ORIGINAL ARTICLES. Frank R Greer, Sharon P Marshall, Rebecca R Severson, David A Smith, Martin J Shearer, Daniel G Pace, Pieter H Joubert
|
|
|
- Joshua Jennings
- 6 years ago
- Views:
Transcription
1 300 Arch Dis Child 1998;79: ORIGINAL ARTICLES Department of Pediatrics, University of Wisconsin, Madison, Wisconsin 53715, USA F R Greer S P Marshall Department of Pediatrics, Franciscan-Skemp Medical Center, La Crosse, Wisconsin 54601, USA R R Severson D A Smith The Haemophilia Centre, St Thomas s Hospital, London SE1 7EH, UK M J Shearer Clinical Pharmacology, HoVmann-La Roche Inc, Nutley, New Jersey 07110, USA DGPace P H Joubert Correspondence to: Dr F R Greer, Perinatal Center, 202 South Park Street, Madison, WI 53715, USA. Accepted 7 April 1998 A new mixed micellar preparation for oral vitamin K prophylaxis: randomised controlled comparison with an intramuscular formulation in breast fed infants Frank R Greer, Sharon P Marshall, Rebecca R Severson, David A Smith, Martin J Shearer, Daniel G Pace, Pieter H Joubert Abstract Objective To compare a new oral preparation of vitamin K 1 (Konakion MM) containing lecithin and glycocholic acid with a standard intramuscular (IM) preparation during the first 8 weeks of life in exclusively breast fed infants. Methods Infants were randomised at birth to the IM group (1 mg vitamin K) or the oral group (2 mg given at birth and repeated at 7 and 30 days of life). Prothrombin time (INR), plasma vitamin K 1, and PIVKA II (undercarboxylated prothrombin) were monitored at 14, 30, and 56 days of age. Results Seventy nine infants were randomised to the oral group and 77 to the IM group. Sixty seven infants in each group completed eight weeks of the study. Prothrombin times did not diver between the two groups. Mean (SD) plasma vitamin K 1 values (in ng/ml) decreased in both groups over time, but were higher in the oral group at 14 and 56 days: 2.0 (1.6) v 1.3 (1.1) at 14 days; 0.5 (0.3) v 0.5 (0.7) at 30 days; and 0.5 (0.8) v 0.2 (0.2) at 56 days of life. PIVKA II was raised (> 0.1 AU/ml) in cord blood in 47% of the infants. By 14 days, only one infant in each group had a raised PIVKA II value and both of these initially had high concentrations of PIVKA II in cord blood. At 30 days, there were no raised PIVKA II values. At 56 days, there were no raised PIVKA II values in the oral group, although three infants in the IM group had raised values. Conclusions Plasma vitamin K concentrations were at least equal or significantly higher in babies given oral vitamin K supplements compared with IM treated babies at the time points measured. Through the first 8 weeks of life, multiple doses of the new oral preparation maintain haemostasis and vitamin K status in breast fed infants at least equal to that of the intramuscular preparation. (Arch Dis Child 1998;79: ) Keywords: vitamin K 1 deficiency; breast feeding; haemorrhagic disease Vitamin K 1 deficiency can cause a bleeding disorder in infants through the first 3 months of life. It is mainly a problem in breast fed infants, particularly those who have disorders of intestinal absorption (biliary atresia, α-1 antitrypsin deficiency, etc). 1 2 The eycacy of newborn intramuscular vitamin K prophylaxis for prevention of this bleeding problem has been well established. However, in some Western countries where oral prophylaxis of vitamin K has been promoted over the traditional intramuscular route, the problem of haemorrhagic disease of the newborn has become a significant concern once again. 3 8 This probably reflects the variable intestinal absorption of presently available preparations. 9 There is a new oral formulation of vitamin K available for prophylactic use in newborns. This new preparation, Konakion MM, is prepared with the phospholipid lecithin and the bile salt glycocholic acid, because vitamin K requires emulsification and the presence of bile salts for its absorption. Limited studies have shown that this mixed micelle solution has greater reliability of absorption in both normal newborns and children with chronic cholestasis However, it has not been compared directly with the intramuscular form in newborn infants except in one small study during the first few weeks of life, in which infants received a single dose of Konakion MM. 11 The purpose of our study was to make this comparison in breast feeding infants. We hypothesised that multiple doses of Konakion MM given during the 1st month of life to exclusively breast feeding infants would be well tolerated, and that their vitamin K 1 status would not diver from that of breast feeding infants who received a single intramuscular dose of vitamin K 1 at birth. Methods Our study was carried out at two private hospitals in southern Wisconsin: Meriter Hospital in Madison and Franciscan-Skemp Medical Center in La Crosse, where most of the infants are breast feeding at the time of discharge. All mothers participating in the study were recruited before delivery. At both hospitals, a
2 Infant oral vitamin K prophylaxis 301 single 1 mg injection of vitamin K 1 is given routinely after birth to all infants. Inclusion criteria included a gestational age of > 37 weeks or < 42 weeks, a birth weight > 2500 g, singleton, mother planning to breast feed her infant until at least 2 months of age, absence of major congenital or malabsorptive disorders, no maternal history of anticonvulsant treatment during pregnancy, and an Apgar score of > 7 at five minutes. Written informed consent was obtained from all mothers and the protocols were approved by the human subject committees at both hospitals. Immediately after birth, infants were randomised to receive either a standard 1 mg intramuscular injection of vitamin K 1 (Konakion) or a 2 mg oral dose of the experimental preparation (Konakion MM). Randomisation was accomplished using a computer generated list, randomised in blocks of four. Because of the diverent methods of drug administration, subjects and clinical study personnel were not blinded to the diverent treatments. Both drugs were supplied in single dose glass vials by HoVmann-La Roche. The intramuscular vitamin K was given according to standard hospital practice. The oral preparation was given by drawing up 0.2 ml (2 mg) of the medication in a 1 ml tuberculin syringe, and dispensing it on to the back of the infant s tongue. The infant was observed for five minutes and if any spitting was noted, the infant was given a dose of intramuscular vitamin K and dropped from the study. The oral dose was repeated during home visits by registered nurses, at 7 and 30 days of age, to give a total dose of 6 mg in the 1st month of life. If an infant in the oral group dropped out before completing the eight week study, an intramuscular dose of vitamin K 1 was given. All mothers were expected to breast feed their infants exclusively. Solid foods were not introduced. Mothers were supplied with a limited quantity of Similac PM 60/40 without added vitamin K 1 (supplied by Ross Laboratories, Columbus, Ohio, USA) for emergency use only. If more than one bottle a week of the formula was used, infants were withdrawn from the study. Potentially adverse events pertaining to all major organ systems were monitored. BLOOD SAMPLING AND LABORATORY METHODS Cord blood samples (5 ml) were collected in EDTA coated glass tubes from all placentas. Additional venous or capillary (heel prick) blood samples (3 4 ml) were collected at 2, 4, and 8 weeks after birth into glass tubes containing EDTA. A topical anaesthetic cream (EMLA) was used for blood sampling. Plasma vitamin K 1 concentrations and prothrombin time were measured at 14, 30, and 56 days after birth. Vitamin K 1 was not measured in cord blood, where it is generally undetectable with the assay used in our study. PIVKA II (protein induced in the absence of vitamin K 1 or des-gamma-carboxy-prothrombin) was measured in all blood samples. Plasma for vitamin K 1 and PIVKA II was separated by centrifugation from red cells and stored at 20 C until analysis. Laboratory personnel were blinded to the infant s randomisation. Vitamin K 1 was assayed in plasma by a modification 12 of an earlier multistage procedure. 13 After extraction of 0.5 ml of plasma with hexane, the lipid extract was purified by normal phase, high performance liquid chromatography (HPLC), and analysed with reversed phase HPLC, using electrochemical detection in the redox mode. Menaquinone-6 was used as the internal standard. The lower limit of detection was 0.05 ng/ml. The intraassay precision for three plasma pools at mean measured concentrations of 0.30, 0.76, and 3.66 ng/ml was 5.0%, 7.2%, and 3.3%, respectively. The interassay precision over the period of our study was 11.3%, 10.2%, and 9.8%, respectively, for the same plasma pools. The normal ranges in adults are: fasting, ng/ml (mean, 0.41) and non-fasting, ng/ml (mean, 0.66), with a low value being < 0.15 ng/ml. PIVKA II was determined by an enzyme linked immunosorbent assay (ELISA) using a commercial kit (Eitest mono P-II; Eisai Co Ltd, Tokyo, Japan) with monoclonal antibodies specific for the inactive des-gamma-carboxy form of prothrombin. 14 Results using this assay are expressed as AU/ml (arbitrary units/ml), with one AU corresponding to 1 µg of purified prothrombin. The maximum concentration measured in a group of normal, healthy adults was < 0.1 AU/ml. Therefore, this value was taken as the cut ov point (or upper limit of normal) of the assay and a value > 0.1 AU/ml was reported as PIVKA II antigen positive. The calibration plots of the log of optical density v PIVKA II concentration were linear over the range of standards ranging between and 2.0 AU/ml. For our study, three quality control plasma pools corresponding to low ( 0.1 AU/ml), middle ( 0.5 AU/ml), and high ( 1.0 AU/ml) PIVKA II values were prepared by mixing a plasma pool taken from patients undergoing warfarin treatment with normal plasma. These were then assayed in duplicate in each assay run. The interassay precision for these three pools was 9.8% for the low pool, 6.2% for the middle pool, and 9.0% for the high pool. With this assay, adult patients on stable anticoagulant treatment have PIVKA II values of AU/ml with a mean of 40 AU/ml. Capillary whole blood prothrombin times (PTs) were measured during the home visits with a Biotrak coagulation monitor (Boehringer Mannheim, Indianapolis, Indiana, USA), which has been used and validated in adults and small children. This monitor uses a disposable plastic reagent cartridge and one drop of whole blood. The PT is determined by the amount of time required for the blood to flow by capillary action through a channel between the initial reservoir and a mixing chamber that is coated with rabbit brain thromboplastin. The cessation of blood flow (clotting) is sensed by variation in the light scatter caused by movement of erythrocytes. The change in light scattering is detected by a photodetector and converted into an electrical
3 302 Greer, Marshall, Severson, Smith, Shearer, Pace, et al Table 1 Mean (SD) vitamin K 1 plasma concentrations and INR values Oral Intramuscular p value* Vitamin K 1 (ng/ml) 14 days 2.1 (1.6) (n = 66) 1.3 (1.1) (n = 69) < days 0.5 (0.3) (n = 64) 0.5 (0.7) (n = 65) NS 56 days 0.5 (0.8) (n = 63) 0.2 (0.2) (n = 66) < INR 14 days 0.96 (0.33) (n = 69) 0.95 (0.25) (n = 70) NS 30 days 0.86 (0.18) (n = 66) 0.84 (0.14) (n = 71) NS 56 days 0.83 (0.18) (n = 64) 0.84 (0.13) (n = 67) NS *Wilcoxon 2 sample test. Table 3 Table 2 Number of infants with positive PIVKA II (> 0.1 AU/ml) Oral Intramuscular Cord blood 35 (44%) 40 (52%) 14 days days days 0 3 One infant in the intramuscular group was positive at both 14 and 56 days. Cord blood values ranged from < 0.1 to 66 AU/ml. signal that is analysed by a microprocessor and displayed on a screen as the PT and the international normalised ratio (INR). Infant haematocrits (autoanalyser, hospital haematology laboratory) were measured on the day of Biotrak measurements to ensure that the haematocrit was in the range recommended for the methodology (> 23% and < 53%). If Biotrak readings for PT were < 9 seconds or > 14 seconds (INR > 1.37), the PT was to be repeated immediately in the central coagulation laboratory after collection of a new sample by venepuncture. However, values for PT in our study never fell outside of this range on the Biotrak. To test the use of the Biotrak in newborn infants, 10 samples of cord blood were collected from 10 diverent elective caesarian section deliveries. One of these samples was discarded as the haematocrit was > 53%. PT was measured simultaneously on the Biotrak and in the hospital coagulation laboratory. None of the paired samples had an INR diverence of more than 0.33 INR units, the mean being STATISTICAL METHODS For statistical purposes, the following hypothesis was to be tested: oral Konakion MM is as evective as intramuscular Konakion in maintaining plasma vitamin K 1 concentrations and plasma prothrombin times at 30 days. The Wilcoxon test was used, with a value of p < 0.05 being considered significant. We estimated that the probability of detecting plasma Subjects with positive PIVKA IIs > 14 days Subject Birth 14 days 30 days 56 days 102 (oral) PIVKA (AU/ml) <0.1 <0.1 Vitamin K (ng/ml) ND (IM) PIVKA (AU/ml) < Vitamin K (ng/ml) ND (IM) PIVKA (AU/ml) < 0.1 < 0.1 < Vitamin K (ng/ml) ND < (IM) PIVKA (AU/ml) 0.21 <0.1 < Vitamin K (ng/ml) ND Positive values in bold. IM, intramuscular; ND, not done. PIVKA II at 30 days after intramuscular administration of Konakion is small (as little as 5/ neonates), so practical limitations did not allow us to use this diverence as a study outcome, because more than a thousand infants would need to be enrolled. Because the analysis was an intent to treat one, dropouts were not replaced. Randomisation was continued until a minimum of 60 evaluable infants (completing the eight week study period) was obtained in each treatment group. Infants who completed at least two weeks of the study were included in the analysis. Results One hundred and fifty six infants (77 boys) were enrolled in the study, 79 in the oral group (mean (SD) birth weight, 3.6 (0.6) kg) and 77 in the IM group (mean (SD) birth weight, 3.5 (0.4) kg). Sixty seven infants in each group completed a full eight weeks of the study. Seven infants in the oral and one infant in the IM vitamin K groups dropped out before two weeks and could not be included in the data analysis. An additional 14 infants completed two weeks but less than eight weeks of the study, and were included in the analysis. All but two infants were white, there being one Hispanic infant in each group. Table 1 shows the results of the vitamin K 1 plasma concentrations. The concentrations were highest on day 14 in both groups, although the concentrations in the oral group were significantly higher than the IM group. By day 30, there was no significant diverence between the two groups, failing to disprove the null hypothesis. With an n of 120, the power to detect a diverence of 0.30 ng/ml between the two groups was 85%. Whereas the concentrations in the oral group had stabilised by 30 days, they continued to fall in the IM group until 56 days. At 56 days, the concentrations in the oral group were significantly higher than the IM group. Table 2 shows the results of the PIVKA II analysis. Note the very high percentage of positive PIVKA II values at the time of birth 47% of all infants. Eleven infants had cord blood values > 2.0 AU/ml. This is in agreement with high detection rates in other studies There were only two positive PIVKA II values (> 0.1 AU/ml) at 14 days (one in each group), none at 28 days, and three at 56 days (all in the IM group). Two of the positive values were in the same patient (patient 230) in the IM group (table 3). The limited number of subjects in this study precludes estimates of relative event rates of positive PIVKA II in these populations. Table 3 shows the four subjects with raised PIVKA II values and their simultaneously measured vitamin K 1 values. At 56 days, the three raised PIVKA II values were associated with low vitamin K 1 (< 0.15 ng/ml). However the raised PIVKA II values at 14 days (patients 102 and 230) were associated with normal concentrations of vitamin K 1 and raised concentrations of PIVKA II at birth. Figure 1 shows the percentage of infants with low plasma vitamin K 1 concentrations (< 0.15 ng/ml) at 30 and 56 days of age. It
4 Infant oral vitamin K prophylaxis 303 % of subjects % Oral IM 5.80% 11.10% 31.80% 0 30 days 56 days Figure 1 Percentage of infants with low plasma vitamin K 1 concentrations at 30 and 56 days. should be noted that no infants had low vitamin K 1 values at 14 days. By 56 days, 21 of 28 of the low vitamin K 1 values were in the IM group, but only two of these infants had raised PIVKA II values (table 3). A total of seven infants in the oral group had low vitamin K 1 concentrations, but there were no positive PIVKA II values in this group at 56 days. Table 1 shows the INR data for the prothrombin times. Although there is a fall in both groups between days 14 and 30, the groups did not diver significantly at any time point. No infant had a haematocrit that was out of the range reported for use with the Biotrak coagulation monitor. There were no major adverse events attributed to the vitamin K preparations used in this study, at least during the first 2 months of life. There were no bleeding episodes of any kind. Eight infants dropped out in the first two weeks of the study. Two of these developed intercurrent illnesses not related to the vitamin K administration. Of the remaining six infants, five were removed for protocol violations and one withdrew consent. An additional 14 infants dropped out after two weeks. One of these had an intercurrent illness, six were dropped for protocol violations, and seven mothers withdrew consent for their infants to continue in the study. Infants tolerated the oral medication very well. No infant spat the drug after administration, thus requiring removal from the study. Discussion The use of oral vitamin K 1 for prophylaxis of the newborn infant has become more widespread following a report of an association between intramuscular vitamin K 1 in the newborn and childhood cancer. 19 However, other investigators in Europe and elsewhere have not confirmed the association, although a very small increased incidence of childhood leukaemia cannot be ruled out. The American Academy of Pediatrics has recommended continued use of intramuscular prophylaxis, because there is no licensed product for oral prophylaxis in the USA. 6 In Europe, the preparation studied in our report is now approved for use in some countries, although a preliminary report from Switzerland indicates several treatment failures in breast fed infants with cholestatic liver disease. 29 In Switzerland, only two doses are given, both during the 1st week of life, even though more than two doses are recommended for breast feeding infants. 30 Obviously, there is a need for an oral vitamin K product. This is the first study in infants to compare directly the new oral Konakion MM with a standard intramuscular preparation. Our study has shown clearly that Konakion MM, when given three times in the first 30 days of life, maintains blood concentrations of vitamin K 1 for the first 2 months of life at a concentration equal to or greater than that achieved with standard intramuscular prophylaxis. In fact, a third oral dose given at 30 days maintains the blood concentration of vitamin K 1 for 56 days, by which time the blood concentration in the intramuscular group has decreased significantly (table 1). This is important because late haemorrhagic disease after the administration of intramuscular preparations usually occurs during the first 3 months of life, and is often associated with intestinal malabsorption. 1 We speculate that, when given in three doses, the mixed micellar form of vitamin K 1 would prevent most cases of late haemorrhagic disease, especially as it has been used to maintain vitamin K status in older children with malabsorption secondary to cholestasis. 10 However, in the normal newborn population, giving three doses of vitamin K 1 to breast feeding infants, two of which would be given after hospital discharge in the USA, is problematic and a public health issue. Poor compliance in administering the three doses has already been reported. The most interesting findings in this study resulted from the PIVKA II analysis. We confirmed the high incidence of positive PIVKA II values in cord blood reported in other studies (table 2). Eleven cord blood samples (7.5%) had PIVKA II values that exceeded 2.0 AU/ml, a value that is more than 20 times the normal background concentration. Three cord blood samples had PIVKA II values that exceeded 10.0 AU/ml. However, why only half of the infants have low PIVKA II at birth, despite low vitamin K 1 plasma concentrations, is puzzling. The raised PIVKA II has been interpreted as a subclinical vitamin K deficiency in the newborn and goes along with the undetectable concentrations of vitamin K 1 in cord plasma with the assay used in this study. 18 PIVKA II is the partially carboxylated form of the prothrombin molecule. Both vitamin K 1 and a liver enzyme, glutamyl carboxylase, are required for the formation of the active carboxylated form of prothrombin. 1 Whether the very high rate of raised PIVKA II in cord blood represents a delay in maturation of the fetal liver enzyme glutamyl carboxylase, and/or is truly a result of inadequate transport of vitamin K 1 across the placenta, remains a question for further study. PIVKA values did not diver significantly between the two groups, although one could speculate on the meaning of three positive values in the IM group at day 56 compared with none in the oral group, especially as these three positive values were associated with low plasma vitamin K 1 concentrations (table 3). Unfortunately, our relatively small number of subjects does not allow us to predict accurately
5 304 Greer, Marshall, Severson, Smith, Shearer, Pace, et al the incidence of positive PIVKA II in this population. This would require many thousands of infants in each group. However, three of 67 positive PIVKA II values at day 56 in the breast feeding IM group would represent a rate of positive PIVKA II values of 450/ in this group at this time. These 450 infants would be at risk of haemorrhagic disease. This figure is very high compared with an incidence of late haemorrhagic disease of the newborn between 1/ and 1/ infants, dependent on vitamin K prophylaxis. 7 There are no comparable data at 2 months of life in exclusively breast fed term infants using the PIVKA II assay. 33 In this study of 156 infants, there were five positive PIVKA II values in four infants between 14 and 56 days of life (table 3). Infant 230, with a PIVKA II value of 66.1 AU/ml (confirmed with duplicate determinations), had the highest cord blood value ever measured in our laboratory. After an intramuscular injection, the value declined to 0.36 AU/ml at 14 days and was down to normal by day 30 (< 0.1 AU/ml). However, by 56 days, the value in this infant had increased to 0.2 AU/ml, reflecting the return of an abnormal vitamin K 1 status. From the values in cord blood and on day 14, it is possible to calculate that the half life of PIVKA II in this infant was 45.0 hours. This agrees with the value (46 hours) found in a previous study. 18 A second infant (table 3) also had a very high PIVKA II value at birth (10.8 AU/ml). This infant was in the oral group and the PIVKA II value was still raised at 14 days (0.11 AU/ml). The calculated half life of PIVKA II in this infant was 50.8 hours. Thus, the two raised PIVKA II values at day 14 can be explained by the very high values at birth (> 10 AU/ml). A third infant (data not shown) had a PIVKA II value of 15.0 in cord blood, which decreases to < 0.1 AU/ml by 14 days, the calculated half life again being about 45 hours. As seen in table 3, raised PIVKA II values did not necessarily occur with low vitamin K 1 values. In the two subjects with the highest cord blood concentrations of PIVKA II, the raised values at 14 days are associated with normal vitamin K 1 concentrations, reflecting the half life of PIVKA II of between 45 and 50 hours. On the other hand, the three raised PIVKA II values in the IM group at 56 days are all associated with low vitamin K 1 plasma concentrations. In one of these patients (patient 19), the vitamin K 1 concentration is low at 30 days without PIVKA II being raised. This infant had an adequate vitamin K 1 status at birth, which decreased to a subclinical deficiency state between 30 and 56 days, with a raised PIVKA II. It appears that neither plasma vitamin K 1 nor PIVKA II are ideal screening tools for subclinical vitamin K deficiency at birth. Many infants have low plasma vitamin K 1 concentrations, particularly those who are exclusively breast fed after birth. 34 All infants are born with low vitamin K 1 plasma concentrations, and half of the infants in our study had detectable PIVKA II at this time. Thus, sensitivities are high and specificities are low for predicting haemorrhagic disease of the newborn. Furthermore, the relatively long half life of PIVKA II might also limit its usefulness as a screening test before 1 month of age, as discussed above. By 30 days of life, raised PIVKA II values are found in less than 10% of infants with low plasma vitamin K 1 concentrations (fig 1). In adults, the correlation between PIVKA II and vitamin K 1 concentrations has been reported to be weak (r = 0.15; p < 0.05). 35 From our study, we can conclude the following about breast feeding infants receiving three oral vitamin K prophylaxis doses with Konakion MM: (1) plasma vitamin K concentrations were equal or significantly higher in orally supplemented babies compared with intramuscularly treated babies at the time points measured; and (2) up to 8 weeks of life, multiple doses of the new oral preparation maintain haemostasis and vitamin K status in breast fed infants at values that are equal to those achieved by the intramuscular preparation. This study was funded by a grant from HoVmann-La Roche Inc, Nutley, New Jersey, USA and Basel, Switzerland. 1 Greer FR. Vitamin K deficiency and hemorrhage in infancy. Clin Perinatol 1995;22: Loughnan PM, McDougall PN. Epidemiology of late onset haemorrhagic disease: a pooled data analysis. J Paediatr Child Health 1993;29: Barton JS, McNinch AW, Tripp JH. Oral vitamin K prophylaxis and frequency of late vitamin K deficiency bleeding. Lancet 1994;343: Ekelund H. Late haemorrhagic disease in Sweden Acta Paediatr Scand 1991;80: Loughnan PM, McDougall PN. The eycacy of oral vitamin K 1 : implications for future prophylaxis to prevent haemorrhagic disease of the newborn. J Paediatr Child Health 1993;29: Vitamin K Ad Hoc Task Force. Controversies concerning vitamin K and the newborn. American Academy of Pediatrics. Pediatrics 1993;91: von Kries R, Göbel U. Repeated oral vitamin K prophylaxis in Germany. Acceptance and eycacy. In: Sutor AH, Hathaway W, eds. Vitamin K in infancy. Stuttgart, Germany: FK Schattauer, l995: Hansen KN, Ebbesen F. Neonatal vitamin K prophylaxis in Denmark: three years experience with oral administration during the first three months of life compared with one oral administration at birth. Acta Paediatr 1996;85: McNinch AW, Upton C, Samuels M, et al. Plasma concentrations after oral or intramuscular vitamin K 1 in neonates. Arch Dis Child 1985;60: Amédée-Manesme O, Lambert WE, Alagille D, et al. Pharmacokinetics and safety of a new solution of vitamin K 1 in children with cholestasis. J Pediatr Gastroenterol Nutr 1992; 14: Schubiger G, Tönz O, Grüter J, et al. Vitamin K 1 concentration in breast-fed neonates after oral or intramuscular administration of a single dose of a new mixed-micellar preparation of phylloquinone. J Pediatr Gastroenterol Nutr 1993;16: McCarthy PT, Harrington DJ, Shearer MJ. Assay of phylloquinone in plasma by HPLC with electrochemical detection. In: McCormick DB, Suttie JW, Wagner C, eds. Methods in enzymology 1997;282: Shearer MJ. Phylloquinone (vitamin K 1 ) in serum or plasma by HPLC. In: Fidanza F, ed. Nutritional status assessment a manual for population studies. London: Chapman & Hall, 1991: Motahara K, Kuroki Y, Kan H, Endo F, Matsuda I. Detection of vitamin K deficiency by use of an enzyme-linked immunosorbent assay for circulating abnormal prothrombin. Pediatr Res 1985;19: McCurdy SA, White RH. Accuracy and precision of a portable anticoagulation monitor in a clinical setting. Arch Intern Med 1992;152: Massicotte P, Marzinotto V, Vegh P, Adams M, Andrew M. Home monitoring of warfarin therapy in children with a whole blood prothrombin monitor. J Pediatr 1995;127: Belle M, Leclercq M, Vignal B, et al. Application of an ELISA test to Vitamin K deficient conditions using a new monoclonal antibody against human des-gamma-carboxyprothrombin (DCP). In: Sutor AH, Hathaway W, eds. Vitamin K in infancy. Stuttgart, Germany: FK Schattauer, 1995:
6 Infant oral vitamin K prophylaxis Cox A, McCarthy PT, Harrington DJ, Shearer MJ. Human vitamin K status assessment: value of plasma des-gammacarboxyprothrombin and vitamin K 1. Int J Vitam Nutr Res 1998;66: Golding J, Greenwood R, Birmingham K, et al. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ 1992;305: Ekelund H, Finnström O, Gunnarskog J, et al. Administration of vitamin K to newborn infants and childhood cancer. BMJ 1993;307: KlebanoV MA, Read JS, Mills JL, et al. The risk of childhood cancer after neonatal exposure to vitamin K. N Engl J Med 1993;329: Olsen JH, Hertaz H, Blinkenberg K, Verder H. Vitamin K regimens and incidence of childhood cancer in Denmark. BMJ 1994;308: Von Kreis R, Göbel U, Hachmeister A, Kaletsh U, Michaelis J. Vitamin K and childhood cancer: a population based case-control study in Lower Saxony, Germany. BMJ 1996; 313: Ansell P, Bull D, Roman E. Childhood leukemia and intramuscular vitamin K: findings from a case control study. BMJ 1996;313: McKinney PA, Juszczak E, Findlay E, Smith K. Casecontrol study of childhood leukaemia and cancer in Scotland: findings for neonatal intramuscular vitamin K. BMJ 1998;316: Passmore SJ, Draper G, Brownbill P, Kroll M. Ecological studies of relation between hospital policies on neonatal vitamin K administration and subsequent occurrence of childhood cancer. BMJ 1998;316: Passmore SJ, Draper G, Brownbill P, Kroll M. Case-control studies of relation between childhood cancer and neonatal vitamin K administration. BMJ 1998;316: Parker L, Cole M, Craft W, Hey EN. Neonatal vitamin K administration and childhood cancer in the north of England: retrospective case-control study. BMJ 1998;316: Baenziger O, Braegger CP, Fanconi S. Oral vitamin K prophylaxis for newborn infants: safe enough? Lancet 1996; 348: Schubiger G, Tönz O, Grüter J, Shearer MJ. Vitamin K and PIVKA II concentrations in breast-fed infants after prophylaxis with diverent preparations of vitamin K. In: Sutor AH, Hathaway W, eds. Vitamin K in infancy. Stuttgart, Germany: FK Schattauer, l995: Croucher C, Azzopardi D. Compliance with recommendations for giving vitamin K to newborn infants. BMJ 1994; 308: Hill RJ. The uptake of the third oral vitamin K dose in general practice. NZ Med J 1994;107: Von Kries R, Greer FR, Suttie JW. Assessment of vitamin K status of the newborn infant. J Pediatr Gastroenterol Nutr 1993;16: Greer, FR, Marshall S, Suttie JW. Improving the vitamin K status of breast-feeding infants with maternal vitamin K supplements. Pediatrics 1997;99: Sokol LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr 1996;63: Arch Dis Child: first published as /adc on 1 October Downloaded from on 29 June 2018 by guest. Protected by copyright.
Nottingham Neonatal Service Clinical Guidelines
 Nottingham Neonatal Service Clinical Guidelines Title: VITAMIN K IN THE NEWBORN: Prophylaxis against vitamin K deficiency bleeding in infants Version: 5 (received and circulated July 2015) Date: V4 March
Nottingham Neonatal Service Clinical Guidelines Title: VITAMIN K IN THE NEWBORN: Prophylaxis against vitamin K deficiency bleeding in infants Version: 5 (received and circulated July 2015) Date: V4 March
Ecological studies of relation between hospital policies on neonatal vitamin K administration and subsequent occurrence of childhood cancer
 prophylaxis; to our colleagues Charles Stiller and Tim Vincent for advice and help in using records from the National Registry of Childhood Tumours; and to Dr Joan Andrews of the Welsh Office and Dr Mary
prophylaxis; to our colleagues Charles Stiller and Tim Vincent for advice and help in using records from the National Registry of Childhood Tumours; and to Dr Joan Andrews of the Welsh Office and Dr Mary
Downloaded from:
 Fear, NT; Roman, E; Ansell, P; Simpson, J; Day, N; Eden, OB; On Behalf Of The Uk Childhood Cancer Study Group (Including Peto, (2003) : a report from the United Kingdom Childhood Cancer Study. British
Fear, NT; Roman, E; Ansell, P; Simpson, J; Day, N; Eden, OB; On Behalf Of The Uk Childhood Cancer Study Group (Including Peto, (2003) : a report from the United Kingdom Childhood Cancer Study. British
EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS
 EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS R.K. Sharma N. Marwaha P. Kumar A. Narang ABSTRACT Intramuscular administration of vitamin K for prophylaxis against hemorrhagic disease
EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS R.K. Sharma N. Marwaha P. Kumar A. Narang ABSTRACT Intramuscular administration of vitamin K for prophylaxis against hemorrhagic disease
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/24702
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/24702
Konakion MM. Phytomenadione Composition. Properties and effects. Pharmacokinetics
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו בנובמבר 2015 Konakion MM Phytomenadione Composition Active ingredient: phytomenadione (synthetic vitamin K 1 ). Ampoules MM 10 mg/ml in a bile
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו בנובמבר 2015 Konakion MM Phytomenadione Composition Active ingredient: phytomenadione (synthetic vitamin K 1 ). Ampoules MM 10 mg/ml in a bile
Summary of Product Characteristics
 NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Konakion MM Paediatric Ampoules 2 mg/ 0.2 ml oral solution or solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule
NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Konakion MM Paediatric Ampoules 2 mg/ 0.2 ml oral solution or solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule
Personal Practice. Vitamin K During Infancy: Current Status and Recommendations
 Personal Practice Vitamin K During Infancy: Current Status and Recommendations Meharban Singh Vitamin K is usually the first vitamin given at birth. Dietary deficiency of vitamin K in healthy subjects
Personal Practice Vitamin K During Infancy: Current Status and Recommendations Meharban Singh Vitamin K is usually the first vitamin given at birth. Dietary deficiency of vitamin K in healthy subjects
Number of preventable vitamin K deficiency bleeding cases
 preventable vitamin K deficiency bleeding cases No. 2017/04Ae, The Hague, April 11, 2017 Background document to: Vitamin K for infants No. 2017/04e, The Hague, April 11, 2017 preventable vitamin K deficiency
preventable vitamin K deficiency bleeding cases No. 2017/04Ae, The Hague, April 11, 2017 Background document to: Vitamin K for infants No. 2017/04e, The Hague, April 11, 2017 preventable vitamin K deficiency
Effects of oral and intramuscular vitamin K prophylaxis on vitamin K1, PIVKA-II, and clotting factors in breast fed infants
 1250 University of Nijmegen, The Netherlands, Paediatrics E A M Cornelissen L A A Kollee R A De Abreu J M Van Baal L A H Monnens Haematology B Verbruggen Paediatrics, Amakusa-Sumoto Hospital, Kumamoto,
1250 University of Nijmegen, The Netherlands, Paediatrics E A M Cornelissen L A A Kollee R A De Abreu J M Van Baal L A H Monnens Haematology B Verbruggen Paediatrics, Amakusa-Sumoto Hospital, Kumamoto,
Vitamin K Deficiency Bleeding (VKDB) in Infancy*
 Thromb Haemost 1999; 81: 456 61 1999 Schattauer Verlag, Stuttgart Scientific and Standardization Committee Communications Vitamin K Deficiency Bleeding (VKDB) in Infancy* On behalf of the ISTH Pediatric/Perinatal
Thromb Haemost 1999; 81: 456 61 1999 Schattauer Verlag, Stuttgart Scientific and Standardization Committee Communications Vitamin K Deficiency Bleeding (VKDB) in Infancy* On behalf of the ISTH Pediatric/Perinatal
L ate onset vitamin K deficiency bleeding (VKDB) is a
 F546 ORIGINAL ARTICLE Intracranial haemorrhage due to late onset vitamin K deficiency bleeding in Hanoi province, Vietnam N Danielsson, D P Hoa, N V Thang, T Vos, P M Loughnan... See end of article for
F546 ORIGINAL ARTICLE Intracranial haemorrhage due to late onset vitamin K deficiency bleeding in Hanoi province, Vietnam N Danielsson, D P Hoa, N V Thang, T Vos, P M Loughnan... See end of article for
Vitamin K. Amina Ziyad Elaf Sohaib
 Vitamin K Amina Ziyad Elaf Sohaib What is Vitamin K? Fat soluble compound Necessary for the synthesis of several proteins required for blood clotting 1) Vit K 1 (Phylloquinone) - natural form - found in
Vitamin K Amina Ziyad Elaf Sohaib What is Vitamin K? Fat soluble compound Necessary for the synthesis of several proteins required for blood clotting 1) Vit K 1 (Phylloquinone) - natural form - found in
2. What you need to know before you are given Konakion MM. Package Leaflet: Information for the patient
 Package Leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start taking this medicine
Package Leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start taking this medicine
Vitamin K in Cystic Fibrosis
 Vitamin K in Cystic Fibrosis Martin J. Shearer PhD, MRCPath Consultant Clinical Scientist Honorary Senior Lecturer Centre for Haemostasis and Thrombosis, Guy s and St. Thomas Foundation Trust London, UK
Vitamin K in Cystic Fibrosis Martin J. Shearer PhD, MRCPath Consultant Clinical Scientist Honorary Senior Lecturer Centre for Haemostasis and Thrombosis, Guy s and St. Thomas Foundation Trust London, UK
The vitamin K debacle: cut the Gordian knot but first do no harm
 Arch Dis Child 1998;79:295 299 295 Annotations The vitamin K debacle: cut the Gordian knot but first do no harm As clinicians who have contributed to the data and the discussions about vitamin K prophylaxis
Arch Dis Child 1998;79:295 299 295 Annotations The vitamin K debacle: cut the Gordian knot but first do no harm As clinicians who have contributed to the data and the discussions about vitamin K prophylaxis
25 OH Vitamin D Rapid Test
 INSTRUCTION FOR USE REF:GDB 7120-25T 25 OH Vitamin D Rapid Test A Rapid Sandwich Immunochromatographic Test for Quantitative Detection of total 25-OH Vitamin D in human finger-prick blood For In Vitro
INSTRUCTION FOR USE REF:GDB 7120-25T 25 OH Vitamin D Rapid Test A Rapid Sandwich Immunochromatographic Test for Quantitative Detection of total 25-OH Vitamin D in human finger-prick blood For In Vitro
Maternal and Infant Nutrition Briefs
 Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA. Date
 CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA 350 300 250 Number 200 150 100 50 0 1/01/1997 1/01/1998 1/01/1999 1/01/2000 31/12/2000 31/12/2001 31/12/2002 Date July 2004 Reported number of perinatally exposed
CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA 350 300 250 Number 200 150 100 50 0 1/01/1997 1/01/1998 1/01/1999 1/01/2000 31/12/2000 31/12/2001 31/12/2002 Date July 2004 Reported number of perinatally exposed
Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System
 Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System Grace DeSantis, PhD Lisa Croner, PhD Michealle Havenhill, BA Shari Kipp, BSc (Honours) MBA Inverness Medical,
Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System Grace DeSantis, PhD Lisa Croner, PhD Michealle Havenhill, BA Shari Kipp, BSc (Honours) MBA Inverness Medical,
Package leaflet: Information for the patient. Konakion MM Ampoules 10mg/ml solution for injection and oral solution Phytomenadione (vitamin K 1 )
 Package leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection and oral solution Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start
Package leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection and oral solution Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start
Effects of Vitamin K-Deficient Diets and Fasting on Blood Coagulation Factors in Conventional and Germ-Free Rats
 Effects of Vitamin K-Deficient Diets and Fasting on Blood Coagulation Factors in Conventional and Germ-Free Rats Kiyohisa UCHIDA, Yasuharu NOMURA, Haruto TAKASE, Toshio HARAUCHI, Toshio YOSHIZAKI and Hiroshi
Effects of Vitamin K-Deficient Diets and Fasting on Blood Coagulation Factors in Conventional and Germ-Free Rats Kiyohisa UCHIDA, Yasuharu NOMURA, Haruto TAKASE, Toshio HARAUCHI, Toshio YOSHIZAKI and Hiroshi
Running Head: CLASSIFYING SMALL FOR GESTATIONAL AGE INFANTS 1
 Running Head: CLASSIFYING SMALL FOR GESTATIONAL AGE INFANTS 1 Classifying Small for Gestational Age Infants with Consideration for Multiple Variables Laura Beth Cook, SN and Thelma Patrick, PhD, RN The
Running Head: CLASSIFYING SMALL FOR GESTATIONAL AGE INFANTS 1 Classifying Small for Gestational Age Infants with Consideration for Multiple Variables Laura Beth Cook, SN and Thelma Patrick, PhD, RN The
Compounds with vitamin K activity all have a common. Vitamin K in Parenteral Nutrition. Metabolic Function
 GASTROENTEROLOGY 2009;137:S105 S118 Vitamin K in Parenteral Nutrition MARTIN J. SHEARER Centre for Haemostasis and Thrombosis, St Thomas Hospital, London, United Kingdom Vitamin K (as phylloquinone and
GASTROENTEROLOGY 2009;137:S105 S118 Vitamin K in Parenteral Nutrition MARTIN J. SHEARER Centre for Haemostasis and Thrombosis, St Thomas Hospital, London, United Kingdom Vitamin K (as phylloquinone and
Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel Approach
 653738JHLXXX10.1177/0890334416653738Journal of Human LactationSimpson et al research-article2016 Original Research: Brief Report Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel
653738JHLXXX10.1177/0890334416653738Journal of Human LactationSimpson et al research-article2016 Original Research: Brief Report Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel
Creatine phosphokinase levels in the newborn
 Archives of Disease in Childhood, 1979, 54, 362-366 Creatine phosphokinase levels in the newborn and their use in screening for Duchenne muscular dystrophy L. M. DRUMMOND University ofauckland School ofmedicine
Archives of Disease in Childhood, 1979, 54, 362-366 Creatine phosphokinase levels in the newborn and their use in screening for Duchenne muscular dystrophy L. M. DRUMMOND University ofauckland School ofmedicine
z_4-/01/11 ,c.,u V clf..rm-t PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route
 PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route PGD Ref: p G- D f j Effective From: Feb 2.0 }9 Expiry: Feb 2. 0 2 J by Paediatrics
PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route PGD Ref: p G- D f j Effective From: Feb 2.0 }9 Expiry: Feb 2. 0 2 J by Paediatrics
DATE: 28 May 2015 CONTEXT AND POLICY ISSUES
 TITLE: Neonatal Vitamin K Administration for the Prevention of Hemorrhagic Disease: A Review of the Clinical Effectiveness, Comparative Effectiveness, and Guidelines DATE: 28 May 2015 CONTEXT AND POLICY
TITLE: Neonatal Vitamin K Administration for the Prevention of Hemorrhagic Disease: A Review of the Clinical Effectiveness, Comparative Effectiveness, and Guidelines DATE: 28 May 2015 CONTEXT AND POLICY
Cord blood bilirubin used as an early predictor of hyperbilirubinemia
 International Journal of Contemporary Pediatrics Ramamoorthy K et al. Int J Contemp Pediatr. 218 Jul;5(4):128-1285 http://www.ijpediatrics.com pissn 2349-3283 eissn 2349-3291 Original Research Article
International Journal of Contemporary Pediatrics Ramamoorthy K et al. Int J Contemp Pediatr. 218 Jul;5(4):128-1285 http://www.ijpediatrics.com pissn 2349-3283 eissn 2349-3291 Original Research Article
Correlation between the levels of anti- A/B IgM/IgG antibodies and cord blood bilirubin levels in haemolytic disease of the new-born
 Original article Correlation between the levels anti- A/B IgM/IgG antibodies and cord blood bilirubin levels in haemolytic disease the new-born C HEMACHANDRIKA 1* TK SHAANTHI GUNASINGH 2 1Pressor & Head,
Original article Correlation between the levels anti- A/B IgM/IgG antibodies and cord blood bilirubin levels in haemolytic disease the new-born C HEMACHANDRIKA 1* TK SHAANTHI GUNASINGH 2 1Pressor & Head,
Jaundice Protocol. Early identification and referral of liver disease in infants. fighting childhood liver disease. fighting childhood liver disease
 fighting childhood liver disease Jaundice Protocol Early identification and referral of liver disease in infants fighting childhood liver disease 36 Great Charles Street Birmingham B3 3JY Telephone: 0121
fighting childhood liver disease Jaundice Protocol Early identification and referral of liver disease in infants fighting childhood liver disease 36 Great Charles Street Birmingham B3 3JY Telephone: 0121
Maternofoetal transfer of Cytomegalovirus IgG antibodies in Maiduguri, North Eastern Nigeria
 ISPUB.COM The Internet Journal of Microbiology Volume 9 Number 1 Maternofoetal transfer of Cytomegalovirus IgG antibodies in Maiduguri, North Eastern Nigeria T Adisa, D Bukbuk, T Harry Citation T Adisa,
ISPUB.COM The Internet Journal of Microbiology Volume 9 Number 1 Maternofoetal transfer of Cytomegalovirus IgG antibodies in Maiduguri, North Eastern Nigeria T Adisa, D Bukbuk, T Harry Citation T Adisa,
2011 UGANDA DHS - ADDENDUM TO CHAPTER 11
 2011 UGANDA DHS - ADDENDUM TO CHAPTER 11 11.9 VITAMIN A 11.9.1 Background and Methodology Vitamin A is an essential micronutrient for vision, for the maintenance of epithelial cells, and for regulation
2011 UGANDA DHS - ADDENDUM TO CHAPTER 11 11.9 VITAMIN A 11.9.1 Background and Methodology Vitamin A is an essential micronutrient for vision, for the maintenance of epithelial cells, and for regulation
Intracranial hemorrhages and late hemorrhagic disease associated cholestatic liver disease
 Neurol Sci (2013) 34:51 56 DOI 10.1007/s10072-012-0965-5 ORIGINAL ARTICLE Intracranial s and late hemorrhagic disease associated cholestatic liver disease Hüseyin Per Duran Arslan Hakan Gümüş Abdulhakim
Neurol Sci (2013) 34:51 56 DOI 10.1007/s10072-012-0965-5 ORIGINAL ARTICLE Intracranial s and late hemorrhagic disease associated cholestatic liver disease Hüseyin Per Duran Arslan Hakan Gümüş Abdulhakim
25 OH Vitamin D Rapid test
 INSTRUCTION FOR USE REF:GDB 7120-25T 25 OH Vitamin D Rapid test Rapid Whole Blood Vitamin D Test A Rapid Sandwich Immunochromatographic Test for the Quantitative Detection of total 25-OH Vitamin D in human
INSTRUCTION FOR USE REF:GDB 7120-25T 25 OH Vitamin D Rapid test Rapid Whole Blood Vitamin D Test A Rapid Sandwich Immunochromatographic Test for the Quantitative Detection of total 25-OH Vitamin D in human
Coagulation, Haemostasis and interpretation of Coagulation tests
 Coagulation, Haemostasis and interpretation of Coagulation tests Learning Outcomes Indicate the normal ranges for routine clotting screen and explain what each measurement means Recognise how to detect
Coagulation, Haemostasis and interpretation of Coagulation tests Learning Outcomes Indicate the normal ranges for routine clotting screen and explain what each measurement means Recognise how to detect
Mouse C-Peptide ELISA Kit
 Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Midterm Exam MMI 409 Spring 2009 Gordon Bleil
 Midterm Exam MMI 409 Spring 2009 Gordon Bleil Table of contents: (Hyperlinked to problem sections) Problem 1 Hypothesis Tests Results Inferences Problem 2 Hypothesis Tests Results Inferences Problem 3
Midterm Exam MMI 409 Spring 2009 Gordon Bleil Table of contents: (Hyperlinked to problem sections) Problem 1 Hypothesis Tests Results Inferences Problem 2 Hypothesis Tests Results Inferences Problem 3
Calcium, phosphorus, and magnesium concentrations in plasma during first week of life and their relation to type of milk feed
 Archives of Disease in Childhood, 1976, 51, 377. Calcium, phosphorus, and magnesium concentrations in plasma during first week of life and their relation to type of milk feed G. T. LALMAN, R. W. LOGAN,
Archives of Disease in Childhood, 1976, 51, 377. Calcium, phosphorus, and magnesium concentrations in plasma during first week of life and their relation to type of milk feed G. T. LALMAN, R. W. LOGAN,
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN PROTHROMBIN COMPLEX PRODUCTS (CPMP/BPWG/3735/02)
 European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
PARENTERAL NUTRITION- ASSOCIATED LIVER DISEASE IN CHILDREN
 PARENTERAL NUTRITION- ASSOCIATED LIVER DISEASE IN CHILDREN Praveen Goday MBBS CNSC Associate Professor Pediatric Gastroenterology Medical College of Wisconsin Milwaukee, WI Parenteral Nutrition-Associated
PARENTERAL NUTRITION- ASSOCIATED LIVER DISEASE IN CHILDREN Praveen Goday MBBS CNSC Associate Professor Pediatric Gastroenterology Medical College of Wisconsin Milwaukee, WI Parenteral Nutrition-Associated
The excretion of zopiclone into breast milk
 Br. J. clin. Pharmac. (1990), 30, 267-271 The excretion of zopiclone into breast milk I. MATHESON1, H. A. SANDE2 & J. GAILLOT3 'Department of Pharmacotherapeutics, University of Oslo, Oslo, 2Department
Br. J. clin. Pharmac. (1990), 30, 267-271 The excretion of zopiclone into breast milk I. MATHESON1, H. A. SANDE2 & J. GAILLOT3 'Department of Pharmacotherapeutics, University of Oslo, Oslo, 2Department
NEW MK-7 SELECT INTRODUCTION
 NEW MK-7 SELECT INTRODUCTION As we have all known for several years now, the presence of optimal amounts of vitamin D is essential for maintaining optimal bone health and bone calcification, as noted by
NEW MK-7 SELECT INTRODUCTION As we have all known for several years now, the presence of optimal amounts of vitamin D is essential for maintaining optimal bone health and bone calcification, as noted by
Maternal and Infant Nutrition Briefs
 Maternal and Infant Nutrition Briefs A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition March/April 2003 New Guidelines on
Maternal and Infant Nutrition Briefs A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition March/April 2003 New Guidelines on
MANUAL RBP4 (human) Competitive ELISA Kit Cat. No. AG-45A-0010YEK-KI01 For research use only. Not for diagnostic use.
 MANUAL RBP4 (human) Competitive ELISA Kit For research use only. Not for diagnostic use. Version 3 (04-May-2015) Cat. No. AG-45A-0010YEK-KI01 www.adipogen.com Table of Contents 1. Intended Use 3 2. Introduction
MANUAL RBP4 (human) Competitive ELISA Kit For research use only. Not for diagnostic use. Version 3 (04-May-2015) Cat. No. AG-45A-0010YEK-KI01 www.adipogen.com Table of Contents 1. Intended Use 3 2. Introduction
Prevention of Mother to Child Transmission of HIV: Our Experience in South India
 pg 62-66 Original Article Prevention of Mother to Child Transmission of HIV: Our Experience in South India Karthekeyani Vijaya 1, Alexander Glory 2, Solomon Eileen 3, Rao Sarita 4, Rao P.S.S.Sunder 5 1
pg 62-66 Original Article Prevention of Mother to Child Transmission of HIV: Our Experience in South India Karthekeyani Vijaya 1, Alexander Glory 2, Solomon Eileen 3, Rao Sarita 4, Rao P.S.S.Sunder 5 1
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
EDUCATIONAL COMMENTARY ORAL ANTICOAGULANT THERAPEUTIC MONITORING AND POINT-OF-CARE TESTING
 POINT-OF-CARE TESTING Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on
POINT-OF-CARE TESTING Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on
Learning from nature for a healthier start Staged infant formulas with Lacprodan mimic the changing protein profile of breast milk
 Learning from nature for a healthier start Staged infant formulas with Lacprodan mimic the changing protein profile of breast milk By Lotte Neergaard Jacobsen Arla Foods Ingredients. May 013 1 3 4 www.arlafoodsingredients.com
Learning from nature for a healthier start Staged infant formulas with Lacprodan mimic the changing protein profile of breast milk By Lotte Neergaard Jacobsen Arla Foods Ingredients. May 013 1 3 4 www.arlafoodsingredients.com
Vitamin K deficiency bleeding (VKDB) was first discovered
 Preventing Vitamin K Deficiency Bleeding: An Evidence-Based Update for the Midwife BY KELSEY SCHERER Vitamin K deficiency bleeding (VKDB) was first discovered in 1894 when spontaneous bleeding was observed
Preventing Vitamin K Deficiency Bleeding: An Evidence-Based Update for the Midwife BY KELSEY SCHERER Vitamin K deficiency bleeding (VKDB) was first discovered in 1894 when spontaneous bleeding was observed
Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review
 OPEN Journal of Perinatology (2016) 36, S29 S34 2016 Nature America, Inc. All rights reserved 0743-8346/16 www.nature.com/jp SYSTEMATIC REVIEWS Vitamin K prophylaxis for prevention of vitamin K deficiency
OPEN Journal of Perinatology (2016) 36, S29 S34 2016 Nature America, Inc. All rights reserved 0743-8346/16 www.nature.com/jp SYSTEMATIC REVIEWS Vitamin K prophylaxis for prevention of vitamin K deficiency
Dr Graham P Taylor Reader in Communicable Diseases
 HIV in Pregnancy Joint RCOG/BHIVA Multidisciplinary Conference Dr Graham Taylor Imperial College London Friday 20 January 2012, Royal College of Obstetricians and Gynaecologist, London Prevention of post-partum
HIV in Pregnancy Joint RCOG/BHIVA Multidisciplinary Conference Dr Graham Taylor Imperial College London Friday 20 January 2012, Royal College of Obstetricians and Gynaecologist, London Prevention of post-partum
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: Alere HIV Combo WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
Routine preoperative coagulation tests: are they necessary?
 Routine preoperative coagulation tests: are they necessary? Dr Azzah Alzahrani MD Pediatrics Hematology /Oncology PSMMS Outline Introduction. Brief review of hemostatic mechanisms. A clinical aspect of
Routine preoperative coagulation tests: are they necessary? Dr Azzah Alzahrani MD Pediatrics Hematology /Oncology PSMMS Outline Introduction. Brief review of hemostatic mechanisms. A clinical aspect of
Feeding the Small for Gestational Age Infant. Feeding the Small for Gestational Age Infant
 Feeding the Small for Gestational Age Infant Feeding the Small for Gestational Age Infant What s the right strategy? Infants born small-for-gestational age (SGA) are at higher risk for adult diseases.
Feeding the Small for Gestational Age Infant Feeding the Small for Gestational Age Infant What s the right strategy? Infants born small-for-gestational age (SGA) are at higher risk for adult diseases.
Prevention and Management of Hypoglycaemia of the Breastfed Newborn Reference Number:
 This is an official Northern Trust policy and should not be edited in any way Prevention and Management of Hypoglycaemia of the Breastfed Newborn Reference Number: NHSCT/10/293 Target audience: Midwifery,
This is an official Northern Trust policy and should not be edited in any way Prevention and Management of Hypoglycaemia of the Breastfed Newborn Reference Number: NHSCT/10/293 Target audience: Midwifery,
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1
 VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
Biochemistry of Vitamin K
 Lecture 4 Biochemistry of Vitamin K The Objectives Types and chemistry of vitamin K Sources and daily requirements Functions Synthesis of γ-carboxyglutamate in: Prothrombin and blood clotting factors Osteocalcin
Lecture 4 Biochemistry of Vitamin K The Objectives Types and chemistry of vitamin K Sources and daily requirements Functions Synthesis of γ-carboxyglutamate in: Prothrombin and blood clotting factors Osteocalcin
When should I transfuse platelets and plasma for children? Dr Liz Chalmers. Consultant Paediatric Haematologist Royal Hospital for Children Glasgow
 When should I transfuse platelets and plasma for children? Dr Liz Chalmers Consultant Paediatric Haematologist Royal Hospital for Children Glasgow When should I transfuse platelets and plasma in children?
When should I transfuse platelets and plasma for children? Dr Liz Chalmers Consultant Paediatric Haematologist Royal Hospital for Children Glasgow When should I transfuse platelets and plasma in children?
Wales Neonatal Network Guideline CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER
 CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER A plan of care is written for all babies to be born to a woman with HIV infection. These are placed in the maternal notes within the Baby Pack
CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER A plan of care is written for all babies to be born to a woman with HIV infection. These are placed in the maternal notes within the Baby Pack
D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity.
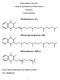
 D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity. 1. Structures VITAMIN K1; Phytonadione; 2-Methyl-3-phytyl-1,4-naphthoquinone VITAMIN K2; Menaquinone-6 and 7; N=6,7 n 2. Function
D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity. 1. Structures VITAMIN K1; Phytonadione; 2-Methyl-3-phytyl-1,4-naphthoquinone VITAMIN K2; Menaquinone-6 and 7; N=6,7 n 2. Function
Guideline scope Neonatal parenteral nutrition
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Neonatal parenteral nutrition The Department of Health in England has asked NICE to develop a new guideline on parenteral nutrition in
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Neonatal parenteral nutrition The Department of Health in England has asked NICE to develop a new guideline on parenteral nutrition in
Standard of Newborn Care in the Age of Birth Plans. Stephanie Deal, MD Tiffany McKee-Garrett, MD
 Standard of Newborn Care in the Age of Birth Plans Stephanie Deal, MD Tiffany McKee-Garrett, MD Disclosure We have no relevant financial relationships with the manufacturers(s) of any commercial products(s)
Standard of Newborn Care in the Age of Birth Plans Stephanie Deal, MD Tiffany McKee-Garrett, MD Disclosure We have no relevant financial relationships with the manufacturers(s) of any commercial products(s)
Sultan Qaboos University. College of Agricultural & Marine Sciences. Vitamin K. Lecture Summary
 Sultan Qaboos University College of Agricultural & Marine Sciences Vitamin K Lecture Summary Name: Mohsin Mohammed Taqi Mohsin Al-Saleh ID #: M020944-99 0 Introduction: Vitamin K is a group of structurally
Sultan Qaboos University College of Agricultural & Marine Sciences Vitamin K Lecture Summary Name: Mohsin Mohammed Taqi Mohsin Al-Saleh ID #: M020944-99 0 Introduction: Vitamin K is a group of structurally
Increasing access to oxytocin for the prevention of postpartum haemorrhage: Inhaled Oxytocin A Phase I Study. Disala Fernando, MD
 Increasing access to oxytocin for the prevention of postpartum haemorrhage: Inhaled Oxytocin A Phase I Study Disala Fernando, MD The following clinical trial (NCT02542813) was funded entirely by the pharmaceutical
Increasing access to oxytocin for the prevention of postpartum haemorrhage: Inhaled Oxytocin A Phase I Study Disala Fernando, MD The following clinical trial (NCT02542813) was funded entirely by the pharmaceutical
VTE in Children: Practical Issues
 VTE in Children: Practical Issues Wasil Jastaniah MBBS,FAAP,FRCPC Consultant Pediatric Hem/Onc/BMT May 2012 Top 10 Reasons Why Pediatric VTE is Different 1. Social, ethical, and legal implications. 2.
VTE in Children: Practical Issues Wasil Jastaniah MBBS,FAAP,FRCPC Consultant Pediatric Hem/Onc/BMT May 2012 Top 10 Reasons Why Pediatric VTE is Different 1. Social, ethical, and legal implications. 2.
Virtual Mentor American Medical Association Journal of Ethics December 2009, Volume 11, Number 12:
 Virtual Mentor American Medical Association Journal of Ethics December 2009, Volume 11, Number 12: 969-973. HEALTH LAW Testing Newborns for HIV Kristin E. Schleiter, JD, LLM Perinatal HIV refers to infection
Virtual Mentor American Medical Association Journal of Ethics December 2009, Volume 11, Number 12: 969-973. HEALTH LAW Testing Newborns for HIV Kristin E. Schleiter, JD, LLM Perinatal HIV refers to infection
A New Method for Maturity Determination in Newborn Infants
 A New Method for Maturity Determination in Newborn Infants by Charles Osayande Eregie Institute of Child Health, University of Benin, Benin City, Nigeria Summary A two-part study was conducted in several
A New Method for Maturity Determination in Newborn Infants by Charles Osayande Eregie Institute of Child Health, University of Benin, Benin City, Nigeria Summary A two-part study was conducted in several
Flecainide excretion in human breast milk
 Flecainide excretion in human breast milk Healthy human volunteers who intended not to breast feed were placed on a regimen of 100 mg oral flecainide every 12 hours for 51/2 days beginning 1 day after
Flecainide excretion in human breast milk Healthy human volunteers who intended not to breast feed were placed on a regimen of 100 mg oral flecainide every 12 hours for 51/2 days beginning 1 day after
ANTIRETROVIRAL (ART) DRUG INFORMATION FOR HEALTH CARE PROFESSIONAL
 ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
ENTERAL NUTRITION Identifying risk of patients for enteral feeding problems: Low risk: Moderate risk: High risk:
 ENTERAL NUTRITION Statement of best practice Feeding with mother s own breastmilk is protective against sepsis, NEC and death All mothers should be informed about this and strongly encouraged to express
ENTERAL NUTRITION Statement of best practice Feeding with mother s own breastmilk is protective against sepsis, NEC and death All mothers should be informed about this and strongly encouraged to express
Circumcision bleeding complications: Neonatal intensive care infants compared to. those in the normal newborn nursery
 Circumcision bleeding complications: Neonatal intensive care infants compared to those in the normal newborn nursery Abigail R. Litwiller MD 1, David M. Haas MD, MS 2 1 Department of OB/GYN, University
Circumcision bleeding complications: Neonatal intensive care infants compared to those in the normal newborn nursery Abigail R. Litwiller MD 1, David M. Haas MD, MS 2 1 Department of OB/GYN, University
Should infants with perinatal thrombosis be screened for thrombophilia and treated by anticoagulants?
 Should infants with perinatal thrombosis be screened for thrombophilia and treated by anticoagulants? Shoshana Revel-Vilk, MD MSc Pediatric Hematology Center, Pediatric Hematology/Oncology Department,
Should infants with perinatal thrombosis be screened for thrombophilia and treated by anticoagulants? Shoshana Revel-Vilk, MD MSc Pediatric Hematology Center, Pediatric Hematology/Oncology Department,
Paediatric Anticoagulant Guidelines. Bhavee Patel, Gareth Thomas
 Paediatric Anticoagulant Guidelines Author: Bhavee Patel, Gareth Thomas Specialty: Paediatrics Date Approved: 18 th July 2012 Approved by: W&CH Quality & Safety Group Date for Review: June 2015 PAEDIATRIC
Paediatric Anticoagulant Guidelines Author: Bhavee Patel, Gareth Thomas Specialty: Paediatrics Date Approved: 18 th July 2012 Approved by: W&CH Quality & Safety Group Date for Review: June 2015 PAEDIATRIC
Johann Hari. Truths 2/29/2016. From the street to the NICU. Treatment works
 From the street to the NICU Richard Christensen, PA, CAS Johann Hari Treatment works Truths Disconnect with pregnant women seeking treatment Disconnect between community and science Medication is not a
From the street to the NICU Richard Christensen, PA, CAS Johann Hari Treatment works Truths Disconnect with pregnant women seeking treatment Disconnect between community and science Medication is not a
Surveillance report Published: 9 January 2017 nice.org.uk
 Surveillance report 2017 Caesarean section (2011) NICE guideline CG132 Surveillance report Published: 9 January 2017 nice.org.uk NICE 2017. All rights reserved. Contents Surveillance decision... 3 Reason
Surveillance report 2017 Caesarean section (2011) NICE guideline CG132 Surveillance report Published: 9 January 2017 nice.org.uk NICE 2017. All rights reserved. Contents Surveillance decision... 3 Reason
Esther Briganti. Fetal And Maternal Health Beyond the Womb: hot topics in endocrinology and pregnancy. Endocrinologist and Clinician Researcher
 Fetal And Maternal Health Beyond the Womb: hot topics in endocrinology and pregnancy Esther Briganti Endocrinologist and Clinician Researcher Director, Melbourne Endocrine Associates Associate Professor,
Fetal And Maternal Health Beyond the Womb: hot topics in endocrinology and pregnancy Esther Briganti Endocrinologist and Clinician Researcher Director, Melbourne Endocrine Associates Associate Professor,
Factor X deficiency. Information for families. Great Ormond Street Hospital for Children NHS Foundation Trust
 Factor X deficiency Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 1 Factor X (previously known as the Stuart-Prower factor) deficiency is a type of clotting disorder.
Factor X deficiency Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 1 Factor X (previously known as the Stuart-Prower factor) deficiency is a type of clotting disorder.
Prolonged Neonatal Jaundice
 Prolonged Neonatal Jaundice Ahmed Laving KPA Annual Scientific Conference 2018 Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Case Presentation
Prolonged Neonatal Jaundice Ahmed Laving KPA Annual Scientific Conference 2018 Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Case Presentation
WHAT IT MEANS or WHY YOU DO IT
 WHAT IT MEANS or WHY YOU DO IT Dr. Patrick Sauer Billings Clinic Pediatrics Objective Increase understanding of prenatal tests Increase understanding of routine newborn procedures Increase knowledge to
WHAT IT MEANS or WHY YOU DO IT Dr. Patrick Sauer Billings Clinic Pediatrics Objective Increase understanding of prenatal tests Increase understanding of routine newborn procedures Increase knowledge to
Surveillance report Published: 8 June 2017 nice.org.uk. NICE All rights reserved.
 Surveillance report 2017 Antenatal and postnatal mental health: clinical management and service guidance (2014) NICE guideline CG192 Surveillance report Published: 8 June 2017 nice.org.uk NICE 2017. All
Surveillance report 2017 Antenatal and postnatal mental health: clinical management and service guidance (2014) NICE guideline CG192 Surveillance report Published: 8 June 2017 nice.org.uk NICE 2017. All
220 SUBJECT INDEX. D Diarrhea and sodium balance, 74 weanling, 161,179,208,212; see also Infection
 Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
Subject Index Acid balance, see ph Allergy, food, see also Immunity and beikost, 143-144 and breast milk, 91,143 and formula, 89-90 Antidiuretic hormone, 66 67 Antigens, see also Immunity determinants,
OB Well Baby Nursery Admission (Term) [ ] For specialty focused order sets for your patient, refer to: General
![OB Well Baby Nursery Admission (Term) [ ] For specialty focused order sets for your patient, refer to: General OB Well Baby Nursery Admission (Term) [ ] For specialty focused order sets for your patient, refer to: General](/thumbs/85/91909911.jpg) OB Well Baby Nursery Admission (Term) [3040000234] For specialty focused order sets for your patient, refer to: 3040000424 Neonatal Circumcision Order Set 3040000522 Neonatal Herpes Viral Order Set 3040000524
OB Well Baby Nursery Admission (Term) [3040000234] For specialty focused order sets for your patient, refer to: 3040000424 Neonatal Circumcision Order Set 3040000522 Neonatal Herpes Viral Order Set 3040000524
human Total Cathepsin B Catalog Number: DY2176
 human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
Post - caesarean section pyrexia and its relation of rupture of membranes and prophylactic antibiotics
 MOJ Women s Health Research Article Open Access Post - caesarean section pyrexia and its relation of rupture of membranes and prophylactic antibiotics Abstract Objectives: To determine the incidence of
MOJ Women s Health Research Article Open Access Post - caesarean section pyrexia and its relation of rupture of membranes and prophylactic antibiotics Abstract Objectives: To determine the incidence of
Vitamins. Nafith Abu Tarboush, DDS, MSc, PhD
 Vitamins Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Vitamins Organic compounds required by an organism in tiny amounts as a vital nutrient Cannot be synthesized
Vitamins Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Vitamins Organic compounds required by an organism in tiny amounts as a vital nutrient Cannot be synthesized
Haemoglobin and ferritin concentrations in children aged 12 and 18 months
 Arch Dis Child 1999;80:153 157 153 Haemoglobin and ferritin concentrations in children aged 12 and 18 months Andrea SherriV, Alan Emond, Neil Hawkins, Jean Golding, the ALSPAC Children in Focus Study Team
Arch Dis Child 1999;80:153 157 153 Haemoglobin and ferritin concentrations in children aged 12 and 18 months Andrea SherriV, Alan Emond, Neil Hawkins, Jean Golding, the ALSPAC Children in Focus Study Team
SWISS SOCIETY OF NEONATOLOGY. Neonatal gastric perforation
 SWISS SOCIETY OF NEONATOLOGY Neonatal gastric perforation September 2002 2 Zankl A, Stähelin J, Roth K, Boudny P and Zeilinger G, Children s Hospital of Aarau (ZA, SJ, RK, ZG) and Institute of Pathology
SWISS SOCIETY OF NEONATOLOGY Neonatal gastric perforation September 2002 2 Zankl A, Stähelin J, Roth K, Boudny P and Zeilinger G, Children s Hospital of Aarau (ZA, SJ, RK, ZG) and Institute of Pathology
Congenital hypothyroidism and your child
 Congenital hypothyroidism and your child Contributed by Sirisha Kusuma. B Consultant Pediatric Endocrinologist Rainbow Children s hospital What is Thyroid? The thyroid is a small butterfly shaped endocrine
Congenital hypothyroidism and your child Contributed by Sirisha Kusuma. B Consultant Pediatric Endocrinologist Rainbow Children s hospital What is Thyroid? The thyroid is a small butterfly shaped endocrine
Histamine and granulocytes in the umbilical cord blood of infants at birth
 Br. J. Pharmac. (1970), 40, 310-316. Histamine and granulocytes in the umbilical cord blood of infants at birth R. G. MITCHELL AND J. F. PORTER Department of Child Health, University of Aberdeen, Foresterhill,
Br. J. Pharmac. (1970), 40, 310-316. Histamine and granulocytes in the umbilical cord blood of infants at birth R. G. MITCHELL AND J. F. PORTER Department of Child Health, University of Aberdeen, Foresterhill,
The hexavalent DTaP/IPV/Hib/HepB combination vaccine
 The hexavalent combination vaccine Information for registered healthcare practitioners about the neonatal selective immunisation programme for babies at high risk (babies born to hepatitis B positive mothers)
The hexavalent combination vaccine Information for registered healthcare practitioners about the neonatal selective immunisation programme for babies at high risk (babies born to hepatitis B positive mothers)
Paediatric Clinical Chemistry
 Paediatric Clinical Chemistry Dr N Oosthuizen Dept Chemical Pathology UP 2011 Paediatric biochemistry The child is not a miniature adult Physiological development Immature organ systems Growing individual
Paediatric Clinical Chemistry Dr N Oosthuizen Dept Chemical Pathology UP 2011 Paediatric biochemistry The child is not a miniature adult Physiological development Immature organ systems Growing individual
Rat cholesterol ELISA Kit
 Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion , version 1.1
 EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
Chapter 11. Lecture and Animation Outline
 Chapter 11 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Chapter 11 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
SWISS SOCIETY OF NEONATOLOGY. Spontaneous intestinal perforation or necrotizing enterocolitis?
 SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
Reference Number: Revision No: Review Cycle:
 Policy and Procedure for the management of patients presenting with excessive anticoagulation (INR>5.0) while on warfarin at the Cork University Hospital Group. Reference Number: Revision No: Review Cycle:
Policy and Procedure for the management of patients presenting with excessive anticoagulation (INR>5.0) while on warfarin at the Cork University Hospital Group. Reference Number: Revision No: Review Cycle:
Point of Care Testing for INR using CoaguChek XS Plus
 Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
Study design: Multicenter, randomized, controlled, cross-over, blinded PK comparison
 Brand Name 1, 2 : Rixubis Generic Name 1, 2 : Coagulation factor IX recombinant Manufacturer 5 : Baxter Drug Class 1, 2, 3 : Antihemophilic agent Labeled Uses 1, 2 : Hemophilia B hemorrhage, routine prophylaxis,
Brand Name 1, 2 : Rixubis Generic Name 1, 2 : Coagulation factor IX recombinant Manufacturer 5 : Baxter Drug Class 1, 2, 3 : Antihemophilic agent Labeled Uses 1, 2 : Hemophilia B hemorrhage, routine prophylaxis,
