Gastrointestinal side effects after intravenous erythromycin
|
|
|
- Frederick Henry
- 5 years ago
- Views:
Transcription
1 Br. J. clin. Pharmac. (1986), 21, Gastrointestinal side effects after intravenous erythromycin lactobionate K. M. DOWNEY & D. M. CHAPUT DE SAINTONGE Department of Pharmacology and Therapeutics, The London Hospital Medical College, Turner Street, London El 2AD 1 Ten healthy normal volunteers received an intravenous infusion of erythromycin lactobionate over 60 min to a total dose of 800 mg (n = 9), and 524 mg (n = 1). 2 Blood samples were collected at 10 min intervals for 100 min and gastric contents aspirated, via a nasogastric tube, from pre-dose to 105 min after start of infusion. 3 Incidence and severity of three gastrointestinal symptoms (nausea, stomach discomfort and feelings of hunger), two CNS symptoms (dizziness and faintness) and a 'control' symptom (back pain) were measured using 100 mm visual analogue scales. 4 Rate of infusion and plasma erythromycin concentration correlated with nausea (P < 0.001) and stomach discomfort (P < 0.001); plasma erythromycin concentration was also correlated with dizziness (P < 0.05). 5 Concentrations of active erythromycin in the aspirate were ph dependent. In one subject the concentration of erythromycin in the aspirate exceeded that in the plasma by 100 fold. Bile staining of samples containing the highest levels of microbiologically active erythromycin makes the origin of the erythromycin in these samples uncertain. Keywords erythromycin side effects intravenous gastric aspirate Introduction Gastrointestinal disturbance after oral erythromycin is well known. Similar disturbance after intravenous erythromycin occurs in up to 95% of subjects (Putzi et al., 1983) and is sometimes serious enough to require cessation of therapy (Klika & Goodman, 1982). The reasons for the unwanted effects are not clear but may involve the development of high erythromycin concentrations in the gut (Marlin et al., 1983), possibly in the gastric secretions where basic drugs concentrate. The aim of this study was to measure erythromycin concentrations in plasma and in gastric secretions after an intravenous dose of erythromycin, to determine whether high concentrations do develop within the stomach and to relate these concentrations to gastrointestinal disturbance. 295 Methods Ten healthy male volunteers aged years (mean 32 years) and weighing kg (mean 75 kg) were studied. Informed written consent was obtained and the study had the approval of the local Ethics Committee. Subjects who had fasted overnight were intubated, via the nose, with a Portex Ryles tube (16 FG) which was screened into the most dependent part of the stomach. Each subject then received a control infusion of 0.9% w/v saline via an indwelling venous cannula, over 15 min. This was followed immediately by an intravenous infusion of erythromycin lactobionate (Erythrocin i.v. lactobionate, Abbott Laboratories) at a concentration of 4 g l-1 in 0.9% w/v saline, over 1 h, to a total dose of 800 mg. The initial rate of infusion was 3.6 mg min7l and this was approximately doubled every
2 296 K. M. Downey & D. M. Chaput de Saintonge 15 min to give rates of 7, 14.6 and 28.4 mg min-. All infusions were given with the subject in the supine position. Blood samples (5 ml) were collected prior to erythromycin infusion and every 10 min for 100 min from the start of infusion, via an indwelling venous cannula in the other arm. Overnight gastric contents were aspirated and discarded. Gastric contents were manually aspirated over 10 min sampling periods until 105 min after the start of erythromycin infusion. Aliquots of aspirate from each sampling period were adjusted to ph > 7 with sodium bicarbonate solution (2 ml of 8.4% w/v). Where possible aspirate ph was measured using ph indicator sticks (Spezialindikator, Merck). Side effects were measured using 100 mm visual analogue scales during the control infusion and every 10 min for 100 min from the start of erythromycin infusion. The scales recorded severity of the symptoms of nausea, stomach discomfort and feelings of hunger (Blenk et al., 1980), dizziness and faintness (Austin et al., 1980), and the 'control' symptom of back pain. The latter was chosen as it has not been reported as an erythromycin side effect and was not an obvious distractor. Subjects were observed for other side effects and the severity of these recorded. Erythromycin concentrations in both plasma and gastric aspirate were determined by microbiological assay using Micrococcus lutea NCTC 8340 as the test organism. Prior to assay all aspirate samples and standards were extracted into phosphate buffer (ph 7.3) using a modification of the extraction reported by Tserng & Wagner (1976). Where necessary samples were diluted to fall within the linear range of the assay ( mg 1-1). Coefficients of variation were less than 6% and less than 16% for plasma and aspirate samples respectively. Statistical analysis was carried out using Page's L test (1965), a significance test for linear ranks which makes no assumptions regarding distribution or interpersonal correspondence of symptom scores. The critical value for significance was taken as P < Results Back pain was felt by five out of ten subjects but this was unrelated to any other factor and was alleviated by subjects changing their position. Eight out of ten subjects experienced stomach discomfort. Six subjects felt nausea with two retching and one vomiting. Eight subjects felt some degree of faintness or dizziness. One subject became very faint and was unable to continue with the study, this subject consequently received only 524 mg of erythromycin. For those subjects who experienced a symptom the median score for the visual analogue scales together with the range of their individual scores is given in Figure 1, with the exception of the 'control' symptom. Rate of infusion correlated significantly with the severity of stomach discomfort (P < 0.001, n = 10) and nausea (P < 0.001, n = 10). Significant correlation was also found between plasma erythromycin concentration and stomach discomfort (P < 0.001, n = 9), nausea (P < 0.001, n = 9) and dizziness (P < 0.05, n = 9). The mean plasma erythromycin concentration and 95% confidence limits for each sampling time are shown in Figure 2. Maximum stomach discomfort occurred at the time of peak plasma erythromycin concentration in two subjects, 10 min before in four subjects, and 20 min before peak plasma erythromycin concentration in a further two. Maximum nausea occurred at the time of peak plasma erythromycin concentration in two subjects and 10 min before plasma erythromycin peak in three subjects. Concentrations of active erythromycin in the aspirate were generally below those in the plasma, but increased towards and sometimes exceeded the plasma erythromycin concentration as the aspirate ph tended towards neutrality (Table 1). Concentration of erythromycin in the plasma was exceeded by that in the aspirate on one occasion in one subject, on two in six subjects, and on three occasions in a further subject. The erythromycin aspirate/plasma concentration ratio was between 1.0 and 8.7 (mean 2.1) on 13 of these 16'occasions, but reached as high as 112 on one occasion. Of these 16 aspirate samples containing a higher active erythromycin concentration than the plasma, 15 were bile stained (Table 1), making the origin of the erythromycin in these samples uncertain. Maximum nausea was experienced by three subjects at a time of bile staining and by two subjects 5 min before bile staining. Maximum stomach discomfort, however, showed no clear relationship with the time of bile staining. Loss of erythromycin activity appears negligible over the ph range 5-8. Thus only where the aspirate sample ph was within this range, can the measured concentration be assumed to reflect total erythromycin. However, it was not possible to detect correlation between concentration of active unhydrolysed erythromycin and either nausea (n = 5) or stomach discomfort (n = 5) where aspirate ph was above 5.5.
3 GI side effects after i. v. erythromycin 297 a Start of infusion End of infusion 40r- b CD E c 0 Cu C._ C c c.)_ 4 E 0 w 0) 0 In Figure 1 Median score (O-*) and range of scores (v-m-) for visual analogue scales for the symptoms of dizziness (a), faintness (b), feelings of hunger (c), nausea (d), and stomach discomfort (e), for those subjects experiencing these symptoms following intravenous erythromycin lactobionate 800 mg (n 9), and 524 = mg (n 1). = Discussion I I I I Severity of stomach discomfort, nausea and dizziness were each found to correlate significantly with plasma erythromycin concentration, which l 40I 60I 80I Figure 2 Plasma erythromycin concentration (mean and 95% confidence limits) following intravenous erythromycin lactobionate 800 mg (n = 9), and 524 mg (n= 1). is contrary to the findings of Blenk et al. (1980) who were unable to detect any correlation between serum erythromycin levels and incidence or severity of abdominal symptoms. This discrepancy may be due in part to the differing methods of side-effect assessment, the current study using visual analogue scales as opposed to direct questioning by an observer. It may be argued that prior knowledge by the subjects of likely symptoms and even the method of recording, which itself suggests symptoms, may have led to an increase in symptom incidence. Prior knowledge of symptoms was unavoidable if true 'informed consent' was to be obtained. The method of recording may, if it is more sensitive than that used previously, lead to an apparent increase in symptom reporting. The aim of the study was to determine whether high erythromycin concentrations developed in gastric secretions after an intravenous dose. Lee et al. (1956) recovered erythromycin, after an intravenous dose, from the intestine of bile fistula rats suggesting entry via the intestine wall. Erythromycin found in the stomach of rats (Lee et al., 1956) was suggested to have entered through the stomach. Holland & Quay (1976) demonstrated secretion of erythromycin into the lumen of rabbit jejunal sections which proceeded against a concentration gradient. The present study shows that as suggested, erythromycin clearly enters the gastric contents.
4 298 K. M. Downey & D. M. Chaput de Saintonge Table 1 Individual values for plasma erythromycin concentration, aspirate erythromycin concentration and aspirate ph, following intravenous erythromycin lactobionate 800 mg (n = 9), and 524 mg (n = 1) Subject Plasma erythromycin concentration (mg L') ND ND ND ND ND Mean s.e. mean Subject Aspirate erythromycin concentration (mg L') ND ND ND 34.0 ND ND ND ND Aspirate ph 1 ND ND ND ND ND ND ND ND 3.6 ND ND ND **7.0 * ND 7.2 ND **7.3 **7.8 *7.3 *ND *2.0 *2.0 *2.2 *ND **ND ND *ND ND ND ND ND 6 ND ND ND ND ND ND ND ND ND **ND **ND *2.2 * *5.7 *3.9 ** ** * *7.6 **7.5 *7.6 * *1.6 * **7.4 **7.4 * **7.4 **ND ND = No data. * = Bile staining present. ** = Maximum bile staining for that subject. - = Not detectable or lower than lowest reliable determination. The inability of the assay method to detect micro- erythromycin in the aspirate samples was of biologically inactive hydrolysis products, means gastric or biliary origin. However, bile staining that total erythromycin present in a number of of 15 out of 16 aspirate samples having eryaspirate samples (where ph < 5.5) may have thromycin concentrations greater than in the been considerably higher than the observed plasma suggests that it is likely to be preery,3hromycin concentration. However, no cor- dominantly of biliary origin. The mean eryration could be detected between total erythro- thromycin aspirate/plasma concentration ratio ihycin in those samples with ph > 5.5 and either of 2.1 is lower than the mean erythromycin bile/ severity of stomach discomfort or nausea, but serum concentration ratio of 8.52, seen by sample size was small (n = 5). Chevlan et al. (1979), after intravenous ery- The reflux of bile during this study means that thromycin. The sample having an erythromycin it is not possible to say for certain whether the aspirate/plasma concentration ratio of 112 was
5 more 'bile stained' than the others, and this ratio is far greater than the largest erythromycin bile/ serum concentration ratio of 23.2 previously reported (Chevlan etal., 1979). The dosage, rate of infusion and plasma levels attained were all greater in the present study. The complexity of variables involved in the measurement of aspirate erythromycin concentrations; fluctuations in ph, the acid degradation of erythromycin and bile reflux, have served to cloud the interpretation of the amount of the total erythromycin entering the stomach from References GI side effects after i. v. erythromycin 299 the plasma. However, a number of subjects had observed erythromycin concentrations in their gastric aspirate many times greater than the corresponding plasma erythromycin concentration. We would like to thank Abbott Laboratories Limited who supplied the erythromycin and provided financial support for the studies, Sister G. Keenan of the Surgical Unit, The London Hospital for performing the intubations and gastric aspirations, Mr C. Glanville for technical assistance, and Miss W. Smith and Mrs C. Studd for secretarial assistance. Austin, K. L., Mather, L. E., Philpot, C. R. & McDonald, P. J. (1980). Intersubject and dose related variability after intravenous administration of erythromycin. Br. J. clin. Pharmac., 10, Blenk, H., Blenk, B., Jahneke, G., Simm, K., Lucke, B. V. & LaFranier, D. (1980). Erythromycin infusions for treatment of infections in the ear, nose and throat region. J. int. med. Res., 8, Suppl. 2, Chevlan, P., Hamilton-Miller, J. M. T. & Brumfitt, W. (1979). Biliary excretion of erythromycin after parenteral administration. Br. J. clin. Pharmac., 8, Holland, D. R. & Quay, J. F. (1976). Intestinal secretion of erythromycin base. J. pharm. Sci., 65, Klika, L. J. & Goodman, J. M. (1982). Gastrointestinal tract symptoms from intravenously administered erythromycin. J. Am. med. Ass., 248, Lee, Gheng-Chun., Anderson, R. C. & Chen, K. K. (1956). Distribution and excretion of radioactivity in rats receiving N-methyl-"4C-erythromycin. J. Pharmac. exp. Ther., 117, Marlin, G. E., Thompson, P. J., Jenkins, C. R., Burgess, K. R. & LaFranier, D. A. J. (1983). Study of serum levels, venous irritation and gastrointestinal side-effects with erythromycin lactobionate in patients which bronchopulmonary infection. Hum. Tox., 2, Page, E. B. (1965). Ordered hypothesis for multiple treatments: A significance test for linear ranks. J. Am. statistical Ass., March, Putzi, R., Blaser, J., Luthy, R., Wehrli, R. & Siegenthaler, W. (1983). Side effects due to the intravenous infusion of erythromycin lactobionate. Infection, 11, Simpson, J. S. (1963). Microbiological assay using large plate methods. In Analytical microbiology. Vol 1. ed. Kavanagh, F., pp London: Academic Press. Tserng, K. & Wagner, J. G. (1976). Fluorimetric determination of erythromycin and erythromycin propionate in whole blood or plasma and correlation of result with microbiological assay. Analyt. Chem., 48, (Received 17 October 1984, accepted 15 October 1985)
Gastric, intestinal and colonic absorption of metoprolol in
 Br. J. clin. Pharmac. (1985), 19, 85S-89S Gastric, intestinal and colonic absorption of metoprolol in the rat J. DOMENECH', M. ALBA', J. M. MORERA', R. OBACH' & J. M. PLA DELFINA2 'Department of Pharmaceutics,
Br. J. clin. Pharmac. (1985), 19, 85S-89S Gastric, intestinal and colonic absorption of metoprolol in the rat J. DOMENECH', M. ALBA', J. M. MORERA', R. OBACH' & J. M. PLA DELFINA2 'Department of Pharmaceutics,
Flecainide pharmacokinetics in healthy volunteers: the influence of urinary ph
 Br. J. clin. Pharmac. (1985), 20, 333-338 Flecainide pharmacokinetics in healthy volunteers: the influence of urinary ph A. JOHNSTON, S. WARRNGTON' & P. TURNER Department of Clinical Pharmacology, St Bartholomew's
Br. J. clin. Pharmac. (1985), 20, 333-338 Flecainide pharmacokinetics in healthy volunteers: the influence of urinary ph A. JOHNSTON, S. WARRNGTON' & P. TURNER Department of Clinical Pharmacology, St Bartholomew's
s. J. RUNE, M.D., AND F. W. HENRIKSEN, M.D.
 GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. CARBON DOXDE TENSONS N TlE PROXMAL PART OF THE CANNE GASTRONTESTNAL TRACT s. J. RUNE, M.D., AND F. W. HENRKSEN,
GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. CARBON DOXDE TENSONS N TlE PROXMAL PART OF THE CANNE GASTRONTESTNAL TRACT s. J. RUNE, M.D., AND F. W. HENRKSEN,
What location in the gastrointestinal (GI) tract has tight, or impermeable, junctions between the epithelial cells?
 CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350)
 MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
Preliminary studies of the pharmacokinetics and pharmacodynamics
 Br. J. clin. Pharmac. (1987), 23, 137-142 Preliminary studies of the pharmacokinetics and pharmacodynamics of prochlorperazine in healthy volunteers WENDY B. TAYLOR & D. N. BATEMAN Wolfson Unit of Clinical
Br. J. clin. Pharmac. (1987), 23, 137-142 Preliminary studies of the pharmacokinetics and pharmacodynamics of prochlorperazine in healthy volunteers WENDY B. TAYLOR & D. N. BATEMAN Wolfson Unit of Clinical
NEXIUM INTRAVENOUS esomeprazole sodium
 NEXIUM INTRAVENOUS esomeprazole sodium Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about NEXIUM Intravenous (IV). It does not contain
NEXIUM INTRAVENOUS esomeprazole sodium Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about NEXIUM Intravenous (IV). It does not contain
Routes of drug administration
 Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Routes of drug administration Definition:- A route of administration in pharmacy is the path by which a drug is taken into the body. Classification:- The various routes of administrations are classified
Chapter 29 Gastrointestinal Intubation
 Chapter 29 Gastrointestinal Intubation Intubation Intubation: placement of a tube into a body structure Types of intubation Orogastric: mouth to stomach Nasogastric: nose to stomach Nasointestinal: nose
Chapter 29 Gastrointestinal Intubation Intubation Intubation: placement of a tube into a body structure Types of intubation Orogastric: mouth to stomach Nasogastric: nose to stomach Nasointestinal: nose
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
Drug CHAPTER 2. Pharmacologic Principles. NDEG 26A Eliza Rivera-Mitu, RN, MSN. Pharmacology. Drug Names. Pharmacologic Principles. Drug Names (cont'd)
 CHAPTER 2 Pharmacologic Principles NDEG 26A Eliza Rivera-Mitu, RN, MSN Drug Any chemical that affects the physiologic processes of a living organism Pharmacology The study or science of drugs Drug Names
CHAPTER 2 Pharmacologic Principles NDEG 26A Eliza Rivera-Mitu, RN, MSN Drug Any chemical that affects the physiologic processes of a living organism Pharmacology The study or science of drugs Drug Names
Chapter 2 Rationale and Objective
 Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
PK-UK Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability. Bath, November 2014
 PK-UK 2014 Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability Bath, November 2014 Prof. Dr. Jennifer Dressman Dressman Bath 2014 Why IVIVC
PK-UK 2014 Challenges and benefits of using PBPK to evaluate an IVIVC for drugs with nonideal solubility and/or permeability Bath, November 2014 Prof. Dr. Jennifer Dressman Dressman Bath 2014 Why IVIVC
PARENTERAL NUTRITION THERAPY
 UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
MOVICOL Junior Powder for Solution (macrogol 3350)
 MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
Pharmaceutics I صيدالنيات 1. Unit 2 Route of Drug Administration
 Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Pharmaceutics I صيدالنيات 1 Unit 2 Route of Drug Administration 1 Routs of Drug administration The possible routes of drug entry into the body may be divided into two classes: Parenteral Rout Enteral Rout
Slide 1. Slide 2. Slide 3. Drug Action and Handling. Lesson 2.1. Lesson 2.1. Drug Action and Handling. Drug Action and Handling.
 Slide 1 Drug Action and Handling Chapter 2 1 Slide 2 Lesson 2.1 Drug Action and Handling 1. Differentiate dose, potency, and efficacy in the context of the actions of drugs. 2. Explain the pharmacologic
Slide 1 Drug Action and Handling Chapter 2 1 Slide 2 Lesson 2.1 Drug Action and Handling 1. Differentiate dose, potency, and efficacy in the context of the actions of drugs. 2. Explain the pharmacologic
In vitro and in vivo deconvolution assessment of drug release kinetics from oxprenolol Oros preparations
 Br. J. clin. Pharmac. (1985), 19, 151S-162S In vitro and in vivo deconvolution assessment of drug release kinetics from oxprenolol Oros preparations F. LANGENBUCHER & J. MYSICKA Pharmaceutical Development,
Br. J. clin. Pharmac. (1985), 19, 151S-162S In vitro and in vivo deconvolution assessment of drug release kinetics from oxprenolol Oros preparations F. LANGENBUCHER & J. MYSICKA Pharmaceutical Development,
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350)
 MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection)
 Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection) DESCRIPTION Mucomist Respirator Solution is a derivative of the amino acid, cysteine. It acts mostly
Mucomist Respirator Solution, Acetylcysteine USP 200 mg/ ml Solution for inhalation (not for injection) DESCRIPTION Mucomist Respirator Solution is a derivative of the amino acid, cysteine. It acts mostly
Effect of Histamine H2-receptor Antagonists on. Acetaminophen and its Metabolites in Human Plasma
 Jpn. J. Pharm. Health Care Sci. Effect of Histamine H2-receptor Antagonists on Acetaminophen and its Metabolites in Human Plasma Hiroki Itoh* and Masaharu Takeyama Department of Clinical Pharmacy, Oita
Jpn. J. Pharm. Health Care Sci. Effect of Histamine H2-receptor Antagonists on Acetaminophen and its Metabolites in Human Plasma Hiroki Itoh* and Masaharu Takeyama Department of Clinical Pharmacy, Oita
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
Dietetic Outcomes in Home Parenteral Nutrition
 Dietetic Outcomes in Home Parenteral Nutrition Dr Alison Culkin Research Dietitian Intestinal Failure & Home Parenteral Nutrition St Mark s Hospital PENG Meeting November 2011 The Dietetic Outcomes Model
Dietetic Outcomes in Home Parenteral Nutrition Dr Alison Culkin Research Dietitian Intestinal Failure & Home Parenteral Nutrition St Mark s Hospital PENG Meeting November 2011 The Dietetic Outcomes Model
Total Parenteral Nutrition and Enteral Nutrition in the Home. Original Policy Date 12:2013
 MP 1.02.01 Total Parenteral Nutrition and Enteral Nutrition in the Home Medical Policy Section Durable Medical Equipment Issue Original Policy Date Last Review Status/Date Return to Medical Policy Index
MP 1.02.01 Total Parenteral Nutrition and Enteral Nutrition in the Home Medical Policy Section Durable Medical Equipment Issue Original Policy Date Last Review Status/Date Return to Medical Policy Index
Issues in Enteral Feeding: Aspiration
 Issues in Enteral Feeding: Aspiration A webinar for HealthTrust Members February 11, 2019 Co-sponsored by HealthTrust and V NOS Continuing Education Provider Presented by: Kathleen Stoessel, RN, BSN, MS
Issues in Enteral Feeding: Aspiration A webinar for HealthTrust Members February 11, 2019 Co-sponsored by HealthTrust and V NOS Continuing Education Provider Presented by: Kathleen Stoessel, RN, BSN, MS
Using a technique by which it is possible to study gastro-intestinal absorption
 531 J. Physiol. (I956) I34, 53I-537 THE ABSORPTION OF GLUCOSE BY THE INTACT RAT BY P. C. REYNELL AND G. H. SPRAY From the Nuffield Department of Clinical Medicine, University of Oxford (Received 30 May
531 J. Physiol. (I956) I34, 53I-537 THE ABSORPTION OF GLUCOSE BY THE INTACT RAT BY P. C. REYNELL AND G. H. SPRAY From the Nuffield Department of Clinical Medicine, University of Oxford (Received 30 May
principles. laboratory [Stehle & Fraser, 1935] and contains 200 pressor units and (Received 20 November 1940)
![principles. laboratory [Stehle & Fraser, 1935] and contains 200 pressor units and (Received 20 November 1940) principles. laboratory [Stehle & Fraser, 1935] and contains 200 pressor units and (Received 20 November 1940)](/thumbs/92/110006249.jpg) .#Lil-RAFY 4 233 J. Physiol. (I94I) IOO, 233-238 4 V>6x2.492.8:577.I52 I THE RATIO BETWEEN ANTIDIURETIC AND PRESSOR ACTIVITIES OF POSTERIOR PITUITARY EXTRACT SUBJECTED TO MILD HYDROLYSIS BY A. M. FRASER
.#Lil-RAFY 4 233 J. Physiol. (I94I) IOO, 233-238 4 V>6x2.492.8:577.I52 I THE RATIO BETWEEN ANTIDIURETIC AND PRESSOR ACTIVITIES OF POSTERIOR PITUITARY EXTRACT SUBJECTED TO MILD HYDROLYSIS BY A. M. FRASER
Histamine released locally after intradermal antigen challenge in man
 Br. J. clin Pharmac. (1984), 18, 915-919 Histamine released locally after intradermal antigen challenge in man D. J. HAVY, P. W. IND, A. MIYATAK, M. J. BROWN, J. MACDRMOT & C. T. DOLLRY Department of Clinical
Br. J. clin Pharmac. (1984), 18, 915-919 Histamine released locally after intradermal antigen challenge in man D. J. HAVY, P. W. IND, A. MIYATAK, M. J. BROWN, J. MACDRMOT & C. T. DOLLRY Department of Clinical
Enteral and parenteral nutrition in GI failure and short bowel syndrome
 Enteral and parenteral nutrition in GI failure and short bowel syndrome Alastair Forbes University College London Intestinal failure Inadequate functional intestine to allow health to be maintained by
Enteral and parenteral nutrition in GI failure and short bowel syndrome Alastair Forbes University College London Intestinal failure Inadequate functional intestine to allow health to be maintained by
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
NUTRITION. Elizabeth Viner Smith & Catherine Jones Foundations of Critical Care Nursing September 2017
 NUTRITION Elizabeth Viner Smith & Catherine Jones Foundations of Critical Care Nursing September 2017 Step One Competency 1.19 Factors contributing to nutritional impairment in critical illness. Nutritional
NUTRITION Elizabeth Viner Smith & Catherine Jones Foundations of Critical Care Nursing September 2017 Step One Competency 1.19 Factors contributing to nutritional impairment in critical illness. Nutritional
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
ESPEN Congress The Hague 2017
 ESPEN Congress The Hague 2017 Meeting nutritional needs of acute care patients Feeding acute pancreatitis patients J. Luttikhold (NL) FEEDING ACUTE PANCREATITIS PATIENTS Joanna Luttikhold, MD PhD Registrar
ESPEN Congress The Hague 2017 Meeting nutritional needs of acute care patients Feeding acute pancreatitis patients J. Luttikhold (NL) FEEDING ACUTE PANCREATITIS PATIENTS Joanna Luttikhold, MD PhD Registrar
Altered GI absorption in special populations: An industry perspective
 Altered GI absorption in special populations: An industry perspective Cordula Stillhart and Neil J. Parrott F. Hoffmann La Roche Ltd, Basel (CH) UNGAP WG meeting, Leuven (B) 8 March 2018 Current challenges
Altered GI absorption in special populations: An industry perspective Cordula Stillhart and Neil J. Parrott F. Hoffmann La Roche Ltd, Basel (CH) UNGAP WG meeting, Leuven (B) 8 March 2018 Current challenges
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
Methods. Effect oforal cimetidine on gastric acid secretion. Wistar rats (12) weighing g were housed
 Br. J. Pharmac. (1982), 76,551-555 THE EFFECT OF CIMETIDINE ON BASAL GASTRIC ACID SECRETION IN THE RAT N.S. BROUGHTON' & J.F. MORRIS Department of Human Anatomy, South Parks Road, Oxford OX1 3QX 1 The
Br. J. Pharmac. (1982), 76,551-555 THE EFFECT OF CIMETIDINE ON BASAL GASTRIC ACID SECRETION IN THE RAT N.S. BROUGHTON' & J.F. MORRIS Department of Human Anatomy, South Parks Road, Oxford OX1 3QX 1 The
2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Macrovic Junior powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Macrovic Junior powder for oral solution
Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets
 Journal of Pharmaceutical and Biomedical Analysis 16 (1998) 785 792 Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets Maria Aletrari *, Popi Kanari, Dora
Journal of Pharmaceutical and Biomedical Analysis 16 (1998) 785 792 Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets Maria Aletrari *, Popi Kanari, Dora
Introduction. The oxytocin/ / vasopressin receptor antagonist, atosiban, delays the gastric emptying of a semisolid meal compared to saline in human
 The oxytocin/ / vasopressin receptor antagonist, atosiban, delays the gastric emptying of a semisolid meal compared to saline in human Bodil Ohlsson, et. al., Sweden BMC Gastroenterology, 2006 Presented
The oxytocin/ / vasopressin receptor antagonist, atosiban, delays the gastric emptying of a semisolid meal compared to saline in human Bodil Ohlsson, et. al., Sweden BMC Gastroenterology, 2006 Presented
PRODUCT INFORMATION. DESCRIPTION Addaven is a concentrated trace element solution for infusion which is clear and colourless to slightly yellow.
 PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID
 GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
What can you expect after your ERCP?
 ERCP Explained and respond to bed rest, pain relief and fasting to rest the gut with the patient needing to stay in hospital for only a few days. Some patients develop severe pancreatitis and may require
ERCP Explained and respond to bed rest, pain relief and fasting to rest the gut with the patient needing to stay in hospital for only a few days. Some patients develop severe pancreatitis and may require
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults
 NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
The excretion of zopiclone into breast milk
 Br. J. clin. Pharmac. (1990), 30, 267-271 The excretion of zopiclone into breast milk I. MATHESON1, H. A. SANDE2 & J. GAILLOT3 'Department of Pharmacotherapeutics, University of Oslo, Oslo, 2Department
Br. J. clin. Pharmac. (1990), 30, 267-271 The excretion of zopiclone into breast milk I. MATHESON1, H. A. SANDE2 & J. GAILLOT3 'Department of Pharmacotherapeutics, University of Oslo, Oslo, 2Department
STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA)
 STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA) DEFINITION OF ENTERAL FEEDING INTOLERANCE Gastrointestinal feeding intolerance are usually defined as: High gastric
STRATEGIES TO IMPROVE ENTERAL FEEDING TOLERANCE. IS IT WORTH IT? ENGELA FRANCIS RD(SA) DEFINITION OF ENTERAL FEEDING INTOLERANCE Gastrointestinal feeding intolerance are usually defined as: High gastric
PRODUCT INFORMATION UROCIT -K. Wax matrix tablets
 PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
A. Incorrect! Histamine is a secretagogue for stomach acid, but this is not the only correct answer.
 Pharmacology - Problem Drill 21: Drugs Used To Treat GI Disorders No. 1 of 10 1. Endogenous secretagogues for stomach acid include: #01 (A) Histamine (B) Gastrin (C) PGE1 (D) A and B (E) A, B and C Histamine
Pharmacology - Problem Drill 21: Drugs Used To Treat GI Disorders No. 1 of 10 1. Endogenous secretagogues for stomach acid include: #01 (A) Histamine (B) Gastrin (C) PGE1 (D) A and B (E) A, B and C Histamine
Prevent gastric distention and vomiting after surgery
 Remove toxic and unwanted substances from the stomach Administration of enteral nutrition, drugs and so on It favors lung expansion in mechanically unconscious and ventilated subjects Aspiration gastric
Remove toxic and unwanted substances from the stomach Administration of enteral nutrition, drugs and so on It favors lung expansion in mechanically unconscious and ventilated subjects Aspiration gastric
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
The pharmacokinetics of nedocromil sodium, a new drug for the treatment of reversible obstructive airways disease, in.
 Br. J. clin. Pharmac. (1987), 24, 493-501 The pharmacokinetics of nedocromil sodium, a new drug for the treatment of reversible obstructive airways disease, in human volunteers and patients with reversible
Br. J. clin. Pharmac. (1987), 24, 493-501 The pharmacokinetics of nedocromil sodium, a new drug for the treatment of reversible obstructive airways disease, in human volunteers and patients with reversible
rabbit, 45 min for dog) and more slowly for dehydrocholic acid (25- decrease, questioning the mechanism by which bile acids increase bile
 J. Physiol. (1972), 224, pp. 259-269 259 With 6 text-ftgure8 Printed in Great Britain SPECIES DIFFERENCES IN THE CHOLERETIC RESPONSE TO BILE SALTS BY CURTIS D. KLAASSEN From the Clinical Pharmacology and
J. Physiol. (1972), 224, pp. 259-269 259 With 6 text-ftgure8 Printed in Great Britain SPECIES DIFFERENCES IN THE CHOLERETIC RESPONSE TO BILE SALTS BY CURTIS D. KLAASSEN From the Clinical Pharmacology and
Bioavailability and Related Pharmacokinetics in Man of Orally Administered L-5-Hydroxytryptophan in Steady State
 Acra pharmacol. et roxicol. 1980, 46, 257-262. From the University Department of Neurology, the Research Laboratory for etabolic Disorders of the University Department of Clinical Chemistry, Aarhus Kornrnunehospital,
Acra pharmacol. et roxicol. 1980, 46, 257-262. From the University Department of Neurology, the Research Laboratory for etabolic Disorders of the University Department of Clinical Chemistry, Aarhus Kornrnunehospital,
Basic Fluid and Electrolytes
 Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems
 Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Addaven concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Addaven contains: 1 ml 1 ampoule (10 ml) Chromic
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Addaven concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Addaven contains: 1 ml 1 ampoule (10 ml) Chromic
The gallbladder stores bile made by the liver. Bile helps you digest fats. During digestion, bile moves from your gall bladder to the small
 14-8-2017 The gallbladder stores bile made by the liver. Bile helps you digest fats. During digestion, bile moves from your gall bladder to the small intestine. Strattera is used to treat indications.
14-8-2017 The gallbladder stores bile made by the liver. Bile helps you digest fats. During digestion, bile moves from your gall bladder to the small intestine. Strattera is used to treat indications.
SUMMARY OF THE PRODUCT CHARACTERISTICS. The content of electrolyte ions per sachet when made up to 125 ml of solution.
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Molaxole powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains following active substances Macrogol
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Molaxole powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains following active substances Macrogol
Introducing Pharmacokinetics and Pharmacodynamics. Janice Davies Pharmacist Room 23 Maudland Building
 Introducing Pharmacokinetics and Pharmacodynamics Janice Davies Pharmacist Room 23 Maudland Building JADavies5@uclan.ac.uk 1 elearn 2 DVD Any problems / questions? 3 Learning outcomes Define and discuss
Introducing Pharmacokinetics and Pharmacodynamics Janice Davies Pharmacist Room 23 Maudland Building JADavies5@uclan.ac.uk 1 elearn 2 DVD Any problems / questions? 3 Learning outcomes Define and discuss
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
Antibiotics.. Tetracyclines. Aminoglycoside. Macrolides. Chloramphenicol
 Antibiotics. Tetracyclines. Aminoglycoside. Macrolides. Chloramphenicol Antibiotics as disturber with the biosynthesis of protein These antibiotics all target the bacterial ribosome and interfere in the
Antibiotics. Tetracyclines. Aminoglycoside. Macrolides. Chloramphenicol Antibiotics as disturber with the biosynthesis of protein These antibiotics all target the bacterial ribosome and interfere in the
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration Nager A L, Wang V J
 Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration Nager A L, Wang V J Record Status This is a critical abstract of an economic evaluation that
Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration Nager A L, Wang V J Record Status This is a critical abstract of an economic evaluation that
Paper tests for occult blood in faeces and some observations on the fate of swallowed red cells
 J. clin. Path. (1961), 14, 436 Paper tests for occult blood in faeces and some observations on the fate of swallowed red cells R. G. HUNTSMAN AND J. LIDDELL From the Department of Clinical Pathology, Guy's
J. clin. Path. (1961), 14, 436 Paper tests for occult blood in faeces and some observations on the fate of swallowed red cells R. G. HUNTSMAN AND J. LIDDELL From the Department of Clinical Pathology, Guy's
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
Stabilization Algorithm
 VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders Stabilization Algorithm Stabilization Pocket Card 1 Patient and Time Information Clinical Institute Withdrawal Assessment
VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders Stabilization Algorithm Stabilization Pocket Card 1 Patient and Time Information Clinical Institute Withdrawal Assessment
MOVICOL HALF PI December MOVICOL-Half. Powder for Solution (macrogol 3350) Potassium 5.4 mmol/l. Bicarbonate 17 mmol/l
 MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
MOVICOL -Half Powder for Solution (macrogol 3350) Product Name: Product Description: MOVICOL-Half Each sachet of MOVICOL-Half contains: Macrogol 3350 6.563 g Sodium chloride 175.4 mg Sodium bicarbonate
Walther(3), who used animals with permanent fistulae of the pancreatic
 OBSERVATIONS ON PANCREATIC SECRETION. BY G. V. ANREP, JOAN L. LUSH AND M. GRACE PALMER. (From the Institute of Physiology, University College, London.) THE experiments of Terroine (1, 2) and his collaborators,
OBSERVATIONS ON PANCREATIC SECRETION. BY G. V. ANREP, JOAN L. LUSH AND M. GRACE PALMER. (From the Institute of Physiology, University College, London.) THE experiments of Terroine (1, 2) and his collaborators,
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems
 Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Section 5.2: Pharmacokinetic properties
 Section 5.2: Pharmacokinetic properties SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
Section 5.2: Pharmacokinetic properties SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350)
 MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
MOVICOL Junior Chocolate Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Chocolate Flavour Product Description: Each sachet of MOVICOL Junior Chocolate contains: Macrogol 3350
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/016/95-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS COLISTIN SUMMARY REPORT (1) 1. Colistin
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/016/95-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS COLISTIN SUMMARY REPORT (1) 1. Colistin
Introduction & Expectations Learning Objectives Etiology & Pathophysiology of Nausea & Vomiting Pharmacology of Dimenhydrinate...
 Primary Care Paramedic Dimenhydrinate (Gravol) Certification Learner Package www.lhsc.on.ca/bhp 1 Table of Contents Introduction & Expectations... 4 Learning Objectives... 4 Etiology & Pathophysiology
Primary Care Paramedic Dimenhydrinate (Gravol) Certification Learner Package www.lhsc.on.ca/bhp 1 Table of Contents Introduction & Expectations... 4 Learning Objectives... 4 Etiology & Pathophysiology
Scope. History. History. Incretins. Incretin-based Therapy and DPP-4 Inhibitors
 Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
GASTRIC FLORA OF FASTING HEALTHY SUBJECTS AND ITS RELATIONS TO ph
 GASTRIC FLORA OF FASTING HEALTHY SUBJECTS AND ITS RELATIONS TO ph Pages with reference to book, From 113 To 116 Rakhshanda Baqai, Sarwar J. Zuberi, Pirzada M.A. Siddiqui ( PMRC Research Centre, Jinnah
GASTRIC FLORA OF FASTING HEALTHY SUBJECTS AND ITS RELATIONS TO ph Pages with reference to book, From 113 To 116 Rakhshanda Baqai, Sarwar J. Zuberi, Pirzada M.A. Siddiqui ( PMRC Research Centre, Jinnah
clearing activity is produced and destroyed in the rat. Both the
 THE SITES AT WHICH PLASMA CLEARING ACTIVITY IS PRODUCED AND DESTROYED IN THE RAT. By G. H. JEFFRIES. From the Sir William Dunn School of Pathology, Oxford. (Received for publication 25th June 1954.) CLEARING
THE SITES AT WHICH PLASMA CLEARING ACTIVITY IS PRODUCED AND DESTROYED IN THE RAT. By G. H. JEFFRIES. From the Sir William Dunn School of Pathology, Oxford. (Received for publication 25th June 1954.) CLEARING
R. B. VAUGHAN. M.B., B.S., Ph.D. Clinical Research Department, Pfizer Limited tion of the indanyl ester. Serum levels were also
 Postgraduate Medical Journal (July 1972) 48, 422-426. The pharmacokinetics of an oral form of carbenicillin in patients with renal failure R. R. BAILEY* M.D.(N.Z.), M.R.A.C.P., M.R.C.P. J. B. EASTWOOD
Postgraduate Medical Journal (July 1972) 48, 422-426. The pharmacokinetics of an oral form of carbenicillin in patients with renal failure R. R. BAILEY* M.D.(N.Z.), M.R.A.C.P., M.R.C.P. J. B. EASTWOOD
PDP 406 CLINICAL TOXICOLOGY
 PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year Mr.D.Raju.M.Pharm., Lecturer INTRODUCTION It is the process of freeing of a person or object of some contaminating substance from intestine which further
PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year Mr.D.Raju.M.Pharm., Lecturer INTRODUCTION It is the process of freeing of a person or object of some contaminating substance from intestine which further
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
COMPOSITION. A film coated tablet contains. Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar (Film coated tablets) Irbesartan
 Rotazar (Film coated tablets) Irbesartan Rotazar 75 mg, 150 mg, 300 mg COMPOSITION A film coated tablet contains Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar 75 mg, 150 mg, 300 mg PHARMACOLOGICAL
Rotazar (Film coated tablets) Irbesartan Rotazar 75 mg, 150 mg, 300 mg COMPOSITION A film coated tablet contains Active ingredient: irbesartan 75 mg, 150 mg or 300 mg. Rotazar 75 mg, 150 mg, 300 mg PHARMACOLOGICAL
Geoffrey Axiak M.Sc. Nursing (Manch.), B.Sc. Nursing, P.G. Dip. Nutrition & Dietetics Clinical Nutrition Practice Nurse
 The Percutaneous Endoscopic Gastrostomy Geoffrey Axiak M.Sc. Nursing (Manch.), B.Sc. Nursing, P.G. Dip. Nutrition & Dietetics Clinical Nutrition Practice Nurse What is a P.E.G.? Percutaneous Endoscopic
The Percutaneous Endoscopic Gastrostomy Geoffrey Axiak M.Sc. Nursing (Manch.), B.Sc. Nursing, P.G. Dip. Nutrition & Dietetics Clinical Nutrition Practice Nurse What is a P.E.G.? Percutaneous Endoscopic
SOUTHERN WEST MIDLANDS NEWBORN NETWORK
 SOUTHERN WEST MIDLANDS NEWBORN NETWORK Hereford, Worcester, Birmingham, Sandwell & Solihull Title : Person Responsible for Review : Management of Gastro-Intestinal Stomata In Neonates R. Wragg & G.Jawaheer
SOUTHERN WEST MIDLANDS NEWBORN NETWORK Hereford, Worcester, Birmingham, Sandwell & Solihull Title : Person Responsible for Review : Management of Gastro-Intestinal Stomata In Neonates R. Wragg & G.Jawaheer
Feeding Protocols Enteral or Parenteral. AM Poleÿ 2012
 Practical aspects on Feeding Protocols Enteral or Parenteral AM Poleÿ 2012 Enteral Feeding Facts A reduction in mortality Prophylaxis for stress ulcers Full-strength Time to start enteral nutrition If
Practical aspects on Feeding Protocols Enteral or Parenteral AM Poleÿ 2012 Enteral Feeding Facts A reduction in mortality Prophylaxis for stress ulcers Full-strength Time to start enteral nutrition If
5 Application of the ESR online-method for the monitoring of nanocapsule digestion
 5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
CYP2C19-Proton Pump Inhibitors
 CYP2C19-Proton Pump Inhibitors Cameron Thomas, Pharm.D. PGY2 Clinical Pharmacogenetics Resident St. Jude Children s Research Hospital February 1, 2018 Objectives: CYP2C19-PPI Implementation Review the
CYP2C19-Proton Pump Inhibitors Cameron Thomas, Pharm.D. PGY2 Clinical Pharmacogenetics Resident St. Jude Children s Research Hospital February 1, 2018 Objectives: CYP2C19-PPI Implementation Review the
MEDICATION GUIDE. BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use
 MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Fundamentals of Pharmacology for Veterinary Technicians Chapter 4
 (A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
(A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
NORMAL MAN. Tidd, 1978). These metabolites included xanthanoic. acid, hydroxyxanthanoic acid, hydroxypropantheline,
 Br. J. clin. Pharmac. (1979), 7, 89-93 PHARMACOKINETICS OF PROPANTHELINE BROMIDE IN NORMAL MAN C.W. VOSE, G.C. FORD, S.J.W. GRIGSON, N.J. HASKINS, M. PROUT, P.M. STEVENS, D.A. ROSE & R.F. PALMER Department
Br. J. clin. Pharmac. (1979), 7, 89-93 PHARMACOKINETICS OF PROPANTHELINE BROMIDE IN NORMAL MAN C.W. VOSE, G.C. FORD, S.J.W. GRIGSON, N.J. HASKINS, M. PROUT, P.M. STEVENS, D.A. ROSE & R.F. PALMER Department
NON-FATAL STRANGULATION DOCUMENTATION FORM
 NON-FATAL STRANGULATION DOCUMENTATION FORM Patient Name: Date: Medical Record Number: _ Time: is a serious event that often occurs in the context of intimate partner violence (IPV). Many times strangulation
NON-FATAL STRANGULATION DOCUMENTATION FORM Patient Name: Date: Medical Record Number: _ Time: is a serious event that often occurs in the context of intimate partner violence (IPV). Many times strangulation
Package leaflet: Information for the patient. Lacosamide G.L. 10 mg/ml solution for infusion lacosamide
 Package leaflet: Information for the patient Lacosamide G.L. 10 mg/ml solution for infusion lacosamide Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Lacosamide G.L. 10 mg/ml solution for infusion lacosamide Read all of this leaflet carefully before you start using this medicine because it contains important
WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)?
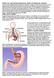 WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)? The term gastroesophageal reflux describes the movement (or reflux) of stomach contents back up into the esophagus, the muscular tube that extends from the
WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)? The term gastroesophageal reflux describes the movement (or reflux) of stomach contents back up into the esophagus, the muscular tube that extends from the
A Layperson s Guide to Pediatric Formulation Development
 A Layperson s Guide to Pediatric Formulation Development Karen C. Thompson PhD Senior Principal Scientist Preclinical Development Merck Research Laboratory West Point, Pennsylvania Martin J. Gartland PhD
A Layperson s Guide to Pediatric Formulation Development Karen C. Thompson PhD Senior Principal Scientist Preclinical Development Merck Research Laboratory West Point, Pennsylvania Martin J. Gartland PhD
(Received for publication, May 28, 1946)
 REMOVAL OF PLASMA PHOSPHOLIPIDES AS A FUNCTION OF THE LIVER: THE EFFECT OF EXCLUSION OF THE LIVER ON THE TURNOVER RATE OF PLASMA PHOSPHOLIPIDES AS MEASURED WITH RADIOACTIVE PHOSPHORUS BY C. ENTENMAN, I.
REMOVAL OF PLASMA PHOSPHOLIPIDES AS A FUNCTION OF THE LIVER: THE EFFECT OF EXCLUSION OF THE LIVER ON THE TURNOVER RATE OF PLASMA PHOSPHOLIPIDES AS MEASURED WITH RADIOACTIVE PHOSPHORUS BY C. ENTENMAN, I.
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
DRUG ELIMINATION II BILIARY EXCRETION MAMMARY, SALIVARY AND PULMONARY EXCRETION
 DRUG ELIMINATION II BILIARY EXCRETION MAMMARY, SALIVARY AND PULMONARY EXCRETION ROUTE OF DRUG ADMINISTRATION AND EXTRAHEPATIC DRUG METABOLISM The decline in plasma concentration after drug administration
DRUG ELIMINATION II BILIARY EXCRETION MAMMARY, SALIVARY AND PULMONARY EXCRETION ROUTE OF DRUG ADMINISTRATION AND EXTRAHEPATIC DRUG METABOLISM The decline in plasma concentration after drug administration
Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog
 Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
