Peptides. Prof. Dr. Mohamed Fawzy Ramadan Hassanien Zagazig University, Egypt
|
|
|
- Brendan Park
- 6 years ago
- Views:
Transcription
1 Peptides Prof. Dr. Mohamed Fawzy Ramadan Hassanien Zagazig University, Egypt
2 -Introduction to peptides -Peptide Bond -Peptide Bond Formation -Characteristics of Peptide Bonds -Peptide classes -Physical Properties of Peptides -Individual Peptides -Food-derived peptides with biological activity -Food Applications of bioactive peptides -Peptides in molecular biology
3 -Peptides (from the Greek word means "digested") are short polymers of amino acid (monomers) linked by peptide bonds, the covalent chemical bonds formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule. -Peptides are distinguished from proteins on the basis of size, typically containing fewer than 50 monomer (AA) units. -The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond. There are also tripeptides, tetrpeptides, etc. -Amino acids which have been incorporated into a peptide are termed "residues"; every peptide has a N-terminus and C- terminus residue on the ends of the peptide.
4 -A polypeptide is a long, continuous, and unbranched peptide. -Proteins consist of one or more polypeptides arranged in a biologically functional way and are often bound to cofactors, or other proteins. -Long peptides such as amyloid beta can be considered proteins, whereas small proteins such as insulin can be considered peptides.
5 Peptide Bond
6 Peptide Bond Formation -Amino acids are linked together by condensation reaction between carboxylic and amino groups from two different amino acids (with elimination of water). -The amide bond formed is called peptide bond. -The product is called a peptide, and named according to the number of amino acids involved: e.g. dipeptide (2), tripeptide (3), decapeptide (10). -Big peptides (> 50 amino acids) are called polypeptides.
7 Peptide bonds -Peptide bonds are formed by a condensation reaction of carboxylic group of an amino acid and amino group of another amino acid with removal of water molecule.
8 Characteristics of Peptide Bonds Peptide bonds are strong with partial double bond character: They are not broken by usual denaturing agents like heating or high salt concentration. They can be broken by: Prolonged exposure to strong acid or base at elevated temperatures. Specific enzymes such as digestive enzymes. Peptide bonds are rigid and planner resisting free rotation, therefore they stabilize protein structure
9 Characteristics of Peptide Bonds Peptide bonds are uncharged but polar: Peptide bonds contain polar hydrogen atoms of amino groups (with a partial positive charge) and polar oxygen atoms of carboxyl groups (with a partial negative charge). This allows hydrogen bonds to form between peptide bonds in different parts of the chain.
10 -Peptides are formed by binding amino acids together through an amide linkage. On the other hand, peptide hydrolysis results in free amino acids. -Functional groups not involved in the peptide synthesis reaction should be blocked. The protecting or blocking groups must be removed after synthesis under conditions which retain the stability of the newly formed peptide bonds. -Peptides are denoted by the number of amino acid residues as di-, tri-, tetrapeptides and the term oligopeptides is used for those with 10 or less amino acid residues.
11 -Higher molecular weight peptides are called polypeptides. -The transition of polypeptide to protein is rather undefined, but the limit is commonly assumed to be at a molecular weight of about 10 kdal, i.e., about 100 amino acid residues are needed in the chain for it to be called a protein. -The first three letters of the amino acids are used as symbols to simplify designation of peptides. Thus, the peptide shown can also be given as: Ala Ser Gly or ASG
12 A tetrapeptide (example Val-Gly-Ser-Ala) with green marked amino end (L-Valine) and blue marked carboxyl end (L-Alanine).
13 -One-letter symbols are used for amino acid sequences of long peptide chains. -In compounds in which a functional group of the side chain is involved, the bond is indicated by a perpendicular line. -The tripeptide glutathione (glutamyl-cysteinyl-glycine) is given as an illustration along with its corresponding disulfide, oxidized glutathione. -The amino acid residue with the free amino group is always placed on the left. The amino acids of the chain ends are denoted as N-terminal and C-terminal amino acid residues. -The peptide linkage direction in cyclic peptides is indicated by an arrow, i.e., CO NH-
14 Peptide classes -Peptides are divided into several classes, depending on how they are produced: 1- Milk peptides Milk peptides are formed from milk proteins by enzymatic breakdown by digestive enzymes or by the proteinases formed by lactobacilli during the fermentation of milk. 2-Ribosomal peptides -Ribosomal peptides are synthesized by translation of mrna. They are often subjected to proteolysis to generate the mature form. These function, typically in higher organisms, as hormones and signaling molecules. -Some organisms produce peptides as antibiotics, such as microcins. Since they are translated, the amino acid residues involved are restricted to those utilized by the ribosome. However, these peptides frequently have post-translational modifications, such as hydroxylation, sulfonation, and disulfide formation.
15 3- Nonribosomal peptides Peptide classes -These peptides are assembled by enzymes that are specific to each peptide, rather than by the ribosome. -The most common non-ribosomal peptide is glutathione, which is a component of the antioxidant defenses of most aerobic organisms. -Other non-ribosomal peptides are most common in plants, and fungi and are synthesized by enzyme complexes called nonribosomal peptide synthetases. -These peptides are often cyclic and can have highly-complex cyclic structures, although linear non-ribosomal peptides are also common.
16 4-Peptones Peptide classes -Peptones are derived from animal milk or meat digested by proteolytic digestion. -In addition to containing small peptides, the resulting spray-dried material includes fats, metals, salts, vitamins and many other biological compounds. -Peptone is used in nutrient media for growing bacteria and fungi. 5-Peptide fragments -Peptide fragments refer to fragments of proteins that are used to identify or quantify the source protein. -Often these are the products of enzymatic degradation performed in the laboratory on a controlled sample, but can also be samples that have been degraded by natural effects.
17 Physical Properties of Peptides
18 1- Dissociation -The isoelectric points (pi) for some peptides are listed in the table. -The acidity of the free carboxyl groups and the basicity of the free amino groups are lower in peptides than in the corresponding free amino acids. -The amino acid sequence also has an influence (e. g., Gly- Asp/Asp-Gly).
19 2- Sensory Properties Taste threshold values of various peptides (tested in aqueous solution at ph 6 7); bi -bitter
20 2- Sensory Properties -While the taste quality of amino acids does depend on configuration, peptides, except for the sweet dipeptide esters of aspartic acid, are neutral or bitter in taste with no relationship to configuration. -Bitter tasting peptides can occur in food after proteolytic reactions. For example, the bitter taste of cheese is a consequence of ripening. -Therefore, the wide use of proteolytic enzymes to achieve welldefined modifications of food proteins, without producing a bitter taste, causes some problems.
21 2- Sensory Properties Bitter taste of dipeptide A B: dependence of recognition threshold value (mmol/l) on side chain hydrophobicity (0: sweet or neutral taste) -As with amino acids, the taste intensity is influenced by the hydrophobicity of the side chains. -The taste intensity does not appear to be dependent on amino acid sequence.
22 2- Sensory Properties Peptides with a salty taste -Some peptides exhibit a salty taste, e.g. ornithyl-β-alanine hydrochloride and may be used as substitutes for sodium chloride.
23 2- Sensory Properties Effect of HCl on the salty taste of Orn-β-Ala -The intensity of the salty taste of Orn-β-Ala depends on the ph.
24 Individual Peptides Peptides are widespread in nature. They are often involved in specific biological activities (peptide hormones, peptide toxins, peptide antibiotics).
25 1-Glutathione -Glutathione (L-glutamyl-L-cysteinyl-glycine) is widespread in animals, plants and microorganisms. -Beef (200), broccoli (140), spinach (120), chicken (95), potatoes (71), paprika (49), tomatoes (49) and oranges (40) are especially rich in glutathione (mg/kg). -A noteworthy feature is the binding of glutamic acid through its carboxyl group. -The peptide is the coenzyme of glyoxalase.
26 -It is involved in active transport of amino acids and, due to its ready oxidation, is also involved in many redoxtype reactions. -It influences the rheological properties of wheat flour dough through thiol-disulfide interchange with wheat gluten. -High concentrations of reduced glutathione in flour bring about reduction of protein disulfide bonds and a corresponding decrease in molecular weight of some of the protein constituents of dough gluten.
27 2-Lysine Peptides A number of peptides including lysine have been shown to be as good as lysine in rat growth feeding tests. -These peptides substantially retard and delay the browning reaction with glucose, hence they are suitable for lysine fortification of sugar-containing foods which must be heat treated.
28 2-Lysine Peptides Browning of some lysine derivatives (0.1 M lysine or lysine derivative, 0.1 M glucose in 0.1 M phosphate buffer at ph 6.5 at 100 C in sealed tubes 1 Lys, 2 Ala-Lys, 3 Gly Lys-Gly, 4 Glu Lys, 5 Lys-Glu)
29 3- Nisin -This peptide is formed by several strains of Streptococcus lactis. -It contains a number of unusual amino acids, namely dehydroalanine, dehydro-β-methyl-alanine, lanthionine, β-methyl-lanthionine, and therefore also five thioether bridges.
30 -Nisin is active against Gram-positive microorganisms (lactic acid bacteria, Streptococci, Bacilli, Clostridia). -Nisin begins to act against the cytoplasmic membrane as soon as the spore has germinated. Hence, its action is more pronounced against spores than against vegetative cells. -Nisin is permitted as a preservative in several countries. It is used to suppress anaerobes in cheese and cheese products, especially in hard cheese and processed cheese to inhibit butyric acid fermentation. - The use of nisin in the canning of vegetables allows mild sterilization conditions.
31 4- Carnosine, Anserine and Balenine -These peptides are noteworthy since they contain a β-amino acid, β-alanine, bound to L-histidine or 1-methyl- or 3-ethyl-L-histidine, and are present in meat extract.
32 Occurrence of carnosine, anserine and balenine (%) in meat
33 -Carnosine is predominant in beef muscle tissue, while anserine is predominant in chicken meat. -Balenine is a characteristic constituent of whale muscle. -These peptides are used analytically to identify the meat extract. -Their physiological roles are not clear. -They may also be involved in generating exhausted muscle, i.e. in the muscle regaining its excitability and ability to contract.
34 Food-derived peptides with biological activity
35 -Many peptides that are released in vitro or in vivo from animal or plant proteins are bioactive and have health-promoting functions in humans beyond normal and adequate nutrition. -Different health effects have been attributed to food-derived peptides, including antimicrobial properties, blood pressurelowering effects, cholesterol-lowering ability, antioxidant activities, enhancement of mineral absorption and/or bioavailability and cyto- or immuno-modulatory effects. -Numerous products are already on the market or under development by food companies that exploit the potential of food-derived bioactive peptides which ascribe scientifically evidenced health claims to consumption of these functional foods.
36 Examples of bioactive peptides from food Many peptides of plant and animal origin with relevant bioactive potential have been discovered, with by far the most being isolated from milk-based products. Candidate proteins containing these latent biological activities are found in milk, eggs, meat and fish as well as in different plant protein sources such as soy and wheat.
37 Overview of benefical effects of bioactive peptides from food proteins -A wide range of activities has been described, including antimicrobial properties, blood pressure-lowering effects, cholesterol-lowering ability, antioxidant activities and enhancement of mineral absorption/bioavailability. -Moreover, some peptides are multi-functional and can exert more than one of the effects mentioned.
38 Antimicrobial peptides -Antimicrobial peptides have been identified from many protein hydrolysates, especially from milk. -The most well studied are the lactoferricins, which are derived from bovine and human lactoferrin. -Additionally, a few antibacterial peptides have been identified from α S1 -casein and α S2 -casein. -Antimicrobial peptides act against different Gram-positive and Gram-negative bacteria (Escherichia, Helicobacter, Listeria, Salmonella and Staphylococcus), yeasts and fungi. -The disruption of normal membrane permeability is at least partly responsible for the antibacterial mechanism of lactoferricins.
39 Antioxidant peptides -Antioxidant properties that prevent enzymatic (lipoxygenase) and non-enzymatic peroxidation of essential fatty acids have been found in peptides derived from milk proteins. -Most of the peptides identified are encoded in the sequence of α-casein. -The addition of a leucine or proline residue to the Nterminus of a His-His dipeptide, for example, can enhance antioxidant activity and facilitate further synergy with non-peptide antioxidants like BHT or BHA (synthetic antioxidants).
40 Food Applications of Bioactive Peptides -A large number of the bioactive peptides found naturally in traditional foods that have been consumed long before the term bioactive was established. -Many of these peptides are released from the host proteins by fermentation of milk, including cheese ripening. -Many peptides are generated by enzymatic reactions in the gut after ingestion of foods containing precursor proteins (e.g. after drinking a glass of milk).
41 Food Applications -Bioactive peptides are fundamental constituents of many products or ingredients marketed as Functional Foods or Nutraceuticals. -In these products the bioactive peptides are either added or enriched by modification of the usual manufacturing process (e.g. by changing process parameters or starter cultures used). -Some of these products, however, are traditional foods now offered with a different marketing strategy. -The following Table lists some examples of commercially available functional foods and food ingredients that carry bioactive peptides and includes the health claim connected with the respective product.
42 Examples of commercially available functional foods or food ingredients carrying bioactive peptides.
43 Peptides in molecular biology -Peptides have recently received importance in molecular biology for several reasons. -The first is that peptides allow the creation of peptide antibodies in animals without the need to purify the protein of interest. This involves synthesizing antigenic peptides of sections of the protein of interest. These will then be used to make antibodies in a rabbit or mouse against the protein. -Another reason is that peptides have become instrumental in mass spectrometry, allowing the identification of proteins of interest based on peptide masses and sequence. In this case, the peptides are most often generated by in-gel digestion after electrophoric separation of the proteins.
44 Peptides in molecular biology -Peptides have recently been used in the study of protein structure and function. -For example, synthetic peptides can be used as probes to see where protein-peptide interactions occur. -Inhibitory peptides are also used in clinical research to examine the effects of peptides on the inhibition of cancer proteins and other diseases.
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Polypeptides. Dr. Mamoun Ahram Summer, 2017
 Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Amino Acids and Proteins (2) Professor Dr. Raid M. H. Al-Salih
 Amino Acids and Proteins (2) Professor Dr. Raid M. H. Al-Salih 1 Some important biologically active peptides 2 Proteins The word protein is derived from Greek word, proteios which means primary. As the
Amino Acids and Proteins (2) Professor Dr. Raid M. H. Al-Salih 1 Some important biologically active peptides 2 Proteins The word protein is derived from Greek word, proteios which means primary. As the
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Raghad Abu Jebbeh. Amani Nofal. Mamoon Ahram
 ... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Chemistry 20 Chapter 14 Proteins
 Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Tala Saleh. Tamer Barakat. Diala Abu-Hassan
 13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
The Structure and Func.on of Macromolecules Proteins GRU1L6
 The Structure and Func.on of Macromolecules Proteins GRU1L6 Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure
The Structure and Func.on of Macromolecules Proteins GRU1L6 Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
! Proteins are involved functionally in almost everything: " Receptor Proteins - Respond to external stimuli. " Storage Proteins - Storing amino acids
 Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
BIOB111 - Tutorial activity for Session 14
 BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Dr. Nafith Abu Tarboush
 11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
1. Denaturation changes which of the following protein structure(s)?
 Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Enzymes for Flavour Development in Dairy Substrates. Presented by: Blanca Camarasa Senior Business Manager
 Enzymes for Flavour Development in Dairy Substrates Presented by: Blanca Camarasa Senior Business Manager Cheese composition Cheese consists of proteins and fat from milk, usually the milk of cows, goat,
Enzymes for Flavour Development in Dairy Substrates Presented by: Blanca Camarasa Senior Business Manager Cheese composition Cheese consists of proteins and fat from milk, usually the milk of cows, goat,
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Structure of -amino acids. Stereoisomers of -amino acids. All amino acids in proteins are L-amino acids, except for glycine, which is achiral.
 amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
Lecture Notes 2: Protiens
 Lecture Notes 2: Protiens BY/ARSHED ABD ALI SHIHAD Proteins and Amino Acids What Are Proteins? Large molecules Made up of chains of amino acids Are found in every cell in the body Are involved in most
Lecture Notes 2: Protiens BY/ARSHED ABD ALI SHIHAD Proteins and Amino Acids What Are Proteins? Large molecules Made up of chains of amino acids Are found in every cell in the body Are involved in most
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
LAB 4 Macromolecules
 LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
BIOLOGICAL MOLECULES. Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds.
 BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
Practice Problems 3. a. What is the name of the bond formed between two amino acids? Are these bonds free to rotate?
 Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
PEPTIDES and PROTEINS
 PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
Chapter 2. Chemical Composition of the Body
 Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
The Basics: A general review of molecular biology:
 The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the
The building blocks of life.
 The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Chapter 3. Table of Contents. Section 1 Carbon Compounds. Section 2 Molecules of Life. Biochemistry
 Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
LIP I I P D I S & PROTEINS
 LIPIDS & PROTEINS I. LIPIDS: Foods: butter, oil, Crisco, lard Commonly called fats & oils Contain more C-H bonds and less O atoms than carbohydrates. Ex: C 57 H 110 O 6 Nonpolar; therefore repel water
LIPIDS & PROTEINS I. LIPIDS: Foods: butter, oil, Crisco, lard Commonly called fats & oils Contain more C-H bonds and less O atoms than carbohydrates. Ex: C 57 H 110 O 6 Nonpolar; therefore repel water
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Cells N5 Homework book
 1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
1 Cells N5 Homework book 2 Homework 1 3 4 5 Homework2 Cell Ultrastructure and Membrane 1. Name and give the function of the numbered organelles in the cell below: A E B D C 2. Name 3 structures you might
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
6/15/2015. Biological Molecules. Outline. Organic Compounds. Organic Compounds - definition Functional Groups Biological Molecules. What is organic?
 Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
130327SCH4U_biochem April 09, 2013
 Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Chemical Composition of the Cell. B. Balen
 Chemical Composition of the Cell B. Balen Table 2-2 Molecular Biology of the Cell ( Garland Science 2008) 1. Water the most abundant substance in the cell! Where did it come from? several hypothesis: -
Chemical Composition of the Cell B. Balen Table 2-2 Molecular Biology of the Cell ( Garland Science 2008) 1. Water the most abundant substance in the cell! Where did it come from? several hypothesis: -
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
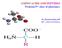 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
1.4. Lipids - Advanced
 1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
BIOLOGICALLY IMPORTANT MOLECULES
 BIOLOGICALLY IMPORTANT MOLECULES ( use with printout from zerobio website) Note: images from internet and used for educational purposes only CARBOHYDRATES: MONOSACCHARIDES H GLUCOSE FRUCTOSE GALACTOSE
BIOLOGICALLY IMPORTANT MOLECULES ( use with printout from zerobio website) Note: images from internet and used for educational purposes only CARBOHYDRATES: MONOSACCHARIDES H GLUCOSE FRUCTOSE GALACTOSE
Four Classes of Biological Macromolecules. Biological Macromolecules. Lipids
 Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Quantity Per Serving. 27 grams
 Whey Protein Isolate Nutrient Details Certain nutrients are included or omitted in our Regular Whey Isolate formula for specific reasons backed by scientific research and development Nutrient Type Protein
Whey Protein Isolate Nutrient Details Certain nutrients are included or omitted in our Regular Whey Isolate formula for specific reasons backed by scientific research and development Nutrient Type Protein
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino acids & Protein Structure Chemwiki: Chapter , with most emphasis on 16.3, 16.4 and 16.6
 Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
Organic molecules are molecules that contain carbon and hydrogen.
 Organic Chemistry, Biochemistry Introduction Organic molecules are molecules that contain carbon and hydrogen. All living things contain these organic molecules: carbohydrates, lipids, proteins, and nucleic
Organic Chemistry, Biochemistry Introduction Organic molecules are molecules that contain carbon and hydrogen. All living things contain these organic molecules: carbohydrates, lipids, proteins, and nucleic
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
The source of protein structures is the Protein Data Bank. The unit of classification of structure in SCOP is the protein domain.
 UNIT 14 PROTEINS DEFINITION A large molecule composed of one or more chains of amino acids in a specific order; the order is determined by the base sequence of nucleotides in the gene that codes for the
UNIT 14 PROTEINS DEFINITION A large molecule composed of one or more chains of amino acids in a specific order; the order is determined by the base sequence of nucleotides in the gene that codes for the
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
