Relative importance of dissolved versus trophic bioaccumulation of copper in marine copepods
|
|
|
- Robert Spencer
- 5 years ago
- Views:
Transcription
1 MARINE ECOLOGY PROGRESS SERIES Vol. 231: , 2002 Published April 22 Mar Ecol Prog Ser Relative importance of dissolved versus trophic bioaccumulation of copper in marine copepods Sung Il Chang, John R. Reinfelder* Department of Environmental Sciences, Rutgers University, 14 College Farm Road, New Brunswick, New Jersey 08901, USA ABSTRACT: In order to evaluate the relative contribution of water and food to the accumulation of copper in herbivorous marine zooplankton, the accumulation and loss rates of Cu in coastal copepods (Acartia sp. and Temora sp.) were measured and applied to a kinetic bioaccumulation model. Experiments were performed to measure stable Cu uptake from the dissolved phase in field-collected copepods acclimated for 2 to 3 d to a low Cu diet. The copepods were exposed to a range of free-cu ion concentrations ([Cu 2+ ] = M, M, M), but a significant Cu accumulation rate was only observed in copepods exposed to M Cu 2+. Based on the Cu uptake rate measured at M Cu 2+, a free ion-specific uptake rate constant of dissolved Cu of l g 1 d 1 was estimated. Efflux rate constants of Cu in copepods estimated from depuration experiments were ± and ± d 1 in laboratory-fed and unacclimated copepods, respectively. Application of these Cu uptake and efflux parameters, and those for Cu trophic transfer from previous studies, to a kinetic bioaccumulation model shows that food is the dominant source of Cu (>75% of total accumulation) at free-cu ion concentrations ranging from to M. At free-cu concentrations M, dissolved uptake becomes a significant portion (>20%) of total Cu accumulation accounting for almost 60% of the total Cu in copepods at M Cu 2+. These results suggest that herbivorous marine zooplankton accumulate Cu mainly by trophic transfer, but that dissolved uptake could be important in contaminated waters. KEY WORDS: Copper Bioaccumulation Dissolved uptake Kinetic model Zooplankton Copepods Resale or republication not permitted without written consent of the publisher INTRODUCTION *Corresponding author. reinfelder@envsci.rutgers.edu The bioavailability and trophic transfer of metals in aquatic food chains has received considerable attention in recent years (Wang et al. 1996, Fisher et al. 2000, Lee et al. 2000) in an effort to better understand the geochemical cycling of metals and the nutritional and toxicological effects of metals in aquatic organisms. A quantitative analysis of the pathways of metal bioaccumulation is also important to the development of water quality criteria, since levels based only on dissolved metal concentrations in natural waters and sediments may not be appropriate to assess the full extent and impact of metal contamination in aquatic environments. For many aquatic invertebrates, metal accumulation from ingested food is the main route of overall bioaccumulation (Luoma et al. 1992, Fisher & Reinfelder 1995, Wang et al. 1996, Munger & Hare 1997). The bioavailability of metals associated with food particles can therefore be important to the assessment of the potential impacts of metal contamination in such organisms. The relative importance of dissolved versus trophic accumulation of metals in herbivorous zooplankton depends on the chemical properties of each metal and the physiology and activity of the zooplankton. These geochemical and biological properties can be used to quantify the 2 routes of accumulation separately in a bioenergetic-based kinetic model. Essential information includes accumulation rates of ingested metals, Inter-Research
2 180 Mar Ecol Prog Ser 231: , 2002 uptake rates for the dissolved phase, and depuration rates of metals in consumer animals (Landrum et al. 1992, Thomann et al. 1995, Wang & Fisher 1997). Kinetic bioaccumulation models have been applied to the bioavailability and trophic transfer of a variety of essential (Co, Se, and Zn) and non-essential (Ag, Am, Cd, and Hg) trace metals in copepods (Wang & Fisher 1998, Fisher et al. 2000), mussels (Wang et al. 1996, Wang & Fisher 1997), and other marine and freshwater animals. These efforts have been supported by substantial data on the bioaccumulation of such metals in marine phytoplankton and aquatic herbivore organisms (Fisher & Reinfelder 1995). However, despite its potential toxicity and elevated concentrations in impacted coastal waters (Moffett et al. 1997), Cu has been little studied with regard to its bioaccumulation by herbivorous zooplankton or other marine consumers (Chang & Reinfelder 2000). There is an indication that Cu concentrations in some polluted estuarine waters are high enough to affect the survival and production of marine copepods (Sunda et al. 1987), but a quantitative study of the pathways of Cu accumulation in such marine herbivores has not been done. The paucity of data may be due to the lack of a readily available, long-lived Cu radioisotope or a stable isotope of low mass abundance. Our objectives were to measure Cu influx and efflux rates in marine copepods and to determine the relative importance of dissolved and trophic pathways to overall Cu bioaccumulation. With the use of trace metal clean culture techniques and trace-metal-buffered seawater, we attempted to measure gross uptake and loss rates of stable Cu in copepods. Another objective was to relate Cu accumulation in copepods to the concentration of free Cu 2+, which is proportional to that of total inorganic Cu and represents the bioavailable pool. Copper accumulation and toxicity in marine phytoplankton (Sunda & Guillard 1976, Anderson & Morel 1978, Sunda & Huntsman 1983) and invertebrates (Zamuda & Sunda 1982, Sanders et al. 1983, Sunda et al. 1987) has been related to the free-cu ion concentration, and mechanistic relationships between Cu accumulation and free or kinetically labile Cu pools have been inferred. It is not known which chemical species of Cu are taken up by copepods, but since deleterious effects were observed at free-cu concentrations of to M (Sunda et al. 1987, Sharp & Stearns 1997), we measured Cu accumulation over the range of to M. MATERIALS AND METHODS Diatom cultures. Axenic cultures of the marine diatom Thalassiosira weissflogii (Actin) were maintained in trace-metal-buffered artificial seawater media (Aquil, Price et al. 1988/89) under continuous (24 h) illumination (200 µmol quanta m 2 s 1 PAR) at 18 C. In these culture media, which include macronutrients (N, P, and Si), vitamins, and trace metals, the concentrations of free and inorganic trace metal pools are maintained constant by excess concentrations of the chelating agent EDTA (ethylenediaminetetraacetic acid). Free-Cu ion concentrations were controlled by adding a 1:1 (mol:mol) Cu:EDTA solution (Sunda & Huntsman 1995, Chang & Reinfelder 2000). Speciation calculations of trace-metal concentrations in the media were made using the MINEQL speciation program (Westall et al. 1976). Copepods. Copepods were collected with a plankton net (63 µm Nylon mesh) from Cheesequake Creek near Raritan Bay, New Jersey, USA. Immediately following collection, adult copepods were separated from nauplii by sieving with a 350 µm Nylon netting and transferred to Cheesequake Creek water (S = 24) that had been passed through 20 µm Nylon mesh. Microscopic examination showed that the mixed assemblage contained ~90% Acartia sp. and ~10% Temora sp. (by number). Thus while the results obtained here are not strictly species-specific, they are most representative of Cu interactions with Acartia sp. Copepods were acclimated in diluted, trace-metal-free synthetic ocean water (SOW, S = 25) at 18 C for 2 or 3 d prior to Cu uptake experiments, during which time they were fed the diatom Thalassiosira weissflogii grown at pcu 14.8 (pcu = log[cu 2+ ]). Copper uptake in copepods from the dissolved phase. Experiments were performed to examine timedependent copper uptake from the dissolved phase into marine copepods over a range of free-cu ion concentrations (pcu = to 9.79). The EDTA-buffered Cu uptake media were prepared exactly as those for the diatom cultures, including 0.2 µm filtration, except that the SOW was diluted to S = 25 to minimize the possible effects of salinity change on metal uptake and copepod physiology. The same concentrations of macro-nutrients (N, P, and Si), vitamins, EDTA, and other micro-nutrient trace elements (Zn, Fe, Mo, and Se) as used in the diatom culture media were added to the copepod uptake media. After adding a 1:1 (mol:mol) Cu:EDTA solution, the uptake media were equilibrated for at least 24 h at 18 C prior to the experiments. The trace metal speciation calculations for the diluted Aquil media are presented in Table 1. Copepods in the acclimation media were collected on a 350 µm Nylon netting and transferred to uptake media in a plastic beaker. This media was then divided into small plastic bottles, each of which held 50 to 60 individual copepods in 40 ml Cu uptake media. These bottles were maintained at 18 C in the dark for 8 to 24 h.
3 Chang & Reinfelder: Bioaccumulation of copper in marine copepods 181 Table 1. Total metal concentrations required to achieve specified free metal concentrations (pm = log[m z+ ]) in diluted salt (S = 25) Aquil media. Metal speciation calculated with MINEQL (Westall 1976) Metal Total concentration (M) pm Cu Fe Mn Zn At each sampling time, copepods were separated from uptake media using a 350 µm Nylon netting and transferred to 10 ml trace-metal-free SOW in a plastic beaker. The copepods were then collected on preweighed 3 µm polycarbonate membrane filters and sequentially rinsed with 10 ml of 1 mm EDTA in tracemetal-free SOW and 10 ml of SOW to remove adsorbed Cu from the surfaces of the copepods. The copepods were also rinsed with 10 ml of 0.7 N ammonium formate to remove inorganic sea-salts. The filter with copepods was dried at 60 C overnight and weighed with a microelectronic balance (copepod dry weight = mg per individual copepod). Efflux rates of copper from laboratory-fed and unacclimated copepods. Laboratory-fed copepods were fed diatoms grown in Aquil as described above, except that the free-cu ion concentration was M. Mid-exponential growth phase cells (cell densities of cells ml 1 ) were collected on 3 µm polycarbonate (PC) membrane filters and rinsed sequentially with 10 ml of 1 mm EDTA in trace-metalfree SOW and 10 ml SOW to remove adsorbed Cu from the cell surfaces. Diatoms were resuspended in 500 ml of diluted (S = 25) SOW in a 500 ml PC bottle and 600 to 700 copepods were added. The feeding suspension was maintained at 18 C. After 1 d of feeding, the copepods were collected on a 350 µm Nylon netting, rinsed with SOW, and transferred to 100 ml of particle-free (0.2 µm filtered) SOW and allowed to clear their guts for 1 h. They were then transferred to 500 ml of particle-free SOW and allowed to depurate in the absence of food for 2 d at 18 C. At each sampling time, 50 to 60 individual copepods were removed from the bottle by filtering 40 ml of depuration media through a 350 µm Nylon mesh. After transferring the copepods to 30 ml of SOW, they were immediately collected on preweighed 3 µm PC membrane filters and rinsed with 10 ml of 0.7 N ammonium formate solution to remove inorganic salts. The filter with the copepods was dried at 60 C overnight and weighed with a microelectronic balance. Additional experiments were conducted to examine the efflux rates of copper from copepods that accumulated Cu from the dissolved phase and by trophic transfer in their natural environment (unacclimated copepods). For these studies, freshly collected copepods were immediately transferred to the diluted SOW and allowed to depurate Cu in the absence of food for 3 d at 18 C. The rates with which trace metals are eliminated from aquatic invertebrates show first-order dependence on the concentration of the metal in the organism (Reinfelder et al. 1997, Wang & Fisher 1998). The efflux of Cu from copepods was therefore fit to a firstorder, exponential loss model in order to calculate Cu loss rate constants: Cu t = Cu 0 e kt where Cu t is the concentration of Cu (µg g 1 dry wt) retained in the copepods at time t (d), Cu 0 is the initial Cu concentration in the copepods, and k is the Cu efflux rate constant (d 1 ). Sample digestion and Cu analysis. For sample digestion, PC filters with copepods were placed in 30 ml fused-quartz crucibles and 3 ml concentrated nitric acid (OPTIMA grade, Fisher) was added. After 2 d incubation at room temperature, filters were removed and the solution was slowly evaporated on a hot plate at 90 C. Copper in the residue was extracted with 1 ml of 1% nitric acid (OPTIMA grade), and the extract was transferred to a 1.25 ml acid-cleaned, micro-centrifuge tube. The sample was centrifuged at g for 3 min at room temperature to spin down any particles in the extract and copper concentrations in the supernatant were determined by graphite furnace atomic absorption spectrometry (GFAAS, Perkin- Elmer 4100 ZL) using an external calibration method. Quality assurance samples (NIST SRM 1643d, trace elements in water ) were analyzed with each set of samples (certified value = 20.5 ± 3.8 µg l 1 ; measured value = 20.1 ± 0.9 µg l 1, n = 8) and Cu concentrations were corrected for method blanks. RESULTS Copper uptake from the dissolved phase The measurement of stable Cu accumulation in labacclimated copepods (fed low-cu diatoms) was not sensitive enough to determine dissolved Cu uptake rates at pcu = and pcu = (Fig. 1). Copepods exposed to pcu = and showed an initial lack of significant accumulation of dissolved Cu followed by an apparent and transitory increase. Since the variability of Cu concentrations in copepods at different time points was similar to that within replicates
4 182 Mar Ecol Prog Ser 231: , 2002 sampled at the same time, no significant Cu accumulation rate could be determined. A significant Cu uptake rate was observed in copepods exposed to pcu = 9.79 (F = 1097, p < 0.01). Dissolved Cu uptake in copepods exposed to M free Cu showed rapid uptake during the first 2 h followed by slower, constant accumulation thereafter (Fig. 1). Although EDTA rinsing results (Table 2) suggest that most of the accumulated Cu was internalized by the copepods, the initial rapid uptake during the first 2 h at pcu = 9.79 could be due to nonsteady-state surface accumulation (apparently not removable by 1 mm EDTA). The Cu uptake rate at pcu = 9.79 was therefore calculated using data from 2h onward. Very little mortality (= 5%) of copepods occurred during the Cu uptake experiments and was not corrected for in rate calculations. The free Cuspecific uptake rate constant of dissolved Cu in copepods was estimated using the Cu uptake rate measured at pcu = 9.79 (110 µg g 1 d 1 ) and was found to have a value of l g 1 d 1. Efflux rate of copper from copepods fed cultured diatoms and natural foods Fig. 1. Accumulation of dissolved Cu in copepods. Copepods were exposed to dissolved Cu at pcu = (M), (d), 9.79 (j), where pcu = log[cu 2+ ]. Data points are means ±1 SD Fig. 2. Efflux of Cu from copepods fed diatoms grown with M free Cu (j) and unacclimated Cheesequake Creek copepods (d). Data points are means ± 1 SD The depuration of Cu from both laboratory-fed (diatoms grown at pcu = 12.79) and unacclimated copepods was fairly slow, with a slightly faster release rate during the first 5 h from the cultured diatom-fed copepods (Fig. 2). By treating the copepods as a single-compartment organism, the slow phase of depuration, representing physiological loss of Cu, can be used to determine the Cu efflux rate constant. Copper loss rate constants were calculated as the linear regression slopes of natural log-transformed depuration data. We used Cu loss data for 5 48 and 0 72 h to estimate the efflux rate constants of Cu in copepods fed cultured diatoms and unacclimated copepods, respectively. Copepod mortality was 10 to 15% during the efflux experiments and was not corrected for in loss rate calculations. Significant exponential loss rates were observed in the lab-fed (0.056 d 1 ; F = 34, p < 0.05) and unacclimated (0.076 d 1 ; F = 46, p < 0.05) copepods and these were not significantly different from each other (p > 0.99) by the Tukey- Kramer test of difference of slopes (Sokal & Rohlf 1981). The maximum potential re-accumulation of Cu lost during the depuration experiments was evaluated using the dissolved Cu concentration that would have been obtained if all Cu lost from the copepods in 3 d was in the dissolved phase at the beginning of the depuration and the dissolved Cu uptake rate constant determined above. Based on bioassays with diatoms, the maximum total dissolved Cu the depuration copepods could have been exposed to corresponds to a free Cu of < M (<10 6 µg l 1 ). Thus the maximum dissolved Cu uptake rate in the depurating copepods could have been <0.01 µg g 1 d 1. Since this is much lower than the observed minimum Cu loss rate (1.2 µg g 1 d 1 ), Cu recycling was considered unimportant in these experiments. Table 2. Surface-adsorbed Cu (removable with 1 mm EDTA) in copepods exposed to dissolved Cu uptake media for 3 h. Values are mean ± SD (n = 3) Cu concentrations with and without EDTA rinsing pcu Cu in copepods (µg g 1 dry wt) Percent Rinsed with Rinsed with surfaceseawater only seawater plus bound EDTA ± ± ± ±
5 Chang & Reinfelder: Bioaccumulation of copper in marine copepods 183 DISCUSSION Accumulation of dissolved copper by copepods Copepods acclimated to a diet of diatoms grown at low free Cu (pcu = 14.79) accumulated dissolved Cu at pcu = 9.79, but not at pcu = or Loss of Cu accumulated by the copepods prior to collection or during acclimation may have obscured dissolved Cu uptake below a [Cu 2+ ] of M. However, there does not appear to have been a rapid exchange of surface adsorbed Cu during the dissolved phase accumulation experiments since only 0 to 11% of Cu in the copepods was in a surface exchangeable pool (Table 2). The rapid accumulation of dissolved Cu in copepods at pcu = 9.79 was therefore mainly associated with internal tissues or strongly bound to the copepods surfaces. At the highest exposure concentration, Cu accumulation in the copepods did not reach a steady-state in 24 h (Fig. 1), indicating an absence of Cu regulation over this time scale. The free-cu ion-specific rate constant of dissolved Cu accumulation in copepods measured here can be compared with those for other metals by conversion to a total metal-specific uptake rate constant with the ratio of free:total Cu in natural waters (total Cu values of the EDTA-buffered media are unrealistically high). The ratio of [Cu 2+ ]:Cu T varies from in the surface waters of oligotrophic open ocean surface waters (Coale & Bruland 1988) to in harbor surface waters (Moffett et al. 1997). Since our copepods were collected from a tributary of Raritan Bay, New Jersey, we used the measured speciation of dissolved Cu in Raritan Bay (99.4% of total dissolved Cu is bound by organic ligands; Sunda et al. 1987) and an inorganic side reaction coefficient (α ) of 13 (Byrne et al. 1988) to estimate a [Cu 2+ ]:Cu T of The calculated total Cu-specific uptake rate constant is therefore 5.1 l g 1 d 1 for these coastal waters. The uncertainty associated with this calculation notwithstanding, dissolved accumulation of Cu appears to be faster than that measured in estuarine copepods (Wang & Fisher 1998) for Cd (0.69 l g 1 d 1 ), slightly slower than that for Ag (10 l g 1 d 1 ) and similar to that for Zn (3.3 l g 1 d 1 ). Efflux of copper from laboratory-fed and unacclimated copepods The efflux of Cu from copepods fed laboratorygrown diatoms showed a slightly faster rate within the first few hours than thereafter (Fig. 2), as was observed for other trace metals in laboratory-fed, marine copepods (Wang & Fisher 1998). This initial loss, which may include Cu egestion, was not seen in the unacclimated copepods. Copper efflux rate constants for unacclimated copepods, which accumulated Cu from both the dissolved phase and food, and copepods fed cultured diatoms were not significantly different, indicating that the efflux rate of Cu is controlled primarily by copepod physiology and not by food type or route of exposure. The Cu efflux rate constants measured in this study (0.06 to 0.08 d 1 ) are similar to that of the copepod Calanus hyperboreus in the Greenland Sea (0.05 d 1 ; Ritterhoff & Zauke 1997a) and for Zn in Temora longicornis from the Long Island Sound (0.08 d 1 ; Wang & Fisher 1998). They are, however, lower than those of Ag (0.3 d 1 ), Cd (0.3 d 1 ), and Co (0.3 d 1 ) in T. longicornis (Wang & Fisher 1998) and somewhat lower than those of Pb (0.23 d 1 ) and Zn (0.17 d 1 ) in Greenland Sea Calanus hyperboreus (Ritterhoff & Zauke 1997c). Based on these limited data, it appears that the efflux kinetics of metals in marine copepods do not vary greatly across taxa or among different metals. This is similar to what has been found for metal efflux in marine bivalves (Wang et al. 1996, Reinfelder et al. 1997). However, metal elimination rates in marine copepods are an order of magnitude higher than in marine bivalves (0.01 to 0.03 d 1 ; Wang et al. 1996, Reinfelder et al. 1997). Our estimates of Cu efflux rate constants in copepods are at the low end of the range among the metals tested, indicating that assimilated Cu is associated with relatively slowly exchanging pools in these animals. The slow elimination of dissolved Cu from copepods may result from its strong tendency to bind protein (Bryan 1984). Copper is referred to as a borderline metal that exhibits chemical reactivity with sulfur-, nitrogen-, and oxygen-bearing functional groups (Turner et al. 1981). The binding strength of Cu to sulfur-nitrogen ligands, such as cysteine or the metalloenzyme carbonic anhydrase, is greatest among the borderline metals which include Cd, Co(II), Fe(II), Ni, and Zn (Williams 1981). The slow turnover of Cu in copepods may also indicate a significant biological requirement for and/or regulation of this essential metal (see below) as has been noted for other marine invertebrates (Bryan 1984, Langston & Spence 1995). Whether or not Cu is regulated or required by marine copepods, the remineralization of Cu by copepods via physiological turnover is sufficiently slow that it is not likely to be a significant pathway for the release of Cu from suspended particles into the dissolved phase. Kinetic model of the bioaccumulation of Cu in copepods Kinetic bioaccumulation models are bioenergetically based models in which the concentration of a metal in
6 184 Mar Ecol Prog Ser 231: , 2002 Table 3. Kinetic parameters and their values used to model Cu bioaccumulation in marine copepods Parameter Symbol Value Units Dissolved Cu uptake rate constant k u l g 1 d 1 (free ion specific) Free Cu ion concentration Cu 2+ w µg l 1 Copepod ingestion rate IR 0.44 a d 1 Cu assimilation efficiency AE 0.4 b Unitless Cu concentrations of diatom food Cu f c µg g 1 Cu efflux rate constant k e 0.08 d 1 Copepod growth rate g 0.02 d d 1 a Estimated from the ingestion rates for Acartia sp. in the Lower Hudson River Estuary from Lonsdale et al. (1996) b From Chang & Reinfelder (2000) c Based on diatom-cu-free-cu ion concentration algorithms in Chang & Reinfelder (2000) d Copepod growth rate was calculated as half of the geometric mean egg production rates for female Acartia sp. in the Lower Hudson River Estuary of Londsdale et al. (1996) 1 or more compartments of an organism is controlled by uptake and elimination. With such a model, different pathways of metal accumulation can be quantified separately and the relative importance of metal uptake from the dissolved phase and ingested food assessed (Thomann et al. 1995, Wang & Fisher 1997, 1998, Reinfelder et al. 1998). Assuming that copepods accumulate Cu in a single compartment, the steady-state concentration of Cu in copepods can be described by the following equations: Cu ss,w = (k u Cu w )/(k ew + g) Cu ss,f = (AE IR Cu f )/(k ef + g) Cu ss = Cu ss,w + Cu ss,f where Cu ss,w and Cu ss,f are the concentrations of Cu (µg g 1 ) in copepods due to uptake from the dissolved phase and food, respectively, k u is the uptake rate constant (l g 1 d 1 ) from the dissolved phase, Cu w and Cu f are the dissolved (µg l 1 ) and food (µg mg 1 ) Cu concentrations, respectively, k ew and k ef are efflux rate constants (d 1 ) following uptake from the dissolved phase and food, respectively, g is the copepod growth rate constant (d 1 ), AE is the assimilation efficiency (%) of ingested Cu, IR is the copepod ingestion rate (d 1 ), and Cu ss is the total concentration of Cu (µg g 1 ) in a copepod. The kinetic parameters shown in Table 3 were used to model Cu accumulation in copepods over a range of free-cu ion concentrations. The ingestion rate of 0.44 d 1 was estimated using the average ingestion rate for Acartia sp. in the Lower Hudson River Estuary measured by Londsdale et al. (1996), assuming a carbon content of 3.7 µg C per copepod. The AE (fraction of ingested Cu that is retained by the copepods following gut clearance) is that measured in copepods by Chang & Reinfelder (2000). The AE of Cu in copepods (like that of other metals) is proportional to the cytoplasm fraction of Cu in diatom cells and the cytoplasm, and total cellular Cu concentrations in diatoms increase linearly with the free-cu concentration of the growth media (Chang & Reinfelder 2000). The AE of Cu in copepods is assumed here to be constant, but may increase somewhat at higher free-cu concentrations (Chang & Reinfelder 2000). The Cu content of food (Cu f ) is based on a diatom diet and the relationships between Cu accumulation in a marine diatom and the free-cu concentration determined by Chang & Reinfelder (2000). In these relationships, diatom Cu was found to increase more rapidly with increasing free-cu concentration above M free Cu. We used an overall Cu efflux rate constant (k e ) to describe the loss of Cu from copepods regardless of uptake pathway with a value of 0.08 d 1, the higher of the 2 measured values. Copepod growth rate was calculated as half of the geometric mean egg production rates for female Acartia sp. in the Lower Hudson River Estuary determined by Londsdale et al. (1996), assuming an equal number of male and female copepods (adult male copepods do not exhibit significant growth). Over a free-cu concentration range of 10 7 to 10 2 µg l 1 ( to M), model-predicted steady-state copepod Cu concentrations varied from 5 to 2000 µg g 1 dry wt (Fig. 3a). Copper concentrations in copepods collected from Cheesequake Creek were 20 to 30 µg g 1, which, based on the model results, corresponds to a free-cu concentration range of 10 5 to 10 4 µg l 1 ( to M). This bioassay result is consistent with free-cu concentrations determined by electrochemical methods in harbor estuaries of the northeast US (Moffett et al. 1997, Kozelka & Bruland 1998). Copepods living in such impacted waters have significantly more Cu than copepods living in cleaner waters such as the Fram Strait and Greenland Sea (4 to 8 µg g 1 ; Ritterhoff & Zauke 1997b). However, it is noteworthy that our harbor estuary copepods have only a factor of 2 or 3 more Cu than the estimated (for decapod crustaceans with no haemocyanin) enzymatic requirement of 7 to 15 µg g 1 (White & Rainbow 1985, Depledge & Bjerregaard 1989). The model results also show that Cu accumulation from food is the main pathway in waters with moderate
7 Chang & Reinfelder: Bioaccumulation of copper in marine copepods 185 Fig. 3. (a) Model predicted Cu content and (b) percent total Cu in copepods attributable to accumulation from water (s) or food (d) to low dissolved free-cu ion concentrations ( 10 4 µg l 1 or M Cu 2+ ). Thus, food accounts for >75% of overall Cu accumulation at free-cu ion concentrations ranging from 10 7 to 10 4 µg l 1 ( to M, Fig. 3b). As a result of the relatively shallow slope of the diatom-cu free-cu concentration relationship (Chang & Reinfelder 2000), over the 1000-fold increase in free-cu concentration from 10 7 to 10 4 µg l 1, Cu accumulation in copepods from food increases by only a factor of 7 (Fig. 3a). Above a free-cu ion concentration of 10 4 µg l 1, Cu accumulation from food increases more sharply with increasing free Cu, but the fraction of total Cu accumulation from food decreased from 75 to 41% (Fig. 3b). At free-cu concentrations > 10 4 µg l 1 ( M), dissolved Cu is a significant (>20%) source of Cu to copepods (Fig. 3). It is well recognized that organic complexation dominates the speciation of Cu in coastal and oceanic surface waters and buffers the concentration of free dissolved Cu (Coale & Bruland 1988, 1990, Kozelka & Bruland 1998). However, small increases in total dissolved Cu can titrate Cu-complexing organic ligands, resulting in large increases in free-dissolved- Cu ion concentrations (Moffett 1995, Moffett et al. 1997). For example, free-cu ion concentrations in polluted harbors can reach as high as M (Ahner et al. 1997). Under such conditions, there could be a greater contribution of the dissolved phase than trophic transfer to Cu accumulation in copepods, especially if the increase in dissolved Cu occurs over a time scale that is shorter than that required for prey phytoplankton to reach steady-state with the dissolved phase. Eventually, elevated free-cu concentrations will lead to higher phytoplankton Cu concentrations and higher Cu accumulation in consumer organisms such as copepods via trophic transfer. Sunda et al. (1987) suggested that the concentration of free Cu in some polluted estuarine waters (~10 11 M) is high enough to affect the survival and reproduction of copepods. We observed very little mortality of copepods (~5%) during 24 h exposure to high free-cu ion concentrations (pcu = 9.79), which indicates that toxic effects on the survival of copepods may not result from short-term dissolved Cu exposures. Copper toxicity in copepods may result from chronic exposure to high dissolved Cu or from Cu obtained via trophic transfer as has been shown for Ag and Cd (Hook & Fisher 2001). The importance of trophic transfer to the accumulation of metals (most notably Se and Zn) has been demonstrated in a number of marine herbivores (Wang et al. 1996, Fisher et al. 2000). The present results show that trophic transfer is also of major importance to the accumulation of Cu in marine copepods. This is so because the bioaccumulation of Cu in copepods is characterized by low efflux rates, a relatively high AE, and a negligible contribution of dissolved uptake at free-cu ion concentrations that are typical of most natural waters. Acknowledgements. This work was supported by Hatch/ McIntyre-Stennis grant and NJ SeaGrant project R/E to J.R.R. We thank 3 anonymous reviewers for their comments. LITERATURE CITED Ahner BA, Morel FMM, Moffett JW (1997) Trace metal control of phytochelatin production in coastal waters. Limnol Oceanogr 42: Anderson DM, Morel FMM (1978) Copper sensitivity of Gonyaulax tamarensis. Limnol Oceanogr 23: Bryan GW (1984) Pollution due to heavy metals and their
8 186 Mar Ecol Prog Ser 231: , 2002 compounds. In: Kinne O (ed) Marine ecology, Vol 5. John Wiley & Sons, Chichester, p Byrne RH, Lump LR, Cantrell KJ (1988) The influence of temperature and ph on trace metal speciation in seawater. Mar Chem 25: Chang SI, Reinfelder JR (2000) Bioaccumulation, subcellular distribution, and trophic transfer of copper in a coastal marine diatom. Environ Sci Technol 34: Coale KH, Bruland KW (1988) Copper complexation in the northeast Pacific. Limnol Oceanogr 33: Coale KH, Bruland KW (1990) Spatial and temporal variability in copper complexation in the North Pacific. Deep-Sea Res 47: Depledge MH, Bjerregaard P (1989) Haemolymph protein composition and copper levels in decapod crustaceans. Helgol Meeresunters 43: Fisher NS, Reinfelder JR (1995) The trophic transfer of metals in marine systems. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. IUPAC, Wiley, Chichester, p Fisher NS, Stupakoff I, Sañudo-Wilhelmy S, Wang WX, Teyssié JL, Fowler SW, Crusius J (2000) Trace metals in marine copepods: a field test of a bioaccumulation model coupled to laboratory uptake kinetics data. Mar Ecol Prog Ser 194: Hook SE, Fisher NS (2001) Reproductive toxicity of metals in calanoid copepods. Mar Biol 138: Kozelka PB, Bruland KW (1998) Chemical speciation of dissolved Cu, Zn, Cd, Pb in Narragansett Bay, Rhode Island. Mar Chem 60: Landrum PF, Lee H, Lydy MJ (1992) Toxicokinetics in aquatic systems: model comparisons and use in hazard assessment. Environ Toxicol Chem 11: Langston WJ, Spence SK (1995) Biological factors involved in metal concentrations observed in aquatic organisms. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester, p Lee BG, Griscom SB, Lee JS, Choi HJ, Koh CH, Luoma SN, Fisher NS (2000) Influence of dietary uptake and acidvolatile sulfide on bioavailability of metals to sedimentdwelling organisms. Science 287: Lonsdale DJ, Cosper EM, Kim WS, Doall M, Divadeenam A, Jonasdottir SH (1996) Food web interactions in the plankton of Long Island bays, with preliminary observations on brown tide effects. Mar Ecol Prog Ser 134: Luoma SN, Johns C, Fisher NS, Steinberg NA, Oremland RS, Reinfelder JR (1992) Determination of selenium bioavailability to a benthic bivalve from particulate and solute pathways. Environ Sci Technol 26: Moffett JW (1995) The spatial and temporal variability of copper complexation by strong organic ligands in the Sargasso Sea. Deep-Sea Res 42: Moffett JW, Brand LE, Croot PL, Barbeau KA (1997) Cu speciation and cyanobacterial distribution in harbors subject to anthropogenic Cu inputs. Limnol Oceanogr 42: Munger C, Hare L (1997) Relative importance of water and food as cadmium sources to an aquatic insect (Chaoborus puntipennis): implications for predicting Cd bioaccumulation in nature. Environ Sci Technol 31: Price NM, Harrison GI, Herring JG, Hudson RJ, Nirel PM, Palenik B, Morel FMM (1988/89) Preparation and chemistry of the artificial algal culture medium Aquil. Biol Oceanogr 6: Reinfelder JR, Wang WX, Luoma SN, Fisher NS (1997) Assimilation efficiencies and turnover rates of trace elements Editorial responsibility: Otto Kinne (Editor), Oldendorf/Luhe, Germany in marine bivalves: a comparison of oyster, clams, and mussels. Mar Biol 129: Reinfelder JR, Fisher NS, Luoma SN, Nichols JW, Wang WX (1998) Trace element trophic transfer in aquatic organisms: a critique of the kinetic model approach. Sci Total Environ 219: Ritterhoff J, Zauke GP (1997a) Evaluation of trace metal toxicokinetics in Greenland Sea copepod and amphipod collectives from semi-static experiments on board ship. Polar Biol 17: Ritterhoff J, Zauke GP (1997b) Trace metals in field samples of zooplankton from the Fram Strait and the Greenland Sea. Sci Total Environ 199: Ritterhoff J, Zauke GP (1997c) Bioaccumulation of trace metals in Greenland Sea copepod and amphipod collectives on board ship: verification of toxicokinetic model parameters. Aquat Toxicol 40:63 78 Sanders BM, Jenkins KD, Sunda WG, Costlow JD (1983) Free cupric ion activity in seawater: effects on metallothionein and growth in crab larvae. Science 222:53 55 Sharp AA, Stearns DE (1997) Sublethal effects of cupric ion activity on the grazing behaviour of three calanoid copepods. Mar Pollut Bull 34: Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, San Francisco Sunda WG, Guillard RRL (1976) The relationship between cupric ion activity and the toxicity of copper to phytoplankton. J Mar Res 34: Sunda WG, Huntsman SA (1983) The effect of competitive interactions between manganese and copper on cellular manganese and growth in estuarine and oceanic species of the diatom Thalassiosira. Limnol Oceanogr 28: Sunda WG, Huntsman SA (1995) Regulation of copper concentration in the oceanic nutricline by phytoplankton uptake and regeneration cycles. Limnol Oceanogr 40: Sunda WG, Tester PA, Huntsman SA (1987) Effects of cupric and zinc ion activities on the survival and reproduction of marine copepods. Mar Biol 94: Thomann RV, Mahony JE, Mueller R (1995) Steady-state model of biota sediment accumulation factor for metals in two marine bivalves. Environ Toxicol Chem 14: Turner DR, Whitfield M, Dickson AG (1981) The equilibrium speciation of dissolved components in freshwater and seawater at 25 C and 1 atm pressure. Geochim Cosmochim Acta 45: Wang WX, Fisher NS (1997) Modeling metal bioavailability for marine mussels. Rev Environ Contam Toxicol 151: Wang WX, Fisher NS (1998) Accumulation of trace elements in a marine copepod. Limnol Oceanogr 43: Wang WX, Fisher NS, Luoma SN (1996) Kinetic determinations of trace element bioaccumulation in the mussel Mytilus edulis. Mar Ecol Prog Ser 140: Westall JC, Zachary JL, Morel FMM (1976) MINEQL, Computer program of thermodynamic calculation. Tech Note 18, RM Parsons Laboratory, MIT, Cambridge, MA White SL, Rainbow PS (1985) On the metabolic requirement for Cu and Zn in molluscs and crustaceans. Mar Environ Res 160: Williams RJP (1981) Physico-chemical aspects of inorganic element transfer through membranes. Phil Trans R Soc Lond B Biol Sci 294:57 74 Zamuda CD, Sunda WG (1982) Bioavailability of dissolved Cu to the American oyster Crassostrea virginica. I. Importance of chemical speciation. Mar Biol 66:77 82 Submitted: April 2, 2001; Accepted: November 8, 2001 Proofs received from author(s): March 26, 2002
LONG-TERM GOALS OBJECTIVES
 Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Uptake of trace metals by aquatic invertebrates
 Uptake of trace metals by aquatic invertebrates Principles Organisms take up trace metals. Aquatic invertebrates take up metals from solution and diet. Metals are then accumulated or excreted. Some accumulated
Uptake of trace metals by aquatic invertebrates Principles Organisms take up trace metals. Aquatic invertebrates take up metals from solution and diet. Metals are then accumulated or excreted. Some accumulated
Award Number: N
 Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Trace Metal Speciation: Equilibrium and Kinetic Considerations on Biological Effects, Phytoplankton Uptake and Sorption Processes in Coastal Waters (Field and Laboratory Studies) Kenneth W. Bruland University
Uptake of Aqueous and Dietary Metals by Mussel Perna viridis with Different Cd Exposure Histories
 Environ. Sci. Technol. 2005, 39, 9363-9369 Uptake of Aqueous and Dietary Metals by Mussel Perna viridis with Different Cd Exposure Histories DALIN SHI AND WEN-XIONG WANG* Department of Biology, The Hong
Environ. Sci. Technol. 2005, 39, 9363-9369 Uptake of Aqueous and Dietary Metals by Mussel Perna viridis with Different Cd Exposure Histories DALIN SHI AND WEN-XIONG WANG* Department of Biology, The Hong
Trace metals in the ocean Lecture January 23, 2006
 Trace metals in the ocean 12.097 Lecture January 23, 2006 Metals of interest Required for metabolic functions Mn, Fe, Co, Ni, Cu, Zn Deficiency limits production (photosynthetic ability) Excess limits
Trace metals in the ocean 12.097 Lecture January 23, 2006 Metals of interest Required for metabolic functions Mn, Fe, Co, Ni, Cu, Zn Deficiency limits production (photosynthetic ability) Excess limits
Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling
 Limnol. Oceanogr., 45(7), 2000, 1501 1516 2000, by the American Society of Limnology and Oceanography, Inc. Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling
Limnol. Oceanogr., 45(7), 2000, 1501 1516 2000, by the American Society of Limnology and Oceanography, Inc. Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling
Limnol. Oceanogr., 40(l), 1995, , by the American Society of Limnology and Oceanography, Inc.
 Limnol. Oceanogr., 40(l), 1995, 132-137 0 1995, by the American Society of Limnology and Oceanography, Inc. Regulation of copper concentration in the oceanic nutricline by phytoplankton uptake and regeneration
Limnol. Oceanogr., 40(l), 1995, 132-137 0 1995, by the American Society of Limnology and Oceanography, Inc. Regulation of copper concentration in the oceanic nutricline by phytoplankton uptake and regeneration
The interaction between zinc deficiency and copper toxicity as it affects the silicic acid uptake mechanisms in Thalassiosira pseudonana l
 Limnol. Oceanogr., 26(l), 1981, 67-73 The interaction between zinc deficiency and copper toxicity as it affects the silicic acid uptake mechanisms in Thalassiosira pseudonana l John G. Rueter, Jr.,2 and
Limnol. Oceanogr., 26(l), 1981, 67-73 The interaction between zinc deficiency and copper toxicity as it affects the silicic acid uptake mechanisms in Thalassiosira pseudonana l John G. Rueter, Jr.,2 and
The importance of trace metal nutrients for marine phytoplankton and bacteria along Line-P. Jay T. Cullen, Maite Maldonado (UBC), Erin Lane (UBC)
 The importance of trace metal nutrients for marine phytoplankton and bacteria along Line-P Jay T. Cullen, Maite Maldonado (UBC), Erin Lane (UBC) Outline Trace Metals and Marine Biogeochemical Cycles Iron
The importance of trace metal nutrients for marine phytoplankton and bacteria along Line-P Jay T. Cullen, Maite Maldonado (UBC), Erin Lane (UBC) Outline Trace Metals and Marine Biogeochemical Cycles Iron
The trophic transfer of Cd, Cr. and Se in the barnacle Balanus amphitrite from planktonic food
 Vol. 187: 191-201, 1999 MARINE ECOLOGY PROGRESS SERIES Mar Ecol Prog Ser Published October 14 The trophic transfer of Cd, Cr. and Se in the barnacle Balanus amphitrite from planktonic food Wen-Xiong Wang*,
Vol. 187: 191-201, 1999 MARINE ECOLOGY PROGRESS SERIES Mar Ecol Prog Ser Published October 14 The trophic transfer of Cd, Cr. and Se in the barnacle Balanus amphitrite from planktonic food Wen-Xiong Wang*,
In steady state, new production = carbon export
 In steady state, new production = carbon export Where does primary production go? Export Bacteria Dissolved organic matter Grazing What other components of the biological pump are important? The majority
In steady state, new production = carbon export Where does primary production go? Export Bacteria Dissolved organic matter Grazing What other components of the biological pump are important? The majority
elucidate the role of trace metals in the ecology of the oceans and the
 CHAPTER I I. INTRODUCTION Microalgae acquire nutrients from their environment in order to sustain their growth and division. The classification of nutrients is made on the basis of their quantitative requirements
CHAPTER I I. INTRODUCTION Microalgae acquire nutrients from their environment in order to sustain their growth and division. The classification of nutrients is made on the basis of their quantitative requirements
Jennifer Grant Lee. B.S., Yale University (1986) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY.
 CADMIUM: A TOXIN AND A NUTRIENT FOR MARINE PHYTOPLANKTON by Jennifer Grant Lee B.S., Yale University (1986) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at
CADMIUM: A TOXIN AND A NUTRIENT FOR MARINE PHYTOPLANKTON by Jennifer Grant Lee B.S., Yale University (1986) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at
Copper and Cadmium Accumulation in Populations of Littorina saxatilis from the Isle of Man Having Differential Tolerance to Zinc and Lead
 International Journal of Animal and Veterinary Sciences 1(2): 92-98, 2009 ISSN: 2041-2908 Maxwell Scientific Organization, 2009 Submitted Date: September 11, 2009 Accepted Date: October 09, 2009 Published
International Journal of Animal and Veterinary Sciences 1(2): 92-98, 2009 ISSN: 2041-2908 Maxwell Scientific Organization, 2009 Submitted Date: September 11, 2009 Accepted Date: October 09, 2009 Published
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS
 SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
SPECIAL ASPECTS OF ASSESSING THE ELEMENTAL COMPOSITION OF PHYTOPLANKTON AND SESTON USING NEUTRON ACTIVATION ANALYSIS Nekhoroshkov P. S., Frontasyeva M. V. Joint Institute for Nuclear Research, FLNP, SNAAAR
Dennis A. Apeti, Larry Robinson and Elijah Johnson Environmental Sciences Institute, Florida Agricultural and Mechanical University
 American Journal of Environmental Sciences 1 (3): 179-186, 25 ISSN 1553-345X 25 Science Publications Relationships between Heavy Metal Concentrations in the American Oyster (Crassostrea virginica) and
American Journal of Environmental Sciences 1 (3): 179-186, 25 ISSN 1553-345X 25 Science Publications Relationships between Heavy Metal Concentrations in the American Oyster (Crassostrea virginica) and
The incorporation of zinc and iron into the frustule of the marine diatom Thalassiosira pseudonana
 Limnol. Oceanogr., 45(7), 2000, 1517 1524 2000, by the American Society of Limnology and Oceanography, Inc. The incorporation of zinc and iron into the frustule of the marine diatom Thalassiosira pseudonana
Limnol. Oceanogr., 45(7), 2000, 1517 1524 2000, by the American Society of Limnology and Oceanography, Inc. The incorporation of zinc and iron into the frustule of the marine diatom Thalassiosira pseudonana
SEA URCHIN SPERM BIOASSAY OF COPPER ASSOCIATED WITH NATURAL SEAWATER ORGANIC CONCENTRATES. P. A. Dinnel, J. M. Link and Q. J.
 FRI UW 8207 June 1982 SEA URCHIN SPERM BIOASSAY OF COPPER ASSOCIATED WITH NATURAL SEAWATER ORGANIC CONCENTRATES By P. A. Dinnel, J. M. Link and Q. J. Stober This work was supported by The College of Ocean
FRI UW 8207 June 1982 SEA URCHIN SPERM BIOASSAY OF COPPER ASSOCIATED WITH NATURAL SEAWATER ORGANIC CONCENTRATES By P. A. Dinnel, J. M. Link and Q. J. Stober This work was supported by The College of Ocean
The effects of Cu and Fe availability on the growth and Cu : C ratios of marine diatoms
 Limnol. Oceanogr., 53(6), 2008, 2451 2461 E 2008, by the American Society of Limnology and Oceanography, Inc. The effects of Cu and Fe availability on the growth and Cu : C ratios of marine diatoms Amber
Limnol. Oceanogr., 53(6), 2008, 2451 2461 E 2008, by the American Society of Limnology and Oceanography, Inc. The effects of Cu and Fe availability on the growth and Cu : C ratios of marine diatoms Amber
Modelling the bioavailability of trace elements
 FEDERAL INSTITUTE FOR RISK ASSESSMENT Modelling the bioavailability of trace elements Dr. Jorge Numata Federal Institute for Risk Assessment (BfR) Unit 33: Epidemiology, Biostatistics and Mathematical
FEDERAL INSTITUTE FOR RISK ASSESSMENT Modelling the bioavailability of trace elements Dr. Jorge Numata Federal Institute for Risk Assessment (BfR) Unit 33: Epidemiology, Biostatistics and Mathematical
Canadian Sediment Quality Guidelines for the Protection of Aquatic Life
 Canadian Sediment Quality Guidelines for the Protection of Aquatic Life Zinc (Zn) is an essential trace element that can be toxic to aquatic biota at elevated concentrations. Zinc enters aquatic systems
Canadian Sediment Quality Guidelines for the Protection of Aquatic Life Zinc (Zn) is an essential trace element that can be toxic to aquatic biota at elevated concentrations. Zinc enters aquatic systems
Alkaline phosphatase inhibition by copper: Implications to phosphorus nutrition and use as a biochemical marker of toxicity1
 Notes 743 Limnol. Oceanogr., 28(4), 1983, 743-748 @ 1983, hy the merican Society of Limnology Ind Oceanography, Inc. lkaline phosphatase inhibition by copper: Implications to phosphorus nutrition and use
Notes 743 Limnol. Oceanogr., 28(4), 1983, 743-748 @ 1983, hy the merican Society of Limnology Ind Oceanography, Inc. lkaline phosphatase inhibition by copper: Implications to phosphorus nutrition and use
Ingestion of microplastics by zooplankton in the western English Channel
 Ingestion of microplastics by zooplankton in the western English Channel Alice Wilson McNeal 1 ; Matthew Cole 2 ; James Clark 1 ; Pennie Lindeque 1 1. Plymouth Marine Laboratory. 2. University of Exeter.
Ingestion of microplastics by zooplankton in the western English Channel Alice Wilson McNeal 1 ; Matthew Cole 2 ; James Clark 1 ; Pennie Lindeque 1 1. Plymouth Marine Laboratory. 2. University of Exeter.
NOTES. Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the marine diatom Thalassiosira oceanica
 NOTES Limnol. Oceanogr., 52(6), 2007, 2710 2714 2007, by the American Society of Limnology and Oceanography, Inc. E Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the
NOTES Limnol. Oceanogr., 52(6), 2007, 2710 2714 2007, by the American Society of Limnology and Oceanography, Inc. E Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the
Respiration: Allometric Relationship
 Metabolism Defined as: all energy transformations, chemical reactions and pathways that make possible the properties of living organisms Measured as: the Respiration Rate, assumes all organism s energy
Metabolism Defined as: all energy transformations, chemical reactions and pathways that make possible the properties of living organisms Measured as: the Respiration Rate, assumes all organism s energy
The interaction between inorganic iron and cadmium uptake in the marine diatom Thalassiosira oceanica
 Limnol. Oceanogr., 53(5), 2008, 178 1789 E 2008, by the American Society of Limnology and Oceanography, Inc. The interaction between inorganic iron and cadmium uptake in the marine diatom Thalassiosira
Limnol. Oceanogr., 53(5), 2008, 178 1789 E 2008, by the American Society of Limnology and Oceanography, Inc. The interaction between inorganic iron and cadmium uptake in the marine diatom Thalassiosira
The Influence of Iron on the Cellular Quota of Prochlorococcus
 The Influence of Iron on the Cellular Quota of Prochlorococcus Mak Saito Department of Marine Chemistry and Geochemistry Woods Hole Oceanographic Institution Woods Hole MA 02543 phone: (508) 289-2393 fax:
The Influence of Iron on the Cellular Quota of Prochlorococcus Mak Saito Department of Marine Chemistry and Geochemistry Woods Hole Oceanographic Institution Woods Hole MA 02543 phone: (508) 289-2393 fax:
Effect of Acartia grazing on the size distribution of phytoplankton in Academy Bay, Galapagos
 Effect of Acartia grazing on the size distribution of phytoplankton in Academy Bay, Galapagos Jonathan Herzog University of Washington School of Oceanography 1 NON-TECHNICAL SUMMARY The goal of this experiment
Effect of Acartia grazing on the size distribution of phytoplankton in Academy Bay, Galapagos Jonathan Herzog University of Washington School of Oceanography 1 NON-TECHNICAL SUMMARY The goal of this experiment
MARINE ECOLOGY PROGRESS SERIES Mar. Ecol. Prog. Ser. Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey
 Vol. 110: 259-269,1994 MARINE ECOLOGY PROGRESS SERIES Mar. Ecol. Prog. Ser. 1 Published July 21 Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey D. A. Hutchins*, K. W.
Vol. 110: 259-269,1994 MARINE ECOLOGY PROGRESS SERIES Mar. Ecol. Prog. Ser. 1 Published July 21 Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey D. A. Hutchins*, K. W.
Copper Removal from the Water Column
 Copper Removal from the Water Column G. Allen Burton and Michelle Hudson School for Environment and Sustainability University of Michigan for the International Copper Association Brussels, Belgium Final
Copper Removal from the Water Column G. Allen Burton and Michelle Hudson School for Environment and Sustainability University of Michigan for the International Copper Association Brussels, Belgium Final
EFFECTS OF FREE CU 2 AND ZN 2 IONS ON GROWTH AND METAL ACCUMULATION IN FRESHWATER ALGAE
 Environmental Toxicology and Chemistry, Vol. 16, No. 2, pp. 220 229, 1997 1997 SETAC Printed in the USA 0730-7268/97 $6.00.00 EFFECTS OF FREE CU 2 AND ZN 2 IONS ON GROWTH AND METAL ACCUMULATION IN FRESHWATER
Environmental Toxicology and Chemistry, Vol. 16, No. 2, pp. 220 229, 1997 1997 SETAC Printed in the USA 0730-7268/97 $6.00.00 EFFECTS OF FREE CU 2 AND ZN 2 IONS ON GROWTH AND METAL ACCUMULATION IN FRESHWATER
EOSC Biology 3. Zooplankton Measurements
 EOSC 473-573 Biology 3 Zooplankton Measurements Zooplankton Biomass and Abundance Two Quantitative Procedures: Biomass Determination (mg C per m 3 or per m 2 ) Abundance (# individuals per m 3 or per m
EOSC 473-573 Biology 3 Zooplankton Measurements Zooplankton Biomass and Abundance Two Quantitative Procedures: Biomass Determination (mg C per m 3 or per m 2 ) Abundance (# individuals per m 3 or per m
JORIND 9(2) December, ISSN
 SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
Microplastic ingestion: the role of taste
 Microplastic ingestion: the role of taste Renske Vroom 1,2, Claudia Halsband 1, Ellen Besseling 2, Bart Koelmans 2 1 Akvaplan-niva, Fram Centre, Tromsø, Norway 2 Wageningen University and Research Centre,
Microplastic ingestion: the role of taste Renske Vroom 1,2, Claudia Halsband 1, Ellen Besseling 2, Bart Koelmans 2 1 Akvaplan-niva, Fram Centre, Tromsø, Norway 2 Wageningen University and Research Centre,
Patterns of Trace Metals Accumulation in Different Trophic Levels of Lake Kailana, Jodhpur (India)
 Sengupta, M. and Dalwani, R. (Editors). 28. Proceedings of Taal27: The 12 th World Lake Conference: 373-377 Patterns of Trace Metals Acmulation in Different Trophic Levels of Lake Kailana, Jodhpur (India)
Sengupta, M. and Dalwani, R. (Editors). 28. Proceedings of Taal27: The 12 th World Lake Conference: 373-377 Patterns of Trace Metals Acmulation in Different Trophic Levels of Lake Kailana, Jodhpur (India)
Feeding Measurements
 Feeding Measurements Measures of Feeding Rates (or specific mortality rate) Clearance Rate Concept Clearance Rate (F) = Volume cleared of prey pred -1 time -1 predator prey A consumer may have different
Feeding Measurements Measures of Feeding Rates (or specific mortality rate) Clearance Rate Concept Clearance Rate (F) = Volume cleared of prey pred -1 time -1 predator prey A consumer may have different
Report for using aquatic plant as phytoremediation for removing heavy metals
 Report for using aquatic plant as phytoremediation for removing heavy metals Vu Thi Dieu Huong (M2) 1. INTRODUCTION Charophytes are submerged macrophytes grown in wide range of water bodies and its existence
Report for using aquatic plant as phytoremediation for removing heavy metals Vu Thi Dieu Huong (M2) 1. INTRODUCTION Charophytes are submerged macrophytes grown in wide range of water bodies and its existence
OFFCHANNEL MARSH HABITATS Base of aquatic food web Juvenile Chinook diet inferred from natural abundance stable isotopes
 OFFCHANNEL MARSH HABITATS Base of aquatic food web Juvenile Chinook diet inferred from natural abundance stable isotopes Tawnya D. Peterson (OHSU) & Estuary Partnership s EMP team http://www.flycraftangling.com
OFFCHANNEL MARSH HABITATS Base of aquatic food web Juvenile Chinook diet inferred from natural abundance stable isotopes Tawnya D. Peterson (OHSU) & Estuary Partnership s EMP team http://www.flycraftangling.com
Nutrients. Classification of Elements or Nutrients (Wally Broecker):
 Nutrients Nutrients are generally considered to be elements or compounds (e.g. N is a nutrient, NO3 and NH4 are types of N compound that are also nutrients) that are needed for biological growth. Classification
Nutrients Nutrients are generally considered to be elements or compounds (e.g. N is a nutrient, NO3 and NH4 are types of N compound that are also nutrients) that are needed for biological growth. Classification
Nickel limitation and zinc toxicity in a urea-grown diatom
 Limnol. Oceanogr., 53(6), 2008, 2462 2471 E 2008, by the American Society of Limnology and Oceanography, Inc. Nickel limitation and zinc toxicity in a urea-grown diatom Eric S. Egleston Department of Civil
Limnol. Oceanogr., 53(6), 2008, 2462 2471 E 2008, by the American Society of Limnology and Oceanography, Inc. Nickel limitation and zinc toxicity in a urea-grown diatom Eric S. Egleston Department of Civil
Zooplankton Grazing and Secondary Production in the Indian Ocean
 Zooplankton Grazing and Secondary Production in the Indian Ocean Michael Landry Integrative Oceanography Division Scripps Institution of Oceanography University of California, San Diego SIBER Workshop
Zooplankton Grazing and Secondary Production in the Indian Ocean Michael Landry Integrative Oceanography Division Scripps Institution of Oceanography University of California, San Diego SIBER Workshop
The role of unchelated Fe in the iron nutrition of phytoplankton
 400 Notes Limnol. Oceanogr., 53(1), 2008, 400 404 E 2008, by the American Society of Limnology and Oceanography, Inc. The role of unchelated Fe in the iron nutrition of phytoplankton Abstract The important
400 Notes Limnol. Oceanogr., 53(1), 2008, 400 404 E 2008, by the American Society of Limnology and Oceanography, Inc. The role of unchelated Fe in the iron nutrition of phytoplankton Abstract The important
Plankton Lab 11/14 Integrated Science 1 Redwood High School Name: Period:
 Plankton Lab 11/14 Integrated Science 1 Redwood High School Period: Introduction Plankton (which comes from the Greek word for drifting) are often defined as organisms that float at or near the surface
Plankton Lab 11/14 Integrated Science 1 Redwood High School Period: Introduction Plankton (which comes from the Greek word for drifting) are often defined as organisms that float at or near the surface
A Survey of Cadmium in Pacific Oysters: Distribution, Influencing Factors and Ways to Minimize Concentrations. Aimee Christy
 A Survey of Cadmium in Pacific Oysters: Distribution, Influencing Factors and Ways to Minimize Concentrations Aimee Christy Pacific Shellfish Institute Olympia, WA Problem Identification 1999 - Hong Kong
A Survey of Cadmium in Pacific Oysters: Distribution, Influencing Factors and Ways to Minimize Concentrations Aimee Christy Pacific Shellfish Institute Olympia, WA Problem Identification 1999 - Hong Kong
Determination of available nutrients in soil using the Agilent 4200 MP-AES
 Determination of available nutrients in soil using the Agilent 4200 MP-AES Application note Agriculture Author Dharmendra Vummiti Agilent Technologies, India Introduction Multielement testing of soil samples
Determination of available nutrients in soil using the Agilent 4200 MP-AES Application note Agriculture Author Dharmendra Vummiti Agilent Technologies, India Introduction Multielement testing of soil samples
Uptake and efflux of 64 Cu by the marine cyanobacterium Synechococcus (WH7803)
 Limnol. Oceanogr., 48(1), 2003, 179 188 2003, by the American Society of Limnology and Oceanography, Inc. Uptake and efflux of Cu by the marine cyanobacterium Synechococcus (WH7803) Peter L. Croot 1 Analytical
Limnol. Oceanogr., 48(1), 2003, 179 188 2003, by the American Society of Limnology and Oceanography, Inc. Uptake and efflux of Cu by the marine cyanobacterium Synechococcus (WH7803) Peter L. Croot 1 Analytical
Primary Productivity and Lake Health: Examination of Phytoplankton Growth Rate Regulations in Keuka Lake via Short-term Microcosm Experiments
 Primary Productivity and Lake Health: Examination of Phytoplankton Growth Rate Regulations in Keuka Lake via Short-term Microcosm Experiments Extended Abstract Rochester Academy of Sciences Student Scientific
Primary Productivity and Lake Health: Examination of Phytoplankton Growth Rate Regulations in Keuka Lake via Short-term Microcosm Experiments Extended Abstract Rochester Academy of Sciences Student Scientific
Bio-energetics and Bio-energy: Blue-mussels as source for raw materials
 Bio-energetics and Bio-energy: Blue-mussels as source for raw materials Daniel Pleissner, Ph.D.-student, Institute of Biology Kerteminde Seminar, Dec. 21st Bio-production and Bio-energetics: Bio-reactor
Bio-energetics and Bio-energy: Blue-mussels as source for raw materials Daniel Pleissner, Ph.D.-student, Institute of Biology Kerteminde Seminar, Dec. 21st Bio-production and Bio-energetics: Bio-reactor
Trace metal control of phytochelatin production in coastal waters. Notes 601. Submitted: 18 July 1995 Accepted: 14 May 1996 Amended: 18 December 1996
 Notes 601 kowski and A. Woodhead [eds.], Primary productivity and biogeochemical cycles in the sea. Plenum. COLLOS, Y. 1987. Calculations of N uptake rates by phytoplankton assimilating one or several
Notes 601 kowski and A. Woodhead [eds.], Primary productivity and biogeochemical cycles in the sea. Plenum. COLLOS, Y. 1987. Calculations of N uptake rates by phytoplankton assimilating one or several
Growth responses of Ulva prolifera to inorganic and organic. nutrients: Implications for macroalgal blooms in the
 1 2 3 Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China 4 5 Hongmei Li a, Yongyu Zhang a *, Xiurong Han b, Xiaoyong
1 2 3 Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China 4 5 Hongmei Li a, Yongyu Zhang a *, Xiurong Han b, Xiaoyong
Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures
 Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures V. Héquet 1, P. Ricou 1, I. Lecuyer 2 and P. Le Cloirec 1 1 Ecole des Mines de Nantes, Dept. Systèmes Energétiques
Removal of Cu 2+ and Zn 2+ in Aqueous Solutions by Sorption onto Fly Ash and Fly Ash Mixtures V. Héquet 1, P. Ricou 1, I. Lecuyer 2 and P. Le Cloirec 1 1 Ecole des Mines de Nantes, Dept. Systèmes Energétiques
Free cupric ion concentration and Cu(I1) speciation in a eutrophic lake
 Limnol. Oceanogr., 38(6), 1993, 1200-1213 0 1993, by the American Society of Limnology and Oceanography, Inc. Free cupric ion concentration and Cu(I1) speciation in a eutrophic lake HanBin Xue and Laura
Limnol. Oceanogr., 38(6), 1993, 1200-1213 0 1993, by the American Society of Limnology and Oceanography, Inc. Free cupric ion concentration and Cu(I1) speciation in a eutrophic lake HanBin Xue and Laura
Matrix Reference Materials - SCP SCIENCE
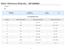 EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
EnviroMAT SS-1 Catalogue No.: 140-025-001 EnviroMAT Contaminated Soil Lot No.: SC0063618 100 g TOTAL DIGESTION VALUES Elements Reference Value (mg/kg) Confidence Interval (mg/kg) Tolerance Interval (mg/kg)
Application of improved scientific approaches in support of risk assessment within the European REACH and Biocides Regulations: A case study on metals
 Application of improved scientific approaches in support of risk assessment within the European REACH and Biocides Regulations: A case study on metals Koen Oorts, Katrien Delbeke, Chris Schlekat, Jasim
Application of improved scientific approaches in support of risk assessment within the European REACH and Biocides Regulations: A case study on metals Koen Oorts, Katrien Delbeke, Chris Schlekat, Jasim
The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil
 Department of Earth and Environmental Sciences Division of Soil and Water Management The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil Erik Smolders
Department of Earth and Environmental Sciences Division of Soil and Water Management The implementation of bioavailability in defining PNEC values for trace metals and metalloids in soil Erik Smolders
Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction. Project Progress Report. Jianmin Wang NUTC R201
 Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction Project Progress Report by Jianmin Wang NUTC R21 Disclaimer The contents of this report reflect the
Leaching Behavior of Coal Combustion Products and the Environmental Implication in Road Construction Project Progress Report by Jianmin Wang NUTC R21 Disclaimer The contents of this report reflect the
Matching analytical methods with EQS values for water and sediment
 Matching analytical methods with EQS values for water and sediment Frank Van Assche International Zinc Association 168 Avenue de Tervueren 1150 Brussels contents Monitoring metals in water total versus
Matching analytical methods with EQS values for water and sediment Frank Van Assche International Zinc Association 168 Avenue de Tervueren 1150 Brussels contents Monitoring metals in water total versus
AD-Net Research Colloquium Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD)
 AD-Net Research Colloquium 11-12 Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD) Cynthia Carliell-Marquet Honorary Senior Research Fellow, University of Birmingham Senior Process Designer,
AD-Net Research Colloquium 11-12 Sept 2017 Choosing Trace Elements to Maximise Benefits (to AD) Cynthia Carliell-Marquet Honorary Senior Research Fellow, University of Birmingham Senior Process Designer,
Zinc isotopes in the Seine River water, France: a probe of. anthropogenic contamination
 Zinc isotopes in the Seine River water, France: a probe of anthropogenic contamination Jiubin Chen*, Jérôme Gaillardet and Pascale Louvat Equipe Géochimie et Cosmochimie, Institut de Physique du Globe
Zinc isotopes in the Seine River water, France: a probe of anthropogenic contamination Jiubin Chen*, Jérôme Gaillardet and Pascale Louvat Equipe Géochimie et Cosmochimie, Institut de Physique du Globe
How to assess effects data sets for metals hazard identification and risk characterization.
 How to assess effects data sets for metals hazard identification and risk characterization. OECD Meeting, 7-8 September 2011 K Delbeke Aims Metal characteristics, critical to assessing environmental effects
How to assess effects data sets for metals hazard identification and risk characterization. OECD Meeting, 7-8 September 2011 K Delbeke Aims Metal characteristics, critical to assessing environmental effects
CHAPTER 3 GUT PHYSIOLOGY, CHEMISTRY AND NUTRITION
 CHAPTER 3 GUT PHYSIOLOGY, CHEMISTRY AND NUTRITION Peter G.C. Campbell, Susan J. Clearwater, Paul B. Brown, Nicholas S. Fisher, Christer Hogstrand, Glenn R. Lopez, Lawrence M. Mayer, Joseph S. Meyer v.
CHAPTER 3 GUT PHYSIOLOGY, CHEMISTRY AND NUTRITION Peter G.C. Campbell, Susan J. Clearwater, Paul B. Brown, Nicholas S. Fisher, Christer Hogstrand, Glenn R. Lopez, Lawrence M. Mayer, Joseph S. Meyer v.
Toxicity and Bioaccumulation of Copper, Zinc, and Cadmium in Some Aquatic Organisms
 Bull. Environ. Contam. Toxicol. (2000) 64:740-747 2000 Springer-Verlag New York Inc. DOI: 10.1007/s001280000066 Toxicity and Bioaccumulation of Copper, Zinc, and Cadmium in Some Aquatic Organisms M. A.
Bull. Environ. Contam. Toxicol. (2000) 64:740-747 2000 Springer-Verlag New York Inc. DOI: 10.1007/s001280000066 Toxicity and Bioaccumulation of Copper, Zinc, and Cadmium in Some Aquatic Organisms M. A.
Upper ocean total organic carbon at BATS Remember DOC = ~98% of the TOC.
 Upper ocean total organic carbon at BATS Remember DOC = ~98% of the TOC. Note the build up in DOC through the spring and summer, with subsequent export the following winter. Figure courtesy of Craig Carlson,
Upper ocean total organic carbon at BATS Remember DOC = ~98% of the TOC. Note the build up in DOC through the spring and summer, with subsequent export the following winter. Figure courtesy of Craig Carlson,
A Survey of Trace Metals in Biota of the Mersey Estuary W. J. Langston, N. D. Pope, and G. R. Burt
 A Survey of Trace Metals in Biota of the Mersey Estuary -1993 W. J. Langston, N. D. Pope, and G. R. Burt Plymouth Marine Laboratory Citadel Hill Plymouth PL1 2PB United Kingdom April 1994 This study was
A Survey of Trace Metals in Biota of the Mersey Estuary -1993 W. J. Langston, N. D. Pope, and G. R. Burt Plymouth Marine Laboratory Citadel Hill Plymouth PL1 2PB United Kingdom April 1994 This study was
AG - 1 AQUACULTURE: A TRACE MINERAL PERSPECTIVE FOR FISH AND CRUSTACEANS
 AG - 1 AQUACULTURE: A TRACE MINERAL PERSPECTIVE FOR FISH AND CRUSTACEANS AQUACULTURE: TRACE MINERALS AVAILABILITY OF TRACE MINERALS TO FISH AND SHRIMP FROM WATER ENVIRONMENT Fish Appear to Be More Tolerant
AG - 1 AQUACULTURE: A TRACE MINERAL PERSPECTIVE FOR FISH AND CRUSTACEANS AQUACULTURE: TRACE MINERALS AVAILABILITY OF TRACE MINERALS TO FISH AND SHRIMP FROM WATER ENVIRONMENT Fish Appear to Be More Tolerant
TECHNICAL MEMORANDUM 6: APPLICATION OF ECOS3 FOR SIMULATION OF SELENIUM FATE AND TRANSPORT
 TECHNICAL MEMORANDUM 6: APPLICATION OF ECOS3 FOR SIMULATION OF SELENIUM FATE AND TRANSPORT IN NORTH SAN FRANCISCO BAY Final Report, February 21 Point Sources (Refineries, POTWs, Other Dischargers) Contribute
TECHNICAL MEMORANDUM 6: APPLICATION OF ECOS3 FOR SIMULATION OF SELENIUM FATE AND TRANSPORT IN NORTH SAN FRANCISCO BAY Final Report, February 21 Point Sources (Refineries, POTWs, Other Dischargers) Contribute
Factors Affecting Photosynthesis!
 Factors Affecting Photosynthesis! Temperature Eppley (1972) Light Sverdrup s Critical Depth Model Nutrients Limitations Uptake Kinetics Temperature! The oceans vary much less than the land does, both seasonally
Factors Affecting Photosynthesis! Temperature Eppley (1972) Light Sverdrup s Critical Depth Model Nutrients Limitations Uptake Kinetics Temperature! The oceans vary much less than the land does, both seasonally
Toxicity of Cu 2+, Cd 2+ and Pb 2+ Metal Ions in Chironomids and Factors that Affect Metal Accumulation
 2011 International Conference on Environment Science and Engineering IPCBEE vol.8 (2011) (2011) IACSIT Press, Singapore Toxicity of Cu 2+, Cd 2+ and Pb 2+ Metal Ions in Chironomids and Factors that Affect
2011 International Conference on Environment Science and Engineering IPCBEE vol.8 (2011) (2011) IACSIT Press, Singapore Toxicity of Cu 2+, Cd 2+ and Pb 2+ Metal Ions in Chironomids and Factors that Affect
BIOL 347L Laboratory Three
 Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid
 5th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2015) Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid S.W. SONG 1*, L. WANG
5th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2015) Speciation Analysis and Removal of Heavy Metals Zn, Cu, Cd from Sludge by Organic Acid S.W. SONG 1*, L. WANG
Domoic acid: The synergy of iron, copper, and the toxicity of diatoms
 Limnol. Oceanogr., 50(6), 2005, 1908 1917 2005, by the American Society of Limnology and Oceanography, Inc. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms Mark L. Wells 1 Institute
Limnol. Oceanogr., 50(6), 2005, 1908 1917 2005, by the American Society of Limnology and Oceanography, Inc. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms Mark L. Wells 1 Institute
Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System
 Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System Michael H. Paller (Savannah River National Laboratory) Anna Sophia Knox (Savannah River National Laboratory) Coral Springs,
Using DGT to Measure Bioavailable Metals in a Constructed Wetland Treatment System Michael H. Paller (Savannah River National Laboratory) Anna Sophia Knox (Savannah River National Laboratory) Coral Springs,
Assimilation and regeneration of trace elements by marine copepods
 Limnol. Oceanogr., 41(l), 1996, 70-8 1 0 1996, by the American Society of Limnology a Oceanography, Inc. I Assimilation a regeneration of trace elements by marine copepods Wen-Xiong Wang Marine Sciences
Limnol. Oceanogr., 41(l), 1996, 70-8 1 0 1996, by the American Society of Limnology a Oceanography, Inc. I Assimilation a regeneration of trace elements by marine copepods Wen-Xiong Wang Marine Sciences
of elements ingested by marine planktonic bivalve larvae
 Limnol. Oceanogr., 39(l), 1994, 12-20 0 1994, by the American Society of Limnology and Oceanography, Inc. The assimilation bivalve larvae of elements ingested by marine planktonic John R. Reinfeldeer and
Limnol. Oceanogr., 39(l), 1994, 12-20 0 1994, by the American Society of Limnology and Oceanography, Inc. The assimilation bivalve larvae of elements ingested by marine planktonic John R. Reinfeldeer and
ISSN (Print), ISSN (online) 1 Okeke O.R. and 2 Okeke M.U.
 ESTIMATION OF THE DIETARY INTAKES OF HEAVY METALS BY CHILDREN, ADOLESCENTS, ADULTS AND SENIORS CONSUMING CHICKEN MEATS WITHIN AWKA AND ENUGU METROPOLIS AND ITS ENVIRONS. 1 Okeke O.R. and 2 Okeke M.U. 1
ESTIMATION OF THE DIETARY INTAKES OF HEAVY METALS BY CHILDREN, ADOLESCENTS, ADULTS AND SENIORS CONSUMING CHICKEN MEATS WITHIN AWKA AND ENUGU METROPOLIS AND ITS ENVIRONS. 1 Okeke O.R. and 2 Okeke M.U. 1
Iron and zinc effects on silicic acid and nitrate uptake kinetics in three high-nutrient, low-chlorophyll (HNLC) regions
 MARINE ECOLOGY PROGRESS SERIES Vol. 252: 1533, 2003 Published April 30 Mar Ecol Prog Ser Iron and zinc effects on silicic acid and nitrate uptake kinetics in three high-nutrient, low-chlorophyll (HNLC)
MARINE ECOLOGY PROGRESS SERIES Vol. 252: 1533, 2003 Published April 30 Mar Ecol Prog Ser Iron and zinc effects on silicic acid and nitrate uptake kinetics in three high-nutrient, low-chlorophyll (HNLC)
Estimating the Grazing Impact of Marine Micro-zooplankton *
 Marine Biology 67, 283-288 (1982) MARINE BIOLOGY 9 Springer-Verlag 1982 Estimating the Grazing Impact of Marine Micro-zooplankton * M. R. Landry and R. P. Hassett School of Oceanography, WB-10, University
Marine Biology 67, 283-288 (1982) MARINE BIOLOGY 9 Springer-Verlag 1982 Estimating the Grazing Impact of Marine Micro-zooplankton * M. R. Landry and R. P. Hassett School of Oceanography, WB-10, University
by Oguguah, N. M. and Olakolu, F. C. Marine Biology section, Fisheries Resources Department, Nigerian Institute for Oceanography and Marine Research
 Heavy Metal (Pd, Cd, Fe, Zn And Mn) Levels in Shrimp By-Catch SppFrom Industrial Shrimp Trawl Fishery in Nigerian Coastal Waters by Oguguah, N. M. and Olakolu, F. C. Marine Biology section, Fisheries Resources
Heavy Metal (Pd, Cd, Fe, Zn And Mn) Levels in Shrimp By-Catch SppFrom Industrial Shrimp Trawl Fishery in Nigerian Coastal Waters by Oguguah, N. M. and Olakolu, F. C. Marine Biology section, Fisheries Resources
POTENTIAL ENVIRONMENTAL CHALLENGES OF HYPER-INTENSIVE BIOFLOC GROWOUT SYSTEMS
 POTENTIAL ENVIRONMENTAL CHALLENGES OF HYPER-INTENSIVE BIOFLOC GROWOUT SYSTEMS John W. Leffler and Jeff F. Brunson South Carolina Department of Natural Resources Marine Resources Research Institute Waddell
POTENTIAL ENVIRONMENTAL CHALLENGES OF HYPER-INTENSIVE BIOFLOC GROWOUT SYSTEMS John W. Leffler and Jeff F. Brunson South Carolina Department of Natural Resources Marine Resources Research Institute Waddell
ENVE 424 Anaerobic Treatment
 ENVE 424 Anaerobic Treatment Lecture 6 Toxic substances in anaerobic treatment 2012 2013 Fall 01 Nov 2012 Assist. Prof. A. Evren Tugtas Basic Fundamentals Inhibition Competitive Inhibition Uncompetitive
ENVE 424 Anaerobic Treatment Lecture 6 Toxic substances in anaerobic treatment 2012 2013 Fall 01 Nov 2012 Assist. Prof. A. Evren Tugtas Basic Fundamentals Inhibition Competitive Inhibition Uncompetitive
Lab 3: Inorganic Plant Nutrients: Nitrogen, Phosphorus, Silicate
 Introduction Lab 3: Inorganic Plant Nutrients: Nitrogen, Phosphorus, Silicate Compounds of nitrogen and phosphorus are major cellular components of organisms. Since the availability of these elements may
Introduction Lab 3: Inorganic Plant Nutrients: Nitrogen, Phosphorus, Silicate Compounds of nitrogen and phosphorus are major cellular components of organisms. Since the availability of these elements may
Sources of nutrients to the surface mixed layer of the ocean
 Sources of nutrients to the surface mixed layer of the ocean What are nutrients anyway? (to a chemist that is.) Where do they come from? Preformed (recycled, delivered from elsewhere) Biosynthesized Nutrient
Sources of nutrients to the surface mixed layer of the ocean What are nutrients anyway? (to a chemist that is.) Where do they come from? Preformed (recycled, delivered from elsewhere) Biosynthesized Nutrient
Growth retardation and altered isotope composition as delayed effects of PCB exposure in Daphnia magna
 Supporting Information Growth retardation and altered isotope composition as delayed effects of PCB exposure in Daphnia magna Caroline Ek a, *, Zandra Gerdes a, Andrius Garbaras b, Margaretha Adolfsson-Erici
Supporting Information Growth retardation and altered isotope composition as delayed effects of PCB exposure in Daphnia magna Caroline Ek a, *, Zandra Gerdes a, Andrius Garbaras b, Margaretha Adolfsson-Erici
Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka
 , 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
, 152-158 Use of A Multi-ionic Extractant to Determine Available P, K, Na, Ca, and Mg in Acid Soils of Sri Lanka W.S. Madurapperuma and D. Kumaragamage 1 Postgraduate Institute of Agriculture University
Plant Nutrients in Mineral Soils
 The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
The Supply and Availability of Plant Nutrients in Mineral Soils Plant Nutrients in Mineral Soils Factors Controlling the Growth of Higher Plants 1. Light 2. Mechanical Support. Heat. Air 5. Water 6. Nutrients
Comparative Biochemistry and Physiology, Part C
 Comparative Biochemistry and Physiology, Part C 149 (2009) 531 537 Contents lists available at ScienceDirect Comparative Biochemistry and Physiology, Part C journal homepage: www.elsevier.com/locate/cbpc
Comparative Biochemistry and Physiology, Part C 149 (2009) 531 537 Contents lists available at ScienceDirect Comparative Biochemistry and Physiology, Part C journal homepage: www.elsevier.com/locate/cbpc
Temperature effects on phytoplankton growth in continuous culture1
 932 Notes lation of marine phytoplankton. Limnol. Oceanogr. 17 : 37 l-382. MACISAAC, J. J., AND R. C. DUGDALE. 1972. Interactions of light and inorganic nitrogen in controlling nitrogen uptake in the sea.
932 Notes lation of marine phytoplankton. Limnol. Oceanogr. 17 : 37 l-382. MACISAAC, J. J., AND R. C. DUGDALE. 1972. Interactions of light and inorganic nitrogen in controlling nitrogen uptake in the sea.
Asian Journal of Ecotoxicology. Cristaria plicata Cu Zn. Cu Zn. 8d p<0.05. Cu. Cu Zn. Zn. X52
 2010 5 2 169-175 Asian Journal of Ecotoxicology Vol 5, 2010 No 2, 169-175 Cu Zn 1 2 1 * 2 1 100084 2 100049 Cristaria plicata Cu Zn Cu Zn 8d p > p > > p< 005 4
2010 5 2 169-175 Asian Journal of Ecotoxicology Vol 5, 2010 No 2, 169-175 Cu Zn 1 2 1 * 2 1 100084 2 100049 Cristaria plicata Cu Zn Cu Zn 8d p > p > > p< 005 4
The Relationship of Calcium Intake, Source, Size, Solubility In Vitro and In Vivo, and Gizzard Limestone Retention in Laying Hens 1
 The Relationship of Calcium Intake, Source, Size, Solubility In Vitro and In Vivo, and Gizzard Limestone Retention in Laying Hens 1 BINGFAN ZHANG and CRAIG N. COON2 Department of Animal Science, University
The Relationship of Calcium Intake, Source, Size, Solubility In Vitro and In Vivo, and Gizzard Limestone Retention in Laying Hens 1 BINGFAN ZHANG and CRAIG N. COON2 Department of Animal Science, University
DIVERSITY AND ABUNDANCE OF ZOOPLANKTON IN CORAL COMMUNITIES AT KO SAK, CHONBURI PROVINCE FOLLOWING THE 2010 CORAL BLEACHING EVENT
 I_I0030 1 DIVERSITY AND ABUNDANCE OF ZOOPLANKTON IN CORAL COMMUNITIES AT KO SAK, CHONBURI PROVINCE FOLLOWING THE 2010 CORAL BLEACHING EVENT Arporn Sompaonoi,* Sittiporn Pengsakun, Wanlaya Klinthong, Makamas
I_I0030 1 DIVERSITY AND ABUNDANCE OF ZOOPLANKTON IN CORAL COMMUNITIES AT KO SAK, CHONBURI PROVINCE FOLLOWING THE 2010 CORAL BLEACHING EVENT Arporn Sompaonoi,* Sittiporn Pengsakun, Wanlaya Klinthong, Makamas
Cadmium: A nutrient for the marine diatom Thalassiosira weissjlogii
 Limnol. Oceanogr., 40(6), 1995, 1056-1063 0 1995, by the American Society of Limnology and Oceanography, Inc. Cadmium: A nutrient for the marine diatom Thalassiosira weissjlogii Jennl$er G. Lee and Samantha
Limnol. Oceanogr., 40(6), 1995, 1056-1063 0 1995, by the American Society of Limnology and Oceanography, Inc. Cadmium: A nutrient for the marine diatom Thalassiosira weissjlogii Jennl$er G. Lee and Samantha
Carbon & Energy Utilization OCN 621
 Carbon & Energy Utilization OCN 621 Lecture Outline 1) Materials Balance Approach defined 2) Sloppy Feeding 3) Egestion: as relates to Assimilation efficiency 4) Metabolism: Allometric Equation 1) Temperature
Carbon & Energy Utilization OCN 621 Lecture Outline 1) Materials Balance Approach defined 2) Sloppy Feeding 3) Egestion: as relates to Assimilation efficiency 4) Metabolism: Allometric Equation 1) Temperature
Philip S. Oshida and Jean LWright EFFECTS OF HEXAVALENT CHROMIUM ON SEA URCHIN EMBRYOS AND BRITTLE STARS
 Philip S. Oshida and Jean LWright EFFECTS OF HEXAVALENT CHROMIUM ON SEA URCHIN EMBRYOS AND BRITTLE STARS A goal of the Project's investigations into the chronic effects of exposure to chromium on marine
Philip S. Oshida and Jean LWright EFFECTS OF HEXAVALENT CHROMIUM ON SEA URCHIN EMBRYOS AND BRITTLE STARS A goal of the Project's investigations into the chronic effects of exposure to chromium on marine
Metal uptake by phytoplankton during a bloom in South San Francisco Bay: Implications for metal cycling in estuaries
 Notes 1007 SARGENT, J. R., AND S. FALK-PETERSEN. 1988. The lipid biochemistry of Calanoid copepods. Hydrobiol. 167/168: 101-114. ~ AND R. J. HENDERSON. 1986. Lipids, p. 59-108. In E. D. S. Corner and S.
Notes 1007 SARGENT, J. R., AND S. FALK-PETERSEN. 1988. The lipid biochemistry of Calanoid copepods. Hydrobiol. 167/168: 101-114. ~ AND R. J. HENDERSON. 1986. Lipids, p. 59-108. In E. D. S. Corner and S.
Interactive Toxic Effect and Distribution of Heavy Metals in Phytoplankton
 Interactive Toxic Effect and Distribution of Heavy Metals in Phytoplankton Hideo Okamura and lsao Aoyama* Division of Ecological Chemistry, Research Institute for Bioresources, Okayama University, 2-2-1,
Interactive Toxic Effect and Distribution of Heavy Metals in Phytoplankton Hideo Okamura and lsao Aoyama* Division of Ecological Chemistry, Research Institute for Bioresources, Okayama University, 2-2-1,
The combined effect of Cu and Zn on Selenastrum capricornutum
 Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1983 The combined effect of Cu and Zn on Selenastrum capricornutum Helmer Colonia-Roque Portland State University
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1983 The combined effect of Cu and Zn on Selenastrum capricornutum Helmer Colonia-Roque Portland State University
CERTIFICATE OF ANALYSIS. tel: fax:
 CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
CERTIFICATE OF ANALYSIS 300 Technology Drive Christiansburg, VA 24073. USA inorganicventures.com tel: 800.669.6799. 540.585.3030 fax: 540.585.3012 info@inorganicventures.com 1.0 ACCREDITATION / REGISTRATION
STANDARD METHODS FOR CHEMICAL ELEMENTS IN FOOD OF ANIMAL ORIGIN
 EN 13804 Performance criteria, general considerations and sample preparation 2013 EN 13805 Pressure digestion 2014 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
EN 13804 Performance criteria, general considerations and sample preparation 2013 EN 13805 Pressure digestion 2014 EN 13806 Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS)
Effects of changes in salinity and osmolality on the rate of uptake of zinc by three crabs of different ecologies
 MARINE ECOLOGY PROGRESS SERIES Vol. 244: 205 217, 2002 Published November 29 Mar Ecol Prog Ser Effects of changes in salinity and osmolality on the rate of uptake of zinc by three crabs of different ecologies
MARINE ECOLOGY PROGRESS SERIES Vol. 244: 205 217, 2002 Published November 29 Mar Ecol Prog Ser Effects of changes in salinity and osmolality on the rate of uptake of zinc by three crabs of different ecologies
Analysis of trace elements in nutraceuticals in compliance with USP chapter <2232> Elemental Contaminants in Dietary Supplements
 APPLICATION NOTE 43445 Analysis of trace elements in nutraceuticals in compliance with USP chapter Elemental Contaminants in Dietary Supplements Authors Sanja Asendorf, Application Specialist, Thermo
APPLICATION NOTE 43445 Analysis of trace elements in nutraceuticals in compliance with USP chapter Elemental Contaminants in Dietary Supplements Authors Sanja Asendorf, Application Specialist, Thermo
