Review Article Targeting the Hepcidin-Ferroportin Axis in the Diagnosis and Treatment of Anemias
|
|
|
- Anastasia Robinson
- 6 years ago
- Views:
Transcription
1 Hindawi Publishing Corporation Advances in Hematology Volume 2010, Article ID , 9 pages doi: /2010/ Review Article Targeting the Hepcidin-Ferroportin Axis in the Diagnosis and Treatment of Anemias Elizabeta Nemeth David Geffen School of Medicine at UCLA, CHS , LeConte Avenue, Los Angeles, CA , USA Correspondence should be addressed to Elizabeta Nemeth, enemeth@mednet.ucla.edu Received 2 November 2009; Accepted 23 November 2009 Academic Editor: Stefano Rivella Copyright 2010 Elizabeta Nemeth. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The hepatic peptide hormone hepcidin regulates dietary iron absorption, plasma iron concentrations, and tissue iron distribution. Hepcidin acts by causing the degradation of its receptor, the cellular iron exporter ferroportin. The loss of ferroportin decreases iron flow into plasma from absorptive enterocytes, from macrophages that recycle the iron of senescent erythrocytes, and from hepatocytes that store iron, thereby lowering plasma iron concentrations. Malfunctions of the hepcidin-ferroportin axis contribute to the pathogenesis of different anemias. Deficient production of hepcidin causes systemic iron overload in iron-loading anemias such as beta-thalassemia; whereas hepcidin excess contributes to the development of anemia in inflammatory disorders and chronic kidney disease, and may cause erythropoietin resistance. The diagnosis of different forms of anemia will be facilitated by improved hepcidin assays, and the treatment will be enhanced by the development of hepcidin agonists and antagonists. 1. Hepcidin-Ferroportin Interaction Regulates Iron Homeostasis Hepcidin is a small peptide hormone secreted by hepatocytes, circulating in blood plasma and excreted in urine [1]. Like other peptide hormones, hepcidin is synthesized initially as a larger 84-amino acid preprohepcidin then processed in hepatocytes by the signal peptidase to 60-amino acid prohepcidin that lacks iron-regulatory activity [2]. Prior to secretion, prohormone convertases cleave prohepcidin at a polybasic motif to generate the mature bioactive 25-amino acid hepcidin [3]. Other cell types including macrophages and adipocytes also contain hepcidin mrna but their local and systemic contribution to the production of bioactive hepcidin has not been established with certainty. Hepcidin plays an essential role in maintaining iron homeostasis, and the dysregulation of its production underlies many iron disorders. Chronic excess of hepcidin causes iron-restricted anemia [4], whereas hepcidin deficiency results in iron overload with iron deposition in the liver and other parenchyma [5]. Hepcidin acts by regulating the cellular concentration of its receptor, ferroportin. Ferroportin is the sole known cellular iron exporter and is essential for iron homeostasis [6]. This multispanning membrane protein is expressed in tissues which transport large amounts of iron (Figure 1): duodenal enterocytes which absorb dietary iron, macrophages of the spleen and liver which recycle iron from old erythrocytes, hepatocytes which store and release iron according to body needs, and placental trophoblast which transports iron from maternal to fetal circulation [7 9]. When ferroportin is located in the cell membrane it allows efflux of iron from the cells into plasma. Hepcidin binding to the extracellular face of ferroportin triggers internalization and degradation of the ligand-receptor complex [10]. Removal of ferroportin from the membrane stops cellular iron export leading to decreased supply of iron into plasma (Figure 1). Without the constant iron influx, the plasma iron pool is rapidly depleted by the iron-consuming cells, most prominently erythroid precursors. In mice, a single injection of synthetic hepcidin caused a rapid drop in serum iron [11], and this lasted for 2 days, presumably until sufficient amount of ferroportin was resynthesized. Decreased ferroportin concentration in cell membranes, as seen during chronic overproduction of hepcidin, can lead to iron-restricted erythropoiesis. Interestingly, ferroportin is also expressed in erythroid precursor cells [12], but its
2 2 Advances in Hematology physiological role or the effect of hepcidin on developing erythrocytes remains to be determined. 2. Regulation of Hepcidin Hepcidin is homeostatically regulated by iron and erythropoietic activity. Increased plasma and stored iron stimulate hepcidin production, which in turn blocks dietary iron absorption and further iron loading (Figure 1). Hepcidin is suppressed in iron deficiency [13], allowing increased absorption of dietary iron and replenishment of iron stores. The feedback loop between iron and hepcidin ensures the stability of plasma iron concentrations. As would be expected for the iron-regulatory hormone, hepcidin production is also regulated by the process which consumes most iron, erythropoiesis [14]. Increased erythropoietic activity suppresses hepcidin production which allows the release of stored iron from macrophages and hepatocytes, and increased iron absorption, all resulting in greater supply of iron for hemoglobin synthesis. Hepcidin production is also pathologically increased in inflammation and infection [15]. Resultant hypoferremia may represent a host defense strategy to limit iron availability to microorganisms, but can also lead to iron dysregulation and iron-restricted anemia in inflammatory diseases. 3. Molecular Mechanisms of Hepcidin Regulation 3.1. Iron. Hepcidin is likely regulated by both circulating iron-transferrin and intracellular iron stores. Although the respective mechanisms of sensing extracellular and intracellular iron are not well understood, they both appear to utilize the bone morphogenetic protein (BMP) pathway to alter hepcidin expression. Several BMPs have been shown to increase hepcidin production in vitro and in vivo [16], but BMP6 has recently emerged as the principal endogenous BMP regulating hepcidin. BMP6 knockout mice develop severe iron overload but no other significant abnormalities [17, 18]. In other biological settings, BMP signaling is known to be modulated by coreceptors and antagonists. Hemojuvelin (HJV), a GPI-linked membrane protein, appears to be the co-receptor specialized for iron regulation [19]. The soluble form of hemojuvelin acts as an antagonist, probably by binding BMPs, but the biological role of this interaction has not beendocumented yet [20]. HJV mutations in humans or mice result in severe iron overload similar to that caused by ablation of hepcidin, without any other apparent problems [21]. Additional molecules, including a protease TMPRSS6 [22] and a large multifunctional transmembrane protein neogenin [23], were shown to interact with HJV and modify its cell-surface expression. Whether these molecules or their interaction with HJV is modulated by iron or other signals remains to be determined. The mechanism by which intracellular iron regulates hepcidin expression is still unclear. However, expression of BMP6 mrna was recently shown to increase with iron loading in mice, raising the possibility that BMP6 is a signal reflecting iron stores [24]. Hepcidin regulation by extracellular iron is better understood, and a tentative model is emerging but needs further experimental support. Sensing of iron-transferrin concentrations apparently depends on transferrin receptor 2 (TfR2) and HFE, two molecules which are mutated in the adult form of hereditary hemochromatosis. TfR2 is a homolog of TfR1, but is primarily expressed in the liver, the site of hepcidin expression. Binding of iron-transferrin to TfR2 stabilizes the protein [25], which results in elevated ERK1/2 and Smad1/5/8 signaling [26]. HFE, a protein similar to MHC I type molecules, appears to function as a shuttle between TfR1 and TfR2 depending on iron-transferrin concentrations [27]. Because the binding sites of HFE and iron-transferrin on TfR1 overlap, higher iron-transferrin concentrations displace HFE from TfR1 allowing HFE to associate with TfR2, which presumably potentiates signaling pathways downstream of TfR2. HFE and TfR2, however, do not appear to be required for hepcidin regulation by iron stores, as mice and humans with HFE and TfR2 mutations are still capable of decreasing hepcidin levels after iron depletion [28] Erythropoiesis. Increased erythropoietic activity is a potent suppressor of hepcidin production. A single injection of erythropoietin in humans caused a dramatic decrease in serum hepcidin within 24 hours [29], and a mouse model showed a dose-dependent decrease in hepcidin mrna after erythropoietin administration [30]. Epo by itself does not appear to be a direct regulator of hepcidin expression because pretreatment of mice with carboplatin, a cytotoxic inhibitor of erythropoiesis, completely abrogated the effect of Epo on hepcidin [14]. Similarly, mouse models of anemias caused by bleeding or hemolysis showed that hepcidin suppression depended on intact erythropoietic activity [14, 31]. How erythropoiesis affects hepcidin production is not clear, but the mediators could include the production of soluble factors by the erythroid precursors in the bone marrow, decreased circulating or stored iron, and hypoxia. Two proteins produced by erythroid precursors, growth differentiation factor 15 (GDF15) and twisted gastrulation protein (TWSG1), have been proposed to mediate hepcidin suppression in anemias with ineffective erythropoiesis [32, 33]. GDF15, a member of the TGF-β superfamily, and TWSG1, a BMP-binding protein, are both produced by developing erythroblasts. The two proteins were shown to suppress hepcidin mrna in vitro [32, 33]. Very high levels of GDF15 were detected in β-thalassemia and congenital dyserythropoietic anemia type I, and elevated Twsg1 expression was found in a mouse model of thalassemia. However, physiologic hepcidin suppression in response to bleeding or to anemias with effective erythropoiesis is likely mediated by other mechanisms. Whatever the signal, erythroid activity appears to suppress hepcidin, at least in part, by modulating the BMP pathway. Erythropoietin administration in mice, which caused the expected decrease in hepcidin levels, was also found to reduce Smad signaling [30]. The physiological relevance of hepcidin regulation by hypoxia is still uncertain. Alterations of the HIF pathway in
3 Advances in Hematology 3 Inflammation Spleen Liver Hepcidin Hepcidin Fpn Hepcidin Fpn Plasma Fe-Tf RBC Fpn Bone marrow Duodenum Iron signal Erythropoietic signal Figure 1: Hepcidin-ferroportin interaction determines the flow of iron into plasma. Hepcidin concentration is in turn regulated by iron, erythropoietic activity, and inflammation. vivo can affect hepcidin expression [34] but whether HIF regulates hepcidin transcription directly or mostly indirectly is still unresolved. It is possible that the main effect of hypoxia on iron homeostasis is to increase erythropoietin production in the kidney, which would lead to proliferation of erythroblasts and suppression of hepcidin by putative erythroid factors Inflammation. Hepcidin synthesis is rapidly increased by infection and inflammation, causing retention of iron in macrophages and decreased iron absorption [15]. The resulting hypoferremia is presumably a component of innate immune responses that deprive invading microbes of iron and other essential nutrients. Serum hepcidin was found to be greatly increased in patients with inflammation defined as CRP > 10 mg/dl, patients with sepsis, burns, inflammatory bowel disease, and multiple myeloma [13, 35 37]. Of inflammatory mediators regulating hepcidin, IL-6 was shown to be a prominent inducer in vitro and in vivo, and it stimulates hepcidin transcription through a STAT-3 dependent mechanism [38, 39]. Other cytokines such as IL- 1 may also directly regulate hepcidin production, at least in mice, as IL-6 knockout mice with chronic inflammation increased hepcidin mrna similarly to wild-type mice [40]. Direct regulation of hepcidin synthesis in myeloid cells by microbial molecules acting through toll-like receptors has also been proposed [41] but it is not clear how much this mechanism contributes to hepcidin production locally or systemically, and whether it also applies to hepatocytes. 4. The Role of Hepcidin in Anemias Hepcidin expression is the result of the interplay between multiple stimuli, including iron, inflammation, and erythropoiesis. Accordingly, hepcidin concentrations in different forms of anemia vary widely, and may have diagnostic potential in differentiating between the various types of anemia. Furthermore, in cases where hepcidin is a causative factor in anemia, hepcidin-targeted therapies may improve treatment options for the patients Iron Deficiency Anemia (IDA). In pure iron deficiency anemia, serum and urinary hepcidin concentrations are greatly decreased and are frequently undetectable by currently available assays [13]. The low expression is presumably due to the lack of transcriptional stimulation by iron as well as active suppression by erythroid factors. Hepcidin appears to be a sensitive indicator of iron deficiency even in the absence of anemia. Decreased hepcidin, together with low transferrin saturation and serum ferritin, is observed prior to a detectable decrease in Hb or Hct ([13], E. Nemeth unpublished). Hepcidin measurements could improve the screening of blood donors, in whom deferral is currently based on low Hct or Hb levels, the relatively late sequelae of iron deficiency. Identifying donors who are already irondeficient prior to the blood donation should reduce the frequency of frank iron deficiency and anemia in blood donors. Hepcidin is readily detectable in urine [13] and thus may be an excellent candidate for the development of a simple
4 4 Advances in Hematology field test for iron deficiency. Apart from the possible use for easy screening of infants and small children, a fieldfriendly test would be particularly useful in areas which lack access to clinical laboratories. Accurate detection of iron deficiency has become an important issue in endemic malarial regions. The Pemba trial, a randomized controlled trial in Zanzibar [42] which evaluated the impact of iron, folic acid, and zinc supplementation on morbidity and mortality in young children, found an increased risk of severe illness and death in children supplemented with iron/folic acid. Furthermore, the risk appeared to be restricted to ironreplete children. Thus, a simple, quick hepcidin test would help distinguish between iron-deficient/anemic children who can benefit from iron supplementation, and iron-replete or already infected children in whom iron supplementation might be harmful Iron-Refractory Iron Deficiency Anemia (IRIDA). Ironrefractory iron deficiency anemia is a hereditary hypochromic, microcytic anemia, refractory to treatment with oral iron, and only partially responsive to parenteral iron. The molecular basis of the disease was only recently described. IRIDA is caused by increased hepcidin production due to mutations in the hepcidin suppressor, TMPRSS6 also called matriptase-2 [43, 44]. The disease is thus a phenotypic opposite of juvenile hemochromatosis caused by disruptive hepcidin mutations. TMPRSS6 is a membrane protease which was reported to act by cleaving membraneassociated HJV [22], which presumably results in decreased BMP pathway activity. When TMPRSS6 is mutated, hepcidin expression is increased, chronically inhibiting iron absorption and resulting in the development of iron deficiency anemia. How TMPRSS6 expression and activity are regulated in relation to iron homeostasis still remains to be determined. Interestingly, TMPRSS6 locus is proving to be highly polymorphic. If some common polymorphisms confer changes in TMPRSS6 expression or activity, this could in turn affect hepcidin expression and the control of iron metabolism. Recently, genome-wide association studies identified association between certain TMPRSS6 variants and serum iron, transferrin saturation, MCV and hemoglobin levels [45, 46]. These findings suggest that even subtle variants in hepcidin regulators have an influence on iron status, erythropoiesis, and associated disorders in the general population. Improved definition of genetic risk factors for iron deficiency or iron overload could help avoid unnecessary burdens associated with chronic treatment Iron-Refractory Anemia Associated with Hepcidinproducing Tumors. Another rare form of iron-refractory iron deficiency anemia is associated with hepatic adenomas. Originally reported in the setting of type 1a glycogen storage disease, patients had large hepatic adenomas associated with moderate to severe unremitting microcytic anemia and hypoferremia, unresponsive to oral iron and only partially responsive to parenteral iron [47]. The anemia and hypoferremia rapidly resolved after tumor resection. A sample of tumor tissue overexpressed hepcidin mrna which was suppressed in the surrounding liver, suggesting autonomous production of hepcidin by the tumor. A patient with a similar presentation in the setting of a large hepatic adenoma but no history of glycogen disorder was recently reported [48] Iron-Loading Anemias. In iron-loading anemias, such as β-thalassemia and congenital dyserythropoietic anemias, urinary and serum hepcidin are severely decreased in the absence of transfusions [49 51]. The low hepcidin in turn allows excessive iron absorption and development of systemic iron overload, similar to hereditary hemochromatosis. The signal causing hepcidin suppression in iron-loading anemias appears to be generated by high erythropoietic activity and outweighs the effects of the resulting iron overload on hepcidin regulation. As mentioned earlier, GDF15 and TWSG1 are two erythroid factors that may contribute to hepcidin suppression in syndromes with ineffective erythropoiesis [32, 33]. Hepcidin diagnostics may be useful for iron-loading anemias to identify the patients at higher risk of iron toxicity due to severely decreased hepcidin levels. Moreover, future hepcidin agonists may be sufficient to prevent the lifethreatening iron overload in these patients. The first promising evidence of the beneficial effect of hepcidin comes from the th3/+ mouse model of β-thalassemia in which moderate transgenic expression of hepcidin resulted in lower spleen and liver iron content, decreased inefficient erythropoiesis in the spleen, lower spleen weight, and even improvement of hematological parameters (Gardenghi et al., submitted). In chronically transfused patients, hepcidin concentrations are much higher than in nontransfused patients, presumably due to both increased iron load and the alleviation of ineffective erythropoiesis [49, 51]. Interestingly, Origa et al. [51] showed that nontransfused patients have liver iron concentrations similar to those of regularly transfused thalassemia major patients. However, because of the different hepcidin levels, the cellular distribution of iron in the liver differed in these two groups. In nontransfused thalassemia, iron was deposited in hepatocytes, whereas higher hepcidin levels in transfused patients resulted in macrophage iron loading. As a consequence of this difference in cellular iron distribution, serum ferritin levels were much lower in nontransfused patients, and did not adequately reflect the patients liver iron load. Considering that high hepcidin shifts iron distribution to macrophages and decreases intestinal iron absorption, it is possible that hepcidin agonists could be useful even in transfused thalassemia patients. Trapping iron in macrophages where it is less toxic may postpone iron deposition and consequent damage in the parenchyma, but this remains to be investigated Anemia of Inflammation. Anemia of inflammation (AI) develops in the setting of many infections and inflammatory disorders, and some malignancies [52]. Elevated hepcidin is believed to be an important mediator of AI but whether hepcidin is a necessary factor in the AI pathogenesis has not yet been established with certainty. IRIDA and mouse models overexpressing hepcidin demonstrate that elevated
5 Advances in Hematology 5 hepcidin is sufficient to cause hypoferremia and anemia [4]. Moderate overproduction of hepcidin in transgenic mice or in mice bearing hepcidin-producing tumors caused an ironrestricted anemia [4, 53]. Transgenic mice also had blunted erythropoietic response to EPO, another characteristic of AI. In humans, elevated hepcidin was observed in a variety of inflammatory disorders including rheumatologic diseases, inflammatory bowel disease, infections, multiple myeloma, and critical illness [13, 35 37, 54, 55]. The AI phenotype presumably develops from hepcidin-mediated inhibition of iron recycling and absorption. Decreased flow of iron into plasma results in hypoferremia, and because most of the iron in plasma is destined for the bone marrow, lower iron availability hinders hemoglobin synthesis and erythrocyte production. Detecting elevated hepcidin in the presence of hypoferremia and anemia could help distinguish AI from IDA. However, mixed anemia is common, for example, in cases of chronic inflammatory illness with coexisting bleeding or malnutrition. In these conditions, the inflammationmediated increase in hepcidin would be opposed by the effects of iron deficiency. Even without blood loss or malnutrition, when inflammation lasts for years, true iron deficiency may also develop because of the inhibition of intestinal iron absorption by hepcidin. The ability of hepcidin measurements to distinguish the contribution of iron deficiency and inflammation in these conditions will have to be carefully evaluated. It is possible that even normal levels of hepcidin in AI or mixed anemia are inappropriate and perpetuate iron restriction. Obesity, itself a chronic inflammatory condition, was reported to be associated with iron deficiency [56], and this can develop despite adequate iron supply and the absence of blood loss. Serum hepcidin concentrations were recently studied in obese premenopausal women [57] and were found to be in the reference range for the healthy iron-replete women. However, when compared to lean volunteers with similar iron deficiency, hepcidin levels were several-fold higher in obese women, indicating they were inappropriately high considering the obese women s iron deficiency. Thus, in very chronic mild inflammatory conditions even a mild hepcidin excess may be sufficient to tip the balance between iron loss and iron uptake and lead to iron deficiency. Studies of interventions that selectively reduce hepcidin will clarify how essential the role of hepcidin is in each type of inflammation-induced anemia. In a mouse model of AI caused by injections of Brucella abortus, hepcidin antagonists (neutralizing monoclonal antibody to hepcidin) in combination with erythropoiesis-stimulating agents restored normal hemoglobin levels [58], even when erythropoiesisstimulating agents alone were ineffective. Considering the variety of other possible inflammatory erythroid pathologies, ranging from hemolysis to bone marrow suppression, not all inflammatory conditions may respond equally to antihepcidin or combination therapies Anemia of Chronic Kidney Disease. Until recently, anemia of chronic kidney disease (CKD) was thought to be primarily due to the deficiency of erythropoietin. Considering that high amounts of erythropoietin are often needed to restore erythropoiesis, this view has been challenged. This, as well as the beneficial effect of high doses of intravenous iron in CKD patients has focused attention on the possible role of hepcidin and iron restriction in the anemia of CKD [59]. Renal excretion is a major route of hepcidin clearance. When kidney function is normal, urinary hepcidin concentrations correlate well with the circulating hepcidin levels, with no apparent regulation of the excretion process. However, loss of kidney function could decrease hepcidin clearance and lead to the accumulation of hepcidin and the development of iron-restrictive anemia. Hepcidin concentrations were indeed reported to be increased in patients with CKD [54, 60]. Although this could be caused by inflammation which frequently accompanies CKD, even patients without significant inflammation had elevated hepcidin which progressively increased with the increasing severity of CKD [60]. Decreased kidney function is the likely factor contributing to this phenomenon, and some studies have reported inverse correlation between glomerular filtration rate and serum hepcidin [60, 61]. Thus, increased hepcidin levels due to decreased renal clearance as well as due to inflammation may be a significant factor contributing to the development of anemia in CKD and should be considered in the development of new therapies for this disease Resistance to Erythropoietin. Hyporesponsiveness to therapeutic erythropoietin has emerged as an important consequence of inflammation, especially in chronic kidney diseases [62, 63], and may be a consequence of iron restriction imposed by high hepcidin. As mentioned before, hepcidinoverexpressing transgenic mice had blunted response to Epo [4], and neutralization of hepcidin by monoclonal antibody in the B. abortus model of AI restored responsiveness to erythropoietin [58]. Hepcidin measurements thus may be useful for predicting patients response to erythropoietin but large studies will be necessary to test this concept. Large pharmacological doses of Epo can sometimes overcome the resistance of AI to erythropoietin [63]. As was discussed before, Epo injections in mice and humans resulted in suppression of hepcidin production [29, 30], and this may be the mechanism by which high Epo levels overcome iron restriction. It is therefore conceivable that administration of anti-hepcidin therapies together with erythropoiesisstimulating agents may improve patients erythropoietic response and enable the use of lower erythropoietin doses to avoid the potential detrimental effects of high Epo concentrations. 5. The Present and the Future of Hepcidin Diagnostics and Therapeutics in Anemias 5.1. Diagnostics. The evaluation of the diagnostic potential of hepcidin has only recently become possible with the development of assays for bioactive mature hepcidin in serum and urine. The methodologies include competitive ELISAs using biotinylated or radioiodinated hepcidin as
6 6 Advances in Hematology Table 1: Diagnostic and therapeutic potenital of hepcidin in different forms of anemia. Condition Expected hepcidin levels Other iron parameters Hepcidin therapy Iron deficiency anemia Low Low Tsat and ferritin Iron-refractory iron deficiency anemia High Low Tsat and ferritin Antagonist Iron-loading anemias Low (unless transfused) High Tsat and ferritin Agonist Anemia of inflammation High Low Tsat, normal-to-elevated ferritin Antagonist Mixed anemia (AI/IDA) Normal Low Tsat, low-to-normal ferritin Antagonist Chronic kidney disease High Variable Antagonist Erythropoietin resistance High Variable Antagonist Tsat = transferrin saturation. tracers [13, 64], and several mass spectrometry-based assays (MALDI-TOF MS, SELDI, and LC MS/MS) using as internal standards isotopically labeled hepcidin or truncated hepcidin variants [55, 65, 66]. Measurements of prohepcidin in serum have also been reported, but these did not correlate with mature hepcidin concentrations indicating that pro-hepcidin is inadequate as a substitute for measuring biologically relevant hepcidin. A summary of potential uses of hepcidin assays for diagnosing different forms of anemia is listed in Table 1. However, the utility of hepcidin for the diagnosis and prognosis of iron disorders is far from understood and needs to be evaluated in larger clinical studies. Some of the problems for interpreting hepcidin levels may include diurnal fluctuations of hepcidin (lower in the morning, higher in the afternoon) [13, 67], and relative sensitivity to the available iron content of the diet [13]. Hepcidin concentrations may also predict which patients could benefit from oral iron therapy. Several studies of healthy volunteers have shown inverse correlation between hepcidin concentrations and radiolabeled iron absorption [68, 69]. If these results are confirmed in appropriate patient populations, patients with elevated hepcidin levels could be treated with parenteral iron and thus avoid weeks of potentially burdensome and unsuccessful oral iron therapy Therapeutics. The potential use of hepcidin-targeted therapeutics in anemias is summarized in Table 1. Although no hepcidin therapies are yet available, several candidates are currently under development. Hepcidin agonists would be useful for preventing or ameliorating iron overload in nontransfused β-thalassemias and other iron-loading anemias. Small peptides based on hepcidin N-terminal region have been shown to act as agonists in mice in vivo (E. Nemeth, unpublished). The small size should allow eventual development of orally available agonists. Hepcidin antagonists would be expected to benefit patients with diseases of hepcidin excess (Table 1), manifested as iron-restricted anemia and eventually as systemic iron deficiency. Several approaches have been undertaken to develop hepcidin antagonists. Hepcidin-neutralizing antibody has already been successfully used in vivo in a mouse model of AI [58]. Apart from directly interfering with hepcidin activity, other agents which target pathways regulating hepcidin production have also been described. Dorsomorphin, a small-molecule inhibitor of BMP signaling, was shown to block hepcidin induction by iron in vivo [70]. Soluble HJV, also acting as an antagonist of BMP signaling, decreased hepcidin baseline expression in mice and concurrently increased liver iron content [71]. Furthermore, some existing therapies may be acting in part by decreasing hepcidin production. Anticytokine therapies such as anti- IL-6 antibody were shown to suppress hepcidin production and improve anemia [72, 73]. Some erythropoiesisstimulating agents, such as prolyl hydroxylase inhibitors, could also be effective hepcidin suppressors, not only by stimulating erythropoiesis, but also by interfering with the HIF pathway [74]. Undoubtedly, future studies will assess the risks and relative benefits of hepcidin-targeted treatment approaches. 6. Conclusion Recent advances in the understanding of the key role of hepcidin-ferroportin interaction in iron homeostasis and its disorders have clarified the pathogenesis of anemias due to iron restriction as well as anemias accompanied by ironloading. While many mechanistic details of hepcidin and ferroportin regulation remain to be worked out, we will soon see medical applications of these advances. In the coming years, the role of hepcidin assays in the diagnosis, prognosis, and therapeutic stratification of anemias will be explored. Therapeutic interventions specifically targeting the hepcidinferroportin axis for the treatment of anemias are also under development. Disclosure Dr. E. Nemeth is a Cofounder and the Chief Scientific Officer of Intrinsic Life Sciences, LLC, a biotech company developing iron-related diagnostics. Acknowledgments This work was supported by the Grant from the National Institute of Health, R01 DK
7 Advances in Hematology 7 References [1] C. H. Park, E. V. Valore, A. J. Waring, and T. Ganz, Hepcidin, a urinary antimicrobial peptide synthesized in the liver, Journal of Biological Chemistry, vol. 276, no. 11, pp , [2] B. Gagliardo, N. Kubat, A. Faye, et al., Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process, Journal of Hepatology, vol. 50, no. 2, pp , [3] E. V. Valore and T. Ganz, Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin, Blood Cells, Molecules, and Diseases, vol. 40, no. 1, pp , [4] C.N.Roy,H.H.Mak,I.Akpan,G.Losyev,D.Zurakowski,and N. C. Andrews, Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation, Blood, vol. 109, no. 9, pp , [5] A. Roettol, G. Papanikolaou, M. Politou, et al., Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis, Nature Genetics, vol. 33, no. 1, pp , [6] A. Donovan, C. A. Lima, J. L. Pinkus, et al., The iron exporter ferroportin/slc40a1 is essential for iron homeostasis, Cell Metabolism, vol. 1, no. 3, pp , [7] S. Abboud and D. J. Haile, A novel mammalian iron-regulated protein involved in intracellular iron metabolism, Journal of Biological Chemistry, vol. 275, no. 26, pp , [8] A. Donovan, A. Brownlie, Y. Zhou, et al., Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter, Nature, vol. 403, no. 6771, pp , [9] A. T. McKie, P. Marciani, A. Rolfs, et al., A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation, Molecular Cell, vol. 5, no. 2, pp , [10] E. Nemeth, M. S. Tuttle, J. Powelson, et al., Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science, vol. 306, no. 5704, pp , [11] S. Rivera, E. Nemeth, V. Gabayan, M. A. Lopez, D. Farshidi, and T. Ganz, Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs, Blood, vol. 106, no. 6, pp , [12] D.-L. Zhang, R. M. Hughes, H. Ollivierre-Wilson, M. C. Ghosh, and T. A. Rouault, A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression, Cell Metabolism, vol. 9, no. 5, pp , [13] T. Ganz, G. Olbina, D. Girelli, E. Nemeth, and M. Westerman, Immunoassay for human serum hepcidin, Blood, vol. 112, no. 10, pp , [14] M. Pak, M. A. Lopez, V. Gabayan, T. Ganz, and S. Rivera, Suppression of hepcidin during anemia requires erythropoietic activity, Blood, vol. 108, no. 12, pp , [15] T. Ganz, Molecular pathogenesis of anemia of chronic disease, Pediatric Blood and Cancer, vol. 46, no. 5, pp , [16] Y. Xia, J. L. Babitt, Y. Sidis, R. T. Chung, and H. Y. Lin, Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin, Blood, vol. 111, no. 10, pp , [17] B. Andriopoulos Jr., E. Corradini, Y. Xia, et al., BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism, Nature Genetics, vol. 41, no. 4, pp , [18] D. Meynard, L. Kautz, V. Darnaud, F. Canonne-Hergaux, H. Coppin, and M.-P. Roth, Lack of the bone morphogenetic protein BMP6 induces massive iron overload, Nature Genetics, vol. 41, no. 4, pp , [19]J.L.Babitt,F.W.Huang,D.M.Wrighting,etal., Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression, Nature Genetics, vol. 38, no. 5, pp , [20] L. Lin, Y. P. Goldberg, and T. Ganz, Competitive regulation of hepcidin mrnaby soluble and cell-associated hemojuvelin, Blood, vol. 106, no. 8, pp , [21] V. Niederkofler, R. Salie, and S. Arber, Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload, Journal of Clinical Investigation, vol. 115, no. 8, pp , [22] L. Silvestri, A. Pagani, A. Nai, I. de Domenico, J. Kaplan, and C. Camaschella, The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin, Cell Metabolism, vol. 8, no. 6, pp , [23] A.-S. Zhang, F. Yang, K. Meyer, et al., Neogenin-mediated hemojuvelin shedding occurs after hemojuvelin traffics to the plasma membrane, Journal of Biological Chemistry, vol. 283, no. 25, pp , [24] L. Kautz, D. Meynard, A. Monnier, et al., Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver, Blood, vol. 112, no. 4, pp , [25] M. B. Johnson, J. Chen, N. Murchison, F. A. Green, and C. A. Enns, Transferrin receptor 2: evidence for ligand-induced stabilization and redirection to a recycling pathway, Molecular Biology of the Cell, vol. 18, no. 3, pp , [26] G. Ramey, J.-C. Deschemin, and S. Vaulont, Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes, Haematologica, vol. 94, no. 6, pp , [27] T. Goswami and N. C. Andrews, Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing, Journal of Biological Chemistry, vol. 281, no. 39, pp , [28] A. Piperno, D. Girelli, E. Nemeth, et al., Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis, Blood, vol. 110, no. 12, pp , [29] D. R. Ashby, D. P. Gale, M Busbridge, et al., Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin, Haematologica. In press. [30] H. Huang, M. Constante, A. Layoun, and M. M. Santos, Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli, Blood, vol. 113, no. 15, pp , [31] M. Vokurka, J. Krijt, K. Sulc, and E. Necas, Hepcidin mrna levels in mouse liver respond to inhibition of erythropoiesis, Physiological Research, vol. 55, no. 6, pp , [32] T. Tanno, N. V. Bhanu, P. A. Oneal, et al., High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin, Nature Medicine, vol. 13, no. 9, pp , 2007.
8 8 Advances in Hematology [33] T. Tanno, P. Porayette, O. Sripichai, et al., Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells, Blood, vol. 114, no. 1, pp , [34] C. Peyssonnaux, A. S. Zinkernagel, R. A. Schuepbach, et al., Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs), Journal of Clinical Investigation, vol. 117, no. 7, pp , [35] G. Semrin, D. S. Fishman, A. Bousvaros, et al., Impaired intestinal iron absorption in Crohn s disease correlates with disease activity and markers of inflammation, Inflammatory Bowel Diseases, vol. 12, no. 12, pp , [36] S. Sharma, E. Nemeth, Y.-H. Chen, et al., Involvement of hepcidin in the anemia of multiple myeloma, Clinical Cancer Research, vol. 14, no. 11, pp , [37] E. Nemeth, E. V. Valore, M. Territo, G. Schiller, A. Lichtenstein, and T. Ganz, Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein, Blood, vol. 101, no. 7, pp , [38] M. V. Verga Falzacappa, M. Vujic Spasic, R. Kessler, J. Stolte, M. W. Hentze, and M. U. Muckenthaler, STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation, Blood, vol. 109, no. 1, pp , [39] E. Nemeth, S. Rivera, V. Gabayan, et al., IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin, Journal of Clinical Investigation, vol. 113, no. 9, pp , [40] S. Rivera, V. Gabayan, and T. Ganz, In chronic inflammation, there exists an IL-6 independent pathway for the induction of hepcidin, vol. 104, no. 11, 2004, Abstract no [41] C. Peyssonnaux, A. S. Zinkernagel, V. Datta, X. Lauth, R. S. Johnson, and V. Nizet, TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens, Blood, vol. 107, no. 9, pp , [42] S. Sazawal, R. E. Black, M. Ramsan, et al., Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial, The Lancet, vol. 367, no. 9505, pp , [43] M. A. Melis, M. Cau, R. Congiu, et al., A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron, Haematologica, vol. 93, no. 10, pp , [44] K. E. Finberg, M. M. Heeney, D. R. Campagna, et al., Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA), Nature Genetics, vol. 40, no. 5, pp , [45] J. C. Chambers, W. Zhang, Y. Li, et al., Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels, Nature Genetics, vol. 41, no. 11, pp , [46] B. Benyamin, M. A. R. Ferreira, G. Willemsen, et al., Common variants in TMPRSS6 are associated with iron status and erythrocyte volume, Nature Genetics, vol. 41, no. 11, pp , [47] D. A. Weinstein, C. N. Roy, M. D. Fleming, M. F. Loda, J. I. Wolfsdorf, and N. C. Andrews, Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease, Blood, vol. 100, no. 10, pp , [48] A. Y. F. Chung, K. W. Leo, G. C. Wong, K. L. Chuah, J. W. Ren, and C. G. L. Lee, Giant hepatocellular adenoma presenting with chronic iron deficiency anemia, American Journal of Gastroenterology, vol. 101, no. 9, pp , [49] S. L. Kearney, E. Nemeth, E. J. Neufeld, et al., Urinary hepcidin in congenital chronic anemias, Pediatric Blood and Cancer, vol. 48, no. 1, pp , [50] E. Nemeth and T. Ganz, Hepcidin and iron-loading anemias, Haematologica, vol. 91, no. 6, pp , [51] R. Origa, R. Galanello, T. Ganz, et al., Liver iron concentrations and urinary hepcidin in β-thalassemia, Haematologica, vol. 92, no. 5, pp , [52] G. E. Cartwright, The anemia of chronic disorders, Seminars in Hematology, vol. 3, no. 4, pp , [53] S.Rivera,L.Liu,E.Nemeth,V.Gabayan,O.E.Sorensen,andT. Ganz, Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia, Blood, vol. 105, no. 4, pp , [54] H. Li, M. J. Rose, L. Tran, et al., Development of a method for the sensitive and quantitative determination of hepcidin in human serum using LC-MS/MS, Journal of Pharmacological and Toxicological Methods, vol. 59, no. 3, pp , [55] E. H. J. M. Kemna, H. Tjalsma, V. N. Podust, and D. W. Swinkels, Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications, Clinical Chemistry, vol. 53, no. 4, pp , [56] L. M. Tussing-Humphreys, H. Liang, E. Nemeth, S. Freels, and C. A. Braunschweig, Excess adiposity, inflammation, and iron-deficiency in female adolescents, Journal of the American Dietetic Association, vol. 109, no. 2, pp , [57] L. M. Tussing-Humphreys, E. Nemeth, G. Fantuzzi, et al., Elevated systemic hepcidin and iron depletion in obese premenopausal females, Obesity.Inpress. [58] B. J. Sasu, M. Hainu, T. C. Boone, et al., Hepcidin, hepcidin antagonists and methods of use, US patent no. US , Amgen Inc.Thousand Oaks, Calif, USA, September [59] B. Young and J. Zaritsky, Hepcidin for clinicians, Clinical Journal of the American Society of Nephrology,vol.4,no.8,pp , [60] J. Zaritsky, B. Young, H. J. Wang, et al., Hepcidin a potential novel biomarker for iron status in chronic kidney disease, Clinical Journal of the American Society of Nephrology, vol. 4, no. 6, pp , [61] D. R. Ashby, D. P. Gale, M. Busbridge, et al., Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease, Kidney International, vol. 75, no. 9, pp , [62] J. W. Adamson, Hyporesponsiveness to erythropoiesis stimulating agents in chronic kidney disease: the many faces of inflammation, Advances in Chronic Kidney Disease, vol. 16, no. 2, pp , [63] J. Elliott, D. Mishler, and R. Agarwal, Hyporesponsiveness to erythropoietin: causes and management, Advances in Chronic Kidney Disease, vol. 16, no. 2, pp , [64] M. Busbridge, C. Griffiths, D. R. Ashby, et al., Development of a novel immunoassay for the iron regulatory peptide hepcidin, British Journal of Biomedical Science, vol. 66, no. 3, pp , [65] A. Castagna, N. Campostrini, F. Zaninotto, and D. Girelli, Hepcidin assay in serum by SELDI-TOF-MS and other approaches, Journal of Proteomics.In press. [66] S. S. Bansal, J. M. Halket, J. Fusova, et al., Quantification of hepcidin using matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry, Rapid Communications in Mass Spectrometry, vol. 23, no. 11, pp , 2009.
9 Advances in Hematology 9 [67] J. J. C. Kroot, J. C. M. Hendriks, C. M. M. Laarakkers, et al., (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies, Analytical Biochemistry, vol. 389, no. 2, pp , [68] M. F. Young, R. P. Glahn, M. Ariza-Nieto, et al., Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women, American Journal of Clinical Nutrition, vol. 89, no. 2, pp , [69] M. Ruivard, F. Laine, T. Ganz, et al., Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin, JournalofHepatology, vol. 50, no. 6, pp , [70] P. B. Yu, C. C. Hong, C. Sachidanandan, et al., Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism, Nature Chemical Biology, vol. 4, no. 1, pp , [71] J. L. Babitt, F. W. Huang, Y. Xia, Y. Sidis, N. C. Andrews, and H. Y. Lin, Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance, Journal of Clinical Investigation, vol. 117, no. 7, pp , [72] N. Nishimoto, Y. Kanakura, K. Aozasa, et al., Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease, Blood, vol. 106, no. 8, pp , [73] H. Kawabata, N. Tomosugi, J. Kanda, Y. Tanaka, K. Yoshizaki, and T. Uchiyama, Anti-interleukin 6 receptor antibody tocilizumab reduces the level of serum hepcidin in patients with multicentric Castleman s disease, Haematologica, vol. 92, no. 6, pp , [74] S. Klaus, M. Arend, P. Fourney, et al., Induction of erythropoiesis and iron utilization by the HIF prolyl hydroxylase inhibitor FG-4592, Journal of the American Society of Nephrology, vol. 16, p. 49A, 2005.
Role of hepcidin in the pathophysiology and diagnosis of anemia
 BLOOD RESEARCH VOLUME 48 ㆍ NUMBER 1 March 2013 REVIEW ARTICLE Role of hepcidin in the pathophysiology and diagnosis of anemia Guido D Angelo Ematologia/Coagulazione, Laboratorio di Chimica-Clinica, Ematologia
BLOOD RESEARCH VOLUME 48 ㆍ NUMBER 1 March 2013 REVIEW ARTICLE Role of hepcidin in the pathophysiology and diagnosis of anemia Guido D Angelo Ematologia/Coagulazione, Laboratorio di Chimica-Clinica, Ematologia
Discovery. Hepcidin Today. Hepcidin: discovery June 2000: Man: Plasma ultrafiltrate Liver Expressed Antimicrobial Peptide
 Hepcidin Today Rachel van Swelm 10 05 2018 ISLH www.radboud ironcenter.com; www.hepcidinanalysis.com Discovery Hepcidin: discovery June 2000: Man: Plasma ultrafiltrate Liver Expressed Antimicrobial Peptide
Hepcidin Today Rachel van Swelm 10 05 2018 ISLH www.radboud ironcenter.com; www.hepcidinanalysis.com Discovery Hepcidin: discovery June 2000: Man: Plasma ultrafiltrate Liver Expressed Antimicrobial Peptide
The Hepcidin-Ferroportin System as a Therapeutic Target in Anemias and Iron Overload Disorders
 UPDATES ON DISORDERS OF IRON UTILIZATION AND DISTRIBUTION The Hepcidin-Ferroportin System as a Therapeutic Target in Anemias and Iron Overload Disorders Tomas Ganz 1 and Elizabeta Nemeth 1 1 Department
UPDATES ON DISORDERS OF IRON UTILIZATION AND DISTRIBUTION The Hepcidin-Ferroportin System as a Therapeutic Target in Anemias and Iron Overload Disorders Tomas Ganz 1 and Elizabeta Nemeth 1 1 Department
Unraveling Mechanisms Regulating Systemic Iron Homeostasis
 UPDATES ON DISORDERS OF IRON UTILIZATION AND DISTRIBUTION Unraveling Mechanisms Regulating Systemic Iron Homeostasis Karin E. Finberg 1 1 Duke University Medical School, Durham, NC Systemic iron balance
UPDATES ON DISORDERS OF IRON UTILIZATION AND DISTRIBUTION Unraveling Mechanisms Regulating Systemic Iron Homeostasis Karin E. Finberg 1 1 Duke University Medical School, Durham, NC Systemic iron balance
Into the matrix: regulation of the iron regulatory hormone hepcidin by matriptase-2
 Emerging Science Into the matrix: regulation of the iron regulatory hormone hepcidin by matriptase-2 Mitchell D Knutson Matriptase-2 is a recently identified membrane-bound, cell-surface serine protease
Emerging Science Into the matrix: regulation of the iron regulatory hormone hepcidin by matriptase-2 Mitchell D Knutson Matriptase-2 is a recently identified membrane-bound, cell-surface serine protease
THE ROLE OF HEMOJUVELIN IN IRON DEFICIENCY AND OVERLOAD
 THE ROLE OF HEMOJUVELIN IN IRON DEFICIENCY AND OVERLOAD Clara Camaschella Vita-Salute University and San Raffaele Scientific Institute Milan, Italy EHA ESH joint Workshop Cascais, Portugal, April 16-18,
THE ROLE OF HEMOJUVELIN IN IRON DEFICIENCY AND OVERLOAD Clara Camaschella Vita-Salute University and San Raffaele Scientific Institute Milan, Italy EHA ESH joint Workshop Cascais, Portugal, April 16-18,
Hepcidin and iron regulation, 10 years later
 Review article Hepcidin and iron regulation, 10 years later Tomas Ganz 1 1 Departments of Medicine and Pathology, David Geffen School of Medicine at UCLA, Los Angeles, CA Introduction Under evolutionary
Review article Hepcidin and iron regulation, 10 years later Tomas Ganz 1 1 Departments of Medicine and Pathology, David Geffen School of Medicine at UCLA, Los Angeles, CA Introduction Under evolutionary
Systemic iron homeostasis is dependent on the hepatic
 LIVER BIOLOGY/PATHOBIOLOGY Evidence for Distinct Pathways of Hepcidin Regulation by Acute and Chronic Iron Loading in Mice Emilio Ramos, 1 Léon Kautz, 4,5 Richard Rodriguez, 2 Michael Hansen, 2 Victoria
LIVER BIOLOGY/PATHOBIOLOGY Evidence for Distinct Pathways of Hepcidin Regulation by Acute and Chronic Iron Loading in Mice Emilio Ramos, 1 Léon Kautz, 4,5 Richard Rodriguez, 2 Michael Hansen, 2 Victoria
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1823 (2012) 1434 1443 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbamcr Review Hepcidin and
Biochimica et Biophysica Acta 1823 (2012) 1434 1443 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbamcr Review Hepcidin and
Iron and hepcidin: a story of recycling and balance
 HAM-WASSERMAN MANUSCRIPT Iron and hepcidin: a story of recycling and balance Clara Camaschella 1 1 Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy To avoid iron deficiency and
HAM-WASSERMAN MANUSCRIPT Iron and hepcidin: a story of recycling and balance Clara Camaschella 1 1 Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy To avoid iron deficiency and
2011 ASH Annual Meeting Targeting the Hepcidin Pathway with RNAi Therapeutics for the Treatment of Anemia. December 12, 2011
 211 ASH Annual Meeting Targeting the Hepcidin Pathway with RNAi Therapeutics for the Treatment of Anemia December 12, 211 Hepcidin is Central Regulator of Iron Homeostasis Hepcidin is liver-expressed,
211 ASH Annual Meeting Targeting the Hepcidin Pathway with RNAi Therapeutics for the Treatment of Anemia December 12, 211 Hepcidin is Central Regulator of Iron Homeostasis Hepcidin is liver-expressed,
Hepcidin Downregulation by Repeated Bleeding Is Not Mediated by Soluble Hemojuvelin
 Physiol. Res. 59: 53-59, 2010 Hepcidin Downregulation by Repeated Bleeding Is Not Mediated by Soluble Hemojuvelin J. KRIJT 1, Y. FUJIKURA 1, L. ŠEFC 1, M. VOKURKA 1, T. HLOBEŇOVÁ 1, E. NEČAS 1 1 Institute
Physiol. Res. 59: 53-59, 2010 Hepcidin Downregulation by Repeated Bleeding Is Not Mediated by Soluble Hemojuvelin J. KRIJT 1, Y. FUJIKURA 1, L. ŠEFC 1, M. VOKURKA 1, T. HLOBEŇOVÁ 1, E. NEČAS 1 1 Institute
Review Article Hepcidin: A Critical Regulator of Iron Metabolism during Hypoxia
 Advances in Hematology Volume 2011, Article ID 510304, 7 pages doi:10.1155/2011/510304 Review Article Hepcidin: A Critical Regulator of Iron Metabolism during Hypoxia Korry J. Hintze 1 and James P. McClung
Advances in Hematology Volume 2011, Article ID 510304, 7 pages doi:10.1155/2011/510304 Review Article Hepcidin: A Critical Regulator of Iron Metabolism during Hypoxia Korry J. Hintze 1 and James P. McClung
Role of Serum Hepcidin levels in the Diagnosis of Iron Deficiency Anemia in Children in Saudi Arabia
 Role of Serum Hepcidin levels in the Diagnosis of Iron Deficiency Anemia in Children in Saudi Arabia Mahmoud Mohamed Elgari*, Al-Oufi F¹, Mohammed alsalmi, M. Kurdi, NA Ibrahim, Abdelgadir Elmugadam College
Role of Serum Hepcidin levels in the Diagnosis of Iron Deficiency Anemia in Children in Saudi Arabia Mahmoud Mohamed Elgari*, Al-Oufi F¹, Mohammed alsalmi, M. Kurdi, NA Ibrahim, Abdelgadir Elmugadam College
Fourth European Symposium on Rare Anaemias. Vita-Salute University - San Raffaele Scientific Institute, Milano
 Fourth European Symposium on Rare Anaemias Clara Camaschella Vita-Salute University - San Raffaele Scientific Institute, Milano Sofia, Bulgaria, November 19-20, 2011 The iron cycle Hepcidin (Jordan et
Fourth European Symposium on Rare Anaemias Clara Camaschella Vita-Salute University - San Raffaele Scientific Institute, Milano Sofia, Bulgaria, November 19-20, 2011 The iron cycle Hepcidin (Jordan et
Hepcidin downregulation by repeated bleeding is not mediated by soluble hemojuvelin
 1 Hepcidin downregulation by repeated bleeding is not mediated by soluble hemojuvelin Jan Krijt, Yuzo Fujikura, Luděk Šefc, Martin Vokurka, Tereza Hlobeňová and Emanuel Nečas Institute of Pathophysiology
1 Hepcidin downregulation by repeated bleeding is not mediated by soluble hemojuvelin Jan Krijt, Yuzo Fujikura, Luděk Šefc, Martin Vokurka, Tereza Hlobeňová and Emanuel Nečas Institute of Pathophysiology
Immunoassay for Human Serum Hepcidin
 Blood First Edition Paper, prepublished online August 8, 28; DOI 1.1182/blood-28-2-139915 Immunoassay for Human Serum Hepcidin Tomas Ganz 1,2, Gordana Olbina 1, Domenico Girelli 3, Elizabeta Nemeth 1,2
Blood First Edition Paper, prepublished online August 8, 28; DOI 1.1182/blood-28-2-139915 Immunoassay for Human Serum Hepcidin Tomas Ganz 1,2, Gordana Olbina 1, Domenico Girelli 3, Elizabeta Nemeth 1,2
The Regulation of Hepcidin and Its Effects on Systemic and Cellular Iron Metabolism
 IRON IN HEMATOLOGY The Regulation of Hepcidin and Its Effects on Systemic and Cellular Iron Metabolism Mark D. Fleming 1 1 Associate Professor of Pathology, Children s Hospital Boston; Harvard Medical
IRON IN HEMATOLOGY The Regulation of Hepcidin and Its Effects on Systemic and Cellular Iron Metabolism Mark D. Fleming 1 1 Associate Professor of Pathology, Children s Hospital Boston; Harvard Medical
Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease
 original article http://www.kidney-international.org & 29 International Society of Nephrology see commentary on page 873 Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in
original article http://www.kidney-international.org & 29 International Society of Nephrology see commentary on page 873 Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in
Iron age: novel targets for iron overload
 IRON HOMEOSTASIS &CHRONIC DISEASE:DISORDERS OF IRON OVERLOAD Iron age: novel targets for iron overload Carla Casu 1 and Stefano Rivella 1,2 1 Department of Pediatrics, Division of Hematology-Oncology,
IRON HOMEOSTASIS &CHRONIC DISEASE:DISORDERS OF IRON OVERLOAD Iron age: novel targets for iron overload Carla Casu 1 and Stefano Rivella 1,2 1 Department of Pediatrics, Division of Hematology-Oncology,
Manipulation of the hepcidin pathway for therapeutic purposes
 Manipulation of the hepcidin pathway for therapeutic purposes Eileen Fung and Elizabeta Nemeth Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, USA REVIEW
Manipulation of the hepcidin pathway for therapeutic purposes Eileen Fung and Elizabeta Nemeth Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, USA REVIEW
Metabolismo del ferro in condizioni normali e patologiche
 Metabolismo del ferro in condizioni normali e patologiche Clara Camaschella Università Vita-Salute e IRCCS San Raffaele, Milano Simposio SIES 41 Congresso Nazionale SIE - Bologna 14-17 ottobre 2007 Metabolismo
Metabolismo del ferro in condizioni normali e patologiche Clara Camaschella Università Vita-Salute e IRCCS San Raffaele, Milano Simposio SIES 41 Congresso Nazionale SIE - Bologna 14-17 ottobre 2007 Metabolismo
Clinical Study Serum Hepcidin Levels and Reticulocyte Hemoglobin Concentrations as Indicators of the Iron Status of Peritoneal Dialysis Patients
 International Nephrology Volume 2012, Article ID 239476, 7 pages doi:10.1155/2012/239476 Clinical Study Serum Hepcidin Levels and Reticulocyte Hemoglobin Concentrations as Indicators of the Iron Status
International Nephrology Volume 2012, Article ID 239476, 7 pages doi:10.1155/2012/239476 Clinical Study Serum Hepcidin Levels and Reticulocyte Hemoglobin Concentrations as Indicators of the Iron Status
Next-Generation Biomarkers for Iron Status
 Applications/End Users Baetge EE, Dhawan A, Prentice AM (eds): Next-Generation Nutritional Biomarkers to Guide Better Health Care. Nestlé Nutr Inst Workshop Ser, vol 84, pp 59 69, (DOI: 10.1159/000436955)
Applications/End Users Baetge EE, Dhawan A, Prentice AM (eds): Next-Generation Nutritional Biomarkers to Guide Better Health Care. Nestlé Nutr Inst Workshop Ser, vol 84, pp 59 69, (DOI: 10.1159/000436955)
Iron Regulation of Hepcidin Despite Attenuated Smad1,5,8 Signaling in Mice Without Transferrin Receptor 2 or Hfe
 GASTROENTEROLOGY 2011;141:1907 1914 Iron Regulation of Hepcidin Despite Attenuated Smad1,5,8 Signaling in Mice Without Transferrin Receptor 2 or Hfe ELENA CORRADINI,* MOLLY ROZIER, DELPHINE MEYNARD,* ADAM
GASTROENTEROLOGY 2011;141:1907 1914 Iron Regulation of Hepcidin Despite Attenuated Smad1,5,8 Signaling in Mice Without Transferrin Receptor 2 or Hfe ELENA CORRADINI,* MOLLY ROZIER, DELPHINE MEYNARD,* ADAM
Iron: a global issue in hematology. Clara Camaschella, MD
 Iron: a global issue in hematology Clara Camaschella, MD Università Vita Salute San Raffaele e IRCCS San Raffaele - Milano Firenze, 18-19 settembre 2015 Clara Camaschella I have nothing to disclose Iron
Iron: a global issue in hematology Clara Camaschella, MD Università Vita Salute San Raffaele e IRCCS San Raffaele - Milano Firenze, 18-19 settembre 2015 Clara Camaschella I have nothing to disclose Iron
Review Article. The liver: conductor of systemic iron balance. Iron in the body. Iron transport to cells and absorption by cells
 Review Article From www.bloodjournal.org by guest on August 17, 2018. For personal use only. The liver: conductor of systemic iron balance Delphine Meynard, Jodie L. Babitt, and Herbert Y. Lin Program
Review Article From www.bloodjournal.org by guest on August 17, 2018. For personal use only. The liver: conductor of systemic iron balance Delphine Meynard, Jodie L. Babitt, and Herbert Y. Lin Program
The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
 Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of β-thalassemia intermedia The Harvard community
Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of β-thalassemia intermedia The Harvard community
Review Article Unexplained Aspects of Anemia of Inflammation
 Advances in Hematology Volume 2010, Article ID 508739, 5 pages doi:10.1155/2010/508739 Review Article Unexplained Aspects of Anemia of Inflammation Elizabeth A. Price 1, 2 and Stanley L. Schrier 1 1 Department
Advances in Hematology Volume 2010, Article ID 508739, 5 pages doi:10.1155/2010/508739 Review Article Unexplained Aspects of Anemia of Inflammation Elizabeth A. Price 1, 2 and Stanley L. Schrier 1 1 Department
Assessing Iron Deficiency in Adults. Chris Theberge. Iron (Fe) deficiency remains as one of the major global public health problems for
 Assessing Iron Deficiency in Adults Chris Theberge Iron (Fe) deficiency remains as one of the major global public health problems for two reasons. It affects about one fourth of the world s population
Assessing Iron Deficiency in Adults Chris Theberge Iron (Fe) deficiency remains as one of the major global public health problems for two reasons. It affects about one fourth of the world s population
Iron and hepcidin: a story of recycling and balance
 Iron and hepcidin: a story of recycling and balance Domenico Girelli (Medicina Generale a indirizzo Immuno-Ematologico e Emocoagulativo, Azienda Ospedaliera Universitaria Integrata VERONA) Special Conference
Iron and hepcidin: a story of recycling and balance Domenico Girelli (Medicina Generale a indirizzo Immuno-Ematologico e Emocoagulativo, Azienda Ospedaliera Universitaria Integrata VERONA) Special Conference
BMP6 and BMP4 expression in patients with cancer-related anemia and its relationship with hepcidin and s-hjv
 BMP6 and BMP4 expression in patients with cancer-related anemia and its relationship with hepcidin and s-hjv Y.J. Shi and X.T. Pan Affiliated Taicang Hospital of Suzhou University, Taicang, Jiangsu, China
BMP6 and BMP4 expression in patients with cancer-related anemia and its relationship with hepcidin and s-hjv Y.J. Shi and X.T. Pan Affiliated Taicang Hospital of Suzhou University, Taicang, Jiangsu, China
Réunion annuelle de pathologie digestive. Iron Metabolism
 Réunion annuelle de pathologie digestive Hopital Cochin-Hôtel Dieu Iron Metabolism 3 Février 2012 Sophie Vaulont Iron metabolism endosome steap IRP Pigeon et ferritin al., 2001, JBC, 276, 7811-7819 use
Réunion annuelle de pathologie digestive Hopital Cochin-Hôtel Dieu Iron Metabolism 3 Février 2012 Sophie Vaulont Iron metabolism endosome steap IRP Pigeon et ferritin al., 2001, JBC, 276, 7811-7819 use
NIH Public Access Author Manuscript Nat Genet. Author manuscript; available in PMC 2011 May 30.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Genet. 2008 May ; 40(5): 569 571. doi:10.1038/ng.130. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA)
NIH Public Access Author Manuscript Published in final edited form as: Nat Genet. 2008 May ; 40(5): 569 571. doi:10.1038/ng.130. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA)
Review Article Crosstalk between Iron Metabolism and Erythropoiesis
 Advances in Hematology Volume 2010, Article ID 605435, 12 pages doi:10.1155/2010/605435 Review Article Crosstalk between Iron Metabolism and Erythropoiesis Huihui Li and Yelena Z. Ginzburg Lindsley F.
Advances in Hematology Volume 2010, Article ID 605435, 12 pages doi:10.1155/2010/605435 Review Article Crosstalk between Iron Metabolism and Erythropoiesis Huihui Li and Yelena Z. Ginzburg Lindsley F.
Microcytic Hypochromic Anemia An Approach to Diagnosis
 Microcytic Hypochromic Anemia An Approach to Diagnosis Decreased hemoglobin synthesis gives rise to microcytic hypochromic anemias. Hypochromic anemias are characterized by normal cellular proliferation
Microcytic Hypochromic Anemia An Approach to Diagnosis Decreased hemoglobin synthesis gives rise to microcytic hypochromic anemias. Hypochromic anemias are characterized by normal cellular proliferation
Disease Pathogenesis and Research Progression of Renal Anemia
 2018 3rd International Conference on Life Sciences, Medicine, and Health (ICLSMH 2018) Disease Pathogenesis and Research Progression of Renal Anemia Yingying Liu, Qi Jiang* Department of Nephrology, China-Japan
2018 3rd International Conference on Life Sciences, Medicine, and Health (ICLSMH 2018) Disease Pathogenesis and Research Progression of Renal Anemia Yingying Liu, Qi Jiang* Department of Nephrology, China-Japan
Review. Laboratory use of hepcidin in renal transplant recipients. Introduction. Lucija Šimetić *1,2, Lada Zibar 3,4
 Review Laboratory use of hepcidin in renal transplant recipients Lucija Šimetić *1,2, Lada Zibar 3,4 1 Department of Clinical Laboratory Diagnostics, Osijek University Hospital, Osijek, Croatia 2 Department
Review Laboratory use of hepcidin in renal transplant recipients Lucija Šimetić *1,2, Lada Zibar 3,4 1 Department of Clinical Laboratory Diagnostics, Osijek University Hospital, Osijek, Croatia 2 Department
Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis
 Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis Qingdu Liu,, Knut Niss, Volker H. Haase J Clin Invest. 2012;122(12):4635-4644. https://doi.org/10.1172/jci63924. Research
Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis Qingdu Liu,, Knut Niss, Volker H. Haase J Clin Invest. 2012;122(12):4635-4644. https://doi.org/10.1172/jci63924. Research
YEAR III Pharm.D Dr. V. Chitra
 YEAR III Pharm.D Dr. V. Chitra Anemia can be defined as a reduction in the hemoglobin,hematocrit or red cell number. In physiologic terms an anemia is any disorder in which the patient suffers from tissue
YEAR III Pharm.D Dr. V. Chitra Anemia can be defined as a reduction in the hemoglobin,hematocrit or red cell number. In physiologic terms an anemia is any disorder in which the patient suffers from tissue
Serum Hepcidin in Haemodialysis Patients: Associations with Iron Status and Microinflammation
 The Journal of International Medical Research 2011; 39: 1961 1967 Serum Hepcidin in Haemodialysis Patients: Associations with Iron Status and Microinflammation Y XU, XQ DING, JZ ZOU, ZH LIU, SH JIANG AND
The Journal of International Medical Research 2011; 39: 1961 1967 Serum Hepcidin in Haemodialysis Patients: Associations with Iron Status and Microinflammation Y XU, XQ DING, JZ ZOU, ZH LIU, SH JIANG AND
HHS Public Access Author manuscript Ann N Y Acad Sci. Author manuscript; available in PMC 2017 March 01.
 New strategies to target iron metabolism for the treatment of beta thalassemia Paraskevi Rea Oikonomidou 1, Carla Casu 1, and Stefano Rivella 1,2 1 Department of Pediatrics, Division of Hematology, Children
New strategies to target iron metabolism for the treatment of beta thalassemia Paraskevi Rea Oikonomidou 1, Carla Casu 1, and Stefano Rivella 1,2 1 Department of Pediatrics, Division of Hematology, Children
In Focus - Micronutrients and Obesity: iron deficiency & obesity.
 In Focus - Micronutrients and Obesity: iron deficiency & obesity. Ana Carla Cepeda López MD PhD Universidad de Monterrey, Vicerrectoría de Ciencias de la Salud, Departamento de Ciencias Básicas. Monterrey,
In Focus - Micronutrients and Obesity: iron deficiency & obesity. Ana Carla Cepeda López MD PhD Universidad de Monterrey, Vicerrectoría de Ciencias de la Salud, Departamento de Ciencias Básicas. Monterrey,
BMP6 Treatment Compensates for the Molecular Defect and Ameliorates Hemochromatosis in Hfe Knockout Mice
 GASTROENTEROLOGY 2010;139:1721 1729 BMP6 Treatment Compensates for the Molecular Defect and Ameliorates Hemochromatosis in Hfe Knockout Mice ELENA CORRADINI,*, PAUL J. SCHMIDT, DELPHINE MEYNARD,* CINZIA
GASTROENTEROLOGY 2010;139:1721 1729 BMP6 Treatment Compensates for the Molecular Defect and Ameliorates Hemochromatosis in Hfe Knockout Mice ELENA CORRADINI,*, PAUL J. SCHMIDT, DELPHINE MEYNARD,* CINZIA
Iron deficiency anemia and porphyrias
 Iron deficiency anemia and porphyrias Fleur Wolff¹, Frédéric Cotton¹, Axelle Gilles² ¹Department of clinical chemistry, Hôpital Erasme, ULB ²Department of hematology, Hôpital Erasme, ULB BHS, November
Iron deficiency anemia and porphyrias Fleur Wolff¹, Frédéric Cotton¹, Axelle Gilles² ¹Department of clinical chemistry, Hôpital Erasme, ULB ²Department of hematology, Hôpital Erasme, ULB BHS, November
Journal of American Science 2018;14(10)
 Hepcidin Level and Iron Status in End Stage Renal Disease (ESRD) Patients Tarek El Baz 1, Fawzy Hamed 1, Amr Mohab 3, Abdallah Mahmoud, Magdy El-Said 1, Osama Khamis 1, Amgad Awad 1, Haytham Sabry 1 and
Hepcidin Level and Iron Status in End Stage Renal Disease (ESRD) Patients Tarek El Baz 1, Fawzy Hamed 1, Amr Mohab 3, Abdallah Mahmoud, Magdy El-Said 1, Osama Khamis 1, Amgad Awad 1, Haytham Sabry 1 and
Liver Iron Modulates Hepcidin Expression During Chronically Elevated Erythropoiesis in Mice
 LIVER BIOLOGY/PATHOBIOLOGY Liver Iron Modulates Hepcidin Expression During Chronically Elevated Erythropoiesis in Mice Vıctor Dıaz, 1,2* Elena Gammella, 3* Stefania Recalcati, 3 Paolo Santambrogio, 4 Arianne
LIVER BIOLOGY/PATHOBIOLOGY Liver Iron Modulates Hepcidin Expression During Chronically Elevated Erythropoiesis in Mice Vıctor Dıaz, 1,2* Elena Gammella, 3* Stefania Recalcati, 3 Paolo Santambrogio, 4 Arianne
Iron-refractory iron deficiency anemia: new molecular mechanisms
 http://www.kidney-international.org & 2009 International Society of Nephrology Iron-refractory iron deficiency anemia: new molecular mechanisms Yujie Cui 1,2, Qingyu Wu 1,3 and Yiqing Zhou 1 1 Cyrus Tang
http://www.kidney-international.org & 2009 International Society of Nephrology Iron-refractory iron deficiency anemia: new molecular mechanisms Yujie Cui 1,2, Qingyu Wu 1,3 and Yiqing Zhou 1 1 Cyrus Tang
Is the acronym IRIDA acceptable for slow responders to iron in the presence of TMPRSS6 mutations?
 The Turkish Journal of Pediatrics 2013; 55: 479-484 Original Is the acronym IRIDA acceptable for slow responders to iron in the presence of TMPRSS6 mutations? Ebru Yılmaz-Keskin 1, Ertan Sal 1, Luigia
The Turkish Journal of Pediatrics 2013; 55: 479-484 Original Is the acronym IRIDA acceptable for slow responders to iron in the presence of TMPRSS6 mutations? Ebru Yılmaz-Keskin 1, Ertan Sal 1, Luigia
Hepcidin as a Novel Biomarker of Congestive Hepatopathy in Advanced Heart Failure Patients
 Christine J. Chung Doris Duke Clinical Research Fellow 7/7/2011 Hepcidin as a Novel Biomarker of Congestive Hepatopathy in Advanced Heart Failure Patients I. Study Purpose and Rationale Background: Heart
Christine J. Chung Doris Duke Clinical Research Fellow 7/7/2011 Hepcidin as a Novel Biomarker of Congestive Hepatopathy in Advanced Heart Failure Patients I. Study Purpose and Rationale Background: Heart
Transferrin Receptors and Hematopoiesis: Review
 Institute of Experimental Morphology, Pathology and Anthropology with Museum Bulgarian Anatomical Society Acta morphologica et anthropologica, 23 Sofia 2016 Transferrin Receptors and Hematopoiesis: Review
Institute of Experimental Morphology, Pathology and Anthropology with Museum Bulgarian Anatomical Society Acta morphologica et anthropologica, 23 Sofia 2016 Transferrin Receptors and Hematopoiesis: Review
Introduction 5/2/2013 IRON RELATED GENES AND OXIDATIVE STRESS IN NON- ALCOHOLIC STEATOHEPATITIS. Iron Physiology. Iron Physiology
 // IRON RELATED GENES AND OXIDATIVE STRESS IN NON- ALCOHOLIC STEATOHEPATITIS DIANA MOYA, MD PEDIATRIC GASTROENTEROLOGY FELLOW DIGESTIVE DISEASES & NUTRITION CENTER MAY,. Iron Physiology. /NAFLD. Iron Metabolism
// IRON RELATED GENES AND OXIDATIVE STRESS IN NON- ALCOHOLIC STEATOHEPATITIS DIANA MOYA, MD PEDIATRIC GASTROENTEROLOGY FELLOW DIGESTIVE DISEASES & NUTRITION CENTER MAY,. Iron Physiology. /NAFLD. Iron Metabolism
Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli
 RED CELLS, IRON, AND ERYTHROPOIESIS Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli Hua Huang, 1 Marco Constante, 1 Antonio Layoun, 1 and Manuela M. Santos 1
RED CELLS, IRON, AND ERYTHROPOIESIS Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli Hua Huang, 1 Marco Constante, 1 Antonio Layoun, 1 and Manuela M. Santos 1
No Disclosures 03/20/2019. Learning Objectives. Renal Anemia: The Basics
 Renal Anemia: The Basics Meredith Atkinson, M.D., M.H.S. Associate Professor of Pediatrics Johns Hopkins School of Medicine 16 March 2019 No Disclosures Learning Objectives At the end of this session the
Renal Anemia: The Basics Meredith Atkinson, M.D., M.H.S. Associate Professor of Pediatrics Johns Hopkins School of Medicine 16 March 2019 No Disclosures Learning Objectives At the end of this session the
HYPOXIA-DEPENDENT HEPCIDIN DOWN-REGULATION: IN VITRO AND IN VIVO STUDIES
 University of Milano-Bicocca School of Medicine and Surgery PhD in Experimental Hematology, XXVII cycle HYPOXIA-DEPENDENT HEPCIDIN DOWN-REGULATION: IN VITRO AND IN VIVO STUDIES Dr. Giulia Ravasi Coordinator:
University of Milano-Bicocca School of Medicine and Surgery PhD in Experimental Hematology, XXVII cycle HYPOXIA-DEPENDENT HEPCIDIN DOWN-REGULATION: IN VITRO AND IN VIVO STUDIES Dr. Giulia Ravasi Coordinator:
Iron Metabolism in Thalassemia and Sickle Cell Disease.
 MEDITERRANEAN JOURNAL OF HEMATOLOGY AND INFECTIOUS DISEASES www.mjhid.org ISSN 2035-3006 Review article Iron Metabolism in Thalassemia and Sickle Cell Disease. Raffaella Mariani, Paola Trombini, Matteo
MEDITERRANEAN JOURNAL OF HEMATOLOGY AND INFECTIOUS DISEASES www.mjhid.org ISSN 2035-3006 Review article Iron Metabolism in Thalassemia and Sickle Cell Disease. Raffaella Mariani, Paola Trombini, Matteo
Anaemia in the ICU: Is there an alternative to using blood transfusion?
 Anaemia in the ICU: Is there an alternative to using blood transfusion? Tim Walsh Professor of Critical Care, Edinburgh University World Health Organisation grading of the severity of anaemia Grade of
Anaemia in the ICU: Is there an alternative to using blood transfusion? Tim Walsh Professor of Critical Care, Edinburgh University World Health Organisation grading of the severity of anaemia Grade of
Iron-Sensing Proteins that Regulate Hepcidin and Enteric Iron Absorption
 Annu. Rev. Nutr. 2010. 30:149 71 First published online as a Review in Advance on April 20, 2010 The Annual Review of Nutrition is online at nutr.annualreviews.org This article s doi: 10.1146/annurev.nutr.012809.104801
Annu. Rev. Nutr. 2010. 30:149 71 First published online as a Review in Advance on April 20, 2010 The Annual Review of Nutrition is online at nutr.annualreviews.org This article s doi: 10.1146/annurev.nutr.012809.104801
Serum hepcidin levels and iron parameters in children with iron deficiency
 VOLUME 47 ㆍ NUMBER 4 ㆍ December 2012 THE KOREAN JOURNAL OF HEMATOLOGY ORIGINAL ARTICLE Serum hepcidin levels and iron parameters in children with iron deficiency Hyoung Soo Choi 1, Sang Hoon Song 2, Jae
VOLUME 47 ㆍ NUMBER 4 ㆍ December 2012 THE KOREAN JOURNAL OF HEMATOLOGY ORIGINAL ARTICLE Serum hepcidin levels and iron parameters in children with iron deficiency Hyoung Soo Choi 1, Sang Hoon Song 2, Jae
Managing peri-operative anaemiathe Papworth way. Dr Andrew A Klein Royal Papworth Hospital Cambridge UK
 Managing peri-operative anaemiathe Papworth way Dr Andrew A Klein Royal Papworth Hospital Cambridge UK Conflicts of interest: Unrestricted educational grants/honoraria from CSL Behring, Brightwake Ltd,
Managing peri-operative anaemiathe Papworth way Dr Andrew A Klein Royal Papworth Hospital Cambridge UK Conflicts of interest: Unrestricted educational grants/honoraria from CSL Behring, Brightwake Ltd,
Faculty of Medicine Dr. Tariq Aladily
 Iron deficiency anemia The most common anemia worldwide Only 10% of ingested iron is absorbed Most dietary iron occurs in meat products Absorbed in duodenum Hepcidin By inhibiting ferroportin, hepcidin
Iron deficiency anemia The most common anemia worldwide Only 10% of ingested iron is absorbed Most dietary iron occurs in meat products Absorbed in duodenum Hepcidin By inhibiting ferroportin, hepcidin
The relationship between systemic iron homeostasis and erythropoiesis
 Editorial The relationship between systemic iron homeostasis and erythropoiesis Gautam Rishi and V. Nathan Subramaniam The Liver Disease and Iron Disorders Research Group, Institute of Health and Biomedical
Editorial The relationship between systemic iron homeostasis and erythropoiesis Gautam Rishi and V. Nathan Subramaniam The Liver Disease and Iron Disorders Research Group, Institute of Health and Biomedical
The role of hypoxia-inducible factors in the regulation of systemic iron homeostasis. Erik Ryan Anderson
 The role of hypoxia-inducible factors in the regulation of systemic iron homeostasis by Erik Ryan Anderson A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of
The role of hypoxia-inducible factors in the regulation of systemic iron homeostasis by Erik Ryan Anderson A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of
Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis
 Iron Metabolism & Its Disorders Articles and Brief Reports Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis Maria
Iron Metabolism & Its Disorders Articles and Brief Reports Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis Maria
Management of anemia in CKD
 Management of anemia in CKD Pierre Cochat, MD PhD Professor of Pediatrics Chair, Pediatrics & Pediatric Surgery Department Head, Center for Rare Renal Diseases Néphrogones Hospices Civils de Lyon & University
Management of anemia in CKD Pierre Cochat, MD PhD Professor of Pediatrics Chair, Pediatrics & Pediatric Surgery Department Head, Center for Rare Renal Diseases Néphrogones Hospices Civils de Lyon & University
Iron depletion in frequently donating whole blood donors. B. Mayer, H. Radtke
 Iron depletion in frequently donating whole blood donors B. Mayer, H. Radtke Iron: relevance oxygen-transporting and storage proteins hemoglobin and myoglobin iron-containing centers in many enzymes mitochondrial
Iron depletion in frequently donating whole blood donors B. Mayer, H. Radtke Iron: relevance oxygen-transporting and storage proteins hemoglobin and myoglobin iron-containing centers in many enzymes mitochondrial
IRON DEFICIENCY / ANAEMIA ANTHONY BEETON
 IRON DEFICIENCY / ANAEMIA ANTHONY BEETON HYPOXIA 1-2 mg IRON Labile iron Body iron ± 3 4 g Liver and the reticuloendothelial system and spleen (approximately 200 300 mg in adult women and 1 g in adult
IRON DEFICIENCY / ANAEMIA ANTHONY BEETON HYPOXIA 1-2 mg IRON Labile iron Body iron ± 3 4 g Liver and the reticuloendothelial system and spleen (approximately 200 300 mg in adult women and 1 g in adult
Up-regulation of hepcidin by interleukin-6 contributes to anemia of inflammation in multicentric Castleman's disease (MCD)
 99 Mini Review Up-regulation of hepcidin by interleukin-6 contributes to anemia of inflammation in multicentric Castleman's disease (MCD) Nakazawa-Soken J. Song and Kazuyuki Yoshizaki Immuno-Medical Science,
99 Mini Review Up-regulation of hepcidin by interleukin-6 contributes to anemia of inflammation in multicentric Castleman's disease (MCD) Nakazawa-Soken J. Song and Kazuyuki Yoshizaki Immuno-Medical Science,
Drugs Used in Anemia
 Drugs Used in Anemia Drugs of Anemia Anemia is defined as a below-normal plasma hemoglobin concentration resulting from: a decreased number of circulating red blood cells or an abnormally low total hemoglobin
Drugs Used in Anemia Drugs of Anemia Anemia is defined as a below-normal plasma hemoglobin concentration resulting from: a decreased number of circulating red blood cells or an abnormally low total hemoglobin
Hematopoiesis, The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid.
 Hematopoiesis, 200 billion new blood cells per day The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid. hematopoietic growth factors, proteins that regulate the proliferation
Hematopoiesis, 200 billion new blood cells per day The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid. hematopoietic growth factors, proteins that regulate the proliferation
Laboratory diagnosis of iron deficiency: The interpretation of automated counting parameters. Dr Wayne Thomas Derriford Hospital, Plymouth
 Laboratory diagnosis of iron deficiency: The interpretation of automated counting parameters. Dr Wayne Thomas Derriford Hospital, Plymouth Why does it matter? Over 30% of the Worlds population are anaemic,
Laboratory diagnosis of iron deficiency: The interpretation of automated counting parameters. Dr Wayne Thomas Derriford Hospital, Plymouth Why does it matter? Over 30% of the Worlds population are anaemic,
ANEMIA & HEMODIALYSIS
 ANEMIA & HEMODIALYSIS The anemia of CKD is, in most patients, normocytic and normochromic, and is due primarily to reduced production of erythropoietin by the kidney and to shortened red cell survival.
ANEMIA & HEMODIALYSIS The anemia of CKD is, in most patients, normocytic and normochromic, and is due primarily to reduced production of erythropoietin by the kidney and to shortened red cell survival.
Quantification of serum hepcidin as a potential biomarker in diagnosis of iron deficiency anaemia
 International Journal of Research in Medical Sciences Vyas S et al. Int J Res Med Sci. 2017 Jul;5(7):2926-2930 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20172546
International Journal of Research in Medical Sciences Vyas S et al. Int J Res Med Sci. 2017 Jul;5(7):2926-2930 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20172546
Hepcidin Regulation of Iron Transport 1 3
 The Journal of Nutrition Symposium: Hepcidin Regulation of Iron Transport Hepcidin Regulation of Iron Transport 1 3 James F. Collins, 4 Marianne Wessling-Resnick, 5 * and Mitchell D. Knutson 6 4 Department
The Journal of Nutrition Symposium: Hepcidin Regulation of Iron Transport Hepcidin Regulation of Iron Transport 1 3 James F. Collins, 4 Marianne Wessling-Resnick, 5 * and Mitchell D. Knutson 6 4 Department
Processing and trafficking of the iron regulatory protein, hemojuvelin
 Oregon Health & Science University OHSU Digital Commons Scholar Archive April 2011 Processing and trafficking of the iron regulatory protein, hemojuvelin Julia Elizabeth Maxson Follow this and additional
Oregon Health & Science University OHSU Digital Commons Scholar Archive April 2011 Processing and trafficking of the iron regulatory protein, hemojuvelin Julia Elizabeth Maxson Follow this and additional
Anemia in -thalassemia patients targets hepatic hepcidin transcript levels independently of iron metabolism genes controlling hepcidin expression
 BRIEF REPORTS Anemia in -thalassemia patients targets hepatic hepcidin transcript levels independently of iron metabolism genes controlling hepcidin expression Emilie Camberlein, 1 Giuliana Zanninelli,
BRIEF REPORTS Anemia in -thalassemia patients targets hepatic hepcidin transcript levels independently of iron metabolism genes controlling hepcidin expression Emilie Camberlein, 1 Giuliana Zanninelli,
Serum soluble transferrin receptor in hypochromic microcytic anaemia
 O r i g i n a l A r t i c l e Singapore Med Med J 2006; J 2006; 47(2) 47(2) : 138 : 1 Serum soluble transferrin receptor in hypochromic microcytic anaemia Jayaranee S, Sthaneshwar P ABSTRACT Introduction:
O r i g i n a l A r t i c l e Singapore Med Med J 2006; J 2006; 47(2) 47(2) : 138 : 1 Serum soluble transferrin receptor in hypochromic microcytic anaemia Jayaranee S, Sthaneshwar P ABSTRACT Introduction:
Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance
 Related Commentary, page 1755 Research article Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance Jodie L. Babitt, 1 Franklin W. Huang, 2 Yin Xia, 1 Yisrael Sidis,
Related Commentary, page 1755 Research article Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance Jodie L. Babitt, 1 Franklin W. Huang, 2 Yin Xia, 1 Yisrael Sidis,
Shaofu Ma 1), Yoshitaka Koshino 2), Satoko Yamamoto 1), Takeo Shimasaki 1), NaohisaTomosugi 1)
 J Kanazawa Med Univ 41 25 31, 2016 Rapid Increase in Serum Iron Level After Oral Iron Intake as an Indicator of Duodenal Iron Absorption and Inverse Regulation of Iron Absorption by Hepcidin Expression
J Kanazawa Med Univ 41 25 31, 2016 Rapid Increase in Serum Iron Level After Oral Iron Intake as an Indicator of Duodenal Iron Absorption and Inverse Regulation of Iron Absorption by Hepcidin Expression
A Broad Clinical Pipeline of Innovative Diabetes / Renal & Oncology Programs. European Business Development Conference Düsseldorf 24 Sept 2013
 A Broad Clinical Pipeline of Innovative Diabetes / Renal & Oncology Programs European Business Development Conference Düsseldorf 24 Sept 2013 Key facts about NOXXON Developing a new class of proprietary
A Broad Clinical Pipeline of Innovative Diabetes / Renal & Oncology Programs European Business Development Conference Düsseldorf 24 Sept 2013 Key facts about NOXXON Developing a new class of proprietary
* imagine if the Hb is free ( e.g. hemolysis ) in the plasma what happens?
 In this lecture we will talk about Some characteristics of RBC. Erythrpoiesis : * During fetal & adult life. * its regulation. RBCs : - Appear under the microscope as circular,unnucleated and biconcave
In this lecture we will talk about Some characteristics of RBC. Erythrpoiesis : * During fetal & adult life. * its regulation. RBCs : - Appear under the microscope as circular,unnucleated and biconcave
The Egyptian Journal of Hospital Medicine (October 2017) Vol. 69 (8), Page
 The Egyptian Journal of Hospital Medicine (October 2017) Vol. 69 (8), Page 2997-3002 Anemia of Chronic Disease Ayman Ali Alhboob 1, Khuludyahyam.Khati 2, SAMAHER Sahal Malibari 3, Bariahyahya Drain 4,
The Egyptian Journal of Hospital Medicine (October 2017) Vol. 69 (8), Page 2997-3002 Anemia of Chronic Disease Ayman Ali Alhboob 1, Khuludyahyam.Khati 2, SAMAHER Sahal Malibari 3, Bariahyahya Drain 4,
Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice
 Related Commentary, page 1424 Research article Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice Shuling Guo, 1 Carla Casu, 2 Sara Gardenghi, 2 Sheri Booten, 1 Mariam Aghajan, 1 Raechel
Related Commentary, page 1424 Research article Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice Shuling Guo, 1 Carla Casu, 2 Sara Gardenghi, 2 Sheri Booten, 1 Mariam Aghajan, 1 Raechel
The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes
 Cantaurus, Vol. 5, -, May 7 McPherson College Division of Science and Technology The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes Callie Crist, Elizabeth
Cantaurus, Vol. 5, -, May 7 McPherson College Division of Science and Technology The Interaction of Alcohol and Iron-Overload in the in-vivo Regulation of Iron Responsive Genes Callie Crist, Elizabeth
Mass spectrometry-based serum Protein Profiling. at population level: serum hepcidin and beyond
 UNIVERSITA DEGLI STUDI DI MILANO-BICOCCA FACOLTA DI MEDICINA E CHIRURGIA DOTTORATO DI RICERCA IN EMATOLOGIA SPERIMENTALE (XXV ciclo) Coordinatore: Prof. Carlo Gambacorti Passerini TESI DI DOTTORATO DI
UNIVERSITA DEGLI STUDI DI MILANO-BICOCCA FACOLTA DI MEDICINA E CHIRURGIA DOTTORATO DI RICERCA IN EMATOLOGIA SPERIMENTALE (XXV ciclo) Coordinatore: Prof. Carlo Gambacorti Passerini TESI DI DOTTORATO DI
The Changing Clinical Landscape of Anemia Management in Patients With CKD: An Update From San Diego Presentation 1
 Presentation 1 The following is a transcript from a web-based CME-certified multimedia activity. Interactivity applies only when viewing the activity online. This activity is supported by educational grants
Presentation 1 The following is a transcript from a web-based CME-certified multimedia activity. Interactivity applies only when viewing the activity online. This activity is supported by educational grants
Hepcidin is a key mediator of anemia of inflammation in Crohn's disease
 Journal of Crohn's and Colitis (203) 7, e286 e29 Available online at www.sciencedirect.com Hepcidin is a key mediator of anemia of inflammation in Crohn's disease Robert J. Basseri a, Elizabeta Nemeth
Journal of Crohn's and Colitis (203) 7, e286 e29 Available online at www.sciencedirect.com Hepcidin is a key mediator of anemia of inflammation in Crohn's disease Robert J. Basseri a, Elizabeta Nemeth
Effective Health Care Program
 Comparative Effectiveness Review Number 83 Effective Health Care Program Biomarkers for Assessing and Managing Iron Deficiency Anemia in Late-Stage Chronic Kidney Disease Executive Summary Background Chronic
Comparative Effectiveness Review Number 83 Effective Health Care Program Biomarkers for Assessing and Managing Iron Deficiency Anemia in Late-Stage Chronic Kidney Disease Executive Summary Background Chronic
Anemia 1: Fourth year Medical Students/ Feb/22/ Abdallah Awidi Abbadi.MD.FRCP.FRCPath Professor
 Anemia 1: Fourth year Medical Students/ Feb/22/ 2018 Abdallah Awidi Abbadi.MD.FRCP.FRCPath Professor Email: abdalla.awidi@gmail.com Kidney EPO O2 Sensor Blood vessel Definition: Anemia is operationally
Anemia 1: Fourth year Medical Students/ Feb/22/ 2018 Abdallah Awidi Abbadi.MD.FRCP.FRCPath Professor Email: abdalla.awidi@gmail.com Kidney EPO O2 Sensor Blood vessel Definition: Anemia is operationally
Ferrata Storti Foundation
 ARTICLES Introduction Iron is an essential nutrient necessary for erythropoiesis and cellular metabolism. Since there is no physiological mechanism for excretion of excess iron, the absorption of dietary
ARTICLES Introduction Iron is an essential nutrient necessary for erythropoiesis and cellular metabolism. Since there is no physiological mechanism for excretion of excess iron, the absorption of dietary
Characterization of iron metabolism and erythropoiesis in erythrocyte membrane defects and thalassemia traits
 Characterization of iron metabolism and erythropoiesis in erythrocyte membrane defects and thalassemia traits Lucie Sulovska a *, Dusan Holub b *, Zuzana Zidova c, Martina Divoka d, Marian Hajduch b, Vladimir
Characterization of iron metabolism and erythropoiesis in erythrocyte membrane defects and thalassemia traits Lucie Sulovska a *, Dusan Holub b *, Zuzana Zidova c, Martina Divoka d, Marian Hajduch b, Vladimir
Arginine as an Example of a Conditionally Essential Nutrient: Sickle Cell Disease & Trauma Claudia R. Morris MD, FAAP
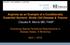 Arginine as an Example of a Conditionally Essential Nutrient: Sickle Cell Disease & Trauma Claudia R. Morris MD, FAAP Examining Special Nutritional Requirements in Disease States, A Workshop April 1, 2018
Arginine as an Example of a Conditionally Essential Nutrient: Sickle Cell Disease & Trauma Claudia R. Morris MD, FAAP Examining Special Nutritional Requirements in Disease States, A Workshop April 1, 2018
COORDINATION OF IRON AND OXYGEN SIGNALING THROUGH POST- TRANSCRIPTIONAL REGULATION OF HYPOXIA INDUCIBLE FACTOR-2ALPHA MCKALE R.
 COORDINATION OF IRON AND OXYGEN SIGNALING THROUGH POST- TRANSCRIPTIONAL REGULATION OF HYPOXIA INDUCIBLE FACTOR-2ALPHA By MCKALE R. DAVIS Bachelor of Science in Nutrition and Dietetics Texas Christian University
COORDINATION OF IRON AND OXYGEN SIGNALING THROUGH POST- TRANSCRIPTIONAL REGULATION OF HYPOXIA INDUCIBLE FACTOR-2ALPHA By MCKALE R. DAVIS Bachelor of Science in Nutrition and Dietetics Texas Christian University
Hemodialysis patients with endstage
 Insights into Achieving Target Hemoglobin Levels: Increasing the Serum Ferritin Parameter Scott Bralow, DO Dr. Scott Bralow is the Medical Director of the Renal Center of Philadelphia. Evidence suggests
Insights into Achieving Target Hemoglobin Levels: Increasing the Serum Ferritin Parameter Scott Bralow, DO Dr. Scott Bralow is the Medical Director of the Renal Center of Philadelphia. Evidence suggests
Rama Nada. -Ensherah Mokheemer. 1 P a g e
 - 3 - Rama Nada -Ensherah Mokheemer - 1 P a g e Don t forget to refer to page index wherever you see * Quick revision: In the previous lecture we said that: - your body contains 4-5g of iron (4g in females
- 3 - Rama Nada -Ensherah Mokheemer - 1 P a g e Don t forget to refer to page index wherever you see * Quick revision: In the previous lecture we said that: - your body contains 4-5g of iron (4g in females
Iron metabolism and medical needs: a view from Academia
 Iron metabolism and medical needs: a view from Academia Paul M. Tulkens Cellular & Molecular Pharmacology & Centre for Clinical Pharmacy Catholic University of Louvain Brussels, Belgium Iron therapy Master
Iron metabolism and medical needs: a view from Academia Paul M. Tulkens Cellular & Molecular Pharmacology & Centre for Clinical Pharmacy Catholic University of Louvain Brussels, Belgium Iron therapy Master
Hepcidin Levels and Iron Status in β- Thalassemia Major Patients with Hepatitis C Virus Infection
 THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 17 (2), 2010 Page: 33-44 Hepcidin Levels and Iron Status in β- Thalassemia Major Patients with Hepatitis C Virus Infection 1 Olfat M Hendy, 1 Maha Allam, 2 Alif
THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 17 (2), 2010 Page: 33-44 Hepcidin Levels and Iron Status in β- Thalassemia Major Patients with Hepatitis C Virus Infection 1 Olfat M Hendy, 1 Maha Allam, 2 Alif
The liver hormone hepcidin is a main regulator
 Serum and Liver Iron Differently Regulate the Bone Morphogenetic Protein 6 (BMP6)-SMAD Signaling Pathway in Mice Elena Corradini, 1,2 Delphine Meynard, 1 Qifang Wu, 1 Shan Chen, 1 Paolo Ventura, 2 Antonello
Serum and Liver Iron Differently Regulate the Bone Morphogenetic Protein 6 (BMP6)-SMAD Signaling Pathway in Mice Elena Corradini, 1,2 Delphine Meynard, 1 Qifang Wu, 1 Shan Chen, 1 Paolo Ventura, 2 Antonello
Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias
 Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias Prem Ponka Department of Physiology Lady Davis Institute, Jewish General Hospital McGill University,
Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias Prem Ponka Department of Physiology Lady Davis Institute, Jewish General Hospital McGill University,
