Structure-Activity Relationship (SAR) of the Phenethylamines: A Focus on the Basics
|
|
|
- Cora Caldwell
- 6 years ago
- Views:
Transcription
1 Structure-Activity Relationship (SAR) of the Phenethylamines: A Focus on the Basics Rob Palmer Toxicology Associates, PLLC Rocky Mountain Poison & Drug Center University of Colorado School of Medicine Disclaimer This is not a complete compendium of all substituted amphetamine derivatives and not all SAR twists and turns are covered I hope to distill the essence of SAR studies into an understandable block The goal is to provide some useful SAR tools for predicting biological activity of a phenethylamine molecule Many references are combined for a given concept What is SAR? The relationship between the threedimensional structure of a molecule and its biological action. What happens to the pharmacology of a molecule when we add to, subtract from or tweak its substituents and geometry? 1
2 Just Nine Little Carbons 2-Carbon Bridge Amine (Basic N) Aromatic Ring Phenethylamine Description Alpha-methylphenethylamine Amphetamine Alpha-methylphenethylamine 1-phenyl-2-aminopropane Phenylisopropylamine Initial SAR β α 2-Phenethylamine Amphetamine 2-Phenethylamine largely lacks CNS effects with systemic administration Little difference in lipophilicity between the two compounds Both are capable of CNS penetration 2
3 Initial SAR 2-Phenethylamine No α-ch 3 group Lacks central effects on systemic administration Rapidly degraded by MAO Amphetamine α-methyl group yields amphetamine Has central effects Poor MAO substrate Stereocenter in the side chain Basic Flavors of Amphetamines Catecholamine Releasers Serotonin Releasers Hallucinogenic Compounds Stereochemistry Reminder How does this work, again? R / S = D / L Absolute Configuration Determined by structure d / l = + / - Optical Activity Experimentally determined) Absolute configuration DOES NOT predict optical activity!!!!! 3
4 Stereochemistry of the α-carbon S (+)-amphetamine is more potent amphetamine-like enantiomer More on what this means in a bit There is a 4 10 fold stereoselective preference depending on assay used Catecholamine Releasers: Ring Substitution ortho- meta- para- Rats trained to distinguish 1 mg/kg (+)-AMP from saline ED 50 for (+)-AMP = 0.42 mg/kg ED 50 for ortho-ch 3 -AMP = 4.1 mg/kg meta- and para- require MUCH higher doses Recall that ortho- is the 2-position, meta- is 3 and para- is 4. Catecholamine Releasers: N-Substitution R 1 R 2 Potency (%) H H 100 H CH H CH 2 CH 3 50 H CH 2 CH 2 CH 3 50 CH 3 CH 3 20 N-Et and N-OH give nearly complete substitution in (+)-AMP trained rats Tertiary amines are dealkylated to more potent secondary amines in vivo Monoamine transport systems can accommodate either primary or N-methyl substituted amines 4
5 Catecholamine Releasers: Benzylic Carbon Oxidation S-(+)-Amphetamine Cathinone Generally good retention of activity ED 50 values are about equal for cathinone and amphetamine Rats trained on saline vs (+)-AMP showed (+/-)-cathinone to be equipotent to (+)-AMP More potent congeners have S- absolute configuration at the α-carbon In (+)-AMP trained rats, S- is about double racemic potency and R- is ~30% Catecholamine Releasers: Diethylpropion Diethylpropion Ethcathione Diethylpropion has low monoamine transporter affinity Prodrug for ethcathinone Ethcathinone has weak 5-HT & DA activity NE effects are fold greater Selective NE releasing agent Catecholamine Releasers: Benzylic Carbon Oxidation Cathinone Ephedrine Norepinephrine Adding a second stereocenter creates diastereomers Pseudoephedrine Ephedrine R-absolute configuration has greatest affinity at adrenergic receptors 5
6 Catecholamine Releasers: Morpholines Phenmetrazine Phendimetrazine Reduction of benzylic ketone and cyclization into morpholine ring Phenmetrazine and phendimetrazine Certainly are CNS stimulants, but structurally not phenethylamines Not further addressed today Catecholamine Releasers: Side-Chain Modification α-ethylamphetamine α-ethylmethamphetamine Simple methyl to ethyl change attenuates amphetamine activity (+)-α-ethyl homolog of AMP does not produce full substitution in (+)-AMP trained rats Racemic α-ethyl METH homolog gives full substitution but had only 10% of the potency of AMP Catecholamine Releasers: Rigid Side Chain Compounds Aminoindan Aminotetralin Cycloheptane 2-Aminoindan (ED mg/kg) fully substitutes for (+)-AMP (0.42 mg/kg) in rats Different investigator saw only 75% of AMP response at triple the dose Variability in tetralin reports, but is only about 12 30% potency of AMP Cycloheptane derivative does not give an AMP-appropriate response 6
7 Catecholamine Releasers: Mechanism of Action An abbreviated (possible) mechanism AMP (a weak base) reduces intracellular ph gradient of synaptic vesicles Once buffering capacity is exceeded, the lack of a proton gradient reduces transmitter uptake driving force Deprotonated catecholamine may diffuse from vesicle along conc gradient Elevated cytosolic monoamine may be released from cell by reversal of uptake pump Catecholamine Releasers: SAR Summary Amphetamine Methamphetamine Using AMP as the model, very little structural alteration is allowed N-CH 3 (METH) increases potency ~2-fold S-configuration at α-carbon is preferred Assume the primary effect of interest is drug-induced efflux of neuronal catecholamines (mostly DA) The optimal structure is probably AMP or METH without aromatic ring substituents Serotonin Releasers Amphetamine is a relatively weak serotonin releaser p-chloroamphetamine para-substitution dramatically increases neuronal serotonin release para-chloroamphetamine (PCA) is the classic example para-chloromethamphetamine received some clinical attention in the early 1970 s as an antidepressant 7
8 Serotonin Releasers: PCA as a 5-HT Neurotoxin PCA is a potent releaser of 5-HT from neuron terminals, but Small doses also cause profound and long-lasting central 5-HT depletion Loss of 5-HT neuronal markers (e.g. tryptophan hydroxylase) Decrease in B max for 5-HT uptake site Loss of 5-HT immunoreactivity Used in some animal models as a 5-HT neurotoxin Mechanism has not been fully elucidated Serotonin Releasers: Fenfluramine and PMA Fenfluramine p-methoxyamphetamine Fenfluramine has some similarities to PCA, but may only deplete 5-HT acutely p-methoxyamphetamine (PMA) has significant indirect adrenergic effects Also an (indirect) 5-HT releaser Isomers similar in potency in releasing labeled 5-HT from rat brain synaptosomes Legally, PMA is usually classified as an hallucinogenic amphetamine Serotonin Releasers: MDA MDA Chemical progenitor to MDMA Clinical effects of MDA described in 1959 Racemic MDA has effects similar to hallucinogenic amphetamines MDA use produced a need to be with and talk to other people as well as a feeling of emotional closeness In contrast to other amphetamines In a dog model, MDA had both amphetamine-like and LSD-like effects Other substituted amphetamines just gave LSD-like effects 8
9 Serotonin Releasers: MDA Optical Isomers S-(+)-MDA Behavioral scoring in mice showed LSDlike effect of racemic MDA are due totally to the R-(-) isomer The S-(+) isomer possesses amphetamine like activity In cats, the S-(+) isomer produces a significant pressor response blocked by Pretreatment with reserpine (depleted endogenous NE stores) Pretreatment with phenoxybenzamine (α-adrenergic antagonist) Serotonin Releasers: MDA Optical Isomers S-(+)-MDA Indirect adrenergic and 5-HT releasing actions are stereoselective for S- In synaptosomes: Potent releaser of 5-HT Moderately potent 5-HT uptake inhibitor Very potent inhibitor of NE uptake Modest effect on inhibition of DA uptake 2 3 fold S- over R- stereoselectivity S-(+)-MDA has some amphetamine-like activity but the primary discriminative cue appears to be serotonergic Serotonin Releasers: N-Substitution R-(-)-MDMA MDMA first synthesized in 1912 In vitro, pharmacology is similar to MDA R-MDMA has higher affinity at 5-HT 2 In vivo, S-MDMA is more potent Discriminative cue (MDMA) is not blocked by ketanserin (5-HT 2 antagonist) In drug discrimination studies, R-(-)-MDA substitutes for hallucinogenic training drugs, but R-(-)-MDMA does not N-Methylation of MDA abolishes R- (hallucinogenic) activity, but has little effect on the S- (5-HT and catecholamine) activity 9
10 Serotonin Releasers: N-Substitution MDEA N-methylation has little effect on 5-HT and catecholamine effects Range of allowable substitutions is limited MDEA is similar to MDMA for 5-HT, NE and DA in vitro DA effects of MDEA weaker than MDMA N-Cyclopropyl-PCA has similar pharmacology to PCA Probably due to N-dealkylation N,N-Dialkylation leads to inactive compounds in vivo (N,N-Dimethyl-MDA) MBDB CAB Serotonin Releasers: Side Chain Modification 2-Carbon bridge optimal for 5-HT release Demonstrated in PCA analog studies MDA and MDMA analogs with two α-ch 3 groups are inactive in humans A single α-ethyl may be tolerated MBDB & CAB (α-ethyl MDMA & PCA) Longer chains are inactive An α-et markedly attenuates DA release Improves 5-HT selectivity, though still somewhat weaker than α-ch 3 compounds Serotonin Releasers: Aromatic Ring Substituents Single substituent at 3- (e.g. CF3 in fenfluramine) or 4- (e.g. PCA and PMA) or at 3,4- (e.g. MDA, MDMA) gives potent 5-HT releasers A B 3-Methoxy-4-methylamphetamine (A) is a potent indirect-acting 5-HT releaser Addition of ortho-och 3 (B) eliminates all effects on 5-HT, NE and DA transporters 10
11 Serotonin Releasers: Mechanism of Action Not just 5-HT uptake inhibitors They also actively cause release of endogenous 5-HT Likely involves 5-HT transporter protein Indirect action with amine transporters (e.g. MDMA-5-HT exchange) is probably also involved Passive diffusion into vesicles and transport out of the neuron by reverse action of the uptake pump Serotonin Releasers: SAR Summary Monosubstitution at the 4-position gives significant 5-HT activity Halogens and OCH 3 have appreciable catecholamine releasing actions A 3-CF 3 group (e.g. fenfluramine) gives relatively 5-HT selective agent A 3,4-dioxole disubstitution gives agents with 5-HT and catecholamine activity (MDA) Primary amine is most potent Extension of α-ch 3 to α-et improves 5-HT action by attenuating catecholamine effects Longer chains are not allowable Hallucinogens Called by various names psychotomimetics, hallucinogens, psychedelics Jaffe argued that psychedelic was the most appropriate moniker The feature that distinguishes the psychedelic agents from other classes of drugs is their capacity to induce states of altered perception, thought and feeling that are not experienced otherwise except in dreams or at times of religious exaltation. 11
12 Hallucinogenic Phenethylamines Mescaline 3,4,5-Trimethoxyamphetamine Mescaline was the starting point of the substituted phenethylamine genre Mescaline begat TMA in 1955 Nearly 200 derivatives have been evaluated for hallucinogenic activity Shulgin s PIHKAL is perhaps the most comprehensive collation of data to date Hallucinogenic Phenethylamines: N-Substitution N-alkylation of hallucinogenic amphetamines dramatically attenuates or even abolishes hallucinogenic activity Both receptor (5-HT 2, 5HT 1C ) affinity and in vivo activity are diminished N-CH 3 reduces hallucinogenic activity by about an order of magnitude N,N-Dialkylation (even with methyl groups), completely abolishes hallucinogenic activity An N-alkyl group larger than CH 3 or incorporation of the nitrogen into a heterocyclic ring leads to inactivity Hallucinogenic Phenethylamines: Aromatic Ring Substitution 3,4,5-Trimethoxy pattern (e.g. mescaline) actually gives lowest potency hallucinogens Moving 3-OCH 3 to the 2 position and/or replacing the 4-OCH 3 with a more hydrophobic group gives highly active hallucinogenic compounds 2,4,5-trisubstituted with 2 and 5 substituents as OCH 3 are most active 12
13 Hallucinogenics based on 2,5-Dimethoxyamphetamine R Trivial Name Est Dose (mg)* H 2,5-DMA OCH 3 TMA OCH 2 CH 3 MEM SCH 3 p-dot 5 10 NO 2 DON CH 3 DOM 3 10 CH 2 CH 3 DOEt 2 6 CH 2 CH 2 CH 3 DOPr Br DOB 1 3 I DOI CF 3 DOTFM Animals Only *Data compiled from Shulgin & Shulgin (1991) Hallucinogenics based on 2,5-Dimethoxyamphetamine 2,5-Dimethoxyamphetamine Are differences a result of electronics? Probably not both alkyl and highly electronegative groups (e.g. NO 2, CF 3 ) are highly active Greatest activity seems to be in congeners with relatively hydrophobic parasubstituent which is fairly resistant to oxidative metabolism Hallucinogenic Phenethylamines: 4-Substituent Orientation A B 4-substituent may force 5-OCH 3 into anti orientation Compound A lacks LSD-like activity while B is as potent as the flexible analog, DOB H-bond donor in the receptor site that needs this orientation of the O lone pair? This orientation of the OCH 3 group is required to make the aromatic system appear electronically similar to the indole nucleus of 5-HT? 13
14 Hallucinogenic Phenethylamines: 4-Substituent Orientation Optimum in the 2,5-dimethoxy-4-n-alkyl series is the n-propyl n-butyl has some activity, but n-pentyl does not Going from n-methyl to n-octyl results in transition from agonist to antagonist Polar 4-substituets (OH, NH 2, CO 2 H) have low 5-HT 2 receptor affinities Hallucinogenic Phenethylamines: 4-Substituent Orientation 3,4,5-Trisubstituted the adjacent OCH 3 groups push the 4-OCH 3 to lie in a plane nearly perpendicular to the aromatic ring plane 4-ethoxy and 4-isopropoxy are quite potent Hallucinogenic Phenethylamines: 4-Substituent Orientation 2,5-dimethoxy series, a 4-alkoxy larger than OCH 3 does not increase potency or binding at the 5-HT 2 receptor 4-Alkoxy group lies in a conformation allowing maximal overlap of oxygen lone pairs with the π-system of the aromatic ring The akyl group attached to the O is forced to lie in the plane of the aromatic ring If S rather than O, alkyl substituent is forced out of ring plane and compounds are potent 14
15 Hallucinogenic Phenethylamines: Ring Poly-Substitutions 2,4- and 2,5-dimethoxyAMP are active in humans (20 60 mg dose) 3,4-DimethoxyAMP is most like catecholamines, but not psychoactive 2,4,6-trimethoxyAMP is almost as potent as 2,4,5-trimethoxyAMP 2,3,4,5-TetramethoxyAMP was reported as active in humans early on, but later study suggests this is not the case DOB 2C-B Hallucinogenic Phenethylamines: Side Chain Modifications Removal of α-ch 3 decreases potency in hallucinogenic amphetamines Many stay within an order of magnitude of α-methylated congener 2C-B and 2C-I are about 1/10 the potency of amphetamine counterparts (DOB & DOI) Hallucinogen-like activity is higher for R- enantiomer (in vitro and in vivo) Stereoselectivity of 3 6 fold (NOT stereoespecificity) The α,α-dimethyl compounds are inactive Hallucinogenic Phenethylamines: Mechanism of Action This is tricky Some in vitro receptor data but difficult model to reproducibly characterize in vivo Probably more direct postsynaptic agonist activity than the other amphetamines Most important receptor seems to be the 5-HT 2 subtype 5-HT 1C is probably #2 5-HT 1A??? Hmmmmm Tryptamines and LSD do hit 5-HT 1A 15
16 Hallucinogenic Phenethylamines: SAR Summary Most active seem to have: Primary amino group 2,5 or 3,5-dimethoxy substituents Hydrophobic group at 4-position such as unbranched alkyl, halogen larger than F or alkylthio group The α-ch 3 gives 2 10 fold greater potency than non-methylated congeners More active enantiomer is R- (levorotatory) Disubstitution at α-carbon kills activity Though α-cyclopropyl has some activity THANKS!! Rob Palmer RPalmer@Toxicologyassoc.com (303)
Adrenergic agonists Sympathomimetic drugs. ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani
 Adrenergic agonists Sympathomimetic drugs ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani 2017-2018 Adrenergic agonists The adrenergic drugs affect receptors that
Adrenergic agonists Sympathomimetic drugs ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani 2017-2018 Adrenergic agonists The adrenergic drugs affect receptors that
Arylalkylamine Drugs of Abuse: An Overview of Drug Discrimination Studies
 PII S0091-3057(99)00045-3 Pharmacology Biochemistry and Behavior, Vol. 64, No. 2, pp. 251 256, 1999 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/99/$ see front matter Arylalkylamine
PII S0091-3057(99)00045-3 Pharmacology Biochemistry and Behavior, Vol. 64, No. 2, pp. 251 256, 1999 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/99/$ see front matter Arylalkylamine
MDMA Stimulus Generalization to the 5-HT 1A Serotonin Agonist 8-Hydroxy-2- (di-n-propylamino)tetralin
 PII S0091-3057(00)00174-X Pharmacology Biochemistry and Behavior, Vol. 66, No. 3, pp. 483 488, 2000 2000 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/00/$ see front matter MDMA
PII S0091-3057(00)00174-X Pharmacology Biochemistry and Behavior, Vol. 66, No. 3, pp. 483 488, 2000 2000 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/00/$ see front matter MDMA
Over the past half-century, substances now
 Structure activity relationships of serotonin 5-T 2A agonists David E. ichols f the 14 known types of serotonin receptors one of the most extensively studied is the 5-T 2A (5-hydroxytryptamine) receptor.
Structure activity relationships of serotonin 5-T 2A agonists David E. ichols f the 14 known types of serotonin receptors one of the most extensively studied is the 5-T 2A (5-hydroxytryptamine) receptor.
Pharmacophore-Based Discovery of Substituted Pyridines as Novel Dopamine Transporter Inhibitors
 Bioorganic& Medicinal Chemistry Letters 13 (2003) 513 517 Pharmacophore-Based Discovery of Substituted Pyridines as Novel Dopamine Transporter Inhibitors Istvan J. Enyedy, a Sukumar Sakamuri, a Wahiduz
Bioorganic& Medicinal Chemistry Letters 13 (2003) 513 517 Pharmacophore-Based Discovery of Substituted Pyridines as Novel Dopamine Transporter Inhibitors Istvan J. Enyedy, a Sukumar Sakamuri, a Wahiduz
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures
 Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Prof. Jose-Luis Jimenez Postulation of Molecular Structures There are several
Lecture 10: MS Interpretation Part 4 Postulation of Molecular Structures CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Prof. Jose-Luis Jimenez Postulation of Molecular Structures There are several
Carboxylic Acid Derivatives Reading Study Problems Key Concepts and Skills Lecture Topics: Structures and reactivity of carboxylic acid derivatives
 Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Central Nervous System Stimulants
 Central ervous System Stimulants 1 CS STIMULATS Are drugs that produce stimulation of CS and enhancement in excitability of different portions of the brains or the spinal cord. 2 Classification 1. Analeptics:
Central ervous System Stimulants 1 CS STIMULATS Are drugs that produce stimulation of CS and enhancement in excitability of different portions of the brains or the spinal cord. 2 Classification 1. Analeptics:
Psychomotor stimulants
 Psych 181: Dr. Anagnostaras Lec 6: Psychomotor stimulants Psychomotor stimulants Large class of diverse compounds Stimulate alertness, arousal ( psycho- ) Stimulate motor activity ( -motor ) Major ones:
Psych 181: Dr. Anagnostaras Lec 6: Psychomotor stimulants Psychomotor stimulants Large class of diverse compounds Stimulate alertness, arousal ( psycho- ) Stimulate motor activity ( -motor ) Major ones:
Neurotransmitters acting on G-protein coupled receptors
 Neurotransmitters acting on G-protein coupled receptors Part 2: Serotonin and Histamine BIOGENIC AMINES Monoamines Diamine Indolamines: Serotonin Basic Neurochemistry. FIGURE 15-1: Chemical structure of
Neurotransmitters acting on G-protein coupled receptors Part 2: Serotonin and Histamine BIOGENIC AMINES Monoamines Diamine Indolamines: Serotonin Basic Neurochemistry. FIGURE 15-1: Chemical structure of
- Neurotransmitters Of The Brain -
 - Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
- Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
Initial Characterization of PMMA as a Discriminative Stimulus 1
 PII S0091-3057(96)00306-1 Pharmacology Biochemistry and Behavior, Vol. 57, Nos. 1/2, pp. 151 158, 1997 Copyright 1997 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/97 $17.00.00
PII S0091-3057(96)00306-1 Pharmacology Biochemistry and Behavior, Vol. 57, Nos. 1/2, pp. 151 158, 1997 Copyright 1997 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/97 $17.00.00
Org/Biochem Final Lec Form, Spring 2012 Page 1 of 6
 Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
Adrenergic Agents. Adrenergic neurotransmitters. Structure and physicochemical properties
 Adrenergic Agents Adrenergic drugs are chemical agents that exert their principal pharmacological and therapeutic effects by either enhancing or reducing the activity of the various components of the sympathetic
Adrenergic Agents Adrenergic drugs are chemical agents that exert their principal pharmacological and therapeutic effects by either enhancing or reducing the activity of the various components of the sympathetic
I. OVERVIEW DIRECT. Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of
 THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
CHEM J-6 June 2014
 CEM1611 2014-J-6 June 2014 rlistat (shown below) is a drug for obesity management which acts by inhibiting the absorption of dietary fats. Indicate all stereogenic centres on the structure below. 6 rlistat
CEM1611 2014-J-6 June 2014 rlistat (shown below) is a drug for obesity management which acts by inhibiting the absorption of dietary fats. Indicate all stereogenic centres on the structure below. 6 rlistat
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Medicinal Chemistry I Examination Name December 15, 2009 Med. Chem. #
 Medicinal Chemistry Examination ame December 15, 2009 Med. Chem. # Part. 80 Points (40 Questions; 2 Points Each) Select the BEST answer for each of the following. f none of the answers are correct, do
Medicinal Chemistry Examination ame December 15, 2009 Med. Chem. # Part. 80 Points (40 Questions; 2 Points Each) Select the BEST answer for each of the following. f none of the answers are correct, do
General Chemistry. Ch. 10
 General Chemistry Ch. 10 Essentials of Organic Chemistry Most biological important molecules are composed of organic compounds. These are mostly produced by biological systems. Organic molecules contain
General Chemistry Ch. 10 Essentials of Organic Chemistry Most biological important molecules are composed of organic compounds. These are mostly produced by biological systems. Organic molecules contain
Carbon s unique bonding pattern arises from the hybridization of the electrons.
 Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
The Nervous System Mark Stanford, Ph.D.
 The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
 PSY 302 Lecture 6: The Neurotransmitters (continued) September 12, 2017 Notes by: Desiree Acetylcholine (ACh) CoA + Acetate Acetyl-CoA (mitochondria) (food, vinegar) + Choline ChAT CoA + ACh (lipids, foods)
PSY 302 Lecture 6: The Neurotransmitters (continued) September 12, 2017 Notes by: Desiree Acetylcholine (ACh) CoA + Acetate Acetyl-CoA (mitochondria) (food, vinegar) + Choline ChAT CoA + ACh (lipids, foods)
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Organic. Carbon Chemistry
 Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
Dr. Tawfiq Almezeiny MBBS, FRCPC ( CCM )
 Dr. Tawfiq Almezeiny MBBS, FRCPC ( CCM ) Cocaine Ephedrine Methamphetamine Methylphenidate CNS Stimulants I. Cocaine, Crack (free base or hydrochloride). II. Amphetamines: D-Amphetamine, Methamphetamine,
Dr. Tawfiq Almezeiny MBBS, FRCPC ( CCM ) Cocaine Ephedrine Methamphetamine Methylphenidate CNS Stimulants I. Cocaine, Crack (free base or hydrochloride). II. Amphetamines: D-Amphetamine, Methamphetamine,
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Adrenergic Receptor Antagonists
 Adrenergic Receptor Antagonists α-adrenergic Receptor Antagonists Unlike the β-adrenergic receptor antagonists, which bear clear structural similarities to the adrenergic agonists E, epinephrine, and isoproterenol,
Adrenergic Receptor Antagonists α-adrenergic Receptor Antagonists Unlike the β-adrenergic receptor antagonists, which bear clear structural similarities to the adrenergic agonists E, epinephrine, and isoproterenol,
University of Groningen. The medicinal chemistry of 2-aminotetralin-derived benzamides Homan, Evert Jan
 University of Groningen The medicinal chemistry of 2-aminotetralin-derived benzamides Homan, Evert Jan IMPORTAT OTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to
University of Groningen The medicinal chemistry of 2-aminotetralin-derived benzamides Homan, Evert Jan IMPORTAT OTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to
MEDCHEM 562. Physicochemical Properties of Drugs: Lecture 2; Kent Kunze. Stereochemistry (Double the trouble. or double the fun?)
 1 MEDCHEM 562 Physicochemical Properties of Drugs: Lecture 2; Kent Kunze Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center or site of
1 MEDCHEM 562 Physicochemical Properties of Drugs: Lecture 2; Kent Kunze Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center or site of
A substance that reduces pain and may or may not have psychoactive properties.
 GLOSSARY OF TERMS AMPHETAMINE-TYPE STIMULANTS (ATS) A group of substances, mostly synthetic, with closely related chemical structure which have, to varying degrees, a stimulating effect on the central
GLOSSARY OF TERMS AMPHETAMINE-TYPE STIMULANTS (ATS) A group of substances, mostly synthetic, with closely related chemical structure which have, to varying degrees, a stimulating effect on the central
Amines. Learning Check. Subclasses of Amines. IUPAC Naming for Amines. Common Naming for Amines. Chapter 16 Amines and Amides
 Amines Chapter 16 Amines and Amides Derivatives of ammonia, NH 3, where one or more hydrogen atoms have been replaced by an aromatic or alkyl group (R) Classified on the basis of molecular structure Primary
Amines Chapter 16 Amines and Amides Derivatives of ammonia, NH 3, where one or more hydrogen atoms have been replaced by an aromatic or alkyl group (R) Classified on the basis of molecular structure Primary
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Identification and Quantification of Psychedelic Phenethylamine 2C Series using SPE and LC-MS/MS
 Identification and Quantification of Psychedelic Phenethylamine 2C Series using SPE and LC-MS/MS UCT Part Numbers: CSXCE106-Clean Screen XCEL I 130mg/6mL SLDA100ID21-3UM - Selectra DA HPLC column, 100
Identification and Quantification of Psychedelic Phenethylamine 2C Series using SPE and LC-MS/MS UCT Part Numbers: CSXCE106-Clean Screen XCEL I 130mg/6mL SLDA100ID21-3UM - Selectra DA HPLC column, 100
Sedative-Hypnotics, Anxiolytic Drugs. A sedative is an agent that lowers excitement and
 Sedative-Hypnotics, Anxiolytic Drugs A sedative is an agent that lowers excitement and activity. A hypnotic is an inducer of sleep. The major use of sedative-hypnotic drugs is the treatment of sleep disorders
Sedative-Hypnotics, Anxiolytic Drugs A sedative is an agent that lowers excitement and activity. A hypnotic is an inducer of sleep. The major use of sedative-hypnotic drugs is the treatment of sleep disorders
Useful reading (refer to Foye s 7 th ed)
 Antidepressants Morten Grøtli Department of Chemistry and Molecular Biology Email: (grotli@chem.gu.se) Useful reading (refer to Foye s 7 th ed) Chaper 18, page 570-631. You are expected to read these pages
Antidepressants Morten Grøtli Department of Chemistry and Molecular Biology Email: (grotli@chem.gu.se) Useful reading (refer to Foye s 7 th ed) Chaper 18, page 570-631. You are expected to read these pages
Metabolic Changes of Drugs and Related Organic Compounds. Oxidative Reactions. Shokhan J. Hamid. 3 rd stage/ 1 st course Lecture 6
 Metabolic Changes of Drugs and Related Organic Compounds Oxidative Reactions 3 rd stage/ 1 st course Lecture 6 Shokhan J. Hamid B. OXIDATION INVOLVING CARBON OXYGEN SYSTEMS: Oxidative O-dealkylation of
Metabolic Changes of Drugs and Related Organic Compounds Oxidative Reactions 3 rd stage/ 1 st course Lecture 6 Shokhan J. Hamid B. OXIDATION INVOLVING CARBON OXYGEN SYSTEMS: Oxidative O-dealkylation of
Organic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins
 rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
CHEM 109A Organic Chemistry
 CEM 09A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter Isomers The arrangement of atoms in space MIDTERM Wednesday Feb. Chapter Sections.8.6 melting, boiling points, conformations
CEM 09A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter Isomers The arrangement of atoms in space MIDTERM Wednesday Feb. Chapter Sections.8.6 melting, boiling points, conformations
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Autonomic Nervous System. Introduction
 Autonomic Nervous System Introduction 1 The nervous system is divided into: 1- the central nervous system (CNS; the brain and spinal cord) 2- the peripheral nervous system (PNS; neuronal tissues outside
Autonomic Nervous System Introduction 1 The nervous system is divided into: 1- the central nervous system (CNS; the brain and spinal cord) 2- the peripheral nervous system (PNS; neuronal tissues outside
UNSATURATED HYDROCARBONS
 hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Stereoselective C,C-bond formation. Cyclizations of biradicals*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1623 1629, 2000. 2000 IUPAC Stereoselective C,C-bond formation. Cyclizations of biradicals* Bernd Giese, Frédérique Barbosa, Christian Stähelin, Stefan Sauer, Philipp
Pure Appl. Chem., Vol. 72, No. 9, pp. 1623 1629, 2000. 2000 IUPAC Stereoselective C,C-bond formation. Cyclizations of biradicals* Bernd Giese, Frédérique Barbosa, Christian Stähelin, Stefan Sauer, Philipp
Dania Ahmad. Tamer Barakat + Dania Ahmad. Faisal I. Mohammed
 16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
Fundamentals of Psychopharmacology. Distribution of Receptors Influences Safety
 Fundamentals of Psychopharmacology Matt Schalles UCSD Cognitive Science mdschalles@ucsd.edu Distribution of Receptors Influences Safety Opioid Receptors Cannabinoid Receptors 1 Brief History of Drug Advances
Fundamentals of Psychopharmacology Matt Schalles UCSD Cognitive Science mdschalles@ucsd.edu Distribution of Receptors Influences Safety Opioid Receptors Cannabinoid Receptors 1 Brief History of Drug Advances
Notes are online at The Neuron
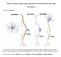 Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
Notes are online at http://cogsci.ucsd.edu/~clovett/neuronotescogs17.pdf A. What is a neuron? The Neuron 1. A neuron is a type of cell that receives and transmits information in the Central Nervous System
KEY NAME (printed very legibly) UT-EID
 BIOLOGY 311C - Brand Spring 2007 KEY NAME (printed very legibly) UT-EID EXAMINATION II Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
BIOLOGY 311C - Brand Spring 2007 KEY NAME (printed very legibly) UT-EID EXAMINATION II Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
Plasma membrane biogenic amine transporters (BATs)
 This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/acsmedchemlett
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/acsmedchemlett
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
C. Correct! A compound that consists only of hydrogen and carbon is termed a hydrocarbon.
 Organic Chemistry - Problem Drill 06: Alkanes and Cycloalkanes No. 1 of 10 1. What is a hydrocarbon? (A) A compound that consists of only carbon. (B) A compound that consists of nonmetals. (C) A compound
Organic Chemistry - Problem Drill 06: Alkanes and Cycloalkanes No. 1 of 10 1. What is a hydrocarbon? (A) A compound that consists of only carbon. (B) A compound that consists of nonmetals. (C) A compound
Can bind to several receptors in the body (nicotinic, muscarinic, everywhere)
 Problems of acetylcholine: 1. Selectivity : Can bind to several receptors in the body (nicotinic, muscarinic, everywhere) 2. Absorption: because any drug that acts as a cholinergic agonist must have a
Problems of acetylcholine: 1. Selectivity : Can bind to several receptors in the body (nicotinic, muscarinic, everywhere) 2. Absorption: because any drug that acts as a cholinergic agonist must have a
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
Structure of Alkenes In ethene (ethylene) each carbon is bonded to 3 other atoms, with zero nonbonding electrons => sp 2 hybridization.
 Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
Stereochemistry. Dr. Sapna Gupta
 Stereochemistry Dr. Sapna Gupta Introduction Stereo left and right handedness Any carbon that has four different groups will show chirality. hirality: the mirror image of the compound will not superimpose
Stereochemistry Dr. Sapna Gupta Introduction Stereo left and right handedness Any carbon that has four different groups will show chirality. hirality: the mirror image of the compound will not superimpose
Level 3 Chemistry, 2007
 Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
A BEGINNER S GUIDE TO BIOCHEMISTRY
 A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
PHARMACOPHORE MODELING
 CHAPTER 9 PHARMACOPHORE MODELING 9.1 PHARMACOPHORES AS VIRTUAL SCREENING TOOLS The pharmacophore concept has been widely used over the past decades for rational drug design approaches and has now been
CHAPTER 9 PHARMACOPHORE MODELING 9.1 PHARMACOPHORES AS VIRTUAL SCREENING TOOLS The pharmacophore concept has been widely used over the past decades for rational drug design approaches and has now been
CHEM 261 Feb. 16, stereogenic center H C C C
 77 EM 261 Feb. 16, 2017 eview of hiral enters 3 quinine - anti-malarial drug from the bark of the tree inchona officinalis malaria is cause by Plasmodium species transmitted by Anopheles mosquito example.
77 EM 261 Feb. 16, 2017 eview of hiral enters 3 quinine - anti-malarial drug from the bark of the tree inchona officinalis malaria is cause by Plasmodium species transmitted by Anopheles mosquito example.
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
Lecture 5: Drug targets (continued)
 Lecture 5: Drug targets (continued) IIa. Enzymes as drug targets (HMG-CoA example) Many drugs are inhibitors of enzymes that catalyze biologically important reactions. The conversion of HMG-CoA to mevalonic
Lecture 5: Drug targets (continued) IIa. Enzymes as drug targets (HMG-CoA example) Many drugs are inhibitors of enzymes that catalyze biologically important reactions. The conversion of HMG-CoA to mevalonic
Ch5: Macromolecules. Proteins
 Ch5: Macromolecules Proteins Essential Knowledge 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule A. Structure and function of polymers are derived
Ch5: Macromolecules Proteins Essential Knowledge 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule A. Structure and function of polymers are derived
metabolism inhibition, this approach causes other adverse effects. The dosage of L-dopa can be brought down further by co-medication of dopamine
 6XPPDU\ Parkinson s disease is a serious neurological disorder of the central nervous system that usually becomes apparent after the age of 55. It concerns the increased deterioration of neurons responsible
6XPPDU\ Parkinson s disease is a serious neurological disorder of the central nervous system that usually becomes apparent after the age of 55. It concerns the increased deterioration of neurons responsible
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
The adrenergic drugs affect receptors that are stimulated by norepinephrine or epinephrine. Some adrenergic drugs act directly on the adrenergic
 Adrenergic drugs The adrenergic drugs affect receptors that are stimulated by norepinephrine or epinephrine. Some adrenergic drugs act directly on the adrenergic receptor (adrenoceptor) by activating it
Adrenergic drugs The adrenergic drugs affect receptors that are stimulated by norepinephrine or epinephrine. Some adrenergic drugs act directly on the adrenergic receptor (adrenoceptor) by activating it
Psychedelic Drugs. Pharmacokinetics. Pharmacodynamics. Anticholinergic PNS. Psychedelics / Hallucinogens
 Psychedelic Drugs Psychedelics / Hallucinogens Psychology 472 Pharmacology of Psychoactive Drugs Listen to the audio lecture while viewing these slides Many Types Broken out by the type of NT it affects
Psychedelic Drugs Psychedelics / Hallucinogens Psychology 472 Pharmacology of Psychoactive Drugs Listen to the audio lecture while viewing these slides Many Types Broken out by the type of NT it affects
ADRENERGIC RECEPTOR ANTAGONISTS: ALPHA RECEPTOR BLOCKERS
 Jack Deuiter, Principles of Drug Action 2, Fall 2000 ADEEGIC ECEPT ATAGISTS: ALPA ECEPT BLCKES General PC/MC: Understand the term "antagonist" and how an antagonist is similar to an agonist, and how an
Jack Deuiter, Principles of Drug Action 2, Fall 2000 ADEEGIC ECEPT ATAGISTS: ALPA ECEPT BLCKES General PC/MC: Understand the term "antagonist" and how an antagonist is similar to an agonist, and how an
IR Spectroscopy Part II
 IR Spectroscopy Part II Carbonyl - compounds For simple aldehydes and ketones, the stretching vibration of the carbonyl group is a strong infrared absorption beetwen 1710 and 1740 cm -1. Alkyl substituents
IR Spectroscopy Part II Carbonyl - compounds For simple aldehydes and ketones, the stretching vibration of the carbonyl group is a strong infrared absorption beetwen 1710 and 1740 cm -1. Alkyl substituents
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Life History of A Drug
 DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
The behavioral pharmacology of hallucinogens
 biochemical pharmacology 75 (2008) 17 33 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/biochempharm Review The behavioral pharmacology of hallucinogens William E. Fantegrossi
biochemical pharmacology 75 (2008) 17 33 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/biochempharm Review The behavioral pharmacology of hallucinogens William E. Fantegrossi
At a Glance. Background Information. Lesson 3 Drugs Change the Way Neurons Communicate
 Lesson 3 Drugs Change the Way Neurons Communicate Overview Students build upon their understanding of neurotransmission by learning how different drugs of abuse disrupt communication between neurons. Students
Lesson 3 Drugs Change the Way Neurons Communicate Overview Students build upon their understanding of neurotransmission by learning how different drugs of abuse disrupt communication between neurons. Students
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Calpain Assays, Nerve injury and Repair
 Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
Proteins. (b) Protein Structure and Conformational Change
 Proteins (b) Protein Structure and Conformational Change Protein Structure and Conformational Change Proteins contain the elements carbon (C), hydrogen (H), oxygen (O2) and nitrogen (N2) Some may also
Proteins (b) Protein Structure and Conformational Change Protein Structure and Conformational Change Proteins contain the elements carbon (C), hydrogen (H), oxygen (O2) and nitrogen (N2) Some may also
Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides
 Educational Goals Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides 1. Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine molecule, be able to give the systematic
Educational Goals Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides 1. Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine molecule, be able to give the systematic
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Sedative-Hypnotics & the Treatment of Hypersomnia October 22, 2018 Pharm 9002 Mark Beenhakker, Pharmacology
 Sedative-Hypnotics & the Treatment of Hypersomnia October 22, 2018 Pharm 9002 Mark Beenhakker, Pharmacology markbeen@virginia.edu Glossary Anxiolytic: decreases anxiety Sedative: (1) decreases activity,
Sedative-Hypnotics & the Treatment of Hypersomnia October 22, 2018 Pharm 9002 Mark Beenhakker, Pharmacology markbeen@virginia.edu Glossary Anxiolytic: decreases anxiety Sedative: (1) decreases activity,
PART IV THE CATALYTIC FUNCTION
 PAT IV TE ATALYTI FUNTIN INTDUTIN The life of a cell, of an organism, depends on the multiplicity of the diverse chemical reactions which constitute metabolism. These reactions are carried out with appreciable
PAT IV TE ATALYTI FUNTIN INTDUTIN The life of a cell, of an organism, depends on the multiplicity of the diverse chemical reactions which constitute metabolism. These reactions are carried out with appreciable
Biochemistry 15 Doctor /7/2012
 Heme The Heme is a chemical structure that diffracts by light to give a red color. This chemical structure is introduced to more than one protein. So, a protein containing this heme will appear red in
Heme The Heme is a chemical structure that diffracts by light to give a red color. This chemical structure is introduced to more than one protein. So, a protein containing this heme will appear red in
The Steric Factor in Medicinal Chemistry Dissymmetric Probes of Pharmacological Receptors
 The Steric Factor in Medicinal Chemistry Dissymmetric Probes of Pharmacological Receptors Alan F. Casy University of Bath Bath, United Kingdom With a contribution by George H. Dewar University of Bath
The Steric Factor in Medicinal Chemistry Dissymmetric Probes of Pharmacological Receptors Alan F. Casy University of Bath Bath, United Kingdom With a contribution by George H. Dewar University of Bath
A review of the collision induced dissociation fragmentation and the metabolism of synthetic cathinone derivatives
 A review of the collision induced dissociation fragmentation and the metabolism of synthetic cathinone derivatives Nichola Cunningham A thesis submitted in fulfilment of the requirements for the degree
A review of the collision induced dissociation fragmentation and the metabolism of synthetic cathinone derivatives Nichola Cunningham A thesis submitted in fulfilment of the requirements for the degree
Stereochemistry 1.1 INTRODUCTION
 1 Stereochemistry 1.1 INTRODUTION The isomers which have same arrangement of atoms but differ only due to different orientation of atoms in space within the molecule are called Stereoisomers and the phenomenon
1 Stereochemistry 1.1 INTRODUTION The isomers which have same arrangement of atoms but differ only due to different orientation of atoms in space within the molecule are called Stereoisomers and the phenomenon
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
A general depiction of olefin metathesis is shown below, where you have two olefins that literally switch partners:
 Class 6 This starts the second 1/3 of our semester, where we talk in detail about a few select reactions. We will spend two classes talking about OLEFIN METATHESIS, mostly because it is a fairly important
Class 6 This starts the second 1/3 of our semester, where we talk in detail about a few select reactions. We will spend two classes talking about OLEFIN METATHESIS, mostly because it is a fairly important
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Chapter 5 Stereochemistry
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith Modified by Dr. Juliet Hahn Chapter 5 Stereochemistry 1 Stereoisomers of 2,3-dibromobutane Figure 5.9 Pair of enantiomers: A and B Pairs of diastereomers:
Organic Chemistry, Fifth Edition Janice Gorzynski Smith Modified by Dr. Juliet Hahn Chapter 5 Stereochemistry 1 Stereoisomers of 2,3-dibromobutane Figure 5.9 Pair of enantiomers: A and B Pairs of diastereomers:
As a result, IV and V are chiral molecules and are enantiomers.
 5.1 lassify each of the following objects as to whether it is chiral or achiral: (a) A screwdriver achiral (d) A tennis shoe chiral (g) A car chiral (b) A baseball bat achiral (e) An ear chiral (h) A hammer
5.1 lassify each of the following objects as to whether it is chiral or achiral: (a) A screwdriver achiral (d) A tennis shoe chiral (g) A car chiral (b) A baseball bat achiral (e) An ear chiral (h) A hammer
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
6.1 Cis Trans Isomers
 6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
THE EXPANDING REACH OF THE DESIGNER DRUG MOVEMENT IN 2011: CHALLENGES FOR FORENSIC TOXICOLOGY & CHEMISTRY
 THE EXPANDING REACH OF THE DESIGNER DRUG MOVEMENT IN 2011: CHALLENGES FOR FORENSIC TOXICOLOGY & CHEMISTRY Barry K Logan PhD, DABFT, National Director Forensic Services, NMS Labs Willow Grove PA History
THE EXPANDING REACH OF THE DESIGNER DRUG MOVEMENT IN 2011: CHALLENGES FOR FORENSIC TOXICOLOGY & CHEMISTRY Barry K Logan PhD, DABFT, National Director Forensic Services, NMS Labs Willow Grove PA History
Omar Ismail. Dana Almanzalji. Faisal Mohammad
 11 Omar Ismail Dana Almanzalji Faisal Mohammad Neuronal classification: Neurons are responsible for transmitting the action potential to the brain. The speed at which the action potential is transmitted
11 Omar Ismail Dana Almanzalji Faisal Mohammad Neuronal classification: Neurons are responsible for transmitting the action potential to the brain. The speed at which the action potential is transmitted
Chem 263 B6 Notes March 30, 2006 Demo-In-Class: O
 hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Autonomic Nervous System
 ANS..??? Autonomic Nervous System Nervous system CNS PNS Autonomic Somatic Symp Parasymp Enteric SOMATIC AUTONOMIC Organ supplied Skeletal muscle Other organs Distal most synapse Nerve fibre Peripheral
ANS..??? Autonomic Nervous System Nervous system CNS PNS Autonomic Somatic Symp Parasymp Enteric SOMATIC AUTONOMIC Organ supplied Skeletal muscle Other organs Distal most synapse Nerve fibre Peripheral
