Characterization of commercially available nanosilver-polymer composite food packaging
|
|
|
- Nathaniel Knight
- 5 years ago
- Views:
Transcription
1 Characterization of commercially available nanosilver-polymer composite food packaging Gregory O. Noonan, Sean Conklin, Laila Ali, Timothy H. Begley Center for Food Safety and Applied Nutrition, US FDA College Park, MD, USA
2 Regulatory Authority 1958 Amendment Food Drug and Cosmetic Act Defines food additive Requires premarket approval of new uses of food additives Provides for a Generally Recognized as Safe (GRAS exemption) 1997 Food and Drug Administration Modernization Act (FDAMA) Established the Notification program for Food Contact Substances (FCS) Establishes proprietary use of FCS to the notifier. Maintains same safety standards as Food Additive Petition. Regulated in Title 21 of the US Code of Federal Regulations (21 CFR)
3 Nanotechnology and Antimicrobials DRAFT Guidance April 2012 Assessing the Effects of Significant Manufacturing Process Changes Including Emerging Technologies FDA does not categorically judge that all nano is inherently benign or harmful. Safety assessments of food substances should be based on data relevant to the physical and chemical properties of the food substance. No regulatory definition of nanomaterial. Antimicrobial US EPA Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) US FDA Federal Food, Drug, and Cosmetic Act (FFDCA) Food Quality Protection Act of 1996 (FQPA), and the Antimicrobial Regulation Technical Corrections Act of 1998 (ARTCA).
4 Research Goals Determine type of polymer and the presence of Ag and determine the form. FTIR ICP-MS Microscopy Measure migration of Ag and determine form. Migration into 3% acetic acid, ICP-MS Microscopy Evaluate antimicrobial claims. ASTM Zone of Inhibition
5 Response (normalized) Polyethylene Thickness Density Ag Conc. Ctrl 91 mm g/cm 3 < 0.5 ng Ag/g PE 63 mm g/cm 3 48 mg Ag/g PE Ag Ctrl-LDPE Ag-LDPE Wavenumber (cm -1 )
6 Response (normalized) Polypropylene Ctrl Thickness Density Ag Conc mm g/cm 3 < 0.5 ng Ag/g PP Ag 1.79 mm g/cm 3 29 mg Ag/g PP Commercial PP Commercial PP with Ag Wavenumber (cm -1 )
7 Repeat Exposure 3 % acetic acid Migration Conditions fresh acid fresh acid 100 C 4 hours Remove acid 100 C 4 hours Remove acid 100 C 4 hours Aliquot 3 + analyze polymer Aliquot 1 Aliquot 2 10 Day Storage 3 % acetic acid fresh acid 100 C 4 hours no acid 40 C 10 days 100 C 4 hours no acid 40 C 10 days Repeat 4 h 100 C Remove acid Aliquot 1 Remove acid Aliquot 2 Aliquot 3
8 Migration Results Sample Silver Concentration (ng/ml) 100% Migration Aliquot 1 Aliquot 2 Aliquot 3 Ag-LDPE Repeat Exposure < Day Storage Underline = <LOQ
9 Migration Results Sample Silver Concentration (ng/ml) 100% Migration Aliquot 1 Aliquot 2 Aliquot 3 Ag-LDPE Repeat Exposure < Day Storage Ag-PP Repeat Exposure Day Storage Underline = <LOQ
10 Migration Results Under these migration conditions would we likely measure diffusion controlled mass transfer from these materials?
11 Extrapolation Migration Estimation/Theory Connolly Volume (Å 3 ) Physicochemical view 2 Log D p 1 1 nm Irganox 1010 D ~ 1.3 x cm 2 /s Assume a nanoparticle with 5 nm radius. 2 nm sphere Connolly Volume ~ 4200 Å 3 D ~ 3 x cm 2 s -1 2 Diffusion Coefficients: A. Simon et al. J Food Nutr. Res (2008) 1 Diffusion Data: A. Reynier et al. Food Addit. Contam.16: (1999)
12 Response (normalized) FDA-Polyethylene Thickness Density Ag Conc. FDA-Ctrl 36 mm g/cm 3 < 0.5 ng Ag/g PE FDA-Ag 43 mm g/cm mg Ag/g PE (Commercial LDPE-Ag = 48 mg/g) FDA-Ctrl FDA-Ag Wavenumber (cm -1 )
13 Migration Results Sample Silver Concentration (ng/ml) 100% Migration Aliquot 1 Aliquot 2 Aliquot 3 FDA Ag-LDPE Repeat Use Ag-LDPE Repeat Use < 0.5 Ag-PP Repeat Use Underline = <LOQ
14 Zone of Inhibition Antimicrobial Properties Inoculate with E. Coli O157:H7 ASTM E : Determining Antimicrobial activity of immobilized Antimicrobial Agents under Dynamic Contact Conditions E. Coli O157:H7 in sterile buffer. + Polymer Sample Shake 1 h Incubate overnight Dilute and Streak Add Polymer Evaluate Cell Growth Incubate Overnight Count colonies
15 Ag-PP Film Zone of Inhibition Positive Control Triclosan (1%) Ag-LDPE Film No Inhibition Inhibition No Inhibition
16 Dynamic Contact Results Polymer Culture Only Control Film (blk) CFU/mL Silver Film Triclosan Film (1%) LDPE 3.5 x x x PP 1.5 x x x 10 4 NA
17 Conclusions Commercial food packaging contained low concentrations (ppm) of silver. Migration (surface desorption) of silver into 3% acetic acid. Extremely small amounts of silver (ng). Repeat exposures showed decreasing migration. None of the polymers exhibited antimicrobial properties (2 different test conditions).
18 Acknowledgements US FDA Laila Ali (Antimicrobial) Timothy Begley (Migration) Sean Conklin (ICP-MS) Tim Duncan (LDPE Films) University of Maryland NISP Lab Dr. Wen-An Chiou Food Environment Research Agency Qasim Chaudhry Emma Bradley Laurence Castle
George Misko is a Partner at Keller and Heckman LLP George joined Keller and Heckman in Mr. Misko's practice focuses on food and drug matters
 George Misko is a Partner at Keller and Heckman LLP George joined Keller and Heckman in 1989. Mr. Misko's practice focuses on food and drug matters and environmental concerns, including pesticide regulation,
George Misko is a Partner at Keller and Heckman LLP George joined Keller and Heckman in 1989. Mr. Misko's practice focuses on food and drug matters and environmental concerns, including pesticide regulation,
FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery
 FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery Laura M. Tarantino, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition (CFSAN)
FDA Oversight of Nanotechnology Applications in Foods, Food Packaging, and Nutrient Delivery Laura M. Tarantino, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition (CFSAN)
Migration and Exposure Considerations Jessica Cooper, PhD
 Migration and Exposure Considerations Jessica Cooper, PhD Chemistry Reviewer US Food and Drug Administration Office of Food Additive Safety Division of Food Contact Notification Jessica.cooper@fda.hhs.gov
Migration and Exposure Considerations Jessica Cooper, PhD Chemistry Reviewer US Food and Drug Administration Office of Food Additive Safety Division of Food Contact Notification Jessica.cooper@fda.hhs.gov
Overview of Regulatory Science of Food Contact Substances
 Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
Family Smoking Prevention and Tobacco Control Act. Comments regarding crop protection agents
 Family Smoking Prevention and Tobacco Control Act (PUBLIC LAW 111-31 JUNE 22, 2009) Comments regarding crop protection agents Presentation Objectives What is the intent of FDA in regulating CPAs? What
Family Smoking Prevention and Tobacco Control Act (PUBLIC LAW 111-31 JUNE 22, 2009) Comments regarding crop protection agents Presentation Objectives What is the intent of FDA in regulating CPAs? What
Pesticide Inert Ingredient Activities
 Pesticide Inert Ingredient Activities CPDA Regulatory Policy Conference Program March 13, 2018 P. V. Shah, Ph.D Chief, Chemistry, Inerts and Toxicology Assessment Branch (CITAB) Registration Division Office
Pesticide Inert Ingredient Activities CPDA Regulatory Policy Conference Program March 13, 2018 P. V. Shah, Ph.D Chief, Chemistry, Inerts and Toxicology Assessment Branch (CITAB) Registration Division Office
Inter-Agency Overlap and Jurisdictional Boundaries
 Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
Inter-Agency Overlap and Jurisdictional Boundaries FDLI Food Advertising, Labeling, and Litigation Conference September 14, 2017 Jessica P. O Connell Covington & Burling LLP jpoconnell@cov.com 1 FDA Approach/Perspective
FDA s Food Additives Program
 FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
Incorporating Computational Approaches into Safety Assessment
 Incorporating Computational Approaches into Safety Assessment Kristi Muldoon Jacobs, Ph.D. Supervisory Toxicologist, DFCN, Office of Food Additive Safety Director (Acting), RIS, Office of Dietary Supplement
Incorporating Computational Approaches into Safety Assessment Kristi Muldoon Jacobs, Ph.D. Supervisory Toxicologist, DFCN, Office of Food Additive Safety Director (Acting), RIS, Office of Dietary Supplement
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D.
 Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
Safety Evaluation for Substances Directly Added to Food
 Safety Evaluation for Substances Directly Added to Food Teresa Croce, PhD Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration How Does FDA Regulate
Safety Evaluation for Substances Directly Added to Food Teresa Croce, PhD Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration How Does FDA Regulate
Harnessing chemical and toxicological data for the evaluation of food ingredients and packaging
 Harnessing chemical and toxicological data for the evaluation of food ingredients and packaging Diane M. Schmit, Tammy Page, Kirk B. Arvidson, Patra Volarath, Leighna Holt US Food and Drug Administration
Harnessing chemical and toxicological data for the evaluation of food ingredients and packaging Diane M. Schmit, Tammy Page, Kirk B. Arvidson, Patra Volarath, Leighna Holt US Food and Drug Administration
An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products
 An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products John Wood Sr. Director, Agency Relations Regulatory Affairs Ecolab Inc. Antimicrobial Regulation: The Statutory Framework FIFRA
An Overview of EPA/FDA Jurisdiction of Food Contact Antimicrobial Products John Wood Sr. Director, Agency Relations Regulatory Affairs Ecolab Inc. Antimicrobial Regulation: The Statutory Framework FIFRA
FDA Food Contact Fundamentals
 FDA Food Contact Fundamentals Deborah Attwood 2017 SPE International Polyolefins Conference March 1, 2017 Legal Highlights for Food 1906: Pure Food and Drug Act 1938: Federal Food, Drug and Cosmetic Act
FDA Food Contact Fundamentals Deborah Attwood 2017 SPE International Polyolefins Conference March 1, 2017 Legal Highlights for Food 1906: Pure Food and Drug Act 1938: Federal Food, Drug and Cosmetic Act
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications
 The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese
 Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese July 2013 By: Dr. Francisco Diez Gonzalez University of Minnesota Dr. Mastura Akhtar Partners: Midwest Dairy Association
Impact of Sodium Reduction on Survival of Listeria monocytogenes in Sliced Process Cheese July 2013 By: Dr. Francisco Diez Gonzalez University of Minnesota Dr. Mastura Akhtar Partners: Midwest Dairy Association
Data Requirements Efficacy Data for FSIS, USDA, FDA
 Study Title Efficacy of High Concentrations of Hypobromous Acid Obtained from HB2 Processing Aid Sprayed on Beef Adipose Tissue Against E. coli O157:H7 and APC Bacteria Data Requirements Efficacy Data
Study Title Efficacy of High Concentrations of Hypobromous Acid Obtained from HB2 Processing Aid Sprayed on Beef Adipose Tissue Against E. coli O157:H7 and APC Bacteria Data Requirements Efficacy Data
Test Report. Report No: BSTDG RR-4 Date: Sep. 22, 2017 Page 1 of 10 :SHENZHEN WANG RUI DA TECHNOLOGY CO., LTD
 Report No: BSTDG1709784330005RR-4 Date: Sep 22, 2017 Page 1 of 10 Applicant Address :SHENZHEN WANG RUI DA TECHNOLOGY CO, LTD :Room 406, Block 4, 1980 Culture Park, Mingxing Rd Shenzhen, Guangdong, China,
Report No: BSTDG1709784330005RR-4 Date: Sep 22, 2017 Page 1 of 10 Applicant Address :SHENZHEN WANG RUI DA TECHNOLOGY CO, LTD :Room 406, Block 4, 1980 Culture Park, Mingxing Rd Shenzhen, Guangdong, China,
AgriTitan TM. Effective, Safe, & Smart. Harvest the Power of Light To Control Bacterial Plant Disease. EcoActive Surfaces, Inc.
 AgriTitan TM Effective, Safe, & Smart Harvest the Power of Light To Control Bacterial Plant Disease 1 What is AgriTitan TM? AgriTitan TM is a proprietary water-based formulation of extremely small particles
AgriTitan TM Effective, Safe, & Smart Harvest the Power of Light To Control Bacterial Plant Disease 1 What is AgriTitan TM? AgriTitan TM is a proprietary water-based formulation of extremely small particles
The Basics of the FDA s Food Contact Notification Process
 The Basics of the FDA s Food Contact Notification Process Seth Goldberg Dr. Mitchell Cheeseman Deborah Attwood November 5, 2015 Copyright 2015 Steptoe & Johnson LLP Moderator: Seth Goldberg Partner in
The Basics of the FDA s Food Contact Notification Process Seth Goldberg Dr. Mitchell Cheeseman Deborah Attwood November 5, 2015 Copyright 2015 Steptoe & Johnson LLP Moderator: Seth Goldberg Partner in
Navigating the Regulatory Environment for Food Contact Applications in the USA. PMA 2014 Annual Meeting
 Navigating the Regulatory Environment for Food Contact Applications in the USA 1 Introduction to FDA and Food Contact Any food additive should be deemed unsafe unless it is used in conformity with a regulation
Navigating the Regulatory Environment for Food Contact Applications in the USA 1 Introduction to FDA and Food Contact Any food additive should be deemed unsafe unless it is used in conformity with a regulation
Purpose of this Document
 Guidance on the Procedures for Joint Food Safety and Inspection Service (FSIS) and Food and Drug Administration (FDA) Approval of Ingredients and Sources of Radiation Used in the Production of Meat and
Guidance on the Procedures for Joint Food Safety and Inspection Service (FSIS) and Food and Drug Administration (FDA) Approval of Ingredients and Sources of Radiation Used in the Production of Meat and
BUNDESINSTITUT FÜR RISIKOBEWERTUNG
 BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
BUNDESINSTITUT FÜR RISIKOBEWERTUNG Mehrfachrückstände von Pflanzenschutzmitteln in Lebensmitteln Teil III Internationale Bewertungskonzepte für Mehrfachrückstände 10.11.2005 Cumulative Risk Assessment:
March 8, DxNow, Inc. Kevin Sly Senior Advisor to DxNow, Inc. 401 Professional Drive, Suite 130 Gaithersburg, Maryland
 March 8, 2018 Kevin Sly Senior Advisor to 401 Professional Drive, Suite 130 Gaithersburg, Maryland 20879-3429 Re: Trade/Device Name: ZyMot ICSI Sperm Separation Device, ZyMot Multi Sperm Separation Device
March 8, 2018 Kevin Sly Senior Advisor to 401 Professional Drive, Suite 130 Gaithersburg, Maryland 20879-3429 Re: Trade/Device Name: ZyMot ICSI Sperm Separation Device, ZyMot Multi Sperm Separation Device
The American Industrial Hygiene Association Stewardship 2015 June 1, 2015
 The American Industrial Hygiene Association Stewardship 2015 June 1, 2015 Devon Wm. Hill Partner Keller and Heckman LLP 1001 G Street NW Suite 500 West Washington, DC 20001 +1 202.434.4279 hill@khlaw.com
The American Industrial Hygiene Association Stewardship 2015 June 1, 2015 Devon Wm. Hill Partner Keller and Heckman LLP 1001 G Street NW Suite 500 West Washington, DC 20001 +1 202.434.4279 hill@khlaw.com
Pesticide Registration of Nanosilver
 Pesticide Registration of Nanosilver Jed Costanza, Ph.D. US EPA Office of Chemical Safety and Pollution Prevention BfR Conference on Nanosilver February 9, 2012 costanza.jed@epa.gov Issues and Presentation
Pesticide Registration of Nanosilver Jed Costanza, Ph.D. US EPA Office of Chemical Safety and Pollution Prevention BfR Conference on Nanosilver February 9, 2012 costanza.jed@epa.gov Issues and Presentation
Pesticide Risk Assessment-- Dietary Exposure
 Pesticide Risk Assessment-- Dietary Exposure Allan Felsot Department of Entomology, WSU-TC Food & Environmental Quality Lab afelsot@tricity.wsu.edu Lecture for 11/17/03 Mandates of the FQPA All tolerances
Pesticide Risk Assessment-- Dietary Exposure Allan Felsot Department of Entomology, WSU-TC Food & Environmental Quality Lab afelsot@tricity.wsu.edu Lecture for 11/17/03 Mandates of the FQPA All tolerances
Module 34: Legal aspects, ADI and GRAS status of food additives
 Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program
 Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
Recipes for Media and Solution Preparation SC-ura/Glucose Agar Dishes (20mL/dish, enough for 8 clones)
 Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
GLP VIRUCIDAL TESTING OF ZEROMOLD PLUS FOR HIV-1
 Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
FDA 510(k) 101 The Basics
 FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
SAFETY ASSESSMENT OF FOOD ADDITIVES
 SAFETY ASSESSMENT OF FOOD ADDITIVES Department of Health and Human Services U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Acronyms to Know Center for Food Safety and Applied
SAFETY ASSESSMENT OF FOOD ADDITIVES Department of Health and Human Services U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Acronyms to Know Center for Food Safety and Applied
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
2014 FDA/JIFSAN Food & Nutrition Webinar
 2014 FDA/JIFSAN Food & Nutrition Webinar Medical Foods Shawne Suggs-Anderson, MMSc, _ RD Infant Formula and Medical Foods Staff ONLDS/CFSAN/FDA September 23, 2014 53 Main Objectives History What is a Medical
2014 FDA/JIFSAN Food & Nutrition Webinar Medical Foods Shawne Suggs-Anderson, MMSc, _ RD Infant Formula and Medical Foods Staff ONLDS/CFSAN/FDA September 23, 2014 53 Main Objectives History What is a Medical
Recent Progress in the Risk Assessment of FCMs. Laurence Castle
 Recent Progress in the Risk Assessment of FCMs Laurence Castle Recent Progress in the Risk Assessment of FCMs TOPICS: 2016 EFSA opinion on FCM 2016 EFSA-WHO Review of the TTC Considerations of Nano Considerations
Recent Progress in the Risk Assessment of FCMs Laurence Castle Recent Progress in the Risk Assessment of FCMs TOPICS: 2016 EFSA opinion on FCM 2016 EFSA-WHO Review of the TTC Considerations of Nano Considerations
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
Medidée Services SA. Nano-Tera.ch. 05 February 2015 part 4. Intro ISO GMP - GLP Pierre-Alain Sommer
 Nano-Tera.ch 05 February 2015 part 4 Intro ISO GMP - GLP Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 Introduction to ISO 13485, cgmp s and GLP s Context
Nano-Tera.ch 05 February 2015 part 4 Intro ISO GMP - GLP Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 Introduction to ISO 13485, cgmp s and GLP s Context
FDA Foods Program Update
 FDA Foods Program Update Comments by Stephen F. Sundlof, D.V.M., Ph.D. Director Center for Food Safety and Applied Nutrition JIFSAN Annual Meeting, College Park, MD March 25, 2010 The Food Safety Working
FDA Foods Program Update Comments by Stephen F. Sundlof, D.V.M., Ph.D. Director Center for Food Safety and Applied Nutrition JIFSAN Annual Meeting, College Park, MD March 25, 2010 The Food Safety Working
K Michael Kolber Regulatory Affairs
 510(k) Number Submitter Name and Address Name: Contact: Address: Consultant, K122105 for the SM I Cardiovascular Patch November 7, 2012 Appendix 4: 510(k) Summary per (21CFRSO7.92) DEC 1 202 K122105 Michael
510(k) Number Submitter Name and Address Name: Contact: Address: Consultant, K122105 for the SM I Cardiovascular Patch November 7, 2012 Appendix 4: 510(k) Summary per (21CFRSO7.92) DEC 1 202 K122105 Michael
The Safety of Convenience-Size Plastic Beverage Bottles
 The Safety of Convenience-Size Plastic Beverage Bottles OVERVIEW To help assure the safety of our food, the U.S. Food and Drug Administration carefully reviews food and beverage packaging materials, including
The Safety of Convenience-Size Plastic Beverage Bottles OVERVIEW To help assure the safety of our food, the U.S. Food and Drug Administration carefully reviews food and beverage packaging materials, including
FSMA, FSVP, and FCS. Deborah Attwood May 12, 2016 Global Food Contact 2016
 FSMA, FSVP, and FCS Deborah Attwood May 12, 2016 Global Food Contact 2016 Food Safety Modernization Act (FSMA) Became law in January 2011 Improves safety and security of food supply through prevention,
FSMA, FSVP, and FCS Deborah Attwood May 12, 2016 Global Food Contact 2016 Food Safety Modernization Act (FSMA) Became law in January 2011 Improves safety and security of food supply through prevention,
Using new scientific knowledge to update regulations in the U.S.
 Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
Guide to the Regulation of Chemical Cleaning Products
 Guide to the Regulation of Chemical Cleaning Products ISSA The Worldwide Cleaning Industry Association 3300 Dundee Road Northbrook, IL 60062 www.issa.com Prepared by Bill Balek ISSA: Director of Legislative
Guide to the Regulation of Chemical Cleaning Products ISSA The Worldwide Cleaning Industry Association 3300 Dundee Road Northbrook, IL 60062 www.issa.com Prepared by Bill Balek ISSA: Director of Legislative
FREEDOM OF INFORMATION SUMMARY
 Date of Approval Letter: September 29, 2000 FREEDOM OF INFORMATION SUMMARY NEW ANIMAL DRUG APPLICATION NADA 141-147 Combination of DECCOX AND CHLORMAX in Cattle Feed (decoquinate and chlortetracycline)
Date of Approval Letter: September 29, 2000 FREEDOM OF INFORMATION SUMMARY NEW ANIMAL DRUG APPLICATION NADA 141-147 Combination of DECCOX AND CHLORMAX in Cattle Feed (decoquinate and chlortetracycline)
The Federal Insecticide, Fungicide and Rodenticide Act (FIFRA)
 July 14, 2015 Mitchell Yergert Director Division of Plant Industry Colorado Department of Agriculture 305 Interlocken Parkway Broomfield, CO 80021 Dear Mr. Yergert, On behalf of Beyond Pesticides, we are
July 14, 2015 Mitchell Yergert Director Division of Plant Industry Colorado Department of Agriculture 305 Interlocken Parkway Broomfield, CO 80021 Dear Mr. Yergert, On behalf of Beyond Pesticides, we are
Aer ODE B3-20. Database System for GEX B3 Dosimetry. Aérial. Optical Dosimetry Equipment. (gamma and/or electron beam versions)
 B3-20 Database System for GEX B3 Dosimetry (gamma and/or electron beam versions) and GEX combine to provide a Genesys 20 version of the flexible 21 CFR Part 11 certified database dosimetry system for use
B3-20 Database System for GEX B3 Dosimetry (gamma and/or electron beam versions) and GEX combine to provide a Genesys 20 version of the flexible 21 CFR Part 11 certified database dosimetry system for use
Premarket Review. FFDCA Section 201(s) FFDCA Section 201(s) (cont.)
 FFDCA Section 201(s) intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food Mitchell
FFDCA Section 201(s) intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food Mitchell
Evaluation of Antibacterial Effect of Odor Eliminating Compounds
 Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
Evaluation of Antibacterial Effect of Odor Eliminating Compounds Yuan Zeng, Bingyu Li, Anwar Kalalah, Sang-Jin Suh, and S.S. Ditchkoff Summary Antibiotic activity of ten commercially available odor eliminating
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
Active and Intelligent Packaging Solutions and Current Developments
 Active and Intelligent Packaging Solutions and Current Developments European Coatings Conference - Packaging and can coatings Corinna Keupp, Sven Sängerlaub Structure History of packaging Problem definition
Active and Intelligent Packaging Solutions and Current Developments European Coatings Conference - Packaging and can coatings Corinna Keupp, Sven Sängerlaub Structure History of packaging Problem definition
Introduction. Existing Applications. Recent Research
 Recent Research of Nano Food Packaging Dr. Nugraha Edhi Suyatma Departemen Ilmu dan Teknologi Pangan & SEAFAST Center INSTITUT PERTANIAN BOGOR Overview Introduction Existing Applications Recent Research
Recent Research of Nano Food Packaging Dr. Nugraha Edhi Suyatma Departemen Ilmu dan Teknologi Pangan & SEAFAST Center INSTITUT PERTANIAN BOGOR Overview Introduction Existing Applications Recent Research
Nutrition Labeling Laws for Food and Supplements Timeline
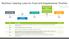 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
FOOD GRADE LUBRICANTS WHAT THEY ARE WHERE TO USE THEM Larry Ludwig, CLS, OMA, CMFS
 FOOD GRADE LUBRICANTS WHAT THEY ARE WHERE TO USE THEM Larry Ludwig, CLS, OMA, CMFS Contents What is food grade Certifications Trends Why Schaeffer s is better Anti-microbial Food Grade Lubricants Wherever
FOOD GRADE LUBRICANTS WHAT THEY ARE WHERE TO USE THEM Larry Ludwig, CLS, OMA, CMFS Contents What is food grade Certifications Trends Why Schaeffer s is better Anti-microbial Food Grade Lubricants Wherever
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels
 SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
April 30, National Organic Standards Board Spring 2012 Meeting Albuquerque, NM. Re. Vaccines from Excluded Methods. Dear Board Members:
 National Organic Standards Board Spring 2012 Meeting Albuquerque, NM Dear Board Members: These comments are submitted on behalf of Beyond Pesticides. Beyond Pesticides, founded in 1981 as a national, grassroots,
National Organic Standards Board Spring 2012 Meeting Albuquerque, NM Dear Board Members: These comments are submitted on behalf of Beyond Pesticides. Beyond Pesticides, founded in 1981 as a national, grassroots,
Guidance for Industry Acidified Foods
 Guidance for Industry Acidified Foods Draft Guidance This guidance document is being distributed for comment purposes only. Although you can comment on any guidance at any time (see 21 CFR 10.115(g)(5)),
Guidance for Industry Acidified Foods Draft Guidance This guidance document is being distributed for comment purposes only. Although you can comment on any guidance at any time (see 21 CFR 10.115(g)(5)),
Overview of the Legal Framework for Medical Device Regulation in the United States
 1 Overview of the Legal Framework for Medical Device Regulation in the United States Ellen J. Flannery This chapter provides an overview of the legal framework for medical device regulation in the United
1 Overview of the Legal Framework for Medical Device Regulation in the United States Ellen J. Flannery This chapter provides an overview of the legal framework for medical device regulation in the United
Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public Hearing;
 4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
Problems with the 1906 Act
 Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Developing a Yeast Cell Assay for Measuring the Toxicity of Inorganic Oxide Nanoparticles
 Developing a Yeast Cell Assay for Measuring the Toxicity of Inorganic Oxide Nanoparticles Citlali Garcia Saucedo Chemical & Environmental Engineering Department University of Arizona May 6 th, 2010 1 Outline
Developing a Yeast Cell Assay for Measuring the Toxicity of Inorganic Oxide Nanoparticles Citlali Garcia Saucedo Chemical & Environmental Engineering Department University of Arizona May 6 th, 2010 1 Outline
Review of Active Solutions for Packaging Applications Jari Vartiainen, Senior Scientist VTT Technical Research Centre of Finland
 VTT TECHNICAL RESEARCH CENTRE OF FINLAND LTD Review of Active Solutions for Packaging Applications Jari Vartiainen, Senior Scientist VTT Technical Research Centre of Finland VTT Technology for business
VTT TECHNICAL RESEARCH CENTRE OF FINLAND LTD Review of Active Solutions for Packaging Applications Jari Vartiainen, Senior Scientist VTT Technical Research Centre of Finland VTT Technology for business
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE. Yuan Xu 18- JAN- 2018
 BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
GHS Safety Data Sheet
 Page 1 of 5 1 PRODUCT AND COMPANY IDENTIFICATION Manufacturer 750 LAKE COOK ROAD SUITE 440 BUFFALO GROVE, IL 60089 Phone: Fax: Email: Web: (847) 215-1144 (847) 215-0577 tech@profileproducts.com www.profileproducts.com
Page 1 of 5 1 PRODUCT AND COMPANY IDENTIFICATION Manufacturer 750 LAKE COOK ROAD SUITE 440 BUFFALO GROVE, IL 60089 Phone: Fax: Email: Web: (847) 215-1144 (847) 215-0577 tech@profileproducts.com www.profileproducts.com
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Chapter 22. Uses and Limitations of Product Labeling for Public Safety
 Chapter 22 Uses and Limitations of Product Labeling for Public Safety Purpose of Product Labeling Labeling is the main method of communication between a manufacturer and the user of the product. The information
Chapter 22 Uses and Limitations of Product Labeling for Public Safety Purpose of Product Labeling Labeling is the main method of communication between a manufacturer and the user of the product. The information
memorandum Venable s FDA Practice Group FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs )
 memorandum TO Valued Dietary Supplement Clients DATE July 5, 2011 FROM Venable s FDA Practice Group RE FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs ) On July 1, 2011, the U.S. Food and
memorandum TO Valued Dietary Supplement Clients DATE July 5, 2011 FROM Venable s FDA Practice Group RE FDA Publishes Draft Guidance on New Dietary Ingredients ( NDIs ) On July 1, 2011, the U.S. Food and
Integrating the Broader Public Health Consequences of Opioid Abuse and Misuse into the Evaluation of New Opioid Products
 Integrating the Broader Public Health Consequences of Opioid Abuse and Misuse into the Evaluation of New Opioid Products Peter Lurie, MD, MPH Associate Commissioner Office of Public Health Strategy and
Integrating the Broader Public Health Consequences of Opioid Abuse and Misuse into the Evaluation of New Opioid Products Peter Lurie, MD, MPH Associate Commissioner Office of Public Health Strategy and
MATERIAL SAFETY DATA SHEET C. E. T. AQUADENT Drinking Water Additive Product Code : CET503 and CET504
 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATIONS AND OF THE COMPANY UNDERTAKING Product Name Product Description Drinking water additive for cats and dogs Manufacturer/Supplier Virbac AH, Inc. Address P.O.
1. IDENTIFICATION OF THE SUBSTANCE/PREPARATIONS AND OF THE COMPANY UNDERTAKING Product Name Product Description Drinking water additive for cats and dogs Manufacturer/Supplier Virbac AH, Inc. Address P.O.
Development of a probabilistic model to establish concentrations of migrants from packaging materials in foods
 Development of a probabilistic model to establish concentrations of migrants from packaging materials in foods Roland Franz (on behalf of all Partners from WP4.2) Fraunhofer Institut Verfahrenstechnik
Development of a probabilistic model to establish concentrations of migrants from packaging materials in foods Roland Franz (on behalf of all Partners from WP4.2) Fraunhofer Institut Verfahrenstechnik
Principles of Toxicology The Study of Poisons
 Principles of Toxicology The Study of Poisons WATER BIOLOGY PHC 6937; Section 4858 Andrew S. Kane, Ph.D. Environmental Health Program College of Public Health & Health Professions KANE@UFL.EDU Toxicology
Principles of Toxicology The Study of Poisons WATER BIOLOGY PHC 6937; Section 4858 Andrew S. Kane, Ph.D. Environmental Health Program College of Public Health & Health Professions KANE@UFL.EDU Toxicology
Detection of nanoparticles in food - an analytical challenge
 Detection of nanoparticles in food - an analytical challenge Stefan Weigel, Ruud Peters IFT International Food Nanoscience Conference July 17, 2010 Chicago, IL, USA Nano and food: a reality Naturally occuring
Detection of nanoparticles in food - an analytical challenge Stefan Weigel, Ruud Peters IFT International Food Nanoscience Conference July 17, 2010 Chicago, IL, USA Nano and food: a reality Naturally occuring
FDA Regulation of Claims on Dietary Supplement and Food Products
 FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Extractables and Leachables from Single Use Bioprocess Systems
 Extractables and Leachables from Single Use Bioprocess Systems 1 Founded in 1980 Medical Devices Food Packaging P harmaceutical Over Extractables Leachables 1,000 Projects Annually IDENTIFICATION QUANTIFICATION
Extractables and Leachables from Single Use Bioprocess Systems 1 Founded in 1980 Medical Devices Food Packaging P harmaceutical Over Extractables Leachables 1,000 Projects Annually IDENTIFICATION QUANTIFICATION
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
SAFETY DATA SHEET MANTIS EC BOTANICAL INSECTICIDE / MITICIDE. 1. Product And Company Identification
 1. Product And Company Identification Product Name: Product Use: Material Number(s): Responsible Party: Botanical Insecticide / Miticide EPA Registration Number: This product has not been registered with
1. Product And Company Identification Product Name: Product Use: Material Number(s): Responsible Party: Botanical Insecticide / Miticide EPA Registration Number: This product has not been registered with
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Safety Data Sheet. 1. Identification. 2. Hazard Identification. 3. Composition / Information on Ingredients. Report Date. 16-Feb-16.
 Page 1 of 5 1. Identification Product Name : SEED SHIELD BEANS Synonyms : None Product Use : Seed Treatment Manufacturer/Supplier : Helena Chemical Company Address : 225 Schilling Blvd. Collierville, TN
Page 1 of 5 1. Identification Product Name : SEED SHIELD BEANS Synonyms : None Product Use : Seed Treatment Manufacturer/Supplier : Helena Chemical Company Address : 225 Schilling Blvd. Collierville, TN
National Organic Program (NOP); Sunset 2017 Amendments to the National List
 This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
U. AUG skeletal dynamics. 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System
 U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
Bayer Environmental Science
 Revision Date: 08/24/2006 SECTION 1. CHEMICAL PRODUCT AND COMPANY INFORMATION Product Name CONTROL SUPER CONCENTRATE MSDS Number 102000015684 EPA Registration No. 72155-56 Bayer Environmental Science 2
Revision Date: 08/24/2006 SECTION 1. CHEMICAL PRODUCT AND COMPANY INFORMATION Product Name CONTROL SUPER CONCENTRATE MSDS Number 102000015684 EPA Registration No. 72155-56 Bayer Environmental Science 2
FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs Hyman, Phelps & McNamara, P.C. Washington, DC
 AIPLA Annual Meeting Joint Biotechnology Committee/ Special Committee on FDA Law Program October 21, 2010 Marriott Wardman Park Hotel Washington, DC FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs
AIPLA Annual Meeting Joint Biotechnology Committee/ Special Committee on FDA Law Program October 21, 2010 Marriott Wardman Park Hotel Washington, DC FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018
 Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
SNuVASivE: 51. 0(k) Premarket Notification Long Lateral Spinal System
 K111410 Page 1 of 2 SNuVASivE: 51 0(k) Premarket Notification Long Lateral Spinal System 510(k) SummaryJU 20201 In accordance with Title 21 of the Code of Federal Regulations, Part 807, and in particular
K111410 Page 1 of 2 SNuVASivE: 51 0(k) Premarket Notification Long Lateral Spinal System 510(k) SummaryJU 20201 In accordance with Title 21 of the Code of Federal Regulations, Part 807, and in particular
Packaging Safety FSSAI Perspective by Kumar Anil Advisor (Standards) Food Safety and Standards Authority of India
 Packaging Safety FSSAI Perspective by Kumar Anil Advisor (Standards) Food Safety and Standards Authority of India Contents FSSAI and its Structure Overview of Standards Development Procedure Current Scenario
Packaging Safety FSSAI Perspective by Kumar Anil Advisor (Standards) Food Safety and Standards Authority of India Contents FSSAI and its Structure Overview of Standards Development Procedure Current Scenario
(Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives)
 ก ก 2553 ก : ก (Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives) : ก ก ก : ก ก ก : 2552 : 2552 1 ก 5 ก : ก (Cochineal
ก ก 2553 ก : ก (Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives) : ก ก ก : ก ก ก : 2552 : 2552 1 ก 5 ก : ก (Cochineal
MATERIAL SAFETY DATA SHEET Consumer Product
 Page 1 of 6 1. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: GENERAL USE: Nail Care PRODUCT DESCRIPTION: PRODUCT FORMULATION NAME: CHEMICAL FAMILY: Nail Care GENERIC NAME: Green Pigment MANUFACTURER
Page 1 of 6 1. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: GENERAL USE: Nail Care PRODUCT DESCRIPTION: PRODUCT FORMULATION NAME: CHEMICAL FAMILY: Nail Care GENERIC NAME: Green Pigment MANUFACTURER
SPINE. 510(k) Summary. Anterior/anterolateral noncervical use (KWQ) (Prodct Cde):Noncervical
 k10 3?I13 SPINE 510(k) Summary FB-22f FB 24f This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Preparation Date: November
k10 3?I13 SPINE 510(k) Summary FB-22f FB 24f This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Preparation Date: November
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry
 Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin
 Product Information DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin Active Matter: 1,3-Dimethylol-5,5-dimethylhydantoin H 3 C CH 3 O HOCH
Product Information DANTOGARD 2000 PRESERVATIVE High performance preservative for Household and Industrial applications DMDM Hydantoin Active Matter: 1,3-Dimethylol-5,5-dimethylhydantoin H 3 C CH 3 O HOCH
FEMA Mission Statement and Critical Objectives
 FEMA 2014-2015 Mission Statement and Critical Objectives Mission Statement: The Flavor and Extract Manufacturers Association furthers the business interests of its members through a sound scientific program
FEMA 2014-2015 Mission Statement and Critical Objectives Mission Statement: The Flavor and Extract Manufacturers Association furthers the business interests of its members through a sound scientific program
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
Effectiveness of Selected Antimicrobial Agents and Their Possible Commercial Implementation
 Effectiveness of Selected Antimicrobial Agents and Their Possible Commercial Implementation Kay Cooksey, Clemson University Abstract Active packaging is defined as a package that senses and responds to
Effectiveness of Selected Antimicrobial Agents and Their Possible Commercial Implementation Kay Cooksey, Clemson University Abstract Active packaging is defined as a package that senses and responds to
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
LIVE INTERACTIVE YOUR DESKTOP. Food Recall Process. Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement
 LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Food Recall Process Cecilia M. Wolyniak Food and Drug Administration Office of Enforcement Wednesday, December 9, 2009 LEGAL ISSUES Code of Federal Regulations
Regulation of Dietary Supplements
 (name redacted) Analyst in Health Policy December 16, 2014 Congressional Research Service 7-... www.crs.gov R43062 Summary Many Americans take dietary supplements (e.g., vitamins, herbs, sports nutrition
(name redacted) Analyst in Health Policy December 16, 2014 Congressional Research Service 7-... www.crs.gov R43062 Summary Many Americans take dietary supplements (e.g., vitamins, herbs, sports nutrition
