Cost-effectiveness of Serotesting Compared with Universal Immunization for Varicella in Refugee Children from Six Geographic Regions
|
|
|
- Juniper Wilcox
- 5 years ago
- Views:
Transcription
1 ORIGINAL ARTICLES Cost-effectiveness of Serotesting Compared with Universal Immunization for Varicella in Refugee Children from Six Geographic Regions Marisol Figueira, Demian Christiansen, and Elizabeth D. Barnett Background: It is unknown whether it is more cost-effective to test for varicella antibody or to immunize without testing in immigrant populations. The reliability of history of varicella disease is also unclear. Methods: The prevalences of varicella antibody in immigrant children from six regions of the world were used in a costeffectiveness model to calculate the antibody prevalence above which it is more cost-effective to test rather than to immunize. History of varicella disease was obtained from chart review. We calculated the positive and negative predictive values of varicella history by age group and region. Results: The prevalence of varicella antibody above which it is more cost-effective to test than to immunize was 34% for children less than 13 years old and 17% for those aged 13 years and older. Overall, the positive predictive value of varicella history was % and the negative predictive value of varicella history was 28 66% among the six geographic regions. Conclusion: Immunization without serotesting was cost-effective in children 5 years of age. Testing prior to immunization was cost-effective in children 5 years of age and older. History of varicella was a good predictor of the presence of antibody to varicella, whereas a negative history was a poor predictor of the absence of antibody to varicella. Immigrant children entering the United States without immunization records, or with records that are not up-to-date by current US immunization standards, must be immunized in order to enter school and are therefore at risk of receiving duplicate or unnecessary vaccines. 1,2 Current practice is to immunize individuals Marisol Figueira, MD: Maxwell Finland Laboratory for Infectious Diseases, Boston Medical Center; Demian Christiansen, MPH: Data Coordinating Center, Boston University School of Public Health; Elizabeth D. Barnett, MD: Maxwell Finland Laboratory for Infectious Diseases, Boston Medical Center, Boston, MA, USA. This study was presented in part at the 6th Conference of the International Society of Travel Medicine, Montreal, Quebec, Canada, 1999, and at the Pediatric Academic Societies and the American Academy of Pediatrics Joint Meeting, Boston, Massachusetts, This study was funded in part by the Joel and Barbara Alpert Children of the City Endowment and the Alden Trust. Apart from this, the authors had no financial or other conflicts of interest to disclose. Correspondence: Marisol Figueira, MD, Maxwell Finland Laboratory for Infectious Diseases, Room 507, Boston Medical Center, 774 Albany Street, Boston, MA 02118, USA J Travel Med 2003; 10: without immunization records rather than test for immunity to vaccine-preventable diseases. Unnecessary immunization may result in additional cost, 3 risk of vaccine adverse events, 4 and inconvenience to families, and is a wasteful strategy in an era plagued by vaccine shortages. There are, to date, no analyses of the cost-effectiveness of serotesting versus presumptive immunization in immigrant populations. Detailed age- and region-specific data on the seroprevalence of vaccine-preventable diseases may be unavailable.in addition,formal cost-effectiveness analyses require precise clinical, epidemiologic and economic information, some or all of which may not be available for immigrant populations. 5 We were interested in determining the most costeffective approach to protecting newly arrived refugee children against varicella by finding the most appropriate strategy combining serotesting and immunization. We chose varicella because: the majority of refugee children who attended our clinic had not received varicella vaccine; we had studied the age-specific varicella seroprevalence for these refugee children; 6 and,in the United States,the only vaccine available is a relatively expensive singleantigen vaccine. We also studied the reliability of varicella history in refugee children, and the feasibility of using history alone to assess the presence or absence of antibody to varicella. 203
2 204 Journal of Travel Medicine, Volume 10, Number 4 Methods Study Population Six hundred and thirty-seven refugee children, 1 to 20 years of age, from six geographic regions (former Yugoslavia, Kosovo, East Africa, Vietnam, Iraq/Kurdistan, and the Caribbean) were seen between March 1996 and January 2000 for a two-visit refugee health assessment at the International Clinic at Boston Medical Center. We recorded demographic information for each subject, including date of birth, birthplace, sex, history of varicella disease, and history of previous immunization. The Institutional Review Board at Boston Medical Center approved this study. The prevalence of varicella antibody from these refugee children was calculated and described previously. 6 We divided children into three age groups (1 to 4, 5 to 12 and 13 to 20 years) based on two factors:first,children 13 years of age and older require two doses of varicella vaccine; second, there was a notable difference in the agespecific prevalence of varicella between children less than 5 years of age and those 5 years of age and older.children less than 1 year of age were excluded because of the presence of maternal antibody to varicella and because varicella vaccine is licensed for children 1 year of age and older. We classified patients who reported an uncertain history of varicella as having a negative history. Patients for whom a history of varicella was not recorded were excluded from the calculation of positive and negative predictive value. Cost-effectiveness Analysis Cost-effectiveness analysis of serotesting versus universal immunization was performed using a model described by Plans Rubio. 5 We used this model to calculate a critical value of prevalence of antibody (p*), defined as the antibody prevalence above which it is more cost-effective to test for antibody than to immunize without testing. The equation used in the model is: S p* V [(1 PV)DAEC] where S is the screening cost for varicella antibody, V is the vaccination cost, PV is the positive predictive value of the ELISA test, C is the vaccine compliance, D is the mean cost of disease, A is the attack rate, and E is the vaccine efficacy. The screening cost for varicella antibody was $17 at Boston Medical Center, and the vaccination cost, based on the CDC contract price of varicella vaccine plus the cost of vaccine administration,was $49.78 per dose. 7 The positive predictive value (PV) of the ELISA test was 98% (Diamedix Corp., Miami, FL, USA). Vaccine compliance for the first dose was 100%; for the second dose, compliance was assumed to be equivalent to attendance at follow-up visits in our clinic (96%). We assumed a mean disease cost of varicella in children 13 years of age of $37, and for those 13 years of age, $42. 8 We assumed an attack rate of 90% in susceptible individuals, vaccine efficacy of one dose of vaccine in children 13 years of age of %, and vaccine efficacy of one dose of vaccine in children 13 years of age of 89%. 8 Statistical Methods We calculated the prevalence of antibody to varicella and 95% confidence intervals (95% CI) for each age group and region of origin using SAS v8.12 (Cary,NC,USA). We calculated the PPV and negative predictive value (NPV) of varicella history with 95% CI for each age group and region. Results Six hundred and thirty-seven refugee children were tested from six regions: 274 from former Yugoslavia, 97 from Kosovo, 155 from East Africa, 40 from Vietnam, 36 from Iraq/Kurdistan, and 35 from the Caribbean. The critical value of antibody prevalence (p*) was 34% in children < 13 years old, who required only one dose of varicella vaccine,and 17% in children 13 years old, who required two doses of varicella vaccine (Fig.). The prevalence of varicella antibody was less than 34% in children 1 to 4 years of age;it was therefore costeffective to immunize without serotesting this group of children. In children 5 to 12 years of age, the prevalence of varicella antibody by region was greater than 34%; thus, serotesting before immunizing was cost-effective. Interpretation of the data from Vietnamese children was limited by small sample size in this age group. In children 13 to 20 years of age,the prevalence of varicella antibody was greater than 17%; therefore, serotesting before immunizing was cost-effective. Sensitivity Analysis The critical value of antibody prevalence is sensitive primarily to variation in screening cost (S), vaccination cost (V) and compliance (C). It is less sensitive to variations in epidemiologic parameters. In children less than 13 years of age (who require one dose of vaccine), if the vaccine cost remains stable at $50 and the screening test cost is increased from $17 to $40, p* increases from 34% to 80%. Likewise, p* increases from 34% to 80% if the screening test cost remains constant at $17 and the vaccine cost is decreased from $50 to $ In both cases, the best strategy would be
3 Figueira et al., Cost-Effectiveness of Serotesting Compared with Universal Immunization % 95% 90% 85% 80% 75% 70% 65% 60% 55% 50% 45% 40% Serotest 35% 30% 25% Immunize 20% 15% 10% 5% 0% (n = 119) 5 12 (n = 301) (n = 217) Age group (years) Prevalence of antibody Former Yugoslavia East Africa Iraq/Kurdistan Kosovo Caribbean Vietnam Figure Prevalence of varicella antibody by age and region with the critical value of prevalence superimposed.
4 206 Journal of Travel Medicine, Volume 10, Number 4 to immunize for varicella rather than to serotest children less than 13 years of age. For children older than 13 years, if the vaccine cost remains stable at $100 ($50 each for two doses of vaccine) and the screening test cost is increased from $17 to $40, p* increases from 17% to 40%.The vaccine cost must be decreased from $100 to $21.50 for two doses of vaccine in order for p* to increase from 17% to 40% when the screening test cost remains constant at $17. The best strategy remains serotesting children older than 13 years of age rather than universally immunizing. For immunization without testing for antibody to be cost-effective in children 13 years of age and older, either the vaccine cost must be very low or the screening test cost very high.for example,to arrive at a critical value of prevalence of 95% or greater, at a vaccine cost of $50 per dose, the screening test cost would have to rise from $17 to $95 or more. Similarly, if the screening test cost remained $17, the vaccine cost would have to decrease from $50 to $9.10 per dose. PPV and NPV of History of Varicella History of varicella was available for 361/637 (56%) charts reviewed. One hundred and sixty-three (45%) reported a positive history of varicella, 166 (46%) reported a negative history of varicella, and 32 (9%) reported an uncertain history of varicella (classified as negative history). The Table shows the PPV (probability of positive serology when history is positive) and NPV (probability of negative serology when history is negative) of varicella history with 95% CI by age group and region of origin. The PPV of a positive history was high among children in all regions except Vietnam. The NPV of a negative history was a poor indicator of the absence of varicella antibodies. Discussion Ideally, policy decisions about new health programs should be based not only on clinical effectiveness, but also on cost-effectiveness. 9 We were interested in developing a policy for varicella immunization for refugee and immigrant children that would limit the number of unnecessary immunizations in a cost-effective way. Our results showed that the most cost-effective strategy for protecting children against varicella is to immunize children under 5 years of age without serotesting, and to serotest children 5 years of age and older prior to immunizing. The diverse geographic, climatic and socioeconomic situations from which our patients come suggest that generalizing to other immigrant populations may be possible. This strategy has the disadvantage that susceptible children will remain susceptible while awaiting results of serotesting, although this will become of less importance as the prevalence of varicella in the United States decreases. Finally, this strategy will be less desirable if children are unlikely to return for a second visit. A negative or uncertain history of varicella is a poor predictor of absence of immunity to varicella in refugee children. The NPV of varicella history ranged between 28% and 66% among children from the six geographic regions studied, similar to results of studies in US populations. Lieu et al found that varicella antibody prevalence in US children 7 to 12 years of age with negative and uncertain histories of varicella ranged from 9% in 7-year-olds to 68% in 11-year-olds. They concluded that serotesting of patients with negative or uncertain histories of varicella would be more costeffective than presumptive vaccination in children 9 to 12 years old, where varicella seroprevalence was greater than 48%. 10 Others have reported that up to 80% of adolescents with negative and uncertain histories of varicella have positive varicella serology, which supports serotesting for previously unknown exposure to varicella, rather than presumptive vaccination. 11 Our study showed similar results, with the NPV of varicella history lower among refugee adolescents than among younger children in all regions (Table). The large number of records with missing information about varicella history was a potential source of bias. Among these patients, the prevalence of antibody was approximately the same as for patients included in the analysis. If we had assumed an uncertain or missing history of varicella to be negative, as is standard clinical practice, and had included them in the analysis, neither PPV nor NPV would change.the increase in the number of subjects with a negative history would result in narrower confidence intervals around values of NPV. The accuracy of histories of varicella was a potential limitation of our study. Possible explanations for difficulty in obtaining accurate varicella history in refugee children could be language barriers, translation problems, lack of knowledge of the disease, and no caregivers present during the health assessment. In general, history of varicella is correlated with immunity in both children and adults. 12 Currently, the standard is to not immunize children with a positive history. 13 We chose to test all individuals, even those with a positive history of varicella, because we were uncertain of the accuracy of varicella history in this refugee population. A positive history of varicella is an acceptable alternative to serotesting children over 5 years of age, and will be even more cost-effective than serotesting all these children,but it will leave a small number of susceptible individuals. If a prevalence of immunity to varicella in the population of 97% is required to prevent the spread of outbreaks, accepting a history of varicella as a surrogate for protection may be inadequate to eliminate varicella in the United States. 14
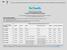
5 Figueira et al., Cost-Effectiveness of Serotesting Compared with Universal Immunization 207 Table Positive and Negative Predictive Values of History of Varicella Stratified by Age Group and Region of Origin Former Age group (years) Yugoslavia East Africa Iraq/Kurdistan Caribbean Vietnam Kosovo 1 4 n PPV% (95% CI) 100 ( ) * 100 ( ) * 0 ( ) 100 ( ) NPV% (95% CI) 96 (89 100) 100 ( ) 100 ( ) 0 ( ) 100 ( ) 80 (56 100) 5 12 n PPV% (95% CI) 94 (89 99) 100 ( ) 100 ( ) 100 ( ) 40 (10 70) 100 ( ) NPV% (95% CI) 54 (43 65) 35 (5 55) 100 ( ) 57 (29 85) 40 (10 70) 72 (58 86) n PPV% (95% CI) 91 (83 99) 100 ( ) 100 ( ) 100 ( ) 66 (43 89) 100 ( ) NPV% (95% CI) 35 (22 49) 25 (6 44) 0 ( ) 20 (0 50) 50 (26 75) 8 (0 17) Overall n PPV% (95% CI) 93 (89 97) 100 ( ) 100 ( ) 100 ( ) 50 (33 67) 100 ( ) NPV% (95% CI) 66 (59 73) 43 (29 57) 60 (37 83) 46 (24 68) 37 (20 54) 28 (18 38) *Infinity indicates that we had no individuals with a positive history and a positive test (resulting in division by 0). Conclusions Varicella immunization without serotesting was costeffective in children less than 5 years of age. In children 5 years of age and older, testing for varicella prior to immunization was cost-effective. A negative history of varicella was a poor indicator of the absence of varicella immunity. Immunizing all patients with a negative history is not cost-effective. A history of varicella was a good indicator of the presence of varicella antibody. Accepting a history of varicella in adolescents is cost-effective, but may result in a small number of susceptible individuals. References 1. American Academy of Pediatrics, Committee on Infectious Diseases. Recommended childhood immunization schedule United States, January December Pediatrics 2003; 111: American Academy of Pediatrics. Immunization in special clinical circumstances. In: Pickering LK, ed Red Book: Report of the Committee on Infectious Diseases, 26th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2003: Feikema SM, Klevens RM, Washington ML, Barker L. Extra immunization among US children. JAMA 2000; 283: American Academy of Pediatrics. Vaccine safety and contraindications. In: Pickering LK, ed Red Book: Report of the Committee on Infectious Diseases, 26th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2003:37 49, Plans Rubio P. Critical value of prevalence for vaccination programmes. The case of hepatitis A vaccination in Spain. Vaccine 1997; 15: Barnett ED, Christiansen D, Figueira M. Seroprevalence of measles, rubella and varicella in refugee children. Clin Infect Dis 2002; 35: Centers for Disease Control and Prevention. Vaccine price list: 8. Lieu TA, Finkler LJ, Sorel ME, Black SB, Shinefield HR. Cost-effectiveness of varicella serotesting versus presumptive vaccination of school-age children and adolescents. Pediatrics 1995; 95: Lieu TA, Cochi SL, Black SB, et al. Cost-effectiveness of a routine varicella vaccination program for US children. JAMA 1994; 271(5): Lieu TA, Black SB, Takahashi H, et al. Varicella serology among school age children with a negative or uncertain history of chickenpox. Pediatr Infect Dis J 1998; 17: Harel Z, Ipp L, Riggs S, et al. Serotesting versus presumptive varicella vaccination of adolescents with a negative and uncertain history of chickenpox. J Adolesc Health 2001; 28: Ross AH,Lencher E,Reittman G.Modification of chickenpox in family contacts by administration of gamma globulin. N Engl J Med 1962; 267: American Academy of Pediatrics. Serologic testing before and after immunization. In: Pickering LK, ed Red Book: Report of the Committee on Infectious Diseases, 26th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2003: Gershon A, Takahashi M, White CJ. Varicella vaccine. In: Plotkin S, Orenstein WA, eds. Vaccines, 3rd edn. Philadelphia: WB Saunders Company, 1999:
Recommended Childhood Immunization Schedu...ates, January - December 2000, NP Central
 Recommended Childhood Immunization Schedule United States, January - December 2000 Vaccines 1 are listed under routinely recommended ages. Solid-colored bars indicate range of recommended ages for immunization.
Recommended Childhood Immunization Schedule United States, January - December 2000 Vaccines 1 are listed under routinely recommended ages. Solid-colored bars indicate range of recommended ages for immunization.
NOTE: The above recommendations must be read along with the footnotes of this schedule.
 Figure 1. Recommended immunization schedule for persons aged 0 through 18 years 2013. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations must be read
Figure 1. Recommended immunization schedule for persons aged 0 through 18 years 2013. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations must be read
3 rd dose. 3 rd or 4 th dose, see footnote 5. see footnote 13. for certain high-risk groups
 Figure 1. Recommended immunization schedule for persons aged 0 through 18 years 2013. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations must be read
Figure 1. Recommended immunization schedule for persons aged 0 through 18 years 2013. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations must be read
Annotated Bibliography:
 Annotated Bibliography: Montana Code Annotated 2009. Montana Legislative Services. September 10, 2010 http://data.opi.mt.gov/bills/mca/20/5/20-5-403.htm. A school may not allow a student to attend unless
Annotated Bibliography: Montana Code Annotated 2009. Montana Legislative Services. September 10, 2010 http://data.opi.mt.gov/bills/mca/20/5/20-5-403.htm. A school may not allow a student to attend unless
Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002
 Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002 Ann R. Fingar, MD, MPH, and Byron J. Francis, MD, MPH Burden of suffering Vaccines are available
Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002 Ann R. Fingar, MD, MPH, and Byron J. Francis, MD, MPH Burden of suffering Vaccines are available
Vaccination Technical Instructions for Civil Surgeons
 Vaccination Technical Instructions for Civil Surgeons Joanna Regan, MD, MPH, FAAP Medical Assessment and Policy (MAP) Team Immigrant, Refugee, and Migrant Health Branch November 14, 2018 National Center
Vaccination Technical Instructions for Civil Surgeons Joanna Regan, MD, MPH, FAAP Medical Assessment and Policy (MAP) Team Immigrant, Refugee, and Migrant Health Branch November 14, 2018 National Center
18 Rubella. Key information. Immunisation Handbook 2017 (2 nd edn, March 2018) 485
 18 Rubella Key information Mode of transmission Incubation period Period of communicability Funded vaccine Dose, presentation, route Funded vaccine indications and schedule Pregnancy Vaccine efficacy/
18 Rubella Key information Mode of transmission Incubation period Period of communicability Funded vaccine Dose, presentation, route Funded vaccine indications and schedule Pregnancy Vaccine efficacy/
Port Gamble S'Klallam Tribe POLICIES/PROCEDURES. Employee Immunity Assessment and Immunization Policy
 Port Gamble S'Klallam Tribe POLICIES/PROCEDURES Employee Immunity Assessment and Immunization Policy Applies To: All Employees subject to the PGST Employee Handbook Purpose The purpose of this policy is
Port Gamble S'Klallam Tribe POLICIES/PROCEDURES Employee Immunity Assessment and Immunization Policy Applies To: All Employees subject to the PGST Employee Handbook Purpose The purpose of this policy is
Vaccine Update Paul A. Offit, MD Division of Infectious Diseases Vaccine Education Center The Children
 Vaccine Update Paul A. Offit, MD Division of Infectious Diseases Vaccine Education Center The Children s Hospital of Philadelphia Perelman School of Medicine The University of Pennsylvania Topics Hep A:
Vaccine Update Paul A. Offit, MD Division of Infectious Diseases Vaccine Education Center The Children s Hospital of Philadelphia Perelman School of Medicine The University of Pennsylvania Topics Hep A:
A Cost-Effectiveness Analysis
 Infectious Diseases in Obstetrics and Gynecology 4:71-76 (1996) (C) 1996 Wiley-Liss, Inc. Strategies for Prevention of Varicella Pneumonia: A Cost-Effectiveness Analysis George A. Macones, Stephanie Ewing,
Infectious Diseases in Obstetrics and Gynecology 4:71-76 (1996) (C) 1996 Wiley-Liss, Inc. Strategies for Prevention of Varicella Pneumonia: A Cost-Effectiveness Analysis George A. Macones, Stephanie Ewing,
PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS
 PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS IMMUNISATION AND HEALTH RECORDS Q: When should I commence my immunisations? A: All immunisation requirements must be completed prior to placement. It is
PLACEMENT OPERATIONS - FREQUENTLY ASKED QUESTIONS IMMUNISATION AND HEALTH RECORDS Q: When should I commence my immunisations? A: All immunisation requirements must be completed prior to placement. It is
Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC
 Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC Assistant Professor, Department of Pediatrics Faculty of Medicine Consultant Pediatric Infectious Diseases NBK Hospital
Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC Assistant Professor, Department of Pediatrics Faculty of Medicine Consultant Pediatric Infectious Diseases NBK Hospital
TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH
 TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH SERIES 95 IMMUNIZATION REQUIRMENTS AND RECOMMENDATIONS FOR NEW SCHOOL ENTERERS 64-95-1. General. 1.1. Scope.
TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH SERIES 95 IMMUNIZATION REQUIRMENTS AND RECOMMENDATIONS FOR NEW SCHOOL ENTERERS 64-95-1. General. 1.1. Scope.
Standard For Recommended Immunization Schedules
 Standard For Recommended Immunization Schedules Section 3: Immunization General Principles Standard #: 03.110 Created by: Province-wide Immunization Program Standards and Quality Approved by: Dr. Predy,
Standard For Recommended Immunization Schedules Section 3: Immunization General Principles Standard #: 03.110 Created by: Province-wide Immunization Program Standards and Quality Approved by: Dr. Predy,
Vaccinology Overview. Complexity of the Vaccine Approval Process Including Lessons Learned
 Vaccinology Overview Complexity of the Vaccine Approval Process Including Lessons Learned Larry K. Pickering, MD, FAAP, FIDSA, FPIDS August 18, 2018 Faculty Disclosure Information In the past 12 months,
Vaccinology Overview Complexity of the Vaccine Approval Process Including Lessons Learned Larry K. Pickering, MD, FAAP, FIDSA, FPIDS August 18, 2018 Faculty Disclosure Information In the past 12 months,
Immunizing the Immunocompromised. Leilani T. Sanchez, MD, DPPS, DPIDSP Crowne Plaza Galleria Manila, 21 February 2013
 Immunizing the Immunocompromised Leilani T. Sanchez, MD, DPPS, DPIDSP Crowne Plaza Galleria Manila, 21 February 2013 WHO World Health Statistics 2012 2 Immunizing the Immunocompromised Leilani T. Sanchez
Immunizing the Immunocompromised Leilani T. Sanchez, MD, DPPS, DPIDSP Crowne Plaza Galleria Manila, 21 February 2013 WHO World Health Statistics 2012 2 Immunizing the Immunocompromised Leilani T. Sanchez
Pertussis in adolescents and adults: should we vaccinate Lee G M, LeBaron C, Murphy T V, Lett S, Schauer S, Lieu T A
 Pertussis in adolescents and adults: should we vaccinate Lee G M, LeBaron C, Murphy T V, Lett S, Schauer S, Lieu T A Record Status This is a critical abstract of an economic evaluation that meets the criteria
Pertussis in adolescents and adults: should we vaccinate Lee G M, LeBaron C, Murphy T V, Lett S, Schauer S, Lieu T A Record Status This is a critical abstract of an economic evaluation that meets the criteria
Healthcare Personnel Immunization Recommendations
 Healthcare Personnel Immunization Recommendations Kathleen Harriman, PhD, MPH, RN California Department of Public Health Immunization Branch Vaccine Preventable Disease Epidemiology Section kathleen.harriman@cdph.ca.gov
Healthcare Personnel Immunization Recommendations Kathleen Harriman, PhD, MPH, RN California Department of Public Health Immunization Branch Vaccine Preventable Disease Epidemiology Section kathleen.harriman@cdph.ca.gov
Epidemiology Update Hepatitis A
 December 2011 Epidemiology Update Hepatitis A Hepatitis A Key Points Between 2000 and 2010, 209 cases of hepatitis A were reported in Hennepin County residents. This represents 30% of the cases reported
December 2011 Epidemiology Update Hepatitis A Hepatitis A Key Points Between 2000 and 2010, 209 cases of hepatitis A were reported in Hennepin County residents. This represents 30% of the cases reported
8: Applicability
 Chapter 14 New Jersey State Sanitary Code Immunization of Pupils in Schools (New Jersey Administrative Code Citation 8:57-4.1 to 8:57-4.20) (Readopted with amendments September 20, 2003. Effective Date:
Chapter 14 New Jersey State Sanitary Code Immunization of Pupils in Schools (New Jersey Administrative Code Citation 8:57-4.1 to 8:57-4.20) (Readopted with amendments September 20, 2003. Effective Date:
Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant
 Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant Reporting Period July 1, 2011 December 31, 2011 Formula Grant Overview The Hepatitis B Foundation received $ 895 in formula funds for the
Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant Reporting Period July 1, 2011 December 31, 2011 Formula Grant Overview The Hepatitis B Foundation received $ 895 in formula funds for the
Immunization Guidelines for the Use of State Supplied Vaccine April 18, 2013
 DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
Immunization Requirements for School Entry - Ohio
 Immunization Requirements for School Entry - Ohio Kindergarten through 12 th Grade Andrew Heffron Cuyahoga County Board of Health This information will help your school better understand Immunization entry
Immunization Requirements for School Entry - Ohio Kindergarten through 12 th Grade Andrew Heffron Cuyahoga County Board of Health This information will help your school better understand Immunization entry
NOTE: The above recommendations must be read along with the footnotes of this schedule.
 Figure 1. Recommended immunization schedule for persons aged 0 through 18 years United States, 2014. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations
Figure 1. Recommended immunization schedule for persons aged 0 through 18 years United States, 2014. (FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE [FIGURE 2]). These recommendations
AMERICAN ACADEMY OF PEDIATRICS
 AMERICAN ACADEMY OF PEDIATRICS Committee on Infectious Diseases Haemophilus influenzae Type b Conjugate Vaccines: Recommendations for Immunization of Infants and Children 2 Months of Age and Older: Update
AMERICAN ACADEMY OF PEDIATRICS Committee on Infectious Diseases Haemophilus influenzae Type b Conjugate Vaccines: Recommendations for Immunization of Infants and Children 2 Months of Age and Older: Update
Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox
 Chickenpox Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox Noelle Bessette, MPH Surveillance Specialist New Jersey Department of Health Vaccine Preventable Disease Program Caused
Chickenpox Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox Noelle Bessette, MPH Surveillance Specialist New Jersey Department of Health Vaccine Preventable Disease Program Caused
Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox
 Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox Noelle Bessette, MPH Surveillance Specialist New Jersey Department of Health Vaccine Preventable Disease Program Chickenpox Caused
Spots and Pox: Contact Tracing and Follow Up for Measles and Chickenpox Noelle Bessette, MPH Surveillance Specialist New Jersey Department of Health Vaccine Preventable Disease Program Chickenpox Caused
Yakima Health District BULLETIN
 Yakima Health District BULLETIN Volume 12, Issue 4 December, 2013 Overview Prevention of Perinatal Hepatitis B Transmission Perinatal transmission of hepatitis B virus (HBV) is preventable through universal
Yakima Health District BULLETIN Volume 12, Issue 4 December, 2013 Overview Prevention of Perinatal Hepatitis B Transmission Perinatal transmission of hepatitis B virus (HBV) is preventable through universal
Utah s Immunization Rule Individual Vaccine Requirements
 Utah s Immunization Rule Individual Vaccine Requirements Which vaccines are required for school entry in Utah? Grades K-6: 5 doses DTaP (4 doses if the 4 th dose was given after the 4 th birthday) 4 doses
Utah s Immunization Rule Individual Vaccine Requirements Which vaccines are required for school entry in Utah? Grades K-6: 5 doses DTaP (4 doses if the 4 th dose was given after the 4 th birthday) 4 doses
Immunization of HCP. Ed Septimus, MD, FACP, FIDSA, FSHEA. Medical Director Infection Prevention and Epidemiology Clinical Services Group, HCA
 Immunization of HCP Ed Septimus, MD, FACP, FIDSA, FSHEA Medical Director Infection Prevention and Epidemiology Clinical Services Group, HCA Professor Internal Medicine Texas A&M Professor, Distinguished
Immunization of HCP Ed Septimus, MD, FACP, FIDSA, FSHEA Medical Director Infection Prevention and Epidemiology Clinical Services Group, HCA Professor Internal Medicine Texas A&M Professor, Distinguished
Prevention of Infections in Mothers & Infants
 Helen Y. Chu, MD MPH Division of Allergy & Infectious Diseases University of Washington Prevention of Infections in Mothers & Infants June 2, 2015 Midwives Association of Washington State Conference Financial
Helen Y. Chu, MD MPH Division of Allergy & Infectious Diseases University of Washington Prevention of Infections in Mothers & Infants June 2, 2015 Midwives Association of Washington State Conference Financial
RUTGERS POLICY. Errors or changes? Contact: Rutgers University Occupational Health Department
 RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
COFM Immunization Policy
 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy This policy applies to all undergraduate medical students attending an Ontario medical
COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy This policy applies to all undergraduate medical students attending an Ontario medical
NJ Department of Health Vaccine Preventable Disease Program
 FOR LOCAL HEALTH DEPARTMENTS AND HEALTH CARE PROVIDERS: CHILD CARE/PRESCHOOL REQUIREMENTS FOR LOCAL HEALTH DEPARTMENTS AND HEALTH CARE PROVIDERS CHILD CARE/PRESCHOOL REQUIREMENTS NJ Department of Health
FOR LOCAL HEALTH DEPARTMENTS AND HEALTH CARE PROVIDERS: CHILD CARE/PRESCHOOL REQUIREMENTS FOR LOCAL HEALTH DEPARTMENTS AND HEALTH CARE PROVIDERS CHILD CARE/PRESCHOOL REQUIREMENTS NJ Department of Health
Perinatal Hepatitis b Prevention
 Perinatal Hepatitis b Prevention Purpose 2 The primary goal of the Perinatal Hepatitis b Prevention Program (PHBPP) is to identify all pregnant women who are infected with hepatitis b and prevent perinatal
Perinatal Hepatitis b Prevention Purpose 2 The primary goal of the Perinatal Hepatitis b Prevention Program (PHBPP) is to identify all pregnant women who are infected with hepatitis b and prevent perinatal
Vaccines. Bacteria and Viruses:
 1 Immunity Resistance to or protection against a specific disease; {power to resist infection. Every day, bacteria, viruses and other germs attack our bodies. But we usually don t get sick. That s because
1 Immunity Resistance to or protection against a specific disease; {power to resist infection. Every day, bacteria, viruses and other germs attack our bodies. But we usually don t get sick. That s because
about VFR Parents and Children
 Pre-CISTM Course, 24 May 2015 : Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! about VFR Parents and Children Stefan Hagmann, MD MSc Associate Professor
Pre-CISTM Course, 24 May 2015 : Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! about VFR Parents and Children Stefan Hagmann, MD MSc Associate Professor
Appendix B: Provincial Case Definitions for Reportable Diseases
 Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Tetanus Revised January 2014 Tetanus 1.0 Provincial Reporting Confirmed cases of disease 2.0 Type of
Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Tetanus Revised January 2014 Tetanus 1.0 Provincial Reporting Confirmed cases of disease 2.0 Type of
Infectious Disease Epidemiology Homework 2 Dr. Selassie Alexis Charles
 Oswego: An Outbreak of Gastrointestinal Illness Following A Church Supper Objectives: After completing this case studym the student should be able to: 1. Define the terms cluster, outbreak, and epidemic.
Oswego: An Outbreak of Gastrointestinal Illness Following A Church Supper Objectives: After completing this case studym the student should be able to: 1. Define the terms cluster, outbreak, and epidemic.
Health Care Worker Vaccinations, 2011: EXTENDED CARE FACILITIES
 Health Care Worker Vaccinations, 2011: EXTENDED CARE FACILITIES Karen K Hoffmann, RN, MS, CIC, FSHEA. Clinical Instructor, Division of Infectious Diseases University of North Carolina at Chapel Hill Associate
Health Care Worker Vaccinations, 2011: EXTENDED CARE FACILITIES Karen K Hoffmann, RN, MS, CIC, FSHEA. Clinical Instructor, Division of Infectious Diseases University of North Carolina at Chapel Hill Associate
Presentation Objectives
 Presentation Objectives Describe the rationale for updating school entry immunization regulations Give a summary of the regulation update and how it will impact immunization requirements Describe how schools
Presentation Objectives Describe the rationale for updating school entry immunization regulations Give a summary of the regulation update and how it will impact immunization requirements Describe how schools
WESTFIELD PUBLIC SCHOOLS 5320 IMMUNIZATION
 WESTFIELD PUBLIC SCHOOLS PUPILS WESTFIELD, NEW JERSEY 5320 Regulations follow Page 1 of 1 5320 IMMUNIZATION In order to safeguard the school community from the spread of certain communicable diseases and
WESTFIELD PUBLIC SCHOOLS PUPILS WESTFIELD, NEW JERSEY 5320 Regulations follow Page 1 of 1 5320 IMMUNIZATION In order to safeguard the school community from the spread of certain communicable diseases and
VACCINE PREVENTABLE DISEASE EPIDEMIOLOGY
 VACCINE PREVENTABLE DISEASE EPIDEMIOLOGY The Twenty-Second Annual Massachusetts Immunization Action Partnership Pediatric Immunization Skills Building Conference October 12, 2017 Marija PopStefanija, MPH,
VACCINE PREVENTABLE DISEASE EPIDEMIOLOGY The Twenty-Second Annual Massachusetts Immunization Action Partnership Pediatric Immunization Skills Building Conference October 12, 2017 Marija PopStefanija, MPH,
AMERICAN ACADEMY OF PEDIATRICS. Measles: Reassessment of the Current Immunization Policy. Commiftee on Infectious Diseases
 AMERICAN ACADEMY OF PEDIATRICS Commiftee on Infectious Diseases Measles: Reassessment of the Current Immunization Policy Since the licensure of measles vaccine in the United States a quarter of a century
AMERICAN ACADEMY OF PEDIATRICS Commiftee on Infectious Diseases Measles: Reassessment of the Current Immunization Policy Since the licensure of measles vaccine in the United States a quarter of a century
Measles: United States, January 1 through June 10, 2011
 Measles: United States, January 1 through June 10, 2011 Preeta K. Kutty, MD, MPH Measles, Mumps, Rubella and Polio Team Division of Viral Diseases Centers for Disease Control and Prevention Atlanta, GA
Measles: United States, January 1 through June 10, 2011 Preeta K. Kutty, MD, MPH Measles, Mumps, Rubella and Polio Team Division of Viral Diseases Centers for Disease Control and Prevention Atlanta, GA
Vaccination Workshop for Immigrants
 National Center for Emerging and Zoonotic Infectious Diseases Vaccination Workshop for Immigrants Zachary White, MPH Public Health Advisor March 12, 2018 Learning Objectives After this workshop, you should
National Center for Emerging and Zoonotic Infectious Diseases Vaccination Workshop for Immigrants Zachary White, MPH Public Health Advisor March 12, 2018 Learning Objectives After this workshop, you should
Management and Reporting of Vaccine Preventable Diseases in Schools. Shirley A. Morales,MPH,CIC
 Management and Reporting of Vaccine Preventable Diseases in Schools Shirley A. Morales,MPH,CIC Presentation Overview Overview of vaccine preventable diseases in Suburban Cook County Reporting Laws and
Management and Reporting of Vaccine Preventable Diseases in Schools Shirley A. Morales,MPH,CIC Presentation Overview Overview of vaccine preventable diseases in Suburban Cook County Reporting Laws and
HEALTH ADVISORY: MEASLES EXPOSURES IN NEW YORK STATE
 December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
DISEASE CONTROL N EWSLETTER
 MINNESOTA DEPARTMENT OF HEALTH DISEASE CONTROL N EWSLETTER Volume 31, Number 2 (pages 13-20) March/April 2003 Recommended Childhood and Adult Immunization Schedules - Minnesota, 2003 In 2003, for the first
MINNESOTA DEPARTMENT OF HEALTH DISEASE CONTROL N EWSLETTER Volume 31, Number 2 (pages 13-20) March/April 2003 Recommended Childhood and Adult Immunization Schedules - Minnesota, 2003 In 2003, for the first
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019
 Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
Critical immunity thresholds for measles elimination
 Critical immunity thresholds for measles elimination Sebastian Funk Centre for the Mathematical Modelling of Infectious Diseases London School of Hygiene & Tropical Medicine 19 October, 2017!"vöå"!(}å!öZ&!
Critical immunity thresholds for measles elimination Sebastian Funk Centre for the Mathematical Modelling of Infectious Diseases London School of Hygiene & Tropical Medicine 19 October, 2017!"vöå"!(}å!öZ&!
Immunization Update: New CDC Recommendations. Blaise L. Congeni M.D. 2012
 Immunization Update: New CDC Recommendations Blaise L. Congeni M.D. 2012 Polysaccharide Vaccines Vaccine Hib capsule polysaccharide PRP (polyribose ribitol phosphate) Not protective in infants
Immunization Update: New CDC Recommendations Blaise L. Congeni M.D. 2012 Polysaccharide Vaccines Vaccine Hib capsule polysaccharide PRP (polyribose ribitol phosphate) Not protective in infants
HIV SCREENING WORKSHOP Exercise
 HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
VACCINE FACT BOOK 2012
 VACCINE FACT BOOK 2012 Preface Prevention is better than cure is a proverb in many other languages as well. This idea is central to the development of vaccines, which have transformed human health since
VACCINE FACT BOOK 2012 Preface Prevention is better than cure is a proverb in many other languages as well. This idea is central to the development of vaccines, which have transformed human health since
Supplementary Online Content
 Supplementary Online Content Jain A, Marshall J, Buikema A, et al. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA. doi:10.1001/jama.2015.3077
Supplementary Online Content Jain A, Marshall J, Buikema A, et al. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA. doi:10.1001/jama.2015.3077
Immunization Policy. "UIC/COD-sponsored graduate education program" is one for which UIC/COD maintains academic responsibility.
 I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
...REPORTS... Childhood Vaccination Against Pneumococcal Otitis Media and Pneumonia: An Analysis of Benefits and Costs
 ...REPORTS... Childhood Vaccination Against Pneumococcal Otitis Media and Pneumonia: An Analysis of Benefits and Costs Derek Weycker, PhD; Erin Richardson, BA; and Gerry Oster, PhD Abstract Objective:
...REPORTS... Childhood Vaccination Against Pneumococcal Otitis Media and Pneumonia: An Analysis of Benefits and Costs Derek Weycker, PhD; Erin Richardson, BA; and Gerry Oster, PhD Abstract Objective:
Many American families are adopting children
 Evaluating Acceptability and Completeness of Overseas Immunization Records of Internationally Adopted Children Joann M. Schulte, DO*; Susan Maloney, MD, MHS ; Jane Aronson, DO ; Pablo San Gabriel, MD,
Evaluating Acceptability and Completeness of Overseas Immunization Records of Internationally Adopted Children Joann M. Schulte, DO*; Susan Maloney, MD, MHS ; Jane Aronson, DO ; Pablo San Gabriel, MD,
Student Health Services 881 Commonwealth Ave, West / Student Information (To be completed by the student) Student Name Last First Middle
 Medical Clearance The following information must be completed on the medical history form, if any information is missing the form will be considered incomplete and will not be processed. If you have questions,
Medical Clearance The following information must be completed on the medical history form, if any information is missing the form will be considered incomplete and will not be processed. If you have questions,
Vaccines Indicated for Infants, Children, and Adolescents Based on Medical and Other Indications
 Vaccines Indicated for Infants, Children, and Adolescents Based on Medical and Other Indications Vaccine Prematurity 1 Altered Immunocompetence 2 (excluding human immunodefi ciency virus [HIV] infection)
Vaccines Indicated for Infants, Children, and Adolescents Based on Medical and Other Indications Vaccine Prematurity 1 Altered Immunocompetence 2 (excluding human immunodefi ciency virus [HIV] infection)
Microbes at Our Doorstep: Emerging issues in infection control and travel-related infections
 Microbes at Our Doorstep: Emerging issues in infection control and travel-related infections Peel November 2, 2016 Learning Objectives At the end of this session, participants will be able to: 1. Identify
Microbes at Our Doorstep: Emerging issues in infection control and travel-related infections Peel November 2, 2016 Learning Objectives At the end of this session, participants will be able to: 1. Identify
Immunization Guidelines For the Use of State Supplied Vaccine July 1, 2011
 DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
Note from the National Guideline Clearinghouse (NGC): The guideline recommendations are presented in the form of tables with footnotes (see below).
 Brief Summary GUIDELINE TITLE Recommended immunization schedules for persons aged 0 through 18 years: United States, 2009. BIBLIOGRAPHIC SOURCE(S) American Academy of Pediatrics Committee on Infectious
Brief Summary GUIDELINE TITLE Recommended immunization schedules for persons aged 0 through 18 years: United States, 2009. BIBLIOGRAPHIC SOURCE(S) American Academy of Pediatrics Committee on Infectious
Immunization: Comments on MacDonald, Halperin, and Rodewald
 Topic Immunization Immunization: Comments on MacDonald, Halperin, and Rodewald DAVID M SALISBURY, CB FRCP FRCPCH FFPH Department of Health, London, UNITED KINGDOM (Published online October 28, 2005) Introduction
Topic Immunization Immunization: Comments on MacDonald, Halperin, and Rodewald DAVID M SALISBURY, CB FRCP FRCPCH FFPH Department of Health, London, UNITED KINGDOM (Published online October 28, 2005) Introduction
Student Health Requirements Master of Arts, Biomedical Sciences Program
 Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
Travel. Program Management. Schedule
 Program Management 69_4 YF (yellow fever) vaccine should be offered to all travellers to and from at-risk areas, unless they belong to the group of individuals for whom YF vaccination is contraindicated.
Program Management 69_4 YF (yellow fever) vaccine should be offered to all travellers to and from at-risk areas, unless they belong to the group of individuals for whom YF vaccination is contraindicated.
CLINICAL GUIDELINES. Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P.
 CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
Early Learning Centre Immunisation Policy Legislation ACT Public Health Regulations (2000)
 Early Learning Centre Immunisation Policy Legislation ACT Public Health Regulations (2000) National Quality Standard / Education and Care Services National Regulations Standard 2.1 Each child s health
Early Learning Centre Immunisation Policy Legislation ACT Public Health Regulations (2000) National Quality Standard / Education and Care Services National Regulations Standard 2.1 Each child s health
Changes for the School Year. The addition of NINTH grade to the requirement for four (4) doses of diphtheria, tetanus, and pertussis.
 February 19, 2013 Dear Immunization Provider: In accordance with South Carolina Code of Laws, Section 44-29-180, and State Regulation 61-8, the 2013-2014 "Required Standards of Immunization for School
February 19, 2013 Dear Immunization Provider: In accordance with South Carolina Code of Laws, Section 44-29-180, and State Regulation 61-8, the 2013-2014 "Required Standards of Immunization for School
Student Verification Pack 2019
 Student Verification Pack 2019 Important Start this Now Page 1 Clinical Placements in NSW Health facilities require students to complete a verification process to ensure the safety of themselves, other
Student Verification Pack 2019 Important Start this Now Page 1 Clinical Placements in NSW Health facilities require students to complete a verification process to ensure the safety of themselves, other
Risk of Seizures following Measles Containing Vaccination in Children Born Preterm or Full-term
 Risk of Seizures following Measles Containing Vaccination in Children Born Preterm or Full-term David L. McClure HSCRN 2018 Annual Meeting 1 Acknowledgements Co-authors Huong McLean Steven Jacobsen Nicola
Risk of Seizures following Measles Containing Vaccination in Children Born Preterm or Full-term David L. McClure HSCRN 2018 Annual Meeting 1 Acknowledgements Co-authors Huong McLean Steven Jacobsen Nicola
I. In accordance with Virginia Code relative to enrollment of certain children in public schools:
 Immunization Requirements I. In accordance with Virginia Code relative to enrollment of certain children in public schools: A. Children not properly immunized in accordance with the Virginia Department
Immunization Requirements I. In accordance with Virginia Code relative to enrollment of certain children in public schools: A. Children not properly immunized in accordance with the Virginia Department
IMMUNIZATION OF PUPILS IN SCHOOL
 R 5320 IMMUNIZATION A. Immunizations on Admission IMMUNIZATION OF IN SCHOOL 1. No Principal shall knowingly admit or retain any pupil who has not submitted acceptable evidence of immunization according
R 5320 IMMUNIZATION A. Immunizations on Admission IMMUNIZATION OF IN SCHOOL 1. No Principal shall knowingly admit or retain any pupil who has not submitted acceptable evidence of immunization according
Hepatitis B Vaccine Biological Page
 Hepatitis B Vaccine Biological Page Section 7: Biological Product Information Standard #: 07.234 Created by: Approved by: Province-wide Immunization Program Standards and Quality Province-wide Immunization
Hepatitis B Vaccine Biological Page Section 7: Biological Product Information Standard #: 07.234 Created by: Approved by: Province-wide Immunization Program Standards and Quality Province-wide Immunization
CHAPTER 23 IMMUNIZATION AND VACCINE DISTRIBUTION
 CHAPTER 23 IMMUNIZATION AND VACCINE DISTRIBUTION BOARD OF HEALTH ROLE AT A GLANCE Promote or provide for the routine immunization of children and adults. Investigate and control the spread of vaccine-preventable
CHAPTER 23 IMMUNIZATION AND VACCINE DISTRIBUTION BOARD OF HEALTH ROLE AT A GLANCE Promote or provide for the routine immunization of children and adults. Investigate and control the spread of vaccine-preventable
13 Mumps. Key information. Immunisation Handbook 2017 (2 nd edn, March 2018) 367
 13 Mumps Key information Mode of transmission Incubation period Period of communicability Funded vaccine Dose, presentation, route Funded vaccine indications and schedule Vaccine efficacy/ effectiveness
13 Mumps Key information Mode of transmission Incubation period Period of communicability Funded vaccine Dose, presentation, route Funded vaccine indications and schedule Vaccine efficacy/ effectiveness
Seropositivity Rates for Measles, Mumps, and Rubella IgG and Associated Cost. with Testing and Revaccination
 CVI Accepts, published online ahead of print on 23 January 2013 Clin. Vaccine Immunol. doi:10.1128/cvi.00503-12 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9
CVI Accepts, published online ahead of print on 23 January 2013 Clin. Vaccine Immunol. doi:10.1128/cvi.00503-12 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9
IOM Committee on Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule
 IOM Committee on Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule Bruce Gellin, MD, MPH Director, National Vaccine Program Office Deputy Assistant Secretary
IOM Committee on Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule Bruce Gellin, MD, MPH Director, National Vaccine Program Office Deputy Assistant Secretary
FAQs about Changes to DHR Immunization Rules and Regulations
 FAQs about Changes to DHR Immunization Rules and Regulations 1. What are the new immunization rules changes? The new immunization rules changes include 1 new definition and changes in 4 vaccine requirements.
FAQs about Changes to DHR Immunization Rules and Regulations 1. What are the new immunization rules changes? The new immunization rules changes include 1 new definition and changes in 4 vaccine requirements.
Immunisation Update for Occupational Health
 Immunisation Update for Occupational Health Dr Gayatri Amirthalingam Immunisation, Hepatitis & Blood Safety department Public Health England 29 th April 2016 Session Outline Epidemiology of vaccine preventable
Immunisation Update for Occupational Health Dr Gayatri Amirthalingam Immunisation, Hepatitis & Blood Safety department Public Health England 29 th April 2016 Session Outline Epidemiology of vaccine preventable
Michael G. DeGroote School of Medicine Visiting Student Electives Program Health Screening Record
 Michael G. DeGroote School of Medicine Visiting Student Electives Program Health Screening Record Thank you for applying to the Visiting Student Electives Program at McMaster University. International
Michael G. DeGroote School of Medicine Visiting Student Electives Program Health Screening Record Thank you for applying to the Visiting Student Electives Program at McMaster University. International
Immunizations are among the most cost effective and widely used public health interventions.
 Focused Issue of This Month Recommended by the Korean Pediatric Society, 2008 Hoan Jong Lee, MD Department of Pediatrics, Seoul National University College of Medicine E mail : hoanlee@snu.ac.kr J Korean
Focused Issue of This Month Recommended by the Korean Pediatric Society, 2008 Hoan Jong Lee, MD Department of Pediatrics, Seoul National University College of Medicine E mail : hoanlee@snu.ac.kr J Korean
ARKANSAS STATE BOARD OF HEALTH
 ARKANSAS STATE BOARD OF HEALTH RULES AND REGULATIONS PERTAINING TO IMMUNIZATION REQUIREMENTS Promulgated Under the Authority of Ark. Code Ann. 20-7-109, 6-18-702, 6-60-501-504, and 20-78-206. By the Arkansas
ARKANSAS STATE BOARD OF HEALTH RULES AND REGULATIONS PERTAINING TO IMMUNIZATION REQUIREMENTS Promulgated Under the Authority of Ark. Code Ann. 20-7-109, 6-18-702, 6-60-501-504, and 20-78-206. By the Arkansas
Immunization Guidelines for the Use of State Supplied Vaccine May 17, 2015
 DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
DTaP / DT DTaP/IPV/Hep B Combination (Pediarix ) Children from 6 weeks of age up to the 7 th birthday Children from 2 months of age up to the 7th birthday: Indicated for the primary doses of DTaP, IPV,
Measles Update. March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief
 Measles Update March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief Colorado Department of Public Health and Environment Presenters have
Measles Update March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief Colorado Department of Public Health and Environment Presenters have
Update on Tuberculosis Skin Testing of Children
 Committee on Infectious Diseases In January 1994, the Committee on Infectious Diseases published detailed guidelines on tuberculin skin testing of infants, children, and adolescents for the detection of
Committee on Infectious Diseases In January 1994, the Committee on Infectious Diseases published detailed guidelines on tuberculin skin testing of infants, children, and adolescents for the detection of
FAQs about Changes to DHR Immunization Rules and Regulations
 FAQs about Changes to DHR Immunization Rules and Regulations 1. What are the new immunization rules changes? The new immunization rules changes include 1 new definition and changes in 4 vaccine requirements.
FAQs about Changes to DHR Immunization Rules and Regulations 1. What are the new immunization rules changes? The new immunization rules changes include 1 new definition and changes in 4 vaccine requirements.
Introduction and overview of the program; new vaccine pipeline and prioritization process
 Immunization for the Modern Family: Western Canada Immunization Forum 2011 Introduction and overview of the program; new vaccine pipeline and prioritization process Monika Naus, MD, MHSc, FRCPC, FACPM
Immunization for the Modern Family: Western Canada Immunization Forum 2011 Introduction and overview of the program; new vaccine pipeline and prioritization process Monika Naus, MD, MHSc, FRCPC, FACPM
Objectives. Immunity. Childhood Immunization Risk of Non-Vaccinated Children 12/22/2015
 Childhood Immunization Risk of Non-Vaccinated Children Bertha P. Rojas, Pharm.D. PGY-1 Pharmacy Resident South Miami Hospital Objectives Understand the definition of herd immunity Identify vaccine-preventable
Childhood Immunization Risk of Non-Vaccinated Children Bertha P. Rojas, Pharm.D. PGY-1 Pharmacy Resident South Miami Hospital Objectives Understand the definition of herd immunity Identify vaccine-preventable
EVALUATION OF THE SCHOOL-BASED HEPATITIS B VACCINATION PROGRAMME IN CATALONIA (SPAIN)
 EVALUATION OF THE SCHOOL-BASED HEPATITIS B VACCINATION PROGRAMME IN CATALONIA (SPAIN) Prof. L. Salleras Department of Public Health University of Barcelona Hospital Clínic Barcelona Hepatitis B vaccine:
EVALUATION OF THE SCHOOL-BASED HEPATITIS B VACCINATION PROGRAMME IN CATALONIA (SPAIN) Prof. L. Salleras Department of Public Health University of Barcelona Hospital Clínic Barcelona Hepatitis B vaccine:
Chancroid Table of Contents
 Subsection: Chancroid Page 1 of 8 Chancroid Table of Contents Chancroid Fact Sheet Subsection: Chancroid Page 2 of 8 Chancroid (Haemophilus ducreyi) Overview (1,2) For a more complete description of chancroid,
Subsection: Chancroid Page 1 of 8 Chancroid Table of Contents Chancroid Fact Sheet Subsection: Chancroid Page 2 of 8 Chancroid (Haemophilus ducreyi) Overview (1,2) For a more complete description of chancroid,
Update/Le point. Principles of measles control* R.H. Henderson,2 C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5
 Update/Le point Principles of measles control* F.T. Cutts,, R.H. Henderson, C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5 WHO's Expanded Programme on Immunization has significantly helped to reduce global
Update/Le point Principles of measles control* F.T. Cutts,, R.H. Henderson, C.J. Clements,3 R.T. Chen,4 & P.A. Patriarca5 WHO's Expanded Programme on Immunization has significantly helped to reduce global
Immunisation Declaration Form - Version 2
 All students undertaking an award within Institute of Health & Nursing Australia with a clinical/work experience placement component are required to ensure immunisations are up to date. Please read the
All students undertaking an award within Institute of Health & Nursing Australia with a clinical/work experience placement component are required to ensure immunisations are up to date. Please read the
EUVAC.NET A surveillance network for vaccine-preventable diseases
 EUVAC.NET A surveillance network for vaccine-preventable diseases Mark Muscat EUVAC.NET Co-ordinator Department of Epidemiology Statens Serum Institut Denmark Email: mmc@ssi.dk Viral Hepatitis Prevention
EUVAC.NET A surveillance network for vaccine-preventable diseases Mark Muscat EUVAC.NET Co-ordinator Department of Epidemiology Statens Serum Institut Denmark Email: mmc@ssi.dk Viral Hepatitis Prevention
Concepts of herd protection and immunity
 Available online at www.sciencedirect.com Procedia in Vaccinology 2 (2010) 134 139 Ninth Global Vaccine Research Forum and Parallel Satellite Symposia, Bamako, Mali, 6-9 December 2009 Concepts of herd
Available online at www.sciencedirect.com Procedia in Vaccinology 2 (2010) 134 139 Ninth Global Vaccine Research Forum and Parallel Satellite Symposia, Bamako, Mali, 6-9 December 2009 Concepts of herd
COFM Immunization Policy 2016
 COFM Immunization Policy 2016 Council of Ontario Faculties of Medicine June 2016 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy 2016
COFM Immunization Policy 2016 Council of Ontario Faculties of Medicine June 2016 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy 2016
Dear Student, Welcome to the University of Chicago!
 Dear Student, Welcome to the University of Chicago! The State of Illinois and University regulations require all students to provide proof of required immunizations prior to registration for classes. In
Dear Student, Welcome to the University of Chicago! The State of Illinois and University regulations require all students to provide proof of required immunizations prior to registration for classes. In
Atlantic Provinces Pediatric Hematology Oncology Network Réseau d oncologie et d hématologie pédiatrique des provinces de l Atlantique
 Atlantic Provinces Pediatric Hematology Oncology Network Réseau d oncologie et d hématologie pédiatrique des provinces de l Atlantique Reviewed and approved by specialists at the IWK Health Centre, Halifax,
Atlantic Provinces Pediatric Hematology Oncology Network Réseau d oncologie et d hématologie pédiatrique des provinces de l Atlantique Reviewed and approved by specialists at the IWK Health Centre, Halifax,
HOW TO COMPLETE YOUR STUDENT IMMUNISATION RECORD FORM
 VERSION 1 DECEMBER 19, 2018 HOW TO COMPLETE YOUR STUDENT IMMUNISATION RECORD FORM SCHOOL OF NURSING, MIDWIFERY AND SOCIAL WORK Bachelor of Nursing Bachelor of Midwifery Bachelor of Nursing/Midwifery Master
VERSION 1 DECEMBER 19, 2018 HOW TO COMPLETE YOUR STUDENT IMMUNISATION RECORD FORM SCHOOL OF NURSING, MIDWIFERY AND SOCIAL WORK Bachelor of Nursing Bachelor of Midwifery Bachelor of Nursing/Midwifery Master
