Ikaclomin Tablets (Clomiphene Citrate 50mg)
|
|
|
- Diane Malone
- 5 years ago
- Views:
Transcription
1 Ikaclomin Tablets (Clomiphene Citrate 50mg) In women : ovulatory failure in patients desiring pregnancy. In men: to improve fertility in: idiopthic oligospermia vavicocele associated oligospermia. # $"! $%!! $"('#!#$&$ $"() '#"('"(' "" ""!"!!" ## "!* " $%" " %# *#!!!!$! ""!"!!" ## "!* $ "! " "+ % ", $#% * #!!!!$! "!!" $ "# + # " #%%"#! $!!"! "##%
2 *!$# #!!! """"#!#"(' $ #" % $"!# ""#!"#"% " # " "! "#$!!" "#!&!!&$!*('!" -!&!!& 0 *('#! 25 C"! The Physicians Prescribing Information has been updated as follows (additions appear as underlined text and deletions as strikethrough): Contraindications Pregnancy; abnormal uterine bleeding, abnormal bleeding of undetermined origin, male and female patients with liver disease or a history of liver dysfunction; uncontrolled thyroid or adrenal dysfunction.; organic intracranial lesion.; Infertile males or females who have either pituitary tumors or pituitary failure with hypogonadotropic hypogonadism. Ovarian cyst: clomiphene citrate should not be given in the presence of an ovarian cyst, except polycystic ovary, since further enlargement of the cyst may occur. Patients should be evaluated for the presence of ovarian cyst prior to each course of treatment. Warnings Good levels of endogenous oestrogen (as estimated from vaginal smears, endometrial biopsy, assay of urinary oestrogen, or endometrial bleeding in response to progesterone) provide a favorable prognosis for ovulatory response induced by clomiphene citrate. A low level of oestrogen, although clinically less favourable, does not preclude successful outcome of therapy. Ikaclomin therapy is ineffective in patients with primary pituitary or primary ovarian failure. Therefore Ikaclomin cannot be expected to substitute for specific treatment of other causes of ovulatory failure, such as thyroid or adrenal disorders. For hyperprolactinaemia there is other preferred specific treatment. Ikaclomin is not first line treatment for low weight related amenorrhoea, with infertility, and has no value if a high FSH blood level is observed following an early menopause. Ovarian Hyperstimulation Syndrome: Ovarian Hyperstimulation Syndrome (OHSS) has been reported in patients receiving clomiphene citrate therapy for ovulation induction. In some cases, OHSS occurred following the cyclic use of clomiphene citrate therapy or when clomiphene citrate was used in combination with gonadotropins. The following symptoms have been reported in association with this syndrome
3 during clomiphene citrate therapy: pericardial effusion, anasarca, hydrothorax, acute abdomen, renal failure, pulmonary oedema, ovarian haemorrhage, deep venous thrombosis, torsion of the ovary and acute respiratory distress. If conception results, rapid progression to the severe form of the syndrome may occur. To minimise the hazard of the abnormal ovarian enlargement associated with clomiphene citrate therapy, the lowest dose consistent with expectation of good results should be used. The patient should be instructed to inform the physician of any abdominal or pelvic pain, weight gain, discomfort or distension after taking clomiphene citrate. Maximal enlargement of the ovary may not occur until several days after discontinuation of the course of clomiphene citrate. Some patients with polycystic ovary syndrome who are unusually sensitive to gonadotropin may have an exaggerated response to usual doses of clomiphene citrate. The patient who complains of abdominal or pelvic pain, discomfort, or distension after taking clomiphene citrate should be examined because of the possible presence of an ovarian cyst or other cause. Due to fragility of enlarged ovaries in severe cases, abdominal and pelvic examination should be performed very cautiously. If abnormal enlargement occurs clomiphene citrate should not be given until the ovaries have returned to pre-treatment size. Ovarian enlargement and cyst formation associated with clomiphene citrate therapy usually regress spontaneously within a few days or weeks after discontinuing treatment. Most of these patients should be managed conservatively. The dosage and/or duration of the next course of treatment should be reduced. Visual Symptoms Patients should be warned that blurring and/or other visual symptoms such as spots or flashes (scintillating scotomata) may occur occasionally (during or shortly after) with clomiphene citrate therapy. These visual disturbances are usually reversible; however, cases of prolonged visual disturbance have been reported including after clomiphene citrate discontinuation. The visual disturbances may be irreversible especially with increased dosage or duration of therapy. These may make activities such as driving or operating machinery more hazardous than usual, particularly under conditions of poor lighting. The significance of these visual symptoms is not understood. If the patient has any visual symptoms, treatment should be discontinued and ophthalmologic evaluation performed.. While their significance is not yet understood (see Adverse Reactions) Patients experiencing any visual symptoms should discontinue treatment and undergo a complete ophthalmological evaluation. Pregnancy Clomiphene citrate is not indicated during pregnancy. Although there is no evidence that clomiphene citrate has a harmful effect on the human foetus, there is evidence that clomiphene citrate has a deleterious effect on rat and rabbit foetuses when given in high doses to the pregnant animal. To avoid inadvertent clomiphene citrate administration during early pregnancy, appropriate tests should be utilised during each treatment cycle to determine whether ovulation occurs. The patient should have a pregnancy test before the next course of clomiphene citrate therapy.
4 Adverse Reactions Symptoms/Signs/Conditions: Adverse effects appeared to be dose related, occurring more frequently at the higher dose and with the longer courses of treatment used in investigational studies. At recommended dosage, adverse effects are not prominent and infrequently interfere with treatment. During the investigational studies, the more commonly reported adverse effects included ovarian enlargement (13.6%), vasomotor flushes (10.4%), abdominal-pelvic discomfort (distention, bloating) (5.5%), nausea and vomiting (2.2%), breast discomfort (2.1%), visual symptoms (1.5%), headache (1.3%) and intermenstrual spotting or menorrhagia (1.3%). Ovarian enlargement: At recommended dosage, abnormal ovarian enlargement is infrequent although the usual cyclic variation in ovarian size may be exaggerated. Similarly, cyclic ovarian pain (mittelschmerz) may be accentuated. With higher or prolonged dosage, more frequent ovarian enlargement and cyst formation may occur, and the luteal phase of the cycle may be prolonged. Rare instances of massive ovarian enlargement are recorded. Such an instance has been described in a patient with polycystic ovary syndrome whose clomiphene citrate therapy consisted of 100 mg daily for 14 days. Abnormal ovarian enlargement usually regresses spontaneously; most of the patients with this condition should be treated conservatively. Eye/Visual Symptoms: Symptoms described usually as blurring or spots or flashes (scintillating scotomata) increase in incidence with increasing total dose. These symptoms appear to be due to intensification and prolongation of after-images. Afterimages as such have also been reported. Symptoms often first appear or are accentuated with exposure to bright-light environment. Ophthalmologically definable scotomata, phosphenes and reduced visual acuity have been reported. There are rare reports of cataracts and optic neuritis. These visual disturbances are usually reversible. However, cases of prolonged visual disturbance have been reported, including after clomiphene citrate have been discontinued. The visual disturbances may be irreversible, especially with increased dosage or duration of therapy. Genitourinary: There are reports of new cases of endometriosis and exacerbation of pre-existing endometriosis during clomiphene citrate therapy. Multiple pregnancies, including simultaneous intrauterine and extrauterine pregnancies, have been reported. There is an increased chance of ectopic pregnancy in women who conceive following clomiphene citrate therapy. Tumours/neoplasms: Isolated reports have been received on the occurrence of endocrine-related or dependent neoplasms or their aggravation. Central nervous system:
5 Convulsions have been reported; patients with a history of seizures may be predisposed. In investigational patients, CNS symptoms/signs, conditions of dizziness, light-headedness/vertigo (0.9%), nervous tension/insonmia (0.8%) and fatigue/depression (0.7%) were reported. After prescription availability, there were isolated additional reports of these conditions and also reports of other conditions such as syncope/fainting, cerebrovascular accident, cerebral thrombosis, psychotic reactions including paranoid psychosis, neurologic impairment, disorientation and speech disturbance. Dermatoses: Dermatitis and rash were reported by investigational patients. Conditions such as rash and urticaria were the most common ones reported after prescription availability but also reported were conditions such as allergic reaction, erythema multiforme, ecchymosis and angioneurotic oedema. Hair thinning (alopecia) has been reported very rarely. Liver function: Bromsulphalein (BSP) retention of greater than 5% was reported in 32 of 141 patients in whom it was measured, including 5 of 43 patients who took approximately the dose of clomiphene citrate now recommended. Retention was usually minimal unless associated with prolonged continuous clomiphene citrate administration or with apparently unrelated liver disease. Other liver function tests were usually normal. In a later study in which patients were given 6 consecutive monthly courses of clomiphene citrate (50 or 100mg daily for 3 days) or matching placebo, BSP tests were done on 94 patients. Values in excess of 5% retention were recorded in 11 patients, 6 of whom had taken drug and 5 placebo. In a separate report, one patient taking 50mg of clomiphene citrate tablets daily developed jaundice on the 19th day of treatment; liver biopsy revealed bile stasis without evidence of hepatitis. Side effects are not prominent at the recommended dosage of clomiphene citrate, and only infrequently interfere with treatment. Side effects tend to occur more frequently at higher doses and during the extended treatment courses used in some early studies. The more common side effects include vasomotor flushes, abdominal discomfort/distension, abnormal uterine bleeding, ovarian enlargement, breast tenderness and visual symptoms. The vasomotor symptoms resemble menopausal `hot flushes' and are not usually severe. They promptly disappear after treatment is discontinued. Abdominal discomfort may resemble ovulatory (mittelschmerz) or premenstrual phenomena, or that due to ovarian enlargement. In addition, nausea and vomiting, nervousness and insomnia, headache, dizziness and lightheadedness, increased urination, depression and fatigue, urticaria and allergic dermatitis, weight gain and reversible hair loss have been reported. Ovarian Enlargement When clomiphene citrate is administered at the recommended dosage, abnormal ovarian enlargement (see Precautions) is infrequent, although the usual cyclic variation in ovarian size may be exaggerated. Similarly, mid-cycle ovarian pain (mittelschmerz) may be accentuated. With
6 prolonged or higher dosage, ovarian enlargement and cyst formation (usually luteal) may occur more often and the luteal phase of the cycle may be prolonged. Patients with polycystic ovary disease may be unusually sensitive to clomiphene therapy. Rare occurrences of massive ovarian enlargement have been reported, for example, in a patient with polycystic ovary syndrome in whom clomiphene citrate therapy consisted of 100 mg daily for 14 days. Since abnormal ovarian enlargement usually regresses spontaneously, most of these patients should be treated conservatively. Visual Disturbances The incidence of visual symptoms usually described as `blurring', spots or flashes (scintillating scotomata) correlates with increases in the total dose. The symptoms disappear within a few days or weeks after clomiphene citrate is discontinued. This may be due to intensification and/or prolongation of after-images. Symptoms often appear first, or are accentuated, upon exposure to a more brightly-lit environment. While measured visual acuity has not generally been affected, in one patient taking 200 mg daily, visual blurring developed on day 7 of treatment progressing to severe diminution of visual acuity by day 10. No other abnormality was coincident and the visual acuity was normal by the day 3 after discontinuation of treatment. Ophthalmologically-definable scotomata and electroretinographic retinal function changes have also been reported. Birth Defects Of 1,803 births following clomiphene citrate administration, 45 infants with birth defects were reported. This represents a cumulative rate of 2.5%. Six cases of Down's Syndrome, one neonatal death with multiple malformations and one case each of the following were reported: exotropia, club foot, tibial torsion, blocked tear duct and hemangioma. The other congenital abnormalities were not described. The investigators did not report that these were presumed to be due to therapy. The cumulative rate of congenital abnormalities does not exceed that reported in the general population. Precautions Multiple Pregnancy: There is an increased chance of multiple pregnancy when conception occurs in relationship to clomiphene citrate therapy. The potential complications and hazards of multiple pregnancy should be discussed with the patient. During the clinical investigation studies, the incidence of multiple pregnancy was 7.9% (186 of 2369 clomiphene citrate associated pregnancies on which outcome was reported). Among these 2369 pregnancies, 165 (6.9%) twin, 11 (0.5%) triplet, 7 (0.3%) quadruplet and 3 (0.13%) quintuplet. Of the 165 twin pregnancies for which sufficient information was available, the ratio of monozygotic twins was 1:5. Ectopic Pregnancy: There is an increased chance of ectopic pregnancy (including tubal and ovarian sites) in women who conceive following clomiphene citrate therapy. Multiple pregnancies, including simultaneous intrauterine and extrauterine pregnancies, have been reported. Uterine Fibroids:
7 Caution should be exercised when using clomiphene citrate in patients with uterine fibroids due to potential for further enlargement of the fibroids. Pregnancy Wastage and Birth Anomalies: The overall incidence of reported birth anomalies from pregnancies associated with maternal clomiphene citrate ingestion (before or after conception) during the investigational studies was within the range of that reported in the published references for the general population. Among the birth anomalies spontaneously reported in the published literature as individual cases, the proportion of neural tube defects has been high among pregnancies associated with ovulation induced by clomiphene citrate, but this has not been supported by data from population based studies. The physician should explain so that the patient understands the assumed risk of any pregnancy whether the ovulation was induced with the aid of clomiphene citrate or occurred naturally. The patient should be informed of the greater pregnancy risks associated with certain characteristics or conditions of any pregnant woman: e.g. age of female and male partner, history of spontaneous abortions, Rh genotype, abnormal menstrual history, infertility history (regardless of cause), organic heart disease, diabetes, exposure to infectious agents such as rubella, familial history of birth anomaly, and other risk factors that may be pertinent to the patient for whom clomiphene citrate is being considered. Based upon the evaluation of the patient, genetic counselling may be indicated. Population based reports have been published on possible elevation of risk of Down's Syndrome in ovulation induction cases and of increase in trisomy defects among spontaneously aborted foetuses from subfertile women receiving ovulation inducing drugs (no women with clomiphene citrate alone and without additional inducing drug). However, as yet, the reported observations are too few to confirm or not confirm the presence of an increased risk that would justify amniocentesis other than for the usual indications because of age and family history. The experience from patients of all diagnosis during clinical investigation of clomiphene citrate shows a pregnancy (single and multiple) wastage or foetal loss rate of 21.4% (abortion rate of 19.0%), ectopic pregnancies, 1.18%, hydatidiform mole, 0.17%, foetus papyraceous, 0.04% and of pregnancies with one or more stillbirths, 1.01%. Clomiphene citrate therapy after conception was reported for 158 of the 2369 delivered and reported pregnancies in the clinical investigations. Of these 158 pregnancies 8 infants (born of 7 pregnancies) were reported to have birth defects. There was no difference in reported incidence of birth defects whether clomiphene citrate was given before the 19th day after conception or between the 20th and 35th day after conception. This incidence is within the anticipated range of general population.
8 Ovarian Cancer: There have been rare reports of ovarian cancer with fertility drugs; infertility itself is a primary risk factor. Epidemiological data suggest that prolonged use of clomiphene citrate may increase this risk. Therefore the recommended duration of treatment should not be exceeded. In the reviewed publications, the incidence of multiple pregnancies was increased during those cycles in which clomiphene citrate was administered. Among the 1,803 pregnancies on which the outcome was reported, 90% were single and 10% twins. Less than 1% of the reported deliveries resulted in triplets or more. Of these multiple pregnancies, 96%-99% resulted in the birth of live infants. Patients and their male partners should be advised of the frequency and potential hazards of multiple pregnancy before starting treatment. Drug Interactions Interactions with other drugs have not been documented. Effects on ability to drive and use machines Patients should be warned that visual symptoms may render such activities as driving a car or operating machinery more hazardous than usual, particularly under conditions of variable lighting.. # % $
Core Safety Profile. Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU. Date of FAR:
 Core Safety Profile Active substance: Urofollitropin Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU P - RMS: UK/H/PSUR/0059/001 Date of FAR: 04.12.2009 4.2 Posology and method
Core Safety Profile Active substance: Urofollitropin Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU P - RMS: UK/H/PSUR/0059/001 Date of FAR: 04.12.2009 4.2 Posology and method
PRODUCT INFORMATION SEROPHENE
 NAME OF THE MEDICINE Non-proprietary name Clomiphene citrate Chemical structure PRODUCT INFORMATION SEROPHENE Clomiphene citrate exists as a mixture of E and Z isomers. The Z-isomer of clomiphene citrate
NAME OF THE MEDICINE Non-proprietary name Clomiphene citrate Chemical structure PRODUCT INFORMATION SEROPHENE Clomiphene citrate exists as a mixture of E and Z isomers. The Z-isomer of clomiphene citrate
Infertility: failure to conceive within one year of unprotected regular sexual intercourse. Primary secondary
 Subfertility Infertility: failure to conceive within one year of unprotected regular sexual intercourse. Primary secondary Infertility affects about 15 % of couples. age of the female. Other factors that
Subfertility Infertility: failure to conceive within one year of unprotected regular sexual intercourse. Primary secondary Infertility affects about 15 % of couples. age of the female. Other factors that
Infertility DR. RAHUL BEVARA
 Infertility DR. RAHUL BEVARA Definitions Infertility is defined as the inability to conceive after one year of unprotected coitus. Affects 10-15% of couples Primary Infertility, that is inability to conceive
Infertility DR. RAHUL BEVARA Definitions Infertility is defined as the inability to conceive after one year of unprotected coitus. Affects 10-15% of couples Primary Infertility, that is inability to conceive
P-RMS: SE/H/PSUR/0039/002
 Core Safety Profile Active substance: Buserelin Pharmaceutical form(s)/strength: 1 mg/ml Solution for injection (Suprefact), 0.1 mg/dose, 0.15 mg/dose Nasal spray, solution (Suprefact, Suprecur),6.3 mg,
Core Safety Profile Active substance: Buserelin Pharmaceutical form(s)/strength: 1 mg/ml Solution for injection (Suprefact), 0.1 mg/dose, 0.15 mg/dose Nasal spray, solution (Suprefact, Suprecur),6.3 mg,
1. NAME OF THE MEDICINAL PRODUCT. Gonapeptyl 0.1 mg/1 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Gonapeptyl 0.1 mg/1 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe of 1 ml solution for injection contains 100 micrograms
1. NAME OF THE MEDICINAL PRODUCT Gonapeptyl 0.1 mg/1 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe of 1 ml solution for injection contains 100 micrograms
Laboratoires Genevirer Menotrophin IU 1.8.2
 Important missing information VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Infertility is when a woman cannot get pregnant (conceive) despite having regular unprotected sexual
Important missing information VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Infertility is when a woman cannot get pregnant (conceive) despite having regular unprotected sexual
Core Safety Profile Triptorelin 1-month/3-month formulation ATC-code: L02A E 04 March 2010
 Core Safety Profile Triptorelin 1-month/3-month formulation ATC-code: L02A E 04 March 2010 4.3 Contraindications Hypersensitivity to GnRH, its analogues or any other component of the medicinal product
Core Safety Profile Triptorelin 1-month/3-month formulation ATC-code: L02A E 04 March 2010 4.3 Contraindications Hypersensitivity to GnRH, its analogues or any other component of the medicinal product
Each 10 mg tablet contains tamoxifen citrate equivalent to 10 mg tamoxifen.
 Page 1 of 11 SCHEDULING STATUS: S4 PROPRIETARY NAMES (and dosage forms): KESSAR 10 (tablets) KESSAR 20 (tablets) COMPOSITION: Each 10 mg tablet contains tamoxifen citrate equivalent to 10 mg tamoxifen.
Page 1 of 11 SCHEDULING STATUS: S4 PROPRIETARY NAMES (and dosage forms): KESSAR 10 (tablets) KESSAR 20 (tablets) COMPOSITION: Each 10 mg tablet contains tamoxifen citrate equivalent to 10 mg tamoxifen.
Infertility History Form
 Date form completed: Infertility History Form Patient s name: _ Age: Date of Birth: Occupation: Partner s name: Age: Date of Birth: Occupation: Prior marriage: Yes No # Prior marriage: Yes No # Attempted
Date form completed: Infertility History Form Patient s name: _ Age: Date of Birth: Occupation: Partner s name: Age: Date of Birth: Occupation: Prior marriage: Yes No # Prior marriage: Yes No # Attempted
IMPORTANT: PLEASE READ
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION PUREGON (follitropin beta) This leaflet is part III of a three-part Product Monograph published when PUREGON was approved for sale in Canada and is
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION PUREGON (follitropin beta) This leaflet is part III of a three-part Product Monograph published when PUREGON was approved for sale in Canada and is
M0BCore Safety Profile. Active substance: Triptorelin Pharmaceutical form(s)/strength: 0,1 mg P-RMS: DE/H/PSUR/0038/002 Date of FAR:
 M0BCore Safety Profile Active substance: Triptorelin Pharmaceutical form(s)/strength: 0,1 mg P-RMS: DE/H/PSUR/0038/002 Date of FAR: 26.05.2014 4.3 Contraindications Hypersensitivity to GnRH, its analogues
M0BCore Safety Profile Active substance: Triptorelin Pharmaceutical form(s)/strength: 0,1 mg P-RMS: DE/H/PSUR/0038/002 Date of FAR: 26.05.2014 4.3 Contraindications Hypersensitivity to GnRH, its analogues
GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch])
![GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch])](/thumbs/88/115281160.jpg) PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about It does not contain all
PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about It does not contain all
Dr Manuela Toledo - Procedures in ART -
 Dr Manuela Toledo - Procedures in ART - Fertility Specialist MBBS FRANZCOG MMed CREI Specialities: IVF & infertility Fertility preservation Consulting Locations East Melbourne Planning a pregnancy - Folic
Dr Manuela Toledo - Procedures in ART - Fertility Specialist MBBS FRANZCOG MMed CREI Specialities: IVF & infertility Fertility preservation Consulting Locations East Melbourne Planning a pregnancy - Folic
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OGEN (AN ESTROGEN HORMONE)?
 PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch])
![GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch])](/thumbs/77/75508754.jpg) GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about GONALf. It does
GONAL-f PEN Recombinant human follicle stimulating hormone (follitropin alfa [rch]) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about GONALf. It does
GONAL-f (follitropin alfa [rch]) solution for injection
![GONAL-f (follitropin alfa [rch]) solution for injection GONAL-f (follitropin alfa [rch]) solution for injection](/thumbs/94/120349310.jpg) (follitropin alfa [rch]) solution for injection Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information.
(follitropin alfa [rch]) solution for injection Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information.
PACKAGE INSERT PREGNYL. (chorionic gonadotropin for injection, USP) 10,000 units/vial. Human Gonadotropin
 PACKAGE INSERT PREGNYL (chorionic gonadotropin for injection, USP) 10,000 units/vial Human Gonadotropin Merck Canada Inc. 16750 route Transcanadienne Kirkland QC Canada H9H 4M7 Date of Revision: July 26,
PACKAGE INSERT PREGNYL (chorionic gonadotropin for injection, USP) 10,000 units/vial Human Gonadotropin Merck Canada Inc. 16750 route Transcanadienne Kirkland QC Canada H9H 4M7 Date of Revision: July 26,
Orgalutran 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
INFERTILITY CAUSES. Basic evaluation of the female
 INFERTILITY Infertility is the inability to conceive after 12 months of unprotected intercourse. There are multiple causes of infertility and a systematic way to evaluate the condition. Let s look at some
INFERTILITY Infertility is the inability to conceive after 12 months of unprotected intercourse. There are multiple causes of infertility and a systematic way to evaluate the condition. Let s look at some
Louise Brown born First IVF baby Born to Lesley Brown, bilateral tubal blockage Natural cycle, single egg fertilization
 Louise Brown born First IVF baby Born to Lesley Brown, bilateral tubal blockage Natural cycle, single egg fertilization IVF 1878 - first reported attempts at IVF 1959 - IVF births in rabbits in USA 1968
Louise Brown born First IVF baby Born to Lesley Brown, bilateral tubal blockage Natural cycle, single egg fertilization IVF 1878 - first reported attempts at IVF 1959 - IVF births in rabbits in USA 1968
UW MEDICINE PATIENT EDUCATION. In Vitro Fertilization How to prepare and what to expect DRAFT
 UW MEDICINE PATIENT EDUCATION In Vitro Fertilization How to prepare and what to expect This handout tells how to prepare for and what to expect when you go through a cycle of in vitro fertilization. It
UW MEDICINE PATIENT EDUCATION In Vitro Fertilization How to prepare and what to expect This handout tells how to prepare for and what to expect when you go through a cycle of in vitro fertilization. It
In Vitro Fertilization What to expect
 Patient Education In Vitro Fertilization What to expect This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this process,
Patient Education In Vitro Fertilization What to expect This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this process,
Intrauterine Insemination - FAQs Q. How Does Pregnancy Occur?
 Published on: 8 Apr 2013 Intrauterine Insemination - FAQs Q. How Does Pregnancy Occur? A. The female reproductive system involves the uterus, ovaries, fallopian tubes, cervix and vagina. The female hormones,
Published on: 8 Apr 2013 Intrauterine Insemination - FAQs Q. How Does Pregnancy Occur? A. The female reproductive system involves the uterus, ovaries, fallopian tubes, cervix and vagina. The female hormones,
PUREGON follitropin beta[rch]
![PUREGON follitropin beta[rch] PUREGON follitropin beta[rch]](/thumbs/86/94247981.jpg) PUREGON follitropin beta[rch] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PUREGON. It does not contain all the available information. It does
PUREGON follitropin beta[rch] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PUREGON. It does not contain all the available information. It does
PUREGON follitropin beta[rch]
![PUREGON follitropin beta[rch] PUREGON follitropin beta[rch]](/thumbs/94/120349294.jpg) PUREGON follitropin beta[rch] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PUREGON. It does not contain all the available information. It does
PUREGON follitropin beta[rch] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PUREGON. It does not contain all the available information. It does
CONSENT FORM FOR TREATMENT WITH OVULATION INDUCTION MEDICATIONS AND INTRAUTERINE INSEMINATIONS
 CONSENT FORM FOR TREATMENT WITH OVULATION INDUCTION MEDICATIONS AND INTRAUTERINE INSEMINATIONS INSTRUCTIONS: This consent form provides a description of the treatment that you are undertaking. Read the
CONSENT FORM FOR TREATMENT WITH OVULATION INDUCTION MEDICATIONS AND INTRAUTERINE INSEMINATIONS INSTRUCTIONS: This consent form provides a description of the treatment that you are undertaking. Read the
Follitropin alfa (rch)/lutropin alfa (rch) Powder for Injection. This leaflet answers some common questions about PERGOVERIS.
 PERGOVERIS 150 IU / 75 IU Follitropin alfa (rch)/lutropin alfa (rch) Powder for Injection Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PERGOVERIS.
PERGOVERIS 150 IU / 75 IU Follitropin alfa (rch)/lutropin alfa (rch) Powder for Injection Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about PERGOVERIS.
Infertility treatment
 In the name of God Infertility treatment Treatment options The optimal treatment is one that provide an acceptable success rate, has minimal risk and is costeffective. The treatment options are: 1- Ovulation
In the name of God Infertility treatment Treatment options The optimal treatment is one that provide an acceptable success rate, has minimal risk and is costeffective. The treatment options are: 1- Ovulation
Pregnyl Human chorionic gonadotrophin (hcg)
 Pregnyl Human chorionic gonadotrophin (hcg) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Pregnyl. It does not contain all the available information.
Pregnyl Human chorionic gonadotrophin (hcg) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Pregnyl. It does not contain all the available information.
Polycystic Ovary Syndrome
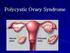 Polycystic Ovary Syndrome Definition: the diagnostic criteria Evidence of hyperandrogenism, biochemical &/or clinical (hirsutism, acne & male pattern baldness). Ovulatory dysfunction; amenorrhoea; oligomenorrhoea
Polycystic Ovary Syndrome Definition: the diagnostic criteria Evidence of hyperandrogenism, biochemical &/or clinical (hirsutism, acne & male pattern baldness). Ovulatory dysfunction; amenorrhoea; oligomenorrhoea
In Vitro Fertilization
 Patient Education In Vitro Fertilization About the treatment This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this
Patient Education In Vitro Fertilization About the treatment This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this
Topic 24: Estrogens and Female Reproductive Drugs
 Topic 24: Estrogens and Female Reproductive Drugs I. Contraceptives A. Estrogen-Progestin Contraceptives Note all of these drugs contain one estrogen (listed first) and one progestin Drug to know: ethinyl
Topic 24: Estrogens and Female Reproductive Drugs I. Contraceptives A. Estrogen-Progestin Contraceptives Note all of these drugs contain one estrogen (listed first) and one progestin Drug to know: ethinyl
FEMALE PATIENT HISTORY
 ew Hope. ew Life. ew Beginnings. A Division of MID-ATLATIC WOME S CARE, PLC FEMALE PATIET HISTORY PLEASE OTE: Infertility patients please complete ALL sections. All other patients, complete section 1.,
ew Hope. ew Life. ew Beginnings. A Division of MID-ATLATIC WOME S CARE, PLC FEMALE PATIET HISTORY PLEASE OTE: Infertility patients please complete ALL sections. All other patients, complete section 1.,
Virginia Center for Reproductive Medicine
 Virginia Center for Reproductive Medicine New Patient Questionnaire Date: Patient Name: Date of Birth: / / Age: Social Security #: Address: Phone: (H) ( ) (W) ( ) Cell Phone: ( ) Pharmacy: ( ) Partner
Virginia Center for Reproductive Medicine New Patient Questionnaire Date: Patient Name: Date of Birth: / / Age: Social Security #: Address: Phone: (H) ( ) (W) ( ) Cell Phone: ( ) Pharmacy: ( ) Partner
Please fill out the following information and have it returned to our office prior to your consultation.
 Please fill out the following information and have it returned to our office prior to your consultation. Patient s Name Partner s Name Address: City: State: Zip: Phone (day#): ( ) (eve#) ( ) (cell) ( )
Please fill out the following information and have it returned to our office prior to your consultation. Patient s Name Partner s Name Address: City: State: Zip: Phone (day#): ( ) (eve#) ( ) (cell) ( )
Package leaflet: Information for the user
 Package leaflet: Information for the user Puregon 150 IU/0.18 ml solution for injection Puregon 300 IU/0.36 ml solution for injection Puregon 600 IU/0.72 ml solution for injection Puregon 900 IU/1.08 ml
Package leaflet: Information for the user Puregon 150 IU/0.18 ml solution for injection Puregon 300 IU/0.36 ml solution for injection Puregon 600 IU/0.72 ml solution for injection Puregon 900 IU/1.08 ml
Pergoveris 150IU/75IU
 Prescribing Information Pergoveris 150IU/75IU 1. NAME OF THE MEDICINAL PRODUCT Pergoveris 150 IU/75 IU powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial
Prescribing Information Pergoveris 150IU/75IU 1. NAME OF THE MEDICINAL PRODUCT Pergoveris 150 IU/75 IU powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial
Dosage and Administration, Recommended Dosing for Induction of Spermatogenesis in Men (2.4) 6/2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Follistim AQ safely and effectively. See full prescribing information for Follistim AQ. Follistim
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Follistim AQ safely and effectively. See full prescribing information for Follistim AQ. Follistim
Neil Goodman, MD, FACE
 Initial Workup of Infertile Couple: Female Neil Goodman, MD, FACE Professor of Medicine Voluntary Faculty University of Miami Miller School of Medicine Scope of Infertility in the United States Affects
Initial Workup of Infertile Couple: Female Neil Goodman, MD, FACE Professor of Medicine Voluntary Faculty University of Miami Miller School of Medicine Scope of Infertility in the United States Affects
Embryo Selection after IVF
 Embryo Selection after IVF Embryo Selection after IVF Many of human embryos produced after in vitro fertilization carry abnormal chromosomes. Placing a chromosomally normal embryo (s) into a normal uterus
Embryo Selection after IVF Embryo Selection after IVF Many of human embryos produced after in vitro fertilization carry abnormal chromosomes. Placing a chromosomally normal embryo (s) into a normal uterus
GPVTS TEACHING APRIL 2016 FERTILITY
 GPVTS TEACHING APRIL 2016 FERTILITY Djavid Alleemudder - Consultant Obstetrics & Gynaecology DEFINITIONS What is the definition of infertility? Failure to conceive after 12 months despite regular, unprotected
GPVTS TEACHING APRIL 2016 FERTILITY Djavid Alleemudder - Consultant Obstetrics & Gynaecology DEFINITIONS What is the definition of infertility? Failure to conceive after 12 months despite regular, unprotected
IVF Patient Information
 What is IVF? IVF (In Vitro Fertilisation) is a treatment by which fertilisation of eggs by sperm takes place outside the body in a dish in an IVF laboratory. An ovary has a pool of immature eggs. In a
What is IVF? IVF (In Vitro Fertilisation) is a treatment by which fertilisation of eggs by sperm takes place outside the body in a dish in an IVF laboratory. An ovary has a pool of immature eggs. In a
Patient Past Medical History
 Patient Past Medical History A. Identifying Data Date this form when completed Your name Partner's name Age Birth date Height Weight Length of marriage (or relationship) How long have you been trying unsuccessfully
Patient Past Medical History A. Identifying Data Date this form when completed Your name Partner's name Age Birth date Height Weight Length of marriage (or relationship) How long have you been trying unsuccessfully
5/5/2010. Infertility FINANCIAL DISCLOSURE. Infertility Definition. Objectives. Normal Human Fertility. Normal Menstrual Cycle
 Infertility FINANCIAL DISCLOSURE I HAVE NO FINANCIAL INTEREST IN ANY OF THE PRODUCTS MENTIONED IN MY PRESENTATION Bryan K. Rone, M.D. University of Kentucky Obstetrics and Gynecology I AM RECEIVING COMPENSATION
Infertility FINANCIAL DISCLOSURE I HAVE NO FINANCIAL INTEREST IN ANY OF THE PRODUCTS MENTIONED IN MY PRESENTATION Bryan K. Rone, M.D. University of Kentucky Obstetrics and Gynecology I AM RECEIVING COMPENSATION
Approach to ovulation induction and superovulation in women with a history of infertility. Anatte E. Karmon, MD
 Approach to ovulation induction and superovulation in women with a history of infertility Anatte E. Karmon, MD Disclosures- Anatte Karmon, MD No financial relationships to disclose 2 Objectives At the
Approach to ovulation induction and superovulation in women with a history of infertility Anatte E. Karmon, MD Disclosures- Anatte Karmon, MD No financial relationships to disclose 2 Objectives At the
The 6 th Scientific Meeting of the Asia Pacific Menopause Federation
 Abnormal uterine bleeding in the perimenopause Perimenopausal menstrual problems are among the most common causes for family practitioner and specialist referral. Often it is due to the hormone changes
Abnormal uterine bleeding in the perimenopause Perimenopausal menstrual problems are among the most common causes for family practitioner and specialist referral. Often it is due to the hormone changes
The Center for Reproductive Health. Patient Questionnaire
 The Center for Reproductive Health Edwin D. Robins, MD Patient Questionnaire Date: Reason for Visit: Patient Name: Last First Middle Date of Birth: Age: Social Security #: Address: City: State: Zip Code:
The Center for Reproductive Health Edwin D. Robins, MD Patient Questionnaire Date: Reason for Visit: Patient Name: Last First Middle Date of Birth: Age: Social Security #: Address: City: State: Zip Code:
Intra uterine insemination (IUI) Information for Patients and Partners
 Intra uterine insemination (IUI) Information for Patients and Partners What is this leaflet about and who is it for? This leaflet is produced to inform couples undergoing IUI (intrauterine insemination)
Intra uterine insemination (IUI) Information for Patients and Partners What is this leaflet about and who is it for? This leaflet is produced to inform couples undergoing IUI (intrauterine insemination)
Contraception. Objectives. Unintended Pregnancy. Unintended Pregnancy in the US. What s the Impact? 10/7/2014
 Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY GROUP
 REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY GROUP NEW PATIENT EVALUATION FORM Name: Age: Partner: Age: Reason for Referral: Date of Appt: Have you ever seen any other physician(s) for this problem? Name:
REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY GROUP NEW PATIENT EVALUATION FORM Name: Age: Partner: Age: Reason for Referral: Date of Appt: Have you ever seen any other physician(s) for this problem? Name:
Menopause & HRT. Rosie & Alex. Image:
 Menopause & HRT Rosie & Alex Image: http://www.keepcalm-o-matic.co.uk/ Menopause The permanent cessation of menstruation for 12 months When does it happen? Average age 51 Image: Nature Medicine - 12, 612-613
Menopause & HRT Rosie & Alex Image: http://www.keepcalm-o-matic.co.uk/ Menopause The permanent cessation of menstruation for 12 months When does it happen? Average age 51 Image: Nature Medicine - 12, 612-613
Patient Information. Marital Status (Single, Married, Life Partner, Divorced, Widowed) CHIEF COMPLAINT
 Patient Information Name Date Home Address City State Zip Phone E-mail Address Cell Phone: Business Address City State Zip Phone Occupation Place of Birth Date of Birth Age Height Weight Soc. Sec. # Sex
Patient Information Name Date Home Address City State Zip Phone E-mail Address Cell Phone: Business Address City State Zip Phone Occupation Place of Birth Date of Birth Age Height Weight Soc. Sec. # Sex
MODUS TABLETS. Medroxyprogesterone Tablets IP
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
Assisted Reproduction. By Dr. Afraa Mahjoob Al-Naddawi
 Assisted Reproduction By Dr. Afraa Mahjoob Al-Naddawi Learning Objectives: By the end of this lecture, you will be able to: 1) Define assisted reproductive techniques (ART). 2) List indications for various
Assisted Reproduction By Dr. Afraa Mahjoob Al-Naddawi Learning Objectives: By the end of this lecture, you will be able to: 1) Define assisted reproductive techniques (ART). 2) List indications for various
WOMEN & INFANTS HOSPITAL Providence, RI CONSENT FOR IN VITRO FERTILIZATION USING A GESTATIONAL CARRIER (PATIENT/INTENDED PARENTS) 1.
 *40675* 40675 MR-838 (9-2017) WOMEN & INFANTS HOSPITAL Providence, RI 02905 CONSENT FOR IN VITRO FERTILIZATION USING A GESTATIONAL CARRIER (PATIENT/INTENDED PARENTS) 1. I, and (Print Patient s name) (Print
*40675* 40675 MR-838 (9-2017) WOMEN & INFANTS HOSPITAL Providence, RI 02905 CONSENT FOR IN VITRO FERTILIZATION USING A GESTATIONAL CARRIER (PATIENT/INTENDED PARENTS) 1. I, and (Print Patient s name) (Print
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elonva 100 micrograms solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elonva 100 micrograms solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains
Read all of this leaflet carefully before you start taking this medicine.
 Moxelle 20mg Tablets Tamoxifen Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor
Moxelle 20mg Tablets Tamoxifen Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor
Package Insert. I-Gest SR Pharmaceutical form Oral tablet with natural micronised progesterone in a sustained release form
 Package Insert I-Gest SR 200 Product Summary 1. Name of the medicinal product I-Gest SR 200 2. Qualitative and quantitative composition Each film coated tablet contains Progesterone IP 200 mg 3. Pharmaceutical
Package Insert I-Gest SR 200 Product Summary 1. Name of the medicinal product I-Gest SR 200 2. Qualitative and quantitative composition Each film coated tablet contains Progesterone IP 200 mg 3. Pharmaceutical
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT GONAL-f 75 IU (5.5 micrograms) powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT GONAL-f 75 IU (5.5 micrograms) powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
Saint Mary s Hospital Department of Reproductive Medicine. Clomid. Information For Patients
 Saint Mary s Hospital Department of Reproductive Medicine Clomid Information For Patients INF/DRM/CL1/32 V1/01/11/2013 What is clomiphene and why is it prescribed? Clomid (clomiphene citrate) is used in
Saint Mary s Hospital Department of Reproductive Medicine Clomid Information For Patients INF/DRM/CL1/32 V1/01/11/2013 What is clomiphene and why is it prescribed? Clomid (clomiphene citrate) is used in
Balancing Female Hormones
 Balancing Female Hormones Food for thoughts: The body is on automatic to self-repair itself. The US health care model is in sad reality - disease management By assisting the body s restorative, regenerative
Balancing Female Hormones Food for thoughts: The body is on automatic to self-repair itself. The US health care model is in sad reality - disease management By assisting the body s restorative, regenerative
Emergency contraception is an occasional method. It should in no instance replace a regular contraceptive method.
 1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS:
 0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
0BCore Safety Profile Active substance: Betaxolol eyedrops Pharmaceutical form(s)/strength: Solution: 5 mg/ml Suspensions: 2.5 and 5 mg/ml P-RMS: HU/H/PSUR/0010/002 Date of FAR: 20.03.2013 4.2 Posology
Patient Information: Patient Name: Date of Birth:
 Weill Cornell Medicine Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine Informed Consent for Oocyte (Egg) Cryopreservation Patient Information: Part 1: I have requested to be treated
Weill Cornell Medicine Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine Informed Consent for Oocyte (Egg) Cryopreservation Patient Information: Part 1: I have requested to be treated
Ovaries: In Sickness and Health. Mr N Pisal Consultant Gynaecologist The Portland Hospital
 Ovaries: In Sickness and Health Mr N Pisal Consultant Gynaecologist The Portland Hospital Topics for discussion How to assess ovarian function? AMH PCOS Ovarian pain Ovarian cysts Ovarian screening Menopause
Ovaries: In Sickness and Health Mr N Pisal Consultant Gynaecologist The Portland Hospital Topics for discussion How to assess ovarian function? AMH PCOS Ovarian pain Ovarian cysts Ovarian screening Menopause
NEW ZEALAND DATA SHEET. LUVERIS 75 IU Powder for Injection. Lutropin alfa (rch)
 NEW ZEALAND DATA SHEET LUVERIS 75 IU Powder for Injection Lutropin alfa (rch) NAME OF THE MEDICINE LUVERIS contains lutropin alfa (rch). # Recombinant-hLH (r-hlh) is a human gonadotropin hormone, composed
NEW ZEALAND DATA SHEET LUVERIS 75 IU Powder for Injection Lutropin alfa (rch) NAME OF THE MEDICINE LUVERIS contains lutropin alfa (rch). # Recombinant-hLH (r-hlh) is a human gonadotropin hormone, composed
trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene
![trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene](/thumbs/95/124190064.jpg) Genox Tamoxifen citrate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Tamoxifen (as citrate) trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene Structural formula:
Genox Tamoxifen citrate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Tamoxifen (as citrate) trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene Structural formula:
First you must understand what is needed for becoming pregnant?
 What is infertility? Infertility means difficulty in becoming pregnant without using contraception. First you must understand what is needed for becoming pregnant? Ovum from the woman to combine with a
What is infertility? Infertility means difficulty in becoming pregnant without using contraception. First you must understand what is needed for becoming pregnant? Ovum from the woman to combine with a
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME 100 micrograms solution for injection 150 micrograms solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 100 micrograms
NEW ZEALAND DATA SHEET 1. PRODUCT NAME 100 micrograms solution for injection 150 micrograms solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 100 micrograms
Common protocols in intra-uterine insemination cycles
 Common protocols in intra-uterine insemination cycles Doç. Dr. Candan İltemir Duvan Turgut Özal Üniversitesi Tıp Fakültesi Kadın Hastalıkları ve Doğum AD Ovulation induction with intra-uterine insemination
Common protocols in intra-uterine insemination cycles Doç. Dr. Candan İltemir Duvan Turgut Özal Üniversitesi Tıp Fakültesi Kadın Hastalıkları ve Doğum AD Ovulation induction with intra-uterine insemination
Polycystic Ovary Syndrome HEATHER BURKS, MD OU PHYSICIANS REPRODUCTIVE MEDICINE SEPTEMBER 21, 2018
 Polycystic Ovary Syndrome HEATHER BURKS, MD OU PHYSICIANS REPRODUCTIVE MEDICINE SEPTEMBER 21, 2018 Learning Objectives At the conclusion of this lecture, learners should: 1) Know the various diagnostic
Polycystic Ovary Syndrome HEATHER BURKS, MD OU PHYSICIANS REPRODUCTIVE MEDICINE SEPTEMBER 21, 2018 Learning Objectives At the conclusion of this lecture, learners should: 1) Know the various diagnostic
Kimberley A. Schroeder, D.O. 115 Baker Drive Tomball, TX
 Kimberley A. Schroeder, D.O. 115 Baker Drive Tomball, TX 77375 281.290.0531 www.feelwellagain.com FEMALE MEDICAL QUESTIONNAIRE (POSTMENOPAUSAL) NAME: DATE OF BIRTH: CHIEF COMPLAINT What is your primary
Kimberley A. Schroeder, D.O. 115 Baker Drive Tomball, TX 77375 281.290.0531 www.feelwellagain.com FEMALE MEDICAL QUESTIONNAIRE (POSTMENOPAUSAL) NAME: DATE OF BIRTH: CHIEF COMPLAINT What is your primary
Information Booklet. Exploring the causes of infertility and treatment options.
 Information Booklet Exploring the causes of infertility and treatment options www.ptafertility.co.za info@ptafertility.co.za +27 12 998 8854 Faith is taking the first step even if you don t see the whole
Information Booklet Exploring the causes of infertility and treatment options www.ptafertility.co.za info@ptafertility.co.za +27 12 998 8854 Faith is taking the first step even if you don t see the whole
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications
 Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
PRETREATMENT ASSESSMENT & MANAGEMENT (MODULE 1 B) March, 2018
 PRETREATMENT ASSESSMENT & MANAGEMENT (MODULE 1 B) March, 2018 Clinical Assessment A thorough clinical evaluation is a prerequisite for ART A thorough clinical evaluation as detailed in the female and male
PRETREATMENT ASSESSMENT & MANAGEMENT (MODULE 1 B) March, 2018 Clinical Assessment A thorough clinical evaluation is a prerequisite for ART A thorough clinical evaluation as detailed in the female and male
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lubion 25 mg solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial (1.119 ml) contains 25 mg of progesterone (theoretical
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lubion 25 mg solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial (1.119 ml) contains 25 mg of progesterone (theoretical
GONAL-F THE COMPLEX PROCESS OF FERTILITY HORMONE DEFICIENCIES CAN LEAD TO INFERTILITY PROBLEMS WHAT IS GONAL-F? HCP FACT SHEET
 HCP FACT SHEET GONAL-F GONAL-f (recombinant follitropin alfa) is prescribed to supplement or replace naturally occurring folliclestimulating hormone (FSH), an essential hormone to treat infertility in
HCP FACT SHEET GONAL-F GONAL-f (recombinant follitropin alfa) is prescribed to supplement or replace naturally occurring folliclestimulating hormone (FSH), an essential hormone to treat infertility in
Female Consultation Questionnaire
 Female Consultation Questionnaire In order to schedule a consultation with the doctor, an overview of your medical history along with a copy of your medical records are requested. Dr. Zouves will review
Female Consultation Questionnaire In order to schedule a consultation with the doctor, an overview of your medical history along with a copy of your medical records are requested. Dr. Zouves will review
Infertility: A Generalist s Perspective
 Infertility: A Generalist s Perspective Learning Objectives Fertility and Lifestyle: Patient education Describe the basic infertility workup Basic treatment strategies unexplained Heather Huddleston, MD
Infertility: A Generalist s Perspective Learning Objectives Fertility and Lifestyle: Patient education Describe the basic infertility workup Basic treatment strategies unexplained Heather Huddleston, MD
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Partner Name: Patient Signature Partner Signature (If applicable) Date (COS/IUI) Witness Name Witness Signature Date
 1. Please place your initials below to indicate which components of controlled ovarian Stimulation (COS) and intrauterine insemination (IUI) treatment you agree to undertake in your upcoming treatment
1. Please place your initials below to indicate which components of controlled ovarian Stimulation (COS) and intrauterine insemination (IUI) treatment you agree to undertake in your upcoming treatment
Questionnaire for Women
 Questionnaire for Women General Information Name Date Address Telephone Home _Work _ Cell Birth date Age _ Occupation Ethnic Background _ Height _ Weight _ Highest Education _ Partner s Name Marriage date
Questionnaire for Women General Information Name Date Address Telephone Home _Work _ Cell Birth date Age _ Occupation Ethnic Background _ Height _ Weight _ Highest Education _ Partner s Name Marriage date
Infertility. Thomas Lloyd and Samera Dean
 Infertility Thomas Lloyd and Samera Dean Infertility Definition Causes Referral criteria Assisted reproductive techniques Complications Ethics What is infertility? Woman Reproductive age Has not conceived
Infertility Thomas Lloyd and Samera Dean Infertility Definition Causes Referral criteria Assisted reproductive techniques Complications Ethics What is infertility? Woman Reproductive age Has not conceived
Progesterone. What is progesterone? Important information. Before taking this medicine
 Progesterone Generic Name: progesterone (proe JESS te rone) Brand Names: First Progesterone MC10, Menopause Formula Progesterone, Prometrium What is progesterone? Progesterone is a female hormone important
Progesterone Generic Name: progesterone (proe JESS te rone) Brand Names: First Progesterone MC10, Menopause Formula Progesterone, Prometrium What is progesterone? Progesterone is a female hormone important
PATIENT INFORMATION LEAFLET. Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate
 PATIENT INFORMATION LEAFLET Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate Read all of this leaflet carefully before you start using this medicine because it contains important
PATIENT INFORMATION LEAFLET Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate Read all of this leaflet carefully before you start using this medicine because it contains important
Read all of this leaflet carefully before you start taking this medicine.
 Package Leaflet: Information for the User Tamoxifen 10mg Tablets Tamoxifen Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If
Package Leaflet: Information for the User Tamoxifen 10mg Tablets Tamoxifen Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal myomectomy in leiomyoma management, 77 Abnormal uterine bleeding (AUB) described, 103 105 normal menstrual bleeding vs., 104
Index Note: Page numbers of article titles are in boldface type. A Abdominal myomectomy in leiomyoma management, 77 Abnormal uterine bleeding (AUB) described, 103 105 normal menstrual bleeding vs., 104
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Fertavid 50 IU/0.5 ml solution for injection Fertavid 75 IU/0.5 ml solution for injection Fertavid 100 IU/0.5 ml solution for
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Fertavid 50 IU/0.5 ml solution for injection Fertavid 75 IU/0.5 ml solution for injection Fertavid 100 IU/0.5 ml solution for
Polycystic Ovary Syndrome (PCOS)
 Mr Nabil Haddad M. OBSTET, GYNAEC, FRCOG Consultant Gynaecologist Patient Information Polycystic Ovary Syndrome (PCOS) Mr Nabil Haddad Consultant Gynaecologist What is Polycystic Ovary Syndrome (PCOS)?
Mr Nabil Haddad M. OBSTET, GYNAEC, FRCOG Consultant Gynaecologist Patient Information Polycystic Ovary Syndrome (PCOS) Mr Nabil Haddad Consultant Gynaecologist What is Polycystic Ovary Syndrome (PCOS)?
WOMEN & INFANTS HOSPITAL Providence, RI CONSENT FOR IVF WITH EMBRYO TRANSFER
 *40639* 40639 WOMEN & INFANTS HOSPITAL Providence, RI 02905 CONSENT FOR IVF WITH EMBRYO TRANSFER I have requested treatment by the physicians and (Print Patient s name) staff of the Women & Infants Fertility
*40639* 40639 WOMEN & INFANTS HOSPITAL Providence, RI 02905 CONSENT FOR IVF WITH EMBRYO TRANSFER I have requested treatment by the physicians and (Print Patient s name) staff of the Women & Infants Fertility
12/13/2017. Important references for PCOS. Polycystic Ovarian Syndrome (PCOS) for the Family Physician. 35 year old obese woman
 Polycystic Ovarian Syndrome (PCOS) for the Family Physician Barbara S. Apgar MD, MS Professor or Family Medicine University of Michigan Ann Arbor, Michigan Important references for PCOS Endocrine Society
Polycystic Ovarian Syndrome (PCOS) for the Family Physician Barbara S. Apgar MD, MS Professor or Family Medicine University of Michigan Ann Arbor, Michigan Important references for PCOS Endocrine Society
Polycystic Ovary Syndrome
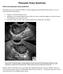 What is the polycystic ovary syndrome? Polycystic Ovary Syndrome The polycystic ovary syndrome (PCOS) is a clinical diagnosis characterized by the presence of two or more of the following features: irregular
What is the polycystic ovary syndrome? Polycystic Ovary Syndrome The polycystic ovary syndrome (PCOS) is a clinical diagnosis characterized by the presence of two or more of the following features: irregular
Western Locality Shared care information ~ Gonadorelin Analogues (Gnrh)
 Western Locality Shared care information ~ Gonadorelin Analogues (Gnrh) Specialist: Please complete the Shared Care letter sending a request to GP (see bottom of the page) April 2013 GP: Please indicate
Western Locality Shared care information ~ Gonadorelin Analogues (Gnrh) Specialist: Please complete the Shared Care letter sending a request to GP (see bottom of the page) April 2013 GP: Please indicate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
41a Pathology: Reproductive System
 41a Pathology: Reproductive System 41a Pathology: Reproductive System! Class Outline" 5 minutes" "Attendance, Breath of Arrival, and Reminders " 10 minutes "Lecture:" 25 minutes "Lecture:" 15 minutes "Active
41a Pathology: Reproductive System 41a Pathology: Reproductive System! Class Outline" 5 minutes" "Attendance, Breath of Arrival, and Reminders " 10 minutes "Lecture:" 25 minutes "Lecture:" 15 minutes "Active
Core Safety Profile. Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% Date of FAR:
 Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
