline Ondansetron QUALITATIVE AND QUANTITATIVE COMPOSITION Each 1 ml of aqueous solution contains 2 mg ondansetron as hydrochloride dihydrate. PHARMACEUTICAL FORM A clear, colourless,
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
SUMMARY OF PRODUCT CHARACTERISTICS. 1. NAME OF THE MEDICINAL PRODUCT Rocuronium Kabi 10 mg/ml solution for injection/infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Rocuronium Kabi 10 mg/ml solution for injection/infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Rocuronium Kabi 10 mg/ml solution for injection/infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME SUXAMETHONIUM CHLORIDE INJECTION B.P. 50 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2 ml of solution contains 100 mg of suxamethonium chloride BP For
NEW ZEALAND DATA SHEET 1. PRODUCT NAME SUXAMETHONIUM CHLORIDE INJECTION B.P. 50 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2 ml of solution contains 100 mg of suxamethonium chloride BP For
MINIMS AMETHOCAINE EYE DROPS
 MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
Suxamethonium Chloride Injection BP PRODUCT INFORMATION
 Suxamethonium Chloride Injection BP Product Information 1 (8) Suxamethonium Chloride Injection BP PRODUCT INFORMATION NAME OF DRUG The Australian Approved Name is suxamethonium chloride. The CAS number
Suxamethonium Chloride Injection BP Product Information 1 (8) Suxamethonium Chloride Injection BP PRODUCT INFORMATION NAME OF DRUG The Australian Approved Name is suxamethonium chloride. The CAS number
This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards.
 MIVACRON Injection (mivacurium chloride) This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. DESCRIPTION MIVACRON (mivacurium
MIVACRON Injection (mivacurium chloride) This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. DESCRIPTION MIVACRON (mivacurium
Core Safety Profile. Date of FAR:
 Core Safety Profile Active substance: Levobupivicaine Pharmaceutical form(s)/strength: Solution for injection, concentrate for solution for infusion, 2,5 mg/ml, 5 mg/ml, 7,5 mg/ml, 0,625 mg/ml, 1,25 mg/ml
Core Safety Profile Active substance: Levobupivicaine Pharmaceutical form(s)/strength: Solution for injection, concentrate for solution for infusion, 2,5 mg/ml, 5 mg/ml, 7,5 mg/ml, 0,625 mg/ml, 1,25 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Page 1 / 6 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Fentadon 50 microgram/ml, solution for injection for dogs SE, DK: Fentadon Vet. 50 microgram/ml, solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS Page 1 / 6 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Fentadon 50 microgram/ml, solution for injection for dogs SE, DK: Fentadon Vet. 50 microgram/ml, solution for injection
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion.
 1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml
 New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
NEW ZEALAND DATA SHEET
 1 ONDANSETRON-CLARIS 2 mg/ml solution for injection Ondansetron-Claris Solution for Injection 2 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Ondansetron-Claris, Solution for Injection, is a clear colourless
1 ONDANSETRON-CLARIS 2 mg/ml solution for injection Ondansetron-Claris Solution for Injection 2 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Ondansetron-Claris, Solution for Injection, is a clear colourless
NIMBEX Injection. This information is intended for U.S. residents only. only. (cisatracurium besylate)
 This information is intended for U.S. residents only. NIMBEX Injection (cisatracurium besylate) only DESCRIPTION CLINICAL PHARMACOLOGY INDICATIONS AND USAGE CONTRAINDICATIONS WARNINGS PRECAUTIONS ADVERSE
This information is intended for U.S. residents only. NIMBEX Injection (cisatracurium besylate) only DESCRIPTION CLINICAL PHARMACOLOGY INDICATIONS AND USAGE CONTRAINDICATIONS WARNINGS PRECAUTIONS ADVERSE
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Shaded areas=not MARKETED 24/2/09
 PACKAGE INSERT SCHEDULING STATUS Schedule 6 PROPRIETARY NAME AND DOSAGE FORM RAPIFEN 2 ml IV injection RAPIFEN 10 ml IV injection COMPOSITION Each ml contains alfentanil hydrochloride 0,544 mg (equivalent
PACKAGE INSERT SCHEDULING STATUS Schedule 6 PROPRIETARY NAME AND DOSAGE FORM RAPIFEN 2 ml IV injection RAPIFEN 10 ml IV injection COMPOSITION Each ml contains alfentanil hydrochloride 0,544 mg (equivalent
SUMMARY OF PRODUCT CHARACTERISTICS. 1 ml solution contains 75 micrograms of sufentanilcitrate, corresponding to 50 micrograms of sufentanil.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Sufentanil Narcomed, 50 microgram / ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 75
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Sufentanil Narcomed, 50 microgram / ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 75
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial.
 ZOVIRAX IV Aciclovir POWDER FOR I.V. INFUSION 1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial. 2. PHARMACEUTICAL FORM Freeze dried powder
ZOVIRAX IV Aciclovir POWDER FOR I.V. INFUSION 1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial. 2. PHARMACEUTICAL FORM Freeze dried powder
NIMBEX (cisatracurium besylate) injection
 NIMBEX (cisatracurium besylate) injection This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. NOT FOR USE IN NEONATES CONTAINS
NIMBEX (cisatracurium besylate) injection This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. NOT FOR USE IN NEONATES CONTAINS
NEUROMUSCULAR BLOCKING AGENTS
 NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
AUSTRALIAN PRODUCT INFORMATION REMIFENTANIL APOTEX (REMIFENTANIL HYDROCHLORIDE) POWDER FOR INJECTION
 AUSTRALIAN PRODUCT INFORMATION REMIFENTANIL APOTEX (REMIFENTANIL HYDROCHLORIDE) POWDER FOR INJECTION 1 NAME OF THE MEDICINE Remifentanil (as hydrochloride) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each
AUSTRALIAN PRODUCT INFORMATION REMIFENTANIL APOTEX (REMIFENTANIL HYDROCHLORIDE) POWDER FOR INJECTION 1 NAME OF THE MEDICINE Remifentanil (as hydrochloride) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each
Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml).
 Ventolin parenteral preparations Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml). Ventolin Solution for
Ventolin parenteral preparations Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml). Ventolin Solution for
Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion
 CSP Drug Substance Propofol Date September 04 2014 Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion CORE SAFETY PROFILE 4.3 Contraindications Propofol
CSP Drug Substance Propofol Date September 04 2014 Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion CORE SAFETY PROFILE 4.3 Contraindications Propofol
Benztropine and trihexyphenidyl: Centrally acting antimuscarinic agents used for treatment of Parkinson disease & extrapyramidal symptoms.
 Scopolamine: Tertiary amine plant alkaloid. Produces peripheral effects similar to those of atropine. Unlike atropine, scopolamine has greater action on the CNS (observed at therapeutic doses). It has
Scopolamine: Tertiary amine plant alkaloid. Produces peripheral effects similar to those of atropine. Unlike atropine, scopolamine has greater action on the CNS (observed at therapeutic doses). It has
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion)
 1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion) 1.3.1 SUMMARY OF PRODUCT CHARACTERISTICS This medicinal product is subject to additional monitoring. This will allow
1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion) 1.3.1 SUMMARY OF PRODUCT CHARACTERISTICS This medicinal product is subject to additional monitoring. This will allow
ZOFRAN TABLETS GlaxoSmithKline
 ZOFRAN TABLETS GlaxoSmithKline Ondansetron QUALITATIVE AND QUANTITATIVE COMPOSITION ZOFRAN tablets 4 mg: Each tablet contains ondansetron 4 mg as hydrochloride dihydrate. ZOFRAN tablets 8 mg: Each tablet
ZOFRAN TABLETS GlaxoSmithKline Ondansetron QUALITATIVE AND QUANTITATIVE COMPOSITION ZOFRAN tablets 4 mg: Each tablet contains ondansetron 4 mg as hydrochloride dihydrate. ZOFRAN tablets 8 mg: Each tablet
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Lidokain Isdin 40 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g cream contains 40 mg lidocaine. Excipients: Propylene glycol
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Lidokain Isdin 40 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g cream contains 40 mg lidocaine. Excipients: Propylene glycol
POLIORIX. Poliomyelitis Vaccine (Inactivated) IP. Inactivated Polio Virus Type 3 (Saukett strain)
 POLIORIX Poliomyelitis Vaccine (Inactivated) IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose (0.5 ml) of the vaccine cultivated on a continuous VERO cell line contains: Inactivated Polio Virus Type
POLIORIX Poliomyelitis Vaccine (Inactivated) IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose (0.5 ml) of the vaccine cultivated on a continuous VERO cell line contains: Inactivated Polio Virus Type
(PP XI) Dr. Samir Matloob
 DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET TRANDATE Injection labetalol hydrochloride injection 5 mg/ml Pharmaceutical Form Solution for injection. Qualitative and Quantitative Composition TRANDATE Injection: 20 ml glass
NEW ZEALAND DATA SHEET TRANDATE Injection labetalol hydrochloride injection 5 mg/ml Pharmaceutical Form Solution for injection. Qualitative and Quantitative Composition TRANDATE Injection: 20 ml glass
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY
 TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
למידע נוסף ניתן לעיין בעלון לרופא באתר משרד הבריאות או ליצור קשר טלפוני בטלפון
 חברת פרמה מדיס שמחה לבשר על תחילת שיווקו של תכשיר חדש. שם התכשיר: NEOSTIGMINE - HAMELN 2.5 MG/ML INJECTION צורת מינון: SOLUTION FOR INJECTION דרך מתן: I.M, I.V, S.C הרכב )חומר פעיל(: NEOSTIGMINE METHYLSULFATE
חברת פרמה מדיס שמחה לבשר על תחילת שיווקו של תכשיר חדש. שם התכשיר: NEOSTIGMINE - HAMELN 2.5 MG/ML INJECTION צורת מינון: SOLUTION FOR INJECTION דרך מתן: I.M, I.V, S.C הרכב )חומר פעיל(: NEOSTIGMINE METHYLSULFATE
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 40 g/l is a solution containing 40 g/l (4%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
WHO PACKAGE INSERT. GlaxoSmithKline Biologicals FluLaval. Dossier First - Chapter 1 to 10 for WHO
 77 WHO PACKAGE INSERT 11 Chapter 4_Annex 4.4-1_ WHO leaflet_en - Page 1 78 1. NAME OF THE MEDICINAL PRODUCT, suspension for injection Influenza vaccine (split virion, inactivated) 2. QUALITATIVE AND QUANTITATIVE
77 WHO PACKAGE INSERT 11 Chapter 4_Annex 4.4-1_ WHO leaflet_en - Page 1 78 1. NAME OF THE MEDICINAL PRODUCT, suspension for injection Influenza vaccine (split virion, inactivated) 2. QUALITATIVE AND QUANTITATIVE
NERVOUS SYSTEM NERVOUS SYSTEM. Somatic nervous system. Brain Spinal Cord Autonomic nervous system. Sympathetic nervous system
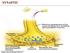 SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
SUMMARY OF PRODUCT CHARACTERISTICS. Flexbumin 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve 50 mg/ml solution for injection for dogs (AT, BE, BG, CY, CZ, DE, EL, ES, HR, HU, IE, IT, LU, NL, PT, RO,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve 50 mg/ml solution for injection for dogs (AT, BE, BG, CY, CZ, DE, EL, ES, HR, HU, IE, IT, LU, NL, PT, RO,
Granisetron Kabi, 1mg/ml, concentrate for solution for injection/infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Granisetron Kabi, 1mg/ml, concentrate for solution for injection/infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains granisetron
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Granisetron Kabi, 1mg/ml, concentrate for solution for injection/infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains granisetron
A specific opioid antagonist, such as naloxone immediately and completely reverses all actions of alfentanil.
 RAPIFEN DATA SHEET NAME OF THE MEDICINE RAPIFEN alfentanil 0.5 mg/ml injection PRESENTATION RAPIFEN is a sterile, preservative free, isotonic, aqueous solution containing alfentanil hydrochloride equivalent
RAPIFEN DATA SHEET NAME OF THE MEDICINE RAPIFEN alfentanil 0.5 mg/ml injection PRESENTATION RAPIFEN is a sterile, preservative free, isotonic, aqueous solution containing alfentanil hydrochloride equivalent
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
GlaxoSmithKline. Renal impairment. Hepatic impairment
 RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS:
 0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
Pharmacology of the Neuromuscular Junction (NMJ)
 Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
NEW ZEALAND DATASHEET
 1. PRODUCT NAME FLUMAZENIL CLARIS 0.1 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 0.1 mg flumazenil 1 5 ml ampoule contains 0.5 mg flumazenil. 1 10 ml ampoule
1. PRODUCT NAME FLUMAZENIL CLARIS 0.1 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 0.1 mg flumazenil 1 5 ml ampoule contains 0.5 mg flumazenil. 1 10 ml ampoule
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
PRODUCT MONOGRAPH. 10 mg/ml. Skeletal Neuromuscular Blocking Agent
 PRODUCT MONOGRAPH Pr ATRACURIUM BESYLATE INJECTION 10 mg/ml Skeletal Neuromuscular Blocking Agent Sandoz Canada Inc. Date of Revision: March 25, 2013 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Control
PRODUCT MONOGRAPH Pr ATRACURIUM BESYLATE INJECTION 10 mg/ml Skeletal Neuromuscular Blocking Agent Sandoz Canada Inc. Date of Revision: March 25, 2013 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Control
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 9 1. NAME OF THE MEDICINAL PRODUCT Magnesium sulfate-kalceks 500 mg/ml solution for injection, BP * 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution
SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 9 1. NAME OF THE MEDICINAL PRODUCT Magnesium sulfate-kalceks 500 mg/ml solution for injection, BP * 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution
Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules.
 Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Outer carton Multi-pack 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve
ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Outer carton Multi-pack 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve
Pharmacology of the Neuromuscular Junction (NMJ)
 Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
MUSCLE RELAXANTS. Mr. D.Raju, M.pharm, Lecturer
 MUSCLE RELAXANTS Mr. D.Raju, M.pharm, Lecturer Muscle Relaxants are classified as: I)Peripherally acting A.Neuromuscular blocking agents:- 1) Depolarizing muscle relaxants. 2) Non-depolarizing muscle relaxants
MUSCLE RELAXANTS Mr. D.Raju, M.pharm, Lecturer Muscle Relaxants are classified as: I)Peripherally acting A.Neuromuscular blocking agents:- 1) Depolarizing muscle relaxants. 2) Non-depolarizing muscle relaxants
Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein of which at least 95% is human albumin.
 1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
Ganglion blocking agents
 Ganglion blocking agents -out of date -Specifically act on the nicotinic receptors of both parasymphatetic and sympathetic ganglia - no selectivity toward PG or SG -These drugs are non-depolarizing, competitive
Ganglion blocking agents -out of date -Specifically act on the nicotinic receptors of both parasymphatetic and sympathetic ganglia - no selectivity toward PG or SG -These drugs are non-depolarizing, competitive
Core Safety Profile. Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV FI/H/PSUR/0010/002 Date of FAR:
 Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
NEW ZEALAND DATA SHEET
 1 PRODUCT NAME Acetylcysteine 200 mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml (as N-acetylcysteine) Each 10 ml ampoule contains 2 g N-acetylcysteine Excipients
1 PRODUCT NAME Acetylcysteine 200 mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml (as N-acetylcysteine) Each 10 ml ampoule contains 2 g N-acetylcysteine Excipients
PRODUCT INFORMATION. NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection
 PRODUCT INFORMATION NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection DESCRIPTION Molecular formulae. Potassium chloride: KCl; sodium chloride: NaCl; calcium chloride dihydrate:
PRODUCT INFORMATION NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection DESCRIPTION Molecular formulae. Potassium chloride: KCl; sodium chloride: NaCl; calcium chloride dihydrate:
QUININE DIHYDROCHLORIDE 6% Sterile Concentrate 600 mg in 10 ml
 QUININE DIHYDROCHLORIDE 6% Sterile Concentrate 600 mg in 10 ml DILUTE BEFORE ADMINISTRATION NAME OF THE MEDICINE Quinine Dihydrochloride BP Chemical Name: - (8S, 9R)-6 -methoxycinchonan-9-ol dihydrochloride.
QUININE DIHYDROCHLORIDE 6% Sterile Concentrate 600 mg in 10 ml DILUTE BEFORE ADMINISTRATION NAME OF THE MEDICINE Quinine Dihydrochloride BP Chemical Name: - (8S, 9R)-6 -methoxycinchonan-9-ol dihydrochloride.
Active ingredients: Metoclopramide Hydrochloride mg Equivalent to metoclopramide hydrochloride anhydrous mg
 Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
RAPIFEN DATA SHEET 1. NAME OF THE MEDICINE 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 3. PHARMACEUTICAL FORM 4. CLINICAL PARTICULARS
 RAPIFEN DATA SHEET 1. NAME OF THE MEDICINE RAPIFEN alfentanil 0.5 mg/ml injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of RAPIFEN contains alfentanil hydrochloride 544 micrograms, equivalent
RAPIFEN DATA SHEET 1. NAME OF THE MEDICINE RAPIFEN alfentanil 0.5 mg/ml injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of RAPIFEN contains alfentanil hydrochloride 544 micrograms, equivalent
FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. (LyophilIzed) 50mg
 FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. AMPHONEX (LyophilIzed) 50mg For Intravenous infusion to hospitalized patients
FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. AMPHONEX (LyophilIzed) 50mg For Intravenous infusion to hospitalized patients
Priorix TM Measles, mumps and rubella vaccine (live, attenuated)
 Priorix TM Measles, mumps and rubella vaccine (live, attenuated) QUALITATIVE AND QUANTITATIVE COMPOSITION Priorix is a lyophilised mixed preparation of the attenuated Schwarz measles, RIT 4385 mumps (derived
Priorix TM Measles, mumps and rubella vaccine (live, attenuated) QUALITATIVE AND QUANTITATIVE COMPOSITION Priorix is a lyophilised mixed preparation of the attenuated Schwarz measles, RIT 4385 mumps (derived
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME Asthalin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Salbultamol respiratory solution 3 PHARMACEUTICAL FORM Asthalin is a plastic ampoule presentation containing a sterile,
New Zealand Datasheet 1 PRODUCT NAME Asthalin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Salbultamol respiratory solution 3 PHARMACEUTICAL FORM Asthalin is a plastic ampoule presentation containing a sterile,
