SWISS BIOTECH DAY 2018
|
|
|
- Chloe Dixon
- 5 years ago
- Views:
Transcription
1 SWISS BIOTECH DAY 2018 Basel, 3 May 2018 Thomas Meier, CEO
2 Disclaimer This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities issued by Santhera Pharmaceuticals Holding AG. Santhera Pharmaceuticals Holding AG makes no representation (either express or implied) that the information and opinions expressed in this presentation are accurate, complete or up to date. Santhera Pharmaceuticals Holding AG disclaims, without limitation, all liability for any loss or damage of any kind, including any direct, indirect or consequential damages, which might be incurred in connection with the information contained in this presentation. This presentation expressly or implicitly contains certain forward-looking statements concerning Santhera Pharmaceuticals Holding AG and its business. Certain of these forward-looking statements can be identified by the use of forward-looking terminology or by discussions of strategy, plans or intentions. Such statements involve certain known and unknown risks, uncertainties and other factors, which could cause the actual results, financial condition, performance or achievements of Santhera Pharmaceuticals Holding AG to be materially different from any expected results, performance or achievements expressed or implied by such forward-looking statements. There can be no guarantee that any of the research and/or development projects described will succeed or that any new products or indications will be brought to market. Similarly, there can be no guarantee that Santhera Pharmaceuticals Holding AG or any future product or indication will achieve any particular level of revenue. In particular, management s expectations could be affected by, among other things, uncertainties involved in the development of new pharmaceutical products, including unexpected preclinical and clinical trial results; unexpected regulatory actions or delays or government regulation generally; the Company s ability to obtain or maintain patent or other proprietary intellectual property protection; competition in general; government, industry, and general public pricing and other political pressures. Santhera Pharmaceuticals Holding AG is providing the information in this new release as of the date of the publication, and does not undertake any obligation to update any forward-looking statements contained herein as a result of new information, future events or otherwise. 3 SBA Annual Conference 3 May 2018
3 Our mission We are focusing on the development of treatments for neuro-ophthalmological, neuromuscular and pulmonary diseases that have a high unmet medical need Focus on rare diseases Neuroophthalmological Neuromuscular Pulmonary THEIR FUTURE OUR FOCUS 3 SBA Annual Conference 3 May 2018
4 Our product pipeline Three different drug candidates covering three therapeutic areas: Neuro-ophthalmological diseases Neuromuscular diseases Pulmonary diseases Santhera Pipeline Drug Preclin. Phase 1 Phase 2 Phase 3 Filing Market Neuro-ophthalmological Diseases Leber s Hereditary Optic Neuropathy Idebenone * Raxone Neuromuscular Diseases Duchenne Muscular Dystrophy (GC non- users) Idebenone * Duchenne Muscular Dystrophy (GC users) Idebenone * Congenital Muscular Dystrophy Pulmonary Diseases Cystic Fibrosis Alpha-1 Antitrypsin Deficiency Non-Cystic Fibrosis Bronchiectasis Primary Ciliary Dyskinesia Omigapil POL6014 POL6014 POL6014 POL6014 To be explored 4 *Raxone (Santhera Pharmaceuticals) is the tradename for idebenone. Raxone (150 mg idebenone) is currently approved for the treatment of visual impairment in adolescent and adult patients with LHON GC: glucocorticoid SBA Annual Conference 3 May 2018
5 Raxone in Leber s Hereditary Optic Neuropathy (LHON) Neuro-ophthalmological Diseases
6 Raxone is the first and only approved treatment for LHON LHON, a rare mitochondrial disease resulting in progressive and severe vision loss Most common in males with a disease onset between years of age Normal vision Days, weeks or months LHON vision Within 1 year > 90% of patients experience vision loss in both eyes Raxone can lead to stabilization or recovery of vision 6 SBA Annual Conference 3 May 2018
7 Raxone improves vision in patients with LHON Prevention of further vision loss by clinically relevant stabilization (CRS) and improvement of visual acuity by a clinically relevant recovery (CRR) are important and meaningful outcomes for patients with LHON Clinical data have shown: 1 in 2 patients who received idebenone experienced a CRS, with vision remaining below logmar 1.0 ** * 1 in 3 patients who have lived with LHON for up to 5 years before treatment achieved a CRR after 6 month idebenone treatment CRS 1.0 logmar (20/200) CRR * ETDRS: early treatment diabetic retinopathy study; ** logmar: logarithm of the minimum angle of resolution 7 SBA Annual Conference 3 May 2018
8 Raxone sales in LHON since marketing authorization Raxone Net Product Sales CHF (mio) mio 22.9 mio Raxone is fully reimbursed in 8 European countries In an additional 12 European countries, Raxone is currently available by special reimbursement schemes In 2017, Israel was granted first approval of Raxone outside the EU Sales guidance for 2018: CHF million 8 SBA Annual Conference 3 May 2018
9 LEROS: An open-label, interventional Phase 4 study to assess the long-term efficacy and safety of Raxone in LHON External natural history controlled, open-label intervention study to assess the efficacy and safety of long-term treatment with idebenone in LHON 250 LHON patients Idebenone 300mg orally, 3 times daily Population Males and females with LHON 12 years of age Onset of symptoms 5 years at baseline Study design Open-label, interventional Phase 4 Treatment Treatment duration Key endpoint Status Single group assignment of idebenone 300mg orally, 3 times daily 24 months Clinically relevant recovery (CRR) of visual acuity Recruiting Centers and locations Countries: Austria Belgium Germany Italy Portugal Spain UK Poland Bulgaria U.S. 9 SBA Annual Conference 3 May 2018
10 Outlook Neuro-ophthalmology business Raxone approved in Europe for LHON Projected sales for 2018 reach profitability for neuroophthalmology business (including post approval studies) Neuroophthalmology Anticipated peak sales potential for Europe: CHF ~50 million p.a. Protection through Orphan Drug Status in Europe until 4Q 2025 Expansion of marketing authorizations to countries outside Europe 10 SBA Annual Conference 3 May 2018
11 Our product pipeline Three different drug candidates covering three therapeutic areas: Neuro-ophthalmological diseases Neuromuscular diseases Pulmonary diseases Santhera Pipeline Drug Pre-clin. Phase 1 Phase 2 Phase 3 Filing Market Neuro-ophthalmological Diseases Leber s Hereditary Optic Neuropathy Idebenone * Raxone Neuromuscular Diseases Duchenne Muscular Dystrophy (GC non- users) Idebenone * Duchenne Muscular Dystrophy (GC users) Idebenone * Congenital Muscular Dystrophy Pulmonary Diseases Cystic Fibrosis Alpha-1 Antitrypsin Deficiency Non-Cystic Fibrosis Bronchiectasis Primary Ciliary Dyskinesia Omigapil POL6014 POL6014 POL6014 POL6014 To be explored 11 *Raxone (Santhera Pharmaceuticals) is the tradename for idebenone. Raxone (150 mg idebenone) is currently approved for the treatment of visual impairment in adolescent and adult patients with LHON GC: glucocorticoid SBA Annual Conference 3 May 2018
12 Raxone in Duchenne Muscular Dystrophy Neuromuscular Diseases
13 Urgent medical need for new therapies in DMD Increasing respiratory muscle weakness in DMD leads to: As respiratory function declines, assisted ventilation is required to alleviate symptoms Decreased lung volumes and flow rates Decreased ability to cough effectively and clear airways from mucus Increased risk of airway infections There are no approved pharmacological therapies for treating respiratory decline ~35,000 patients combined in US and Europe 13 SBA Annual Conference 3 May 2018
14 Santhera studies in DMD Patient eligibility Patients with DMD not using glucocorticoids 40% of patients 10 years and older are not using glucocorticoids and were eligible for the DELOS study: The DELOS study (Phase 3) Patients with DMD using glucocorticoids Patients who are currently using glucocorticoids are eligible to enter the SIDEROS study: The SIDEROS study (Phase 3) Patients not using GCs Idebenone 900mg daily Placebo Patients using GCs Idebenone 900mg daily Placebo Treatment duration: 52 weeks Completed Treatment duration: 78 weeks Ongoing 14 SBA Annual Conference 3 May 2018
15 Regulatory strategy Patients with DMD not using glucocorticoids Successful Phase 3 DELOS trial as basis for regulatory dossier Additional natural history data to establish clinical relevance of treatment effect Additional open-label data with idebenone Best approval pathway in EU and US under consideration Patients with DMD using glucocorticoids Positive SIDEROS Study allows expansion of label to all patients irrespective of GC use status Top-line data available 2H 2020 GC: Glucocorticoid 15 SBA Annual Conference 3 May 2018
16 A Phase 3 double-blind study with idebenone in patients with DMD taking glucocorticoid steroids (SIDEROS) 266 patients using GCs 1:1 Idebenone 900mg daily Placebo Open-label extension offered Population Study design Treatments Treatment duration Key endpoint Status Patients 10 y in respiratory function decline Interventional, placebo controlled, Phase 3, RCT Parallel group assignment to idebenone 300mg orally 3 time daily, or placebo 18 months Change from baseline in forced vital capacity percent predicted (FVC %p) at 18 months Recruiting >60 sites across the EU, U.S. and Israel 10 EU countries 1 non-eu country 18 states across US Status: March 2018 FVC: forced vital capacity; GC: glucocorticoid; RCT: randomized controlled trial 16 SBA Annual Conference 3 May 2018
17 US Expanded Access Program: BreatheDMD A US Expanded Access Program (EAP) in patients with DMD Up to 250 DMD patients Population Objective Treatment Key endpoints Status DMD patients 10 years in respiratory decline Provide access to treatment with idebenone for patients with DMD in the US Idebenone 300mg orally 3 times daily Safety, tolerability, effectiveness and QoL data Enrolling Idebenone 300mg orally 3 times daily Up to 35 sites across the US CA OR WA NV Supported by: ID AZ UT Centers and locations MT WY CO NM ND SD NE TX KS OK MN IA MO AR LA WI IL MS MI IN TN AL KY OH GA WV PA SC FL VA NY NC MD VT NH NJ DE ME MA Please visit for more information 17 SBA Annual Conference 3 May 2018
18 Santhera s disease awareness campaigns in DMD Dedicated website providing information on respiratory function care US website: European website: 18 SBA Annual Conference 3 May 2018
19 Omigapil in Congenital Muscular Dystrophy (CMD) Neuromuscular Diseases 19
20 CMD is a group of inherited neuromuscular diseases CMD is characterized by progressive and potentially life-threatening muscle weakness Affected patients experience weakness in upper and lower extremities and decline in respiratory function Affects both boys and girls equally, with a disease onset frequently at birth or early childhood Prevalence is estimated as ~1 4 per 100, SBA Annual Conference 3 May 2018
21 CALLISTO: Safety and pharmacokinetics of omigapil in CMD Ascending, multiple dose cohort study evaluating the pharmacokinetic profile, safety and tolerability of oral omigapil in pediatric and adolescent patients with CMD 20 patients (5 groups) Omigapil, different doses Population Study design Treatment Treatment duration Centers Key objectives Status 5-16 year-old males and females with a CMD (clinical picture: Ullrich CMD or MDC1A) Phase 1, open-label, sequential group study 5 groups with different omigapil doses 12 weeks Single center in the US (NINDS, NIH) Establish the pharmacokinetic profile, safety and tolerability of omigapil in children and adolescents with CMD Complete 21 SBA Annual Conference 3 May 2018
22 Successful completion of CALLISTO Study NEWS RELEASE Santhera Announces Successful Completion of First Clinical Trial with Omigapil in Patients with Congenital Muscular Dystrophy Pratteln, Switzerland, April 5, 2018 Santhera Pharmaceuticals (SIX: SANN) reports the successful completion of the first clinical trial with omigapil in patients with two forms of congenital muscular dystrophy (CMD) conducted in the US at the National Institutes of Health (NIH). The ascending multiple dose cohort study (CALLISTO) met its primary objective to establish a favorable pharmacokinetic profile of omigapil and demonstrated that the study drug was safe and well tolerated in children and adolescents with CMD. Following further data analysis, the Company will discuss these results with clinical experts and regulatory authorities to prepare for a pivotal trial in patients with CMD. 22 SBA Annual Conference 3 May 2018
23 Outlook: Neuromuscular diseases pipeline Idebenone in DMD Roll-out Expanded Access Program in US Prepare for EU and US regulatory filing, initially for patients not using GCs Continue SIDEROS study in GC users; expected high level readout 2H 2020 Neuromuscular Omigapil in CMD Discuss new study design with clinical expert team Obtain input from US and EU regulators on development plan for 2 CMD subtypes GCs: glucocorticoids 23 SBA Annual Conference 3 May 2018
24 Our product pipeline Three different drug candidates covering three therapeutic areas: Neuro-ophthalmological diseases Neuromuscular diseases Pulmonary diseases Santhera Pipeline Drug Preclin. Phase 1 Phase 2 Phase 3 Filing Market Neuro-ophthalmological Diseases Leber s Hereditary Optic Neuropathy Idebenone * Raxone Neuromuscular Diseases Duchenne Muscular Dystrophy (GC non- users) Idebenone * Duchenne Muscular Dystrophy (GC users) Idebenone * Congenital Muscular Dystrophy Pulmonary Diseases Cystic Fibrosis Alpha-1 Antitrypsin Deficiency Non-Cystic Fibrosis Bronchiectasis Primary Ciliary Dyskinesia Omigapil POL6014 POL6014 POL6014 POL6014 To be explored 24 *Raxone (Santhera Pharmaceuticals) is the tradename for idebenone. Raxone (150 mg idebenone) is currently approved for the treatment of visual impairment in adolescent and adult patients with LHON GC: glucocorticoid SBA Annual Conference 3 May 2018
25 25 POL6014 in Cystic Fibrosis (CF) Pulmonary Diseases
26 Cystic Fibrosis, a rare inherited lung disease CF is a progressive, genetic disease leading to thick mucus in the lung (airway obstruction) This results in persistent lung infections, chronic inflammation and loss of respiratory function Obstruction Infection Genetic Defect Respiratory Failure Respiratory Failure Inflammation The disease is diagnosed in young children, about 70,000 patients live in US & EU Current treatments do not specifically address the chronic, underlying inflammation 26 SBA Annual Conference 3 May 2018
27 Targeting elastase for chronic lung inflammation Inflammation causes excessive production of human neutrophil elastase (hne) Elevated hne levels play a central role in lung tissue damage POL6014, a cyclic peptide, is a reversible, competitive and selective inhibitor of hne The compound has been rationally designed for potency and selectivity The drug is administered via inhalation to achieve high concentrations in the lung Inflammation Chronic inflammation is also present in other so-called neutrophilic lung diseases POL6014 presents an opportunity for a pipeline in a product 27 SBA Annual Conference 3 May 2018
28 Outlook: POL6014 Development Plan POL6014 in CF Start multiple ascending dose trial in CF patients (3Q 2018) Apply for Orphan Drug Designations for CF in EU and US (2H 2018) Prepare for Phase II efficacy trial (2019) Pulmonary Study Phase 1 Phase 2 Single Ascending Dose in healthy volunteers Completed Single Ascending Dose in CF patients Completed Multiple Ascending Dose in CF patients Start 3Q 2018 Phase 2 Efficacy Study in CF patients Start 2H 2019 POL6014 in other pulmonary diseases Explore opportunities in other pulmonary diseases with clear rationale for elastase inhibition 28 SBA Annual Conference 3 May 2018
29 Summary Continue to establish Santhera as specialty pharma company with focus on orphan drugs Successfully expanded pipeline to three therapeutic focus areas of orphan diseases First product (Raxone ) successfully launched in rare neuro-ophthalmological disease (LHON) Positive data from Phase 3 trial as basis for regulatory filing strategy in subset of DMD patients Company adequately funded to execute development and commercial plans 29 SBA Annual Conference 3 May 2018
30 THEIR FUTURE OUR FOCUS
Santhera Pharmaceuticals Company Presentation. September 2018
 Santhera Pharmaceuticals Company Presentation September 2018 Disclaimer This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
Santhera Pharmaceuticals Company Presentation September 2018 Disclaimer This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
Idebenone (Raxone ) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Kristina Nygren, MD CMO, Head of Development Santhera Pharmaceuticals,
 Idebenone (Raxone ) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Kristina Nygren, MD CMO, Head of Development Santhera Pharmaceuticals, Inc Disclaimer This presentation is not and under no circumstances
Idebenone (Raxone ) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Kristina Nygren, MD CMO, Head of Development Santhera Pharmaceuticals, Inc Disclaimer This presentation is not and under no circumstances
Advancing Mitochondrial Medicine. Thomas Meier, PhD CEO
 Advancing Mitochondrial Medicine Thomas Meier, PhD CEO Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
Advancing Mitochondrial Medicine Thomas Meier, PhD CEO Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone. Webcast, 21 November 2018
 Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone Webcast, 21 November 2018 Disclaimer This presentation is not and under no circumstances
Santhera to Acquire Option from Idorsia for Exclusive Sub-License to First-in-class Dissociative Steroid Vamorolone Webcast, 21 November 2018 Disclaimer This presentation is not and under no circumstances
on the Respiratory Health of Boys and Men With Duchenne Muscular Dystrophy (DMD)
 on the Respiratory Health of Boys and Men With Duchenne Muscular Dystrophy (DMD) At Santhera, we re studying a potential new treatment option to slow the progression of respiratory function decline in
on the Respiratory Health of Boys and Men With Duchenne Muscular Dystrophy (DMD) At Santhera, we re studying a potential new treatment option to slow the progression of respiratory function decline in
Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD)
 Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Thomas Meier, PhD February 2018 Agenda Medical need for effective treatment of respiratory illness in DMD Understanding respiratory
Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Thomas Meier, PhD February 2018 Agenda Medical need for effective treatment of respiratory illness in DMD Understanding respiratory
Santhera Enters into Agreement to Acquire Option from Idorsia for Exclusive Sub-License of First-in-class Dissociative Steroid Vamorolone
 Santhera will hold a webcast tomorrow, November 21, 2018 at 13:00 CET, 12:00 GMT, 07:00 EST. Details at the end of statement. Santhera Enters into Agreement to Acquire Option from Idorsia for Exclusive
Santhera will hold a webcast tomorrow, November 21, 2018 at 13:00 CET, 12:00 GMT, 07:00 EST. Details at the end of statement. Santhera Enters into Agreement to Acquire Option from Idorsia for Exclusive
Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone
 Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) April 26 th 2018 Raxone tablets
Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) April 26 th 2018 Raxone tablets
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Retaking NPTE
 The table below lists the requirements for retaking the National Physical Therapy Exam (NPTE) for each jurisdiction. Summary Number of attempts on NPTE limited? 16 27 Number of attempts allowed before
The table below lists the requirements for retaking the National Physical Therapy Exam (NPTE) for each jurisdiction. Summary Number of attempts on NPTE limited? 16 27 Number of attempts allowed before
Workforce Data The American Board of Pediatrics
 Workforce Data 2009-2010 The American Board of Pediatrics Caution. Before using this report as a resource, please read the information below! Please use caution when comparing data in this version of the
Workforce Data 2009-2010 The American Board of Pediatrics Caution. Before using this report as a resource, please read the information below! Please use caution when comparing data in this version of the
How is the introduction of a new medicine regulated in the UK?
 Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) A medicine called Raxone, which
Information provided to Duchenne muscular dystrophy patient organisations regarding Raxone (idebenone) and the Early Access to Medicines Scheme in the UK (EAMS 46555/0001) A medicine called Raxone, which
Uroplasty, Inc. Investor Update Canaccord Genuity Conference December 6, 2011
 Uroplasty, Inc. Investor Update Canaccord Genuity Conference December 6, 2011 Forward Looking Statement This presentation includes forward-looking statements, including financial projections, relating
Uroplasty, Inc. Investor Update Canaccord Genuity Conference December 6, 2011 Forward Looking Statement This presentation includes forward-looking statements, including financial projections, relating
Financial Impact of Lung Cancer in West Virginia
 Financial Impact of Lung Cancer in West Virginia John Deskins, Ph.D. Christiadi, Ph.D. Sara Harper November 2018 Bureau of Business & Economic Research College of Business & Economics West Virginia University
Financial Impact of Lung Cancer in West Virginia John Deskins, Ph.D. Christiadi, Ph.D. Sara Harper November 2018 Bureau of Business & Economic Research College of Business & Economics West Virginia University
THREE BIG IMPACT ISSUES
 THREE BIG IMPACT ISSUES Tim McAfee, MD, MPH Director CDC Office on Smoking and Health Presented at the National Cancer Policy Forum Workshop on Reducing Tobacco-Related Cancer Incidence and Mortality June
THREE BIG IMPACT ISSUES Tim McAfee, MD, MPH Director CDC Office on Smoking and Health Presented at the National Cancer Policy Forum Workshop on Reducing Tobacco-Related Cancer Incidence and Mortality June
The Affordable Care Act and HIV: What are the Implications?
 The Affordable Care Act and HIV: What are the Implications? 2013 National Black AIDS Institute Webinar Series September 18, 2013 Jen Kates, Kaiser Family Foundation The Challenge Figure 1 30 years into
The Affordable Care Act and HIV: What are the Implications? 2013 National Black AIDS Institute Webinar Series September 18, 2013 Jen Kates, Kaiser Family Foundation The Challenge Figure 1 30 years into
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
RECOVERY SUPPORT SERVICES IN STATES
 RECOVERY SUPPORT SERVICES IN STATES An analysis of State recovery support services using the 16 17 Substance Abuse Block Grant (SABG) Behavioral Health Assessment and Plan THIS PROJECT IS BEING SUPPORTED
RECOVERY SUPPORT SERVICES IN STATES An analysis of State recovery support services using the 16 17 Substance Abuse Block Grant (SABG) Behavioral Health Assessment and Plan THIS PROJECT IS BEING SUPPORTED
Black Women s Access to Health Insurance
 FACT SHEET Black Women s Access to Health Insurance APRIL 2018 Data released by the U.S. Census Bureau show that, despite significant health insurance gains since the Affordable Care Act (ACA) was implemented,
FACT SHEET Black Women s Access to Health Insurance APRIL 2018 Data released by the U.S. Census Bureau show that, despite significant health insurance gains since the Affordable Care Act (ACA) was implemented,
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Foreign Educated Physical Therapists
 Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 Graduation from a program equivalent to CAPTE 37 Eligibility to practice in the country in which
Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 Graduation from a program equivalent to CAPTE 37 Eligibility to practice in the country in which
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Foreign Educated PTs and PTAs
 PT Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 from a program equivalent to CAPTE 37 Eligibility to practice in the country in which education
PT Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 from a program equivalent to CAPTE 37 Eligibility to practice in the country in which education
State of California Department of Justice. Bureau of Narcotic Enforcement
 State of California Department of Justice Bureau of Narcotic Enforcement Prescription Drugs in the U.S. At least half of all Americans take one prescription drug regularly, with one in six taking three
State of California Department of Justice Bureau of Narcotic Enforcement Prescription Drugs in the U.S. At least half of all Americans take one prescription drug regularly, with one in six taking three
BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999
 STATE-BY BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999 James Verdier,, Ann Cherlow,, and Allison Barrett Mathematica Policy Research, Inc. Jeffrey Buck and Judith Teich Substance Abuse
STATE-BY BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999 James Verdier,, Ann Cherlow,, and Allison Barrett Mathematica Policy Research, Inc. Jeffrey Buck and Judith Teich Substance Abuse
Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva )
 Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
2016 COMMUNITY SURVEY
 1 Epilepsy Innovation Institute (Ei ) 016 COMMUNITY SURVEY INTRODUCTION From September 8th to November 9th, 016, epilepsy.com hosted a survey that asked the community the following: What are the aspects
1 Epilepsy Innovation Institute (Ei ) 016 COMMUNITY SURVEY INTRODUCTION From September 8th to November 9th, 016, epilepsy.com hosted a survey that asked the community the following: What are the aspects
State Tobacco Control Programs
 State Tobacco Control Programs National Cancer Policy Forum Workshop Reducing Tobacco-Related Cancer Incidence and Mortality Karla S. Sneegas, MPH Chief Program Services Branch CDC Office on Smoking and
State Tobacco Control Programs National Cancer Policy Forum Workshop Reducing Tobacco-Related Cancer Incidence and Mortality Karla S. Sneegas, MPH Chief Program Services Branch CDC Office on Smoking and
USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2
 USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2 Prepared by David Yoo, HanaSoul Consulting, Omaha, Nebraska dcyoo@cox.net
USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2 Prepared by David Yoo, HanaSoul Consulting, Omaha, Nebraska dcyoo@cox.net
Dental ER Visits: Evidence of a Failed System. Shelly Gehshan AACDP Conference April 29, 2012
 Dental ER Visits: Evidence of a Failed System Shelly Gehshan AACDP Conference April 29, 2012 Overview of Pew s s findings Preventable dental conditions were the primary diagnosis in 830,590 visits to hospital
Dental ER Visits: Evidence of a Failed System Shelly Gehshan AACDP Conference April 29, 2012 Overview of Pew s s findings Preventable dental conditions were the primary diagnosis in 830,590 visits to hospital
CHILDHOOD ALLERGIES IN AMERICA
 CHILDHOOD ALLERGIES IN AMERICA Severe Allergic Reactions Causing More Emergency Room Visits for U.S. Children PUBLISHED MARCH 13, 2018 ( 2 ) EXECUTIVE SUMMARY In this report, the Blue Cross Blue Shield
CHILDHOOD ALLERGIES IN AMERICA Severe Allergic Reactions Causing More Emergency Room Visits for U.S. Children PUBLISHED MARCH 13, 2018 ( 2 ) EXECUTIVE SUMMARY In this report, the Blue Cross Blue Shield
Alzheimer s Association Clinical Studies Initiative
 Alzheimer s Association Clinical Studies Initiative Presented at the October 4, 2007 meeting on Recruitment and Retention Challenges and Opportunities For the Alzheimer Disease Centers By Paula Moore Director,
Alzheimer s Association Clinical Studies Initiative Presented at the October 4, 2007 meeting on Recruitment and Retention Challenges and Opportunities For the Alzheimer Disease Centers By Paula Moore Director,
-- Edasalonexent Substantially Slowed Duchenne Muscular Dystrophy Disease Progression through 36 Weeks --
 Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
ADAPTIMMUNE INVESTOR PRESENTATION. August 2016
 ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
Galvus US NDA Approvable - Overview
 Galvus US NDA Approvable - Overview Galvus NDA filed with FDA Jan 2006 PDUFA action date extended by 3 months until February 26th 2007 Significant new clinical data added to NDA - Approximately 1000 patient
Galvus US NDA Approvable - Overview Galvus NDA filed with FDA Jan 2006 PDUFA action date extended by 3 months until February 26th 2007 Significant new clinical data added to NDA - Approximately 1000 patient
Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting
 Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting Kim Martin Association of State and Territorial Health Officials (ASTHO) May 12, 2015 The Need for IIS Interstate Data Exchange States,
Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting Kim Martin Association of State and Territorial Health Officials (ASTHO) May 12, 2015 The Need for IIS Interstate Data Exchange States,
Evidence-Based Policymaking: Investing in Programs that Work
 Evidence-Based Policymaking: Investing in Programs that Work August 4, 2015 The Policy Challenge Though policymakers want to make the best choices, the process often relies on inertia and anecdote Very
Evidence-Based Policymaking: Investing in Programs that Work August 4, 2015 The Policy Challenge Though policymakers want to make the best choices, the process often relies on inertia and anecdote Very
Forward Looking Statements
 Therapeutic products for respiratory diseases September 2008 Forward Looking Statements This presentation may contain forward-looking statements that are based on management s current expectations and
Therapeutic products for respiratory diseases September 2008 Forward Looking Statements This presentation may contain forward-looking statements that are based on management s current expectations and
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are three tables: Types and Limits Specific Limits
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are three tables: Types and Limits Specific Limits
Improving Oral Health:
 Improving Oral Health: New Tools for State Policy Makers Cassie Yarbrough, MPP Lead Public Policy Analyst Health Policy Institute Lansing, MI June 1, 2017 The ADA Health Policy Institute 2017 American
Improving Oral Health: New Tools for State Policy Makers Cassie Yarbrough, MPP Lead Public Policy Analyst Health Policy Institute Lansing, MI June 1, 2017 The ADA Health Policy Institute 2017 American
How to Get Paid for Doing EBD
 How to Get Paid for Doing EBD Robert D. Compton, DDS President Robert Compton, DDS Executive Director DentaQuest Institute Disclosure DentaQuest Institute President DentaQuest Benefits Senior VP & CDO
How to Get Paid for Doing EBD Robert D. Compton, DDS President Robert Compton, DDS Executive Director DentaQuest Institute Disclosure DentaQuest Institute President DentaQuest Benefits Senior VP & CDO
Women s Health Coverage: Stalled Progress
 FACT SHEET Women s Health Coverage: Stalled Progress SEPTEMBER 2018 New data from the U.S. Census Bureau show that 1 in 10 women lack access to health insurance. This year, progress in reducing the number
FACT SHEET Women s Health Coverage: Stalled Progress SEPTEMBER 2018 New data from the U.S. Census Bureau show that 1 in 10 women lack access to health insurance. This year, progress in reducing the number
AMERICAN IMMUNIZATION REGISTRY ASSOCIATION A L I S O N C H I, P R O G R A M D I R E C T O R
 AMERICAN IMMUNIZATION REGISTRY ASSOCIATION A L I S O N C H I, P R O G R A M D I R E C T O R WHAT IS AIRA A way to work together to develop standards, best practices, develop and share strategies and provide
AMERICAN IMMUNIZATION REGISTRY ASSOCIATION A L I S O N C H I, P R O G R A M D I R E C T O R WHAT IS AIRA A way to work together to develop standards, best practices, develop and share strategies and provide
Translating Science. Transforming Lives. ACT DMD Clinical Trial Results
 Translating Science. Transforming Lives ACT DMD Clinical Trial Results FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Translating Science. Transforming Lives ACT DMD Clinical Trial Results FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
SNAP Outreach within Food Banks: A View From The Ground
 SNAP Outreach within Food Banks: A View From The Ground Shana Alford, Feeding America Colleen Heflin, University of Missouri Elaine Waxman, Feeding America FEEDING AMERICA + PARTNER NAME PARTNERSHIP DISCUSSION
SNAP Outreach within Food Banks: A View From The Ground Shana Alford, Feeding America Colleen Heflin, University of Missouri Elaine Waxman, Feeding America FEEDING AMERICA + PARTNER NAME PARTNERSHIP DISCUSSION
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
Arkansas Prescription Monitoring Program
 Arkansas Prescription Monitoring Program FY 2016 Third Quarter Report January-March 2016 Arkansas Prescription Monitoring Program Quarterly Report January March, Fiscal year 2016 Act 304 of 2011 authorized
Arkansas Prescription Monitoring Program FY 2016 Third Quarter Report January-March 2016 Arkansas Prescription Monitoring Program Quarterly Report January March, Fiscal year 2016 Act 304 of 2011 authorized
-- Single Global Phase 3 Trial Expected to Begin in First Half of
 Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
A National and Statewide Perspective on the Opioid Crisis
 Insert CIO Name Here A National and Statewide Perspective on the Opioid Crisis Amy Peeples, MPA Deputy Director, National Center for Injury Prevention and Control Centers for Disease Control and Prevention
Insert CIO Name Here A National and Statewide Perspective on the Opioid Crisis Amy Peeples, MPA Deputy Director, National Center for Injury Prevention and Control Centers for Disease Control and Prevention
Breathtaking science. Developing respiratory drugs to improve health and quality of life. H.C. Wainwright Global Life Sciences Conference April 2018
 Breathtaking science Developing respiratory drugs to improve health and quality of life H.C. Wainwright Global Life Sciences Conference April 2018 www.veronapharma.com Forward-Looking Statements This presentation
Breathtaking science Developing respiratory drugs to improve health and quality of life H.C. Wainwright Global Life Sciences Conference April 2018 www.veronapharma.com Forward-Looking Statements This presentation
How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor
 How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor The Vegetarian Resource Group asks in a 2015 National Survey Conducted
How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor The Vegetarian Resource Group asks in a 2015 National Survey Conducted
Overview of the HHS National Network of Quitlines Initiative
 Overview of the HHS National Network of Quitlines Initiative Prepared for the 2005 National Oral Health Conference 6th Joint Meeting of ASTDD and AAPHD Barbara Z. Park, RDH, MPH May 2, 2005 Background
Overview of the HHS National Network of Quitlines Initiative Prepared for the 2005 National Oral Health Conference 6th Joint Meeting of ASTDD and AAPHD Barbara Z. Park, RDH, MPH May 2, 2005 Background
AMERICA S OPIOID EPIDEMIC AND ITS EFFECT ON THE NATION S COMMERCIALLY-INSURED POPULATION PUBLISHED JUNE 29, 2017
 AMERICA S OPIOID EPIDEMIC AND ITS EFFECT ON THE NATION S COMMERCIALLY-INSURED POPULATION PUBLISHED JUNE 29, 2017 America s Opioid Epidemic and Its Effect on the Nation s Commercially-Insured Population
AMERICA S OPIOID EPIDEMIC AND ITS EFFECT ON THE NATION S COMMERCIALLY-INSURED POPULATION PUBLISHED JUNE 29, 2017 America s Opioid Epidemic and Its Effect on the Nation s Commercially-Insured Population
ANNUAL REPORT EXECUTIVE SUMMARY. The full report is available at DECEMBER 2017
 ANNUAL REPORT EXECUTIVE SUMMARY DECEMBER 2017 The full report is available at www.americashealthrankings.org EXECUTIVE SUMMARY OVERVIEW America s Health Rankings presents its 28th Annual Report, providing
ANNUAL REPORT EXECUTIVE SUMMARY DECEMBER 2017 The full report is available at www.americashealthrankings.org EXECUTIVE SUMMARY OVERVIEW America s Health Rankings presents its 28th Annual Report, providing
37 th Annual J.P. Morgan Healthcare Conference. January 9, 2019
 37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
Figure 1. Illustration of the progression of visual acuity in RESCUE
 Press Release GenSight Biologics reports topline results at Week 48 of the RESCUE Phase III clinical trial of GS010 in subjects within six months of visual loss onset due to Leber Hereditary Optic Neuropathy
Press Release GenSight Biologics reports topline results at Week 48 of the RESCUE Phase III clinical trial of GS010 in subjects within six months of visual loss onset due to Leber Hereditary Optic Neuropathy
Forward Looking Statements
 Therapeutic products for respiratory diseases October 2011 Forward Looking Statements This presentation may contain forward-looking statements that are based on management s current expectations and beliefs
Therapeutic products for respiratory diseases October 2011 Forward Looking Statements This presentation may contain forward-looking statements that are based on management s current expectations and beliefs
Targeted Therapeutics for Inflammatory Disease
 Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women
 MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women For the American Public Health Association s 135 th Annual Meeting Alexandra Stewart, JD Department of Health Policy November 7, 2007 1 The
MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women For the American Public Health Association s 135 th Annual Meeting Alexandra Stewart, JD Department of Health Policy November 7, 2007 1 The
Do Facts Matter? Shelly Gehshan Director, Pew Children s s Dental Campaign
 Agenda Pew Children s Dental Campaign Why dentists might oppose new workforce models Economics of new models in private practice Do Facts Matter? Shelly Gehshan Director, Pew Children s s Dental Campaign
Agenda Pew Children s Dental Campaign Why dentists might oppose new workforce models Economics of new models in private practice Do Facts Matter? Shelly Gehshan Director, Pew Children s s Dental Campaign
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
Improving Cancer Surveillance and Mortality Data for AI/AN Populations
 Improving Cancer Surveillance and Mortality Data for AI/AN Populations Melissa A. Jim, MPH (Diné) Epidemiologist, Cancer Surveillance Branch Assigned to IHS Division of Epidemiology and Disease Prevention
Improving Cancer Surveillance and Mortality Data for AI/AN Populations Melissa A. Jim, MPH (Diné) Epidemiologist, Cancer Surveillance Branch Assigned to IHS Division of Epidemiology and Disease Prevention
Alcobra Ltd. (NASDAQ:ADHD) June Dr. Yaron Daniely President & CEO
 Alcobra Ltd. (NASDAQ:ADHD) June 2015 Dr. Yaron Daniely President & CEO 1 Forward-Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some
Alcobra Ltd. (NASDAQ:ADHD) June 2015 Dr. Yaron Daniely President & CEO 1 Forward-Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some
GLPG1690 FLORA topline results
 GLPG1690 FLORA topline results Webcast presentation 10 August 2017 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the potential activity
GLPG1690 FLORA topline results Webcast presentation 10 August 2017 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the potential activity
Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay. Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007
 Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007 Introduction 1997: Nearly 300,000 children were admitted to
Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007 Introduction 1997: Nearly 300,000 children were admitted to
What is the Objective of the DQA in Developing Performance Measures. Robert Compton, DDS Executive Director
 What is the Objective of the DQA in Developing Performance Measures Robert Compton, DDS Executive Director EBD Champions Conference May 9-10, 2014 DISCLOSURE Disclosure on DentaQuest Benefits ~ 20 million
What is the Objective of the DQA in Developing Performance Measures Robert Compton, DDS Executive Director EBD Champions Conference May 9-10, 2014 DISCLOSURE Disclosure on DentaQuest Benefits ~ 20 million
Expanding Immunizing Pharmacist Services in North Carolina
 Expanding Immunizing Pharmacist Services in North Carolina Ryan Swanson, Pharm.D. Clinical Coordinator Kerr Drug/Kerr Health September 23, 2010 Financial Disclosure No relevant financial relationships
Expanding Immunizing Pharmacist Services in North Carolina Ryan Swanson, Pharm.D. Clinical Coordinator Kerr Drug/Kerr Health September 23, 2010 Financial Disclosure No relevant financial relationships
November 2, Q Financial Results
 November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
Capricor Therapeutics
 Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
SAVARA CORPORATE PRESENTATION (NASDAQ: SVRA) NOVEMBER 2018
 SAVARA CORPORATE PRESENTATION (NASDAQ: SVRA) NOVEMBER 2018 SAFE HARBOR STATEMENT Savara Inc. ( Savara or the Company ) cautions you that statements in this presentation that are not a description of historical
SAVARA CORPORATE PRESENTATION (NASDAQ: SVRA) NOVEMBER 2018 SAFE HARBOR STATEMENT Savara Inc. ( Savara or the Company ) cautions you that statements in this presentation that are not a description of historical
Number of fatal work injuries,
 Number of fatal work injuries, 1992 2010 Number of fatal work injuries 7,000 6,217 6,331 6,632 6,275 6,202 6,238 6,055 6,054 5,920 5,915 6,000 5,534 5,575 5,764 5,734 5,840 5,657 5,214 5,000 4,551 4,690
Number of fatal work injuries, 1992 2010 Number of fatal work injuries 7,000 6,217 6,331 6,632 6,275 6,202 6,238 6,055 6,054 5,920 5,915 6,000 5,534 5,575 5,764 5,734 5,840 5,657 5,214 5,000 4,551 4,690
Improving Access to Oral Health Care for Vulnerable and Underserved Populations
 Improving Access to Oral Health Care for Vulnerable and Underserved Populations Report of the Committee on Oral Health Access to Services Shelly Gehshan Director, Pew Children s Dental Campaign Committee
Improving Access to Oral Health Care for Vulnerable and Underserved Populations Report of the Committee on Oral Health Access to Services Shelly Gehshan Director, Pew Children s Dental Campaign Committee
Calliditas Therapeutics Q2 Report Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies
 Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies Kara J. Milliron, MS, CGC Mark D. Pearlman, MD Disclosure I am a contract genetic counselor with Informed DNA, Inc. The
Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies Kara J. Milliron, MS, CGC Mark D. Pearlman, MD Disclosure I am a contract genetic counselor with Informed DNA, Inc. The
Q1 Results 2018 Webcast presentation 26 April 2018
 Q1 Results 2018 Webcast presentation 26 April 2018 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the progress of our clinical pipeline,
Q1 Results 2018 Webcast presentation 26 April 2018 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the progress of our clinical pipeline,
Managing out-licensing collaborations: a big pharma perspective
 Managing out-licensing collaborations: a big pharma perspective Arne Wörn, PhD, Head Alliance Management IID European PLG Conference, Copenhagen, Sept. 5-6 These materials contain certain forward-looking
Managing out-licensing collaborations: a big pharma perspective Arne Wörn, PhD, Head Alliance Management IID European PLG Conference, Copenhagen, Sept. 5-6 These materials contain certain forward-looking
34 th Annual J.P. Morgan Healthcare Conference
 JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
DARA Reports Year-End 2012 Financial Results
 April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
Acquisition of Novartis Influenza Vaccines Business. 27 th October 2014
 1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
Anti-IL-33 (ANB020) Program
 Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
a call to states: make alzheimer s a policy priority
 a call to states: make alzheimer s a policy priority the compassion to care, the leadership to conquer Alzheimer s is a public health crisis. One in eight Americans aged 65 and older have Alzheimer s disease
a call to states: make alzheimer s a policy priority the compassion to care, the leadership to conquer Alzheimer s is a public health crisis. One in eight Americans aged 65 and older have Alzheimer s disease
Ipsen Acquisition of Clementia Pharmaceuticals. February 25, 2019
 Ipsen Acquisition of Clementia Pharmaceuticals February 25, 2019 Disclaimer & Safe Harbor This presentation includes only summary information and does not purport to be comprehensive. Forward-looking statements,
Ipsen Acquisition of Clementia Pharmaceuticals February 25, 2019 Disclaimer & Safe Harbor This presentation includes only summary information and does not purport to be comprehensive. Forward-looking statements,
The drug 3,4-methylenedioxymethamphetamine
 March 24, 2011 Emergency Department Visits Involving Ecstasy In Brief The number of drug-related emergency department (ED) visits involving 3,4-methylenedioxymethamphetamine (MDMA), commonly known as Ecstasy,
March 24, 2011 Emergency Department Visits Involving Ecstasy In Brief The number of drug-related emergency department (ED) visits involving 3,4-methylenedioxymethamphetamine (MDMA), commonly known as Ecstasy,
Considerations for State Obesity Policy
 Considerations for State Obesity Policy Scott Kahan, MD, MPH Faculty, Johns Hopkins Bloomberg School of Public Health Director, National Center for Weight & Wellness Clinical Director, STOP Obesity Alliance,
Considerations for State Obesity Policy Scott Kahan, MD, MPH Faculty, Johns Hopkins Bloomberg School of Public Health Director, National Center for Weight & Wellness Clinical Director, STOP Obesity Alliance,
2018 Perinatal Hepatitis B Summit. Eva Hansson, RN, MSN Perinatal Hepatitis B Prevention Coordinator
 2018 Perinatal Hepatitis B Summit Eva Hansson, RN, MSN Perinatal Hepatitis B Prevention Coordinator Overview of Summit Summary of perinatal Hepatitis B in the US & Texas ACIP Hepatitis B Prevention recommendations
2018 Perinatal Hepatitis B Summit Eva Hansson, RN, MSN Perinatal Hepatitis B Prevention Coordinator Overview of Summit Summary of perinatal Hepatitis B in the US & Texas ACIP Hepatitis B Prevention recommendations
Q1 What is your age?
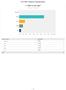 Q What is your age? Answ ered: 9 Skipped: Under 3 3-33 34-37 38-4 4+ Under 3 3-33 34-37 38-4 4+ 68.42% 3 5.79% 3.53% 2 5.26% Total 9 / 35 Q2 What is your gender? Answ ered: 9 Skipped: Male Female Male
Q What is your age? Answ ered: 9 Skipped: Under 3 3-33 34-37 38-4 4+ Under 3 3-33 34-37 38-4 4+ 68.42% 3 5.79% 3.53% 2 5.26% Total 9 / 35 Q2 What is your gender? Answ ered: 9 Skipped: Male Female Male
PS : Comprehensive HIV Prevention Programs for Health Departments
 PS12-1201: Comprehensive HIV Prevention Programs for Health Departments Program Overview Erica K. Dunbar, MPH Program Leader, Health Department Initiatives National Center for HIV/AIDS, Viral Hepatitis,
PS12-1201: Comprehensive HIV Prevention Programs for Health Departments Program Overview Erica K. Dunbar, MPH Program Leader, Health Department Initiatives National Center for HIV/AIDS, Viral Hepatitis,
Arkansas Prescription Monitoring Program
 Arkansas Prescription Monitoring Program FY 2017 Second Quarter Report October December 2016 Arkansas Prescription Monitoring Program Quarterly Report October December, Fiscal year 2017 Act 304 of 2011
Arkansas Prescription Monitoring Program FY 2017 Second Quarter Report October December 2016 Arkansas Prescription Monitoring Program Quarterly Report October December, Fiscal year 2017 Act 304 of 2011
Present value cost-savings to Medicaid over 25 years
 cost-savings from switching would be realized 2 relies on assumptions about the fraction of aggregate medical expenditures avoided and the rate at which they are realized over a 25-year period. In particular,
cost-savings from switching would be realized 2 relies on assumptions about the fraction of aggregate medical expenditures avoided and the rate at which they are realized over a 25-year period. In particular,
Edasalonexent (CAT-1004) Program
 Edasalonexent (CAT-1004) Program Oral small molecule designed to inhibit NF-κB for the treatment of Duchenne muscular dystrophy Joanne M. Donovan, MD, PhD Chief Medical Officer, Catabasis Pharmaceuticals
Edasalonexent (CAT-1004) Program Oral small molecule designed to inhibit NF-κB for the treatment of Duchenne muscular dystrophy Joanne M. Donovan, MD, PhD Chief Medical Officer, Catabasis Pharmaceuticals
August 7, Q Financial Results
 August 7, 2018 Q2 2018 Financial Results 1 Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
August 7, 2018 Q2 2018 Financial Results 1 Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
Report to Congressional Defense Committees
 Report to Congressional Defense Committees The Department of Defense Comprehensive Autism Care Demonstration Quarterly Report to Congress Second Quarter, Fiscal Year 2017 In Response to: Senate Report
Report to Congressional Defense Committees The Department of Defense Comprehensive Autism Care Demonstration Quarterly Report to Congress Second Quarter, Fiscal Year 2017 In Response to: Senate Report
50-STATE REPORT CARD
 JANUARY 2014 The State of Reproductive Health and Rights: 50-STATE REPORT CARD U.S. REPRODUCTIVE HEALTH AND RIGHTS AT A CROSSROADS The status of reproductive health and rights in the U.S. is at an historic
JANUARY 2014 The State of Reproductive Health and Rights: 50-STATE REPORT CARD U.S. REPRODUCTIVE HEALTH AND RIGHTS AT A CROSSROADS The status of reproductive health and rights in the U.S. is at an historic
Vicore Pharma AB. Audiocast, October 2, Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO
 Vicore Pharma AB Audiocast, October 2, 2017 1 Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO FORWARD-LOOKING STATEMENTS This presentation may contain certain forward-looking statements
Vicore Pharma AB Audiocast, October 2, 2017 1 Presentation by Per Jansson, CEO and Ulrike Muscha Steckelings, CSO FORWARD-LOOKING STATEMENTS This presentation may contain certain forward-looking statements
A Personal Story: Turning Tragedy into Triumph. Martha Lopez Anderson Chair, Board of Directors Parent Heart Watch
 A Personal Story: Turning Tragedy into Triumph Martha Lopez Anderson Chair, Board of Directors Parent Heart Watch A Personal Story: Turning Tragedy into Triumph Sean Alexander, a seemingly healthy and
A Personal Story: Turning Tragedy into Triumph Martha Lopez Anderson Chair, Board of Directors Parent Heart Watch A Personal Story: Turning Tragedy into Triumph Sean Alexander, a seemingly healthy and
Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
African American Women and HIV/AIDS: Confronting the Crisis and Planning for Action
 African American Women and HIV/AIDS: Confronting the Crisis and Planning for Action Kellye McKenzie, Senior Program Manager, Racial & Ethnic Health Disparities Joy Mbajah, Program Manager, Prevention Presentation
African American Women and HIV/AIDS: Confronting the Crisis and Planning for Action Kellye McKenzie, Senior Program Manager, Racial & Ethnic Health Disparities Joy Mbajah, Program Manager, Prevention Presentation
Neuropsychological Evaluations of Capacity STEVEN E. ROTHKE, PH.D., ABPP HAYLEY AMSBAUGH, M.S.
 Neuropsychological Evaluations of Capacity STEVEN E. ROTHKE, PH.D., ABPP HAYLEY AMSBAUGH, M.S. Qualifications of Neuropsychologists Doctoral degree in psychology from an accredited university training
Neuropsychological Evaluations of Capacity STEVEN E. ROTHKE, PH.D., ABPP HAYLEY AMSBAUGH, M.S. Qualifications of Neuropsychologists Doctoral degree in psychology from an accredited university training
Lewis & Clark National Estimation and Awareness Study
 Lewis & Clark National Estimation and Awareness Study Prepared by for the Institute for Tourism and Recreation Research and Montana's Tourism Advisory Council Institute for Tourism and Recreation Research
Lewis & Clark National Estimation and Awareness Study Prepared by for the Institute for Tourism and Recreation Research and Montana's Tourism Advisory Council Institute for Tourism and Recreation Research
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
Application of Advanced Practice Nurses Attitudes and Behaviors about Opioid Prescribing for Chronic Pain Survey
 Application of Advanced Practice Nurses Attitudes and Behaviors about Opioid Prescribing for Chronic Pain Survey Pat Bruckenthal, PhD, APRN-BC, ANP Aaron Gilson, MS, MSSW, PhD Conflict of Interest Disclosure
Application of Advanced Practice Nurses Attitudes and Behaviors about Opioid Prescribing for Chronic Pain Survey Pat Bruckenthal, PhD, APRN-BC, ANP Aaron Gilson, MS, MSSW, PhD Conflict of Interest Disclosure
