(AVP) also stimulates the release of ACTH (3, 4). Vasopressin-stimulated. which the pituitary has been transplanted to the kidney capsule
|
|
|
- Adrian Cox
- 5 years ago
- Views:
Transcription
1 Vasopressin Stimulation of Adrenocorticotropin Hormone (ACTH) in Humans In Vivo Bioassay of Corticotropin-releasing Factor (CRF) which Provides Evidence for CRF Mediation of the Diurnal Rhythm of ACTH R. A. Salata, D. B. Jarrett, J. G. Verbalis, and A. G. Robinson Departments ofmedicine and Psychiatry, University ofpittsburgh, Pittsburgh, Pennsylvania Abstract The diurnal response of ACTH release to intravenously administered arginine vasopressin was tested in normal volunteers given consecutively moderate doses of vasopressin every 15 min (0.1, 03, 1.0, and 3.0 IU) at 2200 h and again at 0700 h (PM/AM). This protocol was repeated 4 wk later with the times reversed (AM/PM). A dose-related increase in ACIH secretion was observed in all subjects. When the AM response of the AM/PM protocol was compared with the PM response of the PM/AM protocol, the release of ACTH was greater in the morning (P < 0.05) as evaluated by the following criteria: peak value of ACTH (129.9±30.4 pg/ml in the AM vs. 57.1±20.2 in the PM), area under the curve (689 in the AM vs. 259 in the PM); and, sensitivity of the ACTH dose-response curve (first significant increase in ACTH with 1 IU of vasopressin in the AM but not significant even after 3 IU in the PM). In addition, when the AM vasopressin testing followed a previous evening stimulation (PM/AM protocol), there was a blunted ACTH response compared with the AM/PM protocol. Corticotropin-releasing factor (CRF) is probably the major ACTH secretagogue, but since vasopressin acts synergistically with CRF to produce an augmented release of ACTH, we suggest that the ACTH response to administered vasopressin depends upon the ambient endogenous level of CRF. We interpret our data and published data that CRF produces a lesser release of ACTH in the AM as follows: in the morning endogenous CRF is high and administered CRF produces little further release of ACTH, but administered vasopressin acting synergistically with high endogenous CRF causes a greater release of ACTH; conversely, in the evening endogenous CRF is low and administered CRF causes a greater release of ACHI, but vasopressin (a weak secretagogue by itself) gives a low ACTH response. We conclude that vasopressin stimulation of ACTH secretion can be used as an in vivo bioassay of endogenous CRF, and that there is a diurnal rhythm of CRF in hypophyseal portal blood. Introduction ACTH secretion by the corticotrophs in the anterior pituitary is regulated by corticotropin-releasing factor(s) (CRE)' from the hypothalamus (1, 2). CRF is probably the major ACTH Address correspondence to Dr. Robinson, 930 Scaife Hall, Div. of Endocrinology and Metabolism, University of Pittsburgh, School of Medicine, Pittsburgh, PA Received for publication 13 May 1987 and in revised form 9 October Abbreviation used in this paper: CRF, corticotropin-releasing factor. J. CGn. Invest. The American Society for Clinical Investigation, Inc /88/03/0766/09 $2.00 Volume 81, March 1988, secretagogue, but administration of arginine vasopressin (AVP) also stimulates the release of ACTH (3, 4). Vasopressin-stimulated release of ACTH or cortisol has been described in man (5), in rats with hypothalamic lesions (6), in rats in which the pituitary has been transplanted to the kidney capsule (7), in isolated pituitaries in organ culture (8), and in dispersed anterior pituitary cells (9). It is unclear whether vasopressin has a physiological role in the regulation of ACTH secretion (10, 1 1). CRF has been shown to be a more potent secretagogue of ACTH than vasopressin (9, 12, 13), and the greatest response of vasopressin to release ACTH occurred when vasopressin was co-administered with CRF (13-16). In this situation there appeared to be a synergism between both releasing factors to cause an augmented release of ACTH. It was recently shown in humans that concurrent administration of vasopressin and CRF produced a 30-fold increase in the release of ACTH as compared with administration of CRF alone (14). The well-known diurnal rhythm of plasma cortisol reflects a similar diurnal rhythm of ACTH. ACTH is secreted in a pulsatile fashion with more frequent bursts occurring in the early morning which produce the early morning surge of cortisol (17). Although CRF has not been measured in portal vessels of man, it is hypothesized that the secretory bursts of ACTH result from pulses of CRF secreted into hypophyseal portal blood, with more frequent bursts of CRF occurring in the early morning (17). When CRF was administered to humans, the ACTH and cortisol response was reported to be lower in the morning than in the evening (18-20), but the mechanism of either the augmented sensitivity ofthe pituitary adrenal axis in the evening, or of the blunted sensitivity in the morning, is not known. Glucocorticoids have been shown to blunt or abolish CRF-induced ACTH release (21, 22), and the lower morning response may simply be due to negative feedback. Alternatively, if endogenous CRF is at or near its maximum level in the morning, exogenously administered CRF may cause relatively little augmentation of release of ACTH. Because vasopressin acts synergistically with CRF to produce an augmented release of ACTH (13-16), we hypothesized that the ACTH response to administered vasopressin might depend on the ambient endogenous level of CRF. Thus, if there is a diurnal rhythm of CRF secretion, administered vasopressin should cause a greater release of ACTH in the morning, when endogenous CRF levels would be high, than in the evening, when CRF levels would be low. To evaluate this we measured the changes in plasma cortisol and ACTH in the morning and in the evening after infusion ofgraded boluses of vasopressin in normal human volunteers. We used small graded boluses of vasopressin to obtain a dose-related release ofacth, as has been described with CRF (23). By obtaining a less than maximal ACTH response, synergism between endogenous CRF and exogenous vasopressin might be demonstrated not only by a difference in the maximum level of ACTH, but also by the threshold at which ACTH was released. 766 R. A. Salata, D. B. Jarrett, J. G. Verbalis, and A. G. Robinson
2 Methods Five healthy volunteers (three females and two males) age 23 to 30 were studied twice at 4-wk intervals at the Clinical Research Center of the University ofpittsburgh. All subjects were nonsmokers, were on no medications, and abstained from alcohol for at least 24 h before each study. None of the subjects were depressed as judged by the Beck Depression Index, nor had any previous history of psychopathology. The women were studied between days 3 and 12 of their menstrual cycle. The study was approved by the Institutional Review Board for Biomedical Research of the University of Pittsburgh, and informed consent was obtained before each study. In the first experimental design (PM/AM protocol), the patients had a light dinner and then had nothing further by mouth until the next morning. At 2000 h an intravenous line was inserted into an antecubital vein for infusion of vasopressin and withdrawing of blood. Normal saline, 0.9% NaCI solution, was infused at a rate sufficient to keep the intravenous line patent. Aqueous pitressin (Parke, Davis & Co., Detroit, MI, synthetic vasopressin, 20 U/ml) was diluted in sterile 5% dextrose and water to a final concentration of I U vasopressin per 5 cm3 before infusion. The patients remained supine until testing was completed. At 2200 h, which was designated 0 time, the vasopressin stimulation was initiated with 0.1 U vasopressin infused intravenously over 1 min. At 1 5-min intervals thereafter, graded boluses of vasopressin were infused intravenously over 1 min as follows: 0.3 U at + 15 min, 1.0 U at +30 min, and 3.0 U at +45 min. Blood samples for ACTH, cortisol, and vasopressin levels were obtained at - 15 min, 0 time, every 5 min from 2200 through 2300 h, and then every 15 min for the next 45 min. During the graded vasopressin infusion, blood pressure and pulse were monitored before and after each dose increase. At 0700 h the next day, the graded vasopressin infusion was repeated after the same protocol as the previous evening infusion. The second study (designated AM/PM) was performed 4-8 wk after the first study. The experimental design was exactly as outlined in the first study, except that the time of day of the vasopressin infusion was reversed, with the subjects being tested first in the morning after an overnight fast and then again in the evening of the same day. Blood samples for assay ofacth were collected into chilled plastic tubes containing EDTA and aproptinin and were immediately placed on ice. Within 5 min of collection the blood was centrifuged at 10,000 g for 5 min at 4VC and the plasma supernatant transferred to plastic tubes containing N-ethylmaleimide at a concentration of 0.01 M. The plasma was rapidly frozen and stored at -70'C until assay, which was always within 3 d. Before assay, the plasma samples were thawed at 4VC and extracted using 125 mg/ml silicic acid. The ACTH was eluted from the silicic pellet with acid methanol. This eluate was air dried and then reconstituted in assay buffer. Recovery was monitored using a standard plasma to which a known amount of [35S]ACTH had been added and was 70-80%. Blood for cortisol was collected in Vacutainer (Becton-Dickinson & Co., Orangeburg, NY) tubes containing 143 U heparin, centrifuged, and frozen as above until assayed. Plasma cortisol was measured by a radioimmunoassay using an antibody specific for cortisol (Endocrine Sciences, Tarzana, CA). Plasma was stored at -70 C until the cortisol radioimmunoassay was performed. For assay, the samples were thawed and an aliquot diluted 1:51 in 1 ml of a PBS, 0.1% gelatin solution, ph 7. The diluted samples were capped and incubated in a water bath at 85 C to destroy the endogenous cortisol binding globulin and release the cortisol. The samples were then cooled and 50-Ml aliquots pipetted into 12 X 75-mm culture tubes. A standard response curve was prepared with cortisol over the range 0 to 300 pg. These tubes were air dried at 45 C and then 5 Ml of PBS-gelatin added. An appropriate tracer solution (11 MCi, [ H]cortisol [100 MCi/ mm]) was air dried and diluted in PBS-gelatin; after mixing, an appropriate volume of cortisol antiserum was added. The tubes were mixed and incubated at room temperature for 2 h and then overnight at 4VC. The bound cortisol was separated using 1 ml of a charcoal dextran solution at 4VC. After addition of the charcoal, the tubes were left for 15 min at 4VC and then centrifuged at 2,200 g for 15 min at 4VC. The assay tubes were decanted into scintillation vials into which 10 ml of a Triton X-100-based scintillation cocktail was added. The vials were shaken for 1 h and then counted in an LS 8100 scintillation counter (Beckman Instruments, Inc., Irvine, CA). Plasma cortisol values were generated by computer from the standard response curve using a log-dose-logit transformation which linearizes the standard curve. For ACTH assay we used an antibody provided by Drs. D. Gann and D. Carlson that is a rabbit antibody directed against porcine ACTHI,39. Synthetic human ACTHI,39 supplied by the National Institutes of Health (NIH), Bethesda, MD, was handled in the manner suggested in their protocol. The original ACTH was stored at a concentration > 200 Ag/ml in 0.01 N HCO and aliquots diluted in acidified albumin saline solution (42.6 ml 12 N HC1, 1 g Cohn fraction V, and 8.5 g NaCl per liter of distilled water), and stored at -70'C. ACTH was iodinated with 125I to a specific activity of 200 MCi/Aug using lodo- Gen (Pierce Chemicals, Rockford, IL) (24). The contents of the reaction vessel were transferred to a 0.9 X 25-cm G-25 Sephadex column equilibrated in 0.05 M Na acetate buffer, ph 5.0, with 1% BSA. Fractions of 0.5 ml were collected from the column and the protein peak pooled. Aliquots of 100 Ml were placed in 1.5 ml polypropylene microfuge tubes which were stored at -20'C. Before each assay an aliquot of '25I-ACTH was purified using HPLC. The '251-ACTH peak was then diluted in assay buffer (0.3 M Na phosphate buffer, ph 7.4, containing 0.1% lysozyme and 15,000 kallikrein inhibitor units of aprotinin), to give 2,500 cpm/100 ul, which resulted in a tracer mass of 5 pg/incubation. The assay was set up in 12 X 75 polypropylene tubes using a - final antibody dilution of 1:18,000 with standards over the range of 0 to 5,000 pg/ml in a final volume of 250 ul. The antibody and standards/unknowns were preincubated for 4 h at 4 C before the freshly chromatographed `25I-ACTH was added. After overnight incubation at 4 C, a sheep anti-rabbit gamma globulin second antibody, diluted 1:20 in 4% polyethylene glycol, was added and incubated for 4 h at 4 C. The bound hormone was separated by centrifugation at 10,000 g for 3 min at 4 C. The supernatants were aspirated and the tubes counted in a Gamma 8000 counter (Beckman Instruments, Inc.). The assay has a sensitivity of 5 pg/ml. As with the cortisol assay, the values are generated by computer, using the log dose-logit plot (25). Plasma AVP was assayed as described previously (26). For statistical analysis, pretest values for ACTH and cortisol were defined as the mean of the -15 min and 0 time values. However, when subtracting baseline values of ACTH and cortisol the lowest point of the initial sampling was used, because ACTH and cortisol values fell initially after the pretest values in some subjects, reflecting the normal diurnal fluctuations in these hormones. The area under the curve for ACTH and cortisol responses were determined from each subject's individual values after the baseline was subtracted. Student's paired t tests were utilized to determine at which dose of vasopressin there was a response significantly above baseline, and to analyze peak ACTH and cortisol response and area under the curve. Overall differences between trials in ACTH, cortisol, and vasopressin were analyzed using repeated measures analysis of variance. Correlations were determined with regression analysis by the method of least squares. Results Dose-response relationships ofadministered A VP and plasma ACTH and cortisol. All subjects were monitored closely during the graded vasopressin infusion. A slight but transient increase in blood pressure and pulse was noted only after the 3-U infusion of vasopressin. Additionally, several subjects noted sensations of acral flushing, increased gastrointestinal motility, or nausea after the 3-U bolus, but these sensations all resolved within 30 s. Every subject had an increase of plasma ACTH and of plasma cortisol during every test, and overall there was a sig- Vasopressin Stimulation ofadrenocorticotropin Hormone in Humans 767
3 nificant relationship between the hormone level and the dose of vasopressin administered both for ACTH, P < 0.01, and for cortisol, P < In all subjects there was no significant increase in ACTH and cortisol with the lowest dose of vasopressin administered (0.1 U). The mean plasma ACTH in the morning of the AM/PM protocol (Fig. 1) illustrates the observed stepwise increase in ACTH which allowed definition of threshold response as well as any shift of the dose-response curve to the left or the right. A cumulative effect of administered vasopressin was observed, with each subsequent and larger dose adding to the residual ACTH response produced by the previous dose. The peak ACTH concentration occurred 5-10 min after each dose of vasopressin, but was still elevated by 15 min when the next dose of vasopressin was given. After the last dose of vasopressin the subsequent decrease in the levels of vasopressin demonstrate a physiologic half-life of - 20 min, which is consistent with the observation that the 15-min bolus regimen produced a cumulative effect on release of ACTH. Effect ofvasopressin infusion in the morning in comparison with the evening. The vasopressin infusion in the morning of the AM/PM protocol was compared with the same infusion in the evening of the PM/AM protocol, i.e., first exposure to vasopressin in each case. Plasma ACTH levels for each individual subject are illustrated in Fig. 2. The mean pretest ACTH level in the morning was 37.4±10.1 pg/ml and in the evening 24.4±8.7 pg/ml. The morning level was greater than the evening level in all subjects, although the mean levels were not significantly different (P = 0.36). The mean pretest cortisol levels were higher in the morning in every subject: morning cortisol, 16.4±3.0 mg/dl, and evening cortisol, 2.7±0.6 mg/dl, P < Plasma levels of ACTH in response to the graded vasopressin infusion are shown for each subject in Fig. 2, and were greater in the morning (Fig. 2 A) than in the evening (Fig. 2 B). This difference was statistically significant by the following criteria: the maximum increase ofacth over baseline was 99.6±25.8 pg/ml in the morning and 40.1±16.6 pg/ml in the evening, P < 0.05; the area under the curve determined by cumulative ACTH increase in the morning was 654.4±168.3 and 229.7±82.3 pg/ml in the evening, P < 0.05; and there was a shift in the dose-response increase in ACTH (Fig. 3), with the first significant increase in ACTH noted after 1 U of vasopres <z a.f v MINUTES FROM FIRST INJECTION Figure 1. Mean plasma ACTH response (±SEM) of five healthy subjects after graded vasopressin infusion in the morning of AM/PM protocol. The doses of administered vasopressin were submaximal and the stepwise increase in ACTH allowed definition of a threshold response. Dose of vasopressin: I, 0.1 IU at 0 time; 0.3 IU at +15 min; 1.0 IU at +30 min; and 3.0 IU at +45 min. 214 IN 'E - a Mg o) 12 9 a 3 A AM of AM/PM TRIAL 10.0 B PMof PM/AM TRIAL , -~'~N0. c i / l _10-13U 1D 5UV 4 MuT FoFUIUR -1TIO Figure 2. Plasma ACTH for individual subjects in response to administered vasopressin for the morning of the AM/PM protocol (A) and for the evening of the PM/AM protocol (B). Plasma cortisol levels for the same subjects for the morning of the AM/PM protocol (C), and for the evening of the PM/AM protocol (D). Vasopressin doses as in Fig. 1. sin in the morning (P < 0.05). In the evening, even the increase in ACTH after a 3-U dose just missed significance (P = 0.06) (Table I). The individual levels of cortisol are shown in Fig. 2, C and D. The maximum increase above baseline of cortisol in the morning, 16.6±3.2 mg/dl, was greater than the maximum level in the evening, which 11.2±1.5 mg/dl, but this was not statistically significant. Similarly, the cumulative increment in cortisol in the morning, 175.2±50.3 mg/dl, was greater than, but not statistically different from the evening cumulative increment in cortisol, which was 70.0±13.8 mg/dl. There also was not a significant difference in the overall cortisol response patterns between the two trials. There was, however, a shift in <Z [lpm MAM E 80 Fm ~~~~~~~~~* _ 60 zz 40 <LU LU PRE-TEST 011U U 3.01U VASOPRESSIN DOSE Figure 3. Dose-response increase of ACTH in the morning of the AM/PM protocol versus the evening of the PM/AM protocol. Each bar is the mean of the three ACTH samples for each subject after each dose of AVP. Baseline ACTH levels subtracted for each subject (see text). The ACTH response in the morning is shifted to the left relative to the evening, with the first significant increase in ACTH occurring with 1 U of AVP in the morning. The ACTH response in the evening after 3 U of AVP was P = (* Significant increase in ACTH compared with baseline value, P < 0.05). C D AM of AM/PM TRIAL PM of PM/AM TRIAL 20.0 tal 0.C,n 1m m7x1 MINUTES FROM FIRST INJECTION 768 R. A. Salata, D. B. Jarrett, J. G. Verbalis, and A. G. Robinson
4 Table L Levels ofacth in Plasma during Each offour Tests Described in Text AM/PM Protocol PM/AM Protocol AM response PM response AM response PM response Pretest ACTH 37.4± ± ± ±8.66 Basal ACTH 30.30± ± ± ±12.36 Maximum measurement in ACTH 88.8± ± ± ±37.38 Cumulative ACTH ± ± ± ± Dose at which significant ACTH increase occurred I IU None 0.3 IU 3 IU pg/mi the dose-response curve for cortisol similar to that found for ACTH. A significant increase of cortisol above baseline was noted after the 0.3-U dose of vasopressin in the morning (P < 0.05), while in the evening a significant increase was not observed until after a dose of 3 U of vasopressin (P < 0.01, Fig. 4, Table II). Effect ofprior administration ofvasopressin on subsequent response to vasopressin stimulation. The pretest ACTH levels in the morning without prior exposure to vasopressin, 37.4±10.1 pg/ml, were not significantly different than when vasopressin had been administered the previous evening (34.2±11.6 pg/ml, Fig. 5, A vs. C). However, the ACTH response to administered vasopressin was significantly blunted in the morning (Fig. 5 C), when vasopressin had been administered the prior evening, as compared with the response without prior exposure to vasopressin (Fig. 5 A). The differences were significant by the same three criteria used to compare the morning with the evening responses: (i) the maximum increase of ACTH above baseline was 99.6±25.8 in the morning without prior exposure to vasopressin and 33.6±6.9 pg/ml when vasopressin had been administered the prior evening (P < 0.05); (ii) the area under the curve determined by the cumulative mean incremental ACTH in the morning without prior exposure to vasopressin was 654.4±168.3 pg/ml, whereas after exposure to vasopressin the previous evening the value was 223.1±45.0 pg/ml (P < 0.05); and, (iii) the dose-response curve for the morning without prior exposure to vasopressin was shifted to the left relative to the response curve after exposure to vasopressin, with the first significant increase in ACTH after 1 U of vasopressin in the former and after 3 U of vasopressin in the latter. The ACTH response to administered vasopressin in the evening when there was no prior exposure to vasopressin (PM of the PM/AM protocol, Fig. 5 B) was not significantly different when compared with the ACTH response when vasopressin was administered in the morning of the same day (PM of the AM/PM protocol, Fig. 5 D). The baseline cortisol levels in the morning were similar in both protocols: 16.4±3 mg/dl in the AM/PM protocol, and 12.1±0.9 mg/dl in the PM/AM protocol when vasopressin had been administered the previous evening. Likewise, the baseline cortisol levels in the evening were not different: 2.7±0.6 mg/dl in the PM/AM protocol and 3.2±0.8 mg/dl in the AM/PM protocol when vasopressin had been administered the same morning. However, similar to ACTH, the increment in serum cortisol in the morning was significantly blunted by exposure to vasopressin the previous evening in the PM/AM protocol, as compared with the morning response of AM/PM protocol when no vasopressin was administered previously (Fig. 6). It can also be seen that prior administration of vasopressin in the evening shifted the dose-response curve the next morning to the right, when the morning response of the PM/AM protocol is compared with the morning response of the AM/PM protocol. Again similar to the ACTH response, there was no significant difference in the cortisol levels in the afternoon whether there had or had not been prior exposure to vasopressin within the same 24 h (PM of AM/PM protocol vs. PM of PM/AM protocol, respectively, Fig. 6). ACTH response in relation to basal plasma cortisol. When the cumulative ACTH response for each individual on the two morning trials were compared with the baseline levels of cortisol in plasma, there was a significant and positive correlation Table II. Levels ofcortisol in Plasma during Each offour Tests Described in Text AM/PM protocol PM/AM protocol AM response PM response AM response PM response Pretest cortisol 16.36± ± ± ±0.64 Basal cortisol 14.02± ± ± ±1.69 Maximum increment in cortisol 16.64± ± ± ±3.37 Cumulative cortisol ± ± ± ±30.83 Dose at which significant cortisol increase occurred 0.1 IU 1 IU 1 IU 1 IU mg/dl Vasopressin Stimulation ofadrenocorticotropin Hormone in Humans 769
5 _- JO - -z a: -:: Up< 11 0 Z zo uio <1 Z EL.j E _: 0).oz <2.c cz w zo WEC VASOPRESSIN DOSE Figure 4. Dose-response increase of cortisol in the morning of the AM/PM protocol versus the evening of the PM/AM protocol. Each bar is the mean of the three cortisol samples for each subject after each dose of AVP. Baseline subtracted as in Fig. 3. The dose-response curve for cortisol is significantly shifted to the left in the morning of the AM/PM protocol when compared with the evening of the PM/AM protocol. Cortisol was significantly increased in the AM of the AM/PM protocol after 0.3 U of AVP (*P < 0.05, when compared with baseline value). The increase was not significant until 3 U of AVP in the evening of the PM/AM protocol (+P < 0.05, when compared with baseline value). (P < 0.05). However, there was no significant correlation between the ACTH response and the baseline cortisol in the evening trials when the baseline cortisols were all low. A VP results. When plasma AVP levels after each graded bolus of vasopressin were compared for each of the protocols, the mean values were not significantly different at any time points except in two isolated samples, the 55-min sample for the AM of the AM/PM protocol and the 60-min sample for the PM of the AM/PM protocol. These small differences were not felt to be meaningful because the samples immediately before o / tb I C AM of PM/AM TRIAL B PM of PM/AM TRIAL 0 PM of AMIPM TRIAL Figure S. Effect of prior administration of AVP on subsequent ACTH response to AVP for individual subjects. The ACTH response to vasopressin administered in the morning was significantly blunted if vasopressin had been administered the prior evening, AM of the PM/AM protocol (C) versus AM of the AM/PM protocol (A). There was no difference in the response in the evening regardless of the prior administration of AVP, PM of the PM/AM protocol (B) versus PM of the AM/PM protocol (D). MINUTES FROM FIRST INJECTION Figure 6. Effect of prior administration of AVP on subsequent cortisol response. Mean levels of cortisol (±SEM) for each of the four trials are shown. The cortisol response in the morning of the PM/AM protocol (o) was significantly blunted and the dose-response curve is shifted to the right when compared with the morning response of the AM/PM protocol (o) when no vasopressin was administered previously. There was no significant difference in the cortisol response in the evening of the AM/PM protocol (v) with prior exposure to vasopressin when compared with the evening of the PM/AM protocol (.) without prior vasopressin exposure. and after these points, 50 and 75 min, showed no differences, i.e., there was no trend. More importantly, significant changes in ACTH and cortisol measurements preceded rather than followed the only differences in plasma vasopressin described above. Mean plasma vasopressin after a 1-U bolus was 146.9±13.7 pg/ml while after a 3-U bolus the mean plasma vasopressin was 372.7±31.8 pg/ml. These levels of vasopressin, although much higher than physiological levels of peripherally measured vasopressin, are within the range of what has been measured in monkey portal blood (26). Discussion The 41-amino acid peptide, CRF, is probably the most important physiologic mediator of ACTH secretion in man. However, even before the isolation of CRF it was recognized that more than one physiologically active principle was present in hypothalamic extracts that possessed ACTH releasing activity (3, 4). Recently the hypothalamic peptide that has received the most attention as another potential physiological ACTH secretagogue is vasopressin. Vasopressin is localized anatomically in the external zone of the median eminence in the area of hormone secretion into long portal vessels that feed the anterior pituitary (27, 28). High levels of vasopressin have been measured in pituitary portal blood (27), and the content of vasopressin in the external zone is increased during some states of ACTH hypersecretion, i.e., after adrenalectomy in experimental animals (28-32). In direct studies it has also been demonstrated that vasopressin causes release of ACTH (12-16). When used alone, vasopressin is inferior as a secretagogue ofacth in comparison with CRF (9, 12, 13). However, both in the rat ( 13) and in man ( 14-16) there is clear synergism between the ACTH releasing activity of CRF and vasopressin, with a greater than additive effect on ACTH release when the 770 R. A. Salata, D. B. Jarrett, J. G. Verbalis, and A. G. Robinson
6 two are co-administered. While most of the studies that demonstrated synergism between these two secretagogues used experimental protocols in which both secretagogues were administered exogenously, it is reasonable to hypothesize that the administration of one secretagogue would also act synergistically with ambient concentrations of endogenous secretagogues. Evidence in support of this has been reported. ACTH secretion in response to administered CRF was reported as threefold greater during concurrent administration of hypertonic saline, which caused elevated levels of vasopressin (33, 34). We hypothesized that the converse might also be true; that is, exogenously administered vasopressin would act synergistically with endogenous levels of CRF. Indeed, because vasopressin is such a weak secretagogue by itself, the magnitude of the ACTH response to administered vasopressin might depend to a great extent upon the ambient level ofendogenous CRF in hypophyseal portal blood. This was given as a possible reason for the greater release of ACTH observed when vasopressin was administered to freely moving rats as compared with rats in which endogenous CRF was blocked by chlorpromazinemorphine-nembutal pretreatment or by anti-crf antiserum (13). To test this hypothesis in humans we used a protocol that was designed to produce a dose-response relationship between administered vasopressin and secretion of ACTH. We began with a dose of vasopressin that was estimated to be too low to produce any measurable increase in ACTH (0.1 U) and increased to a maximum of 3 U, which was still considerably less than the 5-10 U that have been used in other published studies of synergism in man (14-16). Because the half-life of vasopressin in man is 2-7 min (35, 36) and ofacth 15 min (37), we increased the administered dose of vasopressin every 15 min. While administration of vasopressin every 15 min did result in some cumulative effect on the level of ACTH, with the response to one dose of vasopressin adding to the residual response produced by the previous dose, nonetheless by starting with a low dose it was possible to develop a dose-response curve and to demonstrate shifts in the curve. There was a lower threshold of ACTH response, shift to the left, when the sensitivity of the axis was increased (morning vs. evening), and a shift to the right with a greater threshold response when the axis was dampened (morning response after exposure to vasopressin the previous evening). However, because the effect of the vasopressin doses on ACTH secretion was cumulative, it should not be anticipated that a single isolated bolus of vasopressin would give results similar to those obtained here at any point in the dose response. To investigate whether endogenous hypophyseal portal levels of CRF could be estimated by synergism with exogenously administered vasopressin, we made use of the wellknown diurnal rhythm of cortisol and ACTH. It is assumed, but not proven, that the diurnal rhythm of ACTH and cortisol is initiated by a diurnal rhythm of CRF, with higher endogenous levels of CRF in the morning and lower levels in the evening (17). This assumption is supported by the results of our studies, and of other recently published reports. In a report in humans with CRF deficiency, pulsatile administration of CRF with more frequent pulses in the morning produced a diurnal pattern of ACTH and cortisol secretion similar to that observed in normal subjects (38), and in another recent report, higher levels of CRF were measured in peripheral plasma in the morning (39). In our study administration of synthetic vasopressin produced a markedly enhanced release of ACTH in the morning as compared with the evening, consistent with synergism of vasopressin with high endogenous CRF in the morning. Note that this result is opposite of that for administration ofgraded boluses of exogenous CRF, where the greater release of ACTH and cortisol was found in the evening (17-20). In those studies there was a negative correlation between basal cortisol levels and both ACTH and cortisol response to CRF stimulation (17-20, 40). In these previous studies the lesser response of ACTH to CRF in the morning was thought possibly to be due to feedback inhibition at the pituitary by the higher level of ambient cortisol levels in the morning. However, in our studies it was apparent that the pituitary was not markedly inhibited in its ability to release ACTH in the morning, because the release of ACTH in response to administered vasopressin was greater in the morning. Thus, one alternate explanation for the results obtained with CRF is that endogenous hypophyseal portal levels of CRF are high in the morning, possibly approaching maximal response, and additional exogenously administered CRF caused little further release of ACTH. With this interpretation, the negative correlation between the ACTH response to CRF and the ambient level of cortisol would be explained, because the high level of cortisol reflected a high level of endogenous CRF (40). In contrast, when vasopressin was administered we found the ACTH release was positively correlated with the ambient level of plasma cortisol. We interpret this to support the concept that high levels of CRF are responsible for the high levels ofcortisol and that vasopressin acted synergistically with the endogenous CRF in hypophyseal portal blood. Therefore, based on the data above it would be reasonable to conclude that CRF is the major hypothalamic regulator of the ACTH and cortisol diurnal rhythm. However, this leaves unexplained the observation (41) that a diurnal rhythm of ACTH release persists even during continuous administration ofcrf. From that study (41) it was suggested that other factors with corticotropin releasing activity (i.e., vasopressin) also participated in the diurnal rhythm of ACTH release. Neither our results nor other published reports would necessarily negate any role of vasopressin in the diurnal rhythm of ACTH, but only suggest that the major secretagogue mediating the diurnal rhythm is CRF. When the AM trial was preceded by a graded vasopressin infusion on the previous evening (PM/AM protocol), a blunted response of both ACTH and cortisol were observed. There are three possible mechanisms for this observation. First, glucocorticoids are known modulators ofcrf-mediated ACTH release (40). They suppress pituitary responsiveness to CRF and possibly also inhibit secretion of CRF through an effect in the hypothalamus (42, 43). Second, it has been shown in vitro that exposure of corticotroph cells to various concentrations of dexamethasone down-regulates the CRF specific receptor on the cells in a dose-dependent fashion (44). Finally, pituitary desensitization to AVP may occur (similar to what has been shown for CRF in in vivo animal studies) independently of any steroid feedback (45). The anterior pituitary contains specific receptors for AVP which are distinct from those for hypothalamic CRF (46, 47), and prior injections of AVP might cause down-regulation of the AVP specific receptor on pituitary cells (47). To determine which, if any, of the explanations is correct will require further study. The site of action of vasopressin to enhance the effect of CRF is presumed to be at the level of the pituitary cortico- Vasopressin Stimulation ofadrenocorticotropin Hormone in Humans 771
7 troph. This assumption is based upon several observations. As described above, vasopressin is localized anatomically within the hypothalamic pituitary portal system in a manner that would allow vasopressin to directly stimulate pituitary corticotrophs (27, 28). Enhancement by AVP of the effect of CRF to release ACTH has been demonstrated in anterior pituitary cell cultures (48) and in isolated pituitary cell lines (44). With isolated cell lines, vasopressin increases the amount of cyclic AMP generated by administered CRF (49). In addition, both CRF and vasopressin receptors have been identified on rat anterior pituitary cells, and these appear to be independent receptors for which the alternate secretagogue is not competitive (44). Initial studies characterizing the vasopressin receptor on the anterior pituitary indicated that the corticotroph secreting activity was via a VI or pressor receptor (50). However, subsequent studies have produced disparate results on this point. In intact animals (e.g., dogs [51] and rats [unpublished results from our laboratory]), the ACTH secreting activity of intravenously administered vasopressin was completely blocked by a specific antagonist to the VI receptor, d(ch2)5 MeTyrAVP, and the effect of vasopressin was mimicked by VI agonists but not by V2 (antidiuretic) agonists (50). But, in studies of rat pituitary in vitro there was a poor correlation between the corticotropin releasing activity of vasopressin analogues and the VI receptor or pressor activity (52, 53). Likewise, the binding of vasopressin to rat anterior pituitary cell membrane preparations was not displaced by a VI antagonist (54). Baertschi and Friedli reported that the activity of vasopressin to act synergistically with CRF to release ACTH from suspensions of rat anterior pituitary cells was not blocked by two antipressor antagonists (55). Thus, there is a discrepency between studies done in vivo and studies in vitro. Studies in vivo have uniformly demonstrated that the ACTH secretagogue activity of vasopressin was related to the pressor action of vasopressin and was inhibited by antipressor antagonists. In vitro, while the enhancement ofcrf action could be obtained with vasopressin, that action of vasopressin was not uniformly related to the pressor effect and was not inhibited by antipressor antagonists. It has been suggested that the vasopressin receptor on the anterior pituitary is distinct from both the VI and the V2 receptors, and is a new class (V3) of receptor (54, 55). Until these issues are resolved, studies such as ours that use the natural hormone, AVP, may provide the most physiologically relevant information. We should emphasize that we used vasopressin as a test substance which gave data to support a diurnal variation of CRF secretion. These observations provide no further insight into the role of vasopressin (or CRF) as a physiologic regulator of ACTH release in adrenal insufficiency or in the variety of stresses that stimulate ACTH release. This subject has recently been reviewed in detail (56). However, one can use the knowledge of the diurnal variation in the ACTH responses to vasopressin and to CRF to interpret other studies in which stimulation of the hypothalamic-pituitary-adrenal axis was produced by more physiologic stimuli. Both administration ofa pyrogen (57) and of insulin (hypoglycemia) (58, 59) have been shown to produce a greater release of cortisol in the evening than in the morning. Because this pattern of response is similar to that obtained with administered CRF and opposite to that obtained with administered vasopressin, we would suggest that CRF is the more likely mediator of these stress responses in humans. In our study each test was done once in each subject and there was a considerable range in the response from subject to subject. This variability in response is probably intrinsic to the subject because (as can be seen from Figs. 1 and 2) the rank order of the subject's response was rather constant among the four times of testing. Because of the variability from person to person, more useful data might be obtained by comparison of the response to administered vasopressin with the response to administered CRF in the same subject. A heightened response to vasopressin coupled with a lower response to CRF would indicate that CRF was the important modulator of the increased ACTH in that particular setting. Such studies might be useful, for example, in defining subsets of Cushing's disease in which CRF hypersecretion was important, or in defining abnormalities ofcrf secretion as might occur in certain patients with depression. In summary, this study confirmed that vasopressin stimulates secretion of ACTH, and demonstrated that exogenously administered vasopressin acts synergistically with endogenous CRF in the release of ACTH. The morning and evening responses ofacth to administered vasopressin are the opposite of those to administered CRF (17-20). The greatest ACTH response was not in the morning after vasopressin infusion, but in the evening after CRF infusion. These data provide indirect evidence that the diurnal rhythm of ACTH and cortisol is secondary to a diurnal rhythm of CRF secretion. The protocol demonstrates that a vasopressin stimulation test may be useful in determining the relative level of CRF secretion, and may be applicable to future research on the pathophysiology of disorders of ACTH secretion. Acknowledgments We acknowledge the support of Jan McWilliams and the nursing staff of The Clinical Research Center, Presbyterian University Hospital, Pittsburgh, PA, Jean Miewald for statistical assistance, and Yvonne Jones and Jane Pennebaker for secretarial assistance. The studies were supported by The General Clinical Research Center NIH grant MOI-RR-00056, NIH grants AM (A. G. Robinson) and MH (D. B. Jarrett), and by the Veterans Administration (J. G. Verbalis). References 1. Saffran, M., A. V. Schally, and B. G. Bentey Stimulation of the release of corticotropin from the adenohypophysis by a neurohypophyseal factor. Endocrinology. 57: Guillemin, R., and B. Rosenberg Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 57: Krieger, D. T., and E. A. Zimmerman The nature ofcrf and its relationship to vasopressin. In Clinical Neuroendocrinology. L. Martini and G. M. Besser, editors. Academic Press, Inc., New York Yates, F. E., and J. W. Maran Stimulation and inhibition of adrenocorticotropin release. In Handbook of Physiology, Section 7: Endocrinology, Vol. 4. E. Knobil and W. H. Sawyer, editors. American Physiological Society, Washington, D.C Brostoff, J., V. H. T. James, and J. Landon Plasma corticosteroid and growth hormone response to lysine-vasopressin in man. J. Clin. Endocrinol. Metab. 28: McCann, S. M Effect of hypothalamic lesions on the adrenal cortical response to stress in the rat. Am. J. Physiol. 175: R. A. Salata, D. B. Jarrett, J. G. Verbalis, and A. G. Robinson
8 7. Sirett, N. E., and H. G. Purves The assay of corticotrophin-releasing factor in ACTH "primed" rats. In Brain-Pituitary-Adrenal Interrelationships. A. Brodish and E. S. Redgate, editors. S. Karger, Basel. pp Fleischer, N., and W. Vale Inhibition of vasopressin-induced ACTH release from the pituitary by glucocorticoids in vitro. Endocrinology. 83: Portanova, R., and G. Sawyers Isolated pituitary cells: CRF-like activity of neurohypophyseal and related polypeptides. Proc. Soc. Exp. Biol. Med. 143: Pearlmutter, A. F., L. Dokar, A. Kong, R. Miller, and M. S. Saffron Is corticotropin-releasing factor modulated vasopressin? Nature (Lond). 283: Gillies, G., and P. J. Lowry Corticotropin-releasing hormone and its vasopressin component. Front Neuroendocrinol. 7: Vale, W., and C. Rivier Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed. Proc. 36: Rivier, C., and W. Vale Interaction of corticotropin-releasing factor and arginine vasopressin on adenocorticotropin secretion in vivo. Endocrinology. 113: DeBold, C. R., W. R. Sheldon, G. S. DeCherney, R. V. Jackson, A. N. Alexander, W. Vale, J. Rivier, and D. N. Orth Arginine vasopressin potentiates adrenocorticotropin release induced by ovine corticotropin-releasing factor. J. Clin. Invest. 73: Lamberts, S. W. J., T. Verleun, R. Oosteron, F. DeJong, and W. H. L. Hackeng Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J. Clin. Endocrinol. Metab. 58: Liu, J. H., K. Muse, P. Contreras, D. Gibbs, W. Vale, J. Rivier and S. C. C. Yen Augmentation of ACTH-releasing activity of synthetic corticotropin releasing factor (CRF) by vasopressin in women. J. Clin. Endocrinol. Metab. 57: Chrousos, G. P., T. H. Schuermeyer, J. Doppman, E. H. Oldfield, H. M. Schulte, P. W. Gold, and D. L. Loriaux Clinical applications of corticotropin-releasing factor. Ann. Intern. Med. 102: DeCherney, G. S., C. R. DeBold, R. V. Jackson, W. R. Sheldon, D. P. Island, and D. N. Orth Diurnal variation in the response of plasma adrenocorticotropin and cortisol to intravenous ovine corticotropin-releasing hormone. J. Clin. Endocrinol. Metab. 61: Schulte, H. M., G. P. Chrousos, E. H. Oldfield, P. W. Gold, G. B. Cutler, Jr., and D. L. Loriaux Ovine corticotropin-releasing factor administration in normal men. Horm. Res. (Basel). 21: Tsukada, T., Y. Nakai, T. Koh, S. Tsujii, and H. Imura Plasma adrenocorticotropin and cortisol responses to intravenous injection of corticotropin-releasing factor in the morning and evening. J. Clin. Endocrinol. Metab. 57: Rivier, C., M. Brownstein, J. Spiess, J. Rivier, and W. Vale In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin B-endorphin and corticosterone. Endocrinology. 110: Copinschi, G., M. Beyloos, D. Bosson, D. Desir, J. Golstein, C. Robyn, P. Linkowski, J. Mendlewicz, and J. R. M. Franckson Immediate and delayed alterations ofadrenocorticotropin and cortisol nyctohemeral profiles after corticotropin-releasing factor in normal man. J. Clin. Endocrinol. Metab. 57: Schurmeyer, T. H., P. C. Avgerinos, P. W. Gold, W. T. Gallucci, T. P. Tomai, G. B. Cutler, Jr., D. L. Loriaux, and G. P. Chrousos Human corticotropin-releasing factor in man: pharmacokinetic properties and dose-response of plasma adrenocorticotropin and cortisol secretion. J. Clin. Endocrinol. Metab. 59: Salacinksi, P. R. P., C. McLean, J. E. C. Sykes, V. V. Clement- Jones, and P. J. Lowry Iodination ofproteins, glycoproteins and peptides using a solid-phase oxidizing agent, 1,3,4,6-Tetrachlor-3,6- diphenyl glycoluril (IODO-GEN). Anal. Biochem. 117: Rodbard, D. W., W. Bridson, and P. L. Rayford Rapid calculation of radioimmunoassay results. J. Lab. Clin. Med. 74: Uretsky, B. F., J. G. Verbalis, T. Generalovich, A. Valdes, and P. S. Reddy Plasma vasopressin response to osmotic and hemodynamic stimuli in heart failure. Am. J. Physiol. 248(Heart Circ. Physiol. 17):H396-H Zimmerman, E. A., P. W. Carmel, M. K. Husain, M. Ferin, M. Tannenbaum, A. G. Frantz, and A. G. Robinson Vasopressin and neurophysin: high concentrations in monkey hypophyseal portal blood. Science (Wash. DC). 182: Stillman, M., S. Recht, S. Rosario, S. Seif, A. G. Robinson, and E. A. Zimmerman The effects of adrenalectomy and glucocorticoid replacement on vasopressin and vasopressin-neurophysin in the zona externa of the median eminence of the rat. Endocrinology. 101: Sawchenko, P. E., L. W. Swanson, and W. W. Vale Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in paraventricular neurosecretory neurons of the adrenalectomized rat. Proc. Nat. Acad. Sci. USA. 81: Tramu, G., C. Croix, and A. Pillez Ability of the CRF immunoreactive neurons of the paraventricular nucleus to produce a vasopressin-like material. Neuroendocrinology. 37: Wolfson, B., R. W. Manning, L. G. Davis, R. Arentzen, and F. Baldino, Jr Co-localization of corticotropin releasing factor and vasopressin mrna in neurons after adrenalectomy. Nature (Lond.). 315: Roth, K. A., E. Weber, and J. D. Berckas Immunoreactive corticotropin releasing factor (CRF) and vasopressin are colocalized in an subpopulation of the immunoreactive cells in the paraventricular nucleus of the hypothalamus. Life Sci. 31: Milsom, S. R., J. V. Conaglen, R. A. Donald, E. A. Espiner, M. G. Nicholls, and J. H. Livesey Augmentation ofthe response to CRF in man: relative contributions of endogenous angiotensin and vasopressin. Clin. Endocrinol. 22: Rittmaster, R. S., G. B. Cutler, Jr., P. W. Gold, D. D. Brandon, T. Tomai, D. L. Loriaux, and G. P. Chrousos The relationship of saline-induced changes in vasopressin secretion to basal and corticotropin-releasing hormone-stimulated adrenocorticotropin and cortisol secretion in man. J. Clin. Endocrinol. Metab. 64(2): Gazis, D., and W. H. Sawyer Estimation of plasma half-lives of vasopressin analogs from vasopressin responses by curvefitting. Proc. Soc. Exp. Biol. Med. 157: Beardwell, C. G Radioimmunoassay of arginine vasopressin in human plasma. J. Clin. Endocrinol. Metab. 33: Sayers, G., and R. H. Travis Adrenocorticotrophic hormone; adrenocortical steroids and their synthetic analogs. In The Pharmacological Basis of Therapeutics. L. S. Goodman and A. Gilman, editors. Macmillan Press, New York Avgerinos, P. C., T. H. Schurmeyer, P. W. Gold, T. P. Tomai, D. L. Loriaux, R. J. Sherins, G. B. Cutler, Jr., and G. P. Chrousos Administration of human corticotropin-releasing hormone in patients with secondary adrenal insufficiency: restoration of the normal cortisol secretory pattern. J. Clin. Endocrinol. Metab. 62: Watabe, T., KK. Tanaka, M. Kumagae, S. Itoh, M. Hasegawa, T. Horiuchi, S. Miyabe, H. Ohno, and N. Shimizu Diurnal rhythm of plasma immunoreactive corticotropin-releasing factor in normal subjects. Life Sci. 40: Hermus, A. R. M. M., G. F. F. M. Pieters, A. G. H. Smals, T. H. J. Benraad, and P. W. C. Kloppenborg Plasma adrenocorticotropin, cortisol and aldosterone responses to corticotropin-releasing factor: modulatory effect of basal cortisol levels. J. Clin. Endocrinol. Metab. 58: Schulte, H. M., G. P. Chrousos, P. W. Gold, J. D. Booth, E. H. Oldfield, G. B. Cutler, Jr., and D. L. Loriaux Continuous ad- Vasopressin Stimulation ofadrenocorticotropin Hormone in Humans 773
MARI NAKAHARA HOTTA, TAMOTSU SHIBASAKI, TOSHIHIRO SUDA, NICHOLAS LING* AND KAZUO SHIZUME
 Endocrinol. Japon. 1985, 32 (1), 113-125 The Use of the Corticotropin-Releasing Hormone Test to Monitor the Recovery of Patients with Cushing's Disease or Cushing's Syndrome Due to an Adrenal Adenoma after
Endocrinol. Japon. 1985, 32 (1), 113-125 The Use of the Corticotropin-Releasing Hormone Test to Monitor the Recovery of Patients with Cushing's Disease or Cushing's Syndrome Due to an Adrenal Adenoma after
Adrenocorticotropin Responses to Corticotropin Releasing Factor and Vasopressin in Spontaneously Hypertensive Rats
 Laboratory Studies Adrenocorticotropin Responses to Corticotropin Releasing Factor and Vasopressin in Spontaneously Hypertensive Rats TERUHIKO HATTORI, KOZO HASHIMOTO, AND ZENSUKE OTA SUMMARY The effects
Laboratory Studies Adrenocorticotropin Responses to Corticotropin Releasing Factor and Vasopressin in Spontaneously Hypertensive Rats TERUHIKO HATTORI, KOZO HASHIMOTO, AND ZENSUKE OTA SUMMARY The effects
Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY
 Excerpted from: Journal Title: Endocrinology. Volume: 89 Issue: 4 October 1971 Pages: 1024-8 Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY Department of Physiology,
Excerpted from: Journal Title: Endocrinology. Volume: 89 Issue: 4 October 1971 Pages: 1024-8 Effect of Orchiectomy on Pituitary Secretion of ACTH MARY D. COYNE AND JULIAN I. KITAY Department of Physiology,
Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat
 J. Biosci., Vol. 15, Number 1, March 1990, pp. 17 21. Printed in India. Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat E. VIJAYAN* and S. M. McCANN Department of Physiology,
J. Biosci., Vol. 15, Number 1, March 1990, pp. 17 21. Printed in India. Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat E. VIJAYAN* and S. M. McCANN Department of Physiology,
The endocrine system is made up of a complex group of glands that secrete hormones.
 1 10. Endocrinology I MEDCHEM 535 Diagnostic Medicinal Chemistry Endocrinology The endocrine system is made up of a complex group of glands that secrete hormones. These hormones control reproduction, metabolism,
1 10. Endocrinology I MEDCHEM 535 Diagnostic Medicinal Chemistry Endocrinology The endocrine system is made up of a complex group of glands that secrete hormones. These hormones control reproduction, metabolism,
significantly suppressed this increase in plasma ACTH.
 Journal of Physiology (1991), 443, pp. 431-439 431 With 3 figures Printed in Great Britain ACTH RELEASE INDUCED IN RATS BY NORADRENALINE IS MEDIATED BY PROSTAGLANDIN E2 BY TATSUO WATANABE, AKIO MORIMOTO,
Journal of Physiology (1991), 443, pp. 431-439 431 With 3 figures Printed in Great Britain ACTH RELEASE INDUCED IN RATS BY NORADRENALINE IS MEDIATED BY PROSTAGLANDIN E2 BY TATSUO WATANABE, AKIO MORIMOTO,
Plasma levels of gonadotropin releasing hormone during menstrual cycle of Macaca radiata
 J. Biosci., Vol. 10, Number 4, December 1986, pp. 423-428. Printed in India. Plasma levels of gonadotropin releasing hormone during menstrual cycle of Macaca radiata N. MATHIALAGAN and A. JAGANNADHA RAO
J. Biosci., Vol. 10, Number 4, December 1986, pp. 423-428. Printed in India. Plasma levels of gonadotropin releasing hormone during menstrual cycle of Macaca radiata N. MATHIALAGAN and A. JAGANNADHA RAO
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone
 Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
Endocrine part one. Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy
 Endocrine part one Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy HORMONES Hormones are chemicals released by a cell or a gland
Endocrine part one Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy HORMONES Hormones are chemicals released by a cell or a gland
Human Obestatin ELISA
 K-ASSAY Human Obestatin ELISA For the quantitative determination of obestatin in human serum and plasma Cat. No. KT-495 For Research Use Only. 1 Rev. 081309 K-ASSAY PRODUCT INFORMATION Human Obestatin
K-ASSAY Human Obestatin ELISA For the quantitative determination of obestatin in human serum and plasma Cat. No. KT-495 For Research Use Only. 1 Rev. 081309 K-ASSAY PRODUCT INFORMATION Human Obestatin
Objectives. Pathophysiology of Steroids. Question 1. Pathophysiology 3/1/2010. Steroids in Septic Shock: An Update
 Objectives : An Update Michael W. Perry PharmD, BCPS PGY2 Critical Care Resident Palmetto Health Richland Hospital Review the history of steroids in sepsis Summarize the current guidelines for steroids
Objectives : An Update Michael W. Perry PharmD, BCPS PGY2 Critical Care Resident Palmetto Health Richland Hospital Review the history of steroids in sepsis Summarize the current guidelines for steroids
HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES
 HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES TERUO NAKAYAMA* Institute of Physiology, School of Medicine, University of Nagoya It is known that electrical
HYPOTHALAMIC ELECTRICAL ACTIVITIES PRODUCED BY FACTORS CAUSING DISCHARGE OF PITUITARY HORMONES TERUO NAKAYAMA* Institute of Physiology, School of Medicine, University of Nagoya It is known that electrical
Feedback Inhibition of Adrenocorticotropic Hormone by Physiological Increases in Plasma Corticosteroids in Conscious Dogs
 Feedback Inhibition of Adrenocorticotropic Hormone by Physiological Increases in Corticosteroids in Conscious Dogs MAUREEN E. KELILIR-WOOD, JEANETTE SHINSAKO, and MARY F. DALIMAN, Department of Physiology
Feedback Inhibition of Adrenocorticotropic Hormone by Physiological Increases in Corticosteroids in Conscious Dogs MAUREEN E. KELILIR-WOOD, JEANETTE SHINSAKO, and MARY F. DALIMAN, Department of Physiology
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES:
 ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES: -In a living organism there must be coordination of number of physiological activities taking place simultaneously such as: movement, respiration,
ENDOCRINOLOGY COORDINATION OF PHYSIOLOGICAL PROCESSES: -In a living organism there must be coordination of number of physiological activities taking place simultaneously such as: movement, respiration,
Art labeling Activity: Figure 16.1
 ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
ADRENOCORTICAL ACTIVITY OF ADENO- HYPOPHYSECTOMIZED
 The Japanese Journal of Physiology- 14, pp.265-269, 1964 ADRENOCORTICAL ACTIVITY OF ADENO- HYPOPHYSECTOMIZED RATS Shinji ITOH AND Makoto YAMAMOTO * Department of Physiology, Hokkaido University School
The Japanese Journal of Physiology- 14, pp.265-269, 1964 ADRENOCORTICAL ACTIVITY OF ADENO- HYPOPHYSECTOMIZED RATS Shinji ITOH AND Makoto YAMAMOTO * Department of Physiology, Hokkaido University School
ULTIMATE BEAUTY OF BIOCHEMISTRY. Dr. Veena Bhaskar S Gowda Dept of Biochemistry 30 th Nov 2017
 ULTIMATE BEAUTY OF BIOCHEMISTRY Dr. Veena Bhaskar S Gowda Dept of Biochemistry 30 th Nov 2017 SUSPECTED CASE OF CUSHING S SYNDROME Clinical features Moon face Obesity Hypertension Hunch back Abdominal
ULTIMATE BEAUTY OF BIOCHEMISTRY Dr. Veena Bhaskar S Gowda Dept of Biochemistry 30 th Nov 2017 SUSPECTED CASE OF CUSHING S SYNDROME Clinical features Moon face Obesity Hypertension Hunch back Abdominal
(Received 9th January 1974)
 RELEASE OF LH AND FSH IN THE NORMAL INTACT RAM BY SYNTHETIC LH-RF AND THE EFFECT OF PRETREATMENT WITH TESTOSTERONE PROPIONATE C. R. N. HOPKINSON, H. C. PANT and R. J. FITZPATRICK Department of Veterinary
RELEASE OF LH AND FSH IN THE NORMAL INTACT RAM BY SYNTHETIC LH-RF AND THE EFFECT OF PRETREATMENT WITH TESTOSTERONE PROPIONATE C. R. N. HOPKINSON, H. C. PANT and R. J. FITZPATRICK Department of Veterinary
ANGIOTENSIN IMMUNOREACTIVITY IN VASOPRESSIN CELLS IN RAT HYPOTHALAMUS AND ITS RELATIVE DEFICIENCY IN HOMOZYGOUS BRATTLEBORO RATS *
 ANGIOTENSIN IMMUNOREACTIVITY IN VASOPRESSIN CELLS IN RAT HYPOTHALAMUS AND ITS RELATIVE DEFICIENCY IN HOMOZYGOUS BRATTLEBORO RATS * D. L. Hoffman, L. Krupp, D. Schrag, G. Nilaver, G. Valiquette, M. M. Kilcoyne,
ANGIOTENSIN IMMUNOREACTIVITY IN VASOPRESSIN CELLS IN RAT HYPOTHALAMUS AND ITS RELATIVE DEFICIENCY IN HOMOZYGOUS BRATTLEBORO RATS * D. L. Hoffman, L. Krupp, D. Schrag, G. Nilaver, G. Valiquette, M. M. Kilcoyne,
The analysis of Glucocorticoid Steroids in Plasma, Urine and Saliva by UPLC/MS/MS
 The analysis of Glucocorticoid Steroids in Plasma, Urine and Saliva by UPLC/MS/MS Brett McWhinney, Supervising Scientist, HPLC Section, Pathology Central, Pathology Queensland Overview 1. Overview of Pathology
The analysis of Glucocorticoid Steroids in Plasma, Urine and Saliva by UPLC/MS/MS Brett McWhinney, Supervising Scientist, HPLC Section, Pathology Central, Pathology Queensland Overview 1. Overview of Pathology
Monday, 7 th of July 2008 ( ) University of Buea MED30. (GENERAL ENDOCRINOLOGY) Exam ( )
 .. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
.. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
Adrenocorticotropic Hormone (ACTH) ELISA
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. ACTH ELISA Kit is intended for the quantitative determination of ACTH Adrenocorticotropic Hormone) in human plasma
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. ACTH ELISA Kit is intended for the quantitative determination of ACTH Adrenocorticotropic Hormone) in human plasma
CHISCG1: Short Synacthen Test for the Investigation of Adrenal Insufficiency
 Pathology at the Royal Derby Hospital Short Synacthen Test Standard Clinical Guidelines Chemical Pathology Department Valid Until 31 st August 2011 Document Code: CHISCG1 Short Synacthen Test for the Investigation
Pathology at the Royal Derby Hospital Short Synacthen Test Standard Clinical Guidelines Chemical Pathology Department Valid Until 31 st August 2011 Document Code: CHISCG1 Short Synacthen Test for the Investigation
Cortisol (serum, plasma)
 Cortisol (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Cortisol 1.2 Alternative names Hydrocortisone, 11β; 17, 21 trihydroxypregn 4 ene 3,20 dione 1.3 NMLC code 1.4 Description
Cortisol (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Cortisol 1.2 Alternative names Hydrocortisone, 11β; 17, 21 trihydroxypregn 4 ene 3,20 dione 1.3 NMLC code 1.4 Description
Ahmed Al Nahari Pediatric Endocrinology Fellow March 11,2016
 Ahmed Al Nahari Pediatric Endocrinology Fellow March 11,2016 Scholar: Review the literature in an evidenced based manner to determine the difference in our clinical setting. Advocate: Develop a better
Ahmed Al Nahari Pediatric Endocrinology Fellow March 11,2016 Scholar: Review the literature in an evidenced based manner to determine the difference in our clinical setting. Advocate: Develop a better
Endocrine Glands: Hormone-secreting organs are called endocrine glands
 University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
ANNALS OF THE NEW YORK ACADEMY OF SCIENCES
 Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES ANGIOTENSIN IMMUNOREACTIVITY IN VASOPRESSIN CELLS IN RAT HYPOTHALAMUS AND ITS RELATIVE DEFICIENCY IN HOMOZYGOUS BRA TTLEBORO RATS * D. L. Hoffman,
Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES ANGIOTENSIN IMMUNOREACTIVITY IN VASOPRESSIN CELLS IN RAT HYPOTHALAMUS AND ITS RELATIVE DEFICIENCY IN HOMOZYGOUS BRA TTLEBORO RATS * D. L. Hoffman,
Adrenal gland consist of: Outer Cortex and Inner Medulla Hormones secreted by Adrenal Cortex are: Glucocorticoid, Mineralocorticoid and Sex Steroids
 1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL MBBS Year III; BMLS & BDS Year 3 ADRENAL
1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL MBBS Year III; BMLS & BDS Year 3 ADRENAL
THE ADRENAL (SUPRARENAL) GLANDS
 THE ADRENAL (SUPRARENAL) GLANDS They are two glands, present above the kidneys. One adrenal gland is sufficient for human beings/mammals (example: we also have two kidneys but one is sufficient). The Adrenal
THE ADRENAL (SUPRARENAL) GLANDS They are two glands, present above the kidneys. One adrenal gland is sufficient for human beings/mammals (example: we also have two kidneys but one is sufficient). The Adrenal
The Third Department of Internal Medicine, University of Tokyo Faculty of Medicine, Hongo, Tokyo 113
 Endocrinol. Japon. 1974, 21 (2), 115 ` 119 A Radioimmunoassay for Serum Dehydroepiandrosterone HISAHIKO SEKIHARA, TOHRU YAMAJI, NAKAAKI OHSAWA AND HIROSHI IBAYASHI * The Third Department of Internal Medicine,
Endocrinol. Japon. 1974, 21 (2), 115 ` 119 A Radioimmunoassay for Serum Dehydroepiandrosterone HISAHIKO SEKIHARA, TOHRU YAMAJI, NAKAAKI OHSAWA AND HIROSHI IBAYASHI * The Third Department of Internal Medicine,
Is the plasma ACTH concentration a reliable parameter in the insulin tolerance test?
 European Journal of Endocrinology (2003) 149 535 541 ISSN 0804-4643 CLINICAL STUDY Is the plasma ACTH concentration a reliable parameter in the insulin tolerance test? K Borm, M Slawik, L Seiler, F Flohr,
European Journal of Endocrinology (2003) 149 535 541 ISSN 0804-4643 CLINICAL STUDY Is the plasma ACTH concentration a reliable parameter in the insulin tolerance test? K Borm, M Slawik, L Seiler, F Flohr,
(Adams 8c Purves 1958), or LATS-protector (LATS-P) (Adams 8c Kennedy. 1967). The failure of the McKenzie (1958) mouse bioassay to detect LATS in
 Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Adrenocortical Insufficiency: Addison's Disease
 280 PHYSIOLOGY CASES AND PROBLEMS Case 49 Adrenocortical Insufficiency: Addison's Disease Susan Oglesby is a 41-year-old divorced mother of two teenagers. She has always been in excellent health. She recently
280 PHYSIOLOGY CASES AND PROBLEMS Case 49 Adrenocortical Insufficiency: Addison's Disease Susan Oglesby is a 41-year-old divorced mother of two teenagers. She has always been in excellent health. She recently
Cortisol (Sheep) ELISA Kit
 Cortisol (Sheep) ELISA Kit Catalog Number KA0919 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
Cortisol (Sheep) ELISA Kit Catalog Number KA0919 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
DIAGNOSIS OF CANINE HYPERADRENOCORTICISM: A CASE-BASED APPROACH Ellen N. Behrend, VMD, PhD, DACVIM
 DIAGNOSIS OF CANINE HYPERADRENOCORTICISM: A CASE-BASED APPROACH Ellen N. Behrend, VMD, PhD, DACVIM Case 1: Signalment: 10 yr old, CM, Miniature poodle; History: Presented for teeth cleaning; PE: Severe
DIAGNOSIS OF CANINE HYPERADRENOCORTICISM: A CASE-BASED APPROACH Ellen N. Behrend, VMD, PhD, DACVIM Case 1: Signalment: 10 yr old, CM, Miniature poodle; History: Presented for teeth cleaning; PE: Severe
A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man *
 Journal of Clinical Investigation Vol. 43, No. 1, 1964 A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man * STEFAN S. FAJANS, JOHN C. FLOYD, JR., RALPH F. KNOPF, AND JEROME W. CONN (From
Journal of Clinical Investigation Vol. 43, No. 1, 1964 A Comparison of Leucine- and Acetoacetate-induced Hypoglycemia in Man * STEFAN S. FAJANS, JOHN C. FLOYD, JR., RALPH F. KNOPF, AND JEROME W. CONN (From
What Current Research Says About Measuring Cortisol and the HPA axis
 What Current Research Says About Measuring Cortisol and the HPA axis Recent research provides a clearer link between stress and its impact on health. Whether that stress is acute or chronic, it can affect
What Current Research Says About Measuring Cortisol and the HPA axis Recent research provides a clearer link between stress and its impact on health. Whether that stress is acute or chronic, it can affect
had no effect on the production of aldosterone, corticosterone, or cortisol after
 INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
INHIBITION OF THE EFFECTS OF ANGIOTENSIN II ON ADRENAL STEROID PRODUCTION BY DIETARY SODIUM BY WARREN W. DAVIS,* LAWRENCE R. BURWELL,t AND FREDERIC C. BARTTERt ENDOCRINOLOGY BRANCH, NATIONAL HEART INSTITUTE,
Endocrine Topic Review. Sethanant Sethakarun, MD
 Endocrine Topic Review Sethanant Sethakarun, MD Definition Cushing's syndrome comprises a large group of signs and symptoms that reflect prolonged and in appropriately high exposure of tissue to glucocorticoids
Endocrine Topic Review Sethanant Sethakarun, MD Definition Cushing's syndrome comprises a large group of signs and symptoms that reflect prolonged and in appropriately high exposure of tissue to glucocorticoids
Where in the adrenal cortex is cortisol produced? How do glucocorticoids inhibit prostaglandin production?
 CASE 35 A 36-year-old woman presents to her gynecologist with complaints of amenorrhea and hirsutism. She has also noticed an increase in her weight (especially in the trunk region) and easy fatigability.
CASE 35 A 36-year-old woman presents to her gynecologist with complaints of amenorrhea and hirsutism. She has also noticed an increase in her weight (especially in the trunk region) and easy fatigability.
Onset and Dose Relationships of ACTH Effects on Blood Pressure in Sheep
 Onset and Dose Relationships of ACTH Effects on Blood Pressure in Sheep BRUCE A. SCOGGINS, KINGSLEY J. ALLEN, JOHN P. COGHLAN, DEREK A. DENTON, DAVID T.W. JANETTE J. TRESHAM, XIAOMING WANG, AND JUDITH
Onset and Dose Relationships of ACTH Effects on Blood Pressure in Sheep BRUCE A. SCOGGINS, KINGSLEY J. ALLEN, JOHN P. COGHLAN, DEREK A. DENTON, DAVID T.W. JANETTE J. TRESHAM, XIAOMING WANG, AND JUDITH
4/23/2018. Endocrine System: Overview. Endocrine System: Overview
 Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
Diabetologia 9 Springer-Verlag 1984
 Diabetologia (1984) 26:203 207 Diabetologia 9 Springer-Verlag 1984 How does glucose regulate the human pancreatic A cell in vivo? C. M. Asplin*, P. M. Hollander** and J. P. Palmer Diabetes Research Center
Diabetologia (1984) 26:203 207 Diabetologia 9 Springer-Verlag 1984 How does glucose regulate the human pancreatic A cell in vivo? C. M. Asplin*, P. M. Hollander** and J. P. Palmer Diabetes Research Center
Kovilvenni, Thiruvarur Dist.,Tamilnadu, India. ABSTRACT:
 A Mathematical Reliability Growth Model using for Acute HPA Axis Responses, Heart Rate and Mood Changes to Psychosocial Stress in Human at Different s of Day S. Lakshmi * and A. Manickam ** * Principal
A Mathematical Reliability Growth Model using for Acute HPA Axis Responses, Heart Rate and Mood Changes to Psychosocial Stress in Human at Different s of Day S. Lakshmi * and A. Manickam ** * Principal
Note: During 30 minute incubation; proceed thru appropriate sections below (e.g. sections II, III and V).
 LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
The physiology of corticotropin-releasing factor (CRF)
 The physiology of corticotropin-releasing factor (CRF) Marie-Charles Raux-Demay, F. Girard To cite this version: Marie-Charles Raux-Demay, F. Girard. The physiology of corticotropin-releasing factor (CRF).
The physiology of corticotropin-releasing factor (CRF) Marie-Charles Raux-Demay, F. Girard To cite this version: Marie-Charles Raux-Demay, F. Girard. The physiology of corticotropin-releasing factor (CRF).
The Acute Effects of Steroid Administration on Pituitary Adrenal Secretion in the Dog *
 Journal of Clinical Investigation Vol. 43, No. 11, 1964 The Acute Effects of Steroid Administration on Pituitary Adrenal Secretion in the Dog * RICHARD H. EGDAHL t (From the Department of Surgery, Medical
Journal of Clinical Investigation Vol. 43, No. 11, 1964 The Acute Effects of Steroid Administration on Pituitary Adrenal Secretion in the Dog * RICHARD H. EGDAHL t (From the Department of Surgery, Medical
Sermorelin as an Alternative to hgh for Treating GH Insufficiency of Aging
 Sermorelin as an Alternative to hgh for Treating GH Insufficiency of Aging Richard F. Walker, Ph.D., R.Ph., Executive Director, Society for Applied Research in Aging (SARA) (www.agesociety.org) SOMATOPAUSE
Sermorelin as an Alternative to hgh for Treating GH Insufficiency of Aging Richard F. Walker, Ph.D., R.Ph., Executive Director, Society for Applied Research in Aging (SARA) (www.agesociety.org) SOMATOPAUSE
DELFIA Tb-DTPA ITC Chelate & Terbium Standard
 AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
AD0035P-2 (en) 1 DELFIA Tb-DTPA ITC Chelate & AD0029 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-DTPA ITC Chelate is optimized for the terbium labelling of proteins and peptides for use
Corticosteroids. Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology
 Corticosteroids Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology Objectives By the end of this lecture you should be
Corticosteroids Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology Objectives By the end of this lecture you should be
norepinephrinee." 2 PNMT activity is stimulated by certain adrenocortical markedly,3' 4 but can be restored to normal by the administration of
 IMPAIRED SECRETION OF EPINEPHRINE IN RESPONSE TO INSULIN AMONG HYPOPHYSECTOMIZED DOGS* BY RICHARD J. WURTMAN, ALFRED CASPER, LARISSA A. POHORECKY, AND FREDERIC C. BARTTER DEPARTMENT OF NUTRITION AND FOOD
IMPAIRED SECRETION OF EPINEPHRINE IN RESPONSE TO INSULIN AMONG HYPOPHYSECTOMIZED DOGS* BY RICHARD J. WURTMAN, ALFRED CASPER, LARISSA A. POHORECKY, AND FREDERIC C. BARTTER DEPARTMENT OF NUTRITION AND FOOD
Radioimmunoassay for plasma renin activity
 Radioimmunoassay for plasma renin activity Manjula S. Kumar, Ph.D.* Division of Sharad Ph.D. Section on Research ' Fellow, Division of D. Deodhar, M.D., Immunopathology Research. The role of the kidney
Radioimmunoassay for plasma renin activity Manjula S. Kumar, Ph.D.* Division of Sharad Ph.D. Section on Research ' Fellow, Division of D. Deodhar, M.D., Immunopathology Research. The role of the kidney
Interrelationship between Angiotensin Catecholamines. Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D.
 Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
Interrelationship between Angiotensin and Catecholamines Tatsuo SATO, M.D., Masaru MAEBASHI, M.D., Koji GOTO, M.D., and Kaoru YOSHINAGA, M.D. SUMMARY Urinary catecholamines were measured with an attempt
2) Storehouse for the hormones produced by the hypothalamus of the brain. 2)
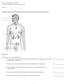 AP 2 Exam Chapter 16 Endocrie Due Wed. night 4/22 or Thurs. morning 4/23 Name: Matching; match the labeled organ with the most appropriate response or identification. Figure 16.1 Using Figure 16.1, match
AP 2 Exam Chapter 16 Endocrie Due Wed. night 4/22 or Thurs. morning 4/23 Name: Matching; match the labeled organ with the most appropriate response or identification. Figure 16.1 Using Figure 16.1, match
Hypothalamus & Pituitary Gland
 Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS
 Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
Glucagon Kit. Procedure for the Radioimmunoassay of Human Plasma Glucagon TABLE OF CONTENTS. For In Vitro Diagnostic Use I. INTRODUCTION...
 TABLE OF CONTENTS I. INTRODUCTION.................................... 1 II. EXPECTED NORMAL VALUES........................ 1 Glucagon Kit III. PRINCIPLE........................................ 2 IV. SPECIMEN
TABLE OF CONTENTS I. INTRODUCTION.................................... 1 II. EXPECTED NORMAL VALUES........................ 1 Glucagon Kit III. PRINCIPLE........................................ 2 IV. SPECIMEN
Uniphasic Insulin Responses to Secretin Stimulation in Man
 Uniphasic Insulin Responses to Stimulation in Man ROGER L. LERNER and DANIEL PORTE, JR. From the University of Washington School of Medicine and Veterans Administration Hospital, Seattle, Washington 98108
Uniphasic Insulin Responses to Stimulation in Man ROGER L. LERNER and DANIEL PORTE, JR. From the University of Washington School of Medicine and Veterans Administration Hospital, Seattle, Washington 98108
Endocrine System Notes
 Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
The endocrine system is complex and sometimes poorly understood.
 1 CE Credit Testing the Endocrine System for Adrenal Disorders and Diabetes Mellitus: It Is All About Signaling Hormones! David Liss, BA, RVT, VTS (ECC) Platt College Alhambra, California For more information,
1 CE Credit Testing the Endocrine System for Adrenal Disorders and Diabetes Mellitus: It Is All About Signaling Hormones! David Liss, BA, RVT, VTS (ECC) Platt College Alhambra, California For more information,
The Endocrine System. PowerPoint Lecture Presentations prepared by Jason LaPres. Lone Star College North Harris
 18 The Endocrine System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris NOTE: Presentations extensively modified for use in MCB 244 & 246 at the University of Illinois
18 The Endocrine System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris NOTE: Presentations extensively modified for use in MCB 244 & 246 at the University of Illinois
General Protocol for RK CGRP (Rat, Mouse) - RIA Kit. (range: pg/ml)
 General Protocol for RK-015-09 CGRP (Rat, Mouse) - RIA Kit (range: 10-1280 pg/ml) introduction This kit is designed to measure a specific peptide and its related peptides by a competitive radioimmunoassay.
General Protocol for RK-015-09 CGRP (Rat, Mouse) - RIA Kit (range: 10-1280 pg/ml) introduction This kit is designed to measure a specific peptide and its related peptides by a competitive radioimmunoassay.
C h a p t e r 3 8 Cushing s Syndrome : Current Concepts in Diagnosis and Management
 C h a p t e r 3 8 Cushing s Syndrome : Current Concepts in Diagnosis and Management Padma S Menon Professor of Endocrinology, Seth G S Medical College & KEM Hospital, Mumbai A clinical syndrome resulting
C h a p t e r 3 8 Cushing s Syndrome : Current Concepts in Diagnosis and Management Padma S Menon Professor of Endocrinology, Seth G S Medical College & KEM Hospital, Mumbai A clinical syndrome resulting
Chapter 11 - Endocrine System
 Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
ENDOCRINE SYSTEM. Endocrine
 ENDOCRINE SYSTEM Endocrine Function Help regulate internal functions Use chemical messengers Recall: Endocrine vs. Exocrine glands Nervous System vs Endocrine System Target Specificity Lock n Key action
ENDOCRINE SYSTEM Endocrine Function Help regulate internal functions Use chemical messengers Recall: Endocrine vs. Exocrine glands Nervous System vs Endocrine System Target Specificity Lock n Key action
Prostaglandin E2 ELISA Kit - Monoclonal
 Prostaglandin E2 ELISA Kit - Monoclonal Cat. No.:DEIA4977 Pkg.Size:96T/480T General Description Prostaglandin E2 (PGE2) is a primary product of arachidonic acid metabolism in many cells. Like most eicosanoids,
Prostaglandin E2 ELISA Kit - Monoclonal Cat. No.:DEIA4977 Pkg.Size:96T/480T General Description Prostaglandin E2 (PGE2) is a primary product of arachidonic acid metabolism in many cells. Like most eicosanoids,
Hypothalamic Control of Posterior Pituitary
 Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
Hypothalamic Control of Posterior Pituitary Hypothalamus neuron cell bodies produce ADH: supraoptic nuclei Oxytocin: paraventricular nuclei Transported along the hypothalamohypophyseal tract Stored in
Effects of Novel Growth Hormone Secretagogues on Growth Hormone Secretion in Farm Animals
 Beef Research Report, 1996 Animal Science Research Reports 1997 Effects of Novel Growth Hormone Secretagogues on Growth Hormone Secretion in Farm Animals Lloyd L. Anderson Iowa State University Follow
Beef Research Report, 1996 Animal Science Research Reports 1997 Effects of Novel Growth Hormone Secretagogues on Growth Hormone Secretion in Farm Animals Lloyd L. Anderson Iowa State University Follow
Standardization of Insulin Immunoassays: Report of the American. Supplemental Information: Complete Materials and Methods
 Standardization of Insulin Immunoassays: Report of the American Diabetes Association s Workgroup Santica Marcovina 1, Ronald R. Bowsher 2, Greg Miller 3, Myrlene Staten 4, Gary Myers 5, Samuel P. Caudill
Standardization of Insulin Immunoassays: Report of the American Diabetes Association s Workgroup Santica Marcovina 1, Ronald R. Bowsher 2, Greg Miller 3, Myrlene Staten 4, Gary Myers 5, Samuel P. Caudill
FOR LABORATORY USE ONLY. <Distributed by> DF Kasumigaseki Place, 3-6-7, Kasumigaseki, Chiyoda-ku Tokyo Japan
 YK191 Rat Urocortin 2 EIA FOR LABORATORY USE ONLY DF Kasumigaseki Place, 3-6-7, Kasumigaseki, Chiyoda-ku Tokyo 100-0013 Japan URL: http://www.sceti.co.jp/export/ e-mail: exp-pet@sceti.co.jp
YK191 Rat Urocortin 2 EIA FOR LABORATORY USE ONLY DF Kasumigaseki Place, 3-6-7, Kasumigaseki, Chiyoda-ku Tokyo 100-0013 Japan URL: http://www.sceti.co.jp/export/ e-mail: exp-pet@sceti.co.jp
Metabolism and Kinetics of Adrenocortical Steroids. -A Consideration on Corticosteroid Therapy-
 Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
Metabolism and Kinetics of Adrenocortical Steroids -A Consideration on Corticosteroid Therapy- Yoshitaka Araki First Department of Internal Medicine, Faculty of Medicine, University of Tokyo, Tokyo I.
Hormonal Proteins And Peptides, Volume XIII: Corticotropin (ACTH)
 Hormonal Proteins And Peptides, Volume XIII: Corticotropin (ACTH) If searching for a book Hormonal Proteins and Peptides, Volume XIII: Corticotropin (ACTH) in pdf format, then you have come on to faithful
Hormonal Proteins And Peptides, Volume XIII: Corticotropin (ACTH) If searching for a book Hormonal Proteins and Peptides, Volume XIII: Corticotropin (ACTH) in pdf format, then you have come on to faithful
Neuroendocrine challenges following hemispherectomy
 Neuroendocrine challenges following hemispherectomy Philip S. Zeitler MD. PhD Professor and Head Section of Endocrinology Children s Hospital Colorado University of Colorado Anschutz Medical Campus I am
Neuroendocrine challenges following hemispherectomy Philip S. Zeitler MD. PhD Professor and Head Section of Endocrinology Children s Hospital Colorado University of Colorado Anschutz Medical Campus I am
ASY-857.1: Synacthen Stimulated 17OH-progesterone Test
 ASY-857.1: Synacthen Stimulated 17OH-progesterone ASY-857.2: Associated Documents a Synacthen Standing Order form (ref 0827/2) G:\Division\NDO\common\ETCProtocols\0827 Standing Order Synacthen 2016.pdf
ASY-857.1: Synacthen Stimulated 17OH-progesterone ASY-857.2: Associated Documents a Synacthen Standing Order form (ref 0827/2) G:\Division\NDO\common\ETCProtocols\0827 Standing Order Synacthen 2016.pdf
Hompes Method. Practitioner Training Level II. Lesson Thirty-one The Adrenals
 Hompes Method Practitioner Training Level II Lesson Thirty-one The Adrenals Health for the People Ltd not for reuse without expressed permission Hompes Method is a trading name of Health For The People
Hompes Method Practitioner Training Level II Lesson Thirty-one The Adrenals Health for the People Ltd not for reuse without expressed permission Hompes Method is a trading name of Health For The People
Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab)
 Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab) Updated 12/3/02 Reagents: ChIP sonication Buffer (1% Triton X-100, 0.1% Deoxycholate, 50 mm Tris 8.1, 150 mm NaCl, 5 mm EDTA): 10 ml 10 % Triton
Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab) Updated 12/3/02 Reagents: ChIP sonication Buffer (1% Triton X-100, 0.1% Deoxycholate, 50 mm Tris 8.1, 150 mm NaCl, 5 mm EDTA): 10 ml 10 % Triton
Subclinical Problems in the ICU:
 Subclinical Problems in the ICU: Corticosteroid Insufficiency C. S. Cutillar, M.D., FPCP, FPSEM Associate Professor Cebu Institute of Medicine H-P-A Axis during Critical Illness CRH ACTH H-P-A Axis during
Subclinical Problems in the ICU: Corticosteroid Insufficiency C. S. Cutillar, M.D., FPCP, FPSEM Associate Professor Cebu Institute of Medicine H-P-A Axis during Critical Illness CRH ACTH H-P-A Axis during
venous blood samples were drawn. The plasma was separated rapidly by centrifugation and assayed within one
 THE ANTIDIURETIC ACTIVITY OF PLASMA OF PATIENTS WITH HEPATIC CIRRHOSIS, CONGESTIVE HEART FAIL- URE, HYPERTENSION AND OTHER CLINICAL DISORDERS' By MARVIN STEIN,2 ROBERT SCHWARTZ, AND I. ARTHUR MIRSKY (From
THE ANTIDIURETIC ACTIVITY OF PLASMA OF PATIENTS WITH HEPATIC CIRRHOSIS, CONGESTIVE HEART FAIL- URE, HYPERTENSION AND OTHER CLINICAL DISORDERS' By MARVIN STEIN,2 ROBERT SCHWARTZ, AND I. ARTHUR MIRSKY (From
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS
 INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
Chapter 13 worksheet
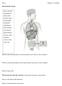 Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
Endocrine Glands. Endocrine glands
 ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
Critical illness and endocrinology. ICU Fellowship Training Radboudumc
 Critical illness and endocrinology ICU Fellowship Training Radboudumc Critical illness Ultimate form of severe physical stress Generates an orchestrated endocrine response to provide the energy for fight
Critical illness and endocrinology ICU Fellowship Training Radboudumc Critical illness Ultimate form of severe physical stress Generates an orchestrated endocrine response to provide the energy for fight
BIOM2010 (till mid sem) Endocrinology. e.g. anterior pituitary gland, thyroid, adrenal. Pineal Heart GI Female
 BIOM2010 (till mid sem) Endocrinology Endocrine system Endocrine gland : a that acts by directly into the which then to other parts of the body to act on (cells, tissues, organs) : found at e.g. anterior
BIOM2010 (till mid sem) Endocrinology Endocrine system Endocrine gland : a that acts by directly into the which then to other parts of the body to act on (cells, tissues, organs) : found at e.g. anterior
X/97/$03.00/0 Vol. 82, No. 6 Journal of Clinical Endocrinology and Metabolism Copyright 1997 by The Endocrine Society
 0021-972X/97/$03.00/0 Vol. 82, No. 6 Journal of Clinical Endocrinology and Metabolism Printed in U.S.A. Copyright 1997 by The Endocrine Society Effectiveness Versus Efficacy: The Limited Value in Clinical
0021-972X/97/$03.00/0 Vol. 82, No. 6 Journal of Clinical Endocrinology and Metabolism Printed in U.S.A. Copyright 1997 by The Endocrine Society Effectiveness Versus Efficacy: The Limited Value in Clinical
Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed.
![Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed. Cambridge CB2 3EG. ['25I]L-thyroxine. Experiments were performed after 24 hr had elapsed.](/thumbs/95/125304458.jpg) J. Physiol. (1971), 212, pp. 447-454 447 With 2 text-ftgurea Printed in Great Britain AN EXAMINATION OF THE EXTENT OF REVERSIBILITY OF THYROXINE BINDING WITHIN THE THYROXINE DISTRIBUTION SPACE IN THE RABBIT
J. Physiol. (1971), 212, pp. 447-454 447 With 2 text-ftgurea Printed in Great Britain AN EXAMINATION OF THE EXTENT OF REVERSIBILITY OF THYROXINE BINDING WITHIN THE THYROXINE DISTRIBUTION SPACE IN THE RABBIT
CLINICAL PHARMACOLOGY
 COSYNTROPIN- cosyntropin injection, powder, lyophilized, for solution Sandoz Inc ---------- cosyntropin for injection For diagnostic use only DESCRIPTION Cosyntropin for Injection is a sterile lyophilized
COSYNTROPIN- cosyntropin injection, powder, lyophilized, for solution Sandoz Inc ---------- cosyntropin for injection For diagnostic use only DESCRIPTION Cosyntropin for Injection is a sterile lyophilized
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
Hormonal regulation of. Physiology Department Medical School, University of Sumatera Utara
 Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Endocrine System. Endocrine vs. Exocrine. Bio 250 Human Anatomy & Physiology
 Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
Insulin Tolerance Test Protocol - RNS Endocrinology
 Page 1 of 7 - RNS Endocrinology Test name Insulin tolerance test. Alternate test names. Related Tests. Indication(s) Investigation of the hypothalamic pituitary axis (HPA) with regard to the release of
Page 1 of 7 - RNS Endocrinology Test name Insulin tolerance test. Alternate test names. Related Tests. Indication(s) Investigation of the hypothalamic pituitary axis (HPA) with regard to the release of
BIO 116 Practice Assignment 1 The Endocrine System and Blood This is not a required assignment but it is recommended.
 BIO 116 Practice Assignment 1 The Endocrine System and Blood This is not a required assignment but it is recommended. 1. Match the following glands of the endocrine system with the appropriate label 1.
BIO 116 Practice Assignment 1 The Endocrine System and Blood This is not a required assignment but it is recommended. 1. Match the following glands of the endocrine system with the appropriate label 1.
Physiological processes controlled by hormones?
 : the study of hormones, their receptors, the intracellular signaling pathways they invoke, and the diseases and conditions associated with them. What are hormones? Major endocrine glands? Fig 7-2 Physiological
: the study of hormones, their receptors, the intracellular signaling pathways they invoke, and the diseases and conditions associated with them. What are hormones? Major endocrine glands? Fig 7-2 Physiological
Chapter 18, Part 2! Chapter 18, Part 2 Endocrine system! The Endocrine System!
 Chapter 18, Part 2! The Endocrine System! SECTION 18-3! The bilobed pituitary gland is an endocrine organ that releases nine peptide hormones! What you need to know for each hormone we cover:! 1. Name
Chapter 18, Part 2! The Endocrine System! SECTION 18-3! The bilobed pituitary gland is an endocrine organ that releases nine peptide hormones! What you need to know for each hormone we cover:! 1. Name
Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges in Female Rats
 Southern Illinois University Carbondale OpenSIUC Honors Theses University Honors Program 5-10-2014 Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges
Southern Illinois University Carbondale OpenSIUC Honors Theses University Honors Program 5-10-2014 Central Progesterone Involvement in Estrogen- Induced Prolactin and Luteinizing Hormone Secretion Surges
Know at the level covered in these notes! SECTION 18-3! The bilobed pituitary gland is an endocrine organ that releases nine peptide hormones!
 Chapter 18, Part 2! The Endocrine System! Know at the level covered in these notes! SECTION 18-3! The bilobed pituitary gland is an endocrine organ that releases nine peptide hormones! What you need to
Chapter 18, Part 2! The Endocrine System! Know at the level covered in these notes! SECTION 18-3! The bilobed pituitary gland is an endocrine organ that releases nine peptide hormones! What you need to
See external label 2 C-8 C Σ=96 tests Cat # 6101Z. Cortisol. Cat # 6101Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
BIOL 347L Laboratory Three
 Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
