(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
|
|
|
- Sarah Kelly
- 5 years ago
- Views:
Transcription
1 (19) United States US 2015O A1 (12) Patent Application Publication (10) Pub. No.: US 2015/ A1 Creaven et al. (43) Pub. Date: Apr. 23, 2015 (54) SYSTEMS, DEVICES AND METHODS FOR TRANSCATHETERVALVE DELVERY (71) Applicant: Medtronic Vascular Galway, Ballybrit (IE) (72) Inventors: Marian Creaven, Ballybrit (IE); Marc Anderson, Ballybrit (IE); Kate Corish, Ballybrit (IE); Declan Costello, Ballybrit (IE); Niall Duffy, Ballybrit (IE); Joshua Dwork, Santa Rosa (CA); John Gallagher, Ballybrit (IE); Patrick Griffin, Ballybrit (IE); Gavin Kenny, Ballybrit (IE): Deirdre McGowan Smyth, Ballybrit (IE); John Milroy, Ballybrit (IE); Jason Quill. Forest Lake, MN (US); Herinaina Rabarimanantsoa Jamous, Ballybrit (IE); Paul Rothstein, Elk River, MN (US); Jeffrey Sandstrom, Scandia, MN (US); Edmond Sheahan, Ballybrit (IE): Frank White, Ballybrit (IE) (21) Appl. No.: 14/519,242 (22) Filed: Oct. 21, 2014 Related U.S. Application Data (60) Provisional application No. 61/893,399, filed on Oct. 21, Publication Classification (51) Int. Cl. A6IF 2/24 ( ) (52) U.S. Cl. CPC... A61F 2/2436 ( ) (57) ABSTRACT A heart Valve therapy system including a delivery device and a stented valve. The delivery device includes an outer sheath, an inner shaft, an optional hub assembly, and a plurality of tethers. In a delivery state, a stent frame of the prosthesis is crimped over the inner shaft and maintained in a compressed condition by the outer sheath. The tethers are connected to the stent frame. In a partial deployment state, the outer sheath is at least partially withdrawn, allowing the stent frame to self expand. Tension in the tethers prevents the stent frame from rapidly expanding and optionally allowing recapture. Upon completion of the stent frame expansion, the tethers are with drawn.
2 Patent Application Publication Apr. 23, 2015 Sheet 1 of 22 US 201S/O A1 F.G. B
3 Patent Application Publication Apr. 23, 2015 Sheet 2 of 22 US 201S/O A1
4 Patent Application Publication Apr. 23, 2015 Sheet 3 of 22 US 201S/O A1
5 Patent Application Publication Apr. 23, 2015 Sheet 4 of 22 US 201S/O A1 R
6 Patent Application Publication Apr. 23, 2015 Sheet 5 of 22 US 201S/O A1 y gs S.ŠS FONx ca G. 48
7 Patent Application Publication Apr. 23, 2015 Sheet 6 of 22 US 201S/O A1 a Sy s
8 Patent Application Publication Apr. 23, 2015 Sheet 7 of 22 US 201S/O A1 58 9) y A. N N F.G. 4E
9 Patent Application Publication Apr. 23, 2015 Sheet 8 of 22 US 201S/O A1
10 Patent Application Publication Apr. 23, 2015 Sheet 9 of 22 US 201S/O A1 F.G. SD
11 Patent Application Publication Apr. 23, 2015 Sheet 10 of 22 US 201S/O A1. s
12 Patent Application Publication Apr. 23, 2015 Sheet 11 of 22 US 201S/O A1
13 Patent Application Publication Apr. 23, 2015 Sheet 12 of 22 US 201S/O A1 2X SSS S3 48 i8 405
14 Patent Application Publication Apr. 23, 2015 Sheet 13 of 22 US 201S/O A1 FG 93
15 Patent Application Publication Apr. 23, 2015 Sheet 14 of 22 US 201S/O A1 F.G. OA
16 Patent Application Publication Apr. 23, 2015 Sheet 15 of 22 US 201S/O A1 FG. OC FG. OD
17 Patent Application Publication Apr. 23, 2015 Sheet 16 of 22 US 201S/O A1 FG. B
18 Patent Application Publication Apr. 23, 2015 Sheet 17 of 22 US 201S/O A1 FG. 2B S. S82 F.G. 12C
19 Patent Application Publication Apr. 23, 2015 Sheet 18 of 22 US 201S/O A1 St. 604-A1V FIG. 13A FG 33
20 Patent Application Publication Apr. 23, 2015 Sheet 19 of 22 US 201S/O A1 y A. SO2 F.G. 4A 694 SA A-1\\ YC YFIG. 14B SS
21 Patent Application Publication Apr. 23, 2015 Sheet 20 of 22 US 201S/O A1 F.G. 5A
22 Patent Application Publication Apr. 23, 2015 Sheet 21 of 22 US 201S/O A1 S s s years
23 Patent Application Publication Apr. 23, 2015 Sheet 22 of 22 US 201S/O A1 F.G. SC FIG. 15D
24 US 2015/ A1 Apr. 23, 2015 SYSTEMS, DEVICES AND METHODS FOR TRANSCATHETERVALVE DELIVERY CROSS REFERENCE TO RELATED APPLICATIONS This Non-Provisional Patent Application claims the benefit of the filing dates of U.S. Provisional Patent Applica tion Ser. No. 61/893,399, filed Oct. 21, 2013, entitled SYS TEMS, DEVICES AND METHODS FORTRANSCATH ETERVALVEDELIVERY the entire teachings of which are incorporated herein by reference. BACKGROUND 0002 The present disclosure relates to delivery devices for implanting transcatheter valves. More particularly, it relates to catheter-based delivery devices and methods for implant ing a prosthetic heart valve with controlled release of the prosthesis from the delivery device Diseased or otherwise deficient heart valves can be repaired or replaced using a variety of different types of heat valve Surgeries. One conventional technique involves an open-heart Surgical approach that is conducted under general anesthesia, during which the heart is stopped and blood flow is controlled by a heart-lung bypass machine More recently, minimally invasive approaches have been developed to facilitate catheter-based implantation of the valve prosthesis on the beating heart, intending to obviate the need for the use of classical sternotomy and cardiopulmo nary bypass. In general terms, an expandable prosthetic valve is compressed about or within a catheter, inserted inside a lumen within the patient, such as the femoral artery, and delivered to a desired location in the heart The heart valve prosthesis employed with catheter based, or transcatheter, procedures generally includes an expandable multi-level frame or stent that supports a valve body having a plurality of leaflets. The frame can be con tracted during percutaneous transluminal delivery, and expanded upon deploymentator within the native valve. One type of valve stent can be initially provided in an expanded or uncrimped condition, then crimped or compressed about a balloon portion of a catheter. The balloon is subsequently inflated to expand and deploy the prosthetic heart valve. With otherstented prosthetic heart valve designs, the stent frame is formed to be self-expanding. With these systems, the valved stent is crimped down to a desired size and held in that compressed state within a sheath for transluminal delivery. Retracting the sheath from this valved stent allows the stent to self-expand to a larger diameter, fixating at the native valve site The actual shape or configuration of any particular transcatheter prosthetic heart valve is dependent, at least to Some extent, upon the valve being replaced or repaired (i.e., mitral valve, tricuspid valve, aortic valve, or pulmonary valve). The stent frame must oftentimes provide and maintain (e.g., elevated hoop strength and resistance to radially com pressive forces) a relatively complex shape in order to achieve desired fixation with the native anatomy. With self-expanding stent designs, the stent frame can experience significant, rapid radial expansion upon deployment from the sheath. Taken in combination, these design features can give rise to delivery concerns. A rapidly expanding stent having one section expanding to a Substantially larger diameter than an adjacent section can cause the prosthetic heart valve to spring off a valve retainer of the delivery device in a relatively un-con trolled fashion. This rapid deployment can, in turn, result in the valve section(s) forcing itself past or beyond the intended anatomical location. For example, exemplary prosthetic mitral valve designs can have an inflow diameter on the order of 60 mm, with the inflow section of the stent frame being perpendicular, or nearly perpendicular, to a shape of the out flow section. During transluminal delivery to the native mitral valve, the stent frame is crimped down to a nearly cylindrical shape, having a diameter on the order of 12 mm. The inflow section of the prosthetic mitral valve is intended to self engage the native annulus, can experience rapid, uncontrolled expansion upon deployment, and may instead thrust past the native annulus and into the left ventricle Although there have been multiple advances in tran scatheter prosthetic heart valves and related delivery systems and techniques, there is a continuing need to provide different delivery tools for controlled deployment of the prosthesis. SUMMARY Some aspects of the present disclosure relate to sys tems for performing a therapeutic procedure on a defective heart valve, and include a delivery device and a stented pros thetic heart valve. The delivery device includes an outer sheath assembly, an inner shaft assembly, and a plurality of tethers. The prosthetic heart valve includes a stent frame that is configured to self-expand from a compressed condition to a normal, expanded condition. In a delivery state of the sys tem, the stent frame is crimped over the inner shaft assembly (for example on to a valve retainer and/or a valve Support), and is constrained in a compressed condition by the outer sheath assembly. Further, the tethers are connected to the stent frame, with at least one end of the tether being routed proximally toward a handle assembly of the delivery device. Arrangement of the stent frame relative to the delivery device defines a proximal portion and a distal portion, with the tethers being connected to the proximal portion or the distal portion. In some embodiments, the tethers are looped about struts or other structures provided by the stent frame. In related embodiments, a free end of each tether is connected to a valve retainer body along the inner shaft assembly; in other embodiments, the both ends of each tether are routed to the handle assembly. In yet other embodiments, a leading end of each tether is directly connected to the stent frame. Regard less, during use in delivering the stented prosthetic heart Valve to a native valve, the system is transitioned to a partial deploy ment state in which the sheath assembly is at least partially retracted from over the stent frame, removing the constrain ing force imparted upon the stent frame. The exposed portion (s) of the stent frame self-expand toward the normal, expanded condition, with a tension in the tethers preventing the corresponding region of the stent frame from rapidly expanding. Upon attaining complete expansion, the delivery device is transitioned to a full deployment state in which the tethers are withdrawn from the stent frame. In some embodi ments, the systems and devices of the present disclosure provide for recapture of an expanded stent frame prior to release of the tethers, for example by re-tensioning the tethers to effectuate at least partial compression or re-collapsing of the corresponding region of the stent frame. The stent frame can then more easily be received within a separate recapture sheath or the delivery sheath.
25 US 2015/ A1 Apr. 23, 2015 BRIEF DESCRIPTION OF THE DRAWINGS 0009 FIG. 1A is a side view of an exemplary stented prosthetic heart valve useful with systems, devices and meth ods of the present disclosure and in a normal, expanded con dition; 0010 FIG.1B is a side view of the prosthetic heart valve of FIG. 1A in a compressed condition; 0011 FIG. 2 is a side view of another exemplary prosthetic heart valve stent useful with systems, devices and methods of the present disclosure and in a normal, expanded condition; 0012 FIG.3A is an exploded perspective view of a stented prosthetic heart valve delivery device in accordance with principles of the present disclosure; 0013 FIG.3B is a side view of the delivery device of FIG. 3A: 0014 FIG. 4A is a simplified, cross-sectional view of a portion of a delivery device in accordance with principles of the present disclosure, loaded with a stented prosthetic heart valve and in a delivery state; 0015 FIGS. 4B-4E illustrate operation of the delivery device of FIG. 4A in transiting to a partial deployment state and a full deployment state; 0016 FIG. 5A is a simplified side view of valve retainer and tether components useful with delivery devices of the present disclosure and in a captured arrangement; 0017 FIG.5B is a side view of the components of FIG.5A and in a released state; 0018 FIG. 5C is a side view of another valve retainer useful with delivery devices of the present disclosure; 0019 FIG. 5D is a side view of another valve retainer useful with delivery devices of the present disclosure; 0020 FIGS. 6A-6C are simplified side views illustrating operation of another embodiment delivery device in accor dance with principles of the present disclosure in conjunction with a prosthetic heart valve stent frame; 0021 FIG. 7A is a side view of a portion of another embodiment prosthetic heart valve stent frame useful with delivery devices of the present disclosure in a compressed condition, along with a tether; 0022 FIG. 7B is a simplified side view of the arrangement of FIG. 23A, with the stent frame in a normal, expanded condition; 0023 FIG. 8A is a perspective view of a portion of another embodiment delivery device in accordance with principles of the present disclosure, along with a portion of prosthetic heart valve stent frame in a compressed condition; 0024 FIG. 8B is a cross-sectional view of a portion of the delivery device of FIG. 8A: 0025 FIG.9A is a perspective view a portion of the assem bly of FIG. 8A in a partial deployment state; 0026 FIG.9B is a perspective view of the arrangement of FIG. 9A and in a Subsequent stage of partial deployment; 0027 FIG. 10A is a perspective view of another embodi ment system in accordance with principles of the present disclosure, including a delivery device loaded with a pros thetic heart valve stent frame and in a delivery state; 0028 FIG. 10B is a perspective view of the system of FIG. 10A in a partial deployment state and immediately prior to full deployment of the stent frame; 0029 FIG. 10C is a perspective view of the prosthetic heart valve of FIG. 10A: 0030 FIG.10D is a perspective view of the system of FIG. 10A and in a delivery state; 0031 FIG. 11A is a perspective view of the system of FIG. 10A and illustrating transitioning of the system toward the arrangement of FIG. 10B; 0032 FIG. 11B is an enlarged, perspective view of a por tion of FIG. 11A: 0033 FIGS. 12A-12C are simplified perspective views of a portion of a control shaft and tether useful with the system of FIG. 11A; and 0034 FIGS. 13A-15D illustrate use of the system of FIG. 11A, including operation of the delivery device in deploying the stent frame to a native mitral valve. DETAILED DESCRIPTION As referred to herein, stented transcatheter pros thetic heart valves useful with and/or as part of the various systems, devices and methods of the present disclosure may assume a wide variety of different configurations, such as a bioprosthetic heart valve having tissue leaflets or a synthetic heart Valve having polymeric, metallic or tissue-engineered leaflets, and can be specifically configured for replacing any of the four valves of the human heart. Thus, the stented prosthetic heart valve useful with the systems, devices, and methods of the present disclosure can be generally used for replacement of a native aortic, mitral, pulmonic or tricuspid valve, or to replace a failed bioprosthesis, such as in the area of an aortic valve or mitral valve, for example In general terms, the stented prosthetic heart valves of the present disclosure include a stent or stent frame having an internal one maintaining a valve structure (tissue or Syn thetic), with the stent frame having a normal, expanded con dition or arrangement and collapsible to a compressed con dition or arrangement for loading within a delivery device. The stent frame is normally constructed to self-deploy or self-expand when release from the delivery device. For example, the Stents or stent frames are Support structures that comprise a number of struts or wire segments arranged rela tive to each other to provide a desired compressibility and strength to the prosthetic heart valve. The struts or wire seg ments are arranged such that they are capable of self-transi tioning from a compressed or collapsed condition to a normal, radially expanded condition. The struts or wire segments can be formed from a shape memory material. Such as a nickel titanium alloy (e.g., NitinolTM). The stent frame can be laser cut from a single piece of material, or can be assembled from a number of discrete components With the above understanding in mind, one simpli fied, non-limiting example of a stented prosthetic heart Valve 30 useful with systems, devices and methods of the present disclosure is illustrated in FIG. 1A. As a point of reference, the prosthetic heart valve 30 is shown in a normal or expanded condition in the view of FIG. 1A, FIG. 1B illustrates the prosthetic heart valve in a compressed condition (e.g., when compressively retained within an outer catheter or sheath as described below). The prosthetic heart valve 30 includes a stent or stent frame 32 and a valve structure 34. The stent frame 32 can assume any of the forms mentioned above, and is generally constructed so as to be self-expandable from the compressed condition (FIG. 1B) to the normal, expanded condition (FIG. 1A) The valve structure 34 can assume a variety of forms, and can be formed, for example, from one or more biocompatible synthetic materials, synthetic polymers, autograft tissue, homograft tissue, Xenograft tissue, or one or more other Suitable materials. In some embodiments, the
26 US 2015/ A1 Apr. 23, 2015 valve structure 34 can be formed, for example, from bovine, porcine, equine, Ovine and/or other Suitable animal tissues. In some embodiments, the valve structure 34 can be formed, for example, from heart Valve tissue, pericardium, and/or other suitable tissue. In some embodiments, the valve structure 34 can include or form one or more leaflets 36. For example, the valve structure 34 can be in the form of a tri-leaflet bovine pericardium valve, a bi-leaflet valve, or another suitable valve. In some constructions, the valve structure 34 can com prise two or three leaflets that are fastened together at enlarged lateral end regions to form commissural joints, with the unattached edges forming coaptation edges of the valve structure 34. The leaflets 36 can be fastened to a skirt that in turn is attached to the frame 32. The upper ends of the com missure points can define an inflow portion 38 corresponding to a first or inflow end 40 of the prosthesis 30. The opposite end of the valve can define an outflow portion 42 correspond ing to a second or outflow end 44 of the prosthesis 30. As shown, the stent frame 32 can have a lattice or cell-like struc ture, and forms or provides crowns 46 and/or eyelets 48 (or other shapes) at the outflow and inflow ends 40, With the but one acceptable construction of FIGS. 1A and 1B, the prosthetic heart valve 30 can be configured (e.g., sized and shaped) for replacing or repairing an aortic valve. Alternatively, other shapes are also envisioned, adapted to mimic the specific anatomy of the valve to be repaired (e.g., stented prosthetic heart valves useful with the present disclo Sure can alternatively be shaped and/or sized for replacing a native mitral, pulmonic ortricuspid valve). For example, FIG. 2 illustrates another non-limiting example of a stent frame 50 portion of another prosthetic heart valve with which the sys tems, devices and methods of the present disclosure are use ful. In the normal or expanded condition of FIG. 2, the stent frame 50 can be sized and shaped for mitral valve implanta tion. Though not shown, the valve structure attached to the stent frame 50 defines an outflow portion 52 arranged at a first or outflow end 54, and an inflow portion 56 arranged at a second or inflow end 58. As compared to the stent frame 32 of FIG. 1A, the inflow portion 56 can exhibit a more pronounced change in shape relative to the corresponding outflow portion 52. Regardless, the stent frame 50 can be forced and con strained to a compressed condition (not shown, butakinto the shape of FIG. 1A) during delivery, and will self-expand to the natural condition of FIG. 2 upon removal of the constraining force(s). As a point of reference, in some constructions, the stent frame 50 is configured to be crimped to a diameter on the order of 12 mm during delivery, and will self-expand to the natural, expanded condition that includes the inflow portion 56 having a diameter on the order of 60 mm. As reflected in FIG. 2, crowns 60 and/or eyelets 62 (or other shapes) can be formed at one or both of the outflow and inflow ends 54, 58. Further, the stent frame 50 can optionally include or carry additional structural components, such as Support arm(s) With the above understanding of the stented pros thetic heart valves in mind, one embodiment of a delivery device 70 for percutaneously delivering the prosthesis is shown in simplified form in FIGS. 3A and 3B. The delivery device 70 includes a delivery sheath assembly 72, an inner shaft assembly 74, a hub assembly 76, one or more tethers 78, and a handle assembly 80. Details on the various components are provided below. In general terms, however, the delivery device 70 combines with a stented prosthetic heart valve (not shown) to form a system for performing a therapeutic proce dure on a defective heart valve of a patient. The delivery device 70 provides a loaded or delivery state in which a stented prosthetic heart valve is coupled to the inner shaft assembly 74 via the hub assembly 76 and compressively retained within a capsule 82 of the delivery sheath assembly 72. For example, the hub assembly 76 can include or provide one or both of a valve support 83 and a valve retainer 84. The valve retainer 84 is configured to selectively receive a corre sponding feature (e.g., posts) provided with the prosthetic heart valve stent frame, whereas the valve support 83 provides an increased diameter (as compared to a diameter of the inner shaft assembly 74) for directly supporting a portion of a length of the stent frame in the compressed condition. The valve support 83 and the valve retainer 84 can be formed as separate components, or can be integrally formed. In yet other embodiments, the hub assembly 76 does not include the valve support 83, or does not include the valve retainer 84. As used throughout this disclosure, then, a "hub assembly compo nent' is in reference to a valve Support, a valve retainer, or both, and the corresponding delivery device need only include one of the valve support or the valve retainer. The tether(s) 78 connect an end of the stented prosthetic heart valve to a remainder of the delivery device 70, for example to the hub assembly 76. The delivery sheath assembly 72 can be manipulated to withdraw the capsule 82 proximally from over the prosthetic heart valve via operation of the handle assem bly 80, permitting the prosthesis to self-expand and partially release from the inner shaft assembly 74 in a partial deploy ment state. In the partial deployment state, the tether(s) 78 maintain connection between the prosthesis and the delivery device 70 (e.g., connection with the hub assembly 76) such that expansion of the corresponding portion or end of the stented prosthetic heart valve is controlled and/or is less than complete in some embodiments. In other embodiments, the tether(s) 78 slowly self-releases from the stent frame as the stent frame expands in a manner that reduces the rate at which expansion occurs (e.g., the tethers 78 effectuate slow release of the stent frame from the delivery device 70). With configu rations in which the tether(s) 78 remains connected to the stent frame upon retraction of the capsule 82, the tether(s) 78 can optionally be subjected to increased tension, causing the corresponding portion of the stent frame to at least partially re-collapse or compress, making recapture of the stent frame possible (e.g., recapture within a separate recapture sheath (not shown) advanced over the delivery sheath assembly 72, or back within the capsule 82). In a deployment state, the tether(s) 78 is removed from engagement with the prosthesis, permitting the stented prosthetic heart valve to completely release or deploy from the delivery device Various features of the components reflected in FIGS. 3A and 3B and as described below can be modified or replaced with differing structures and/or mechanisms. Thus, the present disclosure is in no way limited to the deliv ery sheath assembly 72, the inner shaft assembly 74, the hub assembly 76 or the handle assembly 80 as shown. Any con struction that generally facilitates compressed loading of a stented prosthetic heart valve over an inner shaft via a retract able outer sheath or capsule is acceptable. For example, the capsule 82 may be a discrete component of the delivery sheath assembly 72, or can be more homogeneously formed as part of a continuous outer sheath. The inner shaft assembly 74 can integrally form the hub assembly 76 (including one or both of the valve support 83 and the valve retainer 84) and can terminate in a dilator tip 86. Further, the tether(s) 78 can also assume a wide variety of forms and arrangements relative to
27 US 2015/ A1 Apr. 23, 2015 a remainder of the delivery device 70 as described below. The tether(s) 78 can be a suture, thread, thin wire, or other elon gated, flexible body. Finally, the delivery device 70 can include additional components or features, such as a flush port assembly 88, a recapture sheath (not shown), etc In more general terms, a simplified representation of one embodiment of the delivery device 70 in the delivery state and loaded with a stented prosthetic heart valve 90 (refer enced generally) to provide a system 92 for performing a therapeutic procedure on a defective heart valve is provided in FIG. 4A. For ease of illustration, only the stent frame 50 of the prosthesis 90 is depicted in FIG. 4A. The stent frame 50 is crimped over the inner shaft assembly 74, and is compress ibly held in the compressed condition by the capsule 82. The prosthesis 90 is arranged such that the inflow end 58 is proxi mal the outflow end 54. As loaded to the delivery device 70, then, the inflow end 58 can be viewed as the proximal end of the prosthesis 90, and the outflow end 54 as the distal end. In other embodiments, an orientation of the prosthetic heart valve 90 can be reversed relative to the delivery device 70. One or more of the tethers 78 are connected to the proximal end 58. For example, in the view of FIG. 4A, two of the tethers 78 are provided, it being understood that in other embodi ments, only a single tether 78 or more than two of the tethers 78 is included. Each of the tethers 78 defines a leading end 94 opposite a trailing end (not shown). A leading segment 96 is defined immediately adjacent the leading end 94. With these designations in mind, the leading segment 96 is looped through a portion of the stent frame 50 at or adjacent the proximal end 58, for example through one of the crowns 60 or eyelets 62 (FIG.2). The leading end 94 is connected to the hub assembly 76 (e.g., at a valve retainer such as the valve retainer 84 (FIG. 3A)), with the capsule 82 (or other portion of the delivery sheath assembly 72) serving to capture the leading end 94 to the hub assembly 76. The trailing end of each of the tethers 78 can be positioned in various locations, and in some embodiments is routed proximally to the handle assembly 80 (FIGS. 3A and 3B). In this regard, the hub assembly 76 can form a corresponding number of passageways through which the tethers 78 extend, respectively Following transluminal delivery of the compressed prosthesis 90 to the targeted native valve (via the delivery device 70 in the delivery state of FIG.4A), the delivery device 70 is operated to deploy the stented prosthetic heart valve 90 by proximally retracting the capsule 82. FIG. 4B illustrates an initial stage of deployment in which the capsule 82 has been partially retracted from over the prosthesis 90. As shown, a portion of the prosthesis 90 is still within the capsule 82 (e.g., a distal end 100 of the capsule 82 is distal the proximal end 58 of the stented prosthetic heart valve 90). The now-exposed segment of the prosthesis 90 distal the capsule 82 self-ex pands to or toward the normal or expanded condition. That portion of the prosthesis 90 still within the confines of the capsule 82 remains in the compressed condition and is thus still captured or robustly connected to the delivery device 70. Further, the capsule 82 maintains the captured arrangement of the tethers 78 with the hub assembly 76, and thus with the Stent frame Proximal retraction of the capsule 82 continues. In the partial deployment state of FIG. 4C, the distal end 100 of the capsule 82 is now proximal to the proximal end 58 of the stent frame 50, but is distal the location of connection between the leading end 94 of each of the tethers 78 with the hub assembly 76. Thus, the tethers 78 maintain the connec tion of the prosthesis 90 with the delivery device 70. In this regard, the tethers 78 are held intension (e.g., the trailing end (not shown) of each of the tethers 78 is located at the handle assembly 80 (FIGS. 3A and 3B), and coupled to a correspond ing component(s) provided therewith). The tensioned tethers 78 serve to control expansion of the proximal end 58. More particularly, were the tethers 78 not present, the stent frame 50, and in particular the proximal end 58, would freely self expand to the normal, expanded condition shown in FIG. 2. The tensioned tethers 78 prevent this self-expansion from rapidly occurring. Instead, tension in the tethers 78 is slowly released, allowing the proximal end 58 to more slowly tran sition toward the normal, expanded condition, as generally reflected in FIG. 4D. As described below, the handle assembly 80 optionally includes one or more mechanisms that allow a user to control tension in the tethers Once tension in the tethers 78 has been sufficiently lessened to permit the proximal end 58 to self-expand to the normal, expanded condition, the capsule 82 is further proxi mally retracted, locating the distal end 100 proximal the leading end 94 of each of the tethers 78. As shown in FIG. 4E, the tethers 78 are now no longer captured relative to the hub assembly 76, and can be removed from engagement with the stent frame 50 by, for example, proximally withdrawing the tethers 78 through the delivery device 70. Alternatively, the delivery device 70 can be configured to effectuate release of the tethers 78 from the hub assembly 76 apart from movement of the capsule 82 as described below. Regardless, with embodiments in which the tethers 78 remain robustly con nected with the stent frame 50, as the stent frame 50 is allowed to expand (e.g., the looped connection described above) at any point prior to release of the leading end 94, the tethers 78 can be manipulated to perform a recapture procedure. For example, tension in the tethers 78 can be increased, causing the proximal end 58 to re-collapse or compress (e.g., transi tion from the expanded condition of FIG. 4D to or toward the compressed condition of FIG. 4C). Once the proximal end 58 is re-collapsed, an entirety of the stent frame 50 can more easily be compressed and recaptured relative to the delivery device 70, for example within a separate recapture sheath (not shown) slidably advanced over the capsule 82 (e.g., the deliv ery device 70 can be retracted relative to the recapture sheath to bring the stent frame 50 within the recapture sheath or the recapture sheath can be distally advanced over the stent frame 50) or by advancing the capsule 82 over the stent frame 50. Regardless, in the full deployment state of FIG. 4E, the stented prosthetic heart valve 90 is fully released from the delivery device The hub assembly component(s) and/or the tethers 78 can assume a variety of forms that facilitate temporary coupling there between pursuant to the above descriptions. For example, FIG. 5A illustrates one embodiment of a tether 120 and a valve retainer 122 (that can be provided with the hub assembly 76 of FIG.3A) useful with delivery devices of the present disclosure. The tether 120 forms or provides a ball 124 at a leading end 126thereof. The valve retainer 122 forms or defines a retention hole 128 and a guide slot 130 extending from the hole 128 to a distal end 132 of the valve retainer 122. The ball 124 can be generated in a variety of manners. For example, where the tether 120 is a conventional suture, the ball 124 can be a knot formed in the suture, or can be formed by melting the leading end 126. In other embodiments, the ball 124 is a separately-formed body that is attached (e.g., adhesive, weld, etc.) to the tether 120. Regardless, the reten
28 US 2015/ A1 Apr. 23, 2015 tion hole 128 is sized to selectively receive the ball 124, and the guide slot 130 is sized to accommodate a thickness of the tether 120. As a point of reference, with embodiments includ ing two or more of the tethers 120, the valve retainer 122 will form a corresponding number of the capture retention holes 128/guide slots In the assembled arrangement of FIG. 5A, the cap sule 82 secures the ball 124 within the retention hole 128. With proximal retraction of the capsule 82 to the arrangement of FIG. 5B, the distal end 100 of the capsule 82 is moved proximal the ball 124, allowing the tether 120 to release from the valve retainer 122 as described above In some embodiments, the valve retainer 122 can incorporate various features that assist in loading the prosthe sis (not shown) to the delivery device, and in particular con necting the tether(s) 120 with the valve retainer 122. As a point of reference, where the particular delivery device incor porates a plurality of the tethers 120, it can be difficult to loop the tethers 120 through the stent frame and then hold all of the tethers 120 in place relative to the valve retainer 122 while simultaneously locating the assembly within the capsule 82. With this in mind, in some embodiments, the guide slot 130 is optionally provided and is formed to a width approximating a diameter of the tether 120. With this construction, the tether 120 will befrictionally held within the corresponding slot 130 during loading. Notably, however, the frictional force or inter face between the tether 120 and the valve retainer 122 at the slot 130 is significantly less than the expected radially-out ward force applied onto the tether 120 by the stent frame (not shown) in self-expanding from the compressed condition to the normal, expanded condition. Thus, the tether 120 will readily disengage from the slot 130 during deployment FIG. 5C illustrates another embodiment valve retainer 122A useful with delivery devices of the present disclosure, and is akin to the valve retainer 122 (FIG. 5A) described above. As shown, the valve retainer 122A forms a plurality of the retention holes 128a-128c for retaining a corresponding number of the tethers (not shown). The reten tion holes 128a-128c are offset from one another in a spiral configuration. With this construction, loading of the prosthe sis (not shown) can include looping a first tether (not shown) through the stent frame and locating the corresponding lead ing end within the first retention hole 128a. The capsule (not shown) is then distally advanced over the first retention hole 128a to capture the first tether. However, the remaining reten tion holes 128b. 128c remain uncovered. The process is repeated to sequentially secure second and third tethers (not shown) to the second and third retention holes 128b, 128c FIG. 5D illustrates another embodiment valve retainer 122B useful with delivery devices of the present disclosure, and is akin to the valve retainer 122 (FIG. 5A) described above. As shown, the valve retainer 122B forms or defines a plurality of enlarged retention holes 140 (one of which is shown in FIG. 5D). As compared to dimensions of the retentionhole 128 of FIG.5A, the enlarged retentionholes 140 have an elevated length, but are sized (width) to house the tether leading end (not show, but for example the ball 124 of FIG. 5A). During loading, the capsule (not shown) is advanced partially over the valve retainer 122B so as to cover a proximal segment of each the retention holes 140. A small gap remains between the capsule and the distal end of the each of the retention holes. The tethers (not shown) are then looped through the stent frame (not shown), and the corresponding leading end forced or pushed through the gap and into the covered, proximal segment of the corresponding retention hole The valve retainers or other hub assembly compo nents of the present disclosure can incorporate other features conducive to selectively retaining a leading end of the tether (s). In other embodiments of the present disclosure, the deliv ery device can include one or more features that promote release of the tether leading end from the hub assembly While several of embodiments of the present disclo Sure couple a leading end of the tether(s) to the retainer, in other constructions the tether(s) is not directly connected to the retainer. For example, FIGS. 6A-6C illustrate, in simpli fied form, portions of another delivery device 200 in accor dance with principles of the present disclosure and including a delivery sheath assembly 202 and a plurality of tethers 204. The delivery device 200 is shown relative to a prosthetic heart valve 206 having a stent frame 208, with the delivery device 200 and the prosthesis 206 combining to provide a system 210 in accordance with principles of the present disclosure. In the delivery state of FIG. 6A, each of the tethers 204 is looped through a proximal portion 212 of the stent frame 208 such that the tethers 204 can be viewed as defining opposing, first and second tether segments 214, 216. The tether segments 214, 216 are routed proximally through the delivery sheath assembly 202, for example through passageways in an inner shaft assembly (not shown), to a handle assembly (not shown) of the delivery device In the partial deployment state of FIG. 6B, the deliv ery sheath assembly 202 has been proximally retracted from over the prosthetic heart valve 206, allowing regions of the stent frame 208 to self-expand toward the natural, expanded condition. The tethers 204 remain connected to the stent frame 208 and are under tension, thus impeding rapid, com plete expansion of the proximal portion 212. As tension in the tethers 204 is released, the proximal portion 212 is allowed to self-expand toward the normal condition in a controlled fash ion, as represented by FIG. 6C. Once the stent frame 208 has completely expanded, the tethers 204 can be removed, for example by pulling on either the first or second tether segment 214, 216 of each of the tethers 204. At any point prior to release of the tethers 204 from the stent frame 208, tension in the tethers 204 can be increased to at least partially re-col lapse the proximal portion 212 as part of an optional recapture operation While some embodiments described above gener ally entail looped-type connection of the tether(s) relative to the corresponding prosthetic heart Valve stent frame, in other constructions, the tether can be more robustly connected to, or terminate at, the stent frame. Self-releasing, temporary engagement between the tether leading end and the stent frame can be provided in a variety of manners. One or more tethers can be temporarily connected to the corresponding prosthetic heart valve stent frame in a looped, twisted or wrapped manner. Further, the Stent frame can incorporate one or more additional features that better ensure complete unwinding of the tether upon full deployment of the stent frame. For example, portions of another system 300 in accor dance with principles of the present disclosure are shown in simplified form in FIGS. 7A and 7B. The system300 includes a prosthetic heart valve stent frame 310 useful with delivery devices of the present disclosure. The stent frame 310 includes a conventional cell structure 312 defined, at least in part, by opposing, first and second strut segments 314,316. A
29 US 2015/ A1 Apr. 23, 2015 first barb 318 extends from the first strut segment 314, and a second barb 320 extends from the second strut segment 316. The barbs 318, 320 are sized and shaped such that in the compressed condition of the stent frame 310 reflected by FIG. 7A, the barbs 318, 320 cross over one another to generate a capture Zone 322. In the compressed condition of FIG. 7A, the capture Zone322 is closed, completely bounded, at least in the proximal direction, by the barbs 318,320. The barbs 318, 320 are further configured such that in the normal, expanded condition of the stent frame 310, the capture Zone 322 is open as shown in FIG. 7B With the above construction, with the stent frame 310 in the compressed condition, a tether 324 can be wound or twisted about the stent frame 310 and temporarily secured thereto via the closed capture Zone 322. This connection is shown in FIG. 7A. As the tether 324 allows the stent frame 310 to slowly self-expand toward the normal, expanded con dition as described above, the barbs 318,320 spatially move relative to one another to open the capture Zone 322. As shown in FIG. 7B, then, upon full deployment of the stent frame 310, the tether 324 is released from the capture Zone 322 and freely unwinds from the stent frame Connection between the tether(s), prosthetic heart valve stent frame, and other components of the delivery device can assume a variety forms in accordance with the present disclosure. For example, FIG. 8A illustrates a portion of another system 390, including another embodiment deliv ery device 400 and a prosthetic heart valve stent frame 402. The delivery device 400 includes a delivery sheath assembly 404, an inner shaft assembly 405 (primarily hidden in FIG. 8A, but shown in FIGS. 8B and 9A), a hub assembly includ ing a valve support 406 and a valve retainer 408, and a plu rality of tethers 410. The valve retainer 408 is attached to, or formed by, the inner shaft assembly 405, and forms a plurality of posts 412 (one of which is visible in FIG. 8A). A groove 414 is defined about each of the posts 412, and is sized to permit winding of a corresponding one of the tethers 410 around the post 412. In the delivery state generally reflected by FIG. 8A, each of the tethers 410 extends from the corre sponding post 412 and is looped through the stent frame 402. For example, relative to the tether 410 identified in FIG. 8A, the tether 410 is looped through or around two crowns 416 formed at a proximal portion 418 of the stent frame 402. FIG. 8B is a simplified cross-sectional view of a portion of the delivery device 400, and reflects that the tether 410 is looped about the corresponding post 412, effectively defining first and second tether segments 410a, 410b extending from the post 412. The tether segments 410a, 410b are connected to the stent frame 402 (FIG. 8A) and then routed proximally to and optionally through at least the valve retainer 408. The tether segments 410a, 410b can extend to a handle assembly (not shown) of the delivery device 400 or can be connected to another component of the delivery device 400 adapted to facilitate user control over a tension in the tether With the above construction in mind and returning to FIG. 8A, the delivery sheath assembly 404 serves to retain the tethers 410 relative to the corresponding post 412. As a point of reference, FIG. 8A illustrates a distal end 420 of the delivery sheath assembly 404 as being proximal the posts 412 for ease of illustration. In the delivery state of the delivery device 400, however, the distal end 420 is distal the posts 412 So as to maintain engagement of the tethers 410 with the posts 412. Further, as with previous embodiments, in the delivery state the distal end 420 is located distal the stent frame 402 to constrain the stent frame 402 to the compressed condition FIG.9A depicts the delivery device 400 in the initial stages of a partial deployment state. The distal end 420 of the delivery sheathassembly 404 is proximala significant portion of the stent frame 402, such that stent frame 402 self-expands toward the normal, expanded condition. However, the distal end 420 remains distal each of the tether 410/post 412 inter faces such that each of the tethers 410 remains connected to the corresponding stent frame 402. Tension in the tethers 410 resists rapid expansion of the proximal portion 418. As reflected by FIG. 9B, the tethers 410 allow the proximal portion 418 to slowly attain the normal, expanded condition. The tethers 410 remain connected to the corresponding posts 412 within the delivery sheath assembly 404. Under circum stances where a user desires to recapture the stent frame 402 for re-deployment at another anatomical location or removal from the patient, tension in the tethers 410 can be increased to cause the proximal portion 418 to at least partially re-collapse or compress back toward the compressed condition. This action, in turn, facilitates recapture of an entirety of the stent frame 402 within a separate recapture sheath (not shown), for example by advancing the recapture sheath over the delivery sheath assembly 404; the delivery system 390 can then be retracted to bring the stent frame 402 into the recapture sheath, or the recapture sheath can be advanced over the partially collapsed stent frame 402. In other recapture opera tions, the partially collapsed stent frame 402 can be reinserted back within the delivery sheath assembly While several of the above embodiments connect the tether(s) at or adjacent a proximal portion of the corre sponding prosthetic heart valve stent frame, in other construc tions, a more distal connection can be provided. For example, portions of another embodiment system 500 for performing a therapeutic procedure on a patient s heart are shown in FIGS. 10A-10B. The system 500 includes a delivery device 502 and a prosthetic heart valve 504 (referenced generally). As a point of reference, only a stent frame assembly 506 of the prosthetic heart valve 504 is illustrated in several of the views. FIG. 10A reflects the stent frame assembly 506 loaded to the delivery device 502 and is indicative of a delivery state. FIG. 10B depicts the stent frame assembly 506 partially deployed from the delivery device 502 (and alternatively can be viewed as an initial stage of loading the stent frame assembly 506 to the delivery device 502) The delivery device 502 includes an outer sheath assembly 510, an inner shaft assembly 512, and a plurality of tethers 514. The delivery sheath assembly 510 includes or forms a capsule 516 terminating at a distal end 518. As with previous embodiments, the delivery sheath assembly 510 is coaxially received over the inner shaft assembly 512, and is longitudinally slidable relative to the inner shaft assembly S A identified in FIG. 10B, the inner shaft assembly 512 includes a primary shaft 530 and carries a valve retainer (or other hub assembly component) 532. The primary shaft 530 forms or defines a plurality of side lumens 534 (refer enced generally) sized to receive respective ones of the tethers 514. An optional central guide lumen 536 (referenced gener ally) is also provided, and through which an optional second ary shaft 538 is disposed. The secondary shaft 538 extends distally from the primary shaft 530, and is attached to or forms
30 US 2015/ A1 Apr. 23, 2015 a dilator tip 540. In some embodiments, the dilator tip 540 and the secondary shaft 538 define a common guidewire lumen The valve retainer 532 can assume a variety of forms, and is attached to the primary shaft 530. In general terms, the valve retainer 532 incorporates one or more fea tures commensurate with components of stent frame assem bly 506 that facilitate mounting of the stent frame assembly 506 to the inner shaft assembly 512. For example, and as best shown in FIG. 10B, the valve retainer 532 can form one or more slots 544 sized and shaped to receive a corresponding component of the stent frame assembly The plurality of tethers 514 can assume any of the forms described above (e.g., threads, Sutures, thin wires, etc.), and are slidably disposed within respective ones of the side lumens 534. In some embodiments, the primary shaft 530 routes each of the tethers 514 proximally to a handle assembly (not shown) provided with the delivery device 502, with the handle assembly, in turn, including one or more mechanisms configured to provide user control over ends of each of the tethers 514 and/or tension within the tethers With additional reference to FIG. 10C, the stent frame assembly 506 can assume a variety of forms and some embodiments includes a stent frame 550 and a plurality of support arms 552. The stent frame 550 carries a valve struc ture 553 provided with the prosthetic heart valve 504, and is configured to self-expand from a compressed condition (Such as the compressed condition of FIG. 10A) to the normal, expanded condition (of FIG. 10C). The stent frame 550 fur ther defines various features that promote connection with the delivery device 502 and/or the valve structure 553, as well as desired interface with native anatomy of the heart valve being treated. For example, the stent frame 550 can form or define posts 554 and crowns 556. Relative to an orientation of the stent frame assembly 506 upon mounting to the delivery device 502, the posts 554 are located at a proximal end 558 of the stent frame 550, whereas the crowns 556 are at a distalend 560. The crowns 556 can have various formats, and in some embodiments areakinto an eyelet that defines an aperture 562 (identified for one of the crowns 556 in FIGS. 10A and 10B) The support arms 552 are optionally provided with the stent frame assembly 506, extending outwardly from the stent frame 550. The support arms 552 can each have gener ally curved shape shown, and are configured to interface with structures of the native valve anatomy, Such as the native leaflets Assembly of the system 500 includes locating the stent frame assembly 506 over the inner shaft assembly 512, and then connecting each of the tethers 514 to the distal end 560 of the stent frame 550. For example, each of the tethers 514 is looped through a respective one of the crowns 556 (via the corresponding aperture 562). The tethers 514 are routed through corresponding ones of the side lumens 534 proxi mally to the handle assembly (not shown). The stent frame assembly 506 is crimped or compressed onto the inner shaft assembly 512. For example, the posts 554 can be located within respective ones of the slots 544 provided with the valve retainer 532. In the compressed condition, the stent frame assembly 506 is loaded within the capsule 516 as generally reflected in FIG. 10A and fully shown in FIG. 10D. Any slack in the tethers 514 is removed, and the tethers 514 are then locked. In this held arrangement, the tethers 514 prevent or impede radial self-expansion of the distal end During use, the prosthetic heart valve 504 can be deployed from the delivery device 502 in a progressive fash ion. For example, FIG. 11A illustrates an initial stage of deployment. The capsule 516 has been proximally retracted relative to the stent frame assembly 506, locating the distal end 518 proximal the support arms 552. As shown, once removed from the confines of capsule 516, the support arms 552 self-expand to or toward the normal, expanded condition. While a substantial portion of the stent frame 550 is also now distal the capsule 516 and thus free of the constraints pre sented by the capsule 516, the tethers 514 prevent or impede self-expansion of the stent frame 550, at least at the distal portion 560. As shown in greater detail in FIG. 11B, the tethers 514 remain connected to the stent frame 550 at the crowns 556. Because the tethers 514 are locked, the tethers 514 are placed in tension by the stent frame 550, and prevent overt radial expansion of the distal portion 560. While regions of the stent frame 550 proximal the distal portion 560 may experience some minor expansion, the stent frame 550 is essentially maintained in the compressed condition When desired, tension in the tethers 514 can be progressively lessened, thereby permitting the distal portion 560 to self-expand. In some embodiments, the tethers 514 can be removed by simply pulling on one end of each of the tethers 514 (while the opposite end is unlocked). In other embodiments, the delivery device 502 can incorporate fea tures that promote a more rapid release of the tethers 514. For example, FIG. 12A illustrates a portion of an alternative embodiment primary shaft 570 useful with the delivery device 502 (FIG. 10A) described above, along with one tether 572. The primary shaft 570 forms a plurality of side lumens 574 along with a central guide lumen 576. The tether 572 can have a more rigid instruction (e.g., a thin metal wire), and is arranged relative to a distal side 578 of the shaft 570 to define a looped end 580. Commensurate with the above descrip tions, the looped end 580 is connected to a corresponding feature of the prosthetic heart valve stent frame (not shown), such as the crowns 556 (FIG. 10A). Regardless, first and second segments 582,584 are defined as extensions from the looped end 580, and are routed through respective ones of the side lumens (labeled as 574a, 574b in FIG. 12A) The looped end 580 of the tether 572 can be advanced in the distal direction, for example, to release ten sion developed in the tether 572. In the view of FIG. 12B, the looped end 580 (referenced generally) has been distally advanced from the primary shaft 570. FIG. 12B further reflects that the tether 572 forms a joint 586 along the first segment 582. The joint 586 effectively divides the first seg ment 582 into a proximal region 588 and a distal region 590. The proximal and distal regions 588,590 are connected to one another at the joint 586, with a configuration of the joint 586 being such that when located within the side lumen 574a, the proximal and distal regions 588, 590 cannot separate from one another even in the presence of significant tension along the first segment 582 (i.e., so long as the joint 586 is within the primary shaft 570, the proximal and distal regions 588, 590 remain robustly connected to one another). However, once the joint 586 is distally located beyond the primary shaft 570, the distal region 590 separates from the proximal region 588 as shown in FIG. 12C. Once separated, the tether 572 can completely withdraw from the prosthetic heart valve stent frame (not shown) by proximally retracting the second seg
31 US 2015/ A1 Apr. 23, 2015 ment 584 (it being recalled that the distal region 590 is con nected to the second segment 584 at the looped end 580 (referenced generally)) Returning to FIG. 10A, the system 500 can be con figured to repair any heart valve, and in some embodiments is useful with the aortic valve. With this in mind, FIG. 13A provides a simplified representation of an aortic valve 600 taken from the vantage point of the left ventricle. An ascend ing portion 602 of the aorta is also identified. The aortic valve 600 generally includes three leaflets 604. The system 500 (referenced generally) is delivered through the ascending aorta 602 in various manners, such as a transfemoral delivery approach. A guide wire 606 can be employed to track the delivery device 502 to the aortic valve 600. The dilator tip 540 is positioned slightly beyond the valve 600 as shown. The location of the delivery device 502 relative to the aortic valve 600 of FIG. 13A is further represented from the vantage point of the ascending aorta 602 in FIG. 13B. At this initial stage of the procedure, the capsule 516 remains completely over the prosthetic heart valve (hidden in the view of FIG. 13B), maintaining the stent frame assembly 506 (hidden) in the compressed condition. (0071. The capsule 516 is then proximally retracted relative to the stent frame assembly 506 as shown in FIG. 14A. More particularly, the capsule 516 is refracted a sufficient distance to expose the support arms 552, thus allowing the support arms 552 to self-expand as shown. As a point of reference, the arrangement of FIG. 14A is akin to that of FIG. 11A whereby the distal end 518 of the capsule 516 remains over the proxi mal portion 558 of the stent frame 550, such that the proximal portion 558 remains compressed and connected to delivery device 502. Further, the tethers 514 (FIG. 11A) are placed in tension, preventing the distal portion 560 (FIG. 11A) of the stent frame 550 from self-expanding. The delivery device 502 is then distally advanced until the support arms 552 engage the leaflets 604 as shown in FIG. 14B Subsequently, tension in the tethers 514 is progres sively lessened, allowing the distal portion 560 of the stent frame 550 to radially self-expand as shown in FIG.15A. FIG. 15B provides a sectional view of the aortic valve 600 with the system 500 in the partial deployment state of FIG. 15A. Where desired, the distal portion 560 can be re-collapsed by pulling on the tethers 514, permitting repositioning of the prosthetic heart valve 504 relative to the aortic valve Once the prosthetic heart valve 504 has been satis factorily located relative to the aortic valve 600, the tethers 514 are then withdrawn (FIG. 15C) followed by complete removal of delivery device 502 from the aortic valve 600. FIG. 15D illustrates final deployment of the stent frame assembly 506 to the aortic valve Although the present disclosure has been described with reference to preferred embodiments, workers skilled in the art will recognize that changes can be made in form and detail without departing from the spirit and scope of the present disclosure. For example, while various systems, devices and methods of the present disclosure have made reference to a self-expanding stent frame, features of the present disclosure are useful with other stented prosthetic heart Valve constructions, such a balloon-expandable stent frame. In this regard, the tethered stent frame connections described above can be employed with a balloon-expandable Stent frame, for example to facilitate a recapture operation whereby following expansion of the stent frame by a balloon, the tethers can be tensioned to effectuate at least partial col lapsing of a corresponding region of the stent frame, that in turn promotes insertion (and more complete collapse) of an entirety of the stent frame within a recapture sheath or other component. What is claimed is: 1. A system for performing a therapeutic procedure on a defective heart valve, the system comprising: a prosthetic heart Valve including a stent frame maintaining a valve structure, the stent frame configured to provide a compressed condition and a natural, expanded condi tion; and a delivery device comprising: a handle, an inner shaft extending from the handle and terminating at a distal tip. an outer sheath co-axially disposed over the inner shaft and including a capsule configured to contain the prosthetic heart valve, a first tether defining a length between opposing, first and second ends; wherein the system is configured to transition between: a delivery state in which the prosthetic heart valve is maintained over the inner shaft in the compressed condition by the capsule and the first tether is con nected to the stent frame, wherein the stent frame defines opposing proximal and distal sides relative to the delivery device, a partial deployment state in which the capsule is with drawn from the prosthetic heart valve and the first tether remains engaged with the stent frame and the stent frame expands toward the expanded condition with the proximal side connected to the delivery device via the first tether, the partial deployment state including expansion of the proximal side being con trolled by a tension in the first tether, a full deployment state in which the first tether is released from the stent frame to permit the first tether to be withdrawn from the stent frame. 2. The system of claim 1, wherein the first tether is con nected to the proximal end of the stent frame in the delivery and partial deployment states. 3. The system of claim 1, wherein the first tether is con nected to the distal end of the stent frame in the delivery and partial deployment states. 4. The system of claim 1, wherein the stent frame is a Self-expanding stent frame configured to self-expand from the compressed condition to the normal, expanded condition. 5. The system of claim 1, wherein the stent frame is a balloon-expandable stent frame. 6. The system of claim 1, wherein the system is further configured to provide a recapture operation in the partial deployment state in which a tension in the first tether is increased and causes a corresponding region of the stent frame to partially re-collapse. 7. The system of claim 1, wherein the first tether is looped through a component of the stent frame in the delivery and partial deployment states. 8. The system of claim 1, wherein the system is configured such that the first tether self-releases from the stent frame in the partial deployment state such that the partial deployment State includes a tension in the tether allowing slow release of the stent frame. 9. The system of claim 8, wherein the first tether is wound about a component of the stent frame in the delivery state.
32 US 2015/ A1 Apr. 23, The system of claim 1, wherein the delivery device further includes a biasing member disposed between the stent frame and an end of the first tether, and further wherein the partial deployment state includes a deployment force of the stent frame gradually overcoming a biasing force of the bias ing member. 11. The system of claim 1, wherein the delivery device further includes a hub assembly carried by the inner shaft. 12. The system of claim 11, wherein the hub assembly includes a valve retainer configured to selectively receive a component of the stent frame in at least the delivery state, and further wherein the first tether is coupled to a capture feature of the valve retainer in the delivery and partial deployment States. 13. The system of claim 12, wherein the valve retainer is located proximate the distal tip. 14. The system of claim 12, wherein the valve retainer is located immediately proximate the proximal end of the pros thetic heart valve in the delivery state. 15. The system of claim 12, wherein the prosthetic heart valve defines a length in the compressed condition, and fur ther wherein a longitudinal distance between the valve retainer and the distal tip approximates the length of the prosthetic heart valve. 16. The system of claim 12, wherein the first tether is one of a plurality of tethers provided with the delivery device, each of the plurality of tethers being connected to the proxi mal side of the prosthetic heart valve in the delivery and partial deployment states. 17. The system of claim 16, wherein the valve retainer defines a plurality of capture features, and further wherein each of the capture features is configured to selectively engage a corresponding one of the plurality of tethers. 18. The system of claim 12, wherein the system is config ured such that partial deployment state includes a distalend of the capsule being located distal the capture feature of the valve retainer Such that the capsule retains engagement between the first tether and the valve retainer. 19. The system of claim 12, wherein the capture feature includes a post circumscribed by a groove, the first tether being located within the groove and looped about the post in the delivery and partial deployment states. 20. A method of performing a therapeutic procedure on a defective heart valve of a patient, the method comprising: receiving a system in a delivery state, the system including a prosthetic heart valve loaded to a delivery device, the prosthetic heart valve including a stent frame maintain ing a valve structure, the stent frame defining proximal and distal side, the delivery device including a handle, an inner shaft, an outer sheath forming a capsule, and a first tether, wherein the delivery state includes: the prosthetic heart valve maintained over the inner shaft by the capsule in a compressed condition, and the first tether connected to the stent frame; manipulating the delivery device to guide the prosthetic heart valve through the patient s vasculature and into the defective heart valve; transitioning the system to a partial deployment state by proximally retracting the capsule from the prosthetic heart Valve, the partial deployment state including the prosthetic heart Valve expanding from the compressed condition toward a natural, expanded condition and remaining connected to the delivery device via the first tether; altering a tension in the first tether to allow a corresponding region of the stent frame to slowly expand toward the expanded condition; and transitioning the system to a full deployment state, includ ing removing the first tether from the stent frame. 21. The method of claim 20, further comprising: prior to the step of transitioning the system to a full deploy ment state, increasing a tension in the first tether to partially re-collapse a corresponding region of the stent frame. 22. The method of claim 20, wherein the stent frame is a self-expanding stent frame configured to self-expand from the compressed condition to the natural condition. 23. The method of claim 20, wherein the first tether is connected to the proximal end of the stent frame in the deliv ery and partial deployment states. 24. The method of claim 20, wherein the first tether is connected to the distal end of the stent frame in the delivery and partial deployment states. 25. The method of claim 20, wherein the first tether is looped through a segment of the stent frame in the delivery and partial deployment states. 26. The method of claim 20, wherein the system is config ured such that the first tetherself-releases from the stent frame as part of the step of transitioning the system to a partial deployment state. 27. The method of claim 20, wherein the delivery device includes a plurality of tethers, including the first tether, and further wherein delivery state includes each of the plurality of tethers connected to the stent frame. 28. The method of claim 27, wherein the step of altering a tension in the first tether includes simultaneously altering a tension in each of the plurality of tethers. 29. The method of claim 20, wherein the delivery device further includes a valve retainer carried by the inner shaft, the valve retainer configured to selectively receive a component of the stent frame and forming a capture feature configured to selectively maintain a portion of the first tether in the delivery and partial deployment states. 30. The method of claim 29, wherein the step of transition ing the system to the partial deployment state includes locat ing a distal end of the capsule longitudinally between the prosthetic heart valve and the valve retainer. 31. The method of claim 30, wherein the step of transition ing the system to the full deployment state includes locating the distal end of the capsule proximal the capture feature of the valve retainer. 32. The method of claim 31, wherein the step of transition ing the system to the full deployment state includes the first tether self-releasing from the valve retainer body upon retrac tion of the distal end of the capsule proximal the capture feature of the valve retainer. k k k k k
(12) United States Patent
 (12) United States Patent USOO968,734.4B2 (10) Patent No.: Murray, III et al. (45) Date of Patent: Jun. 27, 2017 (54) TRANSCATHETER PROSTHETIC HEART (58) Field of Classification Search VALVE DELVERY DEVICE
(12) United States Patent USOO968,734.4B2 (10) Patent No.: Murray, III et al. (45) Date of Patent: Jun. 27, 2017 (54) TRANSCATHETER PROSTHETIC HEART (58) Field of Classification Search VALVE DELVERY DEVICE
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(12) (10) Patent No.: US 9,468,773 B1. Anderson et al. (45) Date of Patent: Oct. 18, 2016
 United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
United States Patent US009468773B1 (12) (10) Patent No.: Anderson et al. (45) Date of Patent: Oct. 18, 2016 (54) INTERVENTIONAL MEDICAL SYSTEMS 7,647,109 B2 * 1/2010 Hastings... A61N 1/O587 AND IMPLANTABLE
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090287290A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0287290 A1 Macaulay et al. (43) Pub. Date: Nov. 19, 2009 (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION
(19) United States US 20090287290A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0287290 A1 Macaulay et al. (43) Pub. Date: Nov. 19, 2009 (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003.01.05520A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0105520 A1 Alferness et al. (43) Pub. Date: (54) ANCHOR AND PULL MITRAL VALVE DEVICE AND METHOD (75) Inventors:
(19) United States US 2003.01.05520A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0105520 A1 Alferness et al. (43) Pub. Date: (54) ANCHOR AND PULL MITRAL VALVE DEVICE AND METHOD (75) Inventors:
(12) United States Patent
 (12) United States Patent Jones et al. USOO6833003B2 (10) Patent No.: (45) Date of Patent: Dec. 21, 2004 (54) EXPANDABLE STENT AND DELIVERY SYSTEM (75) Inventors: Donald K. Jones, Lauderhill, FL (US);
(12) United States Patent Jones et al. USOO6833003B2 (10) Patent No.: (45) Date of Patent: Dec. 21, 2004 (54) EXPANDABLE STENT AND DELIVERY SYSTEM (75) Inventors: Donald K. Jones, Lauderhill, FL (US);
58 Field of Search /191,192, wire stent which extends in a path defining a generally
 USOO5882335A United States Patent (19) 11 Patent Number: Leone et al. (45) Date of Patent: Mar 16, 1999 54 RETRIEVABLE DRUG DELIVERY STENT 5,306,250 4/1994 March et al.... 604/104 5,306,286 4/1994 Stack
USOO5882335A United States Patent (19) 11 Patent Number: Leone et al. (45) Date of Patent: Mar 16, 1999 54 RETRIEVABLE DRUG DELIVERY STENT 5,306,250 4/1994 March et al.... 604/104 5,306,286 4/1994 Stack
( ) -- (12) Patent Application Publication (10) Pub. No.: US 2016/ A1. (19) United States. (43) Pub. Date: Oct. 27, 2016.
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310302 A1 NEGLEN et al. US 201603103O2A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (60) SYSTEMIS AND METHODS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310302 A1 NEGLEN et al. US 201603103O2A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (60) SYSTEMIS AND METHODS
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 US 2017.0065412A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0065412 A1 Mesana et al. (43) Pub. Date: (54) STENTED HEART VALVE DEVICES AND (60) Provisional application
US 2017.0065412A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0065412 A1 Mesana et al. (43) Pub. Date: (54) STENTED HEART VALVE DEVICES AND (60) Provisional application
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0071716 A1 Milsom et al. US 2017007 1716A1 (43) Pub. Date: Mar. 16, 2017 (54) (71) (72) (21) (22) (63) (60) METHOD AND APPARATUS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0071716 A1 Milsom et al. US 2017007 1716A1 (43) Pub. Date: Mar. 16, 2017 (54) (71) (72) (21) (22) (63) (60) METHOD AND APPARATUS
III. United States Patent (19) Sheiban 5,226,889. Jul. 13, and at least a pair of inflatable balloons carried on the
 United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
(12) United States Patent (10) Patent No.: US 6,514,280 B1. Gilson (45) Date of Patent: Feb. 4, 2003
 USOO651428OB1 (12) United States Patent (10) Patent No.: US 6,514,280 B1 Gilson (45) Date of Patent: Feb. 4, 2003 (54) DELIVERY CATHETER 5,683,451 A 11/1997 Lenker et al.... 623/1 5,885.258 A * 3/1999
USOO651428OB1 (12) United States Patent (10) Patent No.: US 6,514,280 B1 Gilson (45) Date of Patent: Feb. 4, 2003 (54) DELIVERY CATHETER 5,683,451 A 11/1997 Lenker et al.... 623/1 5,885.258 A * 3/1999
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0189183 A1 Spenser et al. US 20170 189183A1 (43) Pub. Date: Jul. 6, 2017 (54) (71) (72) (73) (21) (22) (60) PROSTHETIC HEART
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0189183 A1 Spenser et al. US 20170 189183A1 (43) Pub. Date: Jul. 6, 2017 (54) (71) (72) (73) (21) (22) (60) PROSTHETIC HEART
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090192591A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0192591 A1 Ryan et al. (43) Pub. Date: (54) MARKERS FOR PROSTHETIC HEART Publication Classification VALVES
US 20090192591A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0192591 A1 Ryan et al. (43) Pub. Date: (54) MARKERS FOR PROSTHETIC HEART Publication Classification VALVES
(12) United States Patent
 US0094.80557B2 (12) United States Patent Pellegrini et al. (10) Patent No.: (45) Date of Patent: US 9.480,557 B2 Nov. 1, 2016 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) STENTS FOR PROSTHETIC
US0094.80557B2 (12) United States Patent Pellegrini et al. (10) Patent No.: (45) Date of Patent: US 9.480,557 B2 Nov. 1, 2016 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) STENTS FOR PROSTHETIC
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070179519A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0179519 A1 Huisun (43) Pub. Date: (54) STENT DELIVERY SYSTEM TO IMPROVE (22) Filed: Jan. 27, 2006 PLACEMENT
US 20070179519A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0179519 A1 Huisun (43) Pub. Date: (54) STENT DELIVERY SYSTEM TO IMPROVE (22) Filed: Jan. 27, 2006 PLACEMENT
(12) United States Patent
 USOO8623074B2 (12) United States Patent Ryan (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR REPLACEMENT PROSTHETIC HEART VALVES (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) Inventor: Timothy
USOO8623074B2 (12) United States Patent Ryan (54) DELIVERY SYSTEMS AND METHODS OF MPLANTATION FOR REPLACEMENT PROSTHETIC HEART VALVES (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) Inventor: Timothy
(12) United States Patent
 US0089.74524B2 (12) United States Patent Yeung et al. (10) Patent No.: (45) Date of Patent: US 8,974,524 B2 Mar. 10, 2015 (54) STENTED TRANSCATHETER PROSTHETIC HEART VALVE DELVERY SYSTEMAND METHOD (71)
US0089.74524B2 (12) United States Patent Yeung et al. (10) Patent No.: (45) Date of Patent: US 8,974,524 B2 Mar. 10, 2015 (54) STENTED TRANSCATHETER PROSTHETIC HEART VALVE DELVERY SYSTEMAND METHOD (71)
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0039210 A1 Yates et al. US 20090039210A1 (43) Pub. Date: Feb. 12, 2009 (54) (76) (21) (22) (60) CPAP HOSE SUPPORT SYSTEM Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0196770 A1 Klintenstedt et al. US 201701.96770A1 (43) Pub. Date: Jul. 13, 2017 (54) (71) (72) (21) (22) (63) (60) (30) CNTAINER
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0196770 A1 Klintenstedt et al. US 201701.96770A1 (43) Pub. Date: Jul. 13, 2017 (54) (71) (72) (21) (22) (63) (60) (30) CNTAINER
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
(19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0188221 A1 Chung et al. US 2014O188221A1 (43) Pub. Date: Jul. 3, 2014 (54) SURGICAL HEART VALVES ADAPTED FOR POST IMPLANT EXPANSION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0188221 A1 Chung et al. US 2014O188221A1 (43) Pub. Date: Jul. 3, 2014 (54) SURGICAL HEART VALVES ADAPTED FOR POST IMPLANT EXPANSION
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
Berry (43) Pub. Date: May 6, (76) Inventor: Bret Berry, Cordova, TN (US) (57) ABSTRACT
 US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
United States Patent (19)
 United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
(12) United States Patent (10) Patent No.: US 6,916,338 B2
 USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150272731A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0272731 A1 Racchini et al. (43) Pub. Date: Oct. 1, 2015 (54) SYSTEMAND METHOD OF STEPPED (52) U.S. Cl. DEPLOYMENT
(19) United States US 20150272731A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0272731 A1 Racchini et al. (43) Pub. Date: Oct. 1, 2015 (54) SYSTEMAND METHOD OF STEPPED (52) U.S. Cl. DEPLOYMENT
(12) United States Patent (10) Patent No.: US 7.690,305 B2
 US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
Sepetka et al. (45) Date of Patent: May 3, ) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson.
 United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
Zenith Alpha T HORACIC ENDOVASCULAR GRAFT
 Device description Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21181-EN-F Modular design The two-piece modular system allows the physician to customize a graft system to fit each
Device description Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21181-EN-F Modular design The two-piece modular system allows the physician to customize a graft system to fit each
11 B N \S44. (12) Patent Application Publication (10) Pub. No.: US 2004/ A1. (19) United States SS 12A
 (19) United States US 20040097.979A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0097979 A1 Svanidze et al. (43) Pub. Date: May 20, 2004 (54) AORTIC VALVE IMPLANTATION DEVICE (76) Inventors:
(19) United States US 20040097.979A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0097979 A1 Svanidze et al. (43) Pub. Date: May 20, 2004 (54) AORTIC VALVE IMPLANTATION DEVICE (76) Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 2008.0021546A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0021546 A1 Patz et al. (43) Pub. Date: Jan. 24, 2008 (54) SYSTEM FOR DEPLOYING BALLOON-EXPANDABLE HEART VALVES
(19) United States US 2008.0021546A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0021546 A1 Patz et al. (43) Pub. Date: Jan. 24, 2008 (54) SYSTEM FOR DEPLOYING BALLOON-EXPANDABLE HEART VALVES
(12) United States Patent
 USOO9498226B2 (12) United States Patent Cage et al. (10) Patent No.: (45) Date of Patent: US 9.498,226 B2 Nov. 22, 2016 (54) EMBOLIC COIL DELIVERY SYSTEM (75) Inventors: Logan Michael Cage, Bloomington,
USOO9498226B2 (12) United States Patent Cage et al. (10) Patent No.: (45) Date of Patent: US 9.498,226 B2 Nov. 22, 2016 (54) EMBOLIC COIL DELIVERY SYSTEM (75) Inventors: Logan Michael Cage, Bloomington,
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
US 2011 0023281A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0023281 A1 Schraga (43) Pub. Date: (54) PEN INJECTION DEVICE CAP WITH Related U.S. Application Data NSSENSECKRELEASE
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
US 2003O130096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0130096 A1 LaCroce (43) Pub. Date: Jul. 10, 2003 (54) BARBELL WITH PLURAL HAND GRIPPING Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States US 2007019.8075A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0198075A1 Levy (43) Pub. Date: Aug. 23, 2007 (54) BFURCATED ANEURYSM TREATMENT Publication Classification
(19) United States US 2007019.8075A1 (12) Patent Application Publication (10) Pub. No.: US 2007/0198075A1 Levy (43) Pub. Date: Aug. 23, 2007 (54) BFURCATED ANEURYSM TREATMENT Publication Classification
United States Patent (19) 11 Patent Number: 5,582,607 Lackman 45 Date of Patent: Dec. 10, 1996
 US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
United States Patent (19) Hensel
 United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 2016.0074165A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0074165 A1 Spence et al. (43) Pub. Date: Mar. 17, 2016 (54) MITRAL REPAIR AND REPLACEMENT (52) U.S. Cl. DEVICES
(19) United States US 2016.0074165A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0074165 A1 Spence et al. (43) Pub. Date: Mar. 17, 2016 (54) MITRAL REPAIR AND REPLACEMENT (52) U.S. Cl. DEVICES
-100 III IIHIIII. United States Patent (19) Fischell et al. 11) Patent Number: 5,492,530 45) Date of Patent: Feb. 20, 1996
 United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
(12) United States Patent
 US0093.20587B2 (12) United States Patent Milsom et al. (10) Patent No.: (45) Date of Patent: US 9,320,587 B2 Apr. 26, 2016 (54) (75) (73) (*) (21) (22) (86) (87) (65) (63) (60) (51) (52) METHOD AND APPARATUS
US0093.20587B2 (12) United States Patent Milsom et al. (10) Patent No.: (45) Date of Patent: US 9,320,587 B2 Apr. 26, 2016 (54) (75) (73) (*) (21) (22) (86) (87) (65) (63) (60) (51) (52) METHOD AND APPARATUS
Portico (St. Jude Medical Inc, St.
 Review Article Portico Transcatheter Heart Valve Apostolos Tzikas 1,2, Michael Chrissoheris 2, Antonios Halapas 2, Konstantinos Spargias 2 1 Interbalkan European Medical Centre, Thessaloniki, 2 Hygeia
Review Article Portico Transcatheter Heart Valve Apostolos Tzikas 1,2, Michael Chrissoheris 2, Antonios Halapas 2, Konstantinos Spargias 2 1 Interbalkan European Medical Centre, Thessaloniki, 2 Hygeia
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0165859 A1 ECKHOUSE et al. US 2012O165859A1 (43) Pub. Date: Jun. 28, 2012 (54) (75) (73) (21) (22) (63) DEVICE AND METHOD INVOLVING
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0165859 A1 ECKHOUSE et al. US 2012O165859A1 (43) Pub. Date: Jun. 28, 2012 (54) (75) (73) (21) (22) (63) DEVICE AND METHOD INVOLVING
EP A2 (19) (11) EP A2 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2012/28
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 474 278 A2 (43) Date of publication: 11.07.2012 Bulletin 2012/28 (1) Int Cl.: A61B 17/80 (2006.01) (21) Application number: 1210644.8 (22) Date of filing:.01.2012
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 474 278 A2 (43) Date of publication: 11.07.2012 Bulletin 2012/28 (1) Int Cl.: A61B 17/80 (2006.01) (21) Application number: 1210644.8 (22) Date of filing:.01.2012
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O316632A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0316632 A1 Gao (43) Pub. Date: Dec. 13, 2012 (54) RETRIEVABLE COVERED STENT FOR (52) U.S. Cl.... 623A1.2 BFURCATION
(19) United States US 2012O316632A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0316632 A1 Gao (43) Pub. Date: Dec. 13, 2012 (54) RETRIEVABLE COVERED STENT FOR (52) U.S. Cl.... 623A1.2 BFURCATION
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 US 2013 O190861A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0190861 A1 Chau et al. (43) Pub. Date: Jul. 25, 2013 (54) PROSTHETIC VALVE FOR REPLACING Publication Classification
US 2013 O190861A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0190861 A1 Chau et al. (43) Pub. Date: Jul. 25, 2013 (54) PROSTHETIC VALVE FOR REPLACING Publication Classification
United States Patent (19)
 United States Patent (19) Galloway 11 Patent Number: 45 Date of Patent: 4,738,667 Apr. 19, 1988 W 54 PREFORMED CATHETER ASSEMBLY 76 Inventor: Niall T. M. Galloway, 3211 Massdale Ave., Durham, N.C. 27707
United States Patent (19) Galloway 11 Patent Number: 45 Date of Patent: 4,738,667 Apr. 19, 1988 W 54 PREFORMED CATHETER ASSEMBLY 76 Inventor: Niall T. M. Galloway, 3211 Massdale Ave., Durham, N.C. 27707
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 (19) United States US 2002O156474A1 (12) Patent Application Publication (10) Pub. No.: US 2002/0156474 A1 Wack et al. (43) Pub. Date: Oct. 24, 2002 (54) POLYAXIAL LOCKING PLATE (76) Inventors: Michael
(19) United States US 2002O156474A1 (12) Patent Application Publication (10) Pub. No.: US 2002/0156474 A1 Wack et al. (43) Pub. Date: Oct. 24, 2002 (54) POLYAXIAL LOCKING PLATE (76) Inventors: Michael
CP STENT. Large Diameter, Balloon Expandable Stent
 CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
(12) United States Patent (10) Patent No.: US 7,108,510 B2
 US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
(19) United States US 20160213850A1 (12) Patent Application Publication (10) Pub. No.: US 2016/0213850 A1 LOOF et al. (43) Pub. Date: Jul. 28, 2016 (54) MEDICAMENT DELIVERY DEVICE Publication Classification
Progress In Transcatheter Aortic Valve Implantation
 Progress In Transcatheter Aortic Valve Implantation Gerald Yong MBBS (Hons) FRACP FSCAI Interventional Cardiologist Royal Perth Hospital Western Australia 4 th APCASH 8 th Sept 2013 Disclosure Statement
Progress In Transcatheter Aortic Valve Implantation Gerald Yong MBBS (Hons) FRACP FSCAI Interventional Cardiologist Royal Perth Hospital Western Australia 4 th APCASH 8 th Sept 2013 Disclosure Statement
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
(19) United States US 2012.0089051A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0089051A1 Draudt et al. (43) Pub. Date: Apr. 12, 2012 (54) BLOOD GLUCOSE METER HAVING INTEGRAL LANCET DEVICE
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0030380 A1 Binmoeller US 20090030.380A1 (43) Pub. Date: Jan. 29, 2009 (54) (75) (73) (21) (22) (86) METHOD AND APPARATUS FOR
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0030380 A1 Binmoeller US 20090030.380A1 (43) Pub. Date: Jan. 29, 2009 (54) (75) (73) (21) (22) (86) METHOD AND APPARATUS FOR
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
(12) United States Patent (10) Patent No.: US 7, B2
 US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
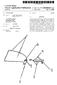 US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
USOO A United States Patent (19) 11 Patent Number: 5,829,589 Nguyen et al. (45) Date of Patent: Nov. 3, 1998
 USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0193020 A1 Krissman et al. US 2014O193020A1 (43) Pub. Date: (54) (71) (72) (73) (21) HEADPHONE ASSEMBLY Applicant: THE KETCHUM
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0193020 A1 Krissman et al. US 2014O193020A1 (43) Pub. Date: (54) (71) (72) (73) (21) HEADPHONE ASSEMBLY Applicant: THE KETCHUM
TABLE OF CONTENTS. 2 (8144 Rev 2)
 1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
(12) United States Patent
 (12) United States Patent Globerman et al. USOO6402777B1 (10) Patent No.: (45) Date of Patent: US 6,402.777 B1 Jun. 11, 2002 (54) RADIOPAQUE STENT MARKERS (75) Inventors: Oren Globerman, Holon; Mordechay
(12) United States Patent Globerman et al. USOO6402777B1 (10) Patent No.: (45) Date of Patent: US 6,402.777 B1 Jun. 11, 2002 (54) RADIOPAQUE STENT MARKERS (75) Inventors: Oren Globerman, Holon; Mordechay
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1. Knott et al. (43) Pub. Date: Sep. 6, 2007
 US 20070204455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0204455A1 Knott et al. (43) Pub. Date: (54) METHOD FOR RETAINING A VASCULAR Related U.S. Application Data
US 20070204455A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0204455A1 Knott et al. (43) Pub. Date: (54) METHOD FOR RETAINING A VASCULAR Related U.S. Application Data
Conventus CAGE PH Surgical Techniques
 Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
M.I.S. MAKE IT SMART IN ONE SYSTEM. Surgical Technique. Hip Knee Spine Navigation
 M.I.S. MAKE IT SMART IN ONE SYSTEM Surgical Technique Hip Knee Spine Navigation M.U.S.T. Mini Open Surgical Technique Hip Knee Spine Navigation 2 C O N T E N T S 1 INTRODUCTION 4 2 SURGICAL TECHNIQUE 5
M.I.S. MAKE IT SMART IN ONE SYSTEM Surgical Technique Hip Knee Spine Navigation M.U.S.T. Mini Open Surgical Technique Hip Knee Spine Navigation 2 C O N T E N T S 1 INTRODUCTION 4 2 SURGICAL TECHNIQUE 5
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0208302 A1 Webster et al. US 200702083 02A1 (43) Pub. Date: Sep. 6, 2007 (54) DEFLECTION CONTROL CATHETERS, SUPPORT CATHETERS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0208302 A1 Webster et al. US 200702083 02A1 (43) Pub. Date: Sep. 6, 2007 (54) DEFLECTION CONTROL CATHETERS, SUPPORT CATHETERS
(12) United States Patent (10) Patent No.: US 6,932,771 B2. Whitmore et al. (45) Date of Patent: Aug. 23, 2005
 USOO6932771B2 (12) United States Patent (10) Patent No.: US 6,932,771 B2 Whitmore et al. (45) Date of Patent: Aug. 23, 2005 (54) TISSUE WARMING DEVICE AND METHOD (56) References Cited (75) Inventors: Willet
USOO6932771B2 (12) United States Patent (10) Patent No.: US 6,932,771 B2 Whitmore et al. (45) Date of Patent: Aug. 23, 2005 (54) TISSUE WARMING DEVICE AND METHOD (56) References Cited (75) Inventors: Willet
(12) United States Patent
 US008556757B2 (12) United States Patent Kilshaw (10) Patent No.: (45) Date of Patent: Oct. 15, 2013 (54) BICYCLE GEAR MECHANISM (76) Inventor: Richard J. Kilshaw, Lake Oswego, OR (US) *) Notice: Subject
US008556757B2 (12) United States Patent Kilshaw (10) Patent No.: (45) Date of Patent: Oct. 15, 2013 (54) BICYCLE GEAR MECHANISM (76) Inventor: Richard J. Kilshaw, Lake Oswego, OR (US) *) Notice: Subject
United States Patent (19)
 United States Patent (19) Turkel et al. 54) COAXAL BONE MARROW BIOPSY CORNG AND ASPRATING NEEDLE ASSEMBLY AND METHOD OF USE THEREOF 75) Inventors: David H. Turkel; Thomas O. Bales, both of Miami; Frank
United States Patent (19) Turkel et al. 54) COAXAL BONE MARROW BIOPSY CORNG AND ASPRATING NEEDLE ASSEMBLY AND METHOD OF USE THEREOF 75) Inventors: David H. Turkel; Thomas O. Bales, both of Miami; Frank
(51) Int Cl.: A61B 17/16 ( ) A61B 17/82 ( )
 (19) TEPZZ 8 Z_ B_T (11) EP 2 8 12 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 03.08.16 Bulletin 16/31 (21) Application number: 13713282. (22)
(19) TEPZZ 8 Z_ B_T (11) EP 2 8 12 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 03.08.16 Bulletin 16/31 (21) Application number: 13713282. (22)
(51) Int Cl.: A61F 2/24 ( )
 (19) (12) EUROPEAN PATENT SPECIFICATION (11) EP 1 441 672 B1 (45) Date of publication and mention of the grant of the patent: 14.09.2011 Bulletin 2011/37 (21) Application number: 02804403.0 (22) Date of
(19) (12) EUROPEAN PATENT SPECIFICATION (11) EP 1 441 672 B1 (45) Date of publication and mention of the grant of the patent: 14.09.2011 Bulletin 2011/37 (21) Application number: 02804403.0 (22) Date of
(12) United States Patent (10) Patent No.: US 6,234,797 B1
 USOO6234797B1 (12) United States Patent (10) Patent No.: Ura (45) Date of Patent: *May 22, 2001 (54) DENTAL IMPLANT AND METHOD FOR 5,322,443 6/1994 Beaty. INSTALLING THE SAME 5,336,225 8/1994 Zang. 5,338,197
USOO6234797B1 (12) United States Patent (10) Patent No.: Ura (45) Date of Patent: *May 22, 2001 (54) DENTAL IMPLANT AND METHOD FOR 5,322,443 6/1994 Beaty. INSTALLING THE SAME 5,336,225 8/1994 Zang. 5,338,197
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003OO69636A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0069636A1 Solem et al. (43) Pub. Date: Apr. 10, 2003 (54) METHOD FOR TREATMENT OF MITRAL INSUFFICIENCY (76)
(19) United States US 2003OO69636A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0069636A1 Solem et al. (43) Pub. Date: Apr. 10, 2003 (54) METHOD FOR TREATMENT OF MITRAL INSUFFICIENCY (76)
(12) United States Patent (10) Patent No.: US 9,332,914 B2
 USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
In Vitro Two-Dimensional Echocardiographic Imaging of a Stented Porcine Bioprosthetic Valve: The Bent Strut Artifact
 C 2008, the Author Journal compilation C 2008, Wiley Periodicals, Inc. DOI: 10.1111/j.1540-8175.2008.00753.x In Vitro Two-Dimensional Echocardiographic Imaging of a Stented Porcine Bioprosthetic Valve:
C 2008, the Author Journal compilation C 2008, Wiley Periodicals, Inc. DOI: 10.1111/j.1540-8175.2008.00753.x In Vitro Two-Dimensional Echocardiographic Imaging of a Stented Porcine Bioprosthetic Valve:
United States Patent (19) Andelin et al.
 United States Patent (19) Andelin et al. 54 BONE MARROW BIOPSY NEEDLE AND METHOD FOR USING THE SAME 76 Inventors: John B. Andelin, 4940 140 Ave., N.W., Lot 62, Williston, N. Dak. 58801-8605; Martin T.
United States Patent (19) Andelin et al. 54 BONE MARROW BIOPSY NEEDLE AND METHOD FOR USING THE SAME 76 Inventors: John B. Andelin, 4940 140 Ave., N.W., Lot 62, Williston, N. Dak. 58801-8605; Martin T.
United States Patent Patent Number: 5,582,598 Chanoch 45) Date of Patent: Dec. 10, 1996
 I I US005582598A United States Patent 19 11 Patent Number: Chanoch 45) Date of Patent: Dec. 10, 1996 54 MEDICATIN DELIVERY PEN WITH 5,114,406 5/1992 Gabriel et al.... 604/232 WARIABLE INCREMENT DSE SCALE
I I US005582598A United States Patent 19 11 Patent Number: Chanoch 45) Date of Patent: Dec. 10, 1996 54 MEDICATIN DELIVERY PEN WITH 5,114,406 5/1992 Gabriel et al.... 604/232 WARIABLE INCREMENT DSE SCALE
(12) Patent Application Publication (10) Pub. No.: US 2002/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: Gray et al. US 2002O173769A1 (43) Pub. Date: Nov. 21, 2002 (54) INFUSION SET FOR A FLUID PUMP (76) (21) (22) (63) (60) Inventors: Larry
(19) United States (12) Patent Application Publication (10) Pub. No.: Gray et al. US 2002O173769A1 (43) Pub. Date: Nov. 21, 2002 (54) INFUSION SET FOR A FLUID PUMP (76) (21) (22) (63) (60) Inventors: Larry
Lindsey, both of Dublin, Ohio 73) Assignee: Medex, Inc., Hilliard, Ohio 21 Appl. No.: 150, Filed: Feb. 1, Int. Cl."...
 United States Patent (19) Patton et al. 54 Y CONNECTOR FOR ANGIOPLASTY PROCEDURE 75 Inventors: William E. Patton; William J. Lindsey, both of Dublin, Ohio 73) Assignee: Medex, Inc., Hilliard, Ohio 21 Appl.
United States Patent (19) Patton et al. 54 Y CONNECTOR FOR ANGIOPLASTY PROCEDURE 75 Inventors: William E. Patton; William J. Lindsey, both of Dublin, Ohio 73) Assignee: Medex, Inc., Hilliard, Ohio 21 Appl.
Piersch (45) Date of Patent: Jun. 29, 1993
 United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
United States Patent (19) 11) USOO5222902A Patent Number: 5,222,902 Piersch (45) Date of Patent: Jun. 29, 1993 54 INTERLOCKING BLOCKS 3,890,738 6/1975 Bassani... 44.6/1 3,900,985 8/1975 Yoen... 44.6/124
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310236A1 Kopelman et al. US 20160310236A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (63) (60) DIRECT FABRICATION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0310236A1 Kopelman et al. US 20160310236A1 (43) Pub. Date: Oct. 27, 2016 (54) (71) (72) (21) (22) (63) (60) DIRECT FABRICATION
(12) United States Patent
 USOO8707549B2 (12) United States Patent Burns et al. () Patent No.: (45) Date of Patent: Apr. 29, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ERGONOMIC GRIPASSEMBLES AND HANDLES FORULTRASOUND
USOO8707549B2 (12) United States Patent Burns et al. () Patent No.: (45) Date of Patent: Apr. 29, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ERGONOMIC GRIPASSEMBLES AND HANDLES FORULTRASOUND
(12) United States Patent (10) Patent No.: US 8,500,773 B2. Nardone et al. (45) Date of Patent: Aug. 6, 2013
 US008500 773B2 (12) United States Patent (10) Patent No.: Nardone et al. (45) Date of Patent: Aug. 6, 2013 (54) SPRING DETACH JOINT FOR DELIVERING 4,827,941. A * 5/1989 Taylor et al.... 600,434 A DETACHABLE
US008500 773B2 (12) United States Patent (10) Patent No.: Nardone et al. (45) Date of Patent: Aug. 6, 2013 (54) SPRING DETACH JOINT FOR DELIVERING 4,827,941. A * 5/1989 Taylor et al.... 600,434 A DETACHABLE
(12) United States Patent (10) Patent No.: US 7,819,889 B2
 patent is extended or adjusted under 35 6,113,622 U.S.C. 154(b) by 1301 days. US007819889B2 (12) United States Patent (10) Patent No.: Healy et al. (45) Date of Patent: *Oct. 26, 2010 (54) DETACHABLE INTRODUCER
patent is extended or adjusted under 35 6,113,622 U.S.C. 154(b) by 1301 days. US007819889B2 (12) United States Patent (10) Patent No.: Healy et al. (45) Date of Patent: *Oct. 26, 2010 (54) DETACHABLE INTRODUCER
U.S.C. 154(b) by 0 days. (21) Appl. No.: 10/064,070 (22) Filed: Jun. 6, 2002 (51) Int. Cl... A61F 2/06 623/1.11, 902, 903, 909 XXX .. 2.
 (12) United States Patent Weiss USOO6676694B1 (10) Patent No.: (45) Date of Patent: Jan. 13, 2004 (54) METHOD FOR INSTALLING ASTENT GRAFT (76) Inventor: Mitchell Weiss, 300 Pinellas St. Radiology Dept.
(12) United States Patent Weiss USOO6676694B1 (10) Patent No.: (45) Date of Patent: Jan. 13, 2004 (54) METHOD FOR INSTALLING ASTENT GRAFT (76) Inventor: Mitchell Weiss, 300 Pinellas St. Radiology Dept.
