Zpracování lidské plazmy
|
|
|
- Jane Wilkerson
- 5 years ago
- Views:
Transcription
1 Zpracování lidské plazmy a bezpečnost koncentrátů plazmových pa pote proteinů ů MUDr. Ivan Vonke, OKH, Nemocnice Č.Budějovice, a.s.
2 Raw material blood Hormons Proteins More than 120 plasma proteins 70-80g/Liter Albumin 60-80% (Transport) Plasma 55% 91% Water Salts White blood cells (Leukocytes,Thrombocytes) Red blood cells (Erythrocytes) y 55% Globulins 20-40% (Immune system) Fibrinogen 4 % 45% (Coagulation)
3 Blood or plasma donation? Plasma can be donated up to 30 times a year (plasmapheresis) Blood can be donated d3ti times a year
4 Recovered versus source plasma Recovered plasma: produced from full blood donations (Red Cross): ml/donation Source Plasma: generated by plasmapheresis ml/donation Predominantly used for plasma products
5 Frozen plasma donations for fractionation
6
7
8 Cohn Fractionation Supernatant 8% ETOH, ph 7, -1 C Supernatant I 25% ETOH, ph 7, -7 C Supernatant II+III 40% ETOH, ph 6.4, -7 C Supernatant IV 40% ETOH, ph 5.4, -10 C Crude Fraction V 40% ETOH, ph 5.2, -9 C Fraction V Precipitate Cohn I Fraction FXIII Fibrinogen Precipitate Crude globulines li Cohn II+III Fraction Ultra/ Diafiltration 12% ETOH ph 4.9, -2 C Supernatant 25% ETOH ph 7, -8 C Immunglobulines Albumin
9 Protein yields from 1,000 l plasma Albumin Immune globulins PCC FIX FVIII AT III Protein C Kg 3-5 Kg 300, ,000 Units 250, ,000 Units 140, ,000 Units 130, , Units 120, ,000 Units WFH homepage
10 F VIII usage for treatment of severe hemophilia A A patient with 60 kg body weigth and severe hemophilia A needs on average: a) Prophylaxis: IU FVIII/year (10-50 Einheiten U/kg every 2-3 days) b) On demand therapy: IU FVIII / year Which corresponds to 100 to >3,000 liter plasma/patient/year > 25 Mil. liter plasma l per year are fractionated t worldwide
11 Today s plasma products are safer than ever based on 3 safety pillars Safe products Donor selection Testing of Virus plasma Reduction donations (Inactivation (incl. PCR) Removal)
12
13
14 Plasma origin Approx. 60 plasmapheresis centers in USA and in Europe (Germany, Austria, Sweden, Czech Republic) All centers - are licensed by local authorities - are iqpp* certified - adhere to standard operating procedures (SOPs) - are subjected to regular inspections * iqpp = International Quality Plasma Program. Standard developed to guarantee safe and high quality plasma
15 The main requirements of iqpp Only plasma sourced from Qualified Donors can be used for manufacture into plasma derived medical products Collection centres should recruit donors from the local community and exclude donors at increased risk of HIV Collection centres should participate in the PPTA National Donor Deferral Registry (NDDR) to ensure that rejected donors do not donate elsewhere Collection centres should recruit qualified staff and implement in-depth initial and on-going training in plasmapheresis and regulatory compliance Facilities present a quality, professional medical appearance and are properly maintained and designed to adequately and safely facilitate donor processing Centres must be in compliance with PPTA Source's Viral Marker Alert limits for HIV, HCV, and HBV (alert limits defined by PPTA for qualified donors as seroconversion rates per 10 5 donations) Centres must satisfy PPTA inspection and compliance requirements Manufacturing systems have been designed, documented, and implemented that Manufacturing systems have been designed, documented, and implemented that consistently guarantee a quality product.
16 Plasma sourcing policies with regard to CJD Donor selection criteria according to US regulations: permanent deferral of any person with CJD and CJD in their family... person with an indication of vcjd and person with this disease in their family... recipient of dura mater or cornea transplants... person who has been treated t with human pituitary growth hormone... person who has undergone brain surgery...person who has received blood transfusion in the UK from 1980 until today Indefinite deferral of any..... person who has resided in the UK for 3 months or more cumulatively from person who spent time in France that adds up to 4 years or more since person who spent a total of 6 months or more from associated with a military base in Belgium, NL or Germany or from in Spain, Portugal, Turkey, Italy or Greece meets these requirements for it s plasma sourced in the US BioLife plasma services SOP # DIS
17 Plasma sourcing policies with regard to CJD Donor selection criteria according to European regulations: permanent deferral of any person with CJD and CJD in their family... person with an indication of vcjd and person with this disease in their family... recipient of dura mater or cornea transplants... person who has been treated with human pituitary growth hormone... person who has resided in the UK for 6 months or more cumulatively from (Please note: Guidelines request only 1 year)... person who has undergone brain surgery...person who has received blood transfusion in the UK from 1980 until today exceeds these requirements for it s plasma sourced in Europe 1.
18
19 Virus marker rates of qualified donors are lower than that requested by PPTA standards d Seroconversion rate per 10 5 donations Virus donor applicants 1 qualified donors 2 requested 3 HIV HBV HCV calculation based on 131,077 donations 2 calculation based on 1,405,375 donations 3 Viral Marker Standard for Qualified Donors by Plasma Protein Therapeutics p Association (PPTA ) (Revised 2/2002)
20
21 Single donation test program for markers of disease Determination by conventional ELISA Assay Requested results HIV-1 antibodies HIV-2 antibodies HCV antibodies HBs antigen non reactive non reactive non reactive non reactive
22 60 days inventory hold Single donation 60 days min. at -20 C Pooling and Fractionation time to receive post donation information o about the donor positive PCR result high risk behavior CJD in the family
23
24
25
26 Virus and assays specific diagnostic windows HIV 21 anti body detection by ELISA virus detection by PCR 11 HBV HCV diagnostic window period PCR 34 PCR ELISA ELISA days after infection
27 PCR diagnostic windows covered by 60 days inventory hold HIV Inventory hold period 21 ELISA PCR 11 HBV HCV 23 PCR 34 PCR ELISA ELISA days after infection
28 PCR test program on 6 viruses (started in Vienna in 1995 with HIV, HBV and HCV) Virus Disease Fully implemented HIV-1+-2 HBV AIDS hepatitis B Jan 1999 Jan 1999 HCV hepatitis C Jan 1999 parvo B19 HAV fifth disease hepatitis A Jan 2000* Aug 2000 * voluntary cut-off: 10 4 parvo units/ml lower than the PPTA standard with cut off 10 5 units parvo / ml
29
30 Identification of a contaminated single donation by a2 2- dimensional pooling system Microtiter plate with 96 donations Virus positive donation Virus negative donation pool together PCR PCR result positive
31
32
33 Identification of a contaminated single donation by 12+8 = 20 additional lpcrt testst 5 16
34
35 Identification of contaminated single donation Virus positive single donation
36
37 Screening of plasma production pools by PCR production pool PCR 2 positive production pool negative production pool destroyed released for fractionation
38 QSEAL certified since Nov as one of the first companies QSEAL Quality Standards of Excellence, Assurance and Leadership Certification process for plasma manufacturer established in 2000 by the Plasma Protein Therapeutics Association (PPTA) based on 4 voluntary industry standards agreed on in 1996 by the PPTA member companies
39 The 4 voluntary industry standards (adopted by the PPTA in 1996 to go beyond authority requirements) Qualified donor standard (at since 1994) to ensure a committed, healthy donor population Viral marker standard ( plasma center relocation in the US) to demonstrate the quality of the donor population Inventory hold standard (at since 1992) to allow for retrieval of plasma if new post-donation donor- related information becomes available NAT testing standard (since 1995) to allow for detection of certain viruses earlier than current serological screening methods. Currently HCV, HIV, and HBV only, PVB19 (cut-off 10 5 /ml)
40 The QSEAL certificate......is evaluated by independent third party inspectors...is issued to a company only when all fractionation facilities meet tthe requested standards d...is issued for a two-year period...is confirmed by regular inspections Newly developed standards will be confirmed at the next regular inspection
41 Why are virus reduction measures needed after all these testing? To meet authority requirements Donor selection and donation testing methods do have limits of detection Virus reduction is the most effective safety measure (on average 100,000-fold virus reduction compared to 100-fold by donor selection or testing) To eliminate i unknown pathogens
42 Virus reduction methods Virus removal inactivation IMP IMP IMP IMP IMP Intermediate product virus particle
43 Demonstration of efficiency of virus inactivation methods by validation studies Man nufacturi ing proc cess dow wn scale ed Starting material virus inactivation step I purification steps virus inactivation step II Final product Addition of test viruses in known amounts Determination of viruses that are still active Reduction factor calculation: R = log (Titer x Volume) before vi step (Titer x Volume) after vi step Calculation overall reduction factor ORF = R1+R2+R3...
44 Regulatory requirements (EMEA CPMP/BWP/269/95 rev.3, 2001) Enveloped viruses At least two robust steps (with complementary mode of action) providing >4 logs inactivation/removal. Non-enveloped viruses At least one robust step providing >4 logs inactivation/removal.
45 Virus partitioning methods Precipitation steps Cryoprecipitation Cohn-fractionation EtOH/pH, PEG Filtration nanofiltration (15-75 nm pore size) Adsorption AlOH adsorption to remove the PTC Chromatographies (batch or columns)
46 Filtration methods (nano filtration 15-75nm pore size) parvob19 : nm ERV: 29 nm HAV: nm HBV: nm TBEV: nm HCV, HGV: nm HIV : nm PVB19 HAV ERV TBEV Fibrinogen FVIII FVIII: 330 kd (25 nm) Fibrinogen: 340 kd (25 nm) IgG : 150 kd (18-20 nm) FIX: 56 kd (14 nm) Albumin: 68 kd (14 nm) Protein C: 56 kd (14 nm) AT3: 58 kd (14 nm) HBV FVII: 50 kd (14 nm) Ig HCV HIV FIX Protein C Albumin FVII AT III
47 Chromatographies Ion exchange chromatography h Anion exchange chromatography (DEAE Sephadex, Q-Sepharose) Kation exchange chromatography h (Source F, S-Sepharose) S Immunoaffinity chromatography monoclonal ab against FVIII, vwf, protein C, heparin Gel permeability chromatography (Sepharose); size exclusion Hydrophobic (C18-Sepharose, Phenylsepharose); binding to hydrophobic proteins Protein A, Protein G protein isolated from Staphylococcus aureus binding to Fc-part of IgG Fc
48 Virus inactivation methods Heat treatments Solvent detergent (SD) treatment Lyophilization Immobilized hydrolases (trypsin, chymotrypsin treatment of Ig) Ethanol
49 Dry heat 100 C; 1 h Virus inactivation heat treatments 100 C; 30 min 80 C; 72 h Vapor heating 7-8% humidity, pressure delta190 mbar S -TIM3: 10h; 60 C S -TIM4: 10h; 60 C plus 1h; 80 C Wet heat = pasteurization 10h; 60 C
50
51 Parvovirus B19 inactivation by vapor heating (FEIBA STIM 4) MMV RF VH1 #1 VH1 #2 VH2 #1 B19V mean RF positive control n.d. - spike control after Lyo h 60 C n.d n.d h 60 C min 80 C
52 Virus inactivation SD treatment Common Solvent Detergent Combinations a) Tri(n-butyl) y)phosphate p (TNBP) + Tween 80 b) TNBP + Triton X-100 or Sodium cholate at room temperature; 6 h Mode of action Solubilization of lipid membranes/virus envelopes
53
54 Inactivation technologies are effective against new emerging pathogens? New emerging pathogens within the last years: WNV SARS H5N1 bird flu
55 West Nile Virus: complete inactivation by vapor heating 8 Vapor Heating of FEIBA (Factor Eight Inhibitor Bypassing Fraction Human) 60min min log10 [T CID50/ml] min 510min temperature profile TBEV (m2;99) TBEV (92) BVDV (m2;01) BVDV (m2;99) SINV (m3;88) WNV (7%,02) Temperat ure [ C] 2 WNV (8%,02) (8%02) 20 1 limit of detection lyo time of heating [min]
56 SARS completely inactivated by vapor heating Inactivation of Coronavirus (Mouse Hepatitis Virus) by Vapor Heating 4 tem perature profile 60 [TCID 50 /ml] log FEIBA* Fibrin Sealant Factor VIII** 40 Temperatur re [ C] 20 1 limit of detection time of heating [min] *...m ean of 2 studies **..m ean of 3 studies
57 H5N1 influenza virus (orthomyxovirus) completely inactivated by vapor heating (>5.1 log 10 ) after 6 hours like other lipid-enveloped viruses TR Kreil et al., Transfusion 47, (2007)
58 Summary and Conclusion Today s plasma products have reached a high level of safety No transmission of HIV and hepatitis viruses occurred since implementation of testing and inactivation technologies There are two groups of safety measures - Donor and plasma screening to avoid contamination of plasma pools - Pathogen inactivation/removal techniques PCR testing of plasma was shown to minimize the risk of virus transmission by shortening the diagnostic window Virus inactivation and removal technologies during manufacturing were demonstrated to be effective against lipid and non-lipid enveloped known and new emerging viruses The Pathogen Safety Program exceeds the QSEAL certification criteria by: -a higher quality of plasma (below requested viral marker rate standard) - an expanded PCR test program (lower PVB19 cut-off and HAV testing)
Source Plasma Safety
 Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
Pathogen Safety and BSE / variant CJD
 Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety Monograph. Baxter BioSurgery Products [North America]
![Pathogen Safety Monograph. Baxter BioSurgery Products [North America] Pathogen Safety Monograph. Baxter BioSurgery Products [North America]](/thumbs/87/95105700.jpg) Pathogen Safety Monograph Baxter BioSurgery Products [North America] In the production of plasma-derived therapeutics, patient safety is of paramount importance. Pathogen Safety Monograph for Baxter BioSurgery
Pathogen Safety Monograph Baxter BioSurgery Products [North America] In the production of plasma-derived therapeutics, patient safety is of paramount importance. Pathogen Safety Monograph for Baxter BioSurgery
Product Monograph. Key Scientific Information
 Product Monograph Key Scientific Information Ko ate -DVI (Double Viral Inactivation) Infusion of more than 2 billion IUs worldwide with no confirmed cases of viral transmission.* Talecris is a leader in
Product Monograph Key Scientific Information Ko ate -DVI (Double Viral Inactivation) Infusion of more than 2 billion IUs worldwide with no confirmed cases of viral transmission.* Talecris is a leader in
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
donors and collecting blood and plasma in for-profit or a not for profit environment
 Remunerated versus non-remunerated donors and collecting blood and plasma in for-profit or a not for profit environment ISBT Amsterdam, June 4, 2013 Jan M. Bult President PPTA PATIENT CENTEREDNESS IT
Remunerated versus non-remunerated donors and collecting blood and plasma in for-profit or a not for profit environment ISBT Amsterdam, June 4, 2013 Jan M. Bult President PPTA PATIENT CENTEREDNESS IT
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Draft Agreed by Biologics Working Party December Draft Agreed by Blood Products Working party December 2010
 15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
IQPP Qualified Donor Standard
 IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
IQPP Qualified Donor Standard Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) IQPP Standards Program. PPTA's Voluntary Standards Program
Meta Analysis of Virus Partitioning in Fractionation
 Meta Analysis of Virus Partitioning in Fractionation Dr. Herbert Dichtelmüller Director Virus Validation Biotest; Dreieich, Germany IPFA/PEI Meeting; Budapest; May 2012 0 Cold Ethanol Fractionation Developed
Meta Analysis of Virus Partitioning in Fractionation Dr. Herbert Dichtelmüller Director Virus Validation Biotest; Dreieich, Germany IPFA/PEI Meeting; Budapest; May 2012 0 Cold Ethanol Fractionation Developed
Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt
 Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt Are plasma and cryoprecipitate still used? They are still largely used for treatment of hereditary bleeding disorders in many resource limited
Magdy El Ekiaby, MD Shabrawishi Hospital BTC & HTC Cairo, Egypt Are plasma and cryoprecipitate still used? They are still largely used for treatment of hereditary bleeding disorders in many resource limited
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Mini Pool Plasma Fractionation: A Pragmatic Approach to Fill in Gaps of Supply and Access
 Mini Pool Plasma Fractionation: A Pragmatic Approach to Fill in Gaps of Supply and Access Magdy El Ekiaby, MD Egypt 7th Annual Bioplasma World Asia 2018 12 to 13 September 2018 Singapore Global FVIII
Mini Pool Plasma Fractionation: A Pragmatic Approach to Fill in Gaps of Supply and Access Magdy El Ekiaby, MD Egypt 7th Annual Bioplasma World Asia 2018 12 to 13 September 2018 Singapore Global FVIII
Viral clearance (inactivation and. Part 3a, Plasma and Plasma Products (Heat and Solvent/Detergent Treatments)
 Part 3a, Plasma and Plasma Products (Heat and Solvent/Detergent Treatments) This article was first published in BioPharm s September 2002 issue. Gail Sofer Viral clearance (inactivation and removal) is
Part 3a, Plasma and Plasma Products (Heat and Solvent/Detergent Treatments) This article was first published in BioPharm s September 2002 issue. Gail Sofer Viral clearance (inactivation and removal) is
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Solvent-Detergent for Virus Inactivation: An Evolving Technology Peter Roberts Bio Products Laboratory Elstree, UK
 Solvent-Detergent for Virus Inactivation: An Evolving Technology Peter Roberts Bio Products Laboratory Elstree, UK Sixth Plasma Product Biotechnology Meeting Menorca, 11-25 May 2009 Summary Background
Solvent-Detergent for Virus Inactivation: An Evolving Technology Peter Roberts Bio Products Laboratory Elstree, UK Sixth Plasma Product Biotechnology Meeting Menorca, 11-25 May 2009 Summary Background
Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB
 Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB Plasma Fractionation Evolved from a medical service to a global manufacturing industry Involves sophisticated industrial process High
Plasma Fractionation Issues for Developing Countries DR YASMIN AYOB Plasma Fractionation Evolved from a medical service to a global manufacturing industry Involves sophisticated industrial process High
TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview
 IPFA Workshop Session 7: REGULATORY CONSIDERATIONS TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview Mary Lavithis, CSL Behring Australia Presented at: IPFA Asia
IPFA Workshop Session 7: REGULATORY CONSIDERATIONS TGA regulation of local manufacturers of plasma derivatives CSL Behring Australia s overview Mary Lavithis, CSL Behring Australia Presented at: IPFA Asia
DHQ Flowcharts v2.0 eff. February 2016
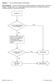 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Guidelines on Viral Inactivation and Removal Procedures Intended to Assure the Viral Safety of Human Blood Plasma Products
 WORLD HEALTH ORGANIZATION ORGANIZATION MONDIALE DE LA SANTE ENGLISH ONLY EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION Geneva, 26 to 30 November 2001 Guidelines on Viral Inactivation and Removal Procedures
WORLD HEALTH ORGANIZATION ORGANIZATION MONDIALE DE LA SANTE ENGLISH ONLY EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION Geneva, 26 to 30 November 2001 Guidelines on Viral Inactivation and Removal Procedures
Plasma for fractionation: South African Plasma Requirements
 Plasma for fractionation: South African Plasma Requirements Dr Jeh-han Omarjee Head: Microbiological Sciences NBI is a "not for profit" company committed to providing safe, cost effective, quality medicinal
Plasma for fractionation: South African Plasma Requirements Dr Jeh-han Omarjee Head: Microbiological Sciences NBI is a "not for profit" company committed to providing safe, cost effective, quality medicinal
INDONESIAN EXPERIENCE IN PRODUCING MINIPOOL CRYOPRECIPITATE. Yuyun SM Soedarmono
 INDONESIAN EXPERIENCE IN PRODUCING MINIPOOL CRYOPRECIPITATE Yuyun SM Soedarmono 1 BACKGROUND Indonesia has more than 240 million population and approximately 25,000 hemophilia A patients Hemophilia A patients
INDONESIAN EXPERIENCE IN PRODUCING MINIPOOL CRYOPRECIPITATE Yuyun SM Soedarmono 1 BACKGROUND Indonesia has more than 240 million population and approximately 25,000 hemophilia A patients Hemophilia A patients
Qualified Donor Standard. Implemented 1997 Revised Version 3.0
 Implemented 1997 Revised 2006 Version 3.0 IQPP Contents I. Background... 3 II. Definitions... 3 III. Standard... 3 IV. Inspection and Compliance Verification... 4 Appendix 1: Questions & Answers... 5 Page
Implemented 1997 Revised 2006 Version 3.0 IQPP Contents I. Background... 3 II. Definitions... 3 III. Standard... 3 IV. Inspection and Compliance Verification... 4 Appendix 1: Questions & Answers... 5 Page
Hepa%%s E Virus Is it a Concern?
 IPFA/PEI 17 th Workshop on Surveillance and Screening of Blood Borne Pathogens 26-27 May 2010, The Regent Esplanade Zagreb Hotel, Zagreb, Croa%a Hepa%%s E Virus Is it a Concern? Keiji Matsubayashi Hokkaido
IPFA/PEI 17 th Workshop on Surveillance and Screening of Blood Borne Pathogens 26-27 May 2010, The Regent Esplanade Zagreb Hotel, Zagreb, Croa%a Hepa%%s E Virus Is it a Concern? Keiji Matsubayashi Hokkaido
HEMOFIL M Antihemophilic Factor (Human) Method M, Monoclonal Purified
 HEMOFIL M Antihemophilic Factor (Human) Method M, Monoclonal Purified Description HEMOFIL M, Antihemophilic Factor (Human) (AHF), Method M, Monoclonal Purified, is a sterile, nonpyrogenic, dried preparation
HEMOFIL M Antihemophilic Factor (Human) Method M, Monoclonal Purified Description HEMOFIL M, Antihemophilic Factor (Human) (AHF), Method M, Monoclonal Purified, is a sterile, nonpyrogenic, dried preparation
Plasma Protein Therapeutics Association
 Plasma Protein Therapeutics Association Mary Gustafson Vice President, Global Regulatory Policy, PPTA MASAC Update, September 20, 2014 Emerging Viruses and Product Safety Issues to be Addressed Presentation
Plasma Protein Therapeutics Association Mary Gustafson Vice President, Global Regulatory Policy, PPTA MASAC Update, September 20, 2014 Emerging Viruses and Product Safety Issues to be Addressed Presentation
New Advances in Transfusion EM I LY CO BERLY, M D
 New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
A comparison of the removal of parvovirus B19 and model parvoviruses from plasma products by virus filtration
 A comparison of the removal of parvovirus B19 and model parvoviruses from plasma products by virus filtration Shirley Stagg, Samantha Fernando, Amy Shackell, Peter Roberts, Joan Dalton Bio Products Laboratory,
A comparison of the removal of parvovirus B19 and model parvoviruses from plasma products by virus filtration Shirley Stagg, Samantha Fernando, Amy Shackell, Peter Roberts, Joan Dalton Bio Products Laboratory,
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
Hyper-Immune Globulin
 Cytomegalovirus Cytogam Intramuscular GamaSTAN S/D Hepatitis B HepaGAM B HyperHEP B S/D Nabi - HB Rabies HyperRAB S/D IMOGAM Rabies - HT Tetanus HyperTET S/D (D) HyperRho S/D RhoGAM UF PLUS Rhophylac WinRho
Cytomegalovirus Cytogam Intramuscular GamaSTAN S/D Hepatitis B HepaGAM B HyperHEP B S/D Nabi - HB Rabies HyperRAB S/D IMOGAM Rabies - HT Tetanus HyperTET S/D (D) HyperRho S/D RhoGAM UF PLUS Rhophylac WinRho
DONOR RECRUITMENT AND PLASMA COLLECTION IN INDONESIA. Yuyun SM Soedarmono Blood Services Committee Ministry of Health of Indonesia 1
 DONOR RECRUITMENT AND PLASMA COLLECTION IN INDONESIA Yuyun SM Soedarmono Blood Services Committee Ministry of Health of Indonesia 1 OUTLINE Introduction Challenges to Fulfill Global Demand of Plasma for
DONOR RECRUITMENT AND PLASMA COLLECTION IN INDONESIA Yuyun SM Soedarmono Blood Services Committee Ministry of Health of Indonesia 1 OUTLINE Introduction Challenges to Fulfill Global Demand of Plasma for
Keeping the Blood Supply Safe Current and Future Strategies. Marissa Li, MD
 Keeping the Blood Supply Safe Current and Future Strategies Marissa Li, MD Overview Pre-collection Donor Screening Collection Visual Inspection Aseptic Processing Leukoreduction of Apheresis Collections
Keeping the Blood Supply Safe Current and Future Strategies Marissa Li, MD Overview Pre-collection Donor Screening Collection Visual Inspection Aseptic Processing Leukoreduction of Apheresis Collections
Quality Assurance Standard. Implemented 1991 Revised Version 3.0
 Implemented 1991 Revised 2006 Version 3.0 IQPP Contents I. Introduction... 3 II. Definitions... 4 III. PPTA Source Quality Assurance Principles... 4 IV. Audits and Compliance Verification... 5 Page 2 IQPP
Implemented 1991 Revised 2006 Version 3.0 IQPP Contents I. Introduction... 3 II. Definitions... 4 III. PPTA Source Quality Assurance Principles... 4 IV. Audits and Compliance Verification... 5 Page 2 IQPP
Ensuring Compliance: Regulatory guidance for virus clearance validation
 Application Note Ensuring Compliance: Regulatory guidance for virus clearance validation Introduction To assure virus safety of biological therapeutics, regulatory guidance advocates an approach in which
Application Note Ensuring Compliance: Regulatory guidance for virus clearance validation Introduction To assure virus safety of biological therapeutics, regulatory guidance advocates an approach in which
SOLVENT-DETERGENT TREATMENT OF IgM-ENRICHED IMMUNOGLOBULIN
 DARU Volume 11, No. 2, 2003 SOLVENT-DETERGENT TREATMENT OF IgM-ENRICHED IMMUNOGLOBULIN * MOJGAN POURMOKHTAR, ** RASSOUL DINARVAND, * KAMRAN MOUSAVI HOSSEINI, * HOURI REZVAN, * MOHAMMAD ALI JALILI * R&D
DARU Volume 11, No. 2, 2003 SOLVENT-DETERGENT TREATMENT OF IgM-ENRICHED IMMUNOGLOBULIN * MOJGAN POURMOKHTAR, ** RASSOUL DINARVAND, * KAMRAN MOUSAVI HOSSEINI, * HOURI REZVAN, * MOHAMMAD ALI JALILI * R&D
Perspectives on self-sufficiency of blood-derived therapies. Global evolution and realities. Albert Farrugia
 TERZO INCONTRO NAZIONALE DEL SISTEMA SANGUE ITALIANO I confini dell autosufficienza. Etica, economia, responsabilità. Perspectives on self-sufficiency of blood-derived therapies. Global evolution and realities.
TERZO INCONTRO NAZIONALE DEL SISTEMA SANGUE ITALIANO I confini dell autosufficienza. Etica, economia, responsabilità. Perspectives on self-sufficiency of blood-derived therapies. Global evolution and realities.
Objectives. Methods. Results. Economic
 Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
CSL Behring LLC Albuminar -25 US Package Insert Albumin (Human) USP, 25% Revised: 01/2008 Page 1
 Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
WHO Parvovirus B19 Genotype Panel
 WHO Parvovirus B19 Genotype Panel Mei-ying W Yu, PhD SoGAT XXII Rome, 14-15 April 2011 1 st WHO International Reference Panel for Parvovirus B19 Genotypes In Oct 2009, a plasma-derived parvovirus B19 (B19V)
WHO Parvovirus B19 Genotype Panel Mei-ying W Yu, PhD SoGAT XXII Rome, 14-15 April 2011 1 st WHO International Reference Panel for Parvovirus B19 Genotypes In Oct 2009, a plasma-derived parvovirus B19 (B19V)
EMEA WORKSHOP ON VIRAL SAFETY OF PLASMA-DERIVED MEDICINAL PRODUCTS WITH PARTICULAR FOCUS ON NON-ENVELOPED VIRUSES. 13 September 2000 REPORT SUMMARY
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 28 March 2001 CPMP/BWP/BPWG/4080/00 EMEA WORKSHOP ON VIRAL SAFETY OF PLASMA-DERIVED MEDICINAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 28 March 2001 CPMP/BWP/BPWG/4080/00 EMEA WORKSHOP ON VIRAL SAFETY OF PLASMA-DERIVED MEDICINAL
Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
Question: 1. Are you currently taking an antibiotic?
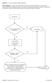 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Blood supply and blood products: Regulatory issues in the pandemic context
 Blood supply and blood products: Regulatory issues in the pandemic context M. Heiden R. Seitz Paul-Ehrlich-Straße 51-59 63225 Langen GERMANY +49 (0) 6103 77 0 +49 (0) 6103 77-1234 Email: pei@pei.de Homepage:
Blood supply and blood products: Regulatory issues in the pandemic context M. Heiden R. Seitz Paul-Ehrlich-Straße 51-59 63225 Langen GERMANY +49 (0) 6103 77 0 +49 (0) 6103 77-1234 Email: pei@pei.de Homepage:
Adverse Reactions Associated with Methylene Blue Inactivated Fresh Frozen Plasma (MB FFP)
 Adverse Reactions Associated with Methylene Blue Inactivated Fresh Frozen Plasma (MB FFP) Constantina Politis Hellenic Coordinating Haemovigilance Centre (SKAE) Background Pathogen inactivation is expected
Adverse Reactions Associated with Methylene Blue Inactivated Fresh Frozen Plasma (MB FFP) Constantina Politis Hellenic Coordinating Haemovigilance Centre (SKAE) Background Pathogen inactivation is expected
HEMOFIL M Antihemophilic Factor (Human), Method M, Monoclonal Purified Nanofiltered
 HEMOFIL M Antihemophilic Factor (Human), Method M, Monoclonal Purified Nanofiltered DESCRIPTION HEMOFIL M, Antihemophilic Factor (Human) (AHF), Method M, Monoclonal Purified, is a sterile, nonpyrogenic,
HEMOFIL M Antihemophilic Factor (Human), Method M, Monoclonal Purified Nanofiltered DESCRIPTION HEMOFIL M, Antihemophilic Factor (Human) (AHF), Method M, Monoclonal Purified, is a sterile, nonpyrogenic,
Human Zoster Immunoglobulin, solution for intramuscular injection.
 Product Information Zoster Immunoglobulin-VF Australia NAME OF THE MEDICINE Human Zoster Immunoglobulin, solution for intramuscular injection. DESCRIPTION Zoster Immunoglobulin-VF is a sterile, preservative-free
Product Information Zoster Immunoglobulin-VF Australia NAME OF THE MEDICINE Human Zoster Immunoglobulin, solution for intramuscular injection. DESCRIPTION Zoster Immunoglobulin-VF is a sterile, preservative-free
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
DATA SHEET RHESONATIV. NAME OF THE MEDICINE Rhesonativ 625 IU/mL, solution for injection
 DATA SHEET RHESONATIV NAME OF THE MEDICINE Rhesonativ 625 IU/mL, solution for injection DESCRIPTION Rhesonativ contains 625 IU of human anti-d immunoglobulin. Rhesonativ is presented as a solution for
DATA SHEET RHESONATIV NAME OF THE MEDICINE Rhesonativ 625 IU/mL, solution for injection DESCRIPTION Rhesonativ contains 625 IU of human anti-d immunoglobulin. Rhesonativ is presented as a solution for
FORM TITLE: Risk Behavior Assessment. 1. Are you a male who has had sex with another male in the preceding five years?
 RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
Immune Globulin Comparison Table
 I. Formulation data Gamunex /IGIVnex Gammagard Liquid Privigen Vivaglobin Formulation Lyophilized Liquid Liquid Liquid Liquid Concentration 5% or 10% upon reconstitution Administration set provided? IgA
I. Formulation data Gamunex /IGIVnex Gammagard Liquid Privigen Vivaglobin Formulation Lyophilized Liquid Liquid Liquid Liquid Concentration 5% or 10% upon reconstitution Administration set provided? IgA
Question: 1. Are you currently taking an antibiotic?
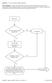 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Using Donor Human Milk in Extremely Premature Infants
 Using Donor Human Milk in Extremely Premature Infants Introduction Scott Eaker, Vice President of Quality and Regulatory Affairs Scott Elster, Chief Executive Officer David Rechtman, MD, Chief Medical
Using Donor Human Milk in Extremely Premature Infants Introduction Scott Eaker, Vice President of Quality and Regulatory Affairs Scott Elster, Chief Executive Officer David Rechtman, MD, Chief Medical
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) CORE SPC FOR HUMAN NORMAL IMMUNOGLOBULIN FOR SUBCUTANEOUS AND INTRAMUSCULAR USE (CPMP/BPWG/282/00)
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 25 July 2002 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) CORE SPC FOR HUMAN NORMAL IMMUNOGLOBULIN
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 25 July 2002 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) CORE SPC FOR HUMAN NORMAL IMMUNOGLOBULIN
Reflection paper on viral safety of plasma-derived medicinal products with respect to hepatitis E virus
 1 2 3 25 June 2015 EMA/CHMP/BWP/723009/2014 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Reflection paper on viral safety of plasma-derived medicinal products with respect to Draft Draft
1 2 3 25 June 2015 EMA/CHMP/BWP/723009/2014 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Reflection paper on viral safety of plasma-derived medicinal products with respect to Draft Draft
Health Care Workers and Viral Hepatitis in Turkey. Bulent Degertekin M.D. Viral Hepatitis Prevention Board Meeting
 Different Aspects of Viral Hepatitis Health Care Workers and Viral Hepatitis in Turkey Bulent Degertekin M.D. Viral Hepatitis Prevention Board Meeting 12 13 November 2009 Istanbul. Turkey Hepatitis B and
Different Aspects of Viral Hepatitis Health Care Workers and Viral Hepatitis in Turkey Bulent Degertekin M.D. Viral Hepatitis Prevention Board Meeting 12 13 November 2009 Istanbul. Turkey Hepatitis B and
Impact of multi-dye multiplex technology on testing algorithm
 Impact of multi-dye multiplex technology on testing algorithm Lydia Blanco. Mª Isabel Gonzalez-Fraile Centro de Hemoterapia y Hemodonación de Castilla y León, España TTI EPIDEMIOLOGICAL DATA IN SPAIN NAT
Impact of multi-dye multiplex technology on testing algorithm Lydia Blanco. Mª Isabel Gonzalez-Fraile Centro de Hemoterapia y Hemodonación de Castilla y León, España TTI EPIDEMIOLOGICAL DATA IN SPAIN NAT
Routine NAT-screening for West Nile Virus Infections in Germany: Being prepared
 Routine NAT-screening for West Nile Virus Infections in Germany: Being prepared R. Thermann, W. K. Roth IPFA / PEI 18 th international workshop on Surveillance and screening of blood borne pathogens Budapest,
Routine NAT-screening for West Nile Virus Infections in Germany: Being prepared R. Thermann, W. K. Roth IPFA / PEI 18 th international workshop on Surveillance and screening of blood borne pathogens Budapest,
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
The testing of Donated Blood and Components at NHSBT
 The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
HEPATITIS VIRUSES. Other causes (not exclusively hepatitis v.)also called sporadic hepatitis: HEPATITIS A(infectious hepatitis)
 Dept.of Microbiology/Virology Assist.prof. Shatha F. Abdullah HEPATITIS VIRUSES Medically important hepatitis v. (liver)are: 1.HAV 2.HBV 3.HCV 4.HDV 5.HEV 6.HGV Other causes (not exclusively hepatitis
Dept.of Microbiology/Virology Assist.prof. Shatha F. Abdullah HEPATITIS VIRUSES Medically important hepatitis v. (liver)are: 1.HAV 2.HBV 3.HCV 4.HDV 5.HEV 6.HGV Other causes (not exclusively hepatitis
APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION*
 11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
Center for Biologics Evaluation and Research
 Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Impact of donor epidemiology and screening strategies on the safety of blood and plasma for fractionation
 IPFA 2 nd Asia Workshop in Yogyakarta Impact of donor epidemiology and screening strategies on the safety of blood and plasma for fractionation Yoshihiko Tani, MD, PhD Japanese Red Cross Osaka Blood Center
IPFA 2 nd Asia Workshop in Yogyakarta Impact of donor epidemiology and screening strategies on the safety of blood and plasma for fractionation Yoshihiko Tani, MD, PhD Japanese Red Cross Osaka Blood Center
Blood transfusion. Dr. J. Potgieter Dept. of Haematology NHLS - TAD
 Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
IVD information *Droppers for the sensitized and control cells. Not for use other than dispensing the sensitized and control cells.
 In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
2014 report. The collection, testing and use of blood and blood components in Europe. M.P. Janssen 1 and G. Rautmann 2
 The collection, testing and use of blood and blood components in Europe 2014 report M.P. Janssen 1 and G. Rautmann 2 1. Julius Center for Health Sciences and Primary Care, University of Utrecht, Utrecht,
The collection, testing and use of blood and blood components in Europe 2014 report M.P. Janssen 1 and G. Rautmann 2 1. Julius Center for Health Sciences and Primary Care, University of Utrecht, Utrecht,
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER
 Verified with regard to factual content 13.12.2011 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER GAMMA anty-hbs 1000 Immunoglobulinum humanum hepatitidis B Human immunoglobulin against Hepatitis
Verified with regard to factual content 13.12.2011 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER GAMMA anty-hbs 1000 Immunoglobulinum humanum hepatitidis B Human immunoglobulin against Hepatitis
Keeping blood transfusions safe:
 ISP-WIV Seminar Diagnosis and surveillance of infectious diseases May 18 th, 2017 Keeping blood transfusions safe: the challenge of emerging infectious diseases Presented by Roland HÜBNER Superior Health
ISP-WIV Seminar Diagnosis and surveillance of infectious diseases May 18 th, 2017 Keeping blood transfusions safe: the challenge of emerging infectious diseases Presented by Roland HÜBNER Superior Health
Weekly Influenza Surveillance Report. Week 11
 Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Purification of viral nucleic acid from serum, plasma, cell-free biological fluids MACHEREY- NAGEL
 Purification of viral nucleic acid from serum, plasma, cell-free biological fluids Purification of viral nucleic acid from serum, plasma, cell-free biological fluids viral RNA: viral DNA: NucleoSpin RNA
Purification of viral nucleic acid from serum, plasma, cell-free biological fluids Purification of viral nucleic acid from serum, plasma, cell-free biological fluids viral RNA: viral DNA: NucleoSpin RNA
Plasma The Patients Perspective. Brian O Mahony, PLUS (Plasma Users) FIODS Conference, San Marino June 2012
 Plasma The Patients Perspective Brian O Mahony, PLUS (Plasma Users) FIODS Conference, San Marino June 2012 1 PLUS (Plasma Users) 2 2 Conditions treated with PDMP s Haemophilia A and B Von Willebrands Disease
Plasma The Patients Perspective Brian O Mahony, PLUS (Plasma Users) FIODS Conference, San Marino June 2012 1 PLUS (Plasma Users) 2 2 Conditions treated with PDMP s Haemophilia A and B Von Willebrands Disease
Infectious Disease Testing. ULTRA Product Line. Safety is not a Matter of Chance
 Infectious Disease Testing ULTRA Product Line Safety is not a Matter of Chance ULTRA Product Line The best answer for HBV, HCV and HIV screening: a global automated solution for safe results. Monolisa
Infectious Disease Testing ULTRA Product Line Safety is not a Matter of Chance ULTRA Product Line The best answer for HBV, HCV and HIV screening: a global automated solution for safe results. Monolisa
SUMMARY OF PRODUCT CHARACTERISTICS. Human proteins g/l g/l of which human immunoglobulin at least to. 180 IU/ml 180 IU/vial
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT UMAN BIG 180 IU/ml Solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. UMAN BIG 180 IU/1
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT UMAN BIG 180 IU/ml Solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. UMAN BIG 180 IU/1
Duration: 12 months May to April
 SPECIALIST CERTIFICATE IN TRANSFUSION SCIENCE PRACTICE PROGRAMME OF STUDY OVERVIEW Example only Duration: 12 months May to April This document serves as a general programme overview only. To ensure you
SPECIALIST CERTIFICATE IN TRANSFUSION SCIENCE PRACTICE PROGRAMME OF STUDY OVERVIEW Example only Duration: 12 months May to April This document serves as a general programme overview only. To ensure you
Overview of Blood Transfusion System of Iran:
 Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
PROPOSAL FOR THE DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA
 PROPOSAL FOR THE DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA SoGAT Clinical Diagnostics II 30 September / 1 October 2009, Istanbul Michael Chudy Julia Kreß C. Micha
PROPOSAL FOR THE DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA SoGAT Clinical Diagnostics II 30 September / 1 October 2009, Istanbul Michael Chudy Julia Kreß C. Micha
Immunologic Products asdhealthcare.com
 Immunologic Products Bivigam Carimune NF Flebogamma DIF Gammagard S/D Low IGA Gammagard Liquid Gammaked Gammaplex Gamunex -C Hizentra HyQvia Octagam Privigen Immunologic Products 800.746.6273 asdhealthcare.com
Immunologic Products Bivigam Carimune NF Flebogamma DIF Gammagard S/D Low IGA Gammagard Liquid Gammaked Gammaplex Gamunex -C Hizentra HyQvia Octagam Privigen Immunologic Products 800.746.6273 asdhealthcare.com
HAV HBV HCV HDV HEV HGV
 Viral Hepatitis HAV HBV HCV HDV HEV HGV Additional well-characterized viruses that can cause sporadic hepatitis, such as yellow fever virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, rubella
Viral Hepatitis HAV HBV HCV HDV HEV HGV Additional well-characterized viruses that can cause sporadic hepatitis, such as yellow fever virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, rubella
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER Octaplex 500 IU powder and solvent for solution for infusion Human prothrombin complex Octaplex 1000 IU powder and solvent for solution for infusion Human prothrombin
PACKAGE LEAFLET: INFORMATION FOR THE USER Octaplex 500 IU powder and solvent for solution for infusion Human prothrombin complex Octaplex 1000 IU powder and solvent for solution for infusion Human prothrombin
Table 1: In vitro reduction factor during Albumin (Human) 25% manufacturing
 Albumin (Human) 25% U.S. License No. 1646 Rx only DESCRIPTION Albumin (Human) 25% is a sterile, liquid preparation of albumin derived from large pools of human plasma. All units of human plasma used in
Albumin (Human) 25% U.S. License No. 1646 Rx only DESCRIPTION Albumin (Human) 25% is a sterile, liquid preparation of albumin derived from large pools of human plasma. All units of human plasma used in
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
FLEXBUMIN 5% Albumin (Human), USP, 5% Solution For intravenous use Initial U.S. Approval: 2005
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FLEXBUMIN 5% safely and effectively. See full prescribing information for FLEXBUMIN 5%. FLEXBUMIN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FLEXBUMIN 5% safely and effectively. See full prescribing information for FLEXBUMIN 5%. FLEXBUMIN
Recombinant Treatments for Bleeding Disorders. An overview of treatments that are considered to have a low risk of viral transmission
 Recombinant Treatments for Bleeding Disorders An overview of treatments that are considered to have a low risk of viral transmission History of bleeding disorder treatments Recombinant products: A significant
Recombinant Treatments for Bleeding Disorders An overview of treatments that are considered to have a low risk of viral transmission History of bleeding disorder treatments Recombinant products: A significant
PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS. File 1 DRAFT FOR CONSULTATION PURPOSES ONLY
 PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS File 1 DRAFT FOR CONSULTATION PURPOSES ONLY ANNEX I INFORMATION REQUIREMENTS A. INFORMATION TO BE PROVIDED TO DONORS 1. Accurate but generally
PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS File 1 DRAFT FOR CONSULTATION PURPOSES ONLY ANNEX I INFORMATION REQUIREMENTS A. INFORMATION TO BE PROVIDED TO DONORS 1. Accurate but generally
Structure and duties of Blood service
 NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
CAUTION: Refer to the Document Library for the most recent version of this document. Cryoprecipitate Transfusion Guideline for Practice.
 Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Human plasma protein 50 mg/ml of which at least 96% is IgG, with a content of antibodies to Hepatitis B virus surface antigen (HBs) of 50 IU/ml
 Hepatect CP 1. NAME OF THE MEDICINAL PRODUCT Hepatect CP 50 IU/ml; solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. Human plasma protein 50 mg/ml of which
Hepatect CP 1. NAME OF THE MEDICINAL PRODUCT Hepatect CP 50 IU/ml; solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human hepatitis B immunoglobulin. Human plasma protein 50 mg/ml of which
Increased Risk Donors for Organ Transplantation
 A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA - UPDATE
 DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA - UPDATE SoGAT Clinical Diagnostics III 12-13 January 2011, London Michael Chudy Julia Kreß Micha Nübling Paul-Ehrlich-Institut
DEVELOPMENT OF AN INTERNATIONAL REFERENCE PREPARATION FOR HEPATITIS D VIRUS RNA - UPDATE SoGAT Clinical Diagnostics III 12-13 January 2011, London Michael Chudy Julia Kreß Micha Nübling Paul-Ehrlich-Institut
Potential Use of Convalescent Plasma During a Flu Pandemic
 Potential Use of Convalescent Plasma During a Flu Pandemic Jay Epstein, MD U.S. Food and Drug Administration ICDRA 2008 Why Discuss Convalescent Plasma? H5N1 infection in Asia has killed 30-80% of patients
Potential Use of Convalescent Plasma During a Flu Pandemic Jay Epstein, MD U.S. Food and Drug Administration ICDRA 2008 Why Discuss Convalescent Plasma? H5N1 infection in Asia has killed 30-80% of patients
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
