Nonrandom contribution of left and right testes to germline transmission from mouse spermatogonial stem cells
|
|
|
- Jesse Porter
- 5 years ago
- Views:
Transcription
1 Biology of Reproduction, 2017, 97(6), doi: /biolre/iox141 Research Article Advance Access Publication Date: 9 November 2017 Research Article Nonrandom contribution of left and right testes to germline transmission from mouse spermatogonial stem cells Mito Kanatsu-Shinohara 1,2,, Honda Naoki 3 and Takashi Shinohara 1 1 Department of Molecular Genetics, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 2 Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology, Kyoto, Japan and 3 Imaging Platform for Spatio-temporal Information, Graduate School of Medicine, Kyoto University, Kyoto, Japan Correspondence: Department of Molecular Genetics, Graduate School of Medicine, Kyoto University, Yoshida Konoe, Sakyo-ku, Kyoto , Japan. Tel: ; Fax: ; mshinoha@virus.kyoto-u.ac.jp Grant Support: Financial support for this research was provided by The Uehara Memorial Foundation, The Takeda Foundation, The Naito Foundation, Grants-in-aid for Scientific Research on Innovative Areas Epigenome Dynamics and Regulation in Germ Cells ( ) and Stem Cell Aging and Disease (17H05639) from The Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Science and Technology Agency (PRESTO), and the Platform for Dynamic Approaches to Living Systems from Japan Agency for Medical Research and Development (AMED). Edited by Dr. Kyle Orwig, PhD, University of Pittsburgh. Received 26 June 2017; Revised 15 August 2017; Accepted 7 November 2017 Abstract Vast amounts of sperm are produced from spermatogonial stem cells (SSCs), which continuously undergo self-renewal. We examined the possible effect of laterality in male germline transmission efficiency of SSCs using a spermatogonial transplantation technique. We transplanted the same number of wild-type and Egfp transgenic SSCs in the same or different testes of individual recipient mice and compared the fertility of each type of recipient by natural mating. Transgenic mice were born within 3 months after transplantation regardless of the transplantation pattern. However, transgenic offspring were born at a significantly increased frequency when wild-type and transgenic SSCs were transplanted separately. In addition, this type of recipient sired significantly more litters that consisted exclusively of transgenic mice, which suggested that left and right testes have different time windows for fertilization. Thus, laterality plays an important role in germline transmission patterns from SSCs. Summary Sentence Nonrandomness of germline transmission pattern of spermatogonial stem cells was examined by spermatogonial transplantation. Key words: spermatogenesis, spermatogonial stem cells, transplantation, testis. Introduction It has been estimated that spermatozoa are ejaculated for fertilization in mice [1]. Both right and left testes contribute to spermatogenesis and generate mature spermatozoa. However, it has remained unknown whether these testes contribute equally to offspring production. Previous studies have shown conflicting observations of fertility after orchidectomy of unilateral testis [2 4], and the effect of laterality in germline transmission remains unknown. On the other hand, ovulation had been considered to occur from alternating ovaries in mono-ovular species since the original observations by Rühl in 1925 [5]. Although more recent studies do not support this conclusion [6], the effect of laterality has been analyzed extensively in females. While ovulation is relatively easily identified by morphological analyses of ovaries, it has been difficult to study the 902 C The Author(s) Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please journals.permissions@oup.com
2 Nonrandom male germline transmission, 2017, Vol. 97, No differences in the contribution of each testis to germline transmission due to lack of appropriate experimental systems. Spermatogonial stem cells (SSCs) provide the foundation of spermatogenesis [7, 8]. In mouse testes, SSCs comprise only % of total germ cells [8, 9]. However, these cells continuously undergo self-renewal and produce sperm for the life span of the male animal. Once SSCs are committed to differentiate, spermatogonia progenitor cells undergo a limited number of mitotic divisions and enter into meiosis, and then transform into spermatids and sperm via spermiogenesis. Sperm produced in the testis are then ejaculated for fertilization after transportation through epididymis and vas deferens. Therefore, any difference in laterality can occur not only in the testis but also in epididymis and vas deferens. Unfortunately, SSCs and committed progenitors are morphologically indistinguishable, but SSCs have a unique ability to reconstitute empty seminiferous tubules of infertile mouse testes after microinjection into the seminiferous tubules [10]. Transplanted SSCs regenerate colonies of germ cells and eventually produce donor cell-derived offspring [11]. We recently developed a robust system to produce offspring using germline stem (GS) cell cultures, in which SSCs can expand indefinitely in vitro in the presence of self-renewal factors, including glial cell line-derived neurotrophic factor [12]. Although the frequency of SSCs in these cultures is 1 2% [13], SSCs in this culture continue to increase their number, which makes it possible to collect a large number of SSCs for biochemical and molecular biological studies. The most remarkable property of GS cells is fertility restoration. We were able to restore fertility to both congenitally infertile WBB6F1-W/W v (W) mice and busulfan-treated infertile mice [14]. Within 4 months after transplantation, 60 and 100% of W and busulfan-treated mice sired donor-derived offspring, respectively. This experimental system created a unique platform to study the effect of candidate factors involved in fertility restoration by SSCs. In this study, we analyzed the effect of laterality on the germline transmission pattern from SSCs. Wild-type (WT) GS cells and those with a homozygous Egfp transgene encoding enhanced green fluorescent protein (EGFP) were separately transplanted into each testicle of W mice. We also produced another type of recipient mice by transplanting both EGFP GS cells and WT GS cells into the same testis. Because W mice do not have endogenous spermatogenesis, all offspring born from W mice must be derived from transplanted donor SSCs. The recipient mice were caged with WT females for at least 7 months, and the genotype of the offspring was examined to determine the origin of SSCs. Materials and methods Cell culture GS cells that express the Egfp transgene were established from a transgenic mouse strain C57BL/6Tg14(act-EGFP)OsbY01 in a DBA/2 background, as previously described [14]. Wild-type GS cells were established from 5 to 10-day-old pup testes using the same procedure. These cells have the same genetic background except for the absence of the transgene. Cells were cultured in Iscove modified Dulbecco Medium (Invitrogen, Carlsbad, CA), which was supplemented with 10 ng/ml human FGF2, 15 ng/ml recombinant rat GDNF (both from Peprotech, London, UK), and 1% fetal bovine serum, as previously described [15]. Cultures were maintained on mitomycin C-treated mouse embryonic fibroblasts. Transplantation Spermatogonial transplantation was carried out as previously described using 4 6-week-old W mice (Japan SLC, Shizuoka, Japan). GS cells were dissociated into single cells by 0.25% trypsin/1 mm EDTA solution, and approximately cells were microinjected into the efferent duct of each testis, as previously described [16]. Each injection filled 75 85% of all seminiferous tubules. The recipient mice were treated with anti-cd4 antibody (GK1.5) intraperitoneally on days 0, 2, and 4 after transplantation to avoid rejection of the donor cells [17]. Recipients were housed with WT B6 females 4 weeks after transplantation to produce offspring. The Institutional Animal Care and Use Committee of Kyoto University approved all of our animal experimentation protocols. Analysis of recipient mice After mating for 7 months, recipients were sacrificed between 8 and 11 months after transplantation. Donor cell colonies in recipient testes were examined under UV light. For evaluation of donor cell colonization levels, testis samples were embedded in paraffin, and histological sections were stained with hematoxylin and eosin. The number of tubules with spermatogenesis, defined by the presence of multiple layers of germ cells in the entire circumference of the tubules, was recorded for one section from each testis. Lectin staining Testis samples were fixed in 2% paraformaldehyde for 3 h. Then they were embedded in Tissue-Tek OCT compound (Sakura Finetek, Tokyo, Japan) for cryosectioning. Lectin staining of cryosections was carried out by treating the samples with 0.1% Triton-X in phosphate-buffered saline (PBS). After immersing the slides in blocking buffer (0.1%Tween 20, 3% bovine serum albumin and 10% goat serum in PBS) for >1 h, samples were incubated with rhodamineconjugated peanut agglutinin (PNA; Vector Laboratories RL-1072, Burlingame, CA). Samples were counterstained with Hoechst (Sigma, St. Louis, MO). Statistical analyses Significant differences between means for single comparisons were determined by a Mann Whitney U-test. Multiple comparison analyses were carried out using ANOVA followed by Tukey Honest Significant Difference test. The method for statistical evaluation of laterality is provided in Supplemental Information. Results Fertility restoration by transplantation of enhanced green fluorescent protein and wild-type GS cells into W mice In our previous study, we used GS cells that have homozygous Egfp transgene to obtain offspring [14]. We used the same GS cells and compared the fertility potential with WT GS cells that do not show fluorescence. Because these two types of GS cells do not show apparent differences in their morphology and proliferation speed during in vitro culture, they were indistinguishable unless they were exposed to UV light. For recipients, we used congenitally infertile W mice. W mice have a very small number of SSCs and undifferentiated spermatogonia, but completely lack differentiated spermatogonia and subsequent stages of germ cells due to mutations in the Kit gene. Therefore, any offspring produced by these mice must be derived from donor cells. We collected the same number of GS cells by trypsin digestion and single-cell suspensions were made. We produced two types of recipients (Figure 1A). In one group of recipients (designated as mixed
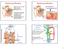
3 904 M. Kanatsu-Shinohara et al., 2017, Vol. 97, No. 6 Figure 1. Transplantation of GS cells into W mice. (A) An experimental scheme showing the transplantation of EGFP or WT GS cells into the seminiferous tubules of congenitally infertile W mice. Recipient males were continuously mated with WT females for offspring production. (B) Offspring produced by a recipient male under UV light. (C) Macroscopic appearance of recipient testis. Green tubules represent donor cell colonization. (D) Testis weight (n = 4 for WT and EGFP, n = 5 for Mix). (E) Histological appearance of the recipient testis. (F) Lectin staining of recipient testis. (G) Quantification of tubules with spermatogenesis. Tubules with PNA + spermatogenic cells were counted. At least 181 tubules in four testes were counted (n = 4 for WT and EGFP, n = 5 for Mix). Bar = 1mm(C), 100 μm (E, F). or M recipients), EGFP and WT GS cells were mixed in a 1:1 ratio, and the cells were transplanted into both sides of six recipient mice. In another group ( separate or S recipients), the same number of recipients underwent transplantation with two different types of cells in separate testes. Three recipients were transplanted with EGFP cells in the right testis, while WT cells were transplanted into the left testis. Four recipients underwent transplantation in the opposite manner. Two months after transplantation, these recipient mice were caged with 3 4 WT females to produce offspring from donor cells. A total of two transplantation experiments were performed. M recipients sired offspring as early as 83 days after transplantation (Table 1). Five of six M recipients became fertile. Similarly, S recipients sired offspring as early as 82 days after transplantation, and six of seven S recipients restored fertility upon transplantation. In both cases, the remaining recipients did not sire progeny for at least 7 months. Although the first transgenic mice were born at comparable speed, the average date of first offspring production from all recipients was somewhat delayed in S recipients (86.8 days vs days). The number of litters produced by S recipients ranged from 1 to 29 (Supplemental Figure S1A), whereas those with M recipients ranged from 16 to 18 (Supplemental Figure S1B). This was because three of the S recipients died within 5 months after transplantation. However, when the experimental animals that survived the whole experimental period were compared, the frequency of offspring production did not show apparent differences between the two types of recipients. In average, M recipients produced 16.8 litters, while S recipients produced 14.2 litters. These values were not significantly different. However, when the total offspring numbers were compared, M recipients sired an average of 9.2 offspring per litter, whereas 7.0 offspring were born from S recipients. This difference was statistically significant. We determined the genotype of the offspring by examining their fluorescence under UV light illumination. Although there is a controversy concerning the effect of laterality on sex ratio and ovulation
4 Nonrandom male germline transmission, 2017, Vol. 97, No Table 1. Offspring production after spermatogonial transplantation. Type Days Period Testis weight (mg) (type) % Spermatogenesis Recipient to first of analysis Litter EGFP + EGFP Total % GFP + / ID offspring (days) number offspring offspring offspring total Right Left Right Left Separate S (GFP) 58.2 (WT) 93.2 (GFP) 81.6 (WT) S ND ND ND ND S ND ND ND ND S (GFP) 63.3 (WT) 89.5 (GFP) 93.5 (WT) S (WT) 56.1 (GFP) 98.7 (WT) 90.2 (GFP) S (WT) 62.3 (GFP) 94.1 (WT) (GFP) Total Mix M ND 100 M M M M Total ND, not determined because these animals did not survive the whole experimental period. [18], we did not find an apparent bias: both left and right testes produced comparable numbers of males and females from S recipients. EGFP and WT SSCs also did not show apparent differences in sex ratio of the offspring. However, we noted a biased transgenic offspring production. Overall, 43.2% of the offspring were transgenic mice from S recipients, while 20.1% of the offspring were transgenic mice from M recipients (Figure 2). On the other hand, the average number of transgenic offspring per litter was 1.8 and 3.0 for M and S recipients, respectively. In both types of recipients, significantly more WT mice were born than transgenic mice (P = ), suggesting that WT SSCs have a competitive advantage over EGFP SSCs. The number of WT offspring per litter was 7.3 and 4.0 for M and S recipients, respectively. This difference between the wild-type litters was also statistically significant (P < ). These results suggested that the kinetics of SSCs in M and S recipients are different. Statistical validation of biased production of offspring by mathematical modeling In addition to the changes in the frequency of transgenic mouse production, we noted that a significant number of litters consisted of only one genotype. M recipient mice sired a total of 84 litters, of which one (1.2%) litter consisted of only transgenic mice (Figure 2). In contrast, S recipients sired 85 litters, of which eight (9.4%) litters consisted of only transgenic mice. Because S recipients contain only one genotype in each testis and sired 12 (14.1%) litters with the wild-type genotype, a total of 20 litters (23.5%) from S recipients were born with only one genotype. Since this finding suggested that left and right testes contribute to offspring production separately, we performed a following statistical analysis to determine whether transgenic or WT offspring were born unevenly from S and M recipients. In this analysis, we quantitatively evaluated the degree of bias of the offspring production in each litter based on a rarity of the offspring production. It should be noted that biased-offspring production patterns in litters with many offspring (e.g., six EGFP/zero WT and one EGFP/seven WT) are considered rare event (low probability), whereas the biases observed in litters with a small number of offspring (e.g., three EGFP/zero WT and zero EGFP/two WT) must be common (high probability), and also that unbiased-offspring production patterns (e.g., one EGFP/two WT and three EGFP/three WT) are considered common (high probability) events. These probabilities were calculated in a null condition where each offspring was evenly selected from a pool of SSC clones at random (Figure 3A) (see Supplemental Information). We then measured the degree of bias of the offspring production by negative log probability, i.e., Shannon information content, which gives a quantitative measurement of the degree of unpredictability. We found that the offspring production from S recipients was more biased than those from M recipients (P = 0.02; Mann Whitney U-test) (Figure 3B and C). Furthermore, we statistically assessed whether the biased offspring production from M recipients did not occur by chance. Under a null hypothesis that each offspring was randomly selected from a pool of SSC clones (Figure 3A), we sampled the offspring birth patterns using a Monte Carlo method and averaged the degree of bias of the offspring production over all litters. By iterating this sampling 1,000,000 times, we obtained a distribution of the average degree of bias, which serves as a null distribution (Figure 3DandE). By comparing this null distribution with actual averages calculated from the real data, we concluded that the offspring production from S recipients was significantly biased (P = 0) (Figure 3D). In contrast, no statistical difference was observed in M recipients (P = 0.21) (Figure 3E). Because there appeared to be litters with relatively high number of offspring with one genotype that were found more frequently in the early phase than in the later phase, we calculated the degree of bias, and also carried out a Monte Carlo method to evaluate the statistical differences in both early and later phases of the experimental period (Supplemental Figure S2A H). However, we found that offspring production from S recipients was still significant in both phases (Supplemental Figure S2C and G). Offspring production patterns of M recipients did not show statistical difference (Supplemental Figure S2D and H). These results suggested that S recipients sired transgenic offspring in a nonrandom pattern from each testis. Analysis of recipient testes after mating experiment We sacrificed all surviving animals after the experimental period and recovered their testes (Figure 1C). The average weights of the testis
5 906 M. Kanatsu-Shinohara et al., 2017, Vol. 97, No. 6 Figure 2. A summary diagram showing the offspring production pattern. All offspring born from S and M recipients are indicated. Each column indicates the offspring born from recipient males. The x-axis indicates the size of the litter. Numbers indicated on the left side of the y-axis represent the date of birth. containing WT, EGFP, and mixed GS cells were 61.9, 53.5, and 58.0 mg, respectively (Figure 1D). Although the value was slightly smaller for testes with EGFP GS cells, there were no significant differences among the three types of testes. All of these testes were much larger than untransplanted control W mouse testes ( 11 mg). Histological sections of the recipient testes showed normal appearing spermatogenesis (Figure 1E). Immunostaining of the recipient testes with an acrosome (PNA) marker also showed normal distribution during spermatogenesis, and no apparent abnormalities or differences were noted among the three types of samples. We also quantified the number of tubules of mature germ cells by staining with PNA staining (Figure 1F). However, comparable numbers of tubules contained multiple layers of germ cells (Figure 1G). These results indicate that
6 Nonrandom male germline transmission, 2017, Vol. 97, No Figure 3. A null model for the analysis and flow to evaluate the degree of bias of offspring production in each litter. Each progeny was assumed to be randomly selected from a pool of EGFP and WT SSC clones. (A) The probability of offspring production patterns from each litter was calculated based on the null model. Each progeny was assumed to be randomly selected from a pool of EGFP and WT SSC clones. The degree of bias of offspring production was evaluated by Shannon information content. Note that the degree of bias changes according to the total number of offspring. For example, the degree of bias in litter 3, which consisted of only two GFP clones, is However, it was 3.69 when all six offspring were WT in litter N. (B, C) Histograms of the information content in S(B) and M (C) recipients. Arrows indicate the average values. Significant difference was found between S and M recipients (P = 0.02; Mann Whitney U-test). (D, E) Statistical tests for the chance of the biased-offspring production. By Monte Carlo sampling in the null model (A), artificial dataset of offspring production patterns were generated following the observed numbers of offspring from recipients, and average of the information contents was calculated according to the same procedure. Probability distributions of the average information content in S (D) and M (E) recipients were obtained by repeating the Monte Carlo sampling 1,000,000 times. Red lines indicate the actual information content average calculated by experimental data. Statistical significance was determined using P values, which were calculated by empirical probabilities greater than the actual information content average (total areas of blue columns above the red bar). While the chance of the biased-offspring production was not statistically significant in mixed conditions (P = 0.21), separate conditions showed statistical significance (P = 0). colonization of the three types of GS cells in testes occurred at the same levels. Discussion The laterality of ovulation has long been studied in females [6, 19 21], and many studies are available on the fertility of orchidectomized human patients, particularly in relation to the treatment of tumors or varicoceles [22]. However, the effect of laterality on the germline transmission pattern has not been analyzed in the male, including experimental animals. One study using mice showed declining fertility after unilateral vasectomy, but not after orchiectomy [3]. Another more recent study showed that left/right differences in vascular architecture are associated with a decrease
7 908 M. Kanatsu-Shinohara et al., 2017, Vol. 97, No. 6 in hemoglobin saturation and increased levels of HIF1A in the left testis, but no effect on fertility has been reported [23]. Because unilateral spermatogonial transplantation could restore fertility [24], it is apparently not necessary to have spermatogenesis in both testes for fertility restoration, which is expected from reports on fertility of hemicastrated mice and humans. However, it is possible that each testis may influence or interact with the other and this possibility has not been evaluated. Despite its importance, an appropriate method to study the effect of laterality for SSCs has not been developed. In this study, we used spermatogonial transplantation and GS cell culture to address this problem. Spermatogonial transplantation is particularly useful because it is the only method to produce offspring from SSCs [10]. However, because SSC frequency is very low ( % in adult testis cells) [8, 9], direct transplantation of fresh testis cells is not very efficient for fertility restoration due to limited number of transplanted SSCs. Although the frequency of SSCs in GS cell culture is 1 2% [13], the concentration of SSCs in this culture is significantly higher than in fresh testis cells ( %), which greatly improves fertility restoration of recipients. In our recent study, we estimated that at least GS cells can restore fertility, which corresponds to SSCs per testis [14]. Thus, the combination of spermatogonial transplantation and GS cell culture techniques allows for assessment of the effect of laterality in a functional manner for the first time. An important finding in this study was the altered germline transmission pattern as a result of the transplantation procedure. The overall efficiency of transgenic offspring production was significantly higher in S recipients compared with M recipients (43.2% vs. 20.1%). Moreover, S recipients produced a total of 20 litters (23.5%) that contained only one genotype. In particular, 8 of 20 litters consisted of only transgenic offspring. Because an average of seven pups were included in a litter from S recipients, the chance of getting such a result would be extremely low if SSCs from both testes contribute randomly to fertilization. Thus, EGFP and WT SSCs not only changed the frequency of transgenic mouse production but also the pattern of their appearance according to the site of transplantation. Indeed, when we carried out mathematical simulation and compared the results with real data, we confirmed that the biased transgenic offspring production from S recipients occurred in a statistically significant manner. Because normal spermatogenesis continued in both testes at the same levels, this result was unexpected. One of the potential factors that are involved in this biased offspring production is competition between SSCs. Competition of spermatogonia was originally suggested in aggregation chimera studies [25], which likely has important implications in humans [26]. By making allophenic chimera mice, it was reported that the genotype of offspring born from male chimeric animals can change during their life time. For example, when C57BL/6 (B6) and C3H embryos were mixed to produce chimeras, male chimeras usually produced more offspring with C3H-derived sperm than B6 sperm and 15% of offspring were derived from B6 germ cells. However, the proportion of B6 germ cells among their progeny continued to decline, although fertility was sustained. It was suggested that such selection might occur at a stage in gametogenesis when successive proliferations are still occurring, i.e., in the diploid spermatogonial period. It was speculated that germ cells of the two strains might compete for available materials with different efficiencies, which confers a selective advantage for the C3H genotype. Although we did not find apparent functional differences between WT and EGFP GS cells during in vitro culture, this example suggests that a slight difference in growth/differentiation rate between WT and EGFP cells may cause competition in M recipient testes. Although spermatogonia competition may occur continuously in the testis, it still does not explain how the recipient could sire progeny with only one genotype from S recipients. It is possible that spermatogonia competition may gradually change the ratio of the transgenic offspring as shown in the chimera studies. However, our results showed that litters with one genotype were suddenly born without gradual change in the ratio of transgenic offspring. Because this occurred throughout the entire experimental period, spermatogonia competition alone does not appear to explain our results. Although we currently do not know the mechanism underlying this phenomenon, it is probably not regulated by the endocrine system because it would affect both sides in a similar manner. In this sense, we speculate that sperm transport through the male reproductive tract is influenced by nerve or vessels, which may distribute unevenly on the right and left side and differentially influenced fertilization patterns. Considering that ovulation is influenced by superior ovarian nerves [27], it is tempting to speculate that a similar mechanism may operate in sperm transport in males. A biased offspring production pattern was recently reported in our studies on lineage tracing of SSCs and may provide a clue to solve this problem [28]. In these experiments, we specifically marked SSCs by introducing a retro- or lentivirus before spermatogonial transplantation and found that offspring with the same viral integration patterns were frequently born in the same litters. The results indicated that a relatively small number of SSCs contribute to offspring production at a time. Given the results of the present experiments and the fact that relatively small numbers of SSCs contribute to a litter, it seems reasonable to speculate that sperm from right and left testes do not simply compete against each other but have different time windows for fertilization. EGFP SSCs may be outcompeted by more dominant WT SSCs in M recipients, but EGFP SSCs in S recipients do not have such competitors in the same testis and have an increased opportunity to participate in fertilization. If many SSCs from both testes compete equally to fertilization, the chance of producing a whole litter with EGFP SSCs would be very low. However, when only a few SSCs in one of the testes contribute to a given litter, the chance of EGFP SSCs to contributing to a whole litter becomes greater. Given these results, it is possible that a novel mutation in the germline may not be transmitted by simple competition of all spermatozoa; germline transmission may occur only when such a small number of SSCs obtain opportunity to fit in the time window for germline transmission. Perhaps, such machinery may prevent rapid expansion of newly generated mutations to the next generation. In addition, because sperm generated from one testis is sufficient for fertilization, it would be efficient to have another testis for subsequent fertilization if accumulation of sperm takes significant amount of time. This would increase the total fertility of mice. Because continuous collection of sperm is difficult from recipient testes and epididymides, these hypotheses are not easy to evaluate, but several approaches may clarify the potential machinery that underlies our observation. For example, it will be possible to identify specific stages of the seminiferous tubules (such as stage VII) and enumerate round spermatids per Sertoli cells to compare the sperm production efficiency between WT and EGFP SSCs. It would be ideal if it becomes possible to monitor the changes in the relative number of stages of seminiferous tubules in each testis as function of time. Absolute copy numbers of sperm genomes transiting the epididymis could be
8 Nonrandom male germline transmission, 2017, Vol. 97, No quantified by Digial Drop PCR. In addition, synchronization of the seminiferous tubules with WIN18,446, which inhibits the biosynthesis of retinoic acid from retinol, may also change the offspring production patterns [29]. Such approaches may help to better unveil novel cellular and molecular machineries that influence testicular laterality. Germline transmission from SSCs involves many steps. Currently, very little is known about the factors that are involved in this process. Because our experimental system is based on cell transplantation, we cannot exclude the possibility that analysis of transplanted SSCs may not fully mimic SSCs under physiological conditions. Nevertheless, analysis based on spermatogonial transplantation is currently the only method to study such process. We think that the early phase ( 3 4 months) may be reflecting the regeneration after transplantation, and the results from later stages probably represent stable spermatogenesis. This is because morphological abnormalities associated with spermatogonial transplantation, such as missing layers of germ cells in the seminiferous tubules, gradually disappear after 3 months [30]. To our knowledge, the current study provides a first functional analysis of effect of laterality on germline transmission patterns in the male and has an important implication in understanding the pattern of male side inheritance of genetic information. Our findings suggest that fertilization resulting from individual testis may have a potential time window, which suggests that regulation of male germline transmission is more sophisticated than is generally considered. Identification of key molecules that underlie this phenomenon is the next obvious goal. Further studies will increase our knowledge of the relationship between male fertility and SSCs, and such studies will also have important implications in genetic variation, disease onset, and human infertility treatment. Supplementary data Supplementary data are available at BIOLRE online. Supplemental Figure S1. Offspring production patterns of recipient mice. All offspring born from individual S (A) or M (B) recipients are shown. Each column indicates the offspring born from each recipient male. The x-axis indicates the size of the litter. Numbers indicated on the left side of the y-axis represent the litter number. Numbers in parenthesis indicate the date of birth after transplantation. Supplemental Figure S2. Comparison of early and late phase of biased offspring production. (A, E) Histograms of the information content in early (A) and late (E) phases of S recipients. (B, F) Histograms of the information content in early (B) and late (F) phases of M recipients. (C, G) Probability distributions of the average information content in early (C) and late (G) phases of S recipients by repeating the Monte Carlo sampling 1,000,000 times. (D, H) Probability distributions of the average information content in early (D) and late (H) phases of M recipients by repeating the Monte Carlo sampling 1,000,000 times. Arrows indicate the average values. Significant difference was found in both early and late phases of S recipients (P = 0; Mann Whitney U-test ), but no difference was found in both phases of M recipients. Acknowledgments We thank Ms Y. Ogata for technical assistance and Dr K. Nakae for giving advice on statistical analysis. References 1. Harper MJK. Sperm and egg transport. In: Austin CR, Short RV (eds.), Germ Cells and Fertilization. Cambridge: Cambridge University Press; 1982: Amelar RD, Dubin L, Hotchkiss RS. Restoration of fertility following unilateral orchiectomy and radiation therapy for testicular tumors. JUrol 1971; 106: Kessler DL, Smith WD, Hamilton MS, Berger RE. Infertility in mice after unilateral vasectomy. Fertil Steril 1985; 43: Ferreira U, Ntto Júnior NR, Esteves SC, Rivero MA, Schirren C. Comparative study of the fertility potential of men with only one testis. Scand J Urol Nephrol 1991; 25: Rühl A. Rgelmassigkeit im wechsel der Ovarial function. Larch Gynak 1925; 124: Baker SJ, Spears N. The role of intra-ovarian interactions in the regulateon of follicle dominance. Hum Reprod Update 1999; 5: de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins CC, Ewing LL (eds.), Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993: Tegelenbosch RAJ, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res 1993; 290: Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994; 91: Brinster RL, Avarbock MR. Germline transmisson of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 1994; 91: Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003; 69: Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol 2013; 29: Kanatsu-Shinohara M, Morimoto H, Shinohara T. Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol Reprod 2016; 94: Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod 2014; 91: Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 1997; 41: Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Honjo T, Shinohara T. Allogeneic offspring produced by male germ line stem cell transplantation into infertile mouse testis. Biol Reprod 2003; 68: James WH. Side of ovulation, hormones and sex ratios. Hum Reprod 2001; 16: Shazly SA, Badee AY, Ali MK, Sobh AM, Aleem AA. The laterality of ovulation: how far does it matter? Eur J Obstet Gynecol Reprod Biol 2013; 167: Wheeler AG. Comparisons of the ovulatory and steroidogenic activities of the left and right ovaries of the ewe. J Reprod Fertil 1978; 53: Wiebold JL, Becker WC. Inequality in function of the right and left ovaries and uterine horns of the mouse. J Reprod Fertil 1987; 79: Garcia-Roig ML, Kirsch AJ. The dilemma of adolescent varicocele. Pediatr Surg Int 2015; 31: Bustamante-Marin XM, Cook MS, Gooding J, Newgard C, Capel B. Leftbased spermatogenic failure in 129/SvJ Dnd1Ter/ + mice correlates with differences in vascular architecture, oxygen availability, and metabolites. Biol Reprod 2015; 93: Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci USA 2001; 98:
9 910 M. Kanatsu-Shinohara et al., 2017, Vol. 97, No Mintz B. Hermaphroditism, sex chromosomal mosaicism and germ cell selection in allophenic mice. J Anim Sci 1968; 27(Suppl 1): Maher GJ, Goriely A, Wilkie AO. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology 2014; 2: Luna F, Cortés M, Flores M, Hemández B, Trujillo A, Domínguez R. The effects of superior ovarian nerve on ovulation in the guinea pig. Reprod Biol Endocrinol 2003; 1: Kanatsu-Shinohara M, Naoki H, Shinohara T. Nonrandom germline transmission of mouse spermatogonial stem cells. Dev Cell 2016;38: Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod 2011; 84: Parreira GG, Ogawa T, Avarbock MR, França LR, Brinster RL, Russell LD. Development of germ cell transplants in mice. Biol Reprod 1998; 59:
Introduction. K. Zohni 1, X. Zhang 1, S.L. Tan 1, P. Chan 2, and M.C. Nagano 1, * ORIGINAL ARTICLE Andrology
 Human Reproduction, Vol.27, No.1 pp. 44 53, 2012 Advanced Access publication on November 14, 2011 doi:10.1093/humrep/der357 ORIGINAL ARTICLE Andrology The efficiency of male fertility restoration is dependent
Human Reproduction, Vol.27, No.1 pp. 44 53, 2012 Advanced Access publication on November 14, 2011 doi:10.1093/humrep/der357 ORIGINAL ARTICLE Andrology The efficiency of male fertility restoration is dependent
Germ Cell Transplantation in Fish
 Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Spermatogenesis occurs by complex interactions between
 Rats produced by interspecies spermatogonial transplantation in mice and in vitro microinsemination Takashi Shinohara*, Megumi Kato, Masanori Takehashi*, Jiyoung Lee*, Shinichiro Chuma, Norio Nakatsuji,
Rats produced by interspecies spermatogonial transplantation in mice and in vitro microinsemination Takashi Shinohara*, Megumi Kato, Masanori Takehashi*, Jiyoung Lee*, Shinichiro Chuma, Norio Nakatsuji,
ICSI with sperm derived from cultured testes (Nature 2011) Establishment of rabbit ips cells (J Biol Chem 2010)
 Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
spermatogonial transplantation (testis/tem cels/spermaoneis/transec mice/fertty)
 Proc. Natl. Acad. Sci. USA Vol. 91, pp. 11303-11307, November 1994 Developmental Biology Germline transmission of donor haplotype following spermatogonial transplantation (testis/tem cels/spermaoneis/transec
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 11303-11307, November 1994 Developmental Biology Germline transmission of donor haplotype following spermatogonial transplantation (testis/tem cels/spermaoneis/transec
Sami Ventelä, 1,2 Hiroshi Ohta, 3 Martti Parvinen, 2 and Yoshitake Nishimune 3
 BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1
 BIOLOGY OF REPRODUCTION 60, 1429 1436 (1999) Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1 Makoto Nagano, Mary R. Avarbock, and Ralph L. Brinster 2 School
BIOLOGY OF REPRODUCTION 60, 1429 1436 (1999) Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes 1 Makoto Nagano, Mary R. Avarbock, and Ralph L. Brinster 2 School
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish
 Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Chapter 36 Active Reading Guide Reproduction and Development
 Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
Name: AP Biology Mr. Croft Chapter 36 Active Reading Guide Reproduction and Development Section 1 1. Distinguish between sexual reproduction and asexual reproduction. 2. Which form of reproduction: a.
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Stem Cell Reports Ar ticle
 Please cite this article in press as: Takashima et al., Functional Differences between GDNF-Dependent and FGF-Dependent Mouse Spermatogonial Stem Cell Self-Renewal, Stem Cell Reports (5), http://dx.doi.org/.6/j.stemcr.5..
Please cite this article in press as: Takashima et al., Functional Differences between GDNF-Dependent and FGF-Dependent Mouse Spermatogonial Stem Cell Self-Renewal, Stem Cell Reports (5), http://dx.doi.org/.6/j.stemcr.5..
Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment
 Iranian Biomedical Journal 11 (1): 15-22 (January 2007) Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment Sayed Hadi Anjamrooz 1,
Iranian Biomedical Journal 11 (1): 15-22 (January 2007) Assessment of Morphological and Functional Changes in the Mouse Testis and Epididymal Sperms Following Busulfan Treatment Sayed Hadi Anjamrooz 1,
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Meiosis & Sexual Reproduction. AP Biology
 Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Meiosis & Sexual Reproduction 2007-2008 Cell division / Asexual reproduction Mitosis produce cells with same information identical daughter cells exact copies clones same amount of DNA same number of chromosomes
Knockout TM SR : ; ; ; : R ; R : A : X(2013) , ,, B. , (Knockout TM
 33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
New Targets for Non-Hormonal Male Contraception. John K. Amory MD, MPH Professor of Medicine University of Washington
 New Targets for Non-Hormonal Male Contraception John K. Amory MD, MPH Professor of Medicine University of Washington Non-Hormonal Male Contraception Definition: An approach to reversible male contraception
New Targets for Non-Hormonal Male Contraception John K. Amory MD, MPH Professor of Medicine University of Washington Non-Hormonal Male Contraception Definition: An approach to reversible male contraception
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Functional and molecular features of the Id4 + germline stem cell population in mouse testes
 Functional and molecular features of the Id4 + germline stem cell population in mouse testes Frieda Chan, 1 Melissa J. Oatley, 1 Amy V. Kaucher, 1 Qi-En Yang, 1 Charles J. Bieberich, 2 Cooduvalli S. Shashikant,
Functional and molecular features of the Id4 + germline stem cell population in mouse testes Frieda Chan, 1 Melissa J. Oatley, 1 Amy V. Kaucher, 1 Qi-En Yang, 1 Charles J. Bieberich, 2 Cooduvalli S. Shashikant,
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Induction of spermatogenic synchrony by retinoic acid in neonatal mice
 EDITOR'S Letter to CORNER the Editor Spermatogenesis 3:1, e23180; January/February/March 2013 2013; 2013 Landes Bioscience EDITOR'S CORNER Induction of spermatogenic synchrony by retinoic acid in neonatal
EDITOR'S Letter to CORNER the Editor Spermatogenesis 3:1, e23180; January/February/March 2013 2013; 2013 Landes Bioscience EDITOR'S CORNER Induction of spermatogenic synchrony by retinoic acid in neonatal
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Spermatogonial stem cells: What does the future hold?
 F, V & V IN OBGYN, 2011, 3 (1): 36-40 Viewpoint Spermatogonial stem cells: What does the future hold? H. TOURNAYE, E. GOOSSENS Research unit Biology of the Testis; Department of Embryology and Genetics;
F, V & V IN OBGYN, 2011, 3 (1): 36-40 Viewpoint Spermatogonial stem cells: What does the future hold? H. TOURNAYE, E. GOOSSENS Research unit Biology of the Testis; Department of Embryology and Genetics;
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
THE EFFECT OF RESTORING GDNF SIGNALING ON SPERMATOGONIAL STEM CELL DIFFERENTIATION
 THE EFFECT OF RESTORING GDNF SIGNALING ON SPERMATOGONIAL STEM CELL DIFFERENTIATION by Andrew J. Laychur A thesis submitted to the Johns Hopkins University in conformity with the requirements for the degree
THE EFFECT OF RESTORING GDNF SIGNALING ON SPERMATOGONIAL STEM CELL DIFFERENTIATION by Andrew J. Laychur A thesis submitted to the Johns Hopkins University in conformity with the requirements for the degree
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception. John K. Amory MD, MPH University of Washington October 30th, 2011
 Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception John K. Amory MD, MPH University of Washington October 30th, 2011 Bisdichloroacetyldiamines (BDADs) Shown to reversibly inhibit
Inhibition of Testicular Retinoic Acid Biosynthesis for Male Contraception John K. Amory MD, MPH University of Washington October 30th, 2011 Bisdichloroacetyldiamines (BDADs) Shown to reversibly inhibit
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1
 BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
BIOLOGY OF REPRODUCTION (2013) 88(5):131, 1 11 Published online before print 27 March 2013. DOI 10.1095/biolreprod.113.108639 Computer Simulation of the Rodent Spermatogonial Stem Cell Niche 1 Dirk G.
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Generation and validation of mtef4-knockout mice.
 Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells 1
 BOR Papers in Press. Published on May 6, 2015 as DOI:10.1095/biolreprod.114.127647 ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells 1 Hiroko Morimoto, 3 Mito Kanatsu-Shinohara,
BOR Papers in Press. Published on May 6, 2015 as DOI:10.1095/biolreprod.114.127647 ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells 1 Hiroko Morimoto, 3 Mito Kanatsu-Shinohara,
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Rac Mediates Mouse Spermatogonial Stem Cell Homing to Germline Niches by Regulating Transmigration through the Blood-Testis Barrier
 Article Rac Mediates Mouse Spermatogonial Stem Cell Homing to Germline Niches by Regulating Transmigration through the Blood-Testis Barrier Seiji Takashima, 1 Mito Kanatsu-Shinohara, 1, * Takashi Tanaka,
Article Rac Mediates Mouse Spermatogonial Stem Cell Homing to Germline Niches by Regulating Transmigration through the Blood-Testis Barrier Seiji Takashima, 1 Mito Kanatsu-Shinohara, 1, * Takashi Tanaka,
Supplementary Materials and Methods
 Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Supplementary Materials and Methods Whole Mount X-Gal Staining Whole tissues were collected, rinsed with PBS and fixed with 4% PFA. Tissues were then rinsed in rinse buffer (100 mm Sodium Phosphate ph
Computer-assisted motility analysis of spermatozoa obtained after spermatogonial stem cell transplantation in the mouse
 Computer-assisted motility analysis of spermatozoa obtained after spermatogonial stem cell transplantation in the mouse Ellen Goossens, Ph.D., Gert De Block, and Herman Tournaye, Ph.D., M.D. Research Centre
Computer-assisted motility analysis of spermatozoa obtained after spermatogonial stem cell transplantation in the mouse Ellen Goossens, Ph.D., Gert De Block, and Herman Tournaye, Ph.D., M.D. Research Centre
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Testosterone Therapy-Male Infertility
 Testosterone Therapy-Male Infertility Testosterone Therapy-Male Infertility Many men are prescribed testosterone for a variety of reasons. Low testosterone levels (Low T) with no symptoms, general symptoms
Testosterone Therapy-Male Infertility Testosterone Therapy-Male Infertility Many men are prescribed testosterone for a variety of reasons. Low testosterone levels (Low T) with no symptoms, general symptoms
Chapter 14 Reproduction Review Assignment
 Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Long-term survival of human spermatogonial stem cells in mouse testes
 FERTILITY AND STERILITY VOL. 78, NO. 6, DECEMBER 2002 Copyright 2002 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. Long-term survival
FERTILITY AND STERILITY VOL. 78, NO. 6, DECEMBER 2002 Copyright 2002 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. Long-term survival
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
follicles and spermatogonia
 5 th World Congress of the International Society for Fertility Preservation Vienna, Austria. November 16-18; 2017 Session 2: Stem cells and in vitro growth of gametes Development, sex differentiation and
5 th World Congress of the International Society for Fertility Preservation Vienna, Austria. November 16-18; 2017 Session 2: Stem cells and in vitro growth of gametes Development, sex differentiation and
Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice)
 Proc. Nati. Acad. Sci. USA Vol. 91, pp. 11298-11302, November 1994 Developmental Biology Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice) RALPH L. BRINSTER*
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 11298-11302, November 1994 Developmental Biology Spermatogenesis following male germ-cell transplantation (spermatogonia/stem cd/testes/ nsc mice) RALPH L. BRINSTER*
Male Reproductive Physiology
 Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Sperm production. Sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production Ductus deferens Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Sperm production. Sperm production. Controlling sperm production. Meiosis. Mitosis. The cells of Leydig in testes secrete
 Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Ductus deferens Sperm production Epididymis The cells of Leydig in testes secrete Seminiferous testosterone (T) tubules T secreted at puberty produces 2 o sex characteristics, spermatogenesis, & maintain
Germ cells and germ cell transplantation
 Int. J. Dev. Biol. 42: 855-860 (1998) EGF, epithelium and Germ cells and germ cell transplantation 855 Germ cells and germ cell transplantation ANNE MCLAREN* Wellcome/CRC Institute of Cancer and Developmental
Int. J. Dev. Biol. 42: 855-860 (1998) EGF, epithelium and Germ cells and germ cell transplantation 855 Germ cells and germ cell transplantation ANNE MCLAREN* Wellcome/CRC Institute of Cancer and Developmental
The Morphological Changes of Adult Mouse Testes after
 Iranian Biomedical Journal 12 (1): 35-42 (January 2008) The Morphological Changes of Adult Mouse Testes after 60 Co γ-radiation Morteza Koruji 1, Mansoureh Movahedin *1, Seyed Javad Mowla 2, Hamid Gourabi
Iranian Biomedical Journal 12 (1): 35-42 (January 2008) The Morphological Changes of Adult Mouse Testes after 60 Co γ-radiation Morteza Koruji 1, Mansoureh Movahedin *1, Seyed Javad Mowla 2, Hamid Gourabi
Bio 3201 Unit 2 REPRODUCTION AND DEVELOPMENT. Cell Division MITOSIS (P )
 Bio 3201 Unit 2 REPRODUCTION AND DEVELOPMENT 31 Hours Cell Division MITOSIS (P. 460-469) 1. Describe mitosis in detail; Specifically describe, in detail, the events of interphase, mitosis and cytokinesis
Bio 3201 Unit 2 REPRODUCTION AND DEVELOPMENT 31 Hours Cell Division MITOSIS (P. 460-469) 1. Describe mitosis in detail; Specifically describe, in detail, the events of interphase, mitosis and cytokinesis
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1
 BIOLOGY OF REPRODUCTION 63, 1185 1191 (2000) Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1 H.L. Boettger-Tong, 2,3 D.S. Johnston,
BIOLOGY OF REPRODUCTION 63, 1185 1191 (2000) Juvenile Spermatogonial Depletion (jsd ) Mutant Seminiferous Tubules Are Capable of Supporting Transplanted Spermatogenesis 1 H.L. Boettger-Tong, 2,3 D.S. Johnston,
The beginning of puberty is marked by the progressive increase in the production of sex hormones.
 Puberty is characterized by the changes that prepare the human body for the ability to reproduce. This stage generally occurs between the ages of 10 and 14 years old. The beginning of puberty is marked
Puberty is characterized by the changes that prepare the human body for the ability to reproduce. This stage generally occurs between the ages of 10 and 14 years old. The beginning of puberty is marked
Spermatogenic Stem Cell System in the Mouse Testis
 Spermatogenic Stem Cell System in the Mouse Testis S. YOSHIDA Division of Germ Cell Biology, National Institute for Basic Biology, Higashiyama, Myodaiji, Okazaki 444-8787, Okazaki, Japan Mouse spermatogenesis
Spermatogenic Stem Cell System in the Mouse Testis S. YOSHIDA Division of Germ Cell Biology, National Institute for Basic Biology, Higashiyama, Myodaiji, Okazaki 444-8787, Okazaki, Japan Mouse spermatogenesis
Development, sex differentiation and clonal expansion of PGCs to create primordial follicles and spermatogonia. Scenarios for in vitro gametogenesis
 5 th World Congress of the International Society for Fertility Preservation Vienna, Austria. November 16-18; 2017 Session 2: Stem cells and in vitro growth of gametes Development, sex differentiation and
5 th World Congress of the International Society for Fertility Preservation Vienna, Austria. November 16-18; 2017 Session 2: Stem cells and in vitro growth of gametes Development, sex differentiation and
The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development in an XX/XY mouse
 Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
Chapter 14 The Reproductive System
 Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal
 What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
What are the main functions of the male reproductive system? 1. Produce sperm 2. Deposit sperm into the female 3. Provide a pathway for the removal of urine Where is sperm produced? -In the 2 testes What
Rejuvenation of Gamete Cells; Past, Present and Future
 Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
Infertility affects 20% of couples, and severe spermatogenic
 Adenovirus-mediated gene delivery and in vitro microinsemination produce offspring from infertile male mice Mito Kanatsu-Shinohara*, Atsuo Ogura, Masaya Ikegawa, Kimiko Inoue, Narumi Ogonuki, Kei Tashiro,
Adenovirus-mediated gene delivery and in vitro microinsemination produce offspring from infertile male mice Mito Kanatsu-Shinohara*, Atsuo Ogura, Masaya Ikegawa, Kimiko Inoue, Narumi Ogonuki, Kei Tashiro,
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY
 FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring
 HUMAN REPRODUCTION Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember: Asexual reproduction:
HUMAN REPRODUCTION Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember: Asexual reproduction:
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
Maintaining the male germline: regulation of spermatogonial stem cells
 133 REVIEW Maintaining the male germline: regulation of spermatogonial stem cells Kyle Caires, Johnathan Broady and Derek McLean Department of Animal Sciences, Center for Reproductive Biology, Washington
133 REVIEW Maintaining the male germline: regulation of spermatogonial stem cells Kyle Caires, Johnathan Broady and Derek McLean Department of Animal Sciences, Center for Reproductive Biology, Washington
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
a. the tail disappears b. they become spermatids c. they undergo capacitation d. they have been stored in the uterus for several days
 (2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
(2 points each) Multiple Choice. Read each question thoroughly before answering. From the choices available, choose the answer that is the most correct. Place all answers on the accompanying answer sheet.
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring
 Quiz: Evolution Human Reproduction Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember:
Quiz: Evolution Human Reproduction Why Reproduce? In order to ensure the continuation of the species and the continuation of life in general by producing offspring Asexual vs Sexual Reproduction Remember:
Long term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device
 Long term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device Mitsuru Komeya 1, 2, Hiroshi Kimura 3, Hiroko Nakamura 3, Tetsuhiro Yokonishi 1,2, Takuya Sato 1, Kazuaki
Long term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device Mitsuru Komeya 1, 2, Hiroshi Kimura 3, Hiroko Nakamura 3, Tetsuhiro Yokonishi 1,2, Takuya Sato 1, Kazuaki
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION
 Bulgarian Journal of Veterinary Medicine (2005), 8, No 4, 227232 RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION S. GEORGIEVA 1, TS. YABLANSKI 1, P.
Bulgarian Journal of Veterinary Medicine (2005), 8, No 4, 227232 RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION S. GEORGIEVA 1, TS. YABLANSKI 1, P.
Unit 4 - Reproduction
 Living Environment Practice Exam- Parts A and B-1 1. Which cell process occurs only in organisms that reproduce sexually? A) mutation B) replication C) meiosis D) mitosis 2. Which sequence represents the
Living Environment Practice Exam- Parts A and B-1 1. Which cell process occurs only in organisms that reproduce sexually? A) mutation B) replication C) meiosis D) mitosis 2. Which sequence represents the
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes
 Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes Spermatogenesis occurs in the testes after puberty. From the testes they are deposited into the epididymas
Functions of male Reproductive System: produce gametes deliver gametes protect and support gametes Spermatogenesis occurs in the testes after puberty. From the testes they are deposited into the epididymas
Development Supplementary information
 Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
Supplemental Materials and Methods Mosaic clonal analysis GSC and SP clones were induced with the FLP/FRT-mediated mitotic recombination technique (Xu and Rubin, 1993) in files with following genotypes:
AP Biology Ch ANIMAL REPRODUCTION. Using only what you already know (you cannot look up anything) complete the chart below.
 AP Biology Ch. 46 - ANIMAL REPRODUCTION Using only what you already know (you cannot look up anything) complete the chart below. I. Overview of Animal Reproduction A. Both asexual and sexual reproduction
AP Biology Ch. 46 - ANIMAL REPRODUCTION Using only what you already know (you cannot look up anything) complete the chart below. I. Overview of Animal Reproduction A. Both asexual and sexual reproduction
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Animal Reproduction Chapter 46. Fission. Budding. Parthenogenesis. Fragmentation 11/27/2017
 Animal Reproduction Chapter 46 Both asexual and sexual reproduction occur in the animal kingdom Sexual reproduction is the creation of an offspring by fusion of a male gamete (sperm) and female gamete
Animal Reproduction Chapter 46 Both asexual and sexual reproduction occur in the animal kingdom Sexual reproduction is the creation of an offspring by fusion of a male gamete (sperm) and female gamete
Unit 2 Physiology and Health Part (a) The Reproductive System HOMEWORK BOOKLET
 Unit 2 Physiology and Health Part (a) The Reproductive System HOMEWORK BOOKLET Name: Homework Date Due Mark % Key Area 1 The structure and function of reproductive organs Key Area 2 Hormonal control of
Unit 2 Physiology and Health Part (a) The Reproductive System HOMEWORK BOOKLET Name: Homework Date Due Mark % Key Area 1 The structure and function of reproductive organs Key Area 2 Hormonal control of
SUPPLEMENTARY INFORMATION
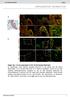 Suppl. Fig. 1 in vivo expression of ISL1 in the human fetal heart. a, Hematoxylin eosin staining showing structures of left atrium and left atrium appendage (*) of a human fetal heart at 11 weeks of gestation.
Suppl. Fig. 1 in vivo expression of ISL1 in the human fetal heart. a, Hematoxylin eosin staining showing structures of left atrium and left atrium appendage (*) of a human fetal heart at 11 weeks of gestation.
TO BE OR NOT TO BE: THE MOLECULAR MECHANISMS THAT REGULATE SPERMATOGONIAL STEM CELL FATE
 TO BE OR NOT TO BE: THE MOLECULAR MECHANISMS THAT REGULATE SPERMATOGONIAL STEM CELL FATE By FRIEDA K. CHAN A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF
TO BE OR NOT TO BE: THE MOLECULAR MECHANISMS THAT REGULATE SPERMATOGONIAL STEM CELL FATE By FRIEDA K. CHAN A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Oocyte and Sperm from ipscs
 Key Points University of Miyazaki, RIKEN, Kyoto University, Shiga University of Medical Science, Hokkaido University Oocyte and Sperm from ipscs of an Endangered Species -Sexual flexibility of endangered
Key Points University of Miyazaki, RIKEN, Kyoto University, Shiga University of Medical Science, Hokkaido University Oocyte and Sperm from ipscs of an Endangered Species -Sexual flexibility of endangered
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
The key role of vitamin A in spermatogenesis
 Review series The key role of vitamin A in spermatogenesis Cathryn A. Hogarth and Michael D. Griswold School of Molecular Biosciences, Washington State University, Pullman. Spermatogenesis in adult mammals
Review series The key role of vitamin A in spermatogenesis Cathryn A. Hogarth and Michael D. Griswold School of Molecular Biosciences, Washington State University, Pullman. Spermatogenesis in adult mammals
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
Bi-potent Gonads. Sex Determination
 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
