Clinical Research Unit Charter
|
|
|
- Norma Haynes
- 6 years ago
- Views:
Transcription
1
2 2. Key Personnel: Oncology CRU: Medical Director: James Abbruzzese, MD Research Practice Manager (RPM): Bonnie Vernarelli, RN, MSN, MBA Research Practice Manager (RPM): Wendy Bloomer, PhD, CCRP Financial Practice Manager (FPM): Chris McIver DCI Clinical Trials Office (CTO): Director of Research Administration and Operations: Karen Kharasch, BS Finance Director: Jane Gaskins, CPA Manager, Cancer Protocol Committee (CPC): Patrick Barrera, BS Manager, Clinical Research Informatics: Tim Steele, BS, MBA Manager, Clinical Trials Operations: Vijaya Chadaram, RN, MSN, CCRP 3. Oncology CRU Disease Groups & Leadership The Oncology CRU currently consists of 13 Disease Groups. Each Disease Group has a trio of leaders representing clinical care, clinical research, and basic research, who will work together to promote and advance the diagnosis, treatment, and continuing care of cancer patients. Disease Group research remains within the scope of the Oncology CRU oversight, policies and procedures. The Disease Groups are supported by a dedicated clinical research support team which includes an Assistant Research Practice Manager (ARPM), clinical nursing staff, regulatory and data personnel and Clinical Trials Office personnel. Disease-Site Director Assoc. Director Clinical Research Assoc. Director Basic Science 1 Brain Tumor Allan Friedman, MD Henry Friedman, MD Darell Bigner, MD, PhD 2 Breast Cancer P. Kelly Marcom, MD Neil Spector, MD Donald McDonnell, PhD 3 Endocrine Cancer Julie Sosa, MD 4 Gastrointestinal Cancer James Abbruzzese, MD & John Strickler, MD Gerard Blobe, MD, PhD Brian Czito, MD 5 Genitourinary Cancer Daniel George, MD Andrew Armstrong, MD Donald McDonnell, PhD 6 Gynecologic Cancer Andrew Berchuck, MD Angeles Secord, MD Donald McDonnell, PhD 7 Head and Neck Cancer David Brizel, MD Walter Lee, MD 8 Hematologic Nelson Chao, MD David Rizzieri, MD Yiping Yang, MD, PhD Malignancies 9 Melanoma TBD April Salama, MD 10 Sarcoma Brian Brigman, MD, PhD Richard Riedel, MD David Kirsch, MD, PhD 11 Thoracic Oncology Thomas D'Amico, MD Jeffrey Crawford, MD & TBD Neal Ready, MD 12 Phase I James Abbruzzese, MD 13 Radiation Oncology Christopher Willett, MD 4. Faculty Advisory Board Composition: The DCI Steering Committee serves as the Advisory Board to the Oncology CRU. The Oncology Page 2 of 5
3 CRU medical director is also the associate director of clinical research within the DCI Steering Committee. Protocols conducted in the Oncology CRU are not evaluated by the Faculty Advisory Board or the Steering Committee. The National Cancer Institute (NCI) approved Data and Safety Monitoring Plan (DSMP) ensures adequate oversight of cancer research in human subjects. Oversight begins with a rigorous review of the scientific merit and safety of each protocol, followed by a multifaceted approach to data and safety monitoring. Protocols are evaluated by individual Disease Groups and the Cancer Protocol Committee (CPC) of the DCI. Within Disease Groups, protocols are evaluated by key investigators and staff, and are subsequently assigned a priority score within the Disease Group portfolio. Protocols approved by the Disease Group leaders are submitted for review via eirb, at which point CPC review occurs. Within the CPC, protocols are independently peer-reviewed for scientific merit, priority, and progress. Scientific merit is assessed by scientific impact, protocol objectives, and study design, among other considerations. Priority within the Oncology CRU and DCI portfolio is assigned based on Disease Group priority, financial/logistical feasibility, patient need, and other considerations. Progress is evaluated by the CPC at least yearly, primarily by monitoring accrual and successful completion of key protocol benchmarks. Only those studies that meet CPC standards for scientific merit and quality get approved. Studies that do not meet key accrual or scientific benchmarks during continuing review are disapproved by the CPC. The DCI Monitoring Team conducts monitoring visits as defined by CPC, on DUHS sponsor-investigator therapeutic intervention and prevention intervention studies that do not have an external monitoring plan to ensure subject safety. Annually, one industry sponsored clinical trial from each oncology disease based group will be randomly selected for monitoring by the DCI Monitoring Team. Additional monitoring of protocols in disease based groups may be prompted by findings from internal monitoring visits, sponsor monitoring findings, unexpected frequency of serious and/or unexpected toxicities, or other concerns and may be initiated upon request of DUHS and DCI leadership, the CPC, the Safety Oversight Committee (SOC), the sponsor, the Principal Investigator, or the IRB. The monitoring team ensures that the study is conducted, recorded and reported in accordance with the protocol, standard operating procedures (SOPs), Good Clinical Practice (GCP), and applicable regulatory requirements. In addition, the oncology CRU also has a Safety Surveillance team to provide consistent, efficient, and well documented processes for collecting, evaluating, and reporting safety data and to ensure adherence with applicable regulations. 5. CRU Governance and Financial Plan: The Oncology CRU reports to the CRU medical director, James Abbruzzese. Financial Plan: Most of the Oncology CRU protocols are housed under the auspices of the DCI; however, there are some that reside within other clinical departments. The bifurcation depends on the staffing assigned to support the protocol. The operating structure within the Oncology CRU (DCI) is delineated into divisions based on disease based tumor groups as listed in Section 3. In addition to the disease groups, the DCI structure also includes the central clinical trials office responsible for oversight and compliance of all Oncology CRU projects. Projects that sit within the DCI are backstopped by the disease based programs using reserves accumulated through study margins Page 3 of 5
4 or philanthropic sources assigned to the division. The Central structure is funded using budgeted subsidies earmarked for infrastructure support. FUNDING SOURCES CENTRAL CLINICAL TRIALS OFFICE: The central services offered by the DCI in support of Oncology trials includes, but is not limited to, CPC, SOC, Safety & Compliance, Biobanking, Biostatistics, Bioinformatics, Informatics, Research Lab Services, and Space Management. DCI allocates the following funding sources to support these services: Indirect Cost Recovery Cancer Center Support Grant (NCI P30) DUHS Margin Share FUNDING SOURCES DISEASE BASED GROUPS: 90% of the program portfolio housed within DCI is sponsored through Pharmaceutical contracts. The remaining 10% includes foundational grants. All residuals (shortfall/surplus) generated by these studies are maintained within the individual divisions. The central business office of the DCI reviews performance quarterly and advises leadership on potential financial risk. In aggregate, the programs generate a small margin annually (cash-basis) which flows into program reserve accounts. These reserves are used to support investigator initiated studies (unfunded/underfunded) and backstop program infrastructure costs; surplus funds accumulated through studies do not flow into discretionary sources under the current model. 6. CRU Stakeholders: Research subjects are our premium stake holders. Other stakeholders include the Federal Government (e.g., Department of Health and Human Services, Food and Drug Administration, National Institute of Health, National Cancer Institute), Duke Cancer Institute, Duke University Health Systems, Duke University School of Medicine, all Duke Oncology investigators, oncology research professionals, sponsors, and both internal and external collaborators. 7. Communication Plan: Open communication from the Oncology CRU Leadership is a guiding principle of our organization. In addition to DOCR and Duke IRB SOPs, Oncology CRU specific SOPs and guidelines are available on the DCI Intranet. An annual DCI retreat was started in May 2012 to discuss the direction of the Oncology CRU and to provide progress updates to the Duke oncology research community. All presentations from the retreats are available on the DCI Intranet. The Oncology CRU RPMs hold monthly ARPM meetings and regulatory coordinator meetings to discuss any significant issues and provide updates as needed. The DCI hosts a monthly Lunch n Learn seminar series focusing on important aspects of cancer protocol development, submission, review, and conduct. It also disseminates information via a subscriber list and, on occasion, in the DOCR monthly Clinical Research Update. The Oncology CRU also develops educational and informational modules as necessary to target specific clinical research needs as they arise. In addition, all Oncology research personnel are encouraged to attend educational programs provided by DOCR and Duke IRB. 8. Oncology CRU Organization Chart: Page 4 of 5
5 Page 5 of 5
ROLE OF PRMS FROM START TO FINISH. Tricia Adrales Bentz, MHA Hollings Cancer Center
 ROLE OF PRMS FROM START TO FINISH Tricia Adrales Bentz, MHA Hollings Cancer Center National Cancer Institute Designation MUSC-HCC received its NCI designation in 2009. The only NCI designated cancer center
ROLE OF PRMS FROM START TO FINISH Tricia Adrales Bentz, MHA Hollings Cancer Center National Cancer Institute Designation MUSC-HCC received its NCI designation in 2009. The only NCI designated cancer center
Fall 2018 Research Training Program Agenda. S E S SI ON 1, Tuesday, S eptember 11, 2018
 S E S SI ON 1, Tuesday, S eptember 11, 2018 7:45 am 8:15 am Sign-In - Refreshments provided, course materials distributed 8 : 1 5 a m - 8 : 2 5 a m Introduction Welcome and overview of the six session
S E S SI ON 1, Tuesday, S eptember 11, 2018 7:45 am 8:15 am Sign-In - Refreshments provided, course materials distributed 8 : 1 5 a m - 8 : 2 5 a m Introduction Welcome and overview of the six session
DEVELOPMENTS IN THE CLINICAL TRIALS ENVIRONMENT Two Initiatives February 27, 2014
 DEVELOPMENTS IN THE CLINICAL TRIALS ENVIRONMENT Two Initiatives February 27, 2014 Karen Arts, Director Canadian Cancer Clinical Trial Network (3CTN) Chair of the Board of N2 Administrative Office: Lawson
DEVELOPMENTS IN THE CLINICAL TRIALS ENVIRONMENT Two Initiatives February 27, 2014 Karen Arts, Director Canadian Cancer Clinical Trial Network (3CTN) Chair of the Board of N2 Administrative Office: Lawson
Outcomes & Opportunities through Collaborating in Clinical Trials
 Outcomes & Opportunities through Collaborating in Clinical Trials Tim Mullett, MD, FACS Professor of Surgery, Division of Cardiothoracic Surgery, Chief, General Thoracic Surgery, University of Kentucky,
Outcomes & Opportunities through Collaborating in Clinical Trials Tim Mullett, MD, FACS Professor of Surgery, Division of Cardiothoracic Surgery, Chief, General Thoracic Surgery, University of Kentucky,
ALS ACT (Accelerated Therapeutics)
 ALS ACT (Accelerated Therapeutics) Request for Proposals: Phase II Clinical development of novel, high-potential treatments for people with ALS Release Date 23 October 2015 Letter of intent due: November
ALS ACT (Accelerated Therapeutics) Request for Proposals: Phase II Clinical development of novel, high-potential treatments for people with ALS Release Date 23 October 2015 Letter of intent due: November
CTA Strengths. Organisational Structure Board. CTA Board CEO. Background to Cancer Trials Australia What we do well An increasing struggle Conclusions
 Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
Alliance Organizational Structure. Trini Ajazi, MM Alliance Chief Administrative Officer Clinical Research Professional Orientation
 Alliance Organizational Structure Trini Ajazi, MM Alliance Chief Administrative Officer Clinical Research Professional Orientation May 10, 2017 NCI-NCTN National Cancer Institute (NCI) National Clinical
Alliance Organizational Structure Trini Ajazi, MM Alliance Chief Administrative Officer Clinical Research Professional Orientation May 10, 2017 NCI-NCTN National Cancer Institute (NCI) National Clinical
Johns Hopkins Clinical Research Network (JHCRN)
 Johns Hopkins Clinical Research Network (JHCRN) A Collaborative Approach to Clinical Research Charles M. Balch, MD ICTR Deputy Director and JHCRN Director Professor of Surgery, Oncology, and Dermatology
Johns Hopkins Clinical Research Network (JHCRN) A Collaborative Approach to Clinical Research Charles M. Balch, MD ICTR Deputy Director and JHCRN Director Professor of Surgery, Oncology, and Dermatology
CANCER CARE AS IT SHOULD BE
 Duke Cancer institute 2012 report CANCER CARE AS IT SHOULD BE Ushering IN a NEW era of cancer research and care at one of THE world s leading academic HEalth systems, Duke MEDICINE has created THE Duke
Duke Cancer institute 2012 report CANCER CARE AS IT SHOULD BE Ushering IN a NEW era of cancer research and care at one of THE world s leading academic HEalth systems, Duke MEDICINE has created THE Duke
Outline. Mission. NCCIH Research Priorities HSR Community Conference September 11, National Center for Complementary and Integrative Health
 NCCIH Research Priorities HSR Community Conference September 11, 2015 Wendy Weber, ND, PhD, MPH Chief, Clinical Research Branch, Division of Extramural Research Outline NCCIH Mission & Strategic Plan Funding
NCCIH Research Priorities HSR Community Conference September 11, 2015 Wendy Weber, ND, PhD, MPH Chief, Clinical Research Branch, Division of Extramural Research Outline NCCIH Mission & Strategic Plan Funding
National Cancer Institute Clinical Trial Cooperative Groups
 National Cancer Institute Clinical Trial Cooperative Groups Perspectives from the National Surgical Adjuvant Breast and Bowel Project Joyce Mull, MPM Director, Regulatory Affairs NSABP Foundation, Inc.
National Cancer Institute Clinical Trial Cooperative Groups Perspectives from the National Surgical Adjuvant Breast and Bowel Project Joyce Mull, MPM Director, Regulatory Affairs NSABP Foundation, Inc.
NCIC CTG Overview and Opportunities. Ralph Meyer Director, NCIC CTG
 NCIC CTG Overview and Opportunities Ralph Meyer Director, NCIC CTG NCIC Clinical Trials Group A research organization A cooperative clinical trials group Previously funded by NCIC Now funded by CCSRI National
NCIC CTG Overview and Opportunities Ralph Meyer Director, NCIC CTG NCIC Clinical Trials Group A research organization A cooperative clinical trials group Previously funded by NCIC Now funded by CCSRI National
Michal Roll PhD, MBA Director, R&D Division
 Michal Roll PhD, MBA Director, R&D Division Dr. Michal Roll Director, R&D Division Ofer (Heart & Brain) Emergency Underground Hospital Arison (Surgery & Oncology) Nursing School Sourasky (Medicine) Lis
Michal Roll PhD, MBA Director, R&D Division Dr. Michal Roll Director, R&D Division Ofer (Heart & Brain) Emergency Underground Hospital Arison (Surgery & Oncology) Nursing School Sourasky (Medicine) Lis
Instructions for Completing the UVMCC Clinical Research Protocol Submission Form UVMCC CLINICAL RESEARCH PROTOCOL SUBMISSION FORM
 Instructions for Completing the UVMCC Clinical Research Protocol Submission Form SUBMITTING THE APPLICATION 1. The PI must complete this application. National Clinical Trials Network (NCTN) Protocols only
Instructions for Completing the UVMCC Clinical Research Protocol Submission Form SUBMITTING THE APPLICATION 1. The PI must complete this application. National Clinical Trials Network (NCTN) Protocols only
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH 2017 REQUEST FOR APPLICATION (RFA)
 LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
R e s e a r c h S t r a t e g y
 Sheffield Teaching Hospitals NHS Foundation Trust Academic Directorate of Specialised Cancer R e s e a r c h S t r a t e g y Academic Directorate of Specialised Cancer Research Strategy Executive Summary
Sheffield Teaching Hospitals NHS Foundation Trust Academic Directorate of Specialised Cancer R e s e a r c h S t r a t e g y Academic Directorate of Specialised Cancer Research Strategy Executive Summary
September News for the Research Community
 September 2016 News for the Research Community Training Opportunities Did You Know? Clinical Research Employee Highlights Partner Resources News for the Research Community Clinical Trials Billing Office
September 2016 News for the Research Community Training Opportunities Did You Know? Clinical Research Employee Highlights Partner Resources News for the Research Community Clinical Trials Billing Office
Diversity Clinical Research Workshop Invited Faculty
 Diversity Clinical Research Workshop Invited Faculty Alex A. Adjei, MD, PhD, FACP Professor and Chair, Department of Medicine Senior Vice President of Clinical Research The Katherine Anne Gioia Chair in
Diversity Clinical Research Workshop Invited Faculty Alex A. Adjei, MD, PhD, FACP Professor and Chair, Department of Medicine Senior Vice President of Clinical Research The Katherine Anne Gioia Chair in
PCPI Update Progress on Strategic Initiatives
 Convened by the American Medical Association The Physician Consortium for Performance Improvement PCPI Update Progress on Strategic Initiatives Kathleen Blake, MD, MPH Vice President, Performance Improvement
Convened by the American Medical Association The Physician Consortium for Performance Improvement PCPI Update Progress on Strategic Initiatives Kathleen Blake, MD, MPH Vice President, Performance Improvement
Making a Difference. Highest quality care. Education. Research. Innovation. OUR MISSION:
 Making a Difference Highest quality care. Education. Research. Innovation. OUR MISSION: To deliver world-class care on a daily basis to our patients using the most advanced technologies available. To conduct
Making a Difference Highest quality care. Education. Research. Innovation. OUR MISSION: To deliver world-class care on a daily basis to our patients using the most advanced technologies available. To conduct
Comprehensive Approach to Oral and Head & Neck Cancer. at the DRAFT PLANNING DOCUMENT
 Comprehensive Approach to Oral and Head & Neck Cancer at the DRAFT PLANNING DOCUMENT 1 VCU Philips Institute for Oral & Craniofacial Molecular l Biology Vision i for the future Comprehensive Institute
Comprehensive Approach to Oral and Head & Neck Cancer at the DRAFT PLANNING DOCUMENT 1 VCU Philips Institute for Oral & Craniofacial Molecular l Biology Vision i for the future Comprehensive Institute
Update on the NCI Cancer Centers Program Henry P. Ciolino, Ph.D. Director, Office of Cancer Centers
 Update on the NCI Cancer Centers Program Henry P. Ciolino, Ph.D. Director, Office of Cancer Centers April 30, 2017 History of the CCSG Funding Opportunity Announcement What hasn t changed What has changed
Update on the NCI Cancer Centers Program Henry P. Ciolino, Ph.D. Director, Office of Cancer Centers April 30, 2017 History of the CCSG Funding Opportunity Announcement What hasn t changed What has changed
Oncology Clinical Trials Conference
 Oncology Clinical Trials Conference Assuring Research Regulatory Compliance Assuring Trial Integrity and Research Subject Wellbeing March 30 and 31, 2017 Newport Beach, CA USA Hyatt Regency Newport Beach
Oncology Clinical Trials Conference Assuring Research Regulatory Compliance Assuring Trial Integrity and Research Subject Wellbeing March 30 and 31, 2017 Newport Beach, CA USA Hyatt Regency Newport Beach
Organ Donation and Transplantation Alliance impact report
 Organ Donation and Transplantation Alliance impact report Connecting OPOs, transplant centers and donor hospitals to education and best practice resources since 2006 Dear Organ Donation and Transplantation
Organ Donation and Transplantation Alliance impact report Connecting OPOs, transplant centers and donor hospitals to education and best practice resources since 2006 Dear Organ Donation and Transplantation
Stephen C. Joseph, M.D., M.P.H.
 MAR 05 1996 MEMORANDUM FOR: ASSISTANT SECRETARY OF THE ARMY (M&RA) ASSISTANT SECRETARY OF THE NAVY (M&RA) ASSISTANT SECRETARY OF THE AIR FORCE (MRAI&E) SUBJECT: DoD Participation in Clinical Cancer Trials
MAR 05 1996 MEMORANDUM FOR: ASSISTANT SECRETARY OF THE ARMY (M&RA) ASSISTANT SECRETARY OF THE NAVY (M&RA) ASSISTANT SECRETARY OF THE AIR FORCE (MRAI&E) SUBJECT: DoD Participation in Clinical Cancer Trials
2018 AFP INTERNATIONAL COMMITTEE DESCRIPTIONS
 2018 AFP INTERNATIONAL COMMITTEE DESCRIPTIONS MEMBERSHIP SERVICES DIVISION Leadership Development and Member Engagement: Ensures AFP services to members and provides support to chapters and chapter services.
2018 AFP INTERNATIONAL COMMITTEE DESCRIPTIONS MEMBERSHIP SERVICES DIVISION Leadership Development and Member Engagement: Ensures AFP services to members and provides support to chapters and chapter services.
ONCOLOGY MEDICAL HOME ACCREDITATION
 2015 Community Oncology Alliance 1 ONCOLOGY MEDICAL HOME ACCREDITATION Panel Moderator: Bo Gamble Director of Strategic Practice Initiatives, Community Oncology Alliance 1 ONCOLOGY MEDICAL HOME ACCREDITATION
2015 Community Oncology Alliance 1 ONCOLOGY MEDICAL HOME ACCREDITATION Panel Moderator: Bo Gamble Director of Strategic Practice Initiatives, Community Oncology Alliance 1 ONCOLOGY MEDICAL HOME ACCREDITATION
Reporting to the IRB Part 2
 Webinar Series Reporting to the IRB Part 2 Frequently Asked Questions December 4, 2013 Presented by: James MacFarlane Director of Board Services About Schulman Associates IRB Established in 1983 US and
Webinar Series Reporting to the IRB Part 2 Frequently Asked Questions December 4, 2013 Presented by: James MacFarlane Director of Board Services About Schulman Associates IRB Established in 1983 US and
Amethyst House Strategic Plan
 Amethyst House Strategic Plan Mission Amethyst House provides a foundation for sober living by partnering with individuals, families and communities impacted by addictions and substance-abuse issues, offering
Amethyst House Strategic Plan Mission Amethyst House provides a foundation for sober living by partnering with individuals, families and communities impacted by addictions and substance-abuse issues, offering
Building a Statewide Cancer Clinical Trials Network
 Building a Statewide Cancer Clinical Trials Network September 25, 2018 Marie L. Rahne, MBA Senior Manager, Minnesota Cancer Clinical Trials Network 7 & 19 Partners Sites 59 Minnesotans Enrolled on Cancer
Building a Statewide Cancer Clinical Trials Network September 25, 2018 Marie L. Rahne, MBA Senior Manager, Minnesota Cancer Clinical Trials Network 7 & 19 Partners Sites 59 Minnesotans Enrolled on Cancer
Request for Applications Basic, Clinical and Population Science Pilot Awards
 Request for Applications Basic, Clinical and Population Science Pilot Awards Spring, 2013 SUMMARY The University of Wisconsin Carbone Cancer Center (UWCCC) is an NCI-designated Comprehensive Cancer Center
Request for Applications Basic, Clinical and Population Science Pilot Awards Spring, 2013 SUMMARY The University of Wisconsin Carbone Cancer Center (UWCCC) is an NCI-designated Comprehensive Cancer Center
UC Brain Tumor Center Molecular Therapeutics Pilot Grant Program 2013/2014
 Introduction An estimated 25,000 new cases of primary brain tumors will be diagnosed in the United States in 2013, and an estimated 170,000 patients will be diagnosed with brain metastases, most often
Introduction An estimated 25,000 new cases of primary brain tumors will be diagnosed in the United States in 2013, and an estimated 170,000 patients will be diagnosed with brain metastases, most often
Office of Clinical Research Annual Report 2017 July 1, June 30, 2017
 Office of Clinical Research Annual Report 2017 July 1, 2016- June 30, 2017 Table of Contents Title Page Number Mission Statement 1 Objective 1 Research Services 1 Research Highlights 2 Figure 1 Number
Office of Clinical Research Annual Report 2017 July 1, 2016- June 30, 2017 Table of Contents Title Page Number Mission Statement 1 Objective 1 Research Services 1 Research Highlights 2 Figure 1 Number
SKCC Protocol Review Committee New Study Application
 Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
LION. Corporate Presentation June 2016 BIOTECHNOLOGIES. Leadership & Innovation in Oncology
 LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
Community Outreach and Dissemination for Prostate Cancer Research
 Community Outreach and Dissemination for Prostate Cancer Research Chanita Hughes Halbert, Ph.D. Department of Psychiatry and Abramson Cancer Center University of Pennsylvania Thomas Anderson, Jr. Abigail
Community Outreach and Dissemination for Prostate Cancer Research Chanita Hughes Halbert, Ph.D. Department of Psychiatry and Abramson Cancer Center University of Pennsylvania Thomas Anderson, Jr. Abigail
Country Participation: An Overview for Interested Investigators
 Country Participation: An Overview for Interested Investigators 1 Introduction This document provides an overview of the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). If you have any
Country Participation: An Overview for Interested Investigators 1 Introduction This document provides an overview of the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). If you have any
HIV QUALITY MANAGEMENT PLAN Updated April 2011
 Idaho Department of Health and Welfare Family Planning, STD and HIV Programs Ryan White Part B Program HIV QUALITY MANAGEMENT PLAN Updated April 2011 QUALITY STATEMENT The Idaho Department of Health and
Idaho Department of Health and Welfare Family Planning, STD and HIV Programs Ryan White Part B Program HIV QUALITY MANAGEMENT PLAN Updated April 2011 QUALITY STATEMENT The Idaho Department of Health and
New NCCIH Funding Opportunities for Mind and Body Clinical Trials. April 24, 2017
 New NCCIH Funding Opportunities for Mind and Body Clinical Trials April 24, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H. Branch Chief, Clinical Research in Complementary and Integrative Health Branch,
New NCCIH Funding Opportunities for Mind and Body Clinical Trials April 24, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H. Branch Chief, Clinical Research in Complementary and Integrative Health Branch,
Excellence in Trial Management
 Excellence in Trial Management COMPANY OVERVIEW Privately held company Founded in 2007 Contract Research Organization HQ in Montréal, Canada Specialized in Clinical Development & Trial Management Client
Excellence in Trial Management COMPANY OVERVIEW Privately held company Founded in 2007 Contract Research Organization HQ in Montréal, Canada Specialized in Clinical Development & Trial Management Client
CONTRIBUTED SESSION 1
 IMPROVING TRANSPARENCY IN CLINICAL TRIAL COSTS FOR PARTICIPANTS: NATIONAL CANCER INSTITUTE (NCI) NATIONAL COVERAGE ANALYSIS INITIATIVE SUPPORTED BY THE NCI S CANCER TRIALS SUPPORT UNIT Lawrence R Ragard
IMPROVING TRANSPARENCY IN CLINICAL TRIAL COSTS FOR PARTICIPANTS: NATIONAL CANCER INSTITUTE (NCI) NATIONAL COVERAGE ANALYSIS INITIATIVE SUPPORTED BY THE NCI S CANCER TRIALS SUPPORT UNIT Lawrence R Ragard
Together 2 Goal Innovator Track: Cardiovascular Disease Cohort. Call for Participation
 Together 2 Goal Innovator Track: Cardiovascular Disease Cohort Call for Participation Cardiovascular Disease (CVD) and Diabetes Approximately 28 million Americans are living with Type 2 diabetes. Due to
Together 2 Goal Innovator Track: Cardiovascular Disease Cohort Call for Participation Cardiovascular Disease (CVD) and Diabetes Approximately 28 million Americans are living with Type 2 diabetes. Due to
SOP-QA-30 V Scope
 Title: Effective Date: 1-4-17 Review Date: 1-4-20 Author: Joanne Rodger, Senior R&D Manager QA Approval: Richard Cowie, QA Manager Approver: Prof Maggie Cruickshank, R&D Director Approver: Prof Steve Heys,
Title: Effective Date: 1-4-17 Review Date: 1-4-20 Author: Joanne Rodger, Senior R&D Manager QA Approval: Richard Cowie, QA Manager Approver: Prof Maggie Cruickshank, R&D Director Approver: Prof Steve Heys,
The future of cancer care... now 1. Strategic Plan
 2017 2021 The future of cancer care... now 1 Strategic Plan 2 Duke Cancer Institute 2017 2021 Strategic Plan Mission: We discover, develop and deliver the future of cancer care... now. Vision: At Duke
2017 2021 The future of cancer care... now 1 Strategic Plan 2 Duke Cancer Institute 2017 2021 Strategic Plan Mission: We discover, develop and deliver the future of cancer care... now. Vision: At Duke
Accelerating Translation at Dana-Farber Cancer Institute*
 Accelerating Translation at Dana-Farber Cancer Institute* *and our partner institutions February 12, 2013 Edward J Benz JR, MD Dana Farber Cancer Institute 1 2 Academic Structure All Dana-Farber faculty
Accelerating Translation at Dana-Farber Cancer Institute* *and our partner institutions February 12, 2013 Edward J Benz JR, MD Dana Farber Cancer Institute 1 2 Academic Structure All Dana-Farber faculty
2018 Media Kit JOURNALS CLASSIFIED ADVERTISING. Table of Contents Click the links above to jump to the desired page
 JOURNALS CLASSIFIED ADVERTISING 2018 Media Kit Journal of Clinical Oncology (JCO) serves its readers as the single most credible, authoritative resource for disseminating significant clinical oncology
JOURNALS CLASSIFIED ADVERTISING 2018 Media Kit Journal of Clinical Oncology (JCO) serves its readers as the single most credible, authoritative resource for disseminating significant clinical oncology
Murtha Cancer Center The DoD Cancer Center of Excellence
 Accelerating Progress against Cancer through Collaboration Center of Excellence Oversight Board September 10, 2013 Colonel Craig D. Shriver, MC, USA. FACS Director 1 Summary Murtha Cancer Center Overview
Accelerating Progress against Cancer through Collaboration Center of Excellence Oversight Board September 10, 2013 Colonel Craig D. Shriver, MC, USA. FACS Director 1 Summary Murtha Cancer Center Overview
African Access Initiative (AAI)
 AAI African Access Initiative (AAI) African Consortium for Cancer Clinical Trials (AC 3 T) Highlights African Access Initiative (AAI) African Consortium for Cancer Clinical Trials (AC 3 T) AAI AAI Approach
AAI African Access Initiative (AAI) African Consortium for Cancer Clinical Trials (AC 3 T) Highlights African Access Initiative (AAI) African Consortium for Cancer Clinical Trials (AC 3 T) AAI AAI Approach
2017 Bon Secours Cancer Institute Summit
 2017 Bon Secours Cancer Institute Summit September 29, 2017 WE SEE CANCER DIFFERENTLY The educational activity is Jointly Provided by the Office of Continuing Medical Education of the University of Virginia
2017 Bon Secours Cancer Institute Summit September 29, 2017 WE SEE CANCER DIFFERENTLY The educational activity is Jointly Provided by the Office of Continuing Medical Education of the University of Virginia
Pandemic Influenza Preparedness Framework. Sharing influenza viruses & access to vaccines and other benefits
 Pandemic Influenza Preparedness Framework Sharing influenza viruses & access to vaccines and other benefits What is PIP? Landmark, innovative public health arrangement to increase global preparedness to
Pandemic Influenza Preparedness Framework Sharing influenza viruses & access to vaccines and other benefits What is PIP? Landmark, innovative public health arrangement to increase global preparedness to
Trends in Oncology: Preparing for Seismic Change. ASCO s Clinical Affairs Department
 Trends in Oncology: Preparing for Seismic Change Association of Northern California Oncologists May 20, 2015 Thomas R. Barr, MBA Director, Business Metrics and Analysis Clinical Affairs Department ASCO
Trends in Oncology: Preparing for Seismic Change Association of Northern California Oncologists May 20, 2015 Thomas R. Barr, MBA Director, Business Metrics and Analysis Clinical Affairs Department ASCO
62 accc-cancer.org May June 2016 OI
 62 accc-cancer.org May June 2016 OI BY MICHAEL A. CALIGIURI, MD; WILLIAM S. DALTON, PHD, MD; LORNA RODRIGUEZ, MD, PHD; THOMAS SELLERS, PHD, MPH; AND CHERYL L. WILLMAN, MD Reshaping Cancer Research & Treatment
62 accc-cancer.org May June 2016 OI BY MICHAEL A. CALIGIURI, MD; WILLIAM S. DALTON, PHD, MD; LORNA RODRIGUEZ, MD, PHD; THOMAS SELLERS, PHD, MPH; AND CHERYL L. WILLMAN, MD Reshaping Cancer Research & Treatment
Structured Pathology Reporting of Cancer Newsletter
 Structured Pathology Reporting of Cancer Newsletter Welcome to the second quarterly edition of the Structured Pathology Reporting of Cancer newsletter. This newsletter is intended to provide information
Structured Pathology Reporting of Cancer Newsletter Welcome to the second quarterly edition of the Structured Pathology Reporting of Cancer newsletter. This newsletter is intended to provide information
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS
 NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
Cancer Center Support Grant
 Cancer Center Support Grant Erin Bank, PhD Associate Director for Programs and Planning UCSF HDFCCC 03.14.2017 HDFCCC Staff Engagement Talk NCI Cancer Centers 1937: NCI established by Roosevelt Annual
Cancer Center Support Grant Erin Bank, PhD Associate Director for Programs and Planning UCSF HDFCCC 03.14.2017 HDFCCC Staff Engagement Talk NCI Cancer Centers 1937: NCI established by Roosevelt Annual
1 st Annual Treatment-Related Adverse Events Symposium: FOCUS ON MANAGEMENT. Friday, October 27, Zuckerman Research Center, New York City
 1 st Annual Treatment-Related Adverse Events Symposium: FOCUS ON MANAGEMENT Friday, October 27, 2017 Zuckerman Research Center, New York City 1 st Annual Treatment-Related Adverse Events Symposium: FOCUS
1 st Annual Treatment-Related Adverse Events Symposium: FOCUS ON MANAGEMENT Friday, October 27, 2017 Zuckerman Research Center, New York City 1 st Annual Treatment-Related Adverse Events Symposium: FOCUS
GATRA/GCCR Fall Conference 14 16, /13/2012. Integration of the Rapid Quality Reporting. System (RQRS) and Patient Navigation
 Reporting System (RQRS) Northside Hospital Cancer Institute GATRA and GCCR 2012 Annual Conference Amy Waits, BS, CTR Northside Hospital: Atlanta, Georgia National Cancer Institute Community Cancer Centers
Reporting System (RQRS) Northside Hospital Cancer Institute GATRA and GCCR 2012 Annual Conference Amy Waits, BS, CTR Northside Hospital: Atlanta, Georgia National Cancer Institute Community Cancer Centers
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center
 FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
Research and Development
 INTRODUCTION Research and Development Cambridgeshire and Peterborough CCG employ a small primary care Research & Development (R&D) Team that sit in the Improving Outcomes Team. The R&D Team support the
INTRODUCTION Research and Development Cambridgeshire and Peterborough CCG employ a small primary care Research & Development (R&D) Team that sit in the Improving Outcomes Team. The R&D Team support the
New NCCIH Funding Opportunities for Natural Product Clinical Trials. May 9, 2017
 New NCCIH Funding Opportunities for Natural Product Clinical Trials May 9, 2017 The Webinar will start at 2:00 p.m. All participants have been placed on mute. We will take questions at the end of the presentation
New NCCIH Funding Opportunities for Natural Product Clinical Trials May 9, 2017 The Webinar will start at 2:00 p.m. All participants have been placed on mute. We will take questions at the end of the presentation
Canadian Prairies Chapter of HIMSS is Coming to Town. December 17, 2018
 Canadian Prairies Chapter of HIMSS is Coming to Town December 17, 2018 Overview What it means to be a HIMSS Member: Prairie Perspectives Guy Paterson, IRG Informatics, Saskatchewan David Pincock, Alberta
Canadian Prairies Chapter of HIMSS is Coming to Town December 17, 2018 Overview What it means to be a HIMSS Member: Prairie Perspectives Guy Paterson, IRG Informatics, Saskatchewan David Pincock, Alberta
PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM
 HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
NCCIH s New Approach to Funding Clinical Trials Informational Webinar. April 18, 2017
 NCCIH s New Approach to Funding Clinical Trials Informational Webinar April 18, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H., Branch Chief, Clinical Research in Complementary and Integrative Health
NCCIH s New Approach to Funding Clinical Trials Informational Webinar April 18, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H., Branch Chief, Clinical Research in Complementary and Integrative Health
Competence by Design. Kenneth A. Harris, MD FRCSC Deputy CEO & Executive Director, Office of Specialty Education February 3, 2016
 Competence by Design Kenneth A. Harris, MD FRCSC Deputy CEO & Executive Director, Office of Specialty Education February 3, 2016 The best health for all. The best care for all. Discussion Topics Overview
Competence by Design Kenneth A. Harris, MD FRCSC Deputy CEO & Executive Director, Office of Specialty Education February 3, 2016 The best health for all. The best care for all. Discussion Topics Overview
Increasing Access to Clinical and Educational Studies
 Increasing Access to Clinical and Educational Studies Ronald E. Myers, PhD 1 ; Audrey Berry, MSN 1 ; Patricia Bradley, PhD 2 ; James Cocroft, MA 1 ; Constantine Daskalakis, ScD 3 ; Ernestine Delmoor, MPH
Increasing Access to Clinical and Educational Studies Ronald E. Myers, PhD 1 ; Audrey Berry, MSN 1 ; Patricia Bradley, PhD 2 ; James Cocroft, MA 1 ; Constantine Daskalakis, ScD 3 ; Ernestine Delmoor, MPH
REPORT. A Model Clinical Trials System for the 21st Century
 www.georgiacore.org www.georgiacancertrials.org REPORT A Model Clinical Trials System for the 21st Century A Response to the Institute of Medicine s 2010 Report A National Cancer Clinical Trials System
www.georgiacore.org www.georgiacancertrials.org REPORT A Model Clinical Trials System for the 21st Century A Response to the Institute of Medicine s 2010 Report A National Cancer Clinical Trials System
National Cancer Institute
 National Cancer Institute U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute Community Oncology Research Program (NCORP) Institute of Medicine Implementing
National Cancer Institute U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute Community Oncology Research Program (NCORP) Institute of Medicine Implementing
CHAPTER House Bill No. 5203
 CHAPTER 2014-165 House Bill No. 5203 An act relating to cancer centers; amending s. 20.435, F.S.; authorizing funds in the Biomedical Research Trust Fund to be used for the Florida Consortium of National
CHAPTER 2014-165 House Bill No. 5203 An act relating to cancer centers; amending s. 20.435, F.S.; authorizing funds in the Biomedical Research Trust Fund to be used for the Florida Consortium of National
THE FUTURE OF DERMATOLOGY THROUGH THE LENS OF MEDICAL INFORMATICS
 This course is no longer being offered for CME credit. is pleased to announce: THE FUTURE OF DERMATOLOGY THROUGH THE LENS OF MEDICAL INFORMATICS CONFERENCE LOCATION MEMORIAL SLOAN KETTERING CANCER CENTER
This course is no longer being offered for CME credit. is pleased to announce: THE FUTURE OF DERMATOLOGY THROUGH THE LENS OF MEDICAL INFORMATICS CONFERENCE LOCATION MEMORIAL SLOAN KETTERING CANCER CENTER
CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING. MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS
 CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS MISSION The Center for Cognitive and Behavioral Brain Imaging is dedicated to pursuing structural and
CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS MISSION The Center for Cognitive and Behavioral Brain Imaging is dedicated to pursuing structural and
Environmental, Health and Safety
 Environmental, Health and Safety Codes of Practice The Environmental, Health and Safety (EHS) Codes of Practice set forth Zimmer EHS requirements for our business functions and facilities worldwide. In
Environmental, Health and Safety Codes of Practice The Environmental, Health and Safety (EHS) Codes of Practice set forth Zimmer EHS requirements for our business functions and facilities worldwide. In
Building a Strong Quality Culture
 Building a Strong Quality Culture Alicia Mozzachio, RPh, MPH Senior Advisor for International Activities Office of Policy for Pharmaceutical Quality Office of Pharmaceutical Quality 1 Agenda Changing an
Building a Strong Quality Culture Alicia Mozzachio, RPh, MPH Senior Advisor for International Activities Office of Policy for Pharmaceutical Quality Office of Pharmaceutical Quality 1 Agenda Changing an
2017 CANCER ANNUAL REPORT
 2017 CANCER ANNUAL REPORT A WORD FROM OUR LEADERSHIP We are pleased to present our 2017 Annual Report highlighting advances in state of the art cancer care at the Roper St. Francis Cancer Program. Our
2017 CANCER ANNUAL REPORT A WORD FROM OUR LEADERSHIP We are pleased to present our 2017 Annual Report highlighting advances in state of the art cancer care at the Roper St. Francis Cancer Program. Our
Scientific Council Fifty-first Session 21/11/2014
 Fifty-first Session 21/11/2014 Lyon, 28 30 January 2015 Auditorium DIRECTOR S RESPONSE TO THE SECTIONS OF IARC MONOGRAPHS (IMO) AND MOLECULAR PATHOLOGY (MPA) REVIEWS, HELD AT IARC IN JANUARY 2014 A number
Fifty-first Session 21/11/2014 Lyon, 28 30 January 2015 Auditorium DIRECTOR S RESPONSE TO THE SECTIONS OF IARC MONOGRAPHS (IMO) AND MOLECULAR PATHOLOGY (MPA) REVIEWS, HELD AT IARC IN JANUARY 2014 A number
Where Sites, Sponsors & CROs Partner for Success
 Where Sites, Sponsors & CROs Partner for Success October 8-11, 2015 Amelia Island, FL The Global Site Solutions Summit brings together research site executives and industry leaders for powerful collaboration
Where Sites, Sponsors & CROs Partner for Success October 8-11, 2015 Amelia Island, FL The Global Site Solutions Summit brings together research site executives and industry leaders for powerful collaboration
UC Davis Comparative Oncology Program and Current Research
 UC Davis Comparative Oncology Program and Current Research Michael S. Kent, DVM, DACVIM (Oncology), DACVIM (Radiation Oncology) Professor Radiation Oncology Director, Center for Companion Animal Health
UC Davis Comparative Oncology Program and Current Research Michael S. Kent, DVM, DACVIM (Oncology), DACVIM (Radiation Oncology) Professor Radiation Oncology Director, Center for Companion Animal Health
Update of The National Vaccine Plan
 Update of The National Vaccine Plan Raymond A. Strikas, MD National Vaccine Program Office Department of Health and Human Services Presentation Outline Statutory basis for the National Vaccine Program
Update of The National Vaccine Plan Raymond A. Strikas, MD National Vaccine Program Office Department of Health and Human Services Presentation Outline Statutory basis for the National Vaccine Program
Duke at. American Society of Clinical Oncology Chicago, Illinois
 Duke at American Society of Clinical Oncology 2017 Chicago, Illinois Duke Medicine Divisions of Hematology Medical Oncology and Hematologic Malignancy & Cellular Therapy Cordially invite you to the Duke
Duke at American Society of Clinical Oncology 2017 Chicago, Illinois Duke Medicine Divisions of Hematology Medical Oncology and Hematologic Malignancy & Cellular Therapy Cordially invite you to the Duke
I. Introduction. II. Program Description
 Advanced Post-Graduate Athletic Training Program Division of Sports Medicine Department of Orthopaedic Surgery Department of Athletics, Physical Education and Recreation I. Introduction The Stanford University
Advanced Post-Graduate Athletic Training Program Division of Sports Medicine Department of Orthopaedic Surgery Department of Athletics, Physical Education and Recreation I. Introduction The Stanford University
Children s Hospital of Philadelphia Committees for the Protection of Human Subjects Policies and Procedures Research That Must Be Reviewed by the IRB
 Page: 1 of 5 I. PURPOSE II. III. IV. To provide guidance on the types of research activities that are subject to review and approval by the Committees for the Protection of Human, which comprise the Institutional
Page: 1 of 5 I. PURPOSE II. III. IV. To provide guidance on the types of research activities that are subject to review and approval by the Committees for the Protection of Human, which comprise the Institutional
Sacramento Transitional Grant Area. Ryan White CARE Program Continuous Quality Improvement Plan
 Sacramento Transitional Grant Area Ryan White CARE Program Continuous Quality Improvement Plan July 2018 March 2020 Table of Contents Introduction... 3 Quality Statement... 5 Vision... 5 Mission... 5 Purpose...
Sacramento Transitional Grant Area Ryan White CARE Program Continuous Quality Improvement Plan July 2018 March 2020 Table of Contents Introduction... 3 Quality Statement... 5 Vision... 5 Mission... 5 Purpose...
DP Program 2 National Comprehensive Cancer Control Program. Objective Reviewer s Tool March 2017
 DP17-1701 Program 2 National Comprehensive Cancer Control Program Objective Reviewer s Tool March 2017 Approach (0-40 total points) Describes the cancer burden in the applicant s jurisdiction? (5) Clearly
DP17-1701 Program 2 National Comprehensive Cancer Control Program Objective Reviewer s Tool March 2017 Approach (0-40 total points) Describes the cancer burden in the applicant s jurisdiction? (5) Clearly
Getting Urologists Involved as Leaders in Major Clinical Trials
 Getting Urologists Involved as Leaders in Major Clinical Trials Society of Academic Urologists Annual Meeting American Urological Association San Francisco, CA May 17 th, 2018 Brian F. Chapin, MD, FACS
Getting Urologists Involved as Leaders in Major Clinical Trials Society of Academic Urologists Annual Meeting American Urological Association San Francisco, CA May 17 th, 2018 Brian F. Chapin, MD, FACS
PET Steering Committee Meeting Minutes. Wednesday, January 20 th, 2016 Time: 1:30-3:30pm
 PET Steering Committee Meeting Minutes Wednesday, January 20 th, 2016 Time: 1:30-3:30pm Committee Members Present: U. Metser (Chair), R. dekemp, M. Freeman, M. Greenberg, D. Hussey, A. Singnurkar, Y. Ung
PET Steering Committee Meeting Minutes Wednesday, January 20 th, 2016 Time: 1:30-3:30pm Committee Members Present: U. Metser (Chair), R. dekemp, M. Freeman, M. Greenberg, D. Hussey, A. Singnurkar, Y. Ung
OFFICE OF THE CHANCELLOR OFFICE OF THE VICE CHANCELLOR FOR RESEARCH
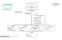 OFFICE OF THE CHANCELLOR STATE OF HAWAII UNIVERSITY OF HAWAII OFFICE OF THE VICE CHANCELLOR FOR RESEARCH UNIVERSITY OF HAWAII CANCER CENTER ORGANIZATION CHART I Grand Total General Funds: 50.00 OFFICE
OFFICE OF THE CHANCELLOR STATE OF HAWAII UNIVERSITY OF HAWAII OFFICE OF THE VICE CHANCELLOR FOR RESEARCH UNIVERSITY OF HAWAII CANCER CENTER ORGANIZATION CHART I Grand Total General Funds: 50.00 OFFICE
Sheila Prindiville, MD, MPH Director Coordinating Center for Clinical Trials National Cancer Institute
 Sheila Prindiville, MD, MPH Director Coordinating Center for Clinical Trials National Cancer Institute NCI and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE) Sheila A. Prindiville, M.D.,
Sheila Prindiville, MD, MPH Director Coordinating Center for Clinical Trials National Cancer Institute NCI and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE) Sheila A. Prindiville, M.D.,
Overview PAUL J. PETRUSKA, M.D.
 Overview The Division of Hematology and Oncology continues to provide excellence in education, patient care, and clinical research. The division is currently staffed with six attending physicians, a certified
Overview The Division of Hematology and Oncology continues to provide excellence in education, patient care, and clinical research. The division is currently staffed with six attending physicians, a certified
Cancer: Pathophysiology, Current Therapies, Clinical Trials and Drug Development One Washington Circle Hotel, Washington, DC October 19-21, 2016
 Cancer: Pathophysiology, Current Therapies, Clinical Trials and Drug Development One Washington Circle Hotel, Washington, DC 20037 October 19-21, 2016 Wednesday, October 19, 2016 7:30 8:30 AM Registration
Cancer: Pathophysiology, Current Therapies, Clinical Trials and Drug Development One Washington Circle Hotel, Washington, DC 20037 October 19-21, 2016 Wednesday, October 19, 2016 7:30 8:30 AM Registration
Cancer Committee Report to the Community
 2014 Cancer Committee Report to the Community 2014 CANCER COMMITTEE MEMBERS Martha Hosford, MD Chairperson & Medical Oncology Jamil Khatri, MD Cancer Conference Coordinator Elizabeth Lowe, MD Breast Program
2014 Cancer Committee Report to the Community 2014 CANCER COMMITTEE MEMBERS Martha Hosford, MD Chairperson & Medical Oncology Jamil Khatri, MD Cancer Conference Coordinator Elizabeth Lowe, MD Breast Program
South West Regional Cancer Program. Cancer Plan
 South West Regional Cancer Program Cancer Plan 2016-2019 1. Cancer System Planning Cancer Care Ontario s role as the government s cancer advisor includes the development and implementation of a provincial
South West Regional Cancer Program Cancer Plan 2016-2019 1. Cancer System Planning Cancer Care Ontario s role as the government s cancer advisor includes the development and implementation of a provincial
Making the Most of Your Cancer Registry
 www.champsods.com Making the Most of Your Cancer Registry Presenter: Toni Hare, Vice President CHAMPS Oncology Data Services Picture of girl here December 11, 2009 Learning Objectives Upon completion of
www.champsods.com Making the Most of Your Cancer Registry Presenter: Toni Hare, Vice President CHAMPS Oncology Data Services Picture of girl here December 11, 2009 Learning Objectives Upon completion of
HEALTHSTREAM LIVING LABS IN ACTION
 HEALTHSTREAM LIVING LABS IN ACTION A CONVERSATION WITH: Mitchel T. Heflin MD, MHS Associate Professor of Medicine, Duke University School of Medicine Eleanor McConnell PhD, RN, GCNS-BC Associate Professor,
HEALTHSTREAM LIVING LABS IN ACTION A CONVERSATION WITH: Mitchel T. Heflin MD, MHS Associate Professor of Medicine, Duke University School of Medicine Eleanor McConnell PhD, RN, GCNS-BC Associate Professor,
Developing a Memory Care Program
 Developing a Memory Care, Senior Living University. All rights reserved. Reproduction or translation of any part of this work, by whatever means, beyond that permitted by Section 107 or 108 of the United
Developing a Memory Care, Senior Living University. All rights reserved. Reproduction or translation of any part of this work, by whatever means, beyond that permitted by Section 107 or 108 of the United
Chapter Affiliation Requirements Workbook
 2019 Chapter Affiliation Requirements Workbook Association for Talent Development (ATD) Chapter Services Dear Chapter Leader, Welcome to the 2019 CARE Planning Workbook, a guiding tool for chapters to
2019 Chapter Affiliation Requirements Workbook Association for Talent Development (ATD) Chapter Services Dear Chapter Leader, Welcome to the 2019 CARE Planning Workbook, a guiding tool for chapters to
Core Competencies. Course Key. HPH 550: Theories of Health Behavior & Communication
 : Contemporary Issues in Public Health : Introduction to the Research Process : Biostatistics I : Biostatistics II : Health Systems Performance : Epidemiology for Public Health : Environmental & Occupational
: Contemporary Issues in Public Health : Introduction to the Research Process : Biostatistics I : Biostatistics II : Health Systems Performance : Epidemiology for Public Health : Environmental & Occupational
PERSONALIZED PSYCHO-ONCOLOGY
 Memorial Sloan Kettering Cancer Center is pleased to present: PERSONALIZED PSYCHO-ONCOLOGY A CASE-BASED APPROACH Saturday, October 12, 2019 Rockefeller Research Laboratories 430 East 67th Street New York,
Memorial Sloan Kettering Cancer Center is pleased to present: PERSONALIZED PSYCHO-ONCOLOGY A CASE-BASED APPROACH Saturday, October 12, 2019 Rockefeller Research Laboratories 430 East 67th Street New York,
APTA EDUCATION STRATEGIC PLAN ( ) BOD Preamble
 APTA EDUCATION STRATEGIC PLAN (2006-2020) BOD 03-06-26-67 Preamble The content of the Education Strategic Plan represents the specific initiatives the American Physical Therapy Association (Association)
APTA EDUCATION STRATEGIC PLAN (2006-2020) BOD 03-06-26-67 Preamble The content of the Education Strategic Plan represents the specific initiatives the American Physical Therapy Association (Association)
Introduction. Current status of 510(k) clinical data requirements. 1 Current Status&Considerations:
 510(k) Current Status&Considerations: Conducting a Well-Controlled Clinical Study When Clinical Data is Required Introduction In an effort to promote innovation while protecting the population at large,
510(k) Current Status&Considerations: Conducting a Well-Controlled Clinical Study When Clinical Data is Required Introduction In an effort to promote innovation while protecting the population at large,
Quality and Fiscal Metrics: What Proves Success?
 Quality and Fiscal Metrics: What Proves Success? 1 Quality and Fiscal Metrics: What Proves Success? Kathleen Kerr Kerr Healthcare Analytics Creating the Future of Palliative Care NHPCO Virtual Event February
Quality and Fiscal Metrics: What Proves Success? 1 Quality and Fiscal Metrics: What Proves Success? Kathleen Kerr Kerr Healthcare Analytics Creating the Future of Palliative Care NHPCO Virtual Event February
London Regional Cancer Program
 London Regional Cancer Program Table of Contents Mission, Vision and Values...1 Key Areas and Directions... 2 Leading in Patient Care and Service Delivery... 2 Improving Quality and Safety... 5 Strengthening
London Regional Cancer Program Table of Contents Mission, Vision and Values...1 Key Areas and Directions... 2 Leading in Patient Care and Service Delivery... 2 Improving Quality and Safety... 5 Strengthening
