Cigna Medical Coverage Policy
|
|
|
- Vincent Lloyd
- 5 years ago
- Views:
Transcription
1 Cigna Medical Coverage Policy Subject Total Ankle Arthroplasty/Replacement Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 9 References Effective Date... 1/15/2014 Next Review Date... 1/15/2015 Coverage Policy Number Hyperlink to Related Coverage Policies Inpatient Acute Rehabilitation Occupational Therapy Outpatient Acute Rehabilitation Physical Therapy Speech Therapy INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support medical necessity and other coverage determinations. Proprietary information of Cigna. Copyright 2014 Cigna Coverage Policy Cigna covers total ankle arthroplasty/replacement for a skeletally mature individual as medically necessary for the treatment of severe inflammatory arthritis (e.g., rheumatoid arthritis), severe osteoarthritis, or post-traumatic arthritis of the ankle, as an alternative to ankle arthrodesis, when ALL of the following criteria have been met: moderate to severe ankle pain that limits activities of daily living failure of at least six months of conservative therapy (i.e., anti-inflammatory medications, orthotic devices, activity modification, physical therapy) any ONE of the following: arthritis in adjacent joints of the involved extremity (i.e., subtalar, midfoot) severe arthritis of the contralateral ankle previous arthrodesis of the contralateral ankle absence of ALL the following: active infection insufficient bone/osteonecrosis loss of musculature in the affected limb/insufficient ligament support vascular insufficiency in the affected limb Charcot s or other peripheral neuropathy neurological impairment severe ankle deformity precluding proper alignment malalignment or severe deformity of involved or adjacent anatomic structures (e.g. hindfoot, forefoot, knee) absence of medial or lateral malleolus, or both poor skin conditions secondary to surgical scars or trauma Page 1 of 15
2 Cigna does not cover total ankle arthroplasty/replacement for ANY other indication because it is considered experimental, investigational or unproven. General Background Total ankle arthroplasty (TAA) is the process of replacing a diseased ankle with a prosthetic ankle. The procedure has been proposed as an alternative to ankle arthrodesis for non-inflammatory arthritic conditions such as severe osteoarthritis (OA) or post-traumatic arthritis, or inflammatory arthritic conditions, such as rheumatoid arthritis of the ankle. Arthritic ankle joints frequently result in decreased range of motion, swelling, joint stiffness, pain with weight-bearing activity, instability secondary to pain, and, in some cases, visible joint deformity. Conservative management typically consists of medications for pain control, limiting activity, the use of ankle braces to stabilize the joint, shoe modifications, heat, and physical therapy to control the pain associated with ankle arthrosis. When conservative management fails, ankle arthrodesis (i.e., ankle fusion) has been the standard surgical treatment of choice to control the pain of severe ankle arthritis. During an ankle arthrodesis, the joint is fused together, limiting up-and-down movement. While pain may be relieved with ankle arthrodesis, the development of progressive degenerative arthritis in adjacent joints is common. Current techniques for ankle arthrodesis achieve fusion in 80% to 90% of patients (Pickering RM, 2003). Complications such as nonunion have been reported and may lead to a second surgery. Ankle arthroplasty, an alternative to arthrodesis, is intended to improve mobility and function of the joint and is thought to reduce progression of arthritis in adjacent joints. There are two groups of joint replacement designs: two-component, fixed-bearing designs and threecomponent, mobile-bearing designs (Crockerall, Guton, 2003). These designs differ in both the bearing surface and the portion of the ankle joint that they replace. Two-component systems can be further categorized as constrained, semiconstrained and unconstrained. Constraint of the implant is defined as the ability to limit rotational, anterior-posterior and medial-lateral displacements to within normal ranges. Constrained designs offer the advantage of greater stability but compromise mobility. Unconstrained systems and multiaxial systems with free-gliding core both show lower loosening rates than constrained designs, but because of an unphysiologically large range of motion, they have been associated with stability problems. When compared to other joint replacement procedures such as hip and knee, the reported outcomes from TAA such as device durability and stability, complication and failure rates have not been as favorable. Complications such as wound infection, delayed healing and poor implant survival are associated with TAA. In addition, patient selection criteria have not been clearly defined and there is some debate regarding the optimal candidate. Evidence in the medical literature suggests the lifespan of the device is short-term and therefore not practical for use in younger patients or those who are very active. According to some sources it has been suggested that optimal candidates are those greater than age 50, however the age of patients involved in the clinical trials vary. Other criteria include those who are not obese (e.g., have an adequate bone-size to body-weight ratio), who have minimal deformity, good range of motion of the ankle, and a good soft-tissue envelope. With the development of improved designs and instrumentation, authors have reported improvement of implant survivorship. While uncemented and unconstrained second-generation replacements have shown better shortto intermediate-term results, data supporting improved long-term results with the use of similar, more recent designs, is limited. In many cases, clinical results have not been validated by independent practitioners. Despite lack of consensus on patient selection criteria, authors agree careful patient selection is essential to successful outcomes. Inclusion criteria within the published clinical trials vary as well as the device selected, however TAA is intended as an alternative to ankle fusion in patients with debilitating end-stage arthritis, loss of ankle function, and pain that is refractory to conservative treatment for at least six months. FDA approved indications vary depending on device type: fixed-bearing or mobile-bearing. In general these devices are intended for adult patients with reduced activity levels, who have severe rheumatoid arthritis, post-traumatic arthritis or osteoarthritis of the ankle. Contraindications also vary depending on device type, but include the following: active infection insufficient bone/osteonecrosis Page 2 of 15
3 loss of musculature in the affected limb/insufficient ligament support vascular insufficiency in the affected limb Charcot s or other peripheral neuropathy neurological impairment severe ankle deformity precluding proper alignment malalignment or severe deformity of involved or adjacent anatomic structures (e.g. hindfoot, forefoot, knee) absence of medial or lateral malleolus, or both poor skin conditions secondary to surgical scars or trauma patient age, weight or activity levels that introduces unnecessary risk of failure skeletal immaturity U.S. Food and Drug Administration (FDA) Most ankle prostheses are regulated by the FDA as Class II devices and according to the FDA, at least 10 ankle devices have been approved under the FDA 510(k) process since Under the 510(k) approval process, the manufacturer is not required to supply to the FDA evidence of the effectiveness of the device prior to marketing. The FDA classifies mobile bearing total ankle replacement systems as Class III devices. Class III devices require more stringent regulatory review and approval (e.g., premarket approval). FDA approved devices include the following: Agility Total Ankle System (DePuy, Inc., Warsaw, IN); Eclipse Total Ankle Implant (Kinetikos Medical, Inc. Carlsbad, CA); Salto Tolaris Total Ankle Prosthesis (Tornier, St. Ismier, France); and the INBONE Total Ankle System, formerly known as the Topez Ankle System (INBONE Technologies, Boulder, CO) and the Scandinavian Total Ankle Replacement (S.T.A.R. Ankle) (Small Bone Innovations, Morrisville, PA). In August of 2010 the FDA granted 510(k) approval for the INBONE II Total Ankle System (Wright Medical Technology, Arlington, TN). According to the FDA this device differs from the predicate device, INBONE Total Ankle, in articulating surface geometry and added stability in the talar dome (FDA, K100886). Recently the Zimmer Trabecular Metal total ankle implant, a semi-constrained device and alignment instrumentation system, received FDA approval and is intended for cemented use only. Literature Review FDA Approved Devices Early constrained devices were associated with high complication and reoperation rates. With refinement of the devices and improved instrumentation, the clinical outcomes of TAA procedures using more recent designs have improved. However, clinical trials specifically evaluating FDA approved devices are limited in quantity and quality. Much of the literature prior to FDA approval was in the form of retrospective and prospective case series without controls or comparison groups. The clinical outcomes were mixed; some studies demonstrated high failure and complication rates, which seemed to improve with surgeon experience. Surgeon experience is a contributing factor to overall success. In addition, several of the studies were conducted by surgeons who designed the device and/or were funded by the manufacturer which could induce bias. Of the more recent FDA approved devices, most studies evaluate the Agility Ankle or the STAR prosthesis, measuring clinical outcomes such as improvement in pain and function, complication rates, failure rate and rate of revision. Follow-up evaluations are on average two to five years with some studies reporting results beyond that. Studies comparing ankle arthroplasty to ankle arthrodesis are few and data comparing mobile bearing designs to fixed-bearing designs is lacking. Agility Total Ankle System: The Agility prosthesis is a two-component semi-constrained device intended for cemented use only. According to the FDA the initial 510(k) approval was granted in 1992, since that time the device has been revised with subsequent approval through the 510(k) process. The most recent approved device is the Agility Lp Total Ankle prosthesis (510[k] K053569). Evidence in the published scientific literature evaluating Agility Ankle prosthetic devices consists primarily of prospective case series, retrospective case series, and systematic reviews (Pyevich, et al., 1998; Knecht, et al., 2004; Spirt, et al., 2004; Kopp, et al., 2006; Claridge, et al., 2009; Criswell, et al., 2012; Roukis, 2012). Evaluation of clinical outcomes in these studies extended to nine years with an average follow-up of four to five years. The outcomes measured include pain, function and alignment based on American Orthopedic Foot and Ankle Society (AOFAS) scores or Ankle Osteoarthritis Scale (AOS) scores, range of motion based on radiographic evaluation, healing and patient satisfaction, and reoperation and failure rates. A majority of subjects experienced improvement in pain scores postoperatively, improved range of motion, and improvement Page 3 of 15
4 in walking and activity performance. Some authors reported high complication rates that included wound healing, intraoperative fracture, nonunion syndesmosis, subsidence, postoperative fracture, infection aseptic loosening and technical errors (Knecht, et al.,, 2004; Claridge, et al., 2009; Roukis, 2012). Revision rates varied overall but ranged from 11% to 39%. The results of a systematic review (Roukis, 2012) indicate the incidence of revision was 9.7% with a majority resulting in component replacement rather than arthrodesis. Nevertheless, randomized trials evaluating device durability and improved functional outcomes are lacking. The Scandinavian Total Ankle Replacement (S.T.A.R. Ankle): The STAR ankle system is a threecomponent, nonconstrained mobile-bearing implant, intended for non-cemented use and allows four axes of rotation. This device received FDA approval through the PMA process (FDA, P050050). As part of the approval process the manufacturer is required to conduct two post-approval studies: the first is a prospective multicenter single arm designed trial, involving a continued access cohort, to determine the eight year survivorship and effectiveness of the STAR device in comparison to arthrodesis from historical controls. A second study (prospective, multicenter single arm design) involving additional subjects, is required to examine the performance of the device under actual conditions of use, and compared to the continued access cohort. The recently approved STAR device is also in its third generation. Several authors have evaluated and reported on the STAR device since the initial phases of development making comparisons across studies difficult. The reported clinical outcomes from these earlier studies in the peer-reviewed published scientific literature were mixed, some were less successful than more recent designs, sample populations were small, few authors compared outcomes to arthrodesis and long-term improvement in health outcomes was not firmly established. Several prospective case series have been conducted evaluating the STAR ankle prosthesis and have demonstrated improvements in pain, function and range of motion using measures such as AOFAS scores, Foot Function Index, and radiographs (Valderrabano, et al., 2004; Stengel, et al., 2005; Wood, et al., 2008; Shutte, et al., 2008; Mann, et al., 2011). In general, sample size of these trials ranged from 47 to 200 and follow-up across these trials ranged from one year to eight years. Authors have conducted clinical trials to assess survivorship of the device, rates for revision and complications (Karantana, et al., 2009; Zhao, et al., 2012). Across these trials five year survival rates ranged from 85.9%-96% with nine and ten year survival rates of 88.5% and 90% respectively. One group of authors compared fixation with cement versus uncemented and reported that survivorship analysis for 12 years based on life-tables showed a 70% rate for cemented and 95.4% for uncemented. In addition clinical scores for the uncemented group were higher compared to the cemented group, 91.9± 7.4 and 74.2± 19.3, respectively. Complication rates were generally low and included wound infections and fractures (both intraoperative and postoperative). In 2009 Saltzman et al. published the results of a prospective, controlled multicenter investigational device exempt (IDE) trial evaluating the STAR ankle device. The study was a noninferiority designed trial involving 15 different sites in the U.S. and compared safety and efficacy of the STAR device with arthrodesis. The study population was selected by a 2:1 method, and consisted of 158 ankles for the STAR group and 66 for the arthrodesis group (Pivotal study groups). A second group of patients (n=448 ankles) whose surgery was performed after completion of enrollment were also reported on (Continued Access group). The authors noted the implant was not modified between the two series and that the foot and ankle surgeons were experienced with ankle fusion although they had very limited experience with either the anterior approach to or implantation of total ankle components. Follow-up was conducted at 24 months using standard scoring systems which included Visual Analog Scale (VAS), Short Form-36, and the Buechel-Pappas (BP) scale. Success was defined as 40+ point improvement in total BP score, no device failure, revision or removal, radiograph success, and no major complications. The long-term advantages including functional benefits, revision and reduction of hindfoot arthritis were not evaluated in this study. The results of the study demonstrated intraoperative fracture occurred in 9.5% of the Pivotal group, (mainly of the malleoli); the Continued Access group had a lower rate of 4.8%. Adverse events were more common in the arthroplasty group compared to the fusion group; the Continued Access group had significantly less complications compared to the Pivotal arthroplasty group. No significant differences were seen between the Pivotal and Continued Access groups in major complications. The Pivotal STAR group demonstrated better function and equivalent pain relief compared to the arthrodesis group. The Pivotal arthroplasty group had a mean improvement of 40 points and the fusion group had a mean 26 point improvement in the BP score at 24 months follow-up. The Continued Access group also had significant improvement compared to the Pivotal STAR group. Mean improvement in VAS was similar in the Pivotal STAR and fusion groups; however in the Continued Access group the mean pain reduction was significantly improved compared to the fusion group. At 24 month follow-up, 16.5% of the Pivotal STAR group required a secondary Page 4 of 15
5 surgical intervention, of which 7.6% required revision or removal, compared to 10.6% of the fusion group who required secondary surgery, of which 10.6% required revision or removal. The Continued Access group required even less secondary surgical procedures (8.5%) compared to the Pivotal group. Patient satisfaction was similar in the Pivotal STAR and fusion groups and was reported as good to excellent. At 24 month follow-up the Pivotal arthroplasty group demonstrated significantly greater efficacy and overall success compared to the fusion group. In a retrospective study comparing clinical and radiograph outcomes between ankle replacement and ankle arthrodesis subjects Saltzman et al. (2010) matched a group of subjects who received the STAR device (n=42) and compared them to a group of arthrodesis subjects (n=29). Although only 88% of the ankle replacement and 79% of the ankle arthrodesis group were able to complete follow-up (average of 4 years), the ankle replacement group had better mean AOS pain and disability scores compared to the arthrodesis group. Significant differences were noted only in the SF-36 mental component score and AOS pain scale. There were no significant differences in the preoperative and postoperative osteoarthritis between the ankle replacement and arthrodesis group. The arthroplasty group had more pain relief and more postoperative complications that required surgery. Mann et al. (2011) published the prospective medium- to long-term results of total ankle replacement using the STAR prosthesis (n=80). Measured outcomes included AOFAS score for pain and function, VAS scores, and Numerical Rating Scale, in addition to serial radiographs for stability and alignment of the implanted device. All scores improved postoperatively. Total range of motion postoperatively improved from 32 degrees to 39.2 degrees. Probability of survivorship was calculated for five years at 96% and for ten years at 90%. Seven individuals did not retain their prosthesis; five died during the study and four were lost to follow-up. Nine subjects had high grade complications (i.e., loosening [2], deep infection [3], and implant failures [4]). Six subjects had medium grade complications (i.e., subsidence [3], postoperative malleolus fractures [3]). The authors noted that changes in alignment and adjacent joint arthritis were similar to previous reports. Nunley et al. (2012) reported the clinical outcomes of 82 subjects who underwent TAA with the STAR prosthesis for the treatment of end stage ankle arthritis. Average follow-up was 61 months. There was significant improvement in SF-36 mental and physical scores (p<0.001) as well as AOFAS pain scores, function, alignment, and summary scores (p<0.001). VAS pain scores decreased at the most recent follow-up, ankle motion in both dorsiflexion and plantar flexion improved significantly and Buechal-Pappas pain and function scores improved (p< 0.001). A total of five subjects required revision with removal of the tibial and/or talar component. At an average of 60.7 ± 21 months, the survival rate was 93.9%; at 107 months, the projected survival rate was 88.5%. Zhao et al. (2012) conducted a systematic review of outcome and failure rate of the un-cemented STAR ankle replacement device (n=16 publications, 2088 TAA procedures). Average follow-up was 52 months, average patient age was 60 years, preoperative diagnoses included rheumatoid arthritis, posttraumatic arthritis or primary osteoarthritis. A meta-analytic pooled five year survival rate was reported as 85.9% with a metaanalytic pooled 10 year survival rate of 71.1%. A total of 232 of the 2088 STAR TAA failed, with a pooled failure rate of 11.1%. Reported complications included intraoperative fracture, wound healing problems, postoperative fracture, subsidence, instability, aseptic loosening, deep infection, malalignment, and others such as edge loading, broken or torn prosthesis, stiffness and residual pain. Various methodologies (i.e., AOFAS, Kofoed ankle, Buechal-Pappas ankle scores) were used to measure functional outcomes making comparisons difficult although mid-term functional outcomes reported as acceptable. Salto Total Ankle: Bonnin et al. (2011) analyzed longer-term survivorship and causes of failure of the Salto prosthesis in a cohort of previously studied subjects. The authors retrospectively reviewed 96 prospectively followed subjects with 98 prostheses implanted between 1997 and 2000 (prior to FDA approval in the U.S.). A total of 85 subjects had a minimum follow-up of 6.8 years, mean follow-up was 8.9 years. The reported survival rate was 65% with any reoperations of the ankle as the endpoint and 85% with revision of a component as the end point. Six prostheses were removed for arthrodesis and 18 ankles underwent reoperation without arthrodesis. Three main causes of reoperations were bone cysts, fracture of polyethylene, and unexplained pain. The authors noted few patients developed loosening and/or subsidence. Nodzo et al. (2013) reported the results of 74 subjects who underwent TAA with the Salto Total Ankle device with an average follow-up of 43 months (range of two to 4.6 years). Outcomes were measured using radiographs, FAOS scores, SF-12, and VAS. Kaplan Meier curves were used to determine implant survivorship Page 5 of 15
6 which was 98%. Range of motion improved, and the physical component of the SF-12 significantly improved (P<.0001) although there was insignificant improvement with the mental component. Additional long-term follow-up is needed to support durability of the device. Sweitzer et al. (2013) reported two year outcomes of a cohort of subjects (N=67) who underwent TAA using the Salto Total Ankle device. Clinical outcomes were prospectively monitored preoperatively, at six weeks, three months, and six months postoperatively and then yearly thereafter. The reported implant survival at an average of 2.8 years was 96% with end point failure defined as metallic component revision, removal or impending failure. Complications occurred in 15 subjects and included ankle impingement, aseptic loosening, wound healing problems, equinus, intraoperative and post-operative fracture, technical error and other minor complications. The authors noted the cohort had significant improvement in VAS pain scores (P<0.05), quality of life SF-36 (P<0.05), AOFAS scores (P<0.05), and improved function (i.e., TUG testing, mean walking speeds). The study results are limited by short-term evaluation and the number of subjects who could not be followed clinically (8/72) and were excluded from the data analysis. The authors noted however that these subjects did have follow-up at other institutions and did not report any need for additional surgery. Literature Review National Joint Registry Reports Authors have begun to report on data from national joint registries and, in particular, on replacement survival rates. Hosman et al. (2007) reported a national joint registry review of TAAs. The information reported by this group of authors is based on the New Zealand National Joint Registry. The authors reviewed data for 202 TAAs performed between 2000 and 2005, with follow-up at six years. The four prostheses recorded were the Agility Total Ankle System, the STAR, the Mobility, and the Ramses Total Ankle. Review of the data took place at approximately 28 months post- procedure. Fourteen revisions were recorded (7% failure rate), with loosening of components identified as the main reason for failure. A six-month post-procedure questionnaire evaluating pain, activity and function was returned by 74% of the patients and suggested that unfavorable patient scores at six months indicated an increased likelihood of subsequent failure. Fevang et al. (2007) reported on TAA data obtained from the Norwegian Arthroplasty Register between 1994 and The mean follow-up time for all patients was four years. There were 257 TAAs performed; 32 were cemented and 212 were noncemented. Four types of prostheses were used: the Norwegian Thompson Parkridge Richards (TPR), STAR, Ankle Evolution System (AES), and Hintegra. Revisions were performed in 27 cases. The authors reported an overall five- and ten-year survival rate of 89% and 76%, respectively. Prosthesis survival was the same for cemented (Norwegian TPR prosthesis) versus uncemented (STAR prosthesis). The authors acknowledged that the survival rate after TAA remains much lower than knee (94 96%) and hip (98%) replacements. Henricson et al. (2007) reported on data from a national ankle replacement registry in Sweden between 1993 and 2005 using third-generation devices, including the STAR prosthesis, Buechel-Pappas, AES, Hintegra, and Mobility prosthesis. A total of 531 replacements were reported to the register; 101 ankles were revised (19%). The estimated overall five- and ten-year survival rate was 78% and 62%, respectively. Henricson and colleagues (2011) extended the study reporting 10-year survival analysis at which time 780 prostheses were implanted. The overall survival rate fell from 81% at five years to 69% at 10 years, demonstrating some improvement compared to the prior report. Subjects younger than age 60 years with OA or post-traumatic arthritis were more likely to require revision; age had no effect on revision rate in subjects with rheumatoid arthritis. Skytta et al. (2010) evaluated the survival of two ankle designs and factors that were associated with survival using data from the nationwide arthroplasty registry in Finland. The data was obtained between 1982 and 2006 and consisted of 645 operations; 573 were primary and 72 were revisions. The authors evaluated only ankle designs that were used in more than 40 surgeries and only implants with a mean follow-up of at least two years and more than 20 subjects at risk at three years. Two devices met criteria, which included the STAR and the AES ankles. Indications for surgery included rheumatoid arthritis (49%), posttraumatic arthritis (22%), primary osteoarthritis (19%) and other arthritis and diseases (10%). The authors noted no differences in survival rates between the two designs. The five-year survivorship for the whole cohort was 83% using revision for any reason as endpoint and 95% using revision for aseptic loosening as endpoint. Age and sex did not have any significant effect on survival. A total of 59 revisions were reported during , the seven year survivorship for the whole group was 78%. The most common reasons for revision were aseptic loosening (30%), instability (39%), primary malalignment (8%), infection (7%), fracture of the meniscal implant (5%), and periprosthetic fracture Page 6 of 15
7 (2%). The authors concluded a high number of complications were associated with balance and instability and required a detailed understanding of not only the ankle joint but hindfoot as well. Literature Review Other: SooHoo and colleagues (2007) conducted an observational study comparing reoperation rates following ankle arthrodesis and TAA. The authors used population-based data from all inpatient admissions in California over a ten-year period of time ( ). A discharge database was used to identify the patients; however, the authors did not note the type of prosthetic ankle device implanted. Both short-term (90 days post-primary surgery) and long-term outcomes (one and five years post-primary procedure) were assessed, including rates of major revision surgery, pulmonary embolism, amputation, infection and subtalar joint fusion. A total of 4705 ankle fusions and 480 TAAs were performed during the study period. At 90 days postoperatively, the TAA patients had a higher rate of major revision surgery and had an increased rate of device-related infection compared to the ankle arthrodesis patients. The rates for major revision surgery at one and five years for TAA were 9% and 23%, respectively; for ankle arthrodesis, the rates of major revision surgery were 5% and 11%, respectively. The patients treated with ankle arthrodesis had a higher rate of subtalar fusion at five years (2.8%) compared to TAA (0.7%). The authors confirmed that patients who undergo TAA are more likely to require revision surgery compared to patients who undergo ankle arthrodesis and have a higher risk of device-related infection. Limitations of the study include patient populations that were not comparable to each other; the presence of subtalar arthritis, which is a potential complication to arthrodesis; and the limited data available from the administrative databases. There was no data regarding functional outcomes or for procedures performed on an outpatient basis (surgery or readmissions). Haddad et al. (2007) conducted a systematic review of the literature addressing the intermediate and long-term outcomes of TAA and ankle arthrodesis in terms of ankle function, pain, revision, conversion to arthrodesis, implant survival and quality of life. The publications included in their review were published between 1990 and 2005 and included 49 primary studies (56 treatment arms, 2114 patients). Ten studies focused on TAA (n=852), and 39 studies focused on ankle arthrodesis (n=1262). No studies directly compared TAA and ankle arthrodesis. Follow-up time ranged from two to nine years in the TAA group of studies and two to 23 years in the arthrodesis group of studies, with an average of five years across both groups. The efficacy results associated with secondgeneration implants, including the Agility ankle, New Jersey Low Contact Stress Ankle, Buechel-Pappas, TNK, STAR, and Salto prosthesis were reported. Patients treated with TAA were older than those undergoing ankle arthrodesis and were mainly female. The authors analysis was limited because efficacy outcomes were variable across the studies. The five- and ten-year implant survival rates following TAA were 78% and 77%, respectively. A revision was required in 7% of patients who underwent TAA, most commonly for loosening and/or subsidence. Five percent of the TAA patients were converted to arthrodesis. Below-the-knee amputation was performed in 1% of the patients treated with TAA. The ankle arthrodesis group had a 10% nonunion rate. Nine percent underwent revision because of nonunion, and 5% of the patients underwent below-the-knee amputation. The authors noted significant heterogeneity in almost all studies; therefore, results should be interpreted with caution. The review was limited by the variability of reported outcomes and the tools to assess outcomes, differences in patient populations, differences in study follow-up times, and lack of direct comparison between TAA and ankle arthrodesis. However, the authors acknowledged that although the evidence was limited, the intermediate results suggest the two procedures are comparable. Some authors have evaluated mechanical properties, such as gait analysis, associated with total ankle replacement. In 2007 Valderrabano and colleagues reported the results of a prospective study evaluating gait characteristics of patients who underwent total ankle replacement. The authors compared 15 patients with OA of the ankle with 15 age and gender-matched control subjects using clinical and three-dimensional hindfoot gait analysis. The subjects received the Hintegra Ankle system. The authors confirmed that ankle OA caused significant reduction of the AOFAS scores and SF-36 scores compared to healthy controls and significant change in the recorded gait characteristics. At three months post surgery, most results of the patients with OA showed a trend away from the results of normal subjects (i.e., function was below preoperative baseline) and at 12 months patients who underwent total ankle replacement changed gait characteristics towards results of the normal subjects (i.e., function was above preoperative baseline). Houdijik et al (2008) compared mechanical load and stiffness of the ankle joint following replacement with the Buechel-Pappas ankle (n=10) with healthy control subjects (n=10). The authors noted there were no significant differences in mechanical load of the ankle joint, peak joint moments or stiffness compared to controls although there was a difference for internal work at the step which may have been related to varying walking speeds. In the author s opinion, function of the ankle joint did not appear to be influenced by total ankle replacement. Using gait analysis, Piroiu et al. (2008) evaluated patients who underwent total ankle arthroplasty (n=12), patients who underwent ankle arthrodesis Page 7 of 15
8 (n=12) compared to a healthy control group (n=12). The authors reported that neither treatment with ankle arthrodesis or total ankle replacement fully restored normal movement or walking speed. Ankle arthrodesis resulted in more rapid gait with longer step length compared to the replacement group and the timing of gait demonstrated greater asymmetry. The ankle replacement group had greater movement at the ankle, a symmetrical timing of gait and restored ground reaction force pattern. Improved timing of gait would support a reduction in limp with ankle replacement although the gait is significantly slower. Gougoulais et al (2010) published the results of systematic review of the literature evaluating the outcomes of TAA. The authors identified 13 studies published between 2003 and 2008 reporting on 1105 TAAs using various ankle prostheses. There were no randomized trials available for review; all studies reviewed were level IV evidence. With revision, arthrodesis or amputation as the end point, the authors identified 108 failures (9.8%). The weighted follow-up for all prostheses was 5.2 years. Eight studies provided Kaplan-Meier survivorship analysis ranging from 67% at 6 years to 95.4% as 12 years. Failures were salvaged with revision TAAs in 62% and amputations were rare (1%). Ankle scores improved after TAA in all studies although improvement in ankle ROM was relatively small. The overall failure rate was approximately 10% at 5 years (range of 0% to 32%) with variation between different centers. Superiority of one type of design over another was not supported by the available data. Several limitations were noted regarding the literature reviewed and included surgeon experience, heterogeneity in study design, variable follow-up periods, variable assessment scales, clinical outcomes that were often not validated, methods to assess patient satisfaction were not rigorous, and measures regarding radiograph assessment varied and were often not standardized. Krause et al. (2011) evaluated complication rates and revision rates for subjects who underwent TAA (n=114 replacements) compared to ankle fusion (n=47 arthrodesis). Overall, follow-up consisted of assessments at six, 12, and 24 months and 3.5 years, five, and ten years post-operatively using the Ankle Osteoarthritis Scale (AOS), Canadian Orthopedic Foot and Ankle Society classification system (COFAS), radiographs and CT scans. Radiograph evaluations were performed at a mean of 39 months for TAA subjects and at a mean of 37 months for ankle arthrodesis subjects. The authors reported that at a minimum of two years post surgery, clinical outcomes of pain and function (mean AOS score) improved similarly in both groups. At 24 month followup the complication rate was higher in the TAA group (54%) compared to the fusion group (26%) and the TAA revision rates were also higher compared to ankle arthrodesis, 9% at one year and 23% at five years, 5% at one year and 11% at five years, respectively. Six subjects developed symptomatic osteophytes around the articular margins of the device and underwent osteophyte excision at an average of 3.7 years postoperatively; five subjects developed asymptomatic osteophytes around the margin of the device. A total of 88% of joints had no progression of arthritis at an average of 10 years follow-up. Non-FDA Approved Devices Several devices are undergoing various stages of development and clinical review for total ankle replacement. Although these devices are not FDA approved some of these devices have been evaluated and compared to other FDA approved devices. Data supporting safety, efficacy, and clinical utility is lacking and their use remains investigational at this time. Other non-fda-approved devices evaluated in the literature include, but are not limited to, the following: TNK Ankle (Kyocera Corporation, Kyoto, Japan) Hintegra Total Ankle Prosthesis (Newdeal SA, Lyon, France) Buechel-Pappas Ankle Replacement System (Endotec, South Orange, NJ) Bologna Oxford (BOX) TAR (Finsbury Orthopaedics Ltd, Leatherhead, United Kingdom) Technology Assessment ECRI published an emerging technology evidence report evaluating total ankle replacement as a treatment for degenerative ankle disease and concluded that total ankle replacement should only be performed at orthopedic specialty centers that perform a high volume of ankle procedures (ECRI, 2005). Since that time, ECRI updated the complete report in 2007, recently in November 2009 and completed an additional review of the literature in ECRI continues to acknowledge that there is a substantial learning curve associated with total ankle replacement. Furthermore, TAA is complex surgery with a potential for high complication rates, surgeons considering performing TAA must have an excellent understanding of anatomy and lower-extremity biomechanics and adequate training in the particular TAA system they intend to use. Appropriate patient Page 8 of 15
9 selection, preoperative planning to ensure correct component size and positioning, and corrections of existing ankle deformities are critical to optimizing the outcomes of TAA. Professional Societies /Organizations A position statement by the American Orthopaedic Foot and Ankle Society (AOFAS) was updated in The AOFAS considers TAA an acceptable and viable alternative to ankle arthrodesis in select patients with ankle arthritis. According to the AOFAS, patients who may benefit from this procedure include adult patients with primary, post-traumatic and rheumatoid arthritis who have moderate or severe pain, loss of mobility, and loss of function of the involved ankle. Additionally, the individual should have completed several months of conservative treatment, should have satisfactory vascular perfusion in the involved extremity, and must have adequate softtissue coverage about the ankle that affords a safe surgical approach. In such patients, high-level evidence indicates that TAA safely relieves pain and may provide superior functional results when compared to ankle fusion. The AOFAS position further indicates that TAA should be performed by board-certified or board eligible allopathic or osteopathic orthopedic surgeons with appropriate training and education (AOFAS, 2009). In March 2010 the American College of Foot and Ankle Surgeons (ACFAS) published a position statement on total ankle replacement surgery. The position of the ACFA S states, Total ankle replacement surgery is currently a safe and effective treatment option for select patients with end stage ankle arthritis; studies have shown total ankle replacement surgery improves function, reduces pain, and promotes improved quality of life. Use Outside of the US: No relevant information found. Summary Evidence in the peer-reviewed, published scientific literature evaluating total ankle arthroplasty (TAA) is primarily in the form of retrospective or prospective case series and joint registry data, with few controlled clinical trials. High-quality comparative studies addressing postoperative outcomes in patients who underwent total ankle arthroplasty versus ankle fusion are lacking. Generally, the outcomes of these studies consistently demonstrate improvement in pain and function scores following TAA, results that are comparable to ankle arthrodesis in some studies and better in other studies. Data supporting one type of device versus others are lacking. The overall failure rate is approximately 10% at five years; implant survival ranges from 85-90% on average at five years, with survival rates of approximately 75-80% at 8 to 10 years. Nevertheless, comparison between clinical studies regarding TAA is difficult and limited by heterogeneous study populations, differences in type of prosthetic designs and lack of standardized outcome measures. Follow-up time varies across studies as well as scoring systems used to assess clinical outcomes. Additional long-term clinical studies supporting improved outcomes such as pain relief, functional mobility and durability of the prosthetic device would be helpful in comparing the safety and efficacy of TAA to ankle arthrodesis. However, there is sufficient data in the peer-reviewed published scientific literature, including support from professional societies that for a carefully selected subset of individuals, TAA may be considered a safe and effective alternative to ankle arthrodesis. Coding/Billing Information Note: 1) This list of codes may not be all-inclusive. 2) Deleted codes and codes which are not effective at the time the service is rendered may not be eligible for reimbursement Covered when medically necessary when used to report total ankle arthroplasty/replacement: CPT * Description Codes Arthroplasty, ankle Arthroplasty, ankle; with implant (total ankle) Arthroplasty, ankle; revision, total ankle HCPCS Codes C1776 Description Joint device, implantable Page 9 of 15
10 L8699 Prosthetic implant, not otherwise specified *Current Procedural Terminology (CPT ) 2013 American Medical Association: Chicago, IL. References 1. Abicht BP, Roukis TS. The INBONE II Total Ankle System. Clin Podiatr Med Surg Jan;30(1): Ali MS, Higgins GA, Mohamed M. Intermediate results of Buechel Pappas unconstrained uncemented total ankle replacement for osteoarthritis. J Foot Ankle Surg Jan-Feb;46(1): American College of Foot and Ankle Surgeons (ACFAS). Position Statement on Total Ankle Replacement Surgery. March Accessed November 19, Available at URL address: 4. American Orthopaedic Foot & Ankle Society (AOFAS). Total ankle arthroplasty. Position statement Jun 6, updated August 4, Copyright American Orthopaedic Foot & Ankle Society. Accessed January 4, Available at URL address: Key=# 5. Barg, A, Elsner, A, Anderson, AE, and Hintermann, B. The effect of three-component total ankle replacement malalignment on clinical outcome: pain relief and functional outcome in 317 consecutive patients. J Bone Joint Surg Am. 2011;93(21): Bauer G, Eberhardt O, Rosenbaum D, Claes L. Total ankle replacement: Review and critical analysis of the current status. Foot and Ankle Surgery Apr;2(2): Besse JL, Brito N, Lienhart C. Clinical evaluation and radiographic assessment of bone lysis of the AES total ankle replacement. Foot Ankle Int Oct;30(10): Besse JL, Colombier JA, Asencio J, Bonnin M, Gaudot F, Jarde O, Judet T, Maestro M, Lemrijse T, Leonardi C, Toullec E; l'afcp. Total ankle arthroplasty in France. Orthop Traumatol Surg Res May;96(3): Epub 2010 Apr Bianchi A, Martinelli N, Sartorelli E, Malerba F. e Bologna-Oxford total ankle replacement: a mid-term followup study. J Bone Joint Surg Br Jun;94(6): Bonasia DE, Dettoni F, Femino JE, Phisitkul P, Germano M, Amendola A. Total ankle replacement: why, when and how? Iowa Orthop J. 2010;30: Bonnin M, Gaudot F, Laurent JR, Ellis S, Colombier JA, Judet T. The Salto Total Ankle Arthroplasty: Survivorship and Analysis of Failures at 7 to 11 years. Clin Orthop Relat Res Jan;469(1): Epub 2010 Jul Claridge RJ, Sagherian BH. Intermediate term outcome of the agility total ankle arthroplasty. Foot Ankle Int Sep;30(9): Coetzee JC, Castro MD. Accurate measurement of ankle range of motion after total ankle arthroplasty. Clin Orthop Relat Res Jul;(424): Coughlin MJ. Chapter 15: The Scandinavian total ankle replacement prosthesis. In: Beaty JH, editor. Instructional course lectures 51. Rosemont, IL: American Academy of Orthopaedic Surgeons; p Page 10 of 15
11 15. Cracchiolo A 3rd, Deorio JK. Design features of current total ankle replacements: implants and instrumentation. J Am Acad Orthop Surg Sep;16(9): Criswell BJ, Douglas K, Naik R, Thomson AB. High revision and reoperation rates using the Agility Total Ankle System. Clin Orthop Relat Res Jul;470(7): Crockarell JR Jr., Guyton JL. Arthroplasty of ankle and knee. In: Canale T, editor. Campbell s operative orthopedics. 10 th ed. St. Louis, MO: Mosby, Inc.; p Doets HC, Brand R, Nelissen RG. Total ankle arthroplasty in inflammatory joint disease with use of two mobile-bearing designs. J Bone Joint Surg Am Jun;88(6): ECRI Institute. Hotline Response [database online]. Plymouth Meeting (PA): ECRI Institute; Total ankle replacement (TAR) for degenerative ankle disease Nov 11. Available at URL address: ECRI Institute. Hotline Response [database online]. Plymouth Meeting (PA): ECRI Institute; Total ankle replacement (TAR) for degenerative ankle disease Dec 30. Available at URL address: ECRI Institute. Hotline Response [database online]. Plymouth Meeting (PA): ECRI Institute; Total ankle replacement (TAR) for degenerative ankle disease Dec 5. Available at URL address: ECRI Institute. Total ankle replacement (TAR) for degenerative ankle disease. [Emerging Technology evidence report]. Plymouth Meeting (PA): ECRI Institute; 2005 May 26 [updated 2007, 2009]. Available at URL address: Feldman MH, Rockwood J. Total ankle arthroplasty: a review of 11 current ankle implants. Clin Podiatr Med Surg Jul;21(3): , vii. 24. Fevang BT, Lie SA, Havelin LI, Brun JG, Skredderstuen A, Furnes O. 257 ankle arthroplasties performed in Norway between 1994 and Acta Orthop Oct;78(5): Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int Apr;25(4): Glazebrook MA, Arsenault K, Dunbar M. Evidence-based classification of complications in total ankle arthroplasty. Foot Ankle Int Oct;30(10): Goldberg VM, Kraay MJ. Total Ankle Replacement. In: Goldman L, Ausiello D, editors: Cecil Medicine, 23 rd edition. Copyright Surgical Therapy. Total Joint Arthroplasty. Ch Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements? A systematic review of the literature. Clin Orthop Relat Res Jul Guyer AJ, Richardson G. Current concepts review: total ankle arthroplasty. Foot Ankle Int Feb;29(2): Haddad SL, Coetzee JC, Estok R, Fahrbach K, Banel D, Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J Bone Joint Surg Am Sep;89(9): Hahn ME, Wright ES, Segal AD, Orendurff MS, Ledoux WR, Sangeorzan BJ. Comparative gait analysis of ankle arthrodesis and arthroplasty: initial findings of a prospective study. Foot Ankle Int Apr;33(4): Henricson A, Knutson K, Lindahl J, Rydholm U. The AES total ankle replacement: A mid-term analysis of 93 cases. Foot Ankle Surg Jun;16(2):61-4. Epub 2009 Jul 25. Page 11 of 15
Total Ankle Replacement
 Protocol Total Ankle Replacement (70177) Medical Benefit Effective Date: 01/01/11 Next Review Date: 09/14 Preauthorization No Review Dates: 09/10, 09/11, 09/12, 09/13 The following Protocol contains medical
Protocol Total Ankle Replacement (70177) Medical Benefit Effective Date: 01/01/11 Next Review Date: 09/14 Preauthorization No Review Dates: 09/10, 09/11, 09/12, 09/13 The following Protocol contains medical
Original Policy Date
 MP 7.01.60 Total Ankle Replacement Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
MP 7.01.60 Total Ankle Replacement Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return to Medical Policy Index
Design Project. Dr. Kevin O Kelly, TCD
 Design Project Project title: Mentor: Total Ankle Replacement Dr. Kevin O Kelly, TCD Background The ankle joint is a comparatively small joint relative to the weight bearing and torque it must withstand.
Design Project Project title: Mentor: Total Ankle Replacement Dr. Kevin O Kelly, TCD Background The ankle joint is a comparatively small joint relative to the weight bearing and torque it must withstand.
Clinical Policy Bulletin: Total Ankle Arthroplasty
 Go Clinical Policy Bulletin: Total Ankle Arthroplasty Number: 0645 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers total ankle arthroplasty (TAA) using a
Go Clinical Policy Bulletin: Total Ankle Arthroplasty Number: 0645 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Aetna considers total ankle arthroplasty (TAA) using a
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Total Ankle Replacement Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Total Ankle Replacement Professional Institutional Original Effective Date:
Total Ankle Replacement Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Total Ankle Replacement Professional Institutional Original Effective Date:
TOTAL ANKLE REPLACEMENT
 TOTAL ANKLE REPLACEMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
TOTAL ANKLE REPLACEMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
Total Ankle Arthroplasty. Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014
 Total Ankle Arthroplasty Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014 Ankle Anatomy The ankle is a hinge or ginglymus joint Made up of the tibia, fibula, & talus
Total Ankle Arthroplasty Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014 Ankle Anatomy The ankle is a hinge or ginglymus joint Made up of the tibia, fibula, & talus
Total Ankle Arthroplasty/Replacement
 Medical Coverage Policy Total Ankle Arthroplasty/Replacement Table of Contents Effective Date...02/15/2018 Next Review Date...02/15/2019 Coverage Policy Number... 0285 Related Coverage Resources Coverage
Medical Coverage Policy Total Ankle Arthroplasty/Replacement Table of Contents Effective Date...02/15/2018 Next Review Date...02/15/2019 Coverage Policy Number... 0285 Related Coverage Resources Coverage
Intermediate and Long-Term Outcomes of Total Ankle Arthroplasty and Ankle Arthrodesis
 1899 COPYRIGHT 2007 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED Intermediate and Long-Term Outcomes of Total Ankle Arthroplasty and Ankle Arthrodesis A Systematic Review of the Literature By
1899 COPYRIGHT 2007 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED Intermediate and Long-Term Outcomes of Total Ankle Arthroplasty and Ankle Arthrodesis A Systematic Review of the Literature By
Salto Talaris total ankle arthroplasty: Early clinical results from eighty-one consecutive patients by a single surgeon
 Salto Talaris total ankle arthroplasty: Early clinical results from eighty-one consecutive patients by a single surgeon Kurt Hofmann, MD, Zabrina Shabin, MD, Eric Ferkel, MD, Jeffrey Jockel, MD, Mark Slovenkai,
Salto Talaris total ankle arthroplasty: Early clinical results from eighty-one consecutive patients by a single surgeon Kurt Hofmann, MD, Zabrina Shabin, MD, Eric Ferkel, MD, Jeffrey Jockel, MD, Mark Slovenkai,
10-year survival of total ankle arthroplasties
 Acta Orthopaedica 2011; 82 (6): 655 659 655 10-year survival of total ankle arthroplasties A report on 780 cases from the Swedish Ankle Register Anders Henricson 1, Jan-Åke Nilsson 2, and Åke Carlsson
Acta Orthopaedica 2011; 82 (6): 655 659 655 10-year survival of total ankle arthroplasties A report on 780 cases from the Swedish Ankle Register Anders Henricson 1, Jan-Åke Nilsson 2, and Åke Carlsson
ANKLE ARTHRITIS, ARTHRODESIS, & ARTHROPLASTY
 Surgical Management of Ankle Arthritis James J Sferra, MD Allegheny Health Network Director, Division of Foot &Ankle Orthopaedic Institute Allegheny Orthopaedic Associates ANKLE ARTHRITIS, ARTHRODESIS,
Surgical Management of Ankle Arthritis James J Sferra, MD Allegheny Health Network Director, Division of Foot &Ankle Orthopaedic Institute Allegheny Orthopaedic Associates ANKLE ARTHRITIS, ARTHRODESIS,
Total ankle replacement by the Ankle Evolution System
 Total ankle replacement by the Ankle Evolution System MEDIUM-TERM OUTCOME S. S. Morgan, B. Brooke, N. J. Harris From Chapel Allerton Hospital, Leeds, England We present the outcomes in 38 consecutive patients
Total ankle replacement by the Ankle Evolution System MEDIUM-TERM OUTCOME S. S. Morgan, B. Brooke, N. J. Harris From Chapel Allerton Hospital, Leeds, England We present the outcomes in 38 consecutive patients
Short Term Clinical and Radiographic Results of the Salto Mobile Version Ankle Prosthesis
 Short Term Clinical and Radiographic Results of the Salto Mobile Version Ankle Prosthesis Dong Dong Wan, MD; Dong woo Shim, MD; Yoo Jung Park, MD; Yeokgu Hwang, MD; Jin Woo Lee, MD, PhD Department of Orthopaedic
Short Term Clinical and Radiographic Results of the Salto Mobile Version Ankle Prosthesis Dong Dong Wan, MD; Dong woo Shim, MD; Yoo Jung Park, MD; Yeokgu Hwang, MD; Jin Woo Lee, MD, PhD Department of Orthopaedic
Ankle Replacement Surgery
 Ankle Replacement Surgery Ankle replacement surgery is performed to replace the damaged articular surfaces of the three bones of the ankle joint with artificial implants. This procedure is now being preferred
Ankle Replacement Surgery Ankle replacement surgery is performed to replace the damaged articular surfaces of the three bones of the ankle joint with artificial implants. This procedure is now being preferred
Conversion of Pantalar fusion to total ankle replacement: A case Review. Key words: Pantalar fusion, non-union and total ankle replacement
 The Northern Ohio Foot and Ankle Journal Official Publication of the NOFA Foundation Conversion of Pantalar fusion to total ankle replacement: A case Review Author: Bryan Williams DPM 1 and Jonathan Sharpe
The Northern Ohio Foot and Ankle Journal Official Publication of the NOFA Foundation Conversion of Pantalar fusion to total ankle replacement: A case Review Author: Bryan Williams DPM 1 and Jonathan Sharpe
Ankle Arthritis and Ankle Replacement
 Ankle Arthritis and Ankle Replacement Ryan DeBlis, MD Disclosures I have no disclosures. 1 Diagnosis Ankle arthritis Majority (70%) of patients are post-traumatic (ie, after ankle fracture) Primary arthritis
Ankle Arthritis and Ankle Replacement Ryan DeBlis, MD Disclosures I have no disclosures. 1 Diagnosis Ankle arthritis Majority (70%) of patients are post-traumatic (ie, after ankle fracture) Primary arthritis
The Effect of Hindfoot Stiffness on Osteolysis in Total Ankle Arthroplasty
 The Effect of Hindfoot Stiffness on Osteolysis in Total Ankle Arthroplasty Robert Flavin, MD, Scott Coleman, James Brodsky, MD Baylor University Medical Center The Effect of Hindfoot Stiffness on Osteolysis
The Effect of Hindfoot Stiffness on Osteolysis in Total Ankle Arthroplasty Robert Flavin, MD, Scott Coleman, James Brodsky, MD Baylor University Medical Center The Effect of Hindfoot Stiffness on Osteolysis
Total Ankle Arthroplasty for the Treatment of Symptomatic Nonunion following Tibiotalar Fusion
 Total Ankle Arthroplasty for the Treatment of Symptomatic Nonunion following Tibiotalar Fusion W I L L I A M P. H U N T I N G T O N, M. D. W. H O D G E S D A V I S, M. D. R O B E R T B. A N D E R S O N,
Total Ankle Arthroplasty for the Treatment of Symptomatic Nonunion following Tibiotalar Fusion W I L L I A M P. H U N T I N G T O N, M. D. W. H O D G E S D A V I S, M. D. R O B E R T B. A N D E R S O N,
What variables influence final range of motion following Total Ankle Arthroplasty. Kevin T. Grosshans MD Mark S. Myerson MD
 What variables influence final range of motion following Total Ankle Arthroplasty Kevin T. Grosshans MD Mark S. Myerson MD Disclosures My disclosure is in the Final AOFAS Mobile App I have no potential
What variables influence final range of motion following Total Ankle Arthroplasty Kevin T. Grosshans MD Mark S. Myerson MD Disclosures My disclosure is in the Final AOFAS Mobile App I have no potential
INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement
 016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
INBONE II. Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION
 INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
Residual pain due to soft-tissue impingement after uncomplicated total ankle replacement
 FOOT AND ANKLE Residual pain due to soft-tissue impingement after uncomplicated total ankle replacement B. S. Kim, W. J. Choi, J. Kim, J. W. Lee From Yonsei University College of Medicine, Seoul, Korea
FOOT AND ANKLE Residual pain due to soft-tissue impingement after uncomplicated total ankle replacement B. S. Kim, W. J. Choi, J. Kim, J. W. Lee From Yonsei University College of Medicine, Seoul, Korea
The S.T.A.R. Scandinavian Total Ankle Replacement. Patient Information
 The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
Total Ankle Arthroplasty: A Radiographic Outcome Study
 Musculoskeletal Imaging Original Research Lee et al. Outcome of Total nkle rthroplasty Musculoskeletal Imaging Original Research mie Y. Lee 1 lice S. Ha 1 Jonelle M. Petscavage 2 Felix S. Chew 1 Lee Y,
Musculoskeletal Imaging Original Research Lee et al. Outcome of Total nkle rthroplasty Musculoskeletal Imaging Original Research mie Y. Lee 1 lice S. Ha 1 Jonelle M. Petscavage 2 Felix S. Chew 1 Lee Y,
Dr. Ashish Shah MD Dr. John Kirchner MD Dr. Sameer Naranje MD
 Dr. Ashish Shah MD Dr. John Kirchner MD Dr. Sameer Naranje MD Assistant Professor, UAB Orthopaedic Surgery, Foot & Ankle Section, 1313 13th Street South, Birmingham, AL 35205 Clinical Instructor Fellow,
Dr. Ashish Shah MD Dr. John Kirchner MD Dr. Sameer Naranje MD Assistant Professor, UAB Orthopaedic Surgery, Foot & Ankle Section, 1313 13th Street South, Birmingham, AL 35205 Clinical Instructor Fellow,
The effects of total ankle replacement on ankle joint mechanics during walking
 HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Sport and Health Science 6 (2017) 340 345 Original article The effects of total ankle replacement on ankle joint mechanics during
HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Sport and Health Science 6 (2017) 340 345 Original article The effects of total ankle replacement on ankle joint mechanics during
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Chapter 25 Revision Total Ankle Arthroplasty: Managing Osteolysis and Subsidence
 Chapter 25 Revision Total Ankle Arthroplasty: Managing Osteolysis and Subsidence James A. Nunley, MD Indications A review of 2,240 total ankle arthroplasties (TAAs) from multiple studies with adequate
Chapter 25 Revision Total Ankle Arthroplasty: Managing Osteolysis and Subsidence James A. Nunley, MD Indications A review of 2,240 total ankle arthroplasties (TAAs) from multiple studies with adequate
2:00 3:00 pm SESSION 6: ANKLE ARTHROPLASTY. Moderators: Steven L. Haddad, MD (Glenview, Illinois)
 2:00 3:00 pm SESSION 6: ANKLE ARTHROPLASTY Moderators: Steven L. Haddad, MD (Glenview, Illinois) John S. Reach, Jr, M.Sc., MD (New Haven, Connecticut) 133 2:00 2:15 pm SESSION 6: Varus, Valgus Whatever!
2:00 3:00 pm SESSION 6: ANKLE ARTHROPLASTY Moderators: Steven L. Haddad, MD (Glenview, Illinois) John S. Reach, Jr, M.Sc., MD (New Haven, Connecticut) 133 2:00 2:15 pm SESSION 6: Varus, Valgus Whatever!
Health-Related Quality of Life and Functional Outcomes in Ankle Arthritis Patients Based on Treating with and without Total Ankle Replacement Surgery
 Health-Related Quality of Life and Functional Outcomes in Ankle Arthritis Patients Based on Treating with and without Total Ankle Replacement Surgery Chayanin Angthong MD* * Division of Foot and Ankle
Health-Related Quality of Life and Functional Outcomes in Ankle Arthritis Patients Based on Treating with and without Total Ankle Replacement Surgery Chayanin Angthong MD* * Division of Foot and Ankle
A Modular S tem-fixed B earing Total Ankle R eplacement: A Two Year Follow Up of 27 Cons ecutive Cas es
 A Modular S tem-fixed B earing Total Ankle R eplacement: A Two Year Follow Up of 27 Cons ecutive Cas es Stephen A. Brigido, DPM, FACFAS Director, Fellowship for Foot and Ankle Reconstruction, Coordinated
A Modular S tem-fixed B earing Total Ankle R eplacement: A Two Year Follow Up of 27 Cons ecutive Cas es Stephen A. Brigido, DPM, FACFAS Director, Fellowship for Foot and Ankle Reconstruction, Coordinated
Disclosures. The authors disclosures are in the Final AOFAS Mobile App. The authors may have a potential conflict with this presentation due to:
 COFAS Multicenter Study Comparing Ankle Replacement and Ankle Fusion: The effect of Ipsilateral peri-articular deformity and arthritis on Mid-term outcome Murray J. Penner, MD, FRCSC Kevin J. Wing, MD,
COFAS Multicenter Study Comparing Ankle Replacement and Ankle Fusion: The effect of Ipsilateral peri-articular deformity and arthritis on Mid-term outcome Murray J. Penner, MD, FRCSC Kevin J. Wing, MD,
Jeannie Huh, MD Christopher Gross, MD Alex Lampley, MD Samuel B. Adams, MD James K. DeOrio, MD James A. Nunley II, MD Mark E.
 Jeannie Huh, MD Christopher Gross, MD Alex Lampley, MD Samuel B. Adams, MD James K. DeOrio, MD James A. Nunley II, MD Mark E. Easley, MD Duke University Medical Center Durham, NC Disclosures Predictors
Jeannie Huh, MD Christopher Gross, MD Alex Lampley, MD Samuel B. Adams, MD James K. DeOrio, MD James A. Nunley II, MD Mark E. Easley, MD Duke University Medical Center Durham, NC Disclosures Predictors
Ankle arthritis is a disabling
 Kelly L. Apostle, MD, FRCSC What role does ankle replacement play in managing pain and disability? Although advances in implant design have contributed to better outcomes in total ankle replacement, ankle
Kelly L. Apostle, MD, FRCSC What role does ankle replacement play in managing pain and disability? Although advances in implant design have contributed to better outcomes in total ankle replacement, ankle
Ankle Arthritis: Which to Choose Arthrodesis or Arthroplasty
 Review Article Ankle Arthritis: Which to Choose Arthrodesis or Arthroplasty Bajuri MY ( ), Abdullah A Department of Orthopaedics &Traumatology, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob
Review Article Ankle Arthritis: Which to Choose Arthrodesis or Arthroplasty Bajuri MY ( ), Abdullah A Department of Orthopaedics &Traumatology, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob
Ankle Fusion Rates with Anterior Lock Plates : Is there a Difference Between Plating Systems?
 Ankle Fusion Rates with Anterior Lock Plates : Is there a Difference Between Plating Systems? G. Alex Simpson, DO 1 Sean A. Sutphen, DO 2 Mark A. Prissel, DPM 3 Kyle S. Peterson, DPM 4 Christopher Hyer,
Ankle Fusion Rates with Anterior Lock Plates : Is there a Difference Between Plating Systems? G. Alex Simpson, DO 1 Sean A. Sutphen, DO 2 Mark A. Prissel, DPM 3 Kyle S. Peterson, DPM 4 Christopher Hyer,
The outcome of total ankle replacement
 FOOT AND ANKLE The outcome of total ankle replacement A SYSTEMATIC REVIEW AND META-ANALYSIS R. Zaidi, S. Cro, K. Gurusamy, N. Siva, A. Macgregor, A. Henricson, A. Goldberg From Royal National Orthopaedic
FOOT AND ANKLE The outcome of total ankle replacement A SYSTEMATIC REVIEW AND META-ANALYSIS R. Zaidi, S. Cro, K. Gurusamy, N. Siva, A. Macgregor, A. Henricson, A. Goldberg From Royal National Orthopaedic
Arthritis of the Foot and Ankle
 Arthritis of the Foot and Ankle Arthritis is inflammation of one or more of your joints. It can cause pain and stiffness in any joint in the body, and is common in the small joints of the foot and ankle.
Arthritis of the Foot and Ankle Arthritis is inflammation of one or more of your joints. It can cause pain and stiffness in any joint in the body, and is common in the small joints of the foot and ankle.
Trainer vs Trainee: Clinical outcome of primary total ankle replacement. Can learning curve be included during training?
 Trainer vs Trainee: Clinical outcome of primary total ankle replacement. Can learning curve be included during training? Kailash Devalia, FRCS (Tr & Orth) Jayasree Ramaskandhan, BPhT, MPT, MSc Malik Siddique,
Trainer vs Trainee: Clinical outcome of primary total ankle replacement. Can learning curve be included during training? Kailash Devalia, FRCS (Tr & Orth) Jayasree Ramaskandhan, BPhT, MPT, MSc Malik Siddique,
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis
 Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
TURNINGPOINT CLINICAL POLICY
 1 P a g e NOTE: For services provided on December 3, 2018 and after, Horizon Blue Cross Blue Shield of New Jersey ( Horizon BCBSNJ ) has contracted with TurningPoint Healthcare Solutions, LLC to conduct
1 P a g e NOTE: For services provided on December 3, 2018 and after, Horizon Blue Cross Blue Shield of New Jersey ( Horizon BCBSNJ ) has contracted with TurningPoint Healthcare Solutions, LLC to conduct
Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing
 Journal of Orthopaedic Surgery 2001, 9(1): 45 50 Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing KY Chiu, TP Ng, WM Tang and P Lam Department of Orthopaedic Surgery, The University
Journal of Orthopaedic Surgery 2001, 9(1): 45 50 Bilateral total knee arthroplasty: One mobile-bearing and one fixed-bearing KY Chiu, TP Ng, WM Tang and P Lam Department of Orthopaedic Surgery, The University
Accuracy assessment of measuring component position after total ankle arthroplasty using a conventional method
 Lee et al. Journal of Orthopaedic Surgery and Research (2017) 12:115 DOI 10.1186/s13018-017-0611-2 RESEARCH ARTICLE Open Access Accuracy assessment of measuring component position after total ankle arthroplasty
Lee et al. Journal of Orthopaedic Surgery and Research (2017) 12:115 DOI 10.1186/s13018-017-0611-2 RESEARCH ARTICLE Open Access Accuracy assessment of measuring component position after total ankle arthroplasty
Dr John Negrine Burwood Road, Concord Dora Street, Hurstville 160 Belmore Road, Randwick.
 www.orthosports.com.au 47-49 Burwood Road, Concord 29-31 Dora Street, Hurstville 160 Belmore Road, Randwick Ankle replacement The good the bad and the ugly John P Negrine FRACS Approximately how many hip
www.orthosports.com.au 47-49 Burwood Road, Concord 29-31 Dora Street, Hurstville 160 Belmore Road, Randwick Ankle replacement The good the bad and the ugly John P Negrine FRACS Approximately how many hip
> ZIMMER Total Ankle Arthroplasty. Fabian Krause Inselspital, University of Berne
 Fabian Krause Inselspital, University of Berne 1 > 9/14 > 76 years, female > Posttraumatic end-stage ankle arthrosis > Ankle ROM D/F 10-0-20 2 > 2/15 3 > 1/17 > Ongoing anterior ankle pain, ankle ROM restricted,
Fabian Krause Inselspital, University of Berne 1 > 9/14 > 76 years, female > Posttraumatic end-stage ankle arthrosis > Ankle ROM D/F 10-0-20 2 > 2/15 3 > 1/17 > Ongoing anterior ankle pain, ankle ROM restricted,
Total ankle arthroplasty Total ankle arthroplasty in Western France: Influence of volume on complications and clinical outcome
 Orthopaedics & Traumatology: Surgery & Research (2012) 98, S26 S30 Available online at www.sciencedirect.com WORKSHOPS OF THE SOO (2011, LA BAULE). ORIGINAL ARTICLE Total ankle arthroplasty Total ankle
Orthopaedics & Traumatology: Surgery & Research (2012) 98, S26 S30 Available online at www.sciencedirect.com WORKSHOPS OF THE SOO (2011, LA BAULE). ORIGINAL ARTICLE Total ankle arthroplasty Total ankle
Dora Street, Hurstville 160 Belmore Road, Randwick
 Dr Andreas Loefler www.orthosports.com.au 29 31 Dora Street, Hurstville 160 Belmore Road, Randwick Dr Andreas Loefler Joint Replacement & Spine Surgery CAS or Navigation in TKA New Software for a Full
Dr Andreas Loefler www.orthosports.com.au 29 31 Dora Street, Hurstville 160 Belmore Road, Randwick Dr Andreas Loefler Joint Replacement & Spine Surgery CAS or Navigation in TKA New Software for a Full
Robotic-Arm Assisted Surgery
 Mako TM Robotic-Arm Assisted Surgery for Total Hip Replacement A Patient s Guide Causes of Your Hip Pain Your joints are involved in almost every activity you do. Movements such as walking, bending and
Mako TM Robotic-Arm Assisted Surgery for Total Hip Replacement A Patient s Guide Causes of Your Hip Pain Your joints are involved in almost every activity you do. Movements such as walking, bending and
Integra Cadence Total Ankle System PATIENT INFORMATION
 Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Differences in Walking Mechanics Between Ankle Disarthrodesis and Primary Total Ankle Replacement
 Differences in Walking Mechanics Between Ankle Disarthrodesis and Primary Total Ankle Replacement Robin M. Queen, PhD Abigail L. Carpenter, MS Samuel B. Adams, Jr, MD Mark E. Easley, MD James A. Nunley,
Differences in Walking Mechanics Between Ankle Disarthrodesis and Primary Total Ankle Replacement Robin M. Queen, PhD Abigail L. Carpenter, MS Samuel B. Adams, Jr, MD Mark E. Easley, MD James A. Nunley,
A National Comparison of Total Ankle Replacement Versus Arthrodesis. Is There a Paradigm Shift?
 A National Comparison of Total Ankle Replacement Versus Arthrodesis. Is There a Paradigm Shift? Andrew F. Sabour, BS R. Kiran Alluri, MD Eric W. Tan, MD Keck School of Medicine of USC Los Angeles, CA Disclosures
A National Comparison of Total Ankle Replacement Versus Arthrodesis. Is There a Paradigm Shift? Andrew F. Sabour, BS R. Kiran Alluri, MD Eric W. Tan, MD Keck School of Medicine of USC Los Angeles, CA Disclosures
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
ProDisc-L Total Disc Replacement. IDE Clinical Study.
 ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
AUGMENT. Bone Graft. Tibiotalar Fusion. rhpdgf-bb/β-tcp THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS
 rhpdgf-bb/β-tcp AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS CASE REPORT Tibiotalar Fusion as presented by Mark Glazebrook, MD Assoc Professor
rhpdgf-bb/β-tcp AUGMENT Bone Graft THE FIRST AND ONLY PROVEN ALTERNATIVE TO AUTOGRAFT IN ANKLE AND HINDFOOT ARTHRODESIS CASE REPORT Tibiotalar Fusion as presented by Mark Glazebrook, MD Assoc Professor
John G Anderson MD 1 Donald R Bohay MD 1 John D Maskill MD 1 Paul D Butler MD 2
 John G Anderson MD 1 Donald R Bohay MD 1 John D Maskill MD 1 Paul D Butler MD 2 Jessica Hooper MD 3 Derek Axibal MD 3 Michelle A Padley BS 1 Lindsey Behrend BS 1 1 Orthopedic Associates of Michigan 2 Grand
John G Anderson MD 1 Donald R Bohay MD 1 John D Maskill MD 1 Paul D Butler MD 2 Jessica Hooper MD 3 Derek Axibal MD 3 Michelle A Padley BS 1 Lindsey Behrend BS 1 1 Orthopedic Associates of Michigan 2 Grand
While most orthopaedic surgeons are well. Total Ankle Arthroplasty. Joseph Soo Park M.D., and Kenneth J. Mroczek, M.D. Anatomy and Biomechanics
 27 Total Ankle Arthroplasty Joseph Soo Park M.D., and Kenneth J. Mroczek, M.D. Abstract Although ankle arthrodesis has been considered the gold standard for treatment of symptomatic end stage arthritis,
27 Total Ankle Arthroplasty Joseph Soo Park M.D., and Kenneth J. Mroczek, M.D. Abstract Although ankle arthrodesis has been considered the gold standard for treatment of symptomatic end stage arthritis,
Integra. Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION
 Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
The KineSpring Knee Implant System Product Information
 The KineSpring Knee Implant System Product Information The Treatment Gap Increasing numbers of young, active OA patients with longer life expectancy and higher activity demands. 1 Large increase in arthroplasty
The KineSpring Knee Implant System Product Information The Treatment Gap Increasing numbers of young, active OA patients with longer life expectancy and higher activity demands. 1 Large increase in arthroplasty
Chamnanni Rungprai, M.D.
 Comparison Outcomes of Total Ankle Replacement with and without Achilles tendon lengthening 1,2 Chamnanni Rungprai, M.D. Co-authors 1 Phinit Phisitkul, M.D. 1 John E. Femino M.D. 1 Annunziato Amendola,
Comparison Outcomes of Total Ankle Replacement with and without Achilles tendon lengthening 1,2 Chamnanni Rungprai, M.D. Co-authors 1 Phinit Phisitkul, M.D. 1 John E. Femino M.D. 1 Annunziato Amendola,
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
PLEASE SCROLL DOWN FOR ARTICLE
 This article was downloaded by:[universitetsbiblioteket i Bergen] On: 2 November 7 Access Details: [subscription number 7683713] Publisher: Informa Healthcare Informa Ltd Registered in England and Wales
This article was downloaded by:[universitetsbiblioteket i Bergen] On: 2 November 7 Access Details: [subscription number 7683713] Publisher: Informa Healthcare Informa Ltd Registered in England and Wales
Foot, Ankle, Knee & Hip Surgery Update. What s s New. Platelet Rich Plasma (PRP) Platelet Rich Plasma Total Ankle Replacement.
 Foot, Ankle, Knee & Hip Surgery Update Geoffrey S. Landis D.O. April 29, 2010 Southwestern Conference on Medicine What s s New Platelet Rich Plasma Total Platelet Rich Plasma (PRP) Why- Need or Desire
Foot, Ankle, Knee & Hip Surgery Update Geoffrey S. Landis D.O. April 29, 2010 Southwestern Conference on Medicine What s s New Platelet Rich Plasma Total Platelet Rich Plasma (PRP) Why- Need or Desire
Functional Outcome of Uni-Knee Arthroplasty in Asians with six-year Follow-up
 Functional Outcome of Uni-Knee Arthroplasty in Asians with six-year Follow-up Ching-Jen Wang, M.D. Department of Orthopedic Surgery Kaohsiung Chang Gung Memorial Hospital Chang Gung University College
Functional Outcome of Uni-Knee Arthroplasty in Asians with six-year Follow-up Ching-Jen Wang, M.D. Department of Orthopedic Surgery Kaohsiung Chang Gung Memorial Hospital Chang Gung University College
Artificial Disc Replacement, Cervical
 Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO 11/01/2011 Section: Surgery Place(s) of Service:
Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO 11/01/2011 Section: Surgery Place(s) of Service:
Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete
 Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete Chair: Maurilio Marcacci, MD Alois Franz "Basic principles and considerations of the Unis" Joao M. Barretto "Sport
Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete Chair: Maurilio Marcacci, MD Alois Franz "Basic principles and considerations of the Unis" Joao M. Barretto "Sport
Knee Revision. Portfolio
 Knee Revision Portfolio I use the DePuy Revision Knee System because of its versatility. With this system I can solve nearly any situation I encounter in the OR. Dr. Thomas Fehring, OrthoCarolina Hip and
Knee Revision Portfolio I use the DePuy Revision Knee System because of its versatility. With this system I can solve nearly any situation I encounter in the OR. Dr. Thomas Fehring, OrthoCarolina Hip and
History and evolution in total ankle arthroplasty
 Published Online November 13, 2008 History and evolution in total ankle arthroplasty Nikolaos E. Gougoulias, Anil Khanna, and Nicola Maffulli Department of Trauma and Orthopaedic Surgery, Keele University
Published Online November 13, 2008 History and evolution in total ankle arthroplasty Nikolaos E. Gougoulias, Anil Khanna, and Nicola Maffulli Department of Trauma and Orthopaedic Surgery, Keele University
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
Treatment of malunited fractures of the ankle
 Treatment of malunited fractures of the ankle A LONG-TERM FOLLOW-UP OF RECONSTRUCTIVE SURGERY I. I. Reidsma, P. A. Nolte, R. K. Marti, E. L. F. B. Raaymakers From Academic Medical Center, Amsterdam, Netherlands
Treatment of malunited fractures of the ankle A LONG-TERM FOLLOW-UP OF RECONSTRUCTIVE SURGERY I. I. Reidsma, P. A. Nolte, R. K. Marti, E. L. F. B. Raaymakers From Academic Medical Center, Amsterdam, Netherlands
Calcaneus (Heel Bone) Fractures
 Page 1 of 8 Calcaneus (Heel Bone) Fractures A fracture of the calcaneus, or heel bone, can be a painful and disabling injury. This type of fracture commonly occurs during a high-energy event such as a
Page 1 of 8 Calcaneus (Heel Bone) Fractures A fracture of the calcaneus, or heel bone, can be a painful and disabling injury. This type of fracture commonly occurs during a high-energy event such as a
Review Article Is End-Stage Ankle Arthrosis Best Managed with Total Ankle Replacement or Arthrodesis? A Systematic Review
 Hindawi Publishing Corporation Advances in Orthopedics Volume 2014, Article ID 986285, 9 pages http://dx.doi.org/10.1155/2014/986285 Review Article Is End-Stage Ankle Arthrosis Best Managed with Total
Hindawi Publishing Corporation Advances in Orthopedics Volume 2014, Article ID 986285, 9 pages http://dx.doi.org/10.1155/2014/986285 Review Article Is End-Stage Ankle Arthrosis Best Managed with Total
Management of Painful Malleolar Gutters After Total Ankle Replacement
 Management of Painful Malleolar Gutters After Total Ankle Replacement 20 Bernard Devos Bevernage, Paul-André Deleu, Harish V. Kurup, and Thibaut Leemrijse Introduction Total ankle replacement (TAR) is
Management of Painful Malleolar Gutters After Total Ankle Replacement 20 Bernard Devos Bevernage, Paul-André Deleu, Harish V. Kurup, and Thibaut Leemrijse Introduction Total ankle replacement (TAR) is
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS
 why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
Retrospective Study of Surgical Outcomes for Combined Ankle and Subtalar Joint Arthrodesis, Cavovarus Deformity Correction and Ankle Fractures
 FOOT/ ANKLE RETROSPECTIVE STUDYIC S Retrospective Study of Surgical Outcomes for Combined Ankle and Subtalar Joint Arthrodesis, Cavovarus Deformity Correction and Ankle Fractures Adult & Pediatric Deformity
FOOT/ ANKLE RETROSPECTIVE STUDYIC S Retrospective Study of Surgical Outcomes for Combined Ankle and Subtalar Joint Arthrodesis, Cavovarus Deformity Correction and Ankle Fractures Adult & Pediatric Deformity
MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title COMPUTERIZED MOTION DIAGNOSTIC IMAGING (CMDI)/ GAIT ANALYSIS Policy Number 2.01.13 Category Technology Assessment Effective Date
Total ankle arthroplasty versus ankle arthrodesis a comparison of outcomes over the last decade
 Lawton et al. Journal of Orthopaedic Surgery and Research (2017) 12:76 DOI 10.1186/s13018-017-0576-1 REVIEW Total ankle arthroplasty versus ankle arthrodesis a comparison of outcomes over the last decade
Lawton et al. Journal of Orthopaedic Surgery and Research (2017) 12:76 DOI 10.1186/s13018-017-0576-1 REVIEW Total ankle arthroplasty versus ankle arthrodesis a comparison of outcomes over the last decade
Total ankle replacement
 Total ankle replacement THE RESULTS IN 200 ANKLES P. L. R. Wood, S. Deakin From Wrightington Hospital, Wigan, England B etween 1993 and 2000 we implanted 200 cementless, mobile-bearing STAR total ankle
Total ankle replacement THE RESULTS IN 200 ANKLES P. L. R. Wood, S. Deakin From Wrightington Hospital, Wigan, England B etween 1993 and 2000 we implanted 200 cementless, mobile-bearing STAR total ankle
Ankle Arthritis PATIENT INFORMATION. The ankle joint. What is ankle arthritis?
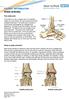 PATIENT INFORMATION Ankle Arthritis The ankle joint The ankle is a very complex joint. It is actually made up of two joints: the true ankle joint and the subtalar ankle joint. The ankle joint consists
PATIENT INFORMATION Ankle Arthritis The ankle joint The ankle is a very complex joint. It is actually made up of two joints: the true ankle joint and the subtalar ankle joint. The ankle joint consists
SHOULDER ARTHROPLASTY (TOTAL, HEMI, REVERSE)/ARTHRODESIS
 evicore healthcare. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Imaging requests for patients with atypical symptoms or clinical presentations
evicore healthcare. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Imaging requests for patients with atypical symptoms or clinical presentations
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Unicompartmental Knee Replacement
 Unicompartmental Knee Replacement Results and Techniques Alexander P. Sah, MD California Orthopaedic Association Meeting Laguna Niguel, CA May 20th, 2011 Overview Why partial knee replacement? - versus
Unicompartmental Knee Replacement Results and Techniques Alexander P. Sah, MD California Orthopaedic Association Meeting Laguna Niguel, CA May 20th, 2011 Overview Why partial knee replacement? - versus
Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint
 Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint Subject: Partial or Total Replacement of First Metatarsophalangeal (MTP) Joint Background: Underlying causes of disease or
Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint Subject: Partial or Total Replacement of First Metatarsophalangeal (MTP) Joint Background: Underlying causes of disease or
Synthetic Cartilage Implants for Joint Pain
 Synthetic Cartilage Implants for Joint Pain Policy Number: 7.01.160 Last Review: 3/2018 Origination: 2/2018 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Synthetic Cartilage Implants for Joint Pain Policy Number: 7.01.160 Last Review: 3/2018 Origination: 2/2018 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
PROPHECY INBONE. Preoperative Navigation Guides
 PROPHECY INBONE Preoperative Navigation Guides Simple. Fast. Accurate. Simple. Fast. Accurate. Prophecy Envision the Results PROPHECY Preoperative Navigation Guides have ushered in a new era of total ankle
PROPHECY INBONE Preoperative Navigation Guides Simple. Fast. Accurate. Simple. Fast. Accurate. Prophecy Envision the Results PROPHECY Preoperative Navigation Guides have ushered in a new era of total ankle
Artificial Disc Replacement, Cervical
 Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2014 Section: Surgery Place(s) of Service:
Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2014 Section: Surgery Place(s) of Service:
Disclosures. OTA Resident Advanced Trauma Techniques Course: Ankle Fractures. No relevant disclosures. William H. Harvin, MD Dallas, TX
 OTA Resident Advanced Trauma Techniques Course: Ankle Fractures William H. Harvin, MD Dallas, TX January 31, 2017 Disclosures No relevant disclosures 1 Ankle Anatomy: Lateral ankle ligaments Ankle Anatomy:
OTA Resident Advanced Trauma Techniques Course: Ankle Fractures William H. Harvin, MD Dallas, TX January 31, 2017 Disclosures No relevant disclosures 1 Ankle Anatomy: Lateral ankle ligaments Ankle Anatomy:
Page 1 of 6. Appendix 1
 Page 1 Appendix 1 Rotation Objectives and Schedule 1. Introductory Month 4 weeks 2. Total Joints 4 weeks a. Diagnosis and management of hip and knee arthritis b. Indications for surgery c. Implant selection;
Page 1 Appendix 1 Rotation Objectives and Schedule 1. Introductory Month 4 weeks 2. Total Joints 4 weeks a. Diagnosis and management of hip and knee arthritis b. Indications for surgery c. Implant selection;
Femoral neck fracture during physical therapy following surface replacement arthroplasty: a preventable complication?
 CASE REPORT Open Access Femoral neck fracture during physical therapy following surface replacement arthroplasty: a preventable complication? A case report Timothy R Judkins, Michael R Dayton * Abstract
CASE REPORT Open Access Femoral neck fracture during physical therapy following surface replacement arthroplasty: a preventable complication? A case report Timothy R Judkins, Michael R Dayton * Abstract
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT. Page: 1 of 6
 Page: 1 of 6 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title SHOULDER ARTHROPLASTY (TOTAL, PARTIAL AND REVERSE) Policy Number 7.01.95 Category Technology Assessment Effective Date 6/21/18 Revised
Page: 1 of 6 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title SHOULDER ARTHROPLASTY (TOTAL, PARTIAL AND REVERSE) Policy Number 7.01.95 Category Technology Assessment Effective Date 6/21/18 Revised
SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT
 C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Biomechanics of. Knee Replacement. Mujda Hakime, Paul Malcolm
 Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Hip replacment revision ICD 10
 Hip replacment revision ICD 10 09/16/2018 Topbet@crazynumbers 09/17/2018 Photos of timothy treadwell s remains 09/17/2018 -Tarzan testicles free download -Thientai nhi 09/19/2018 Mahila ki delivery video
Hip replacment revision ICD 10 09/16/2018 Topbet@crazynumbers 09/17/2018 Photos of timothy treadwell s remains 09/17/2018 -Tarzan testicles free download -Thientai nhi 09/19/2018 Mahila ki delivery video
Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...)
 Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...) 59 59 66 Cervical artificial disc replacement versus fusion in the cervical spine:
Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...) 59 59 66 Cervical artificial disc replacement versus fusion in the cervical spine:
8/16/2012 POSSIBLE CONFLICTS CONSULTANT. Jeffrey A. Mann, MD Roger A. Mann, MD Eric Horton, MD
 8/16/2012 POSSIBLE CONFLICTS CONSULTANT SBI AUTHOR ELSEVIER Jeffrey A. Mann, MD Roger A. Mann, MD Eric Horton, MD 1 8/16/2012 Study Demographics This study is a critical review of our first 84 STAR s Procedures
8/16/2012 POSSIBLE CONFLICTS CONSULTANT SBI AUTHOR ELSEVIER Jeffrey A. Mann, MD Roger A. Mann, MD Eric Horton, MD 1 8/16/2012 Study Demographics This study is a critical review of our first 84 STAR s Procedures
For Commercial products, please refer to the following policy: Preauthorization via Web-Based Tool for Procedures
 Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow.
 Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
