HMG Co-A reductase inhibitors, popularly known as CLINICAL LIVER, PANCREAS, AND BILIARY TRACT
|
|
|
- Theodore McKenzie
- 6 years ago
- Views:
Transcription
1 GASTROENTEROLOGY 2004;126: CLINICAL LIVER, PANCREAS, AND BILIARY TRACT Patients With Elevated Liver Enzymes Are Not at Higher Risk for Statin Hepatotoxicity NAGA CHALASANI,* HISHAM ALJADHEY, JOE KESTERSON, MICHAEL D. MURRAY,, and STEPHEN D. HALL* *Department of Medicine, Indiana University School of Medicine, Indianapolis; Regenstrief Institute for Health Care, Indianapolis; and Purdue University School of Pharmacy, West Lafayette, Indiana See editorial on page Background & Aims: Studies that evaluate the risk of hepatotoxicity from statins in hyperlipidemic subjects with elevated baseline serum transaminases are lacking. We conducted a study to test the hypothesis that patients with elevated baseline liver enzymes have higher risk of statin hepatotoxicity. Methods: Our study consisted of the following 3 cohorts of patients seen between January 1, 1998 and June 31, 2002: Cohort 1: 342 hyperlipidemic patients with elevated baseline enzymes (AST >40 IU/L or ALT >35 IU/L) who were prescribed a statin; cohort 2: 1437 hyperlipidemic patients with normal transaminases who were prescribed a statin; and cohort 3: 2245 patients with elevated liver enzymes but who were not prescribed a statin. The effect of statins on liver biochemistries was assessed over a 6-month period after statins were prescribed. Elevations in liver biochemistries during follow-up were categorized into mild-moderate or severe based on predefined criteria. Results: The incidence of mild-moderate elevations and severe elevations in liver biochemistries in cohort 1 were 4.7% and 0.6%, respectively. Compared with cohort 1, individuals in cohort 2 had lower incidence of mild-moderate elevations (1.9%, P 0.002) but not severe elevations (0.2%, P 0.2). However, between cohorts 1 and 3, there were no differences in the incidence of mild-moderate elevations (4.7% vs. 6.4%, respectively, P 0.2) or severe elevations (0.6% vs. 0.4%, respectively, P 0.6). Statin discontinuation during the follow-up was similar between cohorts 1 and 2 (11.1% vs. 10.7%, respectively, P 0.8). Conclusions: These data suggest that individuals with elevated baseline liver enzymes do not have higher risk for hepatotoxicity from statins. HMG Co-A reductase inhibitors, popularly known as statins, are the most potent lipid lowering agents and are among the most widely prescribed medications. 1 Statins are generally well tolerated but can occasionally lead to muscle and liver toxicity. Hepatotoxicity from statins is generally mild, leading to asymptomatic elevations in transaminases, but rare instances of marked liver injury have been reported in the literature. 2 9 Although it is unknown whether the risk of hepatotoxicity from statins is higher in patients with underlying liver disease, the third report of the National Cholesterol Education Program (NCEP) states that use of statin is contraindicated in patients with active liver disease. 10 Manufacturers recommend that serum liver enzymes should be checked prior to starting a statin and not to prescribe these drugs for patients with persistent elevated transaminases. 11 In clinical practice, gastroenterologists and hepatologists are often consulted to advise referring physicians about the safety of prescribing statins for hyperlipidemic individuals who are found to have elevated baseline serum transaminases. Previous studies have shown that low-dose cerivastatin or pravastatin can be used safely in liver transplant recipients, 12 and the pharmacokinetics of rosuvastatin are not significantly altered in individuals with compensated cirrhosis. 13 However, to our knowledge, there are no studies in the literature that systematically evaluated the effect of statin therapy on liver biochemistries in hyperlipidemic patients with elevated baseline serum transaminases as compared with those with normal baseline liver enzymes or liver disease controls who are not started on statins. We conducted the following study to address some of the uncertainties related to the use of statins in patients with elevated liver enzymes. The main objective of our study was to evaluate whether the individuals with ele- Abbreviations used in this paper: ALT, alanine aminotransferase; AST, aspartate aminotransferase by the American Gastroenterological Association /04/$30.00 doi: /j.gastro
2 1288 CHALASANI ET AL. GASTROENTEROLOGY Vol. 126, No. 5 vated baseline liver enzymes are at higher risk for hepatotoxicity from statins by comparing the course of their liver biochemistries over a 6-month period with individuals with normal baseline liver enzymes who were prescribed a statin and individuals with elevated baseline liver enzymes but who were not prescribed a statin. Two secondary objectives of this study were (1) to compare the frequency of statin discontinuation between individuals with elevated baseline liver enzymes and normal baseline liver enzymes within 6-months after prescribing statins and (2) to evaluate how often practicing physicians comply with the recommendation that liver biochemistries be checked prior to initiating statin therapy. Materials and Methods Patients The Institutional Review Board at Indiana University School of Medicine has approved and administered this study in concordance with the principles of the Declaration of Helsinki. Data were collected from a large academic medical practice located in Indianapolis, which uses the Regenstrief Medical Record System (RMRS). 14 This practice-based electronic record system captures patient information from 3 hospitals on the Indiana University Medical Center campus (Wishard Memorial Hospital, Indiana University Hospital, and Riley Hospital for Children) and from 30 clinics scattered around the inner city of Indianapolis. 14 It captures all diagnostic studies (labs, EKGs, radiology reports, surgical pathology, and others), discharge summaries, and all orders (including prescriptions) in a coded form. 14 This database links patient s prescriptions and their laboratory test results using a unique patient identifier. In so doing, the system can be programmed to identify every patient who develops elevations in liver enzymes after initiating therapy with specified medications. Using the RMRS, we identified 3 cohorts of patients who attended Wishard Memorial Hospital and affiliated primary care clinics between January 1, 1998 and June 31, Wishard Memorial Hospital is a 400-bed academic inner-city hospital located on the Indiana University Medical Center campus. Cohort 1 consisted of individuals with elevated baseline liver enzymes who were prescribed a statin between January 1, 1998 and June 31, 2002 and had serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) values within 6 months before and 6 months after starting the statin agent and absence of any evidence of alcohol abuse, hepatitis B surface antigen, or hepatitis C antibody. Cohort 2 consisted of individuals with normal liver enzymes who were prescribed a statin between January 1, 1998 and June 31, 2002 and had ALT and/or AST values within 6 months before and 6 months after starting the statin agent and absence of any evidence of alcohol abuse, hepatitis B surface antigen, or hepatitis C antibody. Cohort 3 consisted of individuals with elevated liver enzymes seen between January 1, 1998 and June 31, 2002 and who had follow-up ALT and/or AST values measured within the next 6 months and absence of any evidence of alcohol abuse, hepatitis B surface antigen, or hepatitis C antibody and who were not prescribed a statin. In addition, for confirmatory comparative purposes, 2 other control groups were constructed, and the details of these 2 confirmatory cohorts are described in the Results section. Patients with serum AST 40 IU/L or ALT 35 IU/L were considered to have elevated transaminases because the upper limit of normal (ULN) for AST and ALT for our laboratory were 40 IU/L and 35 IU/L, respectively. Absence of alcohol abuse was determined based on the clinic notes, discharge summaries, and other encounter transcripts. The elevations in liver biochemistries during the follow-up were defined and categorized depending on the patient s baseline levels of serum aminotransferases. For this study s purposes, mild-moderate elevations in liver biochemistries were defined as elevations of AST and/or ALT up to 10 times ULN in patients with normal baseline enzymes or up to 10-fold elevations from their baseline values of AST and/or ALT in patients with elevated liver enzymes at baseline. Severe elevations in liver biochemistries were defined as the development of serum bilirubin 3 mg/dl (regardless of their baseline transaminases) or elevations of AST and/or ALT greater than 10 times ULN in patients with normal baseline enzymes or 10-fold elevations from their baseline values of AST and/or ALT in patients with elevated liver enzymes at baseline. We used the RMRS prescription records to determine whether patients continued to take statins during the follow-up period (i.e., 6-month period immediately after starting the statins). Subjects were considered to have discontinued the use of statins if there was no evidence of a prescription refill after their supply of statins ran out and if there was no prescription for an alternate statin. It previously has been shown that most patients attending Wishard Memorial Hospital and the affiliated primary care clinics fill their medicine prescriptions exclusively at the Wishard Hospital pharmacies. 15 During the study period, atorvastatin, simvastatin, pravastatin, and fluvastatin were available at Wishard Hospital pharmacies. To examine how often practicing physicians within our health care system comply with the recommendation that baseline liver enzymes should be checked before starting statins, we examined the proportion of patients in whom AST or ALT levels were available within the prior 6-month period before starting statin therapy. Statistical Analyses Data validity procedures, database management, and statistical analyses were performed using SAS software. Basic descriptive statistics, including means, standard deviations (SD), ranges, and percentages, were used to characterize the study patients. 2 tests for categorical variables and Student t tests or Mann Whitney U tests for continuous variables were
3 May 2004 STATINS IN PEOPLE WITH ELEVATED LIVER ENZYMES 1289 Table 1. Demographic and Selected Characteristics of the Study Cohorts Cohort 1 (n 342) Cohort 2 (n 1437) Cohort 3 (n 2245) Age (yr) Females (%) Ethnicity (B/W) (%) 34/59 42/54 35/48 Weight (lb) AST at baseline (IU/L) ALT at baseline (IU/L) Cholesterol (mg/dl) LDL (mg/dl) HDL (mg/dl) Triglyceride (mg/dl) Serum creatinine (mg) Statin prescribed (%) Atorvastatin Simvastatin Fluvastatin 2 3 NOTE. Data presented as mean SD unless specified otherwise. Cohort 1: individuals with elevated baseline liver enzymes who were placed on a statin; cohort 2: individuals with normal baseline liver enzymes who were placed on a statin; cohort 3: individuals with elevated liver enzymes but not placed on a statin. made for univariate comparisons between the groups. The primary comparisons are between cohort 1 vs. cohort 2 and cohort 1 vs. cohort 3. A P value 0.05 was considered statistically significant. Results Cohort 1 contained 342 patients; cohort 2 contained 1437 patients; and cohort 3 contained 2245 patients. Demographics and selected characteristics of these 3 cohorts are shown in Table 1. Although there were some statistical differences in the demographics among the 3 groups, they did not appear to be clinically significant. Generally, all 3 cohorts consisted of a hyperlipidemic, middle-aged population with a female and white preponderance. Although the body weight, LDL, HDL, and creatinine were similar among the 3 groups, cohort 3 had significantly lower triglyceride and cholesterol levels. As expected, individuals in cohorts 1 and 3 had significantly higher AST and ALT values than those in cohort 2. The mean duration of statin exposure in patients belonging to cohorts 1 and 2 was similar ( and years, respectively, P 0.7). The course of transaminases during the 6-month follow-up period in patients belonging to all 3 cohorts is shown in Table 2. The incidence of mild-moderate elevations and severe elevations in liver biochemistries in cohort 1 was 4.7% and 0.6%, respectively. Compared with cohort 1, individuals in cohort 2 had lower incidence of mild-moderate elevations (1.9%, P 0.002) but not severe elevations (0.2%, P 0.2). However, between cohorts 1 and 3, there were no differences in the incidence of mild-moderate elevations (4.7% vs. 6.4%, respectively, P 0.2) or severe elevations in liver biochemistries (0.6% vs. 0.4%, respectively, P 0.6). For confirmatory purposes and to enhance the generalizability, we have compared the frequency of liver biochemistry abnormalities in cohort 1 to 2 additional control groups. One of them consisted of a subgroup of cohort 3 patients who were age and gender matched to cohort 1 (n 326). Their frequency of mild-moderate elevations (6.1%, P 0.4) or severe elevations (0.9%, P 0.6) was not significantly different than cohort 1 patients. The additional confirmatory cohort consisted of 1111 individuals with detectable hepatitis C antibody with a minimum of 2 or more AST or ALT values and elevated baseline AST or ALT but who were not placed on statins or interferon and were seen during the same period. Compared with cohort 1, individuals in this hepatitis C control group had a significantly higher frequency of mild-moderate (11.1%, P 0.001) or severe elevations in liver biochemistries (5.9%, P 0.001). The likelihood of discontinuing a statin in the 6-month follow-up period was not different between cohorts 1 and 2 (11.1% vs. 10.7%, respectively, P 0.8) (Table 3). The mean duration of exposure ( vs years, respectively, P 0.7) and the Table 2. Frequency of Varying Degrees of Elevations in Liver Biochemistries Over a 6-Month Period in 3 Study Cohorts Cohort 2 (n 1437) Cohort 1 (n 342) Cohort 3 (n 2245) Mild-moderate elevations in liver biochemistries a 1.9% 4.7% 6.4% P P 0.2 Severe elevations in liver biochemistries a 0.2% 0.6% 0.4% NOTE. Cohort 1: individuals with elevated baseline liver enzymes who were placed on a statin. Cohort 2: Individuals with normal baseline liver enzymes who were placed on a statin. Cohort 3: Individuals with elevated liver enzymes but not placed on a statin. a See Materials and Methods section for definitions. P 0.2 P 0.6
4 1290 CHALASANI ET AL. GASTROENTEROLOGY Vol. 126, No. 5 Table 3. Doses and Duration of Statin Therapy Between Cohorts 1 and 2 Cohort 1 (n 342) Cohort 2 (n 1437) P value Atorvastatin (mg/d) Mean dose Median dose Simvastatin (mg/d) Mean dose Median dose Fluvastatin (mg/d) Mean dose Median dose Duration of therapy (yr) Statin discontinuation during follow-up 11.1% 10.7% 0.8 median duration of exposure to statins (0.5 years vs. 0.5 years, respectively) was not different between cohorts 1 and 2. Similarly, the frequency of liver biochemistry monitoring during statin therapy between these 2 cohorts was similar ( vs , respectively, P 0.7) (Table 3). During the study period, atorvastatin (46%) and simvastatin (51%) were the 2 most commonly prescribed statins, and fluvastatin or pravastatin were prescribed for the remaining 3% of the patients (Tables 3 and 4). The median doses of different statins prescribed during the study period are shown in Table 3. Fifty-seven percent of the cohort received the median doses of statin, whereas 17% received the doses that were greater than the median doses. The mean and median doses of various statins were similar between patients belonging to cohorts 1 and 2 (Table 4). We failed to detect any association between the statin used and the proportion of patients developing elevations in liver enzymes or mean change in AST or ALT values or the proportion discontinuing the statin during the follow-up period (Table 4). Furthermore, there was no statistical difference in the incidence of liver enzyme elevations among the patients who received the median dose of the statins and those who received more than the median dose. The incidence of mild-moderate elevations in liver biochemistries in patients prescribed median doses of statins and those prescribed higher doses were 2.5% and 2.9%, respectively (P 0.6). Similarly, the incidence of severe elevations in liver biochemistries in patients on median doses or higher doses of statins was 0.3% and 0.3%, respectively (P 0.9). To determine how often practicing physicians comply with the recommendation that baseline liver enzymes should be checked before starting statins, we have examined the proportion of patients in whom AST or ALT levels were available within the prior 6-month period before starting a statin agent. The proportion of patients in whom transaminase levels were available within 6 months prior to starting a statin were 58% in year 1998, 57% in year 1999, 58% in year 2000, 66% in year 2001, and 63% in year There were no demographic differences between the patients with and without baseline transaminases available prior to starting statins. Interestingly, the proportion of patients who exhibited a serum bilirubin 3 mg/dl during the follow-up period or the proportion of patients who discontinued statins during the follow-up period was similar between patients with and without baseline liver enzymes available prior to starting statins. Furthermore, in comparing the patients who received statins with those who did not, there was not a significant difference in the follow-up levels of AST (P 0.4) or ALT (P 0.8). Discussion To our knowledge, this is the first study to specifically address the question of whether patients with elevated baseline liver enzymes are at higher risk to develop hepatotoxicity from statins. In this study, we have addressed this question by comparing the changes Table 4. Change in Transaminases Within 6 Months After Starting a Statin Stratified by Type of Statin Prescribed Atorvastatin (n 819) Simvastatin (n 904) Other statins a (n 56) P value Median dose per day 10 mg 20 mg 20 mg Mean dose per day Baseline AST (IU/L) Baseline ALT (IU/L) Proportion with elevated AST and/or ALT at baseline 18% 21% 13% 0.2 Incidence of liver biochemistries elevations b 2% 3.1% 4.3% 0.4 Change in AST (IU/L) Change in ALT (IU/L) Proportion that discontinued statin during follow-up (%) 10% 11.5% 8.5% 0.5 NOTE. Table represents cohorts 1 and 2 combined. a Other statins include fluvastatin in 49 patients and pravastatin in 7 patients. b This represents the combination of mild-moderate and severe elevations in liver biochemistries.
5 May 2004 STATINS IN PEOPLE WITH ELEVATED LIVER ENZYMES 1291 in liver biochemistries of individuals with elevated liver enzymes and who were prescribed statins to controls. We found that patients with elevated baseline liver enzymes had higher incidence of mild-moderate but not severe elevations in liver biochemistries than those with normal baseline liver enzymes who were prescribed statins. However, the frequency of mild-moderate or severe elevations in patients with elevated liver enzymes who were prescribed statins was not significantly higher than that of the patients with elevated liver enzymes who were not prescribed a statin. These data show that some individuals with elevated liver enzymes have elevations in their liver biochemistries, regardless of whether or not statins were prescribed (possibly because of the activity of underlying liver disease or because of unknown coexisting pathology). This suggests that individuals with elevated liver enzymes do not have increased susceptibility to hepatotoxicity from statins. We failed to find a relationship between the type of statin used and the frequency of elevations in liver biochemistries among those who were placed on statin therapy. In more than one third of the patients who were initiated on statins, the baseline transaminases were not checked prior to starting statins, and the risk of hepatotoxicity in this group did not appear to be different from those who had their transaminases checked prior to starting statins. Although this observation questions the utility of checking baseline liver enzymes prior to starting statins, it is not sufficient to suggest a change in this recommendation. As long as the recommendation is to periodically monitor transaminases after statins are started, it is then essential to have baseline liver enzymes available for comparative purposes. Because this is a retrospective study of an electronic medical record system, it carries several limitations that require further discussion. First, our definition of statin discontinuation is based on the prescription records rather than actual documentation that a prescribing physician or a patient has discontinued statin therapy because of side effects. In general, a majority of the patients attending our facilities use our outpatient and inpatient pharmacies exclusively and are not likely to take prescription medications obtained from outside pharmacies. Second, our study examined the course of serum transaminases within 6 months after starting statins and therefore may not shed light on what happens to liver enzymes after the 6-month period. However, hepatotoxicity from statins appears to be an early event rather than late phenomenon. 10 Finally, because Wishard Hospital has a formulary, we were restricted to study only those statins approved by the Pharmacy and Therapeutic Committee and were commonly prescribed by the practicing physicians. Despite all these limitations, we believe that our study provides valuable information that is clinically pertinent and provides guidance to clinicians and the policy makers. The regulatory authorities are currently considering whether to make some statins available without a prescription to target patient populations in the United States (lovastatin) and the United Kingdom (simvastatin), 16,17 and our data may assist rational decision making for this important public health issue. In summary, mild-moderate or severe elevations in patients with elevated baseline liver enzymes who were placed on a statin are not significantly higher than those with elevated liver enzymes who were not placed on statin therapy. This shows that some individuals with elevated liver enzymes will have varying degrees of elevations in their liver biochemistries during the follow-up period, regardless of whether or not they were placed on statins. These data suggest that individuals with elevated liver enzymes do not have increased susceptibility to hepatotoxicity from statins. References 1. Kreisberg RA, Oberman A. Medical management of hyperlipidemia/ dyslipidemia. J Clin Endocrinol Metab 2003;88: Bolego C, Baetta R, Bellosta S, Corsini A, Paoletti R. Safety considerations for statins. Curr Opin Lipidol 2002;13: Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis 2002;22: Kinnman N, Hultcrantz R. Lipid lowering medication and hepatotoxicity. J Intern Med 2001;250: Nakad A, Bataille L, Hamoir V, Sempoux C, Horsmans Y. Atorvastatin-induced acute hepatitis with absence of cross-sensitivity with simvastatin. Lancet 1999;353: England JD, Viles A, Labib S. Liver side effects associated with simvastatin therapy. Aust Med J 1991;155: Grimbert S, Pessayre D, Degott C, Benhamou JP. Acute hepatitis induced by HMG-CoA reductase inhibitor, lovastatin. Dig Dis Sci 1994;39: Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol 1999;94: Raveh D, Arnon R, Israeli A, Eisenberg S. Lovastatin induced hepatitis. Isr J Med Sci 1992;28: National Cholesterol Education Program. Third report of the expert panel on detection, evaluation, and treatment of high cholesterol in adults (Adult Treatment Panel III). Website: Accessed July 10, Physician Desk Reference (PDR), 57th edition, Zachoval R, Gerbes AL, Schwandt O, Parhofer KG. Short-term effects of statin therapy in patients with hyperlipoproteinemia after liver transplantation: results of a randomized cross-over trial. J Hepatol 2001;35: Simonson SG, Martin PD, Mitchell P, Schneck DW, Lasseter KC, Warwick MJ. Pharmacokinetics and pharmacodynamics of rosuvastatin in subjects with hepatic impairment. Eur J Clin Pharmacol 2003;58:
6 1292 CHALASANI ET AL. GASTROENTEROLOGY Vol. 126, No McDonald CJ, Overhage JM, Tierney WM, Dexter PR, Martin DK, Suico JG, Zafar A, Schadow G, Blevins L, Glazener T, Meeks- Johnson J, Lemmon L, Warvel J, Porterfield B, Warvel J, Cassidy P, Lindbergh D, Belsito A, Tucker M, Williams B, Wodniak C. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform 1999;54: Murray MD, Loos B, Tu W, Eckert GJ, Zhou XH, Tierney WM. Work patterns of ambulatory care pharmacists with access to electronic guideline-based treatment suggestions. Am J Health Syst Pharm 1999;56: Accessed December 23, Anonymous. Statin set to become next big POM-to-P switch: is this good news for patients? Pharm J 2003:271:705. Received August 28, Accepted February 5, Address requests for reprints to: Naga Chalasani, M.D., Indiana University School of Medicine, WD OPW 2005, 1001 West 10th Street, Indianapolis, Indiana nchalasa@iupui.edu; fax: (317) Supported in part by FDA (FD-T to S.D.H.) and NIH grants (UO-1 DK to N.C.).
Drug-induced hepatotoxicity is a well-recognized adverse. Incidence of Statin Hepatotoxicity in Patients With Hepatitis C
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:902 907 Incidence of Statin Hepatotoxicity in Patients With Hepatitis C SHIRIN KHORASHADI,* NOELLE K. HASSON,* and RAMSEY C. CHEUNG, *Pharmacy Service, and
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:902 907 Incidence of Statin Hepatotoxicity in Patients With Hepatitis C SHIRIN KHORASHADI,* NOELLE K. HASSON,* and RAMSEY C. CHEUNG, *Pharmacy Service, and
How to use statins in patients with chronic liver disease
 REVIEW CME CREDIT MARK W. RUSSO, MD Center for the Study of Hepatitis C, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, New York, NY IRA M. JACOBSON, MD Center for the Study
REVIEW CME CREDIT MARK W. RUSSO, MD Center for the Study of Hepatitis C, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, New York, NY IRA M. JACOBSON, MD Center for the Study
In 2001, the National Cholesterol Education Program
 At a Glance Practical Implications p 330 Author Information p 333 Full text and PDF www.ajpblive.com Lipid Management When Converting Fluvastatin to Pravastatin: Medication Use Evaluation Original Research
At a Glance Practical Implications p 330 Author Information p 333 Full text and PDF www.ajpblive.com Lipid Management When Converting Fluvastatin to Pravastatin: Medication Use Evaluation Original Research
Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization
 The American Journal of Medicine (2005) 118, 618 624 CLINICAL RESEARCH STUDY Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization Evguenia C. Charles,
The American Journal of Medicine (2005) 118, 618 624 CLINICAL RESEARCH STUDY Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization Evguenia C. Charles,
Purpose: To establish a guideline in order to reduce ASCVD risk based the four identified statin benefit groups
 Title: Algorithm Based on AHA-13 Report on Lipids Date Created: 11/30/2015 Date Modified: N/A Date Approved by Board of Directors: 12/08/2015 Clinical Guideline # CGC-CG-12 Purpose: To establish a guideline
Title: Algorithm Based on AHA-13 Report on Lipids Date Created: 11/30/2015 Date Modified: N/A Date Approved by Board of Directors: 12/08/2015 Clinical Guideline # CGC-CG-12 Purpose: To establish a guideline
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPA.171 Effective Date:
 Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin
 Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
Kynamro. Kynamro (mipomersen) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.02 Subject: Kynamro Page: 1 of 5 Last Review Date: December 2, 2016 Kynamro Description Kynamro (mipomersen)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.02 Subject: Kynamro Page: 1 of 5 Last Review Date: December 2, 2016 Kynamro Description Kynamro (mipomersen)
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
High ( 50%) Restrictions mg 20-40mg PA; TS ⱡ 15 ⱡ
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
Juxtapid. Juxtapid (lomitapide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Juxtapid Page: 1 of 6 Last Review Date: September 20, 2018 Juxtapid Description Juxtapid (lomitapide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Juxtapid Page: 1 of 6 Last Review Date: September 20, 2018 Juxtapid Description Juxtapid (lomitapide)
Original paper. Abstract. Abdullah S. Asia 1*, Al-Mahdi A. Modar 2, Hadi M. Ali 3
 Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
Kynamro. Kynamro (mipomersen) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.02 Subject: Kynamro Page: 1 of 5 Last Review Date: September 15, 2017 Kynamro Description Kynamro
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.02 Subject: Kynamro Page: 1 of 5 Last Review Date: September 15, 2017 Kynamro Description Kynamro
MOLINA HEALTHCARE OF CALIFORNIA
 MOLINA HEALTHCARE OF CALIFORNIA HIGH BLOOD CHOLESTEROL IN ADULTS GUIDELINE Molina Healthcare of California has adopted the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel
MOLINA HEALTHCARE OF CALIFORNIA HIGH BLOOD CHOLESTEROL IN ADULTS GUIDELINE Molina Healthcare of California has adopted the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
Pharmacy Drug Class Review
 Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Elevated low-density lipoprotein
 A for Simvastatin and Gemfibrozil Molly Haselden, PharmD, BCPS; Thomas Worrall, PharmD, BCPS; and Dorothy Jenrette, PharmD, BCPS Eliminating gemfibrozil from a statin-containing regimen may be safe and
A for Simvastatin and Gemfibrozil Molly Haselden, PharmD, BCPS; Thomas Worrall, PharmD, BCPS; and Dorothy Jenrette, PharmD, BCPS Eliminating gemfibrozil from a statin-containing regimen may be safe and
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
Introduction. Objective. Critical Questions Addressed
 Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
Praluent. Praluent (alirocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Learning Objectives. Disclosures. Self Assessment Questions. Background
 Association of Hyperuricemia with Liver Dysfunction amongst Adults with Metabolic Disorders in the United States: A Cross Sectional Study Disclosures Dr. Prashant Sakharkar and Dr. Subrata Deb declare(s)
Association of Hyperuricemia with Liver Dysfunction amongst Adults with Metabolic Disorders in the United States: A Cross Sectional Study Disclosures Dr. Prashant Sakharkar and Dr. Subrata Deb declare(s)
RISK FACTORS AND DRUG TO STATIN-INDUCED MYOPATHY
 RISK FACTORS AND DRUG INTERACTION PREDISPOSING TO STATIN-INDUCED MYOPATHY Assist. Prof. Dr. Verawan Uchaipichat Clinical Pharmacy Department Khon Kaen University Advanced Pharmacotherapy 2012 Updated d
RISK FACTORS AND DRUG INTERACTION PREDISPOSING TO STATIN-INDUCED MYOPATHY Assist. Prof. Dr. Verawan Uchaipichat Clinical Pharmacy Department Khon Kaen University Advanced Pharmacotherapy 2012 Updated d
It is the policy of health plans affiliated with PA Health & Wellness that Vytorin is medically necessary when the following criteria are met:
 Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Clinical Policy: Ezetimibe (Zetia) Reference Number: CP.PMN.78 Effective Date: Last Review Date: 02.19
 Clinical Policy: (Zetia) Reference Number: CP.PMN.78 Effective Date: 02.01.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Zetia) Reference Number: CP.PMN.78 Effective Date: 02.01.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Learning Objectives. Patient Case
 Joseph Saseen, Pharm.D., FASHP, FCCP, BCPS Professor and Vice Chair, Department of Clinical Pharmacy University of Colorado Anschutz Medical Campus Learning Objectives Identify the 4 patient populations
Joseph Saseen, Pharm.D., FASHP, FCCP, BCPS Professor and Vice Chair, Department of Clinical Pharmacy University of Colorado Anschutz Medical Campus Learning Objectives Identify the 4 patient populations
Management of Post-transplant hyperlipidemia
 Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia
 ISPUB.COM The Internet Journal of Cardiovascular Research Volume 3 Number 1 Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia V Save, N Patil, G Rajadhyaksha Citation V Save,
ISPUB.COM The Internet Journal of Cardiovascular Research Volume 3 Number 1 Rosuvastatin: An Effective Lipid Lowering Drug against Hypercholesterolemia V Save, N Patil, G Rajadhyaksha Citation V Save,
REACH Risk Evaluation to Achieve Cardiovascular Health
 Dyslipidemia and transplantation History: An 8-year-old boy presented with generalized edema and hypertension. A renal biopsy confirmed a diagnosis of focal segmental glomerulosclerosis (FSGS). After his
Dyslipidemia and transplantation History: An 8-year-old boy presented with generalized edema and hypertension. A renal biopsy confirmed a diagnosis of focal segmental glomerulosclerosis (FSGS). After his
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Guidelines for Screening and Management of Dyslipidemia
 PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
GSK Medicine: Study No.: Title: Rationale: before initiation of treatment, every 4-6 weeks during treatment
 GSK Medicine: Lapatinib Study No.: WWE115270/WEUKSTV4275 Title: Assessment of Physician Compliance to Recommend Liver Function Test (LFT) Monitoring for Lapatinib Patients Rationale: Lapatinib (Tykerb
GSK Medicine: Lapatinib Study No.: WWE115270/WEUKSTV4275 Title: Assessment of Physician Compliance to Recommend Liver Function Test (LFT) Monitoring for Lapatinib Patients Rationale: Lapatinib (Tykerb
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017
 Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
1 DOS CME Course 2011
 Statin Myopathy February 23, 2011 Jinny Tavee, MD Associate Professor Neurological Institute Cleveland Clinic Foundation 1 Case 1 50 y/o woman with hyperlipidemia presents with one year history of deep
Statin Myopathy February 23, 2011 Jinny Tavee, MD Associate Professor Neurological Institute Cleveland Clinic Foundation 1 Case 1 50 y/o woman with hyperlipidemia presents with one year history of deep
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Juxtapid) Reference Number: CP.PHAR.283 Effective Date: 10.01.16 Last Review Date: 08.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Juxtapid) Reference Number: CP.PHAR.283 Effective Date: 10.01.16 Last Review Date: 08.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Ammonia level at admission predicts in-hospital mortality for patients with alcoholic hepatitis
 Gastroenterology Report, 5(3), 2017, 232 236 doi: 10.1093/gastro/gow010 Advance Access Publication Date: 1 May 2016 Original article ORIGINAL ARTICLE Ammonia level at admission predicts in-hospital mortality
Gastroenterology Report, 5(3), 2017, 232 236 doi: 10.1093/gastro/gow010 Advance Access Publication Date: 1 May 2016 Original article ORIGINAL ARTICLE Ammonia level at admission predicts in-hospital mortality
Andrew Cohen, MD and Neil S. Skolnik, MD INTRODUCTION
 2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
ORIGINAL INVESTIGATION. Screening for Statin-Related Toxicity. The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting
 ORIGINAL INVESTIGATION Screening for Statin-Related Toxicity The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting C. Christopher Smith, MD; Lana I. Bernstein, MD; Roger
ORIGINAL INVESTIGATION Screening for Statin-Related Toxicity The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting C. Christopher Smith, MD; Lana I. Bernstein, MD; Roger
Promacta. Promacta (eltrombopag) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.15 Subject: Promacta Page: 1 of 6 Last Review Date: September 15, 2017 Promacta Description Promacta
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.15 Subject: Promacta Page: 1 of 6 Last Review Date: September 15, 2017 Promacta Description Promacta
Joshua Shepherd PA-C, MMS, MT (ASCP)
 Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
ACTEMRA Risk Mitigation Strategy Presenter Name, Degree
 ACTEMRA Risk Mitigation Strategy Presenter Name, Degree Medical Science Liaison Genentech, Inc. 1 Indications and Dosage Rheumatoid Arthritis (RA) (1 of 2) Indication in RA ACTEMRA (tocilizumab) is indicated
ACTEMRA Risk Mitigation Strategy Presenter Name, Degree Medical Science Liaison Genentech, Inc. 1 Indications and Dosage Rheumatoid Arthritis (RA) (1 of 2) Indication in RA ACTEMRA (tocilizumab) is indicated
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2062-8 Program Prior Authorization/Medical Necessity Medication Praluent (alirocumab) P&T Approval Date 5/2015, 8/2015, 9/2015,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2062-8 Program Prior Authorization/Medical Necessity Medication Praluent (alirocumab) P&T Approval Date 5/2015, 8/2015, 9/2015,
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date:
 Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Disclosures No relationships (not even to an employer) No off-label uses. Cholesterol Lowering Guidelines: What now?
 Disclosures No relationships (not even to an employer) No off-label uses Cholesterol Lowering Guidelines: What now?, FACP 1 2 65-year-old white woman Total cholesterol 175mg/dL HDL 54 mg/dl LDL 96 mg/dl
Disclosures No relationships (not even to an employer) No off-label uses Cholesterol Lowering Guidelines: What now?, FACP 1 2 65-year-old white woman Total cholesterol 175mg/dL HDL 54 mg/dl LDL 96 mg/dl
Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States
 Bull. Natl. Inst. Health Sci., 123 Notes # Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States Journal of American Medical Association (JAMA) Statin HMG
Bull. Natl. Inst. Health Sci., 123 Notes # Withdrawal of Cerivastatin Revealed a Flaw of Post-marketing Surveillance System in the United States Journal of American Medical Association (JAMA) Statin HMG
New Guidelines in Dyslipidemia Management
 The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins)
 Drug Class Review on HMG-CoA Reductase Inhibitors () Final Report June 2004 Mark Helfand, MD, MPH Susan Carson, MPH Cathy Kelley, PharmD Oregon Evidence-based Practice Center Oregon Health & Science University
Drug Class Review on HMG-CoA Reductase Inhibitors () Final Report June 2004 Mark Helfand, MD, MPH Susan Carson, MPH Cathy Kelley, PharmD Oregon Evidence-based Practice Center Oregon Health & Science University
Clinical Approach to Achieving Treatment Targets: Case Vignette Discussion
 Clinical Approach to Achieving Treatment Targets: Case Vignette Discussion Om P. Ganda, MD; Kirit Tolia, MD, FACE Postcardiac Follow-up in a 66-Year-Old Man Slide 1. Dr. Ganda: Now we will present a case
Clinical Approach to Achieving Treatment Targets: Case Vignette Discussion Om P. Ganda, MD; Kirit Tolia, MD, FACE Postcardiac Follow-up in a 66-Year-Old Man Slide 1. Dr. Ganda: Now we will present a case
Trade Name FDA-Approved Indication(s) Usual Daily Dose Immediate release nicotinic acid, 500 mg crystalline tablet
 Does extended release nicotinic acid reduce the risk of hepatotoxicity compared to immediate release nicotinic acid? Nicotinic acid (synthetic niacin or 3-pyridinecarboxylic acid) is a water soluble B-complex
Does extended release nicotinic acid reduce the risk of hepatotoxicity compared to immediate release nicotinic acid? Nicotinic acid (synthetic niacin or 3-pyridinecarboxylic acid) is a water soluble B-complex
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Caduet) Reference Number: CP.CPA.237 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Caduet) Reference Number: CP.CPA.237 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
New Guidelines in Dyslipidemia Management
 The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
A mong the 20 leading prescription
 Safety and Statins: Pharmacologic and Clinical Perspectives Michael B. Bottorff, PharmD A mong the 20 leading prescription drugs in the United States, 3 agents atorvastatin (Lipitor), simvastatin (Zocor),
Safety and Statins: Pharmacologic and Clinical Perspectives Michael B. Bottorff, PharmD A mong the 20 leading prescription drugs in the United States, 3 agents atorvastatin (Lipitor), simvastatin (Zocor),
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Kynamro) Reference Number: CP.PHAR.284 Effective Date: 10.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Kynamro) Reference Number: CP.PHAR.284 Effective Date: 10.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
B. Patient has not reached the percentage reduction goal with statin therapy
 Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
Atorvastatin associated liver disease
 Digestive and Liver Disease 38 (2006) 772 777 Brief Clinical Observation Atorvastatin associated liver disease A.T. Clarke, P.R. Mills Gastroenterology Unit, Gartnavel General Hospital, Glasgow, UK Received
Digestive and Liver Disease 38 (2006) 772 777 Brief Clinical Observation Atorvastatin associated liver disease A.T. Clarke, P.R. Mills Gastroenterology Unit, Gartnavel General Hospital, Glasgow, UK Received
New Evidence reports on presentations given at EULAR Safety and Efficacy of Tocilizumab as Monotherapy and in Combination with Methotrexate
 New Evidence reports on presentations given at EULAR 2009 Safety and Efficacy of Tocilizumab as Monotherapy and in Combination with Methotrexate Report on EULAR 2009 presentations Tocilizumab inhibits
New Evidence reports on presentations given at EULAR 2009 Safety and Efficacy of Tocilizumab as Monotherapy and in Combination with Methotrexate Report on EULAR 2009 presentations Tocilizumab inhibits
Antihyperlipidemic Drugs
 Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Drug Class Monograph
 Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
Periodic Rosuvastatin or Atorvastatin Dosing Arrays (PRADA): Patient-Centered Practice
 Drugs R D (2014) 14:221 225 DOI 10.1007/s40268-014-0061-9 ORIGINAL RESEARCH ARTICLE Periodic Rosuvastatin or Atorvastatin Dosing Arrays (PRADA): Patient-Centered Practice Theodore Christou Hesham R. Omar
Drugs R D (2014) 14:221 225 DOI 10.1007/s40268-014-0061-9 ORIGINAL RESEARCH ARTICLE Periodic Rosuvastatin or Atorvastatin Dosing Arrays (PRADA): Patient-Centered Practice Theodore Christou Hesham R. Omar
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins)
 Drug Class Review on HMG-CoA Reductase Inhibitors (Statins) August 2006 Original Report Date: April 2002 Update 1 Report Date: July 2003 Update 2 Report Date: June 2004 Update 3 Report Date: September
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins) August 2006 Original Report Date: April 2002 Update 1 Report Date: July 2003 Update 2 Report Date: June 2004 Update 3 Report Date: September
Primary Prevention Patients aged 85yrs and over
 Rotherham Guideline for the management of Non-Familial Hypercholesterolaemia Type 1 Diabetes Offer lifestyle advice Over 40yrs of age? Diabetic for more than 10 years? Established nephropathy? Other CVD
Rotherham Guideline for the management of Non-Familial Hypercholesterolaemia Type 1 Diabetes Offer lifestyle advice Over 40yrs of age? Diabetic for more than 10 years? Established nephropathy? Other CVD
Mipomersen (ISIS ) Page 2 of 1979 Clinical Study Report ISIS CS3
 (ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
(ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
hyperlipidemia in CKD DR MOJGAN MORTAZAVI ASSOCIATE PROFESSOR OF NEPHROLOGY ISFAHAN KIDNEY DISEASES RESEARCH CENTER
 Management of hyperlipidemia in CKD DR MOJGAN MORTAZAVI ASSOCIATE PROFESSOR OF NEPHROLOGY ISFAHAN KIDNEY DISEASES RESEARCH CENTER Background on Dyslipidemia in CKD In advanced chronic kidney disease (CKD),
Management of hyperlipidemia in CKD DR MOJGAN MORTAZAVI ASSOCIATE PROFESSOR OF NEPHROLOGY ISFAHAN KIDNEY DISEASES RESEARCH CENTER Background on Dyslipidemia in CKD In advanced chronic kidney disease (CKD),
PCSK9 Agents Drug Class Prior Authorization Protocol
 PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction
 Supplement: Tables Supplement Table 1. Study Eligibility Criteria Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction Supplement Table
Supplement: Tables Supplement Table 1. Study Eligibility Criteria Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction Supplement Table
Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention)
 Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention) Policy Statement: October 2010 This policy defines the decision made by NHS Wirral following an evidence review
Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention) Policy Statement: October 2010 This policy defines the decision made by NHS Wirral following an evidence review
Statin intolerance. Pr Franck Boccara, MD, PhD Cardiologie, INSERM UMRS938 CHU St Antoine, UPMC, Paris, France
 Statin intolerance Pr Franck Boccara, MD, PhD Cardiologie, INSERM UMRS938 CHU St Antoine, UPMC, Paris, France Disclosure Statement of Financial Interest I currently have, or have had over the last two
Statin intolerance Pr Franck Boccara, MD, PhD Cardiologie, INSERM UMRS938 CHU St Antoine, UPMC, Paris, France Disclosure Statement of Financial Interest I currently have, or have had over the last two
High ( 50%) Restrictions mg 20-40mg Simvastatin (Zocor) 10mg 20-40mg $1.66 Pravastatin (Pravachol) mg $6.
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/8/2018 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/17, 5/16, 5/15, 2/14, LOB AFFECTED: Medi-Cal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/8/2018 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/17, 5/16, 5/15, 2/14, LOB AFFECTED: Medi-Cal
Conflict of Interest Disclosure. Learning Objectives. Learning Objectives. Guidelines. Update on Lifestyle Guidelines
 Conflict of Interest Disclosure Updates for the Ambulatory Care Pharmacist: Dyslipidemia and CV Risk Assessment No conflicts of interest to disclose 2014 Updates to the Updates in Ambulatory Care Pharmacy
Conflict of Interest Disclosure Updates for the Ambulatory Care Pharmacist: Dyslipidemia and CV Risk Assessment No conflicts of interest to disclose 2014 Updates to the Updates in Ambulatory Care Pharmacy
Promacta. Promacta (eltrombopag) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.15 Subject: Promacta Page: 1 of 6 Last Review Date: September 20, 2018 Promacta Description Promacta
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.15 Subject: Promacta Page: 1 of 6 Last Review Date: September 20, 2018 Promacta Description Promacta
Heart disease is the leading cause of death in. Impact of an EMR Clinical Decision Support Tool on Lipid Management.
 Impact of an EMR Clinical Decision Support Tool on Lipid Management Erik Kelly, BA, Thomas Wasser, PhD, MEd, Julian Diaz Fraga, MD, Jorge J. Scheirer, MD, and Richard L. Alweis, MD ABSTRACT Objective:
Impact of an EMR Clinical Decision Support Tool on Lipid Management Erik Kelly, BA, Thomas Wasser, PhD, MEd, Julian Diaz Fraga, MD, Jorge J. Scheirer, MD, and Richard L. Alweis, MD ABSTRACT Objective:
Cholesterol Management Roy Gandolfi, MD
 Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Pitavastatin 4 mg vs. Pravastatin 40 mg in HIV Dyslipidemia: Post- Hoc Analysis of the INTREPID Trial Based on the Independent CHD Risk Factor for Age
 Pitavastatin 4 mg vs. Pravastatin 40 mg in HIV Dyslipidemia: Post- Hoc Analysis of the INTREPID Trial Based on the Independent CHD Risk Factor for Age Craig A. Sponseller, Masaya Tanahashi, Hideki Suganami,
Pitavastatin 4 mg vs. Pravastatin 40 mg in HIV Dyslipidemia: Post- Hoc Analysis of the INTREPID Trial Based on the Independent CHD Risk Factor for Age Craig A. Sponseller, Masaya Tanahashi, Hideki Suganami,
Juxtapid (lomitapide)
 Juxtapid (lomitapide) Policy Number: 5.01.599 Last Review: 06/2018 Origination: 07/2015 Next Review: 06/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Juxtapid
Juxtapid (lomitapide) Policy Number: 5.01.599 Last Review: 06/2018 Origination: 07/2015 Next Review: 06/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Juxtapid
Major recommendations for statin therapy for ASCVD prevention
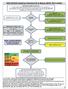 2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
Ocaliva (obeticholic acid tablets)
 Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Ocaliva (obeticholic acid tablets) Policy Number: 5.01.619 Last Review: 11/2018 Origination: 11/2016 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
2013 Cholesterol Guidelines. Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc.
 2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
Statins are among the most widely prescribed medications
 PERSPECTIVES IN CLINICAL HEPATOLOGY s and Hepatotoxicity: Focus on Patients With Fatty Liver Naga Chalasani s are among the most widely prescribed medications in the western world. 1 Their benefit in the
PERSPECTIVES IN CLINICAL HEPATOLOGY s and Hepatotoxicity: Focus on Patients With Fatty Liver Naga Chalasani s are among the most widely prescribed medications in the western world. 1 Their benefit in the
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Disclosures. How do statins work? Statin Pharmacokinetics 9/12/2013 THERAPEUTIC INTERVENTIONS FOR STATIN INTOLERANT PATIENTS
 Disclosures Speakers Bureau- LipoScience Inc. THERAPEUTIC INTERVENTIONS FOR STATIN INTOLERANT PATIENTS Casey Elkins, DNP, NP-C, CLS How do statins work? Bays H, Stein EA. Expert Opin Pharmacother. 2003;4(11):1901-1938.
Disclosures Speakers Bureau- LipoScience Inc. THERAPEUTIC INTERVENTIONS FOR STATIN INTOLERANT PATIENTS Casey Elkins, DNP, NP-C, CLS How do statins work? Bays H, Stein EA. Expert Opin Pharmacother. 2003;4(11):1901-1938.
Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate
 Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate BMJ Jenny H Humphreys, Alexander Warner, Ruth Costello, Mark Lunt, Suzanne M M Verstappen,
Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate BMJ Jenny H Humphreys, Alexander Warner, Ruth Costello, Mark Lunt, Suzanne M M Verstappen,
Setting The setting was secondary care. The economic study was carried out in the USA.
 Cost-effectiveness analysis of combination statin/ezetimibe therapy for the treatment of elevated low-density lipoprotein cholesterol Gryskiewicz K A, Coleman C I, Gillespie E L, White C M Record Status
Cost-effectiveness analysis of combination statin/ezetimibe therapy for the treatment of elevated low-density lipoprotein cholesterol Gryskiewicz K A, Coleman C I, Gillespie E L, White C M Record Status
The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study
 The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study DK Li, YJ Ren, DS Fierer, S Rutledge, OS Shaikh, V Lo
The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study DK Li, YJ Ren, DS Fierer, S Rutledge, OS Shaikh, V Lo
Lipid Panel Management Refresher Course for the Family Physician
 Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Scientific conclusions
 Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Effectiveness of statins in Medicare-eligible patients and patients < 65 years using clinical practice data*
 ORIGINAL PAPER Effectiveness of statins in Medicare-eligible patients and patients < 65 years using clinical practice data* K. M. Fox, 1 S. K. Gandhi, 2 R. L. Ohsfeldt, 3 J. W. Blasetto, 4 M. H. Davidson
ORIGINAL PAPER Effectiveness of statins in Medicare-eligible patients and patients < 65 years using clinical practice data* K. M. Fox, 1 S. K. Gandhi, 2 R. L. Ohsfeldt, 3 J. W. Blasetto, 4 M. H. Davidson
HBV Forum 2 April 18 th 2017 Hilton Amsterdam.
 HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
Pharmacology Challenges: Managing Statin Myalgia
 Clinical Case: RM is a 50 year-old African American woman with a past medical history of type diabetes, dyslipidemia, hypertension and peripheral arterial disease. She had been prescribed simvastatin 80
Clinical Case: RM is a 50 year-old African American woman with a past medical history of type diabetes, dyslipidemia, hypertension and peripheral arterial disease. She had been prescribed simvastatin 80
Achieving Lipid Goals: 2008 Update. Laura Hansen, Pharm.D. Associate Professor, University of Colorado School of Pharmacy
 Achieving Lipid Goals: 2008 Update Laura Hansen, Pharm.D. Associate Professor, University of Colorado School of Pharmacy Discuss relationship between lipid values and coronary events Evaluate clinical
Achieving Lipid Goals: 2008 Update Laura Hansen, Pharm.D. Associate Professor, University of Colorado School of Pharmacy Discuss relationship between lipid values and coronary events Evaluate clinical
Managed Care Trends in Statin Usage GARY R. BAZALO, MS, MBA
 PHARMACY UTILIZATION Managed Care Trends in Statin Usage GARY R. BAZALO, MS, MBA ABSTRACT Purpose HMG-CoA reductase inhibitors ( statins ) have become the drug class of choice for the treatment of hyperlipidemia.
PHARMACY UTILIZATION Managed Care Trends in Statin Usage GARY R. BAZALO, MS, MBA ABSTRACT Purpose HMG-CoA reductase inhibitors ( statins ) have become the drug class of choice for the treatment of hyperlipidemia.
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2
 2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
This document was created on 07 January 2005 and has been printed from
 1 Spontaneous Adverse Event Reporting Information Introduction This document provides information regarding spontaneous adverse event reports received concerning patients taking rosuvastatin, which relates
1 Spontaneous Adverse Event Reporting Information Introduction This document provides information regarding spontaneous adverse event reports received concerning patients taking rosuvastatin, which relates
T REV 21. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 07/2009
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZETIA safely and effectively. See full prescribing information for ZETIA. ZETIA (ezetimibe) Tablets
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZETIA safely and effectively. See full prescribing information for ZETIA. ZETIA (ezetimibe) Tablets
Simvastatin With or Without Ezetimibe in Familial Hypercholesterolemia
 Simvastatin With or Without Ezetimibe in Familial Hypercholesterolemia The trial ClinicalTrials.gov number: NCT00552097 John J.P. Kastelein, MD, PhD* Department of Vascular Medicine Academic Medical Center
Simvastatin With or Without Ezetimibe in Familial Hypercholesterolemia The trial ClinicalTrials.gov number: NCT00552097 John J.P. Kastelein, MD, PhD* Department of Vascular Medicine Academic Medical Center
1. Based on A.S. s labs and presentation, what type of liver injury would you classify her as experiencing?
 Drug Induced Liver Injury Cases Case #1 A.S., a16 year-old female, was found by her pediatrician to be slightly jaundiced during a routine school physical. She denied any history of liver disease, abdominal
Drug Induced Liver Injury Cases Case #1 A.S., a16 year-old female, was found by her pediatrician to be slightly jaundiced during a routine school physical. She denied any history of liver disease, abdominal
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 Ezetimibe-simvastatin 10-80 mg oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Ezetimibe-simvastatin 10-80 mg oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
