This was a multinational, multicenter study conducted at 14 sites in both the United States (US) and Europe (EU).
|
|
|
- Arleen Fletcher
- 5 years ago
- Views:
Transcription
1 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation, 500 Kendall Street, Cambridge, MA TITLE OF STUDY: Protocol TSH : A Study of the Safety and Efficacy of Thryogen (recombinant human TSH) in Detecting Well- Differentiated Thyroid Cancer by Radioiodine Whole Body Scanning and Thyroglobulin Testing INVESTIGATORS AND STUDY CENTER(S) This was a multinational, multicenter study conducted at 14 sites in both the United States (US) and Europe (EU). STUDIED PERIOD First patient enrolled: 8 November 1995 Late patient completed: 4 April 1997 PHASE OF DEVELOPMENT Phase 3, Confirmatory OBJECTIVES Primary Objectives Confirm that Thyrogen is safe and effective in providing adequate thyroid stimulating hormone (TSH) stimulation for the detection of post-thyroidectomy remnants and well-differentiated thyroid cancer by diagnostic radioiodine ( 131 I) imaging. Determine the superior Thyrogen dosing regimen for providing adequate TSH stimulation for the detection of post-thyroidectomy remnants and well-differentiated thyroid cancer by diagnostic 131 I imaging. Confirm that patients experience fewer hypothyroid signs and symptoms after Thyrogen administration than during the thyroid hormone suppression therapy (THST) withdrawal as measured by the Billewicz Scale. Secondary Objectives Examine the benefit of TSH stimulation provided by Thyrogen on the diagnostic utility of thyroglobulin (Tg) testing, both alone and in combination with diagnostic 131 I imaging, to detect thyroid remnants and thyroid cancer in patients who have had a total or near-total thyroidectomy. Establish the kinetic profile of the serum Tg response after Thyrogen administration in patients capable of a Tg response. Collect additional safety data on patients with thyroid cancer following the administration of Thyrogen. Evaluate patient-reported quality of life (QOL) after Thyrogen administration in comparison to QOL during the TSH withdrawal (Hypothyroid) phase using the Standard Form-36 (SF-36) QOL scale. METHODOLOGY This study was a multi-center, open-label, randomized, 2-arm, parallel study that was designed to confirm that Thyrogen is safe and effective when used as an adjunct to diagnostic 131 I imaging and Tg testing for detection of thyroid cancer. To study safety and efficacy, this study compared the whole-body scan (WBS) and Tg level obtained during TSH stimulation to the
2 WBS and Tg level obtained following THST withdrawal. Patients were enrolled into Arm I or Arm II of the study, either at the time of initial WBS or following a total or near-total thyroidectomy (when scheduled for 131 I imaging and a Tg test for the ongoing monitoring of their thyroid cancer). While patients remained on THST with suppressed endogenous serum TSH levels < 0.5 mu/l, single 0.9 mg doses of Thyrogen were administered as intramuscular (IM) injections according to the dosing regimen of the study arm (Thyrogen Phase). The 2 dosing regimens were Arm I: 0.9 mg IM every 24 hours for 2 doses; and Arm II: 0.9 mg IM every 72 hours for 3 doses. Twenty-four hours after the final Thyrogen dose, patients received a diagnostic activity of 4 mci (±10%) (148 MBq) 131 I and underwent diagnostic scanning 48 hours later. Tg levels were measured at 1, 2, 3, and 7 days after the final injection of Thyrogen in all study patients. Subsequently, the patients remained on THST for 2 additional weeks before being withdrawn for the period required for endogenous TSH levels to rise > 25 mu/l (Hypothyroid Phase). Patients had a Tg level measurement and were then given the same diagnostic activity of 4 mci (±10%) (148 MBq) 131 I and underwent diagnostic scanning 48 hours later. An assessment of hypothyroid symptomology was performed during the Pre-Treatment phase, after Thyrogen administration, and after THST withdrawal using 2 validated QOL instruments. NUMBER OF PATIENTS (PLANNED AND ANALYZED) The number of patients planned was 254; the number analyzed was 220. DIAGNOSIS AND MAIN CRITERIA FOR INCLUSION All patients had a history of well-differentiated thyroid cancer (and were scheduled for diagnostic 131 I imaging and a Tg test). These included patients being scanned for the first time after a total or near-total thyroidectomy and patients who were returning as part of the ongoing monitoring of their cancer. All patients who had recently undergone a total or near-total thyroidectomy had surgery at least 6 weeks before study enrollment. It had been at least 4 months since 131 I ablation or 131 I therapy. Patients with non-thyroidal conditions known to decrease radioiodine uptake (e.g., congestive heart failure) or to cause false-positive radioiodine scans were excluded. Patients who were taking drugs that could affect thyroid function were also excluded. At enrollment, suppression of patients TSH levels (<0.5 mu/l) by THST was confirmed. Additionally, for the Tg analysis, patients were required to be successfully ablated and Tg antibody negative. TEST PRODUCT, DOSE, AND MODE OF ADMINISTRATION Thyrogen (thyrotropin alfa), 0.9 mg by IM injection. DURATION OF TREATMENT Arm I: 0.9 mg IM every 24 hours x 2 doses Arm II: 0.9 mg IM every 72 hours x 3 doses REFERENCE THERAPY, DOSE AND MODE OF ADMINISTRATION Thyrogen, 0.9 mg, was administered daily for 2 days via IM injection while patients were continuing their THST. Forty-eight hours after 131 I, patients returned for a WBS (Thyrogen scan). After the Thyrogen scan, patients continued on THST for at least 2 weeks to allow for TSH levels to return to pre-thyrogen treatment levels. The patient was withdrawn from this therapy for a minimum of 2 weeks until adequate levels of endogenous TSH (>25 mu/l) for a WBS were reached. Subsequently, the same tracer 131 I dose (4 mci) was used as the Thyrogen scan was administered and a second WBS (Withdrawal scan) was performed 48 hours later. CRITERIA FOR EVALUATION Criteria for Evaluation Efficacy Anterior and posterior WBS at 48 hours after 131 I administration were evaluated for the presence of post-thyroidectomy thyroid remnants or well differentiated thyroid cancer. Evaluations were made by the independent reviewers using the uptake classification system developed for this study. The consensus of the independent reviewers was used for the efficacy analysis. The primary efficacy analysis was a comparison of the uptake classification rating for the 48-hours WBS from each of the 2 scan series.
3 Criteria for Evaluation Safety Vital signs, hematologic signs, blood chemistry, development of antibodies to Thyrogen, and adverse events (AEs) were evaluated for safety. STATISTICAL METHODS Demographic and Baseline characteristics are presented using descriptive statistics. Treatment group comparisons were made using analysis of variance (ANOVA) adjusting for centers. Comparisons of treatment arms for nominal categorical variables (e.g., sex) were made using the Cochran-Mantel-Haenszel test to allow adjustment for center effects. Statistical Methods Efficacy For statistical analysis of the WBS, the proportion of patients in each uptake classification was presented with a point estimate and a 95% confidence interval (CI) using the Fleiss formula. In addition, a 2-tailed sign test was performed to test whether discordances significantly favored the Thyrogen Phase scan or the Hypothyroid Phase scan. The 2-tailed Fisher s exact test was used to determine whether Arm II was significantly different from Arm I. Descriptive statistics of the kinetic profile of the Tg were presented by study day and for each treatment arm. Treatment arm comparisons were performed using ANOVA on the change of Tg from Baseline (Day-7-Day0) for the common days only (1, 2, 3, and 7 days after final injection of Thyrogen ). In the analysis of the diagnostic utility of a Thyrogen Phase scan alone, a Thyrogen Tg test alone, and the combination of the 2 methods together in the diagnosing the presence of tissue thyroid origin, a 95% CI for the sensitivity and specificity was presented using the Fleiss formula. Hypothyroid Signs and Symptoms (Billewicz Scale and SF-36 Instrument): median change in signs and symptoms from Baseline to the Thyrogen Phase scan were compared to median change from Baseline to the Hypothyroid Phase scan using the Wilcoxon Signed Rank test for within-treatment arm comparison and the Mann-Whitney test for comparisons between-treatment arms. The mean thyroid percent 131 I uptake measured at the time of the Thyrogen Phase scan was compared to the mean thyroidal percent 131 I uptake measured at the time of the Hypothyroid Phase scan using the Wilcoxon Sign Rank test within each treatment arm. Statistical Methods Safety All safety data were summarized for the safety population. All AEs and serious adverse events (SAEs) were tabulated by body system, preferred term, relationship to study drug, and severity. In the event that AEs were reported more than once, the most extreme level of severity and relationship to treatment was tabulated in the summary tables. SUMMARY / CONCLUSIONS In the Intent to Treat (ITT) population, 142 patients had papillary thyroid cancer, 40 patients had papillary/follicular variant thyroid cancer, 39 patients had follicular thyroid cancer, and 8 patients had Hürthle cell thyroid cancer. Of 229 patients, 49 (Arm I {19 patients}; Arm II {30 patients}) had metastatic disease detected during the study. Summary Efficacy Diagnostic 131 I imaging with Thyrogen Compared to Withdrawal: In Arm I scans evaluated by the independent reviewers (IRs), the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 104/113 (92.0%) patients. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 110/113 (97.3%) patients. The Thyrogen Phase and Hypothyroid Phase scans showed a higher uptake classification than the Thyrogen Phase scans. In Arm II scans evaluated by the IRs, the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 99/107 (92.5%) patients. Conversely the Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 102/107 (95.3%) patients. The scan pairs were concordant in 94/107 (87.9%) cases. Of the 13/107 (12.1%) patients with discordant scans, 5 patients Thyrogen Phase scans showed a higher uptake classification than the Hypothyroid Phase scans, and 8 patients Hypothyroid Phase scans showed a higher uptake classification than the Thyrogen Phase scans. Scan Evaluable Intent to Treat Population: In Arm I scans evaluated by the IRs, the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 104/113 (92.0%) patients. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 110/113 (97.3%) patients. The Thyrogen Phase and Hypothyroid Phase scans were concordant in 101/113 (89.4%) patients.
4 In Arm II scans evaluated by the IRs, the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 99/107 (92.5%) patients. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 102/107 (95.3%) patients. The scan pairs were concordant in 94/107 (87.9%) cases. Patients with Positive Diagnostic Scans: Arm I, the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 39/48 (81.3%) cases. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 45/48 (93.8%) cases. 52/60 (86.7%) cases. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 55/60 (91.7%) cases. Patients with Uptake Limited to the Thyroid Bed: In Arm I, the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 33/39 (84.6%) cases. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 36/39 (92.3%) cases. 38/44 (86.4%) cases. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 41/44 (93.2%) cases. Patients with Uptake Outside of the Thyroid Bed: In Arm I the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase scan in 6/9 (66.7%) patients. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 9/9 patients (95% C.I. = %). 14/16 (87.5%) cases (95% C.I. = %). The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 14/16 (87.5%) cases (95% C.I. = %). Patients with Confirmed Metastatic Thyroid Cancer: In Arm I the Thyrogen Phase scan showed a higher or concordant uptake classification to the Hypothyroid Phase Scan in 15/19 (79.8%) patients. Conversely the Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 18/19 (94.7%) patients. 26/30 (86.7%) patients. The Hypothyroid Phase scan showed a higher or concordant uptake classification to the Thyrogen Phase scan in 29/30 (96.7%) patients. Kinetics of the Tg Response to Thyrogen : For Arm I, the highest Tg levels were achieved more than 24 hours after the final Thyrogen injection, with mean values increasing for the first 3 days and declining by the seventh day. Arm II results were consistent with Arm I and indicated that there is little difference in the Thyrogen -stimulated Tg levels at 1, 2, and 3 days after the final Thyrogen injection. For both study arms, Tg levels achieved 2 to 6 weeks after THST withdrawal were consistently higher than levels 1 to 9 days after TSH stimulation by Thyrogen. Diagnostic Utility of Tg Testing with Thyrogen and 131 I imaging: Seventy-nine patients from Arm I were eligible for this diagnostic utility analysis. Fifty (63%) of these 79 patients had the presence of thyroid remnants or cancer identified by the reference standard. Within this group of 79 patients, if a patient had a Tg level > 3 ng/ml while euthyroid on THST and had either a Hypothyroid Phase Tg < 10 ng/ml or a positive Hypothyroid scan, then this patient was rated as a true positive. If a patient had a Tg level > 3 ng/ml while euthyroid on THST but had both a Hypothyroid Phase Tg level < 10 ng/ml and a negative Hypothyroid Phase scan, then this patient was rated as a false positive. Within this group of 79 patients, if a patient had a Tg level < 3 ng/ml while euthyroid on THST, and had both a Hypothyroid Phase Tg level < 10 ng/ml and a negative Hypothyroid scan, then this patient was rated as a true negative. If a patient had a Tg level < 3 ng/ml while euthyroid on THST but had either a Hypothyroid Phase Tg > 10 ng/ml or a positive Hypothyroid Phase scan, then this patient was rated as a false negative. Within this group of 79 patients, 19 patients were rated as true positives, no patients were rated as false positives, 29 were rated as true negatives, and 31 were rated as false negatives. Therefore, a Tg test while patients were euthyroid on THST (using the Tg level of 3 ng/ml) correctly detected 19/50 patients considered to have thyroid remnant or cancer. A Tg test while patients were euthyroid on THST (using the Tg level of 3 ng/ml) did not detect 31/50 patients considered to have thyroid remnant or cancer.
5 Sensitivity is determined by the ratio of true positives to the sum of true positives and false negatives. In this group of patients the ratio is 19/( ) = 38%. As the number of false negative results decreases, the sensitivity becomes better. Negative predictive value is determined by the ratio of true negatives to the sum of true negatives and false negatives. In this group of patients the ratio is 29/( ) = 48%. As the number of false negative results decreases, the negative predictive value becomes better. Specificity is determined by the ratio of true negatives to the sum of true negatives and false positives. In this group of patients the ratio is 29/(29 + 0) = 100%. As the number of false positive results decreases, the specificity becomes better. Positive predictive value is determined by the ratio of true positives to the sum of true positives and false positives. In this group of patients the ratio is 19/(19 + 0) = 100%. As the number of false positive results decreases, the positive predictive value becomes better. Accuracy is determined by the ratio of the sum of true positives and true negatives to the total number of eligible patients. In this group of patients the ratio is ( )/79 = 61%. As the number of true positive and true negative results increases, the accuracy of the test increases. Hypothyroid Signs and Symptoms (Billewicz Scale): Significant (p<0.05) paired differences were observed for hypothyroid signs and symptoms between the Thyrogen Phase and Hypothyroid Phase. The differences favored the Thyrogen Phase, indicating that patients who were administered Thyrogen while clinically euthyroid on THST experienced fewer hypothyroid signs and symptoms than patients who became clinically hypothyroid while undergoing THST withdrawal. Quality of Life (SF-36 Instrument): Significant (p<0.05) paired differences were observed for hypothyroid signs and symptoms between the Thyrogen Phase and Hypothyroid Phase. The differences clearly favored the Thyrogen Phase across all eight domains of the SF-36, indicating that patients who were administered Thyrogen while clinically euthyroid on THST experienced a better QOL than patients who became clinically hypothyroid while undergoing THST withdrawal. The statistically significant differences between the study phases favored the Thyrogen Phase. Evaluation of Percent 131 I Uptake: There was no difference between 48-hour uptake of the Thyrogen Phase scan compared to 48-hour uptake of the Hypothyroid Phase scan in Arm I. However, in Arm II, the Hypothyroid Phase scan uptake was higher than in Arm I (p<0.050, Wilcoxon Signed Rank Test) with uptake measured by a thyroid probe. When making the same comparison using 48-hour uptake measured by region of interest (ROI) techniques, there were no differences observed in either Arm. However, interpretation of these data is limited because of the high proportion of patients who had very minimal thyroid bed uptake. Summary - Safety Results: Adverse Events: No deaths were reported during the study. A total of 4 patients reported 8 SAEs (Arm I: 3 patients; Arm II: 1 patient), which were considered to be not related to Thyrogen by the treating physician. All patients reporting SAEs recovered. During the entire study (i.e., Thyrogen Phase and Hypothyroid Phase), 103/229 patients reported a total of 207 AEs. In Arm I during the Thyrogen Phase, 46/117 patients reported a total of 73 AEs. In Arm II, 33/112 patients reported a total of 69 AEs. During the Hypothyroid Phase, 43/229 patients reported a total of 65 AEs. During the Thyrogen Phase, the most common AEs included headache, nausea and vomiting, asthenia, paresthesia, and pain. In general, these treatmentemergent AEs were mild. No statistical difference (p=0.076, paired t-test) was found between Arm I and Arm II in the occurrence of treatment-emergent AEs. During the Hypothyroid Phase, the most frequently reported treatment-emergent AEs were hypercholesterolemia, hyperlipidemia, and elevated creatinine levels. These treatment-emergent AEs were generally mild and are expected in hypothyroid patients.. Vital Signs, Hematology Parameters, Blood Chemistry Parameters: Greater differences from Baseline values were observed during the Hypothyroid Phase than during the Thyrogen Phase. In general, there was no clear effect of Thyrogen administration on these parameters. Patient Immune Response (Antibody development) to Thyrogen : The results of testing for anitbodies to Thyrogen indicated that no patients developed antibodies specific to Thyrogen. Based on Report Prepared on: 16 October 1997 Synopsis Prepared on: 20 September 2005
CLINICAL PHARMACOLOGY
 THYROGEN Genzyme (thyrotropin alfa for injection) DESCRIPTION Thyrogen (thyrotropin alfa for injection) contains a highly purified recombinant form of human thyroid stimulating hormone (TSH), a glycoprotein
THYROGEN Genzyme (thyrotropin alfa for injection) DESCRIPTION Thyrogen (thyrotropin alfa for injection) contains a highly purified recombinant form of human thyroid stimulating hormone (TSH), a glycoprotein
SCIENTIFIC DISCUSSION
 London, 20 January 2005 Product Name: THYROGEN Procedure No.: EMEA/H/C/220/II/18 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86
London, 20 January 2005 Product Name: THYROGEN Procedure No.: EMEA/H/C/220/II/18 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86
Protocol GTC : A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients.
 Protocol GTC-68-208: A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients. These results are supplied for informational purposes only.
Protocol GTC-68-208: A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients. These results are supplied for informational purposes only.
PRODUCT MONOGRAPH THYROGEN
 PRODUCT MONOGRAPH Pr THYROGEN Thyrotropin alfa for injection Lyophilized Powder for Reconstitution and Intramuscular Injection (0.9 mg/ml) Human Thyroid Stimulating Hormone ATC code: H01AB01 Genzyme Canada
PRODUCT MONOGRAPH Pr THYROGEN Thyrotropin alfa for injection Lyophilized Powder for Reconstitution and Intramuscular Injection (0.9 mg/ml) Human Thyroid Stimulating Hormone ATC code: H01AB01 Genzyme Canada
Thyroid Cancer & rhtsh: When and How?
 Thyroid Cancer & rhtsh: When and How? 8 th Postgraduate Course in Endocrine Surgery Capsis Beach, Crete, September 21, 2006 Quan-Yang Duh, Professor of Surgery, UCSF Increasing Incidence of Thyroid Cancer
Thyroid Cancer & rhtsh: When and How? 8 th Postgraduate Course in Endocrine Surgery Capsis Beach, Crete, September 21, 2006 Quan-Yang Duh, Professor of Surgery, UCSF Increasing Incidence of Thyroid Cancer
Individual Study Table Referring to Part of the Dossier. Volume: Page:
 1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
1 SYNOPSIS (CR002878) Title of Study: The effect of on vasomotor symptoms in healthy postmenopausal women: a double-blind placebo controlled pilot study Investigators: Multiple, see Section 4, Investigators
Allergan Not Applicable AGN A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple Dose, Parallel
 Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
Peripheral Neuropathy Design, Dose Ranging Study of the Safety and Efficacy of AGN 203818 in Patients with Painful Diabetic 203818-004. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Multiple
Although adequate treatment of differentiated thyroid
 Serum Thyroglobulin Concentrations and 131 I Whole-Body Scan Results in Patients with Differentiated Thyroid Carcinoma After Administration of Recombinant Human Thyroid-Stimulating Hormone Alessia David,
Serum Thyroglobulin Concentrations and 131 I Whole-Body Scan Results in Patients with Differentiated Thyroid Carcinoma After Administration of Recombinant Human Thyroid-Stimulating Hormone Alessia David,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Thyrogen) Reference Number: CP.PHAR.95 Effective Date: 03.12 Last Review Date: 08.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of
Clinical Policy: (Thyrogen) Reference Number: CP.PHAR.95 Effective Date: 03.12 Last Review Date: 08.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of
Hydrocodone/Acetaminophen Extended-Release Tablets M Clinical Study Report R&D/09/1109
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Immediate-release Hydrocodone/Acetaminophen M Abbreviated Clinical Study Report R&D/08/1020
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
2. SYNOPSIS Name of Sponsor/Company:
 in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
in patients with refractory partial seizures 14 Jun 2007 2. SYNOPSIS TITLE OF STUDY: Efficacy and safety of BIA 2-093 as adjunctive therapy for refractory partial seizures in a double-blind, randomized,
SYNOPSIS. Risperidone-R064766: Clinical Study Report RIS-INT-24 (FOR NATIONAL AUTHORITY USE ONLY)
 SYNOPSIS Protocol No.: RIS-INT-24 Psychosis in Alzheimer s disease (PAD) analysis Title of Study: Risperidone in the treatment of behavioral disturbances in demented patients: an international, multicenter,
SYNOPSIS Protocol No.: RIS-INT-24 Psychosis in Alzheimer s disease (PAD) analysis Title of Study: Risperidone in the treatment of behavioral disturbances in demented patients: an international, multicenter,
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
2.0 Synopsis. ABT-358 M Clinical Study Report R&D/06/099. (For National Authority Use Only) to Item of the Submission: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
CED-SOS Advice Report 5 EDUCATION AND INFORMATION 2012
 CED-SOS Advice Report 5 EDUCATION AND INFORMATION 2012 Recombinant Humanized Thyroid Stimulating Hormone () Preparation Prior To Radioiodine Ablation in Patients Who Have Undergone Thyroidectomy for Papillary
CED-SOS Advice Report 5 EDUCATION AND INFORMATION 2012 Recombinant Humanized Thyroid Stimulating Hormone () Preparation Prior To Radioiodine Ablation in Patients Who Have Undergone Thyroidectomy for Papillary
Thyrogen (thyrotropin alfa for injection) Billing and Coding Guide
 thyrotropin alfa for injection Thyrogen (thyrotropin alfa for injection) Billing and Coding Guide For Physician s Offices and Hospitals The following is provided for informational purposes only and is
thyrotropin alfa for injection Thyrogen (thyrotropin alfa for injection) Billing and Coding Guide For Physician s Offices and Hospitals The following is provided for informational purposes only and is
This was a multicenter study conducted at 11 sites in the United States and 11 sites in Europe.
 Protocol CAM211: A Phase II Study of Campath-1H (CAMPATH ) in Patients with B- Cell Chronic Lymphocytic Leukemia who have Received an Alkylating Agent and Failed Fludarabine Therapy These results are supplied
Protocol CAM211: A Phase II Study of Campath-1H (CAMPATH ) in Patients with B- Cell Chronic Lymphocytic Leukemia who have Received an Alkylating Agent and Failed Fludarabine Therapy These results are supplied
Present status of the use of recombinant human TSH in thyroid cancer management
 Acta Oncologica ISSN: 0284-186X (Print) 1651-226X (Online) Journal homepage: http://www.tandfonline.com/loi/ionc20 Present status of the use of recombinant human TSH in thyroid cancer management Markus
Acta Oncologica ISSN: 0284-186X (Print) 1651-226X (Online) Journal homepage: http://www.tandfonline.com/loi/ionc20 Present status of the use of recombinant human TSH in thyroid cancer management Markus
Mipomersen (ISIS ) Page 2 of 1979 Clinical Study Report ISIS CS3
 (ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
(ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
PFIZER INC. Study Initiation Date and Primary Completion or Completion Dates: 11 November 1998 to 17 September 1999
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Adalimumab M Clinical Study Report Final R&D/16/0603
 Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Centocor Ortho Biotech Services, LLC
 SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
Differentiated Thyroid Cancer: Initial Management
 Page 1 ATA HOME GIVE ONLINE ABOUT THE ATA JOIN THE ATA MEMBER SIGN-IN INFORMATION FOR PATIENTS FIND A THYROID SPECIALIST Home Management Guidelines for Patients with Thyroid Nodules and Differentiated
Page 1 ATA HOME GIVE ONLINE ABOUT THE ATA JOIN THE ATA MEMBER SIGN-IN INFORMATION FOR PATIENTS FIND A THYROID SPECIALIST Home Management Guidelines for Patients with Thyroid Nodules and Differentiated
SYNOPSIS INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER (FOR NATIONAL AUTHORITY USE ONLY) Volume: Page:
 SYNOPSIS Risperdal Risperidone (R064766) Protocol No.: RIS-USA-150 Part 1 INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER AUTHORITY USE ONLY) Title of Study: A Double-Blind, Placebo-Controlled
SYNOPSIS Risperdal Risperidone (R064766) Protocol No.: RIS-USA-150 Part 1 INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER AUTHORITY USE ONLY) Title of Study: A Double-Blind, Placebo-Controlled
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
PFIZER INC. PROTOCOL TITLE: Efficacy and Safety of the Authentic Recombinant Human Somatropin Genotropin in Children with Familial Short Stature
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
ABT-358 (Paricalcitol) M Clinical Study Report R&D/08/333
 Subjects who satisfied all inclusion criteria and none of the exclusion criteria were eligible to enroll in this study and were assigned randomly in equal numbers to Sequence Group I or II. The sequences
Subjects who satisfied all inclusion criteria and none of the exclusion criteria were eligible to enroll in this study and were assigned randomly in equal numbers to Sequence Group I or II. The sequences
INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER Volume: Page:
 SYNOPSIS Protocol No.: CR004357 Title of Study: A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of 50 and 100 mg eq. of Paliperidone Palmitate in Subjects With
SYNOPSIS Protocol No.: CR004357 Title of Study: A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of 50 and 100 mg eq. of Paliperidone Palmitate in Subjects With
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Thyroid remnant volume and Radioiodine ablation in Differentiated thyroid carcinoma.
 ORIGINAL ARTICLE Thyroid remnant volume and Radioiodine ablation in Differentiated thyroid carcinoma. Md. Sayedur Rahman Miah, Md. Reajul Islam, Tanjim Siddika Institute of Nuclear Medicine & Allied Sciences,
ORIGINAL ARTICLE Thyroid remnant volume and Radioiodine ablation in Differentiated thyroid carcinoma. Md. Sayedur Rahman Miah, Md. Reajul Islam, Tanjim Siddika Institute of Nuclear Medicine & Allied Sciences,
BRL /RSD-101C0D/1/CPMS-704. Report Synopsis
 Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Location of study report in Regulatory Dossier for authorities
 This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been downloaded from www.leo-pharma.com subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
ClinialTrials.gov Identifier: HOE901_4020 Insulin Glargine Date: Study Code: This was a multicenter study that was conducted at 59 US sites
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Success rate of thyroid remnant ablation for differentiated thyroid cancer based on 5550 MBq post-therapy scan
 ORIGINAL ARTICLE Success rate of thyroid remnant ablation for differentiated thyroid cancer based on 5550 MBq post-therapy scan I. Hommel 1 *, G.F. Pieters 1, A.J.M. Rijnders 2, M.M. van Borren 3, H. de
ORIGINAL ARTICLE Success rate of thyroid remnant ablation for differentiated thyroid cancer based on 5550 MBq post-therapy scan I. Hommel 1 *, G.F. Pieters 1, A.J.M. Rijnders 2, M.M. van Borren 3, H. de
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
Adalimumab M Clinical Study Report R&D/12/323
 Methodology (Continued): Sequence DC: first dose with adalimumab ("D") formulation in the syringe and the second dose with current adalimumab formulation ("C") in the prefilled syringe. Subjects recorded
Methodology (Continued): Sequence DC: first dose with adalimumab ("D") formulation in the syringe and the second dose with current adalimumab formulation ("C") in the prefilled syringe. Subjects recorded
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
Study Center(s): The study was conducted at 39 study sites in Japan.
 SYNOPSIS Issue Date: 20 NOVEMBER 2012 Name of Sponsor/Company Janssen Pharmaceutical K. K. Name of Finished Product CONCERTA Name of Active Ingredient(s) Methylphenidate HCl Protocol No.: JNS001-JPN-A01
SYNOPSIS Issue Date: 20 NOVEMBER 2012 Name of Sponsor/Company Janssen Pharmaceutical K. K. Name of Finished Product CONCERTA Name of Active Ingredient(s) Methylphenidate HCl Protocol No.: JNS001-JPN-A01
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
THE AIM OF postsurgical follow-up in patients with differentiated
 0013-7227/01/$03.00/0 The Journal of Clinical Endocrinology & Metabolism 86(12):5686 5690 Printed in U.S.A. Copyright 2001 by The Endocrine Society Prediction of Disease Status by Recombinant Human TSH-Stimulated
0013-7227/01/$03.00/0 The Journal of Clinical Endocrinology & Metabolism 86(12):5686 5690 Printed in U.S.A. Copyright 2001 by The Endocrine Society Prediction of Disease Status by Recombinant Human TSH-Stimulated
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/04/900. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
2. Synopsis Abbott Laboratories Name of Study Drug: Name of Active Ingredient: Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Phase
2.0 Synopsis. Adalimumab M Clinical Study Report R&D/13/1011. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Name of Active Ingredient: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study: A Phase 3 Multicenter
2.0 Synopsis AbbVie Inc. Name of Study Drug: Name of Active Ingredient: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study: A Phase 3 Multicenter
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor/Company: sanofi-aventis Drug substance: Elitek/Fasturtec (rasburicase, SR29142)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
SYNOPSIS. Administration: subcutaneous injection Batch number(s):
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
SYNOPSIS. Issue Date: 25 Oct 2011
 SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
Individual Study Table Referring to Part of Dossier: Volume: Page:
 Synopsis AbbVie Inc. Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
International Czech and Slovak cooperation in the treatment of patients with differentiated thyroid cancer
 Nuclear Medicine Review 2006 Vol. 9, No. 1, pp. 84 88 Copyright 2006 Via Medica ISSN 1506 9680 International Czech and Slovak cooperation in the treatment of patients with differentiated thyroid cancer
Nuclear Medicine Review 2006 Vol. 9, No. 1, pp. 84 88 Copyright 2006 Via Medica ISSN 1506 9680 International Czech and Slovak cooperation in the treatment of patients with differentiated thyroid cancer
IRBES_R_04320 Generic drug name: Irbesartan + amlodipine Date: Study Code: 31 active centers: 20 in Korea, 7 in India and 4 in Philippines.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinicalTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinicalTrials.gov
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/054. (For National Authority Use Only) Referring to Part of Dossier: Volume: Page:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex Sodium Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex Sodium Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
42 yr old male with h/o Graves disease and prior I 131 treatment presents with hyperthyroidism and undetectable TSH. 2 hr uptake 20%, 24 hr uptake 50%
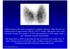 Pinhole images of the neck are acquired in multiple projections, 24hrs after the oral administration of approximately 200 µci of I123. Usually, 24hr uptake value if also calculated (normal 24 hr uptake
Pinhole images of the neck are acquired in multiple projections, 24hrs after the oral administration of approximately 200 µci of I123. Usually, 24hr uptake value if also calculated (normal 24 hr uptake
Correspondence should be addressed to Stan H. M. Van Uum;
 Oncology Volume 2016, Article ID 6496750, 6 pages http://dx.doi.org/10.1155/2016/6496750 Research Article Recombinant Human Thyroid Stimulating Hormone versus Thyroid Hormone Withdrawal for Radioactive
Oncology Volume 2016, Article ID 6496750, 6 pages http://dx.doi.org/10.1155/2016/6496750 Research Article Recombinant Human Thyroid Stimulating Hormone versus Thyroid Hormone Withdrawal for Radioactive
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Study No: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
23-Aug-2011 Lixisenatide (AVE0010) - EFC6014 Version number: 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/573. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
ABC/3TC/ZDV ABC PBO/3TC/ZDV
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Janssen-Cilag EMEA Medical Affairs a division of Janssen Pharmaceuticals N.V.
 SYNOPSIS Issue Date: Final 22 July 2009 [Document No.: EDMS-PSDB-9245102] Name of Sponsor/Company Name of Finished Product Risperdal Consta Name of Active Ingredient(s) Protocol No.: RIS-BMN-3001 Janssen-Cilag
SYNOPSIS Issue Date: Final 22 July 2009 [Document No.: EDMS-PSDB-9245102] Name of Sponsor/Company Name of Finished Product Risperdal Consta Name of Active Ingredient(s) Protocol No.: RIS-BMN-3001 Janssen-Cilag
Case 4: Disseminated bone metastases from differentiated follicular thyroid cancer
 Case 4: Disseminated bone metastases from differentiated follicular thyroid cancer Giuliano Mariani Regional Center of Nuclear Medicine, University of Pisa Medical School, Pisa (Italy) Disseminated bone
Case 4: Disseminated bone metastases from differentiated follicular thyroid cancer Giuliano Mariani Regional Center of Nuclear Medicine, University of Pisa Medical School, Pisa (Italy) Disseminated bone
Austin Radiological Association Nuclear Medicine Procedure THERAPY FOR THYROID CANCER (I-131 as Sodium Iodide)
 Austin Radiological Association Nuclear Medicine Procedure THERAPY FOR THYROID CANCER (I-131 as Sodium Iodide) Overview Indications I-131 therapy for Thyroid Cancer, of the papillo-follicular type, is
Austin Radiological Association Nuclear Medicine Procedure THERAPY FOR THYROID CANCER (I-131 as Sodium Iodide) Overview Indications I-131 therapy for Thyroid Cancer, of the papillo-follicular type, is
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 Study No.: 29060/717 Title: A Double-Blind, Placebo-Controlled, 3-Arm, Fixed-Dose Study of CR Intermittent Dosing (12.5 mg and 25 mg) for Premenstrual Dysphoric Disorder Rationale: In most trials investigating
Study No.: 29060/717 Title: A Double-Blind, Placebo-Controlled, 3-Arm, Fixed-Dose Study of CR Intermittent Dosing (12.5 mg and 25 mg) for Premenstrual Dysphoric Disorder Rationale: In most trials investigating
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
SYNOPSIS Final Clinical Study Report for Study AI444031
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
I-131 ABLATION AND ADJUVANT THERAPY OF THYROID CANCER
 AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS Advances in Medical and Surgical Management of Thyroid Cancer January 23-24, 2015 I-131 ABLATION AND ADJUVANT THERAPY OF THYROID CANCER 2015 Leonard Wartofsky,
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS Advances in Medical and Surgical Management of Thyroid Cancer January 23-24, 2015 I-131 ABLATION AND ADJUVANT THERAPY OF THYROID CANCER 2015 Leonard Wartofsky,
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine (HOE901) Insulin Glulisine (HMR1964)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
2.0 Synopsis. Paricalcitol Capsules M Clinical Study Report R&D/15/0380. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Number of patients: Planned: 403 Randomized: 391 Treated: 390 Efficacy: 363 (Full analysis set) 356 (Per-protocol set)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company : sanofi-aventis
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company : sanofi-aventis
SYNOPSIS. Risperidone-R064766: Clinical Study Report RIS-USA-232 (FOR NATIONAL AUTHORITY USE ONLY)
 SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
SYNOPSIS Protocol No.: RIS-USA-232 Title of Study: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis of Alzheimer's Disease Principal Investigator: M.D.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective: Primary Outcome/Efficacy Variable:
 Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Page: 17 December 2012 (Study M13-692) 22 October 2013 (Study M13-692)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
2.0 Synopsis. Adalimumab R&D/04/118. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective: Primary Outcome/Efficacy Variable:
 Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Studies listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Principal Investigator: Marion, Alan, S, M.D., MDS Pharma Services (US) Inc., 621 Rose Street, PO Box 80837, Lincoln, NE 68502, USA
 SYNOPSIS Issue Date: 06 October 2008 Document No.: EDMS-PSDB-8954363:2. Name of Sponsor/Company Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Name of Finished Product Name of Active Ingredient(s)
SYNOPSIS Issue Date: 06 October 2008 Document No.: EDMS-PSDB-8954363:2. Name of Sponsor/Company Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Name of Finished Product Name of Active Ingredient(s)
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Sponsor / Company: Sanofi Drug substance(s): AMARYL M (1/250 mg) / HOE490
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Bristol-Myers Squibb
 A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
저작권법에따른이용자의권리는위의내용에의하여영향을받지않습니다.
 저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
Clinical Policy Bulletin: Thyrogen (Thyrotropin Alfa)
 Thyrogen (Thyrotropin Alfa) Page 1 of 9 Clinical Policy Bulletin: Thyrogen (Thyrotropin Alfa) Number: 0515 Policy Aetna considers administration of Thyrogen (thyrotropin alfa) medically necessary for the
Thyrogen (Thyrotropin Alfa) Page 1 of 9 Clinical Policy Bulletin: Thyrogen (Thyrotropin Alfa) Number: 0515 Policy Aetna considers administration of Thyrogen (thyrotropin alfa) medically necessary for the
Adjuvant therapy for thyroid cancer
 Carcinoma of the thyroid Adjuvant therapy for thyroid cancer John Hay Department of Radiation Oncology Vancouver Cancer Centre Department of Surgery UBC 1% of all new malignancies 0.5% in men 1.5% in women
Carcinoma of the thyroid Adjuvant therapy for thyroid cancer John Hay Department of Radiation Oncology Vancouver Cancer Centre Department of Surgery UBC 1% of all new malignancies 0.5% in men 1.5% in women
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
SYNOPSIS. Risperidone-R064766: Clinical Study Report RIS-AUS-5 (FOR NATIONAL AUTHORITY USE ONLY)
 SYNOPSIS Protocol No.: RIS-AUS-5 Psychosis in Alzheimer s disease (PAD) analysis Title of Study: Risperidone in the treatment of behavioral and psychological symptoms in dementia: a multicenter, double-blind,
SYNOPSIS Protocol No.: RIS-AUS-5 Psychosis in Alzheimer s disease (PAD) analysis Title of Study: Risperidone in the treatment of behavioral and psychological symptoms in dementia: a multicenter, double-blind,
