Darifenacin: first M3 receptor antagonist for overactive bladder
|
|
|
- Kelley Murphy
- 5 years ago
- Views:
Transcription
1 Darifenacin: first M3 receptor antagonist for overactive bladder Steve Chaplin MSc, MRPharmS and Christopher Chapple BSc, MD, FRCS(Urol) PRODUCT PROFILE Proprietary name: Emselex Constituents: darifenacin Indication: symptomatic treatment of urge incontinence and/or increased urinary frequency and urgency as may occur in patients with overactive bladder (OAB) syndrome Dosage and method of administration: adults: initially 7.5mg daily; may be increased to 15mg daily after two weeks in patients requiring greater symptom relief; elderly initially 7.5mg daily; may be increased to 15mg daily after two weeks for those patients who have an acceptable tolerability profile but require greater symptom relief; children not recommended in patients under 18 Contraindications: patients hypersensitive to darifenacin or its excipients; patients with: urinary retention, gastric retention, uncontrolled narrow-angle glaucoma, myasthenia gravis, severe hepatic impairment (Child-Pugh C), severe ulcerative colitis, toxic megacolon, concomitant treatment with potent CYP3A4 inhibitors Precautions: administer Emselex with caution in patients with: autonomic neuropathy, hiatus hernia, clinically significant bladder outflow obstruction, risk for urinary retention, severe constipation or GI obstructive disorders, eg pyloric stenosis; use Emselex with caution in patients with: narrow-angle glaucoma, risk of decreased GI motility, gastro-oesophageal reflux and/or who are concurrently taking medicinal products, eg oral bisphosphonates, that can cause/exacerbate oesophagitis Pregnancy and lactation: not recommended during pregnancy; caution should be exercised before administering Emselex to a nursing woman Interactions: digoxin, inhibitors of CYP2D6 (eg paroxetine, terbinafine, cimetidine, quinidine) or CYP3A4 (eg ketoconazole, itraconazole, protease inhibitors such as ritonavir), moderate CYP3A4 inhibitors (eg erythromycin, clarithromycin, telithromycin, fluconazole, grapefruit juice), substrates of CYP2D6 (eg flecainide, thioridazine, imipramine) or CYP3A4 (eg midazolam), inducers of CYP3A4 (eg rifampicin, carbamazepine, barbiturates, St John s wort), P-glycoprotein inhibitors (eg ciclosporin, verapamil), antimuscarinic agents (eg oxybutynin, tolterodine, flavoxate) Side-effects: very common: dry mouth, constipation; common: abdominal pain, headache; nausea, dyspepsia; dry eyes Presentation/cost: 7.5mg, 15mg prolonged-release tablets; 7.5mg 28, 26.13; 15mg 28, Darifenacin (Emselex) is the first antimuscarinic agent to target the M3 receptor that is associated with detrusor muscle contraction. In our review Steve Chaplin presents the clinical data relating to its efficacy and adverse effects, and Professor Christopher Chapple comments on its place in the treatment of overactive bladder. The International Continence Society defines overactive bladder (OAB) as urgency, with or without urge incontinence, usually with frequency and nocturia. 1 The prevalence of OAB in the UK is about 20 per cent overall 2,3 and increases with age. 4 OAB has a marked impact on quality of life. 5 A European survey found that many people with OAB symptoms have not been formally diagnosed and, though half of those diagnosed have been prescribed medication at some time, only one-quarter continue to take it. 2 The National Institute for Health and Clinical Excellence (NICE) clinical guideline for the management of urinary incontinence in women includes the management of OAB. 6 (The Scottish Intercollegiate Guidelines Network, 14 Prescriber 19 October
2 Dose N Median Median change Median difference 95% CI p value baseline from baseline from placebo placebo darifenacin 7.5mg od (-3.6,-0.7) placebo darifenacin 15mg od (-4.5, -2.0) <0.001 placebo darifenacin 7.5mg 15mg (-2.9,-0.0) Table 1. Pooled analysis of clinical studies showing efficacy of darifenacin 7.5mg and 15mg once daily in reducing median number of incontinence episodes per week SIGN, has published a management guideline for urinary incontinence in both men and women. 7 ) Initial management comprises lifestyle change (weight loss if overweight, modifying fluid intake, avoiding caffeine), pelvic floor muscle training, bladder training and treatment with an antimuscarinic agent. 6 The drug of first choice is immediate-release oxybutynin; if this is not tolerated, the alternatives are darifenacin (Emselex), solifenacin (Vesicare), tolterodine (Detrusitol) or trospium (Regurin), or modifiedrelease (Lyrinel XL) or transdermal (Kentera) oxybutynin. Propiverine (Detrunorm) may also be considered for urinary frequency. Meta-analysis of clinical trials of antimuscarinic agents has revealed a high placebo response to treatment (about 40 per cent) but antimuscarinic agents improve or stop symptoms in a further 15 per cent of patients; they also offer a modest improvement in quality of life. 8 The technology 9 Darifenacin is an antagonist at muscarinic receptors, with selectivity within the therapeutic range for M3 receptors (the predominant receptor subtype in the bladder). It is licensed for the symptomatic treatment of urge incontinence and/or increased urinary frequency and urgency as may occur in patients with OAB syndrome. The recommended dose is 7.5mg per day initially; this should be assessed after two weeks and may be increased to 15mg per day if required. The dose should be increased in older patients only when tolerability is acceptable. Darifenacin is metabolised by hepatic CYP2D6 and CYP3A4 enzymes. It should be prescribed with caution in patients taking other drugs that inhibit or induce these enzymes and should not be prescribed for patients taking potent inhibitors of CYP3A4 such as verapamil, itraconazole or ritonavir (Norvir). Like other antimuscarinic agents, darifenacin may potentiate the anticholinergic effects of TCAs and drugs for Parkinson s disease. Emselex is a modified-release formulation. Peak plasma levels of darifenacin occur seven hours after administration and steady state levels are achieved on the sixth day. Clinical trials Three similar randomised, fixeddose, double-blind trials have been pooled for analysis; 10,11 a randomised flexible-dose trial has also been published. 12 In the pooled analysis, a total of 1059 patients (85 per cent women, 95 per cent idiopathic OAB) with frequency, urgency and urge incontinence of at least six months duration were randomised to placebo or darifenacin 7.5 or 15mg per day. The primary end-point was the median change in number of incontinence episodes per week. At baseline, the median number of incontinence episodes per week was After 12 weeks, the median change was -8.8 episodes per week for darifenacin 7.5mg per day (2.0 fewer than placebo) 13 and episodes per week for 15mg per day (3.2 fewer than placebo); 13 both differences were statistically significant (see Table 1). Darifenacin also significantly improved secondary end-points including the number of daily voids or urgency episodes ( episodes fewer than placebo), the severity of urgency episodes, incontinence episodes requiring a change of pad ( fewer per week), and at the 15mg per day dose only nocturnal awakenings (0.7 per week). The proportions of patients experiencing at least three dry days per week were 55 per cent at 7.5mg per day and 65 per cent at 15mg per day, both of which were significantly greater than placebo (43 and 48 per cent respectively). 10 Similar findings were reported in a subgroup analysis of 317 patients aged 65 or over Prescriber 19 October
3 In the flexible dose study, treatment with darifenacin was initiated at a dose of 7.5mg per day and increased to 15mg per day after two weeks if additional efficacy was required. Fifty-nine per cent of patients increased their dose; outcomes at each dose were similar after 12 weeks and comparable with those reported in the pooled analysis. A nonblinded uncontrolled extension study involving 716 patients who had participated in two of the 12-week trials showed that the number of incontinence episodes per week was further reduced after two years and over 40 per cent of patients experienced at least a reduction in weekly incontinence episodes of at least 90 per cent. 14 Two small short-term trials suggest that the efficacy of darifenacin may be comparable with that of immediate-release oxybutynin 2.5-5mg three times daily. 15,16 Adverse effects In seven-day studies in healthy volunteers, including older people, darifenacin has been shown to have no effect on cognitive function, cardiac function or vision The adverse anticholinergic effects of darifenacin are dosedependent. 9 In clinical trials, as with other antimuscarinic agents, dry mouth (placebo 8 per cent, 7.5mg per day 20 per cent, 15mg Key points per day 35 per cent) and constipation (6, 15 and 21 per cent respectively) were the commonest adverse events associated with darifenacin. 10 The incidence of cardiovascular and CNS effects was comparable with placebo and there was no evidence of an effect on cardiac conduction. 13 The frequency of discontinuation due to treatment-related adverse events was 1.3 per cent with placebo and 1.2 and 4.5 per cent for 7.5mg and 15mg per day darifenacin. 10 In the nonblinded extension study, 66 per cent of patients completed two years treatment with darifenacin. The commonest adverse events were dry mouth (23 per cent) and constipation (21 per cent). 14 Summary Darifenacin is an antimuscarinic agent for OAB. It reduces the number of incontinence episodes by about one per week and the number of urgency episodes by about one per day, in addition to improving other OAB symptoms. Its common adverse effects are typical of antimuscarinic agents; in clinical trials, darifenacin was not associated with an increased risk of clinically significant adverse cardiovascular or CNS effects. Long-term trials comparing darifenacin with other treatments for OAB are lacking. the prevalence of OAB in the UK is about 20 per cent; drug treatment is one component of management darifenacin is an M3 receptor antagonist for the treatment of OAB it reduces the number of episodes of weekly incontinence and daily urgency, and reduces urgency severity the commonest adverse effects of darifenacin are dry mouth and constipation, affecting about 20 per cent of patients in long-term use darifenacin does not appear to be associated with significant adverse cardiovascular or CNS effects its effectiveness compared with other antimuscarinic agents is uncertain References 1. Abrams P, Cardozo L, Fall M, et al; International Continence Society. The standardisation of terminology of lower urinary tract function: report from the International Continence Society. Neurourol Urodynamics 2002;21: Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 2001;87: McGrother CW, Donaldson MM, Hayward T, et al; The Leicestershire MRC Incontinence Study Team. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing 2006;35: National Collaborating Centre for Women s and Children s Health. Urinary incontinence. The management of urinary incontinence in women. National Collaborating Centre for Women s and Children s Health. RCOG Press. London. October /English. 5. Jackson S. The patient with an overactive bladder symptoms and qualityof-life issues. Urology 1997;50(Suppl 6A): NICE. Urinary incontinence. The management of urinary incontinence in women. Clinical Guideline 40. October ceguidance/pdf/english. 7. Scottish Intercollegiate Guidelines Network. Management of urinary incontinence in primary care. SIGN Guideline No. 79. December ac.uk/pdf/sign79.pdf. 8. Nabi G, Cody JD, Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD DOI: / CD pub2. 9. Novartis Pharmaceuticals UK Ltd. Emselex 7.5mg and 15mg prolongedrelease tablets. Summary of Product Characteristics. May Chapple C, Steers W, Norton P, et al. A pooled analysis of three phase III 18 Prescriber 19 October
4 studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int 2005;95: Foote J, Glavind K, Kralidis G, et al. Treatment of overactive bladder in the older patient: pooled analysis of three phase III studies of darifenacin, an M3 selective receptor antagonist. Eur Urol 2005;48: Steers W, Corcos J, Foote J, et al.an investigation of dose titration with darifenacin, an M3-selective receptor antagonist. BJU Int 2005;95: European Medicines Agency. Emselex EPAR Scientific Discussion. May humandocs/pdfs/epar/emselex/ en6.pdf. 14. Haab F, Corcos J, Siami P, et al. Long-term treatment with darifenacin for overactive bladder: results of a 2- year, open-label extension study. BJU Int 2006;98: Chapple CR, Abrams P. Comparison of darifenacin and oxybutynin in patients with overactive bladder: assessment of ambulatory urodynamics and impact on salivary flow. Eur Urol 2005;48: Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J Urol 2005;23: Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. JUrol2005;173: Serra DB, Affrime MB, Bedigian MP, et al. QT and QTc interval with standard and supratherapeutic doses of darifenacin, a muscarinic M3 selective receptor antagonist for the treatment of overactive bladder. J Clin Pharmacol 2005;45: Kay GG, Wesnes KA. Pharmacodynamic effects of darifenacin, a muscarinic M selective receptor antagonist for the treatment of overactive bladder, in healthy volunteers. BJU Int 2005;96: By Steve Chaplin, a pharmacist who specialises in writing on therapeutics Place in therapy OAB is a term applied to the troublesome urinary storage symptoms of urgency (a sudden and strong desire to void) with or without urgency incontinence, usually with increased frequency of micturition (over eight micturitions per 24 hours) and nocturia. 1 It is a highly prevalent, chronic and debilitating disease that affects men and women of all ages. The symptoms occur in the absence of pathological (eg urinary tract infection, urinary stones or interstitial cystitis) or metabolic factors (eg diabetes mellitus) that would explain them. The prevalence of OAB in Europe and the USA has been estimated. One population-based survey of men and women aged 40 years and older conducted in six countries estimated the prevalence of OAB in Europe to be 15.6 and 17.4 per cent respectively for men and women, with an overall prevalence of 16.6 per cent. 2 The prevalence of OAB was also assessed in a large populationbased survey in the USA: the National Overactive BLadder Evaluation (NOBLE) survey. 3 A sample of 5204 adults over 18 years of age and representative of the US population by sex, age and geographical region was assessed. The overall prevalence of OAB was similar between men (16.0 per cent) and women (16.9 per cent) and was quite similar to the results reported earlier from Europe. 2 Overall, from these studies it can be concluded that of these patients with OAB, approximately one-third are troubled by incontinence (OAB wet ) and two-thirds are not (OAB dry ). Clearly, OAB impacts on all aspects of patients everyday activities, including emotional, physical, social, occupational and domestic functions, and shows similar prevalence to several other chronic medical conditions such as asthma and chronic bronchitis, yet many patients with OAB do not seek medical help for their symptoms. Reasons include embarrassment and misconceptions, such as lack of effective treatment options. Consequently, many patients with OAB resort to elaborate coping strategies in order to manage their symptoms, such as frequent voiding, mapping the location of toilets, restricting fluid intake and/or the use of absorbent pads. OAB therefore accounts for a considerable socioeconomic burden. Muscarinic receptors The symptoms of OAB can be explained by abnormal increases in bladder detrusor muscle contractility during the filling phase of the micturition cycle. Although the exact aetiology of OAB remains largely unknown, it is well recognised that the underlying abnormal detrusor muscle contractions (together with those associated with normal voiding) are at least in part mediated by acetylcholine-mediated stimulation of bladder muscarinic receptors. Consequently, antimuscarinic agents have become the treatment of choice for OAB. 4 However, existing agents of this class are not highly selective for the muscarinic M3 receptor subtype that has been shown to be primarily responsible for mediating human detrusor muscle contraction. 5 This explains their potential to cause a number of side-effects given that five muscarinic receptor subtypes are known to exist and Prescriber 19 October
5 such subtypes are located in many other tissues throughout the body (including the salivary glands, GI tract, heart, CNS and eye). 6 Such effects often give rise to tolerability and safety problems. Antimuscarinic agents selective for the muscarinic M3 receptor might therefore on theoretical grounds be expected to have clinical efficacy in the treatment of OAB with a lower propensity for side-effects and safety concerns related to blockade of other receptor subtypes, including cognitive impairment and tachycardia (primarily M1 and M2 receptor-mediated, respectively). 7 Darifenacin Darifenacin is a novel agent that was identified on the basis of this subtype-selectivity rationale, and represents the first M3 selective receptor antagonist to undergo extensive clinical evaluation for the treatment of patients with OAB. It appears to have similar efficacy to the other agents that are currently available and the presence of two doses allows flexible dosing. Potential advantages over other contemporary agents in terms of increased safety relating to pharmacological selectivity in the context of both the cardiovascular and CNS have been suggested, but definitive comments on this await the results of adequately powered head-to-head clinical studies. To summarise, at this juncture darifenacin has to be considered to be a new agent with similar efficacy to other agents, with the possibility of flexible dosing and similar tolerability to other contemporary agents. References 1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the International Continence Society. Am J Obstet Gynecol 2002;187: Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 2001; 87: Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20: Andersson Ke, Appell R., Awad S. et al.: Pharmacological treatment of urinary incontinence. In: Abrams P, Khoury S, Wein A, editors. Incontinence, 2nd International Consultation on Incontinence. Plymouth: Plymbridge Distributors Ltd, 2002: pp Chess-Williams R, Chapple CR, Yamanishi T, et al. The minor population of M 3 -receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol 2001;21: Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998;50: Andersson K-E. Potential benefits of muscarinic M 3 receptor selectivity. Eur Urol 2002;1(Suppl.): Christopher Chapple is consultant urologist at Sheffield Teaching Hospitals NHS Foundation Trust and visiting professor at Sheffield Hallam University. He is a consultant/investigator/speaker for Astellas (Yamanouchi), Pfizer Inc, Novartis and Schwartz and has acted as a consultant for UCB.
Anticholinergic medication use for female overactive bladder in the ambulatory setting in the United States.
 Página 1 de 6 PubMed darifenacin vs solifenacin Display Settings:, Sorted by Recently Added Results: 5 1. Int Urogynecol J. 2013 Oct 25. [Epub ahead of print] Anticholinergic medication use for female
Página 1 de 6 PubMed darifenacin vs solifenacin Display Settings:, Sorted by Recently Added Results: 5 1. Int Urogynecol J. 2013 Oct 25. [Epub ahead of print] Anticholinergic medication use for female
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Emselex 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Emselex 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Emselex 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Emselex 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
VESIGARD Tablets (Darifenacin hydrobromide)
 Published on: 10 Jul 2014 VESIGARD Tablets (Darifenacin hydrobromide) Composition VESIGARD 7.5 Extended Release Tablets Each tablet contains: Darifenacin (as a hydrobromide).. 7.5 mg Dosage Form Tablets
Published on: 10 Jul 2014 VESIGARD Tablets (Darifenacin hydrobromide) Composition VESIGARD 7.5 Extended Release Tablets Each tablet contains: Darifenacin (as a hydrobromide).. 7.5 mg Dosage Form Tablets
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT EMSELEX 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT EMSELEX 7.5 mg prolonged-release tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 7.5 mg of darifenacin
Management of OAB. Lynsey McHugh. Consultant Urological Surgeon. Lancashire Teaching Hospitals
 Management of OAB Lynsey McHugh Consultant Urological Surgeon Lancashire Teaching Hospitals Summary Physiology Epidemiology Definitions NICE guidelines Evaluation Conservative management Medical management
Management of OAB Lynsey McHugh Consultant Urological Surgeon Lancashire Teaching Hospitals Summary Physiology Epidemiology Definitions NICE guidelines Evaluation Conservative management Medical management
Overactive Bladder beyond antimuscarinics
 Overactive Bladder beyond antimuscarinics Marcus Drake Bristol Urological Institute Conflicts of interest; Allergan, AMS, Astellas, Ferring, Pfizer Getting the diagnosis right Improving treatment Improving
Overactive Bladder beyond antimuscarinics Marcus Drake Bristol Urological Institute Conflicts of interest; Allergan, AMS, Astellas, Ferring, Pfizer Getting the diagnosis right Improving treatment Improving
The International Continence Society
 REPORTS Safety and Tolerability of Tolterodine for the Treatment of Overactive Bladder in Adults Richard G. Roberts, MD, JD; Alan D. Garely, MD; and Tamara Bavendam, MD Abstract This article evaluates
REPORTS Safety and Tolerability of Tolterodine for the Treatment of Overactive Bladder in Adults Richard G. Roberts, MD, JD; Alan D. Garely, MD; and Tamara Bavendam, MD Abstract This article evaluates
Urinary Incontinence for the Primary Care Provider
 Urinary Incontinence for the Primary Care Provider Diana J Scott FNP-BC https://youtu.be/gmzaue1ojn4 1 Assessment of Urinary Incontinence Urge Stress Mixed Other overflow, postural, continuous, insensible,
Urinary Incontinence for the Primary Care Provider Diana J Scott FNP-BC https://youtu.be/gmzaue1ojn4 1 Assessment of Urinary Incontinence Urge Stress Mixed Other overflow, postural, continuous, insensible,
Pharmacologic management of overactive bladder
 REVIEW Pharmacologic management of overactive bladder Sum Lam 1,2 lga Hilas 1,3 1 St. John s University, College of Pharmacy and Allied Health Professions, Department of Clinical Pharmacy Practice, Queens,
REVIEW Pharmacologic management of overactive bladder Sum Lam 1,2 lga Hilas 1,3 1 St. John s University, College of Pharmacy and Allied Health Professions, Department of Clinical Pharmacy Practice, Queens,
The Evidence for Antimuscarinic Agents in Female Mixed Urinary Incontinence
 european urology supplements 5 (2006) 849 853 available at www.sciencedirect.com journal homepage: www.europeanurology.com The Evidence for Antimuscarinic Agents in Female Mixed Urinary Incontinence Stefano
european urology supplements 5 (2006) 849 853 available at www.sciencedirect.com journal homepage: www.europeanurology.com The Evidence for Antimuscarinic Agents in Female Mixed Urinary Incontinence Stefano
Continence PGD transdermal oxybutynin Kentera patch 36mg
 Continence PGD transdermal oxybutynin Kentera patch 36mg Patient group direction for the supply of transdermal oxybutynin Kentera patch 36mg to patients suffering from urinary frequency, urgency or incontinence
Continence PGD transdermal oxybutynin Kentera patch 36mg Patient group direction for the supply of transdermal oxybutynin Kentera patch 36mg to patients suffering from urinary frequency, urgency or incontinence
Report on New Patented Drugs Enablex
 Report on New Patented Drugs Enablex Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Excessive
Report on New Patented Drugs Enablex Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Excessive
Solifenacin significantly improves all symptoms of overactive bladder syndrome
 REVIEW doi: 10.1111/j.1742-1241.2006.01067.x Solifenacin significantly improves all symptoms of overactive bladder syndrome C. R. CHAPPLE, 1 L. CARDOZO, 2 W. D. STEERS, 3 F. E. GOVIER 4 1 Department of
REVIEW doi: 10.1111/j.1742-1241.2006.01067.x Solifenacin significantly improves all symptoms of overactive bladder syndrome C. R. CHAPPLE, 1 L. CARDOZO, 2 W. D. STEERS, 3 F. E. GOVIER 4 1 Department of
Primary Care management of Overactive Bladder (OAB)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Primary Care management of Overactive Bladder (OAB) Prescribing Tips All medicines for OAB have similar dose-related efficacy. More than one agent (up
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Primary Care management of Overactive Bladder (OAB) Prescribing Tips All medicines for OAB have similar dose-related efficacy. More than one agent (up
Management of urinary incontinence in older people Shashi Gadgil BSc, MRCP and Adrian Wagg FRCP
 Management of urinary incontinence in older people Shashi Gadgil BSc, MRCP and Adrian Wagg FRCP Prevalence (%) 40 35 30 25 20 15 10 5 Our series Prescribing in older people gives practical advice for successful
Management of urinary incontinence in older people Shashi Gadgil BSc, MRCP and Adrian Wagg FRCP Prevalence (%) 40 35 30 25 20 15 10 5 Our series Prescribing in older people gives practical advice for successful
Comparison of Symptom Severity and Treatment Response in Patients with Incontinent and Continent Overactive Bladder
 European Urology European Urology 48 (2005) 110 115 Female UrologyöIncontinence Comparison of Symptom Severity and Treatment Response in Patients with Incontinent and Continent Overactive Bladder Martin
European Urology European Urology 48 (2005) 110 115 Female UrologyöIncontinence Comparison of Symptom Severity and Treatment Response in Patients with Incontinent and Continent Overactive Bladder Martin
The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs
 The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs Author Version Date Consultation Date of Ratification By JPG Shaista Hussain Joint Formulary Pharmacist V2 16.09.2014 Homerton University
The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs Author Version Date Consultation Date of Ratification By JPG Shaista Hussain Joint Formulary Pharmacist V2 16.09.2014 Homerton University
ENABLEX (darifenacin hydrobromide)
 ENABLEX (darifenacin hydrobromide) NAME OF DRUG The active ingredient of Enablex is darifenacin hydrobromide. Chemical name: (S)-2-{1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2- diphenylacetamide
ENABLEX (darifenacin hydrobromide) NAME OF DRUG The active ingredient of Enablex is darifenacin hydrobromide. Chemical name: (S)-2-{1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2- diphenylacetamide
Dr. Melissa Kagarise, PA C
 Dr. Melissa Kagarise, PA C This program has been supported by an educational grant from Pfizer Pharmaceuticals PharmCon is accredited by the Accreditation Council for Pharmacy Education as a provider of
Dr. Melissa Kagarise, PA C This program has been supported by an educational grant from Pfizer Pharmaceuticals PharmCon is accredited by the Accreditation Council for Pharmacy Education as a provider of
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based
 Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
Overactive Bladder (OAB) Step Therapy Program Policy Number: Last Review: Origination: Next Review: Policy When Policy Topic is covered:
 Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Origination: 07/2013 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for brand name Overactive Bladder
Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Origination: 07/2013 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for brand name Overactive Bladder
Treatment of overactive bladder in the aging population: focus on darifenacin
 REVIEW Treatment of overactive bladder in the aging population: focus on darifenacin Swati Jha Matthew Parsons Department of Urogynaecology, Birmingham Women s Hospital, Birmingham. UK Abstract: Anticholinergics
REVIEW Treatment of overactive bladder in the aging population: focus on darifenacin Swati Jha Matthew Parsons Department of Urogynaecology, Birmingham Women s Hospital, Birmingham. UK Abstract: Anticholinergics
Urinary Incontinence in Women: Never an Acceptable Consequence of Aging
 Urinary Incontinence in Women: Never an Acceptable Consequence of Aging Catherine A. Matthews, MD Associate Professor Chief, Urogynecology and Pelvic Reconstructive Surgery University of North Carolina,
Urinary Incontinence in Women: Never an Acceptable Consequence of Aging Catherine A. Matthews, MD Associate Professor Chief, Urogynecology and Pelvic Reconstructive Surgery University of North Carolina,
Overactive Bladder (OAB) Step Therapy Program
 Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Last Review: 11/2018 Origination: 7/2013 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Last Review: 11/2018 Origination: 7/2013 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
DRUG FORECAST. Select Conditions of the Lower Urinary Tract
 Transdermal Oxybutynin: Novel Drug Delivery for Overactive Bladder Vitalina Rozenfeld, PharmD, BCPS, Stanley Zaslau, MD, Kathleen New Geissel, PharmD, David Lucas, PhD, and Lili Babazadeh, PharmD INTRODUCTION
Transdermal Oxybutynin: Novel Drug Delivery for Overactive Bladder Vitalina Rozenfeld, PharmD, BCPS, Stanley Zaslau, MD, Kathleen New Geissel, PharmD, David Lucas, PhD, and Lili Babazadeh, PharmD INTRODUCTION
ENABLEX (darifenacin)
 T2004-49 ENABLEX (darifenacin) Extended-Release tablets Rx only Prescribing Information DESCRIPTION ENABLEX (darifenacin) is an extended-release tablet which contains 7.5 mg or 15 mg darifenacin as its
T2004-49 ENABLEX (darifenacin) Extended-Release tablets Rx only Prescribing Information DESCRIPTION ENABLEX (darifenacin) is an extended-release tablet which contains 7.5 mg or 15 mg darifenacin as its
Current drug treatments for female urinary incontinence Tarang Majmudar MRCOG and Mark Slack MMed, FRCOG, FCOG(SA)
 Drug review Incontinence Current drug treatments for female urinary incontinence Tarang Majmudar MRCOG and Mark Slack MMed, FRCOG, FCOG(SA) Skyline Imaging Ltd Several new drug treatments are now marketed
Drug review Incontinence Current drug treatments for female urinary incontinence Tarang Majmudar MRCOG and Mark Slack MMed, FRCOG, FCOG(SA) Skyline Imaging Ltd Several new drug treatments are now marketed
ENABLEX (darifenacin) extended-release tablets Initial U.S. Approval: 2004
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENABLEX safely and effectively. See full prescribing information for ENABLEX. ENABLEX (darifenacin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ENABLEX safely and effectively. See full prescribing information for ENABLEX. ENABLEX (darifenacin)
Drugs for the overactive bladder: are there differences in persistence and compliance?
 Editorial Drugs for the overactive bladder: are there differences in persistence and compliance? Karl-Erik Andersson 1,2 1 Institute for Regenerative Medicine, Wake Forest University School of Medicine,
Editorial Drugs for the overactive bladder: are there differences in persistence and compliance? Karl-Erik Andersson 1,2 1 Institute for Regenerative Medicine, Wake Forest University School of Medicine,
Overactive Bladder Syndrome
 Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
Enablex. Prolonged Release Tablets
 Enablex Prolonged Release Tablets Composition The active ingredient is Darifenacin (as hydrobromide). One prolonged release tablet contains 7.5 mg and 15 mg Darifenacin (as hydrobromide) Indications Enablex
Enablex Prolonged Release Tablets Composition The active ingredient is Darifenacin (as hydrobromide). One prolonged release tablet contains 7.5 mg and 15 mg Darifenacin (as hydrobromide) Indications Enablex
According to the INDICATIONS AND USAGE section of its FDA-approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Karen L. Walles, M.S. Assistant Director, Regulatory Affairs 3
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Karen L. Walles, M.S. Assistant Director, Regulatory Affairs 3
TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH
 TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH CONTENTS Overactive bladder (OAB) Treatment of OAB Beta-3 adrenoceptor agonist (Betmiga ) - Panacea? LASER treatment - a flash in the pan or the
TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH CONTENTS Overactive bladder (OAB) Treatment of OAB Beta-3 adrenoceptor agonist (Betmiga ) - Panacea? LASER treatment - a flash in the pan or the
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline.
 Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Overactive bladder. Information for patients from Urogynaecology
 Overactive bladder Information for patients from Urogynaecology An overactive bladder (OAB) is a very common problem. It can cause distressing symptoms that are difficult to control. These can include
Overactive bladder Information for patients from Urogynaecology An overactive bladder (OAB) is a very common problem. It can cause distressing symptoms that are difficult to control. These can include
URGE MOTOR INCONTINENCE
 URGE MOTOR INCONTINENCE URGE INCONTINENCE COMMONEST TYPE IN ELDERLY WOMEN Causes: 1 - Defects in CNS regulation Stroke Parkinson s disease Dementia (Alzheimer s and other types) Normopressure hydrocephalus
URGE MOTOR INCONTINENCE URGE INCONTINENCE COMMONEST TYPE IN ELDERLY WOMEN Causes: 1 - Defects in CNS regulation Stroke Parkinson s disease Dementia (Alzheimer s and other types) Normopressure hydrocephalus
Overactive Bladder in Clinical Practice
 Overactive Bladder in Clinical Practice Alan J. Wein Christopher Chapple Overactive Bladder in Clinical Practice Authors Alan J. Wein Division of Urology University of Pennsylvania Health System Philadelphia
Overactive Bladder in Clinical Practice Alan J. Wein Christopher Chapple Overactive Bladder in Clinical Practice Authors Alan J. Wein Division of Urology University of Pennsylvania Health System Philadelphia
3/20/10. Prevalence of OAB Men: 16.0% Women: 16.9% Prevalence of OAB with incontinence (OAB wet) Men: 2.6% Women: 9.3% Dry. Population (millions) Wet
 1 Prevalence of OAB Men: 16.0% Women: 16.9% Stewart WF, et al. World J Urol. 2003;20:327-336. Prevalence of OAB with incontinence (OAB wet) Men: 2.6% Women: 9.3% Stewart WF, et al. World J Urol. 2003;20:327-336.
1 Prevalence of OAB Men: 16.0% Women: 16.9% Stewart WF, et al. World J Urol. 2003;20:327-336. Prevalence of OAB with incontinence (OAB wet) Men: 2.6% Women: 9.3% Stewart WF, et al. World J Urol. 2003;20:327-336.
Comparison of Side Effects of Tolterodine and Solifenacinsucinate in Patients with Urinary Incontinence
 ORIGINAL ARTICLE Comparison of Side Effects of Tolterodine and Solifenacinsucinate in Patients with Urinary Incontinence MARYAM RANA, IRAM MOBUSHER ABSTRACT Background: Overactive bladder syndrome is a
ORIGINAL ARTICLE Comparison of Side Effects of Tolterodine and Solifenacinsucinate in Patients with Urinary Incontinence MARYAM RANA, IRAM MOBUSHER ABSTRACT Background: Overactive bladder syndrome is a
Efficacy and Safety of Propiverine and Solifenacin for the Treatment of Female Patients with Overactive Bladder: A Crossover Study
 LUTS () 3, 36 4 ORIGINAL ARTICLE Efficacy and Safety of Propiverine and Solifenacin for the Treatment of Female Patients with Overactive Bladder: A Crossover Study Naoki WADA, Masaki WATANABE, Masafumi
LUTS () 3, 36 4 ORIGINAL ARTICLE Efficacy and Safety of Propiverine and Solifenacin for the Treatment of Female Patients with Overactive Bladder: A Crossover Study Naoki WADA, Masaki WATANABE, Masafumi
Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders
 TNe 10 Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders Stuart B. Bauer, MD President, ICCS Department of Urology Children s Hospital Boston Professor of Urology Harvard
TNe 10 Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders Stuart B. Bauer, MD President, ICCS Department of Urology Children s Hospital Boston Professor of Urology Harvard
Oxytrol Transdermal Drug Delivery System
 Oxytrol Transdermal Drug Delivery System Name of medicine Oxybutynin The structure of oxybutynin 4-(diethylamino)but-2-ynyl (RS)-2-cyclohexyl-2-hydroxy- 2-phenylacetate is given below. Oxybutynin is an
Oxytrol Transdermal Drug Delivery System Name of medicine Oxybutynin The structure of oxybutynin 4-(diethylamino)but-2-ynyl (RS)-2-cyclohexyl-2-hydroxy- 2-phenylacetate is given below. Oxybutynin is an
Comparison of efficacy and tolerability of pharmacological treatment for the overactive bladder in women: A network meta-analysis
 RESEARCH Comparison of efficacy and tolerability of pharmacological treatment for the overactive bladder in women: A network meta-analysis Sivalingam Nalliah, Pou Wee Gan, Premjit K Masten Singh, Piravin
RESEARCH Comparison of efficacy and tolerability of pharmacological treatment for the overactive bladder in women: A network meta-analysis Sivalingam Nalliah, Pou Wee Gan, Premjit K Masten Singh, Piravin
Efficacy and Adverse Effects of Solifenacin in the Treatment of Lower Urinary Tract Symptoms in Patients With Overactive Bladder
 Urol Sci 21;21(1):38 43 ORIGINAL ARTICLE Efficacy and Adverse Effects of Solifenacin in the Treatment of Lower Urinary Tract Symptoms in Patients With Overactive Bladder Yih-Chou Chen, Chia-Yen Chen, Hann-Chorng
Urol Sci 21;21(1):38 43 ORIGINAL ARTICLE Efficacy and Adverse Effects of Solifenacin in the Treatment of Lower Urinary Tract Symptoms in Patients With Overactive Bladder Yih-Chou Chen, Chia-Yen Chen, Hann-Chorng
DETRUSITOL. Presentation. Uses. Tolterodine L-tartrate 1 mg & 2 mg tablets
 DETRUSITOL Tolterodine L-tartrate 1 mg & 2 mg tablets Presentation DETRUSITOL tablets are white, round, biconvex, film-coated tablets. The 1 mg tablet is engraved with arcs above and below the letters
DETRUSITOL Tolterodine L-tartrate 1 mg & 2 mg tablets Presentation DETRUSITOL tablets are white, round, biconvex, film-coated tablets. The 1 mg tablet is engraved with arcs above and below the letters
OXYSPAS Tablets (Oxybutynin chloride)
 Published on: 10 Jul 2014 OXYSPAS Tablets (Oxybutynin chloride) Composition OXYSPAS-2.5 Tablets Each uncoated tablet contains Oxybutynin chloride... 2.5 mg OXYSPAS-5 Tablets Each uncoated tablet contains
Published on: 10 Jul 2014 OXYSPAS Tablets (Oxybutynin chloride) Composition OXYSPAS-2.5 Tablets Each uncoated tablet contains Oxybutynin chloride... 2.5 mg OXYSPAS-5 Tablets Each uncoated tablet contains
Is There a Best Drug for Overactive Bladder in a Patient with Dementia?
 Is There a Best Drug for Overactive Bladder in a Patient with Dementia? Todd Semla, MS, Pharm.D., BCPS, FCCP, AGSF National PBM Clinical Pharmacy Program Manager Mental Health & Geriatrics U.S. Department
Is There a Best Drug for Overactive Bladder in a Patient with Dementia? Todd Semla, MS, Pharm.D., BCPS, FCCP, AGSF National PBM Clinical Pharmacy Program Manager Mental Health & Geriatrics U.S. Department
Comparative Study of Solifenecin Alone Versus Solifenecin with Duloxetine in Patients of Overactive Bladder
 International Journal of Scientific and Research Publications, Volume 3, Issue 1, January 2013 1 Comparative Study of Solifenecin Alone Versus Solifenecin with Duloxetine in Patients of Overactive Bladder
International Journal of Scientific and Research Publications, Volume 3, Issue 1, January 2013 1 Comparative Study of Solifenecin Alone Versus Solifenecin with Duloxetine in Patients of Overactive Bladder
NICE Pathways bring together all NICE guidance, quality standards and other NICE information on a specific topic.
 bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
Report on New Patented Drugs - Vesicare
 Report on New Patented Drugs - Vesicare Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board's Excessive
Report on New Patented Drugs - Vesicare Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board's Excessive
Clinical Medicine Insights: Urology
 Clinical Medicine Insights: Urology original research Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Efficacy and Safety of 10 mg Solifenacin Succinate in
Clinical Medicine Insights: Urology original research Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Efficacy and Safety of 10 mg Solifenacin Succinate in
Presentation Goals 4/14/2015. Pharmacology for Urinary Incontinence in Women. Medications Review anti muscarinic medications Focus on newer meds
 Presentation Goals Pharmacology for Urinary Incontinence in Women Medications Review anti muscarinic medications Focus on newer meds Introduce beta adrenergic medications Current Concepts in Drug Therapy
Presentation Goals Pharmacology for Urinary Incontinence in Women Medications Review anti muscarinic medications Focus on newer meds Introduce beta adrenergic medications Current Concepts in Drug Therapy
The Effects of AntimuscarinicTreatments in Overactive Bladder: A Systematic Review and Meta-Analysis
 European Urology European Urology 48 (2005) 5 26 ReviewöOveractive Bladder The Effects of AntimuscarinicTreatments in Overactive Bladder: A Systematic Review and Meta-Analysis Christopher Chapple a, *,
European Urology European Urology 48 (2005) 5 26 ReviewöOveractive Bladder The Effects of AntimuscarinicTreatments in Overactive Bladder: A Systematic Review and Meta-Analysis Christopher Chapple a, *,
PRUCAPLA Tablets (Prucalopride)
 Published on: 2 May 2018 PRUCAPLA Tablets (Prucalopride) Composition PRUCAPLA 1 mg Each film coated tablet contains: Prucalopride succinate equivalent to Prucalopride 1 mg Excipients. q. s. Color: Titanium
Published on: 2 May 2018 PRUCAPLA Tablets (Prucalopride) Composition PRUCAPLA 1 mg Each film coated tablet contains: Prucalopride succinate equivalent to Prucalopride 1 mg Excipients. q. s. Color: Titanium
Retrospective Analysis of Efficacy and Tolerability of Tolterodine in Children with Overactive Bladder
 European Urology European Urology 45 (2004) 240 244 Retrospective Analysis of Efficacy and Tolerability of Tolterodine in Children with Overactive Bladder A. Raes a,, P. Hoebeke b, I. Segaert a, E. Van
European Urology European Urology 45 (2004) 240 244 Retrospective Analysis of Efficacy and Tolerability of Tolterodine in Children with Overactive Bladder A. Raes a,, P. Hoebeke b, I. Segaert a, E. Van
Objectives. Prevalence of Urinary Incontinence URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS
 URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
Diagnosis and Mangement of Nocturia in Adults
 Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Rational Pharmacotherapy for LUTS in Older People. Dr William Gibson MBChB MRCP
 Rational Pharmacotherapy for LUTS in Older People Dr William Gibson MBChB MRCP Frailty Frailty = state of increased vulnerability resulting from agingassociated decline in reserve and function NOT synonymous
Rational Pharmacotherapy for LUTS in Older People Dr William Gibson MBChB MRCP Frailty Frailty = state of increased vulnerability resulting from agingassociated decline in reserve and function NOT synonymous
FESOTERODINE FUMARATE
 FESOTERODINE FUMARATE TOVIAZ TABLET 1. 0 THERAPEUTIC CATEGORY Over-active Bladder Antimuscarinic Agent 2. 0. DESCRIPTION Fesoterodine fumarate (Toviaz) contains fesoterodine fumarate and is an extendedrelease
FESOTERODINE FUMARATE TOVIAZ TABLET 1. 0 THERAPEUTIC CATEGORY Over-active Bladder Antimuscarinic Agent 2. 0. DESCRIPTION Fesoterodine fumarate (Toviaz) contains fesoterodine fumarate and is an extendedrelease
Safety and Tolerability Profiles of Anticholinergic Agents Used for the Treatment of Overactive Bladder
 REVIEW ARTICLE Drug Saf 2011; 34 (9): 733-754 0114-5916/11/0009-0733/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. Safety and Tolerability Profiles of Anticholinergic Agents Used for the
REVIEW ARTICLE Drug Saf 2011; 34 (9): 733-754 0114-5916/11/0009-0733/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. Safety and Tolerability Profiles of Anticholinergic Agents Used for the
Treatment of OAB in postmenopause. Držislav Kalafatić Department Obstetrics and Gynecology School of Medicine, University of Zagreb
 Treatment of OAB in postmenopause Držislav Kalafatić Department Obstetrics and Gynecology School of Medicine, University of Zagreb Spectrum of overactive bladder Urinary urgency usually accompanied by
Treatment of OAB in postmenopause Držislav Kalafatić Department Obstetrics and Gynecology School of Medicine, University of Zagreb Spectrum of overactive bladder Urinary urgency usually accompanied by
Overactive bladder affects 33 million adults in the United
 Overactive Bladder: Treatment Options in Primary Care Medicine David O. Sussman, DO Overactive bladder is a highly prevalent condition, affecting approximately 33 million adults in the United States. Despite
Overactive Bladder: Treatment Options in Primary Care Medicine David O. Sussman, DO Overactive bladder is a highly prevalent condition, affecting approximately 33 million adults in the United States. Despite
SELF CARE IN URINARY INCONTINENCE
 O P I N I O N SelfCare 2011;2(6):160-166 Advancing the study&understanding of self-care JULIAN SPINKS General Practitioner, Medway Primary Care Trust ABSTRACT Urinary incontinence and its associated urinary
O P I N I O N SelfCare 2011;2(6):160-166 Advancing the study&understanding of self-care JULIAN SPINKS General Practitioner, Medway Primary Care Trust ABSTRACT Urinary incontinence and its associated urinary
An Overview of Overactive Bladder and Its Pharmacological Management with a Focus on Anticholinergic Drugs
 An Overview of Overactive Bladder and Its Pharmacological Management with a Focus on Anticholinergic Drugs George DeMaagd, PharmD, BCPS, and Jeffrey D. Geibig, PharmD Educational Objectives After reviewing
An Overview of Overactive Bladder and Its Pharmacological Management with a Focus on Anticholinergic Drugs George DeMaagd, PharmD, BCPS, and Jeffrey D. Geibig, PharmD Educational Objectives After reviewing
Dr Jonathan Evans Paediatric Nephrologist
 How do I manage a patient with intractable daytime wetting: Dr Jonathan Evans Paediatric Nephrologist Of 107 children aged 11-12 with day-wetting 91 (85%) were dry at 15-16 yr Swithinbank et al BJU 1998
How do I manage a patient with intractable daytime wetting: Dr Jonathan Evans Paediatric Nephrologist Of 107 children aged 11-12 with day-wetting 91 (85%) were dry at 15-16 yr Swithinbank et al BJU 1998
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 BETMIGA 25 mg, prolonged-release tablet B/30 (CIP: 34 009 273 182-5 2) BETMIGA 50 mg, prolonged-release
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 BETMIGA 25 mg, prolonged-release tablet B/30 (CIP: 34 009 273 182-5 2) BETMIGA 50 mg, prolonged-release
Your OAB Doctor Discussion Guide
 Your OAB Doctor Discussion Guide This guide includes your symptom quiz results, conversation tips, and a 1-day bladder diary. All 3 could help your doctor understand your symptoms. Make sure you bring
Your OAB Doctor Discussion Guide This guide includes your symptom quiz results, conversation tips, and a 1-day bladder diary. All 3 could help your doctor understand your symptoms. Make sure you bring
Management of male LUTS in general practice
 17 Management of male LUTS in general practice MARK J. SPEAKMAN AND FAITH MCMEEKIN The initial management of lower urinary tract symptoms in men is usually carried out in primary care. The authors explain
17 Management of male LUTS in general practice MARK J. SPEAKMAN AND FAITH MCMEEKIN The initial management of lower urinary tract symptoms in men is usually carried out in primary care. The authors explain
Overactive Bladder (OAB) and Quality of Life
 Overactive Bladder (OAB) and Quality of Life Dr. Byron Wong MBBS (Sydney), FRCSEd, FRCSEd (Urol), FCSHK, FHKAM (Surgery) Specialist in Urology Central Urology Clinic Hong Kong Continence Society Annual
Overactive Bladder (OAB) and Quality of Life Dr. Byron Wong MBBS (Sydney), FRCSEd, FRCSEd (Urol), FCSHK, FHKAM (Surgery) Specialist in Urology Central Urology Clinic Hong Kong Continence Society Annual
Urinary Incontinence and Overactive Bladder Update NICE Guidelines on UI for women - GP Perspectives
 Urinary Incontinence and Overactive Bladder Update NICE Guidelines on UI for women - GP Perspectives Arun Sahai PhD, FRCS (Urol) Consultant Urological Surgeon & Honorary Senior Lecturer Guy s Hospital
Urinary Incontinence and Overactive Bladder Update NICE Guidelines on UI for women - GP Perspectives Arun Sahai PhD, FRCS (Urol) Consultant Urological Surgeon & Honorary Senior Lecturer Guy s Hospital
Overactive bladder: current understanding and future issues
 DOI: 10.1111/j.1471-0528.2006.01081.x www.blackwellpublishing.com/bjog Review article Overactive bladder: current understanding and future issues I Milsom Department of Obstetrics and Gynecology, Sahlgrenska
DOI: 10.1111/j.1471-0528.2006.01081.x www.blackwellpublishing.com/bjog Review article Overactive bladder: current understanding and future issues I Milsom Department of Obstetrics and Gynecology, Sahlgrenska
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION MIRABEGRON (Myrbetriq Astellas Pharma Canada Inc.) Indication: Overactive Bladder Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that mirabegron be listed
CDEC FINAL RECOMMENDATION MIRABEGRON (Myrbetriq Astellas Pharma Canada Inc.) Indication: Overactive Bladder Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that mirabegron be listed
Subjective Measures of Efficacy: Quality of Life, Patient Satisfaction and Patient-Oriented Goals the Search for Value
 european urology supplements 6 (2007) 438 443 available at www.sciencedirect.com journal homepage: www.europeanurology.com Subjective Measures of Efficacy: Quality of Life, Patient Satisfaction and Patient-Oriented
european urology supplements 6 (2007) 438 443 available at www.sciencedirect.com journal homepage: www.europeanurology.com Subjective Measures of Efficacy: Quality of Life, Patient Satisfaction and Patient-Oriented
Overactive Bladder Syndrome
 Page 1 of 5 Overactive Bladder Syndrome Overactive bladder syndrome is common. Symptoms include an urgent feeling to go to the toilet, going to the toilet frequently, and sometimes leaking urine before
Page 1 of 5 Overactive Bladder Syndrome Overactive bladder syndrome is common. Symptoms include an urgent feeling to go to the toilet, going to the toilet frequently, and sometimes leaking urine before
Internal Plumbing Guide Get an inside look at how your bladder works.
 Please note: When you print out your Internal Plumbing Guide transcript, the complete Prescribing Information for VESIcare (solifenacin succinate) tablets will also print out. Internal Plumbing Guide Get
Please note: When you print out your Internal Plumbing Guide transcript, the complete Prescribing Information for VESIcare (solifenacin succinate) tablets will also print out. Internal Plumbing Guide Get
Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions?
 Review Article SENSORY ACTIONS OF ANTIMUSCARINICS FINNEY et al. Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions? STEVEN M. FINNEY, KARL-ERIK
Review Article SENSORY ACTIONS OF ANTIMUSCARINICS FINNEY et al. Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions? STEVEN M. FINNEY, KARL-ERIK
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Oxybutynin Accord 2.5mg tablets Oxybutynin Accord 5mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mg tablet contains 2.5mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Oxybutynin Accord 2.5mg tablets Oxybutynin Accord 5mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mg tablet contains 2.5mg
Understanding the Benefits and Risks
 LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
Overactive bladder syndrome (OAB)
 Service: Urology Overactive bladder syndrome (OAB) Exceptional healthcare, personally delivered What is OAB? An overactive bladder or OAB is where a person regularly gets a sudden and compelling need or
Service: Urology Overactive bladder syndrome (OAB) Exceptional healthcare, personally delivered What is OAB? An overactive bladder or OAB is where a person regularly gets a sudden and compelling need or
FDA Briefing Document for Nonprescription Drugs Advisory Committee
 FDA Briefing Document for Nonprescription Drugs Advisory Committee Oxybutynin Transdermal System NDA 202211 Proposed Indication: Treats overactive bladder in women Topic: Partial Rx-to-OTC switch for oxybutynin
FDA Briefing Document for Nonprescription Drugs Advisory Committee Oxybutynin Transdermal System NDA 202211 Proposed Indication: Treats overactive bladder in women Topic: Partial Rx-to-OTC switch for oxybutynin
SELECTED POSTER PRESENTATIONS
 SELECTED POSTER PRESENTATIONS The following summaries are based on posters presented at the American Urogynecological Society 2004 Scientific Meeting, held July 29-31, 2004, in San Diego, California. CENTRAL
SELECTED POSTER PRESENTATIONS The following summaries are based on posters presented at the American Urogynecological Society 2004 Scientific Meeting, held July 29-31, 2004, in San Diego, California. CENTRAL
Long-Term Safety, Tolerability and Ef cacy of Extended-Release Tolterodine in the Treatment of Overactive Bladder
 European Urology European Urology 41 (2002) 588±595 Long-Term Safety, Tolerability and Ef cacy of Extended-Release Tolterodine in the Treatment of Overactive Bladder K. Kreder a,*, C. Mayne b, U. Jonas
European Urology European Urology 41 (2002) 588±595 Long-Term Safety, Tolerability and Ef cacy of Extended-Release Tolterodine in the Treatment of Overactive Bladder K. Kreder a,*, C. Mayne b, U. Jonas
BJUI. Validity and reliability of the patient s perception of intensity of urgency scale in overactive bladder
 ; 2010 Lower Urinary Tract PATIENT S PERCEPTION OF INTENSITY OF URGENCY SCALE IN OAB CARTWRIGHT ET AL. BJUI Validity and reliability of the patient s perception of intensity of urgency scale in overactive
; 2010 Lower Urinary Tract PATIENT S PERCEPTION OF INTENSITY OF URGENCY SCALE IN OAB CARTWRIGHT ET AL. BJUI Validity and reliability of the patient s perception of intensity of urgency scale in overactive
Introductory Clinical Pharmacology Chapter 32 Antiparkinsonism Drugs
 Introductory Clinical Pharmacology Chapter 32 Antiparkinsonism Drugs Dopaminergic Drugs: Actions Symptoms of parkinsonism are caused by depletion of dopamine in CNS Amantadine: makes more of dopamine available
Introductory Clinical Pharmacology Chapter 32 Antiparkinsonism Drugs Dopaminergic Drugs: Actions Symptoms of parkinsonism are caused by depletion of dopamine in CNS Amantadine: makes more of dopamine available
Management, Evaluation, and Treatment of Overactive Bladder and Urinary Incontinence
 Management, Evaluation, and Treatment of Overactive Bladder and Urinary Incontinence Arthur Mourtzinos, MD, MBA Co-Vice Chair, Institute of Urology Director, Continence Center Assistant Professor of Urology,
Management, Evaluation, and Treatment of Overactive Bladder and Urinary Incontinence Arthur Mourtzinos, MD, MBA Co-Vice Chair, Institute of Urology Director, Continence Center Assistant Professor of Urology,
Elements for a Public Summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Gout i Gout has a worldwide distribution. In the United Kingdom from 2000 to 2007, the estimated occurrence of gout is 5.9% in
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Gout i Gout has a worldwide distribution. In the United Kingdom from 2000 to 2007, the estimated occurrence of gout is 5.9% in
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
Patient Information. Basic Information on Overactive Bladder Symptoms. pubic bone. urethra. scrotum. bladder. vaginal canal
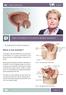 Patient Information English Basic Information on Overactive Bladder Symptoms The underlined terms are listed in the glossary. What is the bladder? pubic bone bladder seminal vesicles prostate rectum The
Patient Information English Basic Information on Overactive Bladder Symptoms The underlined terms are listed in the glossary. What is the bladder? pubic bone bladder seminal vesicles prostate rectum The
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
An awfully overactive bladder By Katie Hayes
 An awfully overactive bladder By Katie Hayes Learning objectives After reading this article you should be able to: Identify signs and symptoms of overactive bladder syndrome Describe the pharmacological
An awfully overactive bladder By Katie Hayes Learning objectives After reading this article you should be able to: Identify signs and symptoms of overactive bladder syndrome Describe the pharmacological
Overactive Bladder: Diagnosis and Approaches to Treatment
 Overactive Bladder: Diagnosis and Approaches to Treatment A Hidden Condition* Many Many patients self-manage by voiding frequently, reducing fluid intake, and wearing pads Nearly Nearly two-thirds thirds
Overactive Bladder: Diagnosis and Approaches to Treatment A Hidden Condition* Many Many patients self-manage by voiding frequently, reducing fluid intake, and wearing pads Nearly Nearly two-thirds thirds
Clinical guidelines for overactive bladder
 International Journal of Urology (2009) 16, 126 142 doi: 10.1111/j.1442-2042.2008.02177.x Guidelines Clinical guidelines for overactive bladder Osamu Yamaguchi, 1 Osamu Nishizawa, 2 Masayuki Takeda, 3
International Journal of Urology (2009) 16, 126 142 doi: 10.1111/j.1442-2042.2008.02177.x Guidelines Clinical guidelines for overactive bladder Osamu Yamaguchi, 1 Osamu Nishizawa, 2 Masayuki Takeda, 3
Urogynecology Office. Can You Hold? An Update on the Treatment of OAB. Can You Hold? Urogynecology Office
 Urogynecology Office Urogynecology Office Can You Hold? An Update on the Treatment of OAB Can You Hold? Karen Noblett, MD Professor and Chair Department of OB/GYN University of California, Riverside Disclosures
Urogynecology Office Urogynecology Office Can You Hold? An Update on the Treatment of OAB Can You Hold? Karen Noblett, MD Professor and Chair Department of OB/GYN University of California, Riverside Disclosures
Overactive Bladder: Identifying Patients at Risk, Implementing New Strategies
 Overactive Bladder: Identifying Patients at Risk, Implementing New Learning Objectives Identify patients with OAB risk factors in order to proactively initiate a discussion about bladder symptoms and establish
Overactive Bladder: Identifying Patients at Risk, Implementing New Learning Objectives Identify patients with OAB risk factors in order to proactively initiate a discussion about bladder symptoms and establish
Efficacy and safety of solifenacin succinate in Korean patients with overactive bladder: a randomised, prospective, double-blind, multicentre study
 doi: 10.1111/j.1742-1241.2008.01898.x ORIGINAL PAPER Efficacy and safety of solifenacin succinate in n patients with overactive bladder: a randomised, prospective, double-blind, multicentre study M.-S.
doi: 10.1111/j.1742-1241.2008.01898.x ORIGINAL PAPER Efficacy and safety of solifenacin succinate in n patients with overactive bladder: a randomised, prospective, double-blind, multicentre study M.-S.
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/ January 2013
 CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
L ower urinary tract symptoms (LUTS) are a major health problem and are prevalent in men aged.45 years1 3.
 OPEN SUBJECT AREAS: UROGENITAL DISEASES DRUG DEVELOPMENT Received 23 October 2013 Accepted 15 January 2014 Published 4 February 2014 Correspondence and requests for materials should be addressed to P.H.
OPEN SUBJECT AREAS: UROGENITAL DISEASES DRUG DEVELOPMENT Received 23 October 2013 Accepted 15 January 2014 Published 4 February 2014 Correspondence and requests for materials should be addressed to P.H.
PFIZER DETRUSITOL* Tolterodine Tartrate
 Disclaimer While these labels are the most current Drug Control Authority (DCA) approved versions of content, these are not necessarily reflective of final printed labeling currently in distribution. LPD
Disclaimer While these labels are the most current Drug Control Authority (DCA) approved versions of content, these are not necessarily reflective of final printed labeling currently in distribution. LPD
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 3Q17 July-August
 BRAND NAME Noctiva GENERIC NAME Desmopressin acetate nasal spray MANUFACTURER Serenity Pharmaceuticals DATE OF APPROVAL March 3, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review
BRAND NAME Noctiva GENERIC NAME Desmopressin acetate nasal spray MANUFACTURER Serenity Pharmaceuticals DATE OF APPROVAL March 3, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review
