Original Policy Date
|
|
|
- Gary Ross
- 5 years ago
- Views:
Transcription
1 MP Genetic Testing for Inherited Thrombophilia Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search12:2013 Return to Medical Policy Index Disclaimer Our medical policies are designed for informational purposes only and are not an authorization, or an explanation of benefits, or a contract. Receipt of benefits is subject to satisfaction of all terms and conditions of the coverage. Medical technology is constantly changing, and we reserve the right to review and update our policies periodically. Description Inherited thrombophilias are a group of disorders that predispose to thrombosis. Genetic testing is available for some of these disorders and could potentially assist in the diagnosis and/or management of patients with thrombosis. Venous thromboembolism. The overall U.S. incidence of venous thromboembolism (VTE) is approximately 1 per 1,000 person-years, and the lifetime clinical prevalence is about 5%, accounting for 100,000 deaths annually. (1) Risk is strongly age-related, with the greatest risk in older populations. VTE also recurs frequently; the estimated cumulative incidence of first VTE recurrence is 30% at 10 years. (1) These figures do not separate patients who had known predisposing conditions from those who do not. Risk factors for thrombosis include a variety of clinical and demographic variables, and at least one risk factor can be identified in approximately 80% of patients with a thrombosis. The following list includes the most important risk factors: Malignancy Immobility Surgery Obesity Pregnancy Hormonal therapy with estrogen/progesterones Systemic lupus erythematosus (SLE), and/or other rheumatologic disorders Myeloproliferative disorders Liver dysfunction Nephrotic syndrome
2 Hereditary factors Treatment of thrombosis involves anticoagulation for a minimum of 3 to 6 months. Following this initial treatment period, patients deemed to be at a continued high risk for recurrent thrombosis may be continued on anticoagulation for longer periods, sometimes indefinitely. Anticoagulation is effective in reducing the subsequent risk of thrombosis, but has its own risks of bleeding. Pregnancy is often considered a special condition because of its frequency and the unique considerations of preventing and treating VTE in this setting. Pregnancy is associated with a 5-10-fold increase in the risk for VTE, and the absolute risk of VTE in pregnancy has been estimated to be 1-2 per 1,000 deliveries. (2) In women with a previous history of pregnancyrelated VTE, the risk of recurrent VTE with subsequent pregnancies is increased greatly at approximately 100-fold. (2) Inherited thrombophilia. Inherited thrombophilias are a group of clinical conditions in which there is a genetic variant defect associated with a predisposition to thrombosis. However, not all patients with a genetic predisposition to thrombosis will develop VTE. The presence of inherited thrombophilia will presumably interact with other VTE risk factors to determine an individual s risk of VTE. There are a number of conditions that fall under the classification of inherited thrombophilias, which arise from genetic variants in the genes involved in defects in the coagulation cascade. Inherited thrombophilias include the following abnormalities: Activated protein C resistance (factor V Leiden mutations) Prothrombin gene mutation Protein C deficiency Protein S deficiency Prothrombin deficiency Hyper-homocysteinemia (MTHFR mutations) The most common type of inherited thrombophilia is a factor V Leiden mutation, which accounts for up to 50% of the inherited thrombophilia syndromes. In unselected patients with an idiopathic thrombosis, the rate of factor V Leiden positivity is in the range of 17-24%, (3) compared to a rate of 5-6% in normal controls. The prothrombin gene mutation is found less commonly, in approximately 5-8% of unselected patients with thrombosis, compared to 2-2.5% of normal controls. (3) Genetic testing for gene variants associated with thrombophilias is available for factor V Leiden, the prothrombin gene mutation, and the MTHFR gene. The use of genetic testing for inherited thrombophilia can be considered in several clinical situations. The clinical situations that will be addressed in this policy include the following: Assessment of the risk for thrombosis in asymptomatic patients (screening for inherited thrombophilia) Evaluation of a patient with established thrombosis, in consideration of change in anticoagulant management based on results
3 Evaluation of close relatives of patients with documented inherited thrombophilia, or with a clinical and family history that is consistent with an inherited thrombophilia Evaluation of patients in other situations that are considered high risk for thrombosis, e.g. pregnancy, planned major surgery, or oral contraceptive use. Regulatory Status More than a dozen commercial laboratories currently offer a wide variety of diagnostic procedures for F2 (prothrombin, coagulation factor II), F5 (coagulation factor V), and MTHFR (5, 10-methylenetetrahydrofolate reductase) genetic testing. These tests are available as laboratory developed procedures under the U.S. Food and Drug Administration (FDA) enforcement discretion policy for laboratory developed tests. Policy Genetic testing for inherited thrombophilia, including testing for factor V Leiden mutations, prothrombin gene mutations, and mutations in the MTHFR gene, is considered investigational. Policy Guidelines Specific CPT codes for this testing became available in 2012: 81240: F2 (prothrombin, coagulation factor II)(e.g., hereditary hypercoagulability) gene analysis, 20210G>A variant 81241: F5 (coagulation Factor V)(e.g., hereditary hypercoagulability) gene analysis, Leiden variant 81291: MTHFR (5, 10-methylenetetrahydrofolate reductase)(e.g., hereditary hypercoagulability) gene analysis, common variants (e.g., 677T, 1298C) Rationale This policy was created in April 2012 with literature review covering the period of 1995 through February LITERATURE REVIEW MTHFR Mutation Testing Mutations in the MTHFR gene are associated with hyperhomocysteinemia, which is in turn associated with an increased risk for venous thromboembolism (VTE). However, the clinical utility of testing for homocysteine levels has not been established. There is a large literature base on the association of homocysteine levels with coronary artery disease (CAD), and clinical trials on the impact of lowering homocysteine levels. This body of evidence indicates that testing or treating for homocysteinemia is not associated with improved outcomes. For the association of MTHFR with VTE, the evidence is not definitive. Some studies have shown an association, but others have not. In one of the larger studies, the MEGA study, there was no association of the MTHFR mutation with recurrent VTE. (4) A randomized controlled trial
4 (RCT) published in abstract form reported that there was no reduction in VTE associated with treatment of hyperhomocysteinemia. (5) Conclusions. There is limited published evidence on the utility of testing for MTHFR mutations in patients with VTE or at risk for VTE. Given the available literature, and the lack of clinical utility for serum homocysteine testing in general, it is unlikely that testing for the MTHFR gene will improve outcomes. Factor V Leiden (FVL) and Prothrombin Mutation Testing Analytic Validity Analytic validity refers to the accuracy of detecting a specific mutation when it is present, and excluding it when absent. For an evidence review reported by the Agency for Healthcare Research and Quality (AHRQ) in 2009, the authors performed a comprehensive literature review of studies of analytic validity. (6) There were 41 studies that compared genetic testing for FVL with a reference standard. The concordance between the tests was high, ranging from %, and was 100% in the majority of studies. This evidence report also reviewed 23 studies on the concordance of prothrombin gene mutations with a reference standard and found that nearly all of the studies reported a 100% concordance. There were 12 studies that reported multiplex methods to test simultaneously for both FVL and the prothrombin gene mutation, and all of these studies reported a 100% concordance with reference standards. Bradley et al. (7) evaluated the analytic validity in individual studies and meta-analyses in the setting of pregnancy-related testing. For studies performed in the U.S., the combined analytic sensitivity and specificity for FVL testing was greater than 99%. For the prothrombin mutation, the analytic sensitivity was 98.4% and the analytic specificity was 99.7%. Conclusions. The analytic validity of genetic testing for inherited thrombophilia is high. Published literature reports that the analytic sensitivity and specificity for FVL testing is greater than 99% and that the analytic sensitivity and specificity for the prothrombin gene mutation is greater than 98%. Clinical Validity The clinical validity, and clinical utility, will be discussed for 4 distinct patient populations. These are: Individuals without a personal history of VTE Individuals with a personal history of VTE Family members of individuals with thrombophilia Pregnant women The clinical validity of testing for inherited thrombophilias is best determined by the predictive ability of the test for future thromboembolic events, both in patients with and without prior thromboembolism. The highest quality evidence for this question consists of prospective cohort studies in which patients with and without the mutation are followed for the development of
5 thromboembolism. A few studies are prospective studies nested within RCTs, in which patients with and without mutations are compared. Individuals without a personal history of VTE Individuals with both FVL and prothrombin mutations have an elevated risk of thrombosis compared to the general population. For individuals with the FVL mutation, the risk may be 2-5- fold higher than the general population. In one study of asymptomatic individuals, those with a FVL mutation had an annual incidence of VTE of 0.45%, compared with an incidence of 0.1% in those without the mutation. (8) For the prothrombin mutation, the risk has also been estimated to be 2-5 times greater than the general population. (9) In a meta-analysis of 79 studies, the combined risk ratio was 3.0. (10) Heterozygosity for prothrombin mutation is also associated with an elevated risk of upper extremity thrombosis, estimated to be 5 times that of the general population. (9) Individuals with a personal history of VTE FVL. The 2009 AHRQ evidence report reviewed the evidence on the risk of recurrence for patients with a history of VTE and the FVL mutation. For individuals with a heterozygous FVL mutation, there were a total of 13 studies that compared the risk of recurrence with a mutation to the risk of recurrence with no mutation. Pooled analysis of these 13 studies yielded an odds ratio of 1.56 (95% confidence interval [CI]: ) for recurrent VTE in patients with the FVL mutation. For patients with a homozygous mutation, there were 7 studies that evaluated risk. The pooled odds ratio for recurrent VTE in these studies was 2.65 (95% CI: ). Not all studies are consistent in reporting an increased risk of recurrent VTE in patients with inherited thrombophilia. For example, the Leiden thrombophilia study (LETS) (11) followed 474 patients who had completed a course of anticoagulation for a mean 0f 7.3 years. All patients were tested for thrombophilia at baseline, with 20% found to have FVL mutation and 6% with a prothrombin mutation. There was not an increased recurrence rate for either patients with a FVL mutation or for patients with a prothrombin mutation. For FVL, there was a mild increase in the risk of recurrence that did not reach statistical significance on multivariate analysis (hazard ratio [HR]: 1.3, 95% CI: ). For the prothrombin mutation, there was no increased risk of recurrence (HR: 0.7, 95% CI: ). Factors that did predict recurrence were mainly clinical variables, such as a provoked versus an unprovoked VTE, gender, and oral contraceptive use. One of the larger RCTs that was included in the AHRQ review was the ELATE study, (12) which was an RCT of 738 patients from 16 clinical centers who were randomized to low-intensity versus conventional-intensity treatment with anticoagulation. All patients were tested for inherited thrombophilias, and the risk of recurrence was calculated in patients with and without inherited thrombophilia. For patients with an FVL mutation, there was not an increased risk of recurrence over a mean follow-up of 2.3 years (HR: 0.7, 95% CI: ). Prothrombin gene mutation. The AHRQ evidence report identified 18 studies that evaluated the risk of recurrence in patients heterozygous for the G20210A prothrombin mutation. Some of these studies included only heterozygotes, while other studies combined both heterozygotes and homozygotes. For the 9 studies that included only heterozygotes, the pooled odds ratio for risk of recurrent VTE was 1.45 (95% CI: ). There were 7 studies that did not specify whether patients were homozygous or heterozygous, the combined odds ratio for these studies was 0.73 (95% CI: ).
6 The prothrombin gene mutation is less common, and therefore, the number of patients evaluated in clinical trials and cohort studies is less than with FVL. In the ELATE trial, (12) the risk of recurrent VTE with the prothrombin mutation could not be calculated because there were no recurrences among 60 patients with the prothrombin mutation. In the LETS study, (11) there were 29 patients with a prothrombin mutation. For patients with a prothrombin mutation, there was no increased risk of recurrence (HR: 0.7, 95% CI: ). Factors that did predict recurrence were mainly clinical variables, such as a provoked versus an unprovoked VTE, gender, and oral contraceptive use. Family members of individuals with thrombophilia FVL. The 2009 AHRQ report identified 9 studies that evaluated the risk of VTE in family members of a proband with a heterozygous mutation. The pooled odds ratio for future VTE was 3.49 (95% CI: ). There were 6 studies that evaluated a total of 48 probands with homozygous FVL mutations. The pooled odds ratio for family members of homozygous individuals was 18 (95% CI: ). In one of the larger, more recent studies of VTE risk in family members, Lijfering et al. (13) pooled results from 5 retrospective family studies of thrombophilia. A total of 2,479 relatives of patients with thrombophilia who were themselves also tested for thrombophilia were included. For relatives with FVL mutations, the annual incidence of thrombosis was 0.49% (95% CI: ). In relatives without thrombophilia, the incidence of VTE was approximately 0.05%/yr, and the adjusted relative risk for VTE in relatives with a FVL mutation was 7.5 (95% CI: ). In patients treated with anticoagulation, the annual risk of major bleeding was 0.29% (95% CI: ). Prothrombin mutation. The evidence on the risk for family members of individuals with a prothrombin mutation is less than for FVL, with 5 studies identified by AHRQ evaluating heterozygotes and only one study evaluating homozygotes. For the heterozygote probands, family members had an odds ratio for future VTE of 1.89 (95% CI: ). In the Lijfering family study, (13) relatives with prothrombin mutations had an annual VTE incidence of 0.34% (95% CI: ). In relatives without thrombophilia, the incidence of VTE was approximately 0.05%/yr, and the adjusted relative risk for VTE in relatives with a prothrombin mutation was 5.2 (95% CI: ). Pregnant patients, and other high-risk situations Pregnancy. The evidence on the risk of recurrent pregnancy loss in women with FVL or prothrombin gene mutation comes from case-control studies and cohort studies that are primarily retrospective. Several case-control studies have reported a higher prevalence of factor V Leiden (FVL) (odds ratio [OR]: 2 5) in women with recurrent, unexplained pregnancy loss compared to controls. (14) Retrospective cohort studies have found a 2- to 3-fold increased risk of pregnancy loss in FVL carriers; homozygous carriers have a 2-fold higher risk than heterozygous carriers. Carriers have the highest risk of pregnancy loss in the second and third trimesters. A 2012 systematic review by Bradley et al. (7) analyzed the evidence on the association of FVL and prothrombin mutations with pregnancy loss. These authors identified the highest quality studies, which were cohort studies that: 1) excluded patients with other causes of VTE, 2) tested eligible women for thrombophilia at baseline, 3) reported on subsequent pregnancy outcomes,
7 and 4) compared rates of pregnancy loss between carriers and non-carriers. Four cohort studies met all these criteria; these studies primarily included patients with FVL mutations. Two of the 4 studies reported a significantly increased rate of recurrence for carriers, and 2 studies did not. Combined analysis of these 4 studies yielded a significantly increased odds ratio (OR) for recurrence of pregnancy loss in carriers (OR: 1.93, 95% CI: ). A number of meta-analyses have concluded that there is also an excess risk of pregnancy loss for patients who are heterozygous for the prothrombin mutation, with an elevated risk in the 2-3 range. (9) Oral contraceptives. Oral contraceptive use alone is associated with an approximately 4-fold increase in risk of thrombosis; in combination with factor V Leiden risk multiplies 34-fold in heterozygotes and more than 100-fold in homozygotes. However, the absolute incidence in one published study is estimated to be 28 thrombotic events per 10,000 per year, (15), 2% of which are estimated to be fatal. Hormone replacement therapy. Women using hormone replacement therapy have a 2- to 4-fold increase in their risk of thrombosis. (14) Absolute risk is low and may be restricted to the first year of use. Limited data suggest that women using selective estrogen receptor modulators (e.g., tamoxifen) may have a similarly increased risk. (14) Conclusions. The clinical validity of genetic testing for thrombophilia has been evaluated by assessing the association between thrombophilia status and VTE in a variety of clinical populations. For the populations discussed here, clinical validity has been reported in numerous case-control and cohort studies. The presence of an FVL or a prothrombin gene mutation is associated with an increased risk for subsequent VTE across a variety of populations studied. However, the magnitude of the association is relatively modest, with odds ratios most commonly between 1 and 2, except for the case of family members of individuals with inherited thrombophilia, in which the odds ratios are somewhat higher. Clinical Utility The clinical utility of genetic testing depends on the ability of testing results to change management that results in improved outcomes. The clinical utility of genetic testing for thrombophilia is considered in the context of the overall risk of thromboembolism and the risk/benefit ratio of treatment, primarily with anticoagulants. The following factors are part of the decision-making process on whether to test: The overall low incidence of thromboembolism in the general population The modest increased risk associated with most forms of inherited thrombophilia, meaning that the absolute risk of thrombosis in patients with inherited thrombophilia is still relatively low. The potential risk of prophylactic treatment, especially the bleeding risk with anticoagulation. This risk may outweigh the benefit in patients with a relatively low absolute risk of thrombosis Individuals without a personal history of VTE No published studies were identified that directly evaluated the clinical utility of screening asymptomatic individuals for inherited thrombophilia. However, it is unlikely that screening
8 asymptomatic individuals will result in a net health benefit, as prophylactic anticoagulation is likely to have more harms than benefits. The risk of major bleeding with full anticoagulation is in the range of 1%/year, therefore the number of major bleeding episodes may far exceed the number of VTEs prevented. Knowledge of thrombophilia status may lead to behaviors that reduce the risk of VTE, such as avoidance of prolonged immobility, but this is unproven. Individuals with a personal history of VTE The MEGA study (16) was a large, population-based, case-control study that evaluated whether testing for thrombophilia in patients with a first episode of VTE was associated with a decrease in the recurrence rate. The MEGA database consisted of 5,051 patients between the ages of years with a first episode of VTE. Researchers identified a total of 197 patients with a recurrence of VTE and matched these patients on age, sex, year of VTE, and geographic region with 324 patients who were free of recurrent VTE. Recurrence rate for VTE was similar in patients who were tested for thrombophilia compared to patients who were not tested (OR: 1.2, 95% CI: ). The presence of FVL or the prothrombin gene mutation was not associated with an increased recurrence rate, with an odds ratio of 0.8 (95% CI: ). One study surveyed 112 primary care physicians about the impact of FVL testing in patients with VTE. (17) A majority of physicians indicated that they would use results in clinical practice, with 82% reporting that they would use results to counsel patients on risk of recurrence and 67% reporting that they would use results to make treatment changes. However, physician confidence in their decisions was not high, including decisions to order FVL testing. Family members of individuals with thrombophilia There are no comparative trials of testing versus no testing in relatives of individuals with thrombophilia. The clinical utility of testing depends on the balance between the benefit of altering management as a result of knowledge of mutation status versus the risk of bleeding with intensification of anticoagulation. This risk benefit is unknown, as previously discussed. The absolute risk of VTE remains low even in patients in inherited thrombophilia, and the potential risks of prophylactic treatment with anticoagulants may outweigh the benefit. Pregnant patients, and other high-risk situations No studies directly evaluated clinical utility of thrombophilia testing in pregnant patients. The clinical utility of testing depends on the efficacy of potential treatments in decreasing fetal loss, versus the risks of treatment. Potential treatments in pregnancy include aspirin, low-dose unfractionated or low molecular-weight heparin, and full-dose heparin. The benefits of these treatments in reducing pregnancy loss are questionable. At least two RCTs have reported that there is not a significant reduction in risk with aspirin or heparin therapy. (18,19) In addition, several meta-analyses also report that there is insufficient evidence to conclude that these interventions reduce recurrent pregnancy loss in patients with FVL or prothrombin mutations. (7) In contrast, the risks of anticoagulation are real, including bleeding, thrombocytopenia, and allergic reactions. There are also additional costs and inconvenience associated with these treatments. Bradley et al. (7) reviewed the evidence on clinical utility and concluded that the evidence is adequate to conclude that there are no safe and effective treatments to reduce recurrent pregnancy loss in women with inherited thrombophilia. They also concluded that the certainty of the evidence was moderate that treatment resulted in a net harm.
9 Conclusions. The clinical utility of testing for FVL or prothrombin mutations has not been demonstrated. While the presence of inherited thrombophilia increases the risk for subsequent VTE events, the increase is modest and the absolute risk of thrombosis remains low. Available prophylactic treatments, such as anticoagulation, have defined risks of major bleeding and other adverse events that may outweigh the reduction in VTE and therefore result in a net harm. The currently available evidence has not defined a role for thrombophilia testing for decisions on the length of anticoagulation treatment. Clinical Input Received through Specialty Medical Societies and Academic Medical Centers While the various physician specialty societies and academic medical centers may collaborate with and make recommendations during this process, through the provision of appropriate reviewers, input received does not represent an endorsement or position statement by the physician specialty societies or academic medical centers, unless otherwise noted. In response to requests, input was received from 4 physician specialty societies (6 reviewers) and 6 academic medical centers, for a total of 12 reviewers, while this policy was under review for July Overall, input was mixed, and there was not uniform consensus that genetic testing for thrombophilia was medically necessary for any of the specific clinical situations included. Several reviewers noted that testing could be useful in isolated instances, but were unable to define the specific criteria that could be used for testing. Summary Genetic testing is available for a number of types of inherited thrombophilia, including mutations in the MTHFR gene, the FVL gene, and the prothrombin gene. For MTHFR testing, the clinical validity and clinical utility of genetic testing is uncertain. Since the clinical utility of testing for elevated serum homocysteine itself has not been established, the utility of genetic testing has also not been established. For FVL and prothrombin gene testing, clinical validity has been established in a variety of clinical situations, by the association of genetic status with subsequent risk of VTE. Increased risk of VTE has been demonstrated for asymptomatic patients, patients with a personal history of VTE, family members of a patient with established inherited thrombophilia, and pregnant women. However, in most reports, the magnitude of this association is modest, resulting in a relatively low absolute rate of VTE even in patients with a genetic mutation. The clinical utility of genetic testing for thrombophilia is less certain. Surveys of physicians indicate that a substantial number order thrombophilia testing with the intent of influencing management, but the specific management changes and the impact of those management changes on outcomes is uncertain. According to the existing evidence and recent guidelines, the presence of inherited thrombophilia is not an important factor in determining the optimum length of anticoagulation in patients with VTE. For other clinical situations, given the low absolute risk of VTE, and the defined risks of anticoagulation, it is not possible to define a clinical situation in which the benefit of testing clearly outweighs the risk. Clinical vetting performed in 2012 did not identify consensus for testing in any of the clinical scenarios that were outlined. Because of the lack of documented clinical utility, and the lack of consensus on clinical vetting as to which populations benefit from testing, genetic testing for inherited thrombophilia is considered investigational. Practice Guidelines and Position Statements
10 There are many guidelines and position statements on testing for thrombophilia published over the last 20 years. These guidelines have evolved with time, often do not agree with each other, and do not typically give specific parameters for when to perform genetic testing. The following are examples of guidelines published in the last 5 years, from the U.S., and developed by major specialty societies. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group published recommendations in 2011 that addressed genetic testing for Factor V Leiden mutations and Prothrombin mutations. (20) This publication included the following recommendations on the clinical utility of genetic testing: There is no evidence that knowledge of FVL/PT mutation status in patients with VTE affects anticoagulation treatment to avoid recurrence There is convincing evidence that anticoagulation beyond three months reduces recurrence of VTE, regardless of mutation status There is no evidence that knowledge of FVL/PT mutation status among asymptomatic family members of patients with VTE leads to anticoagulation aimed at avoiding initial episodes of VTE The American College of Chest Physicians published evidence-based guidelines for the treatment of thromboembolic disease in (10) These guidelines stated the following concerning genetic testing for thrombophilia: The presence of hereditary thrombophilia has not been used as a major factor to guide duration of anticoagulation for VTE in these guidelines because evidence from prospective studies suggests that these factors are not major determinants of the risk of recurrence. The American College of Obstetricians and Gynecologists published clinical management guidelines for inherited thrombophilias in pregnancy in (21) These guidelines stated that testing for inherited thrombophilias is controversial, but could be considered for pregnant women in the following situations: A personal history of venous thromboembolism that was associated with a nonrecurrent risk factor (e.g., fractures, surgery, and prolonged immobilization). A first-degree relative with a history of high-risk thrombophilia or venous thromboembolism before age 50 years in the absence of other risk factors in as much as affected women should receive prophylaxis. The guidelines also state that: Testing for inherited thrombophilias should include FVL, Prothrombin gene mutation, and tests for deficiencies in antithrombin, protein S and protein C Testing for inherited thrombophilias in women who have experienced recurrent fetal loss or placental abruption is not recommended. Medicare National Coverage None
11 References: Heit JA, Silverstein MD, Mohr DN et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001; 86(1): Baglin T, Gray E, Greaves M et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149(2): Bauer KA, Lip GYH. Evaluation of the patient with established venous thrombosis. In: Leung LLK LS, ed. Up-To-Date Online Bezemer ID DC, Vos HL, et al. No association between the common MTHFR 677C>T polymorphism and venous thrombosis: results from the MEGA study. Arch Intern Med 2007; 167: den Heijer M WH, Blom HJ, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: a randomized, placebo-controlled, double-blind trial. Blood 2007; 109: Segal JB, Brotman DJ, Emadi A et al. Outcomes of genetic testing in adults with a history of venous thromboembolism. Evid Rep Technol Assess (Full Rep) 2009; (180): Bradley LA, Palomaki GE, Bienstock J et al. Can Factor V Leiden and prothrombin G20210A testing in women with recurrent pregnancy loss result in improved pregnancy outcomes?: results from a targeted evidence-based review. Genet Med 2012; 14(1): Middeldorp S, Henkens CM, Koopman MM et al. The incidence of venous thromboembolism in family members of patients with factor V Leiden mutation and venous thrombosis. Ann Intern Med 1998; 128(1): Kujovich JL. Prothrombin-Related Thrombophilia. In: Pagon RA, Bird TD, Dolan CR et al., eds. GeneReviews. Seattle (WA):2011. Available online at: Last accessed April Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A metaanalysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost 2009; 102(2): Christiansen SC, Cannegieter SC, Koster T et al. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293(19): Kearon C, Julian JA, Kovacs MJ et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112(12): Lijfering WM, Brouwer JL, Veeger NJ et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113(21):
12 14. Press RD, Bauer KA, Kujovich JL et al. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126(11): Vandenbroucke JP, Koster T, Briet E et al. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet 1994; 344(8935): Coppens M, Reijnders JH, Middeldorp S et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6(9): Hindorff LA, Burke W, Laberge AM et al. Motivating factors for physician ordering of factor V Leiden genetic tests. Arch Intern Med 2009; 169(1): Clark P, Walker ID, Langhorne P et al. SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized controlled trial of low-molecular-weight heparin and low-dose aspirin in women with recurrent miscarriage. Blood 2010; 115(21): Kaandorp SP, Goddijn M, van der Post JA et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010; 362(17): EGAPP Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med 2011; 13(1): ACOG. American College of Obstetrics and Gynecology. Practice bulletin no. 113: inherited thrombophilias in pregnancy. Obstet Gynecol 2010; 116(1): Codes Number Description CPT ICD-9-CM Diagnosis ICD-10-CM (effective 10/1/13) ICD-10-PCS (effectve F2 (prothrombin, coagulation factor II)(e.g., hereditary hypercoagulability) gene analysis, 20210G>A variant F5 (coagulation Factor V)(e.g., hereditary hypercoagulability) gene analysis, Leiden variant MTHFR (5, 10-methylenetetrahydrofolate reductase)(e.g., hereditary hypercoagulability) gene analysis, common variants (e.g., 677T, 1298C) Investigational for all relevent diagnoses Investigational for all relevent diagnoses D68.51 Activated protein C resistance (includes Factor V Leiden mutation) D68.52 Prothrobin gene mutation D68.59 Other primary thrombophilia Not applicable. ICD-10-PCS codes are only
13 10/1/13) used for inpatient services. There are no ICD procedure codes for laboratory tests. Index F2 Prothrombin Coagulation Factor II, Genetic Testing Factor V Leiden, Genetic Testing Hypercoagulability, Genetic Testing MTHFR, Genetic Testing
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Genetic Testing for Inherited Thrombophilia Description Inherited thrombophilias are a group of disorders that predispose
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Genetic Testing for Inherited Thrombophilia Description Inherited thrombophilias are a group of disorders that predispose
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Thrombophilia: To test or not to test
 Kenneth Bauer, MD Harvard Medical School, Boston, MA Professor of Medicine VA Boston Healthcare System Chief, Hematology Section Beth Israel Deaconess Medical Center, Boston, MA Director, Thrombosis Clinical
Kenneth Bauer, MD Harvard Medical School, Boston, MA Professor of Medicine VA Boston Healthcare System Chief, Hematology Section Beth Israel Deaconess Medical Center, Boston, MA Director, Thrombosis Clinical
Thrombophilia. Stephan Moll, MD Medicine, Heme-Coag UNC Chapel Hill, NC. GASCO Atlanta Sept 8 th, Disclosures. Conflicts of interest: NONE
 LA APLA 1 3 ACA Anti-ß2-GP I 2 45 Thrombophilia Stephan Moll, MD Medicine, Heme-Coag UNC Chapel Hill, NC GASCO Atlanta Sept 8 th, 2017 Disclosures Conflicts of interest: NONE Off-label product use discussion:
LA APLA 1 3 ACA Anti-ß2-GP I 2 45 Thrombophilia Stephan Moll, MD Medicine, Heme-Coag UNC Chapel Hill, NC GASCO Atlanta Sept 8 th, 2017 Disclosures Conflicts of interest: NONE Off-label product use discussion:
Thrombophilia. Diagnosis and Management. Kevin P. Hubbard, DO, FACOI
 Thrombophilia Diagnosis and Management Kevin P. Hubbard, DO, FACOI Clinical Professor of Medicine Kansas City University of Medicine and Biosciences-College of Osteopathic Medicine Kansas City, Missouri
Thrombophilia Diagnosis and Management Kevin P. Hubbard, DO, FACOI Clinical Professor of Medicine Kansas City University of Medicine and Biosciences-College of Osteopathic Medicine Kansas City, Missouri
Chapter. A higher risk of recurrent venous thrombosis in men is due to hormonal risk factors in women in thrombophilic families
 Chapter A higher risk of recurrent venous thrombosis in men is due to hormonal risk factors in women in thrombophilic families Willem M. Lijfering Nic J.G.M. Veeger Saskia Middeldorp Karly Hamulyák Martin
Chapter A higher risk of recurrent venous thrombosis in men is due to hormonal risk factors in women in thrombophilic families Willem M. Lijfering Nic J.G.M. Veeger Saskia Middeldorp Karly Hamulyák Martin
Choosing Wisely: If, When, What, and Who to Test for Thrombophilia
 Choosing Wisely: If, When, What, and Who to Test for Thrombophilia Nicole Dodge Zantek, MD, PhD Medical Director, Special Coagulation Laboratory University of Minnesota Disclosures Financial interests
Choosing Wisely: If, When, What, and Who to Test for Thrombophilia Nicole Dodge Zantek, MD, PhD Medical Director, Special Coagulation Laboratory University of Minnesota Disclosures Financial interests
Is Thrombophilia Testing Useful?
 CONSULTATIVE HEMATOLOGY I: COMMON QUESTIONS IN THROMBOSIS CONSULTS Is Thrombophilia Testing Useful? Saskia Middeldorp 1 1 Department of Vascular Medicine, Academic Medical Center, Amsterdam, The Netherlands
CONSULTATIVE HEMATOLOGY I: COMMON QUESTIONS IN THROMBOSIS CONSULTS Is Thrombophilia Testing Useful? Saskia Middeldorp 1 1 Department of Vascular Medicine, Academic Medical Center, Amsterdam, The Netherlands
Scott M. Stevens, MD. Co-Director, Thrombosis Clinic. Associate Professor of Clinical Medicine
 Scott M. Stevens, MD Co-Director, Thrombosis Clinic Intermountain Medical Center Associate Professor of Clinical Medicine The University of Utah School of Medicine No Relevant Financial Relationships Research
Scott M. Stevens, MD Co-Director, Thrombosis Clinic Intermountain Medical Center Associate Professor of Clinical Medicine The University of Utah School of Medicine No Relevant Financial Relationships Research
Inherited Thrombophilia Testing. George Rodgers, MD, PhD Kristi Smock MD
 Inherited Thrombophilia Testing George Rodgers, MD, PhD Kristi Smock MD Prevalence and risk associated with inherited thrombotic disorders Inherited Risk Factor % General Population % Patients w/ Thrombosis
Inherited Thrombophilia Testing George Rodgers, MD, PhD Kristi Smock MD Prevalence and risk associated with inherited thrombotic disorders Inherited Risk Factor % General Population % Patients w/ Thrombosis
Citation for published version (APA): van Vlijmen, E. F. W. (2016). The pill and thrombosis. [Groningen]: Rijksuniversiteit Groningen.
![Citation for published version (APA): van Vlijmen, E. F. W. (2016). The pill and thrombosis. [Groningen]: Rijksuniversiteit Groningen. Citation for published version (APA): van Vlijmen, E. F. W. (2016). The pill and thrombosis. [Groningen]: Rijksuniversiteit Groningen.](/thumbs/96/128289916.jpg) University of Groningen The pill and thrombosis van Vlijmen, Elizabeth Femma Willemien IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
University of Groningen The pill and thrombosis van Vlijmen, Elizabeth Femma Willemien IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
Genetic Tests for the Better Outcome of VTE? 서울대학교병원혈액종양내과윤성수
 Genetic Tests for the Better Outcome of VTE? 서울대학교병원혈액종양내과윤성수 Thrombophilia A hereditary or acquired disorder predisposing to thrombosis Questions Why should we test? Who should we test For what disorders?
Genetic Tests for the Better Outcome of VTE? 서울대학교병원혈액종양내과윤성수 Thrombophilia A hereditary or acquired disorder predisposing to thrombosis Questions Why should we test? Who should we test For what disorders?
Thrombophilia testing: who is it good for? F.R. Rosendaal, Leiden
 Thrombophilia testing: who is it good for? F.R. Rosendaal, Leiden Inaugural meeting of Iranian Society on Thrombosis and Haemostasis Mofid Children Hospital Tehran, 24 December 2015 Chest. 2012;141(2_suppl):e48S-e801S
Thrombophilia testing: who is it good for? F.R. Rosendaal, Leiden Inaugural meeting of Iranian Society on Thrombosis and Haemostasis Mofid Children Hospital Tehran, 24 December 2015 Chest. 2012;141(2_suppl):e48S-e801S
Venous thromboembolism (VTE) consists of deep vein
 Clinical Utility of Factor V Leiden (R506Q) Testing for the Diagnosis and Management of Thromboembolic Disorders Richard D. Press, MD, PhD; Kenneth A. Bauer, MD; Jody L. Kujovich, MD; John A. Heit, MD
Clinical Utility of Factor V Leiden (R506Q) Testing for the Diagnosis and Management of Thromboembolic Disorders Richard D. Press, MD, PhD; Kenneth A. Bauer, MD; Jody L. Kujovich, MD; John A. Heit, MD
THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO
 THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO DISCLAIMER I m a pediatrician I will be discussing this issue primarily from a pediatric perspective
THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO DISCLAIMER I m a pediatrician I will be discussing this issue primarily from a pediatric perspective
Duration of Therapy for Venous Thromboembolism
 Duration of Therapy for Venous Thromboembolism Michael B Streiff, MD FACP Associate Professor of Medicine and Pathology Medical Director, Johns Hopkins Anticoagulation Service Chairman, VTE Guideline Committee
Duration of Therapy for Venous Thromboembolism Michael B Streiff, MD FACP Associate Professor of Medicine and Pathology Medical Director, Johns Hopkins Anticoagulation Service Chairman, VTE Guideline Committee
General. Recommendations. Guideline Title. Bibliographic Source(s) Guideline Status. Major Recommendations
 General Guideline Title Prevention of deep vein thrombosis and pulmonary embolism. Bibliographic Source(s) American College of Obstetricians and Gynecologists (ACOG). Prevention of deep vein thrombosis
General Guideline Title Prevention of deep vein thrombosis and pulmonary embolism. Bibliographic Source(s) American College of Obstetricians and Gynecologists (ACOG). Prevention of deep vein thrombosis
Should Patients with Venous Thromboembolism Be Screened for Thrombophilia?
 REVIEW Should Patients with Venous Thromboembolism Be Screened for Thrombophilia? James E. Dalen, MD, MPH University of Arizona, Tucson. ABSTRACT In the mid-19th century, Virchow identified hypercoagulability
REVIEW Should Patients with Venous Thromboembolism Be Screened for Thrombophilia? James E. Dalen, MD, MPH University of Arizona, Tucson. ABSTRACT In the mid-19th century, Virchow identified hypercoagulability
Are there still any valid indications for thrombophilia screening in DVT?
 Carotid artery stenosis and risk of stroke Are there still any valid indications for thrombophilia screening in DVT? Armando Mansilha MD, PhD, FEBVS Faculty of Medicine of University of Porto Munich, 2016
Carotid artery stenosis and risk of stroke Are there still any valid indications for thrombophilia screening in DVT? Armando Mansilha MD, PhD, FEBVS Faculty of Medicine of University of Porto Munich, 2016
Molecular mechanisms & clinical consequences. of prothrombin mutations. A.J. Hauer
 Molecular mechanisms & clinical consequences of prothrombin mutations A.J. Hauer 07-12-2018 Prothrombin & the coagulation cascade Coagulation factor II, thrombin. Prothrombin is synthesized in the liver
Molecular mechanisms & clinical consequences of prothrombin mutations A.J. Hauer 07-12-2018 Prothrombin & the coagulation cascade Coagulation factor II, thrombin. Prothrombin is synthesized in the liver
Menopausal Hormone Therapy & Haemostasis
 Menopausal Hormone Therapy & Haemostasis The Haematologist Perspective Dr. Batia Roth-Yelinek Coagulation unit Hadassah MC Menopausal Hormone Therapy & Hemostasis Hemostatic mechanism Mechanism of estrogen
Menopausal Hormone Therapy & Haemostasis The Haematologist Perspective Dr. Batia Roth-Yelinek Coagulation unit Hadassah MC Menopausal Hormone Therapy & Hemostasis Hemostatic mechanism Mechanism of estrogen
Optimal Utilization of Thrombophilia Testing
 Optimal Utilization of Thrombophilia Testing Rajiv K. Pruthi, MBBS Special Coagulation Laboratory & Comprehensive Hemophilia Center Division of Hematology/Internal Medicine Dept of Laboratory Medicine
Optimal Utilization of Thrombophilia Testing Rajiv K. Pruthi, MBBS Special Coagulation Laboratory & Comprehensive Hemophilia Center Division of Hematology/Internal Medicine Dept of Laboratory Medicine
Outcomes of Genetic Testing in Adults with a History of Venous Thromboembolism
 Evidence Report/Technology Assessment Number 180 Outcomes of Genetic Testing in Adults with a History of Venous Thromboembolism Prepared for: Agency for Healthcare Research and Quality U.S. Department
Evidence Report/Technology Assessment Number 180 Outcomes of Genetic Testing in Adults with a History of Venous Thromboembolism Prepared for: Agency for Healthcare Research and Quality U.S. Department
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT
 VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
CADTH. August [Draft] Draft for Consultation 1
![CADTH. August [Draft] Draft for Consultation 1 CADTH. August [Draft] Draft for Consultation 1](/thumbs/80/81468248.jpg) CADTH August 2014 [Draft] Effectiveness of Factor V Leiden and Prothrombin Mutation Testing in Patients Presenting with Unprovoked First Thromboembolic Episode: Systematic Review and Economic Analysis
CADTH August 2014 [Draft] Effectiveness of Factor V Leiden and Prothrombin Mutation Testing in Patients Presenting with Unprovoked First Thromboembolic Episode: Systematic Review and Economic Analysis
Laboratory Evaluation of Venous Thrombosis Risk
 Laboratory Evaluation of Venous Thrombosis Risk Dorothy M. Adcock, MD Volume 17, Number 12 December 2003 Objective: The reader will be able to discuss the concepts of risk factor, risk potential and thrombotic
Laboratory Evaluation of Venous Thrombosis Risk Dorothy M. Adcock, MD Volume 17, Number 12 December 2003 Objective: The reader will be able to discuss the concepts of risk factor, risk potential and thrombotic
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Genetics. Risk of Recurrent Venous Thrombosis in Homozygous Carriers and Double Heterozygous Carriers of Factor V Leiden and Prothrombin G20210A
 Genetics Risk of Recurrent Venous Thrombosis in Homozygous Carriers and Double Heterozygous Carriers of Factor V Leiden and Prothrombin G20210A Willem M. Lijfering, MD, PhD; Saskia Middeldorp, MD, PhD;
Genetics Risk of Recurrent Venous Thrombosis in Homozygous Carriers and Double Heterozygous Carriers of Factor V Leiden and Prothrombin G20210A Willem M. Lijfering, MD, PhD; Saskia Middeldorp, MD, PhD;
Diagnosis and management of heritable thrombophilias
 Link to this article online for CPD/CME credits Diagnosis and management of heritable thrombophilias Peter MacCallum, 1 2 Louise Bowles, 2 David Keeling 3 1 Wolfson Institute of Preventive Medicine, Barts
Link to this article online for CPD/CME credits Diagnosis and management of heritable thrombophilias Peter MacCallum, 1 2 Louise Bowles, 2 David Keeling 3 1 Wolfson Institute of Preventive Medicine, Barts
Chapter. Absolute risk of venous and arterial thrombosis in HIV-infected patients and effects of combination antiretroviral therapy
 Chapter Absolute risk of venous and arterial thrombosis in HIV-infected patients and effects of combination antiretroviral therapy Willem M. Lijfering Min Ki ten Kate Herman G. Sprenger Jan van der Meer
Chapter Absolute risk of venous and arterial thrombosis in HIV-infected patients and effects of combination antiretroviral therapy Willem M. Lijfering Min Ki ten Kate Herman G. Sprenger Jan van der Meer
University of Groningen
 University of Groningen The impact of a male or female thrombotic family history on contraceptive counseling van Vlijmen, E. F. W.; Veeger, Nicolaas; Middeldorp, S.; Hamulyak, K.; Prins, M. H.; Nelemans,
University of Groningen The impact of a male or female thrombotic family history on contraceptive counseling van Vlijmen, E. F. W.; Veeger, Nicolaas; Middeldorp, S.; Hamulyak, K.; Prins, M. H.; Nelemans,
Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life
 Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life Sources of Risk for Venous Diseases Pulmonary embolism (thrombus) Bleeding from anticoagulation Mortality from underlying disease Chronic
Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life Sources of Risk for Venous Diseases Pulmonary embolism (thrombus) Bleeding from anticoagulation Mortality from underlying disease Chronic
Clinical implications of deficiencies of protein S, protein C or antithrombin Brouwer, Jan Leendert Pouwel
 University of Groningen Clinical implications of deficiencies of protein S, protein C or antithrombin Brouwer, Jan Leendert Pouwel IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Clinical implications of deficiencies of protein S, protein C or antithrombin Brouwer, Jan Leendert Pouwel IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Potpourri of Hematology Oncology. Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy
 Potpourri of Hematology Oncology Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy Lifestyle Modifications to Decrease the Risk of Colorectal Cancer Estimates for 2018 American
Potpourri of Hematology Oncology Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy Lifestyle Modifications to Decrease the Risk of Colorectal Cancer Estimates for 2018 American
Approach to Thrombosis
 Approach to Thrombosis Theera Ruchutrakool, M.D. Division of Hematology Department of Medicine Siriraj Hospital Faculty of Medicine Mahidol University Approach to Thrombosis Thrombosis: thrombus formation
Approach to Thrombosis Theera Ruchutrakool, M.D. Division of Hematology Department of Medicine Siriraj Hospital Faculty of Medicine Mahidol University Approach to Thrombosis Thrombosis: thrombus formation
Stanford University H IGH VALUE C ARE: OPTIMAL A PPROACH. Andre Kumar MD Tyler Johnson MD Neil Shah MD Jason Hom MD
 Stanford University H IGH VALUE C ARE: OPTIMAL A PPROACH TO T HROMBOPHILIA W ORKUPS & FRESH F ROZEN P LASMA U SE Andre Kumar MD Tyler Johnson MD Neil Shah MD Jason Hom MD Learning Objectives AFTER COMPLETION
Stanford University H IGH VALUE C ARE: OPTIMAL A PPROACH TO T HROMBOPHILIA W ORKUPS & FRESH F ROZEN P LASMA U SE Andre Kumar MD Tyler Johnson MD Neil Shah MD Jason Hom MD Learning Objectives AFTER COMPLETION
CLINICIANS TEST FOR PROTHROMbotic
 CLINICAL REVIEW CLINICIAN S CORNER Predictive Value of Factor V Leiden and Prothrombin G20210A in Adults With Venous Thromboembolism and in Family Members of Those With a Mutation A Systematic Review Jodi
CLINICAL REVIEW CLINICIAN S CORNER Predictive Value of Factor V Leiden and Prothrombin G20210A in Adults With Venous Thromboembolism and in Family Members of Those With a Mutation A Systematic Review Jodi
Recurrence risk after anticoagulant treatment of limited duration for late, second venous thromboembolism
 ARTICLES Coagulation & its Disorders Recurrence risk after anticoagulant treatment of limited duration for late, second venous thromboembolism Tom van der Hulle, Melanie Tan, Paul L. den Exter, Mark J.G.
ARTICLES Coagulation & its Disorders Recurrence risk after anticoagulant treatment of limited duration for late, second venous thromboembolism Tom van der Hulle, Melanie Tan, Paul L. den Exter, Mark J.G.
VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK?
 VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK? Ayman El-Menyar (1), MD, Hassan Al-Thani (2),MD (1)Clinical Research Consultant, (2) Head of Vascular Surgery, Hamad General Hospital
VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK? Ayman El-Menyar (1), MD, Hassan Al-Thani (2),MD (1)Clinical Research Consultant, (2) Head of Vascular Surgery, Hamad General Hospital
Duration of Anticoagulant Therapy. Linda R. Kelly PharmD, PhC, CACP September 17, 2016
 Duration of Anticoagulant Therapy Linda R. Kelly PharmD, PhC, CACP September 17, 2016 Conflicts of Interest No conflicts of interest to report Objectives At the end of the program participants will be
Duration of Anticoagulant Therapy Linda R. Kelly PharmD, PhC, CACP September 17, 2016 Conflicts of Interest No conflicts of interest to report Objectives At the end of the program participants will be
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
The risk of recurrence in women with venous thromboembolism while using estrogens: a prospective cohort study
 Journal of Thrombosis and Haemostasis, 12: 635 640 DOI: 10.1111/jth.12528 ORIGINAL ARTICLE The risk of recurrence in women with venous thromboembolism while using estrogens: a prospective cohort study
Journal of Thrombosis and Haemostasis, 12: 635 640 DOI: 10.1111/jth.12528 ORIGINAL ARTICLE The risk of recurrence in women with venous thromboembolism while using estrogens: a prospective cohort study
Venous Thrombosis in Asia
 Venous Thrombosis in Asia Pantep Angchaisuksiri, M.D. Professor of Medicine, Mahidol University, Thailand Adjunct Associate Professor, University of North Carolina, Chapel Hill, USA Venous Thromboembolism
Venous Thrombosis in Asia Pantep Angchaisuksiri, M.D. Professor of Medicine, Mahidol University, Thailand Adjunct Associate Professor, University of North Carolina, Chapel Hill, USA Venous Thromboembolism
Thrombosis. By Dr. Sara Mohamed Abuelgasim
 Thrombosis By Dr. Sara Mohamed Abuelgasim 1 Thrombosis Unchecked, blood coagulation would lead to dangerous occlusion of blood vessels if the protective mechanisms of coagulation factor inhibitors, blood
Thrombosis By Dr. Sara Mohamed Abuelgasim 1 Thrombosis Unchecked, blood coagulation would lead to dangerous occlusion of blood vessels if the protective mechanisms of coagulation factor inhibitors, blood
Diagnosis and Treatment of Deep Venous Thrombosis and Pulmonary Embolism
 Agency for Healthcare Research and Quality Evidence Report/Technology Assessment Diagnosis and Treatment of Deep Venous Thrombosis and Pulmonary Embolism Summary Number 68 Overview Venous thromboembolism
Agency for Healthcare Research and Quality Evidence Report/Technology Assessment Diagnosis and Treatment of Deep Venous Thrombosis and Pulmonary Embolism Summary Number 68 Overview Venous thromboembolism
DISCLOSURE. Presented by: Merav Sendowski, MD Oregon Health and Science University
 Thrombophilia! DISCLOSURE Presented by: Merav Sendowski, MD Oregon Health and Science University Created by: Thomas Deloughery, MD Oregon Health and Science University Current Relevant Financial Relationship(s)
Thrombophilia! DISCLOSURE Presented by: Merav Sendowski, MD Oregon Health and Science University Created by: Thomas Deloughery, MD Oregon Health and Science University Current Relevant Financial Relationship(s)
THROMBOPHILIA SCREENING
 THROMBOPHILIA SCREENING Introduction The regulation of haemostasis Normally, when a clot occurs, it exactly occurs where it has to be and does not grow more than necessary due to the action of the haemostasis
THROMBOPHILIA SCREENING Introduction The regulation of haemostasis Normally, when a clot occurs, it exactly occurs where it has to be and does not grow more than necessary due to the action of the haemostasis
Predicting the risk of recurrent venous thromboembolism (VTE)
 J Thromb Thrombolysis (2015) 39:353 366 DOI 10.1007/s19-015-1188-4 Predicting the risk of recurrent venous thromboembolism (VTE) Michael B. Streiff Published online: 27 February 2015 Ó Springer Science+Business
J Thromb Thrombolysis (2015) 39:353 366 DOI 10.1007/s19-015-1188-4 Predicting the risk of recurrent venous thromboembolism (VTE) Michael B. Streiff Published online: 27 February 2015 Ó Springer Science+Business
Session Chair: Andrew I. Schafer, MD Speakers: Mary Cushman, MD, MSc; Paolo Prandoni, MD, PhD; and Thomas L. Ortel, MD, PhD
 Thrombosis II Session Chair: Andrew I. Schafer, MD Speakers: Mary Cushman, MD, MSc; Paolo Prandoni, MD, PhD; and Thomas L. Ortel, MD, PhD Inherited Risk Factors for Venous Thrombosis Mary Cushman Venous
Thrombosis II Session Chair: Andrew I. Schafer, MD Speakers: Mary Cushman, MD, MSc; Paolo Prandoni, MD, PhD; and Thomas L. Ortel, MD, PhD Inherited Risk Factors for Venous Thrombosis Mary Cushman Venous
Focus: l embolia polmonare Per quanto la terapia anticoagulante orale? Giulia Magnani 27 Gennaio, 2018
 Focus: l embolia polmonare Per quanto la terapia anticoagulante orale? Giulia Magnani 27 Gennaio, 2018 NO DISCLOSURE Pulmonary Embolism Venous thromboembolism (VT) is the third most common cause of cardiovascular
Focus: l embolia polmonare Per quanto la terapia anticoagulante orale? Giulia Magnani 27 Gennaio, 2018 NO DISCLOSURE Pulmonary Embolism Venous thromboembolism (VT) is the third most common cause of cardiovascular
In which direction, and how aggressively,
 Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Evaluating idiopathic venous thromboembolism: What is necessary, what is not Charles F. S. Locke, MD Johns Hopkins Community
Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Evaluating idiopathic venous thromboembolism: What is necessary, what is not Charles F. S. Locke, MD Johns Hopkins Community
THE INCIDENCE RATE OF A FIRST
 ORIGINAL CONTRIBUTION Thrombophilia, Clinical Factors, and Recurrent Venous Thrombotic Events Sverre C. Christiansen, MD Suzanne C. Cannegieter, MD, PhD Ted Koster, MD, PhD Jan P. Vandenbroucke, MD, PhD
ORIGINAL CONTRIBUTION Thrombophilia, Clinical Factors, and Recurrent Venous Thrombotic Events Sverre C. Christiansen, MD Suzanne C. Cannegieter, MD, PhD Ted Koster, MD, PhD Jan P. Vandenbroucke, MD, PhD
Venous thromboembolism (VTe) affects approximately
 The Journal of The american osteopathic association Duration of Anticoagulation Treatment in Patients With Venous Thromboembolism Scott Kaatz, DO, MSc; Waqas Qureshi, MD; Christopher Fain, DO; and David
The Journal of The american osteopathic association Duration of Anticoagulation Treatment in Patients With Venous Thromboembolism Scott Kaatz, DO, MSc; Waqas Qureshi, MD; Christopher Fain, DO; and David
Infections, inflammation and venous thrombosis; an epidemiological perspective Tichelaar, Ynse
 University of Groningen Infections, inflammation and venous thrombosis; an epidemiological perspective Tichelaar, Ynse IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
University of Groningen Infections, inflammation and venous thrombosis; an epidemiological perspective Tichelaar, Ynse IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
DEEP VENOUS THROMBOSIS AMONG CARRIERS OF FACTOR V LEIDEN AND THE G20210A PROTHROMBIN MUTATION
 DEEP VENOUS THROMBOSIS AMONG CARRIERS OF FACTOR V LEIDEN AND THE G20210A PROTHROMBIN MUTATION THE RISK OF RECURRENT DEEP VENOUS THROMBOSIS AMONG HETEROZYGOUS CARRIERS OF BOTH FACTOR V LEIDEN AND THE G20210A
DEEP VENOUS THROMBOSIS AMONG CARRIERS OF FACTOR V LEIDEN AND THE G20210A PROTHROMBIN MUTATION THE RISK OF RECURRENT DEEP VENOUS THROMBOSIS AMONG HETEROZYGOUS CARRIERS OF BOTH FACTOR V LEIDEN AND THE G20210A
Pulmonary Thromboembolism
 Pulmonary Thromboembolism James Allen, MD Epidemiology of Pulmonary Embolism 1,500,000 new cases per year in the United States Often asymptomatic 300,000 deaths per year DVT or PE present in 10% of ICU
Pulmonary Thromboembolism James Allen, MD Epidemiology of Pulmonary Embolism 1,500,000 new cases per year in the United States Often asymptomatic 300,000 deaths per year DVT or PE present in 10% of ICU
Pharmacy Prior Authorization
 Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Anticoagulant Injectable (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Anticoagulant Injectable (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/188/20915 holds various files of this Leiden University dissertation. Author: Flinterman, Linda Elisabeth Title: Risk factors for a first and recurrent venous
Cover Page The handle http://hdl.handle.net/188/20915 holds various files of this Leiden University dissertation. Author: Flinterman, Linda Elisabeth Title: Risk factors for a first and recurrent venous
Genetics of Arterial and Venous Thrombosis: Clinical Aspects and a Look to the Future
 Genetics of Arterial and Venous Thrombosis: Clinical Aspects and a Look to the Future Paul M Ridker, MD Eugene Braunwald Professor of Medicine Harvard Medical School Director, Center for Cardiovascular
Genetics of Arterial and Venous Thrombosis: Clinical Aspects and a Look to the Future Paul M Ridker, MD Eugene Braunwald Professor of Medicine Harvard Medical School Director, Center for Cardiovascular
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE)
 DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
THROMBOSIS RISK FACTOR ASSESSMENT
 Name: Procedure: Doctor: Date: THROMBOSIS RISK FACTOR ASSESSMENT CHOOSE ALL THAT APPLY EACH RISK FACTOR REPRESENTS 1 POINT Age 41 60 years Minor Surgery Planned History of Prior Major Surgery (< 1 month)
Name: Procedure: Doctor: Date: THROMBOSIS RISK FACTOR ASSESSMENT CHOOSE ALL THAT APPLY EACH RISK FACTOR REPRESENTS 1 POINT Age 41 60 years Minor Surgery Planned History of Prior Major Surgery (< 1 month)
Consultative Coagulation How to Effectively Answer Common Questions About Hemostasis Testing Session #5000
 Consultative Coagulation How to Effectively Answer Common Questions About Hemostasis Testing Session #5000 Dorothy M. (Adcock) Funk, M.D. Karen A. Moser, M.D. Esoterix Coagulation September 20, 2013 Disclosures
Consultative Coagulation How to Effectively Answer Common Questions About Hemostasis Testing Session #5000 Dorothy M. (Adcock) Funk, M.D. Karen A. Moser, M.D. Esoterix Coagulation September 20, 2013 Disclosures
What You Should Know
 1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
UC SF. Division of General Internal Medicine UNIVERSITY OF CALIFORNIA SAN FRANCISCO, DIVISION OF HOSPITAL MEDICINE
 Updates in the Management of Venous Thromboembolism Margaret C. Fang, MD, MPH Associate Professor of Medicine UCSF Division of Hospital Medicine Medical Director, Anticoagulation Clinic Venous Thromboembolism
Updates in the Management of Venous Thromboembolism Margaret C. Fang, MD, MPH Associate Professor of Medicine UCSF Division of Hospital Medicine Medical Director, Anticoagulation Clinic Venous Thromboembolism
Homocysteine is a chemical in
 CARDIOLOGY PATIENT PAGE CARDIOLOGY PATIENT PAGE Homocysteine and MTHFR Mutations Relation to Thrombosis and Coronary Artery Disease Elizabeth A. Varga, MS; Amy C. Sturm, MS; Caron P. Misita, PharmD; Stephan
CARDIOLOGY PATIENT PAGE CARDIOLOGY PATIENT PAGE Homocysteine and MTHFR Mutations Relation to Thrombosis and Coronary Artery Disease Elizabeth A. Varga, MS; Amy C. Sturm, MS; Caron P. Misita, PharmD; Stephan
Low-Molecular-Weight Heparin
 Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO 10/28/2011 Section: Prescription Drugs Place(s) of Service:
Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO 10/28/2011 Section: Prescription Drugs Place(s) of Service:
Prevention and treatment of venous thromboembolic disease
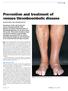 REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
Low-Molecular-Weight Heparin
 Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2016 Section: Prescription Drugs
Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2016 Section: Prescription Drugs
The Multi-Factorial Threshold Model of Thrombotic Risk
 The Multi-Factorial Threshold Model of Thrombotic Risk Richard A. Marlar, PhD and Dorothy M. Adcock, MD The incidence of venous thrombosis in the US is between two and three million per year, resulting
The Multi-Factorial Threshold Model of Thrombotic Risk Richard A. Marlar, PhD and Dorothy M. Adcock, MD The incidence of venous thrombosis in the US is between two and three million per year, resulting
Adaptation of Published Heritable Thrombophilia Testing Guidelines into Local Practice
 Adaptation of Published Heritable Thrombophilia Testing Guidelines into Local Practice Tyler Smith, PGY-5, UBC Hematopathology Supervisor: Dr. A. Lee Collaborators: Dr. D. Pi, Dr. M. Hudoba 2011 CADTH
Adaptation of Published Heritable Thrombophilia Testing Guidelines into Local Practice Tyler Smith, PGY-5, UBC Hematopathology Supervisor: Dr. A. Lee Collaborators: Dr. D. Pi, Dr. M. Hudoba 2011 CADTH
Drug Class Review Newer Oral Anticoagulant Drugs
 Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients. David Liff MD Oklahoma Heart Institute Vascular Center
 DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
Low Molecular Weight Heparin for Prevention and Treatment of Venous Thromboembolic Disorders
 SURGICAL GRAND ROUNDS March 17 th, 2007 Low Molecular Weight Heparin for Prevention and Treatment of Venous Thromboembolic Disorders Guillermo Escobar, M.D. LMWH vs UFH Jayer s sales pitch: FALSE LMW is
SURGICAL GRAND ROUNDS March 17 th, 2007 Low Molecular Weight Heparin for Prevention and Treatment of Venous Thromboembolic Disorders Guillermo Escobar, M.D. LMWH vs UFH Jayer s sales pitch: FALSE LMW is
Duration of Anticoagulation? Peter Verhamme MD, PhD Department of Cardiovascular Medicine University of Leuven Belgium
 Duration of Anticoagulation? Peter Verhamme MD, PhD Department of Cardiovascular Medicine University of Leuven Belgium Disclosures Honoraria and research support: Daiichi-Sankyo, Boehringer Ingelheim,
Duration of Anticoagulation? Peter Verhamme MD, PhD Department of Cardiovascular Medicine University of Leuven Belgium Disclosures Honoraria and research support: Daiichi-Sankyo, Boehringer Ingelheim,
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
Thrombophilia Testing and Venous Thrombosis
 Review Article Dan L. Longo, M.D., Editor Thrombophilia Testing and Venous Thrombosis Jean M. Connors, M.D. Ordering thrombophilia tests is easy; determining whom to test and how to use the results is
Review Article Dan L. Longo, M.D., Editor Thrombophilia Testing and Venous Thrombosis Jean M. Connors, M.D. Ordering thrombophilia tests is easy; determining whom to test and how to use the results is
Corporate Medical Policy
 Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
In the Clinic: Annals Sweta Kakaraparthi 1/23/15
 In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
Causative factors of deep vein thrombosis of lower limb in Indian population
 International Surgery Jthisnal Khadilkar R et al. Int Surg J. 18 Jan;(1):3-3 http://www.ijsurgery.com pissn 39-33 eissn 39-9 Original Research Article DOI: http://dx.doi.org/1.183/39-9.isj17919 Causative
International Surgery Jthisnal Khadilkar R et al. Int Surg J. 18 Jan;(1):3-3 http://www.ijsurgery.com pissn 39-33 eissn 39-9 Original Research Article DOI: http://dx.doi.org/1.183/39-9.isj17919 Causative
Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή
 Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή Ευφροσύνη Δ. Μάναλη Λέκτορας Β Πανεπιστημιακή Πνευμονολογική Κλινική ΓΝΑ «Αττικόν» Εθνικό και Καποδιστριακό Πανεπιστήμιο Αθηνών Existing guidelines
Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή Ευφροσύνη Δ. Μάναλη Λέκτορας Β Πανεπιστημιακή Πνευμονολογική Κλινική ΓΝΑ «Αττικόν» Εθνικό και Καποδιστριακό Πανεπιστήμιο Αθηνών Existing guidelines
Thrombophilias: A Practical Approach
 Disclosures Thrombophilias: A Practical Approach Speaker s Bureau: BMS/Pfizer (Apixaban) PI Altru Health System: EVE Trial Apixaban in Malignancy Keith E Swanson MD North Dakota Family Medicine Conference,
Disclosures Thrombophilias: A Practical Approach Speaker s Bureau: BMS/Pfizer (Apixaban) PI Altru Health System: EVE Trial Apixaban in Malignancy Keith E Swanson MD North Dakota Family Medicine Conference,
Clinical Policy: Dalteparin (Fragmin) Reference Number: CP.PHAR.225
 Clinical Policy: (Fragmin) Reference Number: CP.PHAR.225 Effective Date: 05/16 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Fragmin) Reference Number: CP.PHAR.225 Effective Date: 05/16 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Moderators: Malgorzata Lutwin-Kawalec, MD, Dinesh K Choudhry, MD, FRCA. Institution: Nemours/AI DuPont Hospital for Children, Wilmington, DE
 PBLD Table # 17 A teenager with Factor V Leiden and pectus excavatum for a Nuss procedure: navigating recommendations for testing, perioperative risk of thrombosis and post-operative pain management. Moderators:
PBLD Table # 17 A teenager with Factor V Leiden and pectus excavatum for a Nuss procedure: navigating recommendations for testing, perioperative risk of thrombosis and post-operative pain management. Moderators:
Venous thrombosis is common and often occurs spontaneously, but it also frequently accompanies medical and surgical conditions, both in the community
 Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
Regence. Medical Policy Manual. Date of Origin: May Topic: Genetic Testing for Lipoprotein(a) Variant(s) as a Decision Aid for Aspirin Treatment
 Regence Medical Policy Manual Topic: Genetic Testing for Lipoprotein(a) Variant(s) as a Decision Aid for Aspirin Treatment Date of Origin: May 2013 Section: Genetic Testing Last Reviewed Date: June 2013
Regence Medical Policy Manual Topic: Genetic Testing for Lipoprotein(a) Variant(s) as a Decision Aid for Aspirin Treatment Date of Origin: May 2013 Section: Genetic Testing Last Reviewed Date: June 2013
Laboratory Markers in the Diagnosis of Venous Thromboembolism
 Laboratory Markers in the Diagnosis of Venous Thromboembolism Joseph A. Caprini, MD, Catherine J. Glase, BS, Christopher B. Anderson, Karen Hathaway, BS Department of Surgery Evanston Northwestern Healthcare,
Laboratory Markers in the Diagnosis of Venous Thromboembolism Joseph A. Caprini, MD, Catherine J. Glase, BS, Christopher B. Anderson, Karen Hathaway, BS Department of Surgery Evanston Northwestern Healthcare,
Duration of anticoagulation
 Duration of anticoagulation P. Fontana Service d angiologie et d hémostase Hôpitaux Universitaires de Genève Pomeriggio formativo in coagulazione, Bellinzona, 19.10.2017 Conflict of interest AstraZeneca,
Duration of anticoagulation P. Fontana Service d angiologie et d hémostase Hôpitaux Universitaires de Genève Pomeriggio formativo in coagulazione, Bellinzona, 19.10.2017 Conflict of interest AstraZeneca,
A study of clinical profile of 50 patients deep venous thrombosis at general hospital
 Research Article A study of clinical profile of 50 patients with deep venous thrombosis at general hospital Kasabe P S 1, Jaykar R D 2, Lakhpatre S B 3* 1 Assistant professor, 2 Associate professor, 3
Research Article A study of clinical profile of 50 patients with deep venous thrombosis at general hospital Kasabe P S 1, Jaykar R D 2, Lakhpatre S B 3* 1 Assistant professor, 2 Associate professor, 3
UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS
 UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS Armando Mansilha MD, PhD, FEBVS 16 th National Congress of the Italian Society of Vascular and Endovascular Surgery Bologna, 2017 Disclosure I have the following
UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS Armando Mansilha MD, PhD, FEBVS 16 th National Congress of the Italian Society of Vascular and Endovascular Surgery Bologna, 2017 Disclosure I have the following
Objectives. Venous Thromboembolism (VTE) Prophylaxis. Case VTE WHY DO IT? Question: Who Is At Risk?
 Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
1. SCOPE of GUIDELINE:
 Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Medical Patients: A Population at Risk
 Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea. Original Policy Date
 MP 4.01.10 Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea Medical Policy Section OB/Gyn/Reproduction Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
MP 4.01.10 Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea Medical Policy Section OB/Gyn/Reproduction Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
How long to continue anticoagulation after DVT?
 How long to continue anticoagulation after DVT? Dr. Nihar Ranjan Pradhan M.S., DNB (Vascular Surgery), FVES(UK) Consultant Vascular Surgeon Apollo Hospital, Jubilee Hills, Hyderabad (Formerly Faculty in
How long to continue anticoagulation after DVT? Dr. Nihar Ranjan Pradhan M.S., DNB (Vascular Surgery), FVES(UK) Consultant Vascular Surgeon Apollo Hospital, Jubilee Hills, Hyderabad (Formerly Faculty in
Thursday, February 26, :00 am. Regulation of Coagulation/Disseminated Intravascular Coagulation HEMOSTASIS/THROMBOSIS III
 REGULATION OF COAGULATION Introduction HEMOSTASIS/THROMBOSIS III Regulation of Coagulation/Disseminated Coagulation necessary for maintenance of vascular integrity Enough fibrinogen to clot all vessels
REGULATION OF COAGULATION Introduction HEMOSTASIS/THROMBOSIS III Regulation of Coagulation/Disseminated Coagulation necessary for maintenance of vascular integrity Enough fibrinogen to clot all vessels
Epidemiology of Thrombosis in Patients with Malignancy. Cancer and Venous Thromboembolism. Chew HK, Arch Int Med, Feb Blom et al, JAMA, Feb 2005
 Cancer and Venous Thromboembolism Objectives 1. Epidemiology of thrombosis in patients with malignancy 2. Anticancer agents and thrombosis 3. Current treatment protocols at UHN 4. Prevention of DVT 5.
Cancer and Venous Thromboembolism Objectives 1. Epidemiology of thrombosis in patients with malignancy 2. Anticancer agents and thrombosis 3. Current treatment protocols at UHN 4. Prevention of DVT 5.
