Transmission of Anaplasma marginale by Boophilus microplus: Retention of Vector Competence in the Absence of Vector-Pathogen Interaction
|
|
|
- Samantha Newton
- 5 years ago
- Views:
Transcription
1 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2003, p Vol. 41, No /03/$ DOI: /JCM Copyright 2003, American Society for Microbiology. All Rights Reserved. Transmission of Anaplasma marginale by Boophilus microplus: Retention of Vector Competence in the Absence of Vector-Pathogen Interaction James E. Futse, 1 Massaro W. Ueti, 1 Donald P. Knowles, Jr., 1,2 and Guy H. Palmer 1 * Program in Vector-Borne Diseases, Department of Veterinary Microbiology and Pathology, Washington State University, 1 and Animal Diseases Research Unit, USDA Agricultural Research Service, 2 Pullman, Washington Received 7 March 2003/Returned for modification 16 May 2003/Accepted 27 May 2003 Whether arthropod vectors retain competence for transmission of infectious agents in the long-term absence of vector-pathogen interaction is unknown. We addressed this question by quantifying the vector competence of two tick vectors, with mutually exclusive tropical- versus temperate-region distributions, for genetically distinct tropical- and temperate-region strains of the cattle pathogen Anaplasma marginale. The tropical cattle tick Boophilus microplus, which has been eradicated from the continental United States for over 60 years, was able to acquire and transmit the temperate St. Maries (Idaho) strain of A. marginale. Similarly, the temperateregion tick Dermacentor andersoni efficiently acquired and transmitted the Puerto Rico strain of A. marginale. There were no significant quantitative differences in infection rate or number of organisms per tick following feeding on cattle with persistent infections of either A. marginale strain. In contrast, the significantly enhanced replication of the Puerto Rico strain in the salivary gland of B. microplus at the time of transmission feeding is consistent with adaptation of a pathogen strain to its available vector. However, the transmission of both strains by B. microplus demonstrates that adaptation or continual interaction between the pathogen and vector is not required for retention of vector competence. Importantly, the results clearly show that reestablishment of acaricide-resistant B. microplus in the United States would be associated with A. marginale transmission. Anaplasma marginale is the most globally prevalent tickborne pathogen of cattle, with regions of endemicity on the six populated continents (22). Although A. marginale has a global distribution, the prevalence and incidence are highest in regions where the tropical cattle fever tick Boophilus microplus is endemic (19, 21, 25, 28). Larval, nymphal, and adult stages of this tick all preferentially feed on cattle, and each can efficiently acquire and transmit A. marginale (1, 24). This high vectorial capacity of B. microplus results in most calves in subtropical and tropical regions being infected within the first year of life, and this high incidence represents a severe constraint on animal health and production (11). Remarkably, B. microplus was eradicated from the continental United States by a mandatory acaricide-based program initiated in 1906, and eradication status is maintained by required acaricide treatment of cattle entering or resident within a quarantine zone along the U.S.-Mexico border (10). Since Boophilus eradication, tick transmission of A. marginale in the United States is mediated by Dermacentor andersoni and Dermacentor variabilis, species with less vectorial capacity because larval and nymphal stages prefer small mammals and only the adults acquire and transmit by feeding on cattle (27). Corresponding with this change in the vector species, A. marginale prevalence in the United States since B. microplus eradication is markedly lower than in regions where B. microplus is the primary vector (17, 30, 32). * Corresponding author. Mailing address: Department of Veterinary Microbiology and Pathology, 402 Bustad Hall, Washington State University, Pullman, WA Phone: (509) Fax: (509) gpalmer@vetmed.wsu.edu. The likelihood of B. microplus reestablishing its previous range in the U.S. has been markedly increased by the emergence in Mexico of ticks resistant to multiple acaricides, including those used to maintain the 80-mile quarantine region (3, 4). Reestablishment of B. microplus, with its high vectorial capacity for A. marginale, threatens U.S. cattle herds with no or minimal levels of immunity due to the low transmission rate associated with Dermacentor spp. (17, 23, 30). Assessment of this risk requires determining whether B. microplus has retained vector competence: that is the ability to acquire and transmit to the United States temperate-region strains of A. marginale in the long-term absence of pathogen-vector interaction. A. marginale strains have well-characterized genotypic and phenotypic differences (23): notably, strains naturally transmitted by B. microplus form a distinct genetically defined clade as compared to temperate-region strains transmitted by Dermacentor spp. (5). In the 60 years since eradication of the cattle fever tick in the 1940s, any A. marginale strains dependent upon tick transmission by B. microplus would have been lost from the cattle population, as is suggested by the distinct clade structures. The ability of the tick vector to acquire A. marginale from a persistently infected animal is influenced by the level of rickettsemia during feeding, and not all fed ticks become infected (7). The failure of the ticks to become infected appears to occur at the level of the midgut (26), consistent with the importance of a midgut barrier to infection. Successful acquisition of A. marginale, subsequent to receptor-mediated invasion of the midgut epithelium (5), is thus a first determinant of vector competence. Whether the efficiency of initial acquisition differs among competent vectors of A. marginale is unknown; Downloaded from on January 17, 2019 by guest 3829
2 3830 FUTSE ET AL. J. CLIN. MICROBIOL. however, a markedly reduced ability of Boophilus to acquire temperate-region strains of A. marginale during feeding on persistently infected cattle would result in the reduction or loss of vector competence. Following invasion of the midgut epithelium, A. marginale undergoes initial intracellular replication in the midgut epithelial cells and progresses to invasion of the salivary glands (14). Within the salivary glands, a second round of replication occurs and culminates in development of infectious organisms during transmission feeding (15, 18, 26). In adult male D. andersoni ticks infected with the temperateregion South Idaho strain of A. marginale, replication continues during the first 72 h of transmission feeding, reaching approximately 10 5 organisms per salivary gland (18). This replication is required for transmission, and the levels influence transmission efficiency (9). As a result, differences among vectors in pathogen replication in the salivary gland would be expected to have a significant impact on transmission. Thus, two critical determinants of vector competence are the ability of the ticks to acquire the infection and pathogen replication within the tick salivary gland. In the present study, we examine these two determinants of vector competence for B. microplus and D. andersoni fed on cattle persistently infected with either a tropical-region strain or a temperate-region strain and determine whether pathogen transmission by each vector is restricted to the genetically and geographically defined A. marginale clades. MATERIALS AND METHODS Pathogen and vector strains. B. microplus and D. andersoni naturally transmit the Puerto Rico and St. Maries (Idaho) strains of A. marginale, respectively. The sequence of the St. Maries msp4 gene assigns it to a clade of temperate-region strains (6), while the sequence of the Puerto Rico msp4 gene (obtained from infected ticks [GenBank accession no. AY191827]) places it within a clade of seven strains from regions of B. microplus endemicity as reported by de la Fuente et al. (6). The La Minita strain of B. microplus and the Reynolds Creek strain of D. andersoni, free of A. marginale and other pathogens, were maintained at the USDA Agricultural Research Service tick unit in Moscow, Idaho. Larvae were fed for 14 to 16 days on calves to produce fully engorged nymphs. Once the nymphs engorged, they were removed and induced to molt to an adult stage within 48 h at 26 C and 92% relative humidity. Adult males were used for acquisition feeding, because terminally developed male D. andersoni and B. microplus ticks feed more efficiently than either nymphs or adult females and are at an epidemiologically relevant stage for transmission by both vectors (16). Acquisition feeding on persistently infected cattle. Persistent infection is defined by control of the acute rickettsemia and maintenance of rickettsemia levels of 10 6 infected erythrocytes per ml of blood (7, 8, 12). To develop carriers for tick feeding, Holstein calves (8 to 12 months of age) confirmed to be A. marginale negative by the msp5 competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA) (13, 28) were each inoculated intravenously with 10 9 organisms of either the Puerto Rico (calves 908 and 929) or St. Maries (calves 928 and 931) strain of A. marginale. During acute infection, Giemsa-stained blood smears were examined daily, following resolution of the acute stage, and weekly during persistence. Ticks were fed only after no infected erythrocytes had been observed by light microscopy (minimum of 15,000 erythrocytes examined) for 3 consecutive weeks, consistent with a rickettsemia level of 10 6 organisms per ml of infected blood. The actual rickettsemia level during acquisition feeding was determined by quantitative real-time PCR (see the following section). The rickettsemia level on each day, expressed as mean log 10 ( standard deviation) of A. marginale per milliliter, was determined from triplicate assays and used to calculate the mean rickettsemia level during the complete 7-day period of acquisition feeding. The latter was expressed as the mean log 10 ( standard error) of A. marginale per milliliter. The D. andersoni and B. microplus ticks were simultaneously fed on each calf by using separate cloth patches to ensure exposure of both vectors to the same level of inoculum during persistent infection and to control for individual host differences in age, genetics, and innate immunity. Ticks were allowed to acquisition feed for 7 days, engorged ticks were removed, and the fed ticks were incubated for 48 h at 26 C at 93% relative humidity with a 12-h photoperiod. Incubation for 48 h under these conditions allows the blood meal to be cleared from the midgut, thus preventing false-positive detection of ticks as infected, and allows replication within the tick (7, 18, 26). Ticks of each species were dissected, and DNA was extracted from isolated individual salivary glands and midguts as previously described (18). Transmission feeding on naïve cattle. Eight MSP5 CI-ELISA-negative Holstein calves (calves 919, 920, 945, 948, 951, 952, 954, and 955) were used for the transmission feeding of B. microplus and D. andersoni. Adult male B. microplus or D. andersoni ticks, previously acquisition fed and incubated, were allowed to transmission feed on individual calves for 6 days (B. microplus on calves 919, 945, 952, and 954 and D. andersoni on calves 920, 948, 951, and 955). Fed ticks were removed after the transmission feed, and DNA was extracted from isolated individual salivary glands and midguts. Giemsa-stained blood smears of recipient calves were examined daily for microscopically detectable A. marginale. The genotype of the transmitted strain was identified by cloning and sequencing of msp1 (see the following section). Serum samples of calves were monitored daily by using the MSP5 CI-ELISA to confirm seroconversion. Determination of tick infection rates. Tick infection was determined by PCR of individual ticks followed by Southern hybridization and was confirmed by immunohistochemistry. Amplification of the A. marginale msp5 gene and hybridization with a digoxigenin-labeled msp5 probe were done as previously described (28). A plasmid containing msp5 was used as a positive control (28), and DNA samples isolated from the salivary glands of uninfected D. andersoni or B. microplus ticks were used as negative controls. The PCR-amplified fragments were size separated by gel electrophoresis, visualized in a 1.5% agarose gel following electrophoresis and staining with ethidium bromide, and then, to confirm the identity of the amplicons, hybridized with a digoxigenin-labeled 343-bp msp5 probe by Southern blotting as previously described (28). For immunohistochemical detection, individual ticks were fixed in 10% glutaraldehyde and embedded in paraffin. Replicate 4- m sections were cut from individual ticks, stained with 0.1 g of monoclonal antibody ANA8A per ml, and blocked in hydrogen peroxide before counterstaining with Mayer s hematoxylin. Determination of the A. marginale infection level of tick salivary glands. Real-time PCR for the conserved, single-copy gene msp5 (18, 29, 31) was used to determine the number of organisms in the salivary glands of individual B. microplus and D. andersoni ticks. For real-time amplification, forward 5 -GCCAA GTGATGGTGAATCGAC-3 and reverse 5 -AGAATTAAGCATGTCACCG CTG-3 primers were used, and for detection of target-specific product, a 22-bp msp5 Taqman probe, AACGTTCATGTACCTCATCAA, was generated. The Taqman assay consisted of 55 cycles of 95 C for 10 min, melting at 95 C for 15 s, and annealing at 50 C for 45 s, with extension at 72 C for 7 min. Standard curves were constructed by amplification of 10 7,10 6,10 5,10 4,10 3, and 10 2 copies of full-length msp5 cloned into pmal-2 vector (New England Biolabs). Amplification of the unknown salivary gland DNA samples was conducted simultaneously with a set of standards and, as an internal standard for amplification efficiency, an infected D. andersoni salivary gland containing 10 5 A. marginale organisms (18). Each sample was analyzed in triplicate, and the number of A. marginale organisms was determined by using the standard curve and presented as mean log 10 ( standard deviation) number of A. marginale organisms per salivary gland pair. The mean value for 10 individual ticks was calculated and expressed as the mean log 10 ( standard deviation) number of A. marginale organisms per salivary gland pair. The difference in the mean numbers of A. marginale organisms per salivary gland between the two tick vectors was analyzed by Student s t test. Verification of strain identity. The stability of the A. marginale genotype during persistent infection, replication, and development within the tick and subsequent transmission was confirmed by PCR amplification and sequencing of the msp1 strain marker. DNA was extracted from the persistently infected calves used for acquisition feeding, salivary glands of the infected ticks, and blood of acutely infected cattle following tick transmission. Primers in the conserved regions flanking the strain-specific repeat region of msp1 (forward, 5 -CATTT CCATATACTGTGCAG-3 ; and reverse, 5 -CTTGGAGCGCATCTCTCTTG CC-3 ) were used in PCR amplification as previously described (23). Amplified fragments were cloned into the PCR-4 TOPO vector using the TOPO-TA cloning kit (Invitrogen), and TOP10 Escherichia coli competent cells were transformed. Plasmid DNA was isolated from individual transformed colonies, the presence of inserts was confirmed by EcoRI digestion, and inserts were sequenced in both directions by using the Big Dye kit and ABI Prism automated sequencer. Sequences were compiled and analyzed with VECTOR NTI (Infor- Max).
3 VOL. 41, 2003 TRANSMISSION OF A. MARGINALE BY BOOPHILUS MICROPLUS 3831 TABLE 1. A. marginale infection rate in B. microplus and D. andersoni ticks A. marginale strain a Rickettsemia (mean log 10 SE) b RESULTS % of fed ticks with A. marginale (no. positive/no. fed) B. microplus D. andersoni Puerto Rico Calf (140/155) 84 (131/155) Calf (138/150) 89 (134/150) St. Maries Calf (138/150) 90 (135/150) Calf (134/150) 89 (134/150) a Shown are the A. marginale strain and the number of the persistently infected calf used for acquisition feeding. b Mean rickettsemia (log 10 infected erythrocytes per milliliter of blood) in the persistently infected calf during the 7-day acquisition feeding period. FIG. 1. Detection of A. marginale in salivary glands isolated from individual adult male B. microplus or D. andersoni ticks. DNA of individual tick salivary glands was PCR amplified with msp5 primers and amplicons hybridized with a digoxigenin-labeled msp5 probe. P is the positive control and represents an amplicon of an msp5 plasmid. N is the negative control and represents salivary glands of individual B. microplus or D. andersoni ticks fed on an uninfected calf. Lanes 1 to 20 designate amplicons of individual ticks fed on calf 908, persistently infected with the Puerto Rico strain. A minimum of 150 individual B. microplus and D. andersoni ticks were examined from each feeding on calves with persistent infections with the St. Maries strain or the Puerto Rico strain. Rickettsial levels in persistently infected calves. A. marginale levels were determined in individual persistently infected calves by real-time PCR analysis during the entire period of acquisition feeding. The log 10 mean numbers of infected erythrocytes per milliliter of blood over the 7-day feeding period were 6.23, 5.78, 6.06, and 5.92 for calves 908, 929, 928, and 931, respectively (Table 1), consistent with these levels being below microscopic detection (log 10 7 to 8 infected erythrocytes per ml) and representative of long-term persistent infection (8). Tick infection rates following acquisition feeding. A. marginale infection rates of ticks that had acquisition fed for 7 days on persistently infected calves were determined by PCR amplification of A. marginale msp5 in genomic DNA extracted from isolated individual midguts and salivary gland pairs, followed by Southern hybridization with an msp5-specific probe (Fig. 1). A minimum of 150 individual B. microplus and D. andersoni ticks were examined from each acquisition feeding on calves with persistent infections with the St. Maries strain or the Puerto Rico strain. Tick infection rates were calculated by dividing the number of infected ticks by the total number of fed ticks probed. Using the PCR and Southern hybridization method, which has a detection limit of 10 A. marginale organisms per sample (28), A. marginale infection rates of B. microplus adult males during replicate acquisition feeding experiments were essentially identical for the Puerto Rico strain and the St. Maries strain (Table 1). Similarly, infection rates within D. andersoni adult males did not differ between the Puerto Rico and St. Maries strains (Table 1). There were no statistically significant differences in the infection rates between B. microplus and D. andersoni for either strain (P 0.05). Notably, the infection rates were high in all vector-pathogen combinations (Table 1), supporting the importance of persistently infected cattle as reservoirs. A. marginale levels in infected tick salivary glands following acquisition feeding. The number of A. marginale organisms per salivary gland pair of individual ticks was determined by realtime PCR in triplicate assays. The efficiency of standard curves obtained from amplification of dilution series of the full-length msp5 gene in each experiment was 0.96 to The numbers of A. marginale per salivary gland, expressed as mean log 10 standard deviation, were similar in B. microplus and D. andersoni following acquisition feeding and infection with either the St. Maries or Puerto Rico strain. The levels in the post-acquisition-fed ticks reflected the rickettsemia level of the individual persistently infected calf on which they fed (Table 2). For example, Puerto Rico strain-infected calves 908 and 929 had, TABLE 2. A. marginale levels in infected salivary glands of acquisition-fed and transmission-fed B. microplus and D. andersoni ticks A. marginale strain a and persistently infected calf a Rickettsemia (mean log 10 SE) b Acquisition fed No. of organisms/salivary gland (log 10 SD) c Transmission fed B. microplus D. andersoni B. microplus D. andersoni Puerto Rico Calf Calf St. Maries Calf Calf a Shown are the A. marginale strain and the number of the persistently infected calf used for acquisition feeding. b Data are expressed as the mean number ( standard error) of infected erythrocytes per milliliter of blood in each calf on 7 consecutive days. c Results are represented as the mean ( standard deviation) of triplicate values obtained from salivary glands of 10 individual ticks. d Transmission-fed B. microplus ticks had significantly higher levels of the A. marginale Puerto Rico strain organisms in the salivary glands than those in identically fed D. andersoni ticks (P 0.05).
4 3832 FUTSE ET AL. J. CLIN. MICROBIOL. FIG. 2. Colonization of the temperate-region A. marginale strain in salivary glands of the tropical tick B. microplus. Colonies were detected in B. microplus ticks fed on calves infected with the St. Maries strain by immunohistochemistry with A. marginale-specific monoclonal antibody ANA8A (top panel), but not when an isotype control antibody to an unrelated pathogen, Trypanosoma brucei (middle panel), was used. B. microplus ticks identically fed on uninfected calves were unreactive with the A. marginale-specific antibody ANA8A (bottom panel). The sections were counterstained with hematoxylin; arrows designate the colonies. respectively, log and log organisms per ml during tick feeding, and the levels in salivary glands of acquisition-fed B. microplus were significantly higher in those fed on calf 908 (log organisms per ml) than those fed on calf 929 (log organisms per ml). A similar relationship occurred with acquisition-fed D. andersoni (Table 2). There were no statistically significant differences in the number of salivary gland organisms between acquisition-fed B. microplus and D. andersoni ticks (P 0.05). A. marginale colonization of the salivary gland acinar cells, the site of replication upon transmission feeding, was confirmed for each pathogen strain in both vector species by immunohistochemistry and examination by light microscopy. Colonization by the temperate-region St. Maries strain in the tropical tick B. microplus is shown in Fig. 2. Colonies were detected in salivary gland acinar cells of infected ticks following probing with A. marginale-specific monoclonal antibody ANA8A (Fig. 2, top panel), but not in sequential sections probed with a negative control monoclonal antibody to Trypanosoma brucei, Tryp1E1 (Fig. 2, middle panel). Stained colonies were not seen in control uninfected B. microplus ticks probed with ANA8A under identical conditions (Fig. 2, bottom panel). A. marginale levels in infected tick salivary glands following transmission feeding. In contrast to the results in the acquisition-fed ticks, there were larger numbers of Puerto Rico strain A. marginale organisms in the salivary glands of transmissionfed B. microplus ticks than in identically-transmission-fed D. andersoni ticks. This enhanced replication of the Puerto Rico strain in the salivary glands of transmission-fed B. microplus was observed and was statistically significant (P 0.05) in replicate feedings done with additional calves and ticks of each species. Similarly, there were significantly higher numbers of the St. Maries strain in transmission-fed D. andersoni than in identically-transmission-fed B. microplus (P 0.05). Transmission. B. microplus successfully transmitted both the Puerto Rico and St. Maries strains of A. marginale in replicate experiments. A. marginale-infected erythrocytes were detected microscopically after feeding with B. microplus adult males infected with the Puerto Rico strain (calves 919 and 954) or the St. Maries strain (calves 945 and 952). Similarly, D. andersoni transmitted both strains in replicate experiments: the Puerto Rico strain to calves 920 and 955 and the St. Maries strain to calves 948 and 951. Following microscopic detection of A. marginale infection, all calves seroconverted from negative to positive, as determined with the MSP5 CI-ELISA (15). The identity of the transmitted strain was confirmed based on the msp1 genotype. Sequence analysis confirmed three 87-bp repeats (Fig. 3) of type J/B/B in the St. Maries strain, consistent with the previously described genotype (23). The Puerto strain contained six 84-bp repeats, including one E form, and five novel repeats designated type Z (Fig. 3 [GenBank accession no. AY191826]). Notably, the msp1 genotypes for each strain were identical in samples obtained during persistent infection,
5 VOL. 41, 2003 TRANSMISSION OF A. MARGINALE BY BOOPHILUS MICROPLUS 3833 FIG. 3. MSP1a N-terminal repeat region sequences from the St. Maries and Puerto Rico A. marginale strains. The msp1 genotype was determined for each strain by PCR amplification and sequencing of the 5 repeat region and reported as the amino acid sequence according to the convention of Allred et al. (2). The 28- or 29-amino-acid repeat regions are underlined, and the start of each of repeat is in boldface. The number and sequence of each repeat are used to designate a unique genotype for each strain (2). The nucleotide and derived amino acid sequence of each strain were identical in A. marginale organisms obtained from persistent infection, the salivary glands of fed ticks, and the blood of the acutely infected calves following transmission. in tick salivary glands of both tick vectors, and following transmission in the infected recipient calves. DISCUSSION In the western hemisphere, the predominant vector species of A. marginale, members of the genera Dermacentor and Boophilus, occupy diverse ecological niches, with the latter dependent on warmer temperatures and higher humidity. Because the natural distribution of B. microplus prior to eradication in the 1940s was limited to the southern United States, and D. andersoni does not occur in Puerto Rico, the transmission of the St. Maries (Idaho) and Puerto Rico strains of A. marginale by both tick genera demonstrated that vector competence is retained without requiring continual exposure or coadaptation. Quantitative analysis of vector competence in replicate trials demonstrated that there were no significant differences in the percentage of fed B. microplus ticks that acquired the St. Maries strain versus those that acquired the Puerto Rico strain. Approximately 90% of B. microplus ticks had A. marginale within the salivary gland, regardless of the pathogen strain, at the time of transmission feeding; this is a critical determinant, because colonization of the salivary gland is required for transmission. Similarly, D. andersoni ticks, obtained from Idaho, were efficiently infected with both the tropical- and temperateregion pathogen strains, resulting in colonization of the salivary gland. These data suggest that expansion of B. microplus into its former range within the United States would reestablish a competent vector for existing temperate-region strains of A. marginale, despite its absence for over 60 years. Importantly, B. microplus as a one-host tick with preferential feeding for cattle has a much greater vectorial capacity for A. marginale than the Dermacentor spp. presently responsible for transmission in the United States. Temperate-region strains of A. marginale, typified by the St. Maries strain, form a genetically distinct clade as compared to a cluster of tropical strains isolated from Mexico, the Caribbean region, and Central and South America (6). This genetic distance between the St. Maries and Puerto Rico strains, combined with their isolation from within well-defined regions in which only one of the tick vector species is present, supports the belief that pathogen-vector coadaptation would be strictly limited to B. microplus for the tropical pathogen strain and to D. andersoni for the temperate strain. The only evidence for coadaptation of the pathogen and its vector is seen with the increase in the numbers of A. marginale organisms in the salivary glands at the time of transmission feeding as compared to the numbers during acquisition feeding. This increase is presumed to reflect replication within the salivary gland, although differences in the release of organisms into the saliva during transmission feeding may also influence the number of A. marginale organisms detected in the salivary gland at this time (20). There were statistically significant increases in the numbers of Puerto Rico strain organisms in the salivary gland of B. microplus and in St. Maries strain organisms in D. andersoni following transmission feeding. In contrast, there was no statistically significant increase in organisms between the acquisition feed levels and the transmission feed levels for the St. Maries strain within B. microplus and for the Puerto Rico strain within D. andersoni (Table 2), consistent with only minimal replication within the salivary gland. Because replication and development of infectivity within the salivary gland triggered by tick feeding are key determinants of transmission, adaptation of an A. marginale strain to its sole available vector, resulting in increased replication, may be expected to provide the pathogen strain with a competitive advantage in regions of endemicity where numerous A. marginale strains exist (23). However, this does not appear to limit the ability of a vector to acquire and transmit a strain from outside the region of endemicity, at least in the absence of competition among strains. B. microplus ticks resistant to multiple acaricides have emerged throughout the world, including Mexico and the Caribbean. As a one-host tick that is, a tick that preferentially completes its life cycle on a single host species B. microplus develops acaricide resistance at a much higher rate than do ticks of the three-host Dermacentor spp. (20). The emergence of resistance to the acaricides used to maintain the quarantine zone represents a significant risk that this tick will reestablish itself within the United States (3, 4). Critically, if allowed to reestablish in its former range, the high intrinsic vectorial capacity of B. microplus combined with its having retained vectorial competence for temperate-region strains of A. marginale would be expected to dramatically increase transmission of A. marginale in the U.S. population of cattle. ACKNOWLEDGMENTS This research was supported by USDA-ARS-CRIS D and S ( ), NIH R01 AI44005, and a Fulbright Scholarship to J. E. Futse. The excellent technical assistance of Peter Hetrick, Ralph Horn, Bev Hunter, and Carla Robertson is gratefully acknowledged. REFERENCES 1. Aguirre, D. H., A. B. Gaido, A. E. Vinabal, S. T. De Echaide, and A. A. Guglielmone Transmission of Anaplasma marginale with adult Boophilus microplus ticks fed as nymphs on calves with different levels of rickettsaemia. Parasite 1: Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87: Brockelman, C. R Prevalence and impact of anaplasmosis and babesiosis in Asia. Anaplasmosis Babesiosis Newsl. 20:3. 4. Davey, R. B., and J. E. George In vitro and in vivo evaluations of a
6 3834 FUTSE ET AL. J. CLIN. MICROBIOL. strain of Boophilus microplus (Acari: Ixodidae) selected for resistance to permethrin. J. Med. Entomol. 35: Davey, R. B., and J. E. George Efficacy of macrocyclic lactone endectocides against Boophilus microplus (Acari: Ixodidae) infested cattle using different pour-on application treatment regimes. J. Med. Entomol. 39: De la Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, and K. M. Kocan Differential adhesion of major surface proteins 1a and 1b of the ehrlichial cattle pathogen Anaplasma marginale to bovine erythrocytes and tick cells. Int. J. Parasitol. 31: De la Fuente, J., R. A. Van Den Bussche, J. C. Garcia-Garcia, S. D. Rodriguez, M. A. Garcia, A. A. Guglielmone, A. J. Mangold, L. M. Friche Passos, M. F. Barbosa Ribeiro, E. F. Blouin, and K. M. Kocan Phylogeography of New World isolates of Anaplasma marginale based on major surface protein sequences. Vet. Microbiol. 88: Eriks, I. S., D. Stiller, and G. H. Palmer Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J. Clin. Microbiol. 31: French, D. M., W. C. Brown, and G. H. Palmer Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67: Ge, N. L., K. M. Kocan, E. F. Blouin, and G. L. Murphy Developmental studies of Anaplasma marginale (Rickettsiales:Anaplasmataceae) in male Dermacentor andersoni (Acari:Ixodidae) infected as adults by using nonradioactive in situ hybridization and microscopy. J. Med. Entomol. 33: Graham, O. H., and J. L. Hourrigan Eradication programs for the arthropod parasites of livestock. J. Med. Entomol. 13: Guglielmone, A. A Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 57: Hodgson, J. L Ph.D. thesis. Washington State University, Pullman. 14. Kieser, S. T., I. S. Eriks, and G. H. Palmer Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58: Knowles, D., S. Torioni de Echaide, G. Palmer, T. McGuire, D. Stiller, and T. McElwain Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 34: Kocan, K. M Development of Anaplasma marginale Theiler in Ixodid ticks: coordinated development of rickettsial organism and its tick host, p In J. Sauer and J. A. Hair (ed.), Morphology, physiology, and behavioral biology of ticks. John Wiley & Sons, Inc., New York, N.Y. 17. Kocan, K. M., D. Stiller, W. L. Goff, P. L. Claypool, W. Edwards, S. A. Ewing, T. C. McGuire, J. A. Hair, and S. J. Barron Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am. J. Vet. Res. 53: Kocan, K. M., W. L. Goff, D. Stiller, W. Edwards, S. A. Ewing, P. L. Claypool, T. C. McGuire, J. A. Hair, and S. J. Barron Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am. J. Vet. Res. 54: Lincoln, S. D., J. L. Zaugg, and J. Maas Bovine anaplasmosis: susceptibility of seronegative cows from an infected herd to experimental infection with Anaplasma marginale. J. Am. Vet. Med. Assoc. 190: Löhr, C. V., F. R. Rurangirwa, T. F. McElwain, D. Stiller, and G. H. Palmer Specific expression of Anaplasma marginale major surface protein 2 salivary gland variants occurs in the midgut and is an early event during tick transmission. Infect. Immun. 70: Love, J. N Cryogenic preservation of A. marginale with dimethyl sulfoxide. Am. J. Vet. Res. 33: Mekonnen, S., N. R. Bryson, L. J. Fourie, R. J. Peter, A. M. Spickett, R. J. Taylor, T. Strydom, and I. G. Horak Acaricide resistance profiles of single- and multi-host ticks from communal and commercial farming areas in the Eastern Cape and North-West Provinces of South Africa. Onderstepoort J. Vet. Res. 69: Nicholls, M. J., G. Ibata, and F. V. Rodas Prevalence of antibodies to Babesia bovis and Anaplasma marginale in dairy cattle in Bolivia. Trop. Anim. Health Prod. 12: Palmer, G. H., A. F. Barbet, A. J. Musoke, J. M. Katende, F. R. Rurangirwa, V. Shkap, E. Pipano, W. C. Davis, and T. C. McGuire Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int. J. Parasitol. 18: Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39: Ribeiro, M. F., and J. D. Lima Morphology and development of Anaplasma marginale in midgut of engorged female ticks of Boophilus microplus. Vet. Parasitol. 61: Smith, R. D Current world research on ticks and tick-borne disease of food-producing animals. Interciencia 2: Stiller, D., K. M. Kocan, W. Edwards, S. A. Ewing, and J. A. Barron Detection of colonies of Anaplasma marginale in salivary glands of three Dermacentor spp infected as nymphs or adults. Am. J. Vet. Res. 50: Stiller, D., and M. E. Coan Recent developments in elucidating tick vector relationships for anaplasmosis. Vet. Parasitol. 57: Torioni de Echaide, S., D. P. Knowles, T. C. McGuire, G. H. Palmer, C. E. Suarez, and T. F. McElwain Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay with recombinant major surface protein 5. J. Clin. Microbiol. 36: Visser, E. S., T. C. McGuire, G. H. Palmer, W. C. Davis, V. Shkap, E. Pipano, and D. P. Knowles, Jr The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect. Immun. 60: Zaugg, J. L., D. Stiller, M. E. Coan, and S. D. Lincoln Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am. J. Vet. Res. 47:
7 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2003, p Vol. 41, No /03/$ DOI: /JCM ERRATUM Transmission of Anaplasma marginale by Boophilus microplus: Retention of Vector Competence in the Absence of Vector-Pathogen Interaction James E. Futse, Massaro W. Ueti, Donald P. Knowles, Jr., and Guy H. Palmer Program in Vector-Borne Diseases, Department of Veterinary Microbiology and Pathology, Washington State University, and Animal Diseases Research Unit, USDA Agricultural Research Service, Pullman, Washington Volume 41, no. 8, p , Page 3829, column 1, lines 1 and 2 from bottom: (17, 30, 32) should read (17, 30). Page 3829, column 2, line 15 from bottom: (5) should read (6). Page 3830, column 1, line 15: (9) should read (9, 16). Page 3830, column 2, line 35: (18, 29, 31) should read (18, 29). Page 3830, column 2, lines 37 to 39: forward 5 -GCCAAGTGATGGTGAATCGAC-3 and reverse 5 -AGAATTAAGCAT GTCACCGCTG-3 should read forward 5 -GCCAAGTGATGGTGATATCGAC-3 and reverse 5 -AGAATTAAGCATGT GACCGCTG-3. Page 3831, Table 2, column 2, row 2: should read Page 3832, column 2, line 9 from bottom: (15) should read (13). Page 3833, column 2, line 11: (20) should read (18). Page 3833 and 3834, References: Reference 3 should be deleted; references 4 to 12 should be numbered 3 to 11, respectively; reference 13 should be deleted; and references 14 to 32 should be numbered 12 to 30, respectively. 5354
TRANSMISSION DYNAMICS OF GENETICALLY DISTINCT STRAINS OF. Anaplasma marginale FOLLOWING SUPERINFECTION MARIA FERNANDA BANDEIRA DE MELO GALLETTI
 TRANSMISSION DYNAMICS OF GENETICALLY DISTINCT STRAINS OF Anaplasma marginale FOLLOWING SUPERINFECTION By MARIA FERNANDA BANDEIRA DE MELO GALLETTI A thesis submitted in partial fulfillment of the requirements
TRANSMISSION DYNAMICS OF GENETICALLY DISTINCT STRAINS OF Anaplasma marginale FOLLOWING SUPERINFECTION By MARIA FERNANDA BANDEIRA DE MELO GALLETTI A thesis submitted in partial fulfillment of the requirements
Received 23 March 2005/Accepted 19 April 2005
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2005, p. 3755 3759 Vol. 43, No. 8 0095-1137/05/$08.00 0 doi:10.1128/jcm.43.8.3755 3759.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2005, p. 3755 3759 Vol. 43, No. 8 0095-1137/05/$08.00 0 doi:10.1128/jcm.43.8.3755 3759.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Implication of antigenic variation for vaccine development: the Anaplasma marginale model. Kelly A. Brayton
 Implication of antigenic variation for vaccine development: the Anaplasma marginale model Kelly A. Brayton Order Rickettsiales Family Anaplasmataceae Rickettsiaceae Anaplasma marginale Causes anaplasmosis,
Implication of antigenic variation for vaccine development: the Anaplasma marginale model Kelly A. Brayton Order Rickettsiales Family Anaplasmataceae Rickettsiaceae Anaplasma marginale Causes anaplasmosis,
TRANSOVARIAL TRANSMISSION EFFICIENCY OF BABESIA BOVIS BY RHIPICEPHALUS (BOOPHILUS) MICROPLUS
 TRANSOVARIAL TRANSMISSION EFFICIENCY OF BABESIA BOVIS BY RHIPICEPHALUS (BOOPHILUS) MICROPLUS By Jeanne Marie Howell A dissertation submitted in partial fulfillment of the requirements for the degree of
TRANSOVARIAL TRANSMISSION EFFICIENCY OF BABESIA BOVIS BY RHIPICEPHALUS (BOOPHILUS) MICROPLUS By Jeanne Marie Howell A dissertation submitted in partial fulfillment of the requirements for the degree of
Cattle vaccination studies using novel anti-cattle tick antigens developed during Beef CRC research
 final report Project code: Prepared by: B.AHE.0212 Alicia Tabor, Manuel Rodriguez Valle, Michael McGowan (UQ), David Mayer, Elizabeth Fowler, Catherin Minchin and Bing Zhang (QDAF) The University of Queensland;
final report Project code: Prepared by: B.AHE.0212 Alicia Tabor, Manuel Rodriguez Valle, Michael McGowan (UQ), David Mayer, Elizabeth Fowler, Catherin Minchin and Bing Zhang (QDAF) The University of Queensland;
Identification of vaccine candidate antigens for a Babesia bovis transmission blocking vaccine
 Identification of vaccine candidate antigens for a Babesia bovis transmission blocking vaccine Carlos E. Suarez Animal Disease Research Unit ARS-USDA Department of Veterinary Microbiology and Pathology
Identification of vaccine candidate antigens for a Babesia bovis transmission blocking vaccine Carlos E. Suarez Animal Disease Research Unit ARS-USDA Department of Veterinary Microbiology and Pathology
EVALUATION OF AN ENZYME LINKED IMMUNOSORBENT ASSAY-KIT FOR THE DETECTION OF BABESIA BOVIS-ANTIBODIES IN CATTLE IN ARGENTINA
 EVALUATION OF AN ENZYME LINKED IMMUNOSORBENT ASSAY-KIT FOR THE DETECTION OF BABESIA BOVIS-ANTIBODIES IN CATTLE IN ARGENTINA S.T. DE ECHAIDE*, I.E. ECHAIDE*, A.B. GAIDO**, A.J. MANGOLD*, C.I. LUGARESI*,
EVALUATION OF AN ENZYME LINKED IMMUNOSORBENT ASSAY-KIT FOR THE DETECTION OF BABESIA BOVIS-ANTIBODIES IN CATTLE IN ARGENTINA S.T. DE ECHAIDE*, I.E. ECHAIDE*, A.B. GAIDO**, A.J. MANGOLD*, C.I. LUGARESI*,
Seroprevalence of bovine anaplasmosis caused by Anaplasma marginale in Malaysia.
 Seroprevalence of bovine anaplasmosis caused by Anaplasma marginale in Malaysia. 1 Samantha Pong, 2 Nik Ahmad Irwan Izzauddin Nik Him 1 School of Biological Sciences, Universiti Sains Malaysia Penang 11800,
Seroprevalence of bovine anaplasmosis caused by Anaplasma marginale in Malaysia. 1 Samantha Pong, 2 Nik Ahmad Irwan Izzauddin Nik Him 1 School of Biological Sciences, Universiti Sains Malaysia Penang 11800,
Department of Agriculture, Fisheries and Forestry, Queensland. Improved protection of cattle against anaplasmosis in tick-infested areas of Australia
 final report Project code: B.AHE.0060 Prepared by: Peter Rolls Date published: August 2013 Department of Agriculture, Fisheries and Forestry, Queensland ISBN: 9781925045376 PUBLISHED BY Meat & Livestock
final report Project code: B.AHE.0060 Prepared by: Peter Rolls Date published: August 2013 Department of Agriculture, Fisheries and Forestry, Queensland ISBN: 9781925045376 PUBLISHED BY Meat & Livestock
Sir Arnold Theiler and the discovery of anaplasmosis: A centennial perspective
 Onderstepoort Journal of Veterinary Research, 76:75 79 (2009) Sir Arnold Theiler and the discovery of anaplasmosis: A centennial perspective Department of Veterinary Microbiology and Pathology and School
Onderstepoort Journal of Veterinary Research, 76:75 79 (2009) Sir Arnold Theiler and the discovery of anaplasmosis: A centennial perspective Department of Veterinary Microbiology and Pathology and School
Study of Virulence and Vector Transmission of Babesia bovis by Use of Cloned Parasite Lines
 INFECTION AND IMMUNITY, JUlY 1990, p. 2171-2176 0019-9567/90/072171-06$02.00/0 Copyright X) 1990, American Society for Microbiology Vol. 58, No. 7 Study of Virulence and Vector Transmission of Babesia
INFECTION AND IMMUNITY, JUlY 1990, p. 2171-2176 0019-9567/90/072171-06$02.00/0 Copyright X) 1990, American Society for Microbiology Vol. 58, No. 7 Study of Virulence and Vector Transmission of Babesia
Received 24 June 2010/Returned for modification 27 July 2010/Accepted 30 September 2010
 CLINICAL AND VACCINE IMMUNOLOGY, Dec. 2010, p. 1881 1890 Vol. 17, No. 12 1556-6811/10/$12.00 doi:10.1128/cvi.00257-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Anaplasma marginale
CLINICAL AND VACCINE IMMUNOLOGY, Dec. 2010, p. 1881 1890 Vol. 17, No. 12 1556-6811/10/$12.00 doi:10.1128/cvi.00257-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Anaplasma marginale
Risk Assessment Centre on Food Chain Project link to the Delphi priorities / EFSA Strategy topics
 Biology and control of some exotic, emerging and transboundary vectorborne and zoonotic diseases in Bulgaria and Germany with emphasis of veterinary and public health importance Risk Assessment Centre
Biology and control of some exotic, emerging and transboundary vectorborne and zoonotic diseases in Bulgaria and Germany with emphasis of veterinary and public health importance Risk Assessment Centre
Better Training for Safer Food BTSF
 Better Training for Safer Food BTSF Importation of vector-borne infectious diseases Tanguy Marcotty Institute of Tropical Medicine Importation routes Trade Infected live animals (e.g. H5N1 avian influenza)
Better Training for Safer Food BTSF Importation of vector-borne infectious diseases Tanguy Marcotty Institute of Tropical Medicine Importation routes Trade Infected live animals (e.g. H5N1 avian influenza)
Alberta Health Public Health Notifiable Disease Management Guidelines January 2013
 January 2013 Rickettsial Infections Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) January 2013 January 2013 December
January 2013 Rickettsial Infections Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) January 2013 January 2013 December
Theileriosis and Tick control management in different Mediterranean Livestock Production Systems
 Theileriosis and Tick control management in different Mediterranean Livestock Production Systems Jacinto Gomes Instituto Nacional de Investigação Agrária e Veterinária, I.P., Portugal Huseyin Bilgin Bilgiç,
Theileriosis and Tick control management in different Mediterranean Livestock Production Systems Jacinto Gomes Instituto Nacional de Investigação Agrária e Veterinária, I.P., Portugal Huseyin Bilgin Bilgiç,
Lyme Disease: A Mathematical Approach
 July 21, 2015 Overview Biology of Lyme Disease 1 Biology of Lyme Disease Borrelia burgdoferi Ixodes scapularis Hosts 2 3 Age-Structured Tick Class Model 4 Age-Structured Tick Class & Seasonality Model
July 21, 2015 Overview Biology of Lyme Disease 1 Biology of Lyme Disease Borrelia burgdoferi Ixodes scapularis Hosts 2 3 Age-Structured Tick Class Model 4 Age-Structured Tick Class & Seasonality Model
Ticks. Tick identification SEASONAL OCCURRENCE / LIFE CYCLE. Seasonal occurrence. Life cycle. Ticks: Tick identification
 Ticks Tick identification Authors: Prof Maxime Madder, Prof Ivan Horak, Dr Hein Stoltsz Licensed under a Creative Commons Attribution license. SEASONAL OCCURRENCE / LIFE CYCLE Seasonal occurrence Long
Ticks Tick identification Authors: Prof Maxime Madder, Prof Ivan Horak, Dr Hein Stoltsz Licensed under a Creative Commons Attribution license. SEASONAL OCCURRENCE / LIFE CYCLE Seasonal occurrence Long
Ultrastructural Studies on Plasmodium vivax
 Characterization of Human Malaria Parasites Ultrastructural Studies on Plasmodium vivax For the first time a detailed ultrastructural study was carried out on P. vivax. Fine structural analysis of growth
Characterization of Human Malaria Parasites Ultrastructural Studies on Plasmodium vivax For the first time a detailed ultrastructural study was carried out on P. vivax. Fine structural analysis of growth
Observations on the use of Anaplasma centrale for immunization of cattle against anaplasmosis in Zimbabwe
 Onderstepoort Journal of Veterinary Research, 65:81-86 (1998) Observations on the use of Anaplasma centrale for immunization of cattle against anaplasmosis in Zimbabwe J.A. TURTON 1 2, T.C. KATSANDE 1,
Onderstepoort Journal of Veterinary Research, 65:81-86 (1998) Observations on the use of Anaplasma centrale for immunization of cattle against anaplasmosis in Zimbabwe J.A. TURTON 1 2, T.C. KATSANDE 1,
Received 21 January 2005/Returned for modification 8 March 2005/Accepted 6 May 2005
 INFECTION AND IMMUNITY, Sept. 2005, p. 5388 5394 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5388 5394.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Sequence
INFECTION AND IMMUNITY, Sept. 2005, p. 5388 5394 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5388 5394.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Sequence
INFECTION AND IMMUNITY, Nov. 1998, p Vol. 66, No. 11. Copyright 1998, American Society for Microbiology. All Rights Reserved.
 INFECTION AND IMMUNITY, Nov. 1998, p. 5406 5413 Vol. 66, No. 11 0019-9567/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. CD4 T-Lymphocyte and Immunoglobulin G2 Responses
INFECTION AND IMMUNITY, Nov. 1998, p. 5406 5413 Vol. 66, No. 11 0019-9567/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. CD4 T-Lymphocyte and Immunoglobulin G2 Responses
Blood Smears Only 19 May Sample Preparation and Quality Control
 NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 9 May 205 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 9 May 205 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
CHAPTER 4 RESULTS. showed that all three replicates had similar growth trends (Figure 4.1) (p<0.05; p=0.0000)
 CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
Involvement of roe deer as source of Anaplasma phagocytophilum infection for Ixodes ricinus in a heterogeneous landscape
 Involvement of roe deer as source of Anaplasma phagocytophilum infection for Ixodes ricinus in a heterogeneous landscape Extract of the PhD thesis of Amélie Chastagner A m é l i e C h a s tagner 1*, A
Involvement of roe deer as source of Anaplasma phagocytophilum infection for Ixodes ricinus in a heterogeneous landscape Extract of the PhD thesis of Amélie Chastagner A m é l i e C h a s tagner 1*, A
West Nile Virus. Family: Flaviviridae
 West Nile Virus 1 Family: Flaviviridae West Nile Virus Genus: Flavivirus Japanese Encephalitis Antigenic Complex Complex Includes: Alfuy, Cacipacore, Japanese encephalitis, koutango, Kunjin, Murray Valley
West Nile Virus 1 Family: Flaviviridae West Nile Virus Genus: Flavivirus Japanese Encephalitis Antigenic Complex Complex Includes: Alfuy, Cacipacore, Japanese encephalitis, koutango, Kunjin, Murray Valley
Holarctic distribution of Lyme disease
 Babesia and Ehrlichia epidemiology: key points Holarctic distribution of Lyme disease 1. Always test for babesia and ehrlichia if Lyme disease is suspected 2. Endemic areas perhaps too broad a term; vector-borne
Babesia and Ehrlichia epidemiology: key points Holarctic distribution of Lyme disease 1. Always test for babesia and ehrlichia if Lyme disease is suspected 2. Endemic areas perhaps too broad a term; vector-borne
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR
 Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis!
 Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Serological response to early vaccination against. Babesia bovis and Babesia bigemina in dairy. calves
 Serological response to early vaccination against Babesia bovis and Babesia bigemina in dairy calves by Anthony J. Davis Supervisor: Prof B L Penzhorn Co-supervisor: Prof A A Latif Co-supervisor: Dr J
Serological response to early vaccination against Babesia bovis and Babesia bigemina in dairy calves by Anthony J. Davis Supervisor: Prof B L Penzhorn Co-supervisor: Prof A A Latif Co-supervisor: Dr J
Wendy C. Brown, 1 * Travis C. McGuire, 1 Waithaka Mwangi, 1 Kimberly A. Kegerreis, 1 Henriette Macmillan, 1 Harris A. Lewin, 2 and Guy H.
 INFECTION AND IMMUNITY, Oct. 2002, p. 5521 5532 Vol. 70, No. 10 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.10.5521 5532.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Major
INFECTION AND IMMUNITY, Oct. 2002, p. 5521 5532 Vol. 70, No. 10 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.10.5521 5532.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Major
Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University
 Role of Chemical lexposure in Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University CNV Discovery Reference Genetic
Role of Chemical lexposure in Generating Spontaneous Copy Number Variants (CNVs) Jennifer Freeman Assistant Professor of Toxicology School of Health Sciences Purdue University CNV Discovery Reference Genetic
Malaria. Population at Risk. Infectious Disease epidemiology BMTRY 713 (Lecture 23) Epidemiology of Malaria. April 6, Selassie AW (DPHS) 1
 Infectious Disease Epidemiology BMTRY 713 (A. Selassie, DrPH) Lecture 23 Vector-Borne Disease (Part II) Epidemiology of Malaria Learning Objectives 1. Overview of malaria Global perspectives 2. Identify
Infectious Disease Epidemiology BMTRY 713 (A. Selassie, DrPH) Lecture 23 Vector-Borne Disease (Part II) Epidemiology of Malaria Learning Objectives 1. Overview of malaria Global perspectives 2. Identify
Global Climate Change and Mosquito-Borne Diseases
 Global Climate Change and Mosquito-Borne Diseases Theodore G. Andreadis Center for Vector Biology & Zoonotic Diseases The Connecticut Agricultural Experiment Station New Haven, CT Evidence for Global Climate
Global Climate Change and Mosquito-Borne Diseases Theodore G. Andreadis Center for Vector Biology & Zoonotic Diseases The Connecticut Agricultural Experiment Station New Haven, CT Evidence for Global Climate
Multi-clonal origin of macrolide-resistant Mycoplasma pneumoniae isolates. determined by multiple-locus variable-number tandem-repeat analysis
 JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
Introduction. In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology.
 Introduction In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology. - Genomics: fasten antigen discovery for complex pathogens
Introduction In the past 15 years, several technological advancements have open new perspectives and applications in the field of vaccinology. - Genomics: fasten antigen discovery for complex pathogens
Tickborne Disease Case Investigations
 Massachusetts Department of Public Health Bureau of Infectious Disease and Laboratory Sciences Tickborne Disease Case Investigations Anthony Osinski, MPH May 31, 2018 Factors Associated with Increasing
Massachusetts Department of Public Health Bureau of Infectious Disease and Laboratory Sciences Tickborne Disease Case Investigations Anthony Osinski, MPH May 31, 2018 Factors Associated with Increasing
Emerging vector-borne diseases in the United States: What s next and are we prepared?
 Emerging vector-borne diseases in the United States: What s next and are we prepared? Lyle R. Petersen, MD, MPH Director Division of Vector-Borne Diseases Centers for Disease Control and Prevention IOM
Emerging vector-borne diseases in the United States: What s next and are we prepared? Lyle R. Petersen, MD, MPH Director Division of Vector-Borne Diseases Centers for Disease Control and Prevention IOM
. MONITORING THEILERIA ON THE BASIS OF MICROSCOPIC EXAMINATION
 . MONITORING THEILERIA ON THE BASIS OF MICROSCOPIC EXAMINATION 3.1 Introduction Theileria is a vector borne protozoan parasite that causes theileriosis which is a fatal disease for cows. It is a disease
. MONITORING THEILERIA ON THE BASIS OF MICROSCOPIC EXAMINATION 3.1 Introduction Theileria is a vector borne protozoan parasite that causes theileriosis which is a fatal disease for cows. It is a disease
West Nile Virus. By Frank Riusech
 West Nile Virus By Frank Riusech Disease Etiology: West Nile virus(wnv), genus, flavivirus is positive- stranded RNA arbovirus (arthropod- borne), belonging to the Flaviviridae family. Included in this
West Nile Virus By Frank Riusech Disease Etiology: West Nile virus(wnv), genus, flavivirus is positive- stranded RNA arbovirus (arthropod- borne), belonging to the Flaviviridae family. Included in this
Supplementary methods:
 Supplementary methods: Primers sequences used in real-time PCR analyses: β-actin F: GACCTCTATGCCAACACAGT β-actin [11] R: AGTACTTGCGCTCAGGAGGA MMP13 F: TTCTGGTCTTCTGGCACACGCTTT MMP13 R: CCAAGCTCATGGGCAGCAACAATA
Supplementary methods: Primers sequences used in real-time PCR analyses: β-actin F: GACCTCTATGCCAACACAGT β-actin [11] R: AGTACTTGCGCTCAGGAGGA MMP13 F: TTCTGGTCTTCTGGCACACGCTTT MMP13 R: CCAAGCTCATGGGCAGCAACAATA
Lab Tuesday: Virus Diseases
 Lab Tuesday: Virus Diseases Quiz for Bacterial Pathogens lab (pp 67-73) and Biocontrol of Crown Gall (p. 113-117), Observation of Viral Movement in Plants (p. 119), and Intro section for Viruses (pp. 75-77).
Lab Tuesday: Virus Diseases Quiz for Bacterial Pathogens lab (pp 67-73) and Biocontrol of Crown Gall (p. 113-117), Observation of Viral Movement in Plants (p. 119), and Intro section for Viruses (pp. 75-77).
Transmission of Anaplasma phagocytophilum to Ixodes ricinus Ticks from Sheep in the Acute and Post-Acute Phases of Infection
 INFECTION AND IMMUNITY, Apr. 2003, p. 2071 2078 Vol. 71, No. 4 0019-9567/03/$08.00 0 DOI: 10.1128/IAI.71.4.2071 2078.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Transmission
INFECTION AND IMMUNITY, Apr. 2003, p. 2071 2078 Vol. 71, No. 4 0019-9567/03/$08.00 0 DOI: 10.1128/IAI.71.4.2071 2078.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Transmission
Supplemental Materials and Methods Plasmids and viruses Quantitative Reverse Transcription PCR Generation of molecular standard for quantitative PCR
 Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
Supplemental Materials and Methods Plasmids and viruses To generate pseudotyped viruses, the previously described recombinant plasmids pnl4-3-δnef-gfp or pnl4-3-δ6-drgfp and a vector expressing HIV-1 X4
PROCEEDINGS OF THE ASSOCIATION OF INSTITUTIONS OF TROPICAL VETERINARY MEDICINE
 PROCEEDINGS OF THE ASSOCIATION OF INSTITUTIONS OF TROPICAL VETERINARY MEDICINE DOES CONTROL OF ANIMAL INFECTIOUS RISKS OFFER A NEW INTERNATIONAL PERSPECTIVE? Proceedings of the 12th International conference
PROCEEDINGS OF THE ASSOCIATION OF INSTITUTIONS OF TROPICAL VETERINARY MEDICINE DOES CONTROL OF ANIMAL INFECTIOUS RISKS OFFER A NEW INTERNATIONAL PERSPECTIVE? Proceedings of the 12th International conference
Case Study: West Nile Virus -Taking an Integrated National Public Health Approach to an Emerging Infectious Disease in Canada
 2008/SOM3/HWG/WKSP/003 Case Study: West Nile Virus -Taking an Integrated National Public Health Approach to an Emerging Infectious Disease in Canada Submitted by: Canada Health Working Group Policy Dialogue
2008/SOM3/HWG/WKSP/003 Case Study: West Nile Virus -Taking an Integrated National Public Health Approach to an Emerging Infectious Disease in Canada Submitted by: Canada Health Working Group Policy Dialogue
Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains
 Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains Name: Claire Ostertag-Hill Mentor: Dr. Ling Jin Bovine herpesvirus Bovine herpesvirus-1 (BHV-1) Pathogen
Investigation of the genetic differences between bovine herpesvirus type 1 variants and vaccine strains Name: Claire Ostertag-Hill Mentor: Dr. Ling Jin Bovine herpesvirus Bovine herpesvirus-1 (BHV-1) Pathogen
Prevalence of HPV-16 genomic variant carrying a 63-bp duplicated sequence within the E1 gene in Slovenian women
 Prevalence of HPV-16 genomic variant carrying a 63-bp duplicated sequence within the E1 gene in Slovenian women K E Y WORDS HPV-16, cervical cancer, E6-T350G variant A BSTRACT High-risk HPV, particularly
Prevalence of HPV-16 genomic variant carrying a 63-bp duplicated sequence within the E1 gene in Slovenian women K E Y WORDS HPV-16, cervical cancer, E6-T350G variant A BSTRACT High-risk HPV, particularly
Dengue Virus-Danger from Deadly Little Dragon
 Molecular Medicine Dengue Virus-Danger from Deadly Little Dragon Dr.G.MATHAN Assistant Professor Department of Biomedical Science Bharathidasan University Tiruchirappalli, Tamil Nadu Vector (A carrier)
Molecular Medicine Dengue Virus-Danger from Deadly Little Dragon Dr.G.MATHAN Assistant Professor Department of Biomedical Science Bharathidasan University Tiruchirappalli, Tamil Nadu Vector (A carrier)
Supplemental Figure 1
 Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
Epidemiology of Vector-Borne Diseases Laura C. Harrington, PhD
 Epidemiology of Vector- Borne Diseases Associate Professor Department of Entomology Cornell University 1 Before we begin Review lectures on transmission, arboviruses and malaria Focus on biologically transmitted
Epidemiology of Vector- Borne Diseases Associate Professor Department of Entomology Cornell University 1 Before we begin Review lectures on transmission, arboviruses and malaria Focus on biologically transmitted
Recent outbreaks of chikungunya in Sri Lanka and the role of Asian Tigers
 Recent outbreaks of chikungunya in Sri Lanka and the role of Asian Tigers Introduction CHIK Virus Classification: An ARBOVIRUS Family - Togaviridae Genus Alphavirus ** Enveloped, positive- strand RNA virus.
Recent outbreaks of chikungunya in Sri Lanka and the role of Asian Tigers Introduction CHIK Virus Classification: An ARBOVIRUS Family - Togaviridae Genus Alphavirus ** Enveloped, positive- strand RNA virus.
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia)
 PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
Chronic HIV-1 Infection Frequently Fails to Protect against Superinfection
 Chronic HIV-1 Infection Frequently Fails to Protect against Superinfection Anne Piantadosi 1,2[, Bhavna Chohan 1,2[, Vrasha Chohan 3, R. Scott McClelland 3,4,5, Julie Overbaugh 1,2* 1 Division of Human
Chronic HIV-1 Infection Frequently Fails to Protect against Superinfection Anne Piantadosi 1,2[, Bhavna Chohan 1,2[, Vrasha Chohan 3, R. Scott McClelland 3,4,5, Julie Overbaugh 1,2* 1 Division of Human
Parasites transmitted by vectors
 Parasites transmitted by vectors Often very specific vector-parasite relationships Biomphalaria sp. - Schistosoma mansoni Anopheles sp. Plasmodium falciparum Simulium sp. Onchocerca volvulis Some more
Parasites transmitted by vectors Often very specific vector-parasite relationships Biomphalaria sp. - Schistosoma mansoni Anopheles sp. Plasmodium falciparum Simulium sp. Onchocerca volvulis Some more
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
PARASITOLOGY CASE HISTORY #13 (BLOOD PARASITES) (Lynne S. Garcia)
 PARASITOLOGY CASE HISTORY #13 (BLOOD PARASITES) (Lynne S. Garcia) An epidemiologic survey was undertaken in a small town in Myanmar (Burma) endemic for lymphatic filariasis. Blood specimens were collected
PARASITOLOGY CASE HISTORY #13 (BLOOD PARASITES) (Lynne S. Garcia) An epidemiologic survey was undertaken in a small town in Myanmar (Burma) endemic for lymphatic filariasis. Blood specimens were collected
Antimicrobial therapy of persistent Anaplasma marginale infections
 Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 2005 Antimicrobial therapy of persistent Anaplasma marginale infections Johann Francois Coetzee Iowa State
Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 2005 Antimicrobial therapy of persistent Anaplasma marginale infections Johann Francois Coetzee Iowa State
Manitoba Annual Tick-Borne Disease Report
 Manitoba Annual Tick-Borne Disease Report 2015 January 1, 2008 to December 31, 2015 Communicable Disease Control Public Health Branch Public Health and Primary Health Care Division Manitoba Health, Seniors
Manitoba Annual Tick-Borne Disease Report 2015 January 1, 2008 to December 31, 2015 Communicable Disease Control Public Health Branch Public Health and Primary Health Care Division Manitoba Health, Seniors
Anaplasmosis In Beef Cattle
 B-5098 Anaplasmosis In Beef Cattle Anaplasmosis is an infectious disease of cattle that causes destruction of red blood cells. The disease is caused by a minute parasite, Anaplasma marginale, found in
B-5098 Anaplasmosis In Beef Cattle Anaplasmosis is an infectious disease of cattle that causes destruction of red blood cells. The disease is caused by a minute parasite, Anaplasma marginale, found in
Host-specialization of Bartonella in flea vectors collected from black-tailed prairie dogs
 University of Richmond UR Scholarship Repository Honors Theses Student Research 2014 Host-specialization of Bartonella in flea vectors collected from black-tailed prairie dogs Mark D. Massaro Follow this
University of Richmond UR Scholarship Repository Honors Theses Student Research 2014 Host-specialization of Bartonella in flea vectors collected from black-tailed prairie dogs Mark D. Massaro Follow this
HIV-1 Genemer Detection Kit Ready to Use Amplification Kit for HIV-1 Specific DNA Fragment Analysis
 Product Manual HIV-1 Genemer Detection Kit Ready to Use Amplification Kit for HIV-1 Specific DNA Fragment Analysis For research use only. Not for use in diagnostic procedures for clinical purposes Catalog
Product Manual HIV-1 Genemer Detection Kit Ready to Use Amplification Kit for HIV-1 Specific DNA Fragment Analysis For research use only. Not for use in diagnostic procedures for clinical purposes Catalog
Estimations of the Infective Period for Bluetongue in Cattle
 EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Sanco/B3/AH/R12/1999 Directorate B - Scientific Health Opinions Unit B3 - Management of scientific committees II Estimations of the
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Sanco/B3/AH/R12/1999 Directorate B - Scientific Health Opinions Unit B3 - Management of scientific committees II Estimations of the
ANTIGEN-SPECIFIC CD4 + T CELLS IN ANAPLASMA MARGINALE INFECTION OF CALVES SUSHAN HAN
 ANTIGEN-SPECIFIC CD4 + T CELLS IN ANAPLASMA MARGINALE INFECTION OF CALVES By SUSHAN HAN A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON
ANTIGEN-SPECIFIC CD4 + T CELLS IN ANAPLASMA MARGINALE INFECTION OF CALVES By SUSHAN HAN A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON
Lab Tuesday: Virus Diseases
 Lab Tuesday: Virus Diseases Quiz for Bacterial Pathogens lab (pp 69-75) and Biocontrol of Crown Gall (p. 115-119), Observation of Viral Movement in Plants (p. 121), and Intro section for Viruses (pp. 77-79).
Lab Tuesday: Virus Diseases Quiz for Bacterial Pathogens lab (pp 69-75) and Biocontrol of Crown Gall (p. 115-119), Observation of Viral Movement in Plants (p. 121), and Intro section for Viruses (pp. 77-79).
WHAT WE KNOW ABOUT ANAPLASMOSIS AND BORRELIOSIS AND WHAT WE DO NOT A. Rick Alleman, DVM, PhD, DACVP, DABVP
 WHAT WE KNOW ABOUT ANAPLASMOSIS AND BORRELIOSIS AND WHAT WE DO NOT A. Rick Alleman, DVM, PhD, DACVP, DABVP Anaplasma phagocytophilum Anaplasma phagocytophilum is an intracellular, gram-negative bacterium
WHAT WE KNOW ABOUT ANAPLASMOSIS AND BORRELIOSIS AND WHAT WE DO NOT A. Rick Alleman, DVM, PhD, DACVP, DABVP Anaplasma phagocytophilum Anaplasma phagocytophilum is an intracellular, gram-negative bacterium
Sex is determined by genes on sex chromosomes
 BREVIA Temperature Sex Reversal Implies Sex Gene Dosage in a Reptile Alexander E. Quinn, 1 * Arthur Georges, 1 Stephen D. Sarre, 1 Fiorenzo Guarino, 1 Tariq Ezaz, 2 Jennifer A. Marshall Graves 2 Sex is
BREVIA Temperature Sex Reversal Implies Sex Gene Dosage in a Reptile Alexander E. Quinn, 1 * Arthur Georges, 1 Stephen D. Sarre, 1 Fiorenzo Guarino, 1 Tariq Ezaz, 2 Jennifer A. Marshall Graves 2 Sex is
Manitoba Annual Tick-Borne Disease Report
 Manitoba Annual Tick-Borne Disease Report 2016 January 1, 2016 to December 31, 2016 Communicable Disease Control Active Living, Population and Public Health Branch Active Living, Indigenous Relations,
Manitoba Annual Tick-Borne Disease Report 2016 January 1, 2016 to December 31, 2016 Communicable Disease Control Active Living, Population and Public Health Branch Active Living, Indigenous Relations,
More than 98 countries affected by Leishmaniasis
 More than 98 countries affected by Leishmaniasis ACL In many urban & sub urban areas (8 provinces) Tehran, Mashhad, Neishabur, Shiraz, Kerman, Bam (Sharifi et al. 2011) ZCL In 2012, totally ( ZCL & ACL)
More than 98 countries affected by Leishmaniasis ACL In many urban & sub urban areas (8 provinces) Tehran, Mashhad, Neishabur, Shiraz, Kerman, Bam (Sharifi et al. 2011) ZCL In 2012, totally ( ZCL & ACL)
Kinetoplastids Handout
 Kinetoplastids Handout 1 Kinetoplastids widespread group of flagellated protozoa parasitize virtually all animal groups as well as plants and insects 3 distinct kinetoplastid species cause human disease
Kinetoplastids Handout 1 Kinetoplastids widespread group of flagellated protozoa parasitize virtually all animal groups as well as plants and insects 3 distinct kinetoplastid species cause human disease
Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels of Lungs1
 INFECTION AND IMMUNITY, Sept. 1974, p. 590-596 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 3 Printed in U.S.A. Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels
INFECTION AND IMMUNITY, Sept. 1974, p. 590-596 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 3 Printed in U.S.A. Relationship of Ehrlichia canis-infected Mononuclear Cells to Blood Vessels
Primer DNA sequence (5 to 3 )
 Supplemental Table 1 Oligonucleotide primers used in the study Primer DNA sequence (5 to 3 ) Plasmodium falciparum 18s forward Plasmodium falciparum 18s reverse Plasmodium falciparum MSP1 forward Plasmodium
Supplemental Table 1 Oligonucleotide primers used in the study Primer DNA sequence (5 to 3 ) Plasmodium falciparum 18s forward Plasmodium falciparum 18s reverse Plasmodium falciparum MSP1 forward Plasmodium
And Current Situation
 African Swine Fever Research And Current Situation Luis L. Rodriguez Research Leader Foreign Animal Disease Research Unit Agricultural Research Service, Plum Island Animal Disease Center African Swine
African Swine Fever Research And Current Situation Luis L. Rodriguez Research Leader Foreign Animal Disease Research Unit Agricultural Research Service, Plum Island Animal Disease Center African Swine
Serodiagnosis of Canine Babesiosis
 Serodiagnosis of Canine Babesiosis Xuenan XUAN, DVM, PhD National Research Center for Protozoan Diseases Obihiro University of Agriculture and Veterinary Medicine Obihiro, Hokkaido 080-8555, Japan Tel.:+81-155-495648;
Serodiagnosis of Canine Babesiosis Xuenan XUAN, DVM, PhD National Research Center for Protozoan Diseases Obihiro University of Agriculture and Veterinary Medicine Obihiro, Hokkaido 080-8555, Japan Tel.:+81-155-495648;
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
Mechanism of Immunity to Tick infestation in Livestock
 Veterinary World, 2011, Vol.4(3):131-135 REVIEW Mechanism of Immunity to Tick infestation in Livestock Biswa Ranjan Maharana*, Rubina Kumari Baithalu, Idrees Mehraj Allaie, Chinmoy mishra and Lipismita
Veterinary World, 2011, Vol.4(3):131-135 REVIEW Mechanism of Immunity to Tick infestation in Livestock Biswa Ranjan Maharana*, Rubina Kumari Baithalu, Idrees Mehraj Allaie, Chinmoy mishra and Lipismita
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran
 Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Fig. 1: Quality assessment of formalin-fixed paraffin-embedded (FFPE)-derived DNA and nuclei. (a) Multiplex PCR analysis of unrepaired and repaired bulk FFPE gdna from
Supplementary Figure 1 Supplementary Fig. 1: Quality assessment of formalin-fixed paraffin-embedded (FFPE)-derived DNA and nuclei. (a) Multiplex PCR analysis of unrepaired and repaired bulk FFPE gdna from
Babesia spp. Emerging Transfusion Dilemmas
 Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
Epidemiology and Laboratory Diagnosis of Fungal Diseases
 Medical Mycology (BIOL 4849) Summer 2007 Dr. Cooper Epidemiology of Mycoses Epidemiology and Laboratory Diagnosis of Fungal Diseases Mycosis (pl., mycoses) - an infection caused by a fungus Two broad categories
Medical Mycology (BIOL 4849) Summer 2007 Dr. Cooper Epidemiology of Mycoses Epidemiology and Laboratory Diagnosis of Fungal Diseases Mycosis (pl., mycoses) - an infection caused by a fungus Two broad categories
Micropathology Ltd. University of Warwick Science Park, Venture Centre, Sir William Lyons Road, Coventry CV4 7EZ
 www.micropathology.com info@micropathology.com Micropathology Ltd Tel 24hrs: +44 (0) 24-76 323222 Fax / Ans: +44 (0) 24-76 - 323333 University of Warwick Science Park, Venture Centre, Sir William Lyons
www.micropathology.com info@micropathology.com Micropathology Ltd Tel 24hrs: +44 (0) 24-76 323222 Fax / Ans: +44 (0) 24-76 - 323333 University of Warwick Science Park, Venture Centre, Sir William Lyons
 http://www.ibs.upm.edu.my Challenges in controlling viral diseases of poultry Abdul Rahman Omar Institute of Bioscience Faculty of Veterinary Medicine Universiti Putra Malaysia aro@upm.edu.my Outline of
http://www.ibs.upm.edu.my Challenges in controlling viral diseases of poultry Abdul Rahman Omar Institute of Bioscience Faculty of Veterinary Medicine Universiti Putra Malaysia aro@upm.edu.my Outline of
BLUETONGUE: PATHOGENESIS AND DURATION OF VIREMIA N James MacLachlan
 BLUETONGUE: PATHOGENESIS AND DURATION OF VIREMIA N James MacLachlan LIFE CYCLE OF BLUETONGUE VIRUS INFECTION Described by Spreull in the late 19th century in South Africa with C. imicola Cattle implicated
BLUETONGUE: PATHOGENESIS AND DURATION OF VIREMIA N James MacLachlan LIFE CYCLE OF BLUETONGUE VIRUS INFECTION Described by Spreull in the late 19th century in South Africa with C. imicola Cattle implicated
ABSTRACT Researches on respiratory virosis of cattle
 ABSTRACT Viral respiratory infections of cattle represent a syndrome characterized by acute, subacute or chronic polifactorial inflammation of respiratory tract. These diseases currently known in increasing
ABSTRACT Viral respiratory infections of cattle represent a syndrome characterized by acute, subacute or chronic polifactorial inflammation of respiratory tract. These diseases currently known in increasing
The success story of BVD virus
 The success story of BVD virus BVD MD PI BVDV BVDV-1 BVDV-2 cp ncp Bovine Viral Diarrhea Mucosal Disease Persistently Infected Bovine Viral Diarrhea Virus Bovine Viral Diarrhea Virus Genotype 1 Bovine
The success story of BVD virus BVD MD PI BVDV BVDV-1 BVDV-2 cp ncp Bovine Viral Diarrhea Mucosal Disease Persistently Infected Bovine Viral Diarrhea Virus Bovine Viral Diarrhea Virus Genotype 1 Bovine
Foundations in Microbiology
 Foundations in Microbiology Fifth Edition Talaro Chapter 13 Microbe Human Interactions: Infection and Disease Chapter 13 2 3 Infection a condition in which pathogenic microbes penetrate host defenses,
Foundations in Microbiology Fifth Edition Talaro Chapter 13 Microbe Human Interactions: Infection and Disease Chapter 13 2 3 Infection a condition in which pathogenic microbes penetrate host defenses,
NEXT GENERATION SEQUENCING OPENS NEW VIEWS ON VIRUS EVOLUTION AND EPIDEMIOLOGY. 16th International WAVLD symposium, 10th OIE Seminar
 NEXT GENERATION SEQUENCING OPENS NEW VIEWS ON VIRUS EVOLUTION AND EPIDEMIOLOGY S. Van Borm, I. Monne, D. King and T. Rosseel 16th International WAVLD symposium, 10th OIE Seminar 07.06.2013 Viral livestock
NEXT GENERATION SEQUENCING OPENS NEW VIEWS ON VIRUS EVOLUTION AND EPIDEMIOLOGY S. Van Borm, I. Monne, D. King and T. Rosseel 16th International WAVLD symposium, 10th OIE Seminar 07.06.2013 Viral livestock
Title: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis)
 07-ID-03 Committee: Infectious Diseases Title: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis) Statement of the Problem: The purpose of the recommended
07-ID-03 Committee: Infectious Diseases Title: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis) Statement of the Problem: The purpose of the recommended
PRESENTER: DENNIS NYACHAE MOSE KENYATTA UNIVERSITY
 18/8/2016 SOURCES OF MICROBIAL CONTAMINANTS IN BIOSAFETY LABORATORIES IN KENYA PRESENTER: DENNIS NYACHAE MOSE KENYATTA UNIVERSITY 1 INTRODUCTION Contamination occurs through avoidable procedural errors
18/8/2016 SOURCES OF MICROBIAL CONTAMINANTS IN BIOSAFETY LABORATORIES IN KENYA PRESENTER: DENNIS NYACHAE MOSE KENYATTA UNIVERSITY 1 INTRODUCTION Contamination occurs through avoidable procedural errors
Cryptosporidium is a protozoa in the Phylum Apicomplexa Cryptosporidium Parvum genotype 1
 September, 2010 Cryptosporidium is a protozoa in the Phylum Apicomplexa Cryptosporidium Parvum genotype 1 Livestock not commonly infected but can occur through contamination of feeds by other species,
September, 2010 Cryptosporidium is a protozoa in the Phylum Apicomplexa Cryptosporidium Parvum genotype 1 Livestock not commonly infected but can occur through contamination of feeds by other species,
Alberta Health Public Health Notifiable Disease Management Guidelines July 2012
 July 2012 Typhus - Louseborne Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) July 2012 July 2012 October 2005 Case Definition
July 2012 Typhus - Louseborne Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) July 2012 July 2012 October 2005 Case Definition
Exploring the evolution of MRSA with Whole Genome Sequencing
 Exploring the evolution of MRSA with Whole Genome Sequencing PhD student: Zheng WANG Supervisor: Professor Margaret IP Department of Microbiology, CUHK Joint Graduate Seminar Department of Microbiology,
Exploring the evolution of MRSA with Whole Genome Sequencing PhD student: Zheng WANG Supervisor: Professor Margaret IP Department of Microbiology, CUHK Joint Graduate Seminar Department of Microbiology,
 Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Malaria parasites Malaria parasites are micro-organisms that belong to the genus Plasmodium. There are more than 100 species of Plasmodium, which can infect many animal species such as reptiles, birds,
Chemotherapeutic Efficacy of Oxytetracycline, Enrofloxacin and Imidocarb for the Elimination of Persistent Anaplasma marginale
 Pakistan J. Zool., vol. 44(2), pp. 449-456, 2012. Chemotherapeutic Efficacy of Oxytetracycline, Enrofloxacin and Imidocarb for the Elimination of Persistent Anaplasma marginale Infection in Naturally Infected
Pakistan J. Zool., vol. 44(2), pp. 449-456, 2012. Chemotherapeutic Efficacy of Oxytetracycline, Enrofloxacin and Imidocarb for the Elimination of Persistent Anaplasma marginale Infection in Naturally Infected
Ph.D. Thesis: Protective immune response in P.falciparum malaria 2011 CHAPTER I: Introduction. S.D. Lourembam 16
 CHAPTER I: Introduction S.D. Lourembam 16 1. INTRODUCTION Malaria remains a major global health problem with 300 to 500 million clinical infections and more than a million deaths reported each year. In
CHAPTER I: Introduction S.D. Lourembam 16 1. INTRODUCTION Malaria remains a major global health problem with 300 to 500 million clinical infections and more than a million deaths reported each year. In
in the Gastrointestinal and Reproductive Tracts of Quarter Horse Mares
 Influence of Probiotics on Microflora in the Gastrointestinal and Reproductive Tracts of Quarter Horse Mares Katie Barnhart Research Advisors: Dr. Kimberly Cole and Dr. John Mark Reddish Department of
Influence of Probiotics on Microflora in the Gastrointestinal and Reproductive Tracts of Quarter Horse Mares Katie Barnhart Research Advisors: Dr. Kimberly Cole and Dr. John Mark Reddish Department of
Antibiotic Resistance Lab Report. Bacterial plasmids are small, circular molecules of DNA that can be found in
 Adil Sabir Bio 110H 24 November 2014 Antibiotic Resistance Lab Report I. Introduction Bacterial plasmids are small, circular molecules of DNA that can be found in bacteria (Hass, Richter, Ward, 2014).
Adil Sabir Bio 110H 24 November 2014 Antibiotic Resistance Lab Report I. Introduction Bacterial plasmids are small, circular molecules of DNA that can be found in bacteria (Hass, Richter, Ward, 2014).
Parasitic Protozoa, Helminths, and Arthropod Vectors
 PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 23 Parasitic Protozoa, Helminths, and Arthropod Vectors Parasitic Diseases Protozoan and helminthic parasites are emerging as serious
PowerPoint Lecture Slides for MICROBIOLOGY ROBERT W. BAUMAN Chapter 23 Parasitic Protozoa, Helminths, and Arthropod Vectors Parasitic Diseases Protozoan and helminthic parasites are emerging as serious
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia)
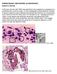 PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
