PREVENTION AND TREATMENT OF VENOUS THROMBO-EMBOLISM (VTE): RISK ASSESSMENT AND PROPHYLAXIS. Type: Clinical Guideline Register No: Status: Public
|
|
|
- Horatio Stewart
- 5 years ago
- Views:
Transcription
1 PREVENTION AND TREATMENT OF VENOUS THROMBO-EMBOLISM (VTE): RISK ASSESSMENT AND PROPHYLAXIS Type: Clinical Guideline Register No: Status: Public Developed in response to: NICE Clinical Guideline 92 (2010 ) update June 2015, NHS Operating Framework 2010/11, House of Commons Select Committee Report (2005), Letter from CMO DOH Risk Assessment Tool (2008) DOH advice on travel related DVT ( ) Contributes to CQC Fundamental Standard 12 Safe Care & Treatment Consulted With Post/Committee/Group Date Rebecca Martin Deputy Chief Medical Officer June 2017 Waseem Nagi Consultant Haematology June 2017 Nicholette Campbell Medication Safety Officer and Lead Pharmacist June 2017 Stephen Hughes Consultant ED June 2017 Jayanthi Venkatesh Consultant Upper GI June 2017 Suthahar Yoganathan Consultant Geriatrician June 2017 Helen Clarke Head of Governance June 2017 Su Ames Patient Safety Officer June 2017 Sarah Moon Specialist Midwife Clinical Guidelines and Audit June 2017 Angela Davis Anti-coagulation Nurse Practitioner June 2017 Professionally Approved By Ellie Makings, Medical Director June 2017 Version Number 4.2 Issuing Directorate Governance Ratified by: DRAG Chairman s Action Ratified on: 29 th October 2017 Exec Group Sign Off November 2017 Implementation Date 6 th November 2017 Next Review Date October 2020 Author/Contact for Information Dr Shereen Elshazly, Consultant Haematologist Policy to be followed by (target staff) All clinical staff Distribution Method Intranet & Website. Related Trust Policies (to be read in conjunction with) Antenatal Thrombo-embolic Prophylaxis Thromboprophylaxis with Caesarean Section Management of Women with VTE, DVT or PE during Antenatal and Postnatal Period Peri-procedural anticoagulation in Adult patients taking Warfarin and novel oral anti-coagulants (NOACS) Mandatory Training Policy Consent Policy Clinical Recording Keeping Policy Clinical Audit Strategy and Policy Document Review History: Version No: Authored/Reviewed by: Active Date: 1.0 Deirdre Miller Deirdre Miller, Clinical Performance Deirdre Miller Dr Shereen Elshazly 6 th November Dr Shereen Elshazly - amended appendices 21 November Helen Clarke - amended reference to patient information 11 December
2 Index 1. Purpose 2. Background 3. Scope 4. Definitions/ Glossary 5. Policy 6. Equality and Diversity 7. Roles and Responsibilities 8. Risk Assessment for VTE 9. General Principles on Thrombo-embolic Prophylaxis 10. Prophylactic Treatment for High Risk Patients 11. Procedure for the Investigation and Management of Deep Vein Thrombosis 12. Investigation of suspected PE and management of patient once a positive diagnosis has been made 13. Information for Patients 14. Ensuring hospital doctors are informed when their patients suffer VTE after discharge 15. Infection Prevention 16. Education and Training 17. Monitoring for Compliance 18. Audit 19. Distribution of this Guideline 20. References Appendix 1 Trust Prescription Chart Appendix 2 Flow diagram patient with signs of DVT Appendix 3 Flow diagram patient with signs of PE Appendix 4 Wells Pulmonary Embolus Clinical Probability 2
3 1.0 Purpose 1.1 This policy has been developed to ensure standardisation of best practice across Mid Essex Hospital Services NHS Trust in the prevention and management of Venous Thromboembolism (VTE) in hospitalised patients. 1.2 This guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer. 1.3 This guideline informs all Trust clinicians of the high incidence of VTE in hospital patients. 1.4 This guideline provides guidance to all health care professionals on reducing the incidence of VTE in hospital patients. 1.5 It provides guidance on risk assessment for VTE for all admitted patientsadmissions. 1.6 It provides general guidance on prophylaxis for reducing the risk of venous thromboembolism. 2.0 Background 2.1 VTE causes more deaths than the combined mortality from breast cancer, road traffic accidents, MRSA and AIDS. Without thromboprophylaxis, one in seven hospitalised patients may develop a deep vein thrombosis (DVT) or pulmonary embolism (PE). Approximately ten per cent of hospital deaths are caused by pulmonary embolism (PE). 2.2 The policy incorporates recommendations from the: The House of Commons Health Committee report on the Prevention of Venous Thromboembolism in hospitalised patients (February 2005) The Government Response to the Health Committee Report (July 2005) The Report of the Independent Expert Working Group to the CMO and Response of CMO NICE Clinical 92- Reducing the risk of venous thromboembolism in patients admitted to hospital, January This version supersedes any previous versions of this document and should be used in conjunction with MEHT policies and procedures as stated on the front sheet. 3.0 Scope 3.1 This policy applies to all permanent, locum, agency and bank clinical staff regardless of grade or profession involved in patient assessment, treatment and care in all patient areas. This includes emergency, elective and day case areas. All professional groups are accountable to their professional bodies at all times. 3
4 4.0 Definitions / Glossary Adult - from aged 18 years AED - Anti-embolism Devices AF - Atrial Fibrillation BNF - British National Formulary- publication of the BMA and the Royal Pharmaceutical Society of Great Britain.(Cross references given in italics) APTT - Activated Partial Thromboplastin Time DOAC Direct Oral Anticoagulant (previously known as NOAC) DVT - Deep Venous Thrombosis INR - International Normalised Ratio (based on the prothrombin time) IPC Intermittent Pneumatic compression IV Intravenous IU - International Units MS - Mitral Stenosis LMWH - Low Molecular Weight Heparin LVF - Left Ventricular Failure PE - Pulmonary Embolus PT - Prothrombin Time SC - Sub-Cutaneous TIA - Transient Ischaemic Attack UF - Unfractionated (sic heparin) VTE - Venous Thrombo-embolic Disease Antiphospholipid Syndrome - an acquired thrombophilic state associated with autoantibodies against phospholipids, diagnosed by the in vitro finding of abnormal phospholipid dependent coagulation assays (i.e. lupus anticoagulant) and, or anticardiolipin antibodies on immuno-assay Bio-prosthetic heart valve - a non-human tissue valve implant Proximal DVT - ileo-femoral thrombosis i.e. above knee Recurrence - three or more episodes Thrombophilia - inherited or acquired disorders of the haemostatic system that may result in an increased risk of thrombosis. 5.0 Policy 5.1 In 2005 a House of Commons Health Committee report identified that 25,000 hospital patients in England die each year from VTE and that many of these deaths are preventable. As recommended in this report, it is MEHT policy that: All medical and surgical patients should be counselled for VTE risk on admission to hospital and should be risk assessed for VTE to determine appropriate prophylaxis All physicians and surgeons should be informed if their patients suffer VTE after discharge from hospital 6.0 Equality and Diversity 6.1 Mid Essex Hospital Services NHS Trust is committed to providing a service that is fair, accessible and meets the needs of all individuals. 4
5 7.0 Roles and Responsibilities: 7.1 Thrombosis Group - the Thrombosis Group has been established with a multidisciplinary representation as recommended by the DOH. The group has the following remit: To promote best practice through local policies based on National Guidance. Lead multi professional audit of the use of thromboprophylaxis and develop and monitor the implementation of any required actions Promote education and training within MEHT Review adverse incidents or near misses involving VTE as reported via the Datix system Report to the Patient Safety Group 7.2 Role of the Senior Line Managers: Senior Line Managers are responsible for: o Supporting professional non-professional groups involved in VTE pathways with resources that enable all aspects of Thrombosis and Anticoagulation including VTE and bleeding risk assessment and data capture to be performed to the standards as set in this policy o Monitoring performance of VTE and bleeding risk assessment and taking action to address low performance 7.3 Role of the Matrons, Ward Sisters and Charge Nurses: Matrons, Ward Sisters and Charge Nurses are responsible for: o Ensuring members of their teams are competent to ensure effective VTE assessment is undertaken where appropriate o Ensuring members of their teams are knowledgeable and competent in the management of VTE prophylaxis or treatment if positive diagnosis is present 7.4 Role of the Senior Clinicians / Consultants: Senior Clinicians / Consultants are responsible for: o Ensuring junior colleagues are knowledgeable and competent in VTE assessment, prophylaxis and management o Monitoring performance of VTE and bleeding risk assessment and taking action where required 7.5 Role of Medical Staff: Medical staff are responsible for: o Taking a comprehensive patient medical history and completing VTE and bleeding risk assessment and arranging appropriate prophylaxis and/or treatment as per policy content o Completing the appropriate VTE risk assessment tool VITAL pack o Reviewing the patient and / or prescribed medication within or at 24 hours and when condition changes occur o Considering further investigation and treatment where appropriate 5
6 o Have a working knowledge of the management of suspected VTE and when a positive diagnosis has been made 7.6 Role of Individual Staff Members: All Staff are responsible for: o Taking positive steps to ensure the appropriate patient VTE assessment is completed accurately o Ensuring any actions identified through monitoring and evaluations are undertaken o Ensuring that any incidents linked with VTE assessment, prophylaxis or management are reported using the Trust s incident reporting procedure (DATIX) 8.0 Risk Assessment for VTE (Refer to the following guidance in relation to Maternity Services: Antenatal Thrombo-embolic Prophylaxis; Thromboprophylaxis with Caesarean Section; Management of Women with VTE, DVT or PE during Antenatal and Postnatal Period) 8.1 All medical and surgical cases on admission (including day surgery cases) should be assessed for risk of VTE using the appropriate specialty risk assessment chart (refer to Appendix 1). All assessments should be undertaken out on day of admission to the hospital. Appropriate prophylaxis should be given to those identified at risk. (Refer to Appendix 1 - Trust Prescription Chart) 8.2 Reassess all patients (medical or surgical) for risk of VTE and bleeding within 24 hours of admission and whenever the clinical situation changes. The re-assessment and reasons for any change of prophylaxis must be documented. 8.3 All VTE risk assessments and prescriptions for thrombo-prophylaxis must be reviewed as part of the post-take ward round to ensure they are concordant with the patient s diagnosis and on-going plan of care. 8.4 Patients whose VTE risk assessments are completed by non-medical staff, i.e. in preoperative settings, must have a senior medical review on the day of their admission. This should be complete on the appropriate chart depending on patient location. 8.5 Some Day case patients are exempt on the basis of an agreed cohort of low risk for thrombosis. Additional low risk cohort groups may be added following decision by the MEHT lead clinician for anticoagulation and agreement and authorisation by the medical director. 9.0 General Principles on Thrombo-embolic Prophylaxis (Refer to the following guidance in relation to Maternity Services: Antenatal Thrombo-embolic Prophylaxis; Thromboprophylaxis with Caesarean Section) 9.1 All hospitalised patients, medical and surgical, should be kept well hydrated and mobile when appropriate. 9.2 In order to reduce risk of failing to administer, clinicians should consider a routine prescribing time for enoxaparin for their patients. 6
7 10.0 Prophylactic Treatment for High-risk Patients (Refer to the following guidance in relation to Maternity Services: Antenatal Thrombo-embolic Prophylaxis) 10.1 Monitoring patients remain the responsibility of the senior clinician. Generally, monitoring is by clinical assessment, without the need for plasma monitoring All staff should ensure that the patient and/or their families or carers are offered verbal and written information before starting VTE prophylaxis on: The risks and possible consequences of VTE The importance of VTE prophylaxis and its possible side effects The correct use of VTE prophylaxis including anti-embolism stockings, or intermittent pneumatic compression devices Reducing their risk of VTE 10.3 All staff should ensure that patients and/or their families or carers are offered verbal and written information on VTE prevention as part of the discharge process Mechanical VTE prophylaxis as follows: Base the choice of mechanical VTE prophylaxis on individual patient factors including clinical condition, surgical procedure and patient preference. Choose any one of: o Anti-embolism stockings (thigh or knee length) o Foot impulse devices o Intermittent pneumatic compression devices (thigh or knee length) o Anti-embolism stockings Do not offer anti-embolism stockings to patients who have: o Suspected or proven peripheral arterial disease o Peripheral arterial bypass grafting o Peripheral neuropathy or other causes of sensory impairment o Any local conditions in which stockings may cause damage, for example fragile 'tissue paper' skin, dermatitis, gangrene or recent skin graft o Known allergy to material of manufacture o Cardiac failure o Severe leg oedema or pulmonary oedema from congestive heart failure o Unusual leg size or shape o Major limb deformity preventing correct fit Ensure that patients who need anti embolism stockings have their legs measured and that the correct size of stocking is provided. Anti embolism stockings should be fitted and patients shown how to use them by staff trained in their use Ensure that patients who develop oedema or postoperative swelling have their legs re measured and anti embolism stockings refitted If arterial disease is suspected, seek expert opinion before fitting anti embolism stockings Use anti embolism stockings that provide graduated compression and produce a calf pressure of mmhg. (This relates to a pressure of mmhg at the ankle and is in line with British Standards for application of compression hosiery 7
8 Encourage patients to wear their anti embolism stockings day and night until they no longer have significantly reduced mobility Remove anti embolism stockings daily for hygiene purposes and to inspect skin condition. In patients with a significant reduction in mobility, poor skin integrity or any sensory loss, inspect the skin two or three times per day, particularly over the heels and bony prominences Discontinue the use of anti embolism stockings if there is marking, blistering or discoloration of the skin, particularly over the heels and bony prominences, or if the patient experiences pain or discomfort. If suitable, offer a foot impulse or intermittent pneumatic compression device as an alternative Show patients how to use anti embolism stockings correctly and ensure they understand that this will reduce their risk of developing VTE Monitor the use of anti embolism stockings and offer assistance if they are not being worn correctly 10.5 Prophylactic treatment in the majority of medical and surgical patients will be Enoxaparin 40mg subcutaneously every 24 hours. If egfr <30 ml/minute/1.73 m 2 reduce dose to 20mg subcutaneously every 24 hours. Dose adjustments may be required in some patients for example morbid obesity If knee or hip replacement surgery is planned oral Rivaroxaban 10mg once a day is given 6-10 hours post-surgery for 2 weeks for the knee and 5 weeks for the hip. For patients already on Anticoagulation, assessment should include indications of anti-coagulations and planned procedures. (Refer to Trust guidelines entitled Peri-procedural anticoagulation in Adult patients taking Warfarin and novel oral anti-coagulants (NOACS); register number 16005) 10.7 Extended post Discharge prophylaxis: NICE currently recommends extended post discharge VTE prophylaxis with LMWH in certain patients group as below: Any bowel resection to remove a malignancy, 28 days post-surgery Surgery for obesity (bypass and gastric banding), 7 days post-surgery Gynaecological surgery for malignant condition, 28 days post-surgery Nephrectomy for the removal of malignancy, 28 days post-surgery Hip fracture, 35 days. Patients in lower limb plaster casts, until cast removal Procedure for the Investigation and Management of Deep Vein Thrombosis (Refer to the following guidance in relation to Maternity Services: Management of Women with VTE, DVT or PE during Antenatal and Postnatal Period) 11.1 This procedure outlines the approach to be taken to investigate and treat deep vein thrombosis (DVT). However, each case will need to be assessed on its own merits and for a variety of reasons may not follow the approach outlined here. This policy is based on BCSH guidelines Lower limb DVT is suspected in patients with leg pain, swelling or tenderness individually or in any combination as follows: In the outpatient, emergency department and medical admissions unit, initial investigations could include DVT screening using the pre-test probability (PTP) 8
9 and / or D- dimer blood tests. D-dimer should not be used to exclude VTE in inpatients with stay >24 hours Objective diagnosis is mandatory. In cases where VTE is suspected, clinical evaluation must be supported by confirmatory imaging. This may include: Doppler ultrasound, venography, and CT pulmonary angiography (CTPA) Baseline blood test should include: full blood count (FBC), coagulation screen (aptt, INR), renal and liver function tests 11.3 Wells et al developed a clinical scoring system, based on the criterion shown in table 1, and this is probably the most widely used internationally for lower limb DVT. Patients suspected of DVT should be examined and a Wells score determined. Those with a low score, zero or less, (info on form) should then have a d-dimer test. Those with a negative d-dimer result (currently less than 250 ng/ml) may be considered as not having a DVT and do not require a Doppler ultrasound scan Those with a low Wells score and a positive d-dimer result (currently 250 ng/ml or higher) should have a Doppler ultrasound scan arranged the same day. If a same day scan is not available then a therapeutic dose of LMWH should be given daily until a reported scan is available Those with a Wells score of 1 or more are considered at higher risk of DVT and a same day Doppler ultrasound scan should be arranged. If a same day scan is not available then a therapeutic dose of LMWH should be given daily until a reported scan is available If a high index of suspicion for DVT remains after a negative scan then consider repeat scanning at an interval of 1 week In pregnant women and patients already on anticoagulant treatment, proceed directly to a doppler ultrasound scan when DVT is suspected. This is also a reasonable approach for those with a previous history of venous thromboembolism (VTE). If the doppler ultrasound scan in pregnancy is negative and pelvic vein thrombosis is suspected discuss further radiological investigation with consultant radiologist If DVT is suspected in areas other than the lower limbs, each case needs to be considered individually and should be discussed with a radiologist to arrange for the most appropriate radiological investigation. Currently, there is no role for d-dimer testing in these settings. Where the appropriate radiology cannot be undertaken on the same day, therapeutic doses of daily LMWH should be started and continued until at least the report is available Whenever a negative scan is reported, but a high index of suspicion remains for a diagnosis of DVT, a repeat scan should be undertaken at an interval of 1 week It is important when requesting d-dimer testing that the Wells score is stated on the request, or it may not be accepted. Similarly, when requesting a Doppler ultrasound the Wells score must be stated, and also the d-dimer where the Wells score is low, on the request. In addition, clinical details should be given on the Doppler ultrasound request, in particular the location of the suspected DVT Following a diagnosis of DVT, treatment with anticoagulation at therapeutic doses as per formulary should be started immediately, unless there is a contraindication to this. 9
10 Consider DOAC s in suitable patients, or therapeutic LMWH, then bridging with Warfarin to start on the same day, unless contraindicated It is preferable, safe and effective to treat pregnant women with LMWH rather than warfarin. Warfarin crosses the placenta. The early risk of warfarin embryopathy is greatest from 6 to 12 weeks gestation and there is the late risk of fetal intracranial haemorrhage. All pregnant women should be treated with LMWH, unless contraindicated, and referred to the hospital anticoagulant clinic for monitoring of (Refer to the following guidance in relation to Maternity Services: Antenatal Thrombo-embolic Prophylaxis; Thromboprophylaxis with Caesarean Section; Management of Women with VTE, DVT or PE during Antenatal and Postnatal Period anticoagulant treatment) A well-established, nurse-led, outpatient DVT service has been in operation at Broomfield Hospital. GPs directly refer cases to this service, which is now based on the emergency assessment unit. Selected outpatients from within the hospital may also be accepted; contact the emergency assessment unit to discuss individual cases An outpatient nurse led anticoagulation clinic is operation at Broomfield hospital, as well as select GP practices. Please ensure patients are referred appropriately for further anti coagulation monitoring after discharge Not all patients will be suitable for outpatient treatment of DVT. Table 2 offers some guidance on unsuitable or high-risk patients, whose care may be more appropriate as an in-patient, but is not exhaustive. Table 2 - Patients that may be unsuitable for outpatient treatment Patients with co-existent serious medical pathology Severe acute venous obstruction Patients in severe pain Significant renal impairment (egfr <30) Patients haemodynamically unstable Known heparin allergy or heparin-associated thrombocytopenia Suspected treatment compliance problems Communication problems (deafness, language difficulties) Patients currently taking warfarin Patients with active bleeding Patients at significant risk of bleeding: o Active peptic ulceration o Liver disease o Uncontrolled hypertension (systolic > 180 mm Hg, diastolic > 110 mm Hg o Angiodysplasia o Recent haemorrhagic stroke (within 1 month) o Thrombocytopenia (platelets <80) 10
11 11.16 In patients with severe acute venous obstruction e.g. critical limb ischaemia, thrombolytic therapy (catheter-directed or systemic) or thrombectomy should be considered. Discuss such cases urgently with consultant interventional radiologist and consultant vascular surgeon. Thrombolysis is not an indication for vena caval (VC) filter insertion (BCSH guidelines 2006). If a VC filter is used, a retrievable one should be considered Vena caval filters are indicated to prevent pulmonary embolism (PE) in patients with VTE who have a contraindication to anticoagulation. Please refer to BCSH Guidelines on the use of vena cava filters (2006) for further information The British Committee for Standards in Haematology published Guidelines on oral anticoagulation with warfarin - 4th edition in 2011 and these should be read in conjunction with this policy. It gives recommendations for the intensity and duration of anticoagulant treatment, as well as recommendations for the reversal of warfarin and management of bleeding complications. It recommends that DVT in patients with cancer be treated with low molecular weight heparin, rather than warfarin. Therefore, these cases should be treated with LMWH at therapeutic doses as per formulary. The guidelines are readily available at Please also refer to the Trust Policy on reversal of warfarin anticoagulation. Advice is also available from the consultant haematologists and the Haemostasis and Thrombosis Nurse Practitioner Always consider the possibility of drug interactions with warfarin treatment; refer to the British National Formulary. Advice may also be sought from the hospital pharmacy Grade II graduated compression stockings (GCS) should be worn for two years following lower limb DVT. Stockings should be checked daily to ensure correct and comfortable fitting. It may be more comfortable for the patient to start wearing these a few days after initiation of anticoagulant treatment, once there has been reduction in swelling. After reduction in leg swelling, a smaller size may be more appropriate. (Refer to updated NICE guidance CG92, Investigation of Suspected PE and Management of Patient on Confirmation of a Positive Diagnosis (Refer to the following guidance in relation to Maternity Services: Management of Women with VTE, DVT or PE during Antenatal and Postnatal Period) 12.1 The diagnosis of pulmonary embolism (PE) may be suspected if patients have symptoms of pleuritic chest pain, haemoptysis or dyspnoea, and severe cases may present with collapse. If a diagnosis is suspected then an urgent CTPA or V/Q scan should be performed the same day The diagnosis of PE may be difficult, and the Wells PE score may aid the diagnostic process (refer to Appendix 2 - Wells Score). Those with a low score and low d-dimer result may be considered, as not having a PE and no further radiological assessment is required Massive Pulmonary Embolism: Massive pulmonary embolus (PE) with haemodynamic instability is likely in the presence of: 11
12 Collapse/hypotension Unexplained hypoxia, and Engorged neck veins, and right ventricular gallop (often) Emergency echocardiography may support the diagnosis by the demonstration of right ventricular strain Thrombolysis is the first line of treatment for massive PE and may be instituted on clinical grounds alone A total dose of 100 mg of Alteplase should be administered in 2 hours as per the following dose regimen: o The total dose should not exceed 1.5 mg/kg in patients with a body weight below 65 kg o Streptokinase may be administered as 250,000 units by IV infusion, over minutes, followed by an infusion at a rate of 100,000 per hour for hours, without laboratory monitoring. It is then followed by anticoagulation with heparin/warfarin NB Streptokinase should never be used again beyond 4 days from the initial use o LMWH and Anti-Xa inhibitors have short half-lives, therefore patients on prophylactic anticoagulant therapy should receive a full dose of therapeutic Low Molecular Weight Heparin The anticoagulant treatment of VTE (PE/DVT) follows the same principles. If a scan is not undertaken on the day of suspected diagnosis then therapeutic doses of LMWH should be given until a reported scan is available Adult patients with confirmed VTE (DVT/PE) should be offered the choice of anticoagulation with a Vitamin K antagonist ie Warfarin or, where appropriate, treatment with a Direct Oral Anticoagulant (DOAC, formerly NOAC) Adult patients with confirmed VTE (DVT/PE) who choose anticoagulation with Warfarin should receive therapeutic doses of LWMH as indicated in the table below until Warfarin has reached therapeutic levels. This can be confirmed when INR is greater than or equal to 2.0 for at least 24 hours, whichever is longer Adult patients with confirmed VTE (DVT/PE) who choose anticoagulation with a DOAC should receive treatment with one of the medications outlined below. Preference should be given to either Rivaroxaban or Apixaban for first line treatment of VTE. Note: there is no requirement for concurrent use of LMWH when patients with confirmed VTE are treated with a either Rivaroxaban or Apixaban Critically ill medical inpatients in which a DOAC s would be a clinically acceptable option, Edoxaban with parenteral anticoagulation with LMWH cover for at least 5 days. (Refer to Appendix 3) 12
13 12.9 DOAC s are not suitable for pregnant or paediatric patients In severe cases, thrombolytic therapy may be considered and this decision should be made at consultant level and in discussion with the interventional radiologists Information for Patients 13.1 All patients must be given verbal and written information on admission about the risks of VTE and the effectiveness of prophylaxis. Mid Essex Hospital Services Preventing Hospital Acquired Blood Clots 13.2 All surgical patients to be advised to consider stopping taking oestrogen based oral contraception or hormone replacing therapy, 4 weeks before elective surgery. If stopped, provide advice on alternative contraceptive methods Surgical Patients must be informed that immobility associated with continuous travel of more than 3 hours in the 4 weeks before surgery or after surgery may increase the risk of VTE On discharge, patients should be given verbal information and the appropriate patient information leaflet Ensuring Hospital Doctors are Informed if their Patients Suffer VTE after Discharge 14.1 It is part of the remit of the Thrombosis Group to ensure there is feedback of information to consultants if a patient suffers VTE after discharge. This can be achieved by: Ensuring that for patients recently discharged from hospital who are now attending the anti-coagulation clinic for DVT/PE, written information is sent to the consultant by the Chair of the Thrombosis Group. The Chair of the Thrombosis Group will send copies of discharge letters for patients re-admitted with DVT/ PE to previous consultant. For patients found to have DVT/PE at post mortem, copies of the report should be sent to the patient s Consultant. The Trust will consider what process should be used for discharged out of area patients developing a DVT 15.0 Infection Prevention 15.1 Staff should follow Universal Precautions and use Aseptic Non-TouchTechnique (ANTT) at all times 16.0 Education and Training 16.1 Staff must refer to the Mandatory Training Policy and the Training Needs Analysis (TNA) what training in relation to thromboembolism is relevant to their role. The Mandatory Training policy identifies how training is monitored and how nonattendance will be followed up and managed. 13
14 16.2 All Directorate Managers / Clinical Directors / Directorate Nurse Managers and Ward / Departmental Managers are responsible for ensuring that relevant staff are familiar with the content of this document, the Mandatory Training Policy and Training Needs Analysis Staff must be competent in the measurement, fitting and monitoring of GES stockings. They must also be competent in educating patients in the use of GES stockings post discharge Monitoring for Compliance 17.1 Regular audit of VTE prophylaxis is undertaken and reported to the Thrombosis Group Data on completion of risk assessment on all patients admitted to hospital is collected and reported to the Department of Health on a monthly basis. The results of the monthly data collection will be reviewed by the Medical Director. The Hospital Thrombosis Group will identify any trends across the organisation and instigate any necessary actions to reduce identified risks Monthly compliance figures are reported to the Clinical Quality Review Group (CQRG) 18.0 Audit 18.1 A rolling programme of audit has been developed by the Thrombosis Group and lead by the Chair of the Thrombosis Group in accordance with the Clinical Audit Strategy and Policy to assess: Appropriate risk assessment to identify patients at risk of VTE on admission, with reassessment at 24 hours; The administration of appropriate prophylaxis including treatment of high risk patients where VTE suspected; The management of patients where a positive diagnosis has been made; Information given to the patient during admission and at discharge; 18.2 The findings of the audit will be reported to the Thrombosis Group, relevant Directorate Governance Meetings and the Patient Safety Group. Where deficiencies are identified an organisational action plan will be developed and progress monitored at subsequent Thrombosis Group meetings Where indicated, Clinical Directors should develop local actions with named leads and timescales to address areas of non-compliance. Progress with these actions will be monitored at Directorate Governance Meetings Any identified learning will be disseminated to relevant staff Communication and implementation of Guideline 19.1 This guideline will be available on MEHT intranet, under Documents; Clinical Guidelines; Trust wide clinical guidelines. 14
15 19.2 This Guideline will be published on the Trust website under Policies and Guidelines and notified in Focus The appropriate risk assessment tool and prophylaxis guide should be visibly displayed as a laminated guide in wards, pre-assessment clinics and theatres References NICE Guidance CG92: Venous Thromboembolism; Reducing the risk for hospital patients (2010), updated June Arya R. (2011). How I manage venous thromboembolism in pregnancy. British Journal of Haematology, 153, Baglin T.P. et al (2006). Guidelines on use of vena cava filters. British Journal of Haematology, 134, Keeling et al. (2004). The diagnosis of deep vein thrombosis in symptomatic outpatients and the potential for clinical assessment and D-dimer assays to reduce the need for diagnostic imaging. British Journal of Haematology, 124, Keeling et al. (2011). Guidelines on oral anticoagulation with warfarin - fourth edition. British Journal of Haematology. Tan et al. (2009). Diagnostic management of clinically suspected acute deep vein thrombosis. British Journal of Haematology, 146, Wells et al.(1995). Accuracy of clinical assessment of deep-vein thrombosis. Lancet, 345, Wells et al. (1997). Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet, 350, Wimperis et al (2010). Standard operating procedure for outpatient management of DVT. Norfolk and Norwich University Hospitals NHS Foundation Trust. NICE Clinical Guideline 92 venous thromboembolism : Reducing the Risk of VTE (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital: January 2010 Department of Health (2008): Risk Assessment for Venous Thrombo-embolism Report of the Independent Expert Working group on the Prevention of VTE CMO letter ( ) announcing recommendations of the Independent Working group. House of Commons Health Committee report 2005 British Standards, 6612:1985 Specification for graduated compression hosiery and 7672:1993 Specification for compression, stiffness and labelling of anti-embolism hosiery.) 15
16 Trust Prescription Chart Appendix 1 16
17 17
18 18
19 Appendix 4 Wells Pulmonary Embolus Clinical Probability WELLS PE CLINICAL PROBABILITY SCORE Clinical Feature Score Patient Score Clinical sign DVT 3 Heart Rate >100/min 1.5 Immobilisation 3 days Or surgery previous 4 weeks 1.5 Previous DVT or PE 1.5 Haemoptysis 1 Malignancy unless in remission >6 months 3 PE as likely or more likely than alternative diagnosis 3 Score of 4 or less = PE unlikely; Score greater than >4 = PE likely WELLS DVT CLINICAL PROBABILITY SCORE Clinical Feature Total Score Patient Score Active cancer (treatment on-going or within previous 6 months 1 or palliative) Paralysis, plaster 1 Bed >3 days or surgery within 4 weeks 1 Tenderness along veins 1 Entire leg swollen 1 Calf swelling >3cm 1 Pitting Oedema 1 Collateral veins 1 Alternative diagnosis to DVT as likely or more likely? -2 Low: 0 or Less; Moderate: 1 or 2; high: 3 or more. Total 19
Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital
 Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Venous thromboembolism - reducing the risk
 Venous thromboembolism - reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital NICE guideline Draft for consultation,
Venous thromboembolism - reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital NICE guideline Draft for consultation,
Venous thromboembolism: reducing the risk
 Issue date: January 2010 Venous thromboembolism: reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital This guideline
Issue date: January 2010 Venous thromboembolism: reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital This guideline
DVT - initial management NSCCG
 Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Suspected Deep Vein Thrombosis (DVT) Pathway for Non Pregnant patients Updated November 2016, with new D-dimer reference range
 Suspected Deep Vein Thrombosis (DVT) Pathway for Non Pregnant patients Updated November 2016, with new D-dimer reference range Suspect a DVT? Complete a Two-level DVT Wells score on ICE system (see page
Suspected Deep Vein Thrombosis (DVT) Pathway for Non Pregnant patients Updated November 2016, with new D-dimer reference range Suspect a DVT? Complete a Two-level DVT Wells score on ICE system (see page
New v1.0 Date: December 2015 Patricia Wain - Associate Director Physical Care. Kenny Laing - Deputy Director of Nursing
 Clinical Venous Thromboembolism: Standing Operating Procedure Document Control Summary Status: Version: Author/Title: Owner/Title: New v1.0 Date: December 2015 Patricia Wain - Associate Director Physical
Clinical Venous Thromboembolism: Standing Operating Procedure Document Control Summary Status: Version: Author/Title: Owner/Title: New v1.0 Date: December 2015 Patricia Wain - Associate Director Physical
Venous Thromboembolism Prophylaxis
 Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Prevention of Venous Thromboembolism
 Prevention of Venous Thromboembolism Reference No: P_CS_05 Version 2.4 Ratified by: LCHS Trust Board Date ratified: 27 th January 2015 Name of originator / author: Medical Director Name of responsible
Prevention of Venous Thromboembolism Reference No: P_CS_05 Version 2.4 Ratified by: LCHS Trust Board Date ratified: 27 th January 2015 Name of originator / author: Medical Director Name of responsible
Deep Vein Thrombosis and Pulmonary Embolism: Patient Information
 Deep Vein Thrombosis and Pulmonary Embolism: Patient Information A Deep Vein Thrombosis (DVT) and a Pulmonary Embolism (PE) are both disorders of unwanted blood clotting. Unwanted blood clots can occur
Deep Vein Thrombosis and Pulmonary Embolism: Patient Information A Deep Vein Thrombosis (DVT) and a Pulmonary Embolism (PE) are both disorders of unwanted blood clotting. Unwanted blood clots can occur
DOAC and NOAC are terms for a novel class of directly acting oral anticoagulant drugs including Rivaroxaban, Apixaban, Edoxaban, and Dabigatran.
 Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
Misunderstandings of Venous thromboembolism prophylaxis
 Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
NICE Guidance: Venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital 1
 The College of Emergency Medicine Patron: HRH The Princess Royal Churchill House Tel +44 (0)207 404 1999 35 Red Lion Square Fax +44 (0)207 067 1267 London WC1R 4SG www.collemergencymed.ac.uk CLINICAL EFFECTIVENESS
The College of Emergency Medicine Patron: HRH The Princess Royal Churchill House Tel +44 (0)207 404 1999 35 Red Lion Square Fax +44 (0)207 067 1267 London WC1R 4SG www.collemergencymed.ac.uk CLINICAL EFFECTIVENESS
PE Pathway. The charts are listed as follows:
 PE Pathway This document comprises 6 simple flow charts to assist clinicians in the investigation and treatment of suspected or confirmed Acute Pulmonary Emboli. The pathway has been put together using
PE Pathway This document comprises 6 simple flow charts to assist clinicians in the investigation and treatment of suspected or confirmed Acute Pulmonary Emboli. The pathway has been put together using
NICE guideline Published: 21 March 2018 nice.org.uk/guidance/ng89
 Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism NICE guideline Published: 21 March 2018 nice.org.uk/guidance/ng89 NICE 2018. All rights
Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism NICE guideline Published: 21 March 2018 nice.org.uk/guidance/ng89 NICE 2018. All rights
pat hways Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16
 pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
Deep Vein Thrombosis
 Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
How to prevent blood clots whilst in hospital and after your return home
 How to prevent blood clots whilst in hospital and after your return home Patient information WHAT What IS is DEEP deep VEIN vein THROMBOSIS? thrombosis? Deep Vein Thrombosis DVT is a blood clot within
How to prevent blood clots whilst in hospital and after your return home Patient information WHAT What IS is DEEP deep VEIN vein THROMBOSIS? thrombosis? Deep Vein Thrombosis DVT is a blood clot within
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM. Gordon Lowe Professor of Vascular Medicine University of Glasgow
 CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM Gordon Lowe Professor of Vascular Medicine University of Glasgow VENOUS THROMBOEMBOLISM Common cause of death and disability 50% hospital-acquired
CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM Gordon Lowe Professor of Vascular Medicine University of Glasgow VENOUS THROMBOEMBOLISM Common cause of death and disability 50% hospital-acquired
Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge
 Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
Venous Thromboembolism Policy (VTE)
 Venous Thromboembolism Policy (VTE) DOCUMENT CONTROL: Version: v3 Ratified by: Clinical Quality Group Date ratified: 2 February 2016 Name of originator/author: Modern Matron St John s Hospice Name of responsible
Venous Thromboembolism Policy (VTE) DOCUMENT CONTROL: Version: v3 Ratified by: Clinical Quality Group Date ratified: 2 February 2016 Name of originator/author: Modern Matron St John s Hospice Name of responsible
NOTE: The first appearance of terms in bold in the body of this document (except titles) are defined terms please refer to the Definitions section.
 TITLE VENOUS THROMBOEMBOLISM PROPHYLAXIS SCOPE Provincial Acute and Sub-Acute Care Facilities APPROVAL AUTHORITY Alberta Health Services Executive Committee SPONSOR Vice President, Quality and Chief Medical
TITLE VENOUS THROMBOEMBOLISM PROPHYLAXIS SCOPE Provincial Acute and Sub-Acute Care Facilities APPROVAL AUTHORITY Alberta Health Services Executive Committee SPONSOR Vice President, Quality and Chief Medical
Hospital Acquired VTE: update on national guidance
 Hospital Acquired VTE: update on national guidance Rebecca Chanda Consultant Pharmacist - Thrombosis and Haemostasis Guy s and St Thomas Hospitals NHS Foundation Trust Chair of the UK Clinical Pharmacy
Hospital Acquired VTE: update on national guidance Rebecca Chanda Consultant Pharmacist - Thrombosis and Haemostasis Guy s and St Thomas Hospitals NHS Foundation Trust Chair of the UK Clinical Pharmacy
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales
 Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
North East Essex Medicines Management Committee
 Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
PULMONARY EMBOLISM (PE): DIAGNOSIS AND TREATMENT
 PULMONARY EMBOLISM (PE): DIAGNOSIS AND TREATMENT OBJECTIVE: To provide a diagnostic algorithm and treatment options for patients with acute pulmonary embolism (PE). BACKGROUND: Venous thromboembolism (VTE)
PULMONARY EMBOLISM (PE): DIAGNOSIS AND TREATMENT OBJECTIVE: To provide a diagnostic algorithm and treatment options for patients with acute pulmonary embolism (PE). BACKGROUND: Venous thromboembolism (VTE)
VTE Prevention Guidelines (Venous Thromboembolism) (Venous Thromboembolism)
 VTE Prevention Guidelines (Venous Thromboembolism) (Venous Thromboembolism) When using this document please ensure that the version you are using is the most up to date either by checking on the Trust
VTE Prevention Guidelines (Venous Thromboembolism) (Venous Thromboembolism) When using this document please ensure that the version you are using is the most up to date either by checking on the Trust
Preventing Blood Clots in Adult Patients
 Manchester Royal Eye Hospital Surgical Services Information for Patients Preventing Blood Clots in Adult Patients This leaflet will give you information on how to reduce the risk of developing blood clots
Manchester Royal Eye Hospital Surgical Services Information for Patients Preventing Blood Clots in Adult Patients This leaflet will give you information on how to reduce the risk of developing blood clots
1. SCOPE of GUIDELINE:
 Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Suspected Deep Vein Thrombosis (DVT) Assessment
 CHI no... First name... DOB... /... /... Last name... Sex: c M c F Address...... Telephone... or attach addressograph label here Hospital/Location: c Hairmyres c Monklands c Wishaw Other (specify)... Ward/Base...
CHI no... First name... DOB... /... /... Last name... Sex: c M c F Address...... Telephone... or attach addressograph label here Hospital/Location: c Hairmyres c Monklands c Wishaw Other (specify)... Ward/Base...
Venous Thromboembolism Policy (VTE)
 Venous Thromboembolism Policy (VTE) DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 5 November 2013 Name of originator/author: Modern Matron St John s Hospice
Venous Thromboembolism Policy (VTE) DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 5 November 2013 Name of originator/author: Modern Matron St John s Hospice
Pathology Service User Guide Haematology
 Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
Venothromboembolism prophylaxis: Trauma and Orthopaedics Clinical guideline, V2
 Clinical Guideline Venothromboembolism prophylaxis: Trauma and Orthopaedics 11/11/11 TEMPORARY GUIDANCE There is no prophylactic tinzaparin available in the Trust currently. Please substitute enoxaparin
Clinical Guideline Venothromboembolism prophylaxis: Trauma and Orthopaedics 11/11/11 TEMPORARY GUIDANCE There is no prophylactic tinzaparin available in the Trust currently. Please substitute enoxaparin
These are guidelines only and can be deviated from if it is thought to be in the patient s best interest.
 Clinical Guideline Venothromboembolism prophylaxis: Trauma and Orthopaedics Venous thromboembolism (VTE) is a recognised complication associated with inactivity and surgical procedures. Therefore, all
Clinical Guideline Venothromboembolism prophylaxis: Trauma and Orthopaedics Venous thromboembolism (VTE) is a recognised complication associated with inactivity and surgical procedures. Therefore, all
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
PULMONARY EMBOLISM MANAGEMENT GUIDELINES
 PULMONARY EMBOLISM MANAGEMENT GUIDELINES This document is adapted from the NICE guidelines titled Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia
PULMONARY EMBOLISM MANAGEMENT GUIDELINES This document is adapted from the NICE guidelines titled Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia
1.0 PATIENT CARE Including Physical Healthcare
 SECTION: 1.0 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.33 NATURE AND SCOPE: SUBJECT: PROCEDURE - TRUST WIDE VENOUS THROMBOEMBOLISM (VTE) This procedure details Venous Thromboembolism
SECTION: 1.0 PATIENT CARE Including Physical Healthcare POLICY /PROCEDURE: 1.33 NATURE AND SCOPE: SUBJECT: PROCEDURE - TRUST WIDE VENOUS THROMBOEMBOLISM (VTE) This procedure details Venous Thromboembolism
INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY
 INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY N.E. Pearce INTRODUCTION Preventable death Cause of morbidity and mortality Risk factors Pulmonary embolism
INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY N.E. Pearce INTRODUCTION Preventable death Cause of morbidity and mortality Risk factors Pulmonary embolism
Pulmonary Embolism Pathway
 Pulmonary Embolism Pathway Ambulatory Care Pathway Dr. A. Zafar, Dr. A. Rehman, Dr. T. Malik September, 2011. Patient Identification Label Pulmonary Embolism Pathway Clinical History Comments Hospital
Pulmonary Embolism Pathway Ambulatory Care Pathway Dr. A. Zafar, Dr. A. Rehman, Dr. T. Malik September, 2011. Patient Identification Label Pulmonary Embolism Pathway Clinical History Comments Hospital
Trust Guideline for the Management of: Adult patients requiring anticoagulation with Warfarin (including reversal)
 (including reversal) A Clinical Guideline recommended for use: In: By: For: Key words: Written by: All Clinical Areas All medical and nursing staff Adult patients requiring anticoagulation with warfarin
(including reversal) A Clinical Guideline recommended for use: In: By: For: Key words: Written by: All Clinical Areas All medical and nursing staff Adult patients requiring anticoagulation with warfarin
Edoxaban for the treatment and prevention of venous thromboembolism (DVT or PE) or stroke prevention in non-valvular AF
 Edoxaban for the treatment and prevention of venous thromboembolism (DVT or PE) or stroke prevention in non-valvular AF Traffic light classification- Amber 2 specialist initiation / recommendation Information
Edoxaban for the treatment and prevention of venous thromboembolism (DVT or PE) or stroke prevention in non-valvular AF Traffic light classification- Amber 2 specialist initiation / recommendation Information
Venous Thromboembolism (VTE)
 Venous Thromboembolism (VTE) Nursing A guide for patients and carers Contents Why do blood clots form in veins?... 1 How common is a deep vein thrombosis (DVT) or pulmonary embolus (PE)?... 2 How are DVTs/
Venous Thromboembolism (VTE) Nursing A guide for patients and carers Contents Why do blood clots form in veins?... 1 How common is a deep vein thrombosis (DVT) or pulmonary embolus (PE)?... 2 How are DVTs/
GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS
 GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS This guideline covers the management of patients being treated with Vitamin K antagonists (VKA): Warfarin Acenocoumarol
GUIDELINES FOR MANAGEMENT OF BLEEDING AND EXCESSIVE ANTICOAGULATION WITH ORAL ANTICOAGULANTS This guideline covers the management of patients being treated with Vitamin K antagonists (VKA): Warfarin Acenocoumarol
GUIDELINE FOR THROMBOPROPHYLAXIS IN ADULT (18 YEARS AND OLDER) IN GENERAL MEDICAL PATIENTS AND INPATIENTS UNDERGOING SURGERY
 GUIDELINE FOR THROMBOPROPHYLAXIS IN ADULT (18 YEARS AND OLDER) IN GENERAL MEDICAL PATIENTS AND INPATIENTS UNDERGOING SURGERY SEE SEPARATE GUIDELINES FOR THROMBOPROPHYLAXIS IN OBSTETRICS This guidance does
GUIDELINE FOR THROMBOPROPHYLAXIS IN ADULT (18 YEARS AND OLDER) IN GENERAL MEDICAL PATIENTS AND INPATIENTS UNDERGOING SURGERY SEE SEPARATE GUIDELINES FOR THROMBOPROPHYLAXIS IN OBSTETRICS This guidance does
Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital Foundation Trust
 MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline. Contents
 Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline Classification: Clinical Guideline Lead Author: Dr Rowena Thomas-Dewing, Consultant Haematologist
Management of haemorrhage in patients taking DOACs/ NOACs (direct/ novel oral anticoagulants) Guideline Classification: Clinical Guideline Lead Author: Dr Rowena Thomas-Dewing, Consultant Haematologist
*Corresponding Author:
 Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Venous thrombosis is common and often occurs spontaneously, but it also frequently accompanies medical and surgical conditions, both in the community
 Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
What You Should Know
 1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
Deep Vein Thrombosis and Pulmonary Embolism: Risks, Prevention & Treatment
 Deep Vein Thrombosis and Pulmonary Embolism: Risks, Prevention & Treatment Other formats If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast,
Deep Vein Thrombosis and Pulmonary Embolism: Risks, Prevention & Treatment Other formats If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast,
Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine
 Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine Venous thromboembolism: pulmonary embolism (PE) deep vein thrombosis (DVT) 1% of all patients admitted to hospital 5% of in-hospital mortality
Dr. Rami M. Adil Al-Hayali Assistant Professor in Medicine Venous thromboembolism: pulmonary embolism (PE) deep vein thrombosis (DVT) 1% of all patients admitted to hospital 5% of in-hospital mortality
MMP016 POLICY FOR PRIMARY THROMBOPROPHYLAXIS FOR PATIENTS ADMITTED TO NHFT
 MMP016 POLICY FOR PRIMARY THROMBOPROPHYLAXIS FOR PATIENTS ADMITTED TO NHFT MMP016 Policy for primary thromboprophylaxis for patients admitted to NHFT (rev Sept 2020) Page 1 of 21 Table of Contents Why
MMP016 POLICY FOR PRIMARY THROMBOPROPHYLAXIS FOR PATIENTS ADMITTED TO NHFT MMP016 Policy for primary thromboprophylaxis for patients admitted to NHFT (rev Sept 2020) Page 1 of 21 Table of Contents Why
Warfarin in Adults : Guidelines for the use of. These guidelines apply to all patients commenced or continuing on warfarin
 Warfarin in Adults : Guidelines for the use of Document Type: Clinical Guideline Clinical Lead: Ian Neilly Author/s: Ian Neilly/Paul Barbieri Directorate: Haematology Approved by Haematology Specialty
Warfarin in Adults : Guidelines for the use of Document Type: Clinical Guideline Clinical Lead: Ian Neilly Author/s: Ian Neilly/Paul Barbieri Directorate: Haematology Approved by Haematology Specialty
Disclosures. DVT: Diagnosis and Treatment. Questions To Ask. Dr. Susanna Shin - DVT: Diagnosis and Treatment. Acute Venous Thromboembolism (VTE) None
 Disclosures DVT: Diagnosis and Treatment None Susanna Shin, MD, FACS Assistant Professor University of Washington Acute Venous Thromboembolism (VTE) Deep Venous Thrombosis (DVT) Pulmonary Embolism (PE)
Disclosures DVT: Diagnosis and Treatment None Susanna Shin, MD, FACS Assistant Professor University of Washington Acute Venous Thromboembolism (VTE) Deep Venous Thrombosis (DVT) Pulmonary Embolism (PE)
Venous Thromboembolism National Hospital Inpatient Quality Measures
 Venous Thromboembolism National Hospital Inpatient Quality Measures Presentation Overview Review venous thromboembolism as a new mandatory measure set Outline measures with exclusions and documentation
Venous Thromboembolism National Hospital Inpatient Quality Measures Presentation Overview Review venous thromboembolism as a new mandatory measure set Outline measures with exclusions and documentation
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Venous Thromboembolism. Prevention
 Venous Thromboembolism Prevention August 2010 Venous Thromboembloism Prevention 1 1 Expected Practice Assess all patients upon admission to the ICU for risk factors of venous thromboembolism (VTE) and
Venous Thromboembolism Prevention August 2010 Venous Thromboembloism Prevention 1 1 Expected Practice Assess all patients upon admission to the ICU for risk factors of venous thromboembolism (VTE) and
Objectives. Venous Thromboembolism (VTE) Prophylaxis. Case VTE WHY DO IT? Question: Who Is At Risk?
 Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Preventing blood clots while you are in hospital and after you leave. Information for patients Pharmacy
 Preventing blood clots while you are in hospital and after you leave Information for patients Pharmacy Why does blood clot? When we cut ourselves, we bleed. To stop us from bleeding too much, chemicals
Preventing blood clots while you are in hospital and after you leave Information for patients Pharmacy Why does blood clot? When we cut ourselves, we bleed. To stop us from bleeding too much, chemicals
Guideline Quick View: Venous Thromboembolism
 Guideline Quick View: Venous Thromboembolism The AORN Guideline Quick View is a key component of Guideline Essentials, a suite of online implementation tools designed to help the perioperative team translate
Guideline Quick View: Venous Thromboembolism The AORN Guideline Quick View is a key component of Guideline Essentials, a suite of online implementation tools designed to help the perioperative team translate
Prevention and treatment of venous thromboembolic disease
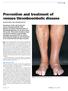 REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144
 Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144 Appendix A: Summary of new evidence from Summary of evidence from previous year Diagnosis Diagnostic
Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144 Appendix A: Summary of new evidence from Summary of evidence from previous year Diagnosis Diagnostic
The Royal College of Emergency Medicine. VTE Risk in Lower Limb Immobilisation in Plaster Cast 2015/2016
 The Royal College of Emergency Medicine Clinical Audits VTE Risk in Lower Limb Immobilisation in Plaster Cast Introduction 2015/2016 EXCELLENCE IN EMERGENCY MEDICINE A significant number of patients attend
The Royal College of Emergency Medicine Clinical Audits VTE Risk in Lower Limb Immobilisation in Plaster Cast Introduction 2015/2016 EXCELLENCE IN EMERGENCY MEDICINE A significant number of patients attend
CLINICAL PROCEDURAL DOCUMENT
 Promoting safe anticoagulation practice APPENDIX 1 to ANTICOAGULATION / VTE POLICY FOR ADULTS PROCEDURAL DOCUMENT Stand-alone document promoting safe anticoagulation practice This document does not cover
Promoting safe anticoagulation practice APPENDIX 1 to ANTICOAGULATION / VTE POLICY FOR ADULTS PROCEDURAL DOCUMENT Stand-alone document promoting safe anticoagulation practice This document does not cover
Deep vein thrombosis: diagnosis, prevention and treatment
 Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients
 Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
CHAPTER 2 VENOUS THROMBOEMBOLISM
 CHAPTER 2 VENOUS THROMBOEMBOLISM Objectives Venous Thromboembolism (VTE) Prevalence Patho-physiology Risk Factors Diagnosis Pulmonary Embolism (PE) Management of DVT/PE Prevention VTE Patho-physiology
CHAPTER 2 VENOUS THROMBOEMBOLISM Objectives Venous Thromboembolism (VTE) Prevalence Patho-physiology Risk Factors Diagnosis Pulmonary Embolism (PE) Management of DVT/PE Prevention VTE Patho-physiology
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised
 Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Pulmonary Embolism. Pulmonary Embolism. Pulmonary Embolism. PE - Clinical
 Pulmonary embolus - a practical approach to investigation and treatment Sam Janes Wellcome Senior Fellow and Respiratory Physician, University College London Background Diagnosis Treatment Common: 50 cases
Pulmonary embolus - a practical approach to investigation and treatment Sam Janes Wellcome Senior Fellow and Respiratory Physician, University College London Background Diagnosis Treatment Common: 50 cases
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Slide 1. Slide 2. Slide 3. Outline of This Presentation
 Slide 1 Current Approaches to Venous Thromboembolism Prevention in Orthopedic Patients Hujefa Vora, MD Maria Fox, RN June 9, 2017 Slide 2 Slide 3 Outline of This Presentation Pathophysiology of venous
Slide 1 Current Approaches to Venous Thromboembolism Prevention in Orthopedic Patients Hujefa Vora, MD Maria Fox, RN June 9, 2017 Slide 2 Slide 3 Outline of This Presentation Pathophysiology of venous
Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension Direct Line tadamson(anhs.
 NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
NHS Grampian Westholme Woodend Hospital Queens Road ABERDEEN AB15 6LS NHS Grampian Date 29 th March 2018 Our Ref FA_Anticoag/MGPG/Mar18 Enquiries to Frances Adamson Extension 56689 Direct Line 01224 556689
THROMBOPROPHYLAXIS IN ORTHOPAEDIC SURGERY. Title of Guideline. (Governance) Version Number 1 Date of Review October 17
 Title of Guideline THROMBOPROPHYLAXIS IN ORTHOPAEDIC SURGERY Contact Name and Job Title (Author) Gary Masterman Deputy Chief Pharmacist (Governance) Division & Specialty Specialist Services Guideline Number
Title of Guideline THROMBOPROPHYLAXIS IN ORTHOPAEDIC SURGERY Contact Name and Job Title (Author) Gary Masterman Deputy Chief Pharmacist (Governance) Division & Specialty Specialist Services Guideline Number
VTE Prophylaxis. NEW NICE guidance. Providing venous thromboembolism (VTE) prophylaxis to all at risk hospital patients
 NEW NICE guidance NICE guidance (MTG19) supports the use of the geko device for people who have a high risk of VTE and for whom pharmacological or other mechanical methods of VTE prevention are impractical
NEW NICE guidance NICE guidance (MTG19) supports the use of the geko device for people who have a high risk of VTE and for whom pharmacological or other mechanical methods of VTE prevention are impractical
Venous Thromboprophylaxis Policy
 SH CP 50 Venous Thromboprophylaxis Policy Version 3 Summary: Policy for the management and prevention of venous thrombosis for patients whilst in the care of Southern Health NHS Foundation Trust. Keywords
SH CP 50 Venous Thromboprophylaxis Policy Version 3 Summary: Policy for the management and prevention of venous thrombosis for patients whilst in the care of Southern Health NHS Foundation Trust. Keywords
THROMBOPROPHYLAXIS POLICY FOR ADULT PATIENTS ADMITTED AS INPATIENTS. 362 Version No: 2 Previous Trust / LHB Ref No:
 Reference No: THROMBOPROPHYLAXIS POLICY FOR ADULT PATIENTS ADMITTED AS INPATIENTS 362 Version No: 2 Previous Trust / LHB Ref No: 362 Documents to read alongside this Policy, Procedure etc (delete as necessary)
Reference No: THROMBOPROPHYLAXIS POLICY FOR ADULT PATIENTS ADMITTED AS INPATIENTS 362 Version No: 2 Previous Trust / LHB Ref No: 362 Documents to read alongside this Policy, Procedure etc (delete as necessary)
Thrombosis Prevention and Anticoagulation Policy
 Thrombosis Prevention and Anticoagulation Policy V7.0 Nov 2017 Page 1 of 32 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership
Thrombosis Prevention and Anticoagulation Policy V7.0 Nov 2017 Page 1 of 32 Table of Contents 1. Introduction... 3 2. Purpose of this Policy... 3 3. Scope... 3 4. Definitions / Glossary... 3 5. Ownership
Trust Guideline for the Management of: Adult patients requiring anticoagulation with Warfarin (including reversal)
 (including reversal) A Clinical Guideline recommended for use: For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author
(including reversal) A Clinical Guideline recommended for use: For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author
Rivaroxaban film coated tablets are available in 2 strengths for this indication: 15mg and 20mg.
 Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
National Quality Improvement Project 2018/2019 VTE Risk in Lower Limb Immobilisation Information Pack
 National Quality Improvement Project 2018/2019 VTE Risk in Lower Limb Immobilisation Information Pack Introduction... 2 Methodology... 3 Flow of data searches to identify audit cases... 3 Data entry information...
National Quality Improvement Project 2018/2019 VTE Risk in Lower Limb Immobilisation Information Pack Introduction... 2 Methodology... 3 Flow of data searches to identify audit cases... 3 Data entry information...
THROMBOSIS RISK FACTOR ASSESSMENT
 Name: Procedure: Doctor: Date: THROMBOSIS RISK FACTOR ASSESSMENT CHOOSE ALL THAT APPLY EACH RISK FACTOR REPRESENTS 1 POINT Age 41 60 years Minor Surgery Planned History of Prior Major Surgery (< 1 month)
Name: Procedure: Doctor: Date: THROMBOSIS RISK FACTOR ASSESSMENT CHOOSE ALL THAT APPLY EACH RISK FACTOR REPRESENTS 1 POINT Age 41 60 years Minor Surgery Planned History of Prior Major Surgery (< 1 month)
Handbook for Venous Thromboembolism
 Handbook for Venous Thromboembolism Gregory Piazza Benjamin Hohlfelder Samuel Z. Goldhaber Handbook for Venous Thromboembolism Gregory Piazza Cardiovascular Division Harvard Medical School Brigham and
Handbook for Venous Thromboembolism Gregory Piazza Benjamin Hohlfelder Samuel Z. Goldhaber Handbook for Venous Thromboembolism Gregory Piazza Cardiovascular Division Harvard Medical School Brigham and
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
 Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM
 PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT
 VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis. By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan
 Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan Objectives Describe the prevalence of PE and DVT as it relates
Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan Objectives Describe the prevalence of PE and DVT as it relates
2 Summary of NICE TA 249: Atrial fibrillation - Dabigatran Etexilate
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354)
 Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
Thromboprophylaxis in Adult General Medical Patients - Guidelines for Management
 Thromboprophylaxis in Adult General Medical Patients - Guidelines for Management Adapted from the Worcestershire Acute Hospitals NHS Trust Guideline WAHT-MED-010 Version: Final Ratified by: Provider Quality
Thromboprophylaxis in Adult General Medical Patients - Guidelines for Management Adapted from the Worcestershire Acute Hospitals NHS Trust Guideline WAHT-MED-010 Version: Final Ratified by: Provider Quality
DIRECT ORAL ANTICOAGULANTS: WHEN TO USE, WHICH TO CHOOSE AND MANAGEMENT OF BLEEDING
 DIRECT ORAL ANTICOAGULANTS: WHEN TO USE, WHICH TO CHOOSE AND MANAGEMENT OF BLEEDING KATHERINE STIRLING CONSULTANT PHARMACIST ANTICOAGULATION AND THROMBOSIS DR LISHEL HORN CONSULTANT HAEMATOLOGIST HAEMOSTASIS
DIRECT ORAL ANTICOAGULANTS: WHEN TO USE, WHICH TO CHOOSE AND MANAGEMENT OF BLEEDING KATHERINE STIRLING CONSULTANT PHARMACIST ANTICOAGULATION AND THROMBOSIS DR LISHEL HORN CONSULTANT HAEMATOLOGIST HAEMOSTASIS
AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS
 The West London Medical Journal 2010 Vol 2 No 4 pp 19-24 AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS Soneji ND Agni NR Acharya MN Anjari
The West London Medical Journal 2010 Vol 2 No 4 pp 19-24 AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS Soneji ND Agni NR Acharya MN Anjari
Venous thromboembolism
 Issue date: April 2007 Venous thromboembolism Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery) NICE clinical guideline 46 Developed
Issue date: April 2007 Venous thromboembolism Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery) NICE clinical guideline 46 Developed
INTRODUCTION Indication and Licensing
 City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
t. Recommendations for periprocedural anticoagulation are available lhrough the American College of Chest Physicians Clinical Practice Guidelines.
 Name or Policy: Policy Number: 3364-133-116 Department: Approving Officer: Responsible Agent: cope: x Management of Anticoagulation with Invasive Procedures Pharmacy Chief Operating Officer Director of
Name or Policy: Policy Number: 3364-133-116 Department: Approving Officer: Responsible Agent: cope: x Management of Anticoagulation with Invasive Procedures Pharmacy Chief Operating Officer Director of
Appendix IV - Prescribing Guidance for Apixaban
 Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
DVT and Pulmonary Embolus. Dr Piers Blombery BSc(Biomed), MBBS (Hons), FRACP, FRCPA Consultant Haematologist Peter MacCallum Cancer Centre
 DVT and Pulmonary Embolus Dr Piers Blombery BSc(Biomed), MBBS (Hons), FRACP, FRCPA Consultant Haematologist Peter MacCallum Cancer Centre Overview Structure of deep and superficial venous system of upper
DVT and Pulmonary Embolus Dr Piers Blombery BSc(Biomed), MBBS (Hons), FRACP, FRCPA Consultant Haematologist Peter MacCallum Cancer Centre Overview Structure of deep and superficial venous system of upper
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
