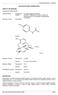
PRODUCT INFORMATION PARADEX NAME OF THE DRUG: Dextropropoxyphene Hydrochloride; Paracetamol. Structural Formulae. Dextropropoxyphene Hydrochloride
|
|
|
- Marvin Charles
- 5 years ago
- Views:
Transcription
1 PRODUCT INFORMATION PARADEX NAME OF THE DRUG: Dextropropoxyphene Hydrochloride; Paracetamol Structural Formulae Dextropropoxyphene Hydrochloride CAS Paracetamol CAS DESCRIPTION: Dextropropoxyphene Hydrochloride 32.5 mg, Mol. Wt , C 22 H 29 NO 2,HCl is (1S, 2R)- 1-benzyl-3-dimethylamino-2-methyl-1-phenylpropyl propionate hydrochloride. Paracetamol 325 mg, Mol. Wt , C 8 H 9 NO 2 is N-(4-hydroxyphenyl) acetamide. Dextropropoxyphene hydrochloride occurs as a white or slightly yellow powder, odourless, and with a bitter taste. It is soluble in 0.3 parts of water, 1 in 1-5 parts of alcohol (95%) and 1 in 0.6 parts of chloroform. It is insoluble in solvent ether. It has a melting point of about 165 o. Paracetamol occurs as white crystals or a white crystalline powder; odourless and with a bitter taste. It is soluble 1 in 70 parts of water, 1 in 7 parts of alcohol (95%), 1 in 13 parts of acetone, 1 in 40 parts of glycerol, and 1 in 9 parts of propylene glycol; it is also soluble in solutions of the alkali hydroxides. It has a melting point of 169 o to 172 o. Excipients: Lactose BP, Starch-wheat, Starch pregelatinised wheat, Silica colloidal anhydrous, Talcpurified, Ethylcellulose, Sodium starch glycollate, Povidone and Magnesium stearate. PARADEX Product Information Full & Marketed Page 1 of 8
2 PHARMACOLOGY: Actions: Dextropropoxyphene hydrochloride is a centrally acting, synthetic opioid analgesic structurally related to methadone. It binds to opioid receptors at many sites within the central nervous system affecting processes for both the physiological perception of pain and the emotional response to pain. There are multiple subtypes of central and peripheral opioid receptors each mediating therapeutic and/or adverse effects of opioid drugs. The potency of dextropropoxyphene hydrochloride is from two thirds to equal that of codeine. Paracetamol is a non-opioid analgesic and an anti-pyretic. The analgesic effect of paracetamol is thought to be due to the inhibition of prostaglandin synthesis in the central nervous system and the periphery and, to a lesser extent, by blocking the pain impulse generation in the periphery. The antipyretic effect is due to a central action on the hypothalamic heat regulating centre to produce peripheral vasodilatation and subsequent heat loss. The combination of dextropropoxyphene with paracetamol produces greater analgesia than that produced by either drug administered alone. Pharmacokinetics: Dextropropoxyphene is readily absorbed from the gastrointestinal tract but is subject to considerable first pass metabolism. Equimolar doses of dextropropoxyphene hydrochloride or dextropropoxyphene napsylate provide similar plasma concentrations. Following administration of 65,130 or 195 mg of dextropropoxyphene hydrochloride, the bioavailability of dextropropoxyphene is equivalent to that of 100, 200 or 300 mg respectively of dextropropoxyphene napsylate. Peak plasma concentrations of dextropropoxyphene are reached in 2 to 2½ hours. After a 65 mg oral dose of dextropropoxyphene hydrochloride, peak plasma levels of 0.05 to 0.1 mcg/ml are achieved. Repeated doses of dextropropoxyphene at six-hour intervals lead to increasing plasma concentrations, with a plateau after the ninth dose at 48 hours. Dextropropoxyphene is metabolized in the liver to yield norpropoxyphene. Dextropropoxyphene has a half-life of 6 to 12 hours, whereas that of norpropoxyphene is 30 to 36 hours. Norpropoxyphene has substantially less central-nervous system-depressant effect than dextropropoxyphene but a greater local anaesthetic effect, which is similar to that of amitriptyline and the antiarrhythmic agents, such as lignocaine and quinidine. In animal studies in which dextropropoxyphene and norpropoxyphene were continually infused in large amounts, intracardiac conduction time (PR and QRS intervals) was prolonged. Any intracardiac conduction delay attributable to high concentrations of norpropoxyphene may be of relatively long duration. Paracetamol is absorbed rapidly and completely from the small intestine after oral administration. Peak plasma paracetamol concentrations occur 30 to 120 minutes after oral administration. It is uniformly distributed throughout most body fluids with an apparent volume of distribution of 1 to 1.2 L/kg. Plasma protein binding is negligible at the usual therapeutic concentrations but increases with increasing concentrations. Approximately 90 to 95% of a dose of paracetamol is metabolised by the hepatic microsomal system. In adults at therapeutic doses, paracetamol is mainly conjugated with glucuronide (45-55%) or sulphate (20-30%). A minor proportion (less than 20%) is PARADEX Product Information Full & Marketed Page 2 of 8
3 metabolised to catechol derivatives. Paracetamol is metabolised differently by infants and children compared with adults, the sulphate conjugate being predominant. Paracetamol is excreted in the urine mainly as the glucuronide and sulphate conjugates. Less than 5% is excreted as unchanged paracetamol with 85-90% of the dose being eliminated within the urine within 24 hours of ingestion. The elimination half-life of paracetamol varies from about 1 to 4 hours. Food delays paracetamol absorption. INDICATIONS: Relief of mild to moderate pain. CONTRAINDICATIONS: Known hypersensitivity to either constituent. Concurrent use of alcohol. Concurrent use of other paracetamol containing products. WARNINGS: DO NOT PRESCRIBE DEXTROPROPOXYPHENE FOR PATIENTS WHO ARE SUICIDAL OR PRONE TO DEPENDENCY. PRESCRIBE DEXTROPROPOXYPHENE WITH CAUTION FOR PATIENTS TAKING TRANQUILLIZERS OR ANTIDEPRESSANT DRUGS AND PATIENTS WHO USE ALCOHOL IN EXCESS. TELL YOUR PATIENTS NOT TO EXCEED THE RECOMMENDED DOSE AND TO AVOID ALCOHOL. Dextropropoxyphene products in excessive doses, either alone or in combination with other CNS depressants, including alcohol, are a major cause of drug-related deaths. Fatalities within the first hour of overdosage are not uncommon. In a survey of deaths due to overdosage conducted in 1975, in approximately 20 percent of the fatal cases, death occurred within the first hour (5 percent occurred within 15 minutes). Dextropropoxyphene should not be taken in doses higher than those recommended by the physician. The judicious prescribing of dextropropoxyphene is essential to the safe use of this drug. In patients who are depressed or suicidal, consideration should be given to the use of non-opioid analgesics. Patients should be cautioned about the Concurrent use of dextropropoxyphene products and alcohol because of potentially serious CNSadditive effects of these agents. Because of its added depressant effects, dextropropoxyphene should be prescribed with caution for those patients whose medical condition requires the concurrent administration of sedatives, tranquillizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Patients should be advised of the additive depressant effects of these combinations. Many of the dextropropoxyphene-related deaths have occurred in patients with previous histories of emotional disturbance or suicidal ideation or attempts as well as histories of misuse of tranquillizers, alcohol, and other CNS-active drugs. Caution should be exercised in prescribing dextropropoxyphene hydrochloride for such patients ( see Management of Overdosage). Some deaths have occurred as a consequence of the accidental ingestion of excessive quantities of dextropropoxyphene alone or in combination with other drugs. Patients taking dextropropoxyphene should be warned not to exceed the dosage recommended by the physician. PARADEX Product Information Full & Marketed Page 3 of 8
4 Drug Dependence Dextropropoxyphene, when taken in higher than recommended doses over long periods of time, can produce drug dependence characterised by psychic dependence and, less frequently, physical dependence and tolerance. Dextropropoxyphene will only partially suppress the withdrawal syndrome in individuals physically dependent on morphine or other narcotics. The abuse liability of dextropropoxyphene is qualitatively similar to that of codeine although quantitatively less and dextropropoxyphene should be prescribed with the same degree of caution appropriate to the use of codeine. Use in Ambulatory Patients Dextropropoxyphene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly. PRECAUTIONS: Use in Patients with Hepatic or Renal Impairment Dextropropoxyphene should be administered with caution to patients with renal or hepatic impairment since higher serum concentrations or delayed elimination may occur. Use in Patients with Respiratory Impairment Dextropropoxyphene should be administered with caution to patients with respiratory impairment as it may depress respiration. Use in Pregnancy Pregnancy (Category C) PARADEX should not be used in pregnant women unless, in the judgement of the physician, the potential benefits outweigh the possible hazards. Dextropropoxyphene may cause respiratory depression in the newborn infant. Safe use in pregnancy has not been established relative to possible adverse effects on foetal development. Instances of withdrawal symptoms in the neonate have been reported following usage during pregnancy. Paracetamol can cross the placenta. However in rats and mice no teratogenic effects have been observed after doses of up to 250 mg/kg. Use in Lactation Low levels of dextropropoxyphene have been detected in human milk. In postpartum studies involving nursing mothers who were given dextropropoxyphene, no adverse effects were noted in infants receiving mother s milk. Paracetamol is excreted in breast milk. The amount available for ingestion by the infant has been reported variously as less than 0.1% of a single 500 mg dose and as 0.04 to 0.23% of a single 650 mg dose. Maternal ingestion of paracetamol in usual analgesic doses does not appear to present a risk to the nursing infant. Use in Children PARADEX is not recommended for use in children. The safety and effectiveness of dextropropoxyphene in children has not been established. Use in the Elderly The elderly are more likely to have age related renal impairment and increased susceptibility to the respiratory depressant effects of opioid analgesics. The rate of dextropropoxyphene metabolism may be reduced in some patients. An increased dosing interval or dose reduction should be considered. PARADEX Product Information Full & Marketed Page 4 of 8
5 Carcinogenicity, Mutagenicity, Impairment of Fertility Clinical toxicity studies in animals have shown that high doses of paracetamol cause testicular atrophy and inhibition of spermatogenesis; the relevance of this finding to use in humans is not known. Drug interactions General Dextropropoxyphene may inhibit the hepatic metabolism of concurrently administered drugs. Should this occur, higher serum concentrations of the concurrently administered drug may result in increased pharmacological and/or adverse effects of that drug. Such occurrences have been reported when dextropropoxyphene has been administered to patients receiving anti-depressants, anti-convulsants, or warfarin like drugs. CNS Depressants including Alcohol Dextropropoxyphene in combination with alcohol, tranquillizers, sedative-hypnotics, and other central nervous system depressants has additive depressant effects, and the patient should be so advised. Patients taking PARADEX should be warned not to exceed the dosage recommended by their physician (see Warnings). CNS Stimulants The convulsant action of dextropropoxyphene may be enhanced by CNS stimulants. Warfarin Concurrent warfarin and dextropropoxyphene administration may increase serum concentrations of warfarin. Paracetamol may affect prothrombin time in patients receiving anticoagulant therapy. Warfarin dosage adjustments may be required. Carbamazepine Concurrent carbamazepine and dextropropoxyphene administration significantly increases carbamazepine concentration and may result in moderate to severe neurotoxicity ( ataxia, nystagmus, diplopia, headache, vomiting, apnoea, seizures, coma). Ritonavir Concurrent ritonavir and dextropropoxyphene administration may increase serum concentrations of dextropropoxyphene resulting in an increased risk of CNS depression or other serious adverse effects. Beta-Blockers Oral bioavailability of metoprolol and propanolol may increase when they are given Concurrently with dextropropoxyphene. Orphenadrine Confusion, anxiety, and tremors have been reported in a few patients receiving dextropropoxyphene Concurrently with orphenadrine. Doxepin Concurrent administration of dextropropoxyphene and doxepin may double steady state doxepin and desmethyldoxepin plasma concentrations. This may increase doxepin toxicity (sedation, lethargy, dry mouth, urinary retention). Chloramphenicol Concurrent administration of paracetamol and chloramphenicol may increase chloramphenicol serum concentrations. Cholestyramine Concurrent administration of paracetamol and cholestyramine can lower plasma paracetamol plasma concentrations. Diflunisal Concurrent administration of paracetamol and diflunisal can increase paracetamol plasma concentrations by up to 50%. Phenytoin Concurrent administration of paracetamol and phenytoin can increase the metabolism of paracetamol by more than 40% and decrease its half-life by about 25%. PARADEX Product Information Full & Marketed Page 5 of 8
6 There is an increased risk of paracetamol hepatoxicity with Concurrent administration of these two drugs. Lamotrigine Concurrent administration of paracetamol and lamotrigine may slightly increase the elimination of lamotrigine. Metyrapone Concurrent administration of paracetamol and metyrapone may decrease the elimination of paracetamol. Probenecid Concurrent administration of paracetamol and probenecid may prolong the half-life and decrease the clearance of paracetamol. The clinical significance of this is unclear. Sulfinpyrazone Concurrent administration of paracetamol and sulfinpyrazone can increase the metabolism of paracetamol. Zidovudine Concurrent administration of paracetamol and zidovudine has been associated with an increased incidence of neutropenia, especially during chronic therapy. ADVERSE REACTIONS: The most commonly reported adverse reactions are dizziness, sedation, nausea, and vomiting. Some of these adverse reactions may be alleviated if the patient lies down. Other less frequent to rarely reported adverse reactions are light headedness, headache, weakness, euphoria, dysphoria, hallucinations, constipation, abdominal pain, hepatic impairment, minor visual disturbances, skin rashes, allergic reactions, thrombocytopenia, leucopenia, pancytopenia, neutropenia, agranulocytosis. Hepatic dysfunction has been reported in association with both dextropropoxyphene and paracetamol. Dextropropoxyphene therapy has been associated with abnormal liver function tests and, more rarely, with instances of reversible jaundice (including cholestatic jaundice). Hepatic necrosis may result from acute paracetamol overdose (see Management of Overdosage). In chronic alcohol abusers this has been reported rarely with short term use of paracetamol doses of 2.5 to 10g/day. Fatalities have occurred. The chronic ingestion of dextropropoxyphene in doses exceeding 720 mg per day has caused toxic psychoses and convulsions. A single dose of 1200 mg of dextropropoxyphene has caused convulsions. Renal papillary necrosis may result from chronic paracetamol use, particularly when the dose is greater than recommended and when combined with aspirin. Subacute painful myopathy has occurred following chronic dextropropoxyphene overdose. DOSAGE & ADMINISTRATION: PARADEX is given orally. The usual adult dose is 1 to 2 tablets four hourly as needed for pain. A dose of 2 tablets will provide 65 mg of dextropropoxyphene hydrochloride and 650 mg of paracetamol. The maximum recommended daily dose of dextropropoxyphene hydrochloride is 390mg. PARADEX Product Information Full & Marketed Page 6 of 8
7 The maximum recommended daily dose of paracetamol is 4g. Consideration should be given to a reduced total daily dosage of PARADEX in patients with hepatic or renal impairment. OVERDOSAGE: There are a disturbing number of fatalities from either accidental or intentional overdosage with dextropropoxyphene, many emphasising the rapidity with which death ensues. The first priority is therefore management of the CNS effects of dextropropoxyphene overdosage. Resuscitative measures should be initiated promptly. Symptoms of Dextropropoxyphene Overdosage The manifestations of acute overdosage with dextropropoxyphene are similar to those of narcotic overdosage. The patient is usually somnolent but may be stuporous or comatose and convulsing. Respiratory depression is characteristic. The ventilatory rate and/or tidal volume is decreased, which results in cyanosis and hypoxia. Pupils, initially pinpoint, may become dilated as hypoxia increases. Cheyne-Stokes respiration and apnoea may occur. Blood pressure and heart rate are usually normal initially, but blood pressure falls and cardiac performance deteriorates, which ultimately results in pulmonary oedema and circulatory collapse, unless the respiratory depression is corrected and adequate ventilation is restored promptly. Cardiac arrhythmias and conduction delay may be present. A combined respiratory metabolic acidosis occurs owing to retained CO 2 (hypercapnoea) and to lactic acid formed during anaerobic glycolysis. Death may occur. Treatment of Dextropropoxyphene Overdosage Attention should be directed first to establishing a patient airway and to restoring ventilation. Mechanically assisted ventilation, with or without oxygen, may be required, and positive pressure respiration may be desirable if pulmonary oedema is present. The narcotic antagonist naloxone will markedly reduce the degree of respiratory depression, and 0.4 to 2 mg should be administered promptly, preferably intravenously. If the desired degree of counteraction with improvement in respiratory functions is not obtained, naloxone should be repeated at 2 to 3 minute intervals. The duration of action of the antagonist may be brief. If no response is observed after 10 mg of naloxone have been administered, the diagnosis of dextropropoxyphene toxicity should be questioned. Naloxone may also be administered by continuous intravenous infusion. Treatment of Dextropropoxyphene Overdosage in Children The usual initial dose of naloxone in children is 0.01mg/kg body weight given intravenously. If this dose does not result in the desired degree of clinical improvement, a subsequent increased dose of 0.1 mg/kg body weight may be administered. If an IV route of administration is not available, naloxone may be administered IM or subcutaneously in divided doses. If necessary, naloxone can be diluted with Sterile Water for Injection. Blood gases, ph, and electrolytes should be monitored in order that acidosis and any electrolyte disturbance present may be corrected promptly. Acidosis, hypoxia, and generalised CNS depression predispose to the development of cardiac arrhythmias. Ventricular fibrillation or cardiac arrest may occur and necessitate the full complement of cardiopulmonary resuscitation (CPR) measures. Respiratory acidosis rapidly subsides as ventilation is restored and hypercapnoea eliminated, but lactic acidosis may require intravenous bicarbonate for prompt correction. Electrocardiographic monitoring is essential. Prompt correction of hypoxia, acidosis, and electrolyte disturbance (when present) will help prevent these cardiac complications and will increase the effectiveness of agents administered to restore normal cardiac function. PARADEX Product Information Full & Marketed Page 7 of 8
8 In addition to the use of a narcotic antagonist, the patient may require careful titration with an anticonvulsant to control convulsions. Analeptic drugs (for example, caffeine or amphetamine) should not be used because of their tendency to precipitate convulsions. General supportive measures, in addition to oxygen, include, when necessary, intravenous fluids, vasopressor-inotropic compounds, and, when infection is likely, antiinfective agents. Gastric lavage may be useful, and activated charcoal can absorb a significant amount of ingested dextropropoxyphene. Dialysis is of little value in poisoning due to dextropropoxyphene. Efforts should be made to determine whether other agents, such as alcohol, barbiturates, tranquillizers, or other CNS depressants, were also ingested, since these increase CNS depression as well as cause specific toxic effects. Symptoms of Paracetamol Overdosage Shortly after oral ingestion of an overdose of paracetamol and for the next 24 hours, anorexia, nausea, vomiting, sweating, general malaise, hypotension and abdominal pain can occur. The most serious adverse effect of the acute overdosage is a dose dependent, potentially fatal hepatic necrosis. In adults, hepatotoxicity may occur after ingestion of 10 to 15g of paracetamol; a dose of 25g or more is potentially fatal. Symptoms during the first 2 days of acute poisoning by paracetamol do not reflect the potential seriousness of the intoxication. The major manifestations of liver failure such as jaundice, hypoglycaemia, and metabolic acidosis may take at least 3 days to develop. Death from hepatic failure may result 3 to 7 days after overdosage. Acute renal failure may accompany the hepatic dysfunction and has been noted in patients who do not exhibit signs of fulminant hepatic failure. Typically, renal impairment is more apparent 6 to 9 days after ingestion of the overdose. Treatment of Paracetamol Overdosage In cases of overdosage, methods of reducing the absorption of ingested drug are important. Gastric lavage is essential even if several hours has elapsed. Prompt administration of activated charcoal by mouth may reduce absorption. Early measurement of paracetamol serum concentration is essential. The antidote, N-acetylcysteine should be administered as early as possible and preferably within 16 hours of the overdose for optimal results, but in any case, within 24 hours. PRESENTATION: Tablets, (white, scored) 20 s, in blister packs STORAGE: Store below 25 C POISON SCHEDULES: S4, except S8 in TAS. SPONSOR: Aspen Pharmacare Australia Pty Ltd 3/34-36 Chandos St St Leonards NSW 2065 AUSTRALIA [ Approved by the Therapeutic Goods Administration: 4 December 1979 ] [ Date of most recent amendment: 2 March 2010 ] PARADEX Product Information Full & Marketed Page 8 of 8
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION DOLOXENE. (dextropropoxyphene napsylate)
 NAME OF THE MEDICINE PRODUCT INFORMATION (dextropropoxyphene napsylate) (dextropropoxyphene napsylate). WARNING: The Administrative Appeals Tribunal is expected to hear an appeal in relation to the continued
NAME OF THE MEDICINE PRODUCT INFORMATION (dextropropoxyphene napsylate) (dextropropoxyphene napsylate). WARNING: The Administrative Appeals Tribunal is expected to hear an appeal in relation to the continued
Molecular formula: Molecular weight: C 8 H 9 NO 2 CAS Registry no.:
 Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES
 PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
2. QUALITATIVE AND QUANTITATIVE COMPOSITION. Each capsule contains PARACETAMOL 500mg For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less. For pack sizes of more than 24 tablets
 SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
PRODUCT INFORMATION LOFENOXAL
 PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other.
 Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE. Chemical structure: CAS 2 Registry Number: DESCRIPTION
 PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS
 SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS Guideline Title Summary of Product Characteristics for Benzodiazepines as Anxiolytics or Hypnotics Legislative basis Directive
SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS Guideline Title Summary of Product Characteristics for Benzodiazepines as Anxiolytics or Hypnotics Legislative basis Directive
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
PRODUCT INFORMATION ANAGRAINE. Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol.
 PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY
 TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
PRODUCT INFORMATION PANADEINE FORTE
 PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL EXTRA PRODUCT INFORMATION
 PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL SINUS DAY & NIGHT TABLETS PRODUCT INFORMATION
 Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION
 RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
European PSUR Work Sharing Project CORE SAFETY PROFILE. Lendormin, 0.25mg, tablets Brotizolam
 European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
Active ingredients: Paracetamol Codeine phosphate. Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
 Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
Lorazepam Tablets, USP
 Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules.
 Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
M0BCore Safety Profile. Active substance: Bromazepam Pharmaceutical form(s)/strength: Tablets 6 mg FR/H/PSUR/0066/001 Date of FAR:
 M0BCore Safety Profile Active substance: Bromazepam Pharmaceutical form(s)/strength: Tablets 6 mg P-RMS: FR/H/PSUR/0066/001 Date of FAR: 26.11.2013 4.3 Contraindications Bromazepam must not be administered
M0BCore Safety Profile Active substance: Bromazepam Pharmaceutical form(s)/strength: Tablets 6 mg P-RMS: FR/H/PSUR/0066/001 Date of FAR: 26.11.2013 4.3 Contraindications Bromazepam must not be administered
DATA SHEET. PANADOL Mini Caps Capsule shaped tablet with a gelatin coating which is one half green and the other half white.
 PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PRESCRIBING INFORMATION LOMOTIL* TABLETS. (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / mg) Anti-Diarrheal Agent
 PRESCRIBING INFORMATION N LOMOTIL* TABLETS (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / 0.025 mg) Anti-Diarrheal Agent Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada
PRESCRIBING INFORMATION N LOMOTIL* TABLETS (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / 0.025 mg) Anti-Diarrheal Agent Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS
 PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS
 AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
PRODUCT INFORMATION PRODEINE 15 H 3 CO. Paracetamol MW Codeine phosphate MW
 PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
NARCAN Injection. 4,5 α Epoxy-3,14 dihydroxy 17 (prop-2-enyl)morphinan-6-one hydrochloride
 NAME OF MEDICINE Naloxone hydrochloride The molecular weight of naloxone hydrochloride is 363.84 and the CAS registry number for the drug substance (naloxone hydrochloride) is 357-08-4. The molecular formula
NAME OF MEDICINE Naloxone hydrochloride The molecular weight of naloxone hydrochloride is 363.84 and the CAS registry number for the drug substance (naloxone hydrochloride) is 357-08-4. The molecular formula
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500mg For the full list of excipients see section6.1.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500mg For the full list of excipients see section6.1.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
Migraleve, Migraleve Pink and Migraleve Yellow Product Information
 Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
Core Safety Profile. Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg BE/H/PSUR/0002/002 Date of FAR:
 Core Safety Profile Active substance: Clotiazepam Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg P-RMS: BE/H/PSUR/0002/002 Date of FAR: 16.06.2011 4.3 Contraindications is contraindicated
Core Safety Profile Active substance: Clotiazepam Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg P-RMS: BE/H/PSUR/0002/002 Date of FAR: 16.06.2011 4.3 Contraindications is contraindicated
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
PRODUCT INFORMATION ACTACODE
 PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
Codeine Phosphate Hemihydrate. Tablet: Solpadol Caplets are white capsule shaped tablets, marked SOLPADOL on one side.
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Solpadol Caplets Solpadol Capsules Solpadol 30mg/500mg Effervescent Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Constituents
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Solpadol Caplets Solpadol Capsules Solpadol 30mg/500mg Effervescent Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Constituents
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
DOMCET Tablet / Suspension (Domperidone maleate + Paracetamol)
 Published on: 23 Sep 2014 DOMCET Tablet / Suspension ( maleate + ) Composition Tablets Each film- coated tablet contains: Maleate equivalent to.. 10 mg.. 325 mg Suspension Each 5 ml of suspension contains:
Published on: 23 Sep 2014 DOMCET Tablet / Suspension ( maleate + ) Composition Tablets Each film- coated tablet contains: Maleate equivalent to.. 10 mg.. 325 mg Suspension Each 5 ml of suspension contains:
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
CLINICAL PHARMACOLOGY
 Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE. Mebeverine hydrochloride
 1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME (strength pharmaceutical form) Methatabs, Tablet, 5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Methadone hydrochloride BP 5 mg Excipient(s)
1. PRODUCT NAME (strength pharmaceutical form) Methatabs, Tablet, 5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Methadone hydrochloride BP 5 mg Excipient(s)
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
BRICANYL INJECTION. terbutaline sulfate PRODUCT INFORMATION
 BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
APO-PARACETAMOL/CODEINE 500/30
 APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
Your Pharmacy Paracetamol Plus, capsule shaped tablets (caplets)
 New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
PRODUCT INFORMATION. Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets
 PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY
 Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE Famotidine. The chemical name for famotidine is N 2 -(aminosulphonyl)-3-[[[2-[(diamino methylene)amino]thiazol-4yl]methyl]thio]propanamidine. Its structural
Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE Famotidine. The chemical name for famotidine is N 2 -(aminosulphonyl)-3-[[[2-[(diamino methylene)amino]thiazol-4yl]methyl]thio]propanamidine. Its structural
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
PRODUCT INFORMATION. AKINETON (biperiden hydrochloride 2 mg tablets)
 PRODUCT INFORMATION AKINETON (biperiden hydrochloride 2 mg tablets) NAME OF THE MEDICINE Non-proprietary name: biperiden hydrochloride Structural formula (biperiden): Chemical name (biperiden): α-5-norbornen-2-yl-α-phenyl-1-piperidine
PRODUCT INFORMATION AKINETON (biperiden hydrochloride 2 mg tablets) NAME OF THE MEDICINE Non-proprietary name: biperiden hydrochloride Structural formula (biperiden): Chemical name (biperiden): α-5-norbornen-2-yl-α-phenyl-1-piperidine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Phenovet 60 mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active substance: Phenobarbital
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Phenovet 60 mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active substance: Phenobarbital
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
NOTOPAIN CAPLETS. Diclofenac Sodium + Paracetamol. Composition. Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg.
 NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.
 Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve 50 mg/ml solution for injection for dogs (AT, BE, BG, CY, CZ, DE, EL, ES, HR, HU, IE, IT, LU, NL, PT, RO,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Tralieve 50 mg/ml solution for injection for dogs (AT, BE, BG, CY, CZ, DE, EL, ES, HR, HU, IE, IT, LU, NL, PT, RO,
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES
 PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PANADOL OSTEO PRODUCT INFORMATION
 PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML)
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET. Each Paracetamol + Codeine contains 500 mg of paracetamol and 8 mg of codeine phosphate.
 NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET. Proprietary (Trade) Name: PANADOL
 CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/ January 2013
 CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
PRODUCT INFORMATION. Codral* Original Cold & Flu Tablets
 PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
CLINICAL PHARMACOLOGY
 robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
Each 5ml of Sinarest LP New Syrup contains: Phenylephrine
 Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
PRODUCT INFORMATION DARAPRIM TABLETS
 PRODUCT INFORMATION DARAPRIM TABLETS NAME OF THE MEDICINE: Pyrimethamine The chemical name of pyrimethamine is 5-(4-Chlorophenyl)-6-ethyl-2,4-pyrimidinediamine, with a molecular formula C 12 H 13 ClN 4
PRODUCT INFORMATION DARAPRIM TABLETS NAME OF THE MEDICINE: Pyrimethamine The chemical name of pyrimethamine is 5-(4-Chlorophenyl)-6-ethyl-2,4-pyrimidinediamine, with a molecular formula C 12 H 13 ClN 4
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Package leaflet: Information for the patient. Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate
 Package leaflet: Information for the patient Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the patient Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate Read all of this leaflet carefully before you start using this medicine because
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30
 PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
Facts About BELBUCA (buprenorphine) Buccal Film
 Facts About BELBUCA (buprenorphine) Buccal Film Indication BELBUCA is a recent FDA-approved medication for the treatment of chronic pain severe enough to require daily, around-the-clock, long-term opioid
Facts About BELBUCA (buprenorphine) Buccal Film Indication BELBUCA is a recent FDA-approved medication for the treatment of chronic pain severe enough to require daily, around-the-clock, long-term opioid
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tipol 250mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 250mg of paracetamol For a full list of
1 NAME OF THE MEDICINAL PRODUCT Tipol 250mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 250mg of paracetamol For a full list of
Core Safety Profile. Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV FI/H/PSUR/0010/002 Date of FAR:
 Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
