catecholamine secretion from cultured, bovine adrenal chromaffin cells
|
|
|
- Constance McKinney
- 5 years ago
- Views:
Transcription
1 Br. J. Pharmac. (1986), 89, Effects of opioid peptides and morphine on histamineinduced catecholamine secretion from cultured, bovine adrenal chromaffin cells Bruce G. Livett & Philip D. Marley' The Russell Grimwade School of Biochemistry, The University of Melbourne, Parkville, Victoria 3052, Australia I The effect of opioid peptides and morphine on histamine-induced catecholamine secretion has been studied in monolayer cultures of dispersed, bovine adrenal chromaffin cells. 2 Histamine-induced a dose-dependent secretion of both adrenaline and noradrenaline with a threshold dose of approximately 5 nm, an EC50 of 150 nm and maximal secretion at 10 AM. 3 Catecholamine secretion induced by I tm histamine was completely dependent on extracellular calcium, was inhibited in a dose-dependent manner by mepyramine (I nm-l M), and was unaffected by cimetidine (10 M) and hexamethonium (0.1 mm). 4 Dynorphin-1-13 (1 nm-201m), metorphamide (O.1 nm-10īm), morphine (I nm-0. mm) and diprenorphine (I nm-0.1 mm) each had no effect on adrenaline or noradrenaline secretion induced by 1 JAM histamine. 5 The characteristics of histamine-induced catecholamine secretion from bovine adrenal chromaffin cells were similar to those reported previously for cat and rat adrenal medulla being calcium-dependent and mediated by HI histamine-receptors. The results with opioid peptides and morphine suggest that endogenous adrenal opioid peptides do not act on the opioid binding sites found on adrenal medullary chromaffin cells to modify their secretory response to histamine. Introduction In the last six years, more than 30 different opioid peptides have been identified in the mammalian adrenal medulla (Lewis & Stem, 1983; Udenfriend & Kilpatrick, 1983). Immunocytochemical studies have shown that these peptides are present both in chromaffin cells and in splanchnic nerve terminals in the adrenal medulla (Schultzberg et al., 1978; Livett et al., 1982). Opioid peptides are released from the adrenal into the circulation in vivo upon stimulation of the splanchnic nerve (Hexum et al., 1980; Chaminade et al., 1984) and can be released in vitro from isolated chromaffin cells by stimulation with nicotinic agonists or high potassium ion concentrations (Livett et al., 1981; Rossier et al., 1981). Adrenal medullary chromaffin cells have been shown to possess highaffinity, stereospecific opioid binding sites (Chavkin et al., 1979; Kumakura et al., 1980; Costa et al., 1981; Lemaire et al., 1981; Saiani & Guidotti 1982; Castanas et al., 1985a,b) suggesting that opioid peptides released from splanchnic nerve terminals or from the 'Author for correspondence. adrenal medullary chromaffin cells may have a physiological role within the adrenal medulla. In previous work, the ability of opioid peptides and opiate alkaloids to modify nicotinic secretory effects on isolated bovine adrenal chromaffin cells has been studied (Kumakura et al., 1980; Costa et al., 1981; 1983; Lemaire et al., 1981; Dean et al., 1982; Saiani & Guidotti 1982; Marley et al., 1986a,b). It was found that opioid receptor agonists are capable of inhibiting nicotinic stimulation of catecholamine (CA) or ATP secretion from adrenal medullary cells. However, the peptides tested were all very weak in these actions and were poorly antagonized by classical opioid receptor antagonists, such as naloxone and diprenophine (Dean et al., 1982; Marley et al., 1986a,b). Furthermore, the actions of opiate alkaloids were also very weak and did not show stereoselectivity (Dean et al., 1982). Consequently, it is unlikely that the classical opioid 'receptors' identified on bovine chromaffin cells by ligand-binding work act physiologically to modify nicotinic stimulation of CA release. In neonatal animals, before adrenal innervation The Macmillan Press Ltd 1986
2 328 B.G. LIVETT & P.D. MARLEY occurs, naloxone (5 mg kg-, s.c.) potentiates the nonneurogenic depletion of catecholamines induced by reserpine, while methadone (2.5 mg kg- ', s.c.) inhibits this response (Chantry et al., 1982). This suggests that opioid compounds may act directly on the adrenal medulla to modify its response to non-neurogenic stimuli. By contrast, in the mature rat, morphine and methadone evoke adrenal catecholamine secretion by a central reflex which results in a depletion of adrenal catecholamines (Yoshizaki, 1973; Slotkin et al., 1980). Similar results have been obtained in adult dogs where morphine acts indirectly because it fails to cause secretion of catecholamines in animals with denervated adrenals (Costa et al., 1981). To see whether the adrenal medullary receptors for opioid peptides have a functional role in vivo in the adult, Costa et al. (1981) carried out experiments in anaesthetized dogs in which the peripheral stump of the splanchnic nerve was stimulated electrically and blood samples taken from the cannulated adrenal vein were analyzed for catecholamines and opioid peptides (Hanbauer et al., 1982). Splanchnic nerve stimulation produced a frequency-dependent secretion both of catecholamines and opioid peptides (Costa et al., 1983; 1984). Injection ofdiprenorphine (a blocker ofadrenal opioid responses), increased further the secretion of low molecular weight opioid peptides and of catecholamines. Hence, an inhibitory regulatory mechanism similar to that proposed from studies on isolated adrenal chromaffin cells in vitro, appears to operate in vivo. The possibility could not be excluded however that the release of catecholamines was inhibited by the secreted adrenal opioids acting on presynaptic receptors located in splanchnic nerve axons. Adrenal CA secretion is, however, not solely under the control of nicotinic receptors on the chromaffin cells. A number of endogenous secretagogues other than acetylcholine are known to play a physiological role in regulating adrenal medullary secretion. It is possible that adrenal opioid peptides may act to modify the CA secretion produced by one of these other secretagogues. In the present study, we have tested the ability of opioid peptides and morphine to modify CA release evoked from cultured, bovine adrenal chromaffin cells by histamine. Methods Preparation of primary cultures of dispersed, bovine, adrenal chromaffin cells Histamine-induced adrenal CA secretion was studied in primary monolayer cultures of bovine isolated adrenal chromaffin cells. Cells were prepared by collagenase digestion of adrenal glands, purified by Percoll (TM) density gradient centrifugation and cultured in 24 well plastic culture dishes pre-coated with rat tail collagen (see Livett 1984; Livett et al., 1986b for full details). Cells were used for catecholamine release experiments after being cultured for 3 days, to allow attachment of the cells. Stimulation of chromaffin cell catecholamine release On the third day after plating, culture dishes were removed from the incubator and equilibrated to room temperature (20-22 C) for 5min. Each well then received either three or four incubations in a medium consisting of (mm): NaCl 154, KCI 2.6, K2HPO4 2.15, KH2PO4 0.85, MgSO4 1.18, D-glucose 10, CaCl2 2.2, bovine serum albumin 0.5%. In experiments where the wells received three incubations, the first two incubations were 5 min washes, and the third a 20 min stimulation period with histamine. In the experiments assessing the calcium-dependence of histamine-induced CA release, all three incubations were performed in medium without CaC12 and containing an additional 2.2 mm MgSO4. In the experiments assessing the effects of drugs on histamine-induced CA release, four incubations were used. The first two were 5 min washes, the third a 5 min preincubation in the presence of drug, and the fourth a 20 min stimulation period with histamine in the presence of drug. The medium from the third (and, where appropriate, the fourth) incubation periods was collected into perchloric acid (PCA, 0.4 M final concentration) and assayed for adrenaline (Ad) and noradrenaline (NA) by separation on a reverse-phase h.p.l.c. column and quantified by electrochemical oxidation (for full details see Livett et al., 1986a; Marley et al., 1986a,b). Cellular CA were extracted from the cultured cells in 0.01 M PCA, after their final incubation, and protein precipitated by acidifying to 0.4 M PCA. The release of each CA is expressed as a fraction (%) of its intracellular content as calculated immediately before the stimulation period. The two opioid peptides used in this study were dissolved in 0.1 M sodium phosphate, ph 7.1, and the concentrations of these stock solutions determined from their absorption at 280 nm assuming a molar extinction coefficient for tyrosine in this buffer of Drugs Dynorphin was from Peninsula Laboratories (San Carlos, CA, U.S.A.). Metorphamide was a gift from Dr E. Weber (Stanford, CA, U.S.A.). Diprenorphine HCI was a gift from Mr I. Mawhinney (C-Vet Ltd., U.K.). Morphine HCI was from Prosana Laboratories (Sydney, Australia). Mepyramine maleate, cimetidine and hexamethonium bromide were from Sigma Chemical Co. (St. Louis, U.S.A.).
3 OPIATE AND HISTAMINE EFFECTS ON CHROMAFFIN CELLS 329 Results Histamine-induced catecholamine secretion Histamine produced a dose-dependent release of both Ad and NA from the chromaffin cells with a threshold concentration of approximately 5 nm, a maximal effect at 10 LM and an ECo of 150 nm (Figure 1). NA and Ad secretion were equally sensitive to histamine; however, the proportion of cell content of each amine released by a given histamine concentration varied between cell preparations. In the cell preparations used for Figures 1, 2 and 4d, 1 IsM histamine induced proportionately more Ad secretion than NA, while in cell preparations used for Figures 4a and 4c, 1 gm histamine induced the same % release of Ad and NA. Histamine-induced secretion of both Ad and NA from the cultured chromaffin cells was completely dependent on extracellular calcium ions. Replacement of CaCl2 in the incubation medium with an equimolar amount of MgSO4 reduced histamine-induced CA secretion to basal levels (Figure 2). The calciumdependency of histamine-induced CA secretion was seen for histamine concentrations from 1 nm-100 tm (data not shown). Mepyramine, a selective HI histamine-receptor antagonist, inhibited histamine-induced CA secretion in a dose-dependent manner (Figure 3). It showed no selectivity for inhibiting Ad versus NA secretion, and abolished 1 gm histamine-induced secretion of both CA at a concentration of0.1-1i M. A concentration of mepyramine (0.1 M) that virtually abolished histamine-induced CA release from cultured chromaffin cells (Figure 4a) had no effect on nicotine-induced CA secretion from parallel cultures (Figure 4b). Histamine-induced CA release was unaffected by high doses of cimetidine (a selective H2 histamine-receptor antagonist, Figure 4c) or of hexamethonium (Figure 4d). Effects of opioid peptides and morphine on histamineinduced catecholamine secretion In previous studies, it was found that C-terminally extended opioid peptides had relatively high affinity for bovine adrenal medullary opioid binding sites, as determined from ligand-binding experiments (Castanas et al., 1985a,b). The larger opioids were also found to be considerably more potent than the pentaand hexa-peptides at inhibiting the nicotinic secretory response of bovine chromaffin cells (Dean et al., 1982; Marley et al., 1986a). In the present study, therefore, two C-terminally extended opioid peptides, dynorphin-1-13 and metorphamide, were tested for their ability to modify histamine-induced CA secretion from the cultured chromaffin cells. These two peptides were the most U)._ a) E 3.0 Z o L-C4 20 C.-a C C = ( 1.0n * a la [Histamine] (M) Figure 1 Concentration-dependence of histamine-induced adrenaline (0) and noradrenaline (0) secretion from monolayer cultures of bovine, isolated, adrenal chromaffin cells. Results are mean for n = 4 (s.e.mean shown by vertical line), and are representative of similar data from two cell preparations. potent of 21 opioid peptides tested on the nicotinic response (Marley et al., 1986a,b) and are both endogenous to the bovine adrenal medulla (Denis et al., 1982; Weber et al., 1983). Dynorphin-l-13, in the concentration range I nm-2014m, and metorphamide, in the range 0.1 nm-1i0m, had no effect on the secretion of Ad or NA from cultured bovine chromaffin cells induced by 1 IBM histamine (Figures 5a and Sb) Ṫhe ligand binding studies of Castanas et al. (1985a,b), have shown that dynorphin-1-13 has a relatively high affinity for bovine adrenal medullary xi - as 2.5 (a.r am) E it cc o 1.5- c CU )0 d ge 0.5- u- - I U I Noradrenaline ri. I qll~meri~ - l Adrenaline Figure 2 Calcium-dependence of I #LM histamine-induced adrenaline and noradrenaline secretion from monolayer cultures of bovine, isolated, adrenal chromaffin cells. Solutions from which CaCl2 was omitted contained an additional 2.2 mm MgSO4. Open columns: basal secretion; horizontally hatched columns: effect of histamine 1 FM; vertically hatched columns: zero Ca2+ basal secretion; solid columns: effect of zero Ca2+ plus histamine 1 #LM. Results are mean for n = 10-14; s.e.mean shown by vertical lines.
4 330 B.G. LIVETT & P.D. MARLEY 120 c 0 o ) (A 0 * ) c 40 _ ) Cu o o [Mepyraminel (M) Figure 3 Inhibition by mepyramine, an Hi-receptor antagonist, of 1 glm histamine-induced adrenaline (Ad) (@) and noradrenaline (NA) (0) secretion from bovine cultured, adrenal chromaffin cells. Results are mean for n = 4-8; s.e.mean shown by vertical lines. In the absence of mepyramine, I glm histamine-induced the secretion of 3.35 (± 0.06)% cell content of NA per 20 min and 2.49 (± 0.03)% cell content Ad per 20 min. and 6 opioid binding sites, has lower affinity for sites and is very poorly recognised by K2 and K3 sites. Metorphamide however, exhibits high affinity for p and sites (studied with guinea-pig brain membranes) and for K sites characterized with [3H]-bremazocine (Weber et al., 1983). Its affinity for the three types of K site identified in bovine adrenal medullary tissue by Castanas et al. (1985a,b) is not known. It was possible therefore that K2 or K3 opioid recognition sites on the adrenal chromaffin cells might modify histamine-induced CA release but that neither dynorphin nor metorphamide might exhibit this action due to their low affinity for these receptors. Consequently, morphine was tested for its ability to modify histamine-induced CA release from the cultured bovine chromaffin cells. Morphine has been shown to have high affinity for bovine adrenal medullary p sites, to have slightly lower affinity for K, sites, and to have fairly low but readily measured affinity for the 6, K2 and K3 sites (Castanas et al., 1985a,b). However, morphine in the concentration range of 1 nm-0. 1 mm had no effect on histamineinduced secretion of chromaffin cell CA (Figure 5c). Since opioid peptides are present in bovine adrenal chromaffin cells in very high concentrations (see Marley & Livett, 1985) and are secreted along with CA upon stimulation of the medullary cells with appropriate stimuli (Livett et al., 1981; Rossier et al., 1981), it is possible that histamine may also induce opioid peptide secretion from the cultured chromaffin cells during CA release. Consequently, any opioid receptors the chromaffin cells possess may be fully occupied and activated during histamine-induced CA release by these secreted endogenous peptides. This may mask any effect of exogenous opioid agonists. To test this possibility, the ability of diprenorphine to modify histamine-induced CA release was assessed. Diprenorphine is a potent opioid-receptor antagonist with very high affinity for all the opioid binding sites characterized in the bovine adrenal medulla (Castanas et al., 1985a,b). However in concentrations of 1 nm-0. 1 mm, diprenorphine failed to affect CA secretion induced by histamine (Figure 5d). Discussion Histamine-induced catecholamine secretion The ability of histamine to induce adrenal CA release is an important physiological mechanism during anaphylactic shock to counter the hypotensive and bronchoconstrictor effects of histamine (see Cession-Fossion & Lecomte 1966; Lish et al., 1966). Histamine has been known for many years to be capable of inducing secretion of adrenomedullary catecholamines when given to -cats or dogs intravenously (Elliot, 1912; Szczygielski, 1932; Wada et al., 1940). In dogs, this action ofhistamine is completely abolished by transection of the splanchnic nerves or by ganglionic blockade, suggesting it is mediated indirectly by a central reflex mechanism (see Wada et al., 1940; Staszewska- Barczak & Vane, 1965) or that histamine in some way potentiates the response of the adrenal medulla to neural drive. In the cat and the rat, however, splanchnicotomy and ganglionic blockade do not reduce the effect of histamine on adrenal CA release (Szczygielski, 1932; Slater & Dresel, 1952; Trendelenburg, 1954; Staszewska-Barczak & Vane, 1965; Cession-Fossion & Lecomte, 1966; Yoshizaki, 1973; Z. Khalil, Marley & Livett - unpublished observations). CA secretion could also be induced in the rat and the cat by injecting or infusing histamine directly into the adrenal gland either in situ or in the isolated perfused gland (Szczygielski, 1932; Yoshizaki, 1973), and this secretion was not prevented by anticholinergic drugs. These data suggest that, in the cat and rat, histamine has a direct action on the adrenal medulla to induce Ad and NA secretion, independently of the cholinergic innervation of the chromaffin cells. This direct action of histamine is dependent on extracellular calcium (Poisner & Douglas, cat), and is mediated by HI histamine-receptors (Emmelin & Muren, 1949; Trendelenburg 1954; Staszewska-Barczak & Vane, 1965; Yoshizaki, 1973; Robinson, cat and rat). In the present study, identical characteristics have been found for histamine-induced CA release from monolayer cultures of bovine, isolated, adrenal
5 OPIATE AND HISTAMINE EFFECTS ON CHROMAFFIN CELLS 331 a b I on D-E.: 0) I rl 30- a13o 2.0. CaJ E 25-0E).'> 20- X 1.0 E 15 = 10 c_) 00a) ino C. ) n Basal 106M 10 6M Histamine Histamine +107M Mepyramine C " a) o% - a) 0.5o ^ o = 00a) Xo - u UO c ra I I 4~~~~~~' Ah Basal 106M 10-6M Histamine Histamine Cimetidine en. o d 3.0 0)c 2a4 0 Cl 0 Basal 5 x 10-6M Nicotine 5x 10-6M Nicotine +107M Mepyramine Basal 106M 106M Histamine Histamine +10-4M Hexamethonium Figure 4 Pharmacology of 1 jum histamine-induced adrenaline (closed columns) and noradrenaline (open columns) secretion from bovine cultured adrenal chromaffin cells. (a) Effect of 0.1I M mepyramine; (b) effect of 0.1 am mepyramine on 5 lm nicotine-induced catecholamine release; (c) effect of im cimetidine; (d) effect of 0.1 mm hexamethonium. Results are mean for n = 6-8; s.e.mean shown by vertical lines. Note change in scale in (b). chromaffin cells. Although histamine has been shown previously to induce CA release from bovine adrenal medulla (Schneider, 1969; Shima et al., 1976), the pharmacology of this action has not been studied. On cultured bovine adrenal chromaffin cells, we have found that histamine induces both Ad and NA secretion in a concentration- and calcium-dependent manner, by an action on Hi-receptors, and independently of the nicotinic receptors (Figures 1-4). It was noteworthy that histamine had a much lower ECm (150 nm) than nicotine (approximately 5 im, see Marley et al., 1986a), but had a much lower efficacy than nicotine (compare Figures 4a and b). Histamine also tended to induce proportionately slightly more Ad secretion than NA secretion (Figures 1, 2, 4) whilst nicotine induced proportionately twice as much NA secretion than A secretion (Figure 4b). Histamine when administered intravenously to anaesthetized animals has been reported to release Ad selectively from the intact adrenal gland of the cat (Staszewska- Barczak & Vane, 1965) the dog (Schaepdryver, 1959; Narita, 1971), and the rat (Z. Khalil, Marley & Livettunpublished observations). Physiologically, histamine may be released by mast cells during anaphylactic reactions and affect adrenal CA secretion via the circulation. However, histamine is also found within the adrenal gland itself (Endo & Ogwa, 1974) and has recently been localised by immunohistochemistry to the noradrenaline-containing chromaffin cells in the rat (Happola et al., 1985). It is possible, therefore that histamine may play a local regulatory role within the adrenal medulla, as well as its better characterised actions on the adrenal during anaphylactic shock. The concentration of histamine used in this study (1 AM) is very comparable to that required to induce contractions of trachea or relaxation of peripheral smooth muscle (see, e.g., Suzuki et al., 1983), and is some five fold higher than histamine concentrations in resting peripheral plasma (Man et al., 1984).
6 332 B.G. LIVETT & P.D. MARLEY a , co Ad M ds= 10 L G-80 NA E 0 60 E O g Basal aa! -C Basal (D20~ ~ ~ ~ ~ e 20- co lo-4 [Metorphamide] (M) [Dynorphin (M) <^, c d.5~~~~~~~o~ C0 ~ A Co.. E Bal ~~~~40 5~~~~~~~~0 40 C--C Basal ,,,, ) o-., [Morphine] (M) [Diprenorphine] (M) Figure 5 Lack of effect of opioid agonists and antagonists on I JM histamine-induced adrenaline (Ad) and noradrenaline (NA) secretion from bovine cultured, adrenal chromaffin cells. (a) Metorphamide, (b) dynorphin- l- 13, (c) morphine, (d) diprenorphine. Results are mean ofn = 6; s.e.mean shown by vertical lines. Control responses to 1 lam histamine were: (a) 1.23 (± 0.07)% cell content NA per 20 min and 2.14 (± 0.09)% cell content Ad per 20 min, n = 12; (b) 1.46 (±0.03)% NA per 20min and 2.37 (±0.04)% Ad per 20min, n = 18; (c) 3.28 (±0.05)% NA per 20min and 2.49 (±0.03)% Ad per 20min, n = 18; and (d) 1.26 (±0.09)% NA per 20min and 1.88 (±0.05)% Ad per 20min, n= 18. Effects of opioid peptides and morphine on histamineinduced catecholamine release Opioid peptides synthesized and stored in adrenal medullary chromaffin cells are secreted concomitantly with CA during stimulation of the medullary cells (see Introduction). Since the chromaffin cells themselves possess binding sites for opioid peptides (Kumokura et al., 1980), these peptides may act to regulate adrenal medullary CA secretion evoked by appropriate physiological stimuli. Previous studies have suggested that they do not inhibit the nicotinic secretory response of these cells in a physiological manner, since this effect of the peptides is very weak and is not blocked by opioid antagonists. The current study shows that opioid peptides and morphine also do not modify histamine-induced CA secretion from adrenal medullary cells. Between them, the three opioid agonists studied can act on all the known opioid binding sites in the bovine adrenal medulla (Castanas et al., 1985a,b). Whether these opioid receptors on adrenal chromaffin cells can modify CA secretion induced by secretogogues other than those acting on histamine or nicotinic receptors remains to be determined. We thank Dr Eckard Weber for his gift ofmetorphamide and Mr I. Mawhinney for his gift of diprenorphine HCI. We thank Mrs Lois Newman for her help in preparing this manuscript and Mr Ken Mitchelhill for his excellent technical assistance. P.D.M. is a Queen Elizabeth II Fellow. The work was supported by project grants from the NH and MRC (Australian) to B.G.L. and P.D.M. References CASTANAS, E., BOURHIM, N., GIRAUD, P., BOUDOUR- ESQUE, F., CANTOU, P. & OLIVER, C. (1985a). Inter actions of opiates with opioid binding sites in the bovine adrenal medulla: I. Interaction with 6 and it sites. J. Neurochem., 45, CASTANAS, E., BOURHIM, N., GIRAUD, P., BOUDOUR-
7 OPIATE AND HISTAMINE EFFECTS ON CHROMAFFIN CELLS 333 ESQUE, F., CANTOU, P. & OLIVER, C. (1985b). Interactions of opiates with opioid binding sites in the bovine adrenal medulla: II. Interaction with K sites. J. Neurochem., 45, CESSION-FOSSION, A. & LECOMTE, J. (1966). Sur les modes de stimulation de la medullo-surrenale du rat. Archs int. Pharmacodyn., 160, CHAMINADE, M., FOUTZ, A.S. & ROSSIER, J. (1984). Corelease of enkephalins and precursors with catecholamines from the perfused cat adrenal gland in situ. J. Physiol., 353, CHANTRY, C.J., SEIDLER, F.J. & SLOTKIN, T.A. (1982). Nonneurogenic mechanism for reserpine-induced release of catecholamines from the adrenal medulla of neonatal rats: possible modulation by opiate receptors. Neuroscience, 7, CHAVKIN, C., COX, B.M. & GOLDSTEIN, A. (1979). Stereospecific opiate binding in bovine adrenal medulla. Mol. Pharmac., 15, COSTA, E., GUIDOTTI, A., HANBAUER, I., HEXUM, T., SAIANI, L., STINE, S. & YANG, H.-Y.T. (1981). Regulation of acetylcholine receptors by endogenous cotransmitters: studies of adrenal medulla. Fedn. Proc., 40, COSTA, E., GUIDOTTI, A., HANBAUER, I. & SAIANI, L. (1983). Modulation of nicotinic receptor function by opiate recognition sites highly selective for Met5-enkephalin-Arg6-Phe7. Fedn. Proc., 42, COSTA, E., GUIDOTTI, A., HANBAUER, I., KAGEYAMA, H., KATAOKA, Y., PANULA, P., QUACH, T.T. & SCHWARTZ, J.P. (1984). Adrenal medulla: Regulation of biosynthesis and secretion of catecholamines and enkephalins. In Catecholamines: Basic and Peripheral Mechanisms. ed. Usdin, E., Carlsson, A., Dahlstrom, A. & Engel, J. pp New York: Alan R. Liss Inc. DEAN, D.M., LEMAIRE, S. & LIVETT, B.G. (1982). Evidence that inhibition of nicotine-mediated catecholamine secretion from adrenal chromaffin cells by enkephalin, P- endorphin, dynorphin-1-13, and opiates is not mediated via specific opiate receptors. J. Neurochem., 38, DENIS, D., DAY, R. & LEMAIRE, S. (1982). Dynorphin in bovine adrenal medulla. II. Isolation, partial characterization and biological activity of two distinct molecules. Int. J. Peptide Protein Res., 19, ELLIOT, T.R. (1912). The control of the suprarenal glands by the splanchnic nerves. J. Physiol., 44, EMMELIN, N. & MUREN, A. (1949). Effects of antihistamine compounds on the adrenaline liberation from the suprarenals. Acta physiol. scand., 17, ENDO, Y. & OGWA, Y. (1974). Distribution of histamine in adrenal gland. Jap. J. Pharmac., 24, HANBAUER, I.,GOVONI, S., MAJANE, E.A., YANG, H.-Y.T. & COSTA, E. (1982). In-vivo regulation of Met'enkephalin like peptides from dog adrenal medulla. Adv. Biochem. Psychopharmac., 30, HAPPOLA, O., SIONILA, S., PAIVARINTA, H., JOH, T.H. & PANULA, P. (1985). Histamine-immunoreactive endocrine cells in the adrenal medulla of the rat. Brain Res., 339, HEXUM, T.D., HANBAUER, I., GOVONI, S., YANG, H.-Y.T. & COSTA, E. (1980). Secretion of enkephalin-like peptides from canine adrenal gland following splanchnic nerve stimulation. Neuropeptides, 1, KUMAKURA, K., KAROUM, F., GUIDOTTI, A. & COSTA, E. (1980). Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature, 283, LEMAIRE, S., LIVETT, B., TSENG, R., MERCIER, P. & LEMAIRE, I. (1981). Studies on the inhibitory action of opiate compounds in isolated bovine adrenal chromaffin cells: non-involvement of stereospecific opiate binding sites. J. Neurochem., 36, LEWIS, R.V. & STERN, A.S. (1983). Biosynthesis of the enkephalins and enkephalin-containing polypeptides. A. Rev. Pharmac. Tox., 23, LISH, P.M., ROBBINS, S.I. & PETERS, E.L. (1966). Specificity of antihistamine drugs and involvement of the adrenergic system in histamine deaths in the guinea-pig. J. Pharmac. exp. Ther., 153, LIVETr, B.G. (1984). Adrenal medullary chromaffin cells in vitro. Physiol. Rev., 64, LIVETT, B.G., DEAN, D.M., WHELAN, L.G., UDENFRIEND, S. & ROSSIER, J. (1981). Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature, 289, LIVETT, B.G., DAY, R., ELDE, R.P. & HOWE, P.R.C. (1982). Co-storage of enkephalins and adrenaline in the bovine adrenal medulla. Neuroscience, 1, LIVETT, B.G., MARLEY, P.D., MITCHELHILL, K.I., WAN, D.C.C. & WHITE, T.D. (1986a). Assessment of adrenal chromaffin cell secretion: presentation of four techniques. In The Secretory Process, Vol. 3. ed. Poisner, A.M. & Trifaro, J. Boca Raton, Florida, U.S.A: CRC Press, (in press). LIVETT, B.G., MITCHELHILL, K.l. & DEAN, D.M. (1986b). Adrenal chromaffin cells: their isolation and culture. In The Secretory Process, Vol. 3. ed. Poisner, A.M. & Trifaro, J. Boca Raton, Florida, U.S.A: CRC Press, (in press). MAN, W.K., RAGO, D., MANOLAS, K., WELBOURN, R.B. & SPENCER, J. (1984). Plasma histamine in the pig: effect of food and pentagastrin. Agents & Actions, 14, MARLEY, P.D. & LIVETT, B.G. (1985). Neuropeptides in the autonomic nervous system. CRC. Crit. Rev. Clin. Neurobiol., 1, MARLEY, P.D., MITCHELHILL, K.I. & LIVETT, B.G. (1986a). Effects of opioid peptides containing the sequence of met5-enkephalin or leu5-enkephalin on nicotine-induced secretion from bovine adrenal chromaffin cells. J. Neurochem., 46, MARLEY, P.D., MITCHELHILL, K.I. & LIVETT, B.G. (1986b). Metorphamide, a novel endogenous adrenal opioid peptide, inhibits nicotine-induced secretion from bovine adrenal chromaffin cells. Brain Res., 363, NARITA, S. (1971). Comparative studies on the adrenal medullary and cortical response to histamine. Tohoku J. exp. Med., 104, POISNER, A.M. & DOUGLAS, W.W. (1966). The need for calcium in adrenomedullary secretion evoked by biogenic amines, polypeptides and muscarinic agents. Proc. Soc. exp. Biol. Med., 123, ROBINSON, R.L. (1982). Histamine-induced catecholamine secretion from the cat adrenal medulla is mediated primarily by the HI receptor. Fedn. Proc., 41, ROSSIER, J., DEAN, D.M., LIVElT, B.G. & UDENFRIEND, S. (1981). Enkephalin congeners and precursors are synthesised and released by primary cultures of adrenal chromaf-
8 334 B.G. LIVElT & P.D. MARLEY fin cells. Life Sci., 28, SAIANI, L. & GUIDOTTI, A. (1982). Opiate receptor-mediated inhibition of catecholamine release in primary cultures of bovine adrenal chromaffin cells. J. Neurochem., 39, SCHAEPDRYVER, A.F. DE. (1959). Physio-pharmacological effects on suprarenal secretion of adrenaline and noradrenaline in dogs. Archs. int. Pharmacodyn., 121, SCHNEIDER, F.H. (1969). Secretion from the cortex-free bovine adrenal medulla. Br. J. Pharmac., 37, SCHULTZBERG, M., LUNDBERG, J.M., HOKFELT, T., TERENIUS, L., BRANDT, J., ELDE, R.P. & GOLDSTEIN, M. (1978). Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience, 3, SHIMA, S., KAWASHIMA, Y., HIRAI, M. & KOUYAMA, H. (1976). Studies on cyclic nucleotides in the adrenal gland. V. Adenylate cyclase in the adrenal medulla. Jap. J. Pharmac., 26, SLATER, I.H. & DRESEL, P.E. (1952). The pressor effect of histamine after autonomic ganglionic blockade. J. Pharmac. exp. Ther., 105, SLOTKIN, T.A., SMITH, P.G., LAU, C. & BAREIS, D.I. (1980). Functional aspects of development of catecholamine biosynthesis and release in the sympathetic nervous system. In Biogenic Amines in Development. ed. Parvez, H. & Parvez, S. pp Amsterdam: Elsevier/North Holland. STASZEWSKA-BARCZAK, J. & VANE, J.R. (1965). The release of catecholamines from the adrenal medulla by histamine. Br. J. Pharmac. Chemother., 25, SUZUKI, S., YAMAUCHI, N., MIYAMOTO,T. &MURANAKA, M. (1983). Gold-induced reduction in reactivity to histamine in isolated guinea pig tracheal rings. J. Allergy Clin. Immunol., 72, SZCZYGIELSKI, J. (1932). Die adrenalinabsondermde Wirkung des Histamins und ihre Beeinflussung durch Nikotin. Archs. exp. Path. Pharmak., 166, TRENDELENBURG, U. (1954). The action of histamine and pilocarpine on the superior cervical ganglion and the adrenal glands of the cat. Br. J. Pharmac. Chemother., 9, UDENFRIEND, S. & KILPATRICK, D.L. (1983). Biochemistry of the enkephalins and enkephalin-containing peptides. Archs. biochem. Biophys., 221, WADA, M., FUZII, K., SIBUTA, M. & LI, M-C. (1940). The action of histamine upon the epinephrine secretion from the suprarenal gland in non-anaesthetized dogs. Tohoki J. exp. Med., 37, WEBER, E., ESCH, F.C., BOHLEN, P., PATERSON, S., COR- BETT, A.D., McKNIGHT, A.T., KOSTERLITZ, H.W., BAR- CHAS, J.D. & EVANS, C.J. (1983). Metorphamide; isolation, structure and biologic activity ofan amidated opioid octapeptide from bovine brain. Proc. natn. Acad. Sci. U.S.A., 80, YOSHIZAKI, T. (1973). Effect of histamine, bradykinin and morphine on adrenaline release from rat adrenal glands. Jap. J. Pharmac., 23, (Received February 22, Revised June 5, Accepted June 9, 1986.)
EFFECT OF MUSCARINE ON RELEASE OF CATECHOLAMINES
 Br. J. Pharmac. (1982), 77, 455-46 EFFECT OF MUSCARINE ON RELEASE OF CATECHOLAMINES FROM THE PERFUSED ADRENAL GLAND OF THE CAT S.M. KIRPEKAR, J.C. PRAT & M.T. SCHIAVONE Department of Pharmacology, Downstate
Br. J. Pharmac. (1982), 77, 455-46 EFFECT OF MUSCARINE ON RELEASE OF CATECHOLAMINES FROM THE PERFUSED ADRENAL GLAND OF THE CAT S.M. KIRPEKAR, J.C. PRAT & M.T. SCHIAVONE Department of Pharmacology, Downstate
EFFECT OF HISTAMINE, BRADYKININ AND MORPHINE ON ADRENALINE RELEASE FROM RAT ADRENAL GLAND
 EFFECT OF HISTAMINE, BRADYKININ AND MORPHINE ON ADRENALINE RELEASE FROM RAT ADRENAL GLAND Toshio YOSHIZAKI Shionogi Research Laboratory, Shionogi & Co., Ltd., Fukushima-ku, Osaka, 553 Japan Accepted March
EFFECT OF HISTAMINE, BRADYKININ AND MORPHINE ON ADRENALINE RELEASE FROM RAT ADRENAL GLAND Toshio YOSHIZAKI Shionogi Research Laboratory, Shionogi & Co., Ltd., Fukushima-ku, Osaka, 553 Japan Accepted March
RELEASE OF MEDULLARY AMINES FROM THE ISOLATED PERFUSED ADRENAL GLAND OF THE DOG
 Brit. J. Pharmacol. (1965), 24, 561-565. RELEASE OF MEDULLARY AMINES FROM THE ISOLATED PERFUSED ADRENAL GLAND OF THE DOG BY MARTHE VOGT From the Agricultural Research Council Institute of Animal Physiology,
Brit. J. Pharmacol. (1965), 24, 561-565. RELEASE OF MEDULLARY AMINES FROM THE ISOLATED PERFUSED ADRENAL GLAND OF THE DOG BY MARTHE VOGT From the Agricultural Research Council Institute of Animal Physiology,
A comparison of the sensitivities of innervated and denervated rat vasa deferentia to agonist drugs
 Br. J. Pharmac. (1970), 39, 748-754. A comparison of the sensitivities of innervated and denervated rat vasa deferentia to agonist drugs A. T. BIRMINGHAM*, G. PATRSON AND J. W6JCICKIt Department of Pharmacology,
Br. J. Pharmac. (1970), 39, 748-754. A comparison of the sensitivities of innervated and denervated rat vasa deferentia to agonist drugs A. T. BIRMINGHAM*, G. PATRSON AND J. W6JCICKIt Department of Pharmacology,
Action of drugs on denervated myoepithelial cells of salivary glands
 Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
marked secretion ofcatecholamines and a subsequent inhibition ofsecretion although the basal secretion shows an initial rise.
 J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
suggesting that the release of noradrenaline from sympathetic fibres was dependent on the concentration of Ca2+ outside the fibre.
 214 J. Phy8iol. (1965), 181, pp. 214-223 With 4 text-figurem Printed in Great Britain THE RELEASE OF NORADRENALINE FROM SYMPATHETIC FIBRES IN RELATION TO CALCIUM CONCENTRATION BY J. H. BURN AND W. R. GIBBONS
214 J. Phy8iol. (1965), 181, pp. 214-223 With 4 text-figurem Printed in Great Britain THE RELEASE OF NORADRENALINE FROM SYMPATHETIC FIBRES IN RELATION TO CALCIUM CONCENTRATION BY J. H. BURN AND W. R. GIBBONS
PHARMACOLOGICAL STUDY OF THE ANOCOCCYGEUS MUSCLE OF
 Br. J. Pharmac. (198). 71, 35-4 PHARMACOLOGICAL STUDY OF TH ANOCOCCYGUS MUSCL OF TH DOG A.R. DHPOUR, M.A. KHOYI, H. KOUTCHKI & M.R. ZARRINDAST Department of Pharmacology, Faculty of Medicine, University
Br. J. Pharmac. (198). 71, 35-4 PHARMACOLOGICAL STUDY OF TH ANOCOCCYGUS MUSCL OF TH DOG A.R. DHPOUR, M.A. KHOYI, H. KOUTCHKI & M.R. ZARRINDAST Department of Pharmacology, Faculty of Medicine, University
THE RELEASE OF CATECHOL AMINES FROM THE
 Brit. J. Pharmacol. (1965), 25, 728-742. THE RELEASE OF CATECHOL AMINES FROM THE ADRENAL MEDULLA BY HISTAMINE BY JANINA STASZEWSKA-BARCZAK* AND J. R. VANE From the Department of Pharmacology, Institute
Brit. J. Pharmacol. (1965), 25, 728-742. THE RELEASE OF CATECHOL AMINES FROM THE ADRENAL MEDULLA BY HISTAMINE BY JANINA STASZEWSKA-BARCZAK* AND J. R. VANE From the Department of Pharmacology, Institute
Effect of the endogenous analgesic dipeptide, kyotorphin, on transmitter release in sympathetic
 Br. J. Pharmac. (1985), 85, 629-634 Effect of the endogenous analgesic dipeptide, kyotorphin, on transmitter release in sympathetic ganglia K. Hirai & Y. Katayama Department of Autonomic Physiology, Medical
Br. J. Pharmac. (1985), 85, 629-634 Effect of the endogenous analgesic dipeptide, kyotorphin, on transmitter release in sympathetic ganglia K. Hirai & Y. Katayama Department of Autonomic Physiology, Medical
EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES
 Brit. J. Pharmacol. (1960), 15, 328. EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES BY B. C. R. STROMBLAD From the Institute of Physiology, Lund, Sweden (RECEIVED FEBRUARY
Brit. J. Pharmacol. (1960), 15, 328. EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES BY B. C. R. STROMBLAD From the Institute of Physiology, Lund, Sweden (RECEIVED FEBRUARY
THE ACTION OF NICOTINE ON THE CILIARY GANGLION
 Brit. J. Pharmnacol. (1952), 7, 665. THE ACTION OF NICOTINE ON THE CILIARY GANGLION BY BRENDA M. SCHOFIELD From the Department of Pharmacology, University of Oxford (Received June 7, 1952) The existing
Brit. J. Pharmnacol. (1952), 7, 665. THE ACTION OF NICOTINE ON THE CILIARY GANGLION BY BRENDA M. SCHOFIELD From the Department of Pharmacology, University of Oxford (Received June 7, 1952) The existing
Effect of cocaine on the affinity of a-adrenoceptors for noradrenaline
 Br. J. Pharmac. (1973), 48, 139-143. Effect of cocaine on the affinity of a-adrenoceptors for noradrenaline I. R. INNES AND R. MAILHOT* Department of Pharmacology and Therapeutics, Faculty of Medicine,
Br. J. Pharmac. (1973), 48, 139-143. Effect of cocaine on the affinity of a-adrenoceptors for noradrenaline I. R. INNES AND R. MAILHOT* Department of Pharmacology and Therapeutics, Faculty of Medicine,
ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES
 Vol. 4, No. 1, September 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 61-66 ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES Kyoji Morita ~)*,
Vol. 4, No. 1, September 1996 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 61-66 ENHANCEMENT BY F-ACTIN OF MGATP-DEPENDENT DOPAMINE UPTAKE INTO ISOLATED CHROMAFFIN GRANULES Kyoji Morita ~)*,
EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE
 Br. J. Pharmac. (1981), 73,829-835 EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE RESPONSES TO CHOLINOMIMETICS IN THE RAT ANOCOCCYGEUS MUSCLE SHEILA A. DOGGRELL Department of Pharmacology & Clinical
Br. J. Pharmac. (1981), 73,829-835 EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE RESPONSES TO CHOLINOMIMETICS IN THE RAT ANOCOCCYGEUS MUSCLE SHEILA A. DOGGRELL Department of Pharmacology & Clinical
Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit
 Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
A NEW TYPE OF DRUG ENHANCEMENT: INCREASED MAXIMUM RESPONSE TO CUMULATIVE NORADREN- ALINE IN THE ISOLATED RAT VAS DEFERENS
 Br. J. Pharmac. Chemother. (1968), 33, 171-176. A NEW TYPE OF DRUG ENHANCEMENT: NCREASED MAXMUM RESPONSE TO CUMULATVE NORADREN- ALNE N THE SOLATED RAT VAS DEFERENS BY A. BARNETT, D. D. GREENHOUSE AND R..
Br. J. Pharmac. Chemother. (1968), 33, 171-176. A NEW TYPE OF DRUG ENHANCEMENT: NCREASED MAXMUM RESPONSE TO CUMULATVE NORADREN- ALNE N THE SOLATED RAT VAS DEFERENS BY A. BARNETT, D. D. GREENHOUSE AND R..
MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE
 Br. J. Pharmac. Chemother. (1968), 32, 539-545. MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE BY K. SHIMAMOTO AND N. TODA From the Department
Br. J. Pharmac. Chemother. (1968), 32, 539-545. MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE BY K. SHIMAMOTO AND N. TODA From the Department
Screening and bioassay of Sympatholytics. Dr. Magdy M. Awny Lecture 4
 Screening and bioassay of Sympatholytics by Dr. Magdy M. Awny Lecture 4 1 They are classified into: Sympatholytics = Antagonist of adrenergic activity Drugs that interfere with the activity of the sympathetic
Screening and bioassay of Sympatholytics by Dr. Magdy M. Awny Lecture 4 1 They are classified into: Sympatholytics = Antagonist of adrenergic activity Drugs that interfere with the activity of the sympathetic
gland, the tongue and the sweat glands of the cat. The submaxillary
 306 547.435-292:6I2.8I7 THE LIBERATION OF ACETYLCHOLINE BY POTASSIUM. BY W. FELDBERG1 AND J. A. GUIMARAIS1,2. (From the National Institute for Medical Research, London, N.W. 3.) (Received November 22,
306 547.435-292:6I2.8I7 THE LIBERATION OF ACETYLCHOLINE BY POTASSIUM. BY W. FELDBERG1 AND J. A. GUIMARAIS1,2. (From the National Institute for Medical Research, London, N.W. 3.) (Received November 22,
ROLE OF ADRENAL HORMONES IN MAINTAINING TISSUE STORES OF NORADRENALINE DURING INCREASED SYMPATHETIC ACTIVITY
 Br. J. Pharmac. Chemother. (1966), 27, 532-535. ROLE OF ADRENAL HORMONES IN MAINTAINING TISSUE STORES OF NORADRENALINE DURING INCREASED SYMPATHETIC ACTIVITY BY V. M. AVAKIAN* AND MARTHE VOGT From the Agricultural
Br. J. Pharmac. Chemother. (1966), 27, 532-535. ROLE OF ADRENAL HORMONES IN MAINTAINING TISSUE STORES OF NORADRENALINE DURING INCREASED SYMPATHETIC ACTIVITY BY V. M. AVAKIAN* AND MARTHE VOGT From the Agricultural
potential, of changes in the ionic environment that are known, from other
 J. Phy8iol. (1967), 191, pp. 107-121 107 With 8 text-fitgurew Printed in Great Britai'n INFLUENCE OF THE IONIC ENVIRONMENT ON THE MEMBRANE POTENTIAL OF ADRENAL CHROMAFFIN CELLS AND ON THE DEPOLARIZING
J. Phy8iol. (1967), 191, pp. 107-121 107 With 8 text-fitgurew Printed in Great Britai'n INFLUENCE OF THE IONIC ENVIRONMENT ON THE MEMBRANE POTENTIAL OF ADRENAL CHROMAFFIN CELLS AND ON THE DEPOLARIZING
THE RELEASE OF ADRENALINE AND NORADRENALINE FROM THE ADRENAL GLAND OF THE CAT BY ACETYLCHOLINE
 Brit. J. Pharmacol. (1957), 12, 422. THE RELEASE OF ADRENALNE AND NORADRENALNE FROM THE ADRENAL GLAND OF THE CAT BY ACETYLCHOLNE BY K. R. BUTTERWORTH* AND MONCA MANN From the Department of Pharmacology,
Brit. J. Pharmacol. (1957), 12, 422. THE RELEASE OF ADRENALNE AND NORADRENALNE FROM THE ADRENAL GLAND OF THE CAT BY ACETYLCHOLNE BY K. R. BUTTERWORTH* AND MONCA MANN From the Department of Pharmacology,
Myocardial Opiate Receptors
 Gen. Physiol. Biophys. 1982, 1, 447-452 Myocardial Opiate Receptors M. E. SAXON, G. R. IVANITSKY, F. F. BELOYARTSEV, V. G. SAFRONOVA, YU. M. KOKOZ and A. A. FREYDIN Institute of Biological Physics, Academy
Gen. Physiol. Biophys. 1982, 1, 447-452 Myocardial Opiate Receptors M. E. SAXON, G. R. IVANITSKY, F. F. BELOYARTSEV, V. G. SAFRONOVA, YU. M. KOKOZ and A. A. FREYDIN Institute of Biological Physics, Academy
Goals and Challenges of Communication. Communication and Signal Transduction. How Do Cells Communicate?
 Goals and Challenges of Communication Reaching (only) the correct recipient(s) Imparting correct information Timeliness Causing the desired effect Effective termination Communication and Signal Transduction
Goals and Challenges of Communication Reaching (only) the correct recipient(s) Imparting correct information Timeliness Causing the desired effect Effective termination Communication and Signal Transduction
PHRM20001: Pharmacology - How Drugs Work!
 PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
Effect of frequency of stimulation on the inhibition by noradrenaline of the acetylcholine output from
 Br. J. Pharmac. (1971), 41, 263-272. Effect of frequency of stimulation on the inhibition by noradrenaline of the acetylcholine output from parasympathetic nerve terminals J. KNOLL AND E. S. VIZI Department
Br. J. Pharmac. (1971), 41, 263-272. Effect of frequency of stimulation on the inhibition by noradrenaline of the acetylcholine output from parasympathetic nerve terminals J. KNOLL AND E. S. VIZI Department
tetrodotoxin. jejunum which inhibited the myogenic spontaneous activity blocked did not reduce the output of acetylcholine.
 J. Phy8iol. (1967), 189, pp. 317-327 317 With 4 text-figure8 Printed in Great Britain INHIBITION OF GASTROINTESTINAL MOVEMENT BY SYMPATHETIC NERVE STIMULATION: THE SITE OF ACTION BY M. D. GERSHON From
J. Phy8iol. (1967), 189, pp. 317-327 317 With 4 text-figure8 Printed in Great Britain INHIBITION OF GASTROINTESTINAL MOVEMENT BY SYMPATHETIC NERVE STIMULATION: THE SITE OF ACTION BY M. D. GERSHON From
Basics of Pharmacology
 Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog
 Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
Tohoku J. exp. Med., 1966, 88, 361-366 Effect of Muscular Exercise on Adrenaline and Noradrenaline Secretion of the Adrenal Gland in the Dog Sennosuke Ohukuzi Deparment of Physiology (Prof. T. Suzuki),
BIOACTIVE PEPTIDES: HORMONES, TRANSMITTERS, OR MODULATORS
 UDK 577.112.85 Pregledni rad Revievo BIOACTIVE PEPTIDES: HORMONES, TRANSMITTERS, OR MODULATORS Oren Zinder Department of Clinical Biochemistry, Rambam Medical Center, Haifa, Israel INTRODUCTION Communication
UDK 577.112.85 Pregledni rad Revievo BIOACTIVE PEPTIDES: HORMONES, TRANSMITTERS, OR MODULATORS Oren Zinder Department of Clinical Biochemistry, Rambam Medical Center, Haifa, Israel INTRODUCTION Communication
Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria
 Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria Shigetoshi CHIBA, M.D., Yasuyuki FURUKAWA, M.D., and Hidehiko WATANABE, M.D. SUMMARY Using the isolated
Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria Shigetoshi CHIBA, M.D., Yasuyuki FURUKAWA, M.D., and Hidehiko WATANABE, M.D. SUMMARY Using the isolated
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION
 Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
DEPLETION AND REPLACEMENT OF THE ADRENALINE AND NORADRENALINE CONTENTS OF THE RAT ADRENAL GLAND, FOLLOWING TREATMENT WITH RESERPINE
 Brit. J. Pharmacol. (1962), 18, 138-149. DEPLETION AND REPLACEMENT OF THE ADRENALINE AND NORADRENALINE CONTENTS OF THE RAT ADRENAL GLAND, FOLLOWING TREATMENT WITH RESERPINE BY B. A. CALLINGHAM AND MONICA
Brit. J. Pharmacol. (1962), 18, 138-149. DEPLETION AND REPLACEMENT OF THE ADRENALINE AND NORADRENALINE CONTENTS OF THE RAT ADRENAL GLAND, FOLLOWING TREATMENT WITH RESERPINE BY B. A. CALLINGHAM AND MONICA
Autonomic Nervous System. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS
 Brit. J. Pharmacol. (1964), 22, 119-125. LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS BY N. ASSARSON AND N. EMMELIN From the Institute of Physiology, University of Lund, Sweden (Received October 8, 1963)
Brit. J. Pharmacol. (1964), 22, 119-125. LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS BY N. ASSARSON AND N. EMMELIN From the Institute of Physiology, University of Lund, Sweden (Received October 8, 1963)
Physiological processes in the GI tract:
 Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
The Nervous System and Metabolism
 = P1: JZP 8 The Nervous System and Metabolism Dendrites Cell body Axon (may be sheathed in myelin) Nucleus Axonal terminals (synapses) Figure 8.1 Basic structure of a nerve cell (neuron). CH 3_ CH 3 CH
= P1: JZP 8 The Nervous System and Metabolism Dendrites Cell body Axon (may be sheathed in myelin) Nucleus Axonal terminals (synapses) Figure 8.1 Basic structure of a nerve cell (neuron). CH 3_ CH 3 CH
Module Objectives: Why to study Organic Pharmaceutical Chemistry? 24-Oct-17
 1 2 Useful Info: Students are encouraged to visit the faculty website: http://pharmacy.uokerbala.edu.iq/ And most importantly the e-learning website: http://elearning.uokerbala.edu.iq Nevertheless, feel
1 2 Useful Info: Students are encouraged to visit the faculty website: http://pharmacy.uokerbala.edu.iq/ And most importantly the e-learning website: http://elearning.uokerbala.edu.iq Nevertheless, feel
Introduction to Autonomic
 Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
CAROTID SINUS REFLEX AND CONTRACTION
 Brit. J. Pharmacol. (1950), 5, 505. CAROTID SINUS REFLEX AND CONTRACTION OF THE SPLEEN BY ROBERT L. DRIVER AND MARTHE VOGT From the Department of Pharmacology, University of Edinburgh (Received July 12,
Brit. J. Pharmacol. (1950), 5, 505. CAROTID SINUS REFLEX AND CONTRACTION OF THE SPLEEN BY ROBERT L. DRIVER AND MARTHE VOGT From the Department of Pharmacology, University of Edinburgh (Received July 12,
T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland
 AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
I. Neural Control of Involuntary Effectors. Chapter 9. Autonomic Motor Nerves. Autonomic Neurons. Autonomic Ganglia. Autonomic Neurons 9/19/11
 Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Lujain Hamdan. Ayman Musleh & Yahya Salem. Mohammed khatatbeh
 12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog
 Br. J. Pharmac. (1971), 41, 1-7 Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog B. N. DAVIES ADi P. G. WITHRINGTON Department of Physiology, Medical College of
Br. J. Pharmac. (1971), 41, 1-7 Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog B. N. DAVIES ADi P. G. WITHRINGTON Department of Physiology, Medical College of
capsaicin-pre-treated rats than in controls, indicating that SP-containing capsaicinsensitive
 Journal of Phy8iology (199), 425, pp. 321-334 321 With 6figurem Printed in Great Britain SUBSTANCE P INCREASES CATECHOLAMINE SECRETION FROM PERFUSED RAT ADRENAL GLANDS EVOKED BY PROLONGED FIELD STIMULATION
Journal of Phy8iology (199), 425, pp. 321-334 321 With 6figurem Printed in Great Britain SUBSTANCE P INCREASES CATECHOLAMINE SECRETION FROM PERFUSED RAT ADRENAL GLANDS EVOKED BY PROLONGED FIELD STIMULATION
ISOLATED CHROMAFFIN GRANULES BY
 Brit. J. Pharmacol. (1957), 12, 61. THE RELEASE OF CATECHOL AMINES FROM ISOLATED CHROMAFFIN GRANULES BY From the Department of Pharmacology, University of Oxford (RECEIVED SEPTEMBER 24, 1956) Release of
Brit. J. Pharmacol. (1957), 12, 61. THE RELEASE OF CATECHOL AMINES FROM ISOLATED CHROMAFFIN GRANULES BY From the Department of Pharmacology, University of Oxford (RECEIVED SEPTEMBER 24, 1956) Release of
Nervous and chemical regulation heart
 Nervous and chemical regulation heart Myogenic heart when separated out from the body keep on beating on their own but when in the body the rate of heart beat is modified through various stimuli such as
Nervous and chemical regulation heart Myogenic heart when separated out from the body keep on beating on their own but when in the body the rate of heart beat is modified through various stimuli such as
THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS
 Br. J. Phannac. Chemother. (1968), 33, 531-536. THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS BY A. L. BARTLET AND T. HASSAN From the Department of Veterinary
Br. J. Phannac. Chemother. (1968), 33, 531-536. THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS BY A. L. BARTLET AND T. HASSAN From the Department of Veterinary
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
2401 : Anatomy/Physiology
 Dr. Chris Doumen Week 11 2401 : Anatomy/Physiology Autonomic Nervous System TextBook Readings Pages 533 through 552 Make use of the figures in your textbook ; a picture is worth a thousand words! Work
Dr. Chris Doumen Week 11 2401 : Anatomy/Physiology Autonomic Nervous System TextBook Readings Pages 533 through 552 Make use of the figures in your textbook ; a picture is worth a thousand words! Work
(Received 14 February 1951)
 510 J. Physiol. (I95I) II4, 5I0-54 PHYSIOLOGICAL SIGNIFICANCE OF THE SWEAT RESPONSE TO ADRENALINE IN MAN BY T. M. CHALMERS jam C. A. KEELE From the Department of Pharmacology, Middlesex Hospital Medical
510 J. Physiol. (I95I) II4, 5I0-54 PHYSIOLOGICAL SIGNIFICANCE OF THE SWEAT RESPONSE TO ADRENALINE IN MAN BY T. M. CHALMERS jam C. A. KEELE From the Department of Pharmacology, Middlesex Hospital Medical
Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type
 Br. J. Pharmac. (1971), 43, 814-818. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type C. GORIDIS AND N. H. NEFF Laboratory of Preclinical Pharmacology, National Institute of
Br. J. Pharmac. (1971), 43, 814-818. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type C. GORIDIS AND N. H. NEFF Laboratory of Preclinical Pharmacology, National Institute of
The Autonomic Nervous System Outline of class lecture for Physiology
 The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM
 ANALELE UNIVERSITATII DIN ORADEA, Fascicula Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentara THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM Gavril Paul, Ioana
ANALELE UNIVERSITATII DIN ORADEA, Fascicula Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentara THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM Gavril Paul, Ioana
A STUDY OF THE ROLE OF BRAIN CATECHOLAMINES IN DRUG INDUCED TREMOR
 Br. J. Pharmac. Chemother. (1967), 3, 349-353. A STUDY OF THE ROLE OF BRAIN CATECHOLAMINES IN DRUG INDUCED TREMOR BY S. L. AGARWAL AND D. BOSE From the Department of Pharmacology, M.G.M. Medical College,
Br. J. Pharmac. Chemother. (1967), 3, 349-353. A STUDY OF THE ROLE OF BRAIN CATECHOLAMINES IN DRUG INDUCED TREMOR BY S. L. AGARWAL AND D. BOSE From the Department of Pharmacology, M.G.M. Medical College,
Department of Physiology, Okayama University Medical School
 The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE
 Brit. J. Pharmacol. (1960), 15, 356. SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE BY N. EMMELIN From the Institute of Physiology, University
Brit. J. Pharmacol. (1960), 15, 356. SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE BY N. EMMELIN From the Institute of Physiology, University
Ouabain distinguishes between nicotinic and muscarinic receptor-mediated catecholamine secretions
 Br. J. Pharmacol. (1989), 96, 470-479 Ouabain distinguishes between nicotinic and muscarinic receptor-mediated catecholamine secretions in perfused adrenal glands of cat Yutaka Yamada, 'Yoshikazu Nakazato
Br. J. Pharmacol. (1989), 96, 470-479 Ouabain distinguishes between nicotinic and muscarinic receptor-mediated catecholamine secretions in perfused adrenal glands of cat Yutaka Yamada, 'Yoshikazu Nakazato
Ch 9. The Autonomic Nervous System
 Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Chapter 41. Lecture 14. Animal Hormones. Dr. Chris Faulkes
 Chapter 41 Lecture 14 Animal Hormones Dr. Chris Faulkes Animal Hormones Aims: To appreciate the variety and roles of hormones in the body To understand the basic types of hormones To understand how hormones
Chapter 41 Lecture 14 Animal Hormones Dr. Chris Faulkes Animal Hormones Aims: To appreciate the variety and roles of hormones in the body To understand the basic types of hormones To understand how hormones
INHIBITION OF THE ACETYLCHOLINE RECEPTOR BY HISTRIONICOTOXIN
 Br. J. Pharmac. (1980), 68, 611-615 INHIBITION OF THE ACETYLCHOLINE RECEPTOR BY HISTRIONICOTOXIN ROGER ANWYL' & TOSHIO NARAHASHI Department of Pharmacology, Northwestern University Medical School, 303
Br. J. Pharmac. (1980), 68, 611-615 INHIBITION OF THE ACETYLCHOLINE RECEPTOR BY HISTRIONICOTOXIN ROGER ANWYL' & TOSHIO NARAHASHI Department of Pharmacology, Northwestern University Medical School, 303
THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM
 Brit. J. Pharinacol. (1963), 20, 171-177. THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM BY G. F. ABERCROMBIE AND B. N. DAVIES From the Department of Physiology,
Brit. J. Pharinacol. (1963), 20, 171-177. THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM BY G. F. ABERCROMBIE AND B. N. DAVIES From the Department of Physiology,
The Nervous System Mark Stanford, Ph.D.
 The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
INTRAVENOUS MORPHINE IN THE
 Brit. J. Pharmacol. (1952), 7, 542. THE FALL OF BLOOD PRESSURE CAUSED BY INTRAVENOUS MORPHINE IN THE RAT AND THE CAT BY A. G. J. EVANS, P. A. NASMYTH, AND H. C. STEWART From the Department of Pharmacology,
Brit. J. Pharmacol. (1952), 7, 542. THE FALL OF BLOOD PRESSURE CAUSED BY INTRAVENOUS MORPHINE IN THE RAT AND THE CAT BY A. G. J. EVANS, P. A. NASMYTH, AND H. C. STEWART From the Department of Pharmacology,
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
ENZYMES AND THEIR SUBSTRATES IN THE ADRENAL GLAND OF THE OX
 Brit. J. Pharmacol. (1951), 6, 318. ENZYMES AND THEIR SUBSTRATES IN THE ADRENAL GLAND OF THE OX BY H. LANGEMANN* From the Department of Pharmacology, University of Oxford- (Received March 19, 1951) Since
Brit. J. Pharmacol. (1951), 6, 318. ENZYMES AND THEIR SUBSTRATES IN THE ADRENAL GLAND OF THE OX BY H. LANGEMANN* From the Department of Pharmacology, University of Oxford- (Received March 19, 1951) Since
University of Groningen. Melatonin on-line Drijfhout, Willem Jan
 University of Groningen Melatonin on-line Drijfhout, Willem Jan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Melatonin on-line Drijfhout, Willem Jan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Glucose-deprivation and nitrogen, when imposed either separately or
 J. Phyaiol. (1969), 202, pp. 197-209 197 With 2 text-ftgurem Printed in Great Britain THE METABOLIC REQUIREMENTS FOR CATECHOLAMINE RELEASE FROM THE ADRENAL MEDULLA BY R. P. RUBIN From the Department of
J. Phyaiol. (1969), 202, pp. 197-209 197 With 2 text-ftgurem Printed in Great Britain THE METABOLIC REQUIREMENTS FOR CATECHOLAMINE RELEASE FROM THE ADRENAL MEDULLA BY R. P. RUBIN From the Department of
HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES
 HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES TERUO NAKAYAMA* Institute of Physiology, School of Medicine, University of Nagoya It is known that electrical
HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES TERUO NAKAYAMA* Institute of Physiology, School of Medicine, University of Nagoya It is known that electrical
Pharmacological significance of biogenic amines in the lungs: noradrenaline and dopanine
 Br. J. Pharmac. (1970), 38, 374-385. Pharmacological significance of biogenic amines in the lungs: noradrenaline and dopanine DOMINGO M. AVIADO AND CHIRAVAT SADAVONGVIVAD* Department of Pharmacology, School
Br. J. Pharmac. (1970), 38, 374-385. Pharmacological significance of biogenic amines in the lungs: noradrenaline and dopanine DOMINGO M. AVIADO AND CHIRAVAT SADAVONGVIVAD* Department of Pharmacology, School
Chapter 6 Communication, Integration, and Homeostasis
 Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Drug Receptor Interactions and Pharmacodynamics
 Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Neurotransmitters. Chemical transmission of a nerve signal by neurotransmitters at a synapse
 Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Vets 111/Biov 111 Cell Signalling-1. Hormones, Neurotransmitters and Local Mediators
 Vets 111/Biov 111 Cell Signalling-1 Hormones, Neurotransmitters and Local Mediators Why do cells need to communicate and signal? In unicellular organisms every cell is capable of the full range of biochemical
Vets 111/Biov 111 Cell Signalling-1 Hormones, Neurotransmitters and Local Mediators Why do cells need to communicate and signal? In unicellular organisms every cell is capable of the full range of biochemical
Autonomic Nervous System (ANS):
 Autonomic Nervous System (ANS): ANS is the major involuntary, unconscious, automatic portion of the nervous system. involuntary voluntary The motor (efferent)portion of the ANS is the major pathway for
Autonomic Nervous System (ANS): ANS is the major involuntary, unconscious, automatic portion of the nervous system. involuntary voluntary The motor (efferent)portion of the ANS is the major pathway for
Receptors and Drug Action. Dr. Subasini Pharmacology Department Ishik University, Erbil
 Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Close to site of release (at synapse); binds to receptors in
 Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
THE LACK OF CROSSED TACHYPHYLAXIS BETWEEN TYRAMINE AND SOME OTHER INDIRECTLY ACTING SYMPATHOMIMETIC AMINES
 Br. J. Pharmac. Chenother. (1967). 30, 631-643. THE LACK OF CROSSED TACHYPHYLAXIS BETWEEN TYRAMINE AND SOME OTHER INDIRECTLY ACTING SYMPATHOMIMETIC AMINES BY M. D. DAY* From the Pharmacology Laboratories,
Br. J. Pharmac. Chenother. (1967). 30, 631-643. THE LACK OF CROSSED TACHYPHYLAXIS BETWEEN TYRAMINE AND SOME OTHER INDIRECTLY ACTING SYMPATHOMIMETIC AMINES BY M. D. DAY* From the Pharmacology Laboratories,
Nervous System Communication. Nervous System Communication. The First Nerve Cells 1/2/11
 Nervous System Communication Nervous System Communication Process information Transfer information to other neurons Generate behavior and experience The First Nerve Cells Developed in primitive animals
Nervous System Communication Nervous System Communication Process information Transfer information to other neurons Generate behavior and experience The First Nerve Cells Developed in primitive animals
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
I. OVERVIEW DIRECT. Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of
 THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
General organization of central and peripheral components of the nervous system
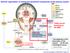 General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
Autonomic nervous system
 Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
blood-vessels of the isolated perfused lungs of the rat. Both Hirakawa
 547.435-292: 547.781.5: 577.174.5: 612.215 THE ACTION OF ADRENALINE, ACETYLCHOLINE, AND HIS- TAMINE ON THE LUNGS OF THE RAT. By P. FoGGIE. From the Physiology Department, University of Edinburgh. (Received
547.435-292: 547.781.5: 577.174.5: 612.215 THE ACTION OF ADRENALINE, ACETYLCHOLINE, AND HIS- TAMINE ON THE LUNGS OF THE RAT. By P. FoGGIE. From the Physiology Department, University of Edinburgh. (Received
INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE
 Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1
 GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
BIOL 2458 A&P II CHAPTER 18 SI Both the system and the endocrine system affect all body cells.
 BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD
 AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
Neuron types and Neurotransmitters
 Neuron types and Neurotransmitters Faisal I. Mohammed. PhD, MD University of Jordan 1 Transmission of Receptor Information to the Brain the larger the nerve fiber diameter the faster the rate of transmission
Neuron types and Neurotransmitters Faisal I. Mohammed. PhD, MD University of Jordan 1 Transmission of Receptor Information to the Brain the larger the nerve fiber diameter the faster the rate of transmission
Interrelationship between Angiotensin Catecholamines. Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D.
 Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
ACETYLCHOLINE IN A SYMPATHETIC GANGLION
 Br. J. Pharmac. (1975), 55, 189-197 STUDIES UPON THE MECHANISM BY WHICH ACETYLCHOLINE RELEASES SURPLUS ACETYLCHOLINE IN A SYMPATHETIC GANGLION B. COLLIER & H.S. KATZ Department of Pharmacology and Therapeutics,
Br. J. Pharmac. (1975), 55, 189-197 STUDIES UPON THE MECHANISM BY WHICH ACETYLCHOLINE RELEASES SURPLUS ACETYLCHOLINE IN A SYMPATHETIC GANGLION B. COLLIER & H.S. KATZ Department of Pharmacology and Therapeutics,
INTERACTIONS BETWEEN COCAINE, TYRAMINE AND NORADRENALINE AT THE NORADRENALINE STORE
 Brit. J. Pharmacol. (1963), 2, 54-549. INTERACTIONS BETWEEN COCAINE, TYRAMINE AND NORADRENALINE AT THE NORADRENALINE STORE BY J. FARRANT* From the Department of Pharmacology, School of Pharmacy, University
Brit. J. Pharmacol. (1963), 2, 54-549. INTERACTIONS BETWEEN COCAINE, TYRAMINE AND NORADRENALINE AT THE NORADRENALINE STORE BY J. FARRANT* From the Department of Pharmacology, School of Pharmacy, University
THE CARDIOVASCULAR EFFECTS OF ERGOMETRINE IN THE EXPERIMENTAL ANIMAL IN VIVO AND IN VITRO
 Br. J. Anaesth. (1974), 46, 473 THE CARDIOVASCULAR EFFECTS OF ERGOMETRINE IN THE EXPERIMENTAL ANIMAL IN VIVO AND IN VITRO M. R. WASSEF, H. LAL AND BARBARA J. PLEUVRY SUMMARY The cardiovascular effects
Br. J. Anaesth. (1974), 46, 473 THE CARDIOVASCULAR EFFECTS OF ERGOMETRINE IN THE EXPERIMENTAL ANIMAL IN VIVO AND IN VITRO M. R. WASSEF, H. LAL AND BARBARA J. PLEUVRY SUMMARY The cardiovascular effects
Integrated Cardiopulmonary Pharmacology Third Edition
 Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Autonomic Regulation of The Cardiovascular system. Cardiac
 Autonomic Regulation of The Cardiovascular system. Cardiac 1. Components of Regulation. 2. Autonomic Component. 3. Receptors. 4. Cardiac, Vascular (Systemic Pulm). 5. Comparative (anatomy). 6. Assessment.
Autonomic Regulation of The Cardiovascular system. Cardiac 1. Components of Regulation. 2. Autonomic Component. 3. Receptors. 4. Cardiac, Vascular (Systemic Pulm). 5. Comparative (anatomy). 6. Assessment.
