CSRC White Papers. An update Dec. 2010
|
|
|
- Horace Tate
- 5 years ago
- Views:
Transcription
1 CSRC White Papers An update Dec Ignacio Rodriguez, MD Clinical Safety Risk Management Roche
2 CSRC White Papers Position papers usually cover challenging areas of cardiovascular safety, describing what is known and unknown, and propose paths forward to address such knowledgee gaps Focus on the available scientific data for a particular matter, explore areas for further evaluation, and present potential approaches to better understand cardiovascular safety issues in drug development No guidance documents Consensus is good but not mandatory Summary of CSRC sponsored Think Tanks CSRC White Paper publication policy available on-line 2
3 Current Status 8 published papers (3 in 2009, 5 in 2010) 4 white papers in progress initiatives in planning phase All involving academia, industry and regulators 3
4 Published papers Cardiac Safety Research Consortium ECG Warehouse: Database specifications and principles of use for algorithm development and testing. Leaders: Am Heart J. 2009;157(5): Paul Kligfield et al Am Heart J. 2009;157(5): Am Heart J.2009;158(3): Am Heart J. 2010;159(5): Am Heart J. 2010; 160(4): Accepted for publication Am Heart J, 2010 Am Heart J. 2010;159(1):17-24 Am Heart J. 2010; 160(4):
5 White Papers in Progress Working Title Troponin Measurements during drug development - Points to consider for monitoring and management of potential cardiotoxicity. Leaders Kristin Newby (newby001@dcri.duke.edu) and Rick Becker (richard.becker@duke.edu) Current Status Open forum held on June 28 th 2010 at the FDA Headquarters Working on the final version Expected publication date: 1Q 2011 Key messages/objectives Determine the role of troponin for assessing potential cardiotoxicity in all phases of drug development Present practical approaches to use serum troponin as a biomarker for detecting potential cardiotoxicity in clinical studies. With recommendations on how to monitor ctn in clinical development, and how to interpret potential signals 5
6 White Papers in Progress Working Title QT/QTc Evaluation for Drugs with Autonomic Effects Leader Christine Garnet Current Status Third draft complete Open discussion ~ 1Q2011 Expected publication date 2 Q 2011 Key messages/objectives Summary of reasonable approaches to evaluate the QT or QTc interval for therapies that have HR and/or autonomic effects. Some methods include: individualizedd QT/RR correction, PK-PD modeling, Holter bin analysis, dynamic beat-to-beat analysis, and QT assessment during constant heart rate (i.e., pacing). At present, there is not enough information the select one as the optimal method. Therefore, the group chose to describe methods that can improve this assessment and encourage further research in the area. 6
7 White Papers in Progress Working Title Non-QT interval ECG evaluation in clinical development (emphasis in PR and QRS) Leader Adel Nada ) Current Status First draft completed Expected publication date 3Q2011 Key messages/objectives Clinical relevance of PR and QRS interval monitoring as safety biomarkers in clinical development Epidemiological evidence and expected variability Expert and consensus state-of-the-artt understanding of how to best profile drug induced PR and QRS liabilities in clinical development 7
8 White Papers in Progress Working Title Scientific discussion on Blood Pressure evaluation in clinical development Leader Jeff Heilbraun Current Status Working on the first draft Expected publication date 2 nd half 2011 Key messages/objectives Relevance of BP as a safety biomarker in clinical development BP assessment methodologies BP monitoring in clinical development Evaluation of BP changes Issues to consider in special populations & indications 8
9 Other initiatives planned Procedures from Think-Tanks TransRadial Pediatric Diabetes Clinical safety options to monitor potential drug-induced changes in left ventricular function Developing Drugs with Preclinical or Early Clinical Cardiovascular Safety Signals: Review of Marketed Compounds that had Signals for Cardiotoxicity Cardiovascular safety monitoring in subpopulations (e.g. blood pressure in pediatrics, cardiotoxicity in oncology, etc) <Add your proposals here> 9
10 Comments Time required to complete the paper Conflicts Goodwill Consensus good to have but no need to have Present areas of controversy and areas where further research is needed No guidance documents or regulatory requirements Great forum for knowledgee sharing! 10
11 Thank you
12 Published papers New precompetitive paradigms: focus on cardiac safety. Finkle J, et al. Am Heart J. 2009;157: Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Rock EP, et al. Am Heart J. 2009;157: Current challenges in the evaluation of cardiac safety during drug development: Translational medicine meets the Critical Path Initiative. Piccini JP, et al. Am Heart J. 2009;158: Planning the safety of atrial fibrillation ablation registry initiative (SAFARI) as a collaborative panstakeholder critical path registry model: A cardiac safety research consortium incubator think tank. Al-Khatib SM, et al. Am Heart J. 2010;159: Evaluation of ventricular arrhythmias in early clinical pharmacology trials and potential consequences for later development. Min SS, et al. Am Heart J. 2010;159: Developing the Safety of Atrial Fibrillation Ablation Registry Initiative (SAFARI) as a collaborative pan-stakeholder critical path registry model: A Cardiac Safety Research Consortium Incubator Think Tank. Am Heart J 2010;160: Electrocardiographic assessment for therapeutic proteins scientific discussion. Rodriguez I, et al. Am Heart J 2010;160:
Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies. Corina Dota, AstraZeneca
 Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies Corina Dota, AstraZeneca CSRC-2 th February 2012 ICH E14 2.2.5 Alternative Strategies to Assess QT/QTc Interval Effects Alternatives
Industry Experience of the Concentration-QTc Relationship in Phase 1 Studies Corina Dota, AstraZeneca CSRC-2 th February 2012 ICH E14 2.2.5 Alternative Strategies to Assess QT/QTc Interval Effects Alternatives
New Biomarkers The Path to Scientific and Regulatory Approval
 New Biomarkers The Path to Scientific and Regulatory Approval Boaz Mendzelevski, MD Cardiac Safety Consultant and Director of Cardiology Medifacts International Medicine is a science of uncertainty and
New Biomarkers The Path to Scientific and Regulatory Approval Boaz Mendzelevski, MD Cardiac Safety Consultant and Director of Cardiology Medifacts International Medicine is a science of uncertainty and
Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety?
 Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety? Hao Zhu, Ph.D. Scientific Lead, QT-IRT, FDA (Dec 10, 2010) Disclaimer: The views expressed in this talk are those of
Interpreting Adult Human Thorough QT Studies: Are They Relevant to Pediatric Safety? Hao Zhu, Ph.D. Scientific Lead, QT-IRT, FDA (Dec 10, 2010) Disclaimer: The views expressed in this talk are those of
QT Assessment: The new paradigm, but now what? Joy Olbertz PharmD, PhD Senior Director, Cardiovascular Safety Services
 QT Assessment: The new paradigm, but now what? Joy Olbertz PharmD, PhD Senior Director, Cardiovascular Safety Services The Evolution of ICH E14 Points to Consider Joint Health Canada/FDA Concept Paper
QT Assessment: The new paradigm, but now what? Joy Olbertz PharmD, PhD Senior Director, Cardiovascular Safety Services The Evolution of ICH E14 Points to Consider Joint Health Canada/FDA Concept Paper
Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience
 Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience Dr. Manfred Wargenau, Institute, Düsseldorf OVERVIEW Clinical background The ICH E14 guideline / review
Design and Analysis of QT/QTc Studies Conceptional and Methodical Considerations Based on Experience Dr. Manfred Wargenau, Institute, Düsseldorf OVERVIEW Clinical background The ICH E14 guideline / review
Breakout #4 Phase 1 ECG:
 Breakout #4 Phase 1 ECG: Potential Role of ECG under CiPA HESI CSRC CiPA Meeting Washington, DC - May 22, 2018 Jose Vicente, PhD Christine Garnett, PharmD Division of Cardiovascular and Renal Products
Breakout #4 Phase 1 ECG: Potential Role of ECG under CiPA HESI CSRC CiPA Meeting Washington, DC - May 22, 2018 Jose Vicente, PhD Christine Garnett, PharmD Division of Cardiovascular and Renal Products
For Discussion Purposes Only
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Step 1 Draft 2 (July 17, 2003) Draft 3 (November 12, 2003), Draft 4 (June 10, 2004)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Step 1 Draft 2 (July 17, 2003) Draft 3 (November 12, 2003), Draft 4 (June 10, 2004)
QT QT Study Design and Statistical Evaluation of QT Prolongation Introduction and QT Interval Correction
 QT QT Study Design and Statistical Evaluation of QT Prolongation Introduction and QT Interval Correction Yasushi Orihashi Clinical Data and Biostatistics Department, Daiichi Sankyo Co., Ltd. email: orihashi.yasushi.c2@daiichisankyo.co.jp
QT QT Study Design and Statistical Evaluation of QT Prolongation Introduction and QT Interval Correction Yasushi Orihashi Clinical Data and Biostatistics Department, Daiichi Sankyo Co., Ltd. email: orihashi.yasushi.c2@daiichisankyo.co.jp
MARS Ambulatory ECG Analysis The power to assess and predict
 GE Healthcare MARS Ambulatory ECG Analysis The power to assess and predict Connecting hearts and minds Prevention starts with knowledge Around the world, heart disease is one of our fastest-growing health
GE Healthcare MARS Ambulatory ECG Analysis The power to assess and predict Connecting hearts and minds Prevention starts with knowledge Around the world, heart disease is one of our fastest-growing health
IQ/CSRC Study Waveform Sharing Program
 IQ/CSRC Study Waveform Sharing Program Presentation for CSRC members August 26, 2015 Borje Darpo, icardiac Cindy Green, DCRI Georg Ferber, Independent Brian Smith, icardiac Jean-Philippe Couderc, THEW
IQ/CSRC Study Waveform Sharing Program Presentation for CSRC members August 26, 2015 Borje Darpo, icardiac Cindy Green, DCRI Georg Ferber, Independent Brian Smith, icardiac Jean-Philippe Couderc, THEW
CSRC COPD Initiative Think Tank meeting Washington DC March 6, 2014
 What is the role of outcome studies in pulmonary drug development and what data can be collected during development to sufficiently characterize an agent so that an outcome study not necessary? CSRC COPD
What is the role of outcome studies in pulmonary drug development and what data can be collected during development to sufficiently characterize an agent so that an outcome study not necessary? CSRC COPD
Second THEW Meeting, April 2009, White Oak Campus
 Second THEW Meeting, April 2009, White Oak Campus 1 Pharmaceutical companies: Abbott, Astra-Zeneca, Inspire Pharmaceuticals, Inc. Johnson&Johnson, Pfizer, Roche, Shire Pharmaceuticals, Wyeth Research.
Second THEW Meeting, April 2009, White Oak Campus 1 Pharmaceutical companies: Abbott, Astra-Zeneca, Inspire Pharmaceuticals, Inc. Johnson&Johnson, Pfizer, Roche, Shire Pharmaceuticals, Wyeth Research.
TQT studies - this is the end!
 TQT studies - this is the end! Borje Darpo MD, PhD Associate Professor of Cardiology Chief Scientific Officer icardiac Technologies Yes as the only way to confidently exclude that a new drug has a clinically
TQT studies - this is the end! Borje Darpo MD, PhD Associate Professor of Cardiology Chief Scientific Officer icardiac Technologies Yes as the only way to confidently exclude that a new drug has a clinically
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Reprinted from FDA s website by Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions and Answers (R1) U.S. Department
Preparing for Changing Cardiac Safety Regulations. Joy Olbertz, PharmD, PhD
 Preparing for Changing Cardiac Safety Regulations Joy Olbertz, PharmD, PhD Questions? Do I still have to assess proarrhythmic potential of my compound in a Thorough QT (TQT) study? If I have a TQT to conduct,
Preparing for Changing Cardiac Safety Regulations Joy Olbertz, PharmD, PhD Questions? Do I still have to assess proarrhythmic potential of my compound in a Thorough QT (TQT) study? If I have a TQT to conduct,
Pathfinder Holter Analyzer CARDIAC DIAGNOSTIC SOLUTIONS
 Pathfinder Holter Analyzer CARDIAC DIAGNOSTIC SOLUTIONS Pathfinder Digital - An Evolution... Del Mar Reynolds rich history of innovation began nearly a half century ago with the development of Holter monitoring
Pathfinder Holter Analyzer CARDIAC DIAGNOSTIC SOLUTIONS Pathfinder Digital - An Evolution... Del Mar Reynolds rich history of innovation began nearly a half century ago with the development of Holter monitoring
Recent Developments in the Analysis of QT Interval Data
 Recent Developments in the Analysis of QT Interval Data Robb Muirhead Statistical Research & Consulting Center Pfizer Global Statistics Graybill Conference VII Colorado State University June 12, 2008 1
Recent Developments in the Analysis of QT Interval Data Robb Muirhead Statistical Research & Consulting Center Pfizer Global Statistics Graybill Conference VII Colorado State University June 12, 2008 1
The QT Interval Safety Endpoint for DR- TB trials. Kelly Dooley & Gary Maartens (Disclaimer: I know very little cardiac electrophysiology)
 The QT Interval Safety Endpoint for DR- TB trials Kelly Dooley & Gary Maartens (Disclaimer: I know very little cardiac electrophysiology) The ECG tracing Physiology of a cardiac myocyte Flow of ions (Na
The QT Interval Safety Endpoint for DR- TB trials Kelly Dooley & Gary Maartens (Disclaimer: I know very little cardiac electrophysiology) The ECG tracing Physiology of a cardiac myocyte Flow of ions (Na
30 Seconds is the Proper Endpoint for AF Ablation YES. Hugh Calkins MD. Professor of Medicine
 30 Seconds is the Proper Endpoint for AF Ablation YES Hugh Calkins MD Professor of Medicine Director of Electrophysiology Johns Hopkins Medical Institutions COI Disclosures Dr Calkins is a consultant to
30 Seconds is the Proper Endpoint for AF Ablation YES Hugh Calkins MD Professor of Medicine Director of Electrophysiology Johns Hopkins Medical Institutions COI Disclosures Dr Calkins is a consultant to
Speed - Accuracy - Exploration. Pathfinder SL
 Speed - Accuracy - Exploration Pathfinder SL 98000 Speed. Accuracy. Exploration. Pathfinder SL represents the evolution of over 40 years of technology, design, algorithm development and experience in the
Speed - Accuracy - Exploration Pathfinder SL 98000 Speed. Accuracy. Exploration. Pathfinder SL represents the evolution of over 40 years of technology, design, algorithm development and experience in the
AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community)
 AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community) Faye Vazvaei, Roche Innovation Center New York The 8th JBF Meeting, 8-9 February 2017 Background
AAPS Views on Bioanalytical Method Validation Harmonization (on Behalf of AAPS Bioanalytical Community) Faye Vazvaei, Roche Innovation Center New York The 8th JBF Meeting, 8-9 February 2017 Background
HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments
 HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments Brian R. Berridge, DVM, PhD, DACVP Committee Co-Chair ILSI Health and Environmental Sciences
HESI Technical Committee on Cardiovascular Safety: Advancing the clinical relevance of preclinical assessments Brian R. Berridge, DVM, PhD, DACVP Committee Co-Chair ILSI Health and Environmental Sciences
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
NIH Public Access Author Manuscript Comput Cardiol (2010). Author manuscript; available in PMC 2011 July 11.
 NIH Public Access Author Manuscript Published in final edited form as: Comput Cardiol (2010). 2010 September 26; 2010: 353 356. Torsadogenic Drug-induced Increased Short-term Variability of JT-area Xiao
NIH Public Access Author Manuscript Published in final edited form as: Comput Cardiol (2010). 2010 September 26; 2010: 353 356. Torsadogenic Drug-induced Increased Short-term Variability of JT-area Xiao
The Maximum Mean Difference
 The Maximum Mean Difference Statistical Problems in Assessing Cardiac Safety Georg Ferber, Novartis Pharma AG, Basel ROeS Seminar Bern, 10-13 Sep 2007 Overview QT prolongation some background The Thorough
The Maximum Mean Difference Statistical Problems in Assessing Cardiac Safety Georg Ferber, Novartis Pharma AG, Basel ROeS Seminar Bern, 10-13 Sep 2007 Overview QT prolongation some background The Thorough
ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS.
 European Medicines Agency London, 25 May 2005 CHMP/ICH/2/04 ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS ICH Step 4 NOTE FOR
European Medicines Agency London, 25 May 2005 CHMP/ICH/2/04 ICH E14 THE CLINICAL EVALUATION OF QT/QTc INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NON-ANTIARRHYTHMIC DRUGS ICH Step 4 NOTE FOR
Guidance for Industry
 Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs U.S. Department of Health and Human Services Food and Drug Administration
Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs U.S. Department of Health and Human Services Food and Drug Administration
QT Studies for Biologics QT assessment in phase I Workshop 2
 QT Studies for Biologics QT assessment in phase I Workshop 2 Philippe L Hostis Joint Conference of European Human Pharmacological Societies and 20th Anniversary of AGAH Berlin, 31st March 2011 QT assessment
QT Studies for Biologics QT assessment in phase I Workshop 2 Philippe L Hostis Joint Conference of European Human Pharmacological Societies and 20th Anniversary of AGAH Berlin, 31st March 2011 QT assessment
Pacing in Drug Testing
 ISHNE International Society for Holter and Noninvasive Electrocardiology 1 st Worldwide Internet Symposium on Drug- Induced QT Prolongation October 1-15, 1 15, 2007 Pacing in Drug Testing I Savelieva,
ISHNE International Society for Holter and Noninvasive Electrocardiology 1 st Worldwide Internet Symposium on Drug- Induced QT Prolongation October 1-15, 1 15, 2007 Pacing in Drug Testing I Savelieva,
The Telemetric and Holter ECG Warehouse (THEW) Initiative
 The Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-Philippe Couderc, PhD THEW HRFUP- Cardiology University of Rochester Medical Center, NY Mission Statement The objective of the Telemetric
The Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-Philippe Couderc, PhD THEW HRFUP- Cardiology University of Rochester Medical Center, NY Mission Statement The objective of the Telemetric
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Pediatric Population Step4
 E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
Interpreting Electrocardiograms (ECG) Physiology Name: Per:
 Interpreting Electrocardiograms (ECG) Physiology Name: Per: Introduction The heart has its own system in place to create nerve impulses and does not actually require the brain to make it beat. This electrical
Interpreting Electrocardiograms (ECG) Physiology Name: Per: Introduction The heart has its own system in place to create nerve impulses and does not actually require the brain to make it beat. This electrical
MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities
 MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities Event Presenter Date EMA Workshop 2017 Tessa de Korte, MSc 2017, October 5 Page 1 Ncardia at a glance Foundation Ncardia
MEA assays using human ipsc-derived cardiomyocytes; challenges and opportunities Event Presenter Date EMA Workshop 2017 Tessa de Korte, MSc 2017, October 5 Page 1 Ncardia at a glance Foundation Ncardia
Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team
 Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team Jiang Liu, Ph.D. Scientific Lead for QT-IRT Division of Pharmacometrics Office of Clinical Pharmacology
Definitive QTc Assessment in Early Phase Trials: Expectations from FDA s Interdisciplinary Review Team Jiang Liu, Ph.D. Scientific Lead for QT-IRT Division of Pharmacometrics Office of Clinical Pharmacology
Evaluation of ventricular arrhythmias in early clinical pharmacology trials and potential consequences for later development
 Cardiac Safety Research Consortium Evaluation of ventricular arrhythmias in early clinical pharmacology trials and potential consequences for later development Sherene S. Min, MD, MPH, a J. Rick Turner,
Cardiac Safety Research Consortium Evaluation of ventricular arrhythmias in early clinical pharmacology trials and potential consequences for later development Sherene S. Min, MD, MPH, a J. Rick Turner,
Collaborative Approaches for Developing Kidney Safety Biomarkers
 Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Study Day 1 Study Days 2 to 9 Sequence 1 Placebo for moxifloxacin Study Days 2 to 8: placebo for pazopanib (placebopaz) 800 mg;
 The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton,
 7 7. A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton, Oxfordshire OX7 5SR. E-mail: admin@bhrs.com. Tel: 01789 867
7 7. A request for a log book extension must be put in writing and sent to BHRS, Unit 6B, Essex House, Cromwell Business Park, Chipping Norton, Oxfordshire OX7 5SR. E-mail: admin@bhrs.com. Tel: 01789 867
IQ-CSRC Prospective Study - Background and Objectives
 IQ-CSRC Prospective Study - Background and Objectives December 12, 2014 Borje Darpo MD, PhD icardiac Technologies Co-chair SoC, CSRC Objectives of the IQ-CSRC Prospective Study The objective of the initiative
IQ-CSRC Prospective Study - Background and Objectives December 12, 2014 Borje Darpo MD, PhD icardiac Technologies Co-chair SoC, CSRC Objectives of the IQ-CSRC Prospective Study The objective of the initiative
FANS Long QT Syndrome Investigation Protocol (including suspected mutation carriers)
 Clinical Features FANS Long QT Syndrome Investigation Protocol (including suspected mutation carriers) History Syncope or presyncope compatible with ventricular tachyarrhythmia, especially relating to
Clinical Features FANS Long QT Syndrome Investigation Protocol (including suspected mutation carriers) History Syncope or presyncope compatible with ventricular tachyarrhythmia, especially relating to
Atrial Fibrillation Ablation: in Whom and How
 Update on Consensus Statement on Management of Atrial Fibrillation: EHRA 2012 Atrial Fibrillation Ablation: in Whom and How Update of HRS/EHRA AF/ECAS Ablation Document 2012 Anne M Gillis MD FHRS Professor
Update on Consensus Statement on Management of Atrial Fibrillation: EHRA 2012 Atrial Fibrillation Ablation: in Whom and How Update of HRS/EHRA AF/ECAS Ablation Document 2012 Anne M Gillis MD FHRS Professor
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS
 MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
MEDICINAL PRODUCTS FOR THE TREATMENT OF ARRHYTHMIAS Guideline Title Medicinal Products for the Treatment of Arrhythmias Legislative basis Directive 75/318/EEC as amended Date of first adoption November
QT prolongation and drug-drug interactions. Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency
 QT prolongation and drug-drug interactions Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency Disposition Introduction to QT prolongation Three relevant DDI scenarios Concluding
QT prolongation and drug-drug interactions Filip Josephson M.D., Ph.D Clinical Assessor Swedish Medical Products Agency Disposition Introduction to QT prolongation Three relevant DDI scenarios Concluding
Editorial. The AMPS Quarterly Issue n.2-2q2009
 The AMPS Quarterly Issue n.2-2q2009 Editors: Fabio Badilini PhD and Martino Vaglio AMPS-QT is a quarterly journal dedicated to all the people and organizations involved in the world of cardiac safety.
The AMPS Quarterly Issue n.2-2q2009 Editors: Fabio Badilini PhD and Martino Vaglio AMPS-QT is a quarterly journal dedicated to all the people and organizations involved in the world of cardiac safety.
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE SCOPE. Clinical guideline for the management of atrial fibrillation
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Clinical guideline for the management of atrial fibrillation 1.1 Short title Atrial fibrillation 2 Background a) The National Institute
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Clinical guideline for the management of atrial fibrillation 1.1 Short title Atrial fibrillation 2 Background a) The National Institute
Syllabus and Program of the Diploma in Cardiac Pacing
 Syllabus and Program of the Diploma in Cardiac Pacing 1. The conductive system of the heart(1 hour): Study the anatomical and functional properties of the different componenets of the conductive system
Syllabus and Program of the Diploma in Cardiac Pacing 1. The conductive system of the heart(1 hour): Study the anatomical and functional properties of the different componenets of the conductive system
EMA/FDA/Health Canada Workshop on Paediatric PAH
 EMA/FDA/Health Canada Workshop on Paediatric PAH Industry Perspective Date: 12/06/2017 * Version: FINAL Industry perspective on partnering with academia and agencies in drug development for children with
EMA/FDA/Health Canada Workshop on Paediatric PAH Industry Perspective Date: 12/06/2017 * Version: FINAL Industry perspective on partnering with academia and agencies in drug development for children with
In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative
 In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative Gary Gintant, AbbVie for the Comprehensive in Vitro ProArrhythmia Assay Group Hot
In Vitro Assessment to Replace the Clinical TQT Study: The Comprehensive In Vitro ProArrhythmia Assay (CiPA) Initiative Gary Gintant, AbbVie for the Comprehensive in Vitro ProArrhythmia Assay Group Hot
IQ-CSRC PROSPECTIVE QTc STUDY Study Design and Choice of Drugs
 IQ-CSRC PROSPECTIVE QTc STUDY Study Design and Choice of Drugs Nenad Sarapa, MD IQ-CSRC meeting, Silver Spring, MD 12 December 2014 Background SAD studies can reliably assess QTc prolongation if proper
IQ-CSRC PROSPECTIVE QTc STUDY Study Design and Choice of Drugs Nenad Sarapa, MD IQ-CSRC meeting, Silver Spring, MD 12 December 2014 Background SAD studies can reliably assess QTc prolongation if proper
CARDIOVASCULAR. THE POWER OFx. Experts. Experience. Execution. A Deeper Dive into Cardiovascular Clinical Development
 THE POWER OFx A Deeper Dive into Cardiovascular Clinical Development As a therapeutically-focused CRO with a long history in supporting drugs, biologics, and medical devices for cardiovascular disease,
THE POWER OFx A Deeper Dive into Cardiovascular Clinical Development As a therapeutically-focused CRO with a long history in supporting drugs, biologics, and medical devices for cardiovascular disease,
Asymptomatic WPW Syndrome; Observation or Ablation? 전남대학교병원순환기내과 박형욱
 Asymptomatic WPW Syndrome; Observation or Ablation? 전남대학교병원순환기내과 박형욱 Let It Be? Vs. Just Do It? Natural history of asymptomatic WPW Incidence of sudden cardiac death in natural history studies involving
Asymptomatic WPW Syndrome; Observation or Ablation? 전남대학교병원순환기내과 박형욱 Let It Be? Vs. Just Do It? Natural history of asymptomatic WPW Incidence of sudden cardiac death in natural history studies involving
New ECG Biomarkers and their Potential Role in CiPA: Results and Implications
 New ECG Biomarkers and their Potential Role in CiPA: Results and Implications HESI CSRC CiPA Meeting Washington, DC - May 21, 2018 Jose Vicente, PhD for the ECG Biomarker Working Group Division of Cardiovascular
New ECG Biomarkers and their Potential Role in CiPA: Results and Implications HESI CSRC CiPA Meeting Washington, DC - May 21, 2018 Jose Vicente, PhD for the ECG Biomarker Working Group Division of Cardiovascular
CASE STUDY: THE CLINICAL BENEFIT OF MOBILE CARDIAC OUTPATIENT TELEMETRY. June 22, 2009
 CASE STUDY: THE CLINICAL BENEFIT OF MOBILE CARDIAC OUTPATIENT TELEMETRY June 22, 2009 Sanjeev Wasson, MD, FACC, Medical Director, Department of Electrophysiology Skagit Valley Medical Center and Skagit
CASE STUDY: THE CLINICAL BENEFIT OF MOBILE CARDIAC OUTPATIENT TELEMETRY June 22, 2009 Sanjeev Wasson, MD, FACC, Medical Director, Department of Electrophysiology Skagit Valley Medical Center and Skagit
Implications of methodological differences in digital electrocardiogram interval measurement B
 Journal of Electrocardiology 39 (2006) S152 S156 www.elsevier.com/locate/jelectrocard Implications of methodological differences in digital electrocardiogram interval measurement B Fabio Badilini, PhD,
Journal of Electrocardiology 39 (2006) S152 S156 www.elsevier.com/locate/jelectrocard Implications of methodological differences in digital electrocardiogram interval measurement B Fabio Badilini, PhD,
Basic Dysrhythmia Interpretation
 Basic Dysrhythmia Interpretation Objectives 2 To understand the Basic ECG To understand the meaning of Dysrhythmia To describe the normal heart conduction system. To describe the normal impulse pathways.
Basic Dysrhythmia Interpretation Objectives 2 To understand the Basic ECG To understand the meaning of Dysrhythmia To describe the normal heart conduction system. To describe the normal impulse pathways.
Future of Diabetes Research in Europe JDRF Perspective
 Future of Diabetes Research in Europe JDRF Perspective IMI-JDRF Diabetes Patient Meeting 20 May 2014 Brussels, Belgium Olivier ARNAUD, Pharm D JDRF European Research Director oarnaud@jdrf.org Future of
Future of Diabetes Research in Europe JDRF Perspective IMI-JDRF Diabetes Patient Meeting 20 May 2014 Brussels, Belgium Olivier ARNAUD, Pharm D JDRF European Research Director oarnaud@jdrf.org Future of
Clinical Policy: Holter Monitors Reference Number: CP.MP.113
 Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
CONTAK RENEWAL CLINICAL SUMMARY
 CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. CLINICAL SUMMARY CONTAK RENEWAL Boston Scientific
CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. CLINICAL SUMMARY CONTAK RENEWAL Boston Scientific
Research Collaborations: Lessons from PeDRA and the SPD
 Research Collaborations: Lessons from PeDRA and the SPD Amy Paller, MD Professor and Chair, Dermatology Professor, Pediatrics Northwestern Univ. Feinberg School of Medicine Chicago, IL No conflicts of
Research Collaborations: Lessons from PeDRA and the SPD Amy Paller, MD Professor and Chair, Dermatology Professor, Pediatrics Northwestern Univ. Feinberg School of Medicine Chicago, IL No conflicts of
Towards a Sustainable Global Infrastructure for Medical Countermeasures
 Towards a Sustainable Global Infrastructure for Medical Countermeasures Institute of Medicine The Public Health Emergency Medical Countermeasures Enterprise: Innovative Strategies to Enhance Products from
Towards a Sustainable Global Infrastructure for Medical Countermeasures Institute of Medicine The Public Health Emergency Medical Countermeasures Enterprise: Innovative Strategies to Enhance Products from
PACES Research Meeting May 12, Boston, MA. Introduction
 PACES Research Meeting May 12, 2015 Boston, MA Introduction On Tuesday May 12 before the annual PACES symposium, a research meeting was held. This was sponsored by Johnson and Johnson. This meeting represents
PACES Research Meeting May 12, 2015 Boston, MA Introduction On Tuesday May 12 before the annual PACES symposium, a research meeting was held. This was sponsored by Johnson and Johnson. This meeting represents
Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials
 CSRC Think Tank: Detection, Assessment and Risk Mitigation of Cardiac Safety Signals in Oncology Drug Development Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials Boaz Mendzelevski,
CSRC Think Tank: Detection, Assessment and Risk Mitigation of Cardiac Safety Signals in Oncology Drug Development Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials Boaz Mendzelevski,
ABCs of ECGs. Shelby L. Durler
 ABCs of ECGs Shelby L. Durler Objectives Review the A&P of the cardiac conduction system Placement and obtaining 4-lead and 12-lead ECGs Overview of the basics of ECG rhythm interpretation Intrinsic
ABCs of ECGs Shelby L. Durler Objectives Review the A&P of the cardiac conduction system Placement and obtaining 4-lead and 12-lead ECGs Overview of the basics of ECG rhythm interpretation Intrinsic
APPROACH TO TACHYARRYTHMIAS
 APPROACH TO TACHYARRYTHMIAS PROF.DR.MD.ZAKIR HOSSAIN PROFESSOR AND HEAD DEPARTMENT OF MEDICINE SZMCH TACHYARRYTHMIA Cardiac arrythmia is a disturbance of electrical rhythm of heart. Cardac arrythmia with
APPROACH TO TACHYARRYTHMIAS PROF.DR.MD.ZAKIR HOSSAIN PROFESSOR AND HEAD DEPARTMENT OF MEDICINE SZMCH TACHYARRYTHMIA Cardiac arrythmia is a disturbance of electrical rhythm of heart. Cardac arrythmia with
Arthur J. Moss, MD Professor of Medicine/Cardiology University of Rochester Medical Center Rochester, NY. DISCLOSURE INFORMATION Arthur J.
 Saving Lives and Preventing Heart Failure: The MADIT Family of Trials Arthur J. Moss, MD Professor of Medicine/Cardiology University of Rochester Medical Center Rochester, NY Update in Electrocardiography
Saving Lives and Preventing Heart Failure: The MADIT Family of Trials Arthur J. Moss, MD Professor of Medicine/Cardiology University of Rochester Medical Center Rochester, NY Update in Electrocardiography
The summit and its purpose
 Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
ECG interpretation basics
 ECG interpretation basics Michał Walczewski, MD Krzysztof Ozierański, MD 21.03.18 Electrical conduction system of the heart Limb leads Precordial leads 21.03.18 Precordial leads Precordial leads 21.03.18
ECG interpretation basics Michał Walczewski, MD Krzysztof Ozierański, MD 21.03.18 Electrical conduction system of the heart Limb leads Precordial leads 21.03.18 Precordial leads Precordial leads 21.03.18
2017 BDKA Review. Regularity Rate P waves PRI QRS Interpretation. Regularity Rate P waves PRI QRS Interpretation 1/1/2017
 1. 2017 BDKA Review 2. 3. 4. Interpretation 5. QT 6. 7. 8. 9. 10. QT 11. 12. 13. 14. 15. 16. 17. 18. QT 19. 20. QT 21. 22. QT 23. 24. Where are pacer spikes? Before the P wave or before the QRS complex?
1. 2017 BDKA Review 2. 3. 4. Interpretation 5. QT 6. 7. 8. 9. 10. QT 11. 12. 13. 14. 15. 16. 17. 18. QT 19. 20. QT 21. 22. QT 23. 24. Where are pacer spikes? Before the P wave or before the QRS complex?
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database
 Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
Author s response to reviews
 Author s response to reviews Title: Multipoint Pacing versus conventional ICD in Patients with a Narrow QRS complex (MPP Narrow QRS trial): study protocol for a pilot randomized controlled trial Authors:
Author s response to reviews Title: Multipoint Pacing versus conventional ICD in Patients with a Narrow QRS complex (MPP Narrow QRS trial): study protocol for a pilot randomized controlled trial Authors:
EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS
 EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS Christine Garnett, PharmD DCRP, FDA April 6, 2016 CSRC/FDA Workshop: The Proarrhythmic Assessment of New Chemical Entities Disclosures
EXPOSURE RESPONSE ANALYSIS TO EVALUATE A DRUG'S EFFECT ON ECG PARAMETERS Christine Garnett, PharmD DCRP, FDA April 6, 2016 CSRC/FDA Workshop: The Proarrhythmic Assessment of New Chemical Entities Disclosures
Personalized medecine Biomarker
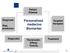 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Data Quality in Small QTc Studies. Marek Malik, London
 Data Quality in Small QTc Studies Marek Malik, London marek.malik@btinternet.com marek.malik@imperial.ac.uk Data Quality in Small QTc Studies DISCLAIMER Views and opinions presented are only my own Data
Data Quality in Small QTc Studies Marek Malik, London marek.malik@btinternet.com marek.malik@imperial.ac.uk Data Quality in Small QTc Studies DISCLAIMER Views and opinions presented are only my own Data
Identifying the Future Needs for Big Data in Medicines Regulation
 Identifying the Future Needs for Big Data in Medicines Regulation Hans Hillege Member of the Committee for Medicinal Products for Human Use (CHMP) for The Netherlands 1 Disclaimer The views and opinions
Identifying the Future Needs for Big Data in Medicines Regulation Hans Hillege Member of the Committee for Medicinal Products for Human Use (CHMP) for The Netherlands 1 Disclaimer The views and opinions
Critical appraisal Gelzer et al., 2009 Randomized controlled trial questions. Introduction. Are the aims clearly stated? Methods
 Critical appraisal Gelzer et al., 2009 Randomized controlled trial questions Introduction Are the aims clearly stated? Methods Is the study design suitable for the aims? Which population was studied?.
Critical appraisal Gelzer et al., 2009 Randomized controlled trial questions Introduction Are the aims clearly stated? Methods Is the study design suitable for the aims? Which population was studied?.
Clinical Cardiac Electrophysiology
 Clinical Cardiac Electrophysiology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of
Clinical Cardiac Electrophysiology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of
La terapia non anticoagulante nel paziente con FA secondo le Linee Guida F. CONROTTO
 La terapia non anticoagulante nel paziente con FA secondo le Linee Guida F. CONROTTO Rhythm or rate control strategy? N Engl J Med 2002;347:1834 40 Rate Control versus Electrical Cardioversion for Persistent
La terapia non anticoagulante nel paziente con FA secondo le Linee Guida F. CONROTTO Rhythm or rate control strategy? N Engl J Med 2002;347:1834 40 Rate Control versus Electrical Cardioversion for Persistent
Collin County Community College
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Perspectives on Evaluating and Managing Cardiovascular Safety Risk for Anticancer Drugs
 Perspectives on Evaluating and Managing Cardiovascular Safety Risk for Anticancer Drugs DCTD/DTP/TPB Myrtle Davis, DVM, Ph.D. Chief, Toxicology and Pharmacology Branch Division of Cancer Treatment and
Perspectives on Evaluating and Managing Cardiovascular Safety Risk for Anticancer Drugs DCTD/DTP/TPB Myrtle Davis, DVM, Ph.D. Chief, Toxicology and Pharmacology Branch Division of Cancer Treatment and
Atrial Fibrillation Ablation Thinktank
 Atrial Fibrillation Ablation Thinktank Mitchell J. Magee, M.D. STS National Database Taskforce CRSTI Dallas, TX FDA, Silver Spring, MD April 27, 2009 STS National Database Current Status Update Adult Cardiac
Atrial Fibrillation Ablation Thinktank Mitchell J. Magee, M.D. STS National Database Taskforce CRSTI Dallas, TX FDA, Silver Spring, MD April 27, 2009 STS National Database Current Status Update Adult Cardiac
HSCRIBE 5 HOLTER ANALYSIS SYSTEM
 HSCRIBE 5 HOLTER ANALYSIS SYSTEM freedom Your patients will be free to go about their miniature devices will let them forget they The H3+ digital recorder gives new meaning to the word compact, while providing
HSCRIBE 5 HOLTER ANALYSIS SYSTEM freedom Your patients will be free to go about their miniature devices will let them forget they The H3+ digital recorder gives new meaning to the word compact, while providing
Developing a Target Product Profile for a Preventive HIV Vaccine
 Developing a Target Product Profile for a Preventive HIV Vaccine Topics to be Covered Overview of TPPs What are they? What is the purpose and benefit of using a TPP? What is usually in a TPP? What are
Developing a Target Product Profile for a Preventive HIV Vaccine Topics to be Covered Overview of TPPs What are they? What is the purpose and benefit of using a TPP? What is usually in a TPP? What are
Onward from Think Tank 1. Salim F Idriss MD PhD, FACC, FHRS
 2 nd nnual CRC Think Tank on Prevention of udden Cardiac Death in the Young Developing a Rational, Reliable, and ustainable National Health Care Resource Onward from Think Tank 1 alim F driss MD PhD, FCC,
2 nd nnual CRC Think Tank on Prevention of udden Cardiac Death in the Young Developing a Rational, Reliable, and ustainable National Health Care Resource Onward from Think Tank 1 alim F driss MD PhD, FCC,
List of Workshops Workshop Title
 List of Workshops 2017 Year 2017 A CANADIAN HEART RHYTHM SOCIETY WORKSHOP: MANAGEMENT OF VENTRICULAR TACHYCARDIA 2017 CANADIAN SOCIETY OF CARDIOVASCULAR NUCLEAR AND CT IMAGING: CORONARY ATHEROSCLEROSIS
List of Workshops 2017 Year 2017 A CANADIAN HEART RHYTHM SOCIETY WORKSHOP: MANAGEMENT OF VENTRICULAR TACHYCARDIA 2017 CANADIAN SOCIETY OF CARDIOVASCULAR NUCLEAR AND CT IMAGING: CORONARY ATHEROSCLEROSIS
Dr.Binoy Skaria 13/07/15
 Dr.Binoy Skaria binoyskaria@hotmail.com binoy.skaria@heartofengland.nhs.uk 13/07/15 Acknowledgement Medtronic, Google images & Elsevier for slides Natalie Ryan, Events Manager, HEFT- for organising the
Dr.Binoy Skaria binoyskaria@hotmail.com binoy.skaria@heartofengland.nhs.uk 13/07/15 Acknowledgement Medtronic, Google images & Elsevier for slides Natalie Ryan, Events Manager, HEFT- for organising the
ICH guideline E14: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic
 25 January 2016 EMA/CHMP/ICH/310133/2008 Committee for Human Medicinal Products ICH guideline E14: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic
25 January 2016 EMA/CHMP/ICH/310133/2008 Committee for Human Medicinal Products ICH guideline E14: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic
Declaration of conflict of interest
 Declaration of conflict of interest There is no any potential conflict of interest. There is not any financial commitments or funds from private companies. Roles of heat shock transcription factor 1 gene
Declaration of conflict of interest There is no any potential conflict of interest. There is not any financial commitments or funds from private companies. Roles of heat shock transcription factor 1 gene
Why to monitor AF ablation success?
 Why to monitor AF ablation success? Why to monitor AF ablation success? CASTLE-AF Primary Endpoint All-cause mortality Worsening heart failure admissions Secondary Endpoints All-cause mortality Worsening
Why to monitor AF ablation success? Why to monitor AF ablation success? CASTLE-AF Primary Endpoint All-cause mortality Worsening heart failure admissions Secondary Endpoints All-cause mortality Worsening
Pediatric Drug Development: Successes and Challenges
 Pediatric Drug Development: Successes and Challenges Lynne Yao, M.D. Director, Division of Pediatric and Maternal Health Office of New Drugs Center for Drug Evaluation and Research U.S. FDA September 23,
Pediatric Drug Development: Successes and Challenges Lynne Yao, M.D. Director, Division of Pediatric and Maternal Health Office of New Drugs Center for Drug Evaluation and Research U.S. FDA September 23,
ECG Changes in Patients Treated with Mild Hypothermia after Cardio-pulmonary Resuscitation for Out-of-hospital Cardiac Arrest
 ECG Changes in Patients Treated with Mild Hypothermia after Cardio-pulmonary Resuscitation for Out-of-hospital Cardiac Arrest R. Schneider, S. Zimmermann, W.G. Daniel, S. Achenbach Department of Internal
ECG Changes in Patients Treated with Mild Hypothermia after Cardio-pulmonary Resuscitation for Out-of-hospital Cardiac Arrest R. Schneider, S. Zimmermann, W.G. Daniel, S. Achenbach Department of Internal
Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use?
 Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use? Helmut Pürerfellner, MD, Assoc. Prof. Saint Elisabeth s Sisters Hospital Academic Teaching Center Linz/Austria
Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use? Helmut Pürerfellner, MD, Assoc. Prof. Saint Elisabeth s Sisters Hospital Academic Teaching Center Linz/Austria
Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution
 Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution Dhananjay D. Marathe, Ph.D. QT-IRT Lead, Office of Clinical Pharmacology US FDA
Recent insights from the FDA QT-IRT on concentration-qtc analysis and requirements for TQT study ( waiver ) substitution Dhananjay D. Marathe, Ph.D. QT-IRT Lead, Office of Clinical Pharmacology US FDA
The QT interval as it relates to the safety of non-cardiac drugs
 European Heart Journal Supplements (2007) 9 (Supplement G), G3 G8 doi:10.1093/eurheartj/sum047 The QT interval as it relates to the safety of non-cardiac drugs Peter R. Kowey 1 * and Marek Malik 2,3 1
European Heart Journal Supplements (2007) 9 (Supplement G), G3 G8 doi:10.1093/eurheartj/sum047 The QT interval as it relates to the safety of non-cardiac drugs Peter R. Kowey 1 * and Marek Malik 2,3 1
Global Pediatric Development: We Are Making Progress. PMDA Perspective. Junko Sato, PhD PMDA
 Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
ICD in a young patient with syncope
 ICD in a young patient with syncope Konstantinos P. Letsas, MD, FESC Second Department of Cardiology Evangelismos General Hospital of Athens Athens, Greece Case presentation A 17-year-old apparently healthy
ICD in a young patient with syncope Konstantinos P. Letsas, MD, FESC Second Department of Cardiology Evangelismos General Hospital of Athens Athens, Greece Case presentation A 17-year-old apparently healthy
Medical management of AF: drugs for rate and rhythm control
 Medical management of AF: drugs for rate and rhythm control Adel Khalifa Sultan Hamad, BMS, MD, FGHRS, FRCP(Canada) Consultant Cardiologist & Interventional Cardiac Electrophysiologist Head of Electrophysiology
Medical management of AF: drugs for rate and rhythm control Adel Khalifa Sultan Hamad, BMS, MD, FGHRS, FRCP(Canada) Consultant Cardiologist & Interventional Cardiac Electrophysiologist Head of Electrophysiology
ISCTM Suicidal Ideation & Behavior Assessment Working Group
 ISCTM Suicidal Ideation & Behavior Assessment Working Group ISCTM Meeting February 19, 2013 Current Co-chairs: Co-Chairs-Elect: Adam Butler Michelle Stewart Phillip Chappell Douglas Feltner Welcome! Brief
ISCTM Suicidal Ideation & Behavior Assessment Working Group ISCTM Meeting February 19, 2013 Current Co-chairs: Co-Chairs-Elect: Adam Butler Michelle Stewart Phillip Chappell Douglas Feltner Welcome! Brief
The NCI Comparative Oncology Trials Consortium (COTC): Structure, Mission and Goals
 National Cancer Institute The NCI Comparative Oncology Trials Consortium (COTC): Structure, Mission and Goals Amy K. LeBlanc, DVM Diplomate ACVIM (Oncology) Director, Comparative Oncology Program Institute
National Cancer Institute The NCI Comparative Oncology Trials Consortium (COTC): Structure, Mission and Goals Amy K. LeBlanc, DVM Diplomate ACVIM (Oncology) Director, Comparative Oncology Program Institute
