CHAPTER 18 - CALCIUM CALCIUM HOMEOSTASIS
|
|
|
- Kenneth Cobb
- 5 years ago
- Views:
Transcription
1 CHAPTER 18 - CALCIUM CALCIUM HOMEOSTASIS CALCIUM BALANCE Normal total plasma Ca++ (bound and free) = mmol/l. Calcium Balance Input Normal diet contains mmol calcium of which about half is absorbed in the upper small intestine mostly under the influence of vitamin D. Of this, approx. 7 mmols is secreted back into the gut in digestive juices so that approx. Thus, mmols appears in the faeces daily. Normal net absorption is therefore 5-10 mmols/day, depending on diet and age. Calcium binders (e.g. phosphate, oxalate, insoluble alkali), decrease absorption. Distribution Once absorbed, blood calcium equilibrates with a large bone reservoir (approx 35,000 mmol calcium in the adult) but only approx. 1% of bone calcium is available for rapid exchange with ECF calcium. Plasma Calcium About half of the total plasma calcium is bound to circulating albumin. What matters physiologically though is the free, or more correctly, the "ionized calcium", which is maintained within narrow limits by the action of parathyroid hormone (PTH). Thus, we can only interpret total plasma calcium levels in the light of plasma albumin. The measured total plasma calcium concentration will be low when plasma albumin is low, so as to maintain ionized plasma calcium normal. A fall or rise in plasma albumin concentration of 10 g/l is normally associated with a change (in the same direction) in the total plasma calcium of about 0.25 mmol/l. Binding of calcium to albumin is also highly ph dependent - alkalosis increases binding and acidosis decreases it. (Hence the hypocalcaemic symptoms of parasthesiae/tetany of hyperventilation-induced respiratory alkalosis). None of the other normally circulating plasma proteins bind plasma significantly, but some of the abnormal ones do, such as myeloma proteins (IgG) and other paraproteins. N.B. Laboratories now report calcium levels corrected for albumin. If requested, free ionized calcium can be measured independent of albumin binding by ion selective electrodes. HORMONES IN IONIZED CALCIUM CONTROL
2 Tight control of plasma ionized calcium is achieved by parathyroid hormone (PTH), the active metabolite of Vitamin D (1,25-OHD), and calcitonin, each acting at one or more of three levels, namely calcium absorption (1,25-OHD), bone resorption (mostly PTH), and renal calcium retention (1, 25-OHD and PTH both). As an approximation, we can say that the (ionized) calcium x phosphate product is reasonably constant, i.e. that if the phosphate rises, then by the law of chemical mass action, calcium will tend to fall. But although this reciprocal relationship does generally exist in plasma, it is not really related to the law of mass action at all, but rather to the action of parathyroid hormone to alter plasma calcium and phosphate in opposite directions. 1. Parathyroid Hormone (PTH). PTH release is stimulated by reduced plasma ionized calcium and its main action is to raise plasma calcium and lower plasma phosphate. Relevant direct actions in this respect are activation of bone osteoclasts to increase bone resorption (releasing both calcium and phosphate) and a renal action to increase calcium reabsorption and decrease renal phosphate (and bicarbonate) reabsorption. PTH also stimulates the conversion of vitamin D to its active 1,25-OHD metabolite in the renal tubules, and so has a further indirect effect on plasma calcium and phosphate through the action of the latter to increase calcium phosphate reabsorption from the gut. The classic biochemical features of excessive PTH action are as follows: Hypercalcaemia (increased bone resorption; increase renal calcium reabsorption; increased gut absorption via PTH stimulating increased 1, 25-OHD). Hypophosphataemia - occurs via increased renal phosphate excretion, and despite tendency of plasma phosphate to be increased by direct PTH action on bone and indirect effect on gut. Because of these conflicting actions, plasma phosphate may rise in some circumstances, particularly where PTH is chronically high enough to cause hypercalcaemic damage to the kidney (phosphate retention), and massive bone resorption. Increased plasma alkaline phosphatase activity (ALP) results from the increased bone osteoblastic activity occurring as a secondary or compensatory response to the primary bone resorption. Excess PTH mostly arises from primary hyperparathyroidism. This is mostly secondary to an adenoma in one of the four parathyroid glands. Then, surgery is the treatment. 2. Vitamin D
3 This fat soluble vitamin is absorbed from the gut, converted first to 25-OHD in the liver, and then to its final metabolically active form, 1,25-OHD, in the renal tubules (some conversion also occurs in bone, placenta, and even pulmonary alveolar macrophages - see relevance below). Renal 1,25 OHD synthesis is stimulated by increased levels of PTH, decreased circulating 1,25-OHD (negative feed-back), and by decreased dietary calcium and/or phosphate; synthesis is decreased when PTH levels are low, and in renal tubular disease. The primary action of physiological doses of 1,25-OHD is on the gut, where it increases the absorption of calcium (and phosphate) thereby maintaining plasma calcium levels. Its most important overall effect on bone is to ensure that newly formed bone matrix or osteoid is calcified. Physiological levels of Vit D promote bone formation, but large doses increase bone resorption. 1,25-OHD also has some effect to increase calcium reabsorption in the renal tubules. The overall effect of 1,25-OHD is therefore to raise both calcium and phosphate levels in the plasma, whereas PTH classically leads to an increase in calcium and a reduction in plasma phosphate. 1,25- OHD is really a hormone. 3. Calcitonin Secreted by the C-cells of the thyroid. It does not lower plasma Ca++ in normal adults, but often has a significant effect to do so in situations of increased calcium turnover, e.g. in children, Paget's disease and malignant hypercalcaemia. Its major actions are to inhibit osteoclastic activity (and decrease renal reabsorption of calcium and phosphate). 4. A number of other hormones can affect calcium homeostasis, including glucocorticoids (increased osteoid catabolism) thyroxine (increased bone turnover), growth hormone (decreased renal calcium reabsorption, increased plasma phosphate), androgens (increased bone growth and calcium deposition), oestrogens (increased osteoblastic activity and therefore increased bone deposition) and glucagon (suppressed bone resorption). HYPOCALCAEMIC STATES Clinical Features. Acute hypocalcaemia is manifest by cramps (and even tetany), and paraesthesiae of the extremities and circum-oral areas, reflecting increased neuromuscular excitability. If severe, Trousseau's and Chvostek's signs may be positive. Hypotension is not uncommon. Chronic hypocalcaemia (especially in the young) may cause significant further changes in a number organs, and give rise to a number of problems, e.g. mental retardation, extrapyramidal symptoms, skeletal malformations (including short stature, poor formation of teeth), dermatitis and hyper-pigmentation.
4 ECG abnormalities include prolonged Q-T interval and ventricular conduction abnormalities. Hypocalcaemia can come about in a number of different ways, and the following will give a background to set the stage for our hierarchic approach to diagnostic-problem solving, particularly of the anatomical site of the condition involved in the individual patient. CAUSES OF HYPOCALCAEMIA. 1. Hypoalbuminaemia.(pseudohypocalcaemia). 2. Hypoparathyroidism. This is the classic form of hypocalcaemia. It usually results from reduced PTH secretion by the parathyroids due to either disease or surgical damage. But there are a number of steps along the pathway from PTH production to its end-organ effect, and failure of PTH action can therefore occur (rarely) by an abnormality at any of these various levels including: (a) Inability of the parathyroid to secrete the PTH molecule (normal parathyroid glands demonstrable, circulating PTH absent, normal response to exogenous PTH). (b) Inability to cleave the PTH molecule to its active form (hypoparathyroidism with normal parathyroid glands demonstrable, high radioimmunoassay levels of PTH in the plasma (but biologically inactive), normal response to exogenous PTH). (c) Failure of end-organ receptor interaction with circulating PTH. (Hypoparathyroidism with normal parathyroid glands, high circulating levels of PTH, no second messenger (C-AMP) or other evidence of biochemical response to exogenous PTH). (d) Lack of response to the C-AMP released from normal PTH-receptor interaction. ("hypoparathyroidism" with normal parathyroids, high levels of circulating PTH (no feed-back), normal adenylate cyclase response to exogenous PTH, but no biochemical response of the cell). Of course, when it comes to diagnostic problem-solving, our first task is to separate ANY form of impaired PTH action from other causes of hypocalcaemia. In this respect, we must bear in mind that the classic biochemical features of failure of PTH action are a low plasma ionized calcium, high plasma phosphate and a normal to low alkaline phosphatase. Treatment: Hypoparathyroidism is usually treated by giving 1,25-OHD (and/or calcium supplements), to maintain plasma calcium at normal (preferably low-normal) levels. However, this needs close monitoring of plasma Ca++ to avoid inducing the more dangerous hypercalcaemia long-term. 3. Vitamin D deficiency
5 This causes rickets in children and osteomalacia in adults. Clinically symptoms may be vague, but more specific ones include bone pain/deformity and (proximal) myopathy. Biochemically, the classic picture is hypocalcaemia (often mild), reduced plasma phosphate, and increased plasma alkaline phosphatase (ALP), although these changes can be subtle. The reason for the variable biochemical picture, particularly for the plasma calcium often being in the normal range, is the variable degree of secondary hyperparathyroidism invoked by the hypocalcaemia. Vitamin D deficiency from poor intake is rare in Australia, not only because diets are usually adequate, but also because of abundant sunlight. However, nutritional vitamin D deficiency may occur in elderly immobile institutionalised patients (especially in the context of previous gastrectomy predisposing to malabsorption), and in dark-skinned migrants, especially covered women. Vitamin D is a fat soluble vitamin, so deficiency may occur in any of the malabsorption syndromes, especially those associated with chronic biliary obstruction. Other liver disease is a theoretical cause of osteomalacia (reduced 25 hydroxylation of absorbed vitamin D) but this is rarely seen in practice. However, the anticonvulsant phenytoin can act via the liver to produce osteomalacia by inducing hepatic microsomal enzyme systems to markedly increase the metabolism of 1,25-OHD, thereby lowering its circulating plasma level. Osteomalacia may also arise because of decreased renal 1- hydroxylation of 25-0HD to reduce production of the active 1,25-OHD (rickets resistant to standard vitamin D therapy, but responsive to 1,25-OHD). Rickets may rarely be due to a decreased receptor responsiveness to 1,25-OHD (resistant to both vitamin D and 1,25-OHD). The classic symptoms of osteomalacia include muscle weakness and bone pain. The latter is characteristically associated with a reduction of bone density on X-ray, radio-translucency and "Looser's zones" or pseudofractures around the pelvic and upper limb girdles. In severe cases there may be symptoms of hypocalcaemia including paraesthesiae, cramps and tetany, and even hypotension. However again, subtle (secondary) hyperparathyroidism often maintains plasma calcium relatively normal, so in some cases all we may have to go on are (i) clinically: vague bone pain, lethargy, and (proximal) muscular weakness; (ii) biochemically: a low-normal calcium and phosphate, and a slight elevation of plasma alkaline phosphatase; and (iii) radiologically: a generalised loss of bone density (osteopenia) without pseudo-fractures. Bone biopsy then becomes important for definitive diagnosis. 4. Renal Disease Renal tubular disease or dysfunction can cause osteomalacia directly via a failure to form 1,25-OHD from its precursor. However, there are several other important aspects of the kidney and calcium. First, renal disease can produce a variable effect on plasma biochemistry/calcium etc. as well as a rather complex bone response. So although most patients with renal failure have mild hypocalcaemia, in some cases plasma calcium can be quite low, partly because phosphate retention (high plasma phosphate) acts to functionally inhibit any remaining capacity of the kidney to convert 25-OHD into its active 1,25-OHD metabolite. Decreased renal reabsorption of calcium, as well as decreased intestinal calcium uptake may aggravate the situation.
6 Secondary hyperparathyroidism (parathyroid gland hyperplasia) as a physiological response to a low calcium in renal disease. This tends to compensate any low calcium levels toward normal, but it cannot always fully compensate because: (i) it cannot exert its full effect on the diseased renal tubules (increased calcium reabsorption); (ii) it cannot be fully effective in stimulating renal tubular conversion of 25-OHD to 1,25-OHD (and hence calcium uptake from the gut); and (iii) uraemia per se causes resistance to the action of PTH on bone. Despite this, many patients who clearly do mount an effective secondary parathyroid response to return plasma calcium levels back to at least low normal. Then, the pathological bony picture tends to become a mixed one i.e. one of osteitis fibrosa cystica (from hyperparathyroidism) and osteomalacia, giving rise to an overall picture of so-called renal osteodystrophy. In this situation, the price for maintaining calcium is one of bone disorganisation, and given the presence of a plasma phosphate, soft tissue calcification can occur as well. Because of this, dietary phosphate restriction and phosphate binders are prescribed early on. Calcium supplements and/or small doses of 1,25-OHD are sometimes also given early in patients with renal disease with a low ionised plasma calcium, so as to avoid these secondary hyperparathyroidism problems. [In some patients with chronic renal disease the parathyroid glands may actually become autonomous, due to hyperplasia eventually going on to the development of micro-adenomata and even macro-adenomata, and then the plasma calcium may actually become elevated above the normal range to give a bone picture dominated by osteitis fibrosis cystica. This situation is sometimes referred to as "tertiary" hyperparathyroidism. One of its dangers then is that, with plasma calcium and phosphate both now being high, extensive soft tissue calcification can occur; and that, in the kidneys, may further aggravate renal damage so as to rapidly worsen the situation. Patchy bone calcification may even occur, giving rise to areas of bone sclerosis strangely super-imposed on a background of demineralisation (from osteomalacia and hyperparathyroidism). These problems may be minimised by giving such patients (early on) phosphate binders orally, to reduce gut phosphate absorption and therefore plasma phosphate, so reducing the high Ca x PO4 product, and therefore the tendency for precipitation of calcium phosphate in tissues. See also Section on Hypercalcaemia.] 5. Other Causes of Hypocalcaemia (a) EDTA contamination of the sample. EDTA is used as an anticoagulant in the collection of some blood samples, because it strongly inhibits the clotting process by chelating (binding) calcium. Therefore, we occasionally find artefactual hypocalcaemia due to blood being taken into the wrong tube, i.e. one containing EDTA. A special tube is needed. (b) Hypomagnesaemia. Low plasma magnesium is often associated with hypocalcaemia, and if severe may actually contribute to it. Mild hypomagnesaemia stimulates PTH in much the same way as hypocalcaemia, but severe hypomagnesaemia (which may be associated with chronic diarrhoea/ malabsorption, alcoholism, and total parenteral nutrition in hospital) impairs the secretion of PTH by the parathyroid gland and may lower target organ PTH sensitivity as well.
7 (c) Acute Pancreatitis. Hypocalcaemia can be seen in severe acute pancreatitis due partly to hypoalbuminaemia, partly to hypomagnesaemia, and partly to the formation of calcium soaps as a result of the action of lipase within the damaged gland. (d) Hungry Bone Syndrome. Important to remember. Following parathyroidectomy, vitamin D therapy for osteomalacia, and in some osteoblastic malignancies, new bone formation may outstrip bone resorption and intestinal uptake of calcium. This characteristically results in a low plasma calcium and phosphate, in association with an increased plasma alkaline phosphatase. HIERARCHIC ANATOMICO-FUNCTIONAL APPROACH TO THE DIAGNOSIS OF HYPOCALCAEMIA As with so many of the metabolic and electrolyte disturbances, hypocalcaemia is often associated with vague symptoms, especially when the condition is mild, e.g. lethargy, proximal muscle weakness, vague bone pain, so you are often only alerted to its presence by investigating the plasma electrolytes. But having found a low plasma calcium, it is imperative to go back and re-examine the patient, not unlike the way we so frequently have to return to obtain more history after physical examination findings in making a final clinical Aetiological diagnosis. And since you have other plasma biochemistry as well, look carefully at the plasma albumin, phosphate, alkaline phosphatase, magnesium, urea and creatinine, because all of these can help build your diagnosis on a hierarchic basis. Thus, you should: Dissecting the problem of a low plasma calcium: First exclude artefacts such as EDTA contamination (blood taken into the wrong tube), and other obvious if unusual situations like the hungry bone syndrome. Next measure: Plasma albumin. If this is abnormal, correct the plasma calcium for any change in it (see above), to ensure that you are indeed dealing with true hypocalcaemia. Measure ionized Ca++ if in any doubt. Next measure: Plasma phosphate level. If increased, you are either dealing with either hypoparathyroidism (check plasma PTH) or renal failure (check creatinine, urea). If the plasma phosphate is normal or decreased:
8 First exclude drugs (e.g. mithramicin, frusemide, citrate) and then further dissect on the basis of plasma alkaline phosphatase. Plasma alkaline phosphatase. If normal, you may well be dealing with pancreatitis, or more subtly, hypomagnesaemia. If plasma alkaline phosphatase elevated, probably osteomalacia. Then, you should again go back to the history and ask about diet, bowel habit (malabsorption), cramps, parasthesiae tetany, bone pain; and radiologically look for the signs of pseudo-fractures, loss of bone density etc., to confirm the diagnosis. Vitamin D and Intact PTH levels. Once you have established, in this hierarchic way, whether you are dealing broadly with hypoparathyroidism or vitamin D deficiency etc. you can then dissect each to further Anatomico- Functional levels. Thus, you can determine whether any defective vitamin D metabolism is due to a lack of vitamin D intake, lack of absorption, decreased 25-hydroxylation in liver disease (uncommon), decreased 1-hydroxylation in renal disease (elevated creatinine), decreased response to the active 1,25-OHD metabolite in the more specific vitamin D-resistant rickets, or an increased clearance of the active 1,25-OHD metabolite either by the induction of microsomal enzymes by drugs such as phenytoin, alcohol etc., or in the nephrotic syndrome where there may be increased clearance from loss of albumin-bound 1,25-OHD via the renal route. In the same way, we have seen how we can dissect the level of any failure of PTH action in terms of parathyroid abnormalities including an inability to secrete the PTH molecule, inability to cleave the PTH molecule to its active form once released, failure of renal receptor interaction with circulating PTH, and failure of adenylate kinase response to PTH receptor interaction. Plasma ionized calcium (not total) also falls with alkalosis, especially acute respiratory alkalosis. This is important not only in diagnosis, but when correcting metabolic acidosis. This is a further reason for avoiding IV. bicarbonate in the correction of metabolic acidosis. HYPERCALCAEMIA - FEATURES Hypercalcaemia also usually presents with vague symptoms. Clinical features of hypercalcaemia depend on the level of plasma calcium, with many patients being asymptomatic even up to levels of 3.0 mmol/l. Nonspecific complaints include nausea, anorexia, vomiting, fatigue and generalised weakness. Hypercalcaemia also alters nerve/muscle function, so that muscular weakness may arise either directly as a proximal myopathy or secondary to impaired neuronal transmission. Other neurological features include irritability, confusion, headaches, and even seizures and coma if plasma calcium level exceeds 3.5 mmol/l. More general symptoms include cardiac dysrhythmias,
9 hypertension, and constipation (from alteration of cardiac/smooth muscle function). Polyuria, polydipsia and thirst may arise from functional alteration of the renal concentrating mechanism. Deposition of calcium in various tissues can be seen in band keratrophy (deposition of calcium near the corneoscleral junction of the eye), and pancreatic and renal calcification. If there is increased renal excretion of calcium salts, renal calculi may form. If hypercalcaemia is associated with removal of mineral from bone, e.g. in hyperparathyroidism, then there will often be associated bone pain, sometimes due to actual pathological fractures. Hypercalcaemia also predisposes to other disorders including pancreatitis and duodenal ulcer. ECG characteristically shows shortened conduction intervals. CAUSES OF HYPERCALCAEMIA 1. Factitious - a mild hypercalcaemia can result from concentration of the blood during collection by use of a tourniquet. Therefore avoid this when collecting blood for calcium estimation. 2. Hyperparathyroidism. Hypercalcaemia may result from primary or "tertiary" hyperparathyroidism. Primary hyperparathyroidism is usually due to a benign adenoma of one of the parathyroid glands (may be associated with multiple endocrine adenomas - MEN 1). Prior to the wide availability of biochemical tests, most of these patients suffered from massive resorption of calcium from bone, due to excessive PTH, resulting not only in severe bony changes (bone cysts and sub-periosteal erosions along the borders of the phalanges and metacarpal bones), but often renal disease as well (nephrocalcinosis, renal stone formation) secondary to the increased urinary excretion of calcium. Nowadays, th condition is picked up earlier with the characteristic biochemical picture of a high plasma calcium, low plasma phosphate (reduced renal phosphate reabsorption), reduced plasma bicarbonate (reduced renal PCT reabsorption of bicarbonate), and a slightly raised plasma alkaline phosphatase. The low phosphate typically differentiates the condition from hypervitaminosis D, where the plasma phosphate is characteristically high. But difficulties arise where the hypercalcaemia of primary hyperparathyroidism leads to secondary impairment of renal function, because this will increase plasma phosphate in its own right. However, with a good history and physical examination, there should be no trouble differentiating these two conditions in practice. Note, too, that although some patients with primary hyperparathyroidism present as renal stone formers (ureteric colic) without much in the way of bony involvement, this is not common, presumably because PTH normally acts to increase calcium reabsorption from the kidney, so that high urinary calcium levels do not tend to occur until the condition is quite advanced, when the plasma calcium itself becomes so high that the filtered load of calcium exceeds the renal calcium reabsorptive capacity.
10 As we have seen, secondary physiological hyperparathyroidism may arise in chronic renal disease from hypocalcaemia secondary to lack of renal conversion of 25OH Vitamin D to its metabolically active form, 1, 25. OH Vit. D. This latter leads to secondary stimulation of the parathyroid in attempted compensation. The parathyroid glands become hyperplastic as a consequence, butcalcium levels remain somewhat below normal at this physiological stage. Tertiary hyperparathyroidism sometimes follows the above, due to the hyperplastic parathyroid glands developing adenomatous change and becoming autonomous with chronic stimulation, hence consequent uncontrolled release of PTH. Hypercalcaemia ensues. The latter can be especially dramatic after renal transplantation for chronic kidney disease. 3. Hypervitimanosis D. Already partly discussed in our differential diagnosis. Causes include overenthusiastic treatment of hypoparathyroidism with 1,25-OHD (regularly monitor plasma calcium); also increased vitamin D sensitivity and/or conversion of 25-OHD to 1,25-OHD as may occur in pulmonary sarcoidosis (probably related to the capacity of pulmonary macrophages to 1-hydroxylate 25-OHD). Note that although 25-OHD has only about 1,000th the activity of the active vitamin D metabolite, 1,25-OHD, it is normally present in plasma in much greater concentrations. Hence, 25 OHD can have effects on calcium homeostasis if significant amounts of Vitamin D are ingested. 4. Malignancy. Hypercalcaemia is not uncommon in patients with malignant disease, particularly in the latter stages. Carcinomas which have a particular propensity to metastasise to bone (e.g. breast, lung, kidney and prostate) are especially likely to cause hypercalcaemia. However, sometimes hypercalcaemia is associated with the primary tumour (e.g. ectopic PTH-like production from tumours such as squamous cell carcinomas of the lung). Hypercalcaemia may also occur in multiple myeloma (partly from the increased level of calcium-binding globulin - check ionized calcium), lymphoma and leukaemia. Actually, the causes of hypercalcaemia in malignancy are complex and often multiple, and include direct invasion of bone, tumour secretion of PTH-like material and/or other factors including prostaglandin E, "osteoclastic activating factor" (OAF), cytokines and vitamin D-like sterols, all of which can increase bone resorption. 5. Other Conditions producing Hypercalcaemia include: (i) Patients with bone disease, particularly Paget's disease, after immobilisation in bed; (ii) So-called milk -(soluble)alkali syndrome where the milk supplies the calcium and the soluble alkali increases its absorption - rarely seen nowadays with the use of nonabsorbable alkalis in the treatment of peptic ulcer. (iii) Occasional endocrine causes including thyrotoxicosis (increased bone turnover), acromegaly (increased gut absorption of calcium) and Addison's disease (mild hypercalcaemia partly due to haemoconcentration from ECF volume depletion);
11 (iv) Drugs, including lithium (stimulates PTH release), thiazide diuretics (ECF volume depletion from thiazides can lead to decreased PCT renal tubular flow rates and this causes an increased proximal tubular reabsorption of calcium as well as sodium). (v) Acute renal failure, particularly the late polyuric phase, may be associated with acute hypercalcaemia, perhaps due to a persistently increased PTH secretion following the initial stimulus of hypocalcaemia during the oliguric phase. HIERARCHIC APPROACH TO THE DIAGNOSIS OF HYPERCALCAEMIA First exclude artefactual hypercalcaemia by making sure that the specimen is taken without a tourniquet (if in doubt repeat), and corrected for any change in plasma albumin and ph. From the history and physical examination, it is usually relatively simple to rule out thyrotoxicosis, renal failure, advanced malignancy, sarcoidosis, Addison's disease, the milk-alkali syndrome and drugs including thiazides and excessive vitamin D. If doubt remains, measure: Plasma phosphate concentration. Decreased or low-normal in patients with primary hyperparathyroidism. This also occurs in some patients with malignancy and others on thiazide diuretic therapy. If you suspect the patient may be taking thiazide diuretics furtively, next measure: Urinary calcium and sodium in the sub-group of patients with decreased or low-normal phosphate. Urinary Ca++ is decreased in patients on chronic thiazide therapy and urinary Na+ increased. If the urinary calcium is increased, you are probably dealing with primary hyperparathyroidism, and a useful next step is to measure the plasma bicarbonate. This tends to be low in primary hyperparathyroidism (PTH inhibits renal PCT reabsorption of bicarbonate) and normal in malignancy. If still in doubt measure: Plasma Intact PTH In some patients with malignancy, hypercalcaemia is due to "ectopic" secretion of PTH-like substances by the tumour, which cross-react on the immuno-assay. If the plasma phosphate is raised, measure:
12 Plasma alkaline phosphatase (ALP). If this is normal you are probably dealing with an increased vitamin D effect, occasionally with malignancy and the milk-alkali syndrome. If the plasma alkaline phosphatase is increased, think of malignancy, hyperparathyroidism as well as the endocrine causes. The above approach of using clinical features together with the plasma phosphate, alkaline phosphatase and urinary calcium usually sorts out most problems. However, in practice difficulties can still arise. First, as we have seen, hypercalcaemia itself may eventually induce renal damage (e.g. by nephrocalcinosis), and with it, phosphate retention, to make differential diagnosis difficult, e.g. between primary and so-called "tertiary" hyperparathyroidism. Moreover, in any situation of renal impairment, plasma PTH tends to be high because of failure of the normal renal excretion of PTH and its metabolites (which can cross-react in the immunoassay for PTH). Nevertheless, the clinical situation, and particularly the mode of onset and progression of the hypercalcaemia in relation to the renal disease, taken together with the different bony pictures of primary versus "tertiary" hyperparathyroidism will usually allow differentiation between the two. Also, we can now measure intact PTH to separate the PTH fragments which accumulate in renal failure. Comment. Most cases are characteristic, and diagnosis is much aided by going back into the history. Try at the first broad level to different hyperparathyroidism from hypervitaminosis D and malignancy; then, if the former, further dissect clinically and biochemically whether there is over-production of intact PTH, and if so, whether this is parathyroid PTH over-production (primary or "tertiary" hyperparathyroidism), or ectopic PTH-like production by a tumour (e.g. squamous cell Ca lung). If the clinical and biochemical features point in directions other than PTH, and if malignancy, drugs, artefacts etc. have been excluded, you should determine whether you are dealing with hypervitaminosis D; and if so, whether this is due to excessive administration of vitamin D (e.g. in hypo-parathyroidism treatment) or to increased conversion of 1,25-OHD to the active metabolite as in sarcoidosis. Follow a hierarchic approach to diagnosis, and keep going back for further clinical information no matter how far down the biochemical line you have travelled. PATH, FUNCT, AND AETIOL DIAGNOSES, and TREATMENT Pathological, Functional, and Aetiological diagnoses- as before. Treatment of hypercalcaemia
13 Removal of the cause. Primary hyperparathyroidism is usually due to a solitary autonomous hyperparathyroid adenoma, which can be cured by surgery. In tertiary hyperparathyroidism, it is sometimes possible to control the calcium levels with cinacalcet, a synthetic agent which increases the sensitivity of the parathyroid calcium-sensing receptor to calcium feedback, so inhibiting PTH release. Other treatments In severe cases, intravenous sodium chloride together with frusemide can be useful, because frusemide causes both calcium and sodium chloride loss and the sodium chloride infusion replaces the sodium losses. Measure urinary K+ and Mg++ losses, and replace these if diuresis continued longer than 24 hrs. IV phosphate administration might seem rational, and was used in the past, but is potentially dangerous. IV calcitonin can be effective, by inhibiting bone resorption to cause increased urinary calcium excretion. Glucocorticoids improve most forms of hypercalcaemia except those related to primary hyperparathyroidism - useful diagnostically as well as in treatment. In more chronic cases, reduction of dietary calcium intake, together with calcium binding agents (e.g. oral phosphate in the form of 'buffered' phosphate) are often very effective (they inhibit gut uptake of calcium). (The use of oral phosphate therapy is contra-indicated in cases of renal impairment, where plasma phosphate is high and there is risk of increasing it still further; that, together with hypercalcaemia can lead to dangerous soft tissue calcification including nephrocalcinosis and further renal damage.) The bisphosphonates (which inhibit osteoclastic bone resorption) are emerging as important treatments, especially in ongoing hypercalcaemia. MCQs & PROBLEM SOLVING A. Mechanisms in Disease 1. A patient is admitted with a diagnosis of chronic renal failure and secondary osteomalacia without hyperparathyroidism. Which of the following would be typical. 1. Plasma calcium elevated above the normal range.
14 2. An elevated plasma albumin. 3. Metabolic alkalosis. 4. Histological picture of 'osteitis fibrosis cystica' on bone biopsy. 5. A low level of circulating of 1-25 dihydroxy vitamin D. 6. A reduction in the plasma phosphate level. 7. Elevated plasma alkaline phosphatase. Mechanisms in Disease 2. You had been seeing a 45 year old (1) patient for ten years (2) with chronic stable renal failure (3) up until four years ago (4). At that stage he had a plasma creatinine 5 times the normal level (6), an elevated plasma phosphate (7), and a persistently low plasma calcium (8) in the presence of a normal plasma albumin (9). You elected at that stage to observe rather than treat (10). After leaving the district for some four years, he now returns and investigations show little change in the plasma creatinine (11), but his plasma calcium is now above the normal range (12). He complains of some bone pain (13) and when you X-ray his hands there are sub-periosteal erosions along the borders of the phalanges and metacarpal bones (14) as well as a generalised decrease in bone density and some bone cysts (15). Which of the following statements is/are likely to be correct: 1. This man has recently developed secondary osteomalacia. 2. It is likely that his plasma phosphate will have risen from its previous level. 3. Bone biopsy will show the classic changes of osteoporosis. 4. He is liable to soft tissue calcification. 5. He should now be given therapeutic doses of 1,25-OHD. 6. In retrospect it may well have been best to treat this man with a small dose of 1,25-OHD when he first developed hypocalcaemia.
15 7. His urinary creatinine excretion (mg per day) will be unchanged from four years ago, and normal. Problem-Solving MCQ 2. A 42 year old housewife (1) presented with a four months history (2) of nausea (3), general malaise, increasing tiredness, lethargy (4), vague headache (5), constipation (6), irritability and vague aches and pains (7), as well as increasing thirst and nocturia (8). There had been no recent weight loss or change in appetite (9), but on direct questioning she did admit to some difficulty in climbing stairs (10), seemingly related to weakness of the lower limbs (11). There had been no shivers or sweats (12) and no respiratory or cardiac symptoms (13). In the past she was known to be normotensive 12 months beforehand (14) and had not had any previous renal disease (15), peptic ulcer (16), or carcinoma of the breast or elsewhere (17). No past history of tuberculosis (18). Social history: non-smoker, non-drinker (19). Happily married, two children (20). Family history: nil relevant. Drug history: no history of drug intake, in particular no spironolactone or related potassium-sparing diuretics such as amiloride (21); no past history of any excessive vitamin B6 consumption (22). Not taking any antacids (23) or potassium supplements (24). On examination: general NAD. Skin normal. BP 180/100 mm Hg (25). Pulse 82/min. JVP normal (26). Intermittent ventricular extra systolic beats (27). Heart otherwise NAD. No bruits in heart, neck or epigastrium (28). No delay of radio-femoral pulses (29). Foot pulses normal (30). No ankle oedema (31). Chest NAD. Abdomen NAD. Bone/joints: mild tenderness to percussion over the dorsal and lumbar spines (32). CNS: hyper-reflexia, bilaterally symmetrical; plantar reflexes downgoing (33). Some difficulty in standing unaided from the sitting position (34), otherwise only minimal generalised weakness (35); no sensory signs or symptoms (36). Mentation, cranial nerves, optic fundi, all NAD (37). The patient was investigated and subsequently had an operation (21/9). The biochemical findings prior to operation (20/9), and on the day after(22/9), were as follows: Solve problem using the four column approach and answer the following questions before viewing the graphic solution at end of the chapter. Which of the following statements is/are correct? 1. The symptoms of thirst and nocturia are probably related to the plasma potassium level. 2. The pre-op. plasma ionised calcium was probably normal when corrected for plasma albumin and ph.
16 3. Given the initial biochemistry, the first step would have been to repeat it, making sure that blood was taken without a tourniquet. 4. An initial normal urinary calcium would exclude a diagnosis of a primary hyperparathyroidism. 5. The low plasma phosphate before operation is explained by the relatively low plasma creatinine. 6. There was clinical and biochemical evidence of ECF volume contraction prior to operation. 7. The raised plasma alkaline phosphatase (ALP) in this situation indicates secondary osteomalacia. 8. The low plasma bicarbonate is more in favour of a diagnosis of hypervitaminosis D than other causes of hypercalcaemia. 9. The pre-op. aches and pains this patient complained of may well have been related to the hypercalcaemia. 10. There is pre-op. evidence to suggest "tertiary" hyperparathyroidism. 11. The anion gap before surgery was higher than normal. 12. The high plasma Cl- pre-operatively suggested isotonic ECF volume depletion. 13. Vitamin B6 was the important vitamin to ask about in the history. 14. It would have been more relevant, in the drug history, to ask about possible intake of thiazide diuretics than the potassium-sparing diuretic group. 15. The sudden fall in plasma calcium post-operatively is well recognised in such cases. 16. Typically, patients with hypercalcaemia of malignancy present with such a biochemical picture. 17. The operation performed on 20/9 was probably a removal of the four parathyroid glands in the neck. A graphic solution is available now for this problem. But try to solve yourself first.
17 PROBLEM SOLUTION-Part1
18 PROBLEM SOLUTION-Part 2
19 PROBLEM SOLUTION-Part 3
20 MCQ ANSWERS A. Mechanisms in disease. No.1. 5 & 7 correct. All other false. No.2. Only 4, 6, 7, correct. B. Problem Solving Case. 3, 9, 14, 15, correct. All others false
Investigations for Disorders of Calcium, Phosphate and Magnesium Homeostasis
 Investigations for Disorders of Calcium, Phosphate and Magnesium Homeostasis Tutorial for Specialist Portfolio Biomedical Scientists 03/02/2014 Dr Petros Kampanis Clinical Scientist 1. Calcium Most abundant
Investigations for Disorders of Calcium, Phosphate and Magnesium Homeostasis Tutorial for Specialist Portfolio Biomedical Scientists 03/02/2014 Dr Petros Kampanis Clinical Scientist 1. Calcium Most abundant
HYPERCALCEMIA. Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences
 HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
HYPERCALCEMIA Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ESSENTIALS OF DIAGNOSIS Serum calcium level > 10.5 mg/dl Serum ionized
Hypocalcemia 6/8/12. Normal value. Physiologic functions. Nephron a functional unit of kidney. Influencing factors in Calcium and Phosphate Balance
 Normal value Hypocalcemia Serum calcium Total mg/dl Ionized mg/dl Cord blood 9.0 ~ 11.5 5.0 ~ 6.o New born (1 st 24 hrs) 9.0 ~ 10.6 4.3 ~ 5.1 24~ 48 hrs 7.0 ~12.0 4.0 ~4.7 Child 8.8 ~10.8 4.8 ~4.92 There
Normal value Hypocalcemia Serum calcium Total mg/dl Ionized mg/dl Cord blood 9.0 ~ 11.5 5.0 ~ 6.o New born (1 st 24 hrs) 9.0 ~ 10.6 4.3 ~ 5.1 24~ 48 hrs 7.0 ~12.0 4.0 ~4.7 Child 8.8 ~10.8 4.8 ~4.92 There
Magnesium Homeostasis
 ECTS PhD Training Course, Rome 3 rd September 2008 Disorders of Calcium, Phosphate h and Magnesium Homeostasis Richard Eastell Professor of Bone Metabolism Academic Unit of Bone Metabolism University of
ECTS PhD Training Course, Rome 3 rd September 2008 Disorders of Calcium, Phosphate h and Magnesium Homeostasis Richard Eastell Professor of Bone Metabolism Academic Unit of Bone Metabolism University of
Clinical biochemistry of calcium and vitamin D
 Clinical biochemistry of calcium and vitamin D Dr Andrew Day Consultant in Clinical Biochemistry and Metabolic Medicine University Hospitals Bristol NHS Trust e-mail: andrew.day@uhbristol.nhs.uk A 48-year
Clinical biochemistry of calcium and vitamin D Dr Andrew Day Consultant in Clinical Biochemistry and Metabolic Medicine University Hospitals Bristol NHS Trust e-mail: andrew.day@uhbristol.nhs.uk A 48-year
Calcium metabolism and the Parathyroid Glands. Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands
 Calcium metabolism and the Parathyroid Glands Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands Calcium is an essential element for contraction of voluntary/smooth
Calcium metabolism and the Parathyroid Glands Calcium, osteoclasts and osteoblasts-essential to understand the function of parathyroid glands Calcium is an essential element for contraction of voluntary/smooth
Southern Derbyshire Shared Care Pathology Guidelines. Primary Hyperparathyroidism
 Southern Derbyshire Shared Care Pathology Guidelines Primary Hyperparathyroidism Please use this Guideline in Conjunction with the Hypercalcaemia Guideline Definition Driven by hyperfunction of one or
Southern Derbyshire Shared Care Pathology Guidelines Primary Hyperparathyroidism Please use this Guideline in Conjunction with the Hypercalcaemia Guideline Definition Driven by hyperfunction of one or
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Colecalciferol Meda 800 IU tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 800 IU
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Colecalciferol Meda 800 IU tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 800 IU
Approach to a patient with hypercalcemia
 Approach to a patient with hypercalcemia Ana-Maria Chindris, MD Division of Endocrinology Mayo Clinic Florida 2013 MFMER slide-1 Background Hypercalcemia is a problem frequently encountered in clinical
Approach to a patient with hypercalcemia Ana-Maria Chindris, MD Division of Endocrinology Mayo Clinic Florida 2013 MFMER slide-1 Background Hypercalcemia is a problem frequently encountered in clinical
Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff.
 Hypercalcaemia Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff. Mild (usually no symptoms) 2.6 3.0 mmol/l Moderate (start to develop symptoms) 3.0 3.4
Hypercalcaemia Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff. Mild (usually no symptoms) 2.6 3.0 mmol/l Moderate (start to develop symptoms) 3.0 3.4
Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff.
 Authoriser: Fiona Davidson Page 1 of 5 Hypercalcaemia Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff. Mild (usually no symptoms) 2.6 3.0 mmol/l Moderate
Authoriser: Fiona Davidson Page 1 of 5 Hypercalcaemia Definition Elevated Adjusted Calcium > 2.6 mmol/l (adjusted for albumin), taken without using a cuff. Mild (usually no symptoms) 2.6 3.0 mmol/l Moderate
Dosage in renal impairment Kalcipos-D chewable tablets should not be used in patients with severe renal impairment.
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D 500 mg/400 IU chewable tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains: calcium 500 mg as
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D 500 mg/400 IU chewable tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains: calcium 500 mg as
PARATHYROID, VITAMIN D AND BONE
 PARATHYROID, VITAMIN D AND BONE G M Kellerman Pathology North Hunter Service 30/01/2015 BIOLOGY OF BONE Bone consists of protein, polysaccharide components and mineral matrix. The mineral is hydroxylapatite,
PARATHYROID, VITAMIN D AND BONE G M Kellerman Pathology North Hunter Service 30/01/2015 BIOLOGY OF BONE Bone consists of protein, polysaccharide components and mineral matrix. The mineral is hydroxylapatite,
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Desunin 4000 IU Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 4000 IU (equivalent
1 NAME OF THE MEDICINAL PRODUCT Desunin 4000 IU Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains colecalciferol (vitamin D 3 ) 4000 IU (equivalent
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D mite 500 mg/200 IU film-coated tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 500 mg
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Kalcipos-D mite 500 mg/200 IU film-coated tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 500 mg
CKD: Bone Mineral Metabolism. Peter Birks, Nephrology Fellow
 CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
Parathyroid hormone (serum, plasma)
 Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
Instrumental determination of electrolytes in urine. Amal Alamri
 Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
5/18/2017. Specific Electrolytes. Sodium. Sodium. Sodium. Sodium. Sodium
 Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Awaisheh. Mousa Al-Abbadi. Abdullah Alaraj. 1 Page
 f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
HYPERPARATHYROIDIS M FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE
 HYPERPARATHYROIDIS M FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE PROFESSOR OF SURGERY J I N N A H S I N D H M E D I C A L U N I V E R S I T Y PREAMBLE Anatomy & physiology of the
HYPERPARATHYROIDIS M FAISAL GHANI SIDDIQUI MBBS; FCPS; PGDIP-BIOMEDICAL ETHICS; MCPS-HPE PROFESSOR OF SURGERY J I N N A H S I N D H M E D I C A L U N I V E R S I T Y PREAMBLE Anatomy & physiology of the
Brunel Health Core Ten Results for Sam Witter. Thank you for submitting a sample of your blood to be tested by Brunel Health.
 Brunel Health Core Ten Results for Sam Witter Dear Sam, Thank you for submitting a sample of your blood to be tested by Brunel Health. We are pleased to say that there was enough viable sample to test
Brunel Health Core Ten Results for Sam Witter Dear Sam, Thank you for submitting a sample of your blood to be tested by Brunel Health. We are pleased to say that there was enough viable sample to test
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM FORTE TABLETS
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM FORTE TABLETS Calcium and Phosphorus with Vitamin D3 Tablets QUALITATIVE AND QUANTITATIVE COMPOSITION Each
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM FORTE TABLETS Calcium and Phosphorus with Vitamin D3 Tablets QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Recikalc-D forte 500 mg/ 800 IU chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each chewable tablet contains calcium
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Recikalc-D forte 500 mg/ 800 IU chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each chewable tablet contains calcium
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES
 Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Figures and tables in this presentation were adopted from various printed and electronic resorces and serve strictly for educational purposes.
 Academic lectures 3rd year of Medical faculty Figures and tables in this presentation were adopted from various printed and electronic resorces and serve strictly for educational purposes. ENDOCRINOLOGY
Academic lectures 3rd year of Medical faculty Figures and tables in this presentation were adopted from various printed and electronic resorces and serve strictly for educational purposes. ENDOCRINOLOGY
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Calcichew-D3 Mite Citron 500 mg/200 IU chewable tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Calcichew-D3 Mite Citron 500 mg/200 IU chewable tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
PRIMARY HYPERPARATHYROIDISM
 PRIMARY HYPERPARATHYROIDISM HYPERPARATHYROIDISM Inappropriate excess secretion of Parathyroid Hormone in Primary Hyperparathyroidism Appropriate Hypersecretion in Secondary Hyperparathyroidism PTH and
PRIMARY HYPERPARATHYROIDISM HYPERPARATHYROIDISM Inappropriate excess secretion of Parathyroid Hormone in Primary Hyperparathyroidism Appropriate Hypersecretion in Secondary Hyperparathyroidism PTH and
hypercalcemia of malignancy hyperparathyroidism PHPT the most common cause of hypercalcemia in the outpatient setting the second most common cause
 hyperparathyroidism A 68-year-old woman with documented osteoporosis has blood tests showing elevated serum calcium and parathyroid hormone (PTH) levels: 11.2 mg/dl (8.8 10.1 mg/dl) and 88 pg/ml (10-60),
hyperparathyroidism A 68-year-old woman with documented osteoporosis has blood tests showing elevated serum calcium and parathyroid hormone (PTH) levels: 11.2 mg/dl (8.8 10.1 mg/dl) and 88 pg/ml (10-60),
David Bruyette, DVM, DACVIM
 VCAwestlaspecialty.com David Bruyette, DVM, DACVIM Disorders of calcium metabolism are common endocrine disorders in both dogs and cats. In this article we present a logical diagnostic approach to patients
VCAwestlaspecialty.com David Bruyette, DVM, DACVIM Disorders of calcium metabolism are common endocrine disorders in both dogs and cats. In this article we present a logical diagnostic approach to patients
CALCIUM BALANCE. James T. McCarthy & Rajiv Kumar
 CALCIUM BALANCE James T. McCarthy & Rajiv Kumar CALCIUM BALANCE TOTAL BODY CALCIUM (~ 1000g in a normal 60 kg adult) - > 99% in bones - ~ 0.6% in the intracellular space - ~ 0.1% in the extracellular space
CALCIUM BALANCE James T. McCarthy & Rajiv Kumar CALCIUM BALANCE TOTAL BODY CALCIUM (~ 1000g in a normal 60 kg adult) - > 99% in bones - ~ 0.6% in the intracellular space - ~ 0.1% in the extracellular space
OMICS Journals are welcoming Submissions
 OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
Calcium and Parathyroid Disorders
 Calcium and Parathyroid Disorders Hussain Mahmud, MD Clinical Assistant Professor of Medicine Division of Endocrinology, Diabetes, and Metabolism University of Pittsburgh Butler Memorial Hospital November
Calcium and Parathyroid Disorders Hussain Mahmud, MD Clinical Assistant Professor of Medicine Division of Endocrinology, Diabetes, and Metabolism University of Pittsburgh Butler Memorial Hospital November
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
PRIMARY HYPERPARATHYROIDISM PRIMARY HYPERPARATHYROIDISM. Hyperparathyroidism Etiology. Common Complex Insidious Chronic Global Only cure is surgery
 ENDOCRINE DISORDER PRIMARY HYPERPARATHYROIDISM Roseann P. Velez, DNP, FNP Francis J. Velez, MD, FACS Common Complex Insidious Chronic Global Only cure is surgery HYPERPARATHYROIDISM PARATHRYOID GLANDS
ENDOCRINE DISORDER PRIMARY HYPERPARATHYROIDISM Roseann P. Velez, DNP, FNP Francis J. Velez, MD, FACS Common Complex Insidious Chronic Global Only cure is surgery HYPERPARATHYROIDISM PARATHRYOID GLANDS
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM TABLETS. Calcium and Phosphorus with Vitamin D3 Tablets
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM TABLETS Calcium and Phosphorus with Vitamin D3 Tablets QUALITATIVE AND QUANTITATIVE COMPOSITION Each uncoated
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory OSTOCALCIUM TABLETS Calcium and Phosphorus with Vitamin D3 Tablets QUALITATIVE AND QUANTITATIVE COMPOSITION Each uncoated
Case 4 Generalised bone pain
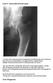 Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Ca, Mg metabolism, bone diseases. Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary
 Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
SUMMARY OF PRODUCT CHARACTERISTICS. One chewable tablet contains 1250 mg calcium carbonate (equivalent to 500 mg calcium).
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [XXX] 500 mg chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains 1250 mg calcium carbonate (equivalent
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [XXX] 500 mg chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One chewable tablet contains 1250 mg calcium carbonate (equivalent
DBL CALCIUM GLUCONATE INJECTION BP
 Description DBL CALCIUM GLUCONATE INJECTION BP DBL Calcium Gluconate Injection BP is a clear, colourless solution containing in each 10 ml, Calcium Gluconate BP 953 mg and Calcium Saccharate U.S.P. 30
Description DBL CALCIUM GLUCONATE INJECTION BP DBL Calcium Gluconate Injection BP is a clear, colourless solution containing in each 10 ml, Calcium Gluconate BP 953 mg and Calcium Saccharate U.S.P. 30
CHRONIC KIDNEY DISEASE (CKD)
 CHRONIC KIDNEY DISEASE (CKD) CKD implies longstanding (more than 3 months), and usually progressive, impairment in renal function. In many instances, no effective means are available to reverse the primary
CHRONIC KIDNEY DISEASE (CKD) CKD implies longstanding (more than 3 months), and usually progressive, impairment in renal function. In many instances, no effective means are available to reverse the primary
Endocrine Regulation of Calcium and Phosphate Metabolism
 Endocrine Regulation of Calcium and Phosphate Metabolism Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C516, Block C, Research Building, School of Medicine Tel: 88208252 Email: wanghuiping@zju.edu.cn
Endocrine Regulation of Calcium and Phosphate Metabolism Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C516, Block C, Research Building, School of Medicine Tel: 88208252 Email: wanghuiping@zju.edu.cn
Parathyoid glands and PTH
 Parathyoid glands and PTH They are four glands located behind the thyroid gland, each one from (20-50) mg in weight, and composed of two types of cells: 1. Cheif cells: that produces PTH. 2-oxyphil cells:
Parathyoid glands and PTH They are four glands located behind the thyroid gland, each one from (20-50) mg in weight, and composed of two types of cells: 1. Cheif cells: that produces PTH. 2-oxyphil cells:
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Vitamins. Vitamins (continued) Lipid-Soluble Vitamins (A, D, E, K) Vitamins Serve Important Roles in Function of Body
 Vitamins Drugs for Nutritional Disorders Organic substances are needed in small amounts Promote growth Maintain health Vitamins Human cells cannot produce vitamins Exception: vitamin D Vitamins or provitamins
Vitamins Drugs for Nutritional Disorders Organic substances are needed in small amounts Promote growth Maintain health Vitamins Human cells cannot produce vitamins Exception: vitamin D Vitamins or provitamins
Thyroid and parathyroid glands
 Thyroid and parathyroid glands Dr. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong May 2007 The thyroid gland straddles the esophagus, just below the larynx, in the neck.
Thyroid and parathyroid glands Dr. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong May 2007 The thyroid gland straddles the esophagus, just below the larynx, in the neck.
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Agents that Affect Bone & Mineral Homeostasis
 Agents that Affect Bone & Mineral Homeostasis 1 Agents that Affect Bone & Mineral Homeostasis Calcium and phosphate are the major mineral constituents of bone. They are also two of the most important minerals
Agents that Affect Bone & Mineral Homeostasis 1 Agents that Affect Bone & Mineral Homeostasis Calcium and phosphate are the major mineral constituents of bone. They are also two of the most important minerals
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
The Parathyroid Glands
 The Parathyroid Glands Bởi: OpenStaxCollege The parathyroid glands are tiny, round structures usually found embedded in the posterior surface of the thyroid gland ([link]). A thick connective tissue capsule
The Parathyroid Glands Bởi: OpenStaxCollege The parathyroid glands are tiny, round structures usually found embedded in the posterior surface of the thyroid gland ([link]). A thick connective tissue capsule
Clinical Approach to Hypercalcemia For the Primary Care Provider
 Clinical Approach to Hypercalcemia For the Primary Care Provider Christina Maser, MD FACS UCSF Fresno Department of Surgery, Endocrine Surgery 2/2/19 Objectives Recognition of pitfalls of diagnosis of
Clinical Approach to Hypercalcemia For the Primary Care Provider Christina Maser, MD FACS UCSF Fresno Department of Surgery, Endocrine Surgery 2/2/19 Objectives Recognition of pitfalls of diagnosis of
Amani Alghamdi. Slide 1
 Minerals in the body Amani Alghamdi Slide 1 The Minerals Small, naturally occurring, inorganic, chemical elements Serve as structural components Minerals classification The minerals present in the body
Minerals in the body Amani Alghamdi Slide 1 The Minerals Small, naturally occurring, inorganic, chemical elements Serve as structural components Minerals classification The minerals present in the body
CAL360 Tablets (Calcium citrate malate + Vitamin D3 )
 Published on: 18 Jan 2016 CAL360 Tablets ( citrate malate + Vitamin D3 ) Composition Each film-coated tablet contains: Citrate Malate equivalent to Elementary 250 mg Cholecalciferol Concentrate (powder
Published on: 18 Jan 2016 CAL360 Tablets ( citrate malate + Vitamin D3 ) Composition Each film-coated tablet contains: Citrate Malate equivalent to Elementary 250 mg Cholecalciferol Concentrate (powder
Osteoporosis. When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of.
 Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Excipient(s) with known effect: One tablet contains 1 mg aspartame (E951), 390 mg sorbitol (E420) and 0.7 mg sucrose
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcichew-D 3 Melon 500 mg/400 IU chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcichew-D 3 Melon 500 mg/400 IU chewable tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate
1. NAME OF THE MEDICINAL PRODUCT. Calciflex-D 3 Citron 500 mg/400 IU film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Calciflex-D 3 Citron 500 mg/400 IU film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate equivalent to 500 mg calcium Cholecalciferol
1. NAME OF THE MEDICINAL PRODUCT Calciflex-D 3 Citron 500 mg/400 IU film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Calcium carbonate equivalent to 500 mg calcium Cholecalciferol
Rahaf AL-Jafari. Marah Qaddourah. Rahmeh Abdullah. Saleem. 1 P a g e
 15 Rahaf AL-Jafari Marah Qaddourah Rahmeh Abdullah Saleem 1 P a g e If you are following with the record you may notice a little bit difference in information sequences. Hormones that function on growth
15 Rahaf AL-Jafari Marah Qaddourah Rahmeh Abdullah Saleem 1 P a g e If you are following with the record you may notice a little bit difference in information sequences. Hormones that function on growth
Chronic kidney disease in cats
 Chronic kidney disease in cats What is chronic kidney disease (CKD)? Chronic kidney disease (CKD) is the name now used to refer to cats with kidney failure (or chronic kidney failure). CKD is one of the
Chronic kidney disease in cats What is chronic kidney disease (CKD)? Chronic kidney disease (CKD) is the name now used to refer to cats with kidney failure (or chronic kidney failure). CKD is one of the
When the level of calcium in the blood falls too low, the parathyroid glands secrete just enough PTH to restore the blood calcium level.
 Hyperparathyroidism Primary hyperparathyroidism is a disorder of the parathyroid glands, also called parathyroids. Primary means this disorder originates in the parathyroids: One or more enlarged, overactive
Hyperparathyroidism Primary hyperparathyroidism is a disorder of the parathyroid glands, also called parathyroids. Primary means this disorder originates in the parathyroids: One or more enlarged, overactive
Contemporary Nutrition 6 th. th ed. Chapter 9 Minerals
 Contemporary Nutrition 6 th th ed. Chapter 9 Minerals Minerals Various functions in the body Major Minerals Require >100 mg /day Calcium, phosphorus Trace Minerals Require < 100 mg/day Iron, zinc Bioavailability
Contemporary Nutrition 6 th th ed. Chapter 9 Minerals Minerals Various functions in the body Major Minerals Require >100 mg /day Calcium, phosphorus Trace Minerals Require < 100 mg/day Iron, zinc Bioavailability
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Hyper and hypocalcaemia. Prof Tricia Tan
 Hyper and hypocalcaemia Prof Tricia Tan Learning Objectives Basic physiology of Ca regulation Case presentations Take home messages Calcium Total body calcium content ~1300g 99% in bone 1% intracellular
Hyper and hypocalcaemia Prof Tricia Tan Learning Objectives Basic physiology of Ca regulation Case presentations Take home messages Calcium Total body calcium content ~1300g 99% in bone 1% intracellular
ELECTROLYTES RENAL SHO TEACHING
 ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
CKD-MBD CKD mineral bone disorder
 CKD Renal bone disease Dr Mike Stone University Hospital Llandough Affects 5 10 % of population Increasingly common Ageing, diabetes, undetected hypertension Associated with: Cardiovascular disease Premature
CKD Renal bone disease Dr Mike Stone University Hospital Llandough Affects 5 10 % of population Increasingly common Ageing, diabetes, undetected hypertension Associated with: Cardiovascular disease Premature
School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR
 1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
1 School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PLB SEMINAR URINARY (RENAL) STONE FORMATION An Overview What are Urinary (Renal)
NEOPLASMS OF THE THYROID PATHOLOGY OF PARATHYROID GLANDS. BY: Shifaa Qa qa
 NEOPLASMS OF THE THYROID PATHOLOGY OF PARATHYROID GLANDS BY: Shifaa Qa qa Neoplasmas of the thyroid thyroid nodules Neoplastic ---- benign, malignant Non neoplastic Solitary nodules ----- neoplastic Nodules
NEOPLASMS OF THE THYROID PATHOLOGY OF PARATHYROID GLANDS BY: Shifaa Qa qa Neoplasmas of the thyroid thyroid nodules Neoplastic ---- benign, malignant Non neoplastic Solitary nodules ----- neoplastic Nodules
The Calcium Conundrum: When, What and How to Give Calcium in Pediatric CKD/ESRD
 The Calcium Conundrum: When, What and How to Give Calcium in Pediatric CKD/ESRD Jess Tower MS RD LD 3/18/19 Children s Mercy Hospital jdtower@cmh.edu 816 460 1067 Disclosures Nothing to disclose 1 How
The Calcium Conundrum: When, What and How to Give Calcium in Pediatric CKD/ESRD Jess Tower MS RD LD 3/18/19 Children s Mercy Hospital jdtower@cmh.edu 816 460 1067 Disclosures Nothing to disclose 1 How
Calcium Chloride 10% Injection 1g in 10 ml (as calcium chloride dihydrate containing 6.8 mmol calcium ions in 10 ml)
 10% Injection 1g in 10 ml (as calcium chloride dihydrate containing 6.8 mmol calcium ions in 10 ml) NAME OF THE MEDICINE Name: Calcium chloride dihydrate Molecular formula: CaCl2.2H20 Molecular weight:
10% Injection 1g in 10 ml (as calcium chloride dihydrate containing 6.8 mmol calcium ions in 10 ml) NAME OF THE MEDICINE Name: Calcium chloride dihydrate Molecular formula: CaCl2.2H20 Molecular weight:
B. Environmental Factors. a. The major risk factor to papillary thyroid cancer is exposure to ionizing radiation, during the first 2 decades of life.
 B. Environmental Factors. a. The major risk factor to papillary thyroid cancer is exposure to ionizing radiation, during the first 2 decades of life. b. Deficiency of dietary iodine: - Is linked with a
B. Environmental Factors. a. The major risk factor to papillary thyroid cancer is exposure to ionizing radiation, during the first 2 decades of life. b. Deficiency of dietary iodine: - Is linked with a
GLOSSARY OF TERMS. produced in response to an antigen to bond with and neutralize that antigen / the body's way of destroying foreign invaders
 TERM 24-hour urine acidosis acquired aemia (prefix) albumin alkalosis anemia antibodies antigen autocrine autoimmune basal ganglion bone turnover calcilytic calcimimetic calcitonin Calcitriol Calcium carbonate
TERM 24-hour urine acidosis acquired aemia (prefix) albumin alkalosis anemia antibodies antigen autocrine autoimmune basal ganglion bone turnover calcilytic calcimimetic calcitonin Calcitriol Calcium carbonate
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Overview. Fluid & Electrolyte Disorders. Water distribution. Introduction 5/10/2014
 Overview Fluid & Electrolyte Disorders Dr Nicola Barlow Clinical Biochemistry Department, City Hospital Introduction Fluid and electrolyte homeostasis Electrolyte disturbances Analytical parameters Methods
Overview Fluid & Electrolyte Disorders Dr Nicola Barlow Clinical Biochemistry Department, City Hospital Introduction Fluid and electrolyte homeostasis Electrolyte disturbances Analytical parameters Methods
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE
 Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
SUMMARY OF PRODUCT CHARACTERISTICS. A 2.5ml single-dose bottle containing IU Cholecalciferol (equivalent to 625 micrograms vitamin D 3 )
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
POTASSIUM DIHYDROGEN PHOSPHATE 13.6% CONCENTRATED INJECTION
 POTASSIUM DIHYDROGEN PHOSPHATE 13.6% CONCENTRATED INJECTION NAME OF THE MEDICINE Potassium Dihydrogen Phosphate Synonyms: potassium biphosphate, potassium acid phosphate, monopotassium phosphate, or monoibasic
POTASSIUM DIHYDROGEN PHOSPHATE 13.6% CONCENTRATED INJECTION NAME OF THE MEDICINE Potassium Dihydrogen Phosphate Synonyms: potassium biphosphate, potassium acid phosphate, monopotassium phosphate, or monoibasic
Fluid and Electrolytes P A R T 4
 Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
CALCIUM AND THYROID METABOLISM. Westmead Primary Exam Group
 CALCIUM AND THYROID METABOLISM Westmead Primary Exam Group THYROID HORMONES Chemistry - principle hormones are: T3 (Triiodothyronine) Can be formed in peripheral tissues by de-iodination of T4 T3 is more
CALCIUM AND THYROID METABOLISM Westmead Primary Exam Group THYROID HORMONES Chemistry - principle hormones are: T3 (Triiodothyronine) Can be formed in peripheral tissues by de-iodination of T4 T3 is more
CHAPTER 27 LECTURE OUTLINE
 CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
HYPERCALCAEMIA 101 FOR THE INTERNIST
 HYPERCALCAEMIA 101 FOR THE INTERNIST Dr Chionh Siok Bee Dept of Medicine, National University Hospital siok_bee_chionh@nuhs.edu.sg Medicine Review Course 18/09/2011 Outline of Talk Definition of hypercalcaemia
HYPERCALCAEMIA 101 FOR THE INTERNIST Dr Chionh Siok Bee Dept of Medicine, National University Hospital siok_bee_chionh@nuhs.edu.sg Medicine Review Course 18/09/2011 Outline of Talk Definition of hypercalcaemia
Hypercalcemia. Hypercalcemia: When to Worry, When to Treat! Mineral Metabolism : A Short Course
 Hypercalcemia: When to Worry, When to Treat! Michael A. Levine has no financial relationships to disclose or Conflicts of Interest to resolve. Michael A. Levine, M.D. This presentation will not involve
Hypercalcemia: When to Worry, When to Treat! Michael A. Levine has no financial relationships to disclose or Conflicts of Interest to resolve. Michael A. Levine, M.D. This presentation will not involve
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Calcium Nephrolithiasis and Bone Health. Noah S. Schenkman, MD
 Calcium Nephrolithiasis and Bone Health Noah S. Schenkman, MD Associate Professor of Urology and Residency Program Director, University of Virginia Health System; Charlottesville, Virginia Objectives:
Calcium Nephrolithiasis and Bone Health Noah S. Schenkman, MD Associate Professor of Urology and Residency Program Director, University of Virginia Health System; Charlottesville, Virginia Objectives:
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Calcium-D-Sandoz 600 mg + 400 IU, effervescent tablets. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 effervescent tablet contains 1500
1 NAME OF THE MEDICINAL PRODUCT Calcium-D-Sandoz 600 mg + 400 IU, effervescent tablets. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 effervescent tablet contains 1500
WHAT IS YOUR DIAGNOSIS?
 WHAT IS YOUR DIAGNOSIS? A 21 month old, female neutered Cockapoo presented with a 5 day history of trembling. The dog had been in the owners possession since a 7 week old puppy, and was up-to-date with
WHAT IS YOUR DIAGNOSIS? A 21 month old, female neutered Cockapoo presented with a 5 day history of trembling. The dog had been in the owners possession since a 7 week old puppy, and was up-to-date with
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia
 BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
Cardiac Pathophysiology
 Cardiac Pathophysiology Evaluation Components Medical history Physical examination Routine laboratory tests Optional tests Medical History Duration and classification of hypertension. Patient history of
Cardiac Pathophysiology Evaluation Components Medical history Physical examination Routine laboratory tests Optional tests Medical History Duration and classification of hypertension. Patient history of
D MADE in EU Brochure Carbocal_UK.indd 1 13/05/14 17:48
 D MADE in EU Brochure Carbocal_UK.indd 1 13/05/14 17:48 Carbocal D 1. NAME OF THE MEDICINAL PRODUCT Carbocal D 600 mg/400 UI orange flavour Chewable Tablets 2. Qualitative and quantitative composition
D MADE in EU Brochure Carbocal_UK.indd 1 13/05/14 17:48 Carbocal D 1. NAME OF THE MEDICINAL PRODUCT Carbocal D 600 mg/400 UI orange flavour Chewable Tablets 2. Qualitative and quantitative composition
Calcium Gluconate Injection 10 ml
 10 ml Calcium gluconate is a supersaturated solution. Supersaturated solutions are prone to precipitation. Examine the vial and do not use if a precipitate is present. NAME OF THE MEDICINE Calcium Gluconate
10 ml Calcium gluconate is a supersaturated solution. Supersaturated solutions are prone to precipitation. Examine the vial and do not use if a precipitate is present. NAME OF THE MEDICINE Calcium Gluconate
20. CALCIUM AND PHOSPHOROUS METABOLISM
 20. CALCIUM AND PHOSPHOROUS METABOLISM Many physiological processes are regulated directly or indirectly by calcium. Furthermore, the main physical structure of vertebrates and other species depend on
20. CALCIUM AND PHOSPHOROUS METABOLISM Many physiological processes are regulated directly or indirectly by calcium. Furthermore, the main physical structure of vertebrates and other species depend on
Case study Group 2 presentation
 Case study Group 2 presentation Patient profile HN 3095-57 Female 60 years old Hometown : Sa Kaeo province Occupation : farmer No drug and food allergy Chief complain Left neck mass 10 years PTA that gradually
Case study Group 2 presentation Patient profile HN 3095-57 Female 60 years old Hometown : Sa Kaeo province Occupation : farmer No drug and food allergy Chief complain Left neck mass 10 years PTA that gradually
Article 30 Referral for Calcitugg (and associated names) Chewable tablets (Calcium carbonate)
 ANNEX I LIST OF THE NAMES, PHARMACEUTICAL FORMS, STRENGTHS, ROUTES OF ADMINISTRATION, MARKETING AUTHORISATION HOLDERS, PACKAGING AND PACKAGE SIZES OF THE MEDICINAL PRODUCT IN THE MEMBER STATES 1 Article
ANNEX I LIST OF THE NAMES, PHARMACEUTICAL FORMS, STRENGTHS, ROUTES OF ADMINISTRATION, MARKETING AUTHORISATION HOLDERS, PACKAGING AND PACKAGE SIZES OF THE MEDICINAL PRODUCT IN THE MEMBER STATES 1 Article
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS
 Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Overview. Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases. People Centred Positive Compassion Excellence
 Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
