KING COUNTY SUPERIOR COURT STATE OF WASHINGTON STATE OF WASHINGTON, 12D I. INTRODUCTION
|
|
|
- Jonathan Fields
- 5 years ago
- Views:
Transcription
1 KING COUNTY SUPERIOR COURT STATE OF WASHINGTON STATE OF WASHINGTON, Plaintiff, JOHNSON & JOHNSON, a New Jersey Corporation; ETHICON, INC., a New Jersey Corporation, a wholly owned subsidiary of JOHNSON & JOHNSON; ETHICON US, LLC, a New Jersey Company, a wholly owned subsidiary of JOHNSON & JOHNSON; and DOES 1 through 100, inclusive, Defendants. 12D OTHER RELIEF I. INTRODUCTION 1.1 Plaintiff, the State of Washington (the State), by and through Attorney General Robert W. Ferguson, Senior Counsel Elizabeth J. Erwin, and Assistant Attorneys General Andrea Alegrett and Leilani Fisher, brings this action against Defendants Johnson & Johnson, Ethicon, Inc., Ethicon US, LLC, and DOES 1 through 100 ("Defendants") for violations of the Consumer Protection Act, RCW ("CPA"). The CPA declares unlawful and prohibits unfair or deceptive acts or practices in the conduct of any trade or commerce. RCW Defendants manufacture, market, and sell surgical mesh medical devices designed to treat common pelvic floor conditions in women, specifically pelvic organ prolapse OTHER RELIEF - 1
2 I and stress urinary incontinence. These are conditions that one-third to one-half of all women 2 will face in their lifetime. Defendants' surgical mesh is composed of woven synthetic 3 polypropylene (plastic) threads that are used to prop up organs that have dropped down or 4 protruded into the vagina. The mesh is inserted into the body through the vagina, pulled 5 through an incision in the vaginal canal, and then anchored permanently in the body. 6 Defendants sold almost 12,000 mesh devices in the State of Washington between 2005 and Defendants marketed their products to doctors as a "new and revolutionary" 9 product that would reduce time in the operating room and increase profits. Defendants made 10 these representations without disclosing to doctors the serious complications their mesh can 11 cause women. Defendants also marketed their mesh products as improvements over traditional 12 repair methods (i.e. native tissue repair) when they knew such claims were inaccurate Defendants knew at all relevant times that the presence of polypropylene in the 14 body and the process of implanting mesh through the vagina could cause severe and 15 unavoidable complications. These unavoidable lifelong complications include for example, 16 permanent loss of sexual function, painful sexual intercourse, near impossibility of removal of 17 the device when complications arise, permanent urinary dysfunction, lifelong risk of infection, 18 and chronic pelvic pain. Defendants knew these complications are caused by the design and 19 placement of the mesh and cannot be avoided by good surgical technique alone Due to the severity and type of complications associated with surgical mesh 21 devices, the impact on a woman's quality of life can be devastating. Some women become 22 permanently disabled or require accommodations from their employers. Women and their 23 partners have suffered loss of physical intimacy. One mesh patient's complaint to Defendants 24 from August 2008, is illustrative of the toll that surgical mesh has taken on people's lives: 25 [I] had all kinds of problems with chronic pain, bleeding, 26 dyspareunia [painful sexual intercourse] (even my husband OTHER RELIEF - 2
3 I I complained of scraping and poking)... The pelvic pain was keeping me awake at night, and the only relief was to sit on a tennis ball. The thought living like that, sitting on a ball, wearing a diaper, splinting my perineum to have a bowel movement, having infrequent and miserable sex, and marital problems was almost more than I could bear. In August 2011, another woman informed Defendants: I experienced excruciating pain from day one. I felt as though my urethra was being strangled, I couldn't pee, walking was out of the question, sitting was agony, & I couldn't lie on my left side due to severe pain... Over the course of the next 14 weeks I visited/was admitted to the [hospital] 10 times... I had no quality of life. My consultant likened the mesh removal as to trying to remove chewing gum from hair. 1.6 In an attempt to treat complications such as these, women often need multiple mesh removal surgeries. However, their suffering may never be alleviated because it is nearly impossible to completely remove mesh from the body. Moreover, the mesh removal surgeries can present additional complications and result in recurrence of the original pelvic floor condition. The suffering by these women is even more egregious considering that the underlying condition the mesh is meant to treat is not life threatening. Further, women have a non-mesh surgical alternative (i.e. the use of native tissue) that has been used for decades. Native tissue repair does not pose the same risks as mesh and can be just as effective for treating pelvic floor conditions. 1.7 Despite Defendants' knowledge of these complications, Defendants' informational and marketing materials misrepresented the safety and effectiveness of their products and omitted known serious risks and complications. Defendants' campaign of deception caused thousands of women in Washington to unknowingly take risks with their bodies. Defendants' conduct deprived doctors and patients of the information necessary to make informed decisions regarding Defendants' products. Defendants' misrepresentations and OTHER RELIEF - 3
4 1 I omissions to consumers are a serious women's health issue. Defendants are thus liable for 2 civil penalties, injunctive relief, restitution, and other appropriate relief under the Washington 3 CPA, as set forth herein. 4 II. PARTIES Plaintiff is the State of Washington. The Attorney General is authorized to 6 commence this action pursuant to RCW and RCW Defendant Johnson & Johnson ("J&J") is a publically-held company that is 8 organized and exists under the laws of the State of New Jersey. Its principal place of business 9 is located at 1 Johnson & Johnson Plaza, New Brunswick, NJ According to its website, 10 J&J is the "world's most comprehensive medical devices business" with "265 operating 11 companies in more than 60 countries," including the United States and the State of 12 Washington. J&J has an open account with the Washington State Department of Revenue, 13 UBI No , and provided a local business location of nd Ave W, University 14 Place, WA Defendant Ethicon Inc. and Defendant Ethicon US, LLC (UBI ) 16 (collectively "Ethicon") are wholly owned subsidiaries of J&J and are located in New Jersey. 17 Ethicon's website proclaims it to be "Part of the Johnson & Johnson Family of Companies." 18" J&J's company structure is divided into three sectors: medical devices, consumer healthcare, 19 and pharmaceuticals. The "Ethicon Franchise" is a business unit in J&J's medical devices 20 sector. Upon information and belief, the Ethicon Franchise is comprised of companies 21 controlled by J&J and include, but are not limited to, Ethicon Inc., Ethicon LLC, Ethicon US, 22 LLC, and Ethicon LTD At J&J's direction and independently, Ethicon designs, develops, promotes, 24 markets, reports, tests, distributes, and sells synthetic polypropylene pelvic floor repair 25 products and trains doctors on these products. Further, J&J together with Ethicon, sold mesh 26 products, placing both their corporate brand names on the packaging for each of their mesh OTHER RELIEF - 4
5 1 products. (See Photographs of Prolift+M, attached as Exhibit A; and Photographs of TVT, 2 attached as Exhibit B.) J&J participated in, controlled, knew about, and approved of, Ethicon's conduct 4 in the development, design, sales, and marketing of Defendants' mesh products, including the 5 training of doctors. J&J is therefore liable for Ethicon's conduct under the Washington CPA J&J, Ethicon, and DOES are collectively referred to as "Defendants." 7 Plaintiff is not aware of the true names and capacities of Defendants sued herein as DOES 1 8 through 100 and therefore sues these defendants by fictitious names. Plaintiff will amend this 9 Complaint to add the true names of the fictitiously named defendants once they are discovered. 10 Acts done by one Defendant were done in furtherance of the business practices of the other. 11 Defendants directed, created, executed, participated in, controlled, had the authority to control 12 or participate in, and had knowledge of the acts and practices set forth in this Complaint. Upon 13 information and belief, all Defendants received significant proceeds from the business 14 practices identified in this Complaint At all relevant times, each Defendant engaged in the business of placing 16 polypropylene mesh medical devices into the stream of commerce by designing, 17 manufacturing, testing, training, marketing, promoting, packaging, and/or selling such devices, 18 including the Prolene Mesh/Prolene Soft Mesh, Gynemesh, Gynemesh PS, TVT, TVT- 19 Obturator (TVT-0), TVT-SECUR (TVT-S), TVT Exact, TVT Abbrevo, Prolift, Prolift+M, 20 Prosima, Artisyn and other polypropylene mesh products unknown at the present 21 ("Polypropylene Mesh Products") At all relevant times, each Defendant acted individually or jointly with every 23 other named Defendant in committing all acts alleged in this Complaint At all relevant times, each Defendant acted (a) as principal; (b) under express or 25 implied agency; and/or (c) with actual or ostensible authority to perform the acts alleged in this 26 Complaint on behalf of every other named Defendant. OTHER RELIEF - 5
6 At all relevant times, one or all of the Defendants acted as the agent of the others and all Defendants acted within the scope of their agency as if actin as the agent of the p g Y g g I other At all relevant times, each Defendant and its employees had awareness of the others' conduct relating to the matters alleged within the Complaint. III. JURISDICTION AND VENUE 3.1 The Attorney General is authorized under RCW , RCW , and RCW to bring suit to enforce the CPA's prohibitions on unfair or deceptive acts or practices in the conduct of trade or commerce. 3.2 This Court has personal jurisdiction over Defendants pursuant to RCW , RCW , and RCW because Defendants have transacted substantial business in this State and the acts alleged have been committed in this State. Defendants knowingly placed their Polypropylene Mesh Products into the stream of commerce through designing, manufacturing, testing, training, marketing, promoting, packaging, and selling such devices. Defendants promoted, marketed, and sold their Polypropylene Mesh Products in the State of Washington and throughout King County. Further, Defendants derived substantial profits from Washington consumers, hospitals, clinics, and health care providers from the sale of their Polypropylene Mesh Products. Their products were ultimately surgically placed in Washington consumers. 3.3 Upon information and belief, Defendants sold approximately 11,728 mesh products in the State of Washington between 2005 and Upon information and belief, Defendants had thousands of sales and marketing contacts to promote Polypropylene Mesh Products in the State of Washington and in King County, including but not limited to: Defendants' sales representative and other employee had contacts with doctors; medical and other trainings offered to Washington licensed-doctors; poster presentations received by doctors; paid consultants promoted Defendants' OTHER RELIEF - 6
7 1 Polypropylene Mesh Products to doctors; Defendants and their agents provided written 2 communications and training videos; and Defendants engaged in direct-to-consumer marketing 3 through brochures, radio advertisements, television advertisements, internet advertisements, 4 phone scripts, websites, and other materials In sum, this action arises from Defendants' purposeful contacts with the State. 6 Therefore exercise of personal jurisdiction over Defendants comports with traditional notions 7 of fair play and substantial justice, and jurisdiction is consistent with the United States 8 Constitution and the Washington State Constitution Venue is proper in King County pursuant to RCW because Defendants 10 transacted business in King County at the time the causes of action in this Complaint arose and 11 continue to do so as of filing this action Defendants' actions in Washington were directed to numerous Washington 13 consumers, doctors, and patients and thus affect the public interest. 14 IV. FACTS Defendants promoted their Polypropylene Mesh Products directly to consumers, 16 including doctors and patients. Defendants mislead consumers through informational and 17 marketing materials that misrepresented the safety and effectiveness of their products and 18 omitted serious risks and complications. Further, Defendants received large numbers of 19 complaints from doctors and patients regarding complications caused by their Polypropylene 20 Mesh Products. Despite this knowledge, Defendants failed to update their informational and 21 promotional materials with these serious complications. 22 A. BACKGROUND OF SUI AND POP TREATMENT Stress urinary incontinence (SUI) and pelvic organ prolapse (POP) are common 24 conditions caused by weakened or damaged tissues and muscles in the pelvic floor area SUI 25 occurs when the muscles or other supporting tissues that control urine flow do not work 26 properly, resulting in involuntary urine leakage during everyday activities such as laughing, OTHER RELIEF - 7
8 1 coughing, or exercise. POP occurs when the muscles or other supporting tissues of the pelvic 2 floor can no longer support the pelvic organs, causing various organs to drop downwards and, 3 in some cases, press into and bulge out of the vagina SUI and POP are conditions that pose lifestyle limitations and, in the case of 5 POP, some mild pain, but they are not life threatening. SUI and POP affect a large percentage 6 of the female population. An estimated thirty to fifty percent of women are affected by 7 11 incontinence, and nearly half of women between the ages of 50 and 79 have some form of 8 m Defendants developed surgical mesh products intended to treat SUI and POP. 10 Defendants' Polypropylene Mesh Products are derived from polypropylene threads that are 11 weaved together. Polypropylene is derived from crude oil and is used to manufacture 12 thousands of consumer and other goods everything from rug backing to automobile parts. 13 Defendants' mesh has openings in its weave known at pores. Tissue grows into and through 14 these pores, which eventually creates a hammock consisting of mesh and tissue to support the 15 organs that need support. The mesh then becomes integrated into the patient's surrounding 16 tissue and is intended to remain permanently implanted in the body Defendants' mesh products are placed transvaginally. "Transvaginal placement" 18 means that the polypropylene mesh is surgically implanted through the vagina for pelvic floor 19 repairs, as opposed to the past practice of surgical repair through a woman's abdomen. For 20 SUI products, the mesh is pulled through an incision in the vagina and placed under the 21 urethra. The mesh lifts the urethra up to stop any involuntary leakage of urine during periods 22 of increased abdominal pressure (such as laughing, coughing, or sneezing). For POP products, 23 mesh is inserted into the body through the vagina, pulled through an incision in the vaginal 24 canal, and placed in different locations in front of or behind the vaginal canal to act as a 25 hammock for the prolapsing organ. Once implanted, the mesh then acts to keep the organ(s) 26 that have dropped onto the vagina from continuing to descend into and out of the vagina. OTHER RELIEF - 8
9 1 Organs that are typically involved in POP procedures include the bladder, small intestine, 2 uterus, rectum, and sometimes the vagina itself POP and SUI may be treated non-surgically through behavioral changes (such 4 as diet, exercise, and weight loss), vaginal and/or tibial stimulation, pelvic floor exercises, or 5 using a removable device called a pessary that is placed into the vagina to support areas of 6 prolapse. Some women, however, may choose surgery for treatment. Surgical options include 7 creating a sling with the patient's own tissue, cadaver tissue, or other tissue, abdominal 8 implantation of mesh, transvaginal implantation of mesh, or a combination of these options. 9 Notably, the U.S. Food and Drug Administration (FDA) has found that use of a patient's native 10 tissue is just as effective as transvaginal mesh and does not pose the same risks as mesh. 11 B. DEFENDANTS' POLYPROPYLENE MESH PRODUCTS Between 2005 and 2015, Defendants sold at least 11,728 Polypropylene Mesh 13 Products in Washington. Beginning in 1998, Defendants began making, marketing, and selling 14 various SUI mesh treatment options. Their first product was called TVT. This was a "tension 15 free vaginal mesh" also called a "mid-urethral" sling. Defendants continue to make, market, 16 and sell similar devices for the treatment of SUI today. Defendants offered, and continue to 17 offer the original TVT in multiple and significant variations, including but not limited to the 18 TVT, TVT-Obturator (TVT-0), TVT-Secur (TVT-S), TVT Exact, and TVT Abbrevo. All 19 references to TVT include all variations. Upon information and belief, Defendants sold 20 thousands of TVT products in Washington, including in King County In 2002, Defendants began making, marketing, and selling Gynemesh for the 22 treatment of SUI and POP. All references to Gynemesh include all variations of Gynemesh, 23 including but not limited to Gynemesh PS Nonabsorbable Prolene Soft Mesh. Upon 24 I information and belief, Defendants sold Gynemesh products in Washington, including in King 25 County In 2005, Defendants began making, marketing, and selling the Prolift System. OTHER RELIEF Fifth Avenue,. Suite 2000
10 1 The Prolift is made from pre-cut pieces of Gynemesh with added "arms" designed to be 2 attached to various fixation points within the patient's pelvis. Defendants sold this product by 3 misrepresenting in their,informational and marketing materials and through their sales 4 representatives that the FDA "approved" the marketing of Prolift when the device was never 5 "approved" by the FDA In 2007, Defendants updated the Prolift System with the Prolift+M System. All 7 references to the Prolift and the Prolift+M Systems include by reference all variations. Upon 8 information and belief, Defendants sold Prolift and Prolift+M products in Washington, 9 including in King County In 2010, Defendants began making, marketing, and selling their Prosima 11 System for the treatment of POP. All references to Prosima include by reference all variations. 12 Upon information and belief, Defendants sold Prosima products in Washington, including in 13 King County In 2012, Defendants began making, marketing, and selling a mesh products 15 called Artisyn, designed to treat POP that is placed through the abdomen rather than 16 transvaginally. Shortly thereafter in 2013, Defendants abandoned their POP mesh products 17 that were placed transvaginally, (i.e. Prolift and Prosima). For the first time, Defendants 18 included the following safety warning in materials for Artisyn: "The safety and effectiveness 19 of this product has not been validated in clinical trials." No such warning was offered to 20 consumers, doctors, and patients for the products sold by Defendants from Rather, 21 during that time period Defendants misled the public by advertising and promoting their other 22 Polypropylene Mesh Products as safe and effective. 23 C. DEFENDANTS MISREPRESENTED THE SAFETY AND EFFECTIVENESS OF THEIR PRODUCTS TO WASHINGTON CONSUMERS Defendants did not conduct human trials prior to the initial sale of their 26 OTHER RELIEF - 10 ATTORNEY GENERAL OF WASIENGTON
11 1 Polypropylene Mesh Products in Defendants destroyed the underlying data they claimed 2 supported the safety and effectiveness of their original SUI products. Defendants never tested 3 the long term safety of their POP mesh products prior to sale. While some mesh products were 4 tested in rats prior to sale, animal results cannot reliably be extrapolated to how mesh devices 5 will perform in women. Many independent studies involving transvaginal mesh have actually 6 demonstrated severe and serious complications associated with the use of polypropylene, 7 including Defendants' products Despite lacking sufficient data, Defendants represented that their products had a 9 "history of safety" and "proven efficacy" while disregarding known serious risks and 10 complications. These misrepresentations were made directly to doctors through educational 11 trainings, sales representative contacts, in person meetings, doctor opinion papers, instructions 12 for use (IFUs), and other informational and marketing materials. IFUs accompany the actual 13 device. They are designed to disclose any relevant hazards, contraindications, side effects, and 14 precautions. As an Ethicon Medical Director admitted, Ethicon should in its IFUs "list each of 15 the adverse reactions that were known" to Ethicon Defendants also made misrepresentations directly to consumers through 17 consumer-directed brochures, radio advertisements, television advertisements, internet 18 advertisements, phone scripts, product advertisements, and through other informational and 19 marketing materials. These misrepresentations include advertising that their products have a 20 "[flow incidence of reported serious complications," have a "small risk of exposure," "allow[] 21 for restoration of sexual function," and remain "soft" and "flexible." Defendants made misrepresentations in their marketing, promotional, 23 informational, and educational materials about complication rates of mesh, citing to studies 24 that did not actually support the Defendants' propositions. Dr. Ulf Ulmsten, creator of the 25 TVT product, sold the rights of the TVT device to Defendants. In marketing the TVT product, 26 Defendants used several studies of Dr. Ulmsten and failed disclose their financial relationship. OTHER RELIEF - 11
12 1 Defendants also failed to disclose that Dr. Ulmsten contractually agreed that he would only get 2 paid by Defendants a specific sum if his future studies produced favorable results regarding the 3 product Many of Defendants' informational and marketing materials advised patients to 5 "talk to their doctors." However, doctors lacked accurate information to give to their patients 6 because Defendants made safety and effectiveness misrepresentations directly to doctors. 7 Defendants withheld key information that would have allowed consumers, doctors, and 8 patients to understand the risks of these products and make informed medical decisions. These 9 misrepresentations are material and have the capacity to deceive a substantial number of 10 consumers in Washington State Defendants Misrepresented the Safety of Their Products by Failing to Disclose Risks and Complications Associated with Pelvic Floor Surgery Defendants represented that their Polypropylene Mesh Products offer "a new 14 and revolutionary surgical procedure" for the treatment of POP and SUI. Yet Defendants 15 failed to disclose that their mesh devices carry similar risks as other pelvic floor surgeries and 16 additional risks caused by the mesh Defendants' informational and marketing materials consistently failed to 18 disclose pelvic floor complications, including but not limited to: abscess, de novo urge 19 incontinence, defecatory dysfunction, dyspareunia (difficult or painful intercourse), dysuria 20 (painful urination), fistula formation (abnormal permanent passageway between two organs), 21 hematoma, hemorrhage, pain to partner during intercourse, permanent urinary dysfunction, 22 recurrence, temporary and transitory pain, urinary tract infection, urinary tract obstruction, 23 vaginal scarring, and worsening incontinence Defendants not only knew that their mesh devices can cause these pelvic floor 25 general complications, but they also knew that such complications can last a lifetime and may 26 develop several years after the initial procedure. And yet, Defendants did not disclose these OTHER RELIEF - 12
13 1 II risks Defendants misrepresented the risks of their mesh products by claiming certain 3 complications were common in all pelvic floor surgeries. For example, Defendants claimed 4 that only patients with compromised immune systems may have healing risks. In fact, any 5 patient who experienced a chronic infection and chronic inflammation from a mesh implant 6 could develop a healing risk due to mesh. Although all of Defendants' products were used in 7 contaminated areas the vagina, Defendants misrepresented that "if the mesh implant is to be 8 used in contaminated areas, it must be only with the understanding that subsequent infection 9 may require its removal." Additionally, Defendants did not disclose, until well after the sale of thousands 11 of devices, that their mesh could cause pelvic pain or pain with intercourse. When Defendants 12 finally did disclose these risks, the disclosures were inadequate. Rather, they stated, "potential 13 adverse reaction are those typically associated with POP repair procedures, including pelvic 14 pain and pain with intercourse. These may resolve with time." In fact, the rate of these 15 complications is much higher in mesh patients than those that receive native tissue repair In order to increase sales of their products and to appear superior to native tissue 17 repair, Defendants represented their products to consumers as having "small risk[s] of 18! exposure," "allow[ing] for restoration. of sexual function," having "low incidence of reported 19 serious complications," and remaining "soft" and "flexible" Defendants Misrepresented Material Complications and Risks Caused By 21 Their Products Defendants misled consumers, doctors, and patients regarding serious risks that 23 are increased by the use of mesh and that are not present in native tissue repair. Such 24 complications cannot be avoided by doctors or patients because they are caused by the 25 products' implantation method and how polypropylene is processed by the body. 26 OTHER RELIEF - 13
14 Defendants represented in informational and marketing materials that their 2 Polypropylene Mesh Products do not potentiate infection. This is a misrepresentation because 3 the transvaginal design of Defendants' Polypropylene Mesh Products presents a risk of chronic 4 infection from bacterial contamination. This risk is greater than the risk of infection posed by 5 non-transvaginal implants because the vagina is a cavity that can never be completely 6 sterilized. Because Defendants' Polypropylene Mesh Products is a fabric made of woven 7 strands bacterial microcolonies can become embedded in the mesh during implantation. The 8 chronic infection can also cause chronic inflammation. Post-surgical infections have been 9 found in mesh removed years after implantation Also, Defendants represented in their informational and marketing materials 11 that their polypropylene mesh products are "inert" meaning that they do not trigger a chronic 12 foreign body reaction or degrade in the body. Defendants further represented in promotional 13 and informational materials that the body does not react to mesh material and that it is 14 biologically compatible in the pelvic floor. This is a misrepresentation because polypropylene 15 incites a chronic foreign body reaction. Polypropylene degrades and oxidizes in the body over 16 time. Chronic foreign body reaction occurs when the immune system continuously fights and 17 tries to remove a foreign object whether a sliver of wood or polypropylene that has entered 18 the human body. This chronic foreign body reaction then causes chronic inflammation The risks of chronic infection, chronic foreign body reaction, and chronic 20 inflammation caused by the Polypropylene Mesh Products trigger a number of adverse 21' reactions in the human body. For example, the mesh hardens, contracts, erodes into other body 22 organs, and becomes so rigid and distorted that complete mesh removal is extremely difficult 23 and often impossible. Other complications that ultimately result from mesh include but are not 24 limited to: chronic infection, chronic foreign body reaction, chronic inflammation, mesh 25 hardening, mesh contracture, erosion into other body organs, mesh exposure (migration of 26 mesh into the vagina), mesh extrusion, mesh degradation (breakdown of mesh particles), OTHER RELIEF - 14
15 1 permanent dyspareunia, chronic pain, vaginal shortening, vaginal stiffness, vaginal distortion, 2 sexual dysfunction, injury to sexual partners, urinary and bowel dysfunction, and other lifelong 3 problems Defendants misrepresented the safety of their Polypropylene Mesh Products by 5 failing to disclose that the mesh cannot be removed effectively upon device failure. Mesh 6 removal is the only treatment option for most continuing mesh complications. Removal often 7 requires multiple surgeries, which may or may not resolve complications, and may in fact 8 result in new problems. One doctor described removing mesh as "akin to taking a hammer and 9 chisel and trying to remove the rebar from a sidewalk, while leaving the cement otherwise 10 intact and not damage the water mains and power lines below." Yet, Defendants failed to 11 disclose the lack of a safe and effective means for removal In recent years, cancer has also been raised as a possible risk of mesh 13 implantation in the body due to the chronic inflammatory reactions incited by mesh. Due to 14 the dormancy period for cancer in humans, the true risk of cancer from the use of Defendants' 15 mesh devices may not be apparent for another decade or more Because mesh remains in the body forever, erosion into the vaginal wall or one 17 of the pelvic organs can occur at any time. Defendants failed to disclose this lifelong risk of 18 erosion despite knowing that "there is no safe time for erosion when permanent materials are 19 used." This omission is significant because erosion is the most common and consistently 20 reported mesh-related complication. Erosion can be debilitating, leading to severe pelvic pain, 21 painful intercourse, or an inability to engage in intercourse Defendants knew prior to sale about each complication identified in this 23 Complaint. For example, an internal document entitled "LIGHTning Critical Strategy," dated 24 September 26, 2006, demonstrates Defendants' knowledge regarding shrinkage and impact on 25 sexual function: 26 OTHER RELIEF - 15
16 Mesh retraction ("shrinkage")... can cause vaginal anatomic distortion, which may eventually have a negative impact on sexual function. Its treatment is difficult. Additionally, the scar plate that forms with in-growth of tissue into the mesh can cause stiffness of the vagina that further impacts sexual function in a negative manner Defendants also knew that claims of softness were "illusory." Nevertheless, Defendants misrepresented that their mesh is "supple," "remains soft and pliable" and has a "bi-directional elastic property [that] allows adaptation to various stresses encountered in the body." Defendants knew the importance that doctors place on pliability and elasticity in the pelvis, which needs to accommodate the flux and movement associated with bladder, bowel and sexual function. Yet, Defendants misrepresented and concealed the risk that mesh can harden and become rigid within the body. This will in turn cause pain, sexual and urinary dysfunction Despite the fact that the foreign body reaction and inflammation triggered by Polypropylene Mesh Products can be chronic and lifelong, Defendants misrepresented in promotional and informational materials that the foreign body response triggered by mesh is only temporary. Defendants stated in physician brochures, physician trainings, and other materials, for examples, that polypropylene mesh response is "transitory." 4.34 These examples are representative of many misrepresentations made by Defendants throughout their promotional and informational materials 3. Defendants Misrepresented the Safety and Effectiveness of Their Pelvic Mesh Products by Marketing their Products as "FDA Approved" 4.35 Defendants misrepresented in their informational and marketing materials that their Polypropylene Mesh Products are "FDA Approved." FDA approved devices undergo a rigorous evaluation of their safety and efficacy. To obtain FDA approval, the safety and effectiveness of a medical device must be demonstrated through adequate clinical trials. OTHER RELIEF - 16
17 1 However, Defendants' mesh products never underwent the FDA approval process. Rather, 2 Defendants' mesh products were "cleared" by the FDA under the 510(k) equivalency process. 3 The 510(k) clearance process does not verify safety or efficacy of the product at issue and does 4 not typically require clinical trials Defendants' Polypropylene Mesh Products have never been approved by the 6 FDA. Yet Defendants instructed their sales representatives to tell doctors that they sold "the 7 only FDA approved partially absorbable pelvic floor mesh." By claiming that their Polypropylene Mesh Products were FDA approved, 9 Defendants misled consumers, doctors, and patients into believing that their mesh devices had 10 been well studied, undergone clinical trials, and were scrutinized robustly by the FDA Defendants Misrepresented the Effectiveness of Their Polypropylene Mesh 12 Products As Compared to Native Tissue Repair Defendants misrepresented the effectiveness of their Polypropylene Mesh 14 Products by claiming their products are superior to traditional pelvic floor repairs while failing 15 to disclose serious complications caused by their products. Defendants' misrepresentations 16 and omissions are material given that Defendants' Polypropylene Mesh Products are in fact not 17 uniformly superior to native tissue repair and, as discussed above, Defendants' mesh may 18' cause irreversible and debilitating long-term complications that are not present with native 19 tissue repair These misrepresentations and omissions are found in informational and 21 1 marketing materials Defendants provided to doctors, i.e. IFUs, doctor brochures, sales 221 representatives, and doctor training sessions, as well as information that Defendants provided 23 directly to consumers and patients through their patient brochures, radio and television 24 advertisements, on Defendants' websites, and in other materials OTHER RELIEF - 17 (206)
18 1 D. DEFENDANTS FAILED TO UPDATE INFORMATIONAL AND MARKETING MATERIALS Defendants failed to update or revise their informational and marketing 4 materials with known risks and complications caused by their Polypropylene Mesh Products. 5 Defendants' failure to update their materials is particularly critical because the device is a 6 permanent implant Defendants knew the problems were associated with their Polypropylene Mesh 8 Products because, among other reasons, Defendants' products generated thousands of 9 complaints from doctors and patients that were submitted directly to Defendants. Defendants 10 received complaints that some women cannot sit or walk comfortably for any extended period 11 of time. Other women informed Defendants that they now suffer from permanent urinary 12 and/or defecatory dysfunction that has resulted in a loss of dignity, inability to function in a 13 work place, and inability to participate in everyday family life Defendants received complaints that some women suffer from permanent 15 dyspareunia, making it impossible to have comfortable sexual relations. For example, one 16 surgeon described a Prolift patient in a February to Defendants: "She will likely 17 lose any coital function as her vaginal length is now 3 cm... This patient will have a 18 permanently destroyed vagina..." While Defendants purportedly had systems in place to update their marketing 20 and informational materials as information came in, Defendants failed to implement changes 21 based on suggestions from key staff doctors. For example, Dr. Meng Chen, a medical director 22 in the complaint review department, informed management on numerous occasions of patient 23 complaints regarding complications associated with their Polypropylene Mesh Products. Dr. 24 Chen proposed adding dyspareunia to the TVT IFU based on patient complaints and how those 25 complications were negatively affecting quality of life. Below is a meeting agenda by 26 Dr. Chen describing her observations from patient complaints: OTHER RELIEF - 18
19 Tape exposure/erosion/extrusion very frequently reported 2. Patients did not feel there were adequate pre-op consent or risk benefit assessment[s] 3. Patient-specific concerns a. b. The three Es The incontinence recurrence C. Post-operative dyspareunia and pain affect quality of life and affect daily routine d. Re-operations-tape excision, removal, re-do sling procedure[s] e. Type and intensity of the post-operative complications disportion[ate] to pre-operative consent-expectations Defendants never acted on Dr. Chen's recommendations. Defendants continued to conceal the material risks of dyspareunia and pain affecting quality of life in their informational and marketing materials In 2005, Dr. Axel Arnaud, an Ethicon medical director and one of the creators of Prolift, also suggested adding the following warning to the Prolift IFU: WARNING: Early clinical experience has shown that the use of mesh through a vaginal approach can occasionally/uncommonly lead to complications such as vaginal erosion and retraction which can result in an anatomical distortion of the vaginal cavity that can interfere with sexual intercourse. Clinical data suggest the risk of such a complication is increased in case of associated hysterectomy. This must be taken in consideration when the procedure is planned in a sexually active woman This warning never made its way into product information that went to consumers and doctors. This is all notwithstanding the fact that Defendants knew about each and every risk and complication stated in this Complaint before selling their SUI products 1997 and their POP products in Importantly, Defendants did not disclose the risk of dyspareunia in their TVT IFU until Not until 2012 did Defendants mention the risk of infection in their TVT brochures or the complication of vaginal scarring and contracture. OTTER RELIEF - 19
20 1 V. FIRST CAUSE OF ACTION 2 Misrepresentations and Omissions Rezardinz Safety Plaintiff realleges and incorporates by reference the allegations set forth in each 4 of the preceding paragraphs of this Complaint Defendants are "persons" within the meaning of the Consumer Protection Act, 6 RCW (1) Defendants conduct "trade" or "commerce" within the meaning of the 8 Consumer Protection Act, RCW (2) Defendants engaged in unfair and/or deceptive acts or practices within the 10 meaning of RCW by representing their Polypropylene Mesh Products were safe. 11 while misrepresenting and omitting risks and complications associated with pelvic floor 12' surgenes Defendants engaged in unfair and/or deceptive acts or practices within the 14 meaning of RCW by representing their Polypropylene Mesh Products were safe 15 while misrepresenting and omitting risks and complications caused by their mesh products Defendants engaged in unfair and/or deceptive acts or practices within the 17 meaning of RCW by representing their Polypropylene Mesh Products were safe by 18 marketing their Polypropylene Mesh Products as "FDA Approved." As alleged herein, these representations are deceptive and/or unfair because 20 Defendants misrepresented material information as alleged in this Complaint to consumers, 21 doctors, and patients; and/or material information was omitted from informational and 22 marketing materials The Consumer Protection Act prohibits unfair or deceptive acts or practices in 24 trade or commence. RCW Defendants' misrepresentations are deceptive because 25 they have the capacity to mislead a substantial number of consumers An act or practice may be unfair if it offends public policy, is immoral, OTHER RELIEF - 20 (206)
21 1 unethical, oppressive, unconscionable, or if it causes injury to consumers. Defendants' acts or 2 practices as alleged in this Complaint are unfair Defendants' conduct affected and continues to affect the public Defendants' acts and practices as alleged in this Complaint violate RCW VI. SECOND CAUSE OF ACTION 7 Misrepresentations or Omissions Regarding Efficacy Plaintiff realleges and incorporates by reference the allegations set forth in each 9 of the preceding paragraphs of this Complaint Defendants are "persons" within the meaning of the Consumer Protection Act, 11 RCW (1) Defendants conduct "trade" or "commerce" within the meaning of the 13 Consumer Protection Act, RCW (2) Defendants engaged in unfair and/or deceptive acts or practices within the 15 meaning of RCW by representing their Polypropylene Mesh Products were effective 16 while misrepresenting their products' effectiveness in comparison to non-mesh alternatives Defendants engaged in unfair and/or deceptive acts or practices within the 18 meaning of RCW by representing their Polypropylene Mesh Products were effective 19 by marketing their Polypropylene Mesh Products as "FDA Approved." As alleged herein, these representations are deceptive and/or unfair because 21 Defendants misrepresented material information as alleged in this Complaint to consumers, 22 doctors, and patients; and/or material information was omitted from informational and 23 marketing materials The Consumer Protection Act prohibits unfair and/or deceptive acts or practices 25 in trade or commence. RCW Defendants' misrepresentations are deceptive 26 because they have the capacity to mislead a substantial number of consumers. OTHER RELIEF - 21
22 An act or practice may be unfair if it offends public policy, is immoral, unethical, oppressive, unconscionable, or if it causes injury to consumers. Defendants' acts or practices as alleged in this Complaint are unfair. 6.9 Defendants engaged in unfair and/or deceptive acts or practices within the meaning of RCW by failing to disclose material information to consumers, doctors, and patients in marketing and informational materials Defendants' conduct affected and continues to affect the public interest Defendants' acts and practices as alleged in this Complaint violate RCW VII. THIRD CAUSE OF ACTION Failure To Update Information Provided To Consumers 7.1 Plaintiff realleges and incorporates by reference the allegations set forth in each of the preceding paragraphs of this Complaint. 7.2 Defendants are "persons" within the meaning of the Consumer Protection Act, RCW (1). 7.3 Defendants conduct "trade" or "commerce" within the meaning of the Consumer Protection Act, RCW (2). 7.4 Defendants engaged in unfair and/or deceptive acts or practices within the meaning of RCW by failing to update their informational and marketing materials with known, material risk and complication information. 7.5 The Consumer Protection Act prohibits unfair and/or deceptive acts or practices in trade or commence. RCW Defendants' misrepresentations are deceptive because they have the capacity to mislead a substantial number of consumers. 7.6 An act or practice may be unfair if it offends public policy, is immoral, unethical, oppressive, unconscionable, or if it causes injury to consumers. Defendants' acts or practices as alleged in this Complaint are unfair. OTHER RELIEF - 22
23 1 7.7 Defendants' conduct affected and continues to affect the public interest Defendants' acts or practices as alleged in this Complaint violate RCW VIII. RELIEF REQUESTED 4 WHEREFORE, Plaintiff, the State of Washington, prays for relief pursuant to each 5 cause of action set forth in this Complaint as follows: 6 A. That the Court adjudge and decree that Defendants have engaged in the acts and 7 practices complained of herein; 8 B. That the Court adjudge and decree that the acts and practices complained of 9 herein constitute unfair and/or deceptive acts or practices in violation of the Consumer 10 Protection Act, RCW 19.86; 11 C. That the Court issue a permanent injunction prohibiting and restraining 12 Defendants and their representatives, successors, assigns, officers, agents, servants, employees, 13 and all other persons acting or claiming to act for, on behalf of, or in active concert or 14 participation with Defendants from continuing or engaging in the unlawful conduct 15 complained of herein, namely, engaging in the business of marketing, advertising, promoting, 16 offering for sale, distributing or selling their Polypropylene Mesh Products in Washington in 17 violation of the Consumer Protection Act, RCW 19.86; 18 D. That the Court assess civil penalties, pursuant to RCW , of two 19 thousand dollars ($2,000) per violation against Defendants for each and every violation of 20 RCW ; 21 E. That the Court make such orders pursuant to RCW as it deems 22 appropriate to provide for restitution to consumers of money or property acquired by 23 Defendants as a result of the conduct complained of herein; 24 F. That the Court make such orders pursuant to RCW to provide that 25 1 Plaintiff, the State of Washington, recovers the costs of this action, including reasonable attorneys' fees; OTHER RELIEF - 23
24 G. That the Court grant Plaintiff leave to amend the Complaint to conform to the 2 evidence presented at trial; and H. That the Court order such other or further relief as the Court may deem just and 0 proper. DATED this day o, ROBERT W. FERGUSON Attorney General 141 TH J. ERWIN, WSBA #16548 Senio ounsel Atto - y for Plaintiff, State of Washington a a_"~ ANDREA M. ALEGRETT, WSBA #50236 Assistant Attorney General Attorney for Plaintiff, State of Washington L ILANI N. FISH, WSBA Assistant Attorney General Attorney for Plaintiff, State of Washington OTHER RELIEF - 24
Pelvic Prolapse. A Patient Guide to Pelvic Floor Reconstruction
 Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Bard: Continence Therapy. Stress Urinary Incontinence. Regaining Control. Restoring Your Lifestyle.
 Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence
 A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE
 493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
Stop Coping. Start Living. Talk to your doctor about pelvic organ prolapse and sacrocolpopexy
 Stop Coping. Start Living Talk to your doctor about pelvic organ prolapse and sacrocolpopexy Did you know? One in three women will suffer from a pelvic health condition in her lifetime. Four of the most
Stop Coping. Start Living Talk to your doctor about pelvic organ prolapse and sacrocolpopexy Did you know? One in three women will suffer from a pelvic health condition in her lifetime. Four of the most
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY NO. COME NOW Plaintiffs by and through their attorneys of record J.
 SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY 1 1 1 1 1 1 WASHINGTON STATE MEDICAL ASSOCIATION, a Washington corporation, JOSEPH O. GEHRETT, JR. M.D., BARBARA K. GEHRETT, M.D., MICHAEL J. KELLY,
SUPERIOR COURT OF THE STATE OF WASHINGTON FOR KING COUNTY 1 1 1 1 1 1 WASHINGTON STATE MEDICAL ASSOCIATION, a Washington corporation, JOSEPH O. GEHRETT, JR. M.D., BARBARA K. GEHRETT, M.D., MICHAEL J. KELLY,
1) What conditions is vaginal mesh used to commonly treat? Vaginal mesh is used to treat two different health issues in women:
 Vaginal Mesh Frequently Asked Questions 1) What conditions is vaginal mesh used to commonly treat? Vaginal mesh is used to treat two different health issues in women: a) stress urinary incontinence (SUI)
Vaginal Mesh Frequently Asked Questions 1) What conditions is vaginal mesh used to commonly treat? Vaginal mesh is used to treat two different health issues in women: a) stress urinary incontinence (SUI)
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... FDA Home Page CDRH Home Page Search A-Z Index
 US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
NOTICE OF FILING. Details of Filing. Kathryn Gill & Ors v Ethicon Sarl & Ors NEW SOUTH WALES REGISTRY - FEDERAL COURT OF AUSTRALIA
 NOTICE OF FILING This document was lodged electronically in the FEDERAL COURT OF AUSTRALIA (FCA) on 13/04/2018 3:35:30 PM AEST and has been accepted for filing under the Court s Rules. Details of filing
NOTICE OF FILING This document was lodged electronically in the FEDERAL COURT OF AUSTRALIA (FCA) on 13/04/2018 3:35:30 PM AEST and has been accepted for filing under the Court s Rules. Details of filing
Case 2:17-cv Document 1 Filed 10/30/17 Page 1 of 10
 Case :-cv-0 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 ANDREA SCHMITT, on her own behalf, and on behalf of all similarly situated individuals,
Case :-cv-0 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 ANDREA SCHMITT, on her own behalf, and on behalf of all similarly situated individuals,
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Case 1:12-cv C Document 1 Filed 06/18/12 Page 1 of 41 PageID 1 UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS ABILENE DIVISION
 Case 1:12-cv-00110-C Document 1 Filed 06/18/12 Page 1 of 41 PageID 1 UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS ABILENE DIVISION LINDA CHANNELL, vs. Plaintiff, COLOPLAST CORPORATION,
Case 1:12-cv-00110-C Document 1 Filed 06/18/12 Page 1 of 41 PageID 1 UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS ABILENE DIVISION LINDA CHANNELL, vs. Plaintiff, COLOPLAST CORPORATION,
SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY. Case No.: Plaintiffs Tammie Aust, Alison Grennan, Jennifer Schill, and Lang You Mau, by and
 FILED FEB PM 1: 1 KING COUNTY SUPERIOR COURT CLERK E-FILED CASE NUMBER: --0-1 SEA SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY TAMMIE AUST, an individual; ALISON GRENNAN, an individual; JENNIFER
FILED FEB PM 1: 1 KING COUNTY SUPERIOR COURT CLERK E-FILED CASE NUMBER: --0-1 SEA SUPERIOR COURT OF WASHINGTON IN AND FOR KING COUNTY TAMMIE AUST, an individual; ALISON GRENNAN, an individual; JENNIFER
Pelvic Support Problems
 AP012, April 2010 ACOG publications are protected by copyright and all rights are reserved. ACOG publications may not be reproduced in any form or by any means without written permission from the copyright
AP012, April 2010 ACOG publications are protected by copyright and all rights are reserved. ACOG publications may not be reproduced in any form or by any means without written permission from the copyright
Stress Urinary Incontinence in Women. What YOU can do about it...
 Stress Urinary Incontinence in Women What YOU can do about it... www.gynecare.com Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 13
Stress Urinary Incontinence in Women What YOU can do about it... www.gynecare.com Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 13
IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY BOWLING GREEN DIVISION ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) )
 IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY BOWLING GREEN DIVISION MARY LINDA GUTHRIE and TROY GUTHRIE, Plaintiffs, v. JOHNSON & JOHNSON, ETHICON, INC., ETHICON WOMEN S HEALTH
IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY BOWLING GREEN DIVISION MARY LINDA GUTHRIE and TROY GUTHRIE, Plaintiffs, v. JOHNSON & JOHNSON, ETHICON, INC., ETHICON WOMEN S HEALTH
Associates, llc, for its Complaint against the defendants, Gary K. DeJohn, Sr. and DeJohn
 DISTRICT COURT, LARIMER COUNTY, COLORADO 201 LaPorte Avenue, Suite 100 Fort Collins, CO 80521 DATE FILED: November 10, 2017 12:55 PM FILING ID: FF4949B297BB2 (970) 494-3500 CASE NUMBER: 2017CV30947 Plaintiff:
DISTRICT COURT, LARIMER COUNTY, COLORADO 201 LaPorte Avenue, Suite 100 Fort Collins, CO 80521 DATE FILED: November 10, 2017 12:55 PM FILING ID: FF4949B297BB2 (970) 494-3500 CASE NUMBER: 2017CV30947 Plaintiff:
Surgical treatment of urinary stress incontinence with tension free vaginal tape
 Surgical treatment of urinary stress incontinence with tension free vaginal tape Gynaecology department 01935 384 385 yeovilhospital.nhs.uk Many surgical operations are available for the treatment of
Surgical treatment of urinary stress incontinence with tension free vaginal tape Gynaecology department 01935 384 385 yeovilhospital.nhs.uk Many surgical operations are available for the treatment of
IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY LOUISVILLE DIVISION ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) )
 IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY LOUISVILLE DIVISION FALISHA MADDOX and WILLIAM MADDOX, Plaintiffs, v. JOHNSON & JOHNSON, ETHICON, INC., ETHICON WOMEN S HEALTH AND UROLOGY,
IN THE UNITED STATES DISTRICT COURT FOR WESTERN DISTRICT OF KENTUCKY LOUISVILLE DIVISION FALISHA MADDOX and WILLIAM MADDOX, Plaintiffs, v. JOHNSON & JOHNSON, ETHICON, INC., ETHICON WOMEN S HEALTH AND UROLOGY,
IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH ) ) ) ) ) ) ) ) ) ) ) ) ) GENERAL JURISDICTION AND COMMON FACTUAL ALLEGATIONS
 Phillip C. Gilbert, OSB No. rd S.E. Avenue Suite A Gresham, Oregon 00-1 Phone: (0-00 Fax: (0-01 pgilbert@teleport.com IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH DAWN D. JOHNSON
Phillip C. Gilbert, OSB No. rd S.E. Avenue Suite A Gresham, Oregon 00-1 Phone: (0-00 Fax: (0-01 pgilbert@teleport.com IN THE CIRCUIT COURT OF THE STATE OF OREGON FOR THE COUNTY OF MULTNOMAH DAWN D. JOHNSON
Case 2:17-cv Document 1 Filed 10/30/17 Page 1 of 10
 Case :-cv-00 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 E.S., by and through her parents, R.S. and J.S., and JODI STERNOFF, both on their own
Case :-cv-00 Document Filed 0/0/ Page of 0 UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WASHINGTON AT SEATTLE 0 E.S., by and through her parents, R.S. and J.S., and JODI STERNOFF, both on their own
ARTIFICIAL MESH REPAIR FOR TREATMENT OF PELVIC ORGAN PROLAPSE
 Pelvic Floor Unit / Department of Gynaecology Ward 17, Singleton Hospital, Sketty, Swansea, SA2 8QA 01792 205666 Secretary Direct Line: 01792 285688. Fax: 01792 285874 ARTIFICIAL MESH REPAIR FOR TREATMENT
Pelvic Floor Unit / Department of Gynaecology Ward 17, Singleton Hospital, Sketty, Swansea, SA2 8QA 01792 205666 Secretary Direct Line: 01792 285688. Fax: 01792 285874 ARTIFICIAL MESH REPAIR FOR TREATMENT
Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566
 Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN
 IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN ULTRATEC, INC. and CAPTEL, INC., v. Plaintiffs, SORENSON COMMUNICATIONS, INC. and CAPTIONCALL, LLC, Defendants. Civil Action No.: 14-cv-66
IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF WISCONSIN ULTRATEC, INC. and CAPTEL, INC., v. Plaintiffs, SORENSON COMMUNICATIONS, INC. and CAPTIONCALL, LLC, Defendants. Civil Action No.: 14-cv-66
FDA and Mesh Complications in Vaginal Surgery
 FDA and Mesh Complications in Vaginal Surgery Response to FDA Safety Communication dated July 13, 2011 To Our Patients and Women of the Community: As many of you are aware, on July 13, 2011, the FDA released
FDA and Mesh Complications in Vaginal Surgery Response to FDA Safety Communication dated July 13, 2011 To Our Patients and Women of the Community: As many of you are aware, on July 13, 2011, the FDA released
Consultation Guide: Specialised gynaecology surgery and complex urogynaecology conditions service specifications
 Consultation Guide: Specialised gynaecology surgery and complex urogynaecology conditions service specifications Consultation guide: Specialised gynaecology surgery and complex urogynaecology conditions
Consultation Guide: Specialised gynaecology surgery and complex urogynaecology conditions service specifications Consultation guide: Specialised gynaecology surgery and complex urogynaecology conditions
UNITED STATES DISTRICT COURT
 Case :-cv-00-spl Document Filed 0// Page of 0 0 Daniel L. Miranda, Esq. SBN 0 MIRANDA LAW FIRM E. Ray Road, Suite #0 Gilbert, AZ Tel: (0) - dan@mirandalawpc.com Robert Tauler, Esq. SBN, (pro hac vice forthcoming)
Case :-cv-00-spl Document Filed 0// Page of 0 0 Daniel L. Miranda, Esq. SBN 0 MIRANDA LAW FIRM E. Ray Road, Suite #0 Gilbert, AZ Tel: (0) - dan@mirandalawpc.com Robert Tauler, Esq. SBN, (pro hac vice forthcoming)
Pelvic Organ Prolapse. Natural Solutions
 Pelvic Organ Prolapse Natural Solutions Bringing your body back to its natural state There is a very common problem affecting millions of women. Many women are too embarrassed to talk to their physicians
Pelvic Organ Prolapse Natural Solutions Bringing your body back to its natural state There is a very common problem affecting millions of women. Many women are too embarrassed to talk to their physicians
Surgical repair of vaginal wall prolapse using mesh
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Surgical repair of vaginal wall prolapse using mesh Vaginal wall prolapse happens when the normal support
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Surgical repair of vaginal wall prolapse using mesh Vaginal wall prolapse happens when the normal support
Case 1:09-cv RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1
 Case 1:09-cv-04115-RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1 John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102-4096
Case 1:09-cv-04115-RMB-AMD Document 1 Filed 08/12/09 Page 1 of 6 PageID: 1 John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102-4096
A Simple Solution. to a Common Problem. Find relief from sudden, unplanned urine leakage.
 A Simple Solution to a Common Problem Find relief from sudden, unplanned urine leakage. A Common Problem Millions of women suffer from stress urinary incontinence (SUI). This condition results in accidental
A Simple Solution to a Common Problem Find relief from sudden, unplanned urine leakage. A Common Problem Millions of women suffer from stress urinary incontinence (SUI). This condition results in accidental
UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF KENTUCKY FRANKFORT DIVISION ) ) ) ) ) ) PLAINTIFF S ORIGINAL COMPLAINT FOR DAMAGES
 UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF KENTUCKY FRANKFORT DIVISION ) MICHELLE MONTGOMERY, ) ) Plaintiff, ) ) v. ) ) BOSTON SCIENTIFIC CORPORATION ) (D/B/A MANSFIELD SCIENTIFIC, INC. )
UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF KENTUCKY FRANKFORT DIVISION ) MICHELLE MONTGOMERY, ) ) Plaintiff, ) ) v. ) ) BOSTON SCIENTIFIC CORPORATION ) (D/B/A MANSFIELD SCIENTIFIC, INC. )
STATE OF WASHINGTON KING COUNTY SUPERIOR COURT
 Hearing Date: December 1, 01, at 1:00 pm WITH ORAL ARGUMENT 1 1 1 1 1 STATE OF WASHINGTON, v. STATE OF WASHINGTON KING COUNTY SUPERIOR COURT Plaintiff, JOHNSON & JOHNSON, a New Jersey Corporation; ETHICON,
Hearing Date: December 1, 01, at 1:00 pm WITH ORAL ARGUMENT 1 1 1 1 1 STATE OF WASHINGTON, v. STATE OF WASHINGTON KING COUNTY SUPERIOR COURT Plaintiff, JOHNSON & JOHNSON, a New Jersey Corporation; ETHICON,
Plaintiff, Comfort Dental Group, Inc. ( Comfort Dental ), by its attorneys, MOYE WHITE LLP, states: INTRODUCTION
 JEFFERSON COUNTY DISTRICT COURT, STATE OF COLORADO Address: 100 Jefferson County Parkway Golden, Colorado 80401 Telephone: (303) 271-6145 Plaintiff: COMFORT DENTAL GROUP, INC., a Colorado Corporation,
JEFFERSON COUNTY DISTRICT COURT, STATE OF COLORADO Address: 100 Jefferson County Parkway Golden, Colorado 80401 Telephone: (303) 271-6145 Plaintiff: COMFORT DENTAL GROUP, INC., a Colorado Corporation,
One Slim Needle One Incision. One Simple Solution for Stress Urinary Incontinence. The Difference is in the Data
 CONTINENCE SOLUTIONS One Slim Needle One Incision ordering information Description US International Order Number Order Number One Simple Solution for Stress Urinary Incontinence MiniArc Single-Incision
CONTINENCE SOLUTIONS One Slim Needle One Incision ordering information Description US International Order Number Order Number One Simple Solution for Stress Urinary Incontinence MiniArc Single-Incision
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF ARIZONA
 Case :-cv-0-djh Document Filed // Page of 0 FREDENBERG BEAMS Daniel E. Fredenberg 00 Christian C. M. Beams 0 N. th Street, Suite 0 Phoenix, Arizona 0 Telephone: 0/- Email: dfredenberg@fblegalgroup.com
Case :-cv-0-djh Document Filed // Page of 0 FREDENBERG BEAMS Daniel E. Fredenberg 00 Christian C. M. Beams 0 N. th Street, Suite 0 Phoenix, Arizona 0 Telephone: 0/- Email: dfredenberg@fblegalgroup.com
Stress Urinary Incontinence in Women
 Stress Urinary Incontinence in Women Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 3 million women in the United Kingdom have urinary
Stress Urinary Incontinence in Women Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 3 million women in the United Kingdom have urinary
Case 2:14-cv Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA
 Case 2:14-cv-02873 Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA TROYLYNN MORRIS CIVIL ACTION NUMBER: INDIVIDUALLY AND ON BEHALF OF Q. B. SECTION:
Case 2:14-cv-02873 Document 1 Filed 12/17/14 Page 1 of 12 UNITED STATES DISTRICT COURT FOR THE EASTEN DISTRICT OF LOUISIANA TROYLYNN MORRIS CIVIL ACTION NUMBER: INDIVIDUALLY AND ON BEHALF OF Q. B. SECTION:
4. Together, defendants CCA and CCC represent the vast majority of chiropractors practicing in Connecticut.
 RETURN DATE JULY 6, 2010 VICTIMS OF CHIROPRACTIC ABUSE, LLC, J.D. OF HARTFORD Plaintiff, at HARTFORD v. CONNECTICUT CHIROPRACTIC ASSOCIATION, INC.; CONNECTICUT CHIROPRACTIC COUNCIL, INC., Defendants JUNE
RETURN DATE JULY 6, 2010 VICTIMS OF CHIROPRACTIC ABUSE, LLC, J.D. OF HARTFORD Plaintiff, at HARTFORD v. CONNECTICUT CHIROPRACTIC ASSOCIATION, INC.; CONNECTICUT CHIROPRACTIC COUNCIL, INC., Defendants JUNE
Surgery for stress incontinence:
 Surgery for stress incontinence: information for you aashara Published February 2005 by the RCOG Contents Key points About this information What is stress incontinence? Do I need an operation? What operation
Surgery for stress incontinence: information for you aashara Published February 2005 by the RCOG Contents Key points About this information What is stress incontinence? Do I need an operation? What operation
Case 2:12-cv KJM-GGH Document 1 Filed 07/02/12 Page 1 of 9 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF CALIFORNIA. (Sacramento Division)
 Case :-cv-0-kjm-ggh Document Filed 0/0/ Page of PAUL W. REIDL (State Bar No. ) Law Office of Paul W. Reidl Eagle Trace Drive Half Moon Bay, CA 0 Telephone: (0) 0-0 Email: paul@reidllaw.com Attorney for
Case :-cv-0-kjm-ggh Document Filed 0/0/ Page of PAUL W. REIDL (State Bar No. ) Law Office of Paul W. Reidl Eagle Trace Drive Half Moon Bay, CA 0 Telephone: (0) 0-0 Email: paul@reidllaw.com Attorney for
FDA & Transvaginal Mesh: What Happened? What s Next?
 FDA & Transvaginal Mesh: What Happened? What s Next? Matthew D. Barber, MD MHS Professor & Vice Chair for Clinical Research Obstetrics Gynecology & Women s Health Institute Disclosures I receive no grants,
FDA & Transvaginal Mesh: What Happened? What s Next? Matthew D. Barber, MD MHS Professor & Vice Chair for Clinical Research Obstetrics Gynecology & Women s Health Institute Disclosures I receive no grants,
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF OREGON PORTLAND DIVISION
 Scott D. Eads, OSB #910400 Email: seads@schwabe.com Nicholas F. Aldrich, Jr., OSB #160306 Email: naldrich@schwabe.com Facsimile: 503.796.2900 Attorneys for Plaintiff AgaMatrix, Inc. IN THE UNITED STATES
Scott D. Eads, OSB #910400 Email: seads@schwabe.com Nicholas F. Aldrich, Jr., OSB #160306 Email: naldrich@schwabe.com Facsimile: 503.796.2900 Attorneys for Plaintiff AgaMatrix, Inc. IN THE UNITED STATES
Case 4:16-cv ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION
 Case 4:16-cv-00140-ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION GLAXO GROUP LIMITED and GLAXOSMITHKLINE CONSUMER HEALTHCARE
Case 4:16-cv-00140-ALM Document 1 Filed 02/29/16 Page 1 of 11 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TEXAS SHERMAN DIVISION GLAXO GROUP LIMITED and GLAXOSMITHKLINE CONSUMER HEALTHCARE
Loss of Bladder Control
 BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
Re: Bayer s false and deceptive marketing for its Men s Multis for prevention of cancer
 June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
Surgery for vaginal vault prolapse. Patient decision aid
 Surgery for vaginal vault prolapse Patient decision aid? i What is vaginal vault prolapse? Vaginal vault prolapse happens when the top of the vagina (the vault) slips from its normal position and sags
Surgery for vaginal vault prolapse Patient decision aid? i What is vaginal vault prolapse? Vaginal vault prolapse happens when the top of the vagina (the vault) slips from its normal position and sags
Case 1:15-cv RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO
 Case 1:15-cv-00270-RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO Civil Action No. 1:15-cv-00270 GEORGE BACA, v. Plaintiff, PARKVIEW
Case 1:15-cv-00270-RBJ Document 1 Filed 02/09/15 USDC Colorado Page 1 of 10 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO Civil Action No. 1:15-cv-00270 GEORGE BACA, v. Plaintiff, PARKVIEW
Women s & Children s Directorate The TVT Operation - a guide for patients
 Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION ) ) ) ) ) ) ) ) ) ) )
 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION EQUAL EMPLOYMENT OPPORTUNITY COMMISSION, Plaintiff, v. MISSION HOSPITAL, INC., Defendant. Civil Action
IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF NORTH CAROLINA ASHEVILLE DIVISION EQUAL EMPLOYMENT OPPORTUNITY COMMISSION, Plaintiff, v. MISSION HOSPITAL, INC., Defendant. Civil Action
Pelvic organ prolapse
 Page 1 of 11 Pelvic organ prolapse Introduction The aim of this leaflet is to give you information about a pelvic organ prolapse, its causes and available treatments but does not replace advice given by
Page 1 of 11 Pelvic organ prolapse Introduction The aim of this leaflet is to give you information about a pelvic organ prolapse, its causes and available treatments but does not replace advice given by
Patient Information. Tension Free Vaginal/ Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust
 Tension Free Vaginal/Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust Patient Information Tension Free Vaginal/ Obturator Tape (TVT) Reference Number: CW 08 011 003 (version date: September
Tension Free Vaginal/Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust Patient Information Tension Free Vaginal/ Obturator Tape (TVT) Reference Number: CW 08 011 003 (version date: September
Karanvir Virk M.D. Minimally Invasive & Pelvic Reconstructive Surgery 01/28/2015
 Karanvir Virk M.D. Minimally Invasive & Pelvic Reconstructive Surgery 01/28/2015 Disclosures I have none Objectives Identify the basic Anatomy and causes of Pelvic Organ Prolapse Examine office diagnosis
Karanvir Virk M.D. Minimally Invasive & Pelvic Reconstructive Surgery 01/28/2015 Disclosures I have none Objectives Identify the basic Anatomy and causes of Pelvic Organ Prolapse Examine office diagnosis
CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT
 CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT Providing for testing standards for cigarette fire safety, for certification of compliance by manufacturers,
CIGARETTE FIRE SAFETY AND FIREFIGHTER PROTECTION ACT Act of Jul. 4, 2008, P.L. 518, No. 42 Cl. 35 AN ACT Providing for testing standards for cigarette fire safety, for certification of compliance by manufacturers,
UNITED STATES DISTRICT COURT CENTRAL DISTRICT OF CALIFORNIA. Plaintiff, COMPLAINT FOR DEFAMATION
 WHITE O'CONNOR CURRY GATTI & AVANZADO LLP Andrew M. White (State Bar No. 60181) Melvin N.A. Avanzado (State Bar No. 137127) 10100 Santa Monica Boulevard Twenty-Third Floor Los Angeles, California 90067-4008
WHITE O'CONNOR CURRY GATTI & AVANZADO LLP Andrew M. White (State Bar No. 60181) Melvin N.A. Avanzado (State Bar No. 137127) 10100 Santa Monica Boulevard Twenty-Third Floor Los Angeles, California 90067-4008
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE
 Case 199-mc-09999 Document 654 Filed 11/09/11 Page 1 of 12 PageID # 61421 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS PHARMACEUTICALS CORPORATION, NOVARTIS AG, NOVARTIS PHARMA
Case 199-mc-09999 Document 654 Filed 11/09/11 Page 1 of 12 PageID # 61421 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE NOVARTIS PHARMACEUTICALS CORPORATION, NOVARTIS AG, NOVARTIS PHARMA
2:12-cv VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN
 2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
Case 5:15-cv Document 1 Filed 06/19/15 Page 1 of 21
 Case 5:15-cv-00510 Document 1 Filed 06/19/15 Page 1 of 21 THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF TEXAS SAN ANTONIO DIVISION MISSION PHARMACAL COMPANY, ) ) Plaintiff, ) ) v. Case No.
Case 5:15-cv-00510 Document 1 Filed 06/19/15 Page 1 of 21 THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF TEXAS SAN ANTONIO DIVISION MISSION PHARMACAL COMPANY, ) ) Plaintiff, ) ) v. Case No.
Patient Information Leaflet
 Patient Information Leaflet MID-URETHRAL SLING OPERATION TENSION-FREE VAGINAL TAPE (TVT) TRANSOBTURATOR TAPE (TOT, TVT-O) This information leaflet has been developed to help your understanding of what
Patient Information Leaflet MID-URETHRAL SLING OPERATION TENSION-FREE VAGINAL TAPE (TVT) TRANSOBTURATOR TAPE (TOT, TVT-O) This information leaflet has been developed to help your understanding of what
Case 1:12-cv RDB Document 1 Filed 09/11/12 Page 1 of 22 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MARYLAND NORTHERN DIVISION
 Case 1:12-cv-02718-RDB Document 1 Filed 09/11/12 Page 1 of 22 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MARYLAND NORTHERN DIVISION MICHELLE NEMPHOS AS Legal Guardian for C.G.N. A Minor under
Case 1:12-cv-02718-RDB Document 1 Filed 09/11/12 Page 1 of 22 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MARYLAND NORTHERN DIVISION MICHELLE NEMPHOS AS Legal Guardian for C.G.N. A Minor under
Robotic Ventral Rectopexy
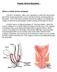 Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Information from the MAUDE Database
 Information from the MAUDE Database Device mesh, surgical, polymeric Regulation Description Surgical mesh. Product Code FTL Regulation Number 878.3300 Device Class 2 Manufacturer ETHICON Medical Device
Information from the MAUDE Database Device mesh, surgical, polymeric Regulation Description Surgical mesh. Product Code FTL Regulation Number 878.3300 Device Class 2 Manufacturer ETHICON Medical Device
Female Urinary Incontinence: What It Is and What You Can Do About It
 Female Urinary Incontinence: What It Is and What You Can Do About It Urogynecology Patient Information Sheet What is Urinary Incontinence? Stress Incontinence is a leakage of urine that occurs, for example,
Female Urinary Incontinence: What It Is and What You Can Do About It Urogynecology Patient Information Sheet What is Urinary Incontinence? Stress Incontinence is a leakage of urine that occurs, for example,
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION. Plaintiff, PURE HEMP COLLECTIVE INC.
 Civil Action No: IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION a Colorado Corporation Plaintiff, v. PURE HEMP COLLECTIVE INC., a Colorado Corporation Defendant.
Civil Action No: IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLORADO UNITED CANNABIS CORPORATION a Colorado Corporation Plaintiff, v. PURE HEMP COLLECTIVE INC., a Colorado Corporation Defendant.
URINARY INCONTINENCE
 Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Tension-free Vaginal Tape (TVT)
 Page 1 of 7 Tension-free Vaginal Tape (TVT) Introduction This leaflet will provide you with basic information about the Tension--free Vaginal Tape (TVT) procedure. What is a TVT? TVT is an operation to
Page 1 of 7 Tension-free Vaginal Tape (TVT) Introduction This leaflet will provide you with basic information about the Tension--free Vaginal Tape (TVT) procedure. What is a TVT? TVT is an operation to
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS
 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS SCOTT RODRIGUES ) Plaintiff ) C.A. 07-10104-GAO ) v. ) ) THE SCOTTS COMPANY, LLC ) Defendant ) AMENDED COMPLAINT and jury trial demand Introduction
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MASSACHUSETTS SCOTT RODRIGUES ) Plaintiff ) C.A. 07-10104-GAO ) v. ) ) THE SCOTTS COMPANY, LLC ) Defendant ) AMENDED COMPLAINT and jury trial demand Introduction
Tension Free vaginal tape. Mrs Ami Shukla, Consultant Urogynaecologist Northampton General Hospital Northampton NN1 5BD
 Tension Free vaginal tape Mrs Ami Shukla, Consultant Urogynaecologist Northampton General Hospital Northampton NN1 5BD What is a TVT procedure? A TVT (Tension Free Vaginal Tape) procedure is an operation
Tension Free vaginal tape Mrs Ami Shukla, Consultant Urogynaecologist Northampton General Hospital Northampton NN1 5BD What is a TVT procedure? A TVT (Tension Free Vaginal Tape) procedure is an operation
The pelvic floor is a system of muscles, ligaments, and tissues that keep your pelvic organs firmly in place.
 The pelvic floor is a system of muscles, ligaments, and tissues that keep your pelvic organs firmly in place. Think of it as the hammock of muscle that holds up your uterus, vagina, bladder, small intestine,
The pelvic floor is a system of muscles, ligaments, and tissues that keep your pelvic organs firmly in place. Think of it as the hammock of muscle that holds up your uterus, vagina, bladder, small intestine,
Voiding Diary. Begin recording upon rising in the morning and continue for a full 24 hours.
 Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Las Vegas Urogynecology
 Las Vegas Urogynecology 7500 Smoke Ranch Road - #200 Las Vegas, NV 89128 Telephone: (702) 233-0727 Fax: (702) 233-4799 Physician's Surgical Procedure Disclosure and Patient s Consent TO THE PATIENT: You
Las Vegas Urogynecology 7500 Smoke Ranch Road - #200 Las Vegas, NV 89128 Telephone: (702) 233-0727 Fax: (702) 233-4799 Physician's Surgical Procedure Disclosure and Patient s Consent TO THE PATIENT: You
Desara and Desara Blue
 Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA
 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA UNITED STATES OF AMERICA v. CRIMINAL NO. UCB, INC., Defendant. VIOLATION 21 U.S.C. 331(k), 352(f)(1), and 333(a)(1) (Causing drugs to be
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA UNITED STATES OF AMERICA v. CRIMINAL NO. UCB, INC., Defendant. VIOLATION 21 U.S.C. 331(k), 352(f)(1), and 333(a)(1) (Causing drugs to be
Adverse Event Reports Relating to Surgical Mesh Implants. Summary of reports received by Medsafe
 Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by Medsafe September 2016 Page 2 of 25 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports
Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by Medsafe September 2016 Page 2 of 25 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports
IN THE SUPREME COURT OF BRITISH COLUMBIA KENNETH KNIGHT IMPERIAL TOBACCO CANADA LIMITED STATEMENT OF CLAIM
 No. Vancouver Registry IN THE SUPREME COURT OF BRITISH COLUMBIA Between: KENNETH KNIGHT Plaintiff AND: IMPERIAL TOBACCO CANADA LIMITED Defendant Brought under the Class Proceedings Act, R.S.B.C. 1996,
No. Vancouver Registry IN THE SUPREME COURT OF BRITISH COLUMBIA Between: KENNETH KNIGHT Plaintiff AND: IMPERIAL TOBACCO CANADA LIMITED Defendant Brought under the Class Proceedings Act, R.S.B.C. 1996,
Treating your prolapse
 Treating your prolapse This leaflet explains what a prolapse is, and how it can be treated and managed. If you have any questions or concerns, please speak to a doctor or nurse caring for you. What is
Treating your prolapse This leaflet explains what a prolapse is, and how it can be treated and managed. If you have any questions or concerns, please speak to a doctor or nurse caring for you. What is
Case 2:15-cv SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246
 Case 2:15-cv-08180-SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246 Elvin Esteves Charles H. Chevalier J. Brugh Lower GIBBONS P.C. One Gateway Center Newark, New Jersey 07102 Tel: (973) 596-4500
Case 2:15-cv-08180-SRC-CLW Document 9 Filed 02/04/16 Page 1 of 19 PageID: 246 Elvin Esteves Charles H. Chevalier J. Brugh Lower GIBBONS P.C. One Gateway Center Newark, New Jersey 07102 Tel: (973) 596-4500
In re: ) ) NOTICE OF CHARGES John E. Marshall, M.D., ) AND ALLEGATIONS; ) NOTICE OF HEARING Respondent. )
 BEFORE THE NORTH CAROLINA MEDICAL BOARD In re: ) ) NOTICE OF CHARGES John E. Marshall, M.D., ) AND ALLEGATIONS; ) NOTICE OF HEARING Respondent. ) The North Carolina Medical Board ( Board ) has preferred
BEFORE THE NORTH CAROLINA MEDICAL BOARD In re: ) ) NOTICE OF CHARGES John E. Marshall, M.D., ) AND ALLEGATIONS; ) NOTICE OF HEARING Respondent. ) The North Carolina Medical Board ( Board ) has preferred
CSA Briefing Note Regarding Joint Application against the University and Re-Commencing Collection of CFS/CFS-O Fees
 CSA Briefing Note Regarding Joint Application against the University and Re-Commencing Collection of CFS/CFS-O Fees The CSA and University of Guelph undergraduate students have been members of the Canadian
CSA Briefing Note Regarding Joint Application against the University and Re-Commencing Collection of CFS/CFS-O Fees The CSA and University of Guelph undergraduate students have been members of the Canadian
Infracoccygeal sacropexy using mesh for uterine prolapse repair
 Infracoccygeal sacropexy using mesh for uterine Issued: January 2009 www.nice.org.uk/ipg280 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Infracoccygeal sacropexy using mesh for uterine Issued: January 2009 www.nice.org.uk/ipg280 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
INGHAM COUNTY. Effective January 1, 2016 as amended November 10, 2015
 INGHAM COUNTY REGULATION TO REQUIRE A LICENSE FOR THE RETAIL SALE OF ELECTROINC SMOKING DEVICES, PROHIBIT SALE OF ELECTROINC SMOKING DEVICESTO MINORS, AND TO RESTRICT LOCATION OF ELECTROINC SMOKING DEVICES
INGHAM COUNTY REGULATION TO REQUIRE A LICENSE FOR THE RETAIL SALE OF ELECTROINC SMOKING DEVICES, PROHIBIT SALE OF ELECTROINC SMOKING DEVICESTO MINORS, AND TO RESTRICT LOCATION OF ELECTROINC SMOKING DEVICES
Latest Treatments for a Leaky Bladder None
 Latest Treatments for a Leaky Bladder None Financial Disclosures Jeremiah McNamara, MD, OBGYN Boulder Women s Care 303-500-1947 Boulder Women s Care Agenda: Prolapse & Urinary Incontinence The Pelvic Floor
Latest Treatments for a Leaky Bladder None Financial Disclosures Jeremiah McNamara, MD, OBGYN Boulder Women s Care 303-500-1947 Boulder Women s Care Agenda: Prolapse & Urinary Incontinence The Pelvic Floor
IN THE UNITED STATES DISTRICT COURT FOR THE CENTRAL DISTRICT OF CALIFORNIA. Civil Action No. 8:14-cv-1322 COMPLAINT DEMAND FOR JURY TRIAL
 1 1 1 1 John B. Sganga, Jr. (SBN 1,1 john.sganga@knobbe.com Sheila N. Swaroop (SBN, sheila.swaroop@knobbe.com Baraa Kahf (SBN 1,1 baraa.kahf@knobbe.com Marissa Calcagno (SBN, marissa.calcagno@knobbe.com
1 1 1 1 John B. Sganga, Jr. (SBN 1,1 john.sganga@knobbe.com Sheila N. Swaroop (SBN, sheila.swaroop@knobbe.com Baraa Kahf (SBN 1,1 baraa.kahf@knobbe.com Marissa Calcagno (SBN, marissa.calcagno@knobbe.com
Laparoscopic Sacrohysteropexy
 Professor Christian Phillips BSc Hons BM DM FRCOG Consultant Gynaecologist and Urogynaecologist Laparoscopic Sacrohysteropexy What is a prolapse? Uterine prolapse is a bulge or lump in the vagina caused
Professor Christian Phillips BSc Hons BM DM FRCOG Consultant Gynaecologist and Urogynaecologist Laparoscopic Sacrohysteropexy What is a prolapse? Uterine prolapse is a bulge or lump in the vagina caused
Adverse Event Reports Relating to Surgical Mesh Implants. Summary of reports received by Medsafe
 Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by Medsafe December 2016 Page 2 of 25 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports
Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by Medsafe December 2016 Page 2 of 25 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports
INFORMED CONSENT TRIGGER FINGER SURGERY
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
Case 1:15-cv ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1. Plaintiff, Defendant. COMPLAINT
 Case 1:15-cv-05526-ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF NEW YORK ---------------------------------------------------------------------X
Case 1:15-cv-05526-ARR-VVP Document 1 Filed 09/23/15 Page 1 of 13 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF NEW YORK ---------------------------------------------------------------------X
Adverse Event Reports Relating to Surgical Mesh Implants
 Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by August 2017 Page 1 of 26 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports received
Adverse Event Reports Relating to Surgical Mesh Implants Summary of reports received by August 2017 Page 1 of 26 Document History Version Number Revision Date Summary of Changes 0 Dec-2013 Reports received
FILED: NEW YORK COUNTY CLERK 06/28/ :49 PM INDEX NO /2017 NYSCEF DOC. NO. 1 RECEIVED NYSCEF: 06/28/2017
 SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK JARVIK HEART, INC., -against- Plaintiff, CALON CARDIO-TECHNOLOGY LTD., STUART MCCONCHIE, JOHN TEAL and ALANI INTINTOLO Defendant. SUMMONS Index
SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK JARVIK HEART, INC., -against- Plaintiff, CALON CARDIO-TECHNOLOGY LTD., STUART MCCONCHIE, JOHN TEAL and ALANI INTINTOLO Defendant. SUMMONS Index
Loss of Bladder Control
 BLADDER HEALTH Loss of Bladder Control Bladder Prolapse AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is the Bladder? The bladder is a hollow, balloon-like organ made mostly
BLADDER HEALTH Loss of Bladder Control Bladder Prolapse AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is the Bladder? The bladder is a hollow, balloon-like organ made mostly
UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF CALIFORNIA. Case No:
 Case :-cv-00-ben-ksc Document Filed 0/0/ PageID. Page of 0 0 THE LAW OFFICE OF PAUL K. JOSEPH, PC PAUL K. JOSEPH (SBN 0) paul@pauljosephlaw.com W. Pt. Loma Blvd., No. 0 San Diego, CA 0 Phone: () -0 Fax:
Case :-cv-00-ben-ksc Document Filed 0/0/ PageID. Page of 0 0 THE LAW OFFICE OF PAUL K. JOSEPH, PC PAUL K. JOSEPH (SBN 0) paul@pauljosephlaw.com W. Pt. Loma Blvd., No. 0 San Diego, CA 0 Phone: () -0 Fax:
Loss of Bladder Control
 BLADDER HEALTH: Bladder Prolapse Loss of Bladder Control Bladder Prolapse Don t Let Bladder Prolapse Keep You from Enjoying Life. What is the Bladder? The bladder is a hollow, balloon-like organ made mostly
BLADDER HEALTH: Bladder Prolapse Loss of Bladder Control Bladder Prolapse Don t Let Bladder Prolapse Keep You from Enjoying Life. What is the Bladder? The bladder is a hollow, balloon-like organ made mostly
REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS
 REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS A. Statement of Purpose: Whereas there exists conclusive evidence that tobacco smoke
REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS A. Statement of Purpose: Whereas there exists conclusive evidence that tobacco smoke
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA LISA SYKES and SETH SYKES, : CIVIL ACTION Individually and as Parents and Natural : Guardians of WESLEY ALEXANDER : NO. SYKES,
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA LISA SYKES and SETH SYKES, : CIVIL ACTION Individually and as Parents and Natural : Guardians of WESLEY ALEXANDER : NO. SYKES,
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Loss of Bladder Control
 BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
An operation for stress incontinence - transobturator tape (TOT, TVT-O)
 INFORMATION FOR PATIENTS An operation for stress incontinence - transobturator tape (TOT, TVT-O) We advise you to take your time to read this leaflet. If you have any questions please write them down on
INFORMATION FOR PATIENTS An operation for stress incontinence - transobturator tape (TOT, TVT-O) We advise you to take your time to read this leaflet. If you have any questions please write them down on
