Zoonotic Potential of the Microsporidia
|
|
|
- Blaze Nash
- 5 years ago
- Views:
Transcription
1 CLINICAL MICROBIOLOGY REVIEWS, July 2005, p Vol. 18, No /05/$ doi: /cmr Copyright 2005, American Society for Microbiology. All Rights Reserved. Zoonotic Potential of the Microsporidia Alexander Mathis, 1 * Rainer Weber, 2 and Peter Deplazes 1 Institute of Parasitology, Vetsuisse Faculty, University of Zurich, 1 and Division of Infectious Diseases and Hospital Epidemiology, University Hospital, 2 Zurich, Switzerland INTRODUCTION The Organisms Diagnostic Techniques Phylogeny Natural History ENTEROCYTOZOON BIENEUSI Infections in Humans Infections in Animals Animal Models Molecular Epidemiology Sources of Human Infections and Transmission ENCEPHALITOZOON ENCEPHALITOZOON CUNICULI Infections in Humans Infections in Animals Rabbits Rodents Carnivores Monkeys Molecular Epidemiology Sources of Human Infections and Transmission ENCEPHALITOZOON HELLEM Infections in Humans Infections in Animals Molecular Epidemiology Sources of Human Infections and Transmission ENCEPHALITOZOON INTESTINALIS Infections in Humans Infections in Animals Molecular Epidemiology Sources of Human Infections and Transmission OTHER MICROSPORIDIA Vittaforma spp Pleistophora spp Trachipleistophora spp Brachiola spp Microsporidium spp Microsporidium ceylonensis Microsporidium africanum CONCLUDING REMARKS ACKNOWLEDGMENT REFERENCES INTRODUCTION Microsporidia are an exceptionally diverse group of organisms, comprising more than 1,200 species which parasitize a wide variety of invertebrate and vertebrate hosts. These organisms have long been known to be causative * Corresponding author. Mailing address: Institute of Parasitology, Winterthurerstr 266a, CH-8057 Zurich, Switzerland. Phone: 41 (0) Fax: 41 (0) alexander.mathis@access.unizh.ch. agents of economically important diseases in insects (silk worms and honey bees) (14), fish (179, 256), and mammals (rabbits, fur-bearing animals, and laboratory rodents) (37), and they emerged as important opportunistic pathogens when AIDS became pandemic (320). Thus, the question of whether animal reservoirs are the sources of human infections is reasonable. The focus of this review is to discuss the most recent perceptions on the zoonotic potential of the various microsporidia with proven vertebrate hosts. Furthermore, we provide a brief 423
2 424 MATHIS ET AL. CLIN. MICROBIOL. REV. FIG. 1. Electron micrograph of the developmental stages of the microsporidian species Encephalitozoon cuniculi in a host cell-derived vacuole in in vitro-cultivated human fibroblast cells. K, nucleus of host cell; M, meront; P, sporont, which divides into two sporoblasts; B, sporoblast (2 m in length), with cross sections of the polar tube; S, mature spore. update on other microsporidia which have no known vertebrate host or an invertebrate host and cause rare infections in humans. The Organisms Microsporidia are unicellular, obligate intracellular eukaryotes. Their life cycle includes a proliferative merogonic stage, followed by a sporogonic stage resulting in characteristically small (1 to 4 m), environmentally resistant, infective spores (Fig. 1) (95). The spores contain a long, coiled tubular extrusion apparatus ( polar tube ), which distinguishes microsporidia from all other organisms and has a crucial role in host cell invasion: Upon extrusion from the spore, it injects the sporoplasm along with its nucleus into the cytoplasm of a new host cell after piercing the plasmalemma of the host cell or the membrane of the phagosomes containing the endocytosed spores (58, 106). Before the onset of the AIDS pandemic, only eight cases of human microsporidial infections had been reported (reviewed in reference 320). In most cases, species identification of the causative agents was not conclusive. In 1985, as early as 2 years after the identification of human immunodeficiency virus (HIV) as the causative agent of AIDS, the microsporidial species Enterocytozoon bieneusi was discovered in HIV-infected patients with chronic diarrhea (76). Subsequently, several new genera and species of microsporidia were found to be important opportunistic pathogens in humans, infecting virtually every organ in the body and a broad spectrum of cell types (217) (Table 1). The most common microsporidial infections in humans are due to E. bieneusi and Encephalitozoon intestinalis. Both species have been found worldwide, mainly in HIV-infected patients with chronic diarrhea but also in immunocompetent persons with acute, self-limited diarrhea. Encephalitozoon cuniculi and Encephalitozoon hellem have been diagnosed, with very few exceptions, in immunocompromised patients and as causing local (e.g., ocular) or disseminated infections. Other rare microsporidial species infecting immunodeficient patients include Vittaforma corneae (formerly Nosema corneum), Pleistophora ronneafiei, Trachipleistophora spp., and Brachiola spp. Furthermore, V. corneae, Trachipleistophora hominis, Brachiola algerae, Nosema ocularum, Microsporidium ceylonensis, and Microsporidium africanum have been described in single cases as agents of ocular infections in immunocompetent persons. Due to the administration of antiretroviral combination therapy, which restores immunity in HIV-infected persons, the number of clinically manifest microsporidial infections has markedly decreased in affluent countries (322). However, it is estimated that two-thirds of all HIV-infected persons live in sub-saharan Africa, where antiretroviral therapy is not widely available due to the costs, and consequently, opportunistic complications continue to cause severe morbidity and mortality. Indeed, a recent study showed that 13% of HIV-positive diarrheic adults in Mali were positive for E. bieneusi, which thus was the most prevalent intestinal parasite in this African country (10). Furthermore, microsporidial infections are increasingly being diagnosed in affluent countries in immunosuppressed patients who undergo organ transplantation (39, 115, 119, 130, 132, 156, 167, 197, 200, 228, 229, 244, 260, 283), as well as causing ocular infections in nonimmunocompromised persons (47, 171, 206, 259, 274, 284). Diagnostic Techniques Considerable progress has been made in improving the repertoire of techniques for detection of microsporidia. Previously, diagnosis was based on laborious electron-microscopic examinations because of the small size of the organisms. The introduction of staining techniques allowed routine, light-microscopic diagnosis of microsporidial spores, at least to the genus level (319). Diagnosis to the species level is achieved by using antibodies (polyclonal or monoclonal) and by molecular methods based on the PCR (reviewed in references 116, 322, and 327). This latter sensitive and specific method has, in addition, the intrinsic advantage that upon further analysis of the PCR products with various methods (restriction fragment length polymorphism, SSCP, or sequencing), identification at the subspecies level (strains or genotypes) can be achieved (86, 154). Serologic tests have been useful in detecting antibodies of E. cuniculi in several species of animals, but the value of detecting antibodies against Encephalitozoon spp. in humans has been controversial because of possible nonspecificity of the tests when spore walls are used as the antigen (322). By employing recombinant antigens of the polar tube of E. cuniculi, a high specificity was recently demonstrated, and the development of
3 VOL. 18, 2005 ZOONOTIC POTENTIAL OF MICROSPORIDIA 425 TABLE 1. Microsporidial species infecting humans Species appropriate serodiagnostic tools seems feasible (298). No tests are available for the serodiagnosis of E. bieneusi. Many but not all microsporidia infecting humans can be continuously cultivated in vitro in various cell lines (184, 306). This facilitates investigations of their basic biology (102, 127) and also allows for easy assessment of drugs (80) or disinfection schemes (118, 147, 150, 173), which has become an issue since human-pathogenic microsporidia have been discovered in surface waters (57, 89, 90, 103, 150, 255, 272). Phylogeny Discovery Host Yr Reference(s) Investigations on the basic biology have unearthed highly exceptional characteristics of the microsporidia. Although they are true eukaryotes (i.e., they possess a typical eukaryotic nucleus, endomembrane system, and cytoskeleton), they also display molecular and cytological characteristics reminiscent of prokaryotes, including features of the translational apparatus, genome size (which is in the range of that of bacteria), and lack No. of confirmed reported patients of recognizable mitochondria, peroxisomes, and typical Golgi membranes (reviewed in reference 189). Because of their prokaryotic features, microsporidia were initially classified within the Archezoa, together with other amitochondriate organisms (Giardia, trichomonads, and Entamoeba) which were thought to have evolved prior to the acquisition of mitochondria by endosymbiosis and consequently to represent the most primitive eukaryotes (42). However, the genome sequence of E. cuniculi revealed that it contains genes related to some mitochondrial functions, implying that microsporidia have retained a mitochondrion-derived organelle (152). Indeed, tiny organelles with double membranes could be demonstrated by using antibodies against a mitochondrial protein (Hsp70) in the human microsporidial parasite T. hominis (330). Furthermore, sophisticated phylogenetic analyses revealed that the microsporidia evolved from within the fungi, being most closely related to the zygomycetes (43, 155). Microsporidia share additional features with fungi, e.g., the presence of chitin (although chitin is also found in other phyla, e.g., mol- Immunocompromised a Immunocompetent b Site(s) of infection (reference[s]) Enterocytozoon bieneusi Human , Intestine, biliary tract, respiratory tract (322) Encephalitozoon hellem Human Eye, respiratory tract, urinary tract, systemic infection (322) Encephalitozoon Human , Intestine, biliary tract, respiratory intestinalis (originally tract, bone, skin, systemic named Septata infection (322) intestinalis) Encephalitozoon cuniculi Vittaforma corneae (originally named Nosema corneum) Rabbit c Systemic infection, eye, respiratory tract, urinary tract, liver, peritoneum, brain (322) Animal host(s) Vertebrates Birds Mammals Mammals Human , Eye, urinary tract (75, 101, 231) Unknown Vittaforma-like d Human Intestine (278) Unknown Pleistophora ronneafiei (originally named Pleistophora sp.) Human , e Muscle (169) Unknown Trachipleistophora hominis Trachipleistophora anthropophthera Brachiola algerae (originally named Nosema algerae) Brachiola connori (originally named Nosema connori) Human Muscle, eye (100, 231) Unknown Human Systemic infection, eye (151, 335) Unknown Mosquito f 1 Eye, muscle (61, 307) Mosquito Human , g Systemic infection (273) Unknown Brachiola vesicularum Human Muscle (33) Unknown Nosema ocularum Human Eye (31) Unknown Microsporidium Human , 12, 36 1 (unknown) Eye (12) Unknown ceylonensis Microsporidium africanum Human (unknown) Eye (223) Unknown a HIV-seropositive persons, AIDS patients, and organ transplant recipients. b Immunocompetent, otherwise healthy. c Two patients with unknown immunostatus, presumably cellular immunodeficiency (see Table 4). d PCR/sequencing results only, needs confirmation. e Cellular immunodeficiency (HIV antibody negative). f Patient was taking immunosuppressive agents for rheumatoid arthritis for decades. g Thymic aplasia.
4 426 MATHIS ET AL. CLIN. MICROBIOL. REV. FIG. 2. Dendrogram generated from the small subunit ribosomal RNA (ssrrna) gene of microsporidian species identified in humans (underlined) and selected other species (Kimura s distance, unweighted pair group method of analysis). Known animal hosts are indicated in brackets; the brewer s yeast Saccharomyces cerevisiae serves as an outgroup. No corresponding gene sequences are known for the human-infecting microsporidian species Pleistophora ronneafiei, Trachipleistophora anthropophthera, Brachiola (formerly Nosema) connori, B. vesicularum, Nosema ocularum, Microsporidium ceylonensis, and M. africanum (Table 1). lusks) and trehalose, similarities between the cell cycles, and the organization of certain genes (35, 211). Therefore, microsporidia are nowadays considered to be highly derived fungi that underwent substantial genetic and functional losses resulting in one of the smallest eukaryotic genomes described to date. The placement of microsporidia among the fungi might have implications for the discovery of new therapeutic strategies. Although microsporidiosis in general can be successfully treated with albendazole and fumagillin, therapy for the most prevalent species, E. bieneusi, is difficult (129, 204). Indeed, a few studies have demonstrated that inhibitors of chitin synthase enzymes are effective against microsporidia (19, 270). Natural History A fundamental question that arose with the discovery of new microsporidial species in humans was that of their natural origin. The phylogram generated with small subunit ribosomal RNA (ssrrna) gene sequences of microsporidia infecting humans (Fig. 2) illustrates their polyphyletic nature. Of particular interest is the fact that the closest relatives of three species infecting humans and belonging to the genera Trachipleistophora and Vittaforma are microsporidia that infect insects, and it is tempting to speculate that the insects might serve as reservoirs for these microsporidia. Indeed, another microsporidian of arthropod origin, Brachiola (formerly Nosema) algerae, was demonstrated to be a causative agent in human infections (61). The two new species of the genus Encephalitozoon, E. intestinalis and E. hellem, are very closely related to the widespread parasite of mammals E. cuniculi. The most prevalent species, E. bieneusi, is most closely related to a fish pathogen. For all four major microsporidial species infecting humans (E. bieneusi and the three Encephalitozoon spp.), animal hosts are known (Table 1; Fig. 2) implying a zoonotic nature of these parasites. Molecular studies have identified phenotypic and/or genetic variability within these species, indicating that they are not uniform. Whereas the significance of this variability remains largely unknown for E. hellem and E. intestinalis, strain variation in E. bieneusi and E. cuniculi provided new insights into the biology, origin and distribution of these parasites and has allowed the question of their zoonotic potential to be addressed. ENTEROCYTOZOON BIENEUSI There are two genera in the family Enterocytozoonidae: (i) Nucleospora, with the two characterized species N. salmonis, an intranuclear microsporidian of salmonid fish (87), and N. secunda, a parasite of a warm-water African fish (180), and (ii) Enterocytozoon, with E. bieneusi, infecting the cytoplasm of enterocytes and other epithelial cells in humans and mammals (76). E. bieneusi, the most common microsporidial species known to cause human disease, was first described as an HIV-associated opportunistic intestinal pathogen in 1985 and was morphologically characterized using electron microscopy (76). In 1996, morphologically identical spores were detected in feces of pigs (74), and subsequently they also were detected in fecal samples and intestinal tissue of other mammals. Infections in Humans Several hundred HIV-infected patients with chronic diarrhea attributed to this organism have been reported from all over the world. The prevalence of E. bieneusi infections among HIV-infected patients reached up to 50% as documented by selected studies in Table 2. Human infections are well documented in all inhabited continents. In most studies, prevalences were significantly higher in patients with chronic diarrhea (92, 99, 128, 271, 324, 332). The prevalences presented in Table 2, however, do not allow for comparative analyses because there were considerable differences with regard to the selection of the patient groups, the patients characteristics (age, sex, sociodemographic data, degree of immunodeficiency, and clinical features), and the specimens investigated (biopsies or stool samples). Furthermore, the improvements of diagnostic methods achieved over the last 15 years have to be considered.
5 VOL. 18, 2005 ZOONOTIC POTENTIAL OF MICROSPORIDIA 427 TABLE 2. Selected studies on prevalence rates of E. bieneusi in HIV-infected persons a Geographic area Specimen; diagnostic method No. of patients examined; patients characteristics Prevalence (%) Yr (reference[s]) Africa Cameroon (Yaunde) Stool; LM 66; chronic diarrhea 9 b 1997 (241) Niger (Niame) Stool; LM 60; 41 with diarrhea (27) Zimbabwe (Harare) Stool; LM 129; hospitalized with diarrhea (300) Zimbabwe Formalin-fixed stool; PCR 74; 45 with diarrhea (41) Zambia (Lusaka) Stool; LM 69; chronic diarrhea (91) Mali (Bamako) Stool; LM, partially 88; 80% with chronic diarrhea (185) confirmed by TEM Tanzania Stool; LM, TEM 86; chronic diarrhea (44) Zimbabwe (Harare) Stool; LM, PCR 88; diarrhea longer than 1 week 18 (LM), 51 (PCR) 1999 (133) Mali (Bamako) Stool; LM, IFAT, PCR 61; diarrhea (10) Americas United States (Washington, D.C.) Intestinal biopsies; EM, TEM 67; chronic unexplained diarrhea (218) United States (Texas) Duodenal biopsies; TEM 55; chronic diarrhea (227) 51; without chronic diarrhea 25 United States (Atlanta, Ga.) Stool; LM 65; diarrhea (128) 65; without diarrhea 2 United States (New York) Intestinal biopsies; TEM 34; AIDS patients 44 b 1994 (160, 161) 194; diarrhea 39 b United States (New York) Duodenal biopsies; PCR, 68; diarrhea (62) confirmation by TEM 43; without diarrhoea 2.3 United States (California) Stool; LM 8,439; diarrhea, yr 1993, 1994, 8.8, 9.7, 6.6, (53) 1995, Brazil (Fortaleza) Stool; LM 79; with diarrhea 6 b 1994 (332) 82; without diarrhea 1 b Brazil (Rio de Janeiro) Stool; LM 13; chronic unexplained diarrhea 46 b 1996 (25) Brazil (Rio de Janeiro) Stools, duodenal or ileal 40; chronic diarrhea (24) biopsies Peru (Lima) Stool; LM 2672; diarrhea (275) Australasia Australia (New South Wales) Duodenal biopsies; LM 109; chronic diarrhea (99) confirmed by EM 71; without diarrhea 1.4 Australia (Victoria) Stool; LM, TEM 139; diarrhea (239) Thailand Stool; LM, TEM 66; chronic diarrhea (313) Thailand (Bangkok) Stool; LM; TEM 288; diarrhea (317) Thailand Stool; LM, TEM 95; children with diarrhea (312) India Stool; LM 120; diarrhea (199) Europe The Netherlands Duodenal biopsies; LM partially confirmed by TEM 55; unexplained diarrhea 38: without diarrhea 27 b 3 b 1991 (92) The Netherlands Stool; LM 143; diarrhea (301) France Duodenal biopsies; LM 66; chronic diarrhea (56) France (Paris) Stool, intestinal biopsies; LM 18; chronic unexplained diarrhea (203) France (Nice) Stool; LM 46; chronic diarrhea (16) Italy (Apulia) Stool; LM 56; diarrhea 2 b 1995 (187) Italy Intestinal biopsies; EM, TEM 72; chronic diarrhea (309) Germany (Cologne) Intestinal biopsies; PCR, 46; diarrhea (110) Southern hybridization Germany (Hamburg) Stool; LM 50; diarrhea, hospitalized patients (271) 47; without diarrhea 4 England (London) Intestinal biopsies; LM, EM 59; diarrhea 14 b 1991 (221) England (northwest) Stool, intestinal biopsies; LM 63; diarrhea (166) confirmed by TEM Spain (Madrid) Stool; LM, confirmation by 48 children; chronic diarrhea (69) PCR Sweden (Stockholm) Duodenal biopsies; LM 72; abdominal symptoms of 3 b 1998 (281) unknown cause Switzerland Stool; LM partially confirmed 164; chronic diarrhea ( ) (324) by TEM and PCR 156; chronic diarrhea ( ) ; without diarrhea 0.4 Portugal Stool; LM, PCR 215; diarrhea 42.8 c 2001 (98) a LM, light microscopy; TEM, transmission electron microscopy. b Intestinal microsporidia, species not stated. c A total of 92 samples were positive for microsporidia; 20 of 69 isolates that were further characterized by PCR were E. bieneusi, and 49 were E. intestinalis. A few studies from affluent countries indicate that the prevalence of E. bieneusi in HIV-infected patients is decreasing (53, 324). Recent studies have shown that administration of antiretroviral combination therapy can result in remission of HIVassociated intestinal microsporidiosis (40, 54, 120, 198, 214). A decrease of 50% in E. bieneusi cases in Switzerland was also interpreted as being associated with antiretroviral therapy (324).
6 428 MATHIS ET AL. CLIN. MICROBIOL. REV. Although predominantly described among adults suffering from immunodeficiency due to HIV infection, E. bieneusi infections were also reported from HIV-negative patients who were immunocompromised due to an underlying disease or due to therapeutic immunosuppression when undergoing organ transplantation (119, 130, 197, 228, 229, 260, 318). Furthermore, a few E. bieneusi infections in HIV-negative, immunocompetent, and otherwise healthy persons that were associated with self-limited diarrhea were reported, mostly in the context of traveler s diarrhea in Europe (9, 71, 114, 181, 182, 209, 242, 268, 282, 314) but also in single cases in Africa (44, 131). Hence, E. bieneusi was detected by PCR in stool samples from 7 of 148 travelers with diarrhea returning to Germany (209). A recent study performed in Spain revealed that 8 of 47 (17%) geriatric persons with diarrhea were infected with E. bieneusi (182), and it was speculated that agerelated diminishment of the immunological capacities might predispose elderly persons to microsporidial infections. On the other hand, no data so far indicate that children might be more susceptible to E. bieneusi infections (300). Over the last decade, evidence has accumulated that E. bieneusi might also persist as an asymptomatic infection in immunocompetent humans. E. bieneusi was recovered in 8 of 990 stool samples from African children who were not considered HIV positive, suggesting enteric carriage among immunocompetent persons in tropical countries (27). Asymptomatic infections in children were reported in another study from Africa (44) and in a study from Asia, where not only healthy orphans (5.9%) but also child-care workers (1.9%) were infected (213). In all these studies, light microscopy, partly combined with transmission electron microscopy for confirmation of positive cases, was the diagnostic method, which might not be sensitive enough to detect subclinical infections under all circumstances. Therefore, more sensitive diagnostic tools such as PCR are required to elucidate the question of whether this parasite is a common organism of the human intestinal flora, causing severe disease only under immunodeficiency, or whether zoonotic transmission is of considerable importance (see below). Infections in Animals Eleven years after its discovery as a human pathogen, E. bieneusi was for the first time detected in animals, namely, in pigs (74), and a prevalence of 35% was determined by PCR in a subsequent investigation (26) of 109 randomly selected pigs from 22 farms located in different parts of Switzerland. A significantly (P 0.05) higher occurrence of E. bieneusi was found in weaned piglets. The feces of three infected pigs, which did not show any clinical signs, were examined weekly by PCR, revealing excretion of E. bieneusi spores in 67% of the samples. Hence, E. bieneusi seems to be a common parasite in asymptomatic pigs. The low pathogenicity of E. bieneusi for pigs was further substantiated by the lack of intestinal lesions in immunosuppressed and immunocompetent gnotobiotic piglets experimentally infected with E. bieneusi of human or macaque origin (159). This study, however, demonstrated that immunosuppression of piglets did lead to an increased excretion of spores. Subsequent studies have confirmed the occurrence of E. bieneusi in pigs with high prevalence (32%) (29) and also in calves (9.5 to 11.5%) (237, 243, 277). The parasite has also been detected in cats (72, 191), dogs (182, 191), a goat (182), a llama (72), a variety of species of wild fur-bearing mammals (beavers, foxes, muskrats, otters, and raccoons) (72, 276), hedgehogs (A. Mathis, unpublished data), and, recently, nonmammalian hosts (chickens and pigeons) (233; M. Haro et al., unpublished data [GenBank accession number AY668953]). Natural infections with E. bieneusi were documented in captive monkeys, namely, rhesus macaques. Prevalences were 16.7% (n 131) in normal, asymptomatic animals, in which the infection persisted for 262 days, and 33.8% (n 53) in animals which were experimentally infected with the simian immunodeficiency virus (186). A screening of 42 wild monkeys from Central Africa (Cameroon) by microscopy and PCR did not yield a single E. bieneusi-positive result (225). Animal Models For various reasons including mass production of the parasite for basic research, development of diagnostic tools, drug screening, and studies on disease pathogenesis, an animal model of enterocytozoonosis is desirable. E. bieneusi of human origin has been established in immunocompromised rhesus monkeys (126, 293), immunosuppressed gnotobiotic piglets (159), Sprague-Dawley rats (2), and New Zealand rabbits (3). In all animals, only chronic asymptomatic infections were observed, similar to the infections in naturally infected, immunocompetent pigs (26). Many attempts to establish E. bieneusi in immunocompetent and immunodeficient mice were unsuccessful (83). Hence, all hitherto-described experimental animal models appear not to be adequate to mimic the pathological intestinal situation in HIV-infected patients. Molecular Epidemiology Analyses of the single internal transcribed spacer of the rrna genes (ITS) have revealed that there is considerable genetic variation within E. bieneusi isolates of human and animal origins, and more than 50 genotypes have so far been described based on subtle differences within this 243-bp sequence. An overview of human-infecting genotypes is provided in Table 3. In contrast to the situation with microsporidia of the genus Encephalitozoon (see below), no other genetic markers are available. However, classification of isolates of E. cuniculi and E. hellem based on ITS sequences has largely been confirmed by data for other genetic loci. Nevertheless, additional independent markers for E. bieneusi are highly desirable in order to clarify the genetic structure of the parasite s populations. Five different ITS genotypes of E. bieneusi infecting humans have been confirmed in independent studies and another 12 were discovered in single studies, with one of these studies accounting for eight of these novel genotypes (275). Limited information is available on the geographic distribution of human-derived genotypes of E. bieneusi. Except for the abovementioned eight genotypes identified in a study in South America (275), all other genotypes have been found in Europe, where most of the studies aiming at genotyping this parasite have been conducted (26, 72, 174, 235, 240). In additions,
7 VOL. 18, 2005 ZOONOTIC POTENTIAL OF MICROSPORIDIA 429 TABLE 3. Enterocytozoon bieneusi genotypes in humans: number of described cases and animal hosts E. bieneusi genotype designation HIV-infected or otherwise immunocompromised patients genotypes A, B, D, and IV have been identified in HIV-infected patients from South America and the United States (193, 275). In single studies from Asia and Africa, genotype A was found in 20 asymptomatic children in Thailand (I. Subrungruang et al., unpublished data [GenBank accession numbers AY to AY357404]) and genotype IV in children with diarrhea in Uganda (292). Of all 17 human-infecting E. bieneusi genotypes identified so far, four seem to have a zoonotic potential, as they have also been discovered in vertebrate hosts (Table 3). For the three genotypes A, B, and C, which account for the overwhelming number of isolates from humans, no animal host is known, and one might speculate that it is simply a matter of time until such hosts will be identified. On the other hand, several lines of evidence suggest that there is a certain degree of host specificity, at least for some of the E. bieneusi genotypes. First, a dendrogram based on ITS sequences of human-infecting E. bieneusi genotypes (confirmed in independent studies) and selected genotypes with animal hosts reveals a clustering of genotypes according to host species, although this grouping is not absolute (Fig. 3). Interestingly, the eight novel E. bieneusi genotypes recently identified in a single study of HIV patients (275) (Table 3) cluster within the branch containing all other No. of reported human cases (reference) Immunocompetent patients Animal host, genotype designation (reference[s]) Genotypes identified in independent studies A 3 (235), 3 (26), 1 (193), 32 (275) B, I 3 (235), 8 (26), 66 (174), 2 (193), 11 (240) C, II 2 (235), 2 (26), 1 (transplant 1 (174) recipient) (260), 9 (174), 7 (transplant recipient) (174), 1 (lymphoma) (174), 1 (72) D 1 (236), 9 (275), 1 (240) Macaque (45, 126); pig, EBITS9 (29); wildlife, IV 9 (174), 1 (transplant recipient), (174), 1 (myeloma) (174), 18 (275), 1 (240) 1 (174), 10 (children with diarrhea, immunostatus not determined; estimated to be 18 29% among these children) (292) WL8 (29, 45, 276) Cat, K (72); cattle, BEB5 (72, 277) Genotypes identified in single studies Q (identical to C, II 1 (72) but with 2 nt insertion) III 3 (174) V 1 (174) Peru3 1 (275) Peru4 1 (275) Pig, EbpC (26); pig E (237); wildlife, WL13 (276) Peru5 3 (275) Wildlife, WL11 (276) Peru6 1 (275) Peru7 8 (275) Peru8 4 (275) Peru10 3 (275) Peru11 6 (275) human-derived genotypes (for a detailed dendrogram, see reference 275). Further evidence for some degree of host specificity originates from experimental animal models using immunodeficient or immunosuppressed animals (see above). With E. bieneusi of human origin, only chronic asymptomatic infections which do not appropriately mimic the pathological intestinal situation in HIV-infected patients were observed in rhesus monkeys, rats, piglets, and rabbits (see above), and many attempts to establish human-derived E. bieneusi in immunodeficient mice were unsuccessful (83). A minor role of animals as sources of human infections is also substantiated by epidemiological data (see below). Taken together, the picture of E. bieneusi with respect to its zoonotic potential is reminiscent of that of another intestinal parasite, Giardia lamblia, which comprises zoonotic as well as nonzoonotic genotypes (205). Sources of Human Infections and Transmission Extensive intestinal and rare respiratory tract involvement as described for patients with disseminated E. bieneusi infections suggest that different modes of transmission are possible, in-
8 430 MATHIS ET AL. CLIN. MICROBIOL. REV. FIG. 3. Dendrogram of ITS sequences of human-infecting E. bieneusi genotypes (confirmed in independent studies) and selected genotypes with animal hosts. All sequences are deposited in GenBank with the same designations (Kimura s distance, unweighted pair group method of analysis). cluding the fecal-oral or oral-oral route, inhalation of aerosols, or ingestion of food contaminated with fecal material. In addition, direct human-to-human transmission is substantiated by studies that implicate male homosexuality or having an HIVinfected cohabitant as risk factors for acquiring intestinal microsporidiosis (148, 316). Person-to-person transmission was also suggested in a study which revealed that 9 of 13 infected orphans, who were HIV negative, were confined to two houses, whereas HIV-positive children inhabiting another house were not infected (213). The detection of E. bieneusi in immunocompetent human carriers indicates that this parasite, or at least some of its genotypes, could naturally persist in the human population. Infections in organ transplant recipients or otherwise immunocompromised HIV-negative patients as well as in immunocompetent individuals were probably unrelated to direct contact with infected patients with AIDS. No seasonal variation was obvious in the prevalence of human intestinal microsporidiosis in Brazil, as had been found for the intestinal parasite Cryptosporidium parvum (332). Despite differences in climate and sociodemographic factors, similar results were obtained in a study in southern California investigating 8,439 HIV-infected patients over a period of 4 years (53). In both studies it was suggested that contaminated drinking water was not likely to be the major source of microsporidial infections. However, other investigators have suggested that water contact may be an independent risk factor for enterocytozoonosis. In a prospective case-control study including 30 HIV-infected patients with intestinal microsporidiosis (28 with E. bieneusi infection and 2 with E. intestinalis infection) and 56 HIV-infected controls (148), swimming in a pool during the preceding 12 months was identified as one of three risk factors for intestinal microsporidiosis (besides male homosexuality and a CD4 lymphocyte count of 100/mm 3 ). Other factors, such as consumption of different sorts of beverages or undercooked food, exposure to animals (cats, dogs, birds, bees, or fish), or recreational activities (swimming in freshwater or in the sea, trips abroad in the past 36 months, or visits to the countryside) were found not to be related to infection risk (148). Another study done in the United States included 12 HIV-infected patients with intestinal microsporidiosis and 54 CD4-matched controls. Risk factors for the acquisition of microsporidia were different kinds of water contacts (recreational swimming in rivers and lakes, drinking unfiltered tap water, or use of humidifiers) and close contact with another HIV-infected person (316). Lastly, a study investigating an urban cohort of HIV-infected patients revealed occupational contact with water or use of a hot tub or spa as risk factors for acquiring intestinal microsporidiosis (64), whereas contact with companion animals was not related to infection risk. Infection risk associated with traveling was suggested for E. bieneusi infections of HIV-infected and noninfected travelers in one study (55) but not in another one (64). A comparative study on diarrheic HIV-infected patients from the Paris area (France), including 26 patients with intestinal microsporidial infection (species not determined) and 28 patients with cryptosporidiosis, revealed that trips to tropical countries were strongly associated only with microsporidial infections (55). It is not known whether particular factors are associated with microsporidial transmission in tropical countries where HIVnegative adult and children were found to be infected (27). Detection of E. bieneusi and confirmation to the species level was achieved by PCR and subsequent sequence analysis of part of the ssrrna gene in surface water but not in groundwater samples (89, 272) and recently, by species-specific PCR, also in zebra mussels from a river (123). Contamination of surface water may be from discharged domestic wastewater or from animal sources. As no genotyping was performed in these studies, the potential infection risk for humans from such sources needs further clarification. ENCEPHALITOZOON Three species of the genus Encephalitozoon have been identified as human pathogens: (i) E. cuniculi, which has a wide host range among mammals (37) and a worldwide distribution in domestic rabbits and is found in distinct geographic areas in carnivores and monkeys; (ii) E. hellem, which was distinguished from E. cuniculi in 1991 (78) and which has been reported in a few cases in avian hosts in the United States and Indonesia;
9 VOL. 18, 2005 ZOONOTIC POTENTIAL OF MICROSPORIDIA 431 TABLE 4. Single cases of human infections due to E. cuniculi confirmed by molecular analyses Immune status and country Clinical manifestations Site(s) of infection and/or specimen E. cuniculi strain Yr Reference(s) HIV-infected patients United Kingdom Abdominal pain, anorexia, nausea, vomiting, Kidney, urine III , 141, 143 fever, cough, renal failure United States Fever, cough, emesis, insomnia, sinus Urine, sputum III , 66, 85 congestion, severe dry eyes, blurred vision Germany Keratoconjunctivitis, sinusitis, rhinitis Urine, sputum stool, nasal discharge, ND a duodenal biopsy Switzerland Headache, visual impairment, cognitive Cerebrospinal fluid, stool, sputum, I impairment, nausea, vomiting urine United States Dizziness, fever, nausea, abdominal pain, Adrenal glands, kidneys, brain, III diarrhea heart, trachea, urinary bladder, spleen, lymph nodes Switzerland None Urine I Mexico Pneumonitis, otitis media Urine, respiratory specimen, stool III , 192 Switzerland None Urine I , 192 Switzerland Conjunctivitis, sinusitis, seizure disorder Urine I Switzerland Renal insufficiency, leucocyturia, Urine I erythrocyturia Switzerland None Urine, respiratory specimen I Italy Chronic sinusitis, bilateral keratoconjunctivitis Nasal epithelium I France Visual impairment Cerebrospinal fluid, urine, sputum, ND stool, duodenal biopsy Chile Cough, fever Bronchoalveolar lavage, ND (III b ) transbronchial biopsy Spain Fever, asthenia, abdominal pain, diarrhea Stool, urine, sputum III Italy Fever, myalgia, poor general condition Kidney, liver, lymph nodes, spleen, adrenal medulla, brain, ovary III HIV-negative patients, immunocompromised (undergoing organ transplantation) Canada Fever, keratoconjunctivitis, allograft Urine, stool, sputum, conjunctival III tenderness, scrapings Mexico Cough, fever, diarrhea, thoracic pain, extreme Liver, kidney III weakness United States Respiratory distress Lung biopsy III HIV-negative patients, otherwise immunocompromised c Switzerland Iris tumor Tumor biopsy I a ND, not determined. b E. cuniculi strain III deduced from clinical and epidemiological findings (see the text). c Idiopathic CD4 T-lymphocytopenia. and (iii) E. intestinalis (originally designated Septata intestinalis), which first was described in 1993 (30) and which was diagnosed in feces of farm animals in Mexico and in gorillas in Africa. All three species have spores that are morphologically indistinguishable. Intraspecies genetic variation has so far been described for E. cuniculi and E. hellem. In E. cuniculi, three strains (I, II, and III) are recognized, which, according to the host of the originally characterized isolates, are also designated rabbit strain, mouse strain, and dog strain. The fourth known species from this genus, E. lacertae (37, 162), was identified in reptiles only and is most closely related to E. cuniculi. ENCEPHALITOZOON CUNICULI Infections in Humans The first Encephalitozoon infection reported in humans, in 1959 (195), as well as a few subsequent cases were diagnosed based on spore morphology only. Therefore, these cases cannot unambiguously be attributed to E. cuniculi, as species differentiation was not possible at that time. Recent findings of E. cuniculi infections, as determined by immunological and/or molecular methods, in several patients (HIV infected, undergoing organ transplantation, or with idiopathic CD4 T-lymphocytopenia) from Europe and from the United States prove the infectivity of E. cuniculi for immunodeficient humans (Table 4). In seroepidemiological studies with enzyme-linked immunosorbent assay (ELISA) and the indirect fluorescent-antibody test (IFAT) using spore antigens of E. cuniculi or parasite cell suspensions, prevalences of up to 42% have been reported for patients with a history of tropical diseases or a stay in tropical countries and for patients with renal diseases, psychiatric disorders, or neurological disorders (15, 142, 146, 261). Despite Western blot analysis, which potentially increases the specificity of the ELISA (146), it is uncertain whether detection of antibodies to E. cuniculi reflects true infections or antigen exposure without establishment of the parasite, cross-reactivity, or reactions due to polyclonal B-cell stimulation, particularly in patients with tropical diseases. More recent studies (50, 121, 135, 165, 224, 302) suffered from the same methodological limitations. However, all these serological studies suggest that human exposure to microsporidia may be common but without clinical significance. In a very recent serodiagnostic study of an
10 432 MATHIS ET AL. CLIN. MICROBIOL. REV. FIG. 4. Rabbit with torticollis (head tilt) due to cerebral infection with E. cuniculi. immunocompetent laboratory worker who was accidentally infected with E. cuniculi when drops containing spores were spilled in his eyes, cross-reactivity of his serum with spore walls of E. hellem and E. intestinalis was demonstrated. However, only little cross-reactivity was observed when recombinant polar tube proteins of these parasites were used as antigens (298). Infections in Animals Beyond the numerous reports of E. cuniculi infections in rabbits, carnivores, and monkeys (see below), the parasite was identified as the causative agent of placentitis and abortion in a horse (220). Serological investigations (IFAT) revealed antibodies against E. cuniculi in goats and cattle (51, 136), but, as outlined above, the test specificity was not ascertained. Rabbits. Encephalitozoonosis in laboratory and pet rabbits is of clinical significance worldwide. E. cuniculi usually causes chronic infections which can persist asymptomatically for years. Severe neurological disease due to granulomatous encephalitis can occur unrelated to the age and sex of the animals (210) (Fig. 4). Until microsporidian-negative rabbit colonies were established, encephalitozoonosis was an important confounding variable in rabbit-based biomedical research on a variety of diseases (reviewed in reference 315). In the past, high prevalences of encephalitozoonosis were recorded for laboratory rabbit colonies (37, 315), but these infections can be controlled by serological screening and high hygienic standards. On the other hand, the disease is still highly endemic in the pet rabbit population. In seroepidemiological surveys in Switzerland and the United Kingdom, specific antibodies against E. cuniculi spores were detected in 7.5% (n 292) and 23% (n 26) of healthy rabbits and in 85% (n 72) and 71% (n 65) of rabbits (mainly kept as pets) with neurological symptoms or with direct contact with symptomatic animals (74, 137, 210), respectively. In rabbits, horizontal transmission by ingestion of spores is regarded to occur most frequently, but intrauterine infection has also been documented (13, 37). After experimental oral infections of rabbits, regular spore excretion in the urine was observed between days 38 and 63 postinfection and later intermittently at very low density (59). Spore excretion was reported in 9 of 11 symptomatic rabbits (60), indicating that such animals may play an important epidemiological role. Based upon serological evidence, it was suggested that wild rabbits (Oryctolagus cuniculus) represent the natural host of E. cuniculi (331). Other studies revealed seroprevalences of 3.9% among 204 wild European rabbits in France (46) and 25% in the wild rabbit population in Western Australia (285). To our knowledge, E. cuniculi infections have so far not been reported for other free ranging lagomorphs. Rodents. E. cuniculi has been diagnosed in the past in numerous cases as a common parasite of laboratory rodents such as mice, rats, hamsters, and guinea pigs (reviewed in references 37 and 315), but nowadays, with high hygienic standards for the maintenance of laboratory rodents being applied, infections with microsporidia should no longer be a significant problem in these animals. On the other hand, rodent models have gained more attention for immunological research in the field of microsporidiosis (reviewed in reference 77). Information about the possible significance of microsporidiosis in rodents kept as pet animals and about the epidemiology of microsporidiosis in wild rodents is scarce. Until 1986, only three reports of E. cuniculi infections in wild rats from Japan and the United Kingdom had been published (37). Recently, we isolated E. cuniculi strain II ( mouse strain ) from one of 30 wild rats (Rattus norvegicus) caught in the city of Zurich, Switzerland (212) (Table 5). In wild mice, specific antibodies against E. cuniculi spores TABLE 5. Hosts and geographical distribution of Encephalitozoon cuniculi strains E. cuniculi strain a Host Geographic area (no. of isolates) Reference(s) I ( rabbit strain ) Rabbit Switzerland (21), United States (3), Germany (1), Australia (1), Italy (1), Japan (1) 86, 113, 153, 192, 210; P. Deplazes and A. Mathis, unpublished data. Human Switzerland (6), Italy (1), United States (1) 73, 238, 321, 334 II ( mouse strain ) Mouse Czech Republic (1), United Kingdom (1), United States (1) 86, 334 Wild rat Switzerland (1) 212 Blue fox Norway (8), Finland (1) 8, 190 III ( dog strain ) Dog United States (10), South Africa (1) 86, 141, 264 Prosimian a As determined by the number of 5 -GTTT-3 repeats in the ITS (86).
11 VOL. 18, 2005 ZOONOTIC POTENTIAL OF MICROSPORIDIA 433 were found in Iceland in 4% and 9% of Apodemus sylvaticus and Mus musculus animals, respectively (140). The authors suggested that mice are a potential reservoir of E. cuniculi for arctic foxes and feral minks. Indeed, recent molecular characterization of E. cuniculi isolates from Norway and Finland (8, 190) revealed that all foxes originating from four different farms were infected with strain II ( mouse strain ) of E. cuniculi (see Molecular Epidemiology below). Carnivores. The clinical manifestation of canine encephalitozoonosis is an encephalitis-nephritis syndrome which had previously been confused with canine distemper (23). Encephalitozoonosis in domestic dogs, which is caused by strain III ( dog strain ) of E. cuniculi, has been described from Tanzania, South Africa, and the United States (23, 37, 252, 264). In domestic cats, only three cases of E. cuniculi infections have been reported (37, 178). In captive carnivores, disseminated infections similar to those found in dogs occurred in fox cubs (215). This disease, which is still a major problem and causes heavy losses of blue foxes in northern European countries (5), is caused by strain II ( mouse strain ) of E. cuniculi (190). Outbreaks of encephalitozoonosis in carnivores in zoos were previously reviewed (37). Few data are available about the disease in wild carnivores. Encephalitozoon-like organisms were detected by light microscopy in brain tissues from a wild hand-reared red fox (Vulpes vulpes) with neurological symptoms from the United Kingdom (331) and from captive wild dog (Lycaon pictus) pups which died of a fatal disease resembling canine distemper 13 days after vaccination with a live attenuated strain of canine distemper virus (303). Serological investigations with ELISA revealed no seropositive animals in 86 wild red foxes from Switzerland (210). In Iceland, seroprevalences were 12% among 372 wild arctic foxes (Alopex lagopus) and 8% in feral mink (Mustela vison) (140). Histopathological findings for a seropositive fox pup with a neurological disorder which died 2 days after capture were consistent with encephalitozoonosis described for farmed foxes. The authors suggested that encephalitozoonosis contributed to the decline of the arctic fox population size by depressing fetal and pup survival and that mice may represent a potential reservoir for microsporidia (140). Indeed, in Greenland, where rodents are absent from the diet of these arctic foxes, none of 230 foxes investigated had antibodies to E. cuniculi (7). In all mentioned reports of encephalitozoonosis in wild carnivores (140, 303, 331), Encephalitozoon-like organisms were detected by light microscopy only. Therefore, species and strain determinations were not conclusive. In dogs and foxes, encephalitozoonosis is being perpetuated in the population by horizontal and vertical transmission (23, 201). Dogs and foxes infected by ingestion of spores showed moderate clinical symptoms, and the chronically infected animals represented the main source of infections for the offspring. In fur farms, food contaminated with spores from infected rodents or rabbits was assumed to be a possible source of infection for foxes and minks (140, 215). Monkeys. Disseminated natural infections resulting in high morbidity and severe encephalitis caused by Encephalitozoonlike organism have been reported for stillborn and young squirrel monkeys (Saimiri sciureus) in the United States (28, 337). Although parasite identification was based on electron microscopy only, which does not allow one to distinguish E. cuniculi from E. hellem, the neuropathological symptoms strongly suggested that E. cuniculi was the species involved. Only recently, strain III ( dog strain ) of E. cuniculi was identified in tamarin colonies (Saguinus imperator, Oedipomidas oedipus, and Leontopithecus rosalia rosalia) in two zoos in Europe, causing severe disseminated infection with high mortality in infants (134, 234, 329). In experimental infections of vervet monkeys with dog-derived E. cuniculi isolates, transmission from infected infants to their nursing dams as well as transplacental transmission was established (297). In naturally infected monkeys, transplacental transmission was shown by the presence of multifocal granulomatous encephalitis with invasion of the brain by Encephalitozoon in stillborn monkeys or monkeys only a few days old, as well as by the reported placentitis in an animal and the isolation of Encephalitozoon-like parasites from placental tissue of a baboon (reviewed in reference 37). The hypothesis that animals with silent infections can perpetuate the disease in a colony is supported by a serological survey of a squirrel monkey colony over 3 years. More than half of these 250 animals tested positive at least once, and asymptomatic young animals were also seropositive (251). Export of such seropositive asymptomatic animals represents a high risk for the introduction of the parasite in other colonies, as was recently observed in an Emperor tamarin colony in Europe (134). So far, no sources of infection have been elucidated for E. cuniculi infections in monkeys, and it is not known whether this microsporidial species is prevalent in free-living monkeys. In contrast to natural infections causing neonatal death or lethal infections in young monkeys, experimental infections of immunocompetent monkeys with dog- or rabbit-derived isolates resulted in asymptomatic infections (84, 254, 297). Therefore, many factors, including host species, E. cuniculi strain, age and immune status of the host, and mode of transmission (e.g., intrauterine or oral), may influence the outcome of infections in monkeys. Molecular Epidemiology In E. cuniculi, the existence of three strains (I, II, and III; also named rabbit strain, mouse strain, and dog strain ) was established using immunological and molecular methods (86). The repeated element 5 -GTTT-3 in the ITS is a reliable and widely used genetic marker: strain I contains three such repeats, strain II contains two repeats, and strain III contains four repeats. A recent multilocus analysis yielded additional markers for the three strains, namely, the genes coding for the polar tube protein and spore wall protein 1 (333). This strain differentiation helped to elucidate the complex epidemiological situation of E. cuniculi infections in different hosts and in different parts of the world. Table 5 shows the host species and the geographical origins of those E. cuniculi isolates which were determined to the strain level and for which comprehensive information about the host animal was available. These data confirm earlier observations which suggested from circumstantial evidence that different strains of E. cuniculi with different host preferences might exist in nature (reviewed in reference 37). Strain II ( mouse strain ) was identified in rodents (mouse and rat) and
Microspora, Pneumocystis & Blastocystis hominis
 PARA 317331 Microspora, Pneumocystis & Blastocystis hominis Nimit Morakote, Ph.D. Microspora A group of organisms: small single-celled, obligate intracellular parasites belonging to Phylum Microspora Early-
PARA 317331 Microspora, Pneumocystis & Blastocystis hominis Nimit Morakote, Ph.D. Microspora A group of organisms: small single-celled, obligate intracellular parasites belonging to Phylum Microspora Early-
Apicomplexa Bowel infection Isosporiasis Blood & Tissue Cryptosporidiosis infection Sarcosporidiasis Toxoplasmosis Cyclosporiasis Babesiasis Malaria
 Apicomplexa Bowel infection Isosporiasis Cryptosporidiosis Sarcosporidiasis Cyclosporiasis Blood & Tissue infection Toxoplasmosis Babesiasis Malaria Life cycle of sporozoa Cryptosporidium spp. C.Parvum
Apicomplexa Bowel infection Isosporiasis Cryptosporidiosis Sarcosporidiasis Cyclosporiasis Blood & Tissue infection Toxoplasmosis Babesiasis Malaria Life cycle of sporozoa Cryptosporidium spp. C.Parvum
First detection of intestinal microsporidia in Northern Nigeria
 Published Quarterly Mangalore, South India ISSN 0972-5997 Volume 4, Issue 3; Jul-Sep 2005 Short Communication First detection of intestinal microsporidia in Northern Nigeria Authors Omalu ICJ Yako AB Duhlinska
Published Quarterly Mangalore, South India ISSN 0972-5997 Volume 4, Issue 3; Jul-Sep 2005 Short Communication First detection of intestinal microsporidia in Northern Nigeria Authors Omalu ICJ Yako AB Duhlinska
Molecular Techniques for Detection, Species Differentiation, and Phylogenetic Analysis of Microsporidia
 CLINICAL MICROBIOLOGY REVIEWS, Apr. 1999, p. 243 285 Vol. 12, No. 2 0893-8512/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Molecular Techniques for Detection, Species
CLINICAL MICROBIOLOGY REVIEWS, Apr. 1999, p. 243 285 Vol. 12, No. 2 0893-8512/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Molecular Techniques for Detection, Species
Review Article Epidemiology of Enterocytozoonbieneusi Infection in Humans
 Journal of Parasitology Research Volume 2012, Article ID 981424, 19 pages doi:10.1155/2012/981424 Review Article Epidemiology of Enterocytozoonbieneusi Infection in Humans Olga Matos, 1 Maria Luisa Lobo,
Journal of Parasitology Research Volume 2012, Article ID 981424, 19 pages doi:10.1155/2012/981424 Review Article Epidemiology of Enterocytozoonbieneusi Infection in Humans Olga Matos, 1 Maria Luisa Lobo,
Giardiasis. Table of Contents
 Table of Contents Case Definition... Error! Bookmark not defined. Reporting Requirements... 2 Etiology... Error! Bookmark not defined. Clinical Presentation... Error! Bookmark not defined. Diagnosis...
Table of Contents Case Definition... Error! Bookmark not defined. Reporting Requirements... 2 Etiology... Error! Bookmark not defined. Clinical Presentation... Error! Bookmark not defined. Diagnosis...
Apicomplexan structure: 1-polar ring, 2-conoid, 3- micronemes, 4-rhoptries, 5-nucleus, 6-nucleolus, 7- mitochondria, 8-posterior ring, 9-alveoli,
 Coccidia Protozoans, phylum Apicomplexa, class Sporozoasida, subclass Coccidiasina. Cryptosporidium parvum Isosporabelli Cyclosporacayetanensis Sarcocystisspp 1 Apicomplexan structure: 1-polar ring, 2-conoid,
Coccidia Protozoans, phylum Apicomplexa, class Sporozoasida, subclass Coccidiasina. Cryptosporidium parvum Isosporabelli Cyclosporacayetanensis Sarcocystisspp 1 Apicomplexan structure: 1-polar ring, 2-conoid,
Lecture-7- Hazem Al-Khafaji 2016
 TOXOPLASMOSIS Lecture-7- Hazem Al-Khafaji 2016 TOXOPLASMOSIS It is a disease caused by Toxoplasma gondii which is a protozoan parasite that is infects a variety of mammals and birds throughout the world.
TOXOPLASMOSIS Lecture-7- Hazem Al-Khafaji 2016 TOXOPLASMOSIS It is a disease caused by Toxoplasma gondii which is a protozoan parasite that is infects a variety of mammals and birds throughout the world.
PUBLIC HEALTH SIGNIFICANCE SEASONAL INFLUENZA AVIAN INFLUENZA SWINE INFLUENZA
 INFLUENZA DEFINITION Influenza is an acute highly infectious viral disease characterized by fever, general and respiratory tract catarrhal manifestations. Influenza has 3 Types Seasonal Influenza Avian
INFLUENZA DEFINITION Influenza is an acute highly infectious viral disease characterized by fever, general and respiratory tract catarrhal manifestations. Influenza has 3 Types Seasonal Influenza Avian
Global Alert & Response (GAR) Leptospirosis. Global Alert & Response (GAR)
 Leptospirosis Leptospirosis, a zoonotic and environmental disease a zoonotic and environmental disease Bacteria hosted in animals' kidneys for months/years Environment contaminated by urine (weeks/months)
Leptospirosis Leptospirosis, a zoonotic and environmental disease a zoonotic and environmental disease Bacteria hosted in animals' kidneys for months/years Environment contaminated by urine (weeks/months)
Human Microsporidial Infections
 CLINICAL MICROBIOLOGY REVIEWS, OCt. 1994, p. 426-461 Vol. 7, No. 4 0893-8512/94/$04.00+0 Copyright 1994, American Society for Microbiology Human Microsporidial Infections RAINER WEBER,"* RALPH T. BRYAN,2
CLINICAL MICROBIOLOGY REVIEWS, OCt. 1994, p. 426-461 Vol. 7, No. 4 0893-8512/94/$04.00+0 Copyright 1994, American Society for Microbiology Human Microsporidial Infections RAINER WEBER,"* RALPH T. BRYAN,2
Q Fever What men and women on the land need to know
 Q Fever What men and women on the land need to know Dr. Stephen Graves Director, Australian Rickettsial Reference Laboratory Director, Division of Microbiology, Pathology North (Hunter) NSW Health Pathology,
Q Fever What men and women on the land need to know Dr. Stephen Graves Director, Australian Rickettsial Reference Laboratory Director, Division of Microbiology, Pathology North (Hunter) NSW Health Pathology,
Parasitic Protozoa, Helminths, and Arthropod Vectors
 PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 23 Parasitic Protozoa, Helminths, and Arthropod Vectors Parasitic Diseases Protozoan and helminthic parasites are emerging as serious
PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 23 Parasitic Protozoa, Helminths, and Arthropod Vectors Parasitic Diseases Protozoan and helminthic parasites are emerging as serious
Giardiasis Surveillance Protocol
 Provider Responsibilities 1. Report all cases to your local health department by completing the provider section of the WVEDSS form within the timeframe indicated: Sporadic case of - should be reported
Provider Responsibilities 1. Report all cases to your local health department by completing the provider section of the WVEDSS form within the timeframe indicated: Sporadic case of - should be reported
Hepatitis E FAQs for Health Professionals
 Hepatitis E FAQs for Health Professionals Index of Questions ± Overview and Statistics What is Hepatitis E? How common is Hepatitis E in the United States? Where is Hepatitis E most common? Are there different
Hepatitis E FAQs for Health Professionals Index of Questions ± Overview and Statistics What is Hepatitis E? How common is Hepatitis E in the United States? Where is Hepatitis E most common? Are there different
Hepatitis E in South Africa. Tongai Maponga
 Hepatitis E in South Africa Tongai Maponga 7th FIDSSA CONGRESS 2017 This is what usually comes to mind History of hepatitis E virus An ET-NANB hepatitis virus later named HEV was first suspected in 1980.
Hepatitis E in South Africa Tongai Maponga 7th FIDSSA CONGRESS 2017 This is what usually comes to mind History of hepatitis E virus An ET-NANB hepatitis virus later named HEV was first suspected in 1980.
Viruse associated gastrointestinal infection
 Viruse associated gastrointestinal infection Dr. Hala Al Daghistani Rotaviruses Rotaviruses are a major cause of diarrheal illness in human (infants), and young animals, including calves and piglets. Infections
Viruse associated gastrointestinal infection Dr. Hala Al Daghistani Rotaviruses Rotaviruses are a major cause of diarrheal illness in human (infants), and young animals, including calves and piglets. Infections
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Giardiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
August 2011 Giardiasis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition August 2011 August 2011 October
Training in Infectious Diseases Modeling. A reflection on vaccination as a disease control measure
 Training in Infectious Diseases Modeling A reflection on vaccination as a disease control measure -Example of Rotavirus disease- Participant s Guide Adapted by Nathalie Elomeiri; Camelia Savulescu; Fernando
Training in Infectious Diseases Modeling A reflection on vaccination as a disease control measure -Example of Rotavirus disease- Participant s Guide Adapted by Nathalie Elomeiri; Camelia Savulescu; Fernando
Alberta Health and Wellness Public Health Disease Under Surveillance Management Guidelines March 2011
 March 2011 Histoplasmosis Case Definition Confirmed Case Clinical illness [1] with laboratory confirmation of infection: Isolation of Histoplasma capsulatum from an appropriate clinical specimen (tissue
March 2011 Histoplasmosis Case Definition Confirmed Case Clinical illness [1] with laboratory confirmation of infection: Isolation of Histoplasma capsulatum from an appropriate clinical specimen (tissue
Brachyspira & Lawsonia
 General Brachyspira & Lawsonia Gram-negative Anaerobic but with aerotolerance Colonize the large intestine of mammals and birds Infections with Brachyspira species are important in pigs Species & Disease
General Brachyspira & Lawsonia Gram-negative Anaerobic but with aerotolerance Colonize the large intestine of mammals and birds Infections with Brachyspira species are important in pigs Species & Disease
Gastroenteritis and viral infections
 Gastroenteritis and viral infections A Large number of viruses are found in the human gut; these include some that are associated with gastroenteritis Rotaviruses Adenoviruses 40/41 Caliciviruses Norwalk-like
Gastroenteritis and viral infections A Large number of viruses are found in the human gut; these include some that are associated with gastroenteritis Rotaviruses Adenoviruses 40/41 Caliciviruses Norwalk-like
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia)
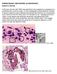 PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
ccess safe drinking wa r is everyone s right Protozoans that cause diarrheal disease
 ccess safe drinking wa r is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
ccess safe drinking wa r is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
West Nile Virus. By Frank Riusech
 West Nile Virus By Frank Riusech Disease Etiology: West Nile virus(wnv), genus, flavivirus is positive- stranded RNA arbovirus (arthropod- borne), belonging to the Flaviviridae family. Included in this
West Nile Virus By Frank Riusech Disease Etiology: West Nile virus(wnv), genus, flavivirus is positive- stranded RNA arbovirus (arthropod- borne), belonging to the Flaviviridae family. Included in this
Rift Valley Fever. What is Rift Valley Fever?
 Rift Valley Fever What is Rift Valley Fever? Rift Valley fever (RVF) is a peracute or acute insect-borne disease of man and animals, historically restricted to Africa. An outbreak of RVF in animals frequently
Rift Valley Fever What is Rift Valley Fever? Rift Valley fever (RVF) is a peracute or acute insect-borne disease of man and animals, historically restricted to Africa. An outbreak of RVF in animals frequently
 Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
RHODOCOCCUS EQUI. Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation
 RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
RHODOCOCCUS EQUI Definition Clinical Signs Transmission Diagnostic Sampling, Testing and Handling Post-mortem Environmental Persistence Specific Control Measures Release of Animals from Isolation Biosecurity
Clinical Significance of Enteric Protozoa in the Immunosuppressed Human Population
 CLINICAL MICROBIOLOGY REVIEWS, Oct. 2009, p. 634 650 Vol. 22, No. 4 0893-8512/09/$08.00 0 doi:10.1128/cmr.00017-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Clinical Significance
CLINICAL MICROBIOLOGY REVIEWS, Oct. 2009, p. 634 650 Vol. 22, No. 4 0893-8512/09/$08.00 0 doi:10.1128/cmr.00017-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Clinical Significance
HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM
 HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, National Institute of Infectious Diseases, Tokyo, Japan, Bach Mai hospital, Vietnam, Military
HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, National Institute of Infectious Diseases, Tokyo, Japan, Bach Mai hospital, Vietnam, Military
For Vets General Information Prevalence and Risk Factors Humans
 For Vets General Information Cryptosporidium spp. are intestinal protozoal parasites of animals and humans that cause the disease cryptosporidiosis. The primary clinical sign of infection is diarrhea,
For Vets General Information Cryptosporidium spp. are intestinal protozoal parasites of animals and humans that cause the disease cryptosporidiosis. The primary clinical sign of infection is diarrhea,
LEC 2, Medical biology, Theory, prepared by Dr. AYAT ALI
 General Characteristics, Structure and Taxonomy of Viruses Viruses A virus is non-cellular organisms made up of genetic material and protein that can invade living cells. They are considered both a living
General Characteristics, Structure and Taxonomy of Viruses Viruses A virus is non-cellular organisms made up of genetic material and protein that can invade living cells. They are considered both a living
Escherichia coli Verotoxigenic Infections
 Revision Dates Case Definition Reporting Requirements Epidemiology/Public Health Management March 2011 May 2018 March 2011 Includes O157:H7 Case Definition Confirmed Case Laboratory confirmation of infection
Revision Dates Case Definition Reporting Requirements Epidemiology/Public Health Management March 2011 May 2018 March 2011 Includes O157:H7 Case Definition Confirmed Case Laboratory confirmation of infection
INFLUENZA-2 Avian Influenza
 INFLUENZA-2 Avian Influenza VL 7 Dec. 9 th 2013 Mohammed El-Khateeb Overview 1. Background Information 2. Origin/History 3. Brief overview of genome structure 4. Geographical Distribution 5. Pandemic Nature
INFLUENZA-2 Avian Influenza VL 7 Dec. 9 th 2013 Mohammed El-Khateeb Overview 1. Background Information 2. Origin/History 3. Brief overview of genome structure 4. Geographical Distribution 5. Pandemic Nature
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Trichinosis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 October 2005 Case Definition
August 2011 Trichinosis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 October 2005 Case Definition
U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Foodborne Pathogenic Microorganisms and Natural Toxins Handbook.
 U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Foodborne Pathogenic Microorganisms and Natural Toxins Handbook Rotavirus 1. Name of the Organism: Rotavirus Rotaviruses are classified
U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Foodborne Pathogenic Microorganisms and Natural Toxins Handbook Rotavirus 1. Name of the Organism: Rotavirus Rotaviruses are classified
Infection Basics. Lecture 12 Biology W3310/4310 Virology Spring 2016
 Infection Basics Lecture 12 Biology W3310/4310 Virology Spring 2016 Before I came here I was confused about this subject. Having listened to your lecture, I am still confused but at a higher level. ENRICO
Infection Basics Lecture 12 Biology W3310/4310 Virology Spring 2016 Before I came here I was confused about this subject. Having listened to your lecture, I am still confused but at a higher level. ENRICO
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASES WITH GLOBAL IMPACT
 Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Earn CE Credits under Continuing Education on
Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Earn CE Credits under Continuing Education on
Cryptosporidiosis. By: Nikole Stewart
 Cryptosporidiosis By: Nikole Stewart Cryptosporidiosis ("Crypto"); Etiological agent- Cryptosporidium (1) Transmission: Transmission occurs via the fecal-oral route when individuals ingest water or food
Cryptosporidiosis By: Nikole Stewart Cryptosporidiosis ("Crypto"); Etiological agent- Cryptosporidium (1) Transmission: Transmission occurs via the fecal-oral route when individuals ingest water or food
LECTURE topics: 1. Immunology. 2. Emerging Pathogens
 LECTURE 23 2 topics: 1. Immunology 2. Emerging Pathogens Benefits of the Normal Flora: 1. Protect us from colonization by other bacteria and fungi (competitive exclusion). 2. Many synthesize vitamins,
LECTURE 23 2 topics: 1. Immunology 2. Emerging Pathogens Benefits of the Normal Flora: 1. Protect us from colonization by other bacteria and fungi (competitive exclusion). 2. Many synthesize vitamins,
Better Training for Safer Food BTSF
 Better Training for Safer Food BTSF Importation of vector-borne infectious diseases Tanguy Marcotty Institute of Tropical Medicine Importation routes Trade Infected live animals (e.g. H5N1 avian influenza)
Better Training for Safer Food BTSF Importation of vector-borne infectious diseases Tanguy Marcotty Institute of Tropical Medicine Importation routes Trade Infected live animals (e.g. H5N1 avian influenza)
Immunodeficiencies HIV/AIDS
 Immunodeficiencies HIV/AIDS Immunodeficiencies Due to impaired function of one or more components of the immune or inflammatory responses. Problem may be with: B cells T cells phagocytes or complement
Immunodeficiencies HIV/AIDS Immunodeficiencies Due to impaired function of one or more components of the immune or inflammatory responses. Problem may be with: B cells T cells phagocytes or complement
Influenza: The past, the present, the (future) pandemic
 Influenza: The past, the present, the (future) pandemic Kristin Butler, MLS (ASCP) cm Department of Clinical Laboratory Sciences Louisiana Health Sciences Center - Shreveport Fall 2017 Objectives 1) Detail
Influenza: The past, the present, the (future) pandemic Kristin Butler, MLS (ASCP) cm Department of Clinical Laboratory Sciences Louisiana Health Sciences Center - Shreveport Fall 2017 Objectives 1) Detail
SEA/CD/154 Distribution : General. Avian Influenza in South-East Asia Region: Priority Areas for Research
 SEA/CD/154 Distribution : General Avian Influenza in South-East Asia Region: Priority Areas for Research World Health Organization Publications of the World Health Organization enjoy copyright protection
SEA/CD/154 Distribution : General Avian Influenza in South-East Asia Region: Priority Areas for Research World Health Organization Publications of the World Health Organization enjoy copyright protection
Coccidia. Eucoccidioside
 Coccidia Kingdom Sub-Kingdom Phylum Class Order Family Genus Species Protista Protozoa Apicomplexa Sporozoasida Eucoccidioside Sarcocystidae Toxoplasma gondii 1 Toxoplasma gondii (life cycle) Sexual cycle
Coccidia Kingdom Sub-Kingdom Phylum Class Order Family Genus Species Protista Protozoa Apicomplexa Sporozoasida Eucoccidioside Sarcocystidae Toxoplasma gondii 1 Toxoplasma gondii (life cycle) Sexual cycle
Chapter 38 Viral Infections
 Chapter 38 Viral Infections Primary Objectives of This Chapter Chapter 38 introduces a wide variety of important human viral diseases and serves as an introduction to Medical Virology. It is considered
Chapter 38 Viral Infections Primary Objectives of This Chapter Chapter 38 introduces a wide variety of important human viral diseases and serves as an introduction to Medical Virology. It is considered
All living creatures share two basic purposes 1. survival 2. reproduction
 Infectious Diseases All living creatures share two basic purposes 1. survival 2. reproduction *Organisms must take nutrients essential for growth and proliferation from the environment. *In many conditions
Infectious Diseases All living creatures share two basic purposes 1. survival 2. reproduction *Organisms must take nutrients essential for growth and proliferation from the environment. *In many conditions
Bacillary Dysentery (Shigellosis)
 Bacillary Dysentery (Shigellosis) An acute bacterial disease involving the large and distal small intestine, caused by the bacteria of the genus shigella. Infectious agent Shigella is comprised of four
Bacillary Dysentery (Shigellosis) An acute bacterial disease involving the large and distal small intestine, caused by the bacteria of the genus shigella. Infectious agent Shigella is comprised of four
POSTGRADUATE INSTITUTE OF MEDICINE UNIVERSITY OF COLOMBO MD (MEDICAL PARASITOLOGY) EXAMINATION JANUARY, 2001 PAPER 1
 JANUARY, 2001 Date: 15 th January 2001 Time: 2.00 p.m. -500 p.m. PAPER 1 Answer all five (5) questions Answer each question in a separate book 1. Discuss the underlying principles relating to the use of
JANUARY, 2001 Date: 15 th January 2001 Time: 2.00 p.m. -500 p.m. PAPER 1 Answer all five (5) questions Answer each question in a separate book 1. Discuss the underlying principles relating to the use of
Avian Influenza. Regional Workshops: Veterinary Discussion. Will Garton
 Avian Influenza Regional Workshops: Veterinary Discussion Will Garton What is Avian Influenza? Influenza virus types A B C BIRDS, MAMMALS (including humans, pigs, horses, mink, sea mammals etc) HUMANS
Avian Influenza Regional Workshops: Veterinary Discussion Will Garton What is Avian Influenza? Influenza virus types A B C BIRDS, MAMMALS (including humans, pigs, horses, mink, sea mammals etc) HUMANS
Pathogen prioritisation
 Pathogen prioritisation Comment on Confidence Score: The confidence score has to be given by who compiles the questionnaire. We propose a very simple scoring system, based on 3 classes: 1. based on experience
Pathogen prioritisation Comment on Confidence Score: The confidence score has to be given by who compiles the questionnaire. We propose a very simple scoring system, based on 3 classes: 1. based on experience
Characteristics of Mycobacterium
 Mycobacterium Characteristics of Mycobacterium Very thin, rod shape. Culture: Aerobic, need high levels of oxygen to grow. Very slow in grow compared to other bacteria (colonies may be visible in up to
Mycobacterium Characteristics of Mycobacterium Very thin, rod shape. Culture: Aerobic, need high levels of oxygen to grow. Very slow in grow compared to other bacteria (colonies may be visible in up to
Weekly Influenza Surveillance Report. Week 11
 Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
ZIKA VIRUS. Epic and aspects of management
 ZIKA VIRUS Epic and aspects of management Classification - Belong to the family Flaviviridae which are mosquitoes borne viruses such as Dengue virus ( DEN V ), West Nile virus ( WN V ), Yellow fever Virus
ZIKA VIRUS Epic and aspects of management Classification - Belong to the family Flaviviridae which are mosquitoes borne viruses such as Dengue virus ( DEN V ), West Nile virus ( WN V ), Yellow fever Virus
Prevalence of Intestinal Parasites in HIV Seropositive Patients with and without Diarrhoea and its Correlation with CD4 Counts
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 10 (2016) pp. 527-532 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.510.058
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 10 (2016) pp. 527-532 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.510.058
- Mycoplasma and Ureaplasma. - Rickettsia
 - Mycoplasma and Ureaplasma - Rickettsia Mycoplasma and Ureaplasma Family: Mycoplasmataceae Genus: Mycoplasma Species: M. pneumoniae Species: M. hominis Species: M. genitalium Genus: Ureaplasma Species:
- Mycoplasma and Ureaplasma - Rickettsia Mycoplasma and Ureaplasma Family: Mycoplasmataceae Genus: Mycoplasma Species: M. pneumoniae Species: M. hominis Species: M. genitalium Genus: Ureaplasma Species:
LEPTOSPIRA SPP Date of document: July 2004
 LEPTOSPIRA SPP Date of document: July 2004 1.- INTRODUCCIÓN The Leptospira genus is a member of the Leptospiraceae family, Spirochaetales order. The classification of the leptospiras is very complex and
LEPTOSPIRA SPP Date of document: July 2004 1.- INTRODUCCIÓN The Leptospira genus is a member of the Leptospiraceae family, Spirochaetales order. The classification of the leptospiras is very complex and
Giardia lamblia (flagellates)
 Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
2018 Science Olympiad: Microbe Mission - Sample Tournament Div C
 2018 Science Olympiad: Microbe Mission - Sample Tournament Div C Section A: Types of cells and their parts 1. Please state if the cell is prokaryotic or eukaryotic. Then label the following molecular components
2018 Science Olympiad: Microbe Mission - Sample Tournament Div C Section A: Types of cells and their parts 1. Please state if the cell is prokaryotic or eukaryotic. Then label the following molecular components
WYANDOT COUNTY 2016 COMMUNICABLE DISEASE REPORT
 WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
Appendix A: Disease-Specific Chapters
 Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Giardiasis Revised Giardiasis Communicable Virulent Health Protection and Promotion Act: Ontario Regulation 558/91 Specification
Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Giardiasis Revised Giardiasis Communicable Virulent Health Protection and Promotion Act: Ontario Regulation 558/91 Specification
Campylobacter ENTERITIS SURVEILLANCE PROTOCOL
 Campylobacter ENTERITIS SURVEILLANCE PROTOCOL Public Health Action 1. Educate providers and laboratories to report stool cultures positive for Campylobacter jejuni or Campylobacter coli from patients within
Campylobacter ENTERITIS SURVEILLANCE PROTOCOL Public Health Action 1. Educate providers and laboratories to report stool cultures positive for Campylobacter jejuni or Campylobacter coli from patients within
WYANDOT COUNTY 2016 COMMUNICABLE DISEASE REPORT
 WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
WYANDOT COUNTY 216 COMMUNICABLE DISEASE REPORT February 217 Wyandot County saw a.87% increase in communicable disease cases from 21 to 216 (11 cases and 116 cases respectively). Numerous infectious diseases
Speaker Talk Summaries
 Back to Zoobiquity Information home page Speaker Talk Summaries Keynote Presentation Michael Lairmore DVM, PhD, DACVP/DACVM University of California Davis School of Veterinary Medicine Lessons in One Health
Back to Zoobiquity Information home page Speaker Talk Summaries Keynote Presentation Michael Lairmore DVM, PhD, DACVP/DACVM University of California Davis School of Veterinary Medicine Lessons in One Health
Trichinellosis. By Michelle Randall
 Trichinellosis By Michelle Randall Disease Name: Trichinellosis Etiological Agent: Trichinella spiralis Transmission: People acquire Trichinellosis by consuming raw or undercooked meat infected with the
Trichinellosis By Michelle Randall Disease Name: Trichinellosis Etiological Agent: Trichinella spiralis Transmission: People acquire Trichinellosis by consuming raw or undercooked meat infected with the
A Just in Time Primer on H1N1 Influenza A and Pandemic Influenza developed by the National Association of State EMS Officials and Revised by the
 A Just in Time Primer on H1N1 Influenza A and Pandemic Influenza developed by the National Association of State EMS Officials and Revised by the Michigan Department of Community Health EMS and Trauma Systems
A Just in Time Primer on H1N1 Influenza A and Pandemic Influenza developed by the National Association of State EMS Officials and Revised by the Michigan Department of Community Health EMS and Trauma Systems
VIRAL GASTRO-ENTERITIS
 VIRAL GASTRO-ENTERITIS Dr Esam Ibraheem Azhar (BSc, MSc, Ph.D Molecular Medical Virology) Asst. Prof. Medical Laboratory Technology Department ١ Gastroenteritis Introduction (1) Paediatric diarrhoea remains
VIRAL GASTRO-ENTERITIS Dr Esam Ibraheem Azhar (BSc, MSc, Ph.D Molecular Medical Virology) Asst. Prof. Medical Laboratory Technology Department ١ Gastroenteritis Introduction (1) Paediatric diarrhoea remains
Western Veterinary Conference 2013
 Western Veterinary Conference 2013 SA283 EMERGING CANINE INFECTIOUS RESPIRATORY DISEASES Stephanie D Janeczko, DVM, MS, Dipl. ABVP (Canine/Feline) ASPCA New York, NY, USA Management of infectious respiratory
Western Veterinary Conference 2013 SA283 EMERGING CANINE INFECTIOUS RESPIRATORY DISEASES Stephanie D Janeczko, DVM, MS, Dipl. ABVP (Canine/Feline) ASPCA New York, NY, USA Management of infectious respiratory
VIRAL AGENTS CAUSING GASTROENTERITIS
 VIRAL AGENTS CAUSING GASTROENTERITIS VIRAL AGENTS CAUSING GASTROENTERITIS Pathogens discussed in our lectures 1. Rotavirus 2. Enteric adenoviruses 3. Caliciviruses 4. Astroviruses 5. Toroviruses Viruses
VIRAL AGENTS CAUSING GASTROENTERITIS VIRAL AGENTS CAUSING GASTROENTERITIS Pathogens discussed in our lectures 1. Rotavirus 2. Enteric adenoviruses 3. Caliciviruses 4. Astroviruses 5. Toroviruses Viruses
West Nile Fever. F. Karup Pedersen 2012
 F. Karup Pedersen 2012 West Nile virus first isolated 1937 in West Nile district, Uganda Japanese encephalitis reconvalescens serum could neutralize West Nile virus Virus antigenically related to Japanese
F. Karup Pedersen 2012 West Nile virus first isolated 1937 in West Nile district, Uganda Japanese encephalitis reconvalescens serum could neutralize West Nile virus Virus antigenically related to Japanese
West Nile Virus. Family: Flaviviridae
 West Nile Virus 1 Family: Flaviviridae West Nile Virus Genus: Flavivirus Japanese Encephalitis Antigenic Complex Complex Includes: Alfuy, Cacipacore, Japanese encephalitis, koutango, Kunjin, Murray Valley
West Nile Virus 1 Family: Flaviviridae West Nile Virus Genus: Flavivirus Japanese Encephalitis Antigenic Complex Complex Includes: Alfuy, Cacipacore, Japanese encephalitis, koutango, Kunjin, Murray Valley
UTSW/BioTel EMS TRAINING BULLETIN October EMS TB Ebola Virus Disease (EVD)
 UTSW/BioTel EMS TRAINING BULLETIN October 2014 EMS TB 14-006 Ebola Virus Disease (EVD) Purpose: 1. To inform & provide management recommendations to UTSW/BioTel EMS System EMS Providers about Ebola Virus
UTSW/BioTel EMS TRAINING BULLETIN October 2014 EMS TB 14-006 Ebola Virus Disease (EVD) Purpose: 1. To inform & provide management recommendations to UTSW/BioTel EMS System EMS Providers about Ebola Virus
Current and Emerging Legionella Diagnostics
 Current and Emerging Legionella Diagnostics Nicole Wolter Centre for Respiratory Diseases and Meningitis (CRDM) National Institute for Communicable Diseases nicolew@nicd.ac.za 7 th FIDSSA Conference, Cape
Current and Emerging Legionella Diagnostics Nicole Wolter Centre for Respiratory Diseases and Meningitis (CRDM) National Institute for Communicable Diseases nicolew@nicd.ac.za 7 th FIDSSA Conference, Cape
Giardiasis (lambliasis)
 SURVEILLANCE REPORT Annual Epidemiological Report for 2016 Giardiasis (lambliasis) Key facts In 2016, 18 985 confirmed giardiasis cases were reported in the EU/EEA, representing an increase of 5.3% from
SURVEILLANCE REPORT Annual Epidemiological Report for 2016 Giardiasis (lambliasis) Key facts In 2016, 18 985 confirmed giardiasis cases were reported in the EU/EEA, representing an increase of 5.3% from
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Campylobacter jejuni
 U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Foodborne Pathogenic Microorganisms and Natural Toxins Handbook Campylobacter jejuni 1. Name of the Organism: Campylobacter jejuni
U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Foodborne Pathogenic Microorganisms and Natural Toxins Handbook Campylobacter jejuni 1. Name of the Organism: Campylobacter jejuni
Introduction. Infections acquired by travellers
 Introduction The number of Australians who travel overseas has increased steadily over recent years and now between 3.5 and 4.5 million exits are made annually. Although many of these trips are to countries
Introduction The number of Australians who travel overseas has increased steadily over recent years and now between 3.5 and 4.5 million exits are made annually. Although many of these trips are to countries
Classical swine fever (CSF) - also known as hog cholera, is a highly contagious multisystemic, haemorrhagic, viral disease of swine.
 Classical swine fever (CSF) - also known as hog cholera, is a highly contagious multisystemic, haemorrhagic, viral disease of swine. Genus PESTIVIRUS Family Flaviviridae Related to BVDV and BDV o Severity
Classical swine fever (CSF) - also known as hog cholera, is a highly contagious multisystemic, haemorrhagic, viral disease of swine. Genus PESTIVIRUS Family Flaviviridae Related to BVDV and BDV o Severity
Coronaviruses cause acute, mild upper respiratory infection (common cold).
 Coronaviruses David A. J. Tyrrell Steven H. Myint GENERAL CONCEPTS Clinical Presentation Coronaviruses cause acute, mild upper respiratory infection (common cold). Structure Spherical or pleomorphic enveloped
Coronaviruses David A. J. Tyrrell Steven H. Myint GENERAL CONCEPTS Clinical Presentation Coronaviruses cause acute, mild upper respiratory infection (common cold). Structure Spherical or pleomorphic enveloped
Appendix E1. Epidemiology
 Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
History of Lyme Disease
 History of Lyme Disease ORIGINS OF THE DISEASE Lyme disease was first recognized in the United States in 1975, following a mysterious outbreak of juvenile rheumatoid arthritis near the community of Lyme,
History of Lyme Disease ORIGINS OF THE DISEASE Lyme disease was first recognized in the United States in 1975, following a mysterious outbreak of juvenile rheumatoid arthritis near the community of Lyme,
Anton van Leeuwenhoek. Protozoa: This is what he saw in his own stool sample. Morphology 10/14/2009. Protozoans that cause diarrheal disease
 Access to safe drinking water is everyone s right Anton van Leeuwenhoek Protozoa: Protozoans that cause diarrheal disease This is what he saw in his own stool sample 1. Giardia lamblia 2. Entameba histolytica
Access to safe drinking water is everyone s right Anton van Leeuwenhoek Protozoa: Protozoans that cause diarrheal disease This is what he saw in his own stool sample 1. Giardia lamblia 2. Entameba histolytica
West Nile Virus Surveillance in mosquito vectors (Culex pipiens)
 West Nile Virus Surveillance in mosquito vectors (Culex pipiens) Dragana Despot, Ivan Aleksić, Nebojša Tačević & Branislav Pešić Institute for Biocides and Medical Ecology, Belgrade, Serbia The virus West
West Nile Virus Surveillance in mosquito vectors (Culex pipiens) Dragana Despot, Ivan Aleksić, Nebojša Tačević & Branislav Pešić Institute for Biocides and Medical Ecology, Belgrade, Serbia The virus West
Vibrio Cholerae (non-o1, non-o139)
 August 2011 Vibrio Cholerae (non-o1, non-o139) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011
August 2011 Vibrio Cholerae (non-o1, non-o139) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011
Zoonosis = an infection or infestation which is shared in nature by man and lower vertebrate animals.
 Zoonosis = an infection or infestation which is shared in nature by man and lower vertebrate animals. For the purposes of this presentation, "zoonotic disease" will be defined as a disease that is caused
Zoonosis = an infection or infestation which is shared in nature by man and lower vertebrate animals. For the purposes of this presentation, "zoonotic disease" will be defined as a disease that is caused
Access to safe drinking water is everyone s right. Protozoans that cause diarrheal disease
 Access to safe drinking water is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
Access to safe drinking water is everyone s right Protozoa: Protozoans that cause diarrheal disease 1. Giardia lamblia 2. Entameba histolytica 3. Cryptosporidium parvum 4. Cyclospora cayetanensis 1 Giardia
Create the Following Chart in your notebook. Fill in as you go through each one.
 Diseases of Africa Create the Following Chart in your notebook. Fill in as you go through each one. History of disease? Affected Population? How do you catch the disease? Symptoms? Prevention / Treatment?
Diseases of Africa Create the Following Chart in your notebook. Fill in as you go through each one. History of disease? Affected Population? How do you catch the disease? Symptoms? Prevention / Treatment?
Viral Agents of Paediatric Gastroenteritis
 Viral Agents of Paediatric Gastroenteritis Dr Carl Kirkwood -------------------- Enteric Virus Research Group Murdoch Childrens Research Institute Royal Children s Hospital Victoria. WHO Collaborating
Viral Agents of Paediatric Gastroenteritis Dr Carl Kirkwood -------------------- Enteric Virus Research Group Murdoch Childrens Research Institute Royal Children s Hospital Victoria. WHO Collaborating
Microbiology of Atypical Pneumonia. Dr. Mohamed Medhat Ali
 Microbiology of Atypical Pneumonia Dr. Mohamed Medhat Ali Pneumonia P n e u m o n i a i s a n infection of the lungs that can be caused by viruses, bacteria, and fungi. Atypical! Pneumonia Symptoms. X-ray
Microbiology of Atypical Pneumonia Dr. Mohamed Medhat Ali Pneumonia P n e u m o n i a i s a n infection of the lungs that can be caused by viruses, bacteria, and fungi. Atypical! Pneumonia Symptoms. X-ray
Detection of Enterocytozoon bieneusi (Microsporidia) by polymerase chain reaction (PCR) using species-specific primer in stool samples of HIV patients
 Indian J Med Res 121, April 2005, pp 215-219 Detection of Enterocytozoon bieneusi (Microsporidia) by polymerase chain reaction (PCR) using species-specific primer in stool samples of HIV patients S. Satheesh
Indian J Med Res 121, April 2005, pp 215-219 Detection of Enterocytozoon bieneusi (Microsporidia) by polymerase chain reaction (PCR) using species-specific primer in stool samples of HIV patients S. Satheesh
Washington State Department of Health (DOH) Cryptosporidium in Drinking Water Position Paper. Purpose
 Washington State Department of Health (DOH) Cryptosporidium in Drinking Water Position Paper Purpose Cryptosporidium is a micro-organism which has gained increased public health significance as a result
Washington State Department of Health (DOH) Cryptosporidium in Drinking Water Position Paper Purpose Cryptosporidium is a micro-organism which has gained increased public health significance as a result
American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX
 Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
(Data from the Travel Health Surveillance Section of the Health Protection Agency Communicable Disease Surveillance Centre)
 Travellers Diarrhoea Introduction Travellers diarrhoea (TD) is a syndrome that commonly affects travellers caused by one of several different organisms, the most common being enterotoxigenic Escherichia
Travellers Diarrhoea Introduction Travellers diarrhoea (TD) is a syndrome that commonly affects travellers caused by one of several different organisms, the most common being enterotoxigenic Escherichia
Situation update pandemic (H1N1) 2009
 Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
Media centre Ebola virus disease
 1 of 6 10/15/2014 10:59 AM Media centre Ebola virus disease Fact sheet N 103 Updated September 2014 Key facts Ebola virus disease (EVD), formerly known as Ebola haemorrhagic fever, is a severe, often fatal
1 of 6 10/15/2014 10:59 AM Media centre Ebola virus disease Fact sheet N 103 Updated September 2014 Key facts Ebola virus disease (EVD), formerly known as Ebola haemorrhagic fever, is a severe, often fatal
Q FEVER Australian and global perspectives including the recent Netherlands outbreak
 Q FEVER Australian and global perspectives including the recent Netherlands outbreak Coxiella burnetii, the causative bacterium for Q Fever is ubiquitous. Thought to be present in every land mass with
Q FEVER Australian and global perspectives including the recent Netherlands outbreak Coxiella burnetii, the causative bacterium for Q Fever is ubiquitous. Thought to be present in every land mass with
Recommended composition of influenza virus vaccines for use in the influenza season
 Recommended composition of influenza virus vaccines for use in the 2006 2007 influenza season This recommendation relates to the composition of vaccines for the forthcoming influenza season in the northern
Recommended composition of influenza virus vaccines for use in the 2006 2007 influenza season This recommendation relates to the composition of vaccines for the forthcoming influenza season in the northern
Communicable diseases. Gastrointestinal track infection. Sarkhell Araz MSc. Public health/epidemiology
 Communicable diseases Gastrointestinal track infection Sarkhell Araz MSc. Public health/epidemiology Communicable diseases : Refer to diseases that can be transmitted and make people ill. They are caused
Communicable diseases Gastrointestinal track infection Sarkhell Araz MSc. Public health/epidemiology Communicable diseases : Refer to diseases that can be transmitted and make people ill. They are caused
David L. Suarez D.V.M., PhD. A.C.V.M.
 David L. Suarez D.V.M., PhD. A.C.V.M. Southeast Poultry Research Laboratory United States National Poultry Research Center The author has no commercial interests in any commercial products presented. U.S.
David L. Suarez D.V.M., PhD. A.C.V.M. Southeast Poultry Research Laboratory United States National Poultry Research Center The author has no commercial interests in any commercial products presented. U.S.
