UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN FRANCISCO DIVISION
|
|
|
- Jocelyn Holt
- 6 years ago
- Views:
Transcription
1 MARK T. JANSEN (SBN ) mjansen@crowell.com PILAR R. STILLWATER (SBN 0) pstillwater@crowell.com GALEN P. SALLOMI (SBN 0) gsallomi@crowell.com Battery Street, rd Floor San Francisco, California 1 Telephone:..00 Facsimile:.. KATHRYN L. CLUNE (pro hac vice application to be filed) kclune@crowell.com ALI H.K. TEHRANI (pro hac vice application to be filed) atehrani@crowell.com 01 Pennsylvania Ave, NW Washington, DC 00 Telephone:..0 Facsimile:.. Attorneys for Plaintiff THE REGENTS OF THE UNIVERSITY OF CALIFORNIA UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN FRANCISCO DIVISION THE REGENTS OF THE UNIVERSITY OF CALIFORNIA, a California Corporation, v. Plaintiff, ATRICURE, INC., a Delaware Corporation, Defendant. Case No. :-cv-0 COMPLAINT FOR PATENT INFRINGEMENT JURY TRIAL DEMANDED Plaintiff The Regents of the University of California ( The Regents or Plaintiff ), by and through its undersigned counsel, complains and alleges against AtriCure, Inc. ( Defendant or AtriCure ), as follows:
2 BACKGROUND AND NATURE OF THE ACTION 1. This is a civil action for patent infringement arising under the patent laws of the United States, U.S.C. 1, et seq., and specifically 1, for Defendant s infringement of The Regents patents covering the now-standard and universally utilized method of treating atrial fibrillation.. Atrial fibrillation (also referred to as AFib or AF ) is the most common type of abnormal heart rhythm. AFib can be an extremely serious condition that severely limits physical activities and significantly increases the risk of other serious heart diseases, stroke, and death. It is estimated that five million people in the United States suffer from AFib currently, and that this number will reach up to million people by 0. Approximately 0,000 new cases of AFib are diagnosed in the U.S. alone each year. These figures are expected to increase as the population ages.. Atrial fibrillation is caused by irregular electrical activity that is triggered typically from specific locations in the pulmonary veins, or near the entrance of the pulmonary veins in the left atrium of the heart. Absent appropriate treatment, the erratic electrical pulses travel from the pulmonary vein into the left atrium, wherein they trigger the onset of AFib, which causes erratic heart muscle contractions and decreases the effectiveness of the heart s ability to pump blood through the patient s body.. Medical researchers spent decades attempting to develop safe and effective nonpharmacologic treatment methods. Dr. Michael Lesh MD, a professor of medicine and a cardiac electrophysiologist at the University of California, San Francisco (or UCSF ), finally solved the problem by inventing the first safe and reliable minimally invasive method of treating AFib.. The treatment method invented by Dr. Lesh (the Patented Method ) involves the formation of a circumferential conduction block at a location where a pulmonary vein extends from the heart s left atrium. The resulting conduction block is intended to block electrical pulses originating within or near the pulmonary vein(s) and to prevent them from entering the left atrium and triggering atrial fibrillation. Dr. Lesh filed several related patent --
3 applications, prosecuted by and on behalf of The Regents, directed to the Patented Method, including the two patents asserted in this action. All of these patents are duly assigned to The Regents (collectively, The Regents Patents ).. AtriCure and the relevant medical community have, at all relevant times, consistently referred to the Patented Method as pulmonary vein isolation, PVI, circumferential PVI, circumferential conduction block, electrical isolation of the pulmonary veins, and other, similar, terms.. The Patented Method has proven highly successful in treating atrial fibrillation. During the early 00s, relevant medical professionals, such as doctors, cardiologists, cardiac electrophysiologists, and cardiothoracic surgeons, universally adopted the Patented Method as the accepted method of treating AFib, either alone, or in combination with other therapy.. Defendant AtriCure has, at all relevant times, been one of the major manufacturers of surgical instruments and related equipment used to treat AFib. AtriCure manufactures, markets, and sells a range of medical devices and related equipment (collectively, AtriCure Devices ) that are used to perform the Patented Method to treat AFib.. AtriCure has, at all relevant times, had knowledge of The Regents Patents, including the two patents asserted in this action. AtriCure is also well aware of the widespread use of AtriCure Devices to perform the Patented Method. Moreover, AtriCure has actively induced, and continues to induce, medical professionals to use AtriCure Devices specifically to practice the Patented Method. In addition, certain of the AtriCure Devices have no substantial use other than to perform the Patented Method, making AtriCure liable for contributory patent infringement in its marketing and sale of those devices. THE PARTIES. Plaintiff The Regents is a California corporation, with a principal place of business located in Oakland, California. The Regents makes up the governing board of the University of California. The Regents maintains a principal, and world-renowned, medical research facility, the University of California, San Francisco, in the City and County of San Francisco. All actions are done in The Regents name, including owning property such as --
4 patents and other intellectual property and entering into contracts.. Defendant AtriCure is a Delaware corporation with corporate headquarters in West Chester, Ohio. AtriCure has at least one office and research facility located in this District. JURISDICTION AND VENUE. This court has original and exclusive subject matter jurisdiction over this controversy pursuant to U.S.C. 1 and (a).. This Court has personal jurisdiction over AtriCure because AtriCure s contacts with the State of California are significant and pervasive, and because AtriCure s contacts with California, as described in this Complaint, directly give rise to this dispute. AtriCure has at least one research facility and office in California, located in this District in San Ramon, Alameda County.. AtriCure has conducted substantial business with individuals, hospitals, and other medical institutions and facilities throughout the State of California, including in this District, and it actively promotes and sells its medical devices and equipment, including the AtriCure Devices that are the subject of this action, throughout California. In doing so, AtriCure regularly transacts business throughout the state, and in this District, in violation of the Asserted Patents, as alleged in this Complaint. Accordingly, this Court may properly exercise personal jurisdiction over AtriCure.. Venue is proper in this District pursuant to U.S.C. 1(b) and (c) and/or 00(b) at least because AtriCure resides in this District, has a regular and established place of business in this District, and has committed acts of infringement in this District. INTRA DISTRICT ASSIGNMENT. This is an intellectual property action to be assigned on a district-wide basis pursuant to Civil Local Rule -(c). THE ASSERTED PATENTS. On December, 00, the United States Patent and Trademark Office ( USPTO ) duly issued United States Patent No.,, ( the Patent ), entitled DEVICE AND METHOD FOR FORMING A CIRCUMFERENTIAL BLOCK IN A PULMONARY VEIN. --
5 The Regents owns by assignment all rights, title and interest in the Patent. A true and correct copy of the Patent is attached hereto as Exhibit 1.. On January, 0, the USPTO duly issued United States Patent No.,0, ( the Patent ), entitled DEVICE AND METHOD FOR FORMING A CIRCUMFERENTIAL BLOCK IN A PULMONARY VEIN. The Regents owns by assignment all rights, title and interest in the Patent. A true and correct copy of the Patent is attached hereto as Exhibit.. The and Patents are referred to collectively as the Asserted Patents. BACKGROUND OF ATRIAL FIBRILLATION. Atrial fibrillation is a type of cardiac arrhythmia that causes an abnormally fast and irregular heart rate. In patients with normal sinus rhythm, the heart is electrically excited to beat in a synchronous, patterned fashion. In patients with a cardiac arrhythmia, however, abnormal regions of cardiac tissue emit erratic electrical signals, disrupting the synchronous beating cycle associated with normally conductive tissue in healthy patients.. Atrial fibrillation occurs in the upper chambers of the heart (i.e., atria). In healthy individuals, the heart s atrial and ventricular chambers (i.e., the lower chambers of the heart) contract in a coordinated fashion, with a normal heart rhythm between 0 and 0 beats per minute.. In patients with AFib, however, the atrial chambers receive such fast and erratic electrical stimulation that they can only quiver and are unable to actively pump blood from the atria to the ventricles. During AFib, the two atria of the heart beat between 0 and 00 times per minute. When this occurs, the atrioventricular node, a part of the electrical pathway between the atria and the ventricles, becomes overloaded with electrical impulses trying to get to the ventricles. As a result, the normal coordination between the atria and ventricles is lost, ventricles develop an irregular heart rhythm, and pumping efficacy is decreased.. As a result of blood not being pumped effectively to the ventricles, blood can pool in the atria, posing a serious health risk. The pooling of blood can lead to coagulation and clotting. Strokes occur when a blood clot travels from the atrium, through the arterial system, to --
6 the brain. People with AFib are five times more likely to suffer a stroke than patients without AFib, and more than % of all strokes occur in patients with AFib. Once AFib is diagnosed, however, treatment can reduce the risk of stroke.. In some patients, the risk of stroke may be reduced with blood thinners to prevent the blood from clotting, and with anti-arrhythmic drugs to restore normal sinus rhythm. These drugs often have serious side effects, such as severe bleeding, dizziness, nausea, bruising, fatigue, lung disease, and ventricular arrhythmias. Further, these drugs often do not prevent further episodes of AFib. If drugs are not effective or well tolerated by a patient, the treatment options include a cardiac ablation procedure, the evolution of which is described more fully below. DR. MICHAEL LESH INVENTS THE PATENTED METHOD TO TREAT ATRIAL FIBRILLATION. Early non-pharmacologic approaches to treat atrial fibrillation were surgical, and involved a complex pattern of surgical incisions in both the left and right atria. The resulting scarred tissue was non-conductive and hence had the potential to block the erratic electrical pulses thought to cause AFib.. The early surgical efforts were reported as having some success in treating patients, but these open heart surgeries were highly invasive with the heart stopped, the chest opened, and the patient placed on a heart-lung machine. They also required a long recovery period, tended to render the left atrium non-functional, and had a high risk of death.. In parallel with the developments of the surgical procedures described above, doctors began to use catheters to ablate cardiac tissue to treat a variety of cardiac arrhythmias. Catheter ablation is a much less invasive procedure than surgery and is performed by cardiac electrophysiologists ( EPs ) in a catheterization lab. EPs are board-certified cardiologists with additional training in treating cardiac arrhythmias. In a catheter ablation procedure, the EP inserts multiple specialized catheters into the patient s veins and arteries. The EP generally guides the catheters into the right atrium of the patient s heart. For procedures involving the left atrium, the EP uses a special catheter to puncture the intra-atrial septum (i.e., the wall separating the left and the right atria) to access the patient s left atrium, where the desired tissue can be ablated. --
7 . In the early 0s, EPs began using catheter ablation in an attempt to treat AFib by mimicking the surgical procedures described above. These catheter procedures typically involved the creation of linear patterns of non-conductive tissue from the inside wall of the heart with a goal to create lesions that were transmural (i.e., through the wall from inside to out). In addition, the lesions needed to be continuous (or nearly so) with no gaps. Because they took many hours to complete, these procedures were very stressful for patients and resulted in safety complications such as perforations of the atrium and excessive radiation exposure.. In the mid-0s, research established that approximately 0% of the erratic electrical pulses causing AFib originated somewhere in the pulmonary veins. Thereafter, treating EPs attempted to cure AFib by locating and ablating the point or points (focus or foci) of origination of the erratic electrical signals within the pulmonary veins. 0. These procedures were of limited success because the exact locations of the originating foci are difficult to identify. In addition, there are often multiple originating foci within each pulmonary vein, causing this methodology to be extremely time-consuming. The procedure also posed safety concerns, the most serious of which was stenosis of the pulmonary veins due to excessive scarring. This stenosis blocked oxygen transmission in the blood, and could lead to serious lung problems and even death. 1. Dr. Lesh invented the solution to this life threatening problem. The Patented Method is directed to forming circumferential conduction blocks at locations where the pulmonary veins extend from a patient s left atrium. The resulting circumferential conduction blocks prevent electrical pulses that originate from within or near the pulmonary veins from entering the left atrium and causing AFib. This allows treatment of AFib without having to identify, locate, or ablate the triggering foci within each pulmonary vein. At the same time, it reduces risk of complications posed by previously-employed methods of treatment.. Beginning in July, Dr. Lesh filed several related patent applications disclosing and covering the Patented Method. The first of these patents was filed on July,, and issued on January, 00, as U.S. Patent No.,0, ( the Patent ) entitled DEVICE AND METHOD FOR FORMING A CIRCUMFERENTIAL BLOCK IN A --
8 PULMONARY VEIN. The Regents owns by assignment all rights, title and interest in the Patent. A true and correct copy of the Patent is attached hereto as Exhibit.. The Asserted Patents claim direct priority from the Patent. More specifically, the Patent is a continuation and the Patent is a continuation-in-part of the Patent.. The Asserted Patents disclose and claim the Patented Method, as demonstrated in representative claims 1 and of the Patent, and claim 1 of the Patent: Claim 1 of the Patent A method for treating atrial arrhythmia in a patient, comprising: forming a circumferential conduction block in a circumferential region of tissue at a location where a pulmonary vein extends from an atrium in the patient, wherein the circumferential conduction block formed is continuous along the circumferential region of tissue, and wherein the circumferential conduction block is formed without contacting the tissue with an ablative fluid medium. Claim of the Patent A method for treating atrial arrhythmia in a left atrium which includes a left posterior atrial wall having a plurality of pulmonary vein ostia, comprising: forming a conduction block around a first ostium of the plurality of pulmonary vein ostia from a portion of the left posterior atrial wall which includes at least one of the other pulmonary vein ostia. Claim 1 of the Patent A method for treating atrial arrhythmia in a heart of a patient, wherein the patient includes a plurality of pulmonary veins and each pulmonary vein extends distally along a lumenal axis from a location in an atrium of the heart, the method comprising: providing a medical device assembly having a distal end portion with an ablation element; positioning the ablation element at one of the locations where one of the pulmonary veins extends from the atrium, wherein the one location is along either a funneling region of a pulmonary vein ostium of the one pulmonary vein or along a region of the one pulmonary vein comprising cardiac tissue upstream from the --
9 pulmonary vein ostium; and using said ablation element to ablate a region of tissue that has a continuous circumferential pattern which extends about the lumenal axis of the one pulmonary vein without substantially repositioning the distal end portion.. The Patented Method, as disclosed and claimed in the Asserted Patents, is performed using a variety of devices in either a surgical procedure or a less-invasive catheterization procedure. The Patented Method has been adopted by surgeons and surgical devices companies, such as AtriCure, as well as by EPs and electrophysiology device companies. ATRICURE S KNOWLEDGE OF THE PATENTED METHOD AND ASSERTED PATENTS. By the early 00s, the Patented Method as claimed in the Asserted Patents had become recognized as the most effective means of treating atrial fibrillation and was the essential element of all non-pharmacological ablation procedures used to treat AFib. In fact, all doctors in the United States that perform surgical or catheter ablation procedures to treat AFib infringe the Asserted Patents, including at least representative claims 1 and of the Patent, and claim 1 of the Patent.. AtriCure claims to be one of the market leaders in the surgical treatment of AFib. AtriCure has performed extensive market research on the procedures and equipment used to treat AFib. AtriCure was at all relevant times aware of the Asserted Patents and knew that the Patented Method was the universally-adopted procedure for treating AFib. Indeed, by no later than 0, AtriCure was sponsoring medical symposia at which leading cardiothoracic surgeons ( Surgeons ) were taught to perform the Patented Method using AtriCure Devices.. The Regents Patents, and in particular the Asserted Patents, are well known in the industry, as evidenced by the fact that they are widely cited in patent applications filed by AtriCure and numerous other medical device companies. According to the USPTO s database, the Patent has been cited as relevant prior art in more than 0 patents and patent applications published before. The asserted Patent has been cited as relevant prior art in more than 0 published U.S. patents, and the asserted Patent has been cited as relevant --
10 prior art in more than 0 published U.S. patents.. According to the USPTO s database, AtriCure cited the Patent in at least patent applications. As the and Patents are a continuation-in-part and a continuation of the Patent, they contain the same disclosure as the Patent cited by AtriCure in its patent applications. AtriCure also applied for and prosecuted at least three U.S. patent applications which have resulted in issued patents or published patent applications that cite the Patent as prior art. Thus, AtriCure maintains a thorough knowledge of all relevant facts, technologies, inventions, published research, and other developments relating to the Patented Method. 0. AtriCure also specifically discussed the Patented Method in its patent applications. For example, as set forth in the following excerpt from AtriCure s own U.S. Patent No.,0,1, AtriCure specifically referenced and explained the universal use of the Patented Method, explaining that the method is designed to: provide a barrier to electrical signals that may otherwise be communicated across the ablated tissue. By way of example only, such a barrier may provide a form of treating atrial fibrillation or other conditions. For instance, where atrial fibrillation is caused by aberrant or erratic electrical signals coming from one or more pulmonary veins to one or both atria of the heart, an ablation may be provided as a barrier between such veins and atria. In other words, one or more ablations may serve to electrically isolate one or more pulmonary veins from the atria. By preventing or substantially preventing aberrant or erratic electrical signals coming from one or more pulmonary veins from reaching the atria, a more desirable sinus rhythm may be maintained. (:- (emphasis added)). 1. The Regents also provided AtriCure additional notice of the Asserted Patents. On February 1,, The Regents advised AtriCure in writing that AtriCure Devices were being marketed and sold to Surgeons for use in practicing the Patented Method as claimed in the Asserted Patents. The Regents letter (attached hereto as Exhibit ), specifically identified the Asserted Patents and explained that they cover the Patented Method, which involve[s] the use of various energy sources... to ablate heart tissue in a circumferential pattern around the pulmonary vein, disrupting the erratic electric pulses that cause atrial fibrillation.. Accordingly, AtriCure had actual knowledge of the Asserted Patents and that the --
11 Asserted Patents cover the Patented Method at all relevant times. ATRICURE MAKES, PROMOTES AND SELLS A WIDE RANGE OF SURGICAL DEVICES THAT DOCTORS USE TO PERFORM THE PATENTED METHOD. During the relevant time period, AtriCure has marketed and sold multiple AtriCure Devices used by Surgeons in violation of the Asserted Patents. At all relevant times, AtriCure was aware that Surgeons used AtriCure Devices to treat AFib and to perform the Patented Method.. AtriCure operates primarily in the United States and Europe. AtriCure reports that it employs approximately 00 people worldwide. AtriCure s sole business is selling devices dedicated to treating AFib. AtriCure s reported revenue for U.S. sales of AFib treatment devices and equipment has ranged from $0 million in to $ million in.. When promoting its AtriCure Devices for treatment of AFib, AtriCure understands, and the relevant medical community understands, that it is promoting the AtriCure Devices to be used specifically to perform the Patented Method. During the relevant time period, AtriCure has marketed and sold a number of ablation catheters specifically for use by Surgeons in performing the Patented Method. These include, but are not limited to, the following: RF Ablation Clamps, including but not limited to: Isolator Synergy Ablation Clamps (OLL / OSL), Isolator Synergy Clamps (EML / EMR), Isolator Synergy Access Clamp (EMT1). Other Ablation Devices and Probes, including but not limited to: Coolrail Linear Pen, Isolator Linear Pen, Isolator Transpolar Pen, AFfirm Bipolar Pacing Probe, CryoICE Cryoablation Probe (CRYO), CryoICE Cryoablation Probe (CRYO), CryoFORM Cryoablation Probe, CryoICE Probe for Cryoanalgesia, COBRA Fusion 0 Ablation System, COBRA Fusion 0 Ablation System, Fusion Magnetic Retriever System. Minimally Invasive Devices, including but not limited to: EPi-Sense Coagulation Device, Subtle Cannula. Other Surgical Devices, including but not limited to: Lumitip Dissection System, --
12 Hercules Universal Stabilizer Arms, Retractors and Blades, Retractors, Atrial Lift System, Rakes and Valve Assistants, Graspers, Scissors, Needle Holders, LiV Accessories. Sensing Equipment, including but not limited to: ORLab System, Ablation Sensing Unit and Switch Matrix, Electrosurgical Unit. Ablation Generators, including but not limited to: RF Generator, CryoICE BOX V.. Numerous AtriCure Devices, including many listed above, are specifically designed for and used by Surgeons only as a material part of performing the Patented Method. With knowledge of the Asserted Patents, AtriCure has knowingly promoted such AtriCure Devices as specifically designed for the purpose of being used by Surgeons to perform the Patented Method. These particular AtriCure Devices, which have no substantial noninfringing uses include, but are not limited to, the following: RF Ablation Clamps, including but not limited to: Isolator Synergy Ablation Clamps (OLL / OSL), Isolator Synergy Clamps (EML / EMR), Isolator Synergy Access Clamp (EMT1).. Not only is AtriCure aware that Surgeons use AtriCure Devices to perform the Patented Method which is covered by the Asserted Patents but it specifically intends for Surgeons to continue that use as it is critical to AtriCure s business. Indeed, AtriCure has stated that its future revenue depends on increasing acceptance by the medical community of the surgical treatment of AFib and the existence, effectiveness and, in particular, the safety of AtriCure s products. See Excerpts of AtriCure s Annual Report attached hereto as Exhibit, at.. At all relevant times, Surgeons have used AtriCure Devices to perform the Patented Method in the United States in violation of the Asserted Patents. AtriCure has at all relevant times promoted, marketed, and advertised the AtriCure Devices to be used by Surgeons to perform the Patented Method. AtriCure was aware of and intended Surgeons to use the AtriCure Devices to specifically perform the Patented Method in violation of the Asserted Patents. --
13 ATRICURE S INFRINGEMENT OF THE ASSERTED PATENTS. AtriCure at all relevant times has induced and contributed to the infringement of the Asserted Patents. With actual knowledge of the Asserted Patents, AtriCure actively encouraged Surgeons to use AtriCure Devices to perform the Patented Method with specific intent to infringe the Asserted Patents. With actual knowledge of the Asserted Patents, AtriCure sold AtriCure Devices that have no substantial non-infringing uses, contributing to the infringement of the Asserted Patents by Surgeons. AtriCure s Synergy Ablation System Is Approved by The FDA for Treatment of AFib to Perform the Patented Method 0. AtriCure makes and sells AtriCure Devices specifically for surgically treating AFib by performing an FDA-approved method that includes circumferential ablation lesions that isolate the pulmonary veins, i.e., the Patented Method. AtriCure s FDA-approved label specifically teaches use of AtriCure Devices according to the Patented Method. 1. AtriCure first sought and obtained FDA approval in 01 to market certain AtriCure Devices, specifically the Synergy Ablation System (which includes the Isolator Synergy Ablation Clamps, Ablation Sensing Units and Switch Matrixes), under the FDA-approved indication that the System, along with radiofrequency and cryoenergy generators, is intended to ablate soft tissue during general surgery using radiofrequency energy. As explained below, in December, the FDA updated the approved indication to specify the Synergy Ablation System s intended and actual use as a tool to ablate cardiac tissue to treat AFib using the Patented Method.. Prior to, AtriCure began actively marketing the Synergy Ablation System to Surgeons to perform a specific pattern of cardiac ablations that requires, as its most important step, pulmonary vein isolation (PVI). In other words, AtriCure routinely promotes and teaches Surgeons to practice the Patented Method using its Synergy Ablation System.. In late December, after being sued by the United States Department of Justice for violations of the False Claim Act for inter alia promoting the non-fda-approved (or off-label ) use of AtriCure Devices to perform the Patented Method, AtriCure submitted a --
14 Premarket Approval Application ( PMA ) to the FDA seeking to expand the devices preexisting indication to include the express approval to market the Synergy Ablation System to Surgeons for purposes of performing the Patented Method. As stated in the application, the goal was to have an updated label specifically for treatment of atrial fibrillation, so as to enable AtriCure to conduct training programs to ensure surgeons learn the optimal surgical technique with the AtriCure Synergy Ablation System. Exhibit at. This surgical technique includes the Patented Method. A copy of relevant excerpts of the AtriCure Synergy Ablation System Panel Pack Executive Summary is attached as Exhibit. (See especially -.). On December,, the FDA granted AtriCure s application and approved the Synergy Ablation System (PMA P00), with an indication to ablate cardiac tissue for the treatment of persistent atrial fibrillation in patients who are undergoing open concomitant coronary artery bypass and/or valve replacement and repair. The FDA-approved label, relevant excerpts of which are attached as Exhibit, includes specific instructions for using the Synergy Ablation System to perform the Patented Method. The image reproduced below, from AtriCure s FDA-approved marketing label, specifically illustrates and promotes using AtriCure Devices to perform the Patented Method by creating the circumferential PVI lesions (highlighted in yellow): Exhibit at.. AtriCure advertises on its website, attached as Exhibit, that [t]he Isolator Synergy Ablation Clamp is the FIRST and ONLY surgical ablation device offered with FDA approval for the treatment of Atrial Fibrillation. AtriCure specifically intends for Surgeons to --
15 use the AtriCure Devices to perform the Patented Method to treat AFib, in violation of the Asserted Patents.. As AtriCure has told its investors [r]egardless of the duration or type of Afib, surgeons will create lesions in the heart tissue surrounding the pulmonary veins to create an electrical barrier between the pulmonary veins and the atrium, or upper chambers of the heart. Exhibit at (emphasis added). AtriCure s Training of Surgeons. At all relevant times, AtriCure has also offered courses teaching Surgeons to use its AtriCure Devices to perform the Patented Method. By way of example, AtriCure has offered and continues to offer an AtriCure Maze IV Surgical Training Course. AtriCure developed, and promoted, the training course in partnership with recognized key opinion leaders in AFib surgery. AtriCure s brochure advertising this program, a true and correct copy of which is attached hereto as Exhibit, depicts and teaches the use of circumferential PVI ablation lesions, according to the Patented Method of the Asserted Patents (highlighted in yellow). Exhibit at.. AtriCure also employs a direct sales force, currently consisting of approximately 0-1 highly trained sales team employees, supporting approximately sales territories in the --
16 United States, teaching the use of and selling AtriCure Devices for treating AFib to medical centers throughout the United States. AtriCure selects these sales employees based on their expertise and reputation in the medical device industry and their knowledge of the requisite cardiac surgery procedures and technologies for performing the Patented Method. AtriCure instructs and expects its large, medically skilled sales force to meet with doctors at leading institutions to provide education specifically on the use of the Synergy System to treat certain AFib patients. Exhibit at ; see also id. at. AtriCure reported that, as of, over 1,0 physicians had been trained to perform the Patented Method. Id. at.. AtriCure s teaching materials, including its brochure for the AtriCure Maze IV Surgical Training Course attached as Exhibit and illustrated below, present an AtriCure sales representative demonstrating how the Isolator Synergy Ablation Clamps are used to create these circumferential PVI ablation lesions: Exhibit at 1. // // --
17 AtriCure s Other Marketing Activities 0. AtriCure has expressly recognized that its business model depends on the increasing acceptance by the medical community of AtriCure Devices as a standard surgical treatment of AFib during open-heart surgical procedures, and also as a sole-therapy minimally invasive procedure. 1. To promote this acceptance, AtriCure creates and distributes promotional materials that further describe and promote the use of AtriCure Devices to perform the Patented Method. As just one example, the below image from the Isolator Synergy Ablation System brochure, a true and correct copy which is attached hereto as Exhibit, specifically demonstrates the use of AtriCure s Synergy Ablation System to perform circumferential PVI, including creation of circumferential ablation lesions around the PV ostia, identified as Right antral PV isolation (right PVI), and left antral PV isolation (left PVI). Id. at. Exhibit at. --
18 . To further promote the use of AtriCure Devices in performing the Patented Method, AtriCure has explained in its annual reports that it also engages in activities such as: investing in clinical trials to validate and promote the use of AtriCure Devices in performing the Patented Method; providing educational grants to institutions to teach the use of AtriCure Devices as an AFib treatment ; sponsoring publication of peer-reviewed articles teaching the use of AtriCure Devices to perform the Patented Method; and forming advisory boards and other consulting relationships with cardiothoracic surgeons and other key opinion leaders in cardiac surgery and other specialties, to oversee AtriCure s surgical training programs and to further promote the use of AtriCure Devices to perform the Patented Method. Exhibit at.. To further market and promote the sale and use of AtriCure Devices, with the specific intent that the Devices are used by Surgeons to perform the Patented Method, AtriCure further explains in its annual reports that it has done and continues to do the following: Funds the publication of articles on the use of AtriCure Devices to perform the Patented Method; Provides grants or other support to renowned Surgeons, medical teaching institutions and other thought leaders, to teach the use of AtriCure Devices to perform the Patented Method; and Engages in its own extensive training of Surgeons on the use of AtriCure Devices to perform the Patented Method. Id.. Upon information and belief, AtriCure promoted and marketed the AtriCure Devices to perform the Patented Method throughout the 00s. The AtriCure Devices were on the market in 01, but were not yet approved for marketing for the treatment of AFib until. As the AtriCure Devices were designed to perform the Patented Method, AtriCure marketed the AtriCure Devices for performing the Patented Method. Indeed, AtriCure was sued for marketing off-label use in a False Claims Act qui tam case in July 0, which the Department of Justice eventually prosecuted. In, AtriCure agreed to pay $. million to resolve the False Claims Act suit based on its marketing of the AtriCure Devices to be used for the Patented Method.. AtriCure s marketing activities alleged herein were performed for the commercial --
19 purpose of selling AtriCure Devices, and were not reasonably related to the development and submission of information necessary to obtain regulatory approval from the FDA, nor were they directed to the collection of information or data necessary for filing an application with the FDA for approval to market any AtriCure Device. The AtriCure Devices promoted by AtriCure, as alleged above, were approved and on the United States market, and being used to perform the Patented Method prior to AtriCure engaging in the marketing and promotional conduct alleged herein.. On February 1,, The Regents wrote to AtriCure and advised it of The Regents concern that AtriCure s products were being marketed and sold to doctors for use in practicing the Patented Method. AtriCure has ignored, and never even acknowledged receipt of, The Regents letter. AtriCure has not in any way changed its marketing or promotional practices so as to stop its infringement of the Asserted Patents. Method. COUNT I: INFRINGEMENT OF THE PATENT. Plaintiff re-alleges here all of the allegations set forth in paragraphs 1- above.. At all relevant times, AtriCure had knowledge of the Patent and the Patented. AtriCure induces others to infringe and/or contributorily infringes one or more claims of the Patent, either literally or under the doctrine of equivalents. 0. Claim of the Patent recites: A method for treating atrial arrhythmia in a left atrium which includes a left posterior atrial wall having a plurality of pulmonary vein ostia, comprising: forming a conduction block around a first ostium of the plurality of pulmonary vein ostia from a portion of the left posterior atrial wall which includes at least one of the other pulmonary vein ostia. 1. The use of the AtriCure Devices by Surgeons to perform the Patented Method on patients with AFib satisfies each and every limitation of claim of the Patent. For example, when Surgeons use AtriCure s Synergy Ablation System including the Isolator Synergy Ablation Clamps to treat AFib, Surgeons use the clamp to form a conduction block around a pulmonary --
20 vein ostia from a portion of the left atrial wall, which includes at least one other pulmonary vein ostia. The Surgeons use of AtriCure s Synergy Ablation System, and related devices, to perform the Patented Method, as described above, satisfies at least Claim of the Patent. At all relevant times, AtriCure knowingly encouraged and intended Surgeons to use AtriCure Devices to perform the Patented Method on patients who have been diagnosed with AFib as described above, in violation of claim.. Upon information and belief, both by manufacturing AtriCure Devices to be used in a manner that AtriCure knows infringes the Patent, and by encouraging Surgeons to use the AtriCure Devices in a manner that AtriCure knows infringes the Patent, AtriCure is inducing infringement of the Patent by Surgeons in violation of U.S.C. 1(b). For example, AtriCure s marketing and promotional materials tout the use of AtriCure Devices to perform the Patented Method in a manner that falls within the scope of at least claim of the Patent.. A subset of AtriCure Devices sold by AtriCure, as set forth in paragraph, are material to performing the Patented Method, according to claim of the Patent.. This subset of AtriCure Devices is not a staple article or commodity of commerce, nor suitable for substantial non-infringing uses. Moreover, by its actual knowledge of the Patent, AtriCure knew that a subset of the AtriCure Devices are especially made or especially adapted for use in a manner that infringes the Patent. Accordingly, AtriCure s sale of the subset of AtriCure Devices set forth in paragraph contributes to the infringement of at least claim of the Patent by Surgeons in violation of U.S.C. 1(c).. AtriCure has profited and will continue to profit from its infringement of the Patent.. AtriCure s infringement of the patent has caused and will continue to cause The Regents substantial monetary harm, for which The Regents is entitled to receive compensatory damages in an amount to be determined at trial, but in no event less than a reasonable royalty.. Further, AtriCure s infringement of the Patent has been willful, deliberate, --
21 and with full knowledge that the use of AtriCure Devices infringes the Patent, justifying an increase in the damages to be awarded to The Regents up to three times the amount found or assessed, in accordance with U.S.C... AtriCure s willful infringement of the Patent, among other actions, renders this an exceptional case, justifying the award to The Regents of its reasonable attorney fees, in accordance with U.S.C.. Method. COUNT II: INFRINGEMENT OF THE PATENT. Plaintiff re-alleges here all of the allegations set forth in paragraphs 1- above. 0. At all relevant times, AtriCure had knowledge of the Patent and the Patented 1. AtriCure induces others to infringe and/or contributorily infringes one or more claims of the Patent, either literally or under the doctrine of equivalents.. Claim 1 of the Patent recites: A method for treating atrial arrhythmia in a heart of a patient, wherein the patient includes a plurality of pulmonary veins and each pulmonary vein extends distally along a lumenal axis from a location in an atrium of the heart, the method comprising: providing a medical device assembly having a distal end portion with an ablation element; positioning the ablation element at one of the locations where one of the pulmonary veins extends from the atrium, wherein the one location is along either a funneling region of a pulmonary vein ostium of the one pulmonary vein or along a region of the one pulmonary vein comprising cardiac tissue upstream from the pulmonary vein ostium; and using said ablation element to ablate a region of tissue that has a continuous circumferential pattern which extends about the lumenal axis of the one pulmonary vein without substantially repositioning the distal end portion.. The use of AtriCure Devices by Surgeons to perform the Patented Method on patients with AFib satisfies each and every limitation of claim 1 of the Patent. For example, when Surgeons use AtriCure s Synergy Ablation System to treat AFib including the Isolator --
22 Synergy Ablation Clamps and other devices, the Isolator Synergy Ablation Claims have an ablation element at their distal ends. Surgeons position the ablation element of the clamps at a location where one of the pulmonary veins extends from the atrium at a funneling region of a pulmonary vein ostium, to ablate a region of tissue with a continuous circumferential pattern, which extends about the lumenal axis of one pulmonary vein, without substantially repositioning the distal end portion. The Surgeons use of AtriCure s Synergy Ablation System, and related AtriCure Devices, to perform the Patented Method, as described above, satisfies at least Claim 1 of the Patent.. At all relevant times, AtriCure knowingly encouraged and intended Surgeons to use AtriCure Devices to perform the Patented Method on patients who have been diagnosed with AFib as described above, in violation of claim 1.. Upon information and belief, both by manufacturing AtriCure Devices to be used in a manner that AtriCure knows infringes the Patent, and by encouraging Surgeons to use the AtriCure Devices in a manner that AtriCure knows infringes the Patent, AtriCure is inducing infringement of the Patent by Surgeons in violation of U.S.C. 1(b). For example, AtriCure s marketing and promotion materials tout the use of AtriCure Devices to perform the Patented Method in a manner that falls within the scope of at least claim 1 of the Patent.. A subset of AtriCure Devices sold by AtriCure, as set forth in paragraph, are material to performing the Patented Method, according to claim 1 of the Patent.. This subset of AtriCure Devices is not a staple article or commodity of commerce, nor suitable for substantial non-infringing uses. Moreover, by its actual knowledge of the Patent, AtriCure knew that a subset of the AtriCure Devices are especially made or especially adapted for use in a manner than infringes the Patent. Accordingly, AtriCure s sale of the subset of AtriCure Devices set forth in paragraph contributes to the infringement of at least Claim 1 of the Patent by Surgeons in violation of U.S.C. 1(c).. AtriCure has profited and will continue to profit from its infringement of the Patent. --
23 . AtriCure s infringement of the Patent has caused and will continue to cause The Regents substantial monetary harm, for which The Regents is entitled to receive compensatory damages in an amount to be determined at trial, but in no event less than a reasonable royalty. 0. Further, AtriCure s infringement of the Patent has been willful, deliberate, and with full knowledge that the use of AtriCure Devices infringes the Patent, justifying an increase in the damages to be awarded to The Regents up to three times the amount found or assessed, in accordance with U.S.C.. 1. AtriCure s willful infringement of the Patent, among other actions, renders this an exceptional case, justifying the award to The Regents of its reasonable attorney fees, in accordance with U.S.C.. PRAYER FOR RELIEF Wherefore, The Regents of the University of California respectfully requests that the Court enter a judgment as follows: A. That AtriCure has infringed the Asserted Patents; B. Awarding The Regents damages, including enhanced damages, pursuant to U.S.C., for AtriCure s infringement of the Asserted Patents, in an amount to be determined at trial, but in no event less than a reasonable royalty; C. Awarding The Regents pre-judgment and post-judgment interest to compensate The Regents for the damages it has sustained; D. Awarding The Regents all of its costs and disbursements incurred in bringing this action; E. Declaring that this is an exceptional case under U.S.C. and awarding The Regents its reasonable attorney fees, costs, and expenses; and // // F. Awarding The Regents any further relief the Court deems just and proper. // --
24 DATED: November, Respectfully submitted, By: /s/ Mark T. Jansen Mark T. Jansen Kathryn L. Clune Pilar R. Stillwater Ali H.K. Tehrani Galen P. Sallomi Attorneys for Plaintiff THE REGENTS OF THE UNIVERSITY OF CALIFORNIA --
25 so triable. DEMAND FOR JURY TRIAL The Regents of the University of California hereby requests a trial by a jury on all issues DATED: November, Respectfully submitted, By: /s/ Mark T. Jansen Mark T. Jansen Kathryn L. Clune Pilar R. Stillwater Ali H.K. Tehrani Galen P. Sallomi Attorneys for Plaintiff THE REGENTS OF THE UNIVERSITY OF CALIFORNIA --
IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN
 IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN ULTRATEC, INC. and CAPTEL, INC., v. Plaintiffs, SORENSON COMMUNICATIONS, INC. and CAPTIONCALL, LLC, Defendants. Civil Action No.: 14-cv-66
IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN ULTRATEC, INC. and CAPTEL, INC., v. Plaintiffs, SORENSON COMMUNICATIONS, INC. and CAPTIONCALL, LLC, Defendants. Civil Action No.: 14-cv-66
Isolator Synergy Ablation System THE ONLY FDA-APPROVED SURGICAL DEVICE TO TREAT ATRIAL FIBRILLATION
 Isolator Synergy Ablation System THE ONLY FDA-APPROVED SURGICAL DEVICE TO TREAT ATRIAL FIBRILLATION WWW.ATRICURE.COM Why the Isolator Synergy Ablation System by AtriCure? / UNIQUE LESION FORMATION A lesion
Isolator Synergy Ablation System THE ONLY FDA-APPROVED SURGICAL DEVICE TO TREAT ATRIAL FIBRILLATION WWW.ATRICURE.COM Why the Isolator Synergy Ablation System by AtriCure? / UNIQUE LESION FORMATION A lesion
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF OREGON PORTLAND DIVISION
 Scott D. Eads, OSB #910400 Email: seads@schwabe.com Nicholas F. Aldrich, Jr., OSB #160306 Email: naldrich@schwabe.com Facsimile: 503.796.2900 Attorneys for Plaintiff AgaMatrix, Inc. IN THE UNITED STATES
Scott D. Eads, OSB #910400 Email: seads@schwabe.com Nicholas F. Aldrich, Jr., OSB #160306 Email: naldrich@schwabe.com Facsimile: 503.796.2900 Attorneys for Plaintiff AgaMatrix, Inc. IN THE UNITED STATES
IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION
 DR. DAVID D. D ALISE, DDS, IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION v. Plaintiff, STRAUMANN USA, LLC, STRAUMANN MANUFACTURING, INC., and STRAUMANN HOLDING
DR. DAVID D. D ALISE, DDS, IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION v. Plaintiff, STRAUMANN USA, LLC, STRAUMANN MANUFACTURING, INC., and STRAUMANN HOLDING
Case 2:15-cv SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246
 Case 2:15-cv-08180-SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246 Elvin Esteves Charles H. Chevalier J. Brugh Lower GIBBONS P.C. One Gateway Center Newark, New Jersey 07102 Tel: (973) 596-4500
Case 2:15-cv-08180-SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246 Elvin Esteves Charles H. Chevalier J. Brugh Lower GIBBONS P.C. One Gateway Center Newark, New Jersey 07102 Tel: (973) 596-4500
Case 1:19-cv UNA Document 1 Filed 03/26/19 Page 1 of 11 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 1:19-cv-00567-UNA Document 1 Filed 03/26/19 Page 1 of 11 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE CAREDX, INC. and THE BOARD OF TRUSTEES OF THE LELAND STANFORD
Case 1:19-cv-00567-UNA Document 1 Filed 03/26/19 Page 1 of 11 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE CAREDX, INC. and THE BOARD OF TRUSTEES OF THE LELAND STANFORD
Case 2:12-cv KJM-GGH Document 1 Filed 07/02/12 Page 1 of 9 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF CALIFORNIA. (Sacramento Division)
 Case :-cv-0-kjm-ggh Document Filed 0/0/ Page of PAUL W. REIDL (State Bar No. ) Law Office of Paul W. Reidl Eagle Trace Drive Half Moon Bay, CA 0 Telephone: (0) 0-0 Email: paul@reidllaw.com Attorney for
Case :-cv-0-kjm-ggh Document Filed 0/0/ Page of PAUL W. REIDL (State Bar No. ) Law Office of Paul W. Reidl Eagle Trace Drive Half Moon Bay, CA 0 Telephone: (0) 0-0 Email: paul@reidllaw.com Attorney for
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION. Plaintiff, PURE HEMP COLLECTIVE INC.
 Civil Action No: IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION a Colorado Corporation Plaintiff, v. PURE HEMP COLLECTIVE INC., a Colorado Corporation Defendant.
Civil Action No: IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION a Colorado Corporation Plaintiff, v. PURE HEMP COLLECTIVE INC., a Colorado Corporation Defendant.
Case 1:09-cv RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1
 Case 1:09-cv-04115-RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1 John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102-4096
Case 1:09-cv-04115-RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1 John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102-4096
IN THE UNITED STATES DISTRICT COURT FOR THE CENTRAL DISTRICT OF CALIFORNIA. Civil Action No. 8:14-cv-1322 COMPLAINT DEMAND FOR JURY TRIAL
 1 1 1 1 John B. Sganga, Jr. (SBN 1,1 john.sganga@knobbe.com Sheila N. Swaroop (SBN, sheila.swaroop@knobbe.com Baraa Kahf (SBN 1,1 baraa.kahf@knobbe.com Marissa Calcagno (SBN, marissa.calcagno@knobbe.com
1 1 1 1 John B. Sganga, Jr. (SBN 1,1 john.sganga@knobbe.com Sheila N. Swaroop (SBN, sheila.swaroop@knobbe.com Baraa Kahf (SBN 1,1 baraa.kahf@knobbe.com Marissa Calcagno (SBN, marissa.calcagno@knobbe.com
Case 1:17-cv ECF No. 1 filed 10/26/17 PageID.1 Page 1 of 8 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF MICHIGAN
 Case 1:17-cv-00939 ECF No. 1 filed 10/26/17 PageID.1 Page 1 of 8 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF MICHIGAN SMILEDIRECTCLUB, LLC, Plaintiff, v. No. MICHIGAN DENTAL ASSOCIATION
Case 1:17-cv-00939 ECF No. 1 filed 10/26/17 PageID.1 Page 1 of 8 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF MICHIGAN SMILEDIRECTCLUB, LLC, Plaintiff, v. No. MICHIGAN DENTAL ASSOCIATION
Case 1:17-cv UNA Document 1 Filed 02/14/17 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 1:17-cv-00159-UNA Document 1 Filed 02/14/17 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC., PF PRISM C.V., and C.P. PHARMACEUTICALS INTERNATIONAL
Case 1:17-cv-00159-UNA Document 1 Filed 02/14/17 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC., PF PRISM C.V., and C.P. PHARMACEUTICALS INTERNATIONAL
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 199-mc-09999 Document 654 Filed 11/09/11 Page 1 of 12 PageID # 61421 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS PHARMACEUTICALS CORPORATION, NOVARTIS AG, NOVARTIS PHARMA
Case 199-mc-09999 Document 654 Filed 11/09/11 Page 1 of 12 PageID # 61421 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS PHARMACEUTICALS CORPORATION, NOVARTIS AG, NOVARTIS PHARMA
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF ARIZONA
 Case :-cv-0-djh Document Filed // Page of 0 FREDENBERG BEAMS Daniel E. Fredenberg 00 Christian C. M. Beams 0 N. th Street, Suite 0 Phoenix, Arizona 0 Telephone: 0/- Email: dfredenberg@fblegalgroup.com
Case :-cv-0-djh Document Filed // Page of 0 FREDENBERG BEAMS Daniel E. Fredenberg 00 Christian C. M. Beams 0 N. th Street, Suite 0 Phoenix, Arizona 0 Telephone: 0/- Email: dfredenberg@fblegalgroup.com
Case 2:17-cv Document 1 Filed 10/30/17 Page 1 of 10
 Case :-cv-0 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 ANDREA SCHMITT, on her own behalf, and on behalf of all similarly situated individuals,
Case :-cv-0 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 ANDREA SCHMITT, on her own behalf, and on behalf of all similarly situated individuals,
Plaintiff, Comfort Dental Group, Inc. ( Comfort Dental ), by its attorneys, MOYE WHITE LLP, states: INTRODUCTION
 JEFFERSON COUNTY DISTRICT COURT, STATE OF COLORADO Address: 100 Jefferson County Parkway Golden, Colorado 80401 Telephone: (303) 271-6145 Plaintiff: COMFORT DENTAL GROUP, INC., a Colorado Corporation,
JEFFERSON COUNTY DISTRICT COURT, STATE OF COLORADO Address: 100 Jefferson County Parkway Golden, Colorado 80401 Telephone: (303) 271-6145 Plaintiff: COMFORT DENTAL GROUP, INC., a Colorado Corporation,
Case 1:17-cv Document 1 Filed 11/07/17 Page 1 of 28 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS BOSTON DIVISION
 Case 1:17-cv-12194 Document 1 Filed 11/07/17 Page 1 of 28 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS BOSTON DIVISION CARIS MPI, INC., Plaintiff, C.A. No. 1:17-CV-12194 v. FOUNDATION
Case 1:17-cv-12194 Document 1 Filed 11/07/17 Page 1 of 28 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS BOSTON DIVISION CARIS MPI, INC., Plaintiff, C.A. No. 1:17-CV-12194 v. FOUNDATION
UNITED STATES DISTRICT COURT CENTRAL DISTRICT OF CALIFORNIA. Plaintiff, COMPLAINT FOR DEFAMATION
 WHITE O'CONNOR CURRY GATTI & AVANZADO LLP Andrew M. White (State Bar No. 60181) Melvin N.A. Avanzado (State Bar No. 137127) 10100 Santa Monica Boulevard Twenty-Third Floor Los Angeles, California 90067-4008
WHITE O'CONNOR CURRY GATTI & AVANZADO LLP Andrew M. White (State Bar No. 60181) Melvin N.A. Avanzado (State Bar No. 137127) 10100 Santa Monica Boulevard Twenty-Third Floor Los Angeles, California 90067-4008
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS SHERMAN DIVISION
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS SHERMAN DIVISION SNYDERS HEART VALVE LLC, Plaintiff, v. ST. JUDE MEDICAL, CARDIOLOGY DIVISION INC.; AND ST. JUDE MEDICAL S.C., INC.,
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS SHERMAN DIVISION SNYDERS HEART VALVE LLC, Plaintiff, v. ST. JUDE MEDICAL, CARDIOLOGY DIVISION INC.; AND ST. JUDE MEDICAL S.C., INC.,
UNITED STATES DISTRICT COURT DISTRICT OF NEW MEXICO PLAINTIFFS FIRST AMENDED COMPLAINT FOR PATENT INFRINGEMENT
 DENTSPLY SIRONA INC. and TULSA DENTAL PRODUCTS LLC d/b/a DENTSPLY SIRONA ENDODONTICS, Plaintiffs, UNITED STATES DISTRICT COURT DISTRICT OF NEW MEXICO V. EDGE ENDO, LLC, 1:17-cv-1041 DEMAND FOR JURY TRIAL
DENTSPLY SIRONA INC. and TULSA DENTAL PRODUCTS LLC d/b/a DENTSPLY SIRONA ENDODONTICS, Plaintiffs, UNITED STATES DISTRICT COURT DISTRICT OF NEW MEXICO V. EDGE ENDO, LLC, 1:17-cv-1041 DEMAND FOR JURY TRIAL
Management strategies for atrial fibrillation Thursday, 20 October :27
 ALTHOUGH anyone who has had to run up a flight of steps or has had a frightening experience is quite familiar with a racing heartbeat, for the more than 2 million Americans who suffer from atrial fibrillation
ALTHOUGH anyone who has had to run up a flight of steps or has had a frightening experience is quite familiar with a racing heartbeat, for the more than 2 million Americans who suffer from atrial fibrillation
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF VIRGINIA ALEXANDRIA DIVISION ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) )
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF VIRGINIA ALEXANDRIA DIVISION THE CLEVELAND CLINIC FOUNDATION 9500 Euclid Avenue Cleveland, OH 44195 and CLEVELAND HEARTLAB, INC., 6701 Carnegie
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF VIRGINIA ALEXANDRIA DIVISION THE CLEVELAND CLINIC FOUNDATION 9500 Euclid Avenue Cleveland, OH 44195 and CLEVELAND HEARTLAB, INC., 6701 Carnegie
UNITED STATES DISTRICT COURT
 Case :-cv-00-spl Document Filed 0// Page of 0 0 Daniel L. Miranda, Esq. SBN 0 MIRANDA LAW FIRM E. Ray Road, Suite #0 Gilbert, AZ Tel: (0) - dan@mirandalawpc.com Robert Tauler, Esq. SBN, (pro hac vice forthcoming)
Case :-cv-00-spl Document Filed 0// Page of 0 0 Daniel L. Miranda, Esq. SBN 0 MIRANDA LAW FIRM E. Ray Road, Suite #0 Gilbert, AZ Tel: (0) - dan@mirandalawpc.com Robert Tauler, Esq. SBN, (pro hac vice forthcoming)
Judicial conflict between Bristol-Myers Squibb Co V. Merck & Co Inc. Keytruda V. Opdivo
 From the SelectedWorks of haitham atiyah Spring April 10, 2016 Judicial conflict between Bristol-Myers Squibb Co V. Merck & Co Inc. Keytruda V. Opdivo haitham atiyah Available at: https://works.bepress.com/haitham_atiyah/3/
From the SelectedWorks of haitham atiyah Spring April 10, 2016 Judicial conflict between Bristol-Myers Squibb Co V. Merck & Co Inc. Keytruda V. Opdivo haitham atiyah Available at: https://works.bepress.com/haitham_atiyah/3/
How does the heart work? The heart is muscle whose main function is a pump; to push blood the rest of your body.
 1 You have a condition called atrial fibrillation. I would like you to learn more about this condition. You should read about it below, and can also watch an Internet program about it. After reading about
1 You have a condition called atrial fibrillation. I would like you to learn more about this condition. You should read about it below, and can also watch an Internet program about it. After reading about
Atrial Fibrillation & Arrhythmias
 Atrial Fibrillation & Arrhythmias Symptoms and Treatments FloridaHospital.com Atrial Fibrillation According to the American Heart Association, Atrial fibrillation (AF) affects an estimated 2.7 million
Atrial Fibrillation & Arrhythmias Symptoms and Treatments FloridaHospital.com Atrial Fibrillation According to the American Heart Association, Atrial fibrillation (AF) affects an estimated 2.7 million
Case 1:16-cv UNA Document 1 Filed 04/22/16 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 1:16-cv-00289-UNA Document 1 Filed 04/22/16 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS AG, NOVARTIS PHARMACEUTICALS CORPORATION, MITSUBISHI
Case 1:16-cv-00289-UNA Document 1 Filed 04/22/16 Page 1 of 10 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS AG, NOVARTIS PHARMACEUTICALS CORPORATION, MITSUBISHI
FILED: NEW YORK COUNTY CLERK 06/28/ :49 PM INDEX NO /2017 NYSCEF DOC. NO. 1 RECEIVED NYSCEF: 06/28/2017
 SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK JARVIK HEART, INC., -against- Plaintiff, CALON CARDIO-TECHNOLOGY LTD., STUART MCCONCHIE, JOHN TEAL and ALANI INTINTOLO Defendant. SUMMONS Index
SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK JARVIK HEART, INC., -against- Plaintiff, CALON CARDIO-TECHNOLOGY LTD., STUART MCCONCHIE, JOHN TEAL and ALANI INTINTOLO Defendant. SUMMONS Index
Arrhythmias. Pulmonary Artery
 Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
X-Plain Atrial Fibrillation Reference Summary
 X-Plain Atrial Fibrillation Reference Summary Introduction Atrial fibrillation is a common heart condition that affects approximately 2.5 million Americans every year. Atrial fibrillation requires immediate
X-Plain Atrial Fibrillation Reference Summary Introduction Atrial fibrillation is a common heart condition that affects approximately 2.5 million Americans every year. Atrial fibrillation requires immediate
Return Date: February 27, 2002
 Return Date: February 27, 2002 Time: 9:30 a.m. COUCH WHITE, LLP 540 Broadway P.O. Box 22222 Albany, New York 12201-2222 (518) 426-4600 Harold N. Iselin, Esq. (H.I. 1428) James J. Barriere, Esq. (J.B. 3206)
Return Date: February 27, 2002 Time: 9:30 a.m. COUCH WHITE, LLP 540 Broadway P.O. Box 22222 Albany, New York 12201-2222 (518) 426-4600 Harold N. Iselin, Esq. (H.I. 1428) James J. Barriere, Esq. (J.B. 3206)
About atrial fibrillation (AFib) Atrial Fibrillation (AFib) What is AFib? What s the danger? Who gets AFib?
 Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Case 1:15-cv RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO
 Case 1:15-cv-00270-RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO Civil Action No. 1:15-cv-00270 GEORGE BACA, v. Plaintiff, PARKVIEW
Case 1:15-cv-00270-RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO Civil Action No. 1:15-cv-00270 GEORGE BACA, v. Plaintiff, PARKVIEW
Understanding Atrial Fibrillation
 Understanding Atrial Fibrillation Todd J. Florin, M.D. Table of Contents The Normal Heart...1 What is Atrial Fibrillation...3 Risks of Afib: Stroke...5 Treatment Options...7 Radiofrequency Ablation...9
Understanding Atrial Fibrillation Todd J. Florin, M.D. Table of Contents The Normal Heart...1 What is Atrial Fibrillation...3 Risks of Afib: Stroke...5 Treatment Options...7 Radiofrequency Ablation...9
Case 1:16-cv UNA Document 1 Filed 11/01/16 Page 1 of 12 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 1:16-cv-01011-UNA Document 1 Filed 11/01/16 Page 1 of 12 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ONYX THERAPEUTICS, INC., v. Plaintiff, DR. REDDY S LABORATORIES,
Case 1:16-cv-01011-UNA Document 1 Filed 11/01/16 Page 1 of 12 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ONYX THERAPEUTICS, INC., v. Plaintiff, DR. REDDY S LABORATORIES,
2:12-cv VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN
 2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY NO. COME NOW Plaintiffs by and through their attorneys of record J.
 SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY 1 1 1 1 1 1 WASHINGTON STATE MEDICAL ASSOCIATION, a Washington corporation, JOSEPH O. GEHRETT, JR. M.D., BARBARA K. GEHRETT, M.D., MICHAEL J. KELLY,
SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY 1 1 1 1 1 1 WASHINGTON STATE MEDICAL ASSOCIATION, a Washington corporation, JOSEPH O. GEHRETT, JR. M.D., BARBARA K. GEHRETT, M.D., MICHAEL J. KELLY,
Case 2:17-cv Document 1 Filed 10/30/17 Page 1 of 10
 Case :-cv-00 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 E.S., by and through her parents, R.S. and J.S., and JODI STERNOFF, both on their own
Case :-cv-00 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 E.S., by and through her parents, R.S. and J.S., and JODI STERNOFF, both on their own
Case 2:14-cv Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA
 Case 2:14-cv-02873 Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA TROYLYNN MORRIS CIVIL ACTION NUMBER: INDIVIDUALLY AND ON BEHALF OF Q. B. SECTION:
Case 2:14-cv-02873 Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA TROYLYNN MORRIS CIVIL ACTION NUMBER: INDIVIDUALLY AND ON BEHALF OF Q. B. SECTION:
IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION ) ) ) ) ) ) ) ) ) ) )
 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION EQUAL EMPLOYMENT OPPORTUNITY COMMISSION, Plaintiff, v. MISSION HOSPITAL, INC., Defendant. Civil Action
IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION EQUAL EMPLOYMENT OPPORTUNITY COMMISSION, Plaintiff, v. MISSION HOSPITAL, INC., Defendant. Civil Action
AF ABLATION Concepts and Techniques
 AF ABLATION Concepts and Techniques Antony F Chu, M.D. Director of Complex Ablation Arrhythmia Services Section Division of Cardiology at the Rhode Island and Miriam Hospital HIGHLIGHTS The main indications
AF ABLATION Concepts and Techniques Antony F Chu, M.D. Director of Complex Ablation Arrhythmia Services Section Division of Cardiology at the Rhode Island and Miriam Hospital HIGHLIGHTS The main indications
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION MEMORANDUM OPINION AND ORDER
 Allergan, Inc. v. Teva Pharmaceuticals USA, Inc. et al Doc. 251 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION ALLERGAN, INC., Plaintiff, v. TEVA PHARMACEUTICALS
Allergan, Inc. v. Teva Pharmaceuticals USA, Inc. et al Doc. 251 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION ALLERGAN, INC., Plaintiff, v. TEVA PHARMACEUTICALS
Case 1:15-cv ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1. Plaintiff, Defendant. COMPLAINT
 Case 1:15-cv-05526-ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF NEW YORK ---------------------------------------------------------------------X
Case 1:15-cv-05526-ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF NEW YORK ---------------------------------------------------------------------X
Catheter Ablation. Patient Education
 Catheter Ablation Patient Education Allina Health System Your heart has four chambers. Two upper chambers (atria) pump blood to the two lower chambers (ventricles). In order for the heart to pump, it requires
Catheter Ablation Patient Education Allina Health System Your heart has four chambers. Two upper chambers (atria) pump blood to the two lower chambers (ventricles). In order for the heart to pump, it requires
ATRIAL FIBRILLATION ANSWERS. A Patient Education Handbook on Electrophysiology
 ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY. Case No.: Plaintiffs Tammie Aust, Alison Grennan, Jennifer Schill, and Lang You Mau, by and
 FILED FEB PM 1: 1 KING COUNTY SUPERIOR COURT CLERK E-FILED CASE NUMBER: --0-1 SEA SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY TAMMIE AUST, an individual; ALISON GRENNAN, an individual; JENNIFER
FILED FEB PM 1: 1 KING COUNTY SUPERIOR COURT CLERK E-FILED CASE NUMBER: --0-1 SEA SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY TAMMIE AUST, an individual; ALISON GRENNAN, an individual; JENNIFER
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) COMPLAINT INTRODUCTION
 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ABBVIE INC. and ABBVIE BIOTECHNOLOGY LTD, v. Plaintiffs, BOEHRINGER INGELHEIM INTERNATIONAL GMBH, BOEHRINGER INGELHEIM PHARMACEUTICALS,
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ABBVIE INC. and ABBVIE BIOTECHNOLOGY LTD, v. Plaintiffs, BOEHRINGER INGELHEIM INTERNATIONAL GMBH, BOEHRINGER INGELHEIM PHARMACEUTICALS,
Special health. guide. Hugh Calkins, M.D., and Ronald Berger, M.D., Ph.D. Guide to Understanding. Atrial Fibrillation WITH
 Hugh Calkins, M.D., and Ronald Berger, M.D., Ph.D. Guide to Understanding Atrial Fibrillation WITH Table of Contents Atrial Fibrillation: An Introduction... 1 How AF Affects the Heart... 2 Who Gets AF?...
Hugh Calkins, M.D., and Ronald Berger, M.D., Ph.D. Guide to Understanding Atrial Fibrillation WITH Table of Contents Atrial Fibrillation: An Introduction... 1 How AF Affects the Heart... 2 Who Gets AF?...
IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION
 Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education
 State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education Introduction Steps to Protect a Child s Right to Special Education: Procedural
State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education Introduction Steps to Protect a Child s Right to Special Education: Procedural
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MISSOURI
 Case 4:08-cv-01915-TCM Document 48 Filed 04/28/2009 Page 1 of 12 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MISSOURI EXPRESS SCRIPTS, INC., ) ) Plaintiff, ) ) vs. ) Cause No.: 4:08-cv-1915 ) WALGREEN
Case 4:08-cv-01915-TCM Document 48 Filed 04/28/2009 Page 1 of 12 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MISSOURI EXPRESS SCRIPTS, INC., ) ) Plaintiff, ) ) vs. ) Cause No.: 4:08-cv-1915 ) WALGREEN
MOTION FOR PRELIMINARY INJUNCTION
 Express Scripts, Inc. v. Walgreen Co. Doc. 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF ILLINOIS EASTERN DIVISION EXPRESS SCRIPTS, INC, a Delaware Corporation, Plaintiff, Case No.
Express Scripts, Inc. v. Walgreen Co. Doc. 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF ILLINOIS EASTERN DIVISION EXPRESS SCRIPTS, INC, a Delaware Corporation, Plaintiff, Case No.
Paper 10 Tel: Entered: September 24, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE
 Trials@uspto.gov Paper 10 Tel: 571-272-7822 Entered: September 24, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD BOSTON SCIENTIFIC CORPORATION, Petitioner, v.
Trials@uspto.gov Paper 10 Tel: 571-272-7822 Entered: September 24, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD BOSTON SCIENTIFIC CORPORATION, Petitioner, v.
Information for patients, parents and guardians. Your child s doctor has recommended that your child has a procedure called an ablation.
 Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
Case 1:09-cv WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA
 Case 1:09-cv-01685-WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA KIMBERLY-CLARK WORLDWIDE, INC., : Plaintiff : v. CIVIL NO.
Case 1:09-cv-01685-WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA KIMBERLY-CLARK WORLDWIDE, INC., : Plaintiff : v. CIVIL NO.
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA LISA SYKES and SETH SYKES, : CIVIL ACTION Individually and as Parents and Natural : Guardians of WESLEY ALEXANDER : NO. SYKES,
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA LISA SYKES and SETH SYKES, : CIVIL ACTION Individually and as Parents and Natural : Guardians of WESLEY ALEXANDER : NO. SYKES,
Less Invasive Ventricular Enhancement For Heart Attack Patients. Revivent TC TransCatheter Ventricular Enhancement System
 For Heart Attack Patients Revivent TC TransCatheter Ventricular Enhancement System This patient booklet is for those who have suffered a heart attack resulting in damage to the left side of the heart causing
For Heart Attack Patients Revivent TC TransCatheter Ventricular Enhancement System This patient booklet is for those who have suffered a heart attack resulting in damage to the left side of the heart causing
Catheter Ablation: Atrial fibrillation (AF) is the most common. Another Option for AF FAQ. Who performs ablation for treatment of AF?
 : Another Option for AF Atrial fibrillation (AF) is a highly common cardiac arrhythmia and a major risk factor for stroke. In this article, Dr. Khan and Dr. Skanes detail how catheter ablation significantly
: Another Option for AF Atrial fibrillation (AF) is a highly common cardiac arrhythmia and a major risk factor for stroke. In this article, Dr. Khan and Dr. Skanes detail how catheter ablation significantly
Case 4:16-cv ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION
 Case 4:16-cv-00140-ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION GLAXO GROUP LIMITED and GLAXOSMITHKLINE CONSUMER HEALTHCARE
Case 4:16-cv-00140-ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION GLAXO GROUP LIMITED and GLAXOSMITHKLINE CONSUMER HEALTHCARE
Associates, llc, for its Complaint against the defendants, Gary K. DeJohn, Sr. and DeJohn
 DISTRICT COURT, LARIMER COUNTY, COLORADO 201 LaPorte Avenue, Suite 100 Fort Collins, CO 80521 DATE FILED: November 10, 2017 12:55 PM FILING ID: FF4949B297BB2 (970) 494-3500 CASE NUMBER: 2017CV30947 Plaintiff:
DISTRICT COURT, LARIMER COUNTY, COLORADO 201 LaPorte Avenue, Suite 100 Fort Collins, CO 80521 DATE FILED: November 10, 2017 12:55 PM FILING ID: FF4949B297BB2 (970) 494-3500 CASE NUMBER: 2017CV30947 Plaintiff:
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF KANSAS ORIGINAL COMPLAINT
 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF KANSAS ALMHA LLC Plaintiff, v. SPECIALTY SURGICAL PRODUCTS, INC. Civil Action No. JURY TRIAL DEMANDED Defendant. ORIGINAL COMPLAINT This is an action
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF KANSAS ALMHA LLC Plaintiff, v. SPECIALTY SURGICAL PRODUCTS, INC. Civil Action No. JURY TRIAL DEMANDED Defendant. ORIGINAL COMPLAINT This is an action
AFFILIATION PROGRAM AGREEMENT
 AFFILIATION PROGRAM AGREEMENT This AFFILIATION PROGRAM AGREEMENT (this Agreement ) is made and entered into by and between FACULTY PHYSICIANS & SURGEONS OF LLUSM dba LOMA LINDA UNIVERSITY FACULTY MEDICAL
AFFILIATION PROGRAM AGREEMENT This AFFILIATION PROGRAM AGREEMENT (this Agreement ) is made and entered into by and between FACULTY PHYSICIANS & SURGEONS OF LLUSM dba LOMA LINDA UNIVERSITY FACULTY MEDICAL
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS
 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS SCOTT RODRIGUES ) Plaintiff ) C.A. 07-10104-GAO ) v. ) ) THE SCOTTS COMPANY, LLC ) Defendant ) AMENDED COMPLAINT and jury trial demand Introduction
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS SCOTT RODRIGUES ) Plaintiff ) C.A. 07-10104-GAO ) v. ) ) THE SCOTTS COMPANY, LLC ) Defendant ) AMENDED COMPLAINT and jury trial demand Introduction
Exhibit 2 RFQ Engagement Letter
 Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Kadlec Regional Medical Center Cardiac Electrophysiology
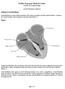 Definition of atrial fibrillation Kadlec Regional Medical Center Cardiac Electrophysiology Atrial Fibrillation Ablation Atrial fibrillation is a heart rhythm disturbance that causes an irregular (and often
Definition of atrial fibrillation Kadlec Regional Medical Center Cardiac Electrophysiology Atrial Fibrillation Ablation Atrial fibrillation is a heart rhythm disturbance that causes an irregular (and often
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES
 UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA
 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA UNITED STATES OF AMERICA v. CRIMINAL NO. UCB, INC., Defendant. VIOLATION 21 U.S.C. 331(k), 352(f)(1), and 333(a)(1) (Causing drugs to be
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA UNITED STATES OF AMERICA v. CRIMINAL NO. UCB, INC., Defendant. VIOLATION 21 U.S.C. 331(k), 352(f)(1), and 333(a)(1) (Causing drugs to be
Case 1:16-cv SEH Document 1 Filed 03/22/16 Page 1 of 12
 Case :-cv-00-seh Document Filed 0// Page of James D. Weakley, Esq. Bar No. 0 WEAKLEY & ARENDT, LLP 0 East Shaw Avenue, Suite Fresno, California 0 Telephone: ( - Facsimile: ( - Jim@walaw-fresno.com Attorneys
Case :-cv-00-seh Document Filed 0// Page of James D. Weakley, Esq. Bar No. 0 WEAKLEY & ARENDT, LLP 0 East Shaw Avenue, Suite Fresno, California 0 Telephone: ( - Facsimile: ( - Jim@walaw-fresno.com Attorneys
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA
 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA R.J. REYNOLDS TOBACCO CO., et al., v. Plaintiffs, No. 1:11-cv-1482 (RJL) UNITED STATES FOOD AND DRUG ADMINISTRATION, et al., Defendants. UNOPPOSED
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA R.J. REYNOLDS TOBACCO CO., et al., v. Plaintiffs, No. 1:11-cv-1482 (RJL) UNITED STATES FOOD AND DRUG ADMINISTRATION, et al., Defendants. UNOPPOSED
Patient Resources: Arrhythmias and Congenital Heart Disease
 Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Radiofrequency ablation for atrial fibrillation in association with other cardiac surgery
 Issue date: May 2005 Radiofrequency ablation for atrial fibrillation in association with other cardiac surgery Understanding NICE guidance information for people considering the procedure, and for the
Issue date: May 2005 Radiofrequency ablation for atrial fibrillation in association with other cardiac surgery Understanding NICE guidance information for people considering the procedure, and for the
Case 3:16-cv PK Document 1 Filed 06/14/16 Page 1 of 11
 Case :-cv-00-pk Document Filed 0// Page of Kevin P. Sullivan, OSB # Sullivan Law Firm 0 Fifth Avenue, Suite 00 Seattle, Washington 0 Telephone: ( 0-00 Facsimile: ( - K.Sullivan@SullivanLawFirm.org 0 VALERIE
Case :-cv-00-pk Document Filed 0// Page of Kevin P. Sullivan, OSB # Sullivan Law Firm 0 Fifth Avenue, Suite 00 Seattle, Washington 0 Telephone: ( 0-00 Facsimile: ( - K.Sullivan@SullivanLawFirm.org 0 VALERIE
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Saudi Council for Health Specialties
 Saudi Council for Health Specialties SAUDI BOARD OF INTERNAL MEDICINE Prince Sultan Cardiac Center (PSCC) Cardiac Electrophysiology & Pacing Training Program 1434 / 2013 1 I. Introduction. II. III. IV.
Saudi Council for Health Specialties SAUDI BOARD OF INTERNAL MEDICINE Prince Sultan Cardiac Center (PSCC) Cardiac Electrophysiology & Pacing Training Program 1434 / 2013 1 I. Introduction. II. III. IV.
Understanding Atrial Fibrillation A guide for patients
 Understanding Atrial Fibrillation A guide for patients Your doctor has determined that you have atrial fibrillation (AF), a common disturbance of the heart s rhythm. This pamphlet will answer many of your
Understanding Atrial Fibrillation A guide for patients Your doctor has determined that you have atrial fibrillation (AF), a common disturbance of the heart s rhythm. This pamphlet will answer many of your
2018 CODING AND REIMBURSEMENT FOR. Cardiac Surgical Ablation and Left Atrial Appendage Management
 2018 CODING AND REIMBURSEMENT FOR Cardiac Surgical Ablation and Left Atrial Appendage Management Introduction This information is shared for educational purposes and current as of January 2018. Healthcare
2018 CODING AND REIMBURSEMENT FOR Cardiac Surgical Ablation and Left Atrial Appendage Management Introduction This information is shared for educational purposes and current as of January 2018. Healthcare
Mission Statement for our Arrhythmia Care
 Mission Statement for our Arrhythmia Care We are dedicated to provide a compassionate and an outstanding care for patients with cardiac arrhythmias. We will be utilizing the cutting edge and the most advanced
Mission Statement for our Arrhythmia Care We are dedicated to provide a compassionate and an outstanding care for patients with cardiac arrhythmias. We will be utilizing the cutting edge and the most advanced
Kadlec Regional Medical Center Cardiac Electrophysiology
 Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
FORM8-K HILLENBRAND,INC.
 UNITEDSTATES SECURITIESANDEXCHANGECOMMISSION Washington,D.C.20549 FORM8-K CURRENTREPORT PursuanttoSection13or15(d)oftheSecuritiesExchangeActof1934 Date of Report (Date of earliest event reported): December18,2015
UNITEDSTATES SECURITIESANDEXCHANGECOMMISSION Washington,D.C.20549 FORM8-K CURRENTREPORT PursuanttoSection13or15(d)oftheSecuritiesExchangeActof1934 Date of Report (Date of earliest event reported): December18,2015
Atrial Fibrillation Ablation: in Whom and How
 Update on Consensus Statement on Management of Atrial Fibrillation: EHRA 2012 Atrial Fibrillation Ablation: in Whom and How Update of HRS/EHRA AF/ECAS Ablation Document 2012 Anne M Gillis MD FHRS Professor
Update on Consensus Statement on Management of Atrial Fibrillation: EHRA 2012 Atrial Fibrillation Ablation: in Whom and How Update of HRS/EHRA AF/ECAS Ablation Document 2012 Anne M Gillis MD FHRS Professor
Blood Thinning in Atrial Fibrillation (AF)
 AF A Blood Thinning in Atrial Fibrillation (AF) Providing information, support and access to established, new or innovative treatments for Atrial Fibrillation www.atrialfibrillation-au.org Glossary Antiarrhythmic
AF A Blood Thinning in Atrial Fibrillation (AF) Providing information, support and access to established, new or innovative treatments for Atrial Fibrillation www.atrialfibrillation-au.org Glossary Antiarrhythmic
Surgical Ablation of Atrial Fibrillation. Gregory D. Rushing, MD. Assistant Professor, Division of Cardiac Surgery
 Surgical Ablation of Atrial Fibrillation Gregory D. Rushing, MD Assistant Professor, Division of Cardiac Surgery Midwestern Conference on Optimizing Electrophysiology Patient Care and Procedural Success
Surgical Ablation of Atrial Fibrillation Gregory D. Rushing, MD Assistant Professor, Division of Cardiac Surgery Midwestern Conference on Optimizing Electrophysiology Patient Care and Procedural Success
Atrial Fibrillation. Why It s Important. Updated on 2007 / 11 / 23. Microlife Corp ww.microlife.com. Page 1
 Atrial Fibrillation Why It s Important Microlife Corp ww.microlife.com Updated on 2007 / 11 / 23 Page 1 CONTENT ORIGINAL PRESENTATION P3 ~ P13 UPDATED ASH ABSTRACT SUBMISSION INFORMATION INTERESTING LINK
Atrial Fibrillation Why It s Important Microlife Corp ww.microlife.com Updated on 2007 / 11 / 23 Page 1 CONTENT ORIGINAL PRESENTATION P3 ~ P13 UPDATED ASH ABSTRACT SUBMISSION INFORMATION INTERESTING LINK
Trademark Use Guidelines for Certified Products and Related Advertising
 Trademark Use Guidelines for Certified Products and Related Advertising Version 2.0. Updated March 2017 2017 CSA Group 1 Introduction CSA Group has developed a significant reputation and goodwill in its
Trademark Use Guidelines for Certified Products and Related Advertising Version 2.0. Updated March 2017 2017 CSA Group 1 Introduction CSA Group has developed a significant reputation and goodwill in its
Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information
 Melbourne Heart Rhythm Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information What is Ventricular Tachycardia? Ventricular tachycardia (VT) is an abnormal rapid heart
Melbourne Heart Rhythm Ventricular Tachycardia in Structurally Normal Hearts (Idiopathic VT) Patient Information What is Ventricular Tachycardia? Ventricular tachycardia (VT) is an abnormal rapid heart
CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT
 CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT Providing for testing standards for cigarette fire safety, for certification of compliance by manufacturers,
CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT Providing for testing standards for cigarette fire safety, for certification of compliance by manufacturers,
City of Carson 701 E. Carson St., Carson, CA Telephone: (310) ; ci.carson.ca.us
 OFFICE USE ONLY Case No. City of Carson 701 E. Carson St., Carson, CA 90745 Telephone: (310) 830-7600; ci.carson.ca.us Application Submittal Date Fee Accepted By SUPPLEMENTAL APPLICATION FOR COMMERCIAL
OFFICE USE ONLY Case No. City of Carson 701 E. Carson St., Carson, CA 90745 Telephone: (310) 830-7600; ci.carson.ca.us Application Submittal Date Fee Accepted By SUPPLEMENTAL APPLICATION FOR COMMERCIAL
Case 3:17-cv JAG Document 4 Filed 03/07/17 Page 1 of 25 PageID# 299
 Case 3:17-cv-00168-JAG Document 4 Filed 03/07/17 Page 1 of 25 PageID# 299 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF VIRGINIA RICHMOND DIVISION LIFENET HEALTH, A Virginia Corporation
Case 3:17-cv-00168-JAG Document 4 Filed 03/07/17 Page 1 of 25 PageID# 299 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF VIRGINIA RICHMOND DIVISION LIFENET HEALTH, A Virginia Corporation
IN THE COURT OF COMMON PLEAS CUYAHOGA COUNTY, OHIO COMPLAINT
 IN THE COURT OF COMMON PLEAS CUYAHOGA COUNTY, OHIO NOTTINGHAM-SPIRK PARTNERS, LLC, 2200 Overlook Road, Cleveland, OH 44106, and NOTTINGHAM-SPIRK DESIGN ASSOCIATES, INC., 2200 Overlook Road, Cleveland,
IN THE COURT OF COMMON PLEAS CUYAHOGA COUNTY, OHIO NOTTINGHAM-SPIRK PARTNERS, LLC, 2200 Overlook Road, Cleveland, OH 44106, and NOTTINGHAM-SPIRK DESIGN ASSOCIATES, INC., 2200 Overlook Road, Cleveland,
Asia-Pacific Electrophysiology Market Outlook to 2020
 Asia-Pacific Electrophysiology Market Outlook to 2020 Reference Code: GDMECR0114DB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 5 1.2 List of Figures...
Asia-Pacific Electrophysiology Market Outlook to 2020 Reference Code: GDMECR0114DB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 5 1.2 List of Figures...
INFORMATION. Atrial Fibrillation. Summary. Information from the
 1. Information from the For more information contact Heartline 1300 362 787 or www.heartfoundation.com.au Atrial Fibrillation INFORMATION Summary What is atrial fibrillation? Atrial fibrillation (AF) is
1. Information from the For more information contact Heartline 1300 362 787 or www.heartfoundation.com.au Atrial Fibrillation INFORMATION Summary What is atrial fibrillation? Atrial fibrillation (AF) is
Purpose: Policy: The Fair Hearing Plan is not applicable to mid-level providers. Grounds for a Hearing
 Subject: Fair Hearing Plan Policy #: CR-16 Department: Credentialing Approvals: Credentialing Committee QM Committee Original Effective Date: 5/00 Revised Effective Date: 1/03, 2/04, 1/05, 11/06, 12/06,
Subject: Fair Hearing Plan Policy #: CR-16 Department: Credentialing Approvals: Credentialing Committee QM Committee Original Effective Date: 5/00 Revised Effective Date: 1/03, 2/04, 1/05, 11/06, 12/06,
AF Today: W. For the majority of patients with atrial. are the Options? Chris Case
 AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
Newer pacemakers also can monitor your blood temperature, breathing, and other factors and adjust your heart rate to changes in your activity.
 Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
Case 3:14-cv JM-WVG Document 1 Filed 11/03/14 Page 1 of 20 IN THE UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF CALIFORNIA
 Case :-cv-0-jm-wvg Document Filed /0/ Page of 0 0 0 CARPENTER LAW GROUP Todd D. Carpenter (CA ) 0 West Broadway, th Floor San Diego, California 0 Telephone:.. Facsimile:.. todd@carpenterlawyers.com PATTERSON
Case :-cv-0-jm-wvg Document Filed /0/ Page of 0 0 0 CARPENTER LAW GROUP Todd D. Carpenter (CA ) 0 West Broadway, th Floor San Diego, California 0 Telephone:.. Facsimile:.. todd@carpenterlawyers.com PATTERSON
IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH ) ) ) ) ) ) ) ) ) ) ) ) ) GENERAL JURISDICTION AND COMMON FACTUAL ALLEGATIONS
 Phillip C. Gilbert, OSB No. rd S.E. Avenue Suite A Gresham, Oregon 00-1 Phone: (0-00 Fax: (0-01 pgilbert@teleport.com IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH DAWN D. JOHNSON
Phillip C. Gilbert, OSB No. rd S.E. Avenue Suite A Gresham, Oregon 00-1 Phone: (0-00 Fax: (0-01 pgilbert@teleport.com IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH DAWN D. JOHNSON
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE. Updated September 2018
 CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
4. Together, defendants CCA and CCC represent the vast majority of chiropractors practicing in Connecticut.
 RETURN DATE JULY 6, 2010 VICTIMS OF CHIROPRACTIC ABUSE, LLC, J.D. OF HARTFORD Plaintiff, at HARTFORD v. CONNECTICUT CHIROPRACTIC ASSOCIATION, INC.; CONNECTICUT CHIROPRACTIC COUNCIL, INC., Defendants JUNE
RETURN DATE JULY 6, 2010 VICTIMS OF CHIROPRACTIC ABUSE, LLC, J.D. OF HARTFORD Plaintiff, at HARTFORD v. CONNECTICUT CHIROPRACTIC ASSOCIATION, INC.; CONNECTICUT CHIROPRACTIC COUNCIL, INC., Defendants JUNE
