Propranolol for infantile haemangiomas: single centre experience of 250 cases and proposed therapeutic protocol
|
|
|
- Gerard Franklin
- 5 years ago
- Views:
Transcription
1 Editor s choice Scan to access more free content 1 Department of Dermatology, Great Ormond Street Hospital for Children, London, UK 2 Department of Plastic and Reconstructive Surgery, Great Ormond Street Hospital for Children, London, UK Correspondence to Dr Lea Solman, Department of Paediatric Dermatology, Great Ormond Street Hospital, Level 6, Southwood Building, London WC1N 3JH, UK; lea.solmankosutic@gosh.nhs. uk LS and AM contributed equally. Received 29 April 2014 Revised 19 July 2014 Accepted 21 July 2014 Published Online First 14 August 2014 To cite: Solman L, Murabit A, Gnarra M, et al. Arch Dis Child 2014;99: Propranolol for infantile haemangiomas: single centre experience of 250 cases and proposed therapeutic protocol Lea Solman, 1 Amera Murabit, 2 Maria Gnarra, 1 John I Harper, 1 Samira B Syed, 1 Mary Glover 1 ABSTRACT Objective To assess the safety and efficacy of systemic propranolol for the treatment of complicated infantile haemangiomas. Design Retrospective review of case notes of paediatric patients treated with propranolol for complicated infantile haemangiomas. Setting Tertiary care children s hospital. Patients All paediatric patients with complicated infantile haemangiomas who commenced treatment with propranolol from July 2008 to December 2011 and have completed treatment for at least 3 months. Results 250 patients were treated with propranolol; 34.4% were premature and 5.6% postmature. Indications for propranolol included: vision compromise (42.0%), bleeding and/or ulceration (30.4%) airway obstruction (8.8%), feeding difficulty (8.4%), risk of permanent disfigurement (4.4%) and other (6%) (nasal obstruction, auditory canal obstruction, large haemangioma, compression of neck structure and spinal cord). Median age at beginning of treatment was 4.5 months. Median age at end of treatment was 16.7 months. Median length of therapy was 11.8 months. Adverse effects (such as wheezing, worsening of ulceration, sleep disturbance, diarrhoea) occurred in 38 patients (15.2%), leading to modifications in management in 26 patients (10.4%). 240 patients (96%) had good to excellent response to treatment. 20 patients (8%) experienced regrowth of the haemangioma on cessation of propranolol and six patients (2.4%) required propranolol to be restarted. Conclusions In appropriately selected patients, propranolol is a safe and effective treatment for infantile haemangiomas. INTRODUCTION Infantile haemangiomas (IHs) are the most common benign vascular tumours of infancy affecting up to 10% of children, with a female to male ratio of 3:1 1 and a higher prevalence in the Caucasian population. 2 Prematurity 3 and low birth weight (<1500 grams) are considered potential predisposing factors. IHs are characterised by a triphasic clinical and histological evolution with rapid growth ( proliferative phase) followed by a plateau period and a slow spontaneous involution (involutive phase). 4 More than 60% of IHs occur on the face, head and neck. 5 Although benign, the involvement of the eyelids, nasal tip, lips and ears may endanger breathing, feeding and vision or lead to irreversible disfigurement. 6 Bleeding, ulceration and subsequent What is already known on this topic? Infantile haemangiomas are the most common paediatric vascular tumours and most do not require intervention. Propranolol has become a first-line treatment in the management of infantile haemangiomas. What this study adds? 1132 Solman L, et al. Arch Dis Child 2014;99: doi: /archdischild Propranolol is a safe and effective treatment of infantile haemangiomas. The adverse effects are mild if the patients are carefully selected, monitored and parents adequately educated about the treatment. We have simplified our treatment protocol, reducing the need for pretreatment blood testing, ECG, echocardiogram, and ongoing monitoring of blood pressure and heart rate. infection can occur in up to 20% of cases. 7 Early recognition and treatment of critical lesions help in preventing or minimising complications. Before 2008, treatment for IH had included systemic and intralesional corticosteroids 8 and α-interferon, 9 which were associated with significant adverse effects. In 2008, the first report of the successful use of propranolol radically changed therapy and, since then, propranolol has become the first-line therapeutic agent in the management of IHs. 10 Propranolol is a non-selective β-adrenergic receptor blocker traditionally used for cardiovascular and anxiety disorders. Although the specific mechanism of action on IH remains largely unknown, propranolol appears to produce clinical improvement by inducing vasoconstriction and apoptosis and decreasing expression of pro-angiogenic factors. 11 While propranolol s mechanism of action on IH is still under investigation, it has been shown to induce a better and faster response in IH management than systemic steroids, and is associated with fewer adverse effects. The purpose of this paper is to present our experience with propranolol for the management of IH in a specialised tertiary care hospital. For this
2 group of patients, a therapeutic protocol was designed when there was very limited experience of propranolol for this indication. 14 As a result of our experience, we have modified this protocol, reducing the need for pretreatment blood testing, ECG, echocardiogram (ECHO), and ongoing monitoring of blood pressure (BP) and heart rate (HR). PATIENTS AND METHODS A retrospective case notes review was performed on 250 paediatric patients treated with propranolol for IH at our hospital from July 2008 to December We identified all patients diagnosed with IH for which propranolol was indicated and prescribed according to our protocol, but only those who had completed their course of treatment at least 3 months before were included. Pretreatment investigations included a full blood count (FBC), urea, creatine, electrolytes, urinalysis, liver function tests (LFT), glucose, ECG, ECHO and clinical photography. Administration of oral liquid propranolol was dosed based on patient weight starting from an initial dose of 1 mg/kg/day, divided in three equal doses, for the first week followed by 2 mg/kg/day, divided in three equal doses, in the second week and then continuous dose adjustments according to the child s weight at each clinical review. The initiation dose for children born prematurely or diagnosed with other comorbidities was 0.5 mg/kg/day divided in three equal doses for the first week, followed by 1 mg/kg/day divided in three equal doses in the second week. Further increases were based on clinical judgement. Children were observed inhospital for 2 4 h with initiation and initial dosage elevation of propranolol therapy ( premature/comorbid patients were observed for at least 4 8 h). Observations included assessment of general well-being, HR and BP monitoring every 30 min. These observations were compared with the age-related referenced data. As outpatients, BP and HR were checked on a weekly basis for the first 4 6 weeks, then fortnightly for the remainder of the therapeutic period. For the measurements taken out of our hospital, we recommended maintaining a BP greater than 70 mm Hg systolic and 35 mm Hg diastolic, with an HR above 100/min and notifying our departmental nurses and/or their local doctor if there are any concerns. Parents/caregivers were made aware of known side effects prior to commencement of therapy, including bradycardia, heart failure, hypotension, cardiac conduction disorder, bronchospasm, peripheral vasoconstriction, weakness and fatigue, sleep disturbance and hypoglycaemia. Parents were advised to stop the propranolol in case of serious adverse effect and to contact a member of our team. Assessment included evaluation by the clinician as to whether there was no response, poor, good or excellent response, plus photographic documentation of IH at each visit. We defined excellent response as 75% 100% improvement, good response as 50% 75% improvement, poor response as 25% 50% improvement and no response as less than 25% improvement. Propranolol therapy was terminated, according to individual clinical response, by halving the dose for 2 weeks and further halving it for another 2 weeks and then stopped, so that propranolol therapy was gradually reduced and stopped over the course of about 1 month. Data regarding patient demographics, haemangioma characteristics, indications for propranolol treatment, associated investigations, management modifications, concomitant therapies, side effects and overall effectiveness were compiled into a separate database and analysed. RESULTS In all, 201 patients were girls and 49 were boys. About a third of patients (86 patients) were born prematurely, and 14 patients were born after 42-week gestation. IHs were located on the face (181 lesions), head and neck (57 lesions), trunk (36 lesions) and extremities (23 lesions). Concomitant visceral lesions were documented in nine patients (3.6%), seven of which were localised to the liver and the remaining two were intraspinal. Primary indications for propranolol included: vision compromise (42.0%), bleeding and/or ulceration (30.4%), airway obstruction (8.8%), feeding difficulty (8.4%), risk of permanent disfigurement (4.4%), nasal obstruction (four patients, 1.6%), auditory canal obstruction (three patients, 1.2%), widespread haemangiomas (four patients, 1.6%), compression of neck structures (three patients, 1.2%) and compression of spinal cord (one patient, 0.4%). Pretreatment blood test investigations and urinalysis did not reveal abnormalities contraindicating propranolol administration in any of our patients. Pretreatment ECG testing showed no signs of cardiac rhythm irregularities (not including sinus tachycardia). Pretreatment ECHO was normal in 208 patients (83.2%), clinically inconsequential in 34 patients (13.6%) and abnormal in eight patients (3.2%). Pathologies included VSD (four patients), large PDA (two patients), aortic coarctation (one patient) and tetralogy of Fallot (one patient). Four patients required a surgical procedure (VSD closure in one patient, aortic coarctation repair in one patient, PDA ligation in one patient and tetralogy of Fallot repair in one patient). The cardiac defects were already known in seven of these patients. One patient presented to the department of paediatric dermatology first with a large segmental facial haemangioma and pretreatment ECHO revealed aortic coarctation. The inhospital monitoring period was uneventful in all of the patients presented in this series. The median age at the beginning of propranolol treatment was 4.5 months (range months). The median age at the end of propranolol treatment was 16.7 months (range months). The median length of propranolol therapy was 11.8 months (range months). In all, 65 patients started their treatment after the age of 6 months. Overall, 29 patients (11.6%) received oral prednisolone. All had presented with functional lesions (obstruction of airway or vision) and prescribed prednisolone either before or in the early stages of usage of propranolol for IH. Table 1 Adverse effects (n=250) Observed adverse effect N (%) Wheezing 17 (6.8) Usually bronchial hyperactivity in the setting of viral respiratory infection Sleep disturbance 9 (3.6) Worsening of the ulceration 4 (1.6) Asymptomatic bradycardia 2 (0.8) Peripheral cyanosis 2 (0.8) Vomiting 2 (0.8) Diarrhoea 1 (0.4) Asymptomatic hypotension 1 (0.4) Solman L, et al. Arch Dis Child 2014;99: doi: /archdischild Drug therapy
3 Adverse events were observed in 38 patients (15.2%) (table 1). Two patients had asymptomatic bradycardia and one patient had asymptomatic hypotension. A total of 26 patients (10.4%) had alterations in therapy including dosage reduction/alterations due to side effects (eight patients, 3.2%), temporary withholding of propranolol (six patients, 2.4%) and premature cessation of treatment (12 patients, 4.8%). Overall, 230 patients (92.0%) did not experience enlargement or recurrence of the lesion after propranolol therapy was stopped. Of the 20 patients (8.0%) who did, 6 (2.4%) required propranolol to be restarted and the other 14 (5.6%) showed reduction in size over time, without restarting propranolol. The average length of treatment in the patients with rebound growth was 46 days shorter than in patients with no rebound growth. Table 2 Treatment protocol Before starting propranolol Full clinical history and examination including HR, BP and SpO 2 Parents education and written information given Clinical photography ECHO and ECG in selected patients (please see table below) ECG If the HR is below normal for age History of arrhythmia or arrhythmia on examination Family history of congenital heart condition, arrhythmia or maternal history of connective tissue disease Dosage regime Week 1 Week 2 Patients with suspected PHACE syndrome Observation and monitoring Weight >3.5 kg and no comorbidities Weight <3.5 kg and/or comorbidities Other investigations* Investigations Blood tests: Thyroid function test Liver function tests Full blood count Abdominal ultrasound MRA brain and neck MRI spine Referrals ENT team Ophthalmology team Continuation of the treatment Reviews Increments Treatment stopping ECG and ECHO Overall, outcome after stopping the propranolol was graded as no response, poor, good or excellent. A total of 240 patients (96.0%) had good or excellent overall outcome. DISCUSSION Our experience has shown propranolol to be an effective treatment for IH. Although systemic β-blockers have the potential to cause adverse effects, the complication rate in our study was low and the adverse effects were not life threatening. In all, 6.8% of our patients experienced wheezing, usually occurring with viral respiratory infection. Wheezing is otherwise common in infancy, with a reported prevalence of 28.5% 32.0%. Adverse events reported in recent literature, including hypoglycaemia and hyperkalaemia, were not experienced in our Patients with history, symptoms or signs of cardiovascular disease, including patients with suspected high output heart failure Patients with large segmental haemangioma of the face and neck and suspected PHACE syndrome 1 mg/kg/day divided into three equal doses 2 mg/kg/day divided into three equal doses 0.5 mg/kg/day and very cautious increase of the dose in small increments until MRA excludes arterial anomalies of head and neck BP and HR immediately before the dose and every 30 min for 2 h BP and HR immediately before the dose and every 30 min for 4 h or longer Group of patients Liver haemangioma Parotid haemangioma PHACE syndrome Liver haemangioma Bleeding haemangiomas More than 10 cutaneous haemangiomas Perianal and perineal haemangioma crossing the midline and/or extending into gluteal cleft Large segmental haemangioma of head and neck with suspected PHACE syndrome Plaque haemangioma in lumbosacral area crossing the midline or perianal/perineal haemangioma extending into gluteal cleft Suspected airway haemangioma Peri-ocular haemangioma 4 6 weeks after starting treatment, then 3 4 monthly Increments greater than 0.5 mg/kg/day should include monitoring of HR and BP for 2 h Usually at months of age but can be longer Gradual dose reduction over 2 4 weeks *Propranolol can be commenced while the investigations/results are pending. BP, blood pressure; ECHO, echocardiogram; ENT, ear, nose and throat; HR, heart rate; MRA, magnetic resonance angiography Solman L, et al. Arch Dis Child 2014;99: doi: /archdischild
4 series. For most of our patients, especially those who were otherwise healthy, our original full therapeutic protocol is unnecessary as neither the pretreatment investigations nor the ongoing community based monitoring of BP and HR leads to deferment or alteration of the course of propranolol therapy. As a result, we have updated our treatment protocol (table 2). Our updated treatment protocol is similar to the recommendations set by experienced group consensus, which was based on comprehensive literature review. 20 The Consensus Report acknowledges that their recommendations are conservative in nature and anticipate revisions. There are minor differences between both protocols. The Consensus Report recommends outpatient initiation of propranolol therapy for patients older than 8 weeks corrected age, with adequate social support and without significant comorbidities. Our protocol recommends that patients are monitored for 2 h in the hospital and for 4 h or longer in patients weighting less than 3.5 kg or with comorbidities. Both protocols are in agreement with regard to pretreatment investigations, target dose, frequency and ongoing monitoring. As well, both protocols emphasise the importance of diagnosing PHACE syndrome, recognising the inherent risks involved in treating PHACE syndrome patients with propranolol and ensuring that that they are managed in close consultation with cardiology department. Our protocol extends even further by recommending when other investigations are indicated, as well as when to consult or refer to other specialties. Moreover, our protocol guides recommendations for the frequency of follow-up, the length of therapy and the appropriate manner to discontinue propranolol at the end of the treatment course. Updated treatment protocol Before initiation of propranolol for IH, screening for risks associated with propranolol use should be performed. 20 The prescribing physician should take complete and thorough history and perform physical examination with special emphasis on the signs and symptoms of cardiovascular and pulmonary disease. Pretreatment ECG and ECHO should be performed only in selected group of patients (table 2). Contraindications for propranolol include cardiogenic shock, sinus bradycardia, hypotension, greater than first-degree heart block, heart failure, bronchial hypersensitivity and hypersensitivity to propranolol hydrochloride. 20 Parents should be given information about the treatment and the possible adverse effects should be explained thoroughly. A leaflet should be provided including the direct contact number of the responsible team. Parents should be informed not to use lignocaine (usually used as a teething gel) and salbutamol. Propranolol has a potential to lower hepatic blood flow and may increase the toxicity of the lignocaine. 21 Salbutamol is β-adrenergic receptor agonist; therefore, an alternative medication (eg, ipratropium bromide) should be used in case of bronchospasm. Parents should be instructed to ensure that their child is fed regularly to avoid prolonged fasts. Propranolol should be discontinued during intercurrent illness, especially in the setting of restricted oral intake. 20 The starting dose of propranolol is 1 mg/kg/day in three divided doses. HR and BP are measured immediately before and then every 30 min for 2 h after administering the propranolol. Blood glucose is not measured routinely, unless the patient is having signs or symptoms of hypoglycaemia. For infants weighing less than 3.5 kg or with comorbidities, it may be appropriate to continue monitoring for 4 h or longer. This procedure is repeated after 1 week when the dose is increased to 2 mg/kg/day. In patients with suspected PHACE syndrome, it is prudent to start with the lower dose of propranolol 0.5 mg/kg/day in three divided doses and very cautiously increase the dose in small increments as propranolol may theoretically increase the risk of the stroke in these patients if arterial anomalies are present. After the magnetic resonance angiography (MRA) of the head and neck has been performed and arterial anomalies of head and neck have been excluded, the dose can be increased to 2 mg/kg/ day. In selected patients, additional investigations and referrals should be performed but the propranolol can be commenced while the results are pending. Thyroid function test should be done in patients with liver, parotid or very large haemangiomas to exclude consumptive hypothyroidism and in patients with suspected PHACE syndrome to exclude congenital hypothyroidism. LFT should be done in patients with liver haemangioma and FBC in patients with bleeding haemangiomas. Patients with more than 10 cutaneous lesions 22 and patients with perianal/ perineal haemangioma crossing the midline and/or extending into a gluteal cleft should have an abdominal ultrasound. MRA of the head and neck should be done in patients with suspected PHACE syndrome. Patients with haemangioma in lumbosacral area or perianal haemangioma extending into gluteal cleft should have MRI of the spine. Patients with peri-ocular haemangioma should be referred to ophthalmology department and patients with suspected airway haemangioma should be referred to the ear, nose and throat team. Ideally, the patient should be reviewed between 4 and 6 weeks after starting treatment, and then approximately 3 4 monthly. Monthly dose adjustment according to the weight of the child is needed unless the haemangioma is responding well to the treatment. Increments that are greater than 0.5 mg/kg/day should include monitoring of the HR and BP for 2 h. Occasionally, higher doses than 2 mg/kg/day of propranolol are required. If any adverse effects occur, the dose of propranolol should be temporarily withheld, lowered or the treatment should be stopped based on the clinical judgement of prescribing physician. The time of cessation of growth of haemangiomas is very variable, and so the age when propranolol can be stopped, without further growth occurring, is also very variable. The treatment is usually stopped at months of age, but substantially longer treatment may be required. When the decision has been made to take the child off propranolol, we recommend gradual dose reduction over 2 4 weeks. Our experience has shown propranolol to be an effective and safe treatment for IH. On the basis of this experience, we have introduced a simplified therapeutic protocol. Acknowledgements Department of Dermatology Clinical Nurse Specialists: Jane Linward and Hilary Kennedy for their assistance. Contributors LS, AM, MG, SBS, JIH and MGl initiated the study design. LS, AM and MG collected the data. LS, AM and MG provided analysis and interpretation of the data. LS and AM drafted the manuscript. MG, SBS, JIH and MGl provided critical revision of the manuscript for important intellectual content. Competing interests None. Patient consent Obtained. Provenance and peer review Not commissioned; externally peer reviewed. REFERENCES 1 Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. JPediatr2007;150: Sundine MJ, Wirth GA. Hemangiomas: an overview. Clin Pediatr (Phila) 2007;46: Amir J, Metzker A, Krikler R, et al. Strawberry hemangioma in preterm infants. Pediatr Dermatol 1986;3: Solman L, et al. Arch Dis Child 2014;99: doi: /archdischild Drug therapy
5 4 Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7 9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol 2005;22: Haggstrom AN, Lammer EJ, Schneider RA, et al. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics 2006;117: Thomson HG, Lanigan M. The Cyrano nose: a clinical review of hemangiomas of the nasal tip. Plast Reconstr Surg 1979;63: Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr 2007; , 89 e1. 8 Greene AK. Corticosteroid treatment for problematic infantile hemangioma: evidence does not support an increased risk for cerebral palsy. Pediatrics 2008;121: Fonseca Junior NL, Cha SB, Cartum J, et al. Therapeutical effectiveness of interferon alpha in a child with craniofacial giant hemangioma: case report. Arq Bras Oftalmol 2008;71: Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358: Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 2010;163: Balma-Mena A, Chakkittakandiyil A, Weinstein M, et al. Propranolol in the management of infantile hemangiomas: clinical response and predictors. J Cutan Med Surg 2012;16: Fuchsmann C, Quintal MC, Giguere C, et al. Propranolol as first-line treatment of head and neck hemangiomas. Arch Otolaryngol Head Neck Surg 2011;137: Manunza F, Syed S, Laguda B, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br J Dermatol 2010;162: Visser CA, Garcia-Marcos L, Eggink J, et al. Prevalence and risk factors of wheeze in Dutch infants in their first year of life. Pediatr Pulmonol 2010;45: Taussig LM, Wright AL, Holberg CJ, et al. Tucson Children s Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003;111:661 75; quiz Maguiness SM, Frieden IJ. Management of difficult infantile haemangiomas. Arch Dis Child 2012;97: Pavlakovic H, Kietz S, Lauerer P, et al. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics 2010;126:e Cavalli R, Buffon RB, de Souza M, et al. Tumor lysis syndrome after propranolol therapy in ulcerative infantile hemangioma: rare complication or incidental finding? Dermatology 2012;224: Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013;131: Bax ND, Tucker GT, Lennard MS, et al. The impairment of lignocaine clearance by propranolol--major contribution from enzyme inhibition. Br J Clin Pharmacol 1985;19: Vredenborg AD, Janmohamed SR, de Laat PC, et al. Multiple cutaneous infantile haemangiomas and the risk of internal haemangioma. Br J Dermatol 2013;169: Arch Dis Child: first published as /archdischild on 14 August Downloaded from Solman L, et al. Arch Dis Child 2014;99: doi: /archdischild on 15 September 2018 by guest. Protected by copyright.
SWISS SOCIETY OF NEONATOLOGY. Multiple infantile hemangiomas in a preterm infant
 SWISS SOCIETY OF NEONATOLOGY Multiple infantile hemangiomas in a preterm infant November 2017 Heschl K, Romaine Arlettaz R, Department of Neonatology (HK, AR), University Hospital Zurich, Switzerland Title
SWISS SOCIETY OF NEONATOLOGY Multiple infantile hemangiomas in a preterm infant November 2017 Heschl K, Romaine Arlettaz R, Department of Neonatology (HK, AR), University Hospital Zurich, Switzerland Title
Summary of the risk management plan (RMP) for Hemangiol (propranolol)
 EMA/122592/2014 Summary of the risk management plan (RMP) for Hemangiol (propranolol) This is a summary of the risk management plan (RMP) for Hemangiol, which details the measures to be taken in order
EMA/122592/2014 Summary of the risk management plan (RMP) for Hemangiol (propranolol) This is a summary of the risk management plan (RMP) for Hemangiol, which details the measures to be taken in order
Infantile Hemangiomas: Not Just Strawberries
 Infantile Hemangiomas: Not Just Strawberries Ilona J. Frieden M.D. Professor of Dermatology & Pediatrics UC San Francisco Conflict of Interests: Consultant: Pierre Fabre Off-label discussion: I do intend
Infantile Hemangiomas: Not Just Strawberries Ilona J. Frieden M.D. Professor of Dermatology & Pediatrics UC San Francisco Conflict of Interests: Consultant: Pierre Fabre Off-label discussion: I do intend
Beta-blockers in the Treatment of Infantile Hemangiomas 5yrs experience
 Beta-blockers in the Treatment of Infantile Hemangiomas 5yrs experience Josef Mališ V.Stará, S.Klovrzová, L.Nováková, M.Kynčl, A.Sukop, B.Kocmichová, Š.Čapková, K.Bláhová Dept. of Pediatric Hematology/Oncology
Beta-blockers in the Treatment of Infantile Hemangiomas 5yrs experience Josef Mališ V.Stará, S.Klovrzová, L.Nováková, M.Kynčl, A.Sukop, B.Kocmichová, Š.Čapková, K.Bláhová Dept. of Pediatric Hematology/Oncology
PAEDIATRIC HOSPITAL LEVEL ESSENTIAL MEDICINES LIST CHAPTER 5: DERMATOLOGY NEMLC 30 JUNE 2016
 PAEDIATRIC HOSPITAL LEVEL ESSENTIAL MEDICINES LIST CHAPTER 5: DERMATOLOGY NEMLC 30 JUNE 2016 PICTURES OF CONDITIONS: The Paediatric Expert Review Committee (ERC) recommended that the Dermatology Chapter
PAEDIATRIC HOSPITAL LEVEL ESSENTIAL MEDICINES LIST CHAPTER 5: DERMATOLOGY NEMLC 30 JUNE 2016 PICTURES OF CONDITIONS: The Paediatric Expert Review Committee (ERC) recommended that the Dermatology Chapter
QUICK REFERENCE GUIDE
 QUICK REFERENCE GUIDE The recommendations in this publication do not indicate an exclusive course of treatment or serve as a standard of care. Variations, taking into account individual circumstances,
QUICK REFERENCE GUIDE The recommendations in this publication do not indicate an exclusive course of treatment or serve as a standard of care. Variations, taking into account individual circumstances,
Propranolol for Infantile Hemangiomas: A Review
 Curr Derm Rep (2012) 1:179 185 DOI 10.1007/s13671-012-0026-6 PEDIATRICS (R SIDBURY, SECTION EDITOR) Propranolol for Infantile Hemangiomas: A Review Marcia Hogeling Published online: 23 September 2012 #
Curr Derm Rep (2012) 1:179 185 DOI 10.1007/s13671-012-0026-6 PEDIATRICS (R SIDBURY, SECTION EDITOR) Propranolol for Infantile Hemangiomas: A Review Marcia Hogeling Published online: 23 September 2012 #
Clinical Policy Bulletin: Infantile Hemangioma
 Infantile Hemangioma Page 1 of 13 Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Infantile Hemangioma Revised April 2014 Number:
Infantile Hemangioma Page 1 of 13 Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Infantile Hemangioma Revised April 2014 Number:
Angina pectoris due to coronary atherosclerosis : Atenolol is indicated for the long term management of patients with angina pectoris.
 Lonet Tablet Description Lonet contains Atenolol, a synthetic β1 selective (cardioselective) adrenoreceptor blocking agent without membrane stabilising or intrinsic sympathomimetic (partial agonist) activity.
Lonet Tablet Description Lonet contains Atenolol, a synthetic β1 selective (cardioselective) adrenoreceptor blocking agent without membrane stabilising or intrinsic sympathomimetic (partial agonist) activity.
Evaluation of hemangioma management in Erbil city
 Evaluation of hemangioma management in Erbil city Received: 30/9/2013 Accepted: 30/1/2014 Introduction Jalal Hamasalih Fattah * Wesam Amjad Kaka ** Abstract Background and objective: The term hemangioma
Evaluation of hemangioma management in Erbil city Received: 30/9/2013 Accepted: 30/1/2014 Introduction Jalal Hamasalih Fattah * Wesam Amjad Kaka ** Abstract Background and objective: The term hemangioma
Quick Reference Guide SUPPORTED BY:
 Quick Reference Guide SUPPORTED BY: 1 The recommendations in this publication do not indicate an exclusive course of treatment or serve as a standard of care. Variations, taking into account individual
Quick Reference Guide SUPPORTED BY: 1 The recommendations in this publication do not indicate an exclusive course of treatment or serve as a standard of care. Variations, taking into account individual
Cleft-Craniofacial Center
 Cleft-Craniofacial Center A Pioneering T eam 2 Welcome to the Cleft-Craniofacial Center at Children s Hospital of Pittsburgh The Cleft-Craniofacial Center at Children s Hospital of Pittsburgh has been
Cleft-Craniofacial Center A Pioneering T eam 2 Welcome to the Cleft-Craniofacial Center at Children s Hospital of Pittsburgh The Cleft-Craniofacial Center at Children s Hospital of Pittsburgh has been
What Syndrome s That? Syndromes Associated with Vascular Anomalies
 What Syndrome s That? Syndromes Associated with Vascular Anomalies Dawn Siegel, MD Medical College of Wisconsin American Academy of Dermatology Meeting San Diego, CA Sat. February 17 th, 2018 Learning
What Syndrome s That? Syndromes Associated with Vascular Anomalies Dawn Siegel, MD Medical College of Wisconsin American Academy of Dermatology Meeting San Diego, CA Sat. February 17 th, 2018 Learning
Western Health Specialist Clinics Access & Referral Guidelines
 Western Health Specialist Clinics Access & Referral Guidelines Paediatric Medicine Clinics at Western Health: Western Health operates the following Specialist Clinic services for patients who require assessment
Western Health Specialist Clinics Access & Referral Guidelines Paediatric Medicine Clinics at Western Health: Western Health operates the following Specialist Clinic services for patients who require assessment
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Core Safety Profile. Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg. Date of FAR:
 Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Anatomy & Physiology
 1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
Screening for Critical Congenital Heart Disease
 Screening for Critical Congenital Heart Disease Caroline K. Lee, MD Pediatric Cardiology Disclosures I have no relevant financial relationships or conflicts of interest 1 Most Common Birth Defect Most
Screening for Critical Congenital Heart Disease Caroline K. Lee, MD Pediatric Cardiology Disclosures I have no relevant financial relationships or conflicts of interest 1 Most Common Birth Defect Most
NUTRITIONAL REQUIREMENTS
 NUTRITION AIMS To achieve growth and nutrient accretion similar to intrauterine rates To achieve best possible neurodevelopmental outcome To prevent specific nutritional deficiencies Target population
NUTRITION AIMS To achieve growth and nutrient accretion similar to intrauterine rates To achieve best possible neurodevelopmental outcome To prevent specific nutritional deficiencies Target population
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
Therapeutic Effect of Propranolol in Mexican Patients with Infantile Hemangioma
 Drugs - Real World Outcomes (2016) 3:25 31 DOI 10.1007/s40801-015-0052-3 ORIGINAL RESEARCH ARTICLE Therapeutic Effect of Propranolol in Mexican Patients with Infantile Hemangioma Saul Castaneda 1,3 Esbeydy
Drugs - Real World Outcomes (2016) 3:25 31 DOI 10.1007/s40801-015-0052-3 ORIGINAL RESEARCH ARTICLE Therapeutic Effect of Propranolol in Mexican Patients with Infantile Hemangioma Saul Castaneda 1,3 Esbeydy
Systemic treatment with propranolol for. Topical treatment with propranolol gel as a supplement to the existing treatment of hemangiomas
 Topical treatment with propranolol gel as a supplement to the existing treatment of hemangiomas Markus Schneider, Andreas Reimer, Hansjoerg Cremer, Peter Ruef Heilbronn, Germany Background: Systemic treatment
Topical treatment with propranolol gel as a supplement to the existing treatment of hemangiomas Markus Schneider, Andreas Reimer, Hansjoerg Cremer, Peter Ruef Heilbronn, Germany Background: Systemic treatment
Completed: 4/11/ :26:32 AM
 Tangtiphaiboontana, Jennifer MS1 Summer Basic, Biomedical, or Clinical and Translational Research Neurological Exam Findings of PHACE Syndrome and Associations with Posterior Fossa Abnormalities and Hemangioma
Tangtiphaiboontana, Jennifer MS1 Summer Basic, Biomedical, or Clinical and Translational Research Neurological Exam Findings of PHACE Syndrome and Associations with Posterior Fossa Abnormalities and Hemangioma
7.2 Part VI.2 Elements for a Public Summary
 7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate).
 VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
abstract ARTICLE BACKGROUND AND OBJECTIVES: Propranolol is first-line therapy for problematic infantile
 Rebound Growth of Infantile Hemangiomas After Propranolol Therapy Sonal D. Shah, MD, a Eulalia Baselga, MD, b Catherine McCuaig, MD, c Elena Pope, MD, d Julien Coulie, MD, e Laurence M. Boon, MD, e Maria
Rebound Growth of Infantile Hemangiomas After Propranolol Therapy Sonal D. Shah, MD, a Eulalia Baselga, MD, b Catherine McCuaig, MD, c Elena Pope, MD, d Julien Coulie, MD, e Laurence M. Boon, MD, e Maria
Drugs used in obstetrics
 Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Cardiac Considerations and Care in Children with Neuromuscular Disorders
 Cardiac Considerations and Care in Children with Neuromuscular Disorders - importance of early and ongoing treatment, management and available able medications. Dr Bo Remenyi Department of Cardiology The
Cardiac Considerations and Care in Children with Neuromuscular Disorders - importance of early and ongoing treatment, management and available able medications. Dr Bo Remenyi Department of Cardiology The
CYANOTIC CONGENITAL HEART DISEASES. PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU
 CYANOTIC CONGENITAL HEART DISEASES PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU DEFINITION Congenital heart diseases are defined as structural and functional problems of the heart that are
CYANOTIC CONGENITAL HEART DISEASES PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU DEFINITION Congenital heart diseases are defined as structural and functional problems of the heart that are
STUDY. Early White Discoloration of Infantile Hemangioma
 STUDY Early White Discoloration of Infantile Hemangioma A Sign of Impending Ulceration Sheilagh M. Maguiness, MD; William Y. Hoffman, MD; Tim H. McCalmont, MD; Ilona J. Frieden, MD Objective: To evaluate
STUDY Early White Discoloration of Infantile Hemangioma A Sign of Impending Ulceration Sheilagh M. Maguiness, MD; William Y. Hoffman, MD; Tim H. McCalmont, MD; Ilona J. Frieden, MD Objective: To evaluate
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating)
 Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate)
 EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
Head and neck cancer - patient information guide
 Head and neck cancer - patient information guide The development of reconstructive surgical techniques in the last 20 years has led to major advances in the treatment of patients with head and neck cancer.
Head and neck cancer - patient information guide The development of reconstructive surgical techniques in the last 20 years has led to major advances in the treatment of patients with head and neck cancer.
Oral Propranolol: A Useful Treatment for Infantile Hemangioma
 J. Biomedical Science and Engineering, 2015, 8, 441-450 Published Online July 2015 in SciRes. http://www.scirp.org/journal/jbise http://dx.doi.org/10.4236/jbise.2015.87041 Oral Propranolol: A Useful Treatment
J. Biomedical Science and Engineering, 2015, 8, 441-450 Published Online July 2015 in SciRes. http://www.scirp.org/journal/jbise http://dx.doi.org/10.4236/jbise.2015.87041 Oral Propranolol: A Useful Treatment
Selected Vascular Birthmarks. Ilona J Frieden M.D. University of California San Francisco
 Selected Vascular Birthmarks Ilona J Frieden M.D. University of California San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY DISCLOSURES Venthera/Biobridge: Consultant Consulting Fees Pfizer Chair
Selected Vascular Birthmarks Ilona J Frieden M.D. University of California San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY DISCLOSURES Venthera/Biobridge: Consultant Consulting Fees Pfizer Chair
Thrombolysis Delivery, Care, and Monitoring. 5 Acute Trusts - 6 Primary Care Trusts Ambulance Trust 4 Local Authorities
 Thrombolysis Delivery, Care, and Monitoring Documentation & Pathways Need to follow locally agreed policies and procedures Follow thrombolysis pathway? Need to complete Sits database Weight Dose matters!
Thrombolysis Delivery, Care, and Monitoring Documentation & Pathways Need to follow locally agreed policies and procedures Follow thrombolysis pathway? Need to complete Sits database Weight Dose matters!
Original Article. Double Aortic Arch in Infants and Children CH XIE, FQ GONG, GP JIANG, SL FU. Key words. Background
 HK J Paediatr (new series) 2018;23:233-238 Original Article Double Aortic Arch in Infants and Children CH XIE, FQ GONG, GP JIANG, SL FU Abstract Key words Background: This study aimed to report the diagnosis,
HK J Paediatr (new series) 2018;23:233-238 Original Article Double Aortic Arch in Infants and Children CH XIE, FQ GONG, GP JIANG, SL FU Abstract Key words Background: This study aimed to report the diagnosis,
ORIGINAL ARTICLE. Propranolol as First-line Treatment of Head and Neck Hemangiomas
 ORIGINAL ARTICLE Propranolol as First-line of Head and Neck Hemangiomas Carine Fuchsmann, MD; Marie-Claude Quintal, MD; Chantal Giguere, MD; Sonia Ayari-Khalfallah, MD; Laurent Guibaud, MD, PhD; Julie
ORIGINAL ARTICLE Propranolol as First-line of Head and Neck Hemangiomas Carine Fuchsmann, MD; Marie-Claude Quintal, MD; Chantal Giguere, MD; Sonia Ayari-Khalfallah, MD; Laurent Guibaud, MD, PhD; Julie
PAEDIATRIC EMQs. Andrew A Mallick Paediatrics.info.
 PAEDIATRIC EMQs Andrew A Mallick Paediatrics.info www.paediatrics.info Paediatric EMQs Paediatrics.info First published in the United Kingdom in 2012. While the advice and information in this book is believed
PAEDIATRIC EMQs Andrew A Mallick Paediatrics.info www.paediatrics.info Paediatric EMQs Paediatrics.info First published in the United Kingdom in 2012. While the advice and information in this book is believed
Clinicians and Facilities: RESOURCES WHEN CARING FOR WOMEN WITH ADULT CONGENITAL HEART DISEASE OR OTHER FORMS OF CARDIOVASCULAR DISEASE!!
 Clinicians and Facilities: RESOURCES WHEN CARING FOR WOMEN WITH ADULT CONGENITAL HEART DISEASE OR OTHER FORMS OF CARDIOVASCULAR DISEASE!! Abha'Khandelwal,'MD,'MS' 'Stanford'University'School'of'Medicine'
Clinicians and Facilities: RESOURCES WHEN CARING FOR WOMEN WITH ADULT CONGENITAL HEART DISEASE OR OTHER FORMS OF CARDIOVASCULAR DISEASE!! Abha'Khandelwal,'MD,'MS' 'Stanford'University'School'of'Medicine'
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology High blood pressure, also known as hypertension, occurs in a large percentage of the adult population. Primary (essential) hypertension
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology High blood pressure, also known as hypertension, occurs in a large percentage of the adult population. Primary (essential) hypertension
Haemangiomas. Information for families. Great Ormond Street Hospital for Children NHS Foundation Trust
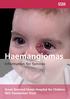 Haemangiomas Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 1 This leaflet explains about haemangiomas and what to expect when your child comes to Great Ormond
Haemangiomas Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 1 This leaflet explains about haemangiomas and what to expect when your child comes to Great Ormond
REFERRAL GUIDELINES VASCULAR SURGERY
 REFERRAL GUIDELINES VASCULAR SURGERY Referral Form: The GP Referral Template is the preferred referral tool (previously known as the Victorian Statewide Referral Form) GP Referral Template IMPORTANT: The
REFERRAL GUIDELINES VASCULAR SURGERY Referral Form: The GP Referral Template is the preferred referral tool (previously known as the Victorian Statewide Referral Form) GP Referral Template IMPORTANT: The
El Hachem et al. Italian Journal of Pediatrics (2017) 43:40 DOI /s
 El Hachem et al. Italian Journal of Pediatrics (2017) 43:40 DOI 10.1186/s13052-017-0357-9 RESEARCH Open Access Safety and effectiveness of oral propranolol for infantile hemangiomas started before 5 weeks
El Hachem et al. Italian Journal of Pediatrics (2017) 43:40 DOI 10.1186/s13052-017-0357-9 RESEARCH Open Access Safety and effectiveness of oral propranolol for infantile hemangiomas started before 5 weeks
FEC-D with HP Fluorouracil, Epirubicin, Cyclophosphamide, Followed by Docetaxel, Trastuzumab, Pertuzumab Neoadjuvant Protocol
 Approved for use in: Neoadjuvant breast cancer: The neoadjuvant treatment of HER2 positive locally advanced, inflammatory or early breast cancer at high risk of recurrence Interim CDF funding from November
Approved for use in: Neoadjuvant breast cancer: The neoadjuvant treatment of HER2 positive locally advanced, inflammatory or early breast cancer at high risk of recurrence Interim CDF funding from November
Propranolol treatment for infantile haemangioma
 Oxford University Hospitals NHS Trust Department of Dermatology Propranolol treatment for infantile haemangioma Information for parents What is a haemangioma? An infantile haemangioma is a collection of
Oxford University Hospitals NHS Trust Department of Dermatology Propranolol treatment for infantile haemangioma Information for parents What is a haemangioma? An infantile haemangioma is a collection of
How to Recognize a Suspected Cardiac Defect in the Neonate
 Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcareprofessionals/education/continuing-medical-nursing-education/neonatalnursing-education-briefs/
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcareprofessionals/education/continuing-medical-nursing-education/neonatalnursing-education-briefs/
Lapatinib and Capecitabine Therapy
 Lapatinib and Capecitabine Therapy This protocol should be read in conjunction with NCCP protocol 00216 Capecitabine Monotherapy. INDICATIONS FOR USE: INDICATION Treatment of adult patients with breast
Lapatinib and Capecitabine Therapy This protocol should be read in conjunction with NCCP protocol 00216 Capecitabine Monotherapy. INDICATIONS FOR USE: INDICATION Treatment of adult patients with breast
PULMONARY VENOLOBAR SYNDROME. Dr.C.Anandhi DNB Resident, Southern Railway Headquarters Hospital.
 PULMONARY VENOLOBAR SYNDROME Dr.C.Anandhi DNB Resident, Southern Railway Headquarters Hospital. Presenting complaint: 10 yrs old girl with recurrent episodes of lower respiratory tract infection from infancy.
PULMONARY VENOLOBAR SYNDROME Dr.C.Anandhi DNB Resident, Southern Railway Headquarters Hospital. Presenting complaint: 10 yrs old girl with recurrent episodes of lower respiratory tract infection from infancy.
PREGNANCY AND CONGENITAL HEART DISEASE
 PREGNANCY AND CONGENITAL HEART DISEASE SIDDHARTH JADHAV M.D. Assistant Professor of Radiology E.B. Singleton Department of Pediatric Radiology Texas Children's Hospital COMMERCIAL DISCLOSURE - None Objectives
PREGNANCY AND CONGENITAL HEART DISEASE SIDDHARTH JADHAV M.D. Assistant Professor of Radiology E.B. Singleton Department of Pediatric Radiology Texas Children's Hospital COMMERCIAL DISCLOSURE - None Objectives
Color Doppler Ultrasound Follow-Up of Infantile Hemangiomas and Peripheral Vascularity in Patients Treated with Propranolol
 Pediatric Dermatology Vol. 32 No. 4 468 475, 2015 Color Doppler Ultrasound Follow-Up of Infantile Hemangiomas and Peripheral Vascularity in Patients Treated with Propranolol Ana M. Kutz, M.D.,* Ligia Aranibar,
Pediatric Dermatology Vol. 32 No. 4 468 475, 2015 Color Doppler Ultrasound Follow-Up of Infantile Hemangiomas and Peripheral Vascularity in Patients Treated with Propranolol Ana M. Kutz, M.D.,* Ligia Aranibar,
Elements for a Public Summary
 VI.2 Elements for a Public Summary Fluticasone propionate / formoterol fumarate are available in pressarised metered dose inhalers (pmdi) under the brand names Flutiform, and in breath actuated inhalers
VI.2 Elements for a Public Summary Fluticasone propionate / formoterol fumarate are available in pressarised metered dose inhalers (pmdi) under the brand names Flutiform, and in breath actuated inhalers
Appropriate prescribing of specialist infant formula feeds
 Appropriate Prescribing of Specialist Infant Formula Feeds Purpose of the guidance These guidelines aim to assist GPs and Health Visitors with information on the appropriate use of infant formula that
Appropriate Prescribing of Specialist Infant Formula Feeds Purpose of the guidance These guidelines aim to assist GPs and Health Visitors with information on the appropriate use of infant formula that
A Clinical Guideline for the use of Intravenous Aminophylline in Acute Severe Asthma in Children
 For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
Dr Doris M. W Kinuthia
 Dr Doris M. W Kinuthia Objectives Normal blood pressures in children Measurement of blood pressure in children Aetiology of Hypertension in children Evaluation of children with hypertension Treatment of
Dr Doris M. W Kinuthia Objectives Normal blood pressures in children Measurement of blood pressure in children Aetiology of Hypertension in children Evaluation of children with hypertension Treatment of
TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol
 Systemic Anti Cancer Treatment Protocol TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol PROTOCOL REF: MPHATCHP (Version No: 1.0) Approved for use in: Neoadjuvant breast: The neoadjuvant
Systemic Anti Cancer Treatment Protocol TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol PROTOCOL REF: MPHATCHP (Version No: 1.0) Approved for use in: Neoadjuvant breast: The neoadjuvant
DERBY-BURTON LOCAL CANCER NETWORK FILENAME Peruse.DOC CONTROLLED DOC NO: CCPG R29
 Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
Pertuzumab + Trastuzumab + Docetaxel (Peruse study) A Multicenter, open-label, single arm study of Pertuzumab in combination with Trastuzumab and a Taxane in first-line treatment of patients with HER2-positive
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Pediatrics. Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment. Overview
 Pediatrics Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment See online here The most common form of cardiac arrhythmia in children is sinus tachycardia which can be caused by
Pediatrics Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment See online here The most common form of cardiac arrhythmia in children is sinus tachycardia which can be caused by
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
Before we are Born: Fetal Diagnosis of Congenital Heart Disease
 Before we are Born: Fetal Diagnosis of Congenital Heart Disease Mohamed Sulaiman, MD Pediatric cardiologist Kidsheart: American Fetal & Children's Heart Center Dubai Healthcare City, Dubai-UAE First Pediatric
Before we are Born: Fetal Diagnosis of Congenital Heart Disease Mohamed Sulaiman, MD Pediatric cardiologist Kidsheart: American Fetal & Children's Heart Center Dubai Healthcare City, Dubai-UAE First Pediatric
Contraindications to time critical surgery; when not to proceed from the perspective of: The Physician A/Prof Peter Morley
 Contraindications to time critical surgery; when not to proceed from the perspective of: The Physician A/Prof Peter Morley British Journal of Surgery 2013; 100: 1045 1049 The risk of 30 day mortality
Contraindications to time critical surgery; when not to proceed from the perspective of: The Physician A/Prof Peter Morley British Journal of Surgery 2013; 100: 1045 1049 The risk of 30 day mortality
Conflict of Interest: none. Neonatal Airway Masses. Neonatal Respiratory Papillomatosis. Paul J. Samuels, MD
 Paul J. Samuels, MD Professor of Anesthesiology and Pediatrics Director of Education Cincinnati Children s Hospital Cincinnati, Ohio Conflict of Interest: none Neonatal Respiratory Papillomatosis Caused
Paul J. Samuels, MD Professor of Anesthesiology and Pediatrics Director of Education Cincinnati Children s Hospital Cincinnati, Ohio Conflict of Interest: none Neonatal Respiratory Papillomatosis Caused
BRICANYL INJECTION. terbutaline sulfate PRODUCT INFORMATION
 BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
Blood pressure parameters for iv amiodarone
 Blood pressure parameters for iv amiodarone Search 17-7-2012 ICU & Fluids - Electrolytes - Nutrition: Edited by Andy S. Binder, MD, Pulmonologist, Critical Care. Contents: Electrolytes : Fluids: Dehydration.
Blood pressure parameters for iv amiodarone Search 17-7-2012 ICU & Fluids - Electrolytes - Nutrition: Edited by Andy S. Binder, MD, Pulmonologist, Critical Care. Contents: Electrolytes : Fluids: Dehydration.
Regional Prenatal Congenital Heart Disease Detection and Practices Lori Erickson MSN, RN, CPNP-PC Ward Family Heart Center
 Regional Prenatal Congenital Heart Disease Detection and Practices Lori Erickson MSN, RN, CPNP-PC Ward Family Heart Center The Children's Mercy Hospital, 2014. 05/14 Objectives Evaluate our regional prenatal
Regional Prenatal Congenital Heart Disease Detection and Practices Lori Erickson MSN, RN, CPNP-PC Ward Family Heart Center The Children's Mercy Hospital, 2014. 05/14 Objectives Evaluate our regional prenatal
Cerebral angiography. Information for families. Great Ormond Street Hospital for Children NHS Foundation Trust
 Cerebral angiography Information for families Great Ormond Street Hospital for Children NHS Foundation Trust This leaflet explains about the cerebral angiography procedure and what to expect when your
Cerebral angiography Information for families Great Ormond Street Hospital for Children NHS Foundation Trust This leaflet explains about the cerebral angiography procedure and what to expect when your
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case
 Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
VENTOLIN RESPIRATOR SOLUTION
 VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
PLASTICS Referral Guidelines
 PLASTICS Referral Guidelines Austin Health PLASTICS Unit holds fortnightly multidisciplinary meetings with ENT/ Maxillary Facial and Oncology Units to discuss and plan the treatment of patients with cancerous
PLASTICS Referral Guidelines Austin Health PLASTICS Unit holds fortnightly multidisciplinary meetings with ENT/ Maxillary Facial and Oncology Units to discuss and plan the treatment of patients with cancerous
Atomoxetine Effective Shared Care Agreement For Attention Deficit Hyperactivity Disorder (ADHD)
 Atomoxetine Effective Shared Care Agreement For Attention Deficit Hyperactivity Disorder (ADHD) Section 1: Shared Care arrangements and responsibilities Section 1.1 Agreement to transfer of prescribing
Atomoxetine Effective Shared Care Agreement For Attention Deficit Hyperactivity Disorder (ADHD) Section 1: Shared Care arrangements and responsibilities Section 1.1 Agreement to transfer of prescribing
Index. cardiology.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A ACHD. See Adult congenital heart disease (ACHD) Adult congenital heart disease (ACHD), 503 512 across life span prevalence of, 504 506
Index Note: Page numbers of article titles are in boldface type. A ACHD. See Adult congenital heart disease (ACHD) Adult congenital heart disease (ACHD), 503 512 across life span prevalence of, 504 506
Infantile Hemangiomas: Complications and Follow-Up
 R E S E A R C H P A P E R Infantile Hemangiomas: Complications and Follow-Up ARZU AKCAY, ZEYNEP KARAKAS, *EBRU TUGRUL SARIBEYOGLU, AYSEGUL UNUVAR, # CAN BAYKAL, MESUT GARIPARDIC, SEMA ANAK, LEYLA AGAOGLU,
R E S E A R C H P A P E R Infantile Hemangiomas: Complications and Follow-Up ARZU AKCAY, ZEYNEP KARAKAS, *EBRU TUGRUL SARIBEYOGLU, AYSEGUL UNUVAR, # CAN BAYKAL, MESUT GARIPARDIC, SEMA ANAK, LEYLA AGAOGLU,
Bevacizumab + Paclitaxel & Carboplatin
 Bevacizumab + Paclitaxel & Carboplatin Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
Bevacizumab + Paclitaxel & Carboplatin Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS:
 0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
The Fetal Cardiology Program
 The Fetal Cardiology Program at Texas Children s Fetal Center About the program Since the 1980s, Texas Children s Fetal Cardiology Program has provided comprehensive fetal cardiac care to expecting families
The Fetal Cardiology Program at Texas Children s Fetal Center About the program Since the 1980s, Texas Children s Fetal Cardiology Program has provided comprehensive fetal cardiac care to expecting families
Core Safety Profile. Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% Date of FAR:
 Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Department of Pediatric Otolarygnology. ENT Specialty Programs
 Department of Pediatric Otolarygnology ENT Specialty Programs Staffed by fellowship-trained otolaryngologists, assisted by pediatric nurse practitioners, ENT (Otolaryngology) at Nationwide Children s Hospital
Department of Pediatric Otolarygnology ENT Specialty Programs Staffed by fellowship-trained otolaryngologists, assisted by pediatric nurse practitioners, ENT (Otolaryngology) at Nationwide Children s Hospital
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
Docetaxel + Carboplatin + Trastuzumab
 Docetaxel + Carboplatin + Trastuzumab Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient
Docetaxel + Carboplatin + Trastuzumab Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient
Cardiology Competency Based Goals and Objectives
 Cardiology Competency Based Goals and Objectives COMPETENCY 1. Patient Care. Provide family centered patient care that is developmentally and age appropriate, compassionate, and effective for the treatment
Cardiology Competency Based Goals and Objectives COMPETENCY 1. Patient Care. Provide family centered patient care that is developmentally and age appropriate, compassionate, and effective for the treatment
CongHeartDis.doc. Андрій Миколайович Лобода
 CongHeartDis.doc Андрій Миколайович Лобода 2015 Зміст 3 Зміст Зміст 4 A child with tetralogy of Fallot is most likely to exhibit: -Increased pulmonary blood flow -Increased pressure in the right ventricle
CongHeartDis.doc Андрій Миколайович Лобода 2015 Зміст 3 Зміст Зміст 4 A child with tetralogy of Fallot is most likely to exhibit: -Increased pulmonary blood flow -Increased pressure in the right ventricle
ABACAVIR HYPERSENSITIVITY REACTION
 ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders
 C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
The Failing Heart in Primary Care
 The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
BETA-BLOCKERS IN CIRRHOSIS.PRO.
 BETA-BLOCKERS IN CIRRHOSIS.PRO. Angela Puente Sánchez. MD PhD Hepatology Unit. Gastroenterology department Marques de Valdecilla University Hospital. Santander INTRODUCTION. Natural history of cirrhosis
BETA-BLOCKERS IN CIRRHOSIS.PRO. Angela Puente Sánchez. MD PhD Hepatology Unit. Gastroenterology department Marques de Valdecilla University Hospital. Santander INTRODUCTION. Natural history of cirrhosis
BRUE and Apnea at Term, how do they relate?
 BRUE and Apnea at Term, how do they relate? Mary Elaine Patrinos, M.D. Attending Neonatologist Rainbow Babies and Children s Hospital Director, Infant Apnea Program Apnea at Term Can it happen? How does
BRUE and Apnea at Term, how do they relate? Mary Elaine Patrinos, M.D. Attending Neonatologist Rainbow Babies and Children s Hospital Director, Infant Apnea Program Apnea at Term Can it happen? How does
PATIENT INFORMATION LEAFLET BILOCOR RANGE
 SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM BILOCOR 5 tablets BILOCOR 10 tablets Please read this leaflet carefully before using BILOCOR 5 / 10 mg tablets Keep this leaflet.
SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM BILOCOR 5 tablets BILOCOR 10 tablets Please read this leaflet carefully before using BILOCOR 5 / 10 mg tablets Keep this leaflet.
: Provide cardiovascular preventive counseling to parents and patients with specific cardiac diseases about:
 Children s Hospital & Research Center Oakland Cardiology Primary Goals for this Rotation 5.13 GOAL: Prevention, Counseling and Screening (Cardiovascular). Understand the role of the pediatrician in preventing
Children s Hospital & Research Center Oakland Cardiology Primary Goals for this Rotation 5.13 GOAL: Prevention, Counseling and Screening (Cardiovascular). Understand the role of the pediatrician in preventing
Panitumumab 6mg/kg Therapy
 Panitumumab 6mg/kg Therapy INDICATIONS FOR USE: INDICATION Treatment of adult patients with wild-type RAS metastatic colorectal cancer (mcrc) ICD10 Protocol Code In first line in combination with FOLFOX
Panitumumab 6mg/kg Therapy INDICATIONS FOR USE: INDICATION Treatment of adult patients with wild-type RAS metastatic colorectal cancer (mcrc) ICD10 Protocol Code In first line in combination with FOLFOX
Michigan Pediatric Cardiac Protocols. Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS
 Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Patient Group Direction for SALBUTAMOL INHALER (Version 02) Valid From 1 October September 2019
 Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
University of Dundee. DOI: /bjd Publication date: Document Version Peer reviewed version
 University of Dundee Propranolol in the treatment of infantile haemangiomas Wedgeworth, E; Glover, M; Irvine, A D; Neri, I; Baselga, E; Clayton, T H; Beattie, P E; Bjerre, J V; Burrows, N P; Foelster-Holst,
University of Dundee Propranolol in the treatment of infantile haemangiomas Wedgeworth, E; Glover, M; Irvine, A D; Neri, I; Baselga, E; Clayton, T H; Beattie, P E; Bjerre, J V; Burrows, N P; Foelster-Holst,
Carboplatin and Fluorouracil
 Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma
 Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
