Clinical Study Synopsis
|
|
|
- Thomas Lee
- 5 years ago
- Views:
Transcription
1 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before making any decisions on their treatment. Healthcare Professionals should always refer to the specific labeling information approved for the patient's country or region. Data in this document or on the related website should not be considered as prescribing advice. The study listed may include approved and non-approved formulations or treatment regimens. Data may differ from published or presented data and are a reflection of the limited information provided here. The results from a single trial need to be considered in the context of the totality of the available clinical research results for a drug. The results from a single study may not reflect the overall results for a drug. The following information is the property of Bayer HealthCare AG. Reproduction of all or part of this report is strictly prohibited without prior written permission from Bayer HealthCare AG. Commercial use of the information is only possible with the written permission of the proprietor and is subject to a license fee. Please note that the General Conditions of Use and the Privacy Statement of bayerhealthcare.com apply to the contents of this file.
2 Study Sponsor: Study Number: 1555 Study Phase: II Clinical Trial Results Synopsis STUDY DESIGN DESCRIPTION Bayer Healthcare AG NCT Official Study Title: Open, non controlled, non randomised, unicenter pilot study to investigate the long-term efficacy of Adalat drops and Adalat retard 10 mg tablets in children with pulmonary hypertension and with peripheral pulmonary stenosis Test Product Therapeutic Area: Cardiology/Coagulation Name of Test Product: Adalat (Nifedipine, BAYA1040) Name of Active Ingredient: Dose and Mode of Administration: Reference Therapy/Placebo Nifedipine Reference Therapy: Not applicable Dose and Mode of Administration: Subjects weighing <5 kg were treated with Adalat drops 0.5% or 2% and subjects weighing >5 kg were treated with Adalat retard 10 mg tablets. The total daily dosage ranged between 0.5 to 2.0 mg/kg body weight/day. The daily dosage of Adalat drops was divided into 4 to 6 regularly administered doses taken with meals. Adalat retard 10 mg tablets were administered twice per day in 12-hour intervals. Not applicable Duration of Treatment: Each subject had an individual dose titration during an inhospital phase for 5 to 7 days. Endpoints of the treatment period were: normalisation of the pulmonary arterial pressure, termination of treatment owing to serious adverse events, e.g. uncontrollable cardiac insufficiency, hypersensitivity to Adalat, withdrawal of parental consent, after correction of congenital defects by operation and limitation of the post-operative treatment period to 1 to 2 years. Studied period: Date of first subjects first visit: 21 Feb 1990 Date of last subjects last visit: 09 Dec 1992 Study Center: One investigational site treated 33 subjects at one center located in Austria. Methodology: This was a unicenter, long-term, open, non-controlled, non randomized observational pilot study without a comparison group. Stabilisation was achieved during a 5 to 7 day inpatient treatment phase with dose titration dependent on efficacy and side effects. Further treatment was carried out at home by the subjects, who collected the study medication at regular intervals Page 1 of 5
3 Indication/ Main Inclusion Criteria: Study Objectives: Evaluation Criteria: from the investigator and reported on the progress during the intervening period. Outpatient control examinations were conducted at 3 to 6 monthly intervals by the investigator which included a physical examination, an ECG, a chest X-ray and Doppler echocardiography. In order to assess the hypertrophy of the right ventricle, an M-Mode Doppler echocardiography was performed. In this mode, the diameter of the ventricular septum, the anterior wall of the right ventricle and the right ventricle were also determined. Two-dimensional echocardiography was also performed in cases of elevated right ventricular pressure. In the subjects with pulmonary hypertension, Doppler echocardiography was performed to study the flow profile. Determination of pressure gradients was also done. Neonates and infants who presented with primary pulmonary hypertension, congenital heart disease with pulmonary hypertension (atrial, ventricular, and combined atrio-ventricular septal defect, ductus arteriosus Botalli persistent, Eisenmenger's syndrome), bronchopulmonary dysplasia, peripheral pulmonary stenoses, or persistent fetal circulation in newborns were included. Subjects suffering from congenital vitium and secondary pulmonary hypertension could be included in the study if they were regarded as primarly inoperable or at increased surgery risk. Overall: To investigate the efficacy and tolerance of Adalat drops and Adalat retard 10 mg tablets in the long-term therapy of neonates and infants with primary and secondary pulmonary hypertension and peripheral pulmonary stenoses and in newborns with persistent fetal circulation. Primary: Reduction in the pulmonary hypertension and regression of right ventricular hypertrophy in subjects with bronchopulmonary dysplasia. Secondary: Improvement in the quality of life, enabling of operability and reduction of the surgical risk. Efficacy (Primary): Endpoints of Adalat therapy were normalization of the pulmonary artery pressure (assessed by Doppler echocardiography), corrective heart surgery and post surgical treatment. The following variables were investigated: Left ventricular end systolic diameter (LVESD), left ventricular end diastolic diameter (LVEDD), shortening fraction (VF%), ventricular septum diameter (SEPT), right ventricular end diastolic diameter (RVEDD), ventricular septum motility (MOTION), acceleration time (TIME), pulmonary artery systolic pressure (PASP), proportion of pre-ejection time to ventricular ejection time (EJECT), ventricular septum defect - gradient (VSD GR), right atrium-right ventricular-gradient (TI), (RA-RV-TI), right Page 2 of 5
4 Statistical Methods: ventricular-mean pulmonary artery gradient (PI), (RV/MPA-PI), systolic and diastolic blood pressure (BPS)(BPD), heart rate (HR), right ventricular systolic pressure (TI), (RVSP), pulmonary artery diastolic pressure (PI), (PADP) and right ventricular hypertrophy (RVHYP). Efficacy (Secondary): -Ability to drink (newborns), -Shortness of breath, -Pleasure of playing, -Limitation in moving, -Enabling of operability and -Reduction of surgical risk. Safety: Safety of Adalat was evaluated by the adverse events (AEs) and their characteristics (intensity, relationship to the study drug, outcome etc.) which occurred during the study. Efficacy (Primary): The primary efficacy parameters were mostly of quantitative character (except for the septum motility and right ventricular hypertrophy). All relevant quantitative variables were analyzed by basic descriptive characteristics of location and dispersion (mean, median, standard deviation, minimum, maximum). Efficacy (Secondary): All secondary parameters were qualitative (categorical). The data of qualitative variables were described by frequency tables. Safety: The safety analyses included the following descriptions: listing of AEs and listing of deaths. Number of Subjects: A total of 20 subjects were planned to be recruited; 33 subjects were enrolled. Results Summary Subject Disposition and Baseline Study Results A total of 33 subjects (18 males, 15 females) were enrolled in the study. All subjects were Caucasians except one Black subject and one Asian subject. All subjects were included into the intent-to-treat analysis. Due to early heart surgery and some cases with infaust prognosis, 9 subjects were left for long-term therapy with follow-up investigations after 3, 6, 9, and 12 months. Only one subject was available after 21 months. The average age of the subjects was 5 months (0 to 15), average weight 4.4 kg (1-8.5) and average height was 60 cm (47 to 45). In 10 cases, complex congenital heart disease was due to Trisomy 21. Eight subjects were diagnosed to have broncho-pulmonal dysplasia and 8 with ventricular septal defect. The daily dose was increased during the in-hospital titration period from a median of 0.5 to 1.0 mg/kg body weight. During the further stages of therapy (i.e. from 3 to 24 months after study start) the average dosage of the study drug remained constant and oscillated around one mg/kg body weight. After 3 months, 9 subjects continued the study; after 15 months there were only 6 subjects and after 21 months only one subject was left. The average duration of the treatment was (3 to 639) days. Page 3 of 5
5 Results Summary Efficacy The variety of diagnoses and their severity, the differing courses, surgical interventions, failure of measurements due to restlessness, small numbers at the beginning and rare cases with late follow-up investigations did not provide the echocardiographic parameters a sensitive tool in this study. Left ventricular end-systolic diameter (VESD): Average values of LVESD measured after 3 months and after 6 months remained constant and did not differ markedly from the average baseline value (= mm). Left ventricular end-diastolic diameter (VEDD): The minimum average value of LVEDD was registered as mm after 3 months of therapy. Later investigations showed a tendency of LVEDD to increase to a maximum value of 25.1 mm after 18 months. Shortening fraction (VF): Before the study, after 3 and 6 months the average VF values oscillated around 40%. After that VF decreased, the minimum average value 33.1% was documented after 12 months. Later investigations showed an increase to a maximum value of 52% after 18 months. Ventricular septum diameter (VSD): During the entire treatment, the measured values of VSD remained almost constant and oscillated around 5.5. Right ventricular end-diastolic diameter (RVEDD): The course of RVEDD during the therapy showed a rather irregular pattern with local peaks of mm (after 6 months), 12.5 mm (after 12 months), mm (after 15 months) and local troughs of 9.57 mm (after 9 months) and 10.0 mm (after 18 months). Septum motility: At baseline, the most frequent categories were "paradoxical" (14 cases), "flattened" (7 cases) and "oblate" (5 cases). In 10 subjects with long term measurements, no change in septum motility was observed. Acceleration Time (AT): Over a period of 12 months from study start, the values of AT increased on the average from 56.6 msec. (before study) to 76.7 msec. (after 12 months). Pulmonary artery systolic pressure (PASP): The average values of PASP decreased monotonously from mmhg at baseline to mmhg after 12 months. After that the values of PASP indicated a certain increase. Relation of pre-ejection time to right ventricular ejection time (EJECT): During the first 12 months of therapy, the EJECT values decreased slightly and monotonously from 0.34 to For the following parameters only a few values were documented: VSD GR, RA-RV-TI, RV-PI, MPA-PI, RVSP and PADP. The available numbers of these parameters were very small and did not allow drawing any conclusion concerning the efficacy. A constant improvement in the secondary efficacy parameters was observed. In 3 children with broncho-pulmonal dysplasia Adalat was stopped after normalization of pulmonary hypertension. In 15 subjects, a corrective operation became feasable by Adalat therapy, leading to a normalized pulmonary circulation in 12 of them. Results Summary Safety A total of 15 AEs was documented in 9 of the 33 subjects: pneumonia (N = 3), cardiac decompensation (N = 2), sepsis (N = 2), anuria for nephrotic syndrome (N = 1), hemorrhagic pneumonia (N = 1), urinary tract infection (N =1), intestinal infection (N = 1), varicella (N = 1), respiratory insufficiency (N = 1), hospitalized in moribund state (N = 1) and severe pulmonary hypertension (N = 1). The relationship to the study drug was assessed as "unlikely" or "not assessable" for all AEs, except one (cardiac decompensation was assessed as "probable"). Seven AEs resolved, 2 remained unchanged, 3 required prolonged hospitalization and 4 caused the subject's Page 4 of 5
6 death. Eight subjects died during the study or within a short period after the end of the study. Conclusions This study was the first investigation of Adalat in pulmonary hypertension. This observational study showed benefit in clinical markers but failed to verify them by quantitative parameters possibly due to a small total number and varying diagnoses. Date Created or Date Last Updated: 23 Aug 2011 Page 5 of 5
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Appendix II: ECHOCARDIOGRAPHY ANALYSIS
 Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
The background of the Cardiac Sonographer Network News masthead is a diagnostic image:
 Number 5 Welcome Number 5 Welcome to the newsletter created just for you: sonographers who perform pediatric echocardiograms in primarily adult echo labs. Each issue features tips on echocardiography of
Number 5 Welcome Number 5 Welcome to the newsletter created just for you: sonographers who perform pediatric echocardiograms in primarily adult echo labs. Each issue features tips on echocardiography of
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
HISTORY. Question: What category of heart disease is suggested by the fact that a murmur was heard at birth?
 HISTORY 23-year-old man. CHIEF COMPLAINT: Decreasing exercise tolerance of several years duration. PRESENT ILLNESS: The patient is the product of an uncomplicated term pregnancy. A heart murmur was discovered
HISTORY 23-year-old man. CHIEF COMPLAINT: Decreasing exercise tolerance of several years duration. PRESENT ILLNESS: The patient is the product of an uncomplicated term pregnancy. A heart murmur was discovered
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Adult Echocardiography Examination Content Outline
 Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Webposting Clinical Trial Results Synopsis
 Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
Study Summary This summary information is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This summary information is not intended to replace
Anatomy & Physiology
 1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
5.8 Congenital Heart Disease
 5.8 Congenital Heart Disease Congenital heart diseases (CHD) refer to structural or functional heart diseases, which are present at birth. Some of these lesions may be discovered later. prevalence of Chd
5.8 Congenital Heart Disease Congenital heart diseases (CHD) refer to structural or functional heart diseases, which are present at birth. Some of these lesions may be discovered later. prevalence of Chd
The impacts of pericardial effusion on the heart function of infants and young children with respiratory syncytial virus infection
 The impacts of pericardial effusion on the heart function of infants and young children with respiratory syncytial virus infection Author(s): Muslim M. Al Saadi, Abdullah S. Al Jarallah Vol. 13, No. 1
The impacts of pericardial effusion on the heart function of infants and young children with respiratory syncytial virus infection Author(s): Muslim M. Al Saadi, Abdullah S. Al Jarallah Vol. 13, No. 1
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
2/4/2011. Nathan Kerner, M.D.
 Nathan Kerner, M.D. Definition Elevated pressures - cut off usually >40 mmhg pulmonary artery systolic pressure (PASP) Usually associated with elevated pulmonary vascular resistance (PVR) measured in dynessec/cm
Nathan Kerner, M.D. Definition Elevated pressures - cut off usually >40 mmhg pulmonary artery systolic pressure (PASP) Usually associated with elevated pulmonary vascular resistance (PVR) measured in dynessec/cm
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Right Ventricle Steven J. Lester MD, FACC, FRCP(C), FASE Mayo Clinic, Arizona
 Right Ventricle Steven J. Lester MD, FACC, FRCP(C), FASE Mayo Clinic, Arizona 1. In which scenario will applying the simplified Bernoulli equation to the peak tricuspid regurgitation velocity and adding
Right Ventricle Steven J. Lester MD, FACC, FRCP(C), FASE Mayo Clinic, Arizona 1. In which scenario will applying the simplified Bernoulli equation to the peak tricuspid regurgitation velocity and adding
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical significance of cardiac murmurs: Get the sound and rhythm!
 Clinical significance of cardiac murmurs: Get the sound and rhythm! Prof. dr. Gunther van Loon, DVM, PhD, Ass Member ECVDI, Dip ECEIM Dept. of Large Animal Internal Medicine Ghent University, Belgium Murmurs
Clinical significance of cardiac murmurs: Get the sound and rhythm! Prof. dr. Gunther van Loon, DVM, PhD, Ass Member ECVDI, Dip ECEIM Dept. of Large Animal Internal Medicine Ghent University, Belgium Murmurs
MITRAL STENOSIS. Joanne Cusack
 MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
Diagnostic approach to heart disease
 Diagnostic approach to heart disease Initial work up History Physical exam Chest radiographs ECG Special studies Echocardiography Cardiac catheterization Echocardiography principles Technique of producing
Diagnostic approach to heart disease Initial work up History Physical exam Chest radiographs ECG Special studies Echocardiography Cardiac catheterization Echocardiography principles Technique of producing
Research Presentation June 23, Nimish Muni Resident Internal Medicine
 Research Presentation June 23, 2009 Nimish Muni Resident Internal Medicine Research Question In adult patients with repaired Tetralogy of Fallot, how does Echocardiography compare to MRI in evaluating
Research Presentation June 23, 2009 Nimish Muni Resident Internal Medicine Research Question In adult patients with repaired Tetralogy of Fallot, how does Echocardiography compare to MRI in evaluating
Pathophysiology: Left To Right Shunts
 Pathophysiology: Left To Right Shunts Daphne T. Hsu, MD dh17@columbia.edu Learning Objectives Learn the relationships between pressure, blood flow, and resistance Review the transition from fetal to mature
Pathophysiology: Left To Right Shunts Daphne T. Hsu, MD dh17@columbia.edu Learning Objectives Learn the relationships between pressure, blood flow, and resistance Review the transition from fetal to mature
2) VSD & PDA - Dr. Aso
 2) VSD & PDA - Dr. Aso Ventricular Septal Defect (VSD) Most common cardiac malformation 25-30 % Types of VSD: According to position perimembranous, inlet, muscular. According to size small, medium, large.
2) VSD & PDA - Dr. Aso Ventricular Septal Defect (VSD) Most common cardiac malformation 25-30 % Types of VSD: According to position perimembranous, inlet, muscular. According to size small, medium, large.
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Trial Study Synopsis
 Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Clinical Trial Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency
Individual Study Table Referring to Item of the Submission: Volume: Page:
 2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
HEMODYNAMIC ASSESSMENT
 HEMODYNAMIC ASSESSMENT INTRODUCTION Conventionally hemodynamics were obtained by cardiac catheterization. It is possible to determine the same by echocardiography. Methods M-mode & 2D echo alone can provide
HEMODYNAMIC ASSESSMENT INTRODUCTION Conventionally hemodynamics were obtained by cardiac catheterization. It is possible to determine the same by echocardiography. Methods M-mode & 2D echo alone can provide
Congenital Heart Defects
 Normal Heart Congenital Heart Defects 1. Patent Ductus Arteriosus The ductus arteriosus connects the main pulmonary artery to the aorta. In utero, it allows the blood leaving the right ventricle to bypass
Normal Heart Congenital Heart Defects 1. Patent Ductus Arteriosus The ductus arteriosus connects the main pulmonary artery to the aorta. In utero, it allows the blood leaving the right ventricle to bypass
HISTORY. Question: What type of heart disease is suggested by this history? CHIEF COMPLAINT: Decreasing exercise tolerance.
 HISTORY 15-year-old male. CHIEF COMPLAINT: Decreasing exercise tolerance. PRESENT ILLNESS: A heart murmur was noted in childhood, but subsequent medical care was sporadic. Easy fatigability and slight
HISTORY 15-year-old male. CHIEF COMPLAINT: Decreasing exercise tolerance. PRESENT ILLNESS: A heart murmur was noted in childhood, but subsequent medical care was sporadic. Easy fatigability and slight
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Doppler-echocardiographic findings in a patient with persisting right ventricular sinusoids
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 1990 Doppler-echocardiographic findings in a patient with persisting right
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 1990 Doppler-echocardiographic findings in a patient with persisting right
ECHO HAWAII. My home. Pulmonary Hypertension and Pulmonary Embolism: Role of Echo U.S.A. Japan. Hawaii Island 1/9/2018
 Pulmonary Hypertension and Pulmonary Embolism: Role of Echo ECHO HAWAII January 15 19, 2018 Kenya Kusunose, MD, PhD, FASE Tokushima University Hospital Japan My home Japan U.S.A Hawaii Island 1 Economy
Pulmonary Hypertension and Pulmonary Embolism: Role of Echo ECHO HAWAII January 15 19, 2018 Kenya Kusunose, MD, PhD, FASE Tokushima University Hospital Japan My home Japan U.S.A Hawaii Island 1 Economy
CONGENITAL HEART DISEASE (CHD)
 CONGENITAL HEART DISEASE (CHD) DEFINITION It is the result of a structural or functional abnormality of the cardiovascular system at birth GENERAL FEATURES OF CHD Structural defects due to specific disturbance
CONGENITAL HEART DISEASE (CHD) DEFINITION It is the result of a structural or functional abnormality of the cardiovascular system at birth GENERAL FEATURES OF CHD Structural defects due to specific disturbance
Pathophysiology: Left To Right Shunts
 Pathophysiology: Left To Right Shunts Daphne T. Hsu, MD dh17@columbia.edu Learning Objectives Learn the relationships between pressure, blood flow, and resistance Review the transition from fetal to mature
Pathophysiology: Left To Right Shunts Daphne T. Hsu, MD dh17@columbia.edu Learning Objectives Learn the relationships between pressure, blood flow, and resistance Review the transition from fetal to mature
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
Congenital heart disease in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery
 Chapter 10 Congenital heart disease in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery Enrico Lopriore MD Regina Bökenkamp MD Marry Rijlaarsdam MD Marieke Sueters MD Frank PHA Vandenbussche
Chapter 10 Congenital heart disease in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery Enrico Lopriore MD Regina Bökenkamp MD Marry Rijlaarsdam MD Marieke Sueters MD Frank PHA Vandenbussche
Case 47 Clinical Presentation
 93 Case 47 C Clinical Presentation 45-year-old man presents with chest pain and new onset of a murmur. Echocardiography shows severe aortic insufficiency. 94 RadCases Cardiac Imaging Imaging Findings C
93 Case 47 C Clinical Presentation 45-year-old man presents with chest pain and new onset of a murmur. Echocardiography shows severe aortic insufficiency. 94 RadCases Cardiac Imaging Imaging Findings C
Pulmonary hypertension in clinical practice: are we focusing on the problem?
 Pulmonary hypertension in clinical practice: are we focusing on the problem? Odd Bech-Hanssen, MD, PhD Cardiology/Clinical Physiology Sahlgrenska University Hospital Gothenburg, Sweden Definition Mean
Pulmonary hypertension in clinical practice: are we focusing on the problem? Odd Bech-Hanssen, MD, PhD Cardiology/Clinical Physiology Sahlgrenska University Hospital Gothenburg, Sweden Definition Mean
P = 4V 2. IVC Dimensions 10/20/2014. Comprehensive Hemodynamic Evaluation by Doppler Echocardiography. The Simplified Bernoulli Equation
 Comprehensive Hemodynamic Evaluation by Doppler Echocardiography Itzhak Kronzon, MD North Shore LIJ/ Lenox Hill Hospital New York, NY Disclosure: Philips Healthcare St. Jude Medical The Simplified Bernoulli
Comprehensive Hemodynamic Evaluation by Doppler Echocardiography Itzhak Kronzon, MD North Shore LIJ/ Lenox Hill Hospital New York, NY Disclosure: Philips Healthcare St. Jude Medical The Simplified Bernoulli
Date: 24 April-2007 Trial ID: GHTUR/E/2 Version: 2 Integrated Clinical Trial Report Status: Final ICTR Synopsis Page: 1 of 5
 ICTR Synopsis Page: 1 of 5 Synopsis TITLE OF TRIAL INDUCTION OF PUBERTY WITH 17 Β-ESTRADIOL IN GIRLS WITH TURNER SYNDROME. AN OPEN RANDOMISED TRIAL TRIAL CODE: GHTUR/E/2 INVESTIGATORS IN TOTAL, 35 PRINCIPAL
ICTR Synopsis Page: 1 of 5 Synopsis TITLE OF TRIAL INDUCTION OF PUBERTY WITH 17 Β-ESTRADIOL IN GIRLS WITH TURNER SYNDROME. AN OPEN RANDOMISED TRIAL TRIAL CODE: GHTUR/E/2 INVESTIGATORS IN TOTAL, 35 PRINCIPAL
Index of subjects. effect on ventricular tachycardia 30 treatment with 101, 116 boosterpump 80 Brockenbrough phenomenon 55, 125
 145 Index of subjects A accessory pathways 3 amiodarone 4, 5, 6, 23, 30, 97, 102 angina pectoris 4, 24, 1l0, 137, 139, 140 angulation, of cavity 73, 74 aorta aortic flow velocity 2 aortic insufficiency
145 Index of subjects A accessory pathways 3 amiodarone 4, 5, 6, 23, 30, 97, 102 angina pectoris 4, 24, 1l0, 137, 139, 140 angulation, of cavity 73, 74 aorta aortic flow velocity 2 aortic insufficiency
Cardiac ultrasound protocols
 Cardiac ultrasound protocols IDEXX Telemedicine Consultants Two-dimensional and M-mode imaging planes Right parasternal long axis four chamber Obtained from the right side Displays the relative proportions
Cardiac ultrasound protocols IDEXX Telemedicine Consultants Two-dimensional and M-mode imaging planes Right parasternal long axis four chamber Obtained from the right side Displays the relative proportions
Echocardiographic Cardiovascular Risk Stratification: Beyond Ejection Fraction
 Echocardiographic Cardiovascular Risk Stratification: Beyond Ejection Fraction October 4, 2014 James S. Lee, M.D., F.A.C.C. Associates in Cardiology, P.A. Silver Spring, M.D. Disclosures Financial none
Echocardiographic Cardiovascular Risk Stratification: Beyond Ejection Fraction October 4, 2014 James S. Lee, M.D., F.A.C.C. Associates in Cardiology, P.A. Silver Spring, M.D. Disclosures Financial none
Pediatric Echocardiography Examination Content Outline
 Pediatric Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 Anatomy and Physiology Normal Anatomy and Physiology 10% 2 Abnormal Pathology and Pathophysiology
Pediatric Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 Anatomy and Physiology Normal Anatomy and Physiology 10% 2 Abnormal Pathology and Pathophysiology
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Giovanni Di Salvo MD, PhD, FESC Second University of Naples Monaldi Hospital
 Giovanni Di Salvo MD, PhD, FESC Second University of Naples Monaldi Hospital VSD is one of the most common congenital cardiac abnormalities in the newborn. It can occur as an isolated finding or in combination
Giovanni Di Salvo MD, PhD, FESC Second University of Naples Monaldi Hospital VSD is one of the most common congenital cardiac abnormalities in the newborn. It can occur as an isolated finding or in combination
Assessing the Impact on the Right Ventricle
 Advances in Tricuspid Regurgitation Congress of the European Society of Cardiology (ESC) Munich, August 25-29, 2012 Assessing the Impact on the Right Ventricle Stephan Rosenkranz, MD Clinic III for Internal
Advances in Tricuspid Regurgitation Congress of the European Society of Cardiology (ESC) Munich, August 25-29, 2012 Assessing the Impact on the Right Ventricle Stephan Rosenkranz, MD Clinic III for Internal
Emergency department diagnosis of atrial and ventricular septal defects, bicuspid aortic valve and pulmonary hypertension
 Crit Ultrasound J (2011) 3:35 39 DOI 10.1007/s13089-011-0061-8 CASE REPORT Emergency department diagnosis of atrial and ventricular septal defects, bicuspid aortic valve and pulmonary hypertension David
Crit Ultrasound J (2011) 3:35 39 DOI 10.1007/s13089-011-0061-8 CASE REPORT Emergency department diagnosis of atrial and ventricular septal defects, bicuspid aortic valve and pulmonary hypertension David
Heart and Lungs. LUNG Coronal section demonstrates relationship of pulmonary parenchyma to heart and chest wall.
 Heart and Lungs Normal Sonographic Anatomy THORAX Axial and coronal sections demonstrate integrity of thorax, fetal breathing movements, and overall size and shape. LUNG Coronal section demonstrates relationship
Heart and Lungs Normal Sonographic Anatomy THORAX Axial and coronal sections demonstrate integrity of thorax, fetal breathing movements, and overall size and shape. LUNG Coronal section demonstrates relationship
HISTORY. Question: What category of heart disease is suggested by this history? CHIEF COMPLAINT: Heart murmur present since early infancy.
 HISTORY 18-year-old man. CHIEF COMPLAINT: Heart murmur present since early infancy. PRESENT ILLNESS: Although normal at birth, a heart murmur was heard at the six week check-up and has persisted since
HISTORY 18-year-old man. CHIEF COMPLAINT: Heart murmur present since early infancy. PRESENT ILLNESS: Although normal at birth, a heart murmur was heard at the six week check-up and has persisted since
ECHOCARDIOGRAPHIC APPROACH TO CONGENITAL HEART DISEASE: THE UNOPERATED ADULT
 ECHOCARDIOGRAPHIC APPROACH TO CONGENITAL HEART DISEASE: THE UNOPERATED ADULT Karen Stout, MD, FACC Divisions of Cardiology University of Washington Medical Center Seattle Children s Hospital NO DISCLOSURES
ECHOCARDIOGRAPHIC APPROACH TO CONGENITAL HEART DISEASE: THE UNOPERATED ADULT Karen Stout, MD, FACC Divisions of Cardiology University of Washington Medical Center Seattle Children s Hospital NO DISCLOSURES
Uptofate Study Summary
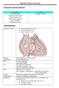 CONGENITAL HEART DISEASE Uptofate Study Summary Acyanotic Atrial septal defect Ventricular septal defect Patent foramen ovale Patent ductus arteriosus Aortic coartation Pulmonary stenosis Cyanotic Tetralogy
CONGENITAL HEART DISEASE Uptofate Study Summary Acyanotic Atrial septal defect Ventricular septal defect Patent foramen ovale Patent ductus arteriosus Aortic coartation Pulmonary stenosis Cyanotic Tetralogy
ARIC HEART FAILURE HOSPITAL RECORD ABSTRACTION FORM. General Instructions: ID NUMBER: FORM NAME: H F A DATE: 10/13/2017 VERSION: CONTACT YEAR NUMBER:
 ARIC HEART FAILURE HOSPITAL RECORD ABSTRACTION FORM General Instructions: The Heart Failure Hospital Record Abstraction Form is completed for all heart failure-eligible cohort hospitalizations. Refer to
ARIC HEART FAILURE HOSPITAL RECORD ABSTRACTION FORM General Instructions: The Heart Failure Hospital Record Abstraction Form is completed for all heart failure-eligible cohort hospitalizations. Refer to
Echocardiographic assessment of the right ventricle in paediatric pulmonary hypertension.
 Echocardiographic assessment of the right ventricle in paediatric pulmonary hypertension. Mark K. Friedberg, MD No disclosures Outline RV response to increased afterload Echo assessment of RV function
Echocardiographic assessment of the right ventricle in paediatric pulmonary hypertension. Mark K. Friedberg, MD No disclosures Outline RV response to increased afterload Echo assessment of RV function
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Congenital heart disease. By Dr Saima Ali Professor of pediatrics
 Congenital heart disease By Dr Saima Ali Professor of pediatrics What is the most striking clinical finding in this child? Learning objectives By the end of this lecture, final year student should be able
Congenital heart disease By Dr Saima Ali Professor of pediatrics What is the most striking clinical finding in this child? Learning objectives By the end of this lecture, final year student should be able
