CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM
|
|
|
- Violet Phelps
- 6 years ago
- Views:
Transcription
1 CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR WITH THE SURGICAL TECHNIQUE. DESCRIPTION The Consensus Orthopedics, Inc. CONSENSUS KNEE SYSTEM consists of a left and a right configuration of the femoral component and the tibial component. The patellar component is designed to fit both right and left patella. It is an unconstrained design and both medial and lateral collateral ligaments must be intact. The femoral component is manufactured from cast CoCrMo (ASTM F75). The two styles offered are the primary and the reduced lateral profile (RLP). When porous coated, interior fixation surfaces are partially coated with CoCrMo beads covered with a Ti layer (ASTM B348). Uncoated and coated (CoCrMo beads without a Ti layer) versions are also available. The tibial component consists of two parts to be assembled at the time of surgery: a baseplate and a plastic insert. The metal tray or baseplate is made of Ti-6Al-4V and coated with commercially pure titanium beads (C.P.Ti, ASTM F67) or is made of CoCrMo (ASTM F75, F799, or F1537). When porous coated, inferior fixation surfaces are partially coated with CoCrMo beads covered with a Ti layer. The baseplate is recessed to allow encapsulation of plastic inserts which form the bearing surface for femoral-tibial articulations. These inserts are manufactured from Ultra-High Molecular Weight Polyethylene (UHMWPE, ASTM F648) or VitalitE, an Ultra-High Molecular Weight Polyethylene blended with Vitamin E. VitalitE inserts are available for use as a congruent and PCL substituting insert only. The two tibial insert variations available for the primary (STD) and RLP CKS are the congruent and PCL substituting. The congruent insert is an anatomic, asymmetric, cruciate retaining design while the PCL substituting is an anatomic, asymmetric, cruciate sacrificing design. The all-polyethylene tibia component is manufactured from UHMWPE. It is a symmetric cruciateretaining tibial component and is designed to articulate with the CONSENSUS KNEE primary femoral component. The patellar component is a round and oval configuration designed to provide more coverage of the resected patella bone. The articular surface of the patella is an offset spherical dome. The patella is provided in two variations all-poly and metal backed. The all-poly version is a one-piece design and is manufactured from UHMWPE or VitalitE, an Ultra-High Molecular Weight Polyethylene blended with Vitamin E. VitalitE patellas are only available in the all-poly variation. The metal backed version is a two-piece design. The UHMWPE dome is attached preassembled to a CoCrMo or titanium backing and partially coated with CoCrMo beads covered with a Ti layer. The CONSENSUS KNEE SYSTEM primary posterior stabilized (P.S.) and reduced lateral profile posterior stabilized (P.S. RLP) femoral components are manufactured from cast CoCrMo. When porous coated, interior fixation surfaces are partially coated with CoCrMo beads covered with a Ti layer. Uncoated and coated (CoCrMo beads without a Ti layer) versions are also available. It is an anatomic, asymmetric, cruciate-sacrificing femoral component similar to the CONSENSUS KNEE primary femoral component and is designed to articulate with the CONSENSUS KNEE P.S. tibial insert thus providing resistance to posterior subluxation of the proximal tibia in the absence of the posterior cruciate ligament. It is also designed to articulate with the CONSENSUS patella components. The CONSENSUS KNEE posterior
2 stabilized (P.S.) tibial insert is manufactured from UHMWPE. It is an anatomic, asymmetric, cruciatesacrificing tibial insert and is designed to articulate with the CONSENSUS primary P.S. femoral component. HOW PRODUCT IS SUPPLIED Each component of the CONSENSUS KNEE SYSTEM is supplied in individually sterilized boxes or packages in a wide range of sizes. Please refer to the current price list, surgical technique, or catalog for the catalog numbers and sizes available. The recommended trial components are used for size determination, preparation, evaluation, trial reduction, and range of motion evaluation. The use of trials preserves the integrity of implants and sterile packaging. INFORMATION FOR USE Indications for the use of the CONSENSUS KNEE SYSTEM must be carefully considered with respect to the patient s entire evaluation and alternative procedures. Patient selection is dependent on age, general health, available bone stock and quality, and any prior surgery or anticipated further surgery. Prosthetic replacement is generally indicated only for patients who have reached skeletal maturity. Total joint replacement in younger patients should be considered only when explicit indications outweigh the associated risks of the surgery and modified demands regarding activity and joint loading are assured. This includes all patients who may or may not have multiple joint involvement, for whom restoration of joint mobility leads to an expectation or greater mobility and an improvement in the quality of life. In using the CONSENSUS KNEE SYSTEM, the surgeon should be aware of the following: The porous-coated metal should not be allowed to contact cloth or other lint-shedding materials prior to implantation. Prostheses should not be re-implanted; therefore, initial size selection is extremely important. Conventional cleaning techniques cannot be relied upon to remove lint, dirt, or body tissue from the porous coating. ASSEMBLY OF COMPONENTS The CONSENSUS KNEE All-Polyethylene tibia requires no assembly. The CONSENSUS KNEE SYSTEM tibial component consists of two parts to be assembled at the time of surgery, the baseplate and the tibial insert, and optional recessed holes to accept bone screws to augment initial fixation. The baseplate is recessed to allow encapsulation of the plastic insert. The tibial insert forms the congruent articular surface, with radii to match the femoral component. The tibial insert is designed to allow up to one femur size larger or smaller to be used interchangeably for maximum intraoperative flexibility. At the time of surgery the appropriate tibial insert will be snapped onto the baseplate. THE SNAP MECHANISM MAY BE ENGAGED ONLY ONCE. DO NOT ATTEMPT TO REINSERT A TIBIAL INSERT A SECOND TIME. Size Compatibility
3 Inter-Component Compatibility Femoral Components RLP STD PS PS RLP All-Poly Yes Yes - - Tibial Inserts (Inserts can be assembled with all baseplates) Congruent Yes Yes - - PCL Substituting Yes Yes - - PS - - Yes Yes Patella Components All-Poly Yes Yes Yes Yes Metal backed Yes Yes Yes Yes INDICATIONS AND USAGE The CONSENSUS KNEE SYSTEM Primary Knee is designed as a system and is not intended for substitution of components from other systems. The indications for use are: A. Primary intervention of rheumatoid arthritis, osteoarthritis, post-traumatic arthritis, or degenerative arthritis. B. Failed osteotomy or unicompartmental replacements. C. Replacement of unsatisfactory cemented or press-fit knee components when sufficient bone stock exists. D. The non-porous (uncoated and coated with CoCr beads without Titanium) components may only be used with cement. E. The porous coated (CoCr beads with Titanium) components may be used with or without cement. INTENDED PERFORMANCE All components of the CONSENSUS KNEE SYSTEM are intended to perform in a safe and effective manner in restoring knee function within the intended use of the product. A. The intended range of flexion/extension of the femur relative to the tibia is from 10 hyperextension to at least 120 flexion. B. The CONSENSUS KNEE SYSTEM is designed to transmit load to the tibia during daily activities including, but not limited to walking, stair climbing, and chair ascent. C. The profile of the CONSENSUS KNEE femoral component is intended to fully cover the resected femoral condyles with anterior-posterior and medial-lateral widths ranging from 37 to 58 mm and 59 to 80 mm respectively.
4 D. The profile of the CONSENSUS KNEE tibial baseplate is intended to fully cover the resected tibial plateau with anterior-posterior and medial-lateral widths ranging from 40 to 56 mm and 62 to 86 mm respectively. E. The CONSENSUS KNEE femoral component and tibial baseplate are designed to reduce stress shielding at the bone-implant interface. F. The matching articulating surfaces at the tibiofemoral articulation are intended to reduce wear over time. G. The mirror finish of the articulating surface of the CONSENSUS KNEE femoral component is intended to reduce wear of the tibial insert. H. The interior fixation surfaces of the nonporous femoral component and nonporous tibial baseplate are grit blasted at the implant-cement-bone interface. I. The interior fixation surfaces of the porous femoral component and porous tibial baseplate are partially coated with CoCrMo beads covered with a Ti layer. CONTRAINDICATIONS A. Any active or suspected latent infection in or about the knee joint. B. Mental or neuromuscular disorders which would create unacceptable risk of prosthesis instability or complications in post-operative care. C. Bone stock compromised by disease, infection, or prior implantation that cannot provide adequate support and fixation of the device. D. Ligamentous or severe muscle laxity or inadequate soft tissue coverage to allow for the normal healing process and for proper mechanics to be re-established. E. Conditions which tend to place increased loads on implants, such as age, weight, and activity level which are incompatible with a satisfactory clinical long-term result. PRECAUTIONS A. Before any implant is used the surgeon should be completely familiar with all aspects of the surgical procedure and the limitations of the device. B. Instruct patients on the limitations of the prosthesis and how to modify their activities accordingly. C. Proper selection of type and placement of the knee components are critical factors in the prevention of unusual stress conditions and their potentially harmful effects on the implant service life. D. The surgeon and O.R. staff must be extremely careful to protect the components from being marred, nicked or notched as a result of contact with metal or any abrasive objects. E. For non-cemented use of porous coated products, the surgeon may need to consider appropriate changes to post-operative patient management.
5 F. When considering non-cemented use of semi-constrained knee components, the surgeon should carefully evaluate the patient for adequate bone quality and other risk factors affecting fixation. WARNINGS A. All CONSENSUS KNEE SYSTEM components are sold sterile. The insert and the double Tyvek packages are subject to ethylene oxide sterilization. Do not use any component if the package has been breached. If packages appear damaged or tampered with, they should be returned to the supplier. B. Do not implant any device that has been used, even if it appears undamaged. C. Take care to protect polished bearing areas and machined surfaces from coming in contact with hard or abrasive surfaces. D. Do not bend or contour an implant, as this may reduce its fatigue strength and may cause immediate or eventual failure under load. E. Never tamper with implants. Tampering may have a detrimental affect on the performance of the implant. Handling of porous coated regions must be avoided as it potentially could result in the compromise of the device effectiveness. F. Consensus Orthopedics, Inc. strongly advises against the use of another manufacturer s component with any CONSENSUS KNEE component. G. Removal of implant may require the use of special instruments to disrupt the bone interface. These techniques may require practice in the laboratory before being attempted clinically. H. Consensus Orthopedics strongly advises not to use any CONSENSUS KNEE components offlabel. ADVERSE EFFECTS A. All prosthetic replacements have the potential for adverse effects, including infection, loosening, fracture breakage, bending of the components, component disassembly, or positional changes of the components. B. Sensitivity reactions to component materials could occur, and should be ruled out preoperatively. C. Total joint replacement surgery is associated with serious complications including, but not limited to: nerve injury, direct arterial injury, false aneurysm, spontaneous vascular occlusion, deep vein thrombosis, ectopic ossification, non-union, dislocation, disassociation, superficial and deep infection, aseptic loosening, component failure, and third party wear associated with UHMWPE. D. Pain due to loosening of the implant, and/or localized pressure associated with incongruencies of the fit, or tissue inflammation of unknown etiology. E. Reoperation may be necessary to correct adverse effects.
6 F. On rare occasions, complications may require arthrodesis, Girdlestone procedure or amputation of the limb. G. Other complications generally associated with surgery, drugs, blood use, or ancillary devices used. H. Postoperative femoral or tibial fracture can occur due to trauma, the presence of defects, or poor bone stock. PATIENT COUNSELING INFORMATION Complications and/or failure of knee prostheses are more likely to occur in patients with unrealistic functional expectations, heavy patients, physically active patients, and/or with patients that fail to follow through with the required rehabilitation program. Excessive physical activity and injury can result in loosening, wear, and/or fracture of the knee implant. The patient must be instructed about all postoperative restrictions, particularly those related to occupational and sports activities and about the possibility that the implant or its components may wear out, fail, or need to be replaced. The implant may not, and is not guaranteed to, last the rest of the patient s life. Because prosthetic joints are not as strong, reliable, or durable as natural, healthy joints, all prosthetic knees may need to be replaced at some point. WARNING: Single Use Only: This product is intended for single use only. Do not attempt to re-use, even if the device appears to be undamaged. Risks include device damage leading to poor performance or failure, patient cross-contamination, inadequate sterilization and general liability. CAUTION: DISPOSAL OF SINGLE-USE IMPLANT DEVICE - THIS DEVICE SHOULD BE REGARDED AS BIO- CONTAMINATED AND HANDLED ACCORDINGLY. PLASTIC OR METAL IMPLANTS SHOULD BE TERMINALLY STERILIZED AND DISPOSED OF FOLLOWING EXISTING HOSPITAL POLICIES AND PROCEDURES. CAUTION: THE CONSENSUS KNEE SYSTEM HAS NOT BEEN EVALUATED FOR SAFETY IN THE MR ENVIRONMENT. THE CONSENSUS KNEE SYSTEM HAS NOT BEEN TESTED FOR HEATING OR MIGRATION IN THE MR ENVIROMENT. PATIENTS SHOULD REGISTER THEIR IMPLANT INFORMATION WITH THE MEDICALERT FOUNDATION ( OR EQUIVALENT ORGANIZATION. CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Consensus Orthopedics, Inc Windfield Way, Suite 100 El Dorado Hills, CA , U.S.A info@consensusortho.com Part No Rev. D, Release Date: 10/2014, DCR# 6754
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC.
 CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
CMA TM Cemented Modular Augmentable. Baseplate. 2 Knee
 CMA TM Cemented Modular Augmentable Baseplate 2 Knee More can be addressed The U2 Cemented Modular Augmentable Baseplate is an optimal solution for tibial bone defect in primary total knee replacement.
CMA TM Cemented Modular Augmentable Baseplate 2 Knee More can be addressed The U2 Cemented Modular Augmentable Baseplate is an optimal solution for tibial bone defect in primary total knee replacement.
Over 20 Years of Proven Clinical Success. Zimmer Natural-Knee II System
 Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Consistency in U2 Knee System over Time
 U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
Knee Revision. Portfolio
 Knee Revision Portfolio I use the DePuy Revision Knee System because of its versatility. With this system I can solve nearly any situation I encounter in the OR. Dr. Thomas Fehring, OrthoCarolina Hip and
Knee Revision Portfolio I use the DePuy Revision Knee System because of its versatility. With this system I can solve nearly any situation I encounter in the OR. Dr. Thomas Fehring, OrthoCarolina Hip and
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Knee Replacement Implants
 Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Biomechanics of. Knee Replacement. Mujda Hakime, Paul Malcolm
 Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
1115 Windfield Way, Suite 100 El Dorado Hills, CA Matthew M. Hull, RAC Phone: (916) / Fax: (916)
 Page 1 of 4 2. 510(k) SUMMARY JUN 2 7 2011 Sponsor Name: 510(k) Contact: Consensus Orthopedics, Inc. 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 Matthew M. Hull, RAC Phone: (916) 355-7156/
Page 1 of 4 2. 510(k) SUMMARY JUN 2 7 2011 Sponsor Name: 510(k) Contact: Consensus Orthopedics, Inc. 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 Matthew M. Hull, RAC Phone: (916) 355-7156/
Constrained Posterior Stabilized (CPS) Surgical Technique
 Constrained Posterior Stabilized (CPS) Surgical Technique Constrained Posterior Stabilized (CPS) Surgical Technique INTRO Introduction The Constrained Posterior Stabilized (CPS) articular surfaces can
Constrained Posterior Stabilized (CPS) Surgical Technique Constrained Posterior Stabilized (CPS) Surgical Technique INTRO Introduction The Constrained Posterior Stabilized (CPS) articular surfaces can
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
INBONE II. Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION
 INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
Description Materials Indications Patient selection factors to be considered include: Contraindications Absolute contraindications include:
 Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 2 Tibial Preparation... 2.1 Tibial Canal Preparation... 2.2 Proximal Tibial Resection... 2.3 Non Offset Tibial Preparation...
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 2 Tibial Preparation... 2.1 Tibial Canal Preparation... 2.2 Proximal Tibial Resection... 2.3 Non Offset Tibial Preparation...
JOINT RULER. Surgical Technique For Knee Joint JRReplacement
 JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR
 CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
StelKast Acetabular. Surgical Protocol and Product Specifications. Including ProForm, Provident, Provident Apical Threaded Hole & Systems
 StelKast Acetabular Surgical Protocol and Product Specifications Including ProForm, Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Protocol Introduction StelKast offers
StelKast Acetabular Surgical Protocol and Product Specifications Including ProForm, Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Protocol Introduction StelKast offers
Constrained Posterior Stabilized (CPS)
 Constrained Posterior Stabilized (CPS) Persona The Personalized Knee Surgical Technique Table of Contents Introduction... 2 Constraint Options Initial Knee Assessment... 3 Femoral Box Cut CPS Tibial Bearing
Constrained Posterior Stabilized (CPS) Persona The Personalized Knee Surgical Technique Table of Contents Introduction... 2 Constraint Options Initial Knee Assessment... 3 Femoral Box Cut CPS Tibial Bearing
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Regenerex Primary Tibial Tray
 Cruciate Retaining and Posterior Stabilized Surgical Technique Addendum to the Vanguard Complete Knee System Knees Hips Extremities Cement and Accessories PMI Technology Contents Microplasty Knee Instrumentation
Cruciate Retaining and Posterior Stabilized Surgical Technique Addendum to the Vanguard Complete Knee System Knees Hips Extremities Cement and Accessories PMI Technology Contents Microplasty Knee Instrumentation
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
Total Knee Replacement
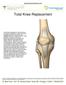 Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
EXACTECH KNEE. Operative Technique ADDENDUM. Metaphyseal Cones. Surgeon focused. Patient driven. TM
 EXACTECH KNEE Operative Technique ADDENDUM Metaphyseal Cones Surgeon focused. Patient driven. TM TABLE OF CONTENTS INTRODUCTION...1 DESCRIPTION...1 DETAILED OPERATIVE TECHNIQUE Initial Preparation and
EXACTECH KNEE Operative Technique ADDENDUM Metaphyseal Cones Surgeon focused. Patient driven. TM TABLE OF CONTENTS INTRODUCTION...1 DESCRIPTION...1 DETAILED OPERATIVE TECHNIQUE Initial Preparation and
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Stephen R Smith Northeast Nebraska Orthopaedics PC. Ligament Preserving Techniques in Total Knee Arthroplasty
 Stephen R Smith Northeast Nebraska Orthopaedics PC Ligament Preserving Techniques in Total Knee Arthroplasty 10-15% have Fair to poor Results? Why? The complication rate is 2.567% If It happens To You
Stephen R Smith Northeast Nebraska Orthopaedics PC Ligament Preserving Techniques in Total Knee Arthroplasty 10-15% have Fair to poor Results? Why? The complication rate is 2.567% If It happens To You
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Persona. The Personalized Knee. Trabecular Metal Tibia. Surgical Technique
 Persona The Personalized Knee Trabecular Metal Tibia Surgical Technique Table of Contents Resect the Tibia... 4 Size and Finish the Tibia... 4 Trial Fit... 6 Component Implantation... 7 Inserter/Implant
Persona The Personalized Knee Trabecular Metal Tibia Surgical Technique Table of Contents Resect the Tibia... 4 Size and Finish the Tibia... 4 Trial Fit... 6 Component Implantation... 7 Inserter/Implant
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Revolution. Unicompartmental Knee System
 Revolution Unicompartmental Knee System While Total Knee Arthroplasty (TKA) is one of the most predictable procedures in orthopedic surgery, many patients undergoing TKA are in fact excellent candidates
Revolution Unicompartmental Knee System While Total Knee Arthroplasty (TKA) is one of the most predictable procedures in orthopedic surgery, many patients undergoing TKA are in fact excellent candidates
Constrained Acetabular Insert
 Constrained Acetabular Insert Surgical Protocol Howmedica Osteonics Constrained Acetabular Insert Surgical Protocol Table of Contents Howmedica Osteonics Constrained Acetabular Insert..........................1
Constrained Acetabular Insert Surgical Protocol Howmedica Osteonics Constrained Acetabular Insert Surgical Protocol Table of Contents Howmedica Osteonics Constrained Acetabular Insert..........................1
Partial Knee Replacement
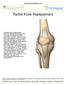 Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
The S.T.A.R. Scandinavian Total Ankle Replacement. Patient Information
 The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
Greater Component Accuracy...
 Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
Surgical Technique. Poly UHMWPE. Bio Hyaluronic Acid. Advancing Materials. Advancing Outcomes.
 Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
Intramedullary Tibial Preparation
 Surgical Technique Intramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Intramedullary tibial preparation Contents Introduction...2 IM tibial highlights...3 Preoperative
Surgical Technique Intramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Intramedullary tibial preparation Contents Introduction...2 IM tibial highlights...3 Preoperative
PARTIAL KNEE REPLACEMENT
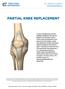 PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Surgical Technique. VISIONAIRE Disposable Instruments for the LEGION Total Knee System
 Surgical Technique VISIONAIRE Disposable Instruments for the LEGION Total Knee System VISIONAIRE and LEGION Disposable instrument technique* Note: All disposable instruments are interchangeable with the
Surgical Technique VISIONAIRE Disposable Instruments for the LEGION Total Knee System VISIONAIRE and LEGION Disposable instrument technique* Note: All disposable instruments are interchangeable with the
CAUTION: Ceramic liners are not approved for use in the United States.
 Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS
 SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
Surgical Technique. VISIONAIRE FastPak Instruments for the LEGION Total Knee System
 Surgical Technique VISIONAIRE FastPak Instruments for the LEGION Total Knee System VISIONAIRE FastPak for LEGION Instrument Technique* Nota Bene The technique description herein is made available to the
Surgical Technique VISIONAIRE FastPak Instruments for the LEGION Total Knee System VISIONAIRE FastPak for LEGION Instrument Technique* Nota Bene The technique description herein is made available to the
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
UTF Stem. reduced. Surgical Protocol
 UTF Stem TM reduced Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Reaming... Canal Broaching...
UTF Stem TM reduced Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Reaming... Canal Broaching...
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
Metha Short Hip Stem System
 Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
- October 2013 English
 TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
THE P.F.C. SIGMA FEMORAL ADAPTER. Surgical Technique
 THE P.F.C. SIGMA FEMORAL ADAPTER Surgical Technique Contents P.F.C. Sigma Femoral Adapter and Revision Knee Surgery Introduction 2 Preoperative Planning 2 Overview 3 Surgical Technique Preparation of the
THE P.F.C. SIGMA FEMORAL ADAPTER Surgical Technique Contents P.F.C. Sigma Femoral Adapter and Revision Knee Surgery Introduction 2 Preoperative Planning 2 Overview 3 Surgical Technique Preparation of the
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
SURGICAL TECHNIQUE. Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM
 SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
BIOLOX delta Option Ceramic Femoral Head System. Product Features and Instructions for Use
 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
BIOLOX delta Option Ceramic Femoral Head System
 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Knees Hips Extremities Cement and Accessories PMI Technology Product Features BIOLOX delta ceramic heads are composed
BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Knees Hips Extremities Cement and Accessories PMI Technology Product Features BIOLOX delta ceramic heads are composed
TOTAL KNEE ARTHROPLASTY (TKA)
 TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement
 Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
CC TRIO VERSAFITCUP. Surgical Technique. each to their own. Hip Knee Spine Navigation
 VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
YOU MAY NEED A KNEE REPLACEMENT
 Knee pain? YOU MAY NEED A KNEE REPLACEMENT Introduction If you re reading this brochure, you or a loved one is likely suffering from a severe knee problem. Serious knee joint conditions can cause pain,
Knee pain? YOU MAY NEED A KNEE REPLACEMENT Introduction If you re reading this brochure, you or a loved one is likely suffering from a severe knee problem. Serious knee joint conditions can cause pain,
Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing
 Journal of Orthopaedic Surgery 2001, 9(1): 45 50 Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing KY Chiu, TP Ng, WM Tang and P Lam Department of Orthopaedic Surgery, The University
Journal of Orthopaedic Surgery 2001, 9(1): 45 50 Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing KY Chiu, TP Ng, WM Tang and P Lam Department of Orthopaedic Surgery, The University
Scorpio NRG PS (cementless)/series 7000 (cementless) Total Knee Investigation
 Scorpio NRG PS (cementless)/series 7000 (cementless) Total Knee Investigation Note: This is an analysis of the Scorpio NRG PS (cless)/series 7000 (cless) Femoral/Tibial Combination. This analysis compares
Scorpio NRG PS (cementless)/series 7000 (cementless) Total Knee Investigation Note: This is an analysis of the Scorpio NRG PS (cless)/series 7000 (cless) Femoral/Tibial Combination. This analysis compares
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Sulzer Orthopedics Joint & Fracture Care
 Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Integra Cadence Total Ankle System PATIENT INFORMATION
 Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Integra. Titan Modular Shoulder System, 2.5
 Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Product Portfolio. Consensus Knee Systems. Consensus Hip Systems
 1115 Windfield Way, Suite 100 El Dorado Hills, Ca 95762 916-355-7100, 916-355-7190 info@consensusortho.com consensusortho.com Product Portfolio Consensus Knee Systems Consensus Hip Systems Product Portfolio
1115 Windfield Way, Suite 100 El Dorado Hills, Ca 95762 916-355-7100, 916-355-7190 info@consensusortho.com consensusortho.com Product Portfolio Consensus Knee Systems Consensus Hip Systems Product Portfolio
Surgical Technique Guide
 Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Surgical Technique Guide FCD: 0423 REV: 03 Introduction The Xtract-All Knee System is a universal extraction system intended to remove knee implant hardware manufactured by a wide range of orthopedic companies.
Unicompartmental Knee Resurfacing
 Disclaimer This movie is an educational resource only and should not be used to manage knee pain. All decisions about the management of knee pain must be made in conjunction with your Physician or a licensed
Disclaimer This movie is an educational resource only and should not be used to manage knee pain. All decisions about the management of knee pain must be made in conjunction with your Physician or a licensed
Zimmer NexGen Trabecular Metal Tibial Tray
 Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Give Bone A Solid Hold Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique
Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Give Bone A Solid Hold Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
