(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
|
|
|
- Aileen Louisa Quinn
- 5 years ago
- Views:
Transcription
1 (19) United States US A1 (12) Patent Application Publication (10) Pub. No.: US 2009/ A1 Macaulay et al. (43) Pub. Date: Nov. 19, 2009 (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR PROSTHETIC HEART VALVES (75) Inventors: Patrick E. Macaulay, Windsor, CA (US); Robert J. Murray, Santa Rosa, CA (US); Trevor Greenan, Santa Rosa, CA (US); Timothy R. Ryan, Shorewood, MN (US); Charles Tabor, Shoreview, MN (US); Matthew J. Birdsall, Santa Rosa, CA (US); Gianfranco Pellegrini, Santa Rosa, CA (US) Correspondence Address: MEDTRONIC, INC. 710 MEDTRONIC PARKWAYNE MINNEAPOLIS, MN (US) (73) Assignee: Medtronic, Inc., Minneapolis, MN (US) (21) Appl. No.: 12/358,084 (22) Filed: Jan. 22, 2009 Related U.S. Application Data (60) Provisional application No. 61/ , filed on Jan. 24, Publication Classification (51) Int. Cl. A6IF 2/06 ( ) (52) U.S. Cl /1.11; 623/1.23 (57) ABSTRACT A delivery system for delivering a stented prosthetic heart valve to a lumen of a patient, the delivery system including a tubular body having a proximal end, a distal end, and a base portion with a plurality of extending elements, wherein each of the extending elements is engageable with a portion of a stent of a prosthetic heart valve. The delivery system further includes a sleeve having an inner area. The sheath is longitu dinally moveable relative to the base portion from a first position where the inner area of the sleeve at least partially covers the extending elements of the base portion to a second position where the extending elements are not positioned within the inner area of the sleeve.
2 Patent Application Publication Nov. 19, 2009 Sheet 1 of 10 US 2009/ A1
3 Patent Application Publication Nov. 19, 2009 Sheet 2 of 10 US 2009/ A1
4 Patent Application Publication Nov. 19, 2009 Sheet 3 of 10 US 2009/ A1 Fig. 4
5 Patent Application Publication Nov. 19, 2009 Sheet 4 of 10 US 2009/ A1
6 Patent Application Publication Nov. 19, 2009 Sheet 5 of 10 US 2009/ A1 Fig. 7
7 Patent Application Publication Nov. 19, 2009 Sheet 6 of 10 US 2009/ A1
8 Patent Application Publication Nov. 19, 2009 Sheet 7 of 10 US 2009/ A1 Z876
9 Patent Application Publication Nov. 19, 2009 Sheet 8 of 10 US 2009/ A a.
10 Patent Application Publication Nov. 19, 2009 Sheet 9 of 10 US 2009/ A
11 ent Application Publication Nov. 19, 2009 Sheet 10 of 10
12 US 2009/ A1 Nov. 19, 2009 DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR PROSTHETIC HEART VALVES CROSS-REFERENCE TO RELATED APPLICATION The present application claims priority to U.S. Pro visional Application No. 61/ , filed Jan. 24, 2008, and titled Delivery Systems and Methods of Implantation for Prosthetic Heart Valves', the entire contents of which is incorporated herein by reference in its entirety. TECHNICAL FIELD 0002 The present invention relates to prosthetic heart valves. More particularly, it relates to delivery systems for percutaneously implanting prosthetic heart valves compris ing a stent. BACKGROUND 0003 Diseased or otherwise deficient heart valves can be repaired or replaced using heart valve Surgery. Typical heart valve Surgeries involve an open-heart Surgical procedure that is conducted under general anesthesia, during which the heart is stopped while blood flow is controlled by a heart-lung bypass machine. This type of valve Surgery is highly invasive and exposes the patient to a number of potentially serious risks. Such as infection, stroke, renal failure, and adverse effects associated with use of the heart-lung machine, for example Recently, there has been increasing interest in mini mally invasive and percutaneous replacement of cardiac valves. Such Surgical techniques involve making a very small opening in the skin of the patient into which a valve assembly is inserted in the body and delivered to the heart via a delivery device similar to a catheter. This technique is often preferable to more invasive forms of Surgery, Such as the open-heart Surgical procedure described above. In the context of pulmo nary valve replacement, U.S. Patent Application Publication Nos. 2003/ A1 and 2003/ A1, both filed by Tower, et al., describe a valved segment of bovine jugular vein, mounted within an expandable stent, for use as a replacement pulmonary valve. The replacement valve is mounted on a balloon catheter and delivered percutaneously via the vascular system to the location of the failed pulmonary valve and expanded by the balloon to compress the valve leaflets against the right ventricular outflow tract, anchoring and sealing the replacement valve. As described in the articles: Percutaneous Insertion of the Pulmonary Valve', Bonhoeffer, et al., Journal of the American College of Cardi ology 2002:39: and Transcatheter Replacement of a Bovine Valve in Pulmonary Position. Bonhoeffer, et al., Circulation 2000; 102: , the replacement pulmonary valve may be implanted to replace native pulmonary valves or prosthetic pulmonary valves located in valved conduits Various types and configurations of prosthetic heart valves are used in percutaneous valve procedures to replace diseased natural human heart valves. The actual shape and configuration of any particular prosthetic heart valve is dependent to some extent upon the valve being replaced (i.e., mitral valve, tricuspid valve, aortic valve, or pulmonary valve). In general, the prosthetic heart valve designs attempt to replicate the function of the valve being replaced and thus will include valve leaflet-like structures used with either bio prostheses or mechanical heart Valve prostheses That is, the replacement valves may include a valved vein segment that is mounted in some manner within an expandable stent to make a stented valve. In order to prepare Such a valve for percutaneous implantation, the stented valve can be initially provided in an expanded or uncrimped con dition, then crimped or compressed around the balloon por tion of a catheter until it is as close to the diameter of the catheter as possible Other percutaneously-delivered prosthetic heart valves have been suggested having a generally similar con figuration, such as by Bonhoeffer, P. et al., Transcatheter Implantation of a Bovine Valve in Pulmonary Position. Cir culation, 2002: 102: , and by Cribier, A. et al. Per cutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation, 2002; 106: , the disclosures of which are incorporated herein by reference. These techniques rely at least partially upon a frictional type of engagement between the expanded Support structure and the native tissue to maintain a position of the delivered prosthesis, although the stents can also become at least partially embedded in the Surrounding tissue in response to the radial force provided by the stent and any balloons used to expand the stent. Thus, with these transcath eter techniques, conventional sewing of the prosthetic heart valve to the patient's native tissue is not necessary. Similarly, in an article by Bonhoeffer, P. et al. titled Percutaneous Insertion of the Pulmonary Valve. JAm Coll Cardiol, 2002: 39: , the disclosure of which is incorporated herein by reference, percutaneous delivery of a biological valve is described. The valve is sutured to an expandable stent within a previously implanted valved or non-valved conduit, or a previously implanted valve. Again, radial expansion of the secondary valve stent is used for placing and maintaining the replacement valve Although there have been advances in percutaneous valve replacement techniques and devices, there is a contin ued desire to provide delivery systems and corresponding valves having features that allow for valve implantation in a minimally invasive and percutaneous manner. SUMMARY The delivery systems and replacement valves of the invention are configured to provide complimentary features that promote optimal placement of the replacement heart valve in a native heart valve, such as the aortic valve, mitral valve, pulmonic valve, and/or tricuspid valve. In some embodiments, the replacement heart valves of the invention are highly amenable to transvascular delivery using a tran sapical approach (either with or without cardiopulmonary bypass and either with or without rapid pacing). The meth odology associated with the present invention can be repeated multiple times, such that several prosthetic heart valves of the present invention can be mounted on top of or within one another, if necessary or desired Heart valves that can be placed in a patient using delivery systems of the invention include a stent to which a valve structure is attached. The stents can include a wide variety of structures and features that can be used alone or in combination with features of other stents. In particular, these stents provide a number of different docking and/or anchor ing structures that are conducive to percutaneous delivery thereof. Many of the structures are thus compressible to a
13 US 2009/ A1 Nov. 19, 2009 relatively small diameter for percutaneous delivery to the heart of the patient, and then are expandable either via removal of external compressive forces (e.g., self-expanding stents), or through application of an outward radial force (e.g., balloon expandable stents). The devices delivered by the delivery systems described herein can be used to deliver stents, valved stents, or other interventional devices such as ASD (atrial septal defect) closure devices, VSD (ventricular septal defect) closure devices, or PFO (patent foramen ovale) occluders Methods for insertion of the replacement heart valves of the invention include delivery systems that can maintain the stent structures in their compressed State during their insertion and allow or cause the stent structures to expand once they are in their desired location. In addition, some delivery methods of the invention can include features that allow the stents to be retrieved for removal or relocation thereof after they have been deployed or partially deployed from the stent delivery systems. The methods may include implantation of the stent structures using either an antegrade or retrograde approach. Further, in many of the delivery approaches of the invention, the Stent structure is rotatable in vivo to allow the stent structure to be positioned in a desired orientation. BRIEF DESCRIPTION OF THE DRAWINGS The present invention will be further explained with reference to the appended Figures, wherein like structure is referred to by like numerals throughout the several views, and wherein: 0013 FIG. 1 is a front view of a portion of a stent posi tioned on a portion of an exemplary delivery system including multiple extending hubs or extending elements; 0014 FIG. 2 is an enlarged view of a portion of the stent and delivery system illustrated in FIG. 1 and further illustrat ing a portion of a sheath positioned over the hubs and crowns of the stent; 0015 FIG.3 is a front view of a stent positioned relative to a distal portion of a delivery system and schematically posi tioned relative to a patient's anatomy, where the stent is being delivered using a retrograde approach: 0016 FIG. 4 is a perspective view of a portion of another exemplary delivery system of the invention; 0017 FIG. 5 is a perspective view of a stent positioned on the portion of the delivery system illustrated in FIG. 4 and further including a collar located over the crowns of the stent; 0018 FIG. 6 is a perspective view of the portion of a delivery system and stent illustrated in FIG. 5, but with the collar in a different position relative to the other components of the delivery system; 0019 FIG. 7 is a perspective view of a portion of another exemplary delivery system of the invention; 0020 FIG. 8 is a perspective view of the portion of a delivery system illustrated in FIG. 7 and further illustrating a portion of a stent positioned on multiple hubs or extending members of the delivery system; 0021 FIG. 9 is a side view of a delivery system that includes the portion of the delivery system illustrated in FIGS. 7 and 8, and further including a stent engaged with extending elements of the delivery system; 0022 FIG.10 is a cross-sectional view taken along section line A-A of the delivery system and stent illustrated in FIG.9; 0023 FIG. 11 is an enlarged view of the circled portion of the delivery system of FIG. 10; 0024 FIG. 12 is a perspective view of a portion of another delivery system of the invention, including a stent that is engaged with features of the delivery system; 0025 FIG. 13 is a schematic cross-sectional view of a portion of the delivery system of FIG. 12; 0026 FIG. 14 is a front view of a stent positioned relative to a distal portion of a delivery system and schematically positioned relative to a patient's anatomy, wherein the stent is being delivered using an antegrade approach; and (0027 FIG. 15 is a front view of another stent being deliv ered to a patient's anatomy using an antegrade approach. DETAILED DESCRIPTION As referred to herein, the prosthetic heart valves used in accordance with the various devices and methods may include a wide variety of different configurations, such as a prosthetic heart Valve having tissue leaflets or a synthetic heart Valve having polymeric, metallic, or tissue-engineered leaflets, and can be specifically configured for replacing any heart valve. That is, while much of the description herein refers to replacement of aortic valves, the prosthetic heart valves of the invention can also generally be used for replace ment of native mitral, pulmonic, or tricuspid valves, for use as a venous valve, or to replace a failed bioprosthesis, such as in the area of an aortic valve or mitral valve, for example Although each of the valves used with the delivery devices and methods described herein would typically include leaflets attached within an interior area of a stent, the leaflets are not shown in many of the illustrated embodiments for clarity purposes. In general, the stents described herein include a Support structure comprising a number of Strut or wire portions arranged relative to each other to provide a desired compressibility and strength to the heart valve. Other details on particular configurations of the stents of the inven tion are also described below; however, in general terms, stents of the invention are generally tubular Support struc tures, and leaflets will be secured to the support structure. The leaflets can be formed from a variety of materials, such as autologous tissue, Xenograph material, or synthetics as are known in the art. The leaflets may be provided as a homog enous, biological valve structure. Such as a porcine, bovine, or equine valve. Alternatively, the leaflets can be provided inde pendent of one another (e.g., bovine or equine pericardial leaflets) and Subsequently assembled to the Support structure of the stent. In another alternative, the stent and leaflets can be fabricated at the same time, such as may be accomplished using high strength nano-manufactured NiTi films produced at Advanced Bio Prosthetic Surfaces (ABPS), for example. The Support structures are generally configured to accommo date three leaflets; however, the replacement prosthetic heart valves of the invention can incorporate more or less than three leaflets In more general terms, the combination of a support structure with one or more leaflets can assume a variety of other configurations that differ from those shown and described, including any known prosthetic heart Valve design. In certain embodiments of the invention, the Support structure with leaflets can be any known expandable prosthetic heart valve configuration, whether balloon expandable, self-ex panding, or unfurling (as described, for example, in U.S. Pat. Nos. 3,671,979; 4,056,854; 4,994,077; 5,332,402:5,370,685; 5,397,351:5,554,185; 5,855,601; and 6,168,614; U.S. Patent Application Publication No. 2004/ ; Bonhoeffer P., et al., Percutaneous Insertion of the Pulmonary Valve'. Pedi
14 US 2009/ A1 Nov. 19, 2009 atric Cardiology, 2002: 39: ; Anderson HR, et al., Transluminal Implantation of Artificial Heart Valves. EUR Heart J., 1992; 13: ; Anderson, J. R., et al., Translu minal Catheter Implantation of New Expandable Artificial Cardiac Valve', EUR Heart J., 1990, 11: (Suppl) 224a: Hil bert S. L., Evaluation of Explanted Polyurethane Trileaflet Cardiac Valve Prosthesis', J Thorac Cardiovascular Surgery, 1989; 94:419-29; Block PC, Clinical and Hemodynamic Follow-Up After Percutaneous Aortic Valvuloplasty in the Elderly. The American Journal of Cardiology, Vol. 62, Oct. 1, 1998; Boudjemline.Y., Steps Toward Percutaneous Aortic Valve Replacement, Circulation, 2002: 105: ; Bon hoeffer, P., Transcatheter Implantation of a Bovine Valve in Pulmonary Position, a Lamb Study, Circulation, 2000:102: ; Boudjemline. Y., Percutaneous Implantation of a Valve in the Descending Aorta In Lambs, EUR Heart J. 2002:23: ; Kulkinski, D., Future Horizons in Sur gical Aortic Valve Replacement: Lessons Learned During the Early Stages of Developing a Transluminal Implantation Technique, ASAIO J, 2004: 50:364-68; the teachings of which are all incorporated herein by reference) Orientation and positioning of the stents of the invention may be accomplished either by self-orientation of the stents (such as by interference between features of the stent and a previously implanted Stent or valve structure) or by manual orientation of the stent to align its features with ana tomical or previous bioprosthetic features, such as can be accomplished using fluoroscopic visualization techniques, for example. For example, when aligning the stents of the invention with native anatomical structures, they should be aligned so as to not block the coronary arteries, and native mitral or tricuspid valves should be aligned relative to the anterior leaflet and/or the trigones/commissures Some embodiments of the support structures of the stents described herein can be a series of wires or wire seg ments arranged so that they are capable of transitioning from a collapsed state to an expanded State. In some embodiments, a number of individual wires comprising the Support structure can be formed of a metal or other material. These wires are arranged in Such a way that a Support structure allows for folding or compressing to a contracted State in which its internal diameter is greatly reduced from its internal diameter in an expanded State. In its collapsed state, Such a Support structure with attached valves can be mounted over a delivery device. Such as a balloon catheter, for example. The Support structure is configured so that it can be changed to its expanded State when desired. Such as by the expansion of a balloon catheter. The delivery systems used for such a stent should be provided with degrees of rotational and axial ori entation capabilities in order to properly position the new stent at its desired location The wires of the support structure of the stents in other embodiments can alternatively be formed from a shape memory material Such as a nickel titanium alloy (e.g., Niti nol). With this material, the support structure is self-expand able from a contracted State to an expanded State, such as by the application of heat, energy, and the like, or by the removal of external forces (e.g., compressive forces). This Support structure can also be compressed and re-expanded multiple times without damaging the structure of the stent. In addition, the Support structure of such an embodiment may be laser cut from a single piece of material or may be assembled from a number of different components. For these types of stent structures, one example of a delivery system that can be used includes a catheter with a retractable sheath that covers the stent until it is to be deployed, at which point the sheath can be retracted to allow the stent to expand. Further details of such embodiments are discussed below Referring now to the Figures, wherein the compo nents are labeled with like numerals throughout the several Figures, and initially to FIGS. 1 and 2, a portion of a delivery system 10 is illustrated as engaged with a stent 12. In particu lar, the delivery system 10 includes multiple hubs 16 that extend from a central base portion 18. These extending hubs 16 are shaped for engagement with the crowns 14 of a distal end of the stent 12 to aid insecuring the stent 12 to the delivery system 10. As shown, the crowns 14 of the stent 12 can hook over the hubs 16, which are generally shaped to match or engage with the compressed inner shape of the stent crowns 14. Thus, the number of hubs 16 that extend from the base portion 18 is preferably the same as the number of crowns 14 on the stent 12 that is engaged with it; however, the number of hubs 16 and number of crowns 14 may be different The proximal end of the stent 12 can be secured to the delivery system 10 in a number of different ways, such as with wires having hook tips that engage with the crowns of proximal end of the stent 12, for example. In this particular example, angled wire tips or protrusions extend at an angle relative to their respective wires, where the angle between each tip and its respective wire can be approximately 90 degrees, for example, although it can be any angle that pro vides for engagement between the wires and the stent crowns In order to keep the crowns 14 of the stent 12 engaged with the hubs 16 at the distal end of the delivery device 10 until it is desired to release the stent, the delivery system 10 is provided with a distal tip 20 that is hollow at its proximal end. In this way, the proximal end of the tip 20 can slide over the base portion 18 and its extending hubs 16 over which stent crowns 14 are positioned, thereby maintaining the system components in this arrangement. In order to release the stent 12 from the delivery system 10, the tip 20 and base portion 18 can be moved relative to each other until the tip 20 no longer covers the stent crowns 14. The crowns 14 will then be able to move outwardly from the hubs 16 to disengage the stent 12 from the hubs FIG. 3 illustrates one exemplary placement of the stent 12 for replacement of an aortic valve that uses the delivery system 10 having a distal end as shown in FIGS. 1 and 2. Alternatively, the delivery system 10 can be used for replacement of other valves and/or other portions of the body in which a stent is to be implanted. Regarding this schematic view of a portion of a patient s heart shown in FIG. 3, the delivery system 10 includes a tip 20 that maintains the stent 12 in a compressed condition at the distal end of the stent, wherein the crowns of the stent 12 (not visible in this Figure) at its distal end are hooked or positioned over the hubs of a sprocket. Alternatively, the crowns of the stent 12 at its distal end may be held in a compressed condition simply by the relatively smaller inner diameter of the tip 20 pressing the stent crowns toward the central axis of the delivery system 10 (i.e., no sprockets are used for this connection). The tip 20 is moveable relative to the crowns of the stent to compress, capture and release the stent crowns, as desired. The proximal end of the stent 12 is attached to the delivery system via multiple wires 22 that are engaged with the stent crowns at the proximal end of the stent 12. These wires 22 are further maintained on the stent crowns with sleeves 24 that surround or partially Surround the wires 22 and their corresponding
15 US 2009/ A1 Nov. 19, 2009 stent crowns. As shown, the area of the stent 12 between its proximal and distal ends is not compressed or held within any type of sheath and therefore is free to expand outwardly, as shown. However, it is contemplated that the stent 12 can be held in its compressed condition at various points during the stent delivery process using a sheath 28. The stent 12 is made of a series of wires that are compressible and expandable through the application and removal of external forces, and may include a series of Nitinol wires that are approximately inches in diameter. That is, the stent 12 is a self-expanding stent The view of this area of a heart in FIG. 3 schemati cally illustrates an aorta 30, mitral valve 32, left ventricle 34, and septum 36. Further, the delivery approach illustrated in this Figure can be considered to be a retrograde delivery approach. In order to deploy the stent via the delivery system 10, the stent 12 is delivered to its desired position in a lumen (e.g. heart valve area) of a patient using a variety of tracking and monitoring techniques. In particular, FIG. 3 shows the stent and delivery system with the stent 12 being attached at both its proximal end and its distal end to the delivery system 10. When it is desired to release the stent 12, the stent is moved relative to the distal tip of the system, thereby expos ing the distal end of the stent 12 and the stent crowns 14 that may be engaged with hubs 16. In this way, the compressive forces that were provided by enclosing the stent 12 within the tip 20 of the delivery system 10 are eliminated and the stent 12 can expand toward its original, expanded condition With the delivery system 10 of the invention, it is contemplated that the user can choose in which sequence the various portions of the stent are deployed or released while other portions are being held or compressed. For example, referring now to FIG. 14, a delivery system 150 is illustrated as it is being used to deliver a stent 152 to an aorta 160 using an antegrade approach. This may be performed either tran sapically or transeptally. Delivery system 150 includes a dis tal tip 154, a guidewire 156, a slideable sleeve 158, and attachment elements 162. With this configuration, the proxi mal or inflow end 164 of the stent 152 is attached to elements 162 which may be wires having hooks or coils and their distal ends, for example. Further, for antegrade delivery using this system, the proximal end 164 of the stent 152 can be released first while maintaining an outflow or distal end 166 of the stent 152 in a compressed state until it is determined that the stent is properly aligned and positioned relative to the native annulus of the aorta 160. The distal end 166 of the stent may then be released, such as by retracting the slideable sleeve 158 until the stent crowns at the distal end 166 are exposed and can thereby move relative to the delivery system Another delivery system 180 is illustrated in FIG.15 as it is being used to deliver a stent 182 to an aorta 198 using an antegrade approach. This may be performed either tran sapically or transeptally. Delivery system 180 includes a dis tal tip 184, a guidewire 186, a slideable sleeve 190, and an outer sheath 188. With this configuration, a portion of the delivery system 180 may include a sprocket with extending hubs (not visible) that can be positioned under the proximal end of the tip 184 to capture the crowns at the distal end 196 of the stent 182. When using this system for antegrade deliv ery of the stent 182 to the area of a native aortic valve 192, a proximal end 194 of the stent 182 can be fully released first, while maintaining the outflow or distal end 196 of the stent 182 in a compressed state. The system can remain in this arrangement until it is determined that the stent 182 is prop erly aligned and positioned relative to the native annulus of the aorta 192. The distal end 196 of the Stent 182 can then be released, such as by retracting the slideable sleeve until the distal end of the stent 182 is exposed, thereby allowing the stent crowns to disengage from the sprocket with extending hubs It is noted that in the above procedure, the stent can be retracted back into a lumen of the delivery system 10 at any point in the process until the wires are disengaged from the stent. Such as for repositioning of the stent if it is determined that the stent has been improperly positioned relative to the patient's anatomy. In this case, the steps described above can be repeated until the desired positioning of the stent is achieved With the delivery systems described herein, full or partial blood flow through the valve can advantageously be maintained during the period when the stented valve is being deployed into the patient but is not yet released from its delivery system. This feature can help to prevent complica tions that may occur when blood flow is stopped or blocked during valve implantation with Some other known delivery systems. In addition, it is possible for the clinician to thereby evaluate the opening and closing of leaflets, examine for any paravalvular leakage and evaluate coronary flow and proper positioning of the valve within the target anatomy before final release of the stented valve FIGS. 4-6 illustrate the distal end of another exem plary delivery system 50 that can be used to deploy a stent 52 in a desired location inapatient. The stent 52 includes a series of wires or wire segments arranged so that they are capable of transitioning from a collapsed State to an expanded State, and is preferably a self-expanding stent comprising a shape memory material. The delivery system 50 is configured so that a stent can be loaded onto the system, at least partially deploying the stent, and then retracting the stent back into the delivery system and relocating it, if desired. In particular, delivery system 50 generally includes a base portion 54 that has multiple elements 56 extending from its outer, generally cylindrical surface. In one embodiment, the outer surface of the base portion 54 comprises multiple flat surfaces around its periphery, with one element 56 extending from each of the flat surfaces. In another embodiment, the outer surface of the base portion 54 has a generally circular outer periphery. The num ber of extending elements 56 preferably is the same as the number of wire structures 60 that extend from a proximal end of the stent 52, although the number of elements 56 can be different than the number of wire structures 60. Each element 56 comprises an angled wedge that tapers from its proximal end toward the distal end of the delivery system 50. All of the elements 56 of a particular delivery system 50 can be the same size and shape as each other, or the elements may be differ ently sized and shaped, as may be desirable for engagement with features of a particular stent Stent 52 further includes a loop or eyelet 62 at the end of each wire structure 60, where each eyelet 62 is sized and shaped for engagement with an extending element 56. All of the eyelets 62 of a particular stent 60 may be the same size and shape as each other, or the eyelets 62 may be differently sized and shaped. In an alternative embodiment, the stent may include crowns at one end that are engageable with the extending elements 56, such as would be the case if the stent did not include eyelets at the end of its wire structures. Once the eyelets 62 (and/or stent crowns) are engaged with their respective elements 56 of the delivery system, a collar 64 can
16 US 2009/ A1 Nov. 19, 2009 be slid at least partially over the eyelets 62, as is best illus trated in FIG. 5. When it is desired to release the stent eyelets 62 from the extending elements 56 of the delivery system 50. the collar 64 can be slid proximally to expose the eyelets 62 and extending elements 56, as is shown in FIG. 6, for example. One advantage provided by this collar 64 is that the outer sheath of the delivery system can be completely with drawn, thereby allowing expansion of the stent to assess acceptable positioning while maintaining precise control and capture of the stent 52 on the extending elements FIGS. 7 and 8 illustrate the distal end of another exemplary delivery system 80 that can be used to deploy a stent 82 in a desired location in a patient. The stent 82 includes a series of wires or wire segments arranged so that they are capable of transitioning from a collapsed State to an expanded state, and is preferably a self-expanding stent comprising a shape-memory material. The delivery system 80 is config ured so that a stent can be loaded onto the system, at least partially deploying the stent, and then retracting the stent back into the delivery system and relocating it, if desired. In particular, delivery system 80 generally includes a base por tion 84 that has multiple elements 86 extending from its outer, generally cylindrical Surface. In one embodiment, the outer surface of the base portion 84 comprises multiple flat surfaces around its periphery, with one element 86 extending from each of the flat surfaces. In another embodiment, the outer surface of the base portion 84 has a generally circular outer periphery. The number of extending elements 86 preferably is the same as the number of wire structures 90 that extend from a proximal end of the stent 82, although the number of ele ments 86 can be different than the number of wire structures 90. Each element 86 comprises an angled wedge that tapers from its proximal end toward the distal end of the delivery system. All of the elements 86 of a particular delivery system 80 can be the same size and shape as each other, or the elements may be differently sized and shaped, as may be desirable for engagement with features of a particular stent. The delivery system 80 further includes a tapered nose por tion.94 that extends from the sprocket 84 toward the distalend of the delivery system Stent 82 further includes a loop or eyelet 92 at the end of each wire structure 90, where each eyelet 92 is sized and shaped for engagement with an extending element 86. All of the eyelets 92 of a particular stent 90 may be the same size and shape as each other, or the eyelets 92 may be differently sized and shaped. In an alternative embodiment, the stent may include crowns at one end that are engageable with the extending elements 86, such as would be the case if the stent did not include eyelets at the end of its wire structures. The stent delivery system 80 further includes a spring-loaded collar 96 that can be configured so that it needs to be actuated to cover the eyelets 92 and the extending elements 86 with which they are engaged, or the collar 96 can be configured so that it needs to be actuated to uncover or release the eyelets 92. In either case, once the eyelets 92 (and/or stent crowns) are all engaged with their respective elements 86 of the delivery system, the collar 96 can be slid at least partially over the eyelets 92. When it is desired to release the stent eyelets 92 from the delivery system 80, the collar 96 can be slid proxi mally to expose the eyelets 92 and extending elements FIGS further illustrate one embodiment of the internal components and configuration of the delivery system 80 and a stent 82 engaged with the extending elements 86. This embodiment is shown without an outer sheath that can be used to maintain the stent in a compressed condition. One exemplary component that can be used for the spring-loaded collar 96 is a spring 98, although a different configuration for a spring can instead be used FIGS. 12 and 13 illustrate the distal end of another exemplary delivery system 100 that can be used to deliver and deploy a stent 102 in a desired location in a patient. The stent 102 includes a series of wires or wire segments arranged so that they are capable of transitioning from a collapsed state to an expanded State, and is preferably a self-expanding stent comprising a shape-memory material. The delivery system 100 is configured so that a stent can be loaded onto the system, at least partially deploying the stent, and then retracting the stent back into the delivery system and relocating it, if desired. In particular, delivery system 100 generally includes a sprocket 104 that includes multiple sprocket teeth 106 extending from its outer, generally cylindrical Surface. The number of sprocket teeth 106 is preferably is the same as the number of wire structures 110 that extend from a proximal end of the stent 102, although the number of teeth 106 can be different than the number of wire structures 110. All of the teeth 106 of a particular delivery system 100 can be the same size and shape as each other, or the teeth 106 may be differ ently sized and shaped, as may be desirable for engagement with features of a particular stent Stent 102 further includes a loop or eyelet 112 at the end of each of its wire structures 110, where each eyelet 112 is sized and shaped for engagement with a sprocket tooth 106. All of the eyelets 112 of a particular stent may be the same size and shape as each other, or the eyelets 112 may be differently sized and shaped. In an alternative embodiment, the stent may include crowns at one end that are engageable with the teeth 106, such as would be the case if the stent did not include eyelets at the end of its wire structures The delivery system 100 further includes a sleeve 118 that is incorporated into the valve cover or sheath. In one exemplary embodiment, the delivery system will include a lock pin on its handle that would limit the travel of the valve cover to provide the additional control over the release of the stent, when desired FIG. 13 further illustrates a portion of the delivery system 100 in cross-section. In particular, delivery system 100 includes a catheter tip 120, an outer shaft 122, an inner member 124, an inner slotted ring 126, a slot 128, and a middle layer 130. A stent 102 is also illustrated in one exem plary manner in which it would be positioned on the delivery system 100 when in its compressed condition Although the number of extending elements, teeth, and/or hubs of the delivery systems described herein can vary, one preferred embodiment includes nine of Such extending elements for engagement with a stent having nine attachment points. The stent attachment points can include stent crowns, eyelets at the end of stent crowns or at the end of extending stent wires, or the like. More or less than nine attachment points and extending elements can be provided; however, the size, shape, and spacing of the elements around a base portion can be adjusted accordingly to achieve a desired configura tion and size for the overall delivery system. Further, the height of the various extending elements can be relatively large or Small, depending on the thickness of the stent wires that will engage with it, the stresses to which the stent will be Subjected during the delivery process, and the like. Because it is often desirable to minimize the diameter of the delivery system for percutaneous delivery of stented valves, the num
17 US 2009/ A1 Nov. 19, 2009 ber of stent wires and corresponding extending elements can be designed or chosen to optimize the quality of the attach ment between the stent and the delivery system, while pro viding a stent that has certain desirable characteristics when implanted in a patient With the various delivery systems of the invention, once the crowns, eyelets, or other stent features are engaged with sprocket teeth or other extending members of a delivery system, the stent can then be moved relative to a sheath of the delivery system to enclose the stent within the sheath by pulling the wires toward the proximal end of the delivery system, by pushing the sheath toward the distal end of the delivery system, or by Some combination of these two move ments. In order to release the stent once the delivery system is positioned within the patient, moving the sheath and stent in opposite directions relative to each other will provide the stent with the freedom to move. When the stent is a self expanding stent, it can expand to a larger diameter once the constraint of the sheath is removed. Other motions can be performed to move the Stent away from the teeth or engaging members with which it is engaged, if desired. The stent is thereby released from the delivery system One exemplary process for apically deploying a stent to the aorta using the delivery systems of the invention is described below. This method can also be used for other delivery approaches, such as for transarterial retrograde delivery. In particular, the stent is loaded onto the delivery system and a sheath is positioned over the stent to maintain it in its compressed condition. The delivery system is then advanced to the area in which the stent will be implanted using known delivery techniques and devices. Once the deliv ery system has been located within the patient so that the stent and/or valve is in its desired position within the aortic valve, the sheath is pulled back toward the proximal end of the delivery system (or the delivery system is moved distally away from the sheath), which allows the stent to expand radially. Alternatively, in a transarterial retrograde delivery approach, the inflow or annular end of the stent can remain compressed while the middle of the stent, along with other features such as petals, are radially expanded. Any minor positional adjustments, if necessary, can be made at this point. However, if it is determined that the stent is not in the desired position, the sheath can be moved back toward the distal end of the delivery system until the entire stent is again enclosed within the sheath, then the delivery system can be reposi tioned until it is in its desired location. The delivery system may also include a stop on its handle or some other portion of its structure that requires a positive action by the user to prevent inadvertent release of the stent from the delivery system Delivering any balloon-expandable stents to an implantation location can be performed percutaneously using modified versions of the delivery systems of the inventions. In general terms, this includes providing a transcatheter assem bly, including a delivery catheter, a balloon catheter, and a guide wire. Some delivery catheters of this type are known in the art, and define alumen within which the balloon catheter is received. The balloon catheter, in turn, defines a lumen within which the guide wire is slideably disposed. Further, the balloon catheter includes a balloon that is fluidly connected to an inflation source. It is noted that if the stent being implanted is the self-expanding type of stent, the balloon would not be needed and a sheath or other restraining means would be used for maintaining the Stent in its compressed State until deploy ment of the stent, as described herein. In any case, for a balloon-expandable stent, the transcatheter assembly is appropriately sized for a desired percutaneous approach to the implantation location. For example, the transcatheter assembly can be sized for delivery to the heart valve via an opening at a carotid artery, a jugular vein, a sub-clavian vein, femoral artery or vein, or the like. Essentially, any percuta neous intercostals penetration can be made to facilitate use of the transcatheter assembly Prior to delivery, the stent is mounted over the bal loon in a contracted State to be as Small as possible without causing permanent deformation of the Stent structure. As compared to the expanded State, the Support structure is com pressed onto itself and the balloon, thus defining a decreased inner diameter as compared to an inner diameter in the expanded state. While this description is related to the deliv ery of a balloon-expandable stent, the same basic procedures can also be applicable to a self-expanding stent, where the delivery system would not include a balloon, but would pref erably include a sheath or some other type of configuration for maintaining the stent in a compressed condition until its deployment With the stent mounted to the balloon, the transcath eter assembly is delivered through a percutaneous opening (not shown) in the patient via the delivery catheter. The implantation location is located by inserting the guide wire into the patient, which guide wire extends from a distal end of the delivery catheter, with the balloon catheter otherwise retracted within the delivery catheter. The balloon catheter is then advanced distally from the delivery catheter along the guide wire, with the balloon and stent positioned relative to the implantation location. In an alternative embodiment, the stent is delivered to an implantation location via a minimally invasive Surgical incision (i.e., non-percutaneously). In another alternative embodiment, the stent is delivered via open heart/chest Surgery. In one embodiment of the stents of the invention, the stent includes a radiopaque, echogenic, or MRI visible material to facilitate visual confirmation of proper placement of the stent. Alternatively, other known Surgical visual aids can be incorporated into the stent. The techniques described relative to placement of the stent within the heart can be used both to monitor and correct the place ment of the stent in a longitudinal direction relative to the length of the anatomical structure in which it is positioned Once the stent is properly positioned, the balloon catheter is operated to inflate the balloon, thus transitioning the stent to an expanded state. Alternatively, where the Sup port structure is formed of a shape memory material, the stent can self-expand to its expanded State The present invention has now been described with reference to several embodiments thereof. The foregoing detailed description and examples have been given for clarity of understanding only. No unnecessary limitations are to be understood therefrom. It will be apparent to those skilled in the art that many changes can be made in the embodiments described without departing from the scope of the invention. Thus, the scope of the present invention should not be limited to the structures described herein. What is claimed is: 1. A delivery system for delivering a stented prosthetic heart valve to a lumen of a patient, the delivery system com prising: a tubular body comprising a proximal end, a distal end, and a base portion comprising a plurality of extending ele
18 US 2009/ A1 Nov. 19, 2009 ments, wherein each of the extending elements is engageable with a portion of a stent of a prosthetic heart valve; and a tip portion comprising an inner area, the tip portion being longitudinally moveable relative to the base portion from a first position where the inner area at least partially covers the extending elements of the base portion to a second position where the extending elements are not positioned within the inner area of the tip portion. 2. The delivery system of claim 1, wherein the base portion is generally cylindrical. 3. The delivery system of claim 1, wherein each of the extending elements tapers in height from a first end toward a distal end of the delivery device. 4. The delivery system of claim 1, in combination with a stent having the same number of stent engagement features at a first end of the stent as the number of extending elements of the base portion. 5. The delivery system of claim 4, wherein the stent engagement features comprise a plurality of stent crowns at a first end of the stent. 6. The delivery system of claim 4, wherein the stent engagement features comprise a plurality of eyelets at the first end of the stent. 7. The delivery system of claim 4, wherein a second end of the stent includes a plurality of stent engagement features for engaging with a plurality of stent engagement devices of the delivery system. 8. The delivery system of claim 7, wherein the plurality of engagement features of the second end of the Stent comprises a plurality of stent crowns at the second end of the stent. 9. The delivery system of claim 7, wherein the plurality of stent engagement devices of the delivery system comprise a plurality of wires that are engageable at their distal ends with the plurality of engagement features at the second end of the Stent. 10. The delivery system of claim 7, wherein the stent engagement features of the second end of the Stent are sequentially disengageable from the plurality of stent engage ment devices prior to the stent engagement features of the first end of the stent being disengaged from the extending ele ments of the base portion. 11. The delivery system of claim 7, wherein the stent engagement features of the first end of the stent are sequen tially disengageable from the extending elements of the base portion prior to the stent engagement features of the second end of the stent being disengaged from the plurality of stent engagement devices. 12. A delivery system for delivering a stented prosthetic heart valve to a lumen of a patient, the delivery system com prising: a tubular body having a proximal end, a distal end, and a base portion comprising a plurality of extending ele ments, wherein each of the extending elements is engageable with a portion of a stent of a prosthetic heart valve; and a sleeve comprising an inner area, the sheath being longi tudinally moveable relative to the base portion from a first position where the inner area of the sleeve at least partially covers the extending elements of the base por tion to a second position where the extending elements are not positioned within the inner area of the sleeve. 13. The delivery system of claim 12, wherein the sleeve is positioned at a proximal end of the stent. 14. The delivery system of claim 12, wherein the sleeve is spring-loaded for movement from a first position to a second position.
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090192591A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0192591 A1 Ryan et al. (43) Pub. Date: (54) MARKERS FOR PROSTHETIC HEART Publication Classification VALVES
US 20090192591A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0192591 A1 Ryan et al. (43) Pub. Date: (54) MARKERS FOR PROSTHETIC HEART Publication Classification VALVES
(12) United States Patent
 US0094.80557B2 (12) United States Patent Pellegrini et al. (10) Patent No.: (45) Date of Patent: US 9.480,557 B2 Nov. 1, 2016 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) STENTS FOR PROSTHETIC
US0094.80557B2 (12) United States Patent Pellegrini et al. (10) Patent No.: (45) Date of Patent: US 9.480,557 B2 Nov. 1, 2016 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) STENTS FOR PROSTHETIC
(12) United States Patent
 USOO8623074B2 (12) United States Patent Ryan (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR REPLACEMENT PROSTHETIC HEART VALVES (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) Inventor: Timothy
USOO8623074B2 (12) United States Patent Ryan (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR REPLACEMENT PROSTHETIC HEART VALVES (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) Inventor: Timothy
(12) United States Patent
 (12) United States Patent Jones et al. USOO6833003B2 (10) Patent No.: (45) Date of Patent: Dec. 21, 2004 (54) EXPANDABLE STENT AND DELIVERY SYSTEM (75) Inventors: Donald K. Jones, Lauderhill, FL (US);
(12) United States Patent Jones et al. USOO6833003B2 (10) Patent No.: (45) Date of Patent: Dec. 21, 2004 (54) EXPANDABLE STENT AND DELIVERY SYSTEM (75) Inventors: Donald K. Jones, Lauderhill, FL (US);
III. United States Patent (19) Sheiban 5,226,889. Jul. 13, and at least a pair of inflatable balloons carried on the
 United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
(12) United States Patent
 (12) United States Patent USOO968,734.4B2 (10) Patent No.: Murray, III et al. (45) Date of Patent: Jun. 27, 2017 (54) TRANSCATHETER PROSTHETIC HEART (58) Field of Classification Search VALVE DELVERY DEVICE
(12) United States Patent USOO968,734.4B2 (10) Patent No.: Murray, III et al. (45) Date of Patent: Jun. 27, 2017 (54) TRANSCATHETER PROSTHETIC HEART (58) Field of Classification Search VALVE DELVERY DEVICE
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 US 2017.0065412A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0065412 A1 Mesana et al. (43) Pub. Date: (54) STENTED HEART VALVE DEVICES AND (60) Provisional application
US 2017.0065412A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0065412 A1 Mesana et al. (43) Pub. Date: (54) STENTED HEART VALVE DEVICES AND (60) Provisional application
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003.01.05520A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0105520 A1 Alferness et al. (43) Pub. Date: (54) ANCHOR AND PULL MITRAL VALVE DEVICE AND METHOD (75) Inventors:
(19) United States US 2003.01.05520A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0105520 A1 Alferness et al. (43) Pub. Date: (54) ANCHOR AND PULL MITRAL VALVE DEVICE AND METHOD (75) Inventors:
58 Field of Search /191,192, wire stent which extends in a path defining a generally
 USOO5882335A United States Patent (19) 11 Patent Number: Leone et al. (45) Date of Patent: Mar 16, 1999 54 RETRIEVABLE DRUG DELIVERY STENT 5,306,250 4/1994 March et al.... 604/104 5,306,286 4/1994 Stack
USOO5882335A United States Patent (19) 11 Patent Number: Leone et al. (45) Date of Patent: Mar 16, 1999 54 RETRIEVABLE DRUG DELIVERY STENT 5,306,250 4/1994 March et al.... 604/104 5,306,286 4/1994 Stack
(12) (10) Patent No.: US 9,468,773 B1. Anderson et al. (45) Date of Patent: Oct. 18, 2016
 United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
(19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
( ) -- (12) Patent Application Publication (10) Pub. No.: US 2016/ A1. (19) United States. (43) Pub. Date: Oct. 27, 2016.
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310302 A1 NEGLEN et al. US 201603103O2A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (60) SYSTEMIS AND METHODS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310302 A1 NEGLEN et al. US 201603103O2A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (60) SYSTEMIS AND METHODS
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070179519A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0179519 A1 Huisun (43) Pub. Date: (54) STENT DELIVERY SYSTEM TO IMPROVE (22) Filed: Jan. 27, 2006 PLACEMENT
US 20070179519A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0179519 A1 Huisun (43) Pub. Date: (54) STENT DELIVERY SYSTEM TO IMPROVE (22) Filed: Jan. 27, 2006 PLACEMENT
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 2015O1124.30A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0112430 A1 Creaven et al. (43) Pub. Date: Apr. 23, 2015 (54) SYSTEMS, DEVICES AND METHODS FOR TRANSCATHETERVALVE
(19) United States US 2015O1124.30A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0112430 A1 Creaven et al. (43) Pub. Date: Apr. 23, 2015 (54) SYSTEMS, DEVICES AND METHODS FOR TRANSCATHETERVALVE
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 2008.0021546A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0021546 A1 Patz et al. (43) Pub. Date: Jan. 24, 2008 (54) SYSTEM FOR DEPLOYING BALLOON-EXPANDABLE HEART VALVES
(19) United States US 2008.0021546A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0021546 A1 Patz et al. (43) Pub. Date: Jan. 24, 2008 (54) SYSTEM FOR DEPLOYING BALLOON-EXPANDABLE HEART VALVES
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(12) United States Patent (10) Patent No.: US 6,514,280 B1. Gilson (45) Date of Patent: Feb. 4, 2003
 USOO651428OB1 (12) United States Patent (10) Patent No.: US 6,514,280 B1 Gilson (45) Date of Patent: Feb. 4, 2003 (54) DELIVERY CATHETER 5,683,451 A 11/1997 Lenker et al.... 623/1 5,885.258 A * 3/1999
USOO651428OB1 (12) United States Patent (10) Patent No.: US 6,514,280 B1 Gilson (45) Date of Patent: Feb. 4, 2003 (54) DELIVERY CATHETER 5,683,451 A 11/1997 Lenker et al.... 623/1 5,885.258 A * 3/1999
(12) United States Patent (10) Patent No.: US 6,210,432 B1
 USOO6210432B1 (12) United States Patent (10) Patent No.: US 6,210,432 B1 Solem et al. (45) Date of Patent: Apr. 3, 2001 (54) DEVICE AND METHOD FOR TREATMENT 5,980,552 * 11/1999 Pinchasik et al.... 623/1.16
USOO6210432B1 (12) United States Patent (10) Patent No.: US 6,210,432 B1 Solem et al. (45) Date of Patent: Apr. 3, 2001 (54) DEVICE AND METHOD FOR TREATMENT 5,980,552 * 11/1999 Pinchasik et al.... 623/1.16
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0071716 A1 Milsom et al. US 2017007 1716A1 (43) Pub. Date: Mar. 16, 2017 (54) (71) (72) (21) (22) (63) (60) METHOD AND APPARATUS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0071716 A1 Milsom et al. US 2017007 1716A1 (43) Pub. Date: Mar. 16, 2017 (54) (71) (72) (21) (22) (63) (60) METHOD AND APPARATUS
(12) United States Patent (10) Patent No.: US 7,819,889 B2
 patent is extended or adjusted under 35 6,113,622 U.S.C. 154(b) by 1301 days. US007819889B2 (12) United States Patent (10) Patent No.: Healy et al. (45) Date of Patent: *Oct. 26, 2010 (54) DETACHABLE INTRODUCER
patent is extended or adjusted under 35 6,113,622 U.S.C. 154(b) by 1301 days. US007819889B2 (12) United States Patent (10) Patent No.: Healy et al. (45) Date of Patent: *Oct. 26, 2010 (54) DETACHABLE INTRODUCER
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 US 2013 O190861A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0190861 A1 Chau et al. (43) Pub. Date: Jul. 25, 2013 (54) PROSTHETIC VALVE FOR REPLACING Publication Classification
US 2013 O190861A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0190861 A1 Chau et al. (43) Pub. Date: Jul. 25, 2013 (54) PROSTHETIC VALVE FOR REPLACING Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
Berry (43) Pub. Date: May 6, (76) Inventor: Bret Berry, Cordova, TN (US) (57) ABSTRACT
 US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0165859 A1 ECKHOUSE et al. US 2012O165859A1 (43) Pub. Date: Jun. 28, 2012 (54) (75) (73) (21) (22) (63) DEVICE AND METHOD INVOLVING
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0165859 A1 ECKHOUSE et al. US 2012O165859A1 (43) Pub. Date: Jun. 28, 2012 (54) (75) (73) (21) (22) (63) DEVICE AND METHOD INVOLVING
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0189183 A1 Spenser et al. US 20170 189183A1 (43) Pub. Date: Jul. 6, 2017 (54) (71) (72) (73) (21) (22) (60) PROSTHETIC HEART
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0189183 A1 Spenser et al. US 20170 189183A1 (43) Pub. Date: Jul. 6, 2017 (54) (71) (72) (73) (21) (22) (60) PROSTHETIC HEART
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O316632A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0316632 A1 Gao (43) Pub. Date: Dec. 13, 2012 (54) RETRIEVABLE COVERED STENT FOR (52) U.S. Cl.... 623A1.2 BFURCATION
(19) United States US 2012O316632A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0316632 A1 Gao (43) Pub. Date: Dec. 13, 2012 (54) RETRIEVABLE COVERED STENT FOR (52) U.S. Cl.... 623A1.2 BFURCATION
United States Patent (19)
 United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
Portico (St. Jude Medical Inc, St.
 Review Article Portico Transcatheter Heart Valve Apostolos Tzikas 1,2, Michael Chrissoheris 2, Antonios Halapas 2, Konstantinos Spargias 2 1 Interbalkan European Medical Centre, Thessaloniki, 2 Hygeia
Review Article Portico Transcatheter Heart Valve Apostolos Tzikas 1,2, Michael Chrissoheris 2, Antonios Halapas 2, Konstantinos Spargias 2 1 Interbalkan European Medical Centre, Thessaloniki, 2 Hygeia
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 US 2002O107531A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0107531A1 Schreck et al. (43) Pub. Date: Aug. 8, 2002 (54) METHOD AND SYSTEM FOR TISSUE Publication Classification
US 2002O107531A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0107531A1 Schreck et al. (43) Pub. Date: Aug. 8, 2002 (54) METHOD AND SYSTEM FOR TISSUE Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
Sepetka et al. (45) Date of Patent: May 3, ) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson.
 United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003OO69636A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0069636A1 Solem et al. (43) Pub. Date: Apr. 10, 2003 (54) METHOD FOR TREATMENT OF MITRAL INSUFFICIENCY (76)
(19) United States US 2003OO69636A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0069636A1 Solem et al. (43) Pub. Date: Apr. 10, 2003 (54) METHOD FOR TREATMENT OF MITRAL INSUFFICIENCY (76)
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0196770 A1 Klintenstedt et al. US 201701.96770A1 (43) Pub. Date: Jul. 13, 2017 (54) (71) (72) (21) (22) (63) (60) (30) CNTAINER
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0196770 A1 Klintenstedt et al. US 201701.96770A1 (43) Pub. Date: Jul. 13, 2017 (54) (71) (72) (21) (22) (63) (60) (30) CNTAINER
-100 III IIHIIII. United States Patent (19) Fischell et al. 11) Patent Number: 5,492,530 45) Date of Patent: Feb. 20, 1996
 United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
USOO A United States Patent (19) 11 Patent Number: 5,829,589 Nguyen et al. (45) Date of Patent: Nov. 3, 1998
 USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
(12) United States Patent
 US0093.20587B2 (12) United States Patent Milsom et al. (10) Patent No.: (45) Date of Patent: US 9,320,587 B2 Apr. 26, 2016 (54) (75) (73) (*) (21) (22) (86) (87) (65) (63) (60) (51) (52) METHOD AND APPARATUS
US0093.20587B2 (12) United States Patent Milsom et al. (10) Patent No.: (45) Date of Patent: US 9,320,587 B2 Apr. 26, 2016 (54) (75) (73) (*) (21) (22) (86) (87) (65) (63) (60) (51) (52) METHOD AND APPARATUS
(12) United States Patent
 (12) United States Patent Globerman et al. USOO6402777B1 (10) Patent No.: (45) Date of Patent: US 6,402.777 B1 Jun. 11, 2002 (54) RADIOPAQUE STENT MARKERS (75) Inventors: Oren Globerman, Holon; Mordechay
(12) United States Patent Globerman et al. USOO6402777B1 (10) Patent No.: (45) Date of Patent: US 6,402.777 B1 Jun. 11, 2002 (54) RADIOPAQUE STENT MARKERS (75) Inventors: Oren Globerman, Holon; Mordechay
United States Patent (19) 11 Patent Number: 5,582,607 Lackman 45 Date of Patent: Dec. 10, 1996
 US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
2 Brigham and Women s Hospital, Boston, MA.
 Chapter 6: History of Transcatheter Aortic Valve Replacement (TAVR) Bryan Piccirillo, MD 1 ; Pinak B. Shah, MD, FACC 2 1 Brigham and Women s Heart and Vascular Center 2 Brigham and Women s Hospital, Boston,
Chapter 6: History of Transcatheter Aortic Valve Replacement (TAVR) Bryan Piccirillo, MD 1 ; Pinak B. Shah, MD, FACC 2 1 Brigham and Women s Heart and Vascular Center 2 Brigham and Women s Hospital, Boston,
(12) United States Patent
 USOO9498226B2 (12) United States Patent Cage et al. (10) Patent No.: (45) Date of Patent: US 9.498,226 B2 Nov. 22, 2016 (54) EMBOLIC COIL DELIVERY SYSTEM (75) Inventors: Logan Michael Cage, Bloomington,
USOO9498226B2 (12) United States Patent Cage et al. (10) Patent No.: (45) Date of Patent: US 9.498,226 B2 Nov. 22, 2016 (54) EMBOLIC COIL DELIVERY SYSTEM (75) Inventors: Logan Michael Cage, Bloomington,
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
(12) Patent Application Publication (10) Pub. No.: US 2001/ A1
 US 20010018611A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0018611 A1 Solem et al. (43) Pub. Date: Aug. 30, 2001 (54) METHOD AND DEVICE FOR TREATMENT Publication Classification
US 20010018611A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0018611 A1 Solem et al. (43) Pub. Date: Aug. 30, 2001 (54) METHOD AND DEVICE FOR TREATMENT Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0030380 A1 Binmoeller US 20090030.380A1 (43) Pub. Date: Jan. 29, 2009 (54) (75) (73) (21) (22) (86) METHOD AND APPARATUS FOR
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0030380 A1 Binmoeller US 20090030.380A1 (43) Pub. Date: Jan. 29, 2009 (54) (75) (73) (21) (22) (86) METHOD AND APPARATUS FOR
CIPG Transcatheter Aortic Valve Replacement- When Is Less, More?
 CIPG 2013 Transcatheter Aortic Valve Replacement- When Is Less, More? James D. Rossen, M.D. Professor of Medicine and Neurosurgery Director, Cardiac Catheterization Laboratory and Interventional Cardiology
CIPG 2013 Transcatheter Aortic Valve Replacement- When Is Less, More? James D. Rossen, M.D. Professor of Medicine and Neurosurgery Director, Cardiac Catheterization Laboratory and Interventional Cardiology
United States Patent (19) Faxon et al.
 United States Patent (19) Faxon et al. US005464395A 11 Patent Number: 45 Date of Patent: Nov. 7, 1995 54 I76) (21) (22) (51) (52) (58) CATHETER FOR DELVERNG ASSESSINGA BODLY PASSAGEWAY Inventors: David
United States Patent (19) Faxon et al. US005464395A 11 Patent Number: 45 Date of Patent: Nov. 7, 1995 54 I76) (21) (22) (51) (52) (58) CATHETER FOR DELVERNG ASSESSINGA BODLY PASSAGEWAY Inventors: David
(12) United States Patent (10) Patent No.: US 6,413,232 B1
 USOO6413232B1 (12) United States Patent (10) Patent No.: US 6,413,232 B1 Townsend et al. (45) Date of Patent: Jul. 2, 2002 (54) ORTHOPEDIC KNEE BRACE HAVING AN 5,807,294. A 9/1998 Cawley et al. ADJUSTABLE
USOO6413232B1 (12) United States Patent (10) Patent No.: US 6,413,232 B1 Townsend et al. (45) Date of Patent: Jul. 2, 2002 (54) ORTHOPEDIC KNEE BRACE HAVING AN 5,807,294. A 9/1998 Cawley et al. ADJUSTABLE
11 B N \S44. (12) Patent Application Publication (10) Pub. No.: US 2004/ A1. (19) United States SS 12A
 (19) United States US 20040097.979A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0097979 A1 Svanidze et al. (43) Pub. Date: May 20, 2004 (54) AORTIC VALVE IMPLANTATION DEVICE (76) Inventors:
(19) United States US 20040097.979A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0097979 A1 Svanidze et al. (43) Pub. Date: May 20, 2004 (54) AORTIC VALVE IMPLANTATION DEVICE (76) Inventors:
Transcatheter Mitral Valve Replacement How Close Are We?
 Transcatheter Mitral Valve Replacement How Close Are We? Gregory Pavlides, MD, PhD, FACC, FESC Professor of Medicine Miscia Chair of Interventional Cardiology Director, Cardiac Catheterization Laboratories,
Transcatheter Mitral Valve Replacement How Close Are We? Gregory Pavlides, MD, PhD, FACC, FESC Professor of Medicine Miscia Chair of Interventional Cardiology Director, Cardiac Catheterization Laboratories,
(12) United States Patent (10) Patent No.: US 6,234,797 B1
 USOO6234797B1 (12) United States Patent (10) Patent No.: Ura (45) Date of Patent: *May 22, 2001 (54) DENTAL IMPLANT AND METHOD FOR 5,322,443 6/1994 Beaty. INSTALLING THE SAME 5,336,225 8/1994 Zang. 5,338,197
USOO6234797B1 (12) United States Patent (10) Patent No.: Ura (45) Date of Patent: *May 22, 2001 (54) DENTAL IMPLANT AND METHOD FOR 5,322,443 6/1994 Beaty. INSTALLING THE SAME 5,336,225 8/1994 Zang. 5,338,197
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1. Knott et al. (43) Pub. Date: Sep. 6, 2007
 US 20070204455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0204455A1 Knott et al. (43) Pub. Date: (54) METHOD FOR RETAINING A VASCULAR Related U.S. Application Data
US 20070204455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0204455A1 Knott et al. (43) Pub. Date: (54) METHOD FOR RETAINING A VASCULAR Related U.S. Application Data
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
(12) United States Patent (10) Patent No.: US 6,916,338 B2
 USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080283526A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0283526 A1 Buchanan (43) Pub. Date: Nov. 20, 2008 (54) ATTACHABLE PACKAGES FOR THIN, (52) U.S. Cl.... 220/234
(19) United States US 20080283526A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0283526 A1 Buchanan (43) Pub. Date: Nov. 20, 2008 (54) ATTACHABLE PACKAGES FOR THIN, (52) U.S. Cl.... 220/234
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 2007019.8075A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0198075A1 Levy (43) Pub. Date: Aug. 23, 2007 (54) BFURCATED ANEURYSM TREATMENT Publication Classification
(19) United States US 2007019.8075A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0198075A1 Levy (43) Pub. Date: Aug. 23, 2007 (54) BFURCATED ANEURYSM TREATMENT Publication Classification
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
(19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
Biliary Metal Stents MAKING A DIFFERENCE TO HEALTH
 Biliary Metal Stents In a fast paced and maturing market, Diagmed Healthcare s Hanarostent has managed to continue to innovate and add unique and clinically superior features to its already premium range.
Biliary Metal Stents In a fast paced and maturing market, Diagmed Healthcare s Hanarostent has managed to continue to innovate and add unique and clinically superior features to its already premium range.
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
United States Patent (19)
 United States Patent (19) Galloway 11 Patent Number: 45 Date of Patent: 4,738,667 Apr. 19, 1988 W 54 PREFORMED CATHETER ASSEMBLY 76 Inventor: Niall T. M. Galloway, 3211 Massdale Ave., Durham, N.C. 27707
United States Patent (19) Galloway 11 Patent Number: 45 Date of Patent: 4,738,667 Apr. 19, 1988 W 54 PREFORMED CATHETER ASSEMBLY 76 Inventor: Niall T. M. Galloway, 3211 Massdale Ave., Durham, N.C. 27707
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
(19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
SS Fifs..."issisi is needle attached tasying with an opening in theside
 United States Patent (19) 11 USOO54376A Patent Number: Bany's et al. Date of Patent: Jun. 20, 1995 (54) METHOD AND APPARATUS FOR 4,757,826 7/1988 Abdulhay... or co-essessor... 128/757 OBTAINING A BIOPSY
United States Patent (19) 11 USOO54376A Patent Number: Bany's et al. Date of Patent: Jun. 20, 1995 (54) METHOD AND APPARATUS FOR 4,757,826 7/1988 Abdulhay... or co-essessor... 128/757 OBTAINING A BIOPSY
United States Patent Patent Number: 5,582,598 Chanoch 45) Date of Patent: Dec. 10, 1996
 I I US005582598A United States Patent 19 11 Patent Number: Chanoch 45) Date of Patent: Dec. 10, 1996 54 MEDICATIN DELIVERY PEN WITH 5,114,406 5/1992 Gabriel et al.... 604/232 WARIABLE INCREMENT DSE SCALE
I I US005582598A United States Patent 19 11 Patent Number: Chanoch 45) Date of Patent: Dec. 10, 1996 54 MEDICATIN DELIVERY PEN WITH 5,114,406 5/1992 Gabriel et al.... 604/232 WARIABLE INCREMENT DSE SCALE
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
50 3 % % 2. ta. SN & (12) United States Patent US 7, B1. Jun. 10, (45) Date of Patent: (10) Patent No.:
 USOO7384414B1 (1) United States Patent Marshall et al. () Patent No.: (45) Date of Patent: US 7,384.414 B1 Jun., 008 (54) SAFETY PEN NEEDLE WITH (75) (73) (*) (1) () (51) (5) (58) (56) NON-INUECTION END
USOO7384414B1 (1) United States Patent Marshall et al. () Patent No.: (45) Date of Patent: US 7,384.414 B1 Jun., 008 (54) SAFETY PEN NEEDLE WITH (75) (73) (*) (1) () (51) (5) (58) (56) NON-INUECTION END
United States Patent (19) Hensel
 United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0297829 A1 Decker et al. US 20150297829A1 (43) Pub. Date: (54) (71) (72) (21) (22) (62) (60) NECTABLE DRUG DELIVERY ARRANGEMENT
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0297829 A1 Decker et al. US 20150297829A1 (43) Pub. Date: (54) (71) (72) (21) (22) (62) (60) NECTABLE DRUG DELIVERY ARRANGEMENT
Paper No Filed: October 23, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 8 571-272-7822 Filed: October 23, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ABIOMED, INC. and ABIOMED R&D, INC., Petitioner, v.
Trials@uspto.gov Paper No. 8 571-272-7822 Filed: October 23, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ABIOMED, INC. and ABIOMED R&D, INC., Petitioner, v.
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: Gray et al. US 2002O173769A1 (43) Pub. Date: Nov. 21, 2002 (54) INFUSION SET FOR A FLUID PUMP (76) (21) (22) (63) (60) Inventors: Larry
(19) United States (12) Patent Application Publication (10) Pub. No.: Gray et al. US 2002O173769A1 (43) Pub. Date: Nov. 21, 2002 (54) INFUSION SET FOR A FLUID PUMP (76) (21) (22) (63) (60) Inventors: Larry
My Choice For Percutaneous Mitral Valve Replacement. Jose Luis Navia, MD.
 My Choice For Percutaneous Mitral Valve Replacement Jose Luis Navia, MD. Disclosure Edwards Lifescienses St. Jude Medical MAQUET NaviGate Consultant, Investigator Consultant, Investigator Consultant, Investigator
My Choice For Percutaneous Mitral Valve Replacement Jose Luis Navia, MD. Disclosure Edwards Lifescienses St. Jude Medical MAQUET NaviGate Consultant, Investigator Consultant, Investigator Consultant, Investigator
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Patent Number: 5,837,006 Ocel et al. (45) Date of Patent: Nov. 17, 1998
 United States Patent (19) 11 USOO5837006A Patent Number: Ocel et al. (45) Date of Patent: Nov. 17, 1998 54 RETRACTION STOPFOR HELICAL FOREIGN PATENT DOCUMENTS MEDICAL LEAD ELECTRODE 0149431 7/1985 European
United States Patent (19) 11 USOO5837006A Patent Number: Ocel et al. (45) Date of Patent: Nov. 17, 1998 54 RETRACTION STOPFOR HELICAL FOREIGN PATENT DOCUMENTS MEDICAL LEAD ELECTRODE 0149431 7/1985 European
(12) United States Patent (10) Patent No.: US 7.690,305 B2
 US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
Percutaneous Valve Interventions. Percutaneous Valve Interventions
 Percutaneous Valve Interventions Stanton J. Rowe President, Percutaneous Valve Interventions Edwards is Best Positioned to Capitalize on Percutaneous Valve Opportunities #1 global valve replacement and
Percutaneous Valve Interventions Stanton J. Rowe President, Percutaneous Valve Interventions Edwards is Best Positioned to Capitalize on Percutaneous Valve Opportunities #1 global valve replacement and
United States Patent (19) Dobben
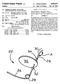 United States Patent (19) Dobben 54 ARTIFICIAL HEART VALVE FOR MPLANTATION IN A BLOOD WESSEL (76 Inventor: Richard L. Dobben, 6355 N. 600 West, Michigan City, Ind. 46360 21 Appl. No.: 342,2 (22 Filed:
United States Patent (19) Dobben 54 ARTIFICIAL HEART VALVE FOR MPLANTATION IN A BLOOD WESSEL (76 Inventor: Richard L. Dobben, 6355 N. 600 West, Michigan City, Ind. 46360 21 Appl. No.: 342,2 (22 Filed:
(12) United States Patent (10) Patent No.: US 7,108,510 B2
 US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 2004O133273A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0133273 A1 Cox (43) Pub. Date: Jul. 8, 2004 (54) APPARATUSES AND METHODS FOR HEART VALVE REPAIR (76) Inventor:
(19) United States US 2004O133273A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0133273 A1 Cox (43) Pub. Date: Jul. 8, 2004 (54) APPARATUSES AND METHODS FOR HEART VALVE REPAIR (76) Inventor:
Vascular complications of embolized core valve
 Vascular complications of embolized core valve Suhail Dohad, MD FACC Director of Endovascular services, Interventional Cardiology Cardiovascular Research Foundation of Southern California, CVMG Director
Vascular complications of embolized core valve Suhail Dohad, MD FACC Director of Endovascular services, Interventional Cardiology Cardiovascular Research Foundation of Southern California, CVMG Director
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150272731A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0272731 A1 Racchini et al. (43) Pub. Date: Oct. 1, 2015 (54) SYSTEMAND METHOD OF STEPPED (52) U.S. Cl. DEPLOYMENT
(19) United States US 20150272731A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0272731 A1 Racchini et al. (43) Pub. Date: Oct. 1, 2015 (54) SYSTEMAND METHOD OF STEPPED (52) U.S. Cl. DEPLOYMENT
United States Patent (19) Annoni
 United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
(19) United States (12) Reissued Patent
 (19) United States (12) Reissued Patent Talaber et al. USOORE430O8E (10) Patent Number: (45) Date of Reissued Patent: Dec. 6, 2011 (54) ORTHOPEDIC IMPLANTASSEMBLY (75) Inventors: David J. Talaber, Livermore,
(19) United States (12) Reissued Patent Talaber et al. USOORE430O8E (10) Patent Number: (45) Date of Reissued Patent: Dec. 6, 2011 (54) ORTHOPEDIC IMPLANTASSEMBLY (75) Inventors: David J. Talaber, Livermore,
Zenith Renu AAA Converter Graft. Device Description Planning and Sizing Deployment Sequence Patient Follow-Up
 Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
United States Patent (19) Bessler et al.
 United States Patent (19) Bessler et al. 54 (75) 73) ARTIFICIAL, HEART WALVE AND METHOD AND DEVICE FOR IMPLANTING THE SAME Inventors: Marc Bessler, Teaneck, N.J.; Timothy A. M. Chuter, Malmö, Sweden Assignee:
United States Patent (19) Bessler et al. 54 (75) 73) ARTIFICIAL, HEART WALVE AND METHOD AND DEVICE FOR IMPLANTING THE SAME Inventors: Marc Bessler, Teaneck, N.J.; Timothy A. M. Chuter, Malmö, Sweden Assignee:
(12) United States Patent (10) Patent No.: US 9,332,914 B2
 USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 20040121286A1 (19) United States (12) Patent Application Publication (10) Pub No: US 2004/0121286 A1 Aravena et al (43) Pub Date: (54) ORGANIC SHAPED INTERFACE FOR Related US Application Data DENTAL
US 20040121286A1 (19) United States (12) Patent Application Publication (10) Pub No: US 2004/0121286 A1 Aravena et al (43) Pub Date: (54) ORGANIC SHAPED INTERFACE FOR Related US Application Data DENTAL
Nit-Occlud. Coil System for PDA Closure IMPLANTATION POCKET GUIDE. Rx only CV / B. Braun Interventional Systems Inc.
 Refer to the Nit-Occlud PDA Instructions for Use for relevant warnings, precautions, complications and contraindications. This device has been designed for single use only. Nit-Occlud Coil System for PDA
Refer to the Nit-Occlud PDA Instructions for Use for relevant warnings, precautions, complications and contraindications. This device has been designed for single use only. Nit-Occlud Coil System for PDA
CP STENT. Large Diameter, Balloon Expandable Stent
 CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
(12) United States Patent (10) Patent No.: US 6,932,771 B2. Whitmore et al. (45) Date of Patent: Aug. 23, 2005
 USOO6932771B2 (12) United States Patent (10) Patent No.: US 6,932,771 B2 Whitmore et al. (45) Date of Patent: Aug. 23, 2005 (54) TISSUE WARMING DEVICE AND METHOD (56) References Cited (75) Inventors: Willet
USOO6932771B2 (12) United States Patent (10) Patent No.: US 6,932,771 B2 Whitmore et al. (45) Date of Patent: Aug. 23, 2005 (54) TISSUE WARMING DEVICE AND METHOD (56) References Cited (75) Inventors: Willet
(51) Int Cl.: A61F 2/24 ( )
 (19) (12) EUROPEAN PATENT SPECIFICATION (11) EP 1 441 672 B1 (45) Date of publication and mention of the grant of the patent: 14.09.2011 Bulletin 2011/37 (21) Application number: 02804403.0 (22) Date of
(19) (12) EUROPEAN PATENT SPECIFICATION (11) EP 1 441 672 B1 (45) Date of publication and mention of the grant of the patent: 14.09.2011 Bulletin 2011/37 (21) Application number: 02804403.0 (22) Date of
United States Patent (1s 3,671,979 Moulopoulos (45) June 27, 1972
 United States Patent (1s Moulopoulos (45) June 27, 1972 (54). CATHETER MOUNTED ARTIFICIAL 2,442,573 6/1948 Stafford... 128/245 X HEART WALVE FOR MPLANTING IN 3,266,487 8/1966 Watkins et al... 128/1 R CLOSE
United States Patent (1s Moulopoulos (45) June 27, 1972 (54). CATHETER MOUNTED ARTIFICIAL 2,442,573 6/1948 Stafford... 128/245 X HEART WALVE FOR MPLANTING IN 3,266,487 8/1966 Watkins et al... 128/1 R CLOSE
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0208302 A1 Webster et al. US 200702083 02A1 (43) Pub. Date: Sep. 6, 2007 (54) DEFLECTION CONTROL CATHETERS, SUPPORT CATHETERS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0208302 A1 Webster et al. US 200702083 02A1 (43) Pub. Date: Sep. 6, 2007 (54) DEFLECTION CONTROL CATHETERS, SUPPORT CATHETERS
(12) United States Patent (10) Patent No.: US 8,137,401 B2
 USOO8137401B2 (12) United States Patent (10) Patent No.: US 8,137,401 B2 Stad et al. (45) Date of Patent: Mar. 20, 2012 (54) INTERVERTEBRAL DEVICE HAVING (56) References Cited EXPANDABLE ENDPLATES U.S.
USOO8137401B2 (12) United States Patent (10) Patent No.: US 8,137,401 B2 Stad et al. (45) Date of Patent: Mar. 20, 2012 (54) INTERVERTEBRAL DEVICE HAVING (56) References Cited EXPANDABLE ENDPLATES U.S.
