T: N: M: EORTC SYSTEMIC THERAPY CHECKLIST. Protocol number: SeqID. Copyright EORTC August 2007 COMPARATIVE PERFORMANCE STATUS TABLE
|
|
|
- Bertram Joseph
- 6 years ago
- Views:
Transcription
1 EORTC SYSTEMIC THERAPY CHECKLIST Copyright EORTC August 2007 (Stick the patient s identification label here) Protocol number: SeqID. T: N: M: WHO / ECOG 0 Normal activity. 1 Symptoms but ambulatory. 2 In bed less than 50% of time. 3 In bed more than 50% of time % bedridden. COMPARATIVE PERFORMANCE STATUS TABLE KARNOFSKY 100 Normal; no complaints, no evidenced of disease 90 Able to carry on normal activities; minor signs of symptoms or disease. 80 Normal activity with effort.. some signs or symptoms of disease 70 Cares for self. Unable to carry on normal activity or to do active work. 60 Requires occasional assistance but is able to care for most of his/her needs 50 Requires considerable assistance and frequent medical care. 40 Disabled; Requires special care and assistance. 30 Severely disabled; hospitalization though death not imminent. 20 Very sick; hospitalization. Death not imminent 10 Moribund, fatal process progressing rapidly
2 TUMOR MEASUREMENTS Check the response evaluation system required by the protocol: WHO RECIST Other: Method of assessment*44444 u u u DATE dd/mm/yy ORGAN INVOLVED LESIONS INITIAL * 1 st. * 2 nd. * 3 rd. * TARGET LESIONS ** MEASUREMENTS MEASUREMENTS MEASUREMENTS MEASUREMENTS u mm X mm u mm X mm u mm X mm u mm X mm u A B C D E F G H I J target lesion NON TARGET LESIONS Non target lesions Present at baseline? Y/N New lesions observed Overall RESPONSE 4 Y / N Y / N Y / N Method of assessment* 4444u u u u DATE 4 TARGET 4 th * 5 th * 6 th * 7 th * 8 th * LESIONS ** MEASUREMENTS MEASUREMENTS MEASUREMENTS MEASUREMENTS MEASUREMENTS mm X mm u mm X mm u mm X mm u mm X mm u mm X mm u A B C D E F G H I J - target lesions non target lesion New lesions Y / N Y / N Y / N Y / N Y / N Overall RESPONSE 4 * METHODS OF ASSESSMENT: 1) Physical Examination 2) Chest X-Ray 3)conventional CT-Scan 4) spiral CT scan 5) MRI 6) Echography 7) Bone scan 8) Tumor Markers 9) Other ** Guidelines for indications to body drawings: Target lesions should be marked TL on the body drawings Non Target lesions should be marked NTL on the body drawings Target lesion : WHO / RECIST: CR/PR/NC/PD Non Target Lesion : WHO: CR/PR/NC/PD RECIST: CR/ non CR non PD/ PD Overall response: WHO / RECIST: CR/PR/NC/PD
3 Height: cm Protocol No:! SeqID: Pre-study Characteristics DATE (DD/MM/YY) Cycle Number Investigators Initials Anti -Tumor Medications (dose in mg) Route Anti Emetics (y/n )see med. Form Weight kg BSA m² Performance status WHO / Karnofsky Blood pressure Pulse Hbg mmol/l / g/dl Platelets 10 9 /L Leucocytes 10 9 /L Granulocytes 10 9 /L Creatinine mg/dl or µmol/l Markers: Transfusions Use grading system required Baseline TOX R TOX R TOX R TOX R TOX R TOX R TOX R TOX R by the protocol DATE (DD/MM/YY) Gastro-intestinal - Nausea Stomatitis/pharyngitis Alopecia Skin: Other - - Rash - Vomiting - Anorexia - Constipation - Diarrhea - nail changes - Infection without neutropenia - Febrile Neutropenia - Infections with Neutropenia Neuropathy - Motor - Sensory - Dizziness Cardio-vascular: - LVEF Edema Other - Fatigue/Asthenia Pain: Pulmonary: specify Other: specify Assessment TOXICITY RELATIONSHIP (R): please use the scale applicable to the trial (See CRFs)
4 Guidelines for toxicity version 3.0 (august 2006) Please add a copy of the required toxicity scale according to the protocol. (see annex in protocol) Electronic version of the NCI-CTC available at: Grade Toxicity Nausea Loss of appetite without alteration in eating habits Oral intake decreased without significant weight loss, dehydration or malnutrition; IV fluids <24 hrs Vomiting 1 episode in 24 hrs 2-5 episodes in 24 hrs; IV fluids < 24 hrs Anorexia Loss of appetite without alteration in Oral intake altered without significant weight eating habits loss or malnutrition; oral nutritional supplements Constipation Diarrhea Mucositis/stomatitis (clinical exam) Select: Anus, Esophagus, Large bowel, Larynx, Oral cavity, Pharynx, Rectum, Small bowel, Stomach, Trachea Mucositis/stomatitis (al/symptomatic) Select: Anus, Esophagus, Large bowel, Larynx, Oral cavity, Pharynx, Rectum, Small bowel, Stomach, Trachea Occasional or intermittent symptoms; occasional use of stool softeners, laxatives, dietary modification, or enema Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline Persistent symptoms with regular use of laxatives or enemas Increase of 4 6 stools per day over baseline; IV fluids <24hrs; moderate increase in ostomy output compared to baseline; not interfering with ADL* Inadequate oral caloric or fluid intake; IV fluids, tube feedings, or TPN 24 hrs 6 episodes in 24 hours; IV fluids or TPN 24 hrs Associated with significant weights loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); IV fluids, tube feedings or TPN. Symptoms ; obstipation with manual evacuation Increase of 7 stools per day over baseline; incontinence; IV fluids 24 hrs; hospitalization; severe increase in ostomy output compared to baseline; Erythema of the mucosa Patchy ulcerations or pseudomembranes Confluent ulcerations or pseudomembranes; bleeding with minor trauma Upper aerodigestive tract sites: Minimal symptoms, normal diet; minimal respiratory symptoms but not interfering with Minimal discomfort, intervention not Upper aerodigestive tract sites: Symptomatic but can eat and swallow modified diet; respiratory symptoms interfering with but not Symptomatic, medical intervention but not Upper aerodigestive tract sites: Symptomatic and unable to adequately aliment or hydrate orally; respiratory symptoms Stool incontinence or other symptoms (e.g., obstruction, toxic megacolon) (e.g., hemodynamic collapse) Tissue necrosis; significant spontaneous bleeding; lifethreatening consequences Symptoms associated with lifethreatening consequences Alopecia Thinning or patchy Complete - - Rash/desquamation Macular or papular eruption or erythema without associated Macular or papular eruption or erythema with pruritus or other associated symptoms; Severe, generalized erythroderma or macular, papular or vesicular eruption; Generalized exfoliative, ulcerative, or bullous dermatitis symptoms localized desquamation or other lesions covering <50% of body surface area (BSA) desquamation covering 50% BSA Nail changes Discoloration; ridging (koilonychia); pitting Partial or complete loss of nail(s); pain in nailbed(s) Interfering with ADL* - Infection with normal ANC or Grade 1 or 2 neutrophils Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection) (ANC <1.0 x 10 9 /L, fever 38.5 C) Infection (documented clinically or microbiologically) with grade 3 or 4 neutropenia neutrophils (ANC <1.0 x 10 9 /L) Neuropathy- motor Neuropathy-sensory Dizziness Cardiac left ventricular diastolic Cardiac left ventricular systolic head and neck limb trunk/genital viscera Fatigue (lethargy, malaise, asthenia) *ADL = Activities of Daily Living. - Localized, local intervention IV antibiotic, antifungal, or antiviral intervention ; interventional acidosis, necrosis) - - Present acidosis, necrosis) - Localized, local intervention IV antibiotic, antifungal, or antiviral intervention ; interventional Asymptomatic, weakness on exam/testing only Asymptomatic; loss of deep tendon reflexes or paresthesia (including tingling) but not interfering with With head movements or nystagmus only; not interfering with Asymptomatic diagnostic finding; intervention not Asymptomatic, resting ejection fraction (EF) <60 50%; shortening fraction (SF) <30 24% Localized to dependent areas, no disability or al impairment 5 10% inter-limb discrepancy in volume or circumference at point of greatest visible difference; swelling or obscuration of anatomic architecture on close inspection; pitting edema Swelling or obscuration of anatomic architecture on close inspection; pitting edema Asymptomatic; clinical or radiographic findings only Mild fatigue over baseline Symptomatic weakness interfering with, but not Sensory alteration or paresthesia (including tingling), interfering with, but not Interfering with, but not interfering with ADL* Asymptomatic, intervention Asymptomatic, resting; EF <50 40%; SF <24 15% Localized facial or neck edema with al impairment >10 30% inter-limb discrepancy in volume or circumference at point of greatest visible difference; readily apparent obscuration of anatomic architecture; obliteration of skin folds; readily apparent deviation from normal anatomic contour Readily apparent obscuration of anatomic architecture; obliteration of skin folds; readily apparent deviation from normal anatomic contour Symptomatic; medical intervention Moderate or causing difficulty performing some ADL* Weakness ; bracing or assistance to walk (e.g., cane or walker) Sensory alteration or paresthesia Interfering with ADL* Symptomatic CHF responsive to intervention Symptomatic CHF responsive to intervention; EF <40 20% SF <15% Generalized facial or neck edema with al impairment (e.g., difficulty in turning neck or opening mouth compared to baseline) >30% inter-limb discrepancy in volume; lymphorrhea; gross deviation from normal anatomic contour; Lymphorrhea; ; gross deviation from normal anatomic contour Symptomatic and unable to aliment adequately orally; interventional Severe fatigue acidosis, necrosis) Life-threatening; disabling (e.g., paralysis) Refractory CHF, poorly controlled; intervention such as ventricular assist device or heart transplant Refractory CHF or poorly controlled; EF <20%; intervention such as ventricular assist device, ventricular reduction surgery, or heart transplant Severe with ulceration or cerebral edema; tracheotomy or feeding tube Progression to malignancy (i.e., lymphangiosarcoma); amputation ; disabling Progression to malignancy (i.e., lymphangiosarcoma); disabling
5 CONCOMITANT MEDICATION Copyright EORTC August 2007 PATIENT NAME: SeqID: REMARKS: Chart number: Protocol number: Page number: (if applicable stick patient s label here) Year: MEDICATION INDICATION ROUTE Dose/unit/rate Start Date Stop Date Ongoing (Generic name) (dd,mm,yy) (dd,mm,yy) Variations in dose should be checked with the nursing file EXAMPLE MEDICATION INDICATION (I) ROUTE Dose/ Start Date Stop Date Ongoing Unit/Rate (Generic name) u (dd,mm,yy) (dd,mm,yy) Medication 1 Indication 1 PO 200 mg bid 2/01/07 n.a. ROUTE: PO - ORAL, SL - SUBLINGUAL, RT - RECTAL, TC - TRANSCUTANEOUS, SC- SUBCUTANEOUS, IV - INTRAVENOUS, IM - INTRAMUSCULAR
RECTUM/SIGMOID COLON/BOWEL,
 EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
Patient Treatment Date/time: Diagnosis: Treatment Regimen:
 Name: Address: DOB: HCRN: Consultant: Ward: Assessment: Pre Systematic Anti Cancer Therapy (SACT) Continuation *This assessment is for patients on multiday regimens Patient Treatment Date/time: Diagnosis:
Name: Address: DOB: HCRN: Consultant: Ward: Assessment: Pre Systematic Anti Cancer Therapy (SACT) Continuation *This assessment is for patients on multiday regimens Patient Treatment Date/time: Diagnosis:
TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS
 TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS Green AEs managed by DTUs REMARK Red Patient referral to provincial hospitals for AE management Note: In the case of patients with events at grade of severity
TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS Green AEs managed by DTUs REMARK Red Patient referral to provincial hospitals for AE management Note: In the case of patients with events at grade of severity
ANAMORELIN FOR CACHEXIA. Series 20 CASE REPORT FORM
 1 ANAMORELIN FOR CACHEXIA Series 20 CASE REPORT FORM Palliative Care Clinical Studies Collaborative (PaCCSC) RAPID Pharmacovigilance in Palliative Care The case report form (CRF) is to be completed in
1 ANAMORELIN FOR CACHEXIA Series 20 CASE REPORT FORM Palliative Care Clinical Studies Collaborative (PaCCSC) RAPID Pharmacovigilance in Palliative Care The case report form (CRF) is to be completed in
WHO (World Health Organization) Toxicity Criteria by Grade
 WHO (World Health Organization) Toxicity Criteria by Grade 化學治療以 WHO Toxicity Criteria 為主放射治療有分急性與慢性副作用以 RTOG Toxicity Criteria 為主 WHO Toxicity Criteria Category Toxicity Grade0 Grade1 Grade2 Grade3 Grade4
WHO (World Health Organization) Toxicity Criteria by Grade 化學治療以 WHO Toxicity Criteria 為主放射治療有分急性與慢性副作用以 RTOG Toxicity Criteria 為主 WHO Toxicity Criteria Category Toxicity Grade0 Grade1 Grade2 Grade3 Grade4
INSTRUCTIONS: 1. Use codetable on page 1 for modifications / termination reasons
 Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
Vincristine-Induced Toxicities Secondary to Azole Antifungal Therapy in Patients with Hematologic Malignancies
 Vincristine-Induced Toxicities Secondary to Azole Antifungal Therapy in Patients with Hematologic Malignancies Kara E. Loth, PharmD PGY2 Oncology Resident Wake Forest University Baptist Medical Center
Vincristine-Induced Toxicities Secondary to Azole Antifungal Therapy in Patients with Hematologic Malignancies Kara E. Loth, PharmD PGY2 Oncology Resident Wake Forest University Baptist Medical Center
Breast Pathway Group Docetaxel in Advanced Breast Cancer
 Breast Pathway Group Docetaxel in Advanced Breast Cancer Indication: First-line palliative treatment, with or without trastuzumab, for advanced breast cancer in patients for whom an anthracycline is not
Breast Pathway Group Docetaxel in Advanced Breast Cancer Indication: First-line palliative treatment, with or without trastuzumab, for advanced breast cancer in patients for whom an anthracycline is not
PREMEDICATIONS: Antiemetic protocol for highly emetogenic chemotherapy. May not need any antiemetic with
 BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
Antiemetic protocol for low-moderately emetogenic chemotherapy (see SCNAUSEA)
 BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
AE Toxicity Grading for Transplant Patients
 AE Toxicity Grading for Transplant Patients Marcie Tomblyn, MD, MS Associate Member Director, BMT Clinical Research Moffitt Cancer Center Objectives Why do we care????? Toxicity vs Adverse Event vs Serious
AE Toxicity Grading for Transplant Patients Marcie Tomblyn, MD, MS Associate Member Director, BMT Clinical Research Moffitt Cancer Center Objectives Why do we care????? Toxicity vs Adverse Event vs Serious
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Breast Pathway Group EC x 4 Docetaxel x 4: Epirubicin & Cyclophosphamide followed by Docetaxel in Early Breast Cancer
 Breast Pathway Group EC x 4 Docetaxel x 4: Epirubicin & Cyclophosphamide followed by Docetaxel in Early Breast Indication: Neoadjuvant therapy for high risk and fit breast cancer patients suitable for
Breast Pathway Group EC x 4 Docetaxel x 4: Epirubicin & Cyclophosphamide followed by Docetaxel in Early Breast Indication: Neoadjuvant therapy for high risk and fit breast cancer patients suitable for
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS
 Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
BMT CTN 0801 Protocol. Chronic GVHD Provider Survey ENROLLMENT
 BMT CTN 0801 Protocol Chronic GVHD Provider Survey ENROLLMENT Instructions: Please score a symptom only if you know or suspect it be related to chronic GVHD. Subjective are acceptable. For example, joint
BMT CTN 0801 Protocol Chronic GVHD Provider Survey ENROLLMENT Instructions: Please score a symptom only if you know or suspect it be related to chronic GVHD. Subjective are acceptable. For example, joint
Cycle 1 PERTuzumab (day 1) and trastuzumab (day 2) loading doses: Drug Dose BC Cancer Administration Guideline
 BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BMT CTN 0801 Protocol. Chronic GVHD Provider Survey. FOLLOW-UP v3.0
 BMT CTN 0801 Protocol Chronic GVHD Provider Survey FOLLOW-UP v3.0 Instructions: Please score a symptom only if you know or suspect it be related to chronic GVHD. Subjective are acceptable. For example,
BMT CTN 0801 Protocol Chronic GVHD Provider Survey FOLLOW-UP v3.0 Instructions: Please score a symptom only if you know or suspect it be related to chronic GVHD. Subjective are acceptable. For example,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy
Safety Considerations and Side Effect Management with Tyverb (Lapatinib)
 Safety Considerations and Side Effect Management with Tyverb (Lapatinib) Prescribing information can be found at the end of the presentation Date of prep: 19-Sep-2011 UK/LPD/0226a/11 HER2 (ErbB2)-positive
Safety Considerations and Side Effect Management with Tyverb (Lapatinib) Prescribing information can be found at the end of the presentation Date of prep: 19-Sep-2011 UK/LPD/0226a/11 HER2 (ErbB2)-positive
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using
 BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced
Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer
 Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for patients in whom anthracyclines are contraindicated or inappropriate Regimen
Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for patients in whom anthracyclines are contraindicated or inappropriate Regimen
BC Cancer Protocol Summary for the Maintenance Therapy of Multiple Myeloma Using Bortezomib for Patients with the High-Risk Chromosome Abnormality
 BC Cancer Protocol Summary for the Maintenance Therapy of Multiple Myeloma Using Bortezomib for Patients with the High-Risk Chromosome Abnormality Protocol Code Tumour Group Contact Physician MYBORMTN
BC Cancer Protocol Summary for the Maintenance Therapy of Multiple Myeloma Using Bortezomib for Patients with the High-Risk Chromosome Abnormality Protocol Code Tumour Group Contact Physician MYBORMTN
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine
 BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
Determining Eligibility for Hospice Care
 Determining Eligibility for Hospice Care Main Number: 203 739-8300 Toll Free Number: 888 357-3334 www.regionalhospicect.org Many people may not understand all that Regional Hospice can offer or they are
Determining Eligibility for Hospice Care Main Number: 203 739-8300 Toll Free Number: 888 357-3334 www.regionalhospicect.org Many people may not understand all that Regional Hospice can offer or they are
Contrast Materials Patient Safety: What are contrast materials and how do they work?
 Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine
 BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAVCAP Breast Dr. Susan Ellard ELIGIBILITY: First line treatment of metastatic
BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAVCAP Breast Dr. Susan Ellard ELIGIBILITY: First line treatment of metastatic
BRAVTPCARB. Protocol Code: Breast. Tumour Group: Dr. Karen Gelmon. Contact Physician:
 BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
Chapter 34. Nursing Care of Patients with Lower Gastrointestinal Disorders
 Chapter 34 Nursing Care of Patients with Lower Gastrointestinal Disorders Lower Gastrointestinal System Small Intestines Large Intestines Rectum Anus Constipation Fecal Mass Held In Rectum Feces Become
Chapter 34 Nursing Care of Patients with Lower Gastrointestinal Disorders Lower Gastrointestinal System Small Intestines Large Intestines Rectum Anus Constipation Fecal Mass Held In Rectum Feces Become
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine
 BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
BC Cancer Protocol Summary for Therapy of Adjuvant Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAJCAP Breast Dr. Stephen Chia ELIGIBILITY: Adjuvant breast cancer therapy
Community and Mental Health Services. Palliative Care. Criteria and
 Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
1/3/2008. Karen Burke Priscilla LeMone Elaine Mohn-Brown. Medical-Surgical Nursing Care, 2e Karen Burke, Priscilla LeMone, and Elaine Mohn-Brown
 Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 20 Caring for Clients with Bowel Disorders Diarrhea Pathophysiology Result from impaired water absorption
Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 20 Caring for Clients with Bowel Disorders Diarrhea Pathophysiology Result from impaired water absorption
ALIMTA Administration and Education Guide
 ALIMTA Administration and Education Guide A comprehensive guide that includes customizable dosing handouts for patients and caregivers ALIMTA is indicated for the initial treatment of patients with locally
ALIMTA Administration and Education Guide A comprehensive guide that includes customizable dosing handouts for patients and caregivers ALIMTA is indicated for the initial treatment of patients with locally
Supplementary Online Content
 Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
Capecitabine plus Docetaxel in Advanced Breast Cancer
 Capecitabine plus Docetaxel in Advanced Breast Cancer Indication: Palliative therapy in Anthracycline-Pretreated Patients with Advanced Breast Cancer Regimen details: Docetaxel 75mg/m 2 IV D1 Capecitabine*
Capecitabine plus Docetaxel in Advanced Breast Cancer Indication: Palliative therapy in Anthracycline-Pretreated Patients with Advanced Breast Cancer Regimen details: Docetaxel 75mg/m 2 IV D1 Capecitabine*
Colon Cancer Surgery
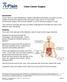 Colon Cancer Surgery Introduction Colon cancer is a life-threatening condition that affects thousands of people. Doctors usually recommend surgery for the removal of colon cancer. If your doctor recommends
Colon Cancer Surgery Introduction Colon cancer is a life-threatening condition that affects thousands of people. Doctors usually recommend surgery for the removal of colon cancer. If your doctor recommends
Therapeutic Enema for Intussusception
 Scan for mobile link. Therapeutic Enema for Intussusception Therapeutic enema is used to help identify and diagnose intussusception, a serious disorder in which one part of the intestine slides into another
Scan for mobile link. Therapeutic Enema for Intussusception Therapeutic enema is used to help identify and diagnose intussusception, a serious disorder in which one part of the intestine slides into another
Colon Cancer , The Patient Education Institute, Inc. oc Last reviewed: 05/17/2017 1
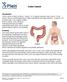 Colon Cancer Introduction Colon cancer is fairly common. About 1 in 15 people develop colon cancer. Colon cancer can be a life threatening condition that affects the large intestine. However, if it is
Colon Cancer Introduction Colon cancer is fairly common. About 1 in 15 people develop colon cancer. Colon cancer can be a life threatening condition that affects the large intestine. However, if it is
GOOVIPPC. Protocol Code: Gynecology. Tumour Group: Paul Hoskins. Contact Physician: James Conklin. Contact Pharmacist:
 BCCA Protocol Summary for Primary Treatment of Stage III less than or equal to 1 cm Visible Residual Invasive Epithelial Ovarian Cancer or Stage I Grade 3 or Stage II Grade 3 Papillary Serous Ovarian Cancer
BCCA Protocol Summary for Primary Treatment of Stage III less than or equal to 1 cm Visible Residual Invasive Epithelial Ovarian Cancer or Stage I Grade 3 or Stage II Grade 3 Papillary Serous Ovarian Cancer
Disclosures. Learning Objectives 4/21/2015. Incorporating Nutrition-Focused Physician Assessment into Malnutrition Diagnosis. None
 Incorporating Nutrition-Focused Physician Assessment into Malnutrition Diagnosis Robert DeChicco MS, RD, LD, CNSC Manager, Nutrition Support Team Center for Human Nutrition Cleveland Clinic Health System,
Incorporating Nutrition-Focused Physician Assessment into Malnutrition Diagnosis Robert DeChicco MS, RD, LD, CNSC Manager, Nutrition Support Team Center for Human Nutrition Cleveland Clinic Health System,
CTCAE & Source Documentation. Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research
 CTCAE & Source Documentation Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research Agenda 1.Definition of AE 2.AE standards 3.AE documentation 4.AE and data quality
CTCAE & Source Documentation Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research Agenda 1.Definition of AE 2.AE standards 3.AE documentation 4.AE and data quality
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine
 BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
Nursing Care of the Dialysis Patient. Adrian Hordon, MSN, RN
 Nursing Care of the Dialysis Patient Adrian Hordon, MSN, RN Understand principles of hemodialysis Recognize different access ports Identify side effects and complications Discuss nursing care for pre and
Nursing Care of the Dialysis Patient Adrian Hordon, MSN, RN Understand principles of hemodialysis Recognize different access ports Identify side effects and complications Discuss nursing care for pre and
Specialist Palliative Care Service Referral Criteria and Guidance
 Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
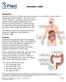 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS OVERVIEW
 Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
Aging Persons with Intellectual Developmental Disorders (IDD): Constipation KEYPOINTS A major medical conditions that commonly is seen among persons with IDD and may lead to serious complications is constipation.
SCORE 0 SCORE 1 SCORE 2 SCORE 3 Asymptomatic and fully active (ECOG 0; KPS or LPS 100%)
 Organ Scoring of Chronic GVHD PERFORMANCE SCORE: KPS ECOG LPS SKIN SCORE % BSA GVHD features to be scored by BSA: applies: Maculopapular rash/erythema Lichen planus-like features Sclerotic features Papulosquamous
Organ Scoring of Chronic GVHD PERFORMANCE SCORE: KPS ECOG LPS SKIN SCORE % BSA GVHD features to be scored by BSA: applies: Maculopapular rash/erythema Lichen planus-like features Sclerotic features Papulosquamous
A Trip Through the GI Tract: Common GI Diseases and Complaints. Jennifer Curtis, MD
 A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x 4 (3-weekly) in Early Breast Cancer
 Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Liposomal Doxorubicin (CAELYX) Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Liposomal Doxorubicin (CAELYX) Gynaecological Cancer PROCTOCOL REF: OPHAGYNCAE (Version No: 1.0) Approved for use in: Advanced ovarian cancer second/third line treatment
Systemic Anti Cancer Treatment Protocol Liposomal Doxorubicin (CAELYX) Gynaecological Cancer PROCTOCOL REF: OPHAGYNCAE (Version No: 1.0) Approved for use in: Advanced ovarian cancer second/third line treatment
Paclitaxel and Trastuzumab Breast Cancer
 Systemic Anti Cancer Treatment Protocol Paclitaxel and Trastuzumab Breast Cancer PROTOCOL REF: MPHAPTRBR (Version No: 1.0) Approved for use in: HER2 positive breast cancer. For adjuvant use in T1 or T2
Systemic Anti Cancer Treatment Protocol Paclitaxel and Trastuzumab Breast Cancer PROTOCOL REF: MPHAPTRBR (Version No: 1.0) Approved for use in: HER2 positive breast cancer. For adjuvant use in T1 or T2
Chapter 19. Assisting With Bowel Elimination. Elsevier items and derived items 2014, 2010 by Mosby, an imprint of Elsevier Inc. All rights reserved.
 Chapter 19 Assisting With Bowel Elimination Normal Bowel Elimination Time and frequency of bowel movements (BMs) vary. To assist with bowel elimination, you need to know these terms: Defecation is the
Chapter 19 Assisting With Bowel Elimination Normal Bowel Elimination Time and frequency of bowel movements (BMs) vary. To assist with bowel elimination, you need to know these terms: Defecation is the
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN)
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
Constipation. What is constipation? What is the criteria for having constipation? What are the different types of constipation?
 What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
Caring for Hospitalized Older Adults With Chemotherapy-Associated Toxicities
 Caring for Hospitalized Older Adults With Chemotherapy-Associated Toxicities Diane G. Cope, PhD, ARNP-BC, AOCNP Oncology Nurse Practitioner Florida Cancer Specialists and Research Institute Fort Myers,
Caring for Hospitalized Older Adults With Chemotherapy-Associated Toxicities Diane G. Cope, PhD, ARNP-BC, AOCNP Oncology Nurse Practitioner Florida Cancer Specialists and Research Institute Fort Myers,
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Specialist Palliative Care Referral for Patients
 Specialist Palliative Care Referral for Patients This guideline covers referrals for patients with progressive terminal illness, whether due to cancer or other disease. For many patients in the late stages
Specialist Palliative Care Referral for Patients This guideline covers referrals for patients with progressive terminal illness, whether due to cancer or other disease. For many patients in the late stages
Listing Form: Heart or Cardiovascular Impairments. Medical Provider:
 Listing Form: Heart or Cardiovascular Impairments Medical Provider: Printed Name Signature Patient Name: Patient DOB: Patient SS#: Date: Dear Provider: Please indicate whether your patient s condition
Listing Form: Heart or Cardiovascular Impairments Medical Provider: Printed Name Signature Patient Name: Patient DOB: Patient SS#: Date: Dear Provider: Please indicate whether your patient s condition
Patient Name Date of Birth Page 1 of 6
 2545 W. Hillcrest Dr. #205 Thousand Oaks, CA 91320 Admissions: 888.822.8938 Fax: 805.273.5246 Dear Medical Professional, This patient is seeking care to address eating disorder behaviors. For the patient
2545 W. Hillcrest Dr. #205 Thousand Oaks, CA 91320 Admissions: 888.822.8938 Fax: 805.273.5246 Dear Medical Professional, This patient is seeking care to address eating disorder behaviors. For the patient
NPAC+PERT+TRAS Regimen
 Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Chapter 22. Bowel Needs. Copyright 2019 by Elsevier, Inc. All rights reserved.
 Chapter 22 Bowel Needs Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 22.1 Define the key terms and key abbreviations in this chapter. Describe normal defecation and the observations to report.
Chapter 22 Bowel Needs Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 22.1 Define the key terms and key abbreviations in this chapter. Describe normal defecation and the observations to report.
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Docetaxel-EC: Docetaxel followed by Epirubicin / Cyclophosphamide in Breast Cancer
 Docetaxel-EC: Docetaxel followed by Epirubicin / Cyclophosphamide in Breast Cancer Indication: Neoadjuvant therapy f HER 2 negative high risk and fit Breast Cancer patients, suitable f a taxane containing
Docetaxel-EC: Docetaxel followed by Epirubicin / Cyclophosphamide in Breast Cancer Indication: Neoadjuvant therapy f HER 2 negative high risk and fit Breast Cancer patients, suitable f a taxane containing
NPAC(W)+PERT+TRAS Regimen
 Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Chapter 14 GASTROINTESTINAL IMPAIRMENT
 Chapter 14 GASTROINTESTINAL IMPAIRMENT Introduction This chapter provides criteria for assessing permanent impairment from entitled conditions of the gastrointestinal tract and the accessory organs of
Chapter 14 GASTROINTESTINAL IMPAIRMENT Introduction This chapter provides criteria for assessing permanent impairment from entitled conditions of the gastrointestinal tract and the accessory organs of
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Robotic Ventral Rectopexy
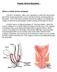 Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
5-FU & Cisplatin + Cetuximab
 5-FU & Cisplatin + Cetuximab Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from either: a) the relevant PCT
5-FU & Cisplatin + Cetuximab Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from either: a) the relevant PCT
Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy
 1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
Arm A: Induction Gemcitabine 1000 mg/m 2 IV once a week for 6 weeks.
 ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
EC-Docetaxel: Epirubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer
 EC-Docetaxel: Epirubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer Indication: Neoadjuvant therapy f high risk and fit Breast Cancer patients, suitable f a taxane containing regimen EC
EC-Docetaxel: Epirubicin / Cyclophosphamide followed by Docetaxel in Breast Cancer Indication: Neoadjuvant therapy f high risk and fit Breast Cancer patients, suitable f a taxane containing regimen EC
Immune-Mediated Adverse Events Management Handbook
 Immune-Mediated Adverse Events Management Handbook Your guide to addressing the immune-mediated adverse events (imaes) associated with patients taking PD-L1 inhibition therapy Indications and Usage IMFINZI
Immune-Mediated Adverse Events Management Handbook Your guide to addressing the immune-mediated adverse events (imaes) associated with patients taking PD-L1 inhibition therapy Indications and Usage IMFINZI
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST. Systemic Anti Cancer Treatment Protocol. EDP + mitotane
 Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
PATIENT HEALTH QUESTIONNAIRE Radiation Oncology
 REVIEWED DATE / INITIALS Safety: Yes No Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: Yes No If YES, please list medication allergies:
REVIEWED DATE / INITIALS Safety: Yes No Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: Yes No If YES, please list medication allergies:
This is a controlled document and therefore must not be changed
 AZACITIDINE NICE TA218 Treatment of adults not eligible for haematopoietic stem cell transplantation who have: Intermediate-2 and high-risk MDS according to the International Prognostic Scoring System
AZACITIDINE NICE TA218 Treatment of adults not eligible for haematopoietic stem cell transplantation who have: Intermediate-2 and high-risk MDS according to the International Prognostic Scoring System
HOMES AND SENIORS SERVICES. APPROVAL DATE: February 2011 REVISION DATE: July 2018
 Page 1 of 7 POLICY: Each resident s level of nutrition and hydration risk will be identified by the Registered Dietitian during the RAI-MDS Admission Assessment and thereafter during the quarterly, significant
Page 1 of 7 POLICY: Each resident s level of nutrition and hydration risk will be identified by the Registered Dietitian during the RAI-MDS Admission Assessment and thereafter during the quarterly, significant
PATIENT HEALTH QUESTIONNAIRE Radiation Oncology
 REVIEWED DATE / INITIALS Safety: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: If YES, please list medication allergies: Do you have
REVIEWED DATE / INITIALS Safety: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: If YES, please list medication allergies: Do you have
CASE-BASED SMALL GROUP DISCUSSION
 MHD I, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION 11 Renal Block Acid- Base Disorders November 7, 2016 MHD I, Session 11, Student Copy Page 2 Case #1 Cc: I have had
MHD I, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION 11 Renal Block Acid- Base Disorders November 7, 2016 MHD I, Session 11, Student Copy Page 2 Case #1 Cc: I have had
Pneumonia. Trachea , The Patient Education Institute, Inc. id Last reviewed: 11/11/2017 1
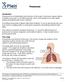 Pneumonia Introduction Pneumonia is an inflammation and infection of the lungs. Pneumonia causes millions of deaths every year. It can affect anybody, but is more dangerous to older adults, babies and
Pneumonia Introduction Pneumonia is an inflammation and infection of the lungs. Pneumonia causes millions of deaths every year. It can affect anybody, but is more dangerous to older adults, babies and
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin)
 SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
SAFETY CONSIDERATIONS WITH YONDELIS (trabectedin) Please see Important Safety Information on pages 14 and 15 and accompanying full Prescribing Information. YONDELIS (trabectedin) STUDY DESIGN INDICATION
Taiwan PI for Velcade. (Followed as USPI version Jun 2017; Local version 1701)
 Taiwan PI for Velcade (Followed as USPI version Jun 2017; Local version 1701) 1 FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE 1.1 Multiple Myeloma Velcade (bortezomib) in combination with other
Taiwan PI for Velcade (Followed as USPI version Jun 2017; Local version 1701) 1 FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE 1.1 Multiple Myeloma Velcade (bortezomib) in combination with other
CASE STUDY: ULCERATIVE COLITIS. Sammi Montag Dietetic Intern
 CASE STUDY: ULCERATIVE COLITIS Sammi Montag Dietetic Intern 2013-2014 PATIENT (CK) INTRODUCTION 26 year old female Chief complaint: bloody diarrhea and abdominal pain Admitting diagnosis: Ulcerative colitis
CASE STUDY: ULCERATIVE COLITIS Sammi Montag Dietetic Intern 2013-2014 PATIENT (CK) INTRODUCTION 26 year old female Chief complaint: bloody diarrhea and abdominal pain Admitting diagnosis: Ulcerative colitis
Irinotecan Capecitabine (14 day regimen) (I-Cap)
 Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
ASSIGNED TREATMENT ARM
 SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital
 Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
Gemcitabine + Capecitabine (ESPAC-4 Trial)
 Gemcitabine + Capecitabine (ESPAC-4 Trial) European Study Group For Pancreatic Cancer - Trial 4. Combination versus single agent chemotherapy in resectable pancreatic ductal and ampullary cancers. ***
Gemcitabine + Capecitabine (ESPAC-4 Trial) European Study Group For Pancreatic Cancer - Trial 4. Combination versus single agent chemotherapy in resectable pancreatic ductal and ampullary cancers. ***
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
IMACS FORM 07b: MYOSITIS DISEASE ACTIVITY ASSESSMENT TOOL, Version
 IMACS FORM 07b: MYOSITIS ASSESSMENT TOOL, Version 2 2005 Subject s IMACS number: ASSESSOR: Date Assessed: Assessment number: The clinical features recorded are based upon the previous 4 weeks and the judgment
IMACS FORM 07b: MYOSITIS ASSESSMENT TOOL, Version 2 2005 Subject s IMACS number: ASSESSOR: Date Assessed: Assessment number: The clinical features recorded are based upon the previous 4 weeks and the judgment
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Eastern Shore MediCann Clinic, LLC
 Eastern Shore MediCann Clinic, LLC New Patient Medical History and Intake Form Medical Marijuana Certification Name Date of Birth Social Security Number Gender: Male Female Address: Street: City: State
Eastern Shore MediCann Clinic, LLC New Patient Medical History and Intake Form Medical Marijuana Certification Name Date of Birth Social Security Number Gender: Male Female Address: Street: City: State
PATIENT INFORMATION FROM YOUR SURGEON & SAGES. Laparoscopic Colon Resection
 Patient Information published on: 03/2004 by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) PATIENT INFORMATION FROM YOUR SURGEON & SAGES Laparoscopic Colon Resection About Conventional
Patient Information published on: 03/2004 by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) PATIENT INFORMATION FROM YOUR SURGEON & SAGES Laparoscopic Colon Resection About Conventional
Carboplatin / Gemcitabine Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
Example Clinician Educational Material for Providers of Immune Effector Cellular Therapy
 Example Clinician Educational Material for Providers of Immune Effector Cellular Therapy Disclaimer: This example is just one of many potential examples of clinician education material that can be provided
Example Clinician Educational Material for Providers of Immune Effector Cellular Therapy Disclaimer: This example is just one of many potential examples of clinician education material that can be provided
Objectives 2/11/2016 HOSPICE 101
 HOSPICE 101 Overview Hospice History and Statistics What is Hospice? Who qualifies for services? Levels of Service The Admission Process Why Not to Wait Objectives Understand how to determine hospice eligibility
HOSPICE 101 Overview Hospice History and Statistics What is Hospice? Who qualifies for services? Levels of Service The Admission Process Why Not to Wait Objectives Understand how to determine hospice eligibility
Carboplatin + Paclitaxel Cancer of the Cervix
 Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
