Covered Mounted CP Stent
|
|
|
- Arabella Hensley
- 6 years ago
- Views:
Transcription
1 Covered Mounted CP Stent COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 4 Right Ventricular Outflow Tract Indication....Page 23 Stent Sizing Charts and Information....Page 33 CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun Interventional Systems Inc. 824 Twelfth Avenue Bethlehem, PA 1818 Customer Service: TEL: (877) FAX: (61) Technical Support TEL: (8) Made in U.S.A. Manufactured by: NuMED, Inc. 288 Main Street Hopkinton, NY USA Telephone: (315) Facsimile: (315) info@numedusa.com Internet:
2 DESCRIPTION The Covered CP Stent is balloon expandable and intended for permanent implant. The Covered CP Stent is composed of heat treated 9% platinum and 1% iridium wire that is arranged in laser welded rows with a zig pattern. The number of zigs in a row can be varied and will impact the strength of the stent as well as the eventual expanded diameter and percentage of stent shortening, while the number of rows will determine the unexpanded length of the stent. The Covered CP Stent has an eptfe covering attached to the stent framework. This covering acts as a fluid barrier creating a fluid tight conduit through the stent length. The NuMED BIB (Balloon in Balloon) Catheter is a triaxial design catheter. Two lumens are used to inflate the balloon while one lumen is for tracking over a guidewire. The radiopaque platinum marker(s) are placed beneath the working area of the balloon. The inner balloon is ½ of the outer balloon diameter and 1 cm shorter. Each balloon inflates to the stated diameter and length at specific pressure. The balloon size is ±1% at Rated Burst Pressure (RBP). The RBP is different for each size. Check the package label for the RBP. It is important that the balloon not be inflated beyond the RBP. The purpose of the double balloon catheter is to apply an incremental inflation for the purpose of opening a vascular channel using a balloon expandable intravascular stent. The inner balloon provides initial expansion of the stent and also acts as a tool to hold the stent in place while the outer balloon is inflated. The outer balloon is then inflated securing the stent against the vessel wall. HOW SUPPLIED Supplied sterilized by ethylene oxide gas. Sterile and non-pyrogenic if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred. MRI SAFETY INFORMATION Nonclinical testing and modeling has demonstrated that the CP Stent is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions: Static magnetic field of 1.5 T and 3 T Maximum spatial gradient magnetic field of 25 gauss/cm (25 T/m) Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2. W/kg for 15 minutes of scanning (Normal Operating Mode) Based on nonclinical testing and modeling, under the scan conditions defined above, the CP Stent is expected to produce a maximum in vivo temperature rise of less than 2 C after 15 minutes of continuous scanning. MR image quality may be compromised if the area of interest is in the same area, or relatively close to the position of the device. In nonclinical testing, the image artifact caused by the device extends approximately 3 mm from the CP Stent when imaged with a spin echo pulse sequence and 6 mm when imaged with a gradient echo pulse sequence and a 3 T MRI System. The lumen of the device was obscured. 2
3 The presence of other implants or medical circumstances of the patient may require lower limits on some or all of the above parameters. WARNING: Radiofrequency heating during MRI scans on overlapped, 1 zig CP Stents has not been evaluated. INSTRUCTIONS FOR USE: Select Stent Size 1. Measure the length of the target stricture to determine the length of stent required. Size the stent length to extend slightly proximal and distal to the stricture. 2. The appropriate stent length should be selected based on covering the entire obstructed segment with a single stent. Note: Should more than one stent be required, place the stent most distal from the puncture site first, followed by placement of the proximal stent in tandem. 3. Measure the diameter of the reference stricture and vessel proximal and distal to the target lesion to determine the appropriate size stent and delivery system. Preparation of Stent Delivery System Visually inspect the balloon/stent assembly to assure proper placement of the stent. Stent Deployment 1. Use of the tools supplied with the stent is necessary to defeat the hemostasis valve without damaging the stent or covering. Once the stent is past the hemostasis valve, the tool must be pulled out of the valve. 2. The system is advanced through the long delivery sheath and over the stiff guidewire into the desired location for implant. 3. After correct positioning of the stent, pull back on the sheath to expose the stent. Confirm proper stent position by a small injection of contrast through the sidearm of sheath or through a second catheter. 4. Expand the stent initially by inflating the inner balloon until it is fully expanded. One may reposition the stent at this point by moving the BIB catheter. The unexpanded outer balloon and expanded inner balloon hold the stent tightly against the BIB catheter. DO NOT deflate the inner balloon before expansion of the outer balloon. This could cause the stent to slip off the balloon catheter. 5. Confirm positioning and inflate the outer balloon to rated diameter. Do not exceed the rated burst pressure. Delivery System Withdrawal 1. Once the stent is expanded, deflate both balloons completely and rotate to ensure the stent is free and properly deployed. If there is a residual waist in the stent, expand only the outer balloon again, making sure not to exceed the rated burst pressure. 2. Remove the balloon catheter and confirm the result with angiography. NOTE: Diameter of the stent may be increased after placement by expanding with a larger diameter balloon. Do not exceed the maximum recommended expanded stent diameter of 24mm for the 8 zig stents, and 3mm for the 1 zig stents. 3
4 COARCTATION INDICATION The Covered CP Stent is indicated for use in the treatment of native and/or recurrent coarctation of the aorta involving the aortic isthmus or first segment of the descending aorta where there is adequate size and patency of at least one femoral artery associated with one or more of the following: acute or chronic aortic wall injury nearly atretic descending aorta of 3 mm or less in diameter a non-compliant stenotic aortic segment found on pre-stent balloon dilation a genetic or congenital syndrome associated with aortic wall weakening or ascending aortic aneurysm CONTRAINDICATIONS Patients too small to allow safe delivery of the stent without compromise to the systemic artery used for delivery; Unfavorable aortic anatomy that does not dilate with high pressure balloon angioplasty; Curved vasculature; Occlusion or obstruction of systemic artery precluding delivery of the stent; Clinical or biological signs of infection; Active endocarditis; Known allergy to aspirin, other antiplatelet agents, or heparin; Pregnancy. CP STENT WARNINGS Radiofrequency heating during MRI scans on overlapped, 1 zig CP Stents has not been evaluated. Coarctation of the aorta involving the aortic isthmus or first segment of the descending aorta should be confirmed by diagnostic imaging. The NuMED CP stent has not been evaluated in patients weighing less than 2kg. As with any type of implant, infection secondary to contamination of the stent may lead to aortitis, or abscess. Over-stretching of the artery may result in rupture or aneurysm formation. Excessive force while crimping may weaken welds of the stent. Crimping the 8 zig stent on a balloon catheter smaller than 12mm, and the 1 zig on a balloon catheter smaller than 26mm, may cause damage to the stent. Excessive handling and manipulation of the covering while crimping the stent may cause the covering to tear off of the stent. Crimping the device in the opposite direction of the folds in the covering may cause the covering to catch while inserting into the hemostasis valve tool and introducer. This could cause the covering to tear off of the stent. Pulling the Covered stent back through the introducer and/or hemostasis valve may cause the covering to catch and tear off of the stent. 4
5 BIB STENT PLACEMENT WARNINGS Do not exceed the RBP. An inflation device with pressure gauge is recommended to monitor pressure. Pressure in excess of the RBP can cause balloon rupture and potential inability to withdraw the catheter through the introducer sheath. Confirm that the distal end of the introducing sheath is at least 2.5cm back from the most proximal image band before inflating the outer balloon. Failure to do so may stretch the outer tubing and severely hinder balloon deflation. Use two appropriate size inflation devices with pressure gauges for inflation. Do not advance the guidewire, balloon catheter, or any other component if resistance is met, without first determining the cause and taking remedial action. This catheter is not recommended for pressure measurement or fluid injection. Do not remove the guidewire from the catheter at any time during the procedure. This device is intended for single use only. Do not resterilize and/or reuse it, as this can potentially result in compromised device performance and increased risk of cross-contamination. PRECAUTIONS Use of an inflation device with pressure gauge is highly recommended during this procedure. The stent is rigid and may make negotiation through vessels difficult. Dilatation procedures should be conducted under fluoroscopic guidance with appropriate x-ray equipment. Guidewires are delicate devices. Care should be exercised while handling to help prevent the possibility of breakage. Careful attention must be paid to the maintenance of tight catheter connections and by aspiration before proceeding to avoid air introduction into the system. The inflation diameter of the balloon used during stent delivery should approximate the diameter of the obstructive vessel and the intended implant site. Under no circumstances should any portion of the catheter system be advanced against resistance. The cause of the resistance should be identified with fluoroscopy and action taken to remedy the problem. If resistance is felt upon removal, the balloon, guidewire, and sheath should be removed as a unit, particularly if balloon rupture or leakage is known or suspected. This may be accomplished by firmly grasping the balloon catheter and sheath as a unit and withdrawing both together, using a gentle twisting motion combined with traction. Proper functioning of the catheter depends on its integrity. Care should be used when handling the catheter. Damage may result from kinking, stretching, or forceful wiping of the catheter. POTENTIAL COMPLICATIONS / ADVERSE EFFECTS NOTE: Circumferential tear of the delivery balloon catheter prior to complete expansion of the stent may cause the balloon to become tethered to the stent, requiring surgical removal. In case of rupture of an adequately sized balloon after stent expansion, it can be withdrawn and a new balloon catheter exchanged over a guidewire to complete expansion of the stent. 5
6 Cardiac catheterization carries certain risks. In addition, potential complications, and related adverse effects associated with implants include, but are not limited to: Femoral Artery injury, thrombosis or pseudoaneurysm Stent Migration Stent Stenosis Stent Fracture Aortic Aneurysm/Pseudoaneurysm Aortic Rupture/Tear Stent Malposition Hematoma Sepsis/infection Thrombosis/Thromboembolism AV fistula formation Death Transitory arrhythmia Endocarditis Bleeding Cell necrosis at the site of implant Cerebrovascular Incident COAST II CLINICAL STUDY INFORMATION COAST II was performed to establish a reasonable assurance of safety and effectiveness of implantation of the Covered CP stent in the native and/or recurrent coarctation of the aorta in the US. The study was a prospective, multi-center, single-arm clinical study that evaluates the Covered CP Stent for treatment of coarctation of the aorta. For effectiveness, each patient serves as his or her own control. For safety, a performance goal was derived from surgical literature. 82 patients were included in the COAST II cohort at 19 investigational sites. 1. Clinical Inclusion and Exclusion Criteria Enrollment in the COAST II study was limited to patients who met the following inclusion criteria: Native or recurrent aortic coarctation*associated with one or more of the following: 1. Acute or chronic aortic wall injury** 2. Nearly atretic descending aorta to 3 mm or less in diameter. 3. Genetic Syndromes associated with aortic wall weakening. Individuals with genetic syndromes such as Marfan Syndrome, Turner s Syndrome or familial bicuspid aortic valve and ascending aortic aneurysm. 4. Advanced age. Men and woman aged 6 years or older. * The significance of aortic obstruction is left to the judgment of the participating investigator. Indications might include mild resting aortic obstruction associated with: Exercise related upper extremity hypertension; Severe coarctation with multiple and/or large arterial collaterals; Single ventricle physiology Left ventricular dysfunction Ascending aortic aneurysm 6
7 **Aortic wall injury might include: Descending aortic aneurysm Descending aortic pseudo-aneurysm Contained aortic wall rupture Non-contained rupture of the aortic wall Patients were not permitted to enroll in the COAST II study if they met any of the following exclusion criteria: 2. Follow-up Schedule a. Patient size too small for safe delivery of the device. The absolute lower limit for inclusion under this protocol is 2 kg. However, serious femoral artery injury can occur in small patients, particularly those in the 2-3 kg range and this risk must be reviewed in detail with parents or guardians of children in this weight range. b. Planned deployment diameter less than 1 mm or greater than 22 mm c. Location requiring covered stent placement across a carotid artery* d. Adults lacking capacity to consent e. Pregnancy *crossing or covering of a subclavian artery is acceptable in certain situations, but only after alternative treatments have been considered. All patients were scheduled to return for follow-up examinations at 1 month, 6 months, 12 months, 24 months, and annually thereafter to 5 years. Adverse events and complications were recorded at all visits. 3. Clinical Endpoints With regards to safety, the following criteria were evaluated. Primary Safety Endpoint: Occurrence of any serious or somewhat serious adverse event attributed to the stent or implantation procedure within 3 days of the catheterization procedure. The following hypothesis was tested using a one-sample, one-sided test of proportions conducted at the.5 level of significance: H: p.18 vs. HA: p <.18 Secondary Safety Endpoint: Proportion of patients experiencing any of the following adverse events related to the device or implant procedure post 1 year Underlying cardiac or non-cardiac disease, aortic wall injury, new aortic aneurysm formation within region of device, stent misplacement, malposition, stent fracture, aortic wall aneurysms, or restenosis requiring reintervention. With regards to effectiveness, the following criteria were evaluated. 7
8 Primary Effectiveness Endpoint #1: Improvement of aortic wall injury and/or aortic arch obstruction by a median increase of at least one grade from pre-implantation baseline to 12 month follow-up using the Severity of Illness Scale (based on upper extremity (UE) systolic BP, UE to lower extremity (LE) systolic BP, and aortic wall injury). The following hypothesis was tested using a one-sided Wilcoxon signed-rank test conducted at the.25 level of significance: H: median change in grade vs. HA: median chang e in grade > Primary Effectiveness Endpoint #2: Aortic wall injury and aortic arch obstruction at Grade 4 or above at the 12 month follow-up, based on the Severity of Illness Scale, with no clinical worsening. The following hypothesis was tested using a one-sample, one-sided test of proportions conducted at the.5 level of significance: H: p.7 vs. HA: p >.7 Secondary Effectiveness Endpoints: Reduction of arm-leg systolic blood pressure gradients to less than 2mmHg and less than 15 mmhg. Reduction of upper extremity blood pressure at 1 year compared to baseline Repair of wall defect with <1% residual endoleak on MRI or CT in patients with aortic wall injury Hospital length of stay compared to length of stay for surgical repair of aortic coarctation. 82 patients were enrolled in COAST II and study accountability is detailed in Table 1. COAST II Patients Safety Cohort Possible n (1%) (1%) Table 1: COAST II Accountability 1 Month 12 Month 24 Month Visit Visit Visit n (%) n (%) n (%) 69 (84%) 67 (81.7%) 3 years Visit n (%) 55 (67.1%) 4 years Visit n (%) 38 (46.3%) 5 years Visit n (%) 22 (26.8%) Effectiveness Cohort (1%) 68 (83%) 66 (8.5%) 54 (65.8%) 37 (45.1%) 21 (25.6%) Subject Demographics Table 2 presents subject demographics and baseline characteristics analyzed for the enrolled subjects. The study population consisted of 52 male and 3 female subjects with a mean age of 18 (range 6 to 67 years). 8
9 Table 2: COAST II Pivotal Cohort Patient Characteristics Number (Percent) or Assessment Median (Range) Prospective (n=29) Legacy (n=53) Total (n=82) Gender Male 21 (72%) 31 (58%) 52 (63%) Female 8 (28%) 22 (42%) 3 (37%) Age, years 2 (6 to 67) 17 (6 to 66) 18 (6 to 67) Primary Indication Repair of aortic wall injury 15 (52%) 34 (64%) 49 (6%) Prevention of aortic wall injury 1 14 (48%) 19 (36%) 33 (4%) 1 Includes 1 patient classified as not having pre-existing aortic wall injury, who was noted to have a small, localized intimal tear with a diameter of < ¼ the aortic diameter. The analysis of safety was based on the implanted cohort of 82 COAST II patients completing their implant procedures. The primary safety outcomes are presented in Table 3. Table 3. Summary of COAST II Outcomes and Pre-Specified Safety Endpoints COAST II Safety Endpoint Event Rate (CI) P Value Primary Serious or Somewhat Serious Adverse Events Attributed to the Stent, Implantation or Catheterization within 3 days of the procedure (includes data from COAST combined with COAST II) 8.2% (5.2%, 12.3%)* <.1 Secondary Proportion of patients experiencing any AEs related to the device or implant procedure post 1 year (among 74 patients followed for at least 1 year) 6.8% (2.2%, 15.1%) # N/A *9% Confidence interval # Confidence interval is not adjusted for multiplicity and is provided to illustrate the variability only. No statistical conclusion should be drawn from this confidence interval. The COAST II primary safety endpoints were met with the occurrence of any serious or somewhat serious adverse event within 3 days post procedure being less than the predefined 18%. The overall incidence and types of adverse events were within expected ranges. Aortic wall injuries were rare and treated appropriately without the need for emergency surgery. The results are durable out to 6 months for each study and recoarctation was treated by transcatheter means when it occurred. Table 4 provides a summary of the adverse events reported under COAST II. 9
10 Patients with adverse events at 3 days Serious or somewhat serious events at 3 days Table 4: Summary of Adverse Events (AEs) for COAST II Stent Related Events 1 (Rates) Stent, Implantation, or Catheterization Related Events 2 (Rates) All Events (Rates) 2 (2.4%) 1 (1.2%) 27 (32.9%) 6 (7.3%) 42 (51.2%) 7 (8.5%) Serious or somewhat serious events at 3 days, excluding stent fracture 1 (1.2%) 6 (7.3%) 7 (8.5%) Serious event at 3 days (.%) Patients with adverse events at 4 12 Months (4.9%) Serious or somewhat serious 3 events at 12 months (3.7%) Serious or somewhat serious 3 events at 12 months, (3.7%) excluding stent fracture Serious event at 12 months 1 (1.2%) Patients with adverse event at 5 24 Months (6.1%) 1 (1.2%) 3 (36.6%) 7 (8.5%) 7 (8.5%) 1 (1.2%) 58 (7.7%) 14 (17.1%) 14 (17.1%) 1 4 (1.2%) (4.9%) 31 6 (37.8%) (73.2%) 1 Includes events that are due to or possible due to stent, and stent fractures. 2 Includes events that are due to or possible due to stent, implantation, or catheterization, and stent fractures Table 5 presents incidence of stent fractures. There were no stent fractures detected at 12 month fluoroscopy. One stent fracture was detected at 24 month fluoroscopy. There was no loss of structural integrity in the detected fracture. 1
11 Table 5: COAST II Pivotal Cohort Safety Data Adverse Events Stent Fracture Percentage of Eligible Subjects Undergoing Fluoroscopy Completed 12 Month Fluoroscopy (n=69) 1 69/81 2 (85%) Completed 24 Month Fluoroscopy (n=65) 2 65/8 4 (81%) Stent Fracture (.%) 1 (1.5%) No loss of structural integrity 1 Loss of structural integrity 1 Among 81 eligible subjects, excludes: 3 patients lost to follow-up at 6 months and 4 patients lost to follow-up at 12 months. An additional 5 patients did not undergo fluoroscopy at 12 months. 2 One patient died prior to 12 month follow-up. 3 Among 8 eligible subjects, excludes: 7 patients previously lost to follow-up, and 2 patients lost to follow-up at 24 months. An additional 6 patients did not undergo fluoroscopy at 24 months. 4 In addition to 1 patient who died prior to 12 month follow-up, 1 patient withdrew consent prior to 24 month visit. Tables 6 through 8 document the stent related, implantation related and catheterization procedure related adverse events in the COAST II trial. Table 6: Stent Related Adverse Events for COAST II Event n (Event Rate) COAST II (n =82) Aortic Aneurysm 2 (2.4 %) Asymmetric Stent Shortening 1 (1.2 %) Left arm numbness and weakness 1 (1.2 %) Table 7: Implantation Related Adverse Events for COAST II Event n (Event Rate) COAST II (n =82) Aneurysmal formation 1 (1.2 %) Chest pain 4 (4.9 %) Chest and back pain 1 (1.2 %) Easy bruising on aspirin 1 (1.2 %) Increased bruising 1 (1.2 %) Right groin pain 1 (1.2 %) Stent malposition 2 (2.4 %) Wound bleeding 1 (1.2 %) 11
12 COAST II (n =82) Table 8: Catheterization Related Adverse Events for COAST II Event n (Event Rate) Aneurysm 1 (1.2 %) Atrial arrhythmia 1 (1.2 %) Brachial plexus injury 1 (1.2 %) Contact skin rash 1 (1.2 %) Corneal abrasion 1 (1.2 %) Discomfort right eye 1 (1.2 %) Dissection of iliac artery 1 (1.2 %) Ecchymosis/groin tenderness 1 (1.2 %) Femoral artery occlusion 1 (1.2 %) Local hematoma groin 2 (2.4 %) Localized groin bruising 1 (1.2 %) Minimal bleeding/cough 1 (1.2 %) Neck swelling 1 (1.2 %) Pulsatile bleeding 1 (1.2 %) Right iliac dissection/pulse loss 1 (1.2 %) Superficial infection of groin 1 (1.2 %) Wide complex non-sustained 1 (1.2 %) tachycardia There were five patients that crossed over from COAST to COAST II. One patient crossed over due to a small aneurysm after dilation, two patients due to a near atretic aorta, one patient due to localized intimal tear after dilation and one patient due to an acute, rapidly expanding aneurysm after dilation. In the COAST II trial two patients experienced two events, representing 2.4% of patients, and both events were resolved using a second Covered CP stent to fully occlude the aneurysm developed with no permanent damage. In the COAST II trial, three patients experienced aortic wall injuries by the 24 month follow-up. These injuries are detailed in Table 9, below. Table 9: COAST II Aortic Wall Injuries by 24 Month Follow-up Date of Catheterization Date of MRI Injury Detected Intervention 11/11/29 11/16/211 1 Neo-intimal proliferation 2/23/21 3/31/211 1 Small aneurysm at 12 m visit 4/15/21 5/12/211 Small aneurysm at 12 m visit Therapy for new aortic wall injury implantation of Covered CP Stent Therapy for new aortic wall injury implantation of Covered CP Stent New Covered CP Stent implanted to occlude aneurysm 1 Aortic wall injury not confirmed by MRI at follow-up visit. Confirmed during reintervention. 12
13 In COAST II, six patients experienced coarctation-related events, representing 7.3% of patients with events. These patients underwent catheter reinterventions. No surgical interventions were completed. Table 1 provides a summary of these interventions. Table 1: COAST II Coarctation-Related Reintervention by 24 months Approximate Indication for Reintervention Procedure Performed Time to Intervention Post-procedure (Months) 7 Persistent hypertension and gradient Radiation of stent across aortic arch 7 Planned re-expansion of stent Radiation of stent 9 Planned re-expansion of stent Radiation of stent 25 Increased gradient with somatic growth; neo-intimal proliferation Therapy for new aortic wall injury - implantation of Covered CP Stent detected in cath lab 14 Aneurysm detected by MRI at 12 m visit 1 Therapy for new aortic wall injury - implantation of Covered CP Stent 13 Aneurysm detected by MRI at 12 m visit 1 Therapy for new aortic wall injury - implantation of Covered CP Stent 1. Presence of aneurysm in this patient was not confirmed by core laboratory review of the MRI In COAST II, two patients experienced non-coarctation related reinterventions that were documented by the 24 month follow-up, representing 2.4% of the patients with events. These patients underwent catheter reinterventions. The time to intervention for one patient was four months, when the patient received a coronary angiogram and graft angiogram to address symptoms of angina. The time to intervention for the second patient was 26 months when the Melody valve was implanted to address a high right ventricle to pulmonary artery conduit gradient. The incidence of stent fracture for covered CP stents (COAST II) was much lower and was not observed to substantially increase over time. Also, relief from blood pressure gradient was maintained through 6 month follow-up and re-intervention was rare. When needed, this was accomplished using transcatheter interventions. 13
14 The key effectiveness outcomes of COAST II are shown in Table 11 through 12. Table 11. Summary of Late Outcomes and Major Pre-Specified Effectiveness Study Endpoints Effectiveness Endpoint Event Rate (CI) P Value Severity of Illness Scale Grade 8%.48 4 or 5 with No Clinical (7.1%, 87%)* Worsening at 12 Month Follow-up COAST II Primary COAST II Secondary Proportion of patients with arm-leg systolic blood pressure differences less than 2mmHg and less than 15 mmhg at 12 month follow-up, compared to baseline Reduction of upper extremity blood pressure at 1 year compared to baseline Complete repair of aortic wall defect with first Covered CP Stent (no residual endoleak during the catheterization procedure Proportion of patients with effective treatment of AWI with no residual aneurysm seen on MRI scanning Hospital length of stay compared to length of stay for surgical repair of aortic coarctation 87% (up from 46% at baseline) (76%, 94%) + 79% (up from 38% at baseline) (68%, 88%) + 12 ± 2mmHg (7mmHg, 17mmHg) +# 47 of 49 (96%) of patients treated for an aortic wall injury 37 of 39 (95%) patients treated for an aortic wall injury 1/39 (2.5%) with a small aneurysm and one patient s MRI could not be evaluated by core lab 1.2 ±.9 days (1. days, 1.4 days) + N/A N/A N/A N/A <.1 *9% Confidence interval + 95% Confidence Interval Mean + standard deviation # Confidence interval is not adjusted for multiplicity and is provided to illustrate the variability only. No statistical conclusion should be drawn from this confidence interval. 14
15 Table 12. COAST II Pivotal Cohort Systolic Blood Pressure Number (Percent) or Median (Range) And Mean ± Standard Deviation Upper Extremity Systolic Blood Pressure (mmhg) Median (range) Mean ± standard deviation Lower Extremity Systolic Blood Pressure (mmhg) Median (range) Mean ± standard deviation Systolic Blood Pressure Difference (mmhg) Median (range) Mean ± standard deviation Systolic Blood Pressure Difference < 1 mmhg < 15 mmhg < 2 mmhg Completed 12 Month Follow-up (n=68) (98 to 166) 125 ± (9 to 199) 124 ± 21 2 (-48 to 38) 1 ± (68%) 54 (79%) 59 (87%) Completed 24 Month Follow-up (n=66) (96 to 158) 126 ± (86 to 156) 125 ± 16 (-35 to 62) 1 ± (79%) 56 (85%) 6 (91%) 1 Among 81 eligible subjects, excludes: 3 patients lost to follow-up at 6 months and 4 patients lost to followup at 12 months. An additional 6 patients missed the 12 month visit. 2 Among 8 eligible subjects, excludes: 7 patients previously lost to follow-up, and 2 patients lost to followup at 24 months. An additional 5 patients missed the 24 month visit. Procedural Data A summary of procedural data of those enrolled patients who underwent cardiac catheterization for the purpose of Coarctation of the Aorta is provided in Table 13. Table 13: COAST II Pivotal Cohort Procedural Data Number (Percent) Total (n=82) Covered CP Stent Implanted 82 (1%) Second Covered CP Stent Implanted 9 (11%) Third Covered CP Stent Implanted 2 (2%) Patient Free of Explant 24 hours after Procedure 82 (1%) 15
16 COAST/COAST II Follow-up Data A.1. Percent of Patients with New Stent Fracture % Stent Fracture (new at each interval) Interval COAST I COAST II 12 months 4/121 1/127 (3.3%) (.8%) 24 months 12/121 5/127 (9.9%) (3.9%) 36 months 2/121 1/127 (1.6%) (.8%) 48 months 1/121 4/127 6 months (8.2%) 9/121 (7.4%) (3.1%) 2/127 (1.6%) A.2. Percent of Patients with Stent Fracture (Cumulative) % Stent Fracture (cumulative) Interval COAST I COAST II 12 months 4/121 1/127 (3%) (.8%) 24 months 16/121 6/127 (13%) (4.7%) 36 months 18/121 7/127 (15%) (5.5%) 48 months 28/121 11/127 (23%) (8.6%) 6 months 37/121 13/127 (31%) (1%) B.1. Percent of Patients with Aortic Wall Injury (New or Progressive)* % patients with AWI Interval COAST I COAST II 1/121 1/ months (.8%) (.8%) 24 months /121 / months 48 months 6 months *All recorded injuries were new, none designated as progressive 3/121 (2.5%) 1/121 (.8%) 1/121 (.8%) /127 /127 2/127 (1.6%) 16
17 B.2. Cumulative Percent of Patients with Aortic Wall Injury (New or Progressive)* % patients with AWI (cumulative) Interval COAST I COAST II 12 months 1/121 1/127 (.8%) (.8%) 24 months 1/121 1/127 (.8%) (.8%) 36 months 4/121 1/127 (3.3%) (.8%) 48 months 5/121 1/127 6 months *All recorded injuries were new, none designated as progressive (4.1%) 6/121 (5%) (.8%) 3/127 (2.4%) C.1. Percent of Patients with Coarctation Related Re-intervention or Surgery* *All patients listed had catheter reinterventions, none had surgery % patients with reintervention or surgery Interval COAST I COAST II 12 months 4/121 9/128 (3.3%) (7%) 24 months 3/121 3/128 (2.5%) (2.3%) 36 months 6/121 3/128 (5%) (2.3%) 48 months 6/121 3/128 (5%) (2.3%) 6 months 2/121 3/128 (1.7%) (2.3%) C.2. Percent of Patients with Coarctation Related Re-intervention or Surgery (Cumulative) % patients with catheter-based re-intervention or surgery (cumulative) Interval Bare Metal Covered Stent 12 months 3/121 9/128 (2.5%) (7%) 24 months 6/121 12/128 (5%) (9.3%) 36 months 12/121 15/128 (9.9%) (11.8%) 48 months 18/121 18/128 6 months (14.9%) 2/121 (16.5%) (14%) 21/128 (16.4%) 17
18 C.3. Percent of Bare Metal Patients with Catheter-Based or Surgical Coarctation- Related Re-intervention % Bare Metal patients with catheter-based re-intervention or surgery Interval Catheterization Surgery 12 months 3/121 (2.5%) (%) 24 months 3/121 (2.5%) (%) 36 months 6/121 (5%) (%) 48 months 6/121 6 months (5%) 2/121 (1.7%) (%) (%) C.4. Percent of Bare Metal Patients with Catheter-Based or Surgical Coarctation- Related Re-intervention (Cumulative) % Bare Metal patients with catheter-based reintervention or surgery (cumulative) Interval Catheterization Surgery 12 months 3/121 (2.5%) (%) 24 months 6/121 (5%) (%) 36 months 12/121 (9.9%) (%) 48 months 18/121 6 months (14.9%) 2/121 (16.5%) (%) (%) C.5. Percent of Covered Stent Patients with Catheter-Based or Surgical Coarctation-Related Re-intervention % Covered Stent patients with re-intervention or surgery Interval Catheterization Surgery 12 months 9/128 (7%) (%) 24 months 3/128 (2.3%) (%) 36 months 3/128 (2.3%) (%) 48 months 3/128 6 months (2.3%) 3/128 (2.3%) (%) (%) 18
19 C.6. Percent of Covered Stent Patients with Catheter-Based or Surgical Coarctation-Related Re-intervention (Cumulative) % Covered Stent patients with re-intervention or surgery (cumulative) Interval Catheterization Surgery 12 months 9/128 (7%) (%) 24 months 12/128 (9.3%) (%) 36 months 15/ months 6 months (11.8%) 18/128 (14%) 21/128 (16.4%) (%) (%) (%) D.1 Percent of Patients with Upper to Lower Extremity Pressure Gradients > 15mmHg % patients with Residual UE to LE pressure gradient > 15mmHg Interval COAST I COAST II 12 months 16/14 16/17 (15.4%) (15%) 24 months 8/95 13/81 (8.4%) (16%) 36 months 6/81 12/72 (7%) (14%) 48 months 6/67 6/53 (9%) (1%) 6 months 7/65 14/47 (11%) (3%) **Note, n is different at each time interval and based on the number of patients with pressures measured at each interval** E.1. Percent of Patients with Proximal SBP >14 % patients with UESBP > 14mmHg 3.% Interval COAST I COAST II 25.% 18/14 26/14 12 months 2.% (17%) (25%) 15.% COAST I 16/95 2/81 24 months 1.% (17%) (25%) 5.% COAST II 6/86 12/88 36 months (7%) (14%).% 8/74 1/64 48 months (11%) (16%) 11/74 12/52 6 months (15%) (23%) **Note, n is different at each time interval and based on the number of patients with pressures measured at each interval** 19
20 F.1 Baseline Characteristics Baseline Characteristics COAST/COAST II Median (Range) Bare Metal Patients (n=128) Bare Metal Re-intervention Patients (n= 2) Age (yrs) 16 (8 to 52) 13 (9 to 2) Weight (kg) 65 (35 to 157) 51.7 (35 to 14) Covered Stent Patients (n=127) Covered Stent Reintervention Patients (n=21) Age (yrs) 19 (6 to 67) 15 (6 to 35) Weight (kg) 68 (17 to 122) 5 (17 to 95) G.1 Systolic Blood Pressure Differences after Initial CoA Stenting SBP difference (AAo- Dao) (mmhg) SBP difference (AAo- Dao) (mmhg) Systolic Blood Pressure Differences after Initial CoA Stenting Median (Range) Bare Metal Non-Reintervention Patients (n=18) Bare Metal Re-intervention Patients (n=2) (-12 to 15) 1.5 (-5 to 12) Covered Stent Non-Reintervention Patients (n=16) Covered Stent Reintervention Patients (n=21) 2 (-12 to 38) 7 (-3 to 2) 2
21 H.1 Stent Diameter Measurements after Initial CoA Stenting Minimum Stent Diameter (mm) Maximum Stent Diameter (mm) Minimum Stent Diameter (mm) Stent Measurements after Initial CoA Stenting Median (Range) Bare Metal No Reintervention or only AWIrelated Re-intervention Patients (n=111) Bare Stent Non-AWI Related Re-intervention Patients (n=17) 14.4 (7.8 to 2.8) 11.7 (8.6 to 16) 16.5 (9.2 to 22.5) 13.5 (9.2 to 19.4) Covered Stent No Reintervention or only AWIrelated Re-intervention Patients (n=11) Covered Stent Non-AWI Related Re-intervention Patients (n=17) 14.8 (9 to 23) 11.5 (7.8 to 16.5) Maximum Stent Diameter (mm) 17 (11 to 25.7) 13.2 (9.2 to 18) 21
22 I.1 Upper Extremity Systolic Blood Pressures Bare Metal Patients Upper Extremity Systolic Blood Pressure (mmhg) (n=67,7) Median (range) Mean ± standard deviation Covered Stent Patients Upper Extremity Systolic Blood Pressure (mmhg) (n=59,5) Median (range) Mean ± standard deviation Bare Metal Patients Upper Extremity Systolic Blood Pressure (mmhg) (n=65,9) Median (range) Mean ± standard deviation Covered Stent Patients Upper Extremity Systolic Blood Pressure (mmhg) (n=47,5) Median (range) Mean ± standard deviation Number (Percent) or Median (Range) and Mean ± Standard Deviation Upper Extremity Systolic Blood Pressures at 4 years Upper and Lower Only Upper Extremity Extremity SBP SBP measured measured 123 (79 to 231) (93 to 174) (7 to 134) (11 to 14) Upper Extremity Systolic Blood Pressures at 5 years Upper and Lower Only Upper Extremity Extremity SBP SBP measured measured 128 (16 to 165) (11 to 159) (1 to 16) (121 to 134)
23 RIGHT VENTRICULAR OUTFLOW TRACT INDICATION Treatment of right ventricle to pulmonary artery (right ventricular outflow tract) conduit disruptions that are identified during conduit pre-dilatation procedures performed in preparation for transcatheter pulmonary valve replacement (TPVR). CONTRAINDICATIONS Patients too small to allow safe delivery of the stent without injury to a systemic vein or to the right side of the heart; Clinical or biological signs of infection; Active endocarditis; Pregnancy. WARNINGS During the Premarket Approval study the Medtronic Melody Valve was used for valve restoration. The safety and effectiveness of the Covered CP Stent for pre-stenting of the right ventricular outflow tract (RVOT) landing zone (i.e., prophylaxis or prevention of either RVOT conduit rupture or TPVR fracture; used as a primary RVOT conduit) in preparation of a transcatheter pulmonary valve replacement (TPVR) has not been evaluated. Radiofrequency heating during MRI scans on overlapped, 1 zig CP Stents has not been evaluated. As with any type of implant, infection secondary to contamination of the stent might lead to endocarditis, or abscess formation. The Covered Stent can migrate from the site of implant potentially causing obstruction to pulmonary artery flow. Over-stretching of the RVOT may result in rupture or aneurysm of the RV-PA conduit or the native pulmonary artery. The inflated diameter of the stent should at least equal the diameter of the intended implant site. When the stent is crimped onto a balloon delivery catheter, the maximum balloon inflation pressure must not exceed the recommended inflation pressure specified in the manufacturer s instructions. Excessive force while crimping may weaken welds of the stent. Crimping the 8 zig stent on a balloon catheter smaller than 12mm, and the 1 zig on a balloon catheter smaller than 26mm, may cause damage to the stent. Excessive handling and manipulation of the covering while crimping the stent may cause the covering to tear off of the stent. Crimping the device in the opposite direction of the folds in the covering may cause the covering to catch while inserting into the hemostasis valve tool and introducer. This could cause the covering to tear off of the stent. Pulling the Covered stent back through the introducer and/or hemostasis valve may cause the covering to catch and tear off of the stent. 23
24 This device is intended for single use only. Do not resterilize and/or reuse it, as this can potentially result in compromised device performance and increased risk of cross-contamination. PRECAUTIONS Use of an inflation device with pressure gauge is highly recommended during this procedure. The stent is rigid and may make negotiation through vessels difficult. Dilatation procedures should be conducted under fluoroscopic guidance with appropriate x-ray equipment. The inflation diameter of the balloon used during stent delivery should approximate the diameter of the obstructive vessel and the intended implant site. Careful attention must be paid to the maintenance of tight catheter connections and by aspiration before proceeding to avoid air introduction into the system. Under no circumstances should any portion of the catheter system be advanced against resistance. The cause of the resistance should be identified with fluoroscopy and action taken to remedy the problem. POTENTIAL COMPLICATIONS / ADVERSE EFFECTS NOTE: Circumferential tear of the delivery balloon catheter prior to complete expansion of the stent may cause the balloon to become tethered to the stent, requiring surgical removal. In case of rupture of an adequately sized balloon after stent expansion, it can be withdrawn and a new balloon catheter exchanged over a guidewire to complete expansion of the stent. Cardiac catheterization carries certain risks. In addition, potential complications, and related adverse effects associated with implants include, but are not limited to: Stent Migration Stent Stenosis Stent Fracture Pseudoaneurysm Vessel Ruptures Stent Malposition Hematoma Sepsis/infection Thrombosis/Thromboembolism AV fistula formation Death Transitory arrhythmia Endocarditis Bleeding Cell necrosis at the site of implant Cerebrovascular Incident PARCS CLINICAL STUDY INFORMATION PARCS was performed to provide information that will support labeling of the Covered CP Stent for the treatment of RV-PA conduit disruption, occurring during cardiac catheterizations aimed at re-dilating the conduit up to 11% of its original size, and then making the conduit competent by the implantation of a transcatheter pulmonary valve. The study was a prospective, multi-center, single arm, non-randomized clinical study trial. 24
25 5 patients were included in the pivotal cohort, and 7 patients were included in the continued access cohort. There were 39 investigational sites. 1. Clinical Inclusion and Exclusion Criteria a. Precatheterization Inclusion Criteria: i. Patient meets institutional criterion for placement of Melody TPV ii. Patient size adequate to receive Melody TPV implantation via venous access using the Ensemble Transcatheter Delivery System iii. RV-PA conduit original size 16mm diameter iv. Patient age between 7 and 75 years b. Catheterization Inclusion Criteria: i. Angiographic evidence for RV-PA conduit disruption including: dissection, aneurysm, pseudo-aneurysm, tears or rupture Recognition and treatment of conduit disruption may occur before, during or after implantation of the Melody TPV Conduit disruption related to prior intervention, identified angiographically before conduit dilation is performed during the Melody implant procedure, can be eligible for CCPS implantation and study inclusion. c. Precatheterization Exclusion Criteria: i. Patient size too small for transvenous placement of the Melody TPV ii. Bloodstream infection, including endocarditis iii. Pregnancy iv. Prisoners and adults lacking the capacity of give consent d. Catheterization Exclusion Criteria: i. Conduit size is not suitable (too small or too large) for a Melody TPV ii. Risk of coronary compression has been identified iii. Lack of angiographic evidence for RV-PA conduit disruption Prophylactic use of study CCPS is prohibited iv. Vessel injury occurring in either the right or left branch pulmonary arteries If injury to branch pulmonary arteries occurs during the catheterization and covered stent usage is indicated, Emergency Use guidelines must be employed. 2. Follow-up Schedule All patients are scheduled to return for follow-up examinations at 6 months. 3. Clinical Endpoints With regards to safety, the following criteria were evaluated. 25
26 Primary Safety Endpoint #1: Patient must have successful coverage of conduit disruption defined as either no residual disruption or contained disruption, followed by successful implantation of the Melody valve. Hypothesis: At least 8% of patients will have successful implantation of the Melody TPV following repair of the RV-PA conduit to a post- procedure SIS level of or 1, using the Covered CP Stent. Table 14: Primary Safety Endpoint #1 Number (Percent) Pivotal CAP Patients with Successful Coverage of Conduit Disruption Followed by Successful Implantation of Melody Valve 1 95% Confidence Interval Implanted (n=5 2 ) 46 (93.9%) (83.1, 98.7) Implanted (n=7 3 ) 62 (89.9%) (8.2, 95.8) 1 Successful coverage defined as no residual disruption or contained disruption 2 Not reported for 1 pivotal patient 3 Not reported for 1 CAP patient Conclusion: Based on the pivotal cohort, successful implantation of the Melody valve is achieved in at least 83% of patients, which exceeds the hypothesized value of 8%. Primary Safety Endpoint #2: Patient must not have any adverse event attributed to the Covered CP Stent within 3 days of the catheterization procedure, as adjudicated by the Data and Safety Monitoring Board. Hypothesis: At least 8% of patients will be free of an adverse event attributed to the Covered CP Stent within 3 days of the catheterization procedure. Table 15: Primary Safety Endpoint #2 Number (Percent) Pivotal CAP Patients with Any Adverse Event Attributed to the Covered CP Stent within 3 Days 1 95% Confidence Interval Implanted (n=5) 1 (2.%) (.1, 1.7) Implanted (n=7) 2 (2.9%) (.4, 9.9) 1 Two adverse events attributed to CCPS in 1 pivotal patient (stent malposition, embolization) 26
27 Level of Security Conclusion: Based on the pivotal cohort, at least 8% of patients were free of an adverse event attributed to the CCPS within 3 days of the catheterization procedure. With regards to effectiveness, the following criteria were evaluated. Primary Effectiveness Endpoint #1: Improvement in the Severity of Illness Score (SIS) is defined as pre-implantation SIS minus post-procedure SIS assessed after Melody Valve insertion (e.g., a change from level 3 at baseline to level 2 postprocedure would represent an improvement of 1 level in SIS). The scale used for the clinical study was developed by the Principal Investigator. Table 16: PARCS Severity of Illness Scale (SIS) Baseline Assessment Level Level 1 Level 2 Level 3 Level 4 No injury or conduit wall disruption: No contrast seen extending more than 2mm outside of or extravasating (leaking) outside of the longitudinal plane of the vascular lumen. (does not indicate the need for CCPS implantation) Contained disruption: Small collection of contrast seen extending outside of the longitudinal plane of the vascular lumen ½ the diameter of the adjacent conduit, indicating the occurrence of an aneurysm, pseudoaneurysm or well contained tear. This category can also be used to describe the unlikely occurrence of a dissection with contrast held in a contained space within the conduit lumen. Partially Contained disruption: Large collection of contrast seen outside the wall of the RV-PA conduit ½ the diameter of the adjacent conduit. Uncontained conduit disruption: Extravasation of contrast into the mediastinum or pleural cavity. N/A 27
28 Table 17: PARCS Severity of Illness Scale (SIS) Post CCPS Implant Assessment Level Level 1 Level 2 Level 3 Level 4 Level of Security No residual disruption: Total occlusion of conduit disruption without contrast seen outside of the longitudinal plane of the vascular lumen. Contained disruption: Small collection of contrast extending outside longitudinal plane of vascular lumen ½ the diameter of the adjacent conduit, c/w persistence of an aneurysm, pseudoaneurysm or well contained tear. This category describes leak into original or new injury. This category should only be used for disruption judged unlikely to need further intervention or surgery. Partially Contained disruption: Large collection of contrast seen outside the wall of the RV-PA conduit > ½ the diameter of the adjacent conduit. Uncontained conduit disruption: Extravasation of contrast into the mediastinum or pleural cavity (likely to require further intervention or surgery). Emergent conduit rupture: Severe conduit rupture resulting in the immediate need for surgery or resulting in death. Hypothesis: Patients will demonstrate a median improvement by at least 1 level from baseline to post-procedure on the severity of illness scale. 28
29 Table 18: Primary Effectiveness Endpoint #1 Number (Percent) Improvement in Severity of Illness Score Improvement in Severity of Illness Score, from Pre- Implantation Baseline to Post-Procedure, Assessed after Melody Valve Insertion Pivotal Implanted (n=5) 3 (6) 8 (17) 31 (66) 3 (6) 2 (4) CAP Implanted (n=7) 3 (5) 8 (12) 47 (71) 7 (11) 1 (2) 1 Baseline SIS not reported for 2 CAP patients; post-procedure SIS not reported for 3 pivotal patients and 2 CAP patients Conclusion: For pivotal patients, there is a median improvement in SIS of 1 level (p<.1, Wilcoxon signed-rank test). Primary Effectiveness Endpoint #2: Procedure Success defined as device and lesion success with the Covered CP Stent implantation without intra-procedural or post-catheterization somewhat serious or serious adverse events attributable to CCPS implantation. Procedure success will be determined 6 months after the implantation procedure, enabling inclusion of repeat cardiac catheterization or surgery to repair a pseudoaneurysm or conduit tear related to incomplete repair of the conduit wall disruption. Hypothesis: The procedure will be successful in at least 75% of patients. Table 19: Primary Efficacy Endpoint #2 Number (Percent) Pivotal CAP Procedure Success Implanted (n=5 1 ) Implanted (n=7 2 ) Both Device and Lesion Success, with No Adverse Event Attributed to the Covered CP Stent 95% Confidence Interval 1 Not reported for 1 pivotal patient 2 Not reported for 1 CAP patient 4 (81.6%) (68., 91.2) 54 (78.3%) (66.7, 87.3) 29
30 Conclusion: Based on the pivotal cohort, procedure success is achieved in at least 68% of patients. This falls short of the hypothesis that procedure success would be achieved in at least 75% of patients. Secondary Effectiveness Endpoint #1: Device Success defined as successful implantation of a Covered CP Stent, either providing complete repair of a conduit disruption or placement in preparation for a second Covered stent overlapping in tandem without adverse event. Table 2: Secondary Effectiveness Endpoint #1 Number (Percent) Device Success Pivotal Implanted (n=5 1 ) CAP Implanted (n=7 2 ) Successful Implantation of First CCPS, Either Providing Complete Repair of Conduit Disruption or Placement in Preparation for Overlapping Tandem Stents, with No Adverse Event Attributed to the Covered CP Stent 95% Confidence Interval 1 Not reported for 1 pivotal patient 2 Not reported for 1 CAP patient 46 (93.9%) (83.1, 98.7) 66 (95.7%) (87.8, 99.1) Secondary Effectiveness Endpoint #2: Lesion Success defined as complete repair of a conduit disruption with a single Covered CP Stent or via planned, tandem covered stent implantations. Table 21: Secondary Effectiveness Endpoint #2 Number (Percent) Lesion Success Successful Coverage of Conduit Disruption with First CCPS or with Subsequent Planned CCPS 95% Confidence Interval 1 Not reported for 1 pivotal patient 2 Not reported for 1 CAP patient Pivotal Implanted (n=5 1 ) 42 (85.7%) (72.8, 94.1) CAP Implanted (n=7 2 ) 56 (81.2%) (69.9, 89.6) 3
Covered CP Stent CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
 Covered CP Stent COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 4 Right Ventricular Outflow Tract Indication..Page
Covered CP Stent COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 4 Right Ventricular Outflow Tract Indication..Page
CP STENT. Large Diameter, Balloon Expandable Stent
 CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA INSTRUCTIONS FOR USE
 NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 5 Right Ventricular Outflow Tract
NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 5 Right Ventricular Outflow Tract
CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
 Mounted CP Stent COARCTATION OF THE AORTA INSTRUCTIONS FOR USE General Instructions.. Page 2 Stent Sizing Charts and Information..Page 21 CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR
Mounted CP Stent COARCTATION OF THE AORTA INSTRUCTIONS FOR USE General Instructions.. Page 2 Stent Sizing Charts and Information..Page 21 CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Transcatheter Pulmonary Valve Replacement Update on progress and outcomes
 Transcatheter Pulmonary Valve Replacement Update on progress and outcomes Barry Love, MD Director, Congenital Cardiac Catheterization Laboratory Mount Sinai Medical Center New York, New York Transcatheter
Transcatheter Pulmonary Valve Replacement Update on progress and outcomes Barry Love, MD Director, Congenital Cardiac Catheterization Laboratory Mount Sinai Medical Center New York, New York Transcatheter
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Talent Abdominal Stent Graft
 Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM
 Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
TYSHAK DILATATION CATHETER. Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician.
 TYSHAK DILATATION CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional Systems Inc. 824 Twelfth
TYSHAK DILATATION CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional Systems Inc. 824 Twelfth
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
CoA Stenting Alexandria Experience
 CoA Stenting Alexandria Experience By Hani Mahmoud Adel, M.D Alexandria University 2/27/2017 CE 2017 Cairo 1 Coarctation of the aorta is the eighth most common form of congenital heart disease & accounts
CoA Stenting Alexandria Experience By Hani Mahmoud Adel, M.D Alexandria University 2/27/2017 CE 2017 Cairo 1 Coarctation of the aorta is the eighth most common form of congenital heart disease & accounts
Introduction 3. What is Peripheral Vascular Disease? 5. What Are Some of the Symptoms of Peripheral Vascular Disease? 6
 Patient Information Table of Contents Introduction 3 What is Peripheral Vascular Disease? 5 What Are Some of the Symptoms of Peripheral Vascular Disease? 6 What Causes Peripheral Vascular Disease? 7 How
Patient Information Table of Contents Introduction 3 What is Peripheral Vascular Disease? 5 What Are Some of the Symptoms of Peripheral Vascular Disease? 6 What Causes Peripheral Vascular Disease? 7 How
Introduction What Causes Peripheral Vascular Disease? How Do Doctors Treat Peripheral Vascular Disease?... 9
 Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
TRANSCATHETER REPLACEMENT OF THE PULMONARY VALVE (PPVI)
 TRANSCATHETER REPLACEMENT OF THE PULMONARY VALVE (PPVI) BASIL D. THANOPOULOS MD, PhD Director Interventional Cardiology of CHD Euroclinic ATHENS - GREECE TRANSCATHETER REPLACEMENT OF THE PULMONARY VALVE
TRANSCATHETER REPLACEMENT OF THE PULMONARY VALVE (PPVI) BASIL D. THANOPOULOS MD, PhD Director Interventional Cardiology of CHD Euroclinic ATHENS - GREECE TRANSCATHETER REPLACEMENT OF THE PULMONARY VALVE
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Finally, the Control You Need to Deliver Accurate Treatment
 Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Coarctation of the Aorta
 Interventional Management of Coarctation of the Aorta Lee Benson MD Professor Pediatrics (Cardiology) Director, Cardiac Diagnostic & Interventional Unit The Hospital for Sick Children Toronto, Canada Outline
Interventional Management of Coarctation of the Aorta Lee Benson MD Professor Pediatrics (Cardiology) Director, Cardiac Diagnostic & Interventional Unit The Hospital for Sick Children Toronto, Canada Outline
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU)
 Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Patient Information Booklet
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Thoracic Aortic Aneurysms Table of Contents Table of Contents Introduction 1 Anatomy of the Thoracic Aorta 2 What Is an Aneurysm?
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Thoracic Aortic Aneurysms Table of Contents Table of Contents Introduction 1 Anatomy of the Thoracic Aorta 2 What Is an Aneurysm?
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
VIRTUS: Trial Design and Primary Endpoint Results
 VIRTUS: Trial Design and Primary Endpoint Results Mahmood K. Razavi, MD St. Joseph Cardiac and Vascular Center Orange, CA, USA IMPORTANT INFORMATION: These materials are intended to describe common clinical
VIRTUS: Trial Design and Primary Endpoint Results Mahmood K. Razavi, MD St. Joseph Cardiac and Vascular Center Orange, CA, USA IMPORTANT INFORMATION: These materials are intended to describe common clinical
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
PERFORMANCE YOU CAN TRUST. EverFlex Self-expanding Peripheral Stent with Entrust Delivery System
 PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
Malperfusion Syndromes Type B Aortic Dissection with Malperfusion
 Malperfusion Syndromes Type B Aortic Dissection with Malperfusion Jade S. Hiramoto, MD, MAS April 27, 2012 Associated with early mortality Occurs when there is end organ ischemia secondary to aortic branch
Malperfusion Syndromes Type B Aortic Dissection with Malperfusion Jade S. Hiramoto, MD, MAS April 27, 2012 Associated with early mortality Occurs when there is end organ ischemia secondary to aortic branch
Access More Patients. Customize Each Seal.
 Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
CONGENITAL HEART DEFECTS IN ADULTS
 CONGENITAL HEART DEFECTS IN ADULTS THE ROLE OF CATHETER INTERVENTIONS Mario Carminati CONGENITAL HEART DEFECTS IN ADULTS CHD in natural history CHD with post-surgical sequelae PULMONARY VALVE STENOSIS
CONGENITAL HEART DEFECTS IN ADULTS THE ROLE OF CATHETER INTERVENTIONS Mario Carminati CONGENITAL HEART DEFECTS IN ADULTS CHD in natural history CHD with post-surgical sequelae PULMONARY VALVE STENOSIS
Patient Brochure. Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland. PK Rev. 0 05/17
 Patient Brochure Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland PK1411100 Rev. 0 05/17 LIFESTREAM Patient Brochure If you or a member of your family has been diagnosed with
Patient Brochure Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland PK1411100 Rev. 0 05/17 LIFESTREAM Patient Brochure If you or a member of your family has been diagnosed with
Advanced Innovation for Exceptional Performance
 Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18
 INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18 www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular Stent Grafts: A treatment for Thoracic Aortic disease Table of
INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18 www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular Stent Grafts: A treatment for Thoracic Aortic disease Table of
Acute dissections of the descending thoracic aorta (Debakey
 Endovascular Treatment of Acute Descending Thoracic Aortic Dissections Nimesh D. Desai, MD, PhD, and Joseph E. Bavaria, MD Acute dissections of the descending thoracic aorta (Debakey type III or Stanford
Endovascular Treatment of Acute Descending Thoracic Aortic Dissections Nimesh D. Desai, MD, PhD, and Joseph E. Bavaria, MD Acute dissections of the descending thoracic aorta (Debakey type III or Stanford
Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System GO TO INDEX COMMONLY BILLED CODES
 2015 Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System COMMONLY BILLED CODES 2015 INDEX For Right Ventricular Outflow Tract Conduit Dysfunction.... 1 ICD-9-CM Diagnosis
2015 Melody Transcatheter Pulmonary Valve and Ensemble Transcatheter Valve Delivery System COMMONLY BILLED CODES 2015 INDEX For Right Ventricular Outflow Tract Conduit Dysfunction.... 1 ICD-9-CM Diagnosis
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English cs Čeština da Dansk nl Nederlands fi Suomi fr Français de Deutsch el Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polski pt Português es Español sv Svenska
INSTRUCTIONS FOR USE FOR: en English cs Čeština da Dansk nl Nederlands fi Suomi fr Français de Deutsch el Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polski pt Português es Español sv Svenska
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
1 Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis. N Engl J Med 352;13, March 31, 2005
 The risk of ischemic stroke in patients with Intracranial Atherosclerotic Disease (ICAD) ranges from 7 to 24%. 1,2 Developed specifically for the treatment of ICAD, the Wingspan Stent System and Gateway
The risk of ischemic stroke in patients with Intracranial Atherosclerotic Disease (ICAD) ranges from 7 to 24%. 1,2 Developed specifically for the treatment of ICAD, the Wingspan Stent System and Gateway
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Neuroform Microdelivery Stent System
 Neuroform Microdelivery Stent System Patient Information Booklet TABLE OF CONTENTS What is the Purpose of This Booklet?...2 What is a Wide Neck, Intracranial Aneurysm?...2 What is the Treatment for Wide
Neuroform Microdelivery Stent System Patient Information Booklet TABLE OF CONTENTS What is the Purpose of This Booklet?...2 What is a Wide Neck, Intracranial Aneurysm?...2 What is the Treatment for Wide
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Understanding aneurysms and flow diversion treatment
 Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Anatomy and Classification of Aortic Dissection
 Aortic dissection is the most common disorder of the aorta that brings a patient to the emergency room. If left untreated, 75 percent of sufferers die in the first two weeks and 90 percent die in the first
Aortic dissection is the most common disorder of the aorta that brings a patient to the emergency room. If left untreated, 75 percent of sufferers die in the first two weeks and 90 percent die in the first
IMAGES. in PAEDIATRIC CARDIOLOGY
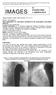 IMAGES in PAEDIATRIC CARDIOLOGY Images Paediatr Cardiol. 2005 Jan-Mar; 7(1): 12 17. PMCID: PMC3232568 Stent implantation for coarctation facilitated by the anterograde trans-septal approach N Sreeram and
IMAGES in PAEDIATRIC CARDIOLOGY Images Paediatr Cardiol. 2005 Jan-Mar; 7(1): 12 17. PMCID: PMC3232568 Stent implantation for coarctation facilitated by the anterograde trans-septal approach N Sreeram and
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Use of covered stents and endograft as a rescue treatment in a patient with a complex form of recurrent aortic coarctation
 TECHNICAL NOTE Use of covered stents and endograft as a rescue treatment in a patient with a complex form of recurrent aortic coarctation Juan C. Parodi, MD, Luis Mariano Ferreira, MD, Horacio Faella,
TECHNICAL NOTE Use of covered stents and endograft as a rescue treatment in a patient with a complex form of recurrent aortic coarctation Juan C. Parodi, MD, Luis Mariano Ferreira, MD, Horacio Faella,
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Percutaneous Intervention for totally Occluded Coarctation Of Aorta. John Jose, Vipin Kumar, Ommen K George Dept Of Cardiology
 Percutaneous Intervention for totally Occluded Coarctation Of Aorta John Jose, Vipin Kumar, Ommen K George Dept Of Cardiology Background Coarctation of aorta (CoA) forms 5-7% of congenital heart diseases
Percutaneous Intervention for totally Occluded Coarctation Of Aorta John Jose, Vipin Kumar, Ommen K George Dept Of Cardiology Background Coarctation of aorta (CoA) forms 5-7% of congenital heart diseases
Performance of the conformable GORE TAG device in Type B aortic dissection from the GORE GREAT real world registry
 University of Milan Thoracic Aortic Research Center Performance of the conformable GORE TAG device in Type B aortic dissection from the GORE GREAT real world registry Santi Trimarchi, MD, PhD Associate
University of Milan Thoracic Aortic Research Center Performance of the conformable GORE TAG device in Type B aortic dissection from the GORE GREAT real world registry Santi Trimarchi, MD, PhD Associate
Patient guide: pfm Nit-Occlud PDA coil occlusion system. Catheter occlusion of. Patent Ductus Arteriosus. with the
 Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING
 Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
Understanding Peripheral
 Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
Figure 1: Revolution TM Peripheral Atherectomy System Diagram. Table 1: Revolution TM Peripheral Atherectomy System Specifications Minimum Burr
 Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Transcather Pulmonary Valve Replacement Using The Melody Valve: Indications, Techniques, Outcomes
 Transcather Pulmonary Valve Replacement Using The Melody Valve: Indications, Techniques, Outcomes Matthew J. Gillespie MD The Children s Hospital of Philadelphia SCAI Fall Fellows Course 2014 December
Transcather Pulmonary Valve Replacement Using The Melody Valve: Indications, Techniques, Outcomes Matthew J. Gillespie MD The Children s Hospital of Philadelphia SCAI Fall Fellows Course 2014 December
Lutonix AV Clinical Trial
 Lutonix AV Clinical Trial Long Term Effects of LUTONIX 035 DCB Catheter 18 month Interim Results Scott O. Trerotola, MD Stanley Baum Professor of Radiology Professor of Surgery Associate Chair and Chief,
Lutonix AV Clinical Trial Long Term Effects of LUTONIX 035 DCB Catheter 18 month Interim Results Scott O. Trerotola, MD Stanley Baum Professor of Radiology Professor of Surgery Associate Chair and Chief,
GORE EXCLUDER AAA Endoprosthesis ANNUAL CLINICAL UPDATE OCTOBER Section I Clinical experience. Section II Worldwide commercial experience
 GORE EXCLUDER AAA Endoprosthesis ANNUAL CLINICAL UPDATE OCTOBER 2018 Abstract This annual clinical update provides a review of the ongoing experience with the GORE EXCLUDER AAA Endoprosthesis used in the
GORE EXCLUDER AAA Endoprosthesis ANNUAL CLINICAL UPDATE OCTOBER 2018 Abstract This annual clinical update provides a review of the ongoing experience with the GORE EXCLUDER AAA Endoprosthesis used in the
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
The clinical update for the Zenith AAA Endovascular Graft has included results from the Zenith AAA Endovascular Graft multi-center clinical study,
 The clinical update for the Zenith AAA Endovascular Graft has included results from the Zenith AAA Endovascular Graft multi-center clinical study, the 36 mm diameter Zenith Flex AAA Endovascular Graft
The clinical update for the Zenith AAA Endovascular Graft has included results from the Zenith AAA Endovascular Graft multi-center clinical study, the 36 mm diameter Zenith Flex AAA Endovascular Graft
GORE TAG Thoracic Endoprosthesis ANNUAL CLINICAL UPDATE SEPTEMBER 2018 Abstract. Section I GORE TAG Device Clinical experience. Section II Conformable
 GORE TAG Thoracic Endoprosthesis ANNUAL CLINICAL UPDATE SEPTEMBER 08 Abstract This annual clinical update provides a review of the ongoing experience with the GORE TAG Thoracic Endoprosthesis There have
GORE TAG Thoracic Endoprosthesis ANNUAL CLINICAL UPDATE SEPTEMBER 08 Abstract This annual clinical update provides a review of the ongoing experience with the GORE TAG Thoracic Endoprosthesis There have
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use
 Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
The Role of LUTONIX 035 DCB in AV Fistula Dysfunction Management in our Practice
 The Role of LUTONIX 035 DCB in AV Fistula Dysfunction Management in our Practice Dr Kate Steiner Consultant Interventional Radiologist East and North Hertfordshire NHS Trust Disclosure Speaker name: Dr
The Role of LUTONIX 035 DCB in AV Fistula Dysfunction Management in our Practice Dr Kate Steiner Consultant Interventional Radiologist East and North Hertfordshire NHS Trust Disclosure Speaker name: Dr
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Carry this card with you at all times. Show this card to any medical professional treating you. Patient Implant Card. Option ELITE Vena Cava Filter.
 Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
RadRx Your Prescription for Accurate Coding & Reimbursement Copyright All Rights Reserved.
 Interventional Radiology Coding Case Studies Prepared by Stacie L. Buck, RHIA, CCS-P, RCC, CIRCC, AAPC Fellow President & Senior Consultant INDICATION: Abdominal aortic aneurysm. INTERVENTIONAL RADIOLOGIST:
Interventional Radiology Coding Case Studies Prepared by Stacie L. Buck, RHIA, CCS-P, RCC, CIRCC, AAPC Fellow President & Senior Consultant INDICATION: Abdominal aortic aneurysm. INTERVENTIONAL RADIOLOGIST:
Cook Medical. Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training
 Cook Medical Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full-thickness,
Cook Medical Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full-thickness,
HOW SHOULD WE FOLLOW PATIENTS AFTER AORTIC ARCH INTERVENTIONS?
 HOW SHOULD WE FOLLOW PATIENTS AFTER AORTIC ARCH INTERVENTIONS? International Symposium on 3D Imaging for Interventional Catheterization in CHD (3DI3 Conference) Martin Bocks, M.D. Pediatric Interventional
HOW SHOULD WE FOLLOW PATIENTS AFTER AORTIC ARCH INTERVENTIONS? International Symposium on 3D Imaging for Interventional Catheterization in CHD (3DI3 Conference) Martin Bocks, M.D. Pediatric Interventional
Thoracic Endovascular Aortic Repair (TEVAR) Indications and Basic Procedure
 Thoracic Endovascular Aortic Repair (TEVAR) Indications and Basic Procedure Tilo Kölbel, MD, PhD University Heart Center Hamburg University Hospital Eppendorf Disclosure Speaker name: Tilo Kölbel, MD I
Thoracic Endovascular Aortic Repair (TEVAR) Indications and Basic Procedure Tilo Kölbel, MD, PhD University Heart Center Hamburg University Hospital Eppendorf Disclosure Speaker name: Tilo Kölbel, MD I
Proven Performance Through Innovative Design *
 Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
Transcatheter Pulmonary Valve Therapy
 Transcatheter Pulmonary Valve Therapy With the Edwards SAPIEN XT Transcatheter Heart Valve For Patients and Caregivers This booklet was created to help you learn more about the Edwards SAPIEN XT Transcatheter
Transcatheter Pulmonary Valve Therapy With the Edwards SAPIEN XT Transcatheter Heart Valve For Patients and Caregivers This booklet was created to help you learn more about the Edwards SAPIEN XT Transcatheter
Santi Trimarchi, MD, PhD Vascular Surgeon Thoracic Aortic Research Center, Director IRCCS Policlinico San Donato University of Milan
 The Gore GREAT Registry: Update about a real life data collection Santi Trimarchi, MD, PhD Vascular Surgeon Thoracic Aortic Research Center, Director IRCCS Policlinico San Donato University of Milan Disclosures
The Gore GREAT Registry: Update about a real life data collection Santi Trimarchi, MD, PhD Vascular Surgeon Thoracic Aortic Research Center, Director IRCCS Policlinico San Donato University of Milan Disclosures
Zenith Renu AAA Converter Graft. Device Description Planning and Sizing Deployment Sequence Patient Follow-Up
 Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
Dual Arterial Access for Stenting of Aortic Coarctation in Patients with Near-Total Descending Aortic Interruption
 DOI 10.1007/s40119-015-0048-9 CASE SERIES Dual Arterial Access for Stenting of Aortic Coarctation in Patients with Near-Total Descending Aortic Interruption Tahir Hamid. Syed Ibrahim Jamallulail. Bernard
DOI 10.1007/s40119-015-0048-9 CASE SERIES Dual Arterial Access for Stenting of Aortic Coarctation in Patients with Near-Total Descending Aortic Interruption Tahir Hamid. Syed Ibrahim Jamallulail. Bernard
2/28/2010. Speakers s name: Paul Chiam. I have the following potential conflicts of interest to report: NONE. Antegrade transvenous transseptal route
 Transcatheter Aortic Valve Implantation Asian perspective Speakers s name: Paul Chiam Paul TL Chiam MBBS, MRCP, FACC I have the following potential conflicts of interest to report: NONE Consultant National
Transcatheter Aortic Valve Implantation Asian perspective Speakers s name: Paul Chiam Paul TL Chiam MBBS, MRCP, FACC I have the following potential conflicts of interest to report: NONE Consultant National
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
FROM THE EVERYDAY TO THE EXTRAORDINARY
 FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
ENG TREO ABDOMINAL STENT-GRAFT SYSTEM TABLE OF CONTENTS
 TREO ABDOMINAL STENT-GRAFT SYSTEM TABLE OF CONTENTS 1. DEVICE DESCRIPTION 28 1.1. Stent-Graft 28 1.1.1. Main Bifurcated Stent-Graft 28 1.1.2. Leg Extensions 28 1.2. Delivery System for the Bifurcated Main
TREO ABDOMINAL STENT-GRAFT SYSTEM TABLE OF CONTENTS 1. DEVICE DESCRIPTION 28 1.1. Stent-Graft 28 1.1.1. Main Bifurcated Stent-Graft 28 1.1.2. Leg Extensions 28 1.2. Delivery System for the Bifurcated Main
Percutaneous Transapical Access for Thoracic Endovascular Repair
 Percutaneous Transapical Access for Thoracic Endovascular Repair Atman P. Shah MD FACC FSCAI Co-Director, Hans Hecht Cardiac Catheterization Laboratory Clinical Director, Section of Cardiology Associate
Percutaneous Transapical Access for Thoracic Endovascular Repair Atman P. Shah MD FACC FSCAI Co-Director, Hans Hecht Cardiac Catheterization Laboratory Clinical Director, Section of Cardiology Associate
Role of Balloons and Stents in Congenital Heart Disease
 Role of Balloons and Stents in Congenital Heart Disease Rui Anjos Lisbon, Portugal No conflict of interest Balloon dilatation in Congenital Heart Disease Balloons in CHD Initially used in the 80s Learning
Role of Balloons and Stents in Congenital Heart Disease Rui Anjos Lisbon, Portugal No conflict of interest Balloon dilatation in Congenital Heart Disease Balloons in CHD Initially used in the 80s Learning
2014 ANNUAL CLINICAL UPDATE. TriVascular Inc. Ovation and Ovation Prime Abdominal Stent Graft Systems
 2014 ANNUAL CLINICAL UPDATE TriVascular Inc. Ovation and Ovation Prime Abdominal Stent Graft Systems 2014 ANNUAL CLINICAL UPDATE TriVascular Inc. Ovation and Ovation Prime Abdominal Stent Graft Systems
2014 ANNUAL CLINICAL UPDATE TriVascular Inc. Ovation and Ovation Prime Abdominal Stent Graft Systems 2014 ANNUAL CLINICAL UPDATE TriVascular Inc. Ovation and Ovation Prime Abdominal Stent Graft Systems
Debate in Management of native COA; Balloon Versus Surgery
 Debate in Management of native COA; Balloon Versus Surgery Dr. Amira Esmat, El Tantawy, MD Professor of Pediatrics Consultant Pediatric Cardiac Interventionist Faculty of Medicine Cairo University 23/2/2017
Debate in Management of native COA; Balloon Versus Surgery Dr. Amira Esmat, El Tantawy, MD Professor of Pediatrics Consultant Pediatric Cardiac Interventionist Faculty of Medicine Cairo University 23/2/2017
BRING LIVING BACK TO LIFE. An Educational Booklet for Recipients of the Abbott Portico Transcatheter Aortic Valve
 BRING LIVING BACK TO LIFE An Educational Booklet for Recipients of the Abbott Portico Transcatheter Aortic Valve TABLE OF CONTENTS Your role in the management of your health is very important. This information
BRING LIVING BACK TO LIFE An Educational Booklet for Recipients of the Abbott Portico Transcatheter Aortic Valve TABLE OF CONTENTS Your role in the management of your health is very important. This information
WallFlex Biliary RX Partially Covered Stent System Prescriptive Information
 Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Single Pass MCA Revascularization with Trevo
 Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Indications for use. Contraindications within the United States
 Indications for use Indications within the United States The GORE TAG Thoracic Endoprosthesis is intended for endovascular repair of all lesions of the descending thoracic aorta, including: Isolated lesions
Indications for use Indications within the United States The GORE TAG Thoracic Endoprosthesis is intended for endovascular repair of all lesions of the descending thoracic aorta, including: Isolated lesions
Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft
 MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
AORTIC DISSECTIONS Current Management. TOMAS D. MARTIN, MD, LAT Professor, TCV Surgery Director UF Health Aortic Disease Center University of Florida
 AORTIC DISSECTIONS Current Management TOMAS D. MARTIN, MD, LAT Professor, TCV Surgery Director UF Health Aortic Disease Center University of Florida DISCLOSURES Terumo Medtronic Cook Edwards Cryolife AORTIC
AORTIC DISSECTIONS Current Management TOMAS D. MARTIN, MD, LAT Professor, TCV Surgery Director UF Health Aortic Disease Center University of Florida DISCLOSURES Terumo Medtronic Cook Edwards Cryolife AORTIC
Angioplasty remains the most common method
 When AVF Angioplasty Fails Defining procedural success and overcoming common problems. By Thomas M. Vesely, MD Angioplasty remains the most common method of treating obstructive vascular stenoses associated
When AVF Angioplasty Fails Defining procedural success and overcoming common problems. By Thomas M. Vesely, MD Angioplasty remains the most common method of treating obstructive vascular stenoses associated
